Atomic Structure: History & Models - High School Chemistry
advertisement

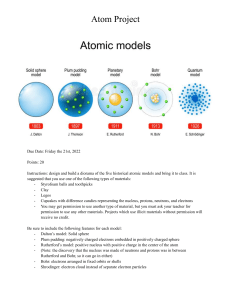
LESSON 4: CONTENT CONTENT STANDARD LEARNING COMPETENCIES How the idea of the atom, along with the idea of the elements evolved At the end of the lesson, you will have to describe: 1. how the concept of the atom evolved from Ancient Greekto the present; and 2. how the concept of the element evolved from AncientGreek to the present At the end of the lesson, you will have to: 1. point out the main ideas in the discovery of the structure of the atom and its subatomic particles (S11/12PS-IIIb-8) 2. cite the contributions of J.J. Thomson, Ernest Rutherford, Henry Moseley, and Niels Bohr to the understanding of the structure of the atom (S11/12PS-IIIb-9) 3. describe the nuclear model of the atom and thelocation of its major components (protons, neutrons, and electrons) (S11/12PS-IIIb-10) 1 A T O M T B A I L O A P C R E G M C What Does an A t o m REALLYLook Like? RECALL KEY TERMS 10) Nucleus – 11) Protons – 12) Niels Bohr – 13) Quantum Model – 5) Joseph John Thomson – 14) James Chadwick – 6) Electrons – 15) Neutrons – 1) 2) 3) 4) Atom – Billiard ball – Robert Brown – John Dalton– 7) Plum-pudding model – 8) Ernest Rutherford – 9) Radioactivity – ATOM The smallest unit of matter as recognized by chemical properties of molecules. composed of protons, neutrons and electrons. very small; typical sizes are around 100 picometers (a ten-billionth of a meter, in the short scale) SEATWORK#3 Sub-atomic Particle of an Atom Electron Proton Neutron is discovered by through the… (what kind of experiment) J. J. Thomson cathode ray tube experiment Ernest Rutherfor d James Chadwick gold foil experimen goldt foil experimen z z ATTRIBUTE OF THE ATOMIC MODEL Conceptualized following cathode ray experiments Has a nucleus Has energy levels or quanta Conceptualized following αparticle experiments Explains why electrons don’t fall into the nucleus Has idea of orbitals First model to use idea of subatomic particles ATOMIC MODEL DALTON THOMSON RUHTERF ORD BOHR QUANTUM nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger– Marsden gold foil experiment. radioactivity The spontaneous decay of atomic nuclei. During radioactivity, alpha particles, beta-rays and gamma rays are emitted. DEMOCRITUS 460 – 370 B.C. ■ There are various basic elements from which all matter is made ■ Everything is composed of small atoms moving in a void ■ Some atoms are round, pointy, oily, have hooks, etc. to account for their properties ■ Ideas rejected by leading philosophers because void = no existence DEMOCRITUS sometimes known as the BILLIARD BALL concept of the atom, wherein the atom is an incredibly small but smooth and whole object. ROBERT BROWN He is known for his idea of the random movement of microscopic particles or "Brownian motion." JohnJODaHltoNn DALTON 1766-1844 IDEA OF AN ATOM ■Introduced his ideas in 1803 that each element is composed of extremely small particles called atoms Proponent: John Dalton This model suggested that atoms: • are the smallest particle of an element • of different elements have different masses • are solid, indestructible units. J. J. THOMSON IDEA OF AN ATOM Conceptualized following cathode ray experiments First model to use idea of subatomic particles J. J. THOMSON IDEA OF AN ATOM Later in 1897, Joseph John Thomson published the idea that electricity was in particles that were part of the atom. Experimenting with cathode rays, he established the mass and charge properties of these particles. These particles were named electrons. In 1904, he came up with the plum-pudding model, which was an idea of what the atom looked like based on his experiments. He would later conclude that the electron was not the only source of mass in the atom. This implied that the atom was composed of other particles. J. J. THOMSON IDEA OF AN ATOM Thomson “Plum Pudding” Model Actual Plum Pudding J. J. THOMSON Thomson’s plum-pudding model, a sphere with a uniformly distributed positive charge and enough embedded electrons to neutralize the positive charge. A plum pudding is a sort of cake with raisins embedded in it. IDEA OF AN ATOM J. J. THOMSON IDEA OF AN ATOM Cathode Ray Tube Experiment ERNEST RUTHERFORD Has a nucleus Conceptualized following α-particle experiments IDEA OF AN ATOM ERNEST RUTHERFORD IDEA OF AN ATOM Ernest Rutherford, a student of Thomson’s, who was among many who studied radioactivity. He concluded that radioactivity occurred due to changes on a subatomic level, or changes within the atom itself. In 1902, he worked in Thomson’s laboratory where he distinguished two kinds of radiation based on their penetrating power: α (alpha) and β (beta). He studied these types of radiation and noticed, from his experiments, that alpha particles would sometimes bounce off at a high angle when made to penetrate a very thin gold foil. ERNEST RUTHERFORD IDEA OF AN ATOM In 1911, Rutherford theorized that the model proposed by Thomson did not explain the deflection of alpha particles. Therefore, he devised his own model with a positive nucleus at the center and electrons revolving like planets at a distance around it. The incredibly dense nucleus explained the occasional deflection experienced by the alpha particles, while the amounts of empty space in between explained why most particles were able to pass through. Rutherford later concluded that the nucleus was composed of positive particles known as protons, which were then thought to be hydrogen nuclei found in other atoms. He suggested the possibility of finding a composite particle (proton + electron) with a negligible electric field that composed the nucleus. Diagram of the Rutherford atomic model. Physicist Ernest Rutherford envisioned the atom as like a miniature solar system, with electrons orbiting around a massive nucleus, and as mostly empty space, with the nucleus occupying only a very small part of the atom. ERNEST RUTHERFORD IDEA OF AN ATOM Gold Foil Experiment NIELS BOHR IDEA OF AN ATOM Has a nucleus Has energy levels or quanta Explains why electrons don’t fall into the nucleus NIELS BOHR proposed that the electrons “jumped” between energy levels IDEA OF AN ATOM NIELS BOHR IDEA OF AN ATOM Niels Bohr, another scientist in Rutherford’s laboratory. He tackled one of the big issues with the Rutherford model in 1913. The system proposed by Rutherford was unstable because, under classical physics, the spinning electrons would tend to be attracted to the positive nucleus and lose energy until they collapse into the center. Bohr proposed that the electrons existed only at fixed distances from the nucleus at set “energy levels,” or quanta. Quanta was first conceptualized mathematically by Max Planck by absorbing or releasing discrete amounts of energy. However, the Bohr model of the atom was still unable to explain why atoms bonded in certain ways to form compounds. For example, carbon formed compounds of CH4 while oxygen formed H2O. NIELS BOHR IDEA OF AN ATOM In the Bohr model of the atom, electrons travel in defined circular orbits around the nucleus. The orbits are labeled by an integer, the quantum number n. Electrons can jump from one orbit to another by emitting or absorbing energy. Schrödinger Werner Heisenberg Ernst IDEA OF AN ATOM QUANTUM MODEL Has a nucleus Has energy levels or quanta Explains why electrons don’t fall into the nucleus Has idea of orbitals Schrödinger Werner Heisenberg Ernst IDEA OF AN ATOM • In quantum mechanics, this branch augmented the Bohr model with new explanations of how matter behaved at a very tiny level that turned it into the quantum model of the atom used today. • The model is based on mathematical equations by several scientists, including Werner Heisenberg and Ernest Schrödinger Quantum model This model uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. Quantum model JAMES CHADWICK James Chadwick was a student of Rutherford’s who built on this possibility in 1926. He worked on radiation emitted by beryllium that took the form of particles heavy enough to displace protons. JAMES CHADWICK These particles were as heavy as protons, but they needed to have a neutral charge that would allow them to smash into the nucleus without being repelled by electrons or protons. He confirmed their existence by measuring their mass and called them NEUTRONS. JAMES CHADWICK The neutron was able to explain the mass unaccounted for by a system of protons and electrons only. It also allowed for more far-reaching advancements in nuclear physics and chemistry. It gave an understanding of isotopes and radioactive decay, and provided the tools to synthesize new elements and radioactive materials. These advancements, for better or for worse, changed the landscape of science because they gave us the ability to derive large amounts of energy from splitting the atom (nuclear fission). JAMES CHADWICK Gold Foil Experiment What Does an A t o m REALLYLook Like? Here are a few atoms using Scanning Transmission Electron Microscopy Single Atom Spectroscopy Here is how the hydrogen orbits look like using a photoionization quantum microscope Hydrogen Atoms under Magnification: Direct Observation of the Nodal Structure of Stark States Ateam of researchers from China’s National Center for Nanoscience & Technology and Renmin University have utilized atomic force microscopy (AFM) to produce a high-resolution image of atoms reaching out to make a link with each other. Research paper: DOI: 10.1126/science.1242603 – “Real-Space Identification of Intermolecular Bonding with Atomic Force Microscopy” (paywall) SEATWORK#3 Sub-atomic Particle of an Atom Electron Proton Neutron is discovered by through the… (what kind of experiment) J. J. Thomson cathode ray tube experiment Ernest Rutherfor d James Chadwick gold foil experimen goldt foil experimen z z ATTRIBUTE OF THE ATOMIC MODEL Conceptualized following cathode ray experiments Has a nucleus Has energy levels or quanta Conceptualized following αparticle experiments Explains why electrons don’t fall into the nucleus Has idea of orbitals First model to use idea of subatomic particles ATOMIC MODEL DALTON THOMSON RUTHER FORD BOHR QUANTUM GRADE 12-MAGSAYSAY 1. DEMOCRATUS:ebdane-sotomayor 2. JOHN DALTON:capunan-agimlod 3. JJ THOMPSON:gian-rodz 4. ERNEST RUTHERFORD:ba-at - marundan 5. NEILS BOHR:pondolanan - lintapan 6. SCHROEDINGER/HEISENBERG:ballener-kenchi 7. JAMES CHADWICK:comaling-bautista 1. DEMOCRATUS: PAGANTUPAN-ALEXA NICOLE 2. JOHN DALTON: RUSSEL JOHN MANLA HILARDES 3. JJ THOMPSON: RICKSON-COMPASION 4. ERNEST RUTHERFORD: MALITOC-GAMAY 5. NEILS BOHR: CANONO-LIGASAN 6. SCHROEDINGER/HEISENBERG: JEFERSONAGIMLOD, R. 7. JAMES CHADWICK:SIBAYAN-CAPUNGAS assignment 1-2) What are quarks? 3-8) What are the “flavors” of quarks? 9-10) Why quarks have “flavors?”