Centennial Honors College Western Illinois University Undergraduate Research Day 2014
advertisement

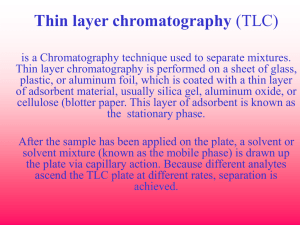
Centennial Honors College Western Illinois University Undergraduate Research Day 2014 Poster Presentation Forensic Analysis of Lipstick Using Thin Layer Chromatography Shelby Crawford Faculty Mentor: Brian J. Bellott Chemistry Thin layer chromatography (TLC) development is a common practice in the chemistry community. For the technique itself, glass or plastic plates are used that contain a thin film of silica gel or alumina. The film is the stationary phase. The mobile phase, or the liquid that is absorbed and moves up the plate, carries components of the sample in question where they are separated and deposited along the trail up the plate according to their composition. The method used to define the visual differences in the sample spotting on the TLC plate, and the individual characteristics of that compound is the retention factor (Rf) formula. In this experiment, the lipstick sample was smeared onto a consistent medium, filter paper, and the dye was extracted by a non-polar solvent, petroleum ether. The remaining liquid was evaporated and methanol was used to dissolve the sample for spotting. Silica gel aluminum plates were used with 250 mL beakers and watch glasses for the developing chamber. Two different solvent systems were used: chloroform: methanol: water and ethyl acetate: methanol: water. Rf values were calculated and collaborated for five trials of TLC developments and used as identifying bands. An unknown trial was then conducted and comparisons led to an accurate matching of all nine lipstick samples.