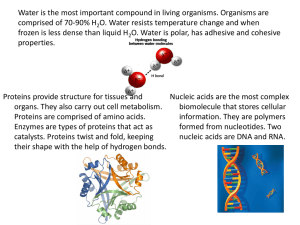
BIOCHEMISTRY (LAB) MODULE I. pH of Common Solutions and Buffer Preparation M1 Lesson 1 - pH of Common Solutions natural water may be either acidic or basic. Cleaners tend to be basic. Not All Liquids Have a pH Value, pH only has meaning in an aqueous solution (in water). Many chemicals, including liquids, do not have pH values. If there's no water, there's no pH. For example, there is no pH value for vegetable oil, gasoline, or pure alcohol. pH of Common Acids Learn the pH of Common Solutions pH is a measure of how acidic or basic a chemical is when it's in an aqueous (water) solution. A neutral pH value (neither an acid nor a base) is 7. Substances with a pH greater than 7 up to 14 are considered bases. Chemicals with a pH lower than 7 down to 0 are considered acids. The closer the pH is to 0 or 14, the greater its acidity or basicity, respectively. Here's a list of the approximate pH of some common chemicals. pH is a measure of how acidic or basic an aqueous solution is. pH usually ranges from 0 (acidic) to 14 (basic). A pH value around 7 is considered neutral. pH is measured using pH paper or a pH meter. Most fruits, vegetables, and body fluids are acidic. While pure water is neutral, Fruits and vegetables tend to be acidic. Citrus fruit, in particular, is acidic to the point where it can erode tooth enamel. Milk is often considered to be neutral since it's only slightly acidic. Milk becomes more acidic over time. The pH of urine and saliva is slightly acidic, around a pH of 6. Human skin, hair, and nails tend to have a pH of around 5. pH Common Acids 0 Hydrochloric Acid (HCl) 1.0 Battery Acid (H2SO4 sulfuric acid) and stomach acid 2.0 Lemon Juice 2.2 Vinegar 3.0 Apples, Soda 3.0 to 3.5 Sauerkraut 3.5 to 3.9 Pickles 4.0 Wine and Beer 4.5 Tomatoes 4.5 to 5.2 Bananas around Acid Rain 5.0 5.0 Milk of Magnesia 11.0 Ammonia 11.5 to Hair Straightening Chemicals 14 Black Coffee Neutral pH Chemicals Distilled water tends to be slightly acidic because of dissolved carbon dioxide and other gases. Pure water is nearly neutral, but rainwater tends to be slightly acidic. Natural water rich in minerals tends to be alkaline or basic. 7.0 10.5 - Pure Water pH of Common Bases Many common cleaners are basic. Usually, these chemicals have a very high pH. Blood is close to neutral but is slightly basic. 12.4 Lime (Calcium Hydroxide) 13.0 Lye 14.0 Sodium Hydroxide (NaOH) Other pH Values Soil pH ranges from 3 to 10. Most plants prefer a pH between 5.5 and 7.5. Stomach acid contains hydrochloric acid and other substances and has a pH value of 1.2. While pure water free of undissolved gases is neutral, not much else is. However, buffer solutions may be prepared to maintain a pH near 7. Dissolving table salt (sodium chloride) in water does not change its pH. pH Common Bases 7.0 to 10 Shampoo There are multiple ways to test the pH of substances. 7.4 Human Blood 7.4 Human Tears 7.8 Egg The simplest method is to use pH paper test strips. You can make these yourself using coffee filters and cabbage juice, use Litmus paper, or other test strips. The color of the test strips corresponds to a pH range. Because the color change depends on the type of indicator dye used to coat the paper, the result needs to be compared against a chart of standard. around Seawater 8 8.3 Baking Soda (Sodium Bicarbonate) around Toothpaste 9 How to Measure pH Another method is to draw a small sample of a substance and apply drops of pH indicator and observe the test change. Many home chemicals are natural pH indicators. M1 Lesson 2 - Buffer Preparation pH test kits are available to test liquids. Usually, these are designed for a particular application, like aquaria or swimming pools. pH test kits are fairly accurate but may be affected by other chemicals in a sample. The most accurate method of measuring pH is using a pH meter. pH meters are more expensive than test papers or kits and require calibration, so they are generally used in schools and labs. pH Equation The equation for calculating pH was proposed in 1909 by Danish biochemist Søren Peter Lauritz Sørensen: pH = -log[H+] where the log is the base-10 logarithm and [H+] stands for the hydrogen ion concentration in units of moles per liter solution. The term "pH" comes from the German word "potenz," which means "power," combined with H, the element symbol for hydrogen, so pH is an abbreviation for "power of hydrogen." Note About Safety Chemicals that have very low or very high pH are often corrosive and can produce chemical burns. It's fine to dilute these chemicals in pure water to test their pH. The value won't be changed, but the risk will be reduced. There are a number of applications in chemistry and biology where changes in pH can have a major negative effect. One example of this exists in the human body; changes to blood pH could have a devastating effect, so a mechanism within the body known as the bicarbonate buffering system keeps your blood's pH in check. In laboratory settings, a buffer solution is used to achieve similar results. The buffer solution maintains a balance in the pH of whatever is being worked with, preventing outside influences from shifting the pH and potentially ruining everything. Buffer Solutions A buffer solution is made up of a weak acid and its conjugate base or a weak base and its conjugate acid. The two components maintain a pH balance that resists change when strong acids or bases are added to it. Buffer Preparation The solution is made by taking a weak acid and adding its conjugate base (which is formed by removing a proton from the same type of acid) or by combining a weak base with its conjugate acid. The use of conjugates is what gives a buffer solution its resistance to pH changes; it creates an equilibrium between the acid and the base which is difficult for other acids or bases to overcome. Even when strong acids or bases are added, the equilibrium between the weak acid/base and its conjugate reduces the impact of the addition on overall solution pH. Examples of Buffers: blood - contains a bicarbonate buffer system TRIS buffer phosphate buffer As stated, buffers are useful over specific pH ranges. For example, here is the pH range of common buffering agents: Buffer pKa citric acid 3.13., 4.76, 6.40 pH range (NaOH), is added to raise the pH of alkaline buffers. Buffering pH Buffer solutions have a wide range of applications, both in the real world and in the lab. A buffered pH is required for most enzymes to function correctly, and buffering is used to ensure proper color concentration when using dyes. Buffer solutions are also used to calibrate equipment, especially pH meters that might be miscalibrated if a buffer is not present. It's worth noting that buffer solutions do not necessarily have a neutral pH, just a balanced one; buffer solutions made from citric acid, ammonia, acetic acid (which is found in vinegar in low concentrations) and other compounds can have pH values as low as 2 or higher than 10. This allows the use of buffer solutions in work with very strong acids or bases. Buffer Capacity 2.1 to 7.4 acetic acid 4.8 3.8 to 5.8 KH2PO4 7.2 6.2 to 8.2 borate 9.24 8.25 to 10.25 CHES 9.3 8.3 to 10.3 When a buffer solution is prepared, the pH of the solution is adjusted to get it within the correct effective range. Typically a strong acid, such as hydrochloric acid (HCl) is added to lower the pH of acidic buffers. A strong base, such as sodium hydroxide solution While buffer solutions are resistant to changes in pH, this doesn't mean that the pH of a buffer solution can't change if enough strong acid or strong base is added. The amount of a strong acid or base that a buffer solution can take before significant pH changes occur is known as the buffer capacity. The capacity differs depending on the core components of the buffer solution and how much of the strong acid or base is added to the solution. If adding a strong acid to the buffer solution, the capacity is equal to the amount of the base in the solution. If adding a strong base, the capacity is equal to the amount of the acid in the solution. MODULE II. Buffer Effects on Solution the K equation because of its constant value. M2 Lesson 2- Effects of Temperature on Buffer Solutions A- + H2O The pH of solutions is also affected by changes in temperatures. Chemical equilibrium exists in all buffer systems that are usually affected by temperature, concentration, and pressure as learned from Le Chatelier’s Principle. This K equation is the key to the buffering effect such that if [OH-] increases, then [HA] and [A-] must change. Some of the HA molecules react with the added OH-, making the solution less basic than without the conjugate base(A-). Thus the buffering effect can be stated as: if K is small, the buffering effect is small because there’s not much HA in the solution. If the value of K increases with temperature, then the buffering effect is stronger at a higher temperature. If K decreases, then the buffering effect is weaker when it's warmer. Thus the pH of any solution is temperature dependent due to this dynamic equilibrium where pK changes with temperature, it follows that pH must also change. In a buffered solution consisting of a mixture of a weak acid and its conjugate base that provides the buffering capacity, its pH changes very little as a small amount of strong acid or base is added into it. The dissociation constant for the weak acid changes with temperature resulting in a small change in pH so that the pK at a particular temperature remains constant. In an unbuffered solution, there is no buffering capacity so that the pH changes significantly as a small amount of strong acid or base is added into it. Effect of increasing the temperature on Buffer Capacity In a buffer solution, as long as dissociation constant changes with temperature, then the concentration of the ions also changes. Consider the following equilibrium reactions: The brackets in the equation means “the concentration of”. H2O is not included in HA + OH- K = [HA] [OH-] / [A-] M2 Lesson 3- Computation of pH of Unbuffered Solutions and Buffered Solutions The pH of solutions is directly measured using pH paper and pH meter. However, this can also be determined by computation using appropriate mathematical formulas and equations such as the Henderson-Hasselbalch Equation. Buffers are chosen with an appropriate pH range for control. This pH range is measured by the HendersonHasselbalch equation derived as follows: we will imagine a buffer composed of acid, HA, and its conjugate base, A-. We know that the acid dissociation constant pKa of the acid is given by this expression: Ka = [H+] [A-] / [HA] The equation can be rearranged as follows: MODULE III. The Cell Introduction [H+] = Ka [HA] / [A-] pH = pKa + log ( [A-] / [HA]) Henderson-Hasselbalch equation Where pH refers to the concentration of H+, pKa is the acid dissociation constant, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the starting acid. From the equilibrium constant K and the initial concentration of the acid, the pH of a buffer solution can be calculated. The equilibrium constant reveals the strength of the weak acid or the buffer. The concentration of [H+] can also be solved using the Ka and the equilibrium equation. pH of the solution can also be calculated from the concentration of [H+] as follows: pH = - log ([H+]) Buffer Effectiveness An effective buffer should be made of an acid and its conjugate base or a base and its conjugate acid where the Ka value is very similar to the desired pH. The exact ratio of the conjugate base to the acid is determined from the Ka value and the Henderson-Hasselbalch equation for the desired pH. The buffer is most effective when the amounts of acid and its conjugate base are approximately equal. LAB Expt 1, 2 - pH & Buffers.pdf Cells are the structural and functional units of all living organisms. The human body is composed of trillions of cells that provide structure for the body, take in nutrients from food, convert nutrients into energy, and carry out specialized functions. Cells share many common features, yet they can look very different. They have adapted over billions of years to a wide array of environments and functional roles. However, all cells rely on the same basic strategies in order to survive: allow necessary substances in and permit others out, maintain their health, and replicate themselves. Cells are the smallest common denominator of life. Some cells are organisms unto themselves; others are part of multicellular organisms. All cells are made from the same major classes of organic molecules: nucleic acids, proteins, carbohydrates, and lipids. Though they are small, together they form tissues that themselves form organs, and eventually entire organisms. Parts of a Eukaryotic Cell M3 Lesson 1 - Types of Cells Types of Cell 1. Cell membrane – It controls what gets in and out of the cell 1. Prokaryotic Cell These are unicellular organisms that do not develop or differentiate into multicellular forms. They are identical and capable of independent existence. 2. Cytoplasm – It is the living substance of the cell Cytosol - It is the fluid portion of the cell. They lack a nucleus and membranous organelles. Organelles – small, membrane-bound compartments. Include all bacteria and archaea (archaebacteria). Mitochondrion – It is the powerhouse of the cell Endoplasmic Reticulum – Responsible for intracellular transport 2. Eukaryotic Cell These cells contain a nucleus and membrane-bound compartments, called organelles, in which specific metabolic activities take place. Include fungi, animals, and plants as well as some unicellular organisms. Golgi body – Modifies, packages and transports proteins Lysosome – Suicide bag of the cell Peroxisome – Detoxifies the cell 3. Nucleus - A rounded structure at the center of the cell that controls the metabolic activities. It contains the DNA. Animal Cell Structurally, plant and animal cells are very similar because they are both eukaryotic cells. However, plant cells are usually larger than animal cells. They also contain structures that are absent in a typical animal cell, such as chloroplasts, plastids cell wall and large vacuoles. On the other hand, there are organelles found in an animal cell that are absent in plant cells. These incudes centrioles, lysosomes (rarely seen in plant cells), microvilli, cilia, and filaments. as part of the same organism or foreign. Some proteins work like fasteners, binding cells together so they can function as a unit. Other membrane proteins serve as communicators, sending and receiving signals from other cells. Parts of the Cell Membrane: Parts of the Cell_08102020-1.pdf M3 Lesson 2 - The Cell Membrane The Cell Membrane 1. Phosphate head Polar Hydrophilic 2. Fatty Acid Tail Non-polar The cell membrane serves as a clear boundary between the cell’s internal and external environment. It is also called plasma membrane or plasmalemma. It is semi-permeable with a framework of fat-based molecules called phospholipids, which prevent hydrophilic substances from entering or escaping the cell. All membranes are phospholipid bilayers with embedded proteins. Some of these proteins act as gatekeepers, determining what substances can and cannot cross the membrane. Others function as markers, identifying the cell Hydrophobic 3. Proteins a) Transmembrane Proteins b) Integral Proteins c) Peripheral Proteins Parts of the Cell Membrane_08072020-1.pdf MODULE IV. Biochemical Processes Introduction M4 Lesson 1 - Processes in Biochemical Systems All molecules in a living cell are lifeless. Molecules move in seemingly random chance or by mere coincidence. Interacting with one another to form complex products that result in wonders each of us can observe. Despite the contradiction, however, this puts up the notion that life has a molecular basis and the cell is considered to be the basic functional unit of life. This tells us that in order to understand the secrets to how living things came as to what it is now, is to fully understand how each of these molecules is vital in fulfilling their roles to perform the mechanism to make the non-living alive! All living organism needs energy to fully function. Energy is what drives biochemical processes. Metabolism is the collective term used as the sum of all of these chemical reactions and it is divided into two types; Anabolism (Building up) and Catabolism (Breaking down). Both processes involve the expense or release of energy, respectively. Processes in Biochemical Systems When we say biochemical systems, this includes all the metabolic processes that the cell undergoes. For students to understand the gist of this topic, they should be familiar with the cell’s organelles and their functions. Take note on each organelle's role in the cell as mentioned. In every biochemical systems, energy is involved, each can be either be through physical or chemical means. These reactions may or may not require energy in the form of ATP. Watch the Video below to have further understanding about this collective biochemical processes. M4 Lesson 2 - Transport Processes Transport Processes A. Active and Passive Transport Passive Transport - From High Concentration to Low Concentration or Along with a Gradient Concentration. Also known as “Downhill transport”. No energy required. Examples: Hemolysis, Diffusion of dissolved sugar in water, and Entry of fructose inside the cell. Active Transport - From Low Concentration to High Concentration or Against a Gradient Concentration. Utilized by Pumps/Carrier Proteins. Also knows as Uphill Transport. Energy requiring. D. Osmosis Basically a diffusion of water molecules from high H2O concentration to low H2O concentration across a semipermeable membrane. Ex: Endocytosis, Exocytosis, and Sodium-Potassium pump B. Dialysis A process of separating molecules in solution by the difference in their rates of diffusion through a semipermeable membrane, such as dialysis tubing, cellophane, and longganisa membrane. You may try the video link below at your own home by using diluted betadine as your iodine source. C. Diffusion It is the movement of a substance from an area of high concentration to an area of low concentration. Diffusion happens in liquids and gases because their particles move randomly from place to place. Diffusion is an important process for living things; it is how substances move in and out of cells. E. Lowering of Surface Tension Lowering Surface Tension is done by surfactants. Surfactants can be further classified into 3: Emulsifiers, Soaps, and Detergents. Surfactants are compounds that lower the surface tension (or interfacial tension) between two liquids, between a gas and a liquid, or between a liquid and a solid. Besides salt. One known biological substance that lowers surface tension is called Bile. Bile can be found from our gallbladder and is used in absorption by emulsifying fats. M4 Biochemical Processes lesson1 ONLINE.pdf MODULE V. Properties of Carbohydrates Introduction Carbohydrates are vital sources of energy for both plants and animals. They also serve as the skeletal structure for plants and as storage for the chemical energy of both plants and animals. In terms of chemical structure, carbohydrates are derivatives of aldehydes and ketones. Their interesting chemical reactions are due to such functional groups. Any material containing carbohydrates yields positive results in the Molisch Test. This test is based on the dehydration of monosaccharides by concentrated sulfuric acid to form furfural derivatives and the subsequent reaction with α-naphthol to form colored complexes. Carbohydrates with a free aldehyde or ketone groups have reducing properties. They are oxidized by alkaline solutions of cupric sulfate (Fehling’s and Benedict's reagents) producing a reddish-brown precipitate because of the reduction of cupric to cuprous ions. Nylander's reagent, an alkaline solution of bismuth sub-nitrate when added to reducing sugars, produces a black precipitate due to the formations of metallic bismuth. Barfoed's reagent, a solution of cupric acetate in a weak acetic acid, serves as another test as it is acted upon by reducing monosaccharides but not by reducing disaccharides. The simplest carbohydrates called monosaccharides consist only of one saccharide unit and thus cannot be hydrolyzed. The most abundant in the group is glucose. Other common monosaccharides are fructose, galactose, ribose, and deoxyribose. Disaccharides consist of monosaccharide units joined by a glycosidic linkage. Common disaccharides include sucrose, lactose, and maltose. Polysaccharides, the most complex of all carbohydrates, composed of many saccharide units, can be hydrolyzed by enzymes or by heating with dilute acids. M2 Lesson 1 M2 Lesson 2 Definition of Carbohydrates.pdf Classifications of Carbohydrates.pdf M5 Lesson 1 - Reactions of Carbohydrates A. Reaction with Acids Molisch’s test is a general for the presence of carbohydrates in a given analyte. This test is named after CzechAustrian botanist Hans Molisch, who is credited with its discovery. Molisch’s test involves the addition of Molisch’s reagent (a solution of ∝-naphthol in ethanol) to the analyte and the subsequent addition of a few drops of concentrated H2SO4 (sulfuric acid) to the mixture. thymol), resulting in the formation of a purple or reddish-purple colored complex. The formation of a purple or a purplishred ring at the point of contact between the H2SO4 and the analyte + Molisch’s reagent mixture confirms the presence of carbohydrates in the analyte. An image detailing a positive result for Molisch’s test is provided below. Moore’s Test is a test for the presence of reducing sugar. When a solution of reducing sugar is heated with an alkali (NaOH), it turns yellow to dark brown solution liberating the odor of caramel. This is due to the liberation of aldehyde which subsequently polymerizes to form a resinous substance. “Caramel” B. Reaction with Alkali C. Reducing Property- only the reducing sugars ( monosaccharides & disaccharides except for sucrose) do respond to this test. The formation of a purple ring at the junction of two liquids shows the visual evidence of Molisch's test with all carbohydrates A positive reaction for Molisch’s test is given by almost all carbohydrates (exceptions include tetroses & trioses). It can be noted that even some glycoproteins and nucleic acids give positive results for this test (since they tend to undergo hydrolysis when exposed to strong mineral acids and form monosaccharides). In Molisch’s test, the carbohydrate (if present) undergoes dehydration upon the introduction of concentrated hydrochloric or sulfuric acid, resulting in the formation of an aldehyde. This aldehyde undergoes condensation along with two phenol-type molecules (such as ∝-naphthol, resorcinol, and 1. Fehling’s solution is a complex compound of Cu2+. When aldehyde compound is treated with Fehling’s solution Cu2+ is reduced to Cu+ and the aldehyde is reduced to acids. During the reaction, a red precipitate is formed. Aromatic aldehydes do not respond to Fehling’s test. An aqueous solution of the compound may be used instead of an alcoholic solution. Formic acid also gives this test. General equation for: RCHO + CuSO4 → R-COOH + Cu2O + H2O Note: The appearance of red precipitate confirms the presence of an aldehydic group. Note: The appearance of shiny silver mirror confirms the presence of reducing sugar The above image shows Fehling’s test with a sucrose solution (-) that remains blue or no reaction with non-reducing sugar while glucose solution (+) gives a brick-red precipitate. Glucose is an example of a reducing sugar while sucrose is a nonreducing sugar. 2. Tollen’s Test: (Silver Mirror Test). Tollen's reagent consists of silver ammonia complex in ammonia solution. Aldehydes react with Tollen's reagent gives a grey-black precipitate or a silver mirror. Always a freshly prepared Tollen’s reagent should be used. Aldehydes are oxidized to the corresponding acid and silver in Tollen's reagent is reduced from the +1 oxidation state to its elemental form. General equation for Tollen's Test: RCHO + AgNO3 + NH4OH → R-COOH + 3NH3 + H2O + 2Ag↓(silver mirror) 3. Nylander’s Test - is a medical test for glucose in the urine, making use of a solution that contains bismuth subnitrate. The solution forms a black precipitate in a positive reaction. To prepare the reagent for the test, dissolve four grams of sodium tartrate in 100 cubic centimeters of a ten percent caustic soda solution. Then add two grams of bismuth subnitrate. Heat the mixture to 50 degrees Celsius and filter after cooling. Preserving the reagent, even for months, leaves it unaltered. The test works on the principle that when a subnitrate of bismuth comes in contact with grape sugar in a boiling alkaline solution, it reduces to black metallic bismuth. The conclusion of the test is that when reducing sugar is present, there is a brown to the black coloration of the solution where the metallic bismuth settles down. Nonreducing sugars such as sucrose do not react with Fehling’s, Tollen’s, Nylander’s, and Benedict’s reagent. It can produce a positive result with the reagent only if it is heated with dilute hydrochloric acid before the test. Once sucrose has been broken down using this method, it produces glucose and fructose, which can be detected by Benedict’s reagent. The 2nd test tube shows a positive result of reducing sugar with Nylander's test, 4. Benedict's Test- A Benedict’s test is used to determine the presence of reducing sugars such as fructose, glucose, maltose, and lactose. It is also used to test for the presence of glucose in urine. In Benedict’s test, a chemical reagent known as Benedict’s reagent or solution is used. This reagent is prepared from sodium carbonate, sodium citrate, and copper (II) sulfate. A positive test with Benedict’s reagent is indicated by a change in color, often from blue to a brick-red precipitate. When testing for the presence of reducing sugars in food, a food sample is dissolved in water and a minimal amount of Benedict’s reagent is added. The mixture is then heated in a water bath. A positive result for the presence of reducing sugars in the food is indicated by the formation of a precipitate and a change in color. Benedict’s reagent contains blue copper (II) ions, which are reduced to copper (I). These are precipitated as red copper (I) oxide, which is not soluble in water. M5 Lesson 2 - Hydrolysis of Carbohydrates Polymers are broken down by hydrolysis, which is essentially the reverse of condensation; The -OH group from water attaches to one monomer and an H attaches to the other. This is a hydrolysis reaction because water (hydro) is used to break (lyse) a bond. When a bond is broken energy is released. Polysaccharides such as starch, glycogen, and dextrin give positive results with iodine test. Starch would not give a positive Fehling's test because starch is a nonreducing polysaccharide and Fehling's is a test for reducing sugar. However, after hydrolysis into monosaccharide by the actions of strong acids, its components (glucose molecules) gives a positive result with Fehling's reagent. Fehling's test is considered positive when the solution turns from blue to orange. To test the presence of starch chemically, an iodine solution is used. If it turns from red to black or blue, the test is positive. M5 Lesson 3 - Specific Reactions of Carbohydrates Specific reactions characterize different carbohydrates. Groups of carbohydrates may be differentiated by their particular reactions with the same reagent. Some examples are: Hexoses which are monosaccharides with six carbon atoms and pentoses which have five carbon atoms are differentiated by the Bial's Orcinol test. The furfural formed from the dehydration of a pentose with orcinol forms a blue-green color solution while that from hexose is a muddy brown solution. Ketoses (carbohydrates with ketone functional group) give cherry red solution within two minutes with Seliwanoff's test, while aldoses are carbohydrates with aldehyde functional group require a longer time. This test involves the reaction of resorcinol and acid on the sugar, forming hydroxymethylfurfural as a result of dehydration. Reducing sugars from osazone crystals when heated with an excess of phenylhydrazine HCl. This reaction serves to identify the sugars by the structure of the crystals and the time required to form them. glycogen, a highly branched complex polysaccharide, gives a pale reddishbrown solution. The difference in the color of the complexes is due to the structure of these polysaccharides. Starch is made up of linear chains of glucose units of amylose which undergo helical formation. A helix containing 6 glucose units is enough to accommodate large molecules like Iodine. Thus, branched polysaccharides like glycogen, give a less intense color of the solution because of interruptions in the helices. Learning Activity 1. A. Bial's Orcinol test Hexoses which are monosaccharides with six carbon atoms and pentoses which have five carbon atoms are differentiated by the Bial's Orcinol test. The furfural formed from the dehydration of a pentose with orcinol forms a bluegreen solution while that from hexose is a muddy brown solution. Watch the youtube presentation and take note of your observation. B. Test for Reducing Sugars Similarly, upon oxidation with nitric acid, hexoses produce crystals that are soluble in dilute acid and water. Galactose in particular produces mucic acid, a dicarboxylic acid, and an isomer of saccharic acid which is identified by its insolubility in acid and water. Reducing sugars form osazone crystals when heated with an excess of phenylhydrazine HCl. This reaction serves to identify the sugars by the structure of the crystals and the time required to form them. Polysaccharides from characteristic colored complexes with Iodine. Starch gives a blue color with Iodine solution. Dextrin a product of partial hydrolysis of starch gives a color red solution and Similarly, upon oxidation with nitric acid, hexoses produce crystals that are soluble in dilute acid and water. Galactose in particular produces mucic acid, a dicarboxylic acid, and an isomer C. Mucic Acid Test of saccharic acid which is identified by its insolubility in acid and water. This is a specific test for galactose and is given by galactose as well as lactose, which is made up of galactose and glucose. Oxidation of most monosaccharides by nitric acid yields soluble dicarboxylic acids. However, oxidation of galactose yields an insoluble mucic acid. Lactose will also yield a mucic acid, due to the hydrolysis of the glycosidic linkage between its glucose and galactose subunits. Being insoluble, galactosaccharic acid crystals separate out. which undergo helical formation. A helix containing 6 glucose units is enough to accommodate large molecules like Iodine. Thus, branched polysaccharide like glycogen, give a less intense color of the solution because of interruptions in the helices. M6 EXPERIMENT 6 M5 Expt. 5 GENERAL Reactions of Carbohydrates SPECIFIC Reactions of Carbohydrates ONLINE.p ONLINE.pdf MODULE VI. Properties of Lipids Introduction D. Test for keto sugar (Seliwanoff's test) Ketoses (carbohydrates with ketone functional group) give cherry red solution within two minutes with Seliwanoff's test, while aldoses are carbohydrates with aldehyde functional group require a longer time. This test involves the reaction of resorcinol and acid on the sugar, forming hydroxymethyl furfural as a result of dehydration. E. Test for polysaccharides Polysaccharide forms characteristic colored complexes with Iodine. Starch gives a blue color with an Iodine solution. Dextrin a product of partial hydrolysis of starch gives a color red solution and glycogen, a highly branched complex polysaccharide, gives a pale reddish-brown solution. The difference in the color of the complexes is due to the structure of these polysaccharides. Starch is made up of linear chains of glucose units of amylose What Are Lipids? Lipids are a group of macromolecules that have a wide variety of functions in living cells. Examples include storing energy, signaling between cells, and forming the cell membrane. They are made from monomers (building blocks) called fatty acids. The functional group attached to each monomer determines the specific type of lipid it will be. Lipids are insoluble in water, making them especially important in cell functions. Unlike water, they are nonpolar. Because of this, they will not mix with water. In fact, they are referred to as hydrophobic, which literally means "water-fearing". If you have ever tried to mix oil and water, you've probably noticed that they remain separate. This is because oil is a lipid and non-polar. Saturated fats have straight carbon chains because they only contain single carbon-carbon bonds (alkane). Saturated fats pack together closely and are solid at room temperature. Saturated fats are typically found in animal products. Butter is a good example. Unsaturated fats have a kink in their chain caused by a double bond or even a triple bond between carbons (alkenes, alkynes). Because of these kinks, unsaturated fats can't pack together very closely, making them liquid at room temperature. They are typically found in plant products. Vegetable oil is a good example. The hydrocarbon chains of both saturated and unsaturated fats are attached to a carboxylic acid functional group. This is what makes them fatty acids. Triglycerides Lipids that store energy is called triglycerides. These molecules have three long chains of fatty acids attached to a glycerol backbone. In many organisms, extra carbohydrates are often stored as triglycerides in fat tissue. Triglycerides are excellent long-term energy storage molecules because they will not mix with water and break down. We can also eat triglycerides (in delicious fried foods, often) and break them down to get energy. M6 Lesson 1- Lipids A lipid is a fat-soluble molecule. To put it another way, lipids are insoluble in water but soluble in at least one organic solvent. The other major classes of organic compounds (nucleic acids (Links to an external site.), proteins, and carbohydrates) are much more soluble in water than in an organic solvent. Lipids are hydrocarbons (molecules consisting of hydrogen and oxygen), but they do not share a common molecule structure. Lipids that contain an ester functional group may be hydrolyzed in water. Waxes, glycolipids, phospholipids, and neutral waxes are hydrolyzable lipids. Lipids that lack this functional group are considered nonhydrolyzable. The nonhydrolyzable lipids include steroids and fat-soluble vitamins A, D, E, and K. M6 Lesson 2-Physical and Chemical Properties of Lipids Lipids are very diverse in both their respective structures and functions. These diverse compounds that make up the lipid family are so grouped because they are insoluble in water. They are also soluble in other organic solvents such as ether, acetone, and other lipids. Lipids serve a variety of important functions in living organisms. They act as chemical messengers, serve as valuable energy sources, provide insulation, and are the main components of membranes. Major lipid groups include fats, phospholipids, steroids, and waxes. Module 7 LAB LIPIDS ONLINE.pdf MODULE VII. Proteins and Amino Acids M7 Lesson 1 - Formation of Polypeptides Peptides and proteins are formed when amino acids are joined together by amide bonds. The amide bond is called a peptide bond. A dipeptide has two amino acids joined together by one peptide bond. Polypeptides have many amino acids, while proteins have more than 40 amino acids. Introduction Peptide bonds are formed by a condensation reaction. Amino acids are joined together and water is released. Amino acids are organic compounds containing an amino group and a carboxyl group. Amino acids are a set of 20 different molecules used to build proteins. Proteins consist of one or more chains of amino acids called polypeptides. The sequence of the amino acid chain causes the polypeptide to fold into a shape that is biologically active. The amino acid sequences of proteins are encoded in the genes. The 20 common amino acids make up proteins in the body are mostly α-amino acids. All the amino acids in the body are L-isomers. alpha (α) carbon, which is covalently linked to both the amino group and the carboxyl group. Also bonded to this carbon are hydrogen and a variable side chain (R group). R group gives each amino acid its identity. Formation of Polypeptides: 1. Remove oxygen from the precedent amino acid and two hydrogens from the subsequent amino acid 2. Place the two groups next to each other. M7 Lesson 2 - Tests for Proteins Xanthoproteic test Biuret test • • • It is the general test for the identification of proteins In the presence of an alkaline solution, Cu2+ ion forms as a complex with the peptide bonds due to the unshared electron pairs in nitrogen, and the oxygen in the water. Once the complex has been formed, the solution turns from blue to purple. • • It is the test for the presence of aromatic amino acids Amino acids containing an aromatic nucleus react with concentrated HNO3 to form a yellow-colored complex on heating, it changes to orange-red color when excess NaOH is added Millon’s test • Ninhydrin test • • • It is the test for the presence of αamino acid in proteins Ninhydrin causes oxidative decarboxylation and deamination of α-amino acids producing an aldehyde, carbon dioxide, and ammonia Nnhydrin is reduced to hydrindantin which reacts with the liberated ammonia and another molecule of ninhydrin • • It is the test for phenolic compounds It is also used to detect the presence of tyrosine – the only amino acid containing a phenol group Tyrosine is first nitrated by nitric acid in the test solution, then the nitrated tyrosine complexes mercury (I) and mercury (II) in the solution to form either a red precipitate or a red solution Hopkins-Cole test • • It is a test for the presence of tryptophan – the only amino acid containing an indole group The indole reacts with glyoxylic acid in the presence of a strong acid to form a violet cyclic product Lead Sulfide test • • • It is a test for the presence of cysteine or cystine The organic sulfur in cysteine or cystine is released as inorganic S8 ions which form lead sulfide The positive result is the formation of black or brown precipitate