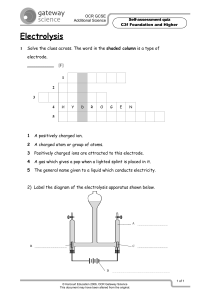
Efficient electrolysis of CO2 in symmetrical solid oxide electrolysis cell with highly active La0.3Sr0.7Fe0.7Ti0.3O3 electrode material Zhiqun Cao a, Bo Wei a, Jipeng Miao a, Zhihong Wang a, b, Zhe Lü a, *, Wenyuan Li c, Yaohui Zhang a, Xiqiang Huang a, Xingbao Zhu a, Qi Feng a a , Yu Sui a Department of Physics, Harbin Institute of Technology, Harbin 150001, China. b School of Chemical Engineering and Technology, Harbin Institute of Technology, Harbin 150001, China. c Mechanical & Aerospace Engineering Department, Benjamin M. Statler College of Engineering & Mineral Resources, West Virginia University, Morgantown, WV 26506-6106, USA. Keywords: Solid oxide electrolysis cell; CO2 reduction; Symmetrical cell; Titanium-substituted lanthanum strontium ferrite Abstract Novel perovskite-type La0.3Sr0.7Fe0.7Ti0.3O3 (LSFT) oxide is investigated as both anode and cathode materials in a symmetrical solid oxide electrolysis cell (SOEC) for direct electrolysis of pure CO2 at 800 ºC. The current density is 521 mA cm-2 at an applied voltage of 2.0 V. The lowest polarization resistance Rp of this symmetrical SOEC is obtained at 2.0 V with a value of as low as 0.08 Ω cm2, which is much lower than that of other perovskite and Ni-cermet cathode. LSFT cathode also shows * Corresponding author E-mail address: lvzhe@hit.edu.cn (Z. Lü) 1 © 2016. This manuscript version is made available under the Elsevier user license http://www.elsevier.com/open-access/userlicense/1.0/ excellent durability in pure CO2 electrolysis, especially at high voltage of 2.0 V. Our results indicate the LSFT is a promising alternative electrode material for CO2 electrolysis. 1. Introduction In recent years, solid oxide electrolysis cells (SOECs) have attracted worldwide attention as a green and renewable energy conversion technology [1]. SOEC can convert electricity and thermal energy into chemical energy with high efficiency for H2 generation through steam electrolysis [2]. Besides, SOECs are also capable of direct electrolyzing greenhouse gas CO2 to CO in cathode chamber and oxygen at anode side. The Ni-YSZ cermet, a conventional anode material of SOFC, is widely used as cathode in SOEC [3, 4]. However, when it comes to CO2 electrolysis, it has been found that the Ni can catalyze the coke formation reaction in high concentrations of CO or hydrocarbon atmospheres [5]. Furthermore, Ni-YSZ cermet requires a high H2 concentration to prevent it from being oxidized to NiO. On the other hand, Ni can be oxidized in the pure and dry CO2 electrolysis mode, leading to degradation of electrode performance [6]. In order to overcome the disadvantages of Ni-YSZ cermet, ceramic oxides have been considered as potential cathodes due to their high redox-stability and reasonable activity. To date, several perovskite oxides have been investigated as potential cathode materials for SOECs [7-12]. Direct electrolysis of CO2 using redox-stable La0.75Sr0.25Cr0.5Mn0.5O3-δ (LSCM) and La0.2Sr0.8TiO3-δ (LSTO) composite cathodes are demonstrated without protective gas of H2 or CO [11, 12]. 2 However, these perovskite materials show large polarization resistances compared to the Ni-YSZ cermet cathode [3, 4, 8]. Moreover, a rapid performance degradation on LSCM cathode occurs when electrolyzing CO2 at an applied voltage of 2.0 V [11]. Therefore, there is an urgent need to develop alternative cathode perovskite materials (such as Fe-based perovskite oxides) for more efficient and stable electrolysis of CO2 [13-14]. Ni-Fe-La0.6Sr0.4Fe0.8Mn0.2O3-δ has been studied for intermediate-temperature CO2 electrolysis with reducing gas CO, which shows a high current density of 2320 mA cm-2 at 1.6 V and 800 ºC using a ca. 300 μm La0.9Sr0.1Ga0.8Mg0.2O3 electrolyte [14]. Raman spectra results confirm almost no coke formation on the cathode surface after electrolysis. In addition, the electrochemical performance of La0.3Sr0.7Fe0.7Cr0.3O3-δ (LSFCr) has been studied as a cathode for electrolysis of CO2 in reversible solid oxide cells. The LSFCr electrode is more active for CO2 reduction than for CO oxidation [15-17]. Yao et al. have reported that the perovskite chromate doped with titanium (La0.75Sr0.25Cr0.5-xFe0.5TixO3-δ, x=0, 0.1, 0.2, 0.3, 0.4, 0.5) can obviously improve the cathode activity for the CO2 reduction in pure CO2 electrolysis [18]. In our previous study [19], perovskite-type oxide La0.3Sr0.7Fe0.7Ti0.3O3 (LSFT) has been investigated as both cathode and anode in symmetrical solid oxide fuel cell. In SOEC mode, it was reported that the oxygen vacancy defect in perovskite cathode plays a key role in the chemical adsorption of CO2 [20-21]. In the case of LSFT cathode, the oxygen vacancy concentration increases with the increasing of temperature via partial reduction of Fe4+ to Fe3+ and Ti4+ to Ti3+ in reducing 3 atmosphere at high temperature. Furthermore, the LSFT electrode exhibits a good phase stability under strong reducing atmosphere (humidified 20% H2/Ar at 800 oC, PO2~1.58*10-20 atm, calculated by Nernst equation) [19]. Actually, during a durability test of symmetrical SOEC for pure CO2 electrolysis with an applied voltage of 2.0 V at 800 oC, CO/CO2 ratio is no more than 10/90 ratio (corresponding to PO2~10-16.6). Consequently, the decomposition or phase separation of LSFT cathode is negligible during co-electrolysis and electrolysis CO2. In this work, LSFT material was employed as electrodes in a symmetrical SOEC for the direct electrolysis of CO2 gas without protective gas (such as CO or H2) during operation. The electrochemical performance of full SOEC with LSFT electrode material as well as the related behaviors during CO2 electrolysis were presented. The related results for mechanism and kinetic of the LSFT cathode will be submitted by a separated paper. 2. Experimental The La0.3Sr0.7Fe0.7Ti0.3O3 (LSFT) powder was synthesized by a conventional solid-state reaction method. The detail of preparation process has been described elsewhere [19]. The effective areas of anode and cathode were 0.28 cm2. The electrochemical impedance spectra (EIS) and the relationship curves between current density and voltage (at the scan rate of 10 mV s-1) were performed at 800 ºC with an electrochemical workstation (Biologic VSP, France). EIS were tested with the static air on the anode side and pure CO2 (purity>99%) on the cathode side. The measurement was carried out at an AC amplitude of 20 mV and a frequency range of 4 400 kHz to 100 mHz. Pure CO2 gas with a flow rate of 14.7 mL min-1 was controlled by a mass flow controller during the test. The composition of outlet gas from the cathode side was analyzed by a mass spectrometer (Ametek LC-D100M, America). 3. Results and discussion Fig. 1 shows the voltage recorded as a function of the current density of the symmetrical cell for the electrolysis of pure CO2 with the applied voltages of 0-2 V at 800 ºC. A constant current density of ca. 10 mA cm-2 is observed in the voltage range of 0.4-0.8 V, which is related to a pumping process of oxygen gas that comes from the sealing leakage or CO2 feeding. In order to further understand the change of electrochemical processes with voltage, the dV/dI-V curve is presented in the inset of Fig. 1. A distinct change in dV/dI-V curve is observed at approximately 0.8 V, which can be considered as the threshold voltage for electrolysis of pure CO2. At the high voltage region (> 0.8 V), the total cell resistance (dV/dI) is consistent with the data obtained from impedance spectra shown in Fig. 2(b). The current-voltage profile plots of the symmetrical SOEC at different flow rates of pure CO2 are plotted in Fig. 1(b). The open circuit voltage (OCV) at the flow rates of 14.7, 29.4 and 58.9 mL min-1 are 0.119, 0.134 and 0.157 V, respectively. It indicates that the oxygen partial pressure caused by oxygen leaking-in decreases with increasing CO2 flow rate. The peak current density at ca. 0.25 V is obtained, which is probably ascribed to reduction of Fe4+ to Fe3+ [16]. To further investigate the electrochemical processes associated with different applied voltages, the AC impedance spectra of symmetrical SOEC for pure CO2 5 electrolysis are presented in Fig. 2. At the OCV condition, the ohmic resistance Rs is approximately 1.91 Ω cm2 which is consistent with the theoretical value calculated by the ionic conductivity of the electrolyte disc and SDC buffer layers. As shown in Fig. 2(a), the Rs increases with applied voltage from OCV to 0.8 V, which is due to the endothermic reaction of CO2 electrolysis at low current density [3]. On the other hand, the significant decrease of Rs at the voltage over 1 V in Fig. 2(b) is attributed to Joule heat effect of the high current density. In addition, the polarization resistances (Rp) are obviously reduced with the voltage increase, demonstrating faster electrode kinetics at higher applied voltage [9, 11]. Additionally, two arcs have been observed in the impedance spectra as Fig. 2(b) shown. The high frequency and low frequency arcs can be attributed to charge transfer process and dissociative adsorption / surface diffusion processes, respectively [4, 20]. More importantly, the lowest polarization resistance Rp of symmetrical SOEC is obtained at 2.0 V and 800 ºC with a value of approximately 0.08 Ω cm2. For comparison, it is significantly lower than other perovskite materials such as La0.75Sr0.25Cr0.5Mn0.5O3-δ (ca. 2.0 Ω cm2 at 2.0 V and 800 ºC) [11], La0.2Sr0.8Ti0.9Mn0.1O3-δ (ca. 1.5 Ω cm2 at 2.0 V and 800 ºC) [20] and La0.65Sr0.3Ce0.05Cr0.5Fe0.5O3-δ (ca. 0.21 Ω cm2 at 2.0 V and 850 ºC) [21]. Furthermore, the polarization resistance of the whole cell (ca. 0.08 Ω cm2 ) is even much lower than the Rp of Ni-YSZ cathode (ca. 0.3 Ω cm2) at 900 ºC and 1.5 V voltage [8], suggesting excellent electrocatalytic activity of LSFT cathode toward CO2 reduction. A short-term durability test of symmetrical SOEC for pure CO2 electrolysis with an applied voltage of 1.5 V at 800 ºC was conducted and the results are shown in Fig. 6 3(a). The current density of SOEC exhibits approximately 5% decrease during 24 h operation. The polarization resistance of the cell slightly increases from 0.21 to 0.23 Ω cm2 after 24 h operation, likely due to the delamination behavior of LSFT oxygen electrode under anodic polarization [22]. The ohmic resistance increases from 2.05 to 2.14 Ω cm2, which is likely attributed to the sintering of Ag current collector or increase of contact resistance between SDC and LSFT. The results show the performance of the symmetrical SOEC based on LSFT electrodes possesses good stability during 24 h test, owing to the good phase stability and high CO2 tolerance of LSFT electrode in pure CO2 atmosphere [23]. Fig. 3(b) shows that the current densities under different voltages are recorded as a function of time for pure CO2 electrolysis at 800 ºC. For a constant voltage stability test within an hour, the degradation rate of LSFT is only 0.013 mA cm-2min-1 at the current densities of 265 mA cm-2. This value is much smaller than La0.75Sr0.25Cr0.5Mn0.5O3-δ (ca. 4.0 mA cm-2min-1, at the current densities of 200 mA cm-2) and La0.2Sr0.8Ti0.9Mn0.1O3-δ, (ca. 2.0 mA cm-2min-1, at the current densities of 220 mA cm-2 ) [11, 20], indicating excellent durability at high applied current densities in pure CO2 electrolysis. The above results are also confirmed by the corresponding variation of outlet gas composition obtained from mass spectrometer as shown in inset of Fig. 3(b). In addition, the corresponding Faraday efficiency with an applied voltage of 2 V reaches around 72 % with a flow rate of 14.7 mL min-1 during pure CO2 electrolysis at 800 oC. The SEM images of the symmetrical SOEC based on LSFT electrodes before and 7 after electrolysis operation with CO2 gas are presented in Fig. 4. The particle size of LSFT electrode is 1~4 μm, and both LSFT|SDC and SDC|YSZ interfaces show well contact without obvious delamination. The electrode average particle sizes exceed couple of micrometers since the sample was calcined at 1400 oC and inadequate ball-milling. The thicknesses of the SDC buffer layer and LSFT electrode are ca. 40 and 20 μm, respectively. Such a thick SDC buffer layer may increase the ohmic resistance of SOEC, so further work is undergoing to reduce the thickness of SDC buffer layer by optimizing the fabrication process. In the case of CO2 electrolysis, Birss has reported porous GDC buffer layer has a poor electrocatalytic activity for CO2 reduction (Rp~25 Ω cm2 at 800 oC) [15]. No significant agglomerations, change of the microstructure and carbon deposition can be observed after electrolysis test. 4. Conclusions In summary, a symmetrical SOEC with novel perovskite La0.3Sr0.7Fe0.7Ti0.7O3 cathode has been prepared and demonstrated for high performance direct electrolysis of pure CO2. The current density is 521 mA cm-2 at an applied voltage of 2.0 V. The values of the current density are mainly limited by the thick YSZ electrolyte (ca. 700 μm in thickness) which accounted for the 95% of total cell resistance. The performance of symmetrical SOEC using LSFT as electrode material can be further improved by decreasing the thickness of YSZ electrolyte. The Rp of symmetrical SOEC at 2.0 V voltage is as low as 0.08 Ω cm2, which is significantly lower than previously reported perovskite materials. The LSFT cathode also shows excellent durability at high applied voltage and high current density compared with that of the 8 LSCM or LSTO cathode. The Faraday efficiency with an applied voltage of 2 V reaches around 72 % with a flow rate of 14.7 mL min-1. No significant agglomeration and carbon deposition on the LSFT cathode have been observed after electrolysis test. Our results indicate LSFT is a very promising electrode material for direct electrolysis of CO2. Conflict of interest The authors declare that there is no conflict of interest. Acknowledgments The authors gratefully acknowledge the financial support of the National Science Foundation of China (No. 51372057, 21373071 and 51402072 ). References [1] A. Hauch, S. Ebbesen, S. Jensen, M. Mogensen, Highly efficient high temperature electrolysis, J. Mater. Chem. 18 (2008) 2331-2340. [2] Q. Liu, C. Yang, X. Dong, F. Chen, Perovskite Sr2Fe1.5Mo0.5O6-δ as electrode materials for symmetrical solid oxide electrolysis cells, Int. J. Hydrogen Energy 35 (2010) 10039-10044. [3] J. Yan, H. Chen, E. Dogdibegovic, J. Stevenson, M. Cheng, X. Zhou, High-efficiency intermediate temperature solid oxide electrolyzer cell for the conversion of carbon dioxide to fuels, J. Power Sources 252 (2014) 79-84. [4] Z. Zhan, L. Zhao, Electrochemical reduction of CO2 in solid oxide electrolysis cells, J. Power Sources 195 (2010) 7250-7254. [5] V. Singh, H. Muroyama, T. Matsui, S. Hashigami, T. Inagaki, K. Eguchi, Feasibility of alternative electrode materials for high temperature CO2 reduction on solid oxide electrolysis 9 cell, J. Power Sources 293 (2015) 642-648. [6] K. Hansen, K. Norrman, M. Mogensen, H2-H2O-Ni-YSZ electrode performance-Effect of segregation to the interface, J. Electrochem. Soc. 151 (2004) A1436-A1444. [7] Y. Xie, J. Xiao, D. Liu, J. Liu, C. Yang, Electrolysis of Carbon Dioxide in a Solid Oxide Electrolyzer with Silver-Gadolinium-Doped Ceria Cathode, J. Electrochem. Soc. 162 (2015) F397-F402. [8] X. Yue, J. Irvine, Alternative Cathode Material for CO2 Reduction by High Temperature Solid Oxide Electrolysis Cells, J. Electrochem. Soc. 159 (2012) F442-F448. [9] X. Yue, J. Irvine, Impedance Studies on LSCM/GDC Cathode for high Temperature CO2 Electrolysis, Electrochem. Solid State Lett. 15 (2012) B31-B34. [10] C. Cheng, G. Kelsall, L. Kleiminger, Reduction of CO2 to CO at Cu-ceria-gadolinia (CGO) cathode in solid oxide electrolyser, J. Appl. Electrochem. 43 (2013) 1131-1144. [11] S. Xu, S. Li, W. Yao, D. Dong, K. Xie, Direct electrolysis of CO2 using an oxygen-ion conducting solid oxide electrolyzer based on La0.75Sr0.25Cr0.5Mn0.5O3-δ, J. Power Sources 230 (2013) 115-121. [12] Y. Li, J. Zhou, D. Dong, Y. Wang, J. Jiang, H. Xiang, K. Xie, Composite fuel electrode La0.2Sr0.8TiO3-δ-Ce0.8Sm0.2O2-δ for electrolysis of CO2 in an oxygen-ion conducting solid oxide electrolyser, Phys. Chem. Chem. Phys. 14 (2012) 15547-15553. [13] T. Shin, J. Myung, M. Verbraeken, G. Kim, J. Irvine, Oxygen deficient layered double perovskite as an active cathode for CO2 electrolysis using a solid oxide conductor, Faraday Discuss. 182 (2015) 227-239. [14] S. Wang, H. Tsuruta, M. Asanuma, T. Ishihara, Ni-Fe-La(Sr)Fe(Mn)O3 as a New Active 10 Cermet Cathode for Intermediate-Temperature CO2 Electrolysis Using a LaGaO3-Based Electrolyte, Adv. Energy Mater. 5 (2015) 1401003 [15] P. Addo, B. Molero-Sánchez, M. Chen, S. Paulson, V. Birss, CO/CO2 Study of High Performance La0.3Sr0.7Fe0.7Cr0.3O3-δ Reversible SOFC Electrodes, Fuel Cells 15 (2015) 689-696. [16] B. Molero-Sánchez, P. Addo, A. Buyukaksoy, S. Paulson, V. Birss, Electrochemistry of La0.3Sr0.7Fe0.7Cr0.3O3-δ as an oxygen and fuel electrode for RSOFCs, Faraday Discuss. 182 (2015) 159-175. [17] B. Molero-Sánchez, J. Prado-Gonjal, D. Ávila-Brande, M. Chen, E. Morán, V. Birss, High performance La0.3Ca0.7Cr0.3Fe0.7O3-δ air electrode for reversible solid oxide fuel cell applications, Int. J. Hydrogen Energy 40 (2015) 1902-1910. [18] W. Yao, T. Duan, Y. Li, L. Yang, K. Xie, Perovskite chromate doped withe titanium for direct carbon dioxide electrolyser, New J. Chem. 39 (2015) 2956-2965. [19] Z. Cao, Y. Zhang, J. Miao, Z. Wang, Z. Lv, Y. Sui, X. Huang, W. Jiang, Titanium-substituted lanthanum strontium ferrite as a novel electrode material for symmetrical solid oxide fuel cell, Int. J. Hydrogen Energy 40 (2015) 16572-16577. [20] W. Qi, Y. Gan, D. Yin, Z. Li, G. Wu, K. Xie, Y. Wu, Remarkable chemical adsorption of manganese-doped titanate for direct carbon dioxide electrolysis, J. Mater. Chem. A. 2 (2014) 6904-6915. [21] Y. Zhang, J. Li, Y. Sun, B. Hua, J. Luo, Highly Active and Redox-Stable Ce-Doped LaSrCrFeO-Based Cathode Catalyst for CO2 SOECs, ACS Appl. Mater. Interfaces 8 (2016) 6457-6463. 11 [22] K. Chen, S. Jiang, Failure mechanism of (La, Sr)MnO3 oxygen electrodes of solid oxide electrolysis cells, Int. J. Hydrogen Energy 36 (2011) 10541-10549. [23] G. Yang, C. Su, Y. Chen, F. Dong, M. Tade, Z. Shao, Cobalt-free SrFe0.9Ti0.1O3-δ as a high-performance electrode material for oxygen reduction reaction on doped ceria electrolyte with favorable CO2 tolerance, J. Eur. Ceram. Soc. 35 (2015) 2531-2539. 12 (a) pure CO2 with a flow rate of 14.7 mL min -1 1.6 1.2 dV/dI ( cm2) Cell voltage (V) 2.0 0.8 0.4 35 30 25 20 15 10 5 0 0.0 -100 EIS data 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2.2 Voltage (V) 0 100 200 300 400 500 600 -2 Current density (mAcm ) -60 -50 -40 -30 -20 -10 0 0.5 10 20 30 40 50 60 (b) Cell Voltage (V) 0.4 0.3 0.2 0.1 -1 (i) 14.7 mL min -1 (ii) 14.7 mL min -1 (iii) 29.4 mL min -1 (iiii) 58.9 mL min 0.157V 0.134V 0.119V 0.090V 0.0 -0.1 -60 -50 -40 -30 -20 -10 0 10 20 30 40 50 60 -2 Current density (mA cm ) Fig. 1. The current density dependence of the voltage profile of the symmetric solid oxide electrolyzer cell for pure CO2 direct electrolysis at 800 ºC: (a) Full range current density-voltage profile with the applied voltages from 0 V to 2.0 V at the flow rate of 14.7 mL min-1. The inset is the dV/dI-V curve of symmetrical SOEC. (b) The details of current density-voltage profiles at different flow rate of pure CO2: (i) from 0 V to 2 V; (ii)-(iiii) from open circuit voltage to 2.0 V. 13 0.5 2 OCV 0.5 V 0.8 V 1.0 V 1.2 0.1Hz 0.9 0.1Hz 0.32Hz 0.6 0.3 0.0 1.5 1.8 2.1 2.4 2 Z( cm ) 2.7 3.0 0.32 Hz 0.1 Hz 0.1 Hz 3 4 5 6 2 Z( cm ) 7 8 9 (b) 0.4 -Z" ( cm ) 1.5 -Z" ( cm 2) 2 -Z" ( cm ) 9 8 (a) 7 OCV 6 0.5 V 0.8 V 5 1.0 V 4 3 2 1 0 0 1 2 1.2 V 1.5 V 1.8 V 2.0 V 0.3 0.2 0.32 Hz 0.1 Hz 0.32 Hz 0.1 0.32 Hz 0.32 Hz 0.0 0.1 Hz -0.1 1.9 0.1 Hz 0.1 Hz 2.0 2.1 2.2 2.3 2.4 2.5 2 Z( cm ) Fig. 2. Typical impedance spectra of symmetrical SOEC under different voltage for pure CO2 electrolysis with the oxygen electrode exposed to static air at 800 ºC: (a) OCV-1 V, (b) 1.2-2.0 V. LSTF SDC anode barrier layer YSZ 14 (a) o Applied voltage:1.5 V in pure CO2 at 800 C 250 0.3 200 After 24 h test As prepared 0.2 2 -Z" ( cm ) -2 Current density (mA cm ) 300 150 100 0.0 50 0 0.1 -0.1 2.0 0 4 2.1 8 2.2 2 Z( cm ) 12 2.3 2.4 20 24 16 28 Time (h) (b) 2.0 V 500 400 1.5 V 300 200 1.0 V 100 0 0.5 V 0 10 20 Mass spectra signal (a.u.) -2 Current density (mA cm ) 600 0.5 V 1.0 V 1.5 V 0V 2.0 V CO CO2 2.0 V 1.5 V 0.5 V 1.0 V 0 10 0V 20 30 40 Time (min) 30 40 50 Time (min) 60 70 50 80 Fig. 3. (a) Short-term durability test of symmetrical SOEC for pure CO2 electrolysis with a 1.5 V applied voltage at 800 ºC. Inset shows the impedance spectra of the electrolyser before and after 24 h stability test at 1.5 V applied voltage in pure CO2. (b) Current density from the electrolysis CO2 of the symmetrical SOEC at 800 ºC and different applied voltage. Inset shows corresponding variation of the outlet gas composition obtained from mass spectrometer with different electrolysis voltage at 800 ºC. 15 (b) (a) 10 μm 10 μm (c) (d) cathode SDC YSZ 10 μm 50 μm Fig. 4. SEM images of the electrodes in symmetrical SOEC: (a) Anode after the operation of electrolysis at 800 ºC. (b) Cathode after the operation of electrolysis at 800 ºC. (c) Cathode|barrier layer|YSZ electrolyte after the operation of electrolysis at 800 ºC. (d) Cathode before the operation of electrolysis at 800 ºC. 16 (a) pure CO2 with a flow rate of 14.7 mL min -1 1.6 1.2 dV/dI ( cm2) Cell voltage (V) 2.0 0.8 0.4 35 30 25 20 15 10 5 0 0.0 -100 EIS data 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2.2 Voltage (V) 0 100 200 300 400 500 600 -2 Current density (mAcm ) [24] 17