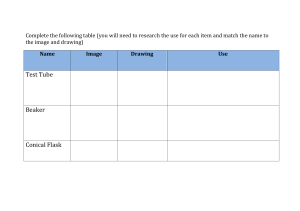
The effect of temperature on the rate of reaction (adapted experiment 6.4) Equipment and materials 10 ml measuring cylinder 25 ml measuring cylinder Stopwatch Paper with cross in the middle 250 ml Conical flask 0.1 M sodium thiosulfate (Na2S2O3 (aq)) 1 M Hydrochloric acid (HCl (aq)) Safety Eye protection should be worn. During this experiment, sulphur dioxide is produced. This is toxic and can trigger asthmatic attacks so the experiment must be completed in a well ventilated room. Once the reaction is completed, the mixture in the conical flask should be poured into the sodium carbonate ‘stop-bath’. Hydrochloric acid is an irritant. Method 1. Place the paper with the ‘X’ under the conical flask so you can see the ‘X’ when you look down at the conical flask. 2. Measure out 15 ml of sodium thiosulfate and add this to the conical flask. 3. Measure out 5 ml of hydrochloric acid. 4. Start the stopclock as you add the acid to the conical flask. 5. Look down at the cross and time how long it takes for the cross to disappear (the precipitate formed by the reaction will make the solution go cloudy). 6. Stop the clock when you can no longer see the ‘X’ and record the time in your results chart. 7. Pour the finished mixture into the stop bath and rinse the conical flask with some water. 8. Repeat steps 1-7 with sodium thiosulfate at a different temperature. Results Temperature of sodium thiosulfate (°C) 20 (room temperature) 40 60 Time for cross to disappear (seconds)