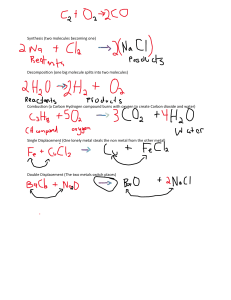
CHEM123 General Chemistry I Periodic Trends in Reactivity Lab Name: ______________________ Introduction: Elements on the periodic table are organized by increasing atomic number. As atomic numbers increases, so does the number the electrons. Electrons, and specifically valence electrons, are important in determining how an atom interacts with other atoms. The elements in a vertical “group” have similar properties because they have the same number of valence electrons and similar electron configurations. This leads to smoothly varying trends in properties such as ionization energy, electronegativity, ionization energy, and atomic radius as one moves both down the groups and across the periods. Thus, the organization of the periodic table is useful for making predictions about an element based on its position in the table. The reactivity of the elements also follow well-defined trends both down the groups and across periods. In this lab, you will explore these trends in reactivity. Part 1: Reactivity of the Metal Groups 1. Observe the reactions of Magnesium and Calcium: Metal Observations: Reaction in Water Magnesium Calcium 2. Watch the video: “Alkali Metals in Water” (on your canvas page under this lab's report) and describe observations: Metal Observations: Reaction in Water Lithium Sodium Potassium Rubidium Cesium Francium prediction: Conclusion Questions: State and EXPLAIN the trend in reactivity for the alkali metals. Why does reactivity increase or decrease down a group in terms of electron shielding? Explain why the alkali metals are more reactive than the alkaline earth metals. 1 Part 2: Activity Series of Some Metals in HCl 1. Obtain a small piece of each of the metals (listed in the data table below). 2. Place each metal in a small test tube 3. Add about 2 mL 1.0M HCl to the test tube and observe. 4. Look for signs of the strength (energy released) of the reaction Metal Observations: Reactions with HCl (acid) Magnesium Aluminum Copper Zinc Conclusion Questions: Arrange the 4 metals from most reactive to least reactive. In general, is there a relationship between the locations of metals on the Periodic Table and their relative activity? Explain. Use your chemistry book or another source to locate a “metal activity series” and compare to your experimental list of metals. Account for any similarities or differences. Part 3 – Metal Activity Series The reactions carried out in this part of the experiment will be used to generate an activity series involving the following six metals: Ca, Cu, Fe, Mg, Sn, and Zn. In the first test, a small piece of each of these metals will be added to separate test tubes containing hydrochloric acid. Any evidence of a reaction (or the absence thereof) will be noted. In the second battery of qualitative tests, each of the given metals will be placed in a variety of different metal ion solutions to determine if a metal displacement reaction occurs. Using the data obtained from these tests, you should be able to order the six metals in order of increasing reactivity (i.e., you should be able to rank them from least active to most active). Please follow the procedure outlined below. 1. Sketch the following table in your notebook. If you prefer, you can construct two separate tables. For instance, one table can be used to record the results of the tests between the various metals and hydrochloric acid. A second table can be used to record the observations that you made when you mixed the six different metals with the various solutions containing metal ions. Regardless of how many tables you construct in your lab notebook, make sure 2 that you leave sufficient room to record detailed observations. Note: The shaded areas in the table shown below indicate tests that need not be (and will not be) performed as no displacement reaction will occur. solns HCl Ca(NO3)2 Cu(NO3)2 FeSO4 Fe(NO3)3 Mg(NO3)2 SnCl4 Zn(NO3)2 metals Ca Cu Fe Mg Sn Zn 2. Reactions with Hydrochloric Acid Obtain six small test tubes. Place 10 drops of 6 M HCl in each of the six test tubes. Examine how Ca, Cu, Fe, Mg, Sn, and Zn react with hydrochloric acid by adding a small piece of each metal to a different test tube. Monitor the solutions for evidence of a chemical reaction. For example, look for the evolution of a gas or a persistent color change. Record your observations. If a reaction occurs, the relative speed of the reaction should be noted (i.e., indicate whether the reaction was immediate, slow, very slow, etc.). If a gas is produced, indicate whether the reaction was vigorous or weak. 3. Metal Displacement Reactions In this last series of tests, you will examine how each of the six metals react when added to solutions containing cations of the other metals. Begin by adding a small piece of calcium metal to six different test tubes. Next, add 5 drops of 0.1 M Cu(NO3)2 to the first test tube, 5 drops of 0.1 M FeSO4 to the second test tube, 5 drops of 0.1 M Fe(NO3)3 to the third test tube, 5 drops of 0.1 M Mg(NO3)2 to the fourth test tube, 5 drops 0.1 M SnCl4 to the fifth test tube, and 5 drops of 0.1 M Zn(NO3)2 to the sixth test tube. Record any changes that occur and note any indication of reaction, including color change, gas evolution, etc. Be sure to make careful observations and record detailed notes. If you observe a color change, document whether it was the color of the solution and/or the metal surface that changed. When you are finished, the test tube contents should be discarded in the appropriate waste contain- er(s). Rinse all of the test tubes before moving on. Repeat this general procedure for each of the remaining 5 metals. Keep in mind that each series will involve slightly different metal solutions as there is no need to react a given metal with a solution containing the same metal cation. For instance, you do not need to add copper to a solution of copper(II) nitrate. When 3 finished this series of tests, you should have data recorded in every cell of your table (or tables) except for those shaded grey in the sample table. In a number of cases, there will be no reaction. A lack of reaction indicates that the metal that was added to the solution is less ac- tive than the metal in solution. Discussion and Analysis: Use the data collected above to help you rank Ca, Cu, Fe, Mg, Sn, and Zn in terms of increasing reactivity. A m o r e r e a c t i v e m e t a l w i l l b e a b l e t o d i s p l a c e a l e s s r e a c t i v e m e t a l i n s o l u t i o n . You should be able to rank most (if not all) of the metals by simply comparing the reactions that occurred when each metal was added to HCl. Clearly, those metals that reacted with HCl to produce hydrogen gas would be considered more active that those that exhibited no reaction. In addition, the metal that reacted the most vigorously in acid would be the most reactive metal. By continuing this line of reasoning you should be able to generate at least a preliminary activity series for the metals under study. Data collected in the metal displacement tests can be used to corroborate your findings and/or aid you in determining the correct ranking (or at least the one supported by your experimental data). In this case, the metal that exhibited no reaction when added to all of the different metal solutions would be con- sidered the least active metal. Conversely, the metal that displaced all of the metals in solution would be considered the most active metal. In your discussion, you should explain in detail how you arrived at your final prediction regarding the relative reactivities of these metals. In other words, you must indicate how you came up with your metal activity series. Overall Conclusion: When elements are organized in the periodic table, various trends appear. Describe some of the trends that you learned about from this lab and in class and discuss, in general, WHY the trends appear across the periods and down the groups. You may want to view some additional properties on the interactive periodic table: 4