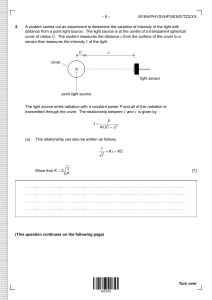
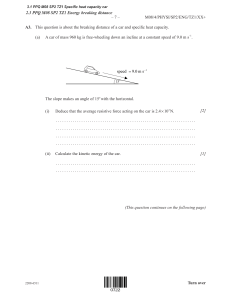
–3– N00/430/H(2) SECTION A Candidates must answer all questions in the spaces provided. A1. Radioactive decay measurement A medical physicist wishes to investigate the decay of a radioactive isotope and determine its decay constant and half-life. A Geiger-Muller counter is used to detect radiation from a sample of the isotope, as shown. Radioactive Radioactive source source (a) Voltage supply and counter Geiger-Muller tube Geiger-Muller tube Define the activity of a radioactive sample. [1] ......................................................................... ......................................................................... Theory predicts that the activity A of the isotope in the sample should decrease exponentially with time t according to the equation A = A0 e− λt , where A0 is the activity at t = 0 and λ is the decay constant for the isotope. (b) Manipulate this equation into a form which will give a straight line if a semi-log graph is plotted with appropriate variables on the axes. State what variables should be plotted. [2] ......................................................................... ......................................................................... ......................................................................... (This question continues on the following page) 880-227 Turn over Page 3 –4– N00/430/H(2) (Question A1 continued) The Geiger-counter detects a proportion of the particles emitted by the source. The physicist records the count-rate R of particles detected as a function of time t and plots the data as a graph of ln R versus t, as shown below. 2 • ln ( R / s −1 ) • • 1 • • 0 (c) 1 2 3 4 5 t / hr Does the plot show that the experimental data are consistent with an exponential law? Explain. [1] ......................................................................... ......................................................................... (d) The Geiger-counter does not measure the total activity A of the sample, but rather the count-rate R of those particles that enter the Geiger tube. Explain why this will not matter in determining the decay constant of the sample. [1] ......................................................................... ......................................................................... (e) [2] From the graph, determine a value for the decay constant λ. ......................................................................... ......................................................................... ......................................................................... (This question continues on the following page) 880-227 Page 4 –5– N00/430/H(2) (Question A1 continued) The physicist now wishes to calculate the half-life. (f) Define the half-life of a radioactive substance. [1] ......................................................................... ......................................................................... (g) [2] Derive a relationship between the decay constant λ and the half-life τ. ......................................................................... ......................................................................... ......................................................................... (h) Hence calculate the half-life of this radioactive isotope. [1] ......................................................................... ......................................................................... 880-227 Turn over Page 5 –2– M01/430/H(2) SECTION A Candidates must answer all questions in the spaces provided. A1. Gas law experiment (data based question) Boyle’s law states that for an ideal gas at constant temperature, pressure is inversely proportional to volume. To test whether or not a real gas obeys Boyle’s law, three students set up the apparatus shown below. Bourdon gauge Tube Gas 200 A 300 100 0 Oil kPa 400 Stopcock Air Oil The gas sample is enclosed in the tube and the length of the gas column can be measured against the scale. The gas pressure in the apparatus can be adjusted by pumping air in or out through the stopcock. The Bourdon gauge indicates ‘gauge pressure’, i.e. the difference in pressure inside and outside the gauge. (a) After each gas adjustment the students wait a few minutes before reading the column length and the Bourdon gauge. Explain why they should not take the readings immediately and what occurs during waiting. [2] .............................................................................. .............................................................................. .............................................................................. (b) Boyle’s law involves the volume V of the gas, yet the students instead measure the length L of the gas column. Why is this acceptable? [1] .............................................................................. .............................................................................. (c) Show algebraically that if Boyle’s law holds, a plot of gas pressure P versus the reciprocal of 1 the column length L (i.e. P versus ) should be of straight line form through the origin. L [2] .............................................................................. .............................................................................. .............................................................................. (This question continues on the following page) 221-171 Page 32 –3– M01/430/H(2) (Question A1 continued) 1 , as shown below. In order L 1 to estimate the uncertainty in the data, the students have repeated the measurement at = 2.8 m −1 L three times, giving a cluster of three data points. The students plot the pressure reading P B on the Bourdon gauge versus 400 Bourdon gauge reading / kPa 300 200 100 0 0 1 2 3 4 1 1 = m −1 Length of gas column L -100 -200 (d) Draw a best-fit straight line for the data points. [1] (e) Determine the intercept value on the Bourdon pressure axis. [1] ......................................................................... (f) From the three repeated measurements reflected by the cluster of points, (i) draw in an error bar on the graph to reflect the experimental uncertainty. [1] (ii) write the pressure value and its uncertainty in the form (value ! uncertainty). [2] ..................................................................... (This question continues on the following page) 221-171 Turn over Page 33 –4– M01/430/H(2) (Question A1 continued) (g) The students note that their graph does not seem to go through the origin (0,0). They each suggest different interpretations of the results of this experiment, as follows: Student 1 concludes that since the graph does not go through the origin, this gas deviates from Boyle’s law behaviour. Student 2 points out that there are random uncertainties in the data. He suggests that within experimental uncertainty the data may reasonably be fitted by a line drawn through the origin. He concludes that the data shows that the gas obeys Boyle’s law within experimental uncertainty. Student 3 says there could be a systematic error somewhere in the readings or the analysis. (i) Discuss the reasoning of student 2 in light of the data. [3] ..................................................................... ..................................................................... ..................................................................... ..................................................................... (ii) Suggest the most likely origin of any systematic error suggested by student 3. Explain this with reference to the particular numerical value found in question (e) of the pressure intercept of the graph. [2] ..................................................................... ..................................................................... ..................................................................... ..................................................................... (iii) If specific adjustment is made for such systematic error, are the data consistent with Boyle’s law? Explain. [2] ..................................................................... ..................................................................... ..................................................................... (iv) Which student’s interpretation is best, and does the gas obey Boyle’s law? [1] ..................................................................... ..................................................................... ..................................................................... 221-171 Page 34 –2– N01/430/H(2) SECTION A Candidates must answer all questions in the spaces provided. A1. This question is about power dissipation in a resistor and the internal resistance of a battery. In the circuit below the variable resistor can be adjusted to have known values of resistance R. The battery has an unknown internal resistance r. r –––– I A R The table below shows the recorded value I of the current in the circuit for different values of R. The last column gives the calculated value of the power P dissipated in the resistor. R/! 0 1.0 2.0 3.0 4.0 6.0 8.0 10.0 (a) I/A !0.01 A 1.50 1.20 1.00 0.86 0.75 0.60 0.50 0.43 P/W 0 1.4 2.0 2.2 2.3 2.2 2.0 Complete the last line of the table by calculating the power dissipated in the variable resistor when its value is 10.0 !. [2] ......................................................................... ......................................................................... ......................................................................... (This question continues on the following page) 881-171 Page 60 –3– N01/430/H(2) (Question A1 continued) (b) If each value of R is known to !10 % determine the absolute uncertainty in the value of P when R = 10.0 !. [3] ......................................................................... ......................................................................... ......................................................................... (c) On the grid below plot a graph of power P against resistance R. (Do not include error bars). [4] (d) It can be shown that the power dissipated in the external resistor is a maximum when the value of its resistance R is equal to the value of the internal resistance r of the battery i.e. R = r. Use this information and your graph to find the value of r. [1] ......................................................................... (e) The manufacturer of the battery gives the value of its internal resistance as 4.50 ! ! 0.01 !. Is the value of r that you obtained from your graph consistent with the manufacturer’s value? Explain. [2] ......................................................................... ......................................................................... ......................................................................... ......................................................................... 881-171 Turn over Page 61 –2– M02/430/H(2) SECTION A Candidates must answer all questions in the spaces provided. A1. This question is about the growth of an electric current in a coil. When a coil is connected to a d.c. power supply the current in the coil does not change instantaneously but takes a finite time to reach a steady value. For a given supply the final, steady value of the current is determined by the resistance (R) of the coil. In the diagram below a coil is connected to a d.c. supply of emf 4.0 V. 4.0 V A S Coil When the switch S is closed an electronic timer is started and the current I is recorded at different values of the time t. The results are shown in the table below. (Uncertainties in measurement are not shown). t /s I /A (a) 0 0 0.2 0.8 0.6 1.6 Plot a graph of current against time. 1.0 1.9 1.4 2.0 1.8 2.0 2.0 2.0 [5] (This question continues on the following page) 222-171 Page 87 –3– M02/430/H(2) (Question A1 continued) (b) What is the steady state value of the current? [1] ......................................................................... (c) Determine the value of the resistance R of the coil. [1] ......................................................................... ......................................................................... (d) By drawing a tangent to the curve at the point (0, 0) on your graph, determine the time it would take for the current to reach its steady state value if it were to continue changing at its initial rate. (This time is known as the time constant of the coil). [2] ......................................................................... ......................................................................... (e) V where L V is the value of the supply potential and L is a property of the coil known as its inductance. Show that the time constant ! for the coil is given by the expression The initial rate at which the current in the coil changes is given by the expression τ= L . R [3] ......................................................................... ......................................................................... ......................................................................... ......................................................................... ......................................................................... (f) Determine the value of the inductance L of the coil. [1] ......................................................................... ......................................................................... 222-171 Turn over Page 88 –3– M03/430/H(2) SECTION A Candidates must answer all questions in the spaces provided. A1. Some students were asked to design and carry out an experiment to determine the specific latent heat of vaporization of water. They set up the apparatus shown below. d.c. supply V A Water Heater g Top-pan balance The current was switched on and maintained constant using the variable resistor. The readings of the voltmeter and the ammeter were noted. When the water was boiling steadily, the reading of the top-pan balance was taken and, simultaneously, a stopwatch was started. The reading of the top-pan balance was taken again after 200 seconds and then after a further 200 seconds. The change in reading of the top-pan balance during each 200 second interval was calculated and an average found. The power of the heater was calculated by multiplying together the readings of the voltmeter and the ammeter. (a) Suggest how the students would know when the water was boiling steadily. [1] ......................................................................... ......................................................................... (b) Explain why a reading of the mass lost in the first 200 seconds and then a reading of the mass lost in the next 200 second interval were taken, rather than one single reading of the mass lost in 400 seconds. [2] ......................................................................... ......................................................................... ......................................................................... (This question continues on the following page) 223-171 Turn over Page 138 –4– M03/430/H(2) (Question A1 continued) The students repeated the experiment for different powers supplied to the heater. A graph of the power of the heater against the mass of water lost (the change in balance reading) in 200 seconds was plotted. The results are shown below. (Error bars showing the uncertainties in the measurements are not shown.) 120 100 80 power / W 60 40 20 0 0 (c) 1 2 3 4 mass / g 5 6 7 8 (i) On the graph above, draw the best-fit straight line for the data points. [1] (ii) Determine the gradient of the line you have drawn. [3] ..................................................................... ..................................................................... ..................................................................... ..................................................................... (This question continues on the following page) 223-171 Page 139 –5– M03/430/H(2) (Question A1 continued) In order to find a value for the specific latent heat of vaporization L, the students used the equation P = mL , where P is the power of the heater and m is the mass of water evaporated per second. (d) Use your answer for the gradient of the graph to determine a value for the specific latent heat of vaporization of water. [3] ......................................................................... ......................................................................... ......................................................................... ......................................................................... (e) The theory of the experiment would suggest that the graph line should pass through the origin. Explain briefly why the graph does not pass through the origin. [2] ......................................................................... ......................................................................... 223-171 Turn over Page 140 –2– N03/430/H(2) SECTION A Candidates must answer all questions in the spaces provided. A1. This question is about an experiment designed to investigate Newton’s second law. In order to investigate Newton’s second law, David arranged for a heavy trolley to be accelerated by small weights, as shown below. The acceleration of the trolley was recorded electronically. David recorded the acceleration for different weights up to a maximum of 3.0 N. He plotted a graph of his results. acceleration heavy trolley pulley weight (a) Describe the graph that would be expected if two quantities are proportional to one another. [2] ......................................................................... ......................................................................... ......................................................................... ......................................................................... (This question continues on the following page) 883-171 Page 161 –3– N03/430/H(2) (Question A1 continued) (b) David’s data are shown below, with uncertainty limits included for the value of the weights. Draw the best-fit line for these data. [2] acceleration 1.40 / m s −2 1.20 1.00 0.80 0.60 0.40 0.20 0.00 0.00 0.50 1.00 1.50 2.00 2.50 weight / N (c) Use the graph to (i) explain what is meant by a systematic error. [2] ..................................................................... ..................................................................... ..................................................................... ..................................................................... (ii) estimate the value of the frictional force that is acting on the trolley. [1] ..................................................................... (iii) estimate the mass of the trolley. [2] ..................................................................... ..................................................................... ..................................................................... ..................................................................... 883-171 Turn over Page 162 –2– M04/431/H(2) SECTION A Answer all the questions in the spaces provided. A1. Data based question. This question is about change of electrical resistance with temperature. The table below gives values of the resistance R of an electrical component for different values of its temperature T. (Uncertainties in measurement are not shown.) T / °C R/ (a) 1.2 2.0 3.5 5.2 6.8 8.1 9.6 3590 3480 3250 3060 2880 2770 2650 On the grid below, plot a graph to show the variation with temperature T of the resistance R. Show values on the temperature axis from T = 0 °C to T = 10 °C . [3] (This question continues on the following page) 224-177 Page 192 –3– M04/431/H(2) (Question A1 continued) (b) (i) (ii) Draw a curve that best fits the points you have plotted. Extend your curve to cover the temperature range from 0 °C to 10 °C . [1] Use your graph to determine the resistance at 0 °C and at 10 °C . [2] Resistance at 0 °C = . . . . . . . . . . . . . . . . . . . . . . . Resistance at 10 °C = . . . . . . . . . . . . . . . . . . . . . . (c) (d) On your graph, draw a straight-line between the resistance values at 0 °C and at 10 °C . This line shows the variation with temperature (between 0 °C and 10 °C ) of the resistance, assuming a linear change. (i) Assuming a linear change of resistance with temperature, use your graph to determine the temperature at which the resistance is 3060 . [1] [1] Temperature = . . . . . . . . . . . . . . . . . . . . . . . . . . °C (ii) Use your answer in (d)(i) to calculate the percentage difference in the temperature for a resistance of 3060 that results from assuming a linear change rather than the non-linear change. [3] ..................................................................... ..................................................................... ..................................................................... (e) In a particular experiment to measure the variation with temperature of the resistance, each measurement of resistance has an uncertainty of ! 30 and the uncertainty in the temperature measurements is ! 0.2 °C . (i) (ii) On your graph in (a), show the uncertainties in the values of R and of T for temperatures of 1.2 °C, 5.2 °C and 9.6 °C . [2] State and explain whether, within the experimental uncertainties, the relationship between resistance and temperature could be linear. [2] ..................................................................... ..................................................................... ..................................................................... 224-177 Turn over Page 193 –2– M04/432/H(2) SECTION A Answer all the questions in the spaces provided. A1. This question is about measuring the permittivity of free space ε 0 . The diagram below shows two parallel conducting plates connected to a variable voltage supply. The plates are of equal areas and are a distance d apart. variable voltage supply + d _ V The charge Q on one of the plates is measured for different values of the potential difference V applied between the plates. The values obtained are shown in the table below. The uncertainty in the value of V is not significant but the uncertainty in Q is !10%. V/V Q / nC !10 % 10.0 30 20.0 80 30.0 100 40.0 160 50.0 180 (This question continues on the following page) 224-180 Page 219 –3– M04/432/H(2) (Question A1 continued) (a) Plot the data points opposite on a graph of V (x-axis) against Q (y-axis). [4] (b) By calculating the relevant uncertainty in Q, add error bars to the data points (10.0, 30) and (50.0, 180). [3] On the graph above, draw the line that best fits the data points and has the maximum permissible gradient. Determine the gradient of the line that you have drawn. [3] (c) ......................................................................... ......................................................................... ......................................................................... ......................................................................... (d) The gradient of the graph is a property of the two plates and is known as capacitance. Deduce the units of capacitance. [1] ......................................................................... (This question continues on the following page) 224-180 Turn over Page 220 –4– M04/432/H(2) (Question A1 continued) The relationship between Q and V for this arrangement is given by the expression Q= ε0 A d V where A is the area of one of the plates. In this particular experiment A = 0.20 ± 0.05 m 2 and d = 0.50 ± 0.01mm. (e) Use your answer to (c) to determine the maximum value of ε 0 that this experiment yields. [4] ......................................................................... ......................................................................... ......................................................................... ......................................................................... ......................................................................... 224-180 Page 221 –2– M05/4/PHYSI/HP2/ENG/TZ1/XX+ SECTION A Answer all the questions in the spaces provided. A1. Data analysis question At high pressures, a real gas does not behave as an ideal gas. For a certain range of pressures, it is suggested that the relation between the pressure P and volume V of one mole of the gas at constant temperature is given by the equation PV = A + BP where A and B are constants. In an experiment to measure the deviation of nitrogen gas from ideal gas behaviour, 1 mole of nitrogen gas was compressed at a constant temperature of 150 K. The volume V of the gas was measured for different values of the pressure P. A graph of the product PV of pressure and volume was plotted against the pressure P and is shown below. (Error bars showing the uncertainties in measurements are not shown). PV PV //×102 N m PP//×106 Pa (a) Draw a line of best fit for the data points. [1] (This question continues on the following page) 2205-6508 0233 Page 246 –3– M05/4/PHYSI/HP2/ENG/TZ1/XX+ (Question A1 continued) (b) Use the graph to determine the values of the constants A and B in the equation PV = A + BP . [5] Constant A . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ................................................................. ................................................................. Constant B . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ............................................................ ............................................................ ............................................................ ............................................................ (c) State the value of the constant B for an ideal gas. [1] ...................................................................... (d) The equation PV = A + BP is valid for pressures up to 6.0 ×107 Pa. (i) [2] Determine the value of PV for nitrogen gas at a pressure of 6.0 ×107 Pa. ................................................................. ................................................................. ................................................................. (ii) Calculate the difference between the value of PV for an ideal gas and nitrogen gas when both are at a pressure of 6.0 ×107 Pa. [2] ................................................................. ................................................................. ................................................................. (e) In the original experiment, the pressure P was measured to an accuracy of 5 % and the volume V was measured to an accuracy of 2 %. Determine the absolute error in the value of the constant A. [3] ...................................................................... ...................................................................... ...................................................................... Turn over 2205-6508 0333 Page 247 –3– M05/4/PHYSI/HP2/ENG/TZ2/XX+ SECTION A Answer all the questions in the spaces provided. A1. The Geiger-Nuttall theory of α -particle emission relates the half-life of the α -particle emitter to the energy E of the α -particle . One form of this relationship is L = 166 1 E2 − 53.5. L is a number calculated from the half-life of the α -particle emitting nuclide and E is measured in MeV. Values of E and L for different nuclides are given below. (Uncertainties in the values are not shown.) Nuclide (a) E / MeV L 1 E 1 2 / MeV − 12 238 U 4.20 17.15 0.488 236 U 4.49 14.87 0.472 234 U 4.82 12.89 0.455 228 Th 5.42 7.78 ........... 208 Rn 6.14 3.16 0.404 212 Po 7.39 –2.75 0.368 Complete the table above by calculating, using the value of E provided, the value of for the nuclide 228 Th. Give your answer to three significant digits. 1 1 E2 [1] (This question continues on the following page) Turn over 2205-6514 0333 Page 281 –4– M05/4/PHYSI/HP2/ENG/TZ2/XX+ (Question A1 continued) 1 The graph below shows the variation with 1 of the quantity L. Error bars have not been E2 added. L 20 16 12 8 4 0 0.2 0.3 0.4 1 1 E2 –4 (b) 208 0.5 / MeV Rn. Label this point R. (i) Identify the data point for the nuclide (ii) On the graph, mark the point for the nuclide 228 Th . Label this point T. (iii) Draw the best-fit straight-line for all the data points. − 12 [1] [1] [1] (This question continues on the following page) 2205-6514 0433 Page 282 –5– M05/4/PHYSI/HP2/ENG/TZ2/XX+ (Question A1 continued) (c) (i) [2] Determine the gradient of the line you have drawn in (b) (iii). ................................................................. ................................................................. ................................................................. (ii) Without taking into consideration any uncertainty in the values for the gradient and for the intercept on the x-axis, suggest why the graph does not agree with the stated relationship for the Geiger-Nuttall theory. [2] ................................................................. ................................................................. ................................................................. ................................................................. (d) On the graph opposite, draw the line that would be expected if the relationship for the Geiger-Nuttall theory were correct. No further calculation is required. (e) U is ± 0.03 MeV. Deduce that this 1 uncertainty is consistent with quoting the value of 1 to three significant digits. E2 ...................................................................... The uncertainty in the measurement of E for [2] 238 [3] ...................................................................... ...................................................................... ...................................................................... Turn over 2205-6514 0533 Page 283 –2– N05/4/PHYSI/HP2/ENG/TZ0/XX+ SECTION A Answer all the questions in the spaces provided. A1. This question is about an electrostatics experiment to investigate how the force between two charges varies with the distance between them. A small charged sphere S hangs vertically from an insulating thread as shown below. S A second identically charged sphere P is brought close to S. S is repelled as shown below. P S force F r The magnitude of the electrostatic force on sphere S is F. The separation between the two spheres is r. (This question continues on the following page) 8805-6502 0233 Page 313 –3– N05/4/PHYSI/HP2/ENG/TZ0/XX+ (Question A1 continued) (a) On the axes below draw a sketch graph to show how, based on Coulomb’s law, you would 1 expect F to vary with 2 . r [2] F 0 1 r2 0 (This question continues on the following page) Turn over 8805-6502 0333 Page 314 –4– N05/4/PHYSI/HP2/ENG/TZ0/XX+ (Question A1 continued) Values of F are determined for different values of r. The variation with 1 of these values is r2 shown below. The estimated uncertainties in these values are negligible. F /×10−3 N 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 (b) 0.0 2.0 4.0 6.0 8.0 10.0 12.0 1 /×103 m −2 r2 (i) Draw the best-fit line for these data points. [2] (ii) Use the graph to explain whether, in the experiment, there are random errors, systematic errors or both. [3] ................................................................. ................................................................. ................................................................. ................................................................. ................................................................. (iii) Calculate the gradient of the line drawn in (b) (i). [2] ................................................................. ................................................................. ................................................................. ................................................................. (This question continues on the following page) 8805-6502 0433 Page 315 –5– N05/4/PHYSI/HP2/ENG/TZ0/XX+ (Question A1 continued) (iv) The magnitude of the charge on each sphere is the same. Use your answer to (b) (iii) to calculate this magnitude. [4] ................................................................. ................................................................. ................................................................. ................................................................. (c) Explain how a graph showing the variation with lg r of lg F can be used to verify the relation between r and F. [3] ...................................................................... ...................................................................... ...................................................................... ...................................................................... ...................................................................... Turn over 8805-6502 0533 Page 316 –2– M06/4/PHYSI/HP2/ENG/TZ1/XX+ sEction a Answer all the questions in the spaces provided. a1. This question is about the rise of water in a capillary tube. A capillary tube is a tube that is open at both ends and has a very narrow bore. A capillary tube is supported vertically with one end immersed in water. Water rises up the tube due to a phenomenon called capillary action. The water in the bore of the tube forms a column of height h as shown below. narrow bore glass wall glass wall h water (This question continues on the following page) 2206-6508 0236 Page 346 –3– M06/4/PHYSI/HP2/ENG/TZ1/XX+ (Question A1 continued) (a) The height h, for a particular capillary tube was measured for different temperatures of the water. The variation with temperature of the height h is shown below. Uncertainties in the measurements are not shown. 17 16 15 14 13 h / cm 12 11 10 9.0 8.0 0 10 20 30 40 50 / °C 60 70 80 90 (i) On the graph above, draw a best-fit line for the data points. [1] (ii) Determine the height h0 of the water column at temperature = 0 °C. [1] .................................................................. .................................................................. (This question continues on the following page) turn over 2206-6508 0336 Page 347 –4– M06/4/PHYSI/HP2/ENG/TZ1/XX+ (Question A1 continued) (b) Explain why the results of this experiment suggest that the relationship between the height h and temperature is of the form h = h0(1 − k) where k is constant. [4] ....................................................................... ....................................................................... ....................................................................... ....................................................................... ....................................................................... ....................................................................... ....................................................................... ....................................................................... (c) Deduce that the value of k is approximately 4.8 10−3 deg C−1. [3] ....................................................................... ....................................................................... ....................................................................... ....................................................................... (This question continues on the following page) 2206-6508 0436 Page 348 –5– M06/4/PHYSI/HP2/ENG/TZ1/XX+ (Question A1 continued) (d) The experiment is repeated using tubes with bores of different radii r but keeping the 1 water temperature constant. The graph below shows the variation with of the height h r for capillary tubes of different radii r for a water temperature of 20 °C. 0.35 0.30 0.25 0.20 h/m 0.15 0.10 0.05 0 0 5.0 10.0 15.0 1 / ×103 m −1 r 20.0 25.0 It is suggested that capillary action is one of the means by which water moves from the roots of a tree to the leaves. A particular tree has a height of 25 m. Use the graph to estimate the radius of the bore of the tubes that would enable water to be raised by capillary action from ground level to the top of the tree. Comment on your answer. Estimate: [4] ............................................................. ............................................................. ............................................................. ............................................................. ............................................................. Comment: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ............................................................. ............................................................. turn over 2206-6508 0536 Page 349 –2– N06/4/PHYSI/HP2/ENG/TZ0/XX+ sEction a Answer all the questions in the spaces provided. a1. A hot object may be cooled by blowing air past it. This cooling process is known as forced convection. In order to investigate forced convection, hot oil was placed in a metal can. The can was placed on an insulating block and air was blown past the can, as shown below. stirrer thermometer lid hot oil current of air metal can insulating block (This question continues on the following page) 8806-6502 0230 Page 382 –3– N06/4/PHYSI/HP2/ENG/TZ0/XX+ (Question A1 continued) The hot oil was stirred continuously and its temperature was taken every minute as it cooled. The graph below shows the variation with time of the temperature of the cooling oil. 120 100 temperature / °C 80 60 40 20 0 0 2 4 6 8 time / minutes 10 12 14 It is thought that the rate R of decrease of temperature depends on the temperature difference between the oil and its surroundings (the excess temperature θ E). The temperature of the surroundings was 26 °C. (a) On the graph above, (i) (ii) draw a straight-line parallel to the time axis to represent the temperature of the surroundings. [1] by drawing a suitable tangent, calculate the rate of decrease of temperature, in °C s–1, for an excess temperature of 50 Celsius degrees (°C). [4] .................................................................. .................................................................. .................................................................. .................................................................. (This question continues on the following page) turn over 8806-6502 0330 Page 383 –4– N06/4/PHYSI/HP2/ENG/TZ0/XX+ (Question A1 continued) (b) In order to investigate the variation with R of θ E, a graph of R against θ E is plotted. The graph below shows four plotted data points. Uncertainties in the points are not included. 0.24 0.20 0.16 R / °C s–1 0.12 0.08 0.04 0.00 0 20 40 60 θE / 80 100 °C (i) Using your answer to (a)(ii), plot the data point corresponding toθθEE = 50 °C. [1] (ii) The uncertainty in the measurement of R at each excess temperature is ±10 %. On the graph, draw error bars to represent the uncertainties in R at excess temperatures of 20 °C and 81 °C. [2] (This question continues on the following page) 8806-6502 0430 Page 384 –5– N06/4/PHYSI/HP2/ENG/TZ0/XX+ (Question A1 continued) (c) (i) Explain why the graph in (b) supports the conclusion that the excess temperature θ E is related to the rate of cooling R by the expression R = kθ E , where k is a constant. [3] .................................................................. .................................................................. .................................................................. .................................................................. (ii) At high excess temperatures, the equation in (i) is thought to become invalid. Discuss whether the graph in (b) provides any evidence for this suggestion. [2] .................................................................. .................................................................. .................................................................. (d) In a second experiment, the data is analysed by plotting a graph of lgR against lgθ E. (lg is the logarithm to the base 10.) (i) On the axes below, draw a sketch graph to show the line that would be obtained. (Note that this is a sketch graph. No data points or values on the axes are required.) [1] lgR lgθ E (ii) Assuming the expression in (c)(i) is correct, state the gradient of the line of the graph. Also, explain how the value of k is obtained. [2] .................................................................. .................................................................. .................................................................. turn over 8806-6502 0530 Page 385 –3– M07/4/PHYSI/HP2/ENG/TZ1/XX+ sEcTion a Answer all the questions in the spaces provided. a1. This question is about thermal energy transfer through a rod. A student designed an experiment to investigate the variation of temperature along a copper rod when each end is kept at a different temperature. In the experiment, one end of the rod is placed in a container of boiling water at 100 °C and the other end is placed in contact with a block of ice at 0.0 °C as shown in the diagram. temperature sensors boiling water 100 °C ice 0 °C copper rod not to scale (This question continues on the following page) Turn over 2207-6508 0337 Page 413 –4– M07/4/PHYSI/HP2/ENG/TZ1/XX+ (Question A1 continued) Temperature sensors are placed at 10 cm intervals along the rod. The final steady state temperature θ of each sensor is recorded, together with the corresponding distance x of each sensor from the hot end of the rod. The data points are shown plotted on the axes below. θ / °C 110 100 90 80 70 60 50 40 30 20 10 0 0 10 20 30 40 50 60 70 80 90 x / cm The uncertainty in the measurement of θ is ±2 °C. The uncertainty in the measurement of x is negligible. (a) (b) On the graph above, draw the uncertainty in the data points for x = 10 cm, x = 40 cm and x = 70 cm. [2] On the graph above, draw the line of best-fit for the data. [1] (This question continues on the following page) 2207-6508 0437 Page 414 –5– M07/4/PHYSI/HP2/ENG/TZ1/XX+ (Question A1 continued) (c) Explain, by reference to the uncertainties you have indicated, the shape of the line you have drawn. [2] ....................................................................... ....................................................................... ....................................................................... (d) (i) Use your graph to estimate the temperature of the rod at x = 55 cm. [1] .................................................................. (ii) Determine the magnitude of the gradient of the line (the temperature gradient) at x = 50 cm. [3] .................................................................. .................................................................. .................................................................. .................................................................. (e) The rate of transfer of thermal energy R through the cross-sectional area of the rod is ∆θ along the rod. At x = 10 cm, R = 43 W and ∆x ∆θ = 1.81°C cm −1. At x = 50 cm the value the magnitude of the temperature gradient is ∆x of R is 25 W. proportional to the temperature gradient Use these data and your answer to d(ii) to suggest whether the rate R of thermal energy transfer is in fact proportional to the temperature gradient. [3] ....................................................................... ....................................................................... ....................................................................... ....................................................................... (This question continues on the following page) Turn over 2207-6508 0537 Page 415 –6– M07/4/PHYSI/HP2/ENG/TZ1/XX+ (Question A1 continued) (f) It is suggested that the variation with x of the temperature θ is of the form θ = θ 0 e − kx where θ 0 and k are constants. State how the value of k may be determined from a suitable graph. [2] ....................................................................... ....................................................................... ....................................................................... 2207-6508 0637 Page 416 –2– M07/4/PHYSI/HP2/ENG/TZ2/XX+ sEction a Answer all the questions in the spaces provided. a1. The question is about investigating a fireball caused by an explosion. When a fire burns within a confined space, the fire can sometimes spread very rapidly in the form of a circular fireball. Knowing the speed with which these fireballs can spread is of great importance to fire-fighters. In order to be able to predict this speed, a series of controlled experiments was carried out in which a known amount of petroleum (petrol) stored in a can was ignited. The radius R of the resulting fireball produced by the explosion of some petrol in a can was measured as a function of time t. The results of the experiment for five different volumes of petroleum are shown plotted below. (Uncertainties in the data are not shown.) 25 Key: 30 10–3 m3 25 10–3 m3 15 10–3 m3 10 10–3 m3 5.0 10–3 m3 20 15 R/m 10 5 0 0 (a) 10 20 30 40 t / ms 50 60 70 The original hypothesis was that, for a given volume of petrol, the radius R of the fireball would be directly proportional to the time t after the explosion. State two reasons why the plotted data do not support this hypothesis. 1. [2] .................................................................. .................................................................. 2. .................................................................. .................................................................. (This question continues on the following page) 2207-6514 0234 Page 449 –3– M07/4/PHYSI/HP2/ENG/TZ2/XX+ (Question A1 continued) (b) The uncertainty in the radius is 0.5 m. The addition of error bars to the data points would show that there is in fact a systematic error in the plotted data. Suggest one reason for this systematic error. [2] ....................................................................... ....................................................................... ....................................................................... ....................................................................... (c) Since the data do not support direct proportionality between the radius R of the fireball and time t, a relation of the form R = kt n is proposed, where k and n are constants. In order to find the value of k and of n, lg(R) is plotted against lg(t). The resulting graph, for a particular volume of petrol, is shown below. (Uncertainties in the data are not shown.) 1.3 1.2 1.1 lg(R) 1.0 0.9 0.8 1 1.1 1.2 1.3 1.4 1.5 lg(t) 1.6 1.7 1.8 1.9 Use this graph to deduce that the radius R is proportional to t0.4. Explain your reasoning. [4] ....................................................................... ....................................................................... ....................................................................... ....................................................................... ....................................................................... ....................................................................... (This question continues on the following page) turn over 2207-6514 0334 Page 450 –4– M07/4/PHYSI/HP2/ENG/TZ2/XX+ (Question A1 continued) (d) It is known that the energy released in the explosion is proportional to the initial volume of petrol. A hypothesis made by the experimenters is that, at a given time, the radius of the fireball is proportional to the energy E released by the explosion. In order to test this hypothesis, the radius R of the fireball 20 ms after the explosion was plotted against the initial volume V of petrol causing the fireball. The resulting graph is shown below. 15 10 R/m 5 0 0 5 10 15 20 25 V / 10–3 m3 30 35 The uncertainties in R have been included. The uncertainty in the volume of petrol is negligible. (i) Describe how the data for the above graph are obtained from the graph in (a). [1] .................................................................. .................................................................. (ii) Draw the line of best-fit for the data points. [2] (iii) Explain whether the plotted data together with the error bars support the hypothesis that R is proportional to V. [2] .................................................................. .................................................................. .................................................................. .................................................................. .................................................................. (This question continues on the following page) 2207-6514 0434 Page 451 –5– M07/4/PHYSI/HP2/ENG/TZ2/XX+ (Question A1 continued) (e) Analysis shows that the relation between the radius R, energy E released and time t is in fact given by R5 = Et2. Use data from the graph in (d) to deduce that the energy liberated by the combustion of 1.0 10–3 m3 of petrol is about 30 MJ. [4] ....................................................................... ....................................................................... ....................................................................... ....................................................................... ....................................................................... turn over 2207-6514 0534 Page 452 –2– N07/4/PHYSI/HP2/ENG/TZ0/XX sEction a Answer all the questions in the spaces provided. a1. As part of a road-safety campaign, the braking distances of a car were measured. A driver in a particular car was instructed to travel along a straight road at a constant speed v. A signal was given to the driver to stop and he applied the brakes to bring the car to rest in as short a distance as possible. The total distance D travelled by the car after the signal was given was measured for corresponding values of v. A sketch-graph of the results is shown below. v 0 0 (a) D State why the sketch graph suggests that D and v are not related by an expression of the form D = mv + c, where m and c are constants. [1] ....................................................................... ....................................................................... (This question continues on the following page) 8807-6502 0233 Page 483 –3– N07/4/PHYSI/HP2/ENG/TZ0/XX (Question A1 continued) (b) It is suggested that D and v may be related by an expression of the form D = av + bv2, where a and b are constants. In order to test this suggestion, the data shown below are used. The uncertainties in the measurements of D and v are not shown. v / m s–1 D/m D / (i .......... v 10.0 14.0 1.40 13.5 22.7 1.68 18.0 36.9 2.05 22.5 52.9 27.0 74.0 2.74 31.5 97.7 3.10 D . v [1] (i) In the table above, state the unit of (ii) D Calculate the magnitude of , to an appropriate number of significant digits, v for v = 22.5 m s–1. [1] .................................................................. .................................................................. (This question continues on the following page) turn over 8807-6502 0333 Page 484 –4– N07/4/PHYSI/HP2/ENG/TZ0/XX (Question A1 continued) (c) D (y-axis) against v (x-axis). Some of the Data from the table are used to plot a graph of v data points are shown plotted below. 3.50 3.00 D / (S.I. units) v 2.50 2.00 1.50 1.00 0.50 0.00 0.00 5.00 10.00 15.00 20.00 25.00 30.00 35.00 –1 v / ms On the graph above, (i) plot the data points for speeds corresponding to 22.5 m s–1 and to 31.5 m s–1. [2] (ii) draw the best-fit line for all the data points. [1] (This question continues on the following page) 8807-6502 0433 Page 485 –5– N07/4/PHYSI/HP2/ENG/TZ0/XX (Question A1 continued) (d) Use your graph in (c) to determine (i) the total stopping distance D for a speed of 35 m s–1. [2] .................................................................. .................................................................. .................................................................. (ii) the intercept on the D axis. v [1] .................................................................. (iii) the gradient of the best-fit line. [2] .................................................................. .................................................................. .................................................................. (e) Using your answers to (d)(ii) and (d)(iii), deduce the equation for D in terms of v. D= (f) [1] .................................................................. The uncertainty in the measurement of the distance D is ±0.3 m and the uncertainty in the measurement of the speed v is ±0.5 m s–1. (i) For the data point corresponding to v = 27.0 m s–1, calculate the absolute uncertainty D in the value of . v [2] .................................................................. .................................................................. .................................................................. (ii) Each of the data points in (b) was obtained by taking the average of several values of D for each value of v. Suggest what effect, if any, the taking of averages will have on the uncertainties in the data points. [2] .................................................................. .................................................................. .................................................................. turn over 8807-6502 0533 Page 486 –2– M08/4/PHYSI/HP2/ENG/TZ1/XX+ Section a Answer all the questions in the spaces provided. a1. This question is about data analysis. Data for the refractive index n of a type of glass and wavelength of the light transmitted through the glass are shown below. Only the uncertainties in the values of n are significant and these uncertainties are shown by error bars. 1.6065 1.6060 1.6055 1.6050 1.6045 n 1.6040 1.6035 1.6030 1.6025 1.6020 1.6015 300 350 400 450 500 550 600 650 /nm (This question continues on the following page) 2208-6508 0233 Page 516 –3– M08/4/PHYSI/HP2/ENG/TZ1/XX+ (Question A1 continued) (a) State why the data do not support the hypothesis that there is a linear relationship between refractive index and wavelength. [1] ....................................................................... ....................................................................... (b) Draw a best-fit line for the data points. (c) The rate of change of refractive index D with wavelength is referred to as the dispersion. λ At any particular value of wavelength, D is defined by λ [2] ∆n D = λ ∆λ Use the graph to determine the value of D at a wavelength of 380 nm. λ [4] ....................................................................... ....................................................................... ....................................................................... ....................................................................... ....................................................................... ....................................................................... (d) It is suggested that the relationship between n and is of the form n = kλ p where k and p are constants. [3] State and explain the graph that you would plot in order to determine the value of p. ....................................................................... ....................................................................... ....................................................................... ....................................................................... ....................................................................... ....................................................................... (This question continues on the following page) turn over 2208-6508 0333 Page 517 –4– M08/4/PHYSI/HP2/ENG/TZ1/XX+ (Question A1 continued) (e) A second suggestion is that the relationship between n and is of the form n = A+ B λ2 where A and B are constants. 1 To test this suggestion, values of n are plotted against values of 2 . The resulting graph λ with the line of best fit is shown below. 1.6065 1.6060 1.6055 1.6050 1.6045 n 1.6040 1.6035 1.6030 1.6025 1.6020 1.6015 1.6010 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 1.1 1.2 1 /×10−15 m −2 λ2 (This question continues on the following page) 2208-6508 0433 Page 518 –5– M08/4/PHYSI/HP2/ENG/TZ1/XX+ (Question A1 continued) (i) Use the graph to determine the value of the constant A. [3] .................................................................. .................................................................. .................................................................. (ii) State the significance of the constant A. [1] .................................................................. .................................................................. turn over 2208-6508 0533 Page 519 –2– M08/4/PHYSI/HP2/ENG/TZ2/XX+ Section a Answer all the questions in the spaces provided. a1. Some data for the resistance R of an electrical component at different temperatures are shown below. t / °c R/Ω 10.0 15.0 25.0 30.0 35.0 40.0 2600 2150 1510 1280 1080 925 A graph of the variation with temperature t of the resistance R of the component is shown below. Error bars have been included. 3400 3200 3000 2800 2600 2400 R/Ω 2200 2000 1800 1600 1400 1200 1000 800 0 5 10 15 20 25 t / °C 30 35 40 45 (This question continues on the following page) 2208-6514 0231 Page 549 –3– M08/4/PHYSI/HP2/ENG/TZ2/XX+ (Question A1 continued) (a) Estimate the (i) uncertainty range in the temperature measurements. [1] .................................................................. (ii) percentage uncertainty in the resistance at 10.0 °C. [2] .................................................................. .................................................................. .................................................................. (b) Use the graph to determine the (i) (ii) most probable resistance of the component at 20.0 °C and at 5.0 °C. Resistance at 20.0 °C ............................................... [1] Resistance at 5.0 °C ............................................... [2] rate of change of resistance with temperature at 20.0 °C. [3] .................................................................. .................................................................. .................................................................. (c) The relationship between resistance and temperature is not linear. Describe, and explain, the evidence for a non-linear relationship that is provided by the graph. [2] ....................................................................... ....................................................................... ....................................................................... (This question continues on the following page) turn over 2208-6514 0331 Page 550 –4– M08/4/PHYSI/HP2/ENG/TZ2/XX+ (Question A1 continued) (d) A student suggests that the relationship between the resistance R and temperature is of the form c R= T where c is a constant and T is the thermodynamic (absolute) temperature. Use data from the table to determine, without drawing a graph, whether this suggestion is correct. [3] ....................................................................... ....................................................................... ....................................................................... 2208-6514 0431 Page 551 –2– N08/4/PHYSI/HP2/ENG/TZ0/XX+ Section a Answer all the questions in the spaces provided. a1. This question is about the mass-radius relation for a certain type of star. The radius R and mass M of ten different stars were measured and the results are shown plotted below. R / R S 2.00 1.75 1.50 1.25 1.00 0.75 0.50 0.25 0.0 0.0 0.4 0.8 1.2 1.6 2.0 M / MS (This question continues on the following page) 8808-6502 0231 Page 580 –3– N08/4/PHYSI/HP2/ENG/TZ0/XX+ (Question A1 continued) The radius is expressed in terms of the Sun’s radius R S and the mass in terms of the Sun’s mass M S. The uncertainty in the measurement of the mass is negligible. measurement of the radius is ±0.05 R S. (a) Draw error bars for the first and the last data points. (b) Using your answer to (a), (i) The uncertainty in the [1] suggest why there might be a linear relationship between R and M for these stars. [2] .................................................................. .................................................................. (ii) determine the equation for this linear relationship. [3] .................................................................. .................................................................. .................................................................. .................................................................. .................................................................. .................................................................. .................................................................. (iii) estimate the maximum value for the mass of this type of star. [1] .................................................................. .................................................................. (c) Suggest why no star of this type can in fact have a mass equal to your answer to (b)(iii). [1] ....................................................................... (This question continues on the following page) turn over 8808-6502 0331 Page 581 –4– N08/4/PHYSI/HP2/ENG/TZ0/XX+ (Question A1 continued) (d) Additional data show that the relation between R and M is in fact not linear, as suggested by the graph below. R / R S 2.00 1.75 1.50 1.25 1.00 0.75 0.50 0.25 0.0 0.0 0.4 0.8 1.2 1.6 2.0 M / MS Uncertainties in the data are not shown. (This question continues on the following page) 8808-6502 0431 Page 582 –5– N08/4/PHYSI/HP2/ENG/TZ0/XX+ (Question A1 continued) (i) Draw a line of best-fit for the data. [1] (ii) The new data suggests that the maximum value for the mass of this type of star is different from your answer in (b)(iii). Estimate this new value. [1] .................................................................. .................................................................. (iii) Suggest why your answer to (d)(ii) is only an estimate. [1] .................................................................. (e) It is hypothesized that the mass–radius relation for a different type of star is R=kM n where k and n are constants. Explain how a graph may be used to (i) verify this hypothesis. [2] .................................................................. .................................................................. .................................................................. .................................................................. (ii) determine the constant n. [1] .................................................................. .................................................................. turn over 8808-6502 0531 Page 583