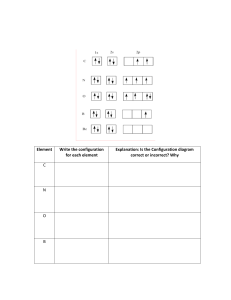
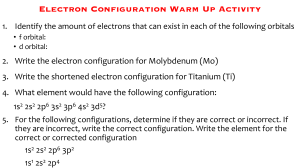
IB Chemistry ATOMIC THEORY Atomic Structure Atomic Structure Atoms are very small ~ 10-10 meters All atoms are made up of three sub-atomic particles: protons, neutrons and electrons The protons and neutrons form a small positively charged nucleus The electrons are in energy levels outside the nucleus Atomic Structure The actual values of the masses and charges of the sub-atomic particles are shown below: A meaningful way to consider the masses of the subatomic particles is to use relative masses Atomic Structure - Definitions Atomic number (Z) is the number of protons in the nucleus of an atom. The number of protons equals the number of electrons in a neutral atom N.B. No. of protons always equals the no. of electrons in any neutral atom of an element. Mass number (A) is the sum of the number of protons and the number of neutrons in the nucleus of an atom. So how can you work out the number of neutrons in an atom? No. of neutrons = Mass number – atomic number Atomic Structure - Example So how can you work out the number of neutrons in an atom? Example No. of neutrons = Mass number – atomic number No. of neutron = Mass No. – Atomic No. = 23 – 11 = 12 Atomic Structure - Questions 1. What are the three sub atomic particles that make 2. 3. 4. 5. up the atom? Draw a representation of the atom and labelling the sub-atomic particles. Draw a table to show the relative masses and charges of the sub-atomic particles. State the atomic number, mass number and number of neutrons of: a) carbon, b) oxygen and c) selenium. Which neutral element contains 11 electrons and 12 neutrons? Atomic Structure - Questions 5. Copy and complete the following table: Summary Slide All atomic masses are relative to the mass of carbon-12. Eg one hydrogen atom weighs 1/12 the mass of a carbon-12 atom. Isotopes Isotopes are atoms of the same element with the same atomic number, but different mass numbers, i.e. they have different numbers of neutrons. Each atom of chlorine contains the following: 35 Cl 17 17 protons 17 electrons 18 neutrons 37 Cl 17 17 protons 17 electrons 20 neutrons The isotopes of chlorine are often referred to as chlorine-35 and chlorine-37 Isotopes Isotopes of an element have the same chemical properties because they have the same number of electrons. When a chemical reaction takes place, it is the electrons that are involved in the reactions. However isotopes of an element have the slightly different physical properties because they have different numbers of neutrons, hence different masses. The isotopes of an element with fewer neutrons will have: Lower masses Lower densities • • faster rate of diffusion lower melting and boiling points Isotopes - Questions 1. Explain what isotopes using hydrogen as an 2. example. One isotope of the element chlorine, contains 20 neutrons. Which other element also contains 20 neutrons? 3. State the number of protons, electrons and neutrons in: a) one atom of carbon-12 b) one atom of carbon-14 c) one atom of uranium-235 d) one atom of uranium-238 Mass Spectrometer The mass spectrometer is an instrument used: To measure the relative masses of isotopes To find the relative abundance of the isotopes in a sample of an element When charged particles pass through a magnetic field, the particles are deflected by the magnetic field, and the amount of deflection depends upon the mass/charge ratio of the charged particle. Mass Spectrometer – 5 Stages Once the sample of an element has been placed in the mass spectrometer, it undergoes five stages. Vaporisation – the sample has to be in gaseous form. If the sample is a solid or liquid, a heater is used to vaporise some of the sample. X (s) X (g) or X (l) X (g) Mass Spectrometer – 5 Stages Ionization – sample is bombarded by a stream of high-energy electrons from an electron gun, which ‘knock’ an electron from an atom. This produces a positive ion: X (g) X + (g) + e- – an electric field is used to accelerate the positive ions towards the magnetic field. The accelerated ions are focused and passed through a slit: this produces a narrow beam of ions. Acceleration Mass Spectrometer – 5 Stages Deflection – The accelerated ions are deflected into the magnetic field. The amount of deflection is greater when: • the mass of the positive ion is less • the charge on the positive ion is greater • the velocity of the positive ion is less • the strength of the magnetic field is greater Mass Spectrometer If all the ions are travelling at the same velocity and carry the same charge, the amount of deflection in a given magnetic field depends upon the mass of the ion. For a given magnetic field, only ions with a particular relative mass (m) to charge (z) ration – the m/z value – are deflected sufficiently to reach the detector. Mass Spectrometer Detection – ions that reach the detector cause electrons to be released in an ioncurrent detector The number of electrons released, hence the current produced is proportional to the number of ions striking the detector. The detector is linked to an amplifier and then to a recorder: this converts the current into a peak which is shown in the mass spectrum. Atomic Structure – Mass Spectrometer Name the five stages which the sample undergoes in the mass spectrometer and make brief notes of what you remember under each stage. Complete Exercise 4, 5 and 6 in the handbook. Any incomplete work to be completed and handed in for next session. Atomic Structure – Mass Spectrometer Isotopes of boron m/z value Relative abundance % 11 10 18.7 81.3 Ar of boron = (11 x 18.7) + (10 x 81.3) (18.7 + 81.3) = 205.7 + 813 100 = 1018.7 = 10.2 100 Mass Spectrometer – Questions A mass spec chart for a sample of neon shows that it contains: 90.9% 0.17% 8.93% 20Ne 21Ne 22Ne Calculate the relative atomic mass of neon You must show all your working! Mass Spectrometer – 90.9% 0.17% 8.93% Questions 20Ne 21Ne 22Ne (90.9 x 20) + (0.17 x 21) + (8.93 x 22) 100 Ar= 20.18 Mass Spectrometer – Questions Calculate the relative atomic mass of lead You must show all your working! 52.3 23.6 22.6 1.5 204 206 207 208 m/e Mass Spectrometer – Questions 1.5% 204Pb 23.6% 206Pb 22.6% 207Pb 52.3% 208Pb (1.5 x 204) + (23.6 x 206) + (22.6 x 207)+(52.3 x 208) 100 306 + 4861.6 + 4678.2 + 10878.4 20724.2 100 100 Ar= 207.24 Energy Levels Electrons go in shells or energy levels. The energy levels are called principle energy levels, 1 to 4. The energy levels contain sub-levels. Principle energy level 1 2 Number of sub-levels 1 2 3 4 3 4 These sub-levels are assigned the letters, s, p, d, f Energy Levels Each type of sub-level can hold a different maximum number of electron. Sub-level s p d f Maximum number of electrons 2 6 10 14 Energy Levels The energy of the sub-levels increases from s to p to d to f. The electrons fill up the lower energy sub-levels first. Looking at this table can you work out in what order the electrons fill the sub-levels? Energy Levels Let’s take a look at the Periodic Table to see how this fits in. Electronic Structure So how do you write it? 1s2 Energy level Sub-level Example For magnesium: 1s2, 2s2, 2p6, 3s2 Number of electrons Electronic Structure The electronic structure follows a pattern – the order of filling the sub-levels is 1s, 2s, 2p, 3s, 3p… After this there is a break in the pattern, as that the 4s fills before 3d. Taking a look at the table below can you work out why this is? • This is because the 4s sub-level is of lower energy than the 3d sub-level. Electronic Structure The order in this the energy levels are filled is called the Aufbau Principle. Example (Sodium – 2, 8, 1) Electronic Structure There are two exceptions to the Aufbau principle. The electronic structures of chromium and copper do not follow the pattern – they are anomalous. Chromium – 1s2, 2s2, 2p6, 3s2, 3p6, 3d5, 4s1 Copper – 1s2, 2s2, 2p6, 3s2. 3p6, 3d10, 4s1 Write the electronic configuration for the following elements: a) hydrogen c) oxygen e) copper b) carbon d) aluminium f) fluorine Electronic Structure – of ions When an atom loses or gains electrons to form an ion, the electronic structure changes: Positive ions: formed by the loss of e1s2 2s2 2p6 3s1 1s2 2s2 2p6 Na atom Na+ ion Negative ions: formed by the gain of e1s2 2s2 2p4 1s2 2s2 2p5 O atom O- ion Electronic Structure – of transition metals With the transition metals it is the 4s electrons that are lost first when they form ions: Titanium (Ti) - loss of 2 e- 1s2 2s2 2p6 3s2 3p6 3d2 4s2 1s2 2s2 2p6 3s2 3p6 3d2 Ti atom Ti2+ ion Chromium (Cr) - loss of 3 e- 1s2 2s2 2p6 3s2 3p6 3d5 4s1 1s2 2s2 2p6 3s2 3p6 3d3 Cr atom Cr3+ ion Electronic Structure - Questions Give the full electronic structure of the following positve ions: a) Mg2+ b) Ca2+ c) Al3+ Give the full electronic structure of the negative ions: a) Clb) Brc) P3- Electronic Structure - Questions Copy and complete the following table: Atomic no. Mass no. No. of No. of No. of protons neutrons electrons Mg 12 Al3+ 27 S2Sc3+ Ni2+ 21 1s2 2s2 2p6 3s2 10 16 16 45 30 Electronic structure 26 Orbitals The energy sub levels are made up of orbitals, each which can hold a maximum of 2 electrons. Different sub-levels have different number of orbitals: Sub-level No. of orbitals Max. no. of electrons s 1 2 p 3 6 d 5 10 f 7 14 Orbitals The orbitals in different sub-levels have different shapes: • s orbitals 1s • p orbitals 2s Orbitals Within a sub-level, the electrons occupy orbitals as unpaired electrons rather than paired electrons. (This is known as Hund’s Rule). We use boxes to represent orbitals: 2p 1s 2s Electronic structure of carbon, 1s2, 2s2, 2p2 Orbitals The arrows represent the electrons in the orbitals. The direction of arrows indiactes the spin of the electron. Paired electrons will have opposite spin, as this reduces the mutual repulsion between the paired electrons. 2p 1s 2s Electronic structure of carbon, 1s2, 2s2, 2p2 Orbitals Using boxes to represent orbitals, give the full electronic structure of the following atoms: a) lithium d) nitrogen b) fluorine e) oxygen 2p 2s 1s c) potassium Orbitals Using boxes to represent orbitals, give the full electronic structure of the following atoms: a) lithium d) nitrogen b) fluorine e) oxygen Electronic structure of lithium: 1s2, 2s1 2p 2s 1s c) potassium Orbitals Using boxes to represent orbitals, give the full electronic structure of the following atoms: b) fluorine e) oxygen 2p 1s 2s Electronic structure of fluorine: 1s2, 2s2, 2p5 c) potassium a) lithium d) nitrogen Orbitals Using boxes to represent orbitals, give the full electronic structure of the following atoms: a) lithium d) nitrogen b) fluorine e) oxygen 4s Electronic structure of potassium: 1s2, 2s2, 2p6, 3s2, 3p6, 4s1 3p 3s 2p 2s 1s c) potassium Orbitals Using boxes to represent orbitals, give the full electronic structure of the following atoms: a) lithium d) nitrogen b) fluorine e) oxygen Electronic structure of nitrogen: 1s2, 2s2, 2p3 2p 1s 2s c) potassium Orbitals Using boxes to represent orbitals, give the full electronic structure of the following atoms: a) lithium d) nitrogen b) fluorine e) oxygen Electronic structure of oxygen: 1s2, 2s2, 2p4 2p 1s 2s c) potassium Ionization Energy Ionization of an atom involves the loss of an electron to form a positive ion. The first ionization energy is defined as the energy required to remove one mole of electrons from one mole of atoms of a gaseous element. The first ionization energy of an atom can be represented by the following general equation: X(g) X+ + e- ΔH > 0 Since all ionizations requires energy, they are endothermic processes and have a positive enthalpy change (ΔH) value. Ionization Energy The value of the first ionization energy depends upon two main factors: The size of the nuclear charge The energy of the electron that has been removed (this depends upon its distance from the nucleus) Ionization Energy As the size of the nuclear charge increases the force of the attraction between the negatively charged electrons and the positively charged nucleus increases. Small nuclear charge Small force of attraction Smaller ionization energy + + Large nuclear charge Large force of attraction Greater ionization energy Ionization energy As the energy of the electron increases, the electron is farther away from the nucleus. As a result the force of attraction between the nucleus and the electron decreases. Electrons closer to positive nucleus Large force of attraction Greater ionization energy + + Electrons further away from positive nucleus Small force of attraction Smaller ionization energy Ionization energy - Questions Write an equation to represent the first ionization of: a) aluminium b) lithium c) sodium Trends across a Period Going across a period, the size of the 1st ionisation energy shows a general increase. This is because the electron comes from the same energy level, but the size of the nuclear charge increases. + + + Going across a Period + Trends across a Period (2 exceptions) The first ionisation of Al is less than that of Mg, despite the increase in the nuclear charge. The reason for this is that the outer electron removed from Al is in a higher sub-level: the electron removed from Al is a 3p electron, whereas that removed from Mg is a 3s. Electronic structure Ionisation energy/kJ mol-1 Na 1s2, 2s2, 2p6, 3s1 494 Mg 1s2, 2s2, 2p6, 3s2 736 Al 1s2, 2s2, 2p6, 3s2, 3p1 577 Si 1s2, 2s2, 2p6, 3s2, 3p2 786 P 1s2, 2s2, 2p6, 3s2, 3p3 1060 S 1s2, 2s2, 2p6, 3s2, 3p4 1000 Cl 1s2, 2s2, 2p6, 3s2, 3p5 1260 Ar 1s2, 2s2, 2p6, 3s2, 3p6 1520 Trends across a Period (2 exceptions) The first ionisation energy of S is less than that of P, despite the increase in the nuclear charge. In both cases the electron removed is from the 3p sublevel. However the 3p electron removed from S is a paired electron, whereas the 3p electron removed from P is an unpaired electron. When the electrons are paired the extra mutual repulsion results in less energy being required to remove an electron, hence a reduction in the ionisation energy. Sulphur Phosphorus 3p 3s 3p 3s Trends across a Period - Questions There is a break in this general trend going across a Period. Look at the table below and point out where the break in the the trend is and try to give an explanation. Electronic structure Ionisation energy/kJ mol-1 Na 1s2, 2s2, 2p6, 3s1 494 Mg 1s2, 2s2, 2p6, 3s2 736 Al 1s2, 2s2, 2p6, 3s2, 3p1 577 Si 1s2, 2s2, 2p6, 3s2, 3p2 786 P 1s2, 2s2, 2p6, 3s2, 3p3 1060 S 1s2, 2s2, 2p6, 3s2, 3p4 1000 Cl 1s2, 2s2, 2p6, 3s2, 3p5 1260 Ar 1s2, 2s2, 2p6, 3s2, 3p6 1520 Clue: which sub-level (s, p, d or f is the outer electron in? Trends across a Period - Questions First ionisation energy/kJ mol-1 Now take a look at the graph below: 3000 2500 2000 1500 1000 500 0 He Ne N H Li 0 C Be B 5 F O P Mg Na 10 Al Si Cl Ar Ca S 15 K 20 25 Atomic number (Z) a) Explain what the graph shows in as much detail as possible b) There is one other break in the general pattern going across a Period. What is it and explain why that is. Trends down a Group + + + Down the Group Ionization energy decreases going down a Group. Going down a Group in the Periodic Table, the electron removed during the first ionization is from a higher energy level and hence it is further from the nucleus. The nuclear charge also increases, but the effect of the increased nuclear charge is reduced by the inner electrons which shield the outer electrons. + Ionization energy - Questions 1. Explain why sodium has a higher first ionization energy than potassium. 2. Explain why the first ionization energy of boron is less than that of beryllium. 3. Why does helium have the highest first ionisation energy of all the elements? 4. Complete Tasks Successive Ionization energy Definition: 2nd i.e. The energy per mole for the process X+(g) X2+(g) +eAnd so on for further successive ionisation energies Successive Ionization energy Successive i.e’s increases because electrons are being removed from increasingly positive ions. Therefore, nuclear attraction is greater. Large jumps seen when electron is removed form a new sublevel closer to the nucleus Successive Ionization energy Large increase between 4th and 3rd shells – electron closer to nucleus 2nd i.e higher than first – electron has greater pull from nucleus Electron Affinity Energy Change per mole for: X (g) + e- X-(g) That is, for the gaseous atoms to gain an electron to form anions Electron Affinity The first e.a is negative (exothermic) because the electron is attracted to the positive charge on the atom’s nucleus. The second e.a is positive (endothermic) because an electron is being added to an ion which is already negative : repulsion occurs