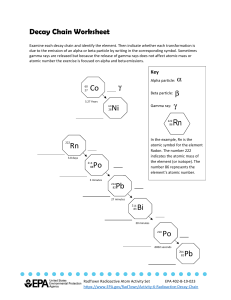
RADIOACTIVE DECAY • Radioisotopes: isotopes that have an unstable nucleus. They emit radiation to attain more stable atomic arrangements. • Radioactivity is the spontaneous disintegration of atomic nuclei. during this process the nucleus emits alpha (α) particles, beta (ß) particles, or gamma ( )rays. – Decay results in an atom of one type, called the parent nuclide, transforming to an atom of a different type, the daughter nuclide. RADIOACTIVE DECAY • Radioactive decay is not affected by chemical reactions or outer electrons. • During radioactive decay, principles of conservation apply: – conservation of energy – conservation of momentum (linear and angular) – conservation of charge – conservation of nucleon number ALPHA DECAY • An alpha particle is identical with the nucleus of a helium atom (4He), consisting of two protons and two neutrons – have a rest mass of 6.64424 × 10-27 kg – have a low penetrating power and a short range (a few centimeters in air). – The most energetic of them (up to 7.5 MeV) will generally fail to penetrate the dead layers of cells covering the skin and can be easily stopped by a sheet of paper – have a charge of +2. ALPHA DECAY • Spontaneous emission of alpha particles occurs in elements of mass number greater than about 150 because they have too many protons to be stable, e.g. uranium, thorium, and plutonium. • During alpha decay the atomic number is reduced by two units and the mass number by four units. ALPHA DECAY • Because of its mass, relatively slow speed, and its positive charge, an alpha particle can easily remove electrons from (cause ionization of) atoms in a substance, and its energy of motion is transferred to the medium. • They have a high linear energy transfer (rapid loss of energy in a small range) which makes them a radiation hazard if ingested. Alpha Decay of Americium-241 to Neptunium-237 BETA DECAY • Two types of radiation can be emitted in this category: – Negative beta decay; an electron is emitted from the nucleus. – Positive beta decay; a positron is emitted from the nucleus. NEGATIVE BETA DECAY • Occurs in very "neutron-rich" elements (mostly that are lighter than lead), e.g. Strontium-90. These elements have too many neutrons and too few protons to be stable. They can become more stable by emitting a beta particle. – Neutron changes into a proton. In the transformation of neutron to proton the nucleus loses a negative charge and emits an electron with charge -1. NEGATIVE BETA DECAY • The atomic number increases but the mass number is unchanged • Electron’s are charged so they will interact with electrons in atoms they come close to and cause ionization. Beta particles cause less ionization than alphas, and have a longer range, typically a few meters in air. • Note: an electron from the outer cloud is never emitted as a beta particle. Negative Beta Decay of Hydrogen-3 to Helium-3 Strontium decay to Yttrium 90 38 Sr Y 90 39 POSITIVE BETA DECAY • Occurs neutron-deficient atoms (mostly that are lighter than Lead) – Proton changes into an neutron and in the transformation the nucleus loses a positive charge and emits a positron (anti-electron), e+. Positron emission Positron Decay of Carbon-11 to Boron-11 ELECTRON CAPTURE • Another type of beta decay which also occurs when the neutron to proton ratio in the nucleus is too small. The nucleus captures an electron which basically turns a proton into a neutron. Atomic number decreases by one but the mass number is unchanged. Electron Capture of Beryllium-7. It decays to Lithium-7. GAMMA RAYS • • • • The most energetic and shortest-λ type of EMR Are waves like X rays and radio waves. Have no mass and no charge Are very penetrating and are best stopped or shielded by dense materials. They also have a long range. • Frequently accompanies alpha and beta emissions and always accompanies nuclear fission. GAMMA RAYS • In many cases, the ejection of an alpha or beta particle completes the radioactive decay process. In some cases the daughter nucleus, when formed, has too much energy to be stable and gets rid of this by the emission of a gamma ray, usually within thousandths of a second. • Emission of gamma ray does not alter the mass number nor the atomic number. • Cause ionization of atoms but in a different way to alpha and beta particles. How? Gamma Decay of Helium-3 EFFECT OF ELECTRIC FIELD ON RADIATION. PENETRATING POWER OF RADIATION