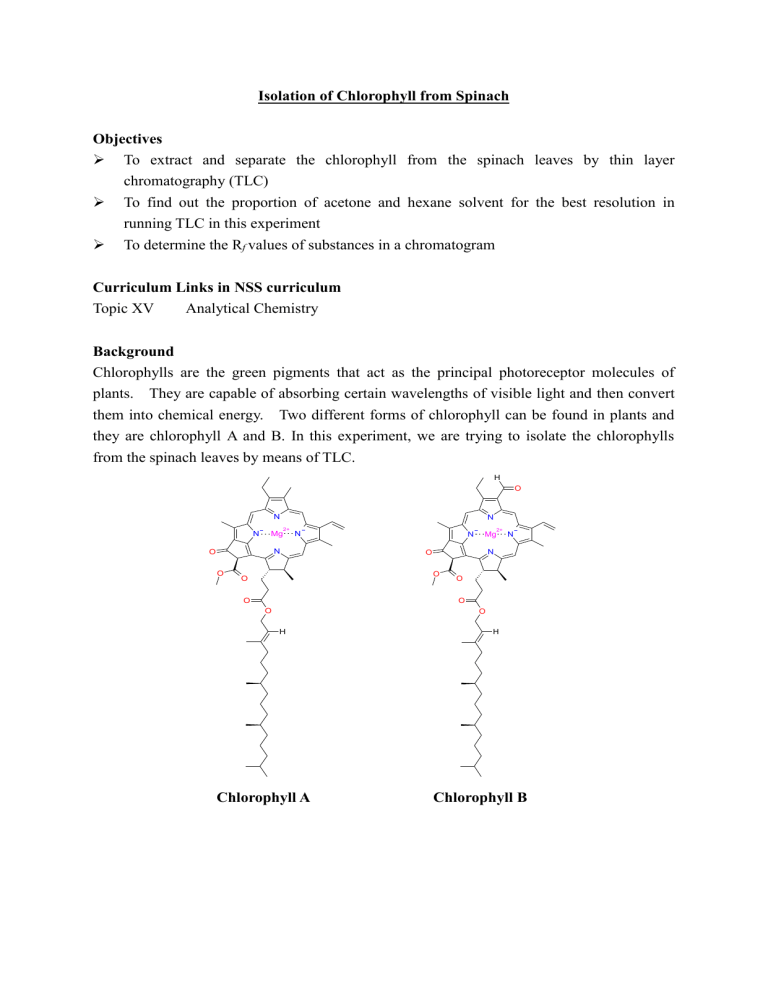
Isolation of Chlorophyll from Spinach Objectives To extract and separate the chlorophyll from the spinach leaves by thin layer chromatography (TLC) To find out the proportion of acetone and hexane solvent for the best resolution in running TLC in this experiment To determine the Rf values of substances in a chromatogram Curriculum Links in NSS curriculum Topic XV Analytical Chemistry Background Chlorophylls are the green pigments that act as the principal photoreceptor molecules of plants. They are capable of absorbing certain wavelengths of visible light and then convert them into chemical energy. Two different forms of chlorophyll can be found in plants and they are chlorophyll A and B. In this experiment, we are trying to isolate the chlorophylls from the spinach leaves by means of TLC. H O N N Mg N 2+ N N O O N O 2+ N N O O O Mg O O O O H Chlorophyll A H Chlorophyll B Materials and Apparatus Spinach leaves Acetone Brine Hexane Anhydrous sodium sulphate Scissors Capillary tubes TLC plates* Mortar and Pestle A pair of forceps 160mm x 16mm screw-cap test tubes Glass container with lid for TLC Droppers Filter paper 25mL conical flasks Filter funnel Paper towel 10mL measuring cylinder 50mL beaker Cotton wool *Cut the TLC plates into small rectangular pieces. The length of each plate should be about the same height of the glass container. Experimental Procedure Part I: Extraction 1. Clean some spinach leaves with water and dry it with a paper towel. Cut 2g of spinach leaves into small pieces with scissors and put into the mortar. Add 3mL of acetone into a mortar and grind the leaves with the pestle. Decant the solution through a filter funnel with a piece of cotton wool to a screw-cap test tube and squeeze the residue for getting more extract. 2. Pour 3 mL of acetone into the mortar and grind the residue with the pestle again. Pour the liquid part into the screw-cap test tube through the filter funnel. Then pour 3 mL of acetone and 5 mL of hexane into the mortar and grind the residue again and then filter the extract into the screw-cap test tube. The residue left over should be very pale green. 3. Add 3 mL of brine into the screw-cap test tube. Put on the cap tightly and shake the screw-cap test tube vigorously to wash the chlorophyll-acetone-hexane solution. Open the screw cap of the test tube occasionally to release the gas pressure. Then set the tube back on the test-tube rack and allow the mixture to separate. The top layer should be a dark green and the bottom layer clear. 4. If emulsion is hard to separate, add some brine to facilitate the separation. By using a dropper, separate and collect the organic layer to a clean screw-cap test tube (or to discard the bottom aqueous layer). 5. Add enough anhydrous sodium sulphate to the solution containing the chlorophyll inside the screw-cap test tube to dry the solution. Then filter the solution with a filter paper into a 25-mL conical flask. Part II: Thin Layer Chromatography 1. Draw a line with a pencil 1 cm from one edge of the plate. Use a capillary tube to take out some chlorophyll solution from the conical flask and lightly dot the solution onto the starting line of the TLC plate. Allow the solvent to completely evaporate off. Then dot more sample onto the same spot. Repeat the above procedures until a colored spot is clearly seen. 2. Put a large strip of filter paper around the interior wall of the glass container and a round paper piece at the bottom. 3. Add a mixture of acetone and hexane in volume ratio 2:8 to the glass container. The depth of the solution in the container should be less than 1 cm. Cover the lid of the container for a few minutes to let the solvent vapors saturate the air in the container. 4. By using a pair of forceps, put the TLC plate vertically into the glass container containing acetone and hexane to let the TLC run. When the solvent almost reaches the top end of the TLC plate, take the plate out and mark the solvent front. 5. Try out other mixtures of acetone and hexane (3:7 and 4:6) to see which mixture can give the best resolution. Find the Rf values. Remarks 1. During the extraction process, the chlorophyll-acetone-hexane solution is washed with brine and shaken vigorously in a screw-cap test tube. It is recommended that the screw cap should be opened frequently to release the gas pressure accumulated from the volatile organic solvent in the test tube. 2. It is recommended that the TLC plate is cut into pieces inside the fumehood with the silica side facing the bottom. 3. The large strip of filter paper surrounding the interior wall of the glass container is used for solvent saturation inside the container, while the round paper piece placed at the bottom is used to prevent the sliding down of the TLC plate positioned vertically in the container. 4. The mixture of acetone and hexane in volume ratio 3:7 should be able to give the best resolution. If lesson time is limited, students may just work on this designated mixture for the TLC analysis. 5. The result of the TLC analysis : As the solvent rises by capillary action up through the TLC plate, the components of the pigment mixture are partitioned between the mobile phase (organic solvent) and the stationary phase (silica plate) due to their different adsorption and solubility strength. The more strongly a given component is adsorbed to the stationary phase, the less easily it is removed by mobile phase. The more weakly a component is adsorbed the faster it will migrate up the TLC plate. On the other hand, the running distance depends on the solubility of the pigment in the solvent. Since the experiment employs a highly non-polar solvent (acetone-hexane mixture), the pigments that are least polar (carotenes) will be best dissolved in the non-polar solvent and will thus have the largest running distance. As chlorophyll A is more non-polar than chlorophyll B (since chlorophyll B contains polar aldehyde group), thus chlorophyll A moves faster with mobile phase to an upper position on TLC plate than chlorophyll B. Questions and suggested answers 1. Why do we need to cut and grind the leaves? Answer: It increases surface area of the leaves for the extraction of chlorophylls. 2. Can ethoxyethane be used as extraction solvent instead of acetone? Explain. Answer: Yes. Ethoxyethane is an organic solvent which is immiscible with water. Besides, it has no reaction with chlorophyll. Upon shaking with the liquid mixture, the organic product will dissolve preferentially in the organic solvent. 3. State the stationary phase and the mobile phase for the thin layer chromatography conducted in this experiment. Answer: Stationary phase – thin layer of silica gel; mobile phase – liquid solvent (the acetone-hexane mixture). 4. Why can a mixture of organic components be separated by thin layer chromatography? Answer: In TLC, silica gel used as the stationary phase can be considered polar while the organic solvent used as the mobile phase is non-polar. Since the components of the mixture differ in polarity, the components in a mixture have different tendencies to adsorb onto the silica gel or dissolve in the organic solvent. The more polar component has a stronger interaction with the silica gel and is therefore adsorbed on the silica gel strongly. The more strongly a compound is adsorbed, the less distance it can travel up the plate. In contrast, the less polar compound moves higher up the plate (resulting in a higher Rf value). 5. Suggest two other types of chromatography. Paper chromatography; high performance liquid chromatography (HPLC). 6. How to recognise chlorophyll A and chlorophyll B from the chromatogram? Answer: Chlorophyll B is more polar than chlorophyll A. Therefore, chlorophyll B should have stronger attraction to silica and consequently shorter running distance up the plate compared with chlorophyll A, i.e. it has lower Rf value. 7. For thin layer chromatography, a capillary tube was used to take some solution and dotted onto the silica plate. How the chromatogram will be affected by the size of the spot? Answer: If the spot is too large, “tailing” may occur (i.e. the diameter of the spot on the chromatogram is large) and thus lead to poor separation of the mixture of compounds.