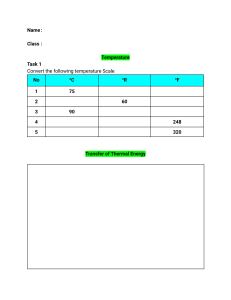
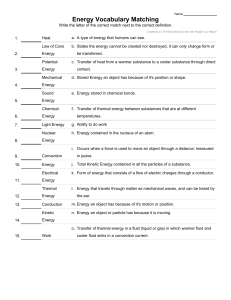
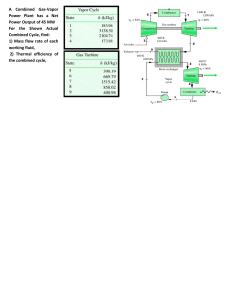
Heat Transfer Chapter 1 1. Define thermal conductivity. Thermal conductivity is a fundamental property of materials that quantifies their ability to conduct heat. It is defined as the rate at which heat is conducted through a unit area of a material, per unit temperature difference across that area. In simpler terms, thermal conductivity represents how well a material can transfer heat through it. Mathematically, the formula for thermal conductivity (k) is given by: k= Q / A⋅ ΔT ⋅t Where: k is the thermal conductivity of the material. Q is the heat conducted through the material. A is the cross-sectional area of the material through which heat flows. ΔT is the temperature difference across the material. t is the time it takes for heat transfer to occur. The SI unit of thermal conductivity is watts per meter per Kelvin (W/(m·K)). 2. Define the convection of heat transfer coefficient. The convection heat transfer coefficient (often denoted as "h") is a parameter used to quantify the rate of heat transfer between a solid surface and a moving fluid (liquid or gas) through convection. It describes how effectively heat is exchanged between the solid surface and the fluid due to their temperature difference and the fluid motion. The convection heat transfer coefficient is used in the convective heat transfer equation: Q = h⋅ A⋅ ΔT Where: Q is the rate of heat transfer between the solid surface and the fluid. h is the convection heat transfer coefficient. A is the surface area over which heat is being transferred. ΔT is the temperature difference between the solid surface and the bulk fluid. The heat transfer coefficient accounts for the efficiency of the heat transfer process, taking into consideration factors such as fluid velocity, fluid properties, surface geometry, and the nature of the fluid flow (laminar or turbulent). Higher values of eh indicates more efficient heat transfer. 3. Discuss the mechanisms of thermal conduction in gases and solids. Let's discuss the mechanisms of thermal conduction in gases and solids. Thermal Conduction in Gases: Thermal conduction in gases occurs through the transfer of kinetic energy from faster-moving molecules to slower-moving ones. Gases consist of molecules that move freely and randomly, colliding with each other. While gases have low densities compared to solids, they can still conduct heat, albeit less efficiently due to the larger gaps between molecules. The mechanism of thermal conduction in gases involves three main steps: 1. Energy Transfer by Collisions: Gas molecules are in constant motion, colliding with each other. When a molecule with higher kinetic energy (temperature) collides with a molecule of lower kinetic energy, it transfers some of its energy. This process continues as energy is gradually transferred from the hotter to the cooler regions. 2. Propagation of Kinetic Energy: As molecules gain kinetic energy from collisions, they move faster and their kinetic energy increases. This kinetic energy is then transferred to nearby molecules through subsequent collisions. Over time, this chain reaction leads to the propagation of heat through the gas. 3. Net Heat Flow: Although individual molecules move randomly, the overall net flow of heat is from the region of higher temperature to the region of lower temperature. This results in the establishment of a temperature gradient within the gas. Thermal Conduction in Solids In solids, thermal conduction occurs primarily through the vibration and interaction of atoms or molecules within a lattice structure. In contrast to gases, solids have a denser arrangement of particles, which allows for more efficient heat transfer through their closely packed structure. The mechanism of thermal conduction in solids involves these steps: 1. Vibration of Particles: At any temperature above absolute zero, particles in solids vibrate around their equilibrium positions. As temperature increases, these vibrations become more vigorous. This vibration corresponds to the internal energy of the solid. 2. Lattice Interaction: As particles vibrate, they interact with neighboring particles through various intermolecular forces (such as van der Waals forces or covalent bonds). When a particle vibrates more intensely, it transfers some of its energy to adjacent particles. This energy transfer occurs in a chain reaction, with energy being propagated through the lattice. 3. Propagation of Energy: The process of energy transfer from particle to particle leads to the propagation of heat through the solid. The lattice vibrations effectively carry thermal energy from the hotter region to the cooler region. 4. Heat Gradient: Similar to gases, a temperature gradient is established in solids, causing heat to flow from the higher-temperature region to the lower-temperature region. In summary, thermal conduction in gases involves the transfer of kinetic energy through molecular collisions, while in solids, it relies on the vibration and interaction of particles within a lattice structure. The efficiency of thermal conduction depends on factors such as the density, arrangement of particles, and material properties. 5. Discuss the mechanism of heat convection. Heat convection is the transfer of heat through the movement of fluids (liquids or gases) due to temperature differences. It occurs as fluid particles carry thermal energy from one place to another through their motion. Heat convection is a vital process in various natural and industrial contexts, such as atmospheric circulation, ocean currents, cooking, and cooling systems. Let's dive into the mechanism of heat convection: 1. Natural Convection: Natural convection occurs when fluid motion is driven by buoyancy forces resulting from temperature differences. The mechanism of natural convection involves the following steps: 1. Heating and Expansion: When a fluid near a heated surface gets warmer, it expands and becomes less dense, causing it to rise. 2. Cooling and Contraction: As the fluid moves away from the heat source, it loses energy to its surroundings, cools down, and contracts, making it denser and causing it to sink. 3. Formation of Convection Cells: The rising and sinking motion creates convection cells, where warmer fluid rises along one path while cooler fluid descends along another path. This circulation allows heat to be transported throughout the fluid. 2. Forced Convection: Forced convection involves external means, such as fans or pumps, to induce fluid motion and enhance heat transfer. The mechanism of forced convection is as follows: 1. Forcing the Fluid: External means introduce a mechanical force that moves the fluid along a specific path. 2. Enhanced Heat Transfer: The continuous movement of fluid helps maintain a higher temperature gradient between the heated surface and the fluid, resulting in faster heat transfer. Turbulence often develops in forced convection, which further enhances heat transfer by disrupting the boundary layer of slow-moving fluid next to the surface. 3. Boundary Layers: In both natural and forced convection, a boundary layer forms at the surface where the fluid velocity is affected by the surface. The boundary layer consists of fluid particles with reduced velocity due to their proximity to the surface. In natural convection, this layer is due to the differences in density, while in forced convection, it's due to the interaction with the moving surface. 4. Effect of Fluid Properties: The properties of the fluid, such as its density, viscosity, and thermal conductivity, influence the convection process. More viscous fluids or those with higher thermal conductivity tend to transfer heat more slowly. These properties also affect whether the convection is in a laminar (smooth, orderly) or turbulent (chaotic) flow regime. In summary, heat convection involves the transfer of heat through the movement of fluids due to temperature differences. Whether its natural convection driven by buoyancy or forced convection aided by external forces, fluid motion plays a crucial role in redistributing heat within a system. 5. When may one expect radiation heat transfer to be important? Radiation heat transfer becomes important in various situations, especially when heat is transferred through electromagnetic waves without requiring a medium for propagation. Here are some scenarios where radiation heat transfer is significant: 1. High Temperatures: At high temperatures, objects emit thermal radiation according to the Stefan-Boltzmann law (E = σT^4), where E is the radiant energy emitted per unit area, σ is the Stefan-Boltzmann constant, and T is the absolute temperature in Kelvin. As temperatures increase, the intensity of radiation becomes substantial. This is why objects like incandescent light bulbs or industrial furnaces radiate heat effectively. 2. Vacuum Conditions: Radiation is the only mode of heat transfer that can occur in a vacuum, where there is no matter to carry heat through conduction or convection. In space, for instance, objects can exchange heat with their surroundings primarily through radiation. This is crucial for understanding how planets and other celestial bodies maintain their temperatures. 4. Transparent Media: Radiation can pass through transparent materials, unlike conduction and convection, which require a medium. For example, solar radiation passes through the Earth's atmosphere and warms the surface, while some of this heat is then radiated back into space. 5. Large Temperature Differences: Radiation heat transfer becomes significant when there are substantial temperature differences between two surfaces. This is evident in situations where an object is heated by a much hotter source, such as a person feeling warm standing near a hot stove. 6. Engineering Applications: Radiation is essential in various engineering applications, such as designing and analyzing thermal systems, building insulation, solar energy systems, heat exchangers, and electronics cooling. In industrial processes like metallurgy, glassmaking, and heat treatment, radiation plays a critical role in achieving specific temperature profiles. 7. Thermal Balance in Space: Objects in space, like satellites or the International Space Station, are exposed to extreme temperature variations due to the absence of an atmosphere. Proper thermal control systems, which include insulation and radiators, are crucial to manage the balance between absorbing solar radiation and radiating excess heat into space. In summary, radiation heat transfer becomes important in situations involving high temperatures, vacuum conditions, transparent media, large temperature differences, and various engineering applications. Understanding and accounting for radiation heat transfer is crucial in a wide range of scientific, industrial, and practical contexts. 6. What is the order of magnitude of thermal conductivity for metals, solid insulating materials, liquids, gases? The thermal conductivity of materials can vary widely based on factors like their composition, structure, temperature, and pressure. Here is approximate order of magnitude ranges for the thermal conductivity of different types of materials: 1. Metals The thermal conductivity of metals is relatively high due to their dense and ordered atomic structures. Typical range: 10 - 500 W/(m·K), depending on the metal and temperature. 2. Solid Insulating Materials Insulating materials have lower thermal conductivity because they are designed to resist heat transfer. Typical range: 0.01 - 10 W/(m·K), with some specialized insulators potentially having even lower values. 3. Liquids The thermal conductivity of liquids is generally lower than that of solids due to their less ordered molecular structure. Typical range: 0.1 - 10 W/(m·K), with some liquids having higher or lower values. 4. Gases The thermal conductivity of gases is usually lower than that of liquids due to their lower density and the larger distances between molecules. Typical range: 0.001 - 0.1 W/(m·K), with gases like air falling within this range. Keep in mind that these ranges are approximate and can vary depending on the specific material and conditions. Additionally, the values provided are for general reference and should not be considered definitive for all materials in each category. It's always a good idea to consult specific material property databases or references for accurate thermal conductivity values for your particular application.