ChC
Roil- 22101
AENCESPATNA
SCIEN
ALL INDIA INSTITUTEOF MEDICAL SCIENCES
PATNA
PRACTICAL MANUAL OF PHYSIOLOGY
EDITED BY:
Dr. Ramji Singh
Dr. Tribhuwan Kumar
Dr. Kamlesh Jha
Dr. Yogesh Kumar
INDEX
Name of Experiment
S.No.
Page No.
General Objective in Physiology
1
Aims of the Physiology Practical Course
Human Lab
1
2
EXAMINATION OF ARTERIAL PULSE
Measurement of Systemic Arterial Blood
4-7
8-12
Pressure
3.
To Study effect of posture and
exercise on
13-15
Blood Pressure
Clinical Physiology
1.
Clinical Examination : General Plan
17-21
2.
Examination of Cardiovascular System
22-26
3.
Examination of Respiratory System
27-30
4.
Examination of Abdomen
31-34
5
Examination of Nervous System
35-42
6
Examination of Cranial Nerves
43-48
7.
Colour Vision
49-51
8.
Perimetry
52-55
9
Normal Electrocardiogram and calculation of
mean electrical axis, duration & amplitude of
56-60
waves & intervals.
10.
Spirometry
11.
Demonstration of EEG, EMG, Nerve
Conduction Velocity, Visual, Sometosensory &
Auditory Evoked Potentials
61-64
65
Sign. of Teacher
Remarks
12
Human Experiments
66-70
13.
Study of Human Fatigue of Mosso's Ergograph
71-72
Haematology Lab
1
3
Compound Microscope
74-78
Equipment and Reagents
79-85
Collection of Blood Samples and Preparation of
86-89
Blood Films
4
ldentification of Various Blood Cells
90-92
5.
Differential Leucocyte Count
93-95
6.
Arneth Count
96-98
7.
Estimation of Haemoglobin
99-102
8.
To determine Blood Groups
103-106
9.
Bleeding and Clotting Time
107-109
10
RBC Counting by Hemocytometry
110-115
11.
To determine total WBC Count
116-119
12.
Absolute Eosinophil Count
120-122
13.
Platelet Count
123-126
14.
Reticulocyte Count
127-129
15.
Osmotic Fragility test of Red Blood Cells
130-132
16.
Haemin Crystals
133-135
17.
Determination of ESR and PCV
136-138
18.
Red Blood Indices
139-140
Experimental Lab
1
Introduction of Experimental Physiology
2.
Tostudy the apparatus and nomenclature
141
142-144
(including Physiograph).
3
To studySkeletal Muscle
145-159
5
To Demonstrate muscle Fatigue
160-162
To study effect of load &length on muscle
163-167
contraction free loaded &after loaded).
6
Tostudy of properties of Cardiac Muscles
168-182
7
To study Smooth muscle in Rabbit
183-188
Stethography
189-190
Nutrition & Dietetics principles for preparation
191-194
9.
of diet
10.
Glucose tolerance tests (Recorded Graph)
195-196
GENERAL OBJECTIVES IN PHYSIOLOGY
The ain ot the course is to develop basic understanding of the functions of the body and their
applications in
nN2n2rennent of patients and to develop skills in assessing the functions of systems of the hody and basic
clinical examination.
At the end of the course the students should be able to.
1 Describe the basic principles of homeostasis, water and
electrolyte balance, acid base balance, energy
balance and temperature regulation.
) Describe the role of various systems of the body, how
they function, the mechanisms that regulate
them and the factors that alter the functions.
3. Outline how pathological factors interfere with the
functions of these systems and how altered
functions of these systems cause disease.
A Describe the physiological basis of various tests used to
assess the functions of these systems and
interpret the results obtained.
5. Mention the names of common chemical agents that alter the
the mechanism of their actions.
functions of these systems and outline
6. Investigate blood for
haemoglobinconcentration, red cell count, white cell count, differential count,
bleeding time, clotting time, blood groups and packed cell volume.
7. Measure body fat, measure blood pressure, lung
volumes, pulmonary ventilation, concentration of
oxVgen and carbon dioxide in alveolar air, metabolic rate, body
temperature, urine flow and specific
gravity of urine
8. Feel arterial pulse and recognise rate, regularity and volume of the
pulse, identify normal heart sounds,
identify waves and intervals in normal E.C.G, record respiratory
perform
movements,
resuscitation and éxamine basic sensory and motor functions and special sensations. cardiorespiratory
9. Having attained the knowledge and skills mentioned above, the
student should view man as a whole
organism and not a collection of systems, apply the knowledge and skills in
understanding and
managing patient problems and keep on continued study of Physiology.
The teaching learning activities include lecture discussions, practical:
classes, tutorials and clinical
demonstrations. Lecture discussions will be delivered by the departmental staff where students are informed
of the topics well in time and are expected to read up based on the
objectives given to them at the beginning
of the course as a book. Practical classes will be conducted in the
laboratory with the aim of developing basic
clinical skills related to Physiology and to demonstrate important physiological principles. Tutorials will be
in
different forms such as free oral question-answer sessions, answer writing sessions, sessions for
students to
clear their doubts and so on as requested by the students.
Clinical demonstrations are conducted to illustrate clinical significance of pre-clinical learning by
bringing
selected patients from the Teaching Hospital or showing relevant video clips and demonstrating the clinical
application of the basic sciences at the end of each section. AIl these activities will be interactive
encouraging
student participation and performance instead of simple delivery of information. The Clinical Departments of
the Faculty will be conducting the clinical demonstrations and, if need arises,
consultants from the Teaching
Hospital will be invited as Visiting Lecturers. In addition, vide0 shows on functions of various systems are
Shown to illustrate their structure and function. Further, there will be formative evaluations at the
end of or
during the course of each section or system. The marks of in-course assessments conducted at the end of each
term will be given to students and the answers will be discussed with the students. The students are given
detailed objectives for the course in physiology and guides for each practical class developed by the
department as teaching material.
1
AIMS OF THE PHYSIOLOGY PRACTICAL COURSE
The students are expected to benefit from the practical classes in the following ways:
1. Learn and acquire skills.
2. Acquire an aptitude for careful observation.
3. Familiarise with nomograms.
4. Gain skillin designing simple experiments.
5. Familiarize with simple statistical concepts.
6. Gain skills in recording an experiments, tabulating and condensing data.
7. Learn to draw valid conclusions from available data.
8. Practice writing a report
9. Practice looking up, indexing, and abstracting journals and tracing the literature
references on a
particular subject.
10. Gain knowledge of concepts of validity, reliability, precision and errors in
measurements.
11. Supplement to oral classes.
12. Apply Physiological learning to health and community problems.
2
HUMAN LAB
3
Experiment-1
EXAMINATION OF ARTERIAL PULSE
The expansion of aorta due to sudden ejection of blood and its transmission in the
arterial system is known as
arterial pulse. This can be felt in superficially placed arteries such as carotid, subclavian, brachial, femoral.
popliteal, posterior tibial and Dorsalis pedis arteries.
Aim: To learn the technique of examining the radial pulse.
Procedure:
Radial pulse is best felt with the tips of three middle fingers slightly
compressing the vessel
the
underlying bone. The subject's arm should be semi-pronated and the wrist slightly flexed. Theagainst
following
observations should be made systematically.
a. Rate (normal 60-100 bpm)
. Rhythm (spacing between successive beats. Normal pulse is regular and
is known as sinus rhythm).
A. Volume (amplitude of the pulse wave or the excursion felt at
the wrist and depends upon stroke.
volume and compliance of arteries).
d. Character (best appreciatedby recording pulse tracings).- rgioa.
e Condition of vessel wall (normally not palpable)
f. Synchronicity: radioradial and radio femoral delay.
Tip: If the rhythm is regular and rate appears normal then multiply 30 seconds rate by 2.
But if
bradycardia or tachycardia is suspected then count for whole one minute.
Carotid
Systoik pek
Dicroticnotch
-Brachial
’dwavo
-Radial
reboLn
(dcubic)
Ulnar
Valvc
Femoral
-Popliteal
Diastolic
Eyectede
(Systolic Phase)
-Posterior tbial
-Dorsalis pedis
Refectedwe
(Diastolic Phase)
Dbservation:
S.
Left
Right
Comment
No
Rate
9bèats/in
Norma
Rhythm
Normal
Volume
Normal
4
Condition of vessel wall
Normal
(n papa blo).
Synchronicity
Synchvorows
chonws
No
nference:
ASsignment:
1. Examine Radial Pulse of the subject. What is
the correct
2. What are the
3. Discuss the
methodology?
precautions while examining radial pulse?
regulation of heart rate in brief? What are the causes of
physiological and pathological
bradycardia and tachycardia?
Ro+e- 88 beat|mn
4. Discuss the following (also learn to examine if
any):
a) Water hammer pulse
b) Pulsus paradoxus
c) Pulsus alterans
d) Pulsus bigeminus
e) Pulsus bisfereins
5. What is pulse deficit?
6. What is the basis of sinus
Charact
Ael
o delay
(R
arrhythmia?
7. What is the basis of thready and
the head
5
(R-F)
bounding pulse?
8. Discuss the above two diagrams. What could be the
What do you mean by PVD?
) By
nproning tha
Adequet
nor ma
importance of dorsalis pedis artery palpation?
vevel
1odponin
For 'bettd
the bone. T5e 30dial
the sadial oe
ecition
Sing
Precoion hìle
Subjed
odial
puloe
shate elax
Pulse
ote couned for on
fulse .bh side be eauid
idle 3
shalo be us ed.
senitfiene)
fge
inciego
coMpahed.
Under cct) 6 A-N:S.
Heart Tate
Tate inc: it
A:NS. Heort
ymheie
ith Inreng
eases
2 decr
porangmothetke ocivitg
Tochcoia- Heon vate
Physiclcg ical
moie than 100 beat
p0 miat.
pothelogical
Beri Beri
Ange Afer eating
Pr
shocK:
Hea
exoycond a- Hooq
ote < 60 beat nin
Potogical
- Atheietes
feon
py
aorhie vgugtin colepsing puda
-(riey
-Obst aund
- Hean' blOCK
aer hommar pulse - Called
Choiocterie d by
PuJse ave
p'd upsfohe 2 clon stke
Dicroie Noch i<
Puse aleans Puloe
(b) pulge
dec
6
parodox - Ms nomer - This is an
ne nomal phanomerorn
ph
appsedak
Gcresitqta
affiult to
lo) uls) bisfetim) (onbn'
palae
b
lean
a
than
foe
defie
phont
st
- Diff b? puae Tae
NeNmaly. e pulst defict
prbnt
sinus
Constentsy changys Sirh ieapr
VanoB o heag vat in
Pule
att
Th ea dy pulee- A
puceptibie
sapa Upube that foclssavcely
ige
a
fine Mcblle thread
Under a
-
frcetll harbat
stog thaatbig pulae due fo a
t hoo a proncunced pulotion that does t eeoily
disappear sith prene
Jmpopnae f Dorsas peclis atfey palpaion i
dorealis pedis
comoy
fo evaluate the pespheal agokie iseoe ice
th onboagit
ob|tegnc.
Peipheal vascula dicea
rwlao
causcd
due
o noo0blo ckas
Spasn in a blood venele,.
7
Experiment-2
Measurement of Systemic Arterial Blood Pressure
Systemic arterial blood pressure can be either end
or
pressure that can be measured onl by invasive Dressure lateral pressure. End pressure is the pertusion
method and so is not suitable for routine
Lateral pressure is the pressure exerted by a column of
measurement.
on the lateral wall of the artery. It
can be
measured indirectly by different methods. In auscultatorvblood
method the observer listens to the korotkoffs
Sounds with a stethoscope and
observes the pressure reading on the
palpatory method the pressure simultaneously
In
at which the pulse annears is
sphygmomanometer.
noted. Oscillometric method
is used in semi
automated and automated devices which detects the
oscillations of amplitude of blood pressure acting on the
arterial wall.
Blood pressure measurement by auscultatory
method with the help of mercury
considered gold standard but its use in clinic setting
is
sphygmomanometer
is being discouraged owing to
of environmental
mercury toxicity. Aneroid
concerns
is being encouraged in clinic
sphygmomanometer
to error and needs frequent
settings but they are susceptible
Loss of calibration occurs
calibration.
roughly and so they need
especially
when the instrument is handled
calibration every two to four weeks.
The blood pressure is usually
as systolic BP/ diastolic BP and
the unit is millimetres of mercury.
Systolic blood pressure (SBP) expressed
is the maximum pressure
during
systole
which can be estimated by the
appearance of korotkoffs sound in auscultatory method and
appearance of pulse in palpatory metnod.
Diastolic blood pressure (DBP).is the minimum
method. Pulse pressure (SBP-DBP) and mean pressure during diastole. It cannot be measured by palpatory
arterial pressure (1/3SBP + 2/3DBP or DBP + 1/3
can be calculated from SBP and
pulse pressure)
DBP.
Aim: To record the Blood
Pressure (BP) of an adult human subject.
The sphygmomanometer:
It consists of an
inflatable rubber tube (or the cuff) connected
through two
(a) A hand air pump
the cuff;
(b)
which can be, by adjusting knob be used either totubings to:
pump air into or release air from
Amercury
manometer (or a spring gauge) that can be read upto 300 mm Hg
The cuff is enclosed in a cloth jacket.
pressure.
Those used in adults should measure 12.5
x 23 cm. The width of
the cuff should be 40% of upper arm
circumference.
Procedure:
(A) Palpatory method:
1.
Ensure that the subject is sitting or lying
comfortably. His arm should be extended and all clothing
removed from it.
2. Tie the cuff on the arm taking
care that:
(a) It is about 3 cm above the cubital fossa;
(b) The brachial artery come under the
middle of the cuff
(c) The tubings are directed inferiorly
(d) The cuff tied tightly, if loose then BP is
falsely recorded high.
3.
Place the manometer at the level of the heart.
4. Palpate the radial artery and inflate the
cuff to apressure about 30 mm above the level at
which the
radial pulse disappears.
8
5 Slowly release the pressure in the cull while continuing to palpate the artery. Note the pressure at
which the pulse reappears.
" This is the systolic pressure as recorded by the palpatory method.
6. Release the pressure in the cuff completely.
3) Auscultatory method
7. Repeat above 4
S. Place the diaphragm of the stethoscope over the brachial artery in the cubital fossa. No sound can be
heard through it now.
9. Slowly release the pressure in the cuff as in step 5 above. Note the pressure at which regular tapping
sounds appear.
This is the Systalic. BP as recorded by the auscultatory method.
10. Continue lowering the pressure in.the cuff.tillL the sound beçome mufled Qr disappears altogether.
Note the pressure
This is Diastolic pressure
Release the pressure in the cuff completely and untie the cuff.
Record the BP in other arm.
ps:
Hold the manometer vertically. (Why?)
Read both systolic and diastolic to nearest 2 mm Hg reading.
Record BP of both arms. Higher reading is reported.
Alcohol, caffeine, tea, smoking etc is stopped 30-45 min before recording(can you suggest why?)
Room should be quite and comfortable. Subject should ideally rest/relax for 5-10 minutes before
recording is done.
Tight clothing over recording arm should be avoided and arm should be at mid chest level.
(Why?)
Pulse pressure(SBP-DBP) indicates volume of pulse
Mean arterial pressure (DBP+1/3 PP) is the average pressure throughout the cardiac cycle.
ommon sources of error:
Inaccurate cuff size and its application
Arm position
Rest period before measurement
Inilation/deflation method
Lack of repeated measures
Time interval between repeated measures
Lack of calibration/maintenance of BP instrument
Body position
Quality of stethoscope
9
Assignment:
1. Record the Blood Pressure by Palpatory and Auscultatory method.
2. What are the precautions while recording Blood Pressure?
3. Joint NationalCommittee Classification (UNCC)of blood pressure and hypertention:
SBP(mm Hg)
DBP(mm Hg)
Normal
<120
<80
Pre HTN
120-139
80-89
Grade I
140-159
90-99
Grade lI
>160
2100
BP classification
HTN
Learn the above table and discuss about BP regulation and factors effecting BP in brief ?
4. Discuss pathophysiology of hypertension. What is meant by essential and secondary
hypertension?
5. Do youthink posture effects the readings? If yes then
justify?
6. What do you mean by
auscultatory gap?
7. Why mercury is used in instrument used for recording BP? Disuss
about the apparatus.
What are the advantages and disadvantages of palpatory and
auscultatory method?
106
62 Mhh
104
108
64
Pulse - 88
ralog - 110
10
2T0
nethod- |O mm
mm
vethod -
)) Piecauion'
"
80 M
.
SUbjet sit comfoaby 5 in before
Aogishoto
L should
be
|orel
Cuff sho.
be
af hegn level
hould be checke
be tiao tro tght1
Long
erm
Hormonal
Chemoreceptor
Boreveeptoy
leflex
too
qe in ftuid
’nt in "aotc
Carofd bedy
’Res
pond to chong
fo chem
cal onpeit
arch
’Ropond to
Pree toad
’ Catechanes
tooistone
ayocordd al Conrofiiy
9Pthophyido
tyerension - Suspainad elovatien.
eons
auog yer tension veons
Classitied
ise in diotie vo|
emenfal 2 seconda
commonet
Lbdue to herid
11
’ Renin- Agioeni
End dfastolie v)?
After load :- Peripherale
Hea9 vate
’
Vo sopr ein
ay to a discant
el sethe/e in the bacy
e
On imedioe
o
by bayoYeceptoY
-Jn
ofte
the
so Und
supit.?ocpoaijien
due t0
3eflex
evply hypert enive
ppear ance
isappean
The peicd
(here
se
mal
i
the
bel o
point
le vet
temox
Palpaoyethod A
|) 94 fves
20 ny
premuse
cæsappeq1ane is calleo
sPhygmonmonomee
Can
poient
ue to i
,.
easonabl.
vey iyh prema
Megtitt.
Disadantae- (i giaie prente Can b deenco
(i) &yotole pren. vecoided is nq occunate
(2- s
enttan aay
scullatory method
Aevanfa-Dyectt diatic p1en. aleo
Accusafe value
Unien folowo by palpaoy wath
pisodvanagethe auscu
12
Experiment-3
To study effect of posture and exercise on Blood Pressure
lim: To Study the effect of posture and exercise on BP.
rocedure:
Allow the subject to lie supine on the examination couch for a few minutes.
Record his BP with a sphygmonmanometer. Do not untie the cuff.
Ask the subject to stand straight and record his BP immediately.
Ask the subject to keep standing for 5 minutes. Record the BP again in the standing position.
Ask the subject to do any form of moderate exercise for about 5 minutes.
Record the BP again in standing
position.
Record the BP in the standing position every 5 minutes till it comes down to normal level.
Assignment:
Observe the changes in the Blood Pressure of the subject before and after exercise.
Observe the changes in the Blood Pressure of the subject in different postures.
: What is the effect of gravity on BP? What will happen if a person stands still for long hours? Discuss about
acute regulation of blood pressure?
SBP-DBP
. What is the effect of exercise on blood pressure?
DBP,PP
. What is orthostatic hypotension?
Pulse
Rate
Systolic |oiast. Pulse
BP.
BP.
Preo
" Supine (^51n
Stondr
meiae
(iS se)
72
100
fter 2mi,
i6
5in
9
30
36
33
78
50
140
9
|16
G94
13
68
|06
3
rgmmeite
Aftes 2min
34
.
74
supia
76
36
60
50
80
65
45
68
66
23
A:
Prem
affect
3
BP.
e to 9-
blorol Colusa.
-d
peren joring for oo log,ob out SoDCasdiac
bioed poels in
R BP toD.
Bovevecepter
incr
yeguloe gP
o
hetþs
aptem
ex
ef
vefkx
vap Onfition
e
caaia
tochy
oput,
cord'ae olput, tochycadia
Othastote Hypoenatn
Phenomena
sg
pearin
of2 Gtorig
blo od moves to loe poy h
veno o etum,
Atso
14|
ce 1led PesBue al
pjensn
B.p.
CLINICAL
PHYSIOLOGY
16
Experiment-1
1. Clinical examination: General Plan
General Principles of clinicalexamination:
medical world the principle that applies ubiquitously is no two
patients are alike!' We the care
eivers therefore, need to practice utmost care in making a
conclusion about any case or scenario.
Adequate assessnment of a patient requires a disciplined
approach. It entails the following two steps in
In
equal measure:
Acquisition of data: This consists of:
" Taking of a detailed yet precise history
"Complete physical examination
"Use of special investigations to reach to a conclusion
" Recording the data and preserving it.
" Processing of this data into a form which allows:
" Drawing conclusion
" Formulation of a differential diagnosis
" Constructing management Plan
" Meaningfulcommunication.
History Taking:
History taking is an art which is a dynamic ongoing process. A
detailed and intelligently-gathered
history is of vital importance in determining the presence of illness, in
assessing its severity, in taking a
diagnosis and in determining the importance of other factors which may
influence the patient's
response to both the illness and its treatment. It should be elaborate yet precise but
not to be based
upon a stereo type format. For beginners a reasonable formàt to start
with could be as follows
1. Back ground information: relates with
patients background information such as their name, age,
marital status, occupation and where they live.
2. Presenting complaints: The patients should be
specifically asked for the problems which compelled
them to see a doctor. It should be recorded in
chronological order.
3. History of Presenting complaint: A brief history of each
complaint regarding its origin, severity,
relieving and aggravating factors etc. should be recorded preferably in the
patient's language rather
than medical jargon.
4. Past history: In this head all the significant past medical
history should be recorded specially
regarding chronic illnesses like diabetes, hypertension, tuberculosis, cardiac condition etc.
5. Personal and social history: In this head history related to the
personal habits, addiction, allergic
condition, bowel and bladder habits, work conditions of the subjects etc is recorded.
6. Family history: History regarding the important clinical
conditions prevailing or prevailed in the
patients family is sought for to figure out any genetic, environmental or stress related condition that
could be having some cause-effect relation with the patient's condition.
7. Treatment history: here one records the details of the
treatment, patient received for his
past/present illnesses. Any particular episode of drug allergy in the past could also be recorded in
this section.
17
GENERAL EXAMINATION
After taking
CAmiation
a detailed
history, the next step in data acquisition is the overall survey and general
of the subject to eet aeross idea about his clinical
condition and the direction of future
pan. The moment a doctor walks to the patient or the patient walks to the
doctor, the general
examination starts and it ends till the patient walks away. It is a continuous process
of the general
diagnostic/ management
Pre-requisite
examination:
Before examining the patient one must
"
"
"
You have introduced
ensure following things
yourself to the patient.
You have taken consent for
examination.
Privacyand dignityof the patient is
maintained.
" The part to be
examined is exposed properly.
"Enough light preferably natural dav light is
available for examination.
" The process of
examination should not make the
"If the patient is female, she
should be
relative
of the
patient.
After ensuring ali the
way-.
patient uncomfortable.
examined in presence of a female attendant,
pre-requisites the patient's general examination could be done
1.
a nurse or a
in the
following
General survey:
section one grossly examines the patient for
some obvious condition or
following points recorded in general survey
anomaly. Generally
In this
" Overall
appearance and mental status (Orientation in time
"
Attitude and behaviour
"
Built and nutrition
"
Posture and gait
"
Height and weight
2.
Vitals:
" Pulse
" Temperature
" Blood Pressure
" Respiration
18
placeand person)
3.
General exanmination Proper:
Placeswhere sought for
Signs
Pallor
conjunctiva (balpebral) nail bed, skin (Palm), Tongue
Cyanosis
Tip of nose, ear lobes, tongue base, lips, skin andnail bed
Clubbipg
Anemic
nail base (see for Schmaroth's window test)
nauncIC, hen ochy ornatela
Icterus
conjunctiva (Bulbar), Tongue, nail bed, Plam
Oedema
shin of tibia, medial malleolus, sacrum (bed ridden patients),dovsurn
Lymph nodes: Submental, Sub-mandibular,pre& post auricular, axilary, supra-clavicular, inguinal and
popliteal lymph nodes.
JVP
Neck
Trachea/ thyroid: Neck
Skin, hair and nail
Breast and genitals (where needed)
All four limbs
Instead of being stereotypic one should adapt a rational' approach while examining. For example one
may proceed from head to toe or toe to head examining various parts of body for requisite information
and then record systematically in the due format. While expressing the findings of general examination
one should present the positive findings and only significant negative findings rather than following a
stereotype pattern.
Once generalexamination is completed one gets a crude idea which system is principally involved and
responsible for the patient's morbidity. By performing the detailed examination of that particular
system and brief review of rest of the systems, one can reach to the stage of provisional diagnosis.
Assignments:
19
borit
Experiment-2
2. Examination of Cardiovascular System
he examination of cardiovascular svstem is of paramount importance in the overall clinical examination.
nong hon-conmmunicable diseases. cardio-vascular disorders are responsible tor hignest numDer of
mortalities and morbidities
in the world.
Examination of cardio-vascular system includes
1.Examination of the arterial pulse
2. Recording of systemic arterial blood
pressure
3.
4.
Assessment of jugular venous pressure and
Examination of the precordium which includes
a.
Inspection
b.
Palpation
C.
d.
Percussion and
Auscultation
Pre-requisite of CVS examination:
he patient should be seated, leaning back to 45°, supported by pillows, with their chest, arms, and ankles (i
appropriate) exposed. Their head should be well supported. In general, the patient should be examined from
right side.
The details of the examination of the arterial pulse and recording of systemic
arterial blood pressure are
discussed in respective sections.
Assessment of jugular venous pressure (JVP
The centre of the right atrium lies 75 cm below the sternal
angle, which is used as the reference point.
The normal JVP is <8 cm of blood (therefore 3 cm
above the
sternal angle). When the JVP is raised it is the vertical distance
from the sternal angle to the upper border of the pulsation
that must be measured.You must add 5 cm to the figure to get
the true JVP.
The pulsation in the internal jugular vein can be seen between
the two heads of the origin of sternocleidomastoid muscle just
above the clavicle when the patient is reclining at 45.This,
therefore, is the position for normal JVP. When the JVP is raised the pulsation rises along the course of
internal jugular vein, which lies along the sternocleidomastoid muscle, and maygo up to the mastoid process.
The venous pulsation should not be confused with any arterial pulsation in that area, remembering that the
venous pulse:
Have 2 or 3 small waves per cardiac cycle.
Better seen than felt.
Moves up & down with respiration.
22
xaminationof thc precordium
nspectio Notable pontsto observe includes
Shavg and symmet1y of the chest.
Apialimpulse.
Any other visible pulsation over precordium,
Prominent veins on the chest wal|
Any sca matk and venous prominence.
Any skeletal defornmity.
Palpation) Before starting the examination, explain what you are going to do and how you will do it,
Daticularly to female patients.f possible, warm your hands by rubbing both hands. By palpation ascertain
ollowing points
Confirm the findings of inspection like shape, symmetry, position of apex beat, presence of sca, and
visible pulsation and its location etc.
" Feel for temperature and texture of the skin
Apex beat: This is the lowermost lateral point at which a definite pulsation can be felt. It is usually at
the fifth ntercostal space medial to the mid-clavicular line.
Thrill: Thrill is the palpable murmur which is an abnormal sound produced at one or more cardiac
valve.
Percuasion: This is not useful and usually not included in the cardiovascular examination. If ever performed, it
Sused to delineate the boundary of cardiac Mness:
Auscultation; This is performed by means of a stethoscope. The bell of the stethoscope is used to detect
ower-pitched sounds; the diaphragm, for higher-pitched sounds.
The four areas for auscultation
Mitral:5th intercostalspace in the mid-axillary line (corresponds to the apex beat)
Tritusnid :5th intercostal space at the left sternal edge
Pulmonary: second intercostal space at the left sternal edge
Aortic,: second intercostal space at the rightt sternal edge
By auscultation note for
" The intensity of the first (S1) and the second (S2) heart sounds;
Any splitting of S1 and S2;
" Athird (S3) heart sounds (if present).
" Extrasounds during systole and diastole
Murmurs(systolic and diastolic)
" Bruits
Normally two heart sound are heard by stethoscope. The first heart sound is produced due to vibrations
caused by closure of the bicuspid and tricuspid valves. It is a low pitched soft sound that coincides with the
carotid pulse peak.The second heart sound is produced due to closure of semilunar valves and is a high
pitched sharp sound.
23
Physiological splitting of the second heart sound may be heard in fit young adults during inspiration
due to decreased intrathoracic pressure during inspiration.
.
Review of other systems:
This includes briefoverview of other systems including respiratory system especially auscultation at lung
bases, abdomen for any pulsation, varicosity and organomegaly and peripheral parts foroedema,
lymphadenopathy and varicose vein etc.
Assignments:
1. Examine Cardiovascular System of the subject.
2. Discuss the events in cardiac cycle diagrammatically?
3. What is the physiological basis of splitting of heart sounds?
4. How do you distinguish arterialand venous pulsations? What are the events in JVP?
S. What is a murmur? What are their types? What are the grades of murmur? Can a murmur be also
physiological?
2) Events in
cordiac
in velation sith ventHce
i) Slo
() Abial
(iv) 9sovofumic
conrotion
O.25
Papid yectio
Vs
slod yeci
Vi rovolumeic
Geneuoly
) Joint diasore
i) Arial
Relaxa ir
(cindde wj
veniculan diatrts)
(3) znd heog sound is due to clasue oh sem÷ lunas valve
Inspiatin in shich venns
24
valve
evente
Dapid, eet
Prescure in
Jug laz
vein
vefte
that in
Jsovolua7e
contio cion
DOwONad displocement h AV valve
C
vein due
AV rolve
blood in
V occumulatic)
doonord displocement t
(5)
vave)
reploca te
a souod
Mu ymur isCoriac
that
sOUnd.
are
TRey
couyd
turbullent fo
Vavulan
mUrmus- when
haa ot is empty
heat
) Cntinds muYMwn
aydible
M) loud but occom poieo
I)
uzmuY
to
25
Can be
tiblent
phyp ciefcal (pegnan
raso
Cane
leyect
Murmw- ohen
’
urent witin he
eddy
is
f0
murnwt
Experiment-3
3.Examination of Respiratory System
nhvsician resots to the detailed exanmination of respiratory system if the subjet complaints are
tive of repiratory ailnent e.g the patients may complain about breathlessness, cOugh with or without
yation dyspnoea on exertion, chest pain etc. along with
nonspecific presentations like fever, rigor,
woakessetc
SNeas exanination of cardiovascula system, examination of respiratory system incudes:
.General assessment
2.Jnspection
5.Palpation
4. Percussion
Auscultation
re-reouisite: the subjects with suspected respiratory ailments are usually examined in seating position with
erect posture with sufficient exposure up to the waist.
General assessment: The general assessment tor suspected respiratory system ailment is more or less same as
hat of cardio-vascular system because of close relation between the twO systems. In general it
includes
ssessment for built and nutrition, oedema, features suggestive of chronic hypoxia like cyanosis, clubbing,
enous engorgement etc.
nspection this includes
Shape of chest- usually it is ellipticalin normal individual. Subjects with chronic hypoxic conditions.may
present with harrelshaned chest.
Symmety
Movement of the chest with respiration, whether.it is symmetricalor not.
Intercostals spaces- for crowding of ribs, any venous prominence or scar.marks etc.
Any visible pulsation or unusual swelling over chest wall.
atpation:
Here findings of the inspection are confirmed. Measurement of antero-posterior and transverse
diameter is done and its ratio is ascertained.
Movement of chest.
Expansion with deep inspiration.
Position of trachea
Position of apex
b e a t tPruthyX
"factile Vocal Fremitus. Ulnar border of the hand is placed over the chest of the subject, along the
intercostal spaces. Subject is asked to repeat words like 1-2-3 or 99 and the intensity of the vibrations
telt is compared alternately in the corresponding areas of thetwo sides over chest wall which should
setalv
normally be equal.
ercusston Note by percussion the degree of resonance in different areas of the chest. Place the middle
nger of the left hand firmly on the part which is to be percussed and strike it with the tip of the middle finger
of the right hand (the pleximeter finger). Note the resonance.
egin in front, by tapping lightly and directly (that is without the pleximeter finger) on the most prominent
point of each clavicle and proceed downwards as indicated above. Thereafter percuss the back (supra-inter
and infra-scapular areas) and the axilary areas.
27
Note for
Hepatic dullness
Cardiac dullness
Other dullness
Auscultation
By Auscultation note the
ofstethoscope,
VOcal resonance- it is analogous to the vocal fermitus done with the help
Breath sound: Normal breath sound could be
Bronhial: heard over larger airway, and
> Vesicular heard over smaller airway all overchest wall.
Abnormal breath sounds are called adventitious or added sound. These are
Wheezes- these are sonorous or musical breath sound produced due to narrowing of airway.
Crepts or rales or crackles- Crackles result from peripheral airways collapse on expiration. Air rapidly
enters these distal airways on inspiration, and the alveoli and small brÓnchi abruptly open, producing
the crackling noise.
> Bronchophony/ aegophony/ Whispring pectoriloquy- these are distorted bronchial resonance sounds
caused by some pathology in the underlying lung area.
Assignments:
1. Examine Respiratory System of the subject. What are the signs and symptoms of respirator
diseases ?
-2. Discuss the following:
i. Barrel shaped chest
ii.
Pes excavatum
ii. Pes carinatum
iv. Harrisons sulcus
V. Ricketic/ascorbatic rosary
vi. Assyrmetrical/flat chest
3.
What is tactile and friction fremitus?
4. What are various types of percussion notes and their importance?
5
Discuss the physiological basis of breath sounds. What is the pathophysiological basis of adde
sounds?
6. Discuss in brief about regulation of respiration. What is the effect of respiration on cardiovascula
system?
7. Discuss the following:
Tidal percussion
ii.
Respiratory failure
ii. Obstructive and restrictive lung diseases
iv.
Silent chest
V.
Tachypnea, Bradypnea, Hyper/hypopnea
vi. Apnea/obstructive sleep apnea/orthopnea
vii. Paroxysmal Nocturnal Dyspnea(PND)
vii. Pulmonary edema and cardiac failure
28
)
c) Breoth
a) Chrone
() Bo)el shoped Che
pi)
.
bre oth out due to
to
Pes
exva um
blopd
inflae
los
Ches
Funne
de frevin a,uhcti
CQs10- ChondMal
prornient
D ceficod
as
Casto chondr al
i
Boaded appoatonce ot
dseose
Assmktical Jflat Chet
cheot - at Unilaeo
expa
boh s)de
|3) Toc4iie fienitey- voice
Vibrat on palpajed
chest oll due to
dee eoeD vibvonis indica ti n h
2
'nc vibratin indi cateo
hyperingk tiH
ypcflacaiet
- vibyoion duè to foiction bJ
Friction
Viseoiely
plewa
be due to inflamotion
percumlCn
la) Dul -
Pleurol effoin
- Pleur al
(b) Resoront
(c)
4hcrentg
Normal inftoo
"gperveonont
pheumohoyax,
(5) Bresth sound- doe to
pomeg
i) Tochealsourd - Harsh (ar
ben boon 0ut
i) BrDnchial sou d Lad 2 igh
S Ppe)
pitchd (lentracheac
hovsh tonAcuo
(in) Bronchioveoiculos soud- scf tes ttan bronchil
|iv) Vesiulos s ouud
29
that
((halo)
rorne
Than
bieag
disockr
auy
hore
Breatherý
In
whe fue even
asthamg
severe
Obstsuction
-Tebesculo
Cart -Fibross,
fuly expano
yreplApneaeNo
a
30
Vi)
PYpereprea
Bxodypnsa
Tachyprea
cve Peern anflo.
chetsilent
-Pophye lu-G
Blo02 fls
kae cloesn
or Cragh
uch
ncfve
(v)
\v)
Rest
toD
(im
(n
Reopivatoy
Lay
Oveg
ml
uni
nor
}
lug hypeeo
du00 become
he don Percun
baCk
opens
pehca)OY
h laps Cxocklos
col (b)
(o
DhozesPothological-
Experiment-4
A. Examination of Abdomen
mination of abdomen is one of the most intomative tools for clinicians to
clinch
orious ongan systems. The general scheme of theabdominal examination consists of. the diagnoses related to
" General examination
Inspection
Palpation
Percussion
Auscultation
Pre-requisite for abdominalexanination:
Abdomen should be exposed adequately before examination.
Clinically abdomen is usually divided into nine regions by two
lateral vertical planes and two horizontal
planes.
Patient is examined in the supine position with legs flexed.
The patient is usually examined from right side the i.e. the
patient.
physician should stand on the right side of the
Before touching the patient one should make sure his
hands are not too cold/ wet especially in winter
As far as possible the subject should be
asked to empty his/her bladder before
season.
examination.
region
Epigastric
Quadrant quadrant
Hypochondrac
Subcoslal line
Right
Laleral abdonitial
region
Umbillcal
Right
upper
Lelt
upper
Left
Joner
oer
quadran! quadrant
region
reglon
Intertubercular
Inguinal region
Midclavidsar
ling
nspection:
Dok for shape,
symmetry, umbilicus, movement pattern, any veins engorgement (prominent vein) and
visible
ulsations, any visible mass, linear white or pink marks which
indicate recent stretching e.g. pregnancy,
Scites, wasting etc.
alpation: (superficial and deep palpation): Subject should lie flat on his back,
breathe quietlyand his face
hould be turned to opposite side.
Dn superficial palpation the findings of the inspection is
confirmed. Normal palpation is pain less and gives as
astic or dough feeling. Assess for any superficial subcutaneous mass, underlying
inflammatory conditions
E. Palpate all the quadrants. On deep palpation Palpate the kidney on both sides (called
ballottement)and
roro Tinding(usually not palpable), palpate spleen and record findings and also examine for
palpable liver.
ermination includes its extent of enlargement, modularity, tenderness and its movement with
respiration.
31
Percussion:
there is dulilness.
ympanC sound all over abdomen eNceDt iver area where
Find boundaries of
Liver:
Spleen:
cavity.
of fluid (Ascites) in peritoneal
provides confirmation of the presence
percussion tenderness of kidneys on costovertebral angle.
Clinically percussion
Asses
Auscultation: Auscultate for
Bowel sound- normal 1-3/minute
Borborygmi - increased bowel sound
Bruits and friction rub
Examination of bladder (only if distended above the pubic symphysis).
Learn the following tips:
instead of asking for it. Also nc
LoOk tor any signs of discomfort or agony on patients face while examining
the abdominal area where on examination subject winces.
Assignments:
what are the structu
1. Examine Abdomen of the subiect. What are various quadrants of abdomen and
related to them. Discuss various signs and symptoms of abdominal diseases?
2. How toexamine the presence of fluid in abdomen?
hepatospleenomegaly.
3. What is hepatospleenomegaly? Enumerate some common causes of
4. Discuss about mesenteric circulation. Discuss about portal hypertension.
5 Learn the physiological roles of: Liver, Kidney, Spleen, Gall bladder and Pancreas.
4-quadront R-
ppe
R- lower
L- wppe, L- (ower
Snepectiong shope - Noimal, flat
Unbiicus.
Roud 2 inver ed
Abdonénal
moveMou - normal
Pulsoion
o pulsoicn
Dialajed vein ’
Gnt
Peis}alois - No cbs erved
Sufoce A SKin- b01mal, swooth
Scor
Hetnia
32
ND
Heunia
een
Palpo
Superficial ;
Temp- omal
Mbsent
Ojuording
Deep :
bsent
Liver Noy polpable
Spleen:
FlidUthvil bsent
dulloem
Per culo
on his bocc
Subje's
Ask subjecq to lie
a
b. Ploce
ulnar
C: PlacQ
flot
d.
Ueing
border
nidline
. band
your |eft
hano on
eight hard, tap
the
flid thil or
side 6
elageent
nfection - hepoie
)
umbas
left hano
Jave is fet
Hepatospleenon egog
Both Liver
2 splecn
CouDeS
ett lu mban
cide
sep sio
b) Chronic lvor ciseage oth potal
ypeeget
c) HIV
(4
Megeic icu? efe
to the vasulotnl
SMall meseie aenig form extensive vancuoture
b) Portal h
ponee
veLn
33|
venos
-hat leads
elevaled
to vel
prenune
tr
*Lver
Plosea pfein Sgr4h
-oter exereton
Splecn- quaity cen)
" Panceay
34
nsuliin
bleoel
Experiment-5
5. Examination of Nervous System
alient features:
" General survey and mental status
Examination of sensory system
. Examination of Motor System
Examination of cranialnerves
Examination of reflexes
eneral survey and mental status:
e subject is surveyed forgeneral mental status including attitude, behaviour, and consciousnes, short term
he long term memory, orientation with time, place and person etc.
Kamination of Sensory system:
ensory system is examined in the following ways
actile localization:
subiect with closed eyes is instructed to respond to the touch by raising his finger or counting
ontaneously. He is than touched with a wisp of cotton asymmetrically considering dermatomes on both
des of body
vise him to report any other sensation (beside touch) which he might feel during the course of the test.
uch the skin surfaces directly, avoiding the hairs.
ctile discrimination:
Duch the patient with the point of a blunt divider, initial keeping the points close together and thereafter,
parating them progressively. Each time ask the patient whether he is being touched at one or two points.
st the different areas of the body systematically.
roprioception:
ex the finger (or toe) of the subject and ask him to subsequently refer to the position of the finger (or toe) as
lown". Similarly demonstrate the extended position of the joints as "Up". Ask the subject to close his eyes.
andomly position his finger (or toe) in the extended or the flexed position andask the subject which way (up
down) has it been positioned.
the subject to keep his eyes closed. Hold one of his hands and move it in various directions through the
finally leaving it in some definite position. (Take care not to leave it iin a position where it touches any part
the body). Ask the subject to put the other limb in a similar position.
the subject to stand with his feet close together and then to close his eyes.
he cannot upright steadily after closing his eyes as in sensory ataxia, then the Romberg's sign is positive.
nesthesia:
the subject to close his eyes. Gradually move a digit or a limb to a newposition, and ask him to inform you
moment he perceives that some movement has Occurred.
Kinesthesia is impaired, the patient will fail to appreciate movements less than 10 degrees at the joints.
ereognosis:
tne subject to close his eyes and place in his palm,object of the some shape but different sizes, e.g. two
atch sticks of different lengths. Ask the subject to say which one is longer.
tne subject to decipher some familiar object such as a coin place on his palm, keeping his eyes closed. In
ereognosis, he fails to do so or does it wrongly.
35
Vibration:
Place the stem of
tuning fork (128 cps) on some superficial bone, e.g. the shin, and ask the
whether he feels thevibrating
sub
vibrations.
Superficial pain:
Prck the patient with sharp pin and ask him if
he/she feels the pain.
Deep pain:
Squeeze amuscle and ask the subject if he feels
the pain.
Varying degrees
of
Temperature:
rm ztest
pain, ranging
from no pain to intense, excessive pain may be felt.
tubes, one with hot and other with cold water.
Touch them in turn to the part oT tne subject to
if he feels hot or
cold.
If the thermal sensibility is
affected. the patient mayfail to distinguish hot and cold.
Tips: Always take the
subject in confidence and demonstrate what you plan to do, unless required the subj
should be instructed to close the eves. For
vibration use 128 Hz tuning fork.
tested and ask him
Assignment:
I. EXamine the Sensory System of the subject.
Discuss the cardinal
What is a
2.
dermatome?
3.. Discuss about
4.
ascending tracts. What are features
neurological signs and symptoms?
of posterior column
disease?
Discuss the pathways involving:
Superficial and deep touch
Vibration
Position
Superficial and deep pain
Temperature
5. What is sensory cortex? What are the
features of its involvement?
6. Learn the following:
Stereognosis, Graphesthesia.
7. What are features of:
Spinal shock
Complete and incomplete transaction of spinal cord.
O Tacfile sencivitT
Face
U linb
L linb
L
R
TnK
R
L
fine touch
6) CTde touch
OTocile localisotim
Tacile diccriminatin
Pain
senstiig
Supeuf pat
Dup
36
pain
Ulnor borde
|3cm
Sntersapula
=3.9cm
Thermal
ensQ
sernootoceon)
vibyotin
sense
<eegneeie(20.)
be
Cts
37
Examination of Motor System
In the examination ot motor system, the nmuscle are examined under following heads
Nutrition
Muscle Tone
MusclePower
Reflexes
Co-ordination
Nutrition (Bulk of the muscle):
Inspect the muscles and note any difference in the bulk of the muscles on the two sides. Ameasurins
may be used to measure the girth of the limbs. Palpate the muscle to note any abnormal hardne
flabbiness.
Tone of muscle:
Flex and extend the limb joints of the subject (passive flexion and extension). The resistance felt reflecte
tone of the muscles (of the extensors and flexor respectively).
Power (Strength of the muscle):
Ask the subject to perform a movement (involving a single or synergistic
group of muscles) while you ar
resistance to the movement. As youdo so, assess the strength of the muscle.
Application of the resistance may not be required if the muscle is too weak or if the movement instruc
itself requires a lot of strength. In these cases, mere observation is adequate.
Muscle power grades:
Grade
O= no contraction
1= Flicker, trace af contraction
2= Active movement with gravity eliminated
3 = Active movement against gravity
4 =Active movement against gravity and some
resistance
5= Active movement against gravity and full resistance(normal strength)
Test the strength of the following muscles:
Abductor pollicis brevis;
Opponenspolicis;
Interossei &lumbricals;
Flexors and extensors of wrist and fingers;
Biceps, triceps &brachioradialis;
Pectoralis, serratus anterior & L. dorsi;
Flexors and extensors of knee:
Extensors, flexors, adductors &abductors of thigh.
38
following
learnthe
tables of spinal segments enumeration:
Upperextremity
C5, C6
Deltoid, infraspinatus, biceps and brachioradialis. Decreased
biceps and brachioradialis
reflexes.Sensory symptoms or loss in
deltoid area or thumb.
Weakness of the triceps, pronator teres, wrist and finger
extensor muscles. Decreased triceps reflex. Sensory
symptoms or loss in the middle
finger.
C7
Weakness of the wrist flexors and intrinsic hand muscles.
C8
Decreased triceps and finger flexor reflex.
Sensory symptoms or loss in the hand
Lower extremity
e
Weakness of the iliopsoas, quadriceps and adductor
3
muscles. Sensory symptoms or loss on the anterior thigh. Decreased or
absent knee
reflex.
ly
Weakness of the anterior tibial, peronei, posterior tibial,and toe
extensor .muscles.
Sensory symptoms or losS on the dorsum of the foot and great toe. Decreased or absent
internal hanstring reflex.
ed
Weakness of the gastrocnemius and toe flexor muscles. Sensory
sole of foot. Decreased or absent Achilles reflex.
S1
symptoms
or loss on
Reflexes:
erks: position the limb in such a way that there is a straight passive stretch but no active
contraction of the
muscle to be tested. Strike the tendon of the muscle with a soft rubber hammer in a single sharp blow.
There is a brief muscle contraction which may or may not be strong enough to move the limb.
the reflexes are feeble, reinforcement (Jendrassik'smanoeuvre) may be required to elicit the reflex. Ask the
Subject to make some strong voluntary muscle contraction not involving the muscle to be tested. e.g. while
testing the knee jerk, asks the subject to clasp his hands and pull them against each other as hard as possible.
Reflex grading:
4 + Brisk, hyperactive, clonus
3+ Less brisk than 4+ but > 2+
2+ Average, normal
1+ Diminished, < normal
ONo response
Elicit the following jerks:
Knee jerk(L2,L3,L4)
Ankle jerk(S1,S2)
Bicep jerk(CS,C6)
Triceps jerk(C6,C7)
Supinator jerk(C5,C6)
Jaw jerk
39
Clonus:
It is the
regular
clonus is seen onlyoscillations
in
Try to elicit the
Ankle clonus
of contraction and relaxation of a muscle elicited on stretching only
hyperetlexia.
following:
Sustair
Patellar clonus
Superticial
reflexes:
These are
reflex
Elicit the followingmuscle
contractions elicited
reflexes:
by firmly stroking the skin of the overlying area.
Plantar(l5,S1)
Abdominal(T8,T9,I10 above andT10-12 below umbilicus)
Scapular
Coordination:
Hold
your finger upri ght in front of the subiect at a
distance of about half a meter. Ask the subject to to
Your Tinger and tip his nose alternately with his
forefinger, if successful, ask him to close his eyes and touch
nose after bringing in his
a
forefinger
from
distance.
Ask the subject to place
the heel of one of his legs on the opposite knee and slide it down the
ankle.
shin towards
Ask the subject to walk
straight bare-footed and note:
If he/ she can walk
straight.
Whether he tends to fall, and if so, in what
direction.
Assignments:
1.
2.
Discus_ the following: Pyramidal and
Extrapyramidal tracts. What are the features of th
involvement?
What do you mean by monosynaptic and
polysynaptic reflex? Give examples of each
demonstrate?
3. What are various
abnormalities of gait and coordination?
4. What is the physiological basis of
reflex? Demonstrate how to test for strength and
5. What are the various types of
rigidity and what
6.
mechanism operates behind them?
Discuss the folowing:
L
R
+++
++
40
reflexes?
Bulk d
()
Muscle
Cm
Mid
id
37 Cm
. Tene
Normae tone
Normal tre
Upper
(ower
Muscle
SYength
LowER UMB
UPER JMB)
Dorsal injeroesei
Dorifleo
Plantarflexor
5
palmar
Knee floxoY
Knee extansor
lumbical
orponens polfces
flexor
5
Hp texor
extenco
5
extensor
Brachioradialis
Adducto
Bicee
Ticepe
TRUNK
Supraspinaous
Detoid
Abdonal
Eriecor
Trapeuo
Jnftaspinoao
Pectoralis
Magor - 55
Sersatus Ante
laY swn dorsi
" Deep Terdon Reftex
O UPpe
imb
Triceps jek (6, co)
spíae
5
" superfii al Reflex
-NN
2
2
PknarReflex
(Ls,s)
2
Abdomninal re flex
supinator jese (cs,cG) I
2
1
41
5
us cll
-5
Bice ps (ek (cs,ce)
(2) Loner
imb
Knee jerk
MUsçLE
(T;- T)
Scapular
2
2
2
Crematic
Bulbo ca vern 0s
Anal
2
eflex
y
2
Neve
Sys
cortico spnt
troc
Brains te m
Retcuspnal t oct
veotibulCapinal
-fbre
(Repicuay
formoie
teoct
Pom Redulla )
Divet synape
patieny o) bold his arn fo tne
(3).(o) sideemp
(b)
offe cqd
iplegic gí- Potient
hemicicle
al k ith abnosnl natro
d heia
oc
(eaknen at one ide m
(e) Roykinsonian
Utre step
flexin at head
slow
cg-crcinatin
Dyomena- Snabil
to con
dance
speech
rereptox ant
srech
tr
paen
peiphosal
o
14) smulopon end
caue
be
Can
Pefion
deg
sle
42
Can
be
Crrsoctisn
Examination of cranial nerves
Examination of cranial nerves constitutes one of the most important tool to reacha provisional diagnosis in
cases of nervous syste disorders. Cranial nerves are examined in the following orders
Olfactory Nerve
.ha ubiect to decipher by smelling only (keeping the eyes closed) familiar substances like clove oil, oil of
peppermint, tincture of asafetida. Avoid ammonia as it can stimulate Vcranial nerve.
Apatient mayfail to smell it at all or may identify it wrongly.
Optic Nerve
Tests for visual acuity (VA):
Snellen's chart:
is a chart displaying s series of test types (usually letters), the top-most being visible to the
normal eye (i.e.
htending an angle of 1 at the nodal point on the eye) at 60 meters and the subsequent lines at 36, 24,
18,12,
ndS meters respectively. The distance (d) is indicated below each line on the chart.
ctant Vision: ask the subject to stand 6 meters (D) away from the Snellen's chart and read
down the chart as
eras he can with one eye, keeping the other eye closed.
Fthe last line that the subject can read is marked (say) 24 meters(d), then his VA is 6/24 (D/d).
or V/A <6/60; ask the subject to stand nearer to the chart (D'). His VA will now be given by D'/d.
for VA <
60. perform the following tests, in that order till the patient responds positively.
fvision is poor (<1/60):
Counting finger: Indicate anumber to the patient with your fingers keeping them at various distances (< 1m)
from his eyes and ask him to count them.
and movements: move your hand in front of the patient's eye and ask hím if he sees it.
Perception of Light and projection of Rays (PL, PR): Shine a torch light in the patient's eye and ask him if he can
See it (PL. If he can, ask him to indicate the direction from which he sees the rays coming (PR).
Near Vision: Visual acuity at the ordinary reading distance is assessed by asking the subject to
read test types
varying sizes, the notation being based on the printer's "point" system. The smaller print used in N%. The
near vision is recorded as the smallest type which the subject can read completely.
Test for field of vision
The confrontation method:
Stand in front of the subject at adistance of about 1meter. For testing his right eye, ask him to shut his
left
eye and look through his right eye at your left eye. Correct him immediately if he takes his eye off
yours. Hold
up a finger of your right hand in a plane midway between the subject and you, almost at full
length to the side
for testing the extent of the nasal field). Keep moving your finger and bring it near in that plane till you
can
ust "with the tail of your eye" catch the movements of the finger. Ask the patient if he too can
see the finger;
he can't bring it nearer till he can. Test the field in other directions too and in the other eye.
he subject's field is declared normal or abnormal by comparison with your own visual field
which is assumed
o be normal.
Tests for colour vision:
ASk the subject to decipher the figures concealed in pseudoisochromatic charts of lshihara.
colour blind, he identifies the figures wrongly.
Oculomotor, Trochlear &Abducent (lI, IV, VI) nerves.
Examination of the pupils: Compare the pupilary sizes in both eyes.
Light reflex:
Pie a torch in one eve of the subiect while shielding his other eye from the light by placing your hand in
ween (alternately, bring alighted torch quickly from lateral side to shine on the eye). Note the pupillary
strction in the same eve (direct light reflex) as well as in the opposite eye (consensual light reflex).
43
Accommodation reflex:
brought in front
Ask the subject to look ata distance and instruct him to look at your finger the moment it is
his eyes. Quickly bring a finger close to his eyes and as he looks at it note:
The convergence of his eyes
The pupillary constriction in his eyes
move it in differer
Make the subject to look in different directions by asking him to look at your fingers as you on his head. A
firnly
Instruct him not to turn his neck and ensure the same by placing your hand
directions.
following movements in each eye:
tell him to report any "double vision" if and when he perceives it. Test the
Abduction
Elevation in full abduction
Depression in full abduction
Adduction
Elevation in full adduction
Depression in full adduction
4. Trigeminal (V) Nerve
Motor functions: Ask the subject to clench his teeth.
prominence on both sides which is better felt th
The masseters and the temporalis stand out with equal
seen.
to the affected side.
Ask the subject to open his mouth. If unilaterally affected, the jaw deviates
Sensory functions:
scalp.
Test for the common sensations(pain, touch and temperature) on the face and the
salt and weak solutions of citrica
Test for the sensation of taste: Use strong solutions of sugar and common
protruded tongue and ask
and quinine. Apply these, one at atime with glass rod to the surfacé of the
the taste by writing
subject to decipher the taste without withdrawing the tongue and indicate
gesticulating. Ask him to rinse the mouth after each test. Quinine should be applied last.
Corneal reflex:
lightly touch the lateral e
Twist some cotton wool into a fine wisp. Ask the subject to look at a distance and
of his cornea at its conjunctival margin with the wisp.
lost.
The eyes reflexly shut unless the muscle ia paralyzed or the corneal sensations are
5. The facial nerve (VII)
while instructing him to resist
Ask the subject to shut both eyes. Now with your fingers try to open his eyes
same. Assess the strength of his resistance.
may fail to shut his eye
The eye can be opened easily on the affected area. In severe cases, the subject
excessive effort to do the same makes his eyeball roll upwards (Bell's phenomenon).
Ask the subject to raise his eyebrows.
He is unable to do on the affected side.
Ask the subject to showhis teeth.
He is unable to do so on the affected side and the mouth is drawn towards the healthy side.
Ask the subject to blow out his cheek. Press gently on each cheek.
When pressed or tapped on the affected side, air escapes easily through the mouth.
Test for the sensation of taste on the anterior 2/3 of the tongue.
6. The Vestibulo-cochlear Nerve (VIll):
o
The Rinne's Test: place the stem of vibrating tuning fork (Frequency 512 Hz) on the mastoid process
Immediately
fork.
tuning
subject and ask him to indicate the moment he ceases to hear the tone of the
the prongs of the tuning fork in front of the subject's ear ask him if he still hears the tone.
If he doesn't, he is either suffering from a conductive deafness (Rinne's test negative) or severe
deafness (Rinne's test false negative).
44
he Weber s test: Placethe sten of a vibrating tuning fork on the centre of the subjet's forehead.
normalsubject hears the tone equally through both his ears. f it is lateralized to one ear, the patient either
as a conductive deafness in that ear o nerve deafness in the opposite car
he Schwbach's
's test (Absolute bone conduction Test). Place the stem of
subject. When he indicates that the tone has
rocess ofthe
ork on yOur Own mastoid and note if you can still hear it.
vibrating tuning fork on the mastoid
stopped, immediately place the stem of the tuning
you canstill hearthe sound, diagnose the subject as having nerve deafness. If you cannot, then declare the
bjectnormalif you are sure (or on the assumption) that your own hearing is normal.
The Glossopharyngeal Nerve (IX):
scabiect to open his mouth. Touch a cotton swab to the posterior
pharyngeal wall, Look for the reflex
ntraction of the stylopharynge al muscle (the gag reflex).
affectedthe reflex is absent (due to damage to its afferent arc).
est for the sensation of taste on the posterior 1/3 of the
tongue.
The Vagus Nerve (X):
ehesubiect to open his mouth and look at the position of the uvula.
nilateral paralysis of the vagus, the uvula is deviated to the normal side.
ck the subject to say "Ah" and look for the elevation of the palatal arches.
nere is no movement of affected side.
est for Gag reflex
affectedthe reflex is affected (due to damage to its efferent arc)
The Accessory Nerve (Xi)
sk the subject to shrug his shoulders while you press them
downwards.
sk the subject to rotate his chin from side to side
against resistance.
affected, the patient is unable to carry out these movements.
D. Hypoglossal Nerve (XI1)
sk the subject to put out his tongue and move it from side to
side.
affected, the tongue deviates to the affected side.
sk the subject to press his cheek with his tongue.
Assess the strength of the tongue by pressing against the
ongue with fingers.
learning Objectives(learn to demonstrate)
Smell
Visual acuity, Field of vision, color,PLR
Pupilary light reflex(PLR)
/IV/VI
Extraocular movements also test lll cranial nerve.
Corneal reflex, jaw movements, sensations of face
VII
Facial expressions, taste sensation,stapedial reflex
VII
Hearing tests(audiometry, Auditory evoked potential?)
X/X
Swallowing, palatal,gagreflex,taste sensation
Speech,voice,tongue
X
Neck and shoulder
XI
Tongue
45
movements
Asvignmet
1
Eamine raniai
Neves of the subiect. Discuss the features of various cranial nerve
isensorynvolvermeny
diseases'
DiscSK in brief the cranial nerve pathways. Which are mixed, pure motor and pure
Discuss abut supranuclear andinfranuclearVlth nerve palsy
4 What
are vanious layers of retina. Discuss about corneal, PLR and accommodation
5 Trace auditory. olfactory and gustatory pathways. How will you test the involved nerves?
nerves?
reflex pathways?
HOw will you distinguish betwveen conductive and sensorineural deafness?
What is the physiological basis of Snellen's chart. Discuss the site where visual acuity is
maximum
why?
S.
Disuss in brief:
Adies pupil
Horners syndrome
Bells palsy
Argyl Robertson pupil
Squint
Brain stem lesions involving cranial
nerves
Due
Pure
Supianuclean Vi th nrve
josion above
nucley
{- Facial
Comen feapuie igroeu por
affected becawee fronyl
kas bilajeal cosicae inevat
due t0 a
pconuclean
MUsclo
palay - Bel's
a, Boh wppes s obe
b
No involvement
pag
foce
"Duteh
Podo
46
ho
vden
qand
call
mer
ia amen
to
topupl
at . foval
NV.
anfrom
47
ence
puplTpbertson
leion
lost sensaticn
is
False
normol
todraon
Side
isside Affected
onlen, expresi
mouth
Bells
PtoigRathei
paly
to
SyndOme
Hornevs
pupl
-:
ag nllen's
voerer
Aes
8
but
Yespecvely
26 at s ceuent
s.
chaellern's
sub
kblaSm R
6.
6om
at
8,12
to ble vis1
ane
teo
series
Rinne's
chsU
eber's
6
c:líanis
ual)
tact
vis
laf
optic
opic
sre
ocdpeyal
(
laoa1
Nv. opic
}pons
oatien
Optic
Pupio
flox
Retina
inlocae
ie
Cente
of
opthalric
br.
Conel
Sqing
Disodes
me
Some
Nerve
n
hicn
(coK
time
direc?
oY
dont
fngion
conroly
Colour Vision
Ain:Io determine the Color Vision of the subiet
Procedure:
Tests for colourvision:
Ask the subject to decipher the figures concealed in pseudoisochromatic charts of Ishihara.
If color blind, he identifies the figures wrongly.
ASSignment:
Record the Visual Acuity of any of your friend and what are the conditiond in which you will test for acuity
of vision. What is the physiological basis of preparing snellens chart?
Check any three of your friends for color blindness.
3.\What do you mean by: Myopia, Hypermetropia , Presbyopia and Astigmatism. What is the correction
adviced?
What is areduced eye?
5.How will you check for acuity and color if no charts are available?
6.Discuss in brief how we see colors.What are various types of abnormalities of color vision. Which color
blindness is most common and which one is Autosomal recessive and why?
learn the following:
WHO Blindness classification
Based on the corrected visual acuity in a better eye
49
Visual acuity
6/6-6/18
Visual acuity
6/18-6/60
Visual impairement
Visual acuity
<6/60-3/60
Severe visual impairement
Visual acuity
<3/60-NPL
Blind
Normal
Perimetry
Aim: To map out the visual fields of a subject (Perimetry)
Perimeter:
a
horizontal axle fixed to a stand. The arc bearing
metallic arc pivoted at one of its ends to a
the
indicate
test object. The arc is graduated in degrees to
sliding disc with 1mmwhite spot serving as the field. On the tip of the axle is a white circular spot which
visual
board, on the
position (isopter) of the test object in the
arc is a vertically disposed circular
rotating
the
to
co-axial
a
Being
diameter of the chart board
serves as the fixation point.
fixed. A metallic scale, half as long as the
chart
is
perimeter
the
which
isopters on the
backside of
graduated to degrees corresponding to the
is
It
board.
chart
the
behind
pressed
is fixed horizontally
placed appropriately on the scale and
when
which
pin,
sliding
the
with
rotated to and fixed
perimeter chart. It is provided
corresponding isopter. The perimeter arc can be together while the
at
chart
the
on
hold
pin-point
punches a
the perimeter arc are locked
on the axle. The chart board and
mounted
in any meridian using a screw
stationary. Thus if the chart paper is properly
remain
it
on
mounted
bringing the corresponding
Scale and the punching-pin
meridian, the chart board rotates with it
particular
a
to
rotated
is
also provided which help in
when the arc
A fixation rod and a chin rest are
punching-pin.
the
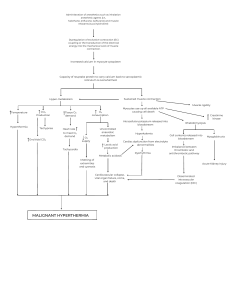
below
chart
fixed at a distance of
meridian of the
the fixation point. The chin-rest is
with
line
in
horizontally
eye
bringing and keeping the
It consists of a
330 cm away from the fixation point.
Perimeter Chart
around it are the
point. The concentric circles
fixation
the
to
corresponds
the same
The central point of the chart
points in the visual field subtending
the
all
of
loci
the
depict
isopters(graduated in degrees) and
graduated in two different scales
of the eyes. The isopters
point
nodal
the
at
axis
visual
used for detailed
visual angle with the
from 0 to 30 degree. The latter is
scale
magnified
other,
a
the
comparison;
and
one from 0 to 90 degrees
degrees, depict the meridian for ready
in
graduated
also
radii,
Two dots
mapping of the central field. The
in the chart with dotted lines.
demarcated
are
eyes
two
the
in
vision
the normal.limits of the field of
correspond to the blind point.
point
central
on either side of the
Procedure
right of the
(Confirmed using a spirit level) and to the
horizontally
arc
the
Place
chart:
1. Mounting the
and allowhim
subject and fix the ch¡rt upright.
perimeter. Explain the procedure to him
the
of
front
in
comfortably
2. Ask the subject to sit
illumination in the room.
Some time to adapt to the
the fixation rod in the
chin on the left chin rest. Place
his
keep
to
subject
the
obstructs the vision of
eye, ask
3. For testing the right
that the tip of fixation rod
so
head
his
of
level
that
the
adjust
comfortably maintain his head in
slot and ask the subject to
can
subject
the
that
so
the chin-rest
the fixation point. Adjust
Instruct him not remove
position.
the central fixation point.
at
gaze
to
subject
the
ask
rod and
4. Renmove the fixation
the course of the test.
throughout
acuity is minor. For
his gaze from it
the impairment of visual
if
avoided
be
should
and
obstruct the field of vision
are preferable.
contact lenses
acuity, glasses are necessary though
visual
of
periphery to the centre.
diminution
greater
object along the arc from the
target
the
Move
chart with the
meridian.
particular
object. Mark this point on the
5. Fix the arc in a
the
sees
he
moment
indicate the
Glasses
Instruct the subject to
centre and instruct the
length of the arc toward the
the
throughout
chart.
the target object
Mark those points too on the
object.
6. Continue to move
the
see
to
fails
stage he
peripheralvision.
subject to indicate if at may
points denoting the limits of
all
Join
meridians.
12
the procedure is same
7. Perform the test at least
right chin rest, thereafter
the
on
chin
his
keep
ask the subject to
8. For testing left eye
punching pin.
as mentioned above.
52
Presentation of results:
Use different colours for demarcating the fields of the right and left eye. Als0 write the following on the
chart:
Name and age of the subject
Date and time of the test
Distance of the test object from the eye (in mm)
Size of the test obËect (in mm)
Colour of the test object
.llumination in the room (type and intensity)
Reliability of the subject (good, fair, or poor)
Tips:Visual field is the entire area seen when the eyes look at the central point. Always ask
the subject
to remove the
spectacles. Instruct subject not to close eyes too tightlv .Constant feedback from
examiners side is required. Be patient while performing the test. Always test for white and color
obiects( can you find any difference for field of vision when testing for colored
objects. If yes can you
give the reason why it happens so?) and learn the importance of
binocular vision.
ssignment:
1. Map out the Visual Field of any of your friend.What are various
conditionds and diseases in which
perimetry is useful? What if no perimeter is available?
2. Locate Blind Spot on the record. What do you mean by
physiological and
Why it is called blind spot?
3. Trace visual pathway and learn the following:
i. Lesion of optic nerve causes
blindness(unilateral or omplete)
ii. Optic chiasma lesion involves fibres originating from nasal half of
pathological blind spot.
retina thus causing bitemporal
hemianopsia(loss of temporal half).
ii. Lesion of otic tract cause homonymous(same)
hemianopsia(half) since it involves fibres
originating on the same side,
iv. Lesion of optic radiation cause quadrantic homonymous defect. Parietal
involvement cause
inferior(pie on floor) and temporal causes superior(pie on sky) lesion.
optic nerve
optic tract
optic
radiations
occipital lobe
53
atrophy.
Draw the labeled diagram of normal optic disc?
Glaucoma, b). Raised ICT and c). Optic nerve
a).
:
discin
optic
examibnation
of
diagrams
the fundus
Draw labeled
Discuss about normal fundus. Also discuss
?
Opthalmoscope
an
What
is
.
Diabetic retinopathy.
findings in :a). Hypertensive and b).
54
duration & amplitude of waves
axis,
electrical
mean
calculation of
Normal Electrocardiogram and
intervals.
Electrocardiogram (ECG)of
Aim. To reord and interpretthe
a human Subject
as recorded on th.
The Electrocardiograph and Electrogram:
electrical activity of the heart
the
of
desciption
obtaining agaphic
perspectives. lt consists o
It is an instrument for
activity from a variety of spatial
the
reflect
to:
to
positioned
amplifier. The amplified signals are fed
body surtface by electrodes
powerful
a
to
connected
of
electrodes or leads
special graph paper with the help
a
on
obtained
a set of recording
is
record
galvanometer. The
writing system based on a
heated stylus.
Procedure:
(A) Recording of the electrocardiogram:
on the bed.
arm
1. Ask the subject to lie supine
respective limbs leads (right arm, left
the
to
LL
and
RL
LA,
jelly for
leads marked RA,
rubbing with electrocardiograph
after
2. Apply firmly the limb
limb,
each
around
straps
right leg, left leg)with rubber
better conduction of electricity.
the help of
designated sites (see below) with
their
on
V6
to
V1
leads marked
3. Position the chest
electrocardiographic jelly and suction cup.
Position of the chest leads:
sternal border;
4" intercostals space: right
V1
4" intercostals space: left sternal
V2
border;
between V2 &V4;
V3
5" intercostals space: left
V4
mid-clavicular line;
axilliaryline;
At the level of V4 left anterior
V5
V6
4. Plugs the
mid-axilliaryline;
At the level of V4 left
machine to the mains and
the machine. Connect the
in
sockets
designated
leads into their
put it on.
and the chart speed so that:
5. Adjust the sensitivity
on the chart paper.
vertical deflection of the stylus
mm
10
a
gives
input
1 mv
runs 2.5 cms.
In 1 sec, the chart paper
and V1 to V6 in
leads I, I, II, aVR, aVL, aVF
in
activity
the
record
selector knob so as to
6. Adjust the lead
that order.
recorded.
to the leads they have
according
strips of the chart paper
7. Label appropriate
electrocardiogram:
(B) Interpretation of the
quick and correct interpretation:
Note the following points for
are present, then
1. If the QRS complexes
Its rate;
Its rhythm;
and S wave)(1.5-2 mv)
Its magnitude (of the R
Itsduration(0.08-0.12 sec)
56
2Theelectrical axis of the
AN0S calculation:
heart( 30 to +110 degree)
: if the P waves are presentthen
Its magnitude(0.05 mv)
Its duration(0.08-0.10 sec)
Its configuration
The duration of PR
interval(0.13-0.16 sec)
4. If the Twaves are present then:
Its amplitude(0.5 mv)
Its duration(0.27 sec)
The duration of the ST
Segment(0.04-0.08 sec)
Any elevation/depression of the ST
segment.
earn the following:
Resting cardiac cells are polarized
On activation: Depolarized
Depolarization causes electrical current which is recorded as ECG/EKG
. It's in the form of vector having
both
magnitude and direction
Positive/upward deflection occurs when the vector is directed toward the positive
electrode of lead
and is downward when directed towards
negative.
Left ventricle has more muscle mass thus it is
dominant vector
W//aVF: Inferior leads
" /aVL:
Left/lateral leads
aVR: cavity
V1: cavity
V1-V2: anterior
V3-V4: anterolateral (septal)
V5-V6: lateral
Repolarization occurs first on epicardial surface and then proceeds towards endocardial surface.
Pwave is best
evaluated
in lead ll.
wave is suggestive of atrial depolarization
US is suggestive of ventricular depolarization: Q(septum), R(majority of ventricle) and S(basal part of
Ventricle).[what is the last portion of heart to be depolarized and which is first to repolarize?]
57
ORS amplitude depends upon eledrical ativity of ventricies
the wave suggestive ot atrial repolari,
TWaVe is suggestive of ventriular repolarization(where is
wave is usually in the same diretion as ORS
" QT interval ventriculat depolarization and repolarization(0 4() 043 sec)[what is corrected
interval?)
conduction(0.13016 sec|What are
PR inteval Atial depolarization and His Bundle
abnormalities of PR interval?]
ST interval
Ventricular repolarization{0.32 sec)
pos5ible explanat
STelevation and I wave inversion is suggestive of Myocardial injury( what is the
What is the total duration of cardiac cycle?
What is the significance of J point?
ST
PR
Segment
Segment
QRS
Complex
PR
Interval
QRS
Interval
JPoint
ST
Interval
0T Interval
Assignment:
intervals and segments.What is the physiologi
1. Record ECG of any of your friend and note various
basis of ECG. Learn to read electrocardiogram?
in the ECG record.
2. Note the voltage and duration of various waves
right a
Axis from the ECG record. What are the reasons for
3. Calculate Heart Rate and Mean Electrical
left axis deviation?
duri
Discuss about heart blocks and ECG tracings expected
4. DiscUss the conducting system of heart?
various types of block?
What is normal axis and various abnormalities?
5
Pacemaker?
arrhythmias and ECG findings of each? What is a
basis
of
pathophysiological
the
6. What is
atrial enlargement
lschemia, Electrolyte abnormalities, Ventricular and
Myocardial
diagnose:
to
Learn
7.
repolarization wave)?
why Twave is erect(though beinga
8. Discuss why aVR is inverted and
posterior wall MI?
9. Howwill you diagnose
What is meant
meant Bipolar and Unipolar leads?
is
What
Electrocardiograph.
10. Discuss about
augmentation?
58
11. Whatare various indications of doing ECG/EKG?
changes are expected in
12. Whatt
recordings before and
after exercise and in old age?
3:o
en you answer:
How to diagnose an old MI
Angiography, Angioplasty, CABG and Echocardiography
elec fied due to
'baois h EG.
2)
Pwa ve =
O.1 mV
TNove
O"3 mV
cardiac
2 duroion : O08 sec
2 durotion
0.O4 sec:
2 duration : O12 sec
3) Heort voe SDO
NO. h Small
bOX e
blw? R-R nave
Lead I
dipole
oveent
IS0O
Q5
Rs wave
pofenial
Leod IIL poqenial
60 beatin
poporplae (6(ewon) (s.ae)
lOx0"|
Elec açs L 72°
Right 42is deiaton
:-30 to Ino)
9f
RS compler in lead I
Predoninont
Left -is deviaton’
predoninont
59
tve
pradouianty
f 9RS conplex is
lea d I
but -ve in aV#
V node ’ Bundle s His -
deJnernvdal pothway
SA
Bundle bvanches
Pusrinje fibre
poseros HemibloK
left onyeo
left 4xís devioion5) Normal
axi< = -30 to +ll0
Abremalty
Rigb axis deviain
synd roe
4) Devtdcardi
Left qis deviatim
3) NPW
hemiblock
anteo
left
2)
3)
wPw
yndrome
dseast
") Obstruive airnay
Potholegícal bogi
4ytne
vythmia efiecs failu
a sA oe do mainton
vatios path
ohich elec impulse
: Avoriaton
ee Pave
P-p interval
dicle
Pacemaar
Oyecaraial
segmen depremi g
ischemja - ST
Jnvevtld T ave
Tall T wave
- Prolongd Tinerval
60
Experiment -6
Spirometry
volumes
Aim:Recordthe lung
pirometer:
and
capacities using Spirometer
metallic cylinders, closed at one end and of two
different diameters, SO that one can be
other without the sides coming in
contact.
The
provided metal tube passing through the bottom and larger one is set with the closed end
consists oftwo
laced within the
ownward. It is
upward
through
to
helevel of rim. The can is filled with the water to near the rim. The other cylinder
is set
in the the
can centre
with the
extending
and is termed the bell.
losed end upward
To it are attached chains
which
over a pulley fastened to an
verhead frame work and thence to weights which counter balance the bell.pass
In
the
end mixtures.
of the bell,Thea
pof tube is provided for drawing samples of air of filling with some oxygen or someclosed
gaseouS
tuhe passing through the outer can serves as breathing pipe.
The outer end is fixed to a mouth piece for
.hiect. When the subject breathes through the mouth piece, he
draws air from and exhale air into the
f the Spirometer which moves up and down with
expiration and inspiration respectively. These
ovements can be recorded using a lever and a kymograph.
rocedure:
Eill the bell with atmospheric air. This can be easily
dane by simply lifting the bell and alow air to be drawn in
through the mouth piece. Ensure
the
Spirometer is leak-proof by pressing the bell slightly while keeping the breathing tube closed;that
it should rise
back to itsoriginal level immediately.
Clean the mouth piece with antiseptic solution and put it in your mouth. Insert its
edge-plate between
vourums and teeth and clench the horizontal plates with
your teeth.
Clamp nose clip on your nose and breathes into the atmosphere. Continue till your
breathing becomes
normal.
Turn the valve of the mouthpiece to connect it to the Spirometer. Record all the
subsequent movements
of the bell using a kymograph.
Breathe normallý for about 30seconds. Keep the drum speed at 1 mm/sec and take the record.
Take a deep breath (maximum inspiration). Turn the kymograph to a faster (20 mm/sec)
your breath as youdo so.
speed, holding
Exhale rapidly and fully.
FVC is recorded.
Scale(for calculating the volumes and capacities):
i.lsmall square(vertical=30 ml) for volume
ii1200 mm/min=20 mm in 1 sec
ii.l sec=20small squares(horizontal) for time
Learn the following:
Lung is soft elastic tissue with the tendency to collapse(then how it is suspended in the thoracic
cavity?)
Chest wallhas the tendency to move outwards
* Torecord static lung volumes and capacities blow as long as you can. Blast to record dynamic lung
volumes and capacities. (What is the difference between static and dynamic mechanics of lung?)
Learn to record MMEFR 25-75%( What is it's importance?)
Capacity means two or more than two lung volumes.
61
lean the importance of FRC, RV and VC.
learn about Dyspenic Index
nstotory
Inspiratory
1eserve
/olume.
copocity
volume
Vital
capocity
Total
Iidot
lung
Copocity
volume
5
Expirotory reserve volume
Functionol
residugl
capacity
Residual volume
Assignment:
Record the various lung volumes and capacities of the
subject by Spirometry.
2. Calculate the FEV, and FVC from the
recorded graph.
3. Discuss in brief the
mechanics of breathing?
1
4.
Learn the following:
The movement of lung and chest wallis
effected in
i.
restrictive lung diseases. Thus all lung volumes
capacities are reduced but the absolute ratio is unchanged.
In obstructive lung
diseases the
to outflow is increa[ed thereby
phase which in turn causes the resistance
decresing the expira
of air resulting in
trapping
increased
end
capacities. The absolute ratio is decreased.
expiratory volumes
What are various restrictive and
obstructive lung diseases. What is the absolute ratio
5. Functional Residual
Capacity(FRC) and Residual Volume(RV) cannot be measured usingtalked of?
is the method to measure
Spirometry. W
them? What is the physiological significance of FRC
and RV?
6. What are various factors
effecting lung volumes and capacities (both
7 What do you
physiological and pathological?)
mean by closing volume?
8. What is a computerized
Spirometry. Learn to read the Spirogram?
Volume (L)
8
7
6
5
IC
TLC
IRV
4
FEV,
FVO
VT
ERV
FAC
3
2
1
RV
1
2
Timo (soc)
62
3
Demonstration of
EEG, EMG, Nerve
Conduction Velocity, Visual, Sometosensory&&Auditory Evoked
Potentials
EEG (ElectroencephaloRram): The electrical activity reco ded from the surface of the scalp, is known as
Electroencephalogram Both the
amplitude and pattern of these electrical signals
on .The
changing,
dependng upon the state of brain activity eg. during excitement, drowsiness, sleep keep
& coma
EEG
eorded fromthe surtace of scalp is the sum total of potentials generated bylarge numbers of neurons in the
EMG (Electromyogram): The process of recording electrical activities of the active muscle by cathode ray
scilloscopeis known as Electromyography (EMG) and the record is known as Electromyogram. At rest a
ormalskeletal muscle is electrically silent When muscle is excited voluntarily or by electrical stimulation of
otor neve a change in membrane potential occurs. Electrical disturbances produced by the excitation of
didual motor unit is called motor unit potential (MUP).
Nerve Conduction Velocity (NCV): Nerve conduction velocity is commonly used in neurophysiological
taries for understanding of normal peripheral nerve structure and function and various
neurological
sces Based on diameter & conduction velocity, nerve fibers have been divided into A, B&C. The nerve
dction involves the study of motor & sensory nerve conduction.
sual Evoked Potential VEP:The electrical potential differences recorded from the scalp in response to
ual stimuli is called visualevoked potential(VEP). It represents resultant response of corticalas wellas sub
ieal areas to photo- stimulation .A normal VEP indicates the intactness of visual system.
Somatosensory Evoked Potential (SEP) : The electrical potential generated mainly by large diameter sensory
bers in the peripheral & central portion of the nervous system in response to a stimulus are called
matosensory evoked potential (SEP) Somatosensory evoked potential (SEP) evaluate the proprioceptive
athways.
rainstem Auditory Evoked Potential (BAEP) :The' potentials recorded from the ear &vertex in response to a
ief auditory stimulation are called brainstem auditory evoked potentials (BAEP) .These are produced within
Dmsec. by a brief click stimulus .It helps to assess conduction through auditory pathway severity of hearing
eficits &brainstem functions.
65
Human experiments
Stethography
Aim: Tostudy the respiratory movements by Stethography and observe the effect of various maneuvers n.
Stethography: It consists of a corrugated rubber tube connected at one end to a metallic cup (Marey's
tambour) through pressure tubing, the other end being closed. It has a hook and chain device for tying it
around the chest wall. Because of the corrugation, there is an increase in its capacity when it is stretched t.
InspiratIon)and therefore a concomitant fall in air pressure within it. The tanmbour is covered with latex or
rubber diaphragms which support the lever on it and is mounted on a stand. The lever moves down when
pressure in the stethograph falls in inspiration and vice-versa.
Procedure:
1.Tie the corrugated rubber tube on the chest of the subject in the 4th intercostal space.
2. Record the normal breathing movements on a kymograph, keeping its speed at 2.5 mm/sec.
3. Take record while asking the subject to: Cough, Swallow, Speak, Hyperventilateand hold breath after ful
inspiration,after full expiration andafter hyper ventilation.
Assignment: 1. Record the chest movements of the subject by Stethography during various maneuvers. 2.
Calculate the Respiratory Rate and Breath Holding Time from the record.
66
Physical Fitness and Exercise Tolerance Tests
Aim: To assess the Physical Fitness of the subject.
Physiologists conside physical fitness as an ability to make physiological adjustments to any stress, especial
incardiovascular functions. Maximum 02 intake (V02max) test is best single method for measuring the
cardiovascular response to exercise.Maximum oxygen intake is measure of maximum amount of oxygen tha
can be taken up by the blood &delivered tothe cells.Exercise can be performed on Bicycle Ergo- meter or
Tread Mill, VO2 max can be calculated by collecting the expired air after graded exercise.The maximum V02
can be predicted by recording the heart rate before &after the exercise. Maximum 02 uptake &maximum
heart rate are reached approximately at the same timne.
Procedure: Determination of Physical Fitness by 1, 3-minute step test.
2. Harvard step test.
3-minute step test: Ask the subject to perform a four steps cadence in sequence up-up-down-down. Female
subjects to perform 22 and male subjects 24 complete steps/min. Exercise for 3minutes. Record the pulse
rate 15 second immediately after the exercise and convert this to HR/min. (Pulse count in 15sec x4). Predicte
VO2 max can be calculated from the equation:
Max V02 (Men)=111.23-(0.42XHR/min)ml/kg/min.
MaxV02 (Women)=65.81-(0.184XHR/min)ml/kg/min
Calculation of energy expenditure: Metabolic Equivalent Task(MET): MET0S a Unit of energy expenditure.Ons
MET is equivalent to resting 02 consumption.For an average man and woman it is 250 8 220 ml/min
respectively. 1MET = 3.5 ml/kg/min.
MET=Vo2 max/3.5.>8 METS: Healthy subjects.
3 minute step test (Men)- Heart rate
18-25
26-35
36-45
46-55
56-65
65+
<79
<81
>83
>87
<86
<88
Good
79-89
81-89
83-96
87-97
86-97
88-96
Above average
90-99
90-99
97-103
98-105
98-103
97-103
Average
100-105
100-107
104-112
106-116
104-112
104-113
Below average
106-116
108-117
113-119
117-122
113-120
114-120
Poor
117-128
118-128
120-130
123-132
121-129
121-130
>128
>128
>130
>132
>129
>130
Age
Excellent
Very poor
68
(Women)- Heart rate
test
step
&minute
18-25
26-35
36-45
46-55
56-65
65+
Age
>90
<85
<88
85-98
88-99
90-102
94-104
95-104
90-102
q9-108
100-111
103-110
105-115
105-112
103-115
109-117
112-119
111-118
116-120
113-118
116-122
118-126
120-126
119-128
121-129
119-128
123-128
127-14-
127-138
129-140 130-135
129-139
129-134
>140
>138
>139
>134
>94
<95
Encellent
AbOveaverage
Average
Belowaverage
Poor
Very poor
>140
>135
<90
Farvard step Test
o Subject tostep up &down on a 41cm plate form 30 times/min.
Counting begin when subjects starts exercise.
With the signal start, ask the subject to place one foot on the platform and subsequently
another foot on the platform.
Ask the subject to straighten the legs and backbone and step down same foot first he used first
to climb up.
Every 2sec give signal up. Ask the subject to use same foot first allthe time.
Perform the exercise as long as he/she can or maximum for 5 min.
After 1min of completion of exercise, pulse rate is counted for 30 sec.
Read score from scoring table for Harvard step test.
Data recording and Analysis:
-minute step test: Recovery heart rate = Predicted VO2 max= MET value Comment to your result=
Harvard step test: Duration of Exercise =Half minute pulse rate value (after one minute of completion of
exercise) =Score from table = Fitness grade
69
Tabie 9: Sono
the ava Steo Teet
Duratien of lest (Minutes) 1otal Hoart Beats 1-1 /2 Mutos Into Recovery (Score Arbitrary Units).
40 44 45 49 50-54 S5.50 60.64 65 69 70-74 75.79 80-84 85-89 90-94 95.
3
19
1
14
13
12
11
11
29
26
24
22
20
19
18
17
16
15
14
23
22
21
20
28
27
25
37
34
33
31
43
41
39
3
41
34
29
27
25
43
3
34
32
53
3 312
84
87
110
5
9
10
17
42
62
57
53
46
72
60
61
57
53
50
17
89
82
75
70
65
61
57
54
51
42
81
84
77
72
68
63
60
57
54
98
82
76
71
67
63
60
56
123
110
100
129
116
105
Normative data for Harvard step test
Gender
Male
Female
Excellent
Above average
> 90
80-90
76-86
>86
Average
65-79.9
Below average
55-64.9
<55
61-75.9
50-60.9
< 50
Assignment:
1. Assess the Physical fitness of the subject by
'3-minute step test' and Harvard Step Tst'.
Bo sal pulse - 90- 92n
Tine- esA beat in inyeval
61
58
|- 1%’ 58
- 2
2- 2
’
fmen nden
70
Sx 24: 36Poüs)
Poor
The Moss's
ergograph
STUDY OF HUMAN EATIGUE OF
MOSSO"S ERGOGRAPH
ispmployed to study the
EQUIPMENT
phenomenon of fatigue and the fators that affet
fatigue
consists of aflat
Mosso's Ergograph: it
b0ard with 2pairs of damps ånd
the forearm of the subject. There is a
I plates to fiz, hold,
hdsteady
pair
of metal tubes
into which indexcurved
seited. The middle finger remains free to be
and
ring fingers are
to athick cord and
carries a lever
hook. Asliding plate
to record muscle
system
ove to and tro
that can
and aastrong cord
exertions on a
e middle tinger,
bearing ahook on which
cylinder. Asling fits over
weights can be hung.
Metronome: it is a clockwork device that
as a
pre-selectedffrequency of upto
to deliver "tick tock'" sounds at
is
athin metal rod
There
trequency.
bearing a scale on which a sliding cip
nset the desired
1. Place the
on atable such that the
r its edge. Explain the
ergograph
weight will hang
proceaure to the subject and seat her/him beside the
table. 2. Fix the forearm
the ergograph, and insert the index and ring
wooden
connected
functions
200/minutes.
PROCEDURES
he string with the cord
kymograph
different
variable interrupter
fingers in the finger
With the middle finger extended
attdcned to, dajuSt the position of the holders.
forearm and
mfortable. Apply a weight of 1- 2 kg on the hook
ek the subiect to flex the middle
finger and check that the
fingers so that the subject is
system works freelv.
30/minute, i.e. one beat every 2 seconds, and set it
maximally and rhythmically, following each beat of theoscillating. Ask the
tinelwithout moving the shoulders)
metronome, and
until fatigue is so
Motivation: Give a rest for 15 minutes and then repeat the great that the weight can no longer be lifted.
whole procedure, telling the subject that
do much better this time.
she/he
Effert of venous occlusion: Effect of arterial
Dper arm and raise the presSure to 4O mm occlusion: After another rest period, apply the BP cuff on the
Hg to stop venous return. Repeat the
cin earlier because of
whole procedure. Fatigue
accumulation
products in the exercising muscles. 7. Effect
of arterial
rlusion: After another period of rest, of.waste
raise the blood pressure to about
160-170 mm Hg to stop arterial
od flow. Tellthe subject to repeat the
muscle contractions. Fatigue sts in much earlier now
not only an accumulation of waste
because there
products but also a deficiency of oxygen and other nutrients.
disct the beat of the
metronome at
ctoontract the flexor muscles
lculation of work done: Calculate the workdone in each case as
shown below:- Work done (in kg meters) =
eight lifted xdistance The distance through which the
is lifted is the sum of all the heights of
weight
ontractions, converted to meters.
Ssignment:
Calculate the work done from Obtained Graph (Normal,
Occlusion and arterial occlusion).
Define fatigue. What are the factors which affect the Venous
of fatigue?
onset
How does Motivation improve muscular
performance?
toel
71
2. Fatiue
Tempo
dlt
L
veversible loss Sh physiol fica Q
skee pal
Controc
facos
Moivoo
Museulan veopOwsl
)MvatmPekae.
Uns
i
l
fgnen
Rnto complefim
improve
Te conected
72
do
ao
wok
HEMATOLOGY
LEARNING OBJECTIVES
Know about -
including their
Various equipment and reagents used for haematology techniques
clinical applications.
while handling it.
" Technique of handling various body fluids and precautions to be taken
blood and
ldentification of various cells/ formed elements (RBC, WBC, Platelets etc.) of
their quantification.
Haemoglobin estimation and concept of various haematological indexes and their
implications.
Concept of haemostasis and its practical implications.
ESRand Osmotic fragility: practical and clinical aspects.
Semen analysis technique and its clinical utility.
73
Chapter
Compound Microscope
Adj
[he
Objectives
To understand the .
Basis of microscopy
Mi
Mechanism of image formation
et
Arrangements for light and magnification
and its appropriate use techniques.
Iris
Trouble shooting and equipment
CO
Introduction:
Microscope is an optical instrument employing asystem of lenses to magnify the objects which are ordinaril, oi
not visible to naked eye. It produces an inverted and magnified image of an object. Its important parts include A
Tube:
It is cvlindrical tube through which the light from an illuminated object traverses enroute its formation of an B
image. Its length determines the mechanical length of the microscope (mechanical length is the distanc#
between the upper part of the objective and the eye piece). It consists of an outer. body tube and an inner
draw tube which can slide inside the outer tube to adjust the mechanical length, eye piece
and lower part
carrying a revolving nose piece.
Eye Piece:
It comprises two co-axial lenses fixed to the ends of a cylindrical tube, which can slide into the upper open end
of the draw tube. It is usually available in magnifications of 12.5X and 10X.
Nose Piece:
It is a plate pivoted to the lower end of the tube. It is fitted with objectives, any of which can at a time be
brought in line with the optical axis by rotating the nose piece. The objectives are so fixed that the
magnification will increase as the user turns the revolving nose piece clockwise.
Objectives:
These are cylindrical in shape and fitted with coaxial lenses. The focal length of the objective is the working
distance of an individual objective such as 16mm for low power, 4mm for high power and 1.6mm for the oil
immersion.
The magnification of the objective is calculated as Mechanical tube length
Focal length of objective
Three objectives are provided:
(a) Low power objective (magnification 10%)
(b) High power objective (magnification 40X)
(c) oil immersion objective (magnification 100X)
74
AdjustmentKnobs:
here are wo adjustable knobs for focusing the image by raising or lowering the body tube. One is for c0arse
ndthe otherfor tine adjustment. Athird knob helps in raising or lowering the condenser.
the platformtfot the glass slide on which the object is mounted. It has an aperture for light in the centre.
irror:
Is hinged below the condenser; it is plane on one side and curved on other. The concave side is used to
flect light from a point source while the plane side is used in diffuse light e.g. in daylight. Mirror can be
nlaced by electrical light source (bulb).
is Diaphragm:
controls the amount of light reaching the condenser.
Condenser:
is system of lenses which tocuses light from the source on the object. The uniformity and the intensity of the
mination of the object can be varied by lowering or raising the condenser. It is raised fully while using the
Olimmersion lens and lowered fully while using the low power objective.
Arm:
ie frame that holds the body tube vertically above the aperture of the stage.
Base:
provides mechanical stability to the instrument.The'arm.is hinged to the base so that the microscope can be
SEt at an angle convenient to the user.
How to use?
Focusing in low power
1. Revolve the nose-piece to bring the low power of the objective above the aperture of the stage.
2. Place the slide to be focused on the stage and adjust its' position so as to bring the object on the slide
directly below the aperture of the objet:
3. Using the course adjustment knob, lower the tube while looking on from the side to ensure that the
objective is close to the slide but does not touch it.
4. Using the course adjustment knob again, raise the tube gradually, this time looking down through the
tube and stop when slide comes in focus.
5. Bring the slide to a sharp focus by using the fine adjustment knob.
Focusing in high power
6. Revolve the noise piece clockwise to bring the high power objective above the object. Focus using the
fine adjustment knob only.
Focusing under oil immersion lens
7. Raise the tube and using dropper, place a drop of cedar wood oil on the area of the slide to be focused.
8. Lower the tube as in step 3 above tillthe tip of the objective dips into the drop of oil. Focus using the
fine adjustment knob only (even as the slide is moved for scanning, a film of oil persists between the
slide and the objective),.
Special Precautions:
1. Never clean the objective with alcohol as the cement which fixes the condenser may dissolve.
Always use xylene for cleaning.
2. Never look into the tube while you lower the optical tube using the course adjustment microscope.
75
Assignments:
1. Fill in the boxes.
9. Eye pioce
Qcculas
Tube
2-Nose piece
3. Obecive
lo3 pouwe
4. Objective
10.NecK
5. (bjectve
n1.
high power
6.
12. CoaTse
s4oge cßp
Adjument
Adjustment
|7. condenset
13. Fine
Lght source
14. gone stond
2. Answer following
questions
a. What is the use of
condenser and Diaphram:
b. Why oil is used for very power (100X)
magnifications and not for other lenses.
C. What precautions to be practiced in
handling microscope. What measures if taken,
prevent inadvertent damages to objective lenses?
Ba) Use of Cordansoh - Collect R focus light
fom lumirofor
into the specinen
that roache the speinMen.
76
b)0
în
for
l00x
gnificotfon,
in orde to
alr
is used
elfminate
due to refroction,
e
ight passes
Ommersion
ol ho
ho
e Precattons
(c)
fron y lonto aiy
tndex
same
hle hordliog miroscobe -
coted
D Rile adjusting the
() 9 shoula be
have
leck
look
to
its
ho
neck 2 bane
acijinti1
coqrse
should
Vover the side R
the cye piece.
to
inadver
tent
damage
prevent
to
Pre Couticn
)Never clean the objecve
ith
alcohol
the condenseh
cement
bj lens the
ay dissolvoo
Use
lo0K
)(i) Never loo
the
77
înto the tube
opfcat twb
ing
whhile
COarse
lOw ers
stent
Chapter 2
Equipment and reagents
OBJECTIVES
To ldentify and understand the -
Various equipment routinely used in haematology labs including pipettes,
slides, Neubauer's chamber, ESR tubes etc.
Various reagents and chemicals including anticoagulants used in the
haematology related experiments and its clinical utility.
Various stains used for basic haematology experiments and their composition.
ntroduction:
or allhaematology related tests and experiments certain basic equipment are essential for all the labs. These
nclude
a. Hemocytometry set: This includes RBC and WBC pipettes, Neubauer'scounting chamber etc.
b. Hemoglobinometer set: This includes Hemoglobinometer pipette, Comparator, Sahli-Adam's
Hemoglobin tube etc.
c. ESR tubes: This includes Westergren's tube and Wintrobe's tube.
d. Micropipette set
e. Miscellaneousitems
Various chemical substances including stains, anticoagulants and diluting fluids are another important
group of things that all must be well versed before working with them in the hematology set up. They
include
a. Staining solutions: Leishman's Stain, Wright stain, Tårk's fluid, Hayem's fluid, N/10 HCI, Dunger's fluid.
b. Anticoagulants: This includesDouble oxalate mixture, EDTA (Ethylene diaminetetracetic acid), Heparin
(Powder or liquid) and Special anticoagulants ACD (Acidcitrate dextrose) etc.
c. Others: Rectified spirit, Normal saline (0.9% Nac), Distilled water, Buffer Solution, glacial (pure) acetic
acid etc.
79
Equipment:
Hemocytometry set:
This is one of the most essential set of basic
equipment required for basic
experiment.
Hemocytometric experiments are those experiments in which one is requiredhemocytometriC
to count a cell or quantify it.
presence in a known volume of bodyfluid.
NEUBADER'S CHAMBER
t00 UNITE
tet Uste
RBC PIPETTE
WBC PIPETTE
Hemoglobinometer set
HernGneter
80
his is an essential set of equipments Used cOmmonly for hemoglobin estimation by Sabli's acid hemnatin
rethod The set includes a comparator, Sahli- Adam's Hemoglobin tube, hemoglobin pipette and a bottle of
10Hcl.
SRtubes:
SAor Enythrocyte Sedimentation Rate is atest the speed of settling down of RBC in its natural suspended
ate is measured. This is one of the essential clinical tool by which many diseases could be prognosticated
eseibed in details elsewhere). Two commonly used tubes for measuring ESR includes
and
a. Westergren's tube
b. Wintrobe's tube
Wintrobe's tube is also used for measuring packed cell Volume (PCV)
25 mm
20 cm
a) Westergren tubc
b)Westergren tubc on the rack
Wintrobe tube
cropipette set
stiy used for experiments where very smallyet accurate amount of chemicals are needed tobe dispensed.
81
Staining solutions:
Leishman's Stain
This is one of the comnnonly used stain in the undergraduate haematology labs. This contains a pair of ari
and basic dye in alcoholic medium to stain various acidophilic and basophilic substances
present in the whis
tbloodcells
The composition of stain includes Leishman power 0.15 gm dissolved in 100 ml of methyl alcohol (Acetor
free). Leishman powder consists of following dyes- Eosin, Methylene blue.Eosine is JISed for
staining RBCs an
Eosinophilic sranules, Methylene Blue is used for staining nucleus and basophilic granules while Meth
Alcohalis used Rs fixative it causes sticking of cells to the slide via protein(of cell membrane) precipitation,
Wright Stain:
Wright's stain is a combination ofacid dye (red 'eosin')_and abasic dye ('methylene blue') for use of stainin
the blood smear. It highlights the differences among the different types. of leukorytes
(immune cells) for easi.
recognition of eosinophils or basophils during the counting process.
Turk's fluid:
It is used for staining WBC's in doing total leukocyte count. It consists of
(i) Glacialacetic acid (3 ml) - to lyses/destroy the membrane of WBC, RBC, and Platelets.
(ii) Gentian violet - 1% (1 ml) - to stain the nucleus of WBC.
(iii) Distilled water- to make the 100 ml.
Hayem's fluid:
It is used for diluting blood for RBC Counting) It consists of:
(i) Sodium Sulphate (2.5 gm)- to prevent rouleax formation.
(ii) Sodium Chloride (0.5 gm) - to maintain isotonicity of the fluid.
(iii) Mercuric Chloride (0.25 gm)- preservative, antibacterial and antifungal.
(iv) Distilled water- 100 ml as solvent.
Dunger's fluid:
It is used for diluting blood for absolute Eosinophil count. It consists of:
Eosin
1
%
(i)
aqueous
solution
(5
ml)
Acetone
(5
ml)
to
inhibit
the
lytic
action
of
(ii)
stain
to
water
(ii) Dist. Water (90 ml) - to lyses the red cells.
on
the
Eosinophil
leukocytes
Reticulocyte diluting fluid:
It is used for reticulocyte staining so that it could be identified with ease. It consists of New Methylene blue,1
gm dissolved in 100 ml of iso-osmotic phosphate buffer (24.3 gm/lof sodium dihydrogen phosphate and 21.3
gm/lof disodium hydrogen phosphate).
Anticoagulants:
These are chemical compounds that delays or prevents blood clotting by various mechanisms. Important
antiucoagulantsused in medical laboratories include
1. Double oxalate mixture - acts by chelating calcium (Potassium oxalate alone causes shrinkage of cells
and Ammonium oxalate alone causes swelling of the cells).4 mg of Potassium oxalate and 6 mg of
Ammonium oxalate taken as mixture can prevent coagulation of 5 ml of blood.
82
FDTA(Ethylene diaminetetracetic acid)
concentration of 1.2
mgot the
prevents coagulation by (helating
anhydrous salt per
alium This requires a
ml
of blood.
: Heparin(Powder or liquid)- acts by inhibiting thrombin
and other stages of clotting factor activation
tive anticoagulant at a
anticoagulants
Special
4 Rlod sugarestimation
concenttation of
10 2I0/ml of
blood.
ACD (Acid citrate dextrose) - used in
blo0od banks, Fluoride and oxalate for
sYnment:
1 Whatarethe differences between
Westergren's tube and Wintrobe's tube used for ESR?
Write uses of Leishman's Stain, Wright's stain, Turk's fluid, Hayem's fluid and Dunger's fluid.
3. Write precautions while utilizing chemicals in Haemtology Lab.
4 Write down the composition of Leishman's Stain and functions of each component.
ointr obe 's tube
westergrens tube
200
mm
long
cifhae as
ongiccngulont
- O.5 ml o}
More
- EsR
diameten
- EDTA
ortog
tiameten
wwecl.
|eme
to limieo length
Oué
fsR meaurement
measurement
ocwrate
.
ioaccuratt
Leishman's staun To san perihaal DUde
blocd
for Tdent'tA
ferant
cels
perípher al blood smear io order to
Trk's fluid- To sain
Used to i lu je blood in
blood
%pkin cout
eo«irbphI
83
RBC
cant
absolutl
3) Pre ouion ot le
usi
chemical
should ear prqecqve Gothg
closed
Dent
mouth
Dont spll
shoe
plrete the chemical
he chemi cal
CCmposiioo
blue
Netyl
(
84
Mormat
heishman's sjain
Basic
4
functta
enpaieai
c4ain acið pat
tye,ain bosic py
dcohot - Fixes the
etone fr ee) s
@cetone
smea
acefone
to side
Caue
Chapter 3
Collection of blood samples and Preparation of
blood films
OBJECTIVES
To understand the
Process ofblood sample collection
Do's and Don'ts of blood collection
procedures
Universal precaution while handling body
fluids
Introduction:
The first step in performing aquality hematological test result for any subject is the specimen collecti
procedure. The venipuncture procedure is complex, requiring both knowledge and skill to perform. Sever
essentialsteps are required for every successful collection procedure.
Two types of blood samples are commonly used for haematological examination
a) Capillary blood
b) Venous blood
a) Capillary blood: use sterilized disposable needle or sterilized heating over the flame. After applyins
spirit, prick the ring finger deep enough on its palmer surface, so there is free flow blood.
b) Venous blood: blood is best withdrawn from an antecubital vein by means of drý disposable plast
syringe.
Difference between the capillary and venous blood are:
1) Capillary blood is obtained by skin puncture from the finger or ear lobes but venous blood is obtained by
vein puncture from any convenient vein.
2) PCV, Hb Content, RBC and platelet count are significantly less in capillary blood but these values are higher
in venous blood.
S
Areas to Avoid When Choosing a Site for Blood Draw:
Certain areas are to be avoided when choosing a site for blood draw which include
Scar tissue - it is difficult to puncture the scar tissue and obtain aspecimen.
Hematoma - may cause erroneous test results. If another site is not avilable, collect the specimen
distal to the hematoma.
Intravenous therapy (IV) /blood transfusions - fluid may dilute the specimen, so collect from the
opposite arm if possible.
Cannula/fistula/heparin lock - In general, blood should not be drawn from an arm with a fistula or
cannula without consulting the attending physician.
Oedematous extremities - tissue fluid accumulation alters test results.
86
do.
to
not
hat
prolongedtourniquet application
void
Avoid massaging,
squeezing, or probing asite
required, Centrifuges must never be
operated without a cover in
Avoidexcessive fist clenching
centrifugation is
If
hattodo
place.
. Proper sterile gloves must be worn
Properr cleaning of the
universal precaution
site with spirit must be
done unless
for handling body
must always
" Subject'sssafety and comfort must be thefluid
priority.
A
contraindicated.
be practiced.
film:
eoarationofblood
finger and touch the
bleeding point at
lsce the narrow edge of the spreader just in one end of the slide.
front of the drop at the angle of
1. Prick the
45° till the blood
spreads along the width of the spreader,.
to
3. Spreadsmoothly the other end of slide. Allow the film to dry in air.
Daur leishman's stain on each slide enough to cover
the smear. Leave it about for 2 minute. Now
add
fow drops of butfer solution. Mix the stain and buffer
slowly by gently blowing air
leave for 10 minutes (in summers put it under moist chamber).
.Wash the slide with tap water gently till the film has apinkish
tinge.
6 Watch it under the mictoscope.
1 Plsce a small drop of whole blood
VERY CLEAN shde. Hold a second
sbde at the angle shown.
2. While mantaining cortact wih the
top shde back to cortact
bgtom shpullwillthepread
by capillary action.
the drop,
3. Mairtain fim contact with the
bottom side and push the top shde in
one motion to produce the smear.
87
intermittently.
Now
Assignment:
1\Wite precautions while collecting blood samples.
2.Write teatures of a good blood filon.
O Precoution chile
Blood sample &
i) Proper Oseptic cond need to be
mainained
i)
near the tp bf the
near the nail
Aveid
pri
c
t
i
n
g
Ralse avoid
it too for auday fron th tp
(ti) wipe off the |st dvop f
blcod ant conaim 'ssue fhtu
(ivy Let
olcoht dy before prickin otherotse bjod
spread,
Foorure of good blood
() Shoulà be
i)
Should
(i)
- (v)
t
not be
streak.
No holes or
.bubble shoul be
shoulo be
nt
Uhlayered
() Shouldnt be too
thiCK On toD in.
(vi) Shoula Covet
82l23
88
Chapter 4
ldentification of various blood cells
OBJECTIVES
To ldentify and understand the Structure and functions of various blood cells
Identification features of various WBCs and RBC
ldentifying features of various cells in blood smear
Introduction:
ldentification and counting various cells is one of the important basic haematological skills required to
learnt for the best interpretation of clinic-pathological conditions and associated haematological changes T
cells are identified on the basis of following characteristics
Cell size
Shape of the cell
Presence or absence of Nucleus
Number of lobes present in the nucleus
Presence or absenceof granules
2 illc roh0
Size and staining characteristics of granules
On the basis of above characteristics detailedfeatures of individual cells could be tabulated as below
Cells
Size
Nucleus
Granules
cytoplasm
-(um)
RBC
7-8
Absent
Absent
Pink or red colour, lighter in the
centre as compared to periphery
periphery.
Neutrophils
Eosinophil
Basophil
10-14
10-14
10-14
1-5 lobes, purple/Fine, few in number, Pink/purple in colour because o
blue colour
pink/purple
Bilobed,
purple/blue colour
Coarse,in
large Not visible because of lar:
number, red or brownnumber of granules.
Bilobed,
Coarse ,Large number, Not visible because of granules.
fine granules.
purple/blue colourdark blue colour
Small
Iymphocyte
90
7-10
Single mass, filling| Absent
almost whole cell
Scanty
A
10-14
Single
covering
nyphocyte
Skyblue colour
mass Absent
2/3
of
cell, purple purple,
course and lumpy
14-18
onocyte
Purple
kidney /
Ground glass hazy blue appearance
colour, Absent
horse
shoe shaped
EcentricEEEcentri
one side
2-4
Platelet
Brown/purple granules Pale cytoplasm.
Absent
ssignment:
1 Name the characteristic features and draw colored and labeled diagram of various cells seen in the
peripheral blood smear.
14e) RBC]:- Round Bodies, Anucles, no
aiscete 'moterials.
2e
to
’
biconcave
gronwles
red oith darker
shape
move
Hb
at
On
size7 um. Central pal or (3e
the
the
cel)
(Gcentral polor
(b) Platelets|- No nudeus , looks rettocfle On fine
adfustment louo power looK (ike s4ained deposít
’stons mouve pink
present in
2 um dfom.
B
(c)
, 5malleot
in periphehal
Neutophills
- Mulilobed (2-6)
nucleus, stim
by
connecteo
thig strardo . Contains fine
pok
that
I0- 14dm . Most aod
Multilobed nucews
ight pink g1onules
91
sme
duo
Silched nodlo nncco0
Sttond
onule)
bu
10-14 Um
Telephoehapad nudeuy
()
B0sopkm:
0bed
Clls
piny
nucau.,lobes ate not distinct
`nCUNded by grorul
be
c
a
u
10-14
on.
LAlue, block
(vi) Smoll lr
Thin
ig
6-9 Üm
cOOIse basephi c
g0nules
hocytes Nuclaus 0ccupie, dmost hole ceil.
}
ccloured
ealoured blue
blwe ytoplom ko cbeewveo
lorgeucleuy
Rim ytoplan
(vi)LoIqe
ymphoyte-Ncleus oceupies 80- 9o 7 }cal
’NUcieu s4an
staons violet , blue
toplon sithout fonve.
(ix) Monoted i
Nudeug 0caupies
) 12-24
50- 60
m
cell
kidny |hose shoe shapd., may be Aouno, ovo
Jtoploorn
in appeohonce.
’NO
gronules
Is
idry shaped
92
Chapter 5
Differential Leucocyte Count
OBJECTIVES
To learn the
Techniques of identification of various WBC
Structure and functions of various White Blood Cells
Find out the relative count of individual WBCs
rroduction:
hite Blood
Cells constitute an important part of human Immune syystem. They are involved in both cellular as
I 2s humoral inmunity. The relative count of various white blood cells are also used as an important bastC
gnostic as welll as prognostic tool for various acute and chronic disease processes.
w to do differential WBC(DLC) count:
hestepsinclude
Preparation of blood smear
Staining of the smear
Fxamination of the stained smear
L.ldentification of different types of leucocytes:
is done by
efore attempting to identify the cells, it is essential to access the quality of the stained smear. This
boking at neutrophils (which can be identified by its morphology alone regardless of its staining). The colour
the neutrophilic granule is used as a standard for assessing the staining of other granulocytes. Besides, a
od theoretical knowledge of the characteristics features of different leucocytes is essential before
oceeding to identify them under the microscope.
2.Counting of the different types of leucocytes:
Aleast a hundreds cells are identified and counted in systematic manner. Counting is recorded using the
columns and 5
ally-bar method. Make the entry of identified WBC's in a box containing 100 squares (20
Ows). Calculate the percentage of various leukocytes.
93
Observations and report:
Subject name: jjowal Age/Sex: 19/M
Date
2
L
2
N
NL
Indication for test:
N N
NL
L
L
L
2
L
N NNL
NN
L
L
L
2.
L
N
?2
MN
N
N
Cells
L
NL
2
2
NNN
N
2 Z
2 2
Count(Tally mark)
N
L
N
2
L
NNNM N
Observed Value
Normal range
Neutrophit
67
50-70%
Eosinophil
2
1-4%
ssre
Basophil
0-1%
Lymphocytes
28
Monocyte
Inference: All
NL
3
20-40%
2-8%
are in noimal
Assignment:
1. Write
differentiating features of various Leucocytes.
2. Enumerate the conditions that increase or decrease the different types of
leucocytes.
94
vaviou) leukucytes i
Je
onules, bosed on stain
Jonule, munilobod ucleun
th
Shapea nuclo)
-
-
Basophi
coarse
block
blocg- se
bilobd nuclery but no1
numoros
vicible due to
staininy
" y t e s - No gronule proent qvonule
-snepyte- Nucleus covers atteast 90 , Rin iro
Ik almost that
RBc (6-9 in)
y
-oN
l u c l exuy corer x anost 80- 9o%:
(10-14 um)
-Monocyte
Nucleus
coVers
Kidney
(12-24
Lm)
insy
So-6ogle
cell.
Nautrophiuta
Petholegical
Yrflomation,Bat
veii se, P'eponcy,
ongicoal ten
ingection , leukemia,
aate hemovvhasd
eosinophilila
cer4ah
(i)
porasie fnfecfon
leUkomia, 'sn dsee
Pascpkiu
Basophilia
HIgy.
infiomatory cord9.
CM
shapeo nuc,
Neutroperia
Physioleical
ve
chinic
Ty phoid, vral
(sd
injecton, apemia.
patie
eosfnopsnia
therapy
Bascpenia
corfissn thaay cosin's
PhOtopeniq
Chronc R
()
infocfon,
MonoykA
MOhGtes
Peycan ds, ACTH therapy
95
imunosuprve thera, AcTH therapy
MOneytopen~a
Chapter6
Arneth Count
OBJECTIVES
To learn the
Techniques of Arneth count
Clinical implications of Arneth Count
Introduction:
Arneth count is the technique by which on the basis of
number
of lobes in the nucleus of
the predominance of immature or
hypermature neutrophile . t
helps in identification of the underlying
pathology responsible for the clinical condc
neutrophils are classified and analysed for
blood. This
information
present.
neutrophi,
Procedure:
1.
It is
Preparation of smear:
same as DLO The stained film is seen under oil
immersion(100x) lens. Only the neutrophils are obServe
and the number of lobes in their nuclei are
counted.
2. Rules of counting the number of lobes:
For two lobes to be called separate
they should be connected only by a
strand of chromatin and
nucleoplasmic connection should exit.
3.
Method of tabulating observations:
The tally-bar method is used and at least a
hundreds
categories.
neutrophils
are counted and divided in following fye
Observations and result:
Subject name:
Age/Sex:
Date:
Cells with various
Indication for test:
Normal value
lobes
Single lobed (Stage 1)
Double lobed (Stage 2)
30%
11||
45%
Four lobed (Stage 4)
18%
Lobed
(stage 5)
96
or
more
Total count
5%
Three lobed (Stage 3)
Five
Count (Tally mark)
2%
34
43
11
2
erence:
sigDments:
Nuite significance of Arneth Count.
Vhat is Arnethindex.
OAneth count c
Usefl
°f formoti erophl
there
of formoti, s staqi
Wçh which
D , N,
NË> o'
hyperociv ity % bone
common Causes ahe
the fodex
Jeater
then ?t
shift
T5is îndiates
mario.
exposdtrm, ho amorhage
X
IV,Y . 2VI
hyractvt
Comon
Cacdi
are -
Perniciouw anemi q
Bone
Yea ter
Poaiot.
bone
. vitomin deficiany
aso
283|23
97
Papeat
MGTrow
t
el
neutphill
an
98
72
sgeo.Different
Anéthindex
number
eont
nCount
neurophl
with
67N,-
Col)ed
lobes
nucleo
is
lc Ce
Counng
number
a
Chapter
Estimation of Haemoglobin
OBJECTIVES
To learn the
Technique of hemoglobin estimation
Clinical implications of hemoglobin content and anaemia
troduction:
Remoglobin estimation is one the most important bed side investigation in clinical haematology.
eemoglobinis an oxygen carrying substance present inside the RBCand its content forms one of the basis for
sfication of anaemia.
Bemoglobincould be estimated by various methods including-
Shli-Adam's Method
ere'smethod
eden's method
introbe's method
Eldane's method
Elliquist method
BSometric method
Sbectrophotometric method
utomated &non-automated hemolglobinometry
kalinehematin method
etermination of Iron content etc.
Out of above Sahli'-Adam's acid hematin method is common,easy-to -do bed side technique though not very
KCurate method of haemoglobin estimation. Spectrophatometric method is one of the most accurate but
ESOurce intensive method of haemoglobin estimation.
Sahlr's Acid hematin method:
Principle: Acidic environment breaks the RBC to release haemoglobin which than combines with the acid(N/10
FC) to form acid hernatin. The colour of the compound is proportional to the haemoglobin content.
aemoglobin is estimated by colorimetric measurement by comparing with astandard comparator.
Procedure:
1. With the help of adropper, pour N/10 HCL in the graduated tube up to 10% or 2gm% mark.
2. Draw exactly 20 cu mm of blood obtained by capllary puncture into the pipette; wipe excess from the
tip of the pipette.
3. Transfer the blood from the pipette into the gradualed tube and rinse the pipette several times
drawing the acid in and blowing it out. Avoid foaming.
4. Mix the content thoroughly with the glass stirrer and allow the solution to stand for 10 minutes.
99
Add distilled water or N/10 HC drop. by.drop unil the color of the solution
matches
color Ensre thorough miving of the solution During color matching, the stirrer should hethe
tdarnat
1i
f
t
e
Since the colot may seemto match over a wide tange of dilutions, it is advisable to take the
thesolution but not completely out of the tube
6
2 reading during the course of diluton, the first when it is just discernibly darker and
when it just gets a shade lighter than the standard
7 Read the Maemoglobin content from the scale provided in the tube
Observations and result:
Subject name
Age/Sex:
Indication for test:
Date 07| 02) 2 3
Observations
Haemoglobin content
darker
tend< to
motch
Inference:
Assignment:
1.
2.
3.
4.
Estimate the Hemoglobin of your own blood.
Write the significance of percentage hemoglobin.
What are the precautions observed during hemoglobinestimation.
What are the functions of hemoglobin.
100
the seo
3
Normal
*|2- 16
gtal
Ou blood <hoc
body
Nermal
7 Hb
m ale.
fore
te
health
|2- I6 l l
than normal
len
i.e Aneniq
i shows the preence
R
i4 shows the
reater than norma
conditon ike pOlycereni a Ver .
nemia may Guse NeaKnen . fo tigu, pale boty.
low
) Precaution dusirg
"finger shouldn
Bloo
from the
baty
Hb ostinator a
o follows
be squeez ed to 4aro
nt te bloo
pipette shoulo bt transferred
in pipet
corHaanig
o nej to prevent
HCe. shoo be
ofter mixig oth Nho nce
left for oteaot Ug-10 moue for complet
Blod
tormaion
Distileo o04e
soure
aca hematin
shoulo be adde? dop by oopP:
ght
gaint nsal
28|3|27
101
Chapter 8
Todetermine Blood Groups
OBJECTIVES
To learn the
Technique of blood grouping
Clinical inmplications of bldd grouping and cross
matching
troduction:
groupingis a classification of blood types based upon the presence or absence of various antigenic
od
stances on RBC surfaces and antibodies in the plasma. Information
about the blood grouping is essential in
seof bloodtransfusion to avoid mismatch transfusion and associated complications.
blood-group systems have been identified by the International Society for Blood Transfusion in addition to
ecommon ABO and Rh systems. Some of them are MNS system, Kell system, Levis system, Duffy system etc.
MofallABO and Rh system is most consistent and prevalent system.
termination of blood group:
inciple:
o6ence of absence of antigen at the surface of RBC membrane and its reactivity with antibodies determines
eblood group of an individual. When the blood is made to react with exOgenously administered antisera,
eantigen cross reacts with the antisera to form clumps which can be examined under microscope to confirm
epresence of particular antigen in the blood.
ocedure:
Pour one drop each of anti-serum A, anti-serum Bandanti-serum Don a clean glass slide with a dropper.
Label the drops on the slide for identification.
Add adrop of the blood to each of dropson the slide and mix with the help ofseparatè applicator sticks.
After waiting for 10 minutes, rock the slide gently back and forth and examine macroscopically for
agglutination. Confirm the findings by microscopic examination under low power after placing cover slide
over the drop; when there is no agglutination, the red cells are wellseparated and evenly distributed;
when agglutination occurs, the cells are massed together in clumps and lose their outline.
The blood group is determined as indicated in the table below:
AntiserumA
Antiserum B
Blood Group
A
B
AB
Agglutination present
Agglutination absent
agglutination is present in Anti Dserum, blood group is Rh +.
O8gutination is absent in Anti Dserum ,blood group is Rh-.)
103
nomed togotter)
ore
cells
(.
somole
utinaion of the blocd
Observations and Result:
wth
Bload
i-B servm
jhayp-[B+vel
Inference:
Assignment:
1. Determine your own blood group.
2. What is Landsteiner's law?.
3. What do you mean by a "universal donor" and a "universal recipient".
4. What is cross-matching of blood and its significance.
5. What is Rh incompatibility and its clinical significance.
6. Enumerate the hazards of mismatched blood transfusion.
R8C
is Ont on the
low sttos wembr an e
be
1s
obsent
Snt in the
the
RBC, the
be piesent
becquse RBC conain
: Thorefore bood con be
(3) Univar sal dono- Per son oith
no
O-ve
Universal ecepien[- persOn th AB ve
plaoma contaim no
because thei
Antibody
28|3|:>
104
Chapter 9
Bleeding and clotting time
OBIECTIVES
To learn the
Technique of blood grouping
Clinical implications of bldd grouping and cross
matching
Landsteiner's law
roduction:
adure:
Aleeding time(Duke method):
Make a4mm deep puncture in the finger under fullasepsis. Note the time.
seconds, remove the drop of blood by applying a clean filter paper. Do not apply pressure.
Don not squeeze the finger.
30
2. After
, Reneat the procedure every 30 seconds until bleeding stops and no further blood spots appears on tne
filter paper. Note the time again.
Bleeding time is the interval between the time of puncture and the time when the bleeding ceases.
It can also be determine by lvy's method,
" Normal range 2-6 minütes.
s dotting time(Wright's method)
1Draw adrop of blood obtained by capillary puncture into a glass capillary tube (about 10 cm long).
Note the time
2. After 1 minute, break off about 1 cm of the tube: very gently separate the broken ends of the tube and
observe whether a strand of clotted blood (made of fibrin threads) connects the broken ends. If no
strand is observed, repeat the procedure every 30 seconds tillastrand of clotted blood appears.
" Clotting time is the time taken for the appearance of a strand of clotted blood (which should be at least
5mm long)after the blood comes in contact with glass.
" It can also be determine by Lee and White method.
" Normal range 3-8 minutes.
signment:
1. Estimate Bleeding Time and Clotting Time of your own blood.
2 What willbe the effect of environmental temperature on Bleeding time and Clotting time?
. What are the conditions in which (1) Bleeding time (2) Clotting time willbe prolonged?
107
Uijausal Anand Choudha y
Dotg :- 31joa]23 (2:4s PM)
Sleeding TIne - 2 min. 30sec
indecrey e
upto
40c -
bloo
Physioloical - meneuropon boo
Patnolcgical Memopbillia
" livor cisene
oejieiang
Dlsiminao in vancutr coajoo
flbrnoganemA
Chistua
108
disaonl
Chapter10
RBC Counting by Hemocytometry
OBJECTIVES
To learn the
Use of hemocytometer for various purposes
Technique of RBC counting
Clinical implications of RBC count
Landsteiner's law
Introduction:
RBC counting is one of the basic
haematologicalprocedure routinely done for bedside assessment of
associated with quantitative change
in RBC content of blood. Though high end
flocytometers are
use for above
is one the best and easy to do technique for RBC
purpose,
hemocytometry
the resource limited settings with fair
i
accuracy.
A brief introduction about the
equipment has already been given at the earlier chapter, some
more detaile ,
being given below about them for the purpose of better
of the technique.
understanding
A. Haemocytometer
disorder
alreadyial
counting especi
Haemocytometer is an apparatus used to do count of various blood cells (RBC, WBC,
It consists of RBC and WBC
pipette and a thick slide (Neubauer's chamber).
1. RBC Pipette
eosinophil and platelet
(0) This
consists of a glass stem having capillary tube in it which
opens in a bulb containing red
to the bulb again there is a
small stem. This small stem is connected to beadand
the ed
colouredmouth piece with the help of a rubber tube.
(ii) The stem has three markings, 0.5, 1.0 and 101.
From the tip of the pipette to the marking 1.0 there ae
10 equal divisions. These are
simple
opposite
divisions not any specific unit like mm, ml, and cu mm.
(iii) The stem has the capacity of one part and
bulb has 100 parts.
(iv) Bead of the pipette serves two purposes one
mixing of the blood with diluting fluid and other act as
an identification mark of.RBC pipette.
2. WBC Pipette
This pipette is similar in shape except size of bulb is
smaller, markings are 0.5, 1.0 and 11 and bulb contains
white bead and color of the mouthpiece is white.
3.
Neubauer's chamber (Counting Chamber)
()
This is a thick glass slide having central platform
divided into two positions with the help of hH
shaped groove or trench.
(iü)
On both the sides of lateral groove there are
raised ridges of a height of 0.1 mm (1/10 mm) trom
the central platform. When a coverslip is placed on the ridges, a
space of 0.1 mm height S
created below the coverslip on the central platform.
(ii)
Counting chamber is made up of ruled area of 3x3 mm size on each central platform. Each
centra
area is further divided by triple lines into 9 squares of equal size (1
square mm each).
110
(N
Four
coner squates ale futhe divided
into 16 quares o Cqual
Used to do total|
leucocyte count
Volume of each big square is 0.1 cu mm (lmm
q0ares
size, Ihese four corner
x0.1 mm)
(vi) Central big square is divided into 25 (medium size) squares each having arm 1/5 mm. Area of
Cach medium size square is 1/25 sq mm (/5 x 1/5) and the volume of each square is 1/250 cu
mm (1/25x1/10).
(vilFurther these medium size squares are divided into 16 small squares of equal size. Area of each
small square is l/20 mm (1/5 x %). The area of each souare js 1/400 sq mm (1/20 X 1/20)
volume IS 1/4000 cu mm (1/400 x 1/10) Total number of smallest squares is big Central squdie
are 400 (25 x 16).
)
RBCS are counted in 5 medium size sauares (R1 R R3 R4. R5), four corners and one central.
MAEMOCYTOMETER (COUNTING CHAMBER)
1mm
Procedure:
Suck blood (obtained by capillary puncture) into the RBC pipette up to the 0.5 mark. (If only a small excess of
blood is drawn in, say up to 0.6 mark,this excess can be removed by tapping against the palm of your hand. If
however, the more than this drawn is, say up to 0.7 mark or more, the procedure must be repeated after .
dleaning the pipette.)
Immediately thereafter, suck Hayem's fluid into the pipette up to 101 mark.
eep the pipette horizontally between the palms and rollgently for about a minute to ensure thorough mixing
of the blood and the diluents.
Pace a cover-slip on the Neubauer's chamber disposing it symmetrically above the ruled area on both sides.
Focus the Neubauer's chamber under low power(X10), of the microscope. Remove the chamber from the
microscope and place it on the table.Do not disturb the focus of the microscope.
DIscard the first 4drops of fluid inthe pipette. Allow a small drop of fluid to form at the tip of the pipette and
BEntly bring this drop in contact with the edge of the cover-slip. The fluid is drawn into the chamber by
epillary action. Charge both side of the Neubauer's chamber. An ideally charged chamber is one which has
Een chargedwith a single adequate sized drop which just about fills the chamber without leaving air-gaps. If
111
the fluid overflows into the gutters, it is called over-charging: if it is insufficient to fill the chamber, itis
cleaning the
under charging. Should the chamtber be under or over charged, repeat procedure (after
chamber
and the cover slip again). The correct size of the drop required by practice. The
charged in parts by two or more smaller drops.
cal en
shouldchanotmbe,be
focus).
Keep the charged Neubauer's chamber under the low power of the microscope (already in
focusthe
time for the cells to settle down in the chamber. Using the fine adjustments, bring into uniformly cells onsomethe
ruled
area and see the distribution of the RBC's in the ruled area. (If the RBC's are not
clean the chamber and recharge it). Focus the chamber now under the high p0Wer (X40) and count the cells in
Alow
the S RBCfields (1/25 mm2 size).
distributed,
The rules of counting In a square bordered by single lines, any cell which is touching the upper or the left border is counted even
though it may lie outside the square.
Acell touching the lower or the right border is not counted even if present within the square;
the square
In squares bordered by triple lines, the middle of the triple lines is assumed to be the boundary of
and the same rules outlined above are followed.
Calculations:
Calculation of the dilution factor
0.5 parts of blood and 100.5 (101-0.5) parts of Hayem's fluid are present in the pipette.
(Which is discarded).
Since 1.0 part of Hayem's fluid in the stem of the pipette does not mix with blood
(0.5+99.5) þarts of
Therefore 0.5 parts of blood mixes with 99.5 narts (100.5-1.0) of Havem's fluid to form 100
the solution.
Therefore, diution factor is 100/0.5-200(Dilution Factor DF)
Calculation of the volume of the fluid examined
The area of 5 RBC fields = 5X (1/5 x 1/5) = 1/5 sq mm
Therefore the volume of fluid on the 5 RBCsquares = 1/50 cumm
Calculation of the RBC count
If 1/50 cu mm of diluted blood contains 'n' RBC's
Then, 1 cu mm of undiluted blood contains:
nx 50 x 200 = 10,000n RBCs
Normal range for RBC Counts:
5-5,5 million /cu mm
Females 4.5-5.0 million /cumm
Infants 6-7 million /cumm
Males
RBCCount
Normal range for reticulocytes 0.2-2% of
Neneofsubject:
jowal
ndicationfortest:
Age/Sex- 19 M
date:
observations:
slel6.
149][68
37
Li
38
21
139
Calculation and result:
Inference:
Assignment:
Write precautions while using various componentsof Haemocytometer.
Enumerate the uses of Haemocytometer.
Determine RBC count of your own blood.
Write conditions in which RBC count is increased.
Write conditions in
113
which RBCcount is decreased.
).Piecoutions oRle
usi
Oke cleon.dy wihout
Roncture shou
be cdoep
jst dop shou
be
Atscode
byoKen tip
ough for free flow
blood .
O Tip
rirette shoulð dip into the blood to prevant ar
bubble furm?.
aker
upto O5
mar K.
Blod
shoula be
,
Tip should be oied
PiPete while
) Blood
heroi se
extra blood
erters tho
houla be dilted irnmodio(oy
Tp pipet sheulà dip thoughout the
Bload sheuld nq contin any cis
While
Chonbes R Coversip - cleaned
Contentt }
2 dhops
bulb must be
h
Stom ao
tlu?
wust be
i bubbl oo shouldo' t
hombn
should o be
Vse of
thaghi
erly
ixed be foro chor
fom the
etters the chambes ohiie chargíg
over | urde4 chargod
Hemocyteme2 i
Used for countng blod cells,i.e, RBCs, leykocy
’
-’
thromboyteo.
Aso used for counti1
bact
Blood -
RBC Count
Voi
S
660
in
mm
S0x660 RBc
buted
undluted, o:
cox660x
t
114
6-6 nion
Aivtfon
4
spOses.
icreac ed
Plydelogical
RBC count
High oude (due
to byponie)
a
eount
NeobOrn bve
esive
ExCe»ive
suJeotig hemeconcentotYon)
Patelegical
L
chheia
h e a yera
ypoxia (oîn emphyenie
seyere
Bdheea
docreqs
Rec:
homdiluon
to
due
volue thon
- Preqnany
to
d e hemtilufon
Ch)ro have looe2
Phystoleg"Ncon
Omern
Pathologfaal
to different anena.
pituit'
poteñor
Case
tuoY.
8)2|23
5
thar nen
loer
havo
îo
goc: AOH secretion
men
Chapter11
To determinetotal WBC Count
ORJECTIVES
To learn the
Use of hemocytometer for total WBC counting
Clinical implications of WBC count
Introduction:
As statedearlier WBCS are integral part of our immune system. Total count of WBC has
got very
relationship with theimmune status of an individual. There are multiple physiological I
and
knowto have its influence upon the total count and differential count of WBC. Bedside
the important tool for bed side diagnostics in clinical settings.
WBC counting is done by haemocytometric method with reasonable accuracy in
pathologicalrelevam
facOnetors,
WBC counting IS
o
resource limited settinoe
Procedure:
It is the same as in the case of the RBC count except that:
1. Turk's fluid is used instead of Hayem's fBujd
2. Fluid is drawn up to 11 instead of 101
3. The fluid is allowed to stand in the pipette for about 8 to 10 minutes before
charging;
4. Counting is done in the WBC squares under lower power (X10)
instead of the RBC squares under hiok
power (X40); the rules of counting arè similar;
5. It is important to distinguish the WBCs (identified by their
refractivity and the presence of nuceusl
from the dust particles.
Calculations:
Area of each WBC square
1x1 =1 sq mm
Depth of the chamber
0.1 mm
Volume of fluid on the 4 WBC squares
4 x1 x0.1= 0.4 cu mm
If the total number of WBCs in 0.4 cumm
of diluted blood
then 1 cu mm of diluted blood contains
n
n/0.4 WBCs
Since, dilution factor is 20) therefore
1 cumm of undiluted blood will contain
n/0.4 x 20
50 n WBCS
Normal range 4,000-11,000/cumm
116
vations:
WBC.
WBC
RT
R
WBE
alculations and result:
Total
gC in al
4 bo
Nos, Tal va
=
I+ 26 + 29 + 25 : 98
.
aNted bloyd
ference:
total, oBc = 98 xdi. foco
Noimal wBc =
signment:
4900 Jm
( og
Determine total WBC count of your own blood.
Write conditions in which WBC count is increased.
Write conditions in which WBC count is decreased.
117
98
98
x 20 =
1
|2 | 3
-o
1
2 2
2
2|2
2
[a2|2|
[2oolo
3 122)
2 o o3
J22
5
29
21
ol 2
1
3
26
1
2
25
6
1
CALCUL ATION :
Total OBC in
all
4
boxes
: 18+ 26+ 29+ 2
=
Nou,
Vo |
4
3
bo e
wBcs
ution
undiluec
bl0od
facton
900 8ea
CRESULT
Normal
wBc caunt
('Normal
118
vange- ioo0-i1000/
Cond2 o
hich RBe WBC Count
is iocreaned
() Newborn
Phyiological- (ii)
)atrn
infont
Physi cat
exerise
Cotbelogisal
(i1) After food înake
(v) Pov
() Accute bocjerial înfection
()Chronic boceial ingectio
(iii) Hemohage
Snglamafy dizorde,
) wBC
count decreaoesi
Physiologica- expoure to severe Cord
PoYhological- i) Snfection- Tjehoid fevon
speais - Consumpti
i) Overwhelming speais
Neutophiis.
exoed
produ ctim
() Cyto twic
tÉre thaag
chlotomphenicol
pYi
119
Chapter 12
Absolute Eosinophil Count
OBJECTIVES
To learn the
Use of hemocytometer for Absolute count of Eosinophil
Clinical implications of AEC
Introduction:
An eosinophil count is a blood test that measures the quantity of eosinophil in
our body. Abnormal
levels are often discovered as part of a routine complete blood count (CBC) test. As the
differential
WBC relies upon the relative percentage of different types of WBC,
sometimes it is difficult to
apparent increase in one type of cell is because of actual increase in the cell
count or a relativesay if
because of actual decrease in the count of other cells. Thus absolute count becomes an
essentiality
counteosinionpghithel ot
increfiasguree
out the exact pathology. Eosinophil is known to increase in many
to
physiological and pathological condit
including allergic conditions, worm infestations, malignancy etc. Hemocytometric
technique is usually uced
bed side estimation of absolute eosinophil
count.
Procedure:
Dur
1. Get a prick in the finger under all aseptic
conditions.
2. Discard first drop of blood and fill the VWBC pipette up to
mark 1.0 with blood írom second drop.
3. Clean the tip of the pipette and suck eosinophil diBating
fluid up to mark 11.
4. Mix the fluid and blood by rotating the
in between palms. Wait-for 15-20 mih for lyses of
pipette
other
cells.
5
Mix the fluid once gain and discard two drops of fluid.
6. Count the eosinophil in 4WBC Squares under low
power. In case of doubt cell can be confirmed under hiph
power.
Calculation:
waaa
Dilution factor = 10
Volume of fluid in which eosinophils are counted
= (1 mm x 1mm x 0.1 mm) x 4 = 0.4 cu mm
No. of eosinophils in 0.4 cu mm volume = X
No. of eosinophils in 0.4 cu mm of diluted blood = X x 10
No. of eosinophils in 1.0 cu mm of undiluted blood= (X x 10 x 0.4) = Xx 25
Normal range 40-440cumm.
Observations:
1
1
120
ations
and,
result:
n 4bovey:
a eocinophill
4 boxes E
(cdiwfon focor 10)
untiluye
8x25=
200 ns
rence:
inophil
200 w
which is n normal
("" Nornal
ement:
coonditions in which eosinophil count is decreased.
in which eosinophil count is increased.
writeconditions
coi
Arite
der: -(az inepenia)
0fosînopb?l
Aplas ic anemia
steroid theapy
- Helminhie infocton
Ascaiama
A1lergie
121
Chapter 13
Platelet Count
OBJECTIVES
To learn the
Technique of platelet counting by direct and indirect
method
Clinical implications of platelet count
oduction:
telets or thrombocytes are one of the blood cell component responsible for initiation and maintenance of
emostatic processes in side our body. They are non-nucleated small cells that has got the ability to form a
porary hemostatic plug in case of vascular injury. Decrease in the platelet count is pathognomonic of many
o andchronic disease processes.
eother cells, platelets can also be counted bedside by hemocytometric methods with reasonable accuracy.
cedure:
RECT METHOD
Draw blood upto 1.0mark in th RBC pipette,and then suck thé dilution fluid (1% ammonium oxalate)upto
101 mark. Mix the solution and wait for 30 minutes. Discard one part of fluid present in stem of pipette.
Charge the Neubauer's chamber and wait for another 30 minutes.
Using the high power objective (x40), count all the platlets seen in the centre square (1 x1mm) whole of
RBC Counting area of the Neubauer's chamber.
ifnbe number of platelets counted
atelets count =n x 1000 platelets/ cu mm of blood
LUTION FACTOR
Final volume/ Initial volume
101-1/1= 100
ULTIPLICATION FACTOR
Dilution factor/Volume
100/0.1= 1000
ELATIVE METHOD
1. Prick the finger through the drop of diluents (ammonium oxalate) so the blood o0zes directly into the
diluents.
2. Take diluted blood on the slide, dry it and stain with Leishman's stain as usual.
3. Introduce a paper having small hole on it, into eye-piece of the microscope(already fixed in eyepiece).
4. Count under high power (40x), the no. of RBCS and Platelets in several fields until 100 RBCS have been
counted.
fnisthe platelet number as %of RBCs.
123
Nootpateets
nx RRS ount (pe Cumm of blood) / 100
The platelets an be cognized by gently altering the focus of the
microsCope
and by teducing the illomination by closing the iris diaphragm. Seen thus,
the
platelets ine adu
retaite bodies, Ihus, they can be differentiated from dust particles.
Nomal tange
with
ap ear
15 A0acs/cumm
Observations:
20
4
jmm
st
3
3
2
2
3
’2|
2
12
3
I6
44 4
30
’21
Calculations and result:
Total oD
NOL:
the 25 sq: box = 25xxx, =
9nmm
90 e s platelets
ituled Blood
=90o
undiluted bl0od
= 90O x
Assignment:
1.
Determine platelet count
of your own blood.
20O
I80000 platelet/nmy
2. Write
conditions in which platelet count is increased.
3. Write
conditions
in which
e s Platelets
: diluton focn
0"S l00= 200
platelet count is decreased.
Learn about thrombopoesis.
Cond?
-
% increased plolet Couoth
Poycethemia
Chonic
Veha
myeloae
leukemi.
- Svon doficieey anemi
splenectond
Snflomoto aisocer- Rheumotoid
124
Doyi )
plate\ef count deceaoed:
(Thyonoayieeniy
a) Smmune medaied
topena pupura
cy
thronbo
Sáiopotbic,
Prinary
0
(i) secondby- Autoifm mune, 1toimmune
keparin)
Po dvced
Snyecion (
125
(Buinidine
tomgo
visus)
Chapter l4
Reticulocyte Count
OBJECTIVES
To learn the
Staining and identification of reticulocytes
Counting the reticulocyte in the given blood sample
Clinical implications of reticulocyte count
troduction:
Reticulocytes are immature red blood cells (RBCs). In the process of erythropoiesis, reticulocytes develop
nmaturein the bone marrow and then circulate for about a day in the blood stream before developing into
ature red blood cells. Normally less than 2% reticulocytes are able to appear in the peripheral blood. But in
ertain conditions their count may increase drastically. Increase in reticulocyte count is also associated with
therapy given for iron deficiency anaemia. Increase in reticulocvte count is used as an index to tne
fectiveness of the therapy,
side reticulocyte count is done by simple staining technique using simple glass slides. It is simple yet.
ctive tool for quick diagnostic and prognostic bed side tool.
rocedure:
Take 2 mlof blood is obtained by vein puncture in a test tube and add the same volume of reticulocyte
staining fluid in the test tube.
Incubate the mixture at 37degree centrifuge for 20 minutes.
The cells are re-suspended in the staining fluid by gentle shaking.
Make a thick smear of the mixture. Let it dry.
In making the reticulocyte count, allred corpuscles that contain blue threads or granules are counted.
In relatively mature reticulocytes, only few blue granules or scattered threads will be found but these
should stillbe classified as reticulocytes.
Express the results as the percentage of reticulocytes in the total population of the red cells (mature
RBCs and reticulocytes).
Hormal range for reticulocytes 0.2-2% of RBC Count
bservations:
Result:
127
Chapter 15
Osmotic Fragility test of Red Blood Cells
OBJECIVES
To learn the
Technique of osmotic fragility test
Clinical implications Osmotic fragility test
Introduction:
Osmotic fragility (0F)
to the degree or proportion of hemolysis that occurs when asarmple of
red
cells are subjected torefers
osmotic stress by being placed in a hypotonic solution. The test depends blood
upon the
membrane integrity
which in turn is determined by multiple factors including cell volume, area to
tatio, membrane structure etc. It is a simple test with high clinical utility in bed side clinicS.
Procedure:
volume
Tube Metho:
I. ill 12 test tubes with serial dilutions of sodium
chloride solutions ranging from 0.28% to 0.72% with
difference of 0.04%
2. Add a drop of blood to each test tube, shake gently
and let it stand for a few minutes.
3. Cehtrifuge the tubes. If the supernatant is tinted
red, haemolysis has occurred. Note the
rangè in
which
haemolysis has occurred.
concntration
IL. Slide Method:
1. Saline solutions of 0.4%, 0.9% and
4.0% strength are prepared.
2. One drop of each solution is put on three
separate slides and one drop of blood is put on each drop of the
solutions.
3. Put acoverslip and observe allthe slides for the shape
of RBC's under high power of
Observations:
Result and inference:
130
microscope.
Chapter 16
lHacnmin Crystals
OBJECTIVES
To learn the
Technique of haemin crystal test
Importance of the test
ntroduction:
aemin crystals are the crystals of haeminchlorhvdrate formed when free
present in a blood stai
ets with chloride (present as NaCl in the blood) in the presence of hacmoglobin
glacial
acctic
acid. Though mostly
nsolete no due to more sophisticated techniques available. once it used to be an
test to deter1nine
important
hether asuspected stain is of blood or not and also to find out if it is of
human or animal
origin.
ocedure:
L Take adrop of blood in the centre of a slide,
allow it to dry.
Put a drop of glacial acetic acid, large enough to cover the blood and heat slide over a smalL
flame while
moving the slide to and fro.
When the acetic_acid has dried up, look for the presence of rhombic and
prismatic crystals of
HeminChlorohydrateunder the microscope.
bservations and result:
’hornid
Selh
Ssignment:
1. What is the significance of Haemin Crystals?
2. How Haemin crystals are formed?
Haeomin
blcod saal
’ Shape h
uoeo to disiguish
from Ghe
colour staín.
aries
baemin
bloed s
human
Rhombid shapad err
133
freoh or dried
Can
different species
be
congfimd
(inpo qont h eeoie
Chapter 17
Determination of ESR and PCV
OBJECTIVES
To learn the
Technique of ESR determination by Wintrobe's and Westergrens
methods.
Determination and use of PCV
Implications of ESR and PCV in clinical diagnostics
Introduction:
The erythrocvte sedimentation rate (ESR) is the ate at which red blood cells in whole blood descend in a
StandardiZcd tube in a period of one hour. It is a common hematology test, and is a non-specitic measure of
inflammation.
PCV Is a test that measures the volume percentage of red blood cells (RBC) in blood. The measurement
depends on the number and size of red bloods cells. It is normally 40.7% to 50.3% for men and 36.1% to 44.3%
tor women. It is a part of a perSon's complete blood count results, along with hemoglobin concentration, white
bloodcell count, and platelet count.
Procedure (Packed Cell Volume):
I. A lle more than 2 ml of blood is obtained by vein puncture and transferred into a vial containing double
Oxalate anticoagulant (a mixture of ammonium and potassiunt Oxalates ratio 3:2).
2.
ml of this blood is filled in a Wintrobe tube with the help of a Wintrobe pipette. Take care that no air
bubble is present in the tube.
3. Centrifuge the tube at 3000 rpm for 30 minutes.
4. Read the upper level of the red sediment of the RBCs.
Procedure (Erythrocyte Sedimentation Rate):
1. Take 0.4 mlof 3.8% sodium citrate solution in a small vial and mix it with L.6 ml of bloodobtained by vein
puncture.
2. Suck the citrated blood slowly into the Westergren's pipette upto zero mark.
3. Fix the tube vertically in the Westergren's rack,taking care that no blood runs out.
4. At the end of one hour, read the level of the interphase between the sedimented RBCS and the supernatant
plasma.
Express the results as
Normal range for Males
Females
Observations and result:
136
.mm in the 1 hour'
0-9 mm in first hour
0-20 mm in first hour.
erence:
signment:
Wne the inmportance of Packed Cell Volume.
Enunmerate the factors aftecting ESR.
ee conditions in which ESR is increased.
Wnte conditions inwhich ESR is decreased.
Smportonce of peV To
detect cordin ohich
ed, cell count inc.
ing blood indiceo, MCV 2 MCHC
or dec..
-dn deerining viscoity of blood.
Focors affectng EsR;
sizo h Rouleou - Lorger the ponicde,
fatet is the faling
> Plasma foctor fbincgen fovoure oulex form Colas
Proten ke flabuin ohich ane
neoplaotic is. ’ina £sR
Toulex
2
137
fom? dependo
hence cdec EsR.
shape
MCH Teandy Touior form
3) Con? inc
hysilogeal
Pegonay (due to emodiutio)
Catologial
Acupe infection (pneumonia)
Chzonc nfecton (Tubesculosi)
Rofocte iglaatie ( Grout)
Malinont discose
Acute non
’Aneia
Cond? decreaning ESR:
Pysaicgical
Pathologica
-polycthe mia
4fbrinagenenda
-’ Heredipy pheroT
’
138
siCkie
anenica.
14/2J2
Chapter I8
Red Blood Indices
roduction:
Indices are the various red blood cell parameters that gives information about the overall cell size, its
anacmia in the
ing potential and features that may lhelp in the
diagnosis and classification of
ANY CaTy
xdure
blood.
, Determine -() the Haemoglobin level (i) the RBC count (ii)the PCV of the given sample of
Calculate using the following formulac:
fL
CV=
RBC count (in millions/cu mm of blood)
Hb (g/dl) X 10
CH=
Pg
RBC count (in millions/cu mm of blood)
Hb (g/dl) X 100
%
CHC=
PCV (%)
omal values
MCV 78-94 Femtoliters
MCH 28-32 picograms
CHC 35 +/-3-%
Jbservations and result:
pcy 45 7
RBC count 6:6 miion|mm
MCV :
45_x10 = 68:1 f
6"6
12:6. x 0 = 19 Pa
66
MCHC = 126x160
28 /"
45
ASSignment:
I. Write the importance of various RBC indices.
2. Write normal ran ges of MCV, MCH and MCHC.
139
Hb: 126 lae
RBc ?ndices is to dejermine the
Oporerce
vS)
RBC
the onount
H°mob
oRBC
cell
to celu
Preoent
the
ovq. Hb conc? per unit vo)" 'b pocKked red coo0
Cell
od
in ord er to be used
for
Normal
MeV
MCH
’ 80-95 fX
27- 32 PA
MCHC’32- 36
140
as
clhfce ?nterprefao
INTRODUCTION TO EXPERIMENTAL PHYSIOLOGY
is
scientific method and so one of the aims of conducting experiments
observation. Often this depends simply upon intelligent use of the sensory
of the
observationis the back bone of
id
aptitude for careful
ouirean
observation
calls for proficiency in special techniques frequently and therefore some
experinents.
will he to learn techniques. These provide proficiency in techniques for subsequent
ments
in all
But
illinpractical work
will grow in the process of learning these techniques and this skill is valuable
cinical,practice and research.
s of
to learn
object of the training is drawn
conclusions
omake logical inferences from observations. Allfacts learnt in any science course are
Physiology is to
results of many experiments. The most important aim of the course in Experimental
in the text
the
given
certain facts
how
verify
knowledge
to
is
acquired
from
scientific
observations
and
stand
information that are
not possible to perform experiments to confirm and verify all the theoretical
is
It
and text books.
edfromlectures
done which will give some
of Experimental Physiology, a select number of experiments will be
course
course in
e
of Physiology If the
standing of the scientific methods as applied to different aspects theoretical
background of each
that
imperative
it
is
objectives,
achieve
these
to
is
Physiology
about that
imental
expected to have read
are
students
Therefore,
experiment.
doing
the
before
ikal is obtained
xperiment before coming to the class.
vation
yield information
only when properly analyzed. Thus the second
t
experiment. An egually important part is to, record
ractical work in laboratory is only a part of an Physiology is to learn proper documentation of the
Experimental
erly. Another aim of the course in
clarify their Own thinKing
to comment on them. Students can
able
be
to
and
observations
and
Tar
edures
if this is done properly, students will gain
and
comments;
and
inference
observations,
recording their
the experiments.
from the experiments than if they stopped with performing
examinations.
experiments before practical
bdrecord will also help to review the
record are:
of the principles to be followed in writing up your
repeat the
information necessary for somebody else to
the
all
contain
must
a
The write-up
experiment if necessary.
observed and so need not
what was done and what was
of
account
an
essentially
is
record
b. The
elaborate on theoretical aspects.
C.
141
virtues.
Legibility, neatness and brevity are three
Experiment -1
(IncludingPhysiograph).
nomenclature
To study the apparatus and
AEVERSINO KEY
SHORT CIACUMNO (SECONDARY) KEY
SUPLE (PRIMARY) KEY
SECONDAAY COIL
PRIMAAY COL
NEEFS HAMMER
HAMMER
DUBOIS RAYMOND INDUCTION COIL WITH NEEF'S
TAP KEY
SCREW LIFT
DEVICE
ORUM-OAIP
LEVER
(locking/
unlocking the
ylinder)
TOP SCAEW
CYLINDER
BRASS AOO
(15 mm DIA)
PENDULUM
CONTACT KNOB
MAIN SPINDLI
-METAL PIECE
ADJUSTING
SCREW
COIL
AEVOLVINO
STRIKERS
tdouble contac
CONTACT BLOCK
ams)
GEAR BOX
LAVELLING
CLUTCH
SCAEW
VIBRATINO VARIABLE INTERAUPTER
KYMOGRAPH
142
AALL ANO
SOCKET
PAIR OF
fLECIA0DEA
PERSPEX
AATH
HOOK FOR FIXING
THE MUSCLE
HORIZONTAL
WAITING LEVER
DAAINAGE TUBE
LUCAS CHAMBER OA MUSCLE TAOUGM
MYOGRAPH B0AAD WITH STAND
HOLES FOR SUSPENDING
WEIGHTS
.AFTER-LOAO SCAEW
STARLINO HEART LEVER
FULCRUM
STIMULATING LEVER
HOOK
19OTONIC MUSCLE LEVER
-WAITING POINT
STEM
PRONGE
TUNING FORK
LEVER
HOOK AND WEIGHT
DOUBLE
MAGNET
CELLULOI0
COILS
CAPILLARY
WAITING
POINT
TERMINALS
AESEAVOIA
SQUARE PERSPEX WITH
ELECTROMAGNETIC TIME MARKER OR SIGNAL MARKER
FOUNTAIN PEN
WAITING POINTS
143
PEN POSITION
CONTAOL
50 HZ FILTER
[AJ
SENSITIVITY
SELECTon
PILOT LAMP
CoUPLEA
HOUSH0
INP,
CAL
SLOT FOR RECEIVING
BIOPOTENNAL COUPLER
RASEINE
CHARTS
MAIN AMPUen
OUT
FUSE
INK WELLS
TO MAINS
GROUND
$TUDENT
PENS
PHYSIoGAAPH
APES
RECEPTABLE
THUMS sCAEW
PEN LIFT CONTROL
PAPER FEED INDOW
RANGE SELECTOR
PAPER DRIVE
SPEED
MAIN SWITCH
SEAAING
SELECTOR
PHYSIOGRAPH STIMULATOR
STAND
PULSESSec
EXT
DELAY mSec
SINGLE
8Y
10
'PULSE^
[B)
130
15
,20
"190
210
So
230
10
250
FYT
NORNAL
PILOT LAMP
TO PHYSIOGRAPH
ECO coUPLER
AVA
STRAJN OAGE COUPLER
a VL
VE
NPUT
CA
INPUT
1 my
OFF
CF
ON
ON
CAI
CF
BALANCE
[C]
PULSE RESPIRATION CoUPLER
TEMPERATURE COUPLER
INPUT
PULSE
INPUT
OFF
OFF
AESPIRATION ON
ON
GAIN
BALANCcE
144
GAIN
Experiment -2
Io study Skeletal Muscle
Nerve
Muscle Preparation in frog
Scatic norve plexS
Vertebit
Colmat1
Muscies of thigh
(Cut and retlected)
Scattc nerve
Aductor magnus
Sartorius
Knee ont
Adductor magnus
Gracilis
Tibia
Gastrocnemius
Tibial1s anterior
longus
muscle
Tendo achilis
emonstration of Nerve Muscle Preparation in Rats: For Nerve Muscle Physiology Teaching
aterials required The equipment required for this practical exercise included Wistar rat, Digital Data
board,
ouisition system, stimulating hook electrodes, force transducer, dissecting instruments, dissection
eights, saline solution, anaesthetic agent
Dbjectives addressed
We addressed the following learning objectives in our study:
1. Determination of subthreshold, threshold, suprathreshold, maximal and supramaximal strength of
stimuli.
Recording simple muscle twitch by stimulating the motor nerve using a supramaximal stimulus
interval may
3. Determination of the effects of two successive stimuli and studying how inter-stimulus
affect muscle contraction
4. Demonstration of the phenomenon of incomplete and complete tetanus
Institute of
Rduit male Wistar rats (body weight 200-250 gm) were obtained from institutional (All India
Medical Sciences. New Delhi, India) central animal facility. The protocol was approved by Institute's Animal
145
Australia) was Usord
" sytem (AD Instruments,
Powetatb
acquisitton Data was displayed in teal time usiDg LabChart" software version 8.1(AD Instrumente
installed on a desktop ystem Data was stored tor offine analysis later. Stimnulating hook
IIsed to deliver cletial stimuli to
described as follows:
the neIve. The detailed proccdure is
Ethics
(ommittee (65//AC 1/2018)
Calibration:
clectro,Adesustralwera) o
ne the tiansduce meaunes conttatuon ctuonoth in Volts by default, it is essential to calibrate the
same betore the start ot the
Transducer channel was iderntified
expeiment to get values in grams.
was done before
of calibration, Two point calibration was done by applying weights
S0n à step up and step down manner..Unit conversion was done to get the subsequent values in
comencement
for data
and zeroing,
from 10-
Newton.
Wistar rats
They were
dark
Animal preparation:
After setting up the data
male
system, animal dissection was performed. Adult
acquisition
facility.
Housing
Iweghing approximately 200-250 grams were procured from the Institute Animal
housed in
2412%C with alight:
departmental animal house in a temperature controlled room at
Ia10 hours and provided ad libitum food and water Six hours of fasting was done betore the cycl
starte oft
dissection. It was weighed and glycopyrrolate injection (0.5 mg/kg IM) was given to prevent secretions.
5-10 min, thiopentone
at a dose of 50 mg/kg was injected intraperitoneally and it was covered to
keep away
from direct light. Once the rat
was anaesthetized, it was placed on dissection board and its limbs were tied
iounting nails with the help of thick cotton thread to prevent any movement taking care not to injure theto
skin. Depth of anesthesia was checked by
eliciting withdrawal retlex.
.After
Animal dissection:
In
anaesthetized rats incision was given on the dorsal side of right thigh extending up to ankle joint. By careful
blunt dissection, gastrocnemius muscle was exposed and subsequently soleus was
separated from its
Surrounding attachments which lie below it. Soleus muscle is flat and red in appearance and lies along the
Surtace of tibia. After the identification of soleus muscle. it should be
detached from
plantaris muscle by dental scalpel. As the tendon of three above mentioned muscles are gastrocnemius and
attached, tendon of
Soleus muscle should be isolated from them without damaging any blood vessels.
Origin 'and insertion of
soleus were
kept intact. A cotton thread was tied-to the tendon of soleus muscle and
other end was attached
to the force transducer. Extending the incision proximally up to the
vertebral column, sciatic nerve and its
branches were exposed and separated from surrounding coninective tissue with the
help of glass seeker. Hook
stimulating electrodes were applied to the sciatic nerve. Liquid paraffin was applied to
exposed nerve to
insulate and prevent evaporative loss. Entire area was covered with gauge piece
moistened with mammalian
ringer. Fig. 1A and 1B showS the representative graphs of the set up for the
experimental protocol.
Stimulation and data acquisition:
The tendon of the soleus muscle was isolated and tied to a force
transducer with the help of a cotton thread.
Sciatic nerve was placed on the stimulating hook electrodes as shown in Fig. 1B.
Square wave pulse of 1 ms
duration with varying strength was delivered.
Recording of threshold stimulus intensity of simple muscle twitch (SMT):
To determine the threshold, square wave pulses of fixed duration and
increasing current intensities was used
to stimulate the sciatic nerve. Current intensity was gradually increased from 0.1V in
increments of 0.1V till a
twitch was
elicited. Minimal stimulus intensity which elicited the simple muscle twitch was
considered as
threshold intensity of SMT. The peak tension generated during a simple muscle twitch was calculated. The
latent period, contraction and relaxation periods were also analyzed.
Recording of suprathreshold, maximal and supramaximal stimulus:
Current intensity beyond the threshold intensity was gradually increased tilla twitch with maximum amplitude
was obtained. Further increase in the current intensity does not increase maximum amplitude of simple
muscle twitch. This value was considered as suprathreshold intensity of SMT and stimulus is supramaximal.
Peak tension generated at the time of contraction was calculated. The latent period, contraction and
relaxation period were analyzed.
146
of two or more
muscle is given two successive
of,effect
ecordin&
stimuli:
second
successive stimuli of suDra threshold intensity the response to the altering
depends upon the
time
are set by
interval between the when
intervals
nulus
two two
frequenCies, In same nerve-muscle preparation
supramaximal
are applied in such a
stimuli.
Inter stimulusstimuli
ckeletal
thatthe second
nner
stimulus falls in the following phases:
L.Completion of first twitch
II.Endof relaxation of the first twitch
IL.During relaxation of the first twitch
IV.Durin8 contraction period of the first
V.During second half of the latent periodtwitch
V.During first half of the latent period
er-stimulusintervals or frequency of stimulation were calculated by analyzing latent period, contraction
A
nter
period and
relaxation period of simple muscle twitch. At each
and duration were
amplitude
phasesas descrribed above.
recorded.
stimuli, twitch
of the two successive
different
The shape of the SMT curve was also analyzed during
enesis of Tetanus:
period of one twitch is
skeletal muscle is repeatedly stimulated at such a
so that,arelaxation
erimposed with its previous one and muscle does frequency,
not get relaxed,
state of sustained contraction is
incompletely
ained which is known as "complete tetanus". Below that frequency muscle relaxation occurs
considerred as "incomplete tetanus". For the genesis of tetanus, sciatic nerve is stimulated at suprait is
tension generated at
Pximal stimulus intensity with increasing frequency. Amplitude of contraction and peak
erent frequencies weere
Sults
calculated. Force-frequency relationship of muscle was plotted and, analyzed.
representative graph records are depicted in Fig. 2 to 6. Simple muscle twitch was recorded and effect of
asing strength of stimuli was observed. Effect of two successive stimuli and genesis of tetanus was
orded in continuation of the same set of experiment. Force-frequency graph was plotted from the obtained
To Demonstrate Simple Muscle twitch and Calculate the different phases.
efnition: Asingle brief electrical stimuliof adequate strength applied to the nerve of askeletal muscle gives
etoa brief contraction of muscle followed by relaxation. This response is known as "Simple muscle twitch".
agram:
PHYSIOLociCAL CURVE
ases of SMT:
Latent phase: Phase between application of stimulus & beginning of recorded response.
Contraction phase: The phase of muscular contraction.
Relaxation phase: The phase of muscular relaxation.
147
Latent phase is due :
(i) Conduction of
impulse along the
(ii) Neuromuscular transmission.
neve.
(ii)Excitation Contraction
(iv) Stretching of series elasticoupling.
(v) Inertia of
Questions:
1.
recording lever.
components of musCle
What are the properties of muscle
demonstrated by this experiment?
ve the normal durations of Latent Phase Contraction Phase &Relaxation Pnaser
3.
What are the causes of Latent
Period?
4. What is
Physiological curve?
S. What is the difference between
isotonic &isometric contraction
0. Can youfind the Tetanizing freguency from
Simple Muscle Twitchr
I. Correlate Simple Muscle Twitch &its action
potential on same time scaler
muscle domonstrate0 Qre
excipblt
conyocfbslit
duva ion lojent phane : I0 ms
Normal
contoc
phase : 20-40 Ms
Relax
Phaoe
.30-SO ms
Tme okon by stimulu to travel along
tho neve to the
neunomusculahU
juncion.
’ Time
for crossinA. NMJ
ime for exdpon- convoc Cawplig
Time
overcoming from ineria d} reot
Time to overcome the v
lever .
iscows estonce
Curve Ps obBuned due to ?nerta s
Tsotonc
,MUscle tension consant
but
’00TK Is done
148
musde.
levet
Ts0metc
-’ Muscle tension
lengh
is constant
but
we Can
teganisable frgueny from imple ancie
Ñtch frd
by the
fomul
Tejonigobil
cOnrac9 period
- ] ’ 4ction poential
Peak 6
contyoc
ension
Muscle
Reloxatib
iniiatfon
Controc?
Appica ion
blo ni+afon
delay
slght
a
is
There
conabn còlled lotent
R onset
U
ction poenti
peiad hich ci
the impulse to
by
taken
time
N J to the
Observed due to the
the
nerve.fíber to
eJever fnerfia
149
viscou
to orercll.
the
(C)
To Demonstrate the effect of temperature on Muscle contraction.
IntHoweve
roductitheon: Frog
IS a Poikilothemic animal, its tissue are adapted to a wide ranpp
teactions, on which function of muscle depends, vary with chanor
ol
chenical
If the
in
of saline is increased above 45 'c then this will cause denaturation of
and below 4'Cthere is
inactivation of enzymes.
temperature
Physiological Significance:
Man being Homeothernic (warm blooded) can maintain the internal body
lemperaluier
mulesrmcleperatpurrotein
temperature within
etrow range inspite of wide variations in environmental temperature.
Efficiency of skeletal muscle contraction increases with increase in surrounding
the physiological limits. This is especially useful during exercise.
a
temperature within
Heat Rigor:
When the temperature of Ringer solution exceeds 45 "C, the muscle proteins get
Actin and Myosin filaments are unable to carry out their functions. Hence, the muscledenaturerd .Due to
remains in a statethsof
permanent contraction, called Heat Rigor.
Diagram:
TIME TRACING
Effect:
Cold ringer increases the duration of all phases of muscle
contraction and decreases the height or
contraction, whereas the warm ringer solution has just the opposite effect.
Questions:
1. Explain the effect of temperature on different phases of simple
muscle twitch?
2.. What happens if temperature is increased above 45 °C and is deceased below 4 C?
3. Why do you record the effect of warm saline before that of cold saline?
4. Whya maximal/supramaximal stimulus be used?
5. Is the effect of temperature on muscle contraction a violation of "All or
none law'?
150
Ringet 501
1oent perrcd dec* due to inc tn concuction velo city b
neve, ncease
foser ovenconing
neusomus culak tansmission note
ver
contac ?
ATPaso
Reldxation period doc. due to faster
yosin
acvafion
selar ohlch occUTs Hue to
contrac?
achvity
viscosy
R decreaseP
MUscle
Muscle
Snc due to
’ Ampltude t contrac in muscle
Achemical" aciv y
in croased enyrotc
opposite effoc are Observed as that in
’ Latent peria inc
oormal
Temp. ine aboe 45'c - ohen temp. ie înc. above 43"45c
uscle remaü
the mUscle protin ae denoturec
SUsaûned contoc? knon as 'Hegt
Temp: decrecned belo 4c- Muscle prfein
ohich prevent
lo
are
nMUscle controc
worm s? sholo be recoled before vecordg te
cfld
s?
be cawe cold
scle
be
151
to Teieve t
muscie
9
(4)4nMovimal
o
Y
supiaaial stfmulus is
Obain vepotured neseul 8ck induc es
an fibere at cach iulaon
Used to siula ol!
152
to
conta(fio
fo Demonstrate SummationsEffect offchangeeof strength of stimuli
orUnit: Askeletal muscle is composed of(Spatial
larpe number of muscle fibres and is supplied by large number
Summation).
envetibres Asingle nerve fibre with all musclefibres supplied by it is called motor unit.
Askeletal muscle contains many such motor units, With a strong stimuli, large number of motor units
ract.
thresholdstimulus:
nulusofineffective magnitude as judged by its failure to elicit acharacteristic response from a muscle.
sholdstimulus:
ulusjust sufficient to elicit aresponse. Below this strength of stimuli no muscle twitch is produced.
maximal:
enthe
strength of the stimulus increases the amplitude of contraction
of
increases as more and more
or unit units contract.
vimal:
is
imul stimulus produces contraction of all motor units of the muscle So the amplitude of contraction
aNmum.
ora-maximal:
Le-maximul stimulus does not increase the amplitude further as all the motor units have alreaay bee
uited with maximal stimulus.
agram:
14
sUPRA
uestions:
1. What is a "Motor unit?
2. What is the mechanism of the graded response to increasing strength of stimulus?
3. Enumerate the factors which affect the threshold of a motor unit.
4.
Will youget a graded response to increasing strength of stimulus if youstimulate
(a)
(b)
(c)
(d)
153
Askeletal muscle
Skeletal muscle fibre
Cardiac muscle
Cardiac muscle fibre
fbre tog tMes
(ohen a
-otor neuron înnervotng all its muscle
e Called
or un
submal
thon here
here
then
the
engt
stinun ine,
Supia oimal sfmul Hhere is no 9oc o
becawse b no, all the oor Urit have been
Fotor
()
strengt
(i) freg
(G)- Seiefal
154
unit activaed
stls
stils
wscle
tinulus
no
the
vesponse
prducod
Ftectoffchange oft
frequency (Temporal Summation)
(a) Effect of two Subminimal stimuli in different phases.
mation: summation means the incIease in activity ofLa lissue produced by multiple stimulaiop. as
with the
d
Ustssue.
effect of a single stimulus. Summation is a
property
nuscular and by
shown both by
of sumnation by muscle are
Sumnation of stimuli: It can berecognized:
demonstrated by tlhe application of multiple sub threshold stimulito
produce muscularfesponse
Sumation of contrackon: It refers to an increase in actual magnitude of contraction as aresult of
ultiple stimulation. It is of two types:
al Wave summation: refers to the condition produced by the passage of successive contracion
overthe same set of muscle fibres.
stimulation additional fibres Dy
(b) Quantal summation: refers to an increase in response due to
stimuli subsequent to the original.
f askeletal muscle is given two successive stimuli of maximal strength,
the response to second
stimulus depends upon how soon after the first stimulus it is given.
there is no response, because the irst
IWhen the second stimulus falls on the first half of latent period.
potential can be generated, however strong
half of latent period is absolutely refractory and no action
the stimulus is applied.
Bgram:
(b) To Demonstrate Beneficial effect.
Effect:
eneficial
from the same point of
by two successive maximal stimuli
obtained
are
responses
separate
tw0
hen
from the previous
in amplitude due to the Beneficial effects
imulation, the second response is greater
esponse.
latent period or
second stimulus falls during the second half of
The height of curve is more when the
"Beneficial
first twitch due to the phenomenon of
contraction period or relaxation period of the
effect".
These benefits are:
stretching of series elastic components of the muscle.
> Increased elasticity due to
> Increase Ca
concentration.
accumulation of C0, and slight lactic acid.
> Decrease in pH due to
> Slight increase in temperature.
155
Graph beneficial effect
Questions:
1.
2.
Detine Summation of Stimuli. Beneficial effect and
Summation of trecIS
What is the
of using maximal/supramaximal stimulus in the experiment of
significance
Effects?
Sumotion
o{
Summation of
simuli
-Cumulotive muscula oY neural effct
prcduret by the frequent repef'tfion ay sfiui
Beneficial effect - hen 2 seperate reoponse ae objeinao by
y succove momal stiuli from same ptstinuatod
te
d reoponso ie
Conc. , der. in DH
Called
SunnaHon
stiul?
In ampliude due to
slight
dtfect
Beneficiai
inc.
Cat
temp.
offec : Procen
Jhich mulple oY vepeated
Can
pxoduee a éponse Pn a ner ve mencle
ofhes part that e stimulo aloe Gant produee
Maximalsuprananal stlus
recruit au the oor unit
156
0Y
, Demonstrate Incomplete &Complete Tetanus.
a
roduction: When askeletal muscle is repeatedly stimulated at such a frequencythat it does not get
re/ax.then the muscle remains in a state of sustained contraction or complete tetanus.
nceto
complete
frequency of stimulation is slower, allow the muscle some time for relaxation but not
BNtion,the state iss called incomplete tetanus.
he
sion developed in tetanus is greater than the tension developed in a twitch.
gram:
slaNALA
levance of tetanus in human being:
clostridium
1. Tetanus produced by
tetani.
tetanised.
2. Cardiac muscle can't be
1eloo'on
estions:
incomplete &complete Tetanus?
1. Define Staircase/ Treppe, Clonus,
skeletal muscle?
to produce complete Tetanus in
frequency
minimum
the
is
What
2.
cardiac muscle, why?
3. Youcan't Tetanise
developed during a complete Tetanus &
4. What is the ratio of tension
157
simple muscle twitch?
Shcose| Teppe - Proqessive ine in the
Jor the fist 2to 3 coyroio
st'uiaod epeate
Clonusis
experimengal
Te tanu -
a stote of
hon a mce
Contocio
partÉal tetons observÌd .
bond?, Relay fe incomplet
SBote of suyained Controc
Muscle.vemain
sa k 4 forced controcl oithout relauofton
iimun feguenay
2)
needed to produce e4anuy is
ninimyn
Coll ed
simple mude
MTF
conro peiod
Cordiac NUscle cont be
oonised
teponse is more than halt ow) duHgbecousete theobsolue
peried (1go - 200 Msec) Hen ce summattbn
cotocfile
hyuscde
n
povsible.
Juig teerus, the ternion dovdlpe
at derell ped oh simple
158
ele ic
Experiment-4
Fatigue
lo Demonstrate muscle
capaity of a cell,
Detinition. tatigue is a
redution of the
whole resulting from temporaty
prolonged exertion
It is a
reversible
phenomenon &passes
In an
working
an organ or an
(a) Lack of
(b)
(o)
are applied for
prepatation, if rhythnic clectrical stimuli
no longer responds to
willlgadually fall to zero &the muscle
nutition
Accumulation of waste metabolite
Depletion ot Acetyl choline stores.
Diagran
Beneliclal
otFect
Umulatiori
Contraction
remainder
Point of stimulus
Questions:
1. Define fatigue?
2. Describe the changes that occur in simple muscle twitch as fatigue sets in?
3. What is the site of fatigue in muscle nerve preparation?
4. What is the site of fatigue in Human beings?
5. What are the factors which hasten fatigue?
6. Why does the baseline rise in a fatigue graph?
7.
What are the causes of recovery? Howcan you hasten recovery?
8. What is contraction remainder?
160
a
off after rest.
isolated nenve musde
anplitude of contaction
is due to
organism as
some time, tha
this
stimulation,
decoaoe Ro peformone |tempot reductCn
ng
} the oorki
Confruousstfanioio
hheepody
pelngud o
O
scie pepr
omaindon
bloetine contoction theosoticay
Can DCCn
Nerve
neu(0mwe. june?
prepfutg
9n s olaeositeMu scle
elevotion
a
in experime nt
But
hcvrmw)e juncon
poimiy
the pinay
neuromusl.
bain
fat
Fator Kohich haoten
tocic acd
nluqnti
ike
iefab];eo
ACcumlotion
eloxeton h
plat
o an incom
sea duifaigu sets
Boele
6)
Tesult
depietim
A
come
hich te
poitin
doon
chone
fresh aretyl
souetion
eplod
Rise in
called
emain ?n pai
conros
fn selaz
Loggenit
for
Case
due
to
AT dupletin
161
point
emande
incomplee
gn e
velox
muscl
conracted StaemefaboGs
accumulatión
Experiment -5
To study effect of load &length on muscle contraction (free loaded & after loaded).
NTRODUCTION
Dertomance of the muscle can be studied in two types of contractions
Eree loaded: the load is constantly being exerted before start of contraction
After loaded: the load starts exerting on the muscle only after the contraction has begun.
he pertomance ot muscle is better in Free loaded condition than in After loaded condition because series
stic elenments of the muscle are stretched during Free loaded condition resulting in increased initial length.
is the
d according to Starling's Law more the length of the muscle.within physiological limits, more efficient
rtormance of the muscle.
AGRAM
Free loaded
cOndition
londed
Gonditlon
Ate
63
Effect
of
Load on In situ Rat Skeletal Muscle preparation Under
Free Loaded and After Loaded
Introduction
Condition
nse to asingle action potential, there occurs a briefcontraction followed by relaxation
in skeletal
(1). The muscle twitch can be produced under free loaded or
In
after
condition, case of free muscle
conditions, the series elastic component is taut initially
when
stretches the muscle in the loaded
relaxed state. This causes increase in both velocity and the height of the load
up to a certain
point which is in accordance with Frank Starling's law. Hence, the height of contractinn
in case of tree
is more
loaded
condition as compared to that of after loaded condition (2). The
of work done by
of
skeletal muscle in-vitro under free loaded and after loaded conditions is one of effect
response localadertled
contraction
dernonstration the most
muscle twitch
important neve-muscle
sciatic
practical for MBBS teaching (3). Traditionally it is done in gastrocnemius
of frog in myograph board using isotonic muscle lever and drummuscle and
preparation
the frequent unavailability of frog for dissection, and the lack of accuracy and
Unfortunately,
traditional set up intrigued us to look
precision of (2).the
nerve
into alternatives to demonstrate this phenomenon.
Materials required:
-kymograph
. niopentone sodium (1 g reconstituted to 10 ml or 100 mg/ml) and 1 ml
tuberculin syringe.
2. Dissection
scissors,
forceps
(curved
artery
forceps
and
toothed
forceps), wooden
board or rat dissection tray with four side hooks for tying limb,
glass dissecting probes or glass seekere
dental scapula, and bone
rongeur.
3. Unyielding-silk thread, warm paraffin wax or
mineral oil, 0.9% normal saline, droppers, cotton, gauze pieces
cloth to pick up rat and gloves.
4. Power Lab 26T with Lab Chart 8
software digital data acquisition system, MLTO015 isotoniC
transducer with
a ML221 bridge amp, MLA270 stimulator cable (BNC to
Micro-Hooks), MLA40 manipulator with stand (AD
Instruments, New South Wales, Australia), rod stand, pulley, pin hook, T
connector for
Connectors (for T connector and isotonic transducer, if required), calibration weights (5 , pulley (if required).
10g and 15g etc.).
and a ruler or measuring tape.
instruments:
dissection
Animal:
Amale
or.female Wistar
rat of weight 200-250 g.
Objectives addressed:
1.
Define free loaded and after loaded conditions of the muscle.
2. Describe the effect of the above on the latent period,
contraction of simple muscle twitch.
contraction phase, relaxation phase and height of
3. Explain the mechanism of difference in the work done by the
muscle under the two conditions.
Principle:
The effect of free loaded and after loaded condition in isotonic mammalian skeletal muscle contraction can be
studied using in-situ nerve-muscle preparation in rat which mimics physiological conditions (intact blood
supply with constant body temperature) very closely unlike the in-vitro nerve-muscle preparation regularly
used for physiological practical demonstration. Using isotonic transducer free loading and after loading at
different weights wasdone in sciatic nerve-soleus muscle in-situ preparation in an anaesthetized rat, and work
done is calculated in each condition from the data collected and compared.
164
perimental protocol:
lt male Wistar rats (body weight
200-250 gm) wee oblained lon intitutional (AI India
Medical Sciences, New Delhi,
Insttu
lndia) central animal facility. The
icsCommittee (65//AEC-1/2018).
protocol was approved by Institute's Animal
detailed procedure is described as follows:
rocedure:
Calibration The calibration of weight or foce and
instruction of the manufacturer.
disolacenent
in the
isotonic transducer ds pe
Animal preparation for the surgery Acclimatize the rat to the
denartmental animal house for one day witn
food (standard rat chow) and water ad libitum, After one day.
keep the rat on overnight fast. Anaestneie
with thiopentone sodium injected
intraperitoneally with a dose of 50 mg/kg body we ght.
Surgery and in-situsciatic nerve-soleus muscle
preparation
Place the rat on a wooden dissection b0ard and tie all the limb with thread to four
corners. Skin one oT
the legs trom knee and down, dissect the soleus muscle free
from surrounding tissue. However, keep the
blood supply intake. Take utmost precaution not to damage or put any drag on the
blood supply of the
muscle.
ace a thread on the distal tendon of the soleus muscle iust proximal to its
attachment to the calcaneus.
Release the distal attachment of the muscle by cutting such that a small piece of calcaneus is still
attached
to the Achilles tendon. Tie the thread to isotonic
transducer with the help of a pulley system iT neeQ be so
as to keep the transducer in line with muscle-tendon unit. and the pull
of the muscle, with the soleus
muscle parallel to tibia bone. This ensures physiological movement. Cut the
gastrocnemius and plantar1s
tendons from that of the soleus. Split the fascia between the soleus and the
gastrocnemius-plantaris about
haltway up the soleus. This should be, however, distal to the blood vessels which supply the belly of the
SOleus. In this way, the movements of the gastrocnemius and plantaris will not interfere with that of
soleus
(5). Clamp the ankle and the middle part of tibia to make the leg
immobile and stable. The origin of the
muscle is immobilized (Fig. 1).
Identify the sciatic nerve and place stimulating electrodes of the PowerLab 26T. Make
sure the soleus
muscle temperature is around 37°Cby pouring pre-warmed 0.9% saline
constantly over the epimysium of
the muscle. Warmed Krebs-Ringer solution may also be used.
However, once the setup is done, warmed
paraffin or mineral oil may be used instead by pouring it after making a container with the
loosened skin of
the hind limb being pulled around the sides of the exposed area of
interest, so as to minimize evaporative
heat loss and distribute heat evenly. A heat lamp may also be used. This is done to
keep the temperature as
close to body temperature as possible. However, make sure that the core
temperature of the animal does
not exceed 38°C.
Instead of using the in-situ sciatic nerve-soleus muscle preparation as above, one
may use in-situ preparation
of gastrocnemius or medial gastrocnemius or extensor digitorum longus or tibialis anterior (9) etc.
Recording of muscle twitch under free loaded and after loaded condition
Example of weights used may be 5 g, 10 g and 15 g. The isotonic transducer is set in free loaded and then
in after loaded condition at different weights. The sequence of free loaded and after loaded conditions may
be randomized,or recordings are taken in al possible sequences.
165
The
stimulus
given is a single square pulse of 1ms duration starting with5 V, and gradually
by 0.2V) to find
Astart delay of 10 ms, maximal repeat
and
maximal
OT maximal voltage.
ampliier range of 5stmulus
m may be taken. Difforent
requnement
sample
A 15
ms
of 1 kHz or 2
kH7 may be used, The above stimulus parameters were
reording
of
muscle twitch using tthe in situ nerve muscle preparation.
muIscle
free loaded and after loaded conditions is done
as
of 1 H
per
the
(5, 7). The
chosen after
pulse at 1 Hz, or 0.2 ms pulse, or 50 s pulse at 0.5V may also be used
trequency
petoTming
and recording
The
increrateased (e.g.,
combinations, however, may be used
at
twitch in
This is taken higher
(eg: 0.5V higher) than the maximal stimulus (5) or double the
Kest duration of about 1-2 min )av be given between two successive stimuli (Fig. 2).
eysis and work done calculation Export the data to data pad of LabChart 8 and excel.
stimulus.
suprarmvoltage
aximal
maximal
Record the maximumtwitch
amplitude or maximal displacement. This is done for free loaded and after
conditions at different weights.
loaded
Calculate the work done in both free loaded and after loaded
Usns this formula: work done in ergs
by
= weight (e) Xmaximum
displacement (Cm) A 9ö1:
6.Sacrificing the
animal
conditions
ATter the data collection, the rat is sacrificed with
the
under anesthesia.
ver dose of anesthetic or neck dislocation while ctill
Results
Representative
records of
displacement by isotonic transducer under free loaded and atter
at 5 gare shown in Fig. 3 and Fig. 4
respectivelv. The maximum displacement and hence
both the conditions are: (a) free loaded
condition: 4290 cm and 2104 ergs, and (b) after
.3962 cm and 1943 ergs. The records presented
here is for understanding purpose only
parameters are different from what is mentioned under
materials & methods.
loaded conditions
work done under
loaded condition:
and the stimulus
Questions:
1.What do you underst£nd by the ternm
optimal load?
2. Explain the term Free/Pre
loaded and After loaded ?
3.Give examples of free and after loading of
muscles in vivo.
4.Examnples of free/pre
1
OPtimu
done
and after loading of cardiac muscles in vivo.
(0ad i- The load
naimum
Freel pre Load :- The
the lever
io
Condition
conne cfed
ptiylooo.
KnonV a
when the
to Musce
eentroctt coaction
Afier load i- when the
Musle contoctn
166
lOad
is
bwe
lod
hang
bofor e te unele
appt'ed aftes te
a Sxample of Pelfoe load
4fter lcad -
Example
a stone | ball
ng
bucket
Cardiac
Venous refusn to the hent
Aftes load
Penipher al
oriac wuscto
h Cardiac
blosd Teictonce.
2r1]2]25
te
167
Experiment -6
To study of properties of Cardiac Muscles
() To Demonstrate the
normal Cardiogram of Frog's heart.
Frog's heart consists of 4 parts
(a) Sinus Venosus
(b) 2Auricles
(c)
1Ventricle
They beats sequentially
Heart beats originates in Sinus
Record of Heart contraction is Venosus under normal condition.
Heart rate can be increased or called Cardiogram.
decreased by various factor.
Supenot vena
(Ava
Right ariun
oft atuum
f
Puimonary
Snus venosu:
veins
inleior vena cava
Venticie
GRAPH
Atrial systole
Atrlal diastole
Ventricle
systole
Ventricle diastofe
Normal (25°C
HR : 24/min
Questions:
1. What is normal cardiogram and labels its component waves?
2. What will happen if warm saline is poured in
(a) Sinus venosus?
(b) ventricle only?
3. Why is it necessary to fix the base of the Heart?
4. Is it necessary to remove Pericardium?
5. Enumerate Physiological causes of increase and decrease in heart rate?
6. What is the cause of bradycardia in athletes?
168
(iü)
whereas cold ringer
Frog's heart.
the effect of temperature on Contraction of heart,
To
Demonstrate
Force
Warm ringer lactate Increases both Rate and
of
opposite effect.
GRAPH
Cold (12C)
al (25C)HA2mn
Warm 35C)
HR35l
Time marker
Questions:
action?
1. What is the effect of Warm Ringer Lactate on Heart Preparation:
its Mechanism of
2. What is the site of action of Warm Ringer lactate on the heart. Give
3. What willhappen if only the ventricle is warned?
4. How warming of Ventricle alone is brought out Experimentaliy?
170
has just
JToDeemonstrate Extrasystole and Compensatory Pause.
pmalytheventricle contracts as a result of an
impulse coming from Sinus venoSus. But if an artificial extra
mulus is given in diastole of ventricle it can contract in
response to this extra stimulus. The next normal
pulsecoming from the sinus to auricles the
ventricles, usually falls in the
period of the extra
ntraction of the
ventricle, therefore, does not respond to it and remains refractory
quiescent till the next normal
pulsereaches it and
ventricle contracts.
periodIof
inactivity is known as Compensatory pause and the extra contraction is called Extrasystole.
e
AGRAM:
Treppe
Early diastole
R0Et
Signal marker
Late diastole
ystole (ES) an compensatary paue (GP)
uestions:
What is the physiological basis and significance of compensatory pause?
Defineectopic beat.
What are the factors predisposing to extra systoles in ahuman subject?
When do extrasystoles assume clinical significance?
HOw will youdifferentiate between atrial and ventricular
extrasystoles?
Dte
171
(iv) To
Demonstrate Stannius preparation of Frog's heart.
Failure of conduction of impulses from
The
conduction of
known as Heart block
one part of the heart to another is
be blocked patially or completely by mechanical pressure
impulses can
tying a thln thread knot
at vaious sile 1hese are known Stanniusigatures and are two in
such
as by
number.
The tSt Stannius Ligature" is tind between the
sinus venosus and atrma
On tying the
the impulse
to beat but ligature
the atria and
from Sinus venosus is prevented from
ventricle stop beating. After some time
reaching the atria. The sinus
the atria start generating
continues
their
impulse,the
impulse
the ventricle and the atrio venticular segment starts beating independent ofown
Sinus hythm but at a reaches
the
slowe
rate th,an that of the Sinus
venosus
The
Second Stannius
Ligature" is ied between the atria and the ventricle around the atrio
ventricular groove
y8 this the inmpulse from atria is prevented from reaching the
ventricle. The atria continues to beat
With its Own rhythm while the ventrice stoo eating After a
short period the ventricle generates its own
impulse and stats beating
independent of atria at a much slower rate than the atrial rhythm. This is
as
known
ldioventricular rhythm.
DIAGRAM
vantcle
White crescent
AnteiE
PosteriCGt yicx
Stelrcase
elfect
Vagl sunulation
172
WCL stmutioh
aga ecepe
UGio ventriçulatytht
(v) To
Demonstrate Simple Cardiac Muscle Twitch after stimulation and to demonstrate refractory period,
Refractory Period is the period during which second stimulus fails to produce response.
In Heart Muscle,
The later part of
GRAPH
contraction period (systole) are Ahsolute Refractory Period.
Relaxation period or Diastole is Relative Refractory Period
electriçal
contraction
Absolute RP
RRA
Time (msec)
Questions:
1. What is meant by
term:
(a)
Absolute Refractory Period?
Relative Refractory Period?
Why the heart
(b)
2.
muscle cannot be
3. What is the rate of
ventricular
(a) Sinus rhythm?
thrown
in to
tetanus?
contraction, when there is
(b) Nodal Rhythm?
(c) ldioventricular Rhythm?
4. What is Heart block?
Discuss it as a hazard to Humans?
5. What is the Importance of
Refractory Period in Heart
Muscle?
174
(v) To
Demonstrate All or None law& Staircase phenomenon.
t o NONE LAW: Heart contracts toits mavimum or it
will not contract at all.
Staircase
An improving effect of previous stimulation on Heart muscle contraction is
Phenomenon:
etve single induction stiuh are ahnlied at auick interval, The first four contracipns are ofobserved
snE amplitude then the prevvous one Tbis effedis kuown as "Staircase Phenomenon".
GRAPH
Questions:
1. What is Allor None Law. Why does
heart obey All or None Law?
2. What is the importance of time interval between successive stimuli while
and Staircase Phenomenon?
3. What is the cause of
Staircase Phenomenon or Trappe?
4. How will you demonstrate experimentally that heart is a
5. What is the
176
significance of
AIlor None Law?
demonstrating All or None Law
functional syncytium?
of Vagus
(vii) To Demonstrate exposure
and
Nerve Crescent
Vagal escape.
SA
fibers end on
(Vagal)
parasympathetic
sympathetic and
muscles.
Alarge number of
supply ventricular
fibers also
tiSsue Sympathetic
rateand
Stimulation of f Vagusdecreases heart
conductionbut
conttaction.
inccreases
Stimulation of sympathetic fibers
heart rate,
node andi conducting
on
has no effect
ventricular
force of
conduction and
contraction
atria.It
venosus andthe
Sinus
(garnglia) thus
junction of between
the
at
located
of nerve cells
group
is
(WCL)
a
line
over
crescentic
same
The white
of WCL will have
(preganglionicfibers ) ends
stimulation
.So
is a region where Vagus nerve
ganglionicfibers arise
post
here
From
synapse.
a
forming
effect as that of Vagus nerve.
Ttypoglossat
Potrohyqd
HScle
Laryngeal
3ranch
Catót20 uranch
Carotid arlery
Pulnonary branch
Vagal. stimulation
Vagal escap
Udla ventriculhm
Questions:
1
difference
Mention the parts of mammalian heart supplied by autonomic nerves .ls there any
between supply of right and left Vagus?
2
What is Vagal escape? what are its causes?
3
What is Vagal tone? How will you assess the degree of vagal tone?
4.
If both sympathetic and parasympathetic nerves to heart are blocked, what will be the effect on heart
rate?
178
() To
Demonstrate effect of
ach drug has its distinct action. An drugs on Frog's heart.
unknown drug can be identified by its action on
biological tissue.
BSERVATIONS:
DRUG
HEART RATE
FORCE OF
VAGAL
CRESCENT
CONTRACTION
STIMULATION
STIMULATION
ADRENALINE
ACETYLCHOLINE
ATROPINE
NICOTINE
Initial then Initial Lthen
normal/
T inhibition
inhibition
Linhibition
inhibition
No inhibition
No inhibition
No inhibition
No inhibition
normal/ 1
QUESTIONS
1. Describe and explain observations in the experiment.
2. How will you differentiate adrenaline from atropine from their action on heart?
3. What is the basis of effects of adrenaline?
4. What is the importance of checking the effects of Vagal and Crescent stimulation after adding the
drug?
5. Which types of adrenergic and cholinergic receptors are present
181
heart?
10te