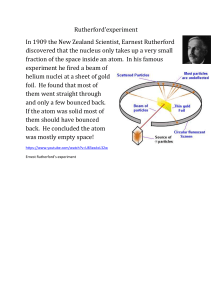
Used cathode ray tubes to cause elements to give off a stream of (-) charged particles Electrons Figured out that (+) charged particles must be in atoms for there wasn’t an overall charge Proposed that Atom = (+) mass with (-) electrons inside. Uses raisin (-) bun (+) model to prove so Ernest Rutherford Discovered that protons are in central nucleus Does an experiment to prove so Ernest Rutherford’s Experiment First image shows what was supposed to happen according to the Raisin Bun model explain and second image shows what actually happened James Chadwick Figured out about another sub atomic particle - Neutron - and added weight to the atom with no charge at all