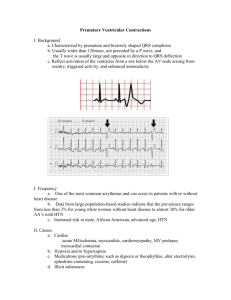
Test 2 Perfusion Notes
Coronary Artery Disease (CAD)
CAD is caused by impaired blood flow to the myocardium, usually d/t accumulation of atherosclerotic
plaque in the coronary arteries.
o Atherosclerosis is a progressive disease characterized by plaque formation that affects midsized
and large arteries.
Abnormal lipid metabolism and injury to (or inflammation, HTN+, infections) of the
cells lining the arteries appear to be key to its development.
LDLs and VLDLs contribute to this condition. HDLs reduce the risk.
Early sclerotic lesions appear as a yellowish, fatty streak on the inner arterial lining. As
time passes, they transform into a fibrous plaque, gradually occluding the vessel lumen.
The final stage of this process is the development of atheromas (complex lesions that
consist of lipids, fibrous tissue, collagen, calcium, cellular debris, and capillaries). These
calcified lesions can ulcerate or rupture, stimulating thrombosis.
Certain vessels have a higher likelihood of being affected, including the coronary arteries
(especially the left anterior descending artery), the renal arteries, the bifurcation of the
carotid arteries, and the branching sections of peripheral arteries.
Manifestations of the sclerotic process do not appear until approximately 75% of
the arterial lumen has been occluded.
In addition to occluding blood flow, atherosclerosis weakens the arterial walls and is a
major cause of aneurysm in vessels such as the aorta and iliac arteries.
May be asymptomatic, or it may lead to angina pectoris, acute coronary syndrome (ACS), MI (heart
attack), dysrhythmias, HF, and sudden death.
o Angina pectoris (or angina) is chest pain (CP) resulting from reduce coronary blood flow, which
causes a temporary imbalance between myocardial blood supply and demand.
Stable angina
Most common and predictable form.
Occurs with a predictable amount of activity, stress, or cold and is a common
manifestation of CAD.
Relieved by rest and nitrates.
Prinzmetal angina
Atypical, occurs unpredictably (unrelated to activity), and at night.
Unstable angina
Occurs with increasing frequency, severity, and duration.
Unpredictable, occurring with decreasing levels of activity or stress, and may
even occur at rest.
Patients with this form are at risk for MI.
Not all myocardial ischemia produces angina. Many patients experience what is known
as asymptomatic or silent myocardial ischemia. This often occurs with exercise and is
associated with a higher relative risk of serious cardiac events.
s/s
Chest pain (substernal or precordial {across the chest wall}; may radiate to
neck, arms, shoulders, or jaw)
Quality (tight, squeezing, constricting, or heavy sensation; may also be
described as burning, aching, choking, dull, or constant)
Associated manifestations (dyspnea, pallor, tachycardia, anxiety, or fear)
Atypical manifestations (indigestion, N/V, or upper back pain)
Precipitating factors (exercise or activity, strong emotion, stress, cold, or
heavy meals)
o
o
Relieving factors (rest, position change, or nitroglycerin {NTG})
Acute coronary syndrome (ACS) refers to any condition that develops because of sudden,
reduced blood flow to the heart. It includes unstable angina and acute myocardial ischemia.
May be precipitated by one or more of the following events: rupture or erosion of
atherosclerotic plaque, formation of a thrombosis, coronary artery spasm, progressive
vessel obstruction following a revascularization procedure, inflammation, or an increase
in myocardial oxygen damage and/or a decrease in supply (i.e., anemia or acute blood
loss).
Most people who are affected by ACS have significant occlusion of one or more coronary
arteries.
Inverted T waves and elevated ST segments = possible.
s/s
Chest pain (usually substernal or epigastric; often radiates to the neck, left
shoulder, and/or left arm; may occur at rest and typically lasts 10-20
minutes)
Pain is more severe and prolonged than that previously experienced by the
patient.
Dyspnea, diaphoresis, pallor, cool skin, HTN-, tachycardia, nausea, and
lightheadedness may be present.
Acute MI (AMI) involves necrosis of myocardial cells and occurs when blood flow to a portion
of the cardiac muscle is blocked (affecting the heart’s ability to maintain effective CO).
Majority of deaths occur during the initial period after symptoms begin.
Heightening public awareness of the manifestations of MI, the importance of immediate
medical assistance, and the value of training in CPR = vital.
If ischemia lasts more than 20-45 minutes, irreversible hypoxemic damage leads to
cellular death and necrosis.
The subendocardium is the first portion of the heart to experience damage during an AMI
(within 20 minutes of injury). If blood flow is restored at this point, it is categorized as a
subendocardial or non-Q-wave infarction. If blood flow is not restored, the damage
progresses to the epicardium within 1-6 hours. When all layers of the myocardium are
affected, it is known as a transmural infarction (significant Q wave).
MI usually affects the left ventricle, because it is the major “workhorse” of the
heart; its muscle mass is greater, as are its oxygen demands.
May also develop d/t cocaine use. The patient may present with an altered LOC,
confusion, restlessness, seizure activity, tachycardia, HTN-, tachypnea, and crackles.
s/s
Substernal or precordial chest pain that may radiate to the neck, jaw,
shoulder(s), or left arm
Tachycardia and tachypnea
Dyspnea and SOB
N/V
Anxiety and impending sense of doom
Diaphoresis
Cool, mottled skin; diminished peripheral pulses
HTN-/HTN+
Palpitations or dysrhythmias
Signs of left-sided HF (FVO s/s)
Decreased LOC
Possible complications include:
Dysrhythmias
PVCs = common after MI
The risk of VF is greatest the first hour after MI.
Bradydysrhythmias (abnormal slow rhythms) may also occur if the
inferior wall of the ventricle is affected.
Pump failure
The risk of HF is greater when large portions of the left ventricle are
infarcted.
Left ventricle infarct = s/s of left-sided HF
Right ventricle infarct = s/s of right-sided HF
Hemodynamic monitoring is often initiated in patients with evidence of
HF.
Cardiogenic shock
Infarct extension
During the first 10-14 days after an MI, patients may experience
extension or reinfarction in the area of the original infarction. This may
cause continuing CP, hemodynamic compromise, and worsening HF.
Structural defects (i.e., necrotic muscle is replaced by scar tissue; regurgitation
can also occur)
Pericarditis
Usually occurs within 2-3 days of the event, causing CP that may be
aching, sharp, and stabbing. Aggravated by movement or deep
breathing. Pericardial friction rub may be heard on auscultation.
Dressler syndrome = hypersensitivity response to necrotic tissue or an
autoimmune disorder; develops days to weeks after an AMI.
Ischemia results when a tissue’s oxygen supply is inadequate to meet its metabolic demands. The ability of
cardiac tissue to satisfy its metabolic demands depends on 2 key factors: coronary perfusion and
myocardial workload.
o Reduced oxygen causes the affected cells to switch from aerobic metabolism to anaerobic
metabolism (which leads to lactic acid buildup in the cells = pain).
o Therapeutic strategies to reduce ischemia-related cardiac injury include the reestablishment of
myocardial perfusion before irreversible damage occurs.
Risk factors
o Male (45 years or older)
o Female (55 years or older)
Once women go through menopause, their risk of CAD is roughly equal to that of men.
Oral contraceptives increase risk; by contrast, estrogen replacement therapy reduces risk.
Risk of CAD and MI is greatest among oral contraceptive users who smoke and are older
than age 35.
o AA, Mexican Americans, American Indians, Alaska Natives, and some Asian Americans.
Low amounts of 25-hydroxyvitamin D are associated with an increased risk of CAD in
White and Chinese populations.
o Genetics (particularly elevated among individuals with a father or brother who was diagnosed by
age 55 or a mother or sister who was diagnosed by age 65)
o Diet
o Socioeconomic factors (i.e., access to healthcare)
o Smoking
o Elevated levels of homocysteine (an amino acid that is a homologue of cysteine)
o Metabolic Syndrome
Emerged as a risk factor for premature CAD equal to smoking.
Patients are said to have this syndrome if they exhibit three or more of these
conditions (which are the direct result of obesity, physical inactivity, and genetics):
Large waistline (40 in. or greater for men; 35 in. or greater for women)
High triglyceride levels (150 mg/dL or greater)
Low HDL levels (less than 40 mg/dL for men and 50 mg/dL for women)
HTN+
Elevated fasting blood glucose (100 mg/dL or greater)
Also elevates a person’s risk of insulin resistance and T2D.
Prevention of CAD focuses on modifiable risk factors, including both lifestyle factors and pathologic
conditions (i.e., HTN+, DM, and hyperlipidemia).
o Diet
An atherogenic diet (i.e., high in saturated and trans fats, cholesterol, and salt; low
in fruits, vegetables, whole grains, and unsaturated fatty acids) promotes CAD.
Nonfat dairy products, fish, and poultry are recommended as primary protein sources.
Soft margarine and vegetable oils should be used instead of butter.
Monosaturated fats (i.e., olive, canola, and peanut oils) lower LDLs and should be
encouraged.
Certain cold water fish (i.e., tuna, salmon, mackerel) = good; high in HDLs.
Soluble fiber (i.e., oats, psyllium, pectin-rich fruit, and beans) and insoluble fiber (i.e.,
whole grains, vegetables, and fruit) = recommended.
Moderate alcohol consumption may provide some health benefits for people with CAD,
particularly middle-age and older adults; consumption should be limited to two
drinks/day (men) and one drink/day (women).
o Exercise
Unless contraindicated, all patients are encouraged to participate in at least 30 minutes of
moderate intensity physical activity 5-6 days each week.
To achieve weight loss and prevent weight gain, experts recommend 60-90 minutes of
moderate intensity exercise daily.
o Obesity
BMI >30 = obesity
People who are obese have higher rates of HTN+, DM, and hyperlipidemia.
Fat distribution also affects the risk for CAD.
Central obesity (intra-abdominal fat). Best indicator = waist circumference. A
waist-to-hip ratio of greater than 0.8 (women) or 0.9 (men) increases the risk for
CAD.
Patients should be informed that high-protein, high-fat weight-loss programs are
not recommended for weight reduction.
o Smoking
o HTN+
Prevalence is higher in AA
HTN+ = consistent SBP greater than 140 mmHg and/or DBP greater than 90 mmHg
o DM
Associated with higher blood lipid levels, a higher incidence of HTN+, and obesity – all
of which are risk factors.
Consistent glucose management = vital
o Hyperlipidemia
LDLs = primary carriers of cholesterol. High LDL levels promote atherosclerosis.
LDLs = less desirable lipoproteins
Optimal = <100 mg/dL
Desirable = 100-129
Borderline high = 130-159
High = 160-189
Very high = > or equal to 190
HDLs = help clear cholesterol from the arteries, transporting it to the liver for excretion.
>60 mg/dL = protective effect
<40 mg/dL (men) and <50 mg/dL (women) = increases risk
Elevated triglyceride levels are another important risk factor.
Desirable = <150 mg/dL
Borderline high = 150-199 mg/dL
High = 200-499 mg/dL
Very high = > or equal to 500 mg/dL
Total cholesterol
Desirable = <200 mg/dL
Borderline high = 200-239 mg/dL
High = > or equal to 240
Collaboration
o Until manifestations of chronic or acute ischemia are experienced, the dx is often presumptive
based on the patient’s hx and presence of risk factors.
o Management of stable angina focuses on maintaining coronary blood flow and cardiac function,
and it may require medical therapy.
o As for CAD, risk factor management is a vital component of care for patients with angina.
o Patients who are experiencing MI require rapid, more aggressive care. Immediate goals include:
Relieving CP
Reducing the extent of myocardial damage
Maintaining cardiovascular stability
Decreasing cardiac workload
Preventing complications
Dx
o Blood lipid profile
Includes measurement of a patient’s total serum cholesterol, as well as HDL, LDL, and
triglyceride levels).
Conducting a blood lipid profile further enables calculation of the patient’s ratio of HDL
to total cholesterol. This ratio should be at least 1:5, with 1:3 being ideal.
Lipoprotein(a) levels may be assessed when patients have a strong family hx of
premature CAD.
o Tests used to identify subclinical (asymptomatic) CAD include:
C-reactive protein (CRP) = elevated
Ankle-brachial blood pressure index (ABI)
Measured via Doppler
ABI of <0.9 in either leg = presence of PAD and a significant risk for CAD
Exercise electrocardiograph (ECG) testing
ST depression by more than 3 mm, if the patient develops CP, or the test is
stopped d/t fatigue, dysrhythmias, etc = positive
Electron bean CT = noninvasive
Myocardial perfusion imaging = costly, not recommended
o Tests used to establish the dx of AMI include:
Creatine kinase (CK)
Normal range (men) = 55-170 units/L
Normal range (women) = 30-135 units/L
Found principally in cardiac and skeletal muscle and the brain.
CK-MB
Normal range = 0-6% of total CK
Troponins
Troponin T (cTnT) normal range = <0.2 ng/mL
Remains elevated for 7-10 days after MI.
Troponin I (cTnI) normal range = 0.1-0.5 ng/mL
Remains elevated for 5-9 days after MI.
These markers are particularly useful when skeletal muscle trauma
contributes to elevated CK levels.
Sensitive enough to detect very small infarcts that do not cause significant
CK elevation.
Myoglobin = one of the first cardiac markers to be detected in the blood after an MI.
CBC
ABG
Electrocardiogram
Classic ECG changes seen in MI = T-wave inversion, ST-segment elevation,
and formation of a Q wave.
Echocardiography (evaluates left ventricular function)
Myocardial nuclear scans
Radionuclide imaging
Hemodynamic monitoring
Conservative management focuses on risk factor modification, including smoking, diet, exercise, and
management of contributing conditions, such as HTN+ and DM.
o RNs should engage in health promotion activities that focus on preventing children,
teenagers, and adults from starting to develop bad habits.
Pharmacologic therapy
o Drugs used to lower cholesterol
Meds to treat hyperlipidemia = expensive, thus, cost-benefit ratio must be considered d/t
the possibility of long-term tx.
Ex: lovastatin (Mevacor), pravastatin (Pravachol), simvastatin (Zocor), fluvastatin
(Lescol), atorvastatin (Lipitor); cholestyramine (Questran), colestipol (Colestid),
colesevelam (Welchol); Nicobid, Nicolar, Niaspan; gemfibrozil (Lopid), fenofibrate
(Tricor), fenofibric acid (Fibricor)
Statins = first-line drugs for treating hyperlipidemia
Safety Alert: In rare cases, statins can cause myopathy, so all patients should be
instructed to report muscle pain and weakness or brown urine. LFTs should also be
monitored during therapy, because statins may increase liver enzyme levels.
o Drugs used to treat angina
Organic nitrates
Ex: NTG
Short-acting and long-acting forms.
Short-acting SL NTG = drug of choice for acute angina.
Rapid-acting NTG is also available as a buccal spray.
Longer-acting NTG preparations (PO, ointment, TD patches) are used
to prevent attacks of angina, not to treat acute attacks.
Tolerance = main problem with long-term tx; can be
limited by a dosing schedule that allows a nitrate-free
period of at least 8-10 hours/day. This is usually scheduled
at night, when angina is less likely to occur.
Beta blockers
Considered first-line drugs for treating stable angina; may be used alone or
with other medications to prevent angina.
Ex: propranolol, metoprolol, nadolol, and atenolol
Safety Alert: Beta-adrenergic blockers are contraindicated in patients with
asthma or severe COPD, because they may cause severe bronchospasm. They
are not used in patients with significant bradycardia or AV conduction blocks,
and they are used cautiously in patients with HF. In addition, beta-adrenergic
blockers should not be used to treat Prinzmetal angina, because they may make
it worse.
Calcium channel blockers
o
Used for long-term prophylaxis because they act too slowly to effective treat
acute attacks of angina.
Not usually prescribed in the initial tx of angina and are used cautiously in
patients with dysrhythmias, HF, or HTN-.
Ex: verapamil, diltiazem, and nifedipine
Patients with angina are also frequently placed on daily aspirin therapy.
80-325 mg/day
Patients who are experiencing AMI may be given a 160-325 mg aspirin
tablet by emergency personnel, with the instruction that the talet is to be
chewed. This initial dose is followed by a daily PO dose of 160-325 mg.
Drugs used to treat MI
Analgesics
Pain relief = vital
Organic nitrates = NTG
Patients may receive up to three 0.4 mg doses of SL NTG at 5minute intervals. IV NTG may then be continued for the first 24-48
hours after AMI to reduce myocardial work.
Morphine sulfate = drug of choice for MI-related pain that is unrelieved by NTG
and for sedation.
Antianxiety agents such as lorazepam (Ativan) may also be administered to
promote rest.
Safety Alert: Ask male patients about use of sildenafil (Viagra) within the prior
24 hours, because combining sildenafil and NTG can precipitate a significant
drop in BP.
Thrombolytics
First-line drugs used to treat AMI when access to a cath lab is not
immediately available.
Administration within 6 hours of MI = best
Not everyone is a candidate for therapy (i.e., bleeding disorders, hx, etc)
Ex: streptokinase (least expensive; IV), anisoylated plasminofen
streptokinase activator complex (APSAC; given via bolus every 2-5 minutes;
expensive), tenecteplase and reteplase (most expensive)
Antidysrythmics
Ventricular dysrhythmias are treated with a class I or class II antidysrythmic.
Symptomatic bradycardia is treated with IV atropine, 0.5-1 mg.
IV metoprolol, amirodorone, or diltiazem may be ordered to treat a-fib or other
SVTs.
Beta blockers
ACE inhibitors
Although it is not known whether ACE inhibitors prevent ischemic events, they
have been demonstrated to decrease the risk of stroke or MI. for this reason, they
are sometimes also prescribed for patients at high risk of CAD and/or MI,
including those with DM or other risk factors.
Anticoagulant and antiplatelet medications
Standard or low-molecular-weight heparin (LMWH) preparations are
often given to patients with AMI.
Ex: aspirin, clopidogrel (Plavix), abciximab (ReoPro), eptifibatide
(Integrilin), tirofiban (Aggrastat)
Other medications
Patients with pump failure and HTN- may receive IV dopamine, a vasopressor,
at low doses (<5 mg/kg/min).
Stool softeners (i.e., docusate {Colace}) prevents straining.
Non-pharmacologic therapy
o Bedrest is prescribed for the first 12 hours after MI. Sitting in a chair is permitted after 12 hours of
being stable. Activity increased as tolerated.
o IV therapy
o Quiet, calm environment. Visitors limited to promote rest.
o Oxygen therapy via NC at 2-5 L
o Liquid diet (may be prescribed for the first 4-12 hours following MI); after that, a low-fat, lowcholesterol, reduced-sodium diet is allowed.
Sodium restrictions may be lifted after 2-3 days if no evidence of HF is present.
Small, frequent feedings are often recommended.
o Drinks containing caffeine, as well as very hot and cold foods, may also be limited.
Revascularization procedures
o Factors that influence the choice of revascularization strategy may include any of the following:
DM, CKD, systolic dysfunction, previous hx of CABG, and type of MI.
o Percutaneous coronary revascularization (PCR)
Similar to procedure used for coronary angiography.
Local anesthesia, short hospital stay (1-2 days).
Percutaneous transluminal coronary angioplasty (PTCA) is typically accompanied by a
stent. Antiplate medications (aspirin and ticlopidine) are given following stent
insertion to reduce the risk of thrombus formation at the site.
Atherectomy procedures remove plaque from identified lesions. Involves three
approaches: directional, rotational, and laser.
Complications following PCR vary and include hematoma, pseudoaneurysm, embolism,
hypersensitivity to the contrast dye, dysrhythmias, bleeding, vessel perforation, and
restenosis or reocclusion of the treated vessel.
o CABG
The internal mammary artery (IMA) in the chest and the saphenous vein in the leg are the
vessels most commonly used.
The IMA is often used to revascularize the left coronary artery because of the
greater oxygen demand on the left ventricle.
Safe and effective. However, angina pain may recur after sx.
Variable option for blockages that cannot be treated with angioplasty.
Many people remain symptom-free for as long as 10-15 years.
Utilizes a cardiopulmonary bypass (CPB) pump – enables surgeons to operate on a quiet
heart and a relatively bloodless field.
Once grafting is completed, CPB is discontinued.
Newer techniques have been developed that allow surgeons to perform CABG
without cardioplegia (stopping the heart) and a use of CPB. Ex: off-pump
coronary artery bypass (OPCAB) – lower morbidity rates as well as faster
recovery rates have been demonstrated for patients undergoing this procedure
compared to CABG.
o Minimally invasive coronary artery sx
Ex: port-access coronary artery bypass (utilizes “ports”); minimally invasive direct
coronary artery bypass (MIDCAB) – CPB is avoided altogether.
o Transmyocardial laser revascularization
o Intra-aortic balloon pump (IABP)
Uses a 30-40 mL balloon that is introduced into the aorta, usually via the femoral artery.
The inflation-deflation sequence is triggered by the ECG pattern. 1:1 ratio.
As the patient’s condition improves, the IABP is weaned to inflate-deflate at varying
intervals (e.g., 1:2, 1:4, 1:8). When mechanical assistance is no longer needed, the
catheter is removed.
o Ventricular assist device (VAD)
Temporarily takes partial or complete control of cardiac function, depending on the type
of device used.
May be used for patients with AMI and/or cardiogenic shock when there is a chance for
recovery of normal heart function after a period of cardiac rest.
May also be used as a bridge to heart transplatation.
Nursing caring for the patient with a VAD is supportive and includes assessing
hemodynamic status and monitoring for complications associated with the device.
Cardiac rehabilitation is a medically supervise program designed to aid people with recovery from MI,
heart attacks, heart surgeries, and percutaneous coronary interventions.
Complementary health approaches
o Diet and exercise programs that emphasize physical conditioning and a low-fat diet rick in
antioxidants have been shown to be effective in managing CAD.
o Supplements of vitamins C, E, B6 and B12, as well as folic acid, may also be beneficial.
o Other potentially helpful complementary health approaches include consumption of red wine or
grape juice, foods containing bioflavonoids, green tea, nuts, and herbal supplements and garlic
(effective only for HTN+).
The RN should emphasize the need for patients to talk to their HCPs because interactions
with prescribed drugs are common.
o Behavioral therapies include relaxation and stress management, guided imagery, treatment of
depression, anger and hostility management, meditation, tai chi, and yoga.
o The Pritkin diet is basically vegetarian, high in complex carbohydrates and fiber, low in
cholesterol, and extremely low in fat (less than 10% of daily calories).
Egg whites and limited amounts of nonfat dairy or soy products are allowed.
Requires 45 minutes of walking daily and recommends multivitamin supplements,
including vitamins C and E and folate.
o The Ornish diet is also vegetarian, although egg whites and a cup of nonfat milk or yogurt per
day are allowed.
No oil or fat is permitted, even for cooking.
Two ounces of alcohol are allowed each day.
Program also calls for stress reduction, emotion-social support systems, daily stretching,
and walking for 1 hour three times a week.
Lifespan considerations
o Women and older adults often present with manifestations of MI different from those of younger
and middle-age men. Early recognition and aggressive treatment = vital.
o Women
More likely to have a “silent” or unrecognized MI or to present in cardiac arrest or
with cardiogenic shock = atypical CP
Many women have epigastric pain, indigestion, N/V, causing them to blame their
discomfort on heartburn of gastroenteritis.
SOB is common, as well are fatigue and weakness of the shoulders and upper arms.
Many women do not realize the true nature of their condition and delay seeking care as a
result. Also, many women ignore CP as they have historically been the caregivers and not
the recipients of care.
It is important for HCPs to stress the importance of quickly seeking medical help for
atypical manifestations of MI. prompt diagnosis and intervention reduce the mortality and
morbidity of MI in women.
o Older adults
Atypical s/s, such as vague complaints of dyspnea, confusion, fainting, dizziness,
abdominal pain, or cough.
Older adults frequently attribute their symptoms to a stoke. The prevalence of silent
ischemia is also greater in older adults.
Many older adults neither seek nor receive prompt treatment, putting them at greater risk
for widespread cardiac damage, complications, and death.
Both patient education about atypical manifestations of MI and prompt diagnosis and
intervention are critical to reducing mortality and morbidity in older adults.
Nursing process
o Nursing care for the patient with known or suspected CAD varies depending on the exact
nature of the patient’s condition.
o Education = critical element.
o Assessment
Obvious signs of distress; signs that may indicate that the patient is experiencing CP or a
loss of perfusion; assess the patient’s current diet, exercise patterns, and medications;
smoking hx and pattern of alcohol intake; hx of heart disease, HTN+, or DM; and family
hx of CAD or other cardiac problems.
VS and heart sounds; strength and equality of peripheral pulses; and skin color and
temperature; current weight; BMI; waist-to-hip ratio; skin color; temperature and
moisture; LOC; cardiac rhythm, bowel sounds and abdominal tenderness.
o Problem statement
Ineffective Health Maintenance
Obesity
Readiness for Enhanced knowledge
Risk for Activity Intolerance
Risk for Impaired Cardiovascular Function
Sedentary Lifestyle
Acute Pain
Anxiety
Risk for Decreased Cardiac Perfusion
Fear
Deficient Knowledge
Ineffective Coping
o Planning
Planning for patients with known or suspected CAD varies depending on individual s/s
and diagnoses.
Planning for patients with symptomatic CAD focuses not only on education, but also on
symptom reduction and/or control.
o Implementation
The focus of nursing care for patients with CAD, angina, and/or MI is on improving
CO, reducing cardiac workload, maximizing function, and teaching the patient how
to care for her-or himself at home while reducing the risk of further cardiac
damage.
Learning of a dx that affects the heart is very frightening for most patients, and they
often require assistance in coping with fear and anxiety.
Promote balanced nutrition
Encourage assessment of food intake and eating patterns to help identify areas
that can be improved.
Discuss dietary recommendations, emphasizing the role of diet in heart disease.
Refer the patient to a clinical dietitian for diet planning and further teaching.
Encourage gradual but progressive dietary changes.
Discourage the use of high-fat, low-carbohydrate, or other fad diets for weight
loss.
Encourage reasonable goals for weight loss and provide information about
weight loss programs and support groups.
Promote effective health maintenance
Discuss risk factors for CAD.
Discuss the immediate benefits of smoking cessation.
Help the patient identify specific sources of psychosocial and physical support
for smoking cessation, dietary modification, and lifestyle change.
Discuss the benefits or regular exercise for CV health and weight loss.
Provide information and teaching about prescribed medications.
Manage acute pain
CP occurs when the oxygen supply to the heart muscle does not meet the
demands.
Pain relief is a priority of care for the patient with AMI.
Assess the patient for verbal/non-verbal signs of pain.
If the patient is hypoxic, administer oxygen at 2-5 L via NC.
Promote physical and psychologic rest, and provide information and emotional
support.
Titrate IV NTG as ordered to relieve CP, maintaining a SBP of greater than 100
mmHg.
Administer 2-4 mg of morphine by IV push for CP as needed.
Monitor tissue perfusion
Assess and document VS.
Assess the patient for changes in LOC. A change in LOC is often the first
manifestation of altered perfusion, because cerebral function depends on a
continuous supply of oxygen.
Auscultate heart and breath sounds.
Monitor the patient’s ECG rhythm continuously.
Monitor the patient’s oxygen saturation levels, and administer as ordered. Also
obtain and assess ABG levels as indicated.
Administer antidysrhythmic medications as needed.
Obtain serial CK, isoenzyme, and troponin levels as ordered.
Plan for invasive hemodynamic monitoring.
Promote effective coping
Denial = common; can eventually interfere with learning and tx adherence.
Establish an environment of caring and trust.
Accept denial as a coping mechanisms, but do not reinforce it.
Note aggressive behaviors, hostility, or anger.
Help the patient to identify positive coping skills used in the past. Reinforce use
of these positive behaviors.
Provide opportunities, as possible, for patients to make decisions about their
plan of care.
Provide privacy for the patient and family members to share their questions and
concerns.
Manage fear
Identify the patient’s level of fear, noting verbal and non-verbal signs.
Acknowledge the patient’s perception of the situation, and allow the patient to
verbalize concerns.
Encourage questions, and provide consistent, factual answers.
Encourage self-care.
Administer anti anxiety medications as ordered.
Teach non-pharmacologic methods of stress reduction.
Promote effective cardiac perfusion
Instruct the patient to keep prescribed NTG tablets always on hand so one can be
taken at the onset of pain.
o
Teach the patient about prescribed medications to maintain myocardial
perfusion and reduce cardiac work. Long-acting organic nitrates, beta
blockers, and CCBs are used to prevent angina attacks, not to treat acute
attacks.
Instruct the patient to take SL NTG before engaging in activities that precipitate
angina.
Encourage the patient to implement and maintain a progressive exercise
program under the supervision of his/her PCP.
Refer the patient to a smoking cessation program.
Space activities to allow rest between them.
Promote adherence to therapeutic regimen
Assess the patient’s knowledge and understanding of CAD and angina.
Teach about angina and atherosclerosis as needed, building on the patient’s
current knowledge base.
Provide written and verbal instructions about prescribed medications.
Stress the importance of taking CP seriously while maintaining a positive
attitude.
Refer the patient to a cardiac rehab program or other organized activities and
support groups for patients with CAD (refer to ‘Patient Teaching on Cardiac
Rehabilitation and Home Care’ on p. 1209).
Evaluation = hemostasis
DVT
DVT typically occurs in the large veins of the lower leg and thigh, and is a result of venous thrombosis
deep in the muscle tissue.
o The deep veins of the legs, primarily in the calves – and of the pelvis provide the most hospitable
environment for venous thrombosis.
It may travel to the lungs; when this occurs, patients develop a life-threatening PE. Because these two
conditions often occur together, they are collectively referred to as a venous thromboembolism (VTE).
Three pathological factors, called Virchow’s triad, are associated with formation of a thrombus:
o Circulatory stasis
o Vascular damage
o Hypercoagulability
Vascular damage stimulates the clotting cascade and the thrombus grows in the direction of blood flow.
This triggers the inflammatory response, causing tenderness, swelling, and erythema in the area of the
thrombus.
Approximately one half of DVTs are asymptomatic. If symptoms are present, they depend on the clot’s
location and size.
Safety Alert: When a thrombus damages the vein or its valves, postthrombotic syndrome may develop.
This condition occurs when damaged valves allow blood to back flow and pool and may result in pain,
edema, discoloration of the skin, and skin lesions. Postthrombotic syndrome may occur at any time
following DVT.
Can be venous or arterial.
o Arterial thrombi tend to occur at sites of arterial plaque rupture.
o Venous thrombi tend to occur at sites where the vein is normal but blood flow is low.
DVT is a common complication of hospitalization, sx, and immobility. Other factors associated include
venous injury, cancer, pregnancy, oral contraceptives or HRT, clotting disorders, obesity, and a personal or
family hx or DVT (refer to p. 1213, box 16-11).
Risk factors
o
Orthopedic procedures (i.e., total hip replacement, traumatic hip fracture repair, total knee
replacement)
o A-fib can cause stroke. The risk of DVT is increased during the first 6 months following dx of afib.
o AMI – older patients with HF, recurrent angina, or ventricular dysrhythmias are most at risk.
o Ischemic stroke
Prevention
o In many cases, prophylactic anticoagulant therapy is prescribed to lower the risk of DVT.
o Elevating the foot of the bed with the knees slightly flexed promotes venous return.
o Early ambulation = key
o Leg exercises such as ankle flexion and extension assist venous flow by muscle compression.
Clinical manifestations
o Often asymptomatic.
o Dull, aching pain in affected extremity, especially when walking.
o Possible tenderness, warmth, and erythema along affected vein.
o Edema of affected extremity.
o Cyanosis of affected extremity.
Patient hx, physical examination, and dx tests are used to establish the dx. Tx focuses on preventing further
clotting or extension of the clot and addressing underlying causes.
Dx
o Laboratory studies that may be ordered include D-dimer, PT (measured as an INR), PTT,
aPTT, bleeding time, and platelet count.
o Duplex venous US
o Plethysmography
o MRI
o Ascending contrast venography
Pharmacologic therapy
o Anticoagulants are the mainstay of tx for venous thrombosis (ex: streptokinase or tissue
plasminogen activator {tPA}).
Heparin and Warfarin
For most patients, anticoagluation is initiated with unfractionated heparin,
although LMWHs may also be used.
Following an initial IV bolus of unfractionated heparin, additional units are
infused over a 24-hour period. The dosage is calculated to maintain the aPTT
at approximately twice the control or normal value. Frequent monitoring of
the infusion is an important nursing responsibility.
Oral anti coagulation with warfarin may be initiated concurrently with heparin
therapy. Overlapping heparin and warfarin therapy for 4-5 days is
important because the full anticoagulant effect of warfarin is delayed.
Warfarin doses are adjusted to maintain an INR >2.0. once this level has
been achieved, the heparin is discontinued, and maintenance dose of
warfarin is prescribed to prevent recurrent thrombosis. Anticoagulation
generally is continued for at least 3 months.
Heparin is the drug of choice for initiating anticoagulant therapy and is
derived from pork. Be culturally competent and sensitive (i.e., Muslims).
LMWH
More effective and carry lower risks for bleeding and thrombocytopenia than
conventional unfractionated heparins.
They do not require the close laboratory monitoring of unfractionated heparins.
Administered SQ in fixed doses one or BID, which makes them appropriate for
both inpatient and outpatient tx.
o
Sx
o
Safety Alert: Some foods and supplements can increase the risk of a bleeding
episode for patients on anticoagulant therapy. Patients should avoid ginger,
garlic, green tea, and ginkgo while taking heparin or warfarin.
Direct thrombin inhibitors
The FDA limits use of these drugs to a few specific situations.
Factor Xa inhibitors
Newer category of anticoagulants.
Work by disrupting the coagulation cascade by directly impairing the function
of Factor Xa.
As effective as warfarin yet offers several advantages over older anticoagulants.
Administered PO
Present a lower risk of interaction with food or other drugs
Do not necessitate frequent INR monitoring
Rapid discontinuation without substitution of another anticoagulant may lead to
serious ischemic events. Also, these drugs should not be used in patients who
are undergoing neuralgia anesthesia or spin puncture, because they increase the
risk of long-term paralysis d/t epidural or spinal hematoma.
NSAIDs (ex: indomethacin {Indocin} or naproxen {Naprosyn}) = reduce inflammation.
DVT is typically treated with conservative measures and anticoagulation. In some cases, sx may
be required.
o Venous thrombectomy is done when thrombi lodge in the femoral vein and their removal is
necessary to prevent PE or gangrene.
o When DVT is recurrent and anticoagulant therapy is contraindicated, a filter may be inserted into
the vena cava to capture emboli from the pelvis and lower extremities, preventing PE.
The Greenfield filter is widely used for its ability to trap emboli within its apex while
maintaining patency of the vena cava. Mortality and morbidity = low.
o Superficial thrombophlebitis of the great saphenous vein can progress to DVT and may be treated
by ligating and dividing the vein where it joins the femoral vein to prevent clot extension into the
deep venous system. Infection can lead to sepsis.
Non-pharmacologic therapy
o With superficial venous thrombosis, applying warm, moist compresses over the affected
vein, resting the extremity, and using anti-inflammatory agents typically provide relief of
symptoms.
o Bedrest
o Legs elevated 15-20 degrees, with the knees slightly flexed above the level of the heart to
promote venous return and discourage venous pooling.
o When permitted, walking = encouraged.
o Avoid crossing legs, prolonged standing/sitting, and wearing tight-fitting garments or
stockings that bind.
o Safety Alert: Elastic antiembolism stockings or pneumatic compression devices are
contraindicated in patients with known DVT, but they are frequently ordered by physicians for use
in the prevention of DVTs. These devices stimulate the muscle-pumping mechanism that promotes
the return of blood to the heart; therefore, they may dislodge a thrombus and cause PE.
Lifespan considerations
o Infants and children
Rare
Asymptomatic and nonspecific s/s: acute pain and swelling of the extremities.
Initial tx typically includes unfractionated or LMWH; over time, the patient is
transitioned to oral warfarin.
o Adolescents and young adults
8x greater risk than infants and children.
Twice as likely to occur in female patients (d/t use of contraceptives containing estrogen
and progestin, often called COCs = birth control pills = increases risk of bleeding).
s/s include swelling, pain, warmth, and tenderness of the extremity.
Young women may experience heavy menstrual bleeding when this occurs, patients
should continue with therapy and receive consoling about managing menstrual flow.
Sexually-active women taking warfarin should use birth control because of the
teratogenic effects of this drug, but it is important that these patients avoid combined
hormonal contraceptives.
o Pregnant women
Risk of DVT and PE are 4-5x higher than non-pregnant women.
Inherited clotting disorders and pregnancy-related changes to the body further increase
the risk.
Venous stasis is increased, especially in the lower extremities, and mobility is decreased.
Risk of developing DVT is greatest before 20 weeks of gestation, peaking at 11-15
weeks. This risk is greater during the PP period than during pregnancy.
More likely to occur in the left leg (believed to be d/t compression of the left iliac vein
by the right iliac artery. In addition, 12% of cases occur in the pelvic veins).
s/s during pregnancy are similar to those of pregnancy in general and include pain
and swelling of the legs, dyspnea, tachycardia, and tachypnea.
Dx relies heavily on the D-dimer test and US. When pelvic DVT is suspected, MRI
may also be used.
Heparin = preferred anticoagulant because it does not cross the placenta. Warfarin
= teratogenic and crosses the placenta.
Patients should be monitored for progressive VTE and heparin allergies for the
duration of therapy.
In patients with planned deliveries, therapy may be discontinued or altered several days
before delivery. Therapy is typically restarted within several hours after delivery. Patients
may also be transitioned from heparin to warfarin during the PP period.
Both heparin and warfarin = safe for BF/lactating mothers.
o Older adults
Age = risk factor and increases 30-fold between age 30-80. Associated with the
development of other risk factors, including venous stasis and conditions that limit
mobility.
Fatality rates = higher
Cancer and cancer therapy are also important risk factors among older adults.
Use of estrogen-containing drugs increases risk in older women.
Asymptomatic, nonspecific s/s. A patient’s known comorbidities may have
symptoms in common with DVT, complicating dx.
Anticoagulant therapy is commonly used to treat older adults with DVT, but they are at
higher risk of bleeding complications.
Multiple comorbidities, decreased kidney function, decreased body weight,
dementia, and increased risk of falls complicate the use of anticoagulant therapy.
Drug monitoring may occur more frequently.
Nursing process
o Assessment
Note c/o leg or calf pain, duration and characteristics of pain, and the effect of pain
on the patient’s ability to walk. PQRST pain assessment!**
Measure diameter or affected extremity.
o Problem statement
Acute Pain
Ineffective Protection
Impaired Physical Mobility
o
o
o
Risk for Ineffective Peripheral Tissue Perfusion
Planning
Pain control
Rest/comfort
No complications
Adequate tissue perfusion
Implementation
Manage pain
Regularly assess pain.
Measure calf and thigh diameter of the affected extremity on admission and
daily thereafter.
Apply warm, moist heat to the affected extremity at least QID using
compresses or an aqua-K pad.
Bedrest, as ordered.
Promote effective peripheral perfusion
Assess the skin of the affected lower leg and foot at least every 8 hours or more
often as indicated.
Elevate the patient’s extremities at all times.
Use mild soaps, solutions, and lotions to clean the affected leg and foot daily.
Use an egg-crate mattress or sheepskin on the bed as needed.
Encourage frequent position changes at least every 2 hours.
Reduce risk for injury
Monitor labs and report values outside normal limits.
Encourage mobility
Encourage active ROM at least every 8 hours and provide passive ROM as
needed.
Encourage frequent position changes, deep breathing and coughing.
Encourage increased fluid and dietary fiber intake.
Assist the patient with and encourage ambulation as allowed.
Encourage diversional activities.
Promote effective cardiopulmonary perfusion
Frequently assess the patient’s respiratory status.
Initiate oxygen therapy, elevate the HOB, and reassure the patient who is
experiencing manifestations of PE.
Safety Alert: Sudden increases in HR, stabbing CP, SOB, and bloody cough may
indicate a DVT has moved through the bloodstream to the lungs, causing a PE. Patients
with a PE may require emergency care.
Evaluation
Patient is able to identify warning signs of DVT and vocalizes risks associated with DVT.
Patient maintains anticoagulant therapy without complications.
Patient collaborates with RN and MD to identify DVT recurrence strategies.
Patient is free of long-term complications.
HF
HF is a condition in which the heart is unable to pump enough blood into circulation to meet the body’s
needs.
o Often caused by a combination of ineffective contraction and relaxation = decreased CO =
decreased perfusion
o Compensatory mechanisms result in vascular congestion, hence the term, congestive heart failure
(CHF).
o
A progressive condition that is frequently a long-term effect of CAD and MI when left ventricular
damage is extensive enough to impair CO.
o May also be the result of a primary cardiac muscle disorder (i.e., cardiomyopathy or myocarditis).
Structural disorders, inflammatory disorders, and HTN+ may also lead to HF.
o Patients with no hx of abnormal myocardial function may present with manifestations of HF
as a result of acute excessive demands placed on the heart by conditions such as FVO,
hyperthyroidism, and massive pulmonary embolus.
Pulmonary edema is a common consequence of HF.
o Pulmonary edema is a sign of severe cardiac decomposition (failure of compensatory mechanisms
to restore tissue perfusion).
o Medical emergency! Immediate tx = necessary!
o Onset = acute or gradual, progressing to severe respiratory distress.
CO= HR x SV
o CO = the performance of cardiac muscle; measured by the amount of blood pumped from
the ventricles in 1 minute.
o SV = the volume of blood ejected with each heartbeat.
Determined by preload and afterload
Preload = pressure
Afterload = resistance
o In addition to CO and SV, ejection fraction (EF) is another important measurement of the heart’s
effectiveness.
Normal range = >60%
Pearson normal range = 50-70%
o When the HR begins to fail, CO, SV, and EF all decrease.
Primary compensatory mechanisms include the following: (1) the Frank-Starling mechanism; (2)
neuroendocrine responses, including activation of the SNS and the renin-angiotensin system; and (3)
myocardial hypertrophy.
o Frank-Starling mechanism: the greater the stretch of cardiac muscle fibers, the greater the force
of contraction.
Increased contractile force = increased CO
Stimulation of stretch receptors in the atria and ventricles leads to the release of
ANP and BNP from stores in the atria (ANP, BNP) and ventricles (BNP). Although
beneficial, these hormones are too weak to completely counteract the vasoconstriction
and the sodium and water retention that occurs in HF.
BNP normal range: 100-300. >300 = HF.
o Neuroendocrine response: decreased CO stimulates the SNS and catecholamine release;
decreased CO and decreased renal perfusion stimulate the renin-angiotensin system.
Increased HR, BP, and contractility; increased vascular resistance and venous return.
The renin-angiotensin system produces additional vasoconstriction and stimulates
the adrenal cortex to produce aldosterone and the posterior pituitary to release
ADH. The effects of these hormones is significant vasoconstriction as well as salt and
water retention, with a resulting increase in vascular volume. Increased ventricular filling
increases the force of contraction, improving CO.
o Vascular hypertrophy: increased cardiac workload causes myocardial muscle to hypertrophy and
ventricles to dilate.
Increased contractile force to maintain CO.
Ventricular remodeling occurs as the heart chambers and myocardium adapt to fluid
volume and pressures increases. This additional stretch initially causes more effective
contractions. Ventricular hypertrophy occurs as existing cardiac muscle cells
enlarge, increase their contractile elements (actin and myosin) and force of
contraction.
o
Although these responses may help I the short-term regulation of CO, it is now recognized that
they also hasten the deterioration of cardiac function.
A rapid HR shortens diastolic filling time, comprises coronary artery perfusion, and
increases myocardial oxygen demand.
Chronic distention eventually causes the ventricular wall to thin and degenerate. It also
exhausts stores of ANP and BNP.
In normal hearts, the cardiac reserve allows the heart to adjust its output to meet the metabolic needs of
the body, increasing the CO by up to 5x the basal level during exercise.
o Patients with HF have minimal to no cardiac reserve.
o At rest, they may be unaffected; however, any stressor (e.g., exercise, illness) taxes their ability to
meet the demand for oxygen and uterine to.
o Manifestations of activity intolerance when the individual is at rest indicate a critical level of
cardiac decompensation.
Classifications
o Systolic vs. diastole failure
Systolic failure occurs when the ventricle fails to contract adequately to eject a sufficient
volume of blood into the arterial system. It is affected by loss of myocardial cells d/t
ischemia and infarction, cardiomyopathy, or inflammation.
Diastole failure occurs when the heart cannot completely relax in diastole, disrupting
normal filling. It results from decreased ventricular compliance caused by hypertrophic
and cellular changes and impaired relaxation of the heart muscle.
o Left-sided vs. right-sided failure
In chronic HF, both ventricles are typically impaired to some degree.
CAD and HTN+ are common causes of left-sided HF, whereas right-sided HF often is
caused by conditions that restrict blood flow to the lungs, such as acute/chronic
pulmonary disease.
Left-sided HF also can lead to right-sided HF.
As left ventricular function fails, CO falls.
Increased pressures impair filling, causing congestion and increased pressures in the
pulmonary vascular system. The manifestations of left-sided HF result from
pulmonary congestion (backward effects) and decreased CO (forward effects).
In right-sided HF, increased pressures in the pulmonary vascular urge or right
ventricular muscle damage impair the right ventricle’s ability to pump blood into
pulmonary circulation. Increased venous pressures cause abdominal organs to become
congested and peripheral tissue edema to develop. Dependent tissues tend to be
affected d/t gravity.
o Low-output vs. high-output failure
Patients with HF resulting from CAD, HTN+, cardiomyopathy, and other primary cardiac
disorders develop low-output failure and manifestations as those previously described.
Patients in hyper metabolic states (e.g., hyperthyroidism, infection, anemia, pregnancy)
require increased CO to maintain blood flow and oxygen to the tissues. Even though Co
is high, the heart is unable to meet increased oxygen demands. This condition is known
as high-output failure.
o Acute vs. chronic failure
Acute = abrupt onset, resulting in suddenly decreased cardiac function and signs of
decreased CO.
Chronic = progressive deterioration as a result of cardiomyopathy, valvular disease, or
CAD.
o Pulmonary edema
Contractility of the left ventricle = severely impaired
Pulmonary hydrostatic pressures rise, ultimately exceeding the osmotic pressure of the
blood. As a result, fluid leading from the pulmonary capillaries contests interstitial spaces
in the tissues, decreasing lung compliance and interfering with gas exchange. As
pressures continue to increase, fluid enters the alveoli.
The prognosis for a patient with HF depends on the underlying cause of the HF and how effectively the
precipitating factors can be treated. 5-year survival rate = roughly 50%; 10-year survival rate = 10-26%.
No cure for HF and symptoms will worsen over time. Patients may experience multiple hospital stays and
be at increased risk for Sickle Cell Disease (SCD).
Risk factors and prevention
o Key risk factors for HF include CAD, smoking, obesity, substance abuse, HTN+, and DM.
Other causes include cardiomyopathy, heart valve disease, dysrhythmias, and
congenital heart defects.
Patients who have had heart attacks = increased risk
Patients with severe lung disease have increased oxygenation demand placed on the
heart.
Sleep apnea is also an important risk factor for developing HTN+ and has been linked to
HF, DM, and stroke.
o Prevention for HF involves controlling risk factors as much as possible and following a tx
regimen.
Patients without heart damage or disease can concentrate on avoiding risky behaviors
such as illicit drug use and smoking and should engage in health-promoting behaviors
such as eating a heart-healthy diet, maintaining a healthy weight, staying physically
active, and reducing stress.
Patients at high-risk or have heart damage may also benefit from these prevention
measures, but they should talk to their HCP about appropriate physical activities and
specific plans.
It is also essential for these patients to take all prescribed medications.
Clinical manifestations (refer to p. 1232, box 16-14)
o The manifestations of systolic failure are those of decreased CO: weakness, fatigue, and
decreased exercise tolerance.
o The manifestations of diastolic failure include SOB, tachypnea, and crackles if the left
ventricle is affected; they include distended neck veins, liver enlargement, anorexia, and
nausea if the right ventricle is affected.
o Left-sided failure
Fatigue and activity intolerance = common early manifestations
Dizziness, syncope
Dyspnea, SOB, cough
Orthopnea (difficultly breathing when supine, prompting the use of 2-3 pillows or a
recline for sleeping)
Cyanosis, dusky color (blue/gray)
Crackles, rales, wheezes in lung bases
S3 gallop = ventricular gallop
o Right-sided failure
Edema in the feet and legs; sacrum is patient is bedridden.
Anorexia
Nausea
RUQ pain from liver engorgement
Distended neck veins
o Other manifestations
Weight gain
Edema
Nocturia
Paroxysmal nocturnal dyspnea (a frightening condition in which the patient awakens at
night acute short of breath.
Severe HF may cause dyspnea at rest as well as with activity, signifying little or no
cardiac reserve.
S4 gallop = atrial gallop
Complications
o Increased abdominal pressure, ascites, and GI problems.
o With prolonged right-sided HF, liver function may be impaired.
o Myocardial distention can precipitate dysrhythmias, further impairing CO.
o Pleural effusions and other pulmonary problems may develop.
Manifestations of pulmonary edema
Respiratory
Tachypnea
Paroxysmal nocturnal dyspnea
Labored respirations
Cough productive of frothy, pink sputum
Dyspnea
Crackles, wheezes
orthopnea
CV
Tachycardia
Cool, clammy skin
HTN_
Hypoxemia
Cyanosis
Ventricular gallop
Neurologic
Restlessness
Feeling of impending doom = panic
Anxiety
o Safety Alert: Pulmonary edema is a medical emergency. Without rapid and effective intervention,
severe tissue hypoxia and acidosis will lead to organ system failure and death.
The main goals of the patient with HF are to slow its progression, reduce cardiac workload, improve
cardiac function, and control fluid retention. Tx strategies are based on the evolution and
progression of HF.
Dx
o Atrial natriuretic peptide (ANP), also called atrial natriuretic hormone, and brain natriuretic
peptide (BNP) = increase.
BNP levels may be elevated in women and patients over age 60 who do not have a dx of
HF. Therefore, they should not be considered as the primary dx tool.
o Serum electrolytes: osmolarity = low; sodium, potassium, and chloride levels = baseline for
evaluating effects of tx.
o UA, BUN, creatinine
o Thyroid function test
o ABG
o CXR
o Electrocardiography
o Echocardiography with Doppler flow studies
Hemodynamic monitoring
o Hemodynamics is the study of forces involved in blood circulation.
Used to assess CV function in the patient who is critically ill or unstable.
Goals = evaluate cardiac and circulatory function and the response to interventions.
Measurements include HR, arterial BP, central venous or right atrial pressure, pulmonary
pressures, and CO.
Valuable, but carries risks. Potential complications include pneumothorax, hemothorax,
bleeding, hematoma, arterial puncture, dysrhythmias, venospasm, infection, air
embolism, thromboembolism, brachial nerve injury, and thoracic nerve injury.
o Intra-arterial pressure monitoring
An indwelling arterial line allows for direct and continuous monitoring of systolic,
diastolic, and MAPs and provides easy access for arterial blood sampling.
Mean arterial pressure (MAP) is the average pressure in the arterial circulation
throughout the cardiac cycle. Reflects the perfusion pressure, an indicator of tissue
perfusion.
MAP = CO x SVR or DBP + PP/3**
MAP normal range = 70-90 mmHg (desirable)
Perfusion to vital organs is severely jeopardized at MAPs <50 mmHg;
MAPs >105 mmHg may indicate HTN+ or vasoconstriction.
Pharmacologic therapy (refer to Medications chart on pgs. 1237-1239)
o ACE inhibitors
Prevent acute coronary events and reduce mortality in HF.
Reduce afterload and improve CO and renal blood flow.
Ex: lisinopril (Prinivil, Zestril), captopril (Capoten), enalapril (Vasotec)
o ARBs
Prevent acute coronary events and reduce mortality in HF.
Block the action of angiotensin II at the receptor rate than interfering with its
production.
Ex: candosartan (Atacand), valsartan (Diovan)
o Beta blockers
Slow HR, reducing BP.
Ex: carvedilol (Coreg), metoprolol (Toprol-XL)
The combination of ACE inhibitors and beta blockers improves patient outcomes**
o Diuretics
Promote excretion of sodium and water.
With the exception of the potassium-sparing diuretics (spironolactone, triamterene,
and amiloride), diuretics also promote potassium excretion, increasing the risk for
hypokalemia**
Ex: spironolactone (Aldactone), furosemide (Lasix), bumetanide (Bumex), HCTZ
(HydroDiuril)
o Vasodilators
Relax smooth muscle in blood vessels, causing dilation.
Nitrates produce both arterial and venous vasodilation. May be given by nasal
spray, SL, PO, or IV.
Sodium nitroprusside = potent; used to treat acute HF. Causes excessive HTN-, so
usually is given along with dopamine or dobutamine to maintain BP.
A new drug for tx of HF in AA = BiDil (a combination of two vasodilators, hydralazine
and isosorbide) in fixed doses.
o Cardiac (Digitalis) glycosides
Digitalis improves myocardial contractility by interfering with ATP. This increased
force of contraction causes the heart to empty more completely, increasing SV and
CO = decreased preload and afterload (reducing cardiac work) = decreasing HR
and oxygen consumption.
Has a positive inotropic effect on the heart = increases strength of myocardial
contraction.
Narrow therapeutic index, meaning therapeutic levels are very close to toxic levels.
Early manifestations of toxicity include anorexia, N/V, headache, altered
vision, and confusion.
A number of cardiac dysrhythmias are associated with toxicity, including
sinus arrest, SVTs and VTs, and high levels of AV block.
Low serum potassium levels increase the risk for toxicity, as do low
magnesium and high calcium levels.
Older adults = increased risk
o Antidysrhythmics
PVCs = frequent, but not associated with an increased risk of VT and fibrillation.
Usually left untreated.
Many depress left ventricular function.
Amiodarone = drug of choice to treat nonsustained VT, which is associated with a
poor prognosis.
o Safety Alert: NSAIDs can interfere with the effectiveness of HF medications. RNs should teach
patients to avoid NSAIDs whenever possible and to check for NSAIDs in any OTC medications
they may use.
Nutrition and activity
o Sodium-restricted diet, generally limited to 1.5-2 g/day
o Exercise intolerance is a common early manifestation of HF.
o Bedrest during acute episodes.
o A moderate, progressive activity program is prescribed to improve myocardial function. Aerobic
exercise should be performed 3-7 days/week; each session should include a 10-15 minute warmup
period, 20-30 minutes of exercise at the recommended intensity, and a cool-down period.
flexibility exercises and weight training should be part of the exercise routine.
Sx
o In end-stage HF, devices to provide circulatory assistance or sx may be required.
o Heart transplantation = only clearly effective surgical tx for end-stage HF; however, its use
is limited by availability of donor hearts.
o Circulatory assistance = intra-aortic balloon pump (IABP) or a left-ventricular assist device
(LVAD)
o Cardiac transplantation
Care is taken to avoid damaging the sinus node of the donor heart and to ensure integrity
of the suture line to prevent postoperative bleeding.
Bleeding = major concern
Chest tube drainage (gently milked, not stripped) is frequently monitored, as are CO,
pulmonary artery pressures, and CVP.
Cardiac tamponade (compression of the heart) can develop. Atrial dysrhythmias =
common.
Gradual rewarding to prevent shock
Infection and rejection = major postoperative concerns
Patients are immediately started on immunosuppressive therapy soon after sx and
are maintained on a regimen that typically includes 1-3 drugs.
Infection control = vital
o Other procedures = cardiomyoplasty and ventricular reduction sx
Complementary health approaches
o Hawthorn = natural ACE inhibitor
Might worsen early disease progression.
Should not be used without consultation.
o Nutritional supplements of CoQ10, magnesium, and thiamine may be used in conjunction with
other treatments.
End-of-life care
o Unless the patient receives a transplant, chronic HF is ultimately terminal.
o The patient and family need honest discussions about the anticipated course of the disease and tx
options.
o
o
o
Discuss advanced directives.
Hospice should be offered when appropriate.
Severe dyspnea = common in the final stages of the disease and may be one of the most
distressing symptoms for HCPs and family members.
o Non-pharmacological measures may be helpful.
Lifespan considerations
o Children
Children with congenital heart defects develop HF.
Typically the result of overcirculation failure or pump failure.
Typically do not have the same treatments as adults.
s/s include dyspnea, diaphoresis, HTN-, and poor feeding/growth.
Pharmacologic tx = diuretics and afterload reducers
Following tx, the child may experience an improvement in symptoms. This is known as
compensated HF. Underlying causes may still exist.
o Pregnant women
30-50% increase in CO and a 40-50% increase in blood volume. HR and SV increase,
too.
Pregnancy = contraindicated in patients with stage III or stage IV HF; also
contraindicated in patients with EF <40%.
Patients with mild HF (stage I, II) may be able to carry and delivery a baby, but
should be counseled about risk before becoming pregnant and monitored carefully
during pregnancy and PP.
ACE inhibitors and ARBs = contraindicated
Diuretics = commonly prescribed, particularly if pulmonary edema is present.
Beta blockers can be used but can result in IUGR for the child.
Women without HF can develop the condition during pregnancy. One common
cause = postpartum cardiomyopathy (PPCM); preeclampsia, chronic HTN+, and
pulmonary HTN+ may also lead to HF.
o Older adults
Prevalence for HF increases with age.
Cardiac function = decreased
Changes can exacerbate existing cardiac conditions; they can also create problems in
patients with no hx of HF.
Older adults typically present with HTN+ and pulmonary edema.
Symptoms = gradual and often accompanied by decreased appetite and weight
loss.
SOB
Less likely to seek tx d/t s/s being attributed to aging.
Pharmacologic tx is similar to that of the general population. Drug interactions and
non-adherence = areas of concern with older adults.
Nursing process
o Health promotion activities reduce the risk for an incidence of HF are directed at lifestyle changes.
o Teach patients about CAD, the primary underlying cause of HF. HTN+ and DM = additional
major causes.
o Reducing the oxygen demand of the heart is a major nursing care goal for the patient in
acute HF. This includes providing rest and carrying out prescribed tx measures to reduce
cardiac work, improve contractility, and manage symptoms.
o Assessment
Review hx and risk factors. Diet and exercise levels. Activity tolerance and DOE. Note
episodes of nocturnal dyspnea and the number of pillows used for sleeping. Current
medications. Respiratory assessment. VS and general appearance. Color of skin and
mucous membranes, JVD, cap refill. Auscultate heart, breath, and bowel sounds.
o
o
o
o
Problem statement
Decreased CO
FVE
Activity Intolerance
Deficient Knowledge
Planning
Adequate oxygenation.
Adequate perfusion.
Meeting body’s energy needs through appropriate/adequate nutrition.
Implementation
A dx of HF produces great fear of death and disability in the patient because the heart is
vital for life. Helping the patient and family to cope with this fear is an important
component of nursing care. Anxiety s/t hypoxia is also anticipated and requires
nursing intervention.
Maintain CO
Encourage rest.
Monitor VS and oxygen saturation as indicated.
Monitor the patient’s BNP levels, reporting trends.
Auscultate heart and breath sounds. Ventricular gallop (S3) = early sign of HF;
atrial gallop (S4) may also be heard.
Administer oxygen as ordered.
Administered medications as ordered.
Monitor fluid volume
Assess the patient’s respiratory status and auscultate lungs sounds at least every
4 hours.
Monitor I/Os. 1 L fluid = 2.2 lb
Record the patient’s abdominal girth every shift. Note c/o loss of appetite.
Monitor and record hemodynamic measurements.
Restrict fluids as ordered.
Monitor activity
Rest periods.
Assist with ADLs as needed.
Plan and implement progressive activity’s (i.e., ROM).
Provide written and verbal information about activity after discharge (refer to p.
1245, box 16-15)
Safety Alert: Patients with unstable stage III or stage IV decompensated HF
should abstain from sexual activity until their condition is stabilized and well
managed.
Provide a low-sodium diet
Consult with a dietitian.
Discuss with the patient the rationale for a low-sodium diet.
Evaluation
HTN+
Classification of BP for adults
o Normal: less than 120/80
o Elevated: (SBP) 120-129; (DBP) <80
o Stage 1: (SBP) 130-139; (DBP) 80-89
o Stage 2: (SBP) at least 140; (DBP) at least 90
o Hypertensive crisis: (SBP) over 180; (DBP) over 120
HTN+ rarely causes symptoms or noticeably limits the patient’s functional health; however, HTN+ is a
major risk factor for CAD, HF, stroke, and renal failure.
Peripheral vascular resistance (PVR) refers to the opposing forces or impedance to blood flow as the
arterial channels become more and more distant from the heart.
o Determined by three factors:
Blood viscosity: greater viscosity, greater resistance.
Length of vessel: longer vessel, greater resistance.
Diameter of vessel: the smaller the diameter, the greater the friction (leading to
greater impedance of blood flow).
Factors affecting arterial BP
o Sympathetic nervous system (SNS) stimulation
The SNS and PNS are the primary mechanisms that regulate BP.
o Circulating epinephrine and norepinephrine (fight or flight response)
o RAAS system
o Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP)
o Adrenomedullin
o Vasopressin or ADH
o Local factors (i.e., inflammatory response)
o Other factors that can affect vessel compliance are the extent of arteriosclerosis (hardening of the
arteries) and the extent of atherosclerosis (plaque accumulation).
o Increased SVR causes HTN+.
The kidneys help maintain BP by excreting or conserving sodium and water.
HTN+ is a major contributor to ESRD**
Primary HTN+
o Formerly known as essential HTN+; is a persistently elevated systemic BP.
o Thought to develop from complex interactions among factors that regulate CP and SVR. The
result of these interactions is sustained increases in blood volume and peripheral resistance.
SNS overstimulation
Altered function of RAAS system
Other chemical medications of vasomotor tone and blood volume, such as ANP
The interaction between insulin resistance, hyperinsulinemia, and endothelial function
(may be the primary cause)
Secondary HTN+
o Elevated BP resulting from an identifiable underlying process, including:
Kidney disease (e.g., renal artery stenosis) or renal function (e.g., glomerulonephritis,
renal failure)
Coarctation of the aorta
Endocrine disorders
Neurologic disorders
Drug use
Pregnancy
Hypothyroidism
Obstructive sleep apnea
Hypertensive crisis
o Also called malignant HTN+ or hypertensive emergency.
o SBP >180 and/or DBP >120
o Immediate tx (within 1 hour) is vital to prevent cardiac, renal, and vascular damage and
reduce morbidity and mortality.
o Cerebral edema often develops.
Risk factors
o Modifiable
High sodium intake
Low potassium calcium, and magnesium intake
Obesity
Excessive alcohol consumption
Insulin resistance
Low activity level
Hypothyroidism
Low vitamin D levels
Depression
Tobacco
o Non-modifiable
Genetics
Age
Family hx
AA
Men
Prevention of HTN+ involves a health lifestyle.
o Strategies include maintaining a health weight and a health diet with reduced salt intake, engaging
in regular physical activity, using stress management techniques, following medication regimens,
and avoiding baths that are not too hot.
o Obtain regular medical care and follow the prescribed tx plan.
Clinical manifestations
o The early stages of primary HTN+ typically are asymptomatic, marked only by elevated BP.
BP elevations = transient
When symptoms do appear, they are usually vague.
Headache, generally in the back of the head and neck, may be present upon
awakening, subsiding during the day.
Other symptoms may include nocturia, confusion, N/V, and visual disturbances.
Examination of the retina of the eye may reveal narrowed arterioles, hemorrhages,
exudates, and papilledema.
o Hypertensive encephalopathy, a syndrome characterized by extremely high BP, altered LOC,
increased ICP, papilledema, and seizures, may develop. Etiology = unclear.
o Proteinuria and microscopy hematuria.
o Patients presenting with hypertensive emergency may have manifestations such as headache,
confusion, swelling of the optic nerve (papilledema), blurred vision, restlessness, and motor
and sensory deficits.
Primary HTN+ cannot be cured; however, it can be controlled.
o Management focuses on reducing BP to <130/80.
o The ultimate goal is to reduce cardiovascular and renal morbidity and mortality.
o The risk for coronary complications (CAD, HF, stroke) decreases when the average BP is
<130/80; when the patient also has DM or renal disease, the tx goal is a BP <129/79.
o Most individuals with HTN+ require a combination or two or more drugs along with lifestyle
change to achieve recommended BP levels.
o Safety Alert: Isometric exercise (e.g., weight training) may not be appropriate for individuals with
HTN+, because it can raise the SBP.
Dx
o The patient with HTN+ is evaluated for the presence of identifiable causes, CV risk factors, and
the presence/absence of target organ damage.
o Before tx is started, the following tests are performed:
ECG
UA
FSBS
Hct
Creatinine
Vitamin D, calcium
Cholesterol and lipid profile (including HDL, LDL, triglycerides)
o Additional tests may include urinary albumin excretion, evaluation of the GFR, and tests for
emerging CV risk factors, such as C-reactive protein and homocysteine levels.
o Also, the following tests may be ordered to differentiate primary and secondary HTN+: renal
function studies and UA; serum potassium; blood chemistry; IV pyelography, renal US, renal
arteriography, and CT/MRI.
Pharmacologic therapy
o Drug classes
Diuretics are the preferred tx for systolic HTN+ in older adults.
Thiazide diuretics, such as HCTZ (HydroDIURIL) = widely used
Diuretics are particularly effective in AA and in patients who are obese, are
older, or have increased plasma volume or low renin activity.
AE = dose related
In addition to hypokalemia, diuretics may affect serum levels of
glucose, triglycerides, urin acid, LDLs, and insulin.
Safety Alert: Patients who are prescribed potassium-sparing diuretics or AVE inhibitors
for HTN+ should not use salt substitutes. These substitutes often substitute potassium for
sodium, which could result in hyperkalemia.
Patients with HF, CAD, or DM may be initially treated with beta blockers (reduce the
risk of complications such as HF and stroke).
Safety Alert: Beta blockers are contraindicated for patients with asthma or
COPD because they promote bronchial constriction.
ACE inhibitors and ARBs are also commonly used in the initial tx of HTN+,
particularly for patients with DM or HF, hx of MI, or CKD.
CCBs (ex: verapamil and diltiazem in particular)
Reflex tachycardia = minimal in verapamil and diltiazem
Direct vasodilators such as hydralazine and minoxidil (reduce PVR with
decreased risk of orthostatic HTN-); however, they are associated with reflex
tachycardia and fluid retention, so they are administered in single-drug tx
regimens.
o Drug regimens
Tx is usually initiated by using a single antihypertensive drug at a low dose. Unless
otherwise indicated, a diuretic is recommended as the initial drug of choice. If the
drug does not work effectively, a different drug from another class of antihypertensive
medications is substituted. If, on the other hand, the drug is tolerated well but does not
lower BP to the desired level, a second drug from another class may be added to the tx
regimen.
Tx of patients with stage 2 HTN+ generally is more aggressive to minimize the risk of
MI, JF, or stroke. Then the patient’s SBP is >160 or DBP >100, immediate therapy (and
possible hospitalization) = vital.
After a year of effective HTN+ control, an effort may be made to reduce the dosage and
number of drugs. This is known as step-down therapy. It is more successful in patients
who have made lifestyle modifications.
Careful BP monitoring is necessary during and after step-down therapy
because BP often rises again to hypertensive levels.
Lifestyle modifications are recommended for all patients who BP falls within the elevated range and for
everyone with intermittent or sustained HTN+.
o These include weight loss, dietary changes, restricted alcohol use and smoking, increased
physical activity, and stress reduction.
o
Dietary approaches focus on reducing sodium intake, maintaining adequate potassium and
calcium intakes, and reducing total and saturated fat intake.
A mild to moderate sodium restriction = good
DASH (Dietary Approaches to Stop HTN+) (p. 1257, box 16-17) focuses on whole
foods rather than individual nutrients. It is rich in fruits and vegetables (up to 10
servings/day) and is low in total and saturated fats.
Grains
Vegetables
Fruits
Fat-free or low-fat milk and milk products
Meats, poultry, and fish
Nuts, seeds, and legumes
Fats and oils
Sweets and added sugars
o Complementary health approaches = reduce feelings of distress and anxiety
Lifespan considerations
o Children and adolescents
Obesity = major risk factor
Prehypertension is defined as having a BP that is between the 90 th and 95th percentile for
the child’s age, sex, and height.
HTN+ is defined as having a BP above the 95th percentile.
Physicians often overlook the problem of HTN+ in this population even though HTN+ is
on the rise.
Children should have their BP checked on a regular basis beginning at age 3.
Atypical s/s or asymptomatic, although changes can be subtle in behavior and school
performance.
Width of bladder: 40%, length of bladder = 80%
Tx = lifestyle modifications, although children with stage II HTN+ or HTN+ that is not
controlled by lifestyle changes may be prescribed medications.
o Older adults
Risk for developing HTN+ increases with age, specifically in AA age 60+.
Many develop systolic HTN+.
Tx should be closely monitored.
Complications r/t polypharmacy may also occur.
Nursing process
o Health promotion teaching and activities focus on modifiable risk actors for HTN+.
P. 1259 Patient Teaching = vital
Healthy eating patterns
Stop smoking
Moderate alcohol consumption
Stress-reducing techniques
Prescribed medication regimen adherence
Monitoring BP regularly and regular visits to the HCP/HTN+ clinic
o Assessment – should focus on the patient interview and examination.
o Problem statement
Ineffective Health Maintenance
Noncompliance
Nutrition: Readiness for Enhanced
FVE
o Planning
o Implementation
Promote health maintenance
o
Promote adherence
Promote balanced nutrition
Monitor fluid volume
Evaluation = maintenance of BP WNL
EKG/ECG
Dysrhythmias = abnormal
o Arise through disruption of the very properties that stimulate and control the heartbeat.
o Ectopic beats interrupt the normal conduction sequence and may not initiate a normal muscle
contraction.
Aging affects cardiac rhythm. The natural pacemaker of the heart loses some of its cells, resulting in a
slightly lower HR. In older adults, the left ventricle tends to increase in size. Heart murmurs =
possible.
Five unique properties of cardiac cells allow effective heart function. Four of these properties are electrical;
the fifth is cardiac muscle’s mechanical response to electrical stimulation. These five properties include the
following:
o Automaticity: ability of pacemaker cells to spontaneously initiate an electrical impulse. SA node
= pacemaker (60-100 BPM)
o Excitability: ability of myocardial cells to respond to stimuli.
o Conductivity: when one cell is stimulated, the impulse spreads rapidly throughout the heart
muscle.
o Refractoriness: the inability of cardiac cells to respond to additional stimuli immediately
following depolarization.
o Contractility: heart muscle responds in an all-or-nothing manner.
Atrial kick = extra bolus of blood into the ventricles before they contract.
Myocardial injury or MI can obstruct or delay impulse conduction. Bundle branch blocks = common
in AMI.
The reentry phenomenon, a phenomenon of normal and slow conduction, is a major cause of
tachydysrhythmia.
Cardiac rhythms are classified according to the site of impulse formation or the site and degree of
conduction block.
o Supraventricular = above ventricles
Usually produces a QRS complex WNL
Ex: sinus rhythms, atrial rhythms, and junctional (arising from the AV junction).
o Ventricular = originate in the ventricles
Fatal if left untreated.
Risk factors
o Hx of heart disease, including CAD, prior heart sx, HTN+, congenital heart disease, MI, and other
heart damage.
o Alterations r/t to the endocrine system (including thyroid problems, DM, and electrolyte
imbalances).
o Sleep apnea
o Alcohol, stimulants (i.e., caffeine and nicotine)
o Medications
Prevention = methods to increase heart health
o Includes maintaining a heart-healthy diet, participating in moderate physical exercise, maintaining
a health weight, following tx recommendations, limiting alcohol and caffeine consumptions,
refraining from tobacco use of any kind, and avoiding medications that can cause dysrhythmias.
Clinical manifestations
o
o
NSR
Impulses originate in the SA (sinus) node and travel through all normal conduction
pathways without delay.
All waveforms are of normal configuration, look alike, and have consistent (fixed)
durations.
Rate = 60-100 BPM
Regular rate and rhythm**
s/s often range from none to SCD.
More severe symptoms tend to occur in patients who have evidence of structural disease.
Common s/s include lightheaded, dizziness, fluttering, a racing or slowing heartbeat,
SOB, chest discomfort or pain, and syncope.
Management = none
Sinus node dysrhythmias
Sinus arrhythmia
Regular rate, irregular rhythm**
Rate increases during inspiration and decreases with expiration.
Common in the very young and very old.
Caused by an increase in vagal tone, by digitalis toxicity, or by morphine
administration.
Management: none
Sinus tachycardia
Rate = >100 BPM
Tachycardia is a normal response to any condition or event that increases the
body’s demand for oxygen and nutrients (i.e., exercise, hypoxia).
Sinus tachycardia may be an early sign of cardiac dysfunction, such as HF.
Common causes include exercise, excitement, anxiety, pain, fever, hypoxia,
hypovolemia, anemia, hyperthyroidism, MI, HF, cardiogenic shock, PE, caffeine
intake, and certain drugs (such as atropine, epinephrine {Adrenalin} or
isoproterenol {Isuprel}).
Manifestations include a rapid pulse rate. The patient may c/o feeling that
the heart is “racing,” SOB, and dizziness. In the presence of heart disease,
sinus tachycardia may precipitate CP.
Very fast rate, regular rhythm**
Management
Tx is only initiated if symptomatic or if patient is at risk for myocardial
damage.
Tx of underlying cause (e.g., hypovolemia, fever, pain).
Possible administration of beta blockers or verapamil.
Sinus bradycardia
Rate = >60 BPM
May result from increased vagal (parasympathetic) activity or from depressed
automaticity d/t injury or ischemia to the SA node.
HR slows during sleep. Other causes include pain, increased ICP, sinus node
disease, AMI, hypothermia, acidosis, and certain drugs.
May be asymptomatic. Manifestations of CO may exist, including decreased
LOC, syncope (fainting), or HTN-. All require intervention.
It is important to assess the patient before treating the rhythm.
Slow rate, regular rhythm**
Management
Tx only if symptomatic.
Possible administration of IV atropine or isoproterenol, and/or
pacemaker therapy.
o
Atrial flutter
Rapid and regular atrial rhythm thought to result from an intra-arterial
reentry mechanism.
Causes include SNS stimulation; thyrotoxicosis; CAD or MI; PE; and abnormal
conduction syndromes (i.e., WPW syndrome).
Older adults with rheumatic heart disease or valvular disease = vulnerable
Patients with this condition may c/o palpitations or a fluttering sensation in
the chest or throat. If the ventricular rate is rapid, manifestations of decreased
CO, such as decreased LOC; HTN-; decreased urinary output and cool,
clammy skin, may be noted.
Atrial kick = lost d/t inadequate filling
ECG characteristics include a “sawtooth” or “picket fence” appearance of
P waves.
Atrial rate = rapid; ~300 BPM
As a protective mechanism, many impulses are blocked at the AV
node, and the ventricular rate is rarely over 150-170 BPM.
Atrial impulses are usually evenly conducted through the AV node
(i.e., 2:1, 4:1, 6:1).
Management
Medications to slow ventricular response, such as beta blockers or
CCBs, followed by a class I antidysrhythmic agent or amiodarone;
synchronized cardioversion on R wave**
Atrial fibrillation
Characterized by disorganized atrial activity without discrete atrial
contractions.
May occur suddenly and recur, or it may persist as a chronic issue.
Commonly associated with HF, rheumatic heart disease, CAD, HTN+, and
hyperthyroidism.
Manifestations r/t the rate of the ventricular response.
With rapid response rates, manifestations of decreased CO such as
HTN-, SOB, fatigue, and angina may develop.
Patients with extensive heart disease may develop syncope or HF.
Peripheral pulses = irregular and of variable amplitude (strength)
ECG characteristics include an irregular rhythm and the absence of
identifiable P waves. The atrial rate is so rapid that it is not measurable. The
ventricular rate varies.
Increased risk for formation of thromboemboli. Organ infarction may occur as a
result; the incidence of stoke = high
“Irregularly irregular”
Management
Synchronized cardioversion; medications to reduce ventricular
response rate (i.e., metoprolol, diltiazem, or digoxin); anticoagulant
therapy to reduce risk of clot formation and stroke.
Ventricular dysrhythmias
Premature ventricular contractions (PVCs)
Beats that occur before the next expected beat of the underlying rhythm.
Usually do not reset the atrial rhythm and are followed by a full compensatory
pause.
Significance in individuals without heart disease. No tx needed.
Frequent, recurrent, or multifocal PVCs may be associated with an
increased risk for lethal dysrhythmias.
May be triggered by anxiety or stress; tobacco, alcohol, caffeine; hypoxia;
acidosis; electrolyte imbalances; SNS stimulation; coronary heart disease; HF;
mechanical stimulation of the heart or repercussion after fibrinolytic therapy.
The incidence and significance of PVCs is greater after MI.
May be isolated or may occur in a specific pattern.
The following are considered warning signs in the patient with acute heart
disease (e.g., AMI):
PVCs that develop within the first 4 hours of an MI
Frequent PVCs (6+/minute)
Couplets, triplets
Multifocal PVCs
R-on-T phenomenon (PVCs falling on the T wave)
In patients with preexisting heart disease, PVCs may indicate drug toxicity.
Variable rate, irregular rhythm**
Management
Tx if symptomatic or in presence of severe heart disease.
Avoiding stimulants (i.e., nicotine, caffeine).
Possible administration of beta blockers or class I or III
antidysrhythmic agents in patients with severe heart disease who
are symptomatic.
PVD
Aka PAD; refers to a category of circulation disorders that may occur when pathologic changes
(arteriosclerosis and atherosclerosis) impair blood supply to peripheral tissues, particularly the lower
extremities.
o Arteriosclerosis = thickening
Arteriosclerosis in the abdominal aorta leads to the development of aneurysms as plaque
erodes the vessel walls.
o Atherosclerosis = plaque (fat and fibrin) buildup
Plaque tends to form at arterial bifurcations. Tissue hypoxia or anoxia results.
Collateral circulation often develops.
Chronic venous insufficiency (CVI) is a disorder of inadequate venous return over a prolonged period.
o Results when venous blood collects and stagnates in the lower leg (venous stasis). Ulcers
develop.
o Venous pressures in the calf and lower leg increase, particularly during ambulation.
o Eventually, there is so little oxygen and nutrients that cells begin to die. Breakdown of RBCs in
the congested tissues causes brown skin pigmentation.
Risk factors
o Over 60 years of age
o Men
o AA
o Hispanic origin
Prevention
o Because PVD arises primarily from atherosclerosis and other disorders that impair CV function,
prevention focuses on preventing these processes and includes maintaining a healthy lifestyle
(e.g., maintaining a healthy weight and healthy diet with regular exercise) and following tx
regimens for chronic illnesses.
o Individuals at risk for PVD may be screened by their HCPs using an ankle-brachial index, or ABI.
o Claudication medications in addition to lifestyle choices may also help slow or even reverse the
progress of PVD symptoms. These include prescribed high BP medications and cholesterol-
lowering medications and may also include drugs to prevent clots. All meds should be taken as
advised.
Clinical manifestations (5 Ps)**
o Pain = primary symptom
o Intermittent claudication (a cramping or aching pain in the calves of the legs, the thighs, and the
buttocks that occurs with a predictable level of activity) is characteristic of PVD. The pain is often
accompanied by weakness and is relieved by rest.
o Rest pain, in contrast, occurs during periods of inactivity.
Described as a burning sensation in the lower legs.
Pain increases when the legs are elevated and decreases when legs are dependent.
o Sensation = diminished, and muscles may atrophy.
o Legs are pale when elevated by dark red (dependent rub or) when dependent. May feel cold
and numb.
o Skin = thin, shiny, hairless with discolored areas
o Toenails may be thickened.
o Areas of skin breakdown and ulceration may be evident.
o Edema may be present.
o Safety Alert: The decreased sensation and skin breakdown that is common during PVD increases
the risk for gangrene and amputation of an extremities. Teach patients with PVD how to assess
their skin for breakdown and other injuries and how to perform foot and leg care.
o Manifestations of CVI include lower extremity edema; itching, dull leg discomfort or pain;
thin, shiny, atrophic skin; cyanosis and brown skin pigmentation of lower leg and foot;
possible weeping; thick, fibrous (hard) SQ tissue; recurrent ulcerations of medial or anterior
ankle.necrosis and fibrosis of SQ tissue cause the affected area of the leg to feel hard and
somewhat leathery.
o Refer to p. 1294, table 16-29 Comparison of arterial and venous leg ulcers**
Management of PVD focuses on slowing the atherosclerotic process and maintaining tissue perfusion.
o Wet dressings = tx
o Bedrest
Hx and physical examination often establish dx of CVI. Careful assessment of the patient’s medical hx =
vital d/t risk of DVT.
o No specific dx tests to confirm dx of CVI.
o Conservative management of venous insufficiency focuses on reducing edema and treating
ulcerations.
Dx
o Although PVD can often be diagnosed on the basis of the hx and physical examination, test may
be ordered to evaluate its extent. These include:
Segmental pressure measurements
Stress testing
Doppler US
Duplex Doppler US
Transcutaneous oximetry
Angiography or magnetic resonance angiography
Pharmacologic therapy
o Medications to inhibit platelet aggregation, such as aspirin or clopidogrel (Plavix).
o Cilostazol (Pletal), a plately inhibitor with vasodilator properties.
o Pentoxifylline (Trental) decreases blood viscosity and increase RBC flexibility.
Non-pharmacologic therapy
o Smoking cessation
o Meticulous foot care (refer to p. 1296 Patient Teaching: Foot and leg care for the patient with
peripheral atherosclerosis)**
o
o
o
o
Elevating HOB (eases rest pain)
Regular, progressively strenuous exercise, such as 30-45 minutes of walking daily = important
Rest at onset of claudication, resuming activity when the pain resolves.
Other measures to slow the process of atherosclerosis, such as controlling DM and HTN+,
lowering cholesterol levels, and weight loss = recommended
Sx
o Revascularization
o Non-surgical procedures (i.e., PTCA, stent placement, atherectomy)
o Surgical procedures include endarterectomy and bypass grafts.
o Risk for post-op complications must be considered.
Complementary health approaches
o Integrative therapies include interventions to improve circulation and reduce stress.
Aromatherapy with rosemary or vetiver; biofeedback; healing or therapeutic touch and
massage; herbals such as ginkgo, garlic, cayenne, hawthorn, and bilberry; and exercise
(i.e., yoga).
In addition, complementary health approaches to reduce atherosclerosis and lower
cholesterol levels may slow the progress of PVD.
Measures such as a very low-fat or vegetarian diet, including antioxidant
nutrients or using vitamin C, vitamin E, or garlic supplements, and traditional
Chinese medicine.
Nursing process
o Nursing care for the patient with CVI is primarily educative and supportive.
Elevate the legs while resting, sleeping.
Walk as much as possible.
When sitting, do not cross legs or allow pressure on the back of knees.
Do not wear anything that pinches or cuts off circulation to the legs.
Wear elastic hose as ordered.
Safety Alert: Teach patients who wear elastic hose or compression stockings to be sure
the tops of the hose do not cut into their legs. This can cut off circulation to the lower
extremities, compounding the damage from PVD.
Keep the skin the feet and legs clean, soft, dry.
o Assessment
Pain and its associated symptom; hx of CAD, PVD, hyperlipidemia, HTN+, or DM;
current medications; smoking hx; diet and activity patterns.
VS, pulses, cap. refill, skin temperature/appearance, hair distribution, movement and
sensation of lower extremities.
o Problem statement
Ineffective Peripheral Tissue Perfusion
Chronic Pain
Impaired Skin Integrity
Activity Intolerance
Disturbed Body Image
Ineffective Health Maintenance
Risk for Infection
Impaired Physical Mobility
o Planning
The patient will stop smoking.
The patient will learn appropriate foot and wound care.
The patient will maintain adherence with medications and wound care.
The patient will maintain activity and exercise as tolerated.
o Implementation
Pain management = priority
o
Promote tissue perfusion
Elevate extremities to promote venous return.
Use a foot cradle and light weight blankets, etc.
Encourage frequent position changes.
Assess peripheral pulses, pain, color, temperature, and cap refill every 4 hours as
needed.
Manage pain
Keep extremities warm.
Teach pain relief and stress reduction techniques.
Assess pain at least every 4 hours using a standard pain scale.
Promote skin integrity
Provide meticulous daily skin care.
Apply a bed cradle.
Provide an egg-crate mattress, flotation pads, sheepskin, or heel protectors.
Encourage activity
Unless contraindicated, encourage gradual increases in duration and intensity of
exercise.
Encourage frequent position changes and active ROM.
Assist with ADLs as needed.
Provide diversional activities during periods of prescribed bedrest.
Evaluation
Pulmonary embolism
Medical emergency. 50% of deaths occur within the first 2 hours following embolization.
Thrombi that affect only the deep veins of the calf rarely embolize to the pulmonary circulation. However,
thrombi often grow proximally to the popliteal and ileofemoral veins. There, they may break loose to
become an embolus.
The impact of a pulmonary embolus depends on the extent to which pulmonary blood flow is obstructed,
the size of the embolus, its nature, and any secondary effects of the obstruction. These effects can vary
widely:
o Obstruction of a large pulmonary artery with sudden death.
o Lung tissue infarction.
o Obstruction of a small segment of the pulmonary circulation with no permanent lung injury.
o Chronic or recurrent, possibly multiple, small emboli with recurring s/s.
In severe cases, this can lead to pulmonary HTN+ and right ventricular HF.
Dead space (areas of the lung that are ventilated by not perfumed) increases. Alveolar surfactant
decreases, increasing risk for atelectasis.
Fat emboli = most common; usually occurs after fracture of long bone (typically the femur) releases
bone marrow fat into circulation. Adipose tissue or liver trauma may also lead to fat emboli.
Risk factors
o Stasis of venous blood flow
o Vessel wall damage
o Altered blood coagulation
o Genetics
o Certain cancers that provide coagulation factors make clot formation more likely.
o Women who use oral contraceptives or estrogen therapy.
o Pregnant, BF
o Smoking
o AA
Prevention
o To begin preventing PE, it is crucial to prevent the clots caused by DVT.
Administering anticoagulants before or after an operation for individuals at risk.
Using compression/support stockings.
Encouraging physical activity asap after sx.
Elevating legs during bedrest.
Taking breaks from sitting, flexing ankles while seated.
Drinking plenty of fluids.
Clinical manifestations
o Hypoxia: restlessness; CP; dyspnea; cyanosis; use of accessory muscles when breathing;
respiratory acidosis; tachycardia; tachypnea; feeling of impending doom.
Clinical therapies
Administer oxygen.
Reposition the patient.
Prepare the patient for possibility of intubation and mechanical ventilation.
Monitor labs.
Maintain a low-stimulus environment.
o Rupture of small aterioles (arterial congestion): auscultation of coarse crackles in the affect
lobe; cough with or without blood; dyspnea.
Clinical therapies
Explain hemoptysis.
Administer oxygen.
Maintain patent airway. Suction as needed.
o Alveolar collapse (atelectasis): hypoxia, dyspnea, productive cough, CP.
Clinical therapies
Administer oxygen.
Reposition the patient.
Promote use of incentive spirometer.
o Inflammatory process: elevated temperature, tachycardia, tachypnea, dehydration, cough.
Clinical therapies
Administer antipyretics.
Conduction lab testing.
Administer ABX.
Because DVT may not be identified until PE occurs, prevention is the primary goal in treating PE.
o When PE occurs, tx = supportive.
Safety Alert: When applying external pneumatic compression boots, be sure to apply the correct size
boots. Compression boots that are too small or too large will be ineffective at preventing DVT and PE.
Ensure that boots of all sizes, including extra-large boots, are on hand.
Dx
o Pulmonary Embolism Rule-out Criteria (PERC), Modified Wells Criteria, Geneva Criteria.
Criteria that indicate risk for PE include the following:
Pulse rate >99 BPM
Pulse oximetry <95% RA
DVT, PE hx
Hemoptysis
Recent sx or trauma requiring hospitalization
Cancer
Unilateral leg swelling or limb pain
If the criteria testing indicates that the patient is at risk for PE, then the patient should
undergo further testing.
o The studies performed to dx pulmonary emboli differ from those used to dx DVT and include the
following:
D-dimer
Chest CT with contrast = principal test to dx PE
Lung scans
Safety Alert: Radioisotopes used in lung scans may cause allergic reactions in some
individuals. Before a lung scan is performed, the RN should ask the patient about any
previous allergic reactions to radioisotopes. In addition, because of the potential harm to
the fetus d/t radiation, female patients should be asked if they are pregnancy or may be
pregnant before beginning the tests. Breastfeeding mothers should be cautioned to refrain
from breastfeeding for 24-48 hours.
CT pulmonary angiography
CXR
Electrocardiography
ABG
ETCO2
Coagulation studies
Pharmacologic therapy
o Anticoagulant therapy = standard
IV heparin bolus
aPTT or PPT is monitored frequently until stabilized.
Heparin therapy is often continued for about 5 days, or until PO anticoagulation therapy
has become fully effective.
Antidote: protamine sulfate**
PO warfarin is initiated at the same time as heparin.
Requires 5-7 days for effectiveness.
Antidote: Vitamin K**
Continued for 2-3 months when few risk factors exist. Long-term therapy = chronic
disorders.
Bleeding = major risk, although hemorrhage = uncommon.
Sx
o When anticoagulant therapy fails or is contraindicated, an umbrella-like filter may be inserted into
the inferior vena cava to trap large emboli while allowing continued blood flow. Inserted via
femoral or jugular vein.
o Safety Alert: If a patient has a vena cava filter inserted and still has many emboli in the legs, the
filter may become clogged, causing severe edema in the lower extremities. Monitor the patient for
edema, and implement preventive measures to reduce or prevent edema.
Lifespan considerations
o Children
Rare
Presenting s/s include tachypnea, tachycardia, CP, SOB, and cough.
Hemoptysis = rare
V/Q scans, CT pulmonary angiography, and magnetic resonance pulmonary angiography
= better for dx; not a D-dimer.
Tx = unfractionated heparin or LMWH
o Pregnant women
Identification of PE = complicated d/t pregnancy
If PE suspected, a bilateral venous compression US of the lower extremities should be
performed.
Wells Criteria should be used with caution.
D-dimer not effective because it increases in the second trimester.
Tx = unfractionated heparin or LMWH.
Warfarin = contraindicated
Insertion of vena cava filter = safe if heparin use is contraindicated
Heparin and warfarin = safe for BF
Nursing process
o Assessment = pain, respiratory, general appearance, oxygen saturation, pulses, skin temperature,
LOC, edema, current medications, hx, risk factors, signs of distress.
o Problem statement
Impaired Gas Exchange
Decreased Cardiac Output
Ineffective Protection
Acute Pain
Anxiety
o Planning
The patient will demonstrate oxygen saturation that remains >94%.
The patient will verbalize fears resulting from respiratory distress.
The patient will obtain relief from pain to allow for adequate rest and comfort.
The patient will demonstrate adequate tissue perfusion.
The patient’s VS will remain WNL.
o Implementation
Compensate for impaired gas exchange
Fowler, high-Fowler with lower extremities dependent
Bedrest
Frequently assess respiratory status
Monitor ABGs
Preserve CO
Report CP or other symptoms of distress
Assess patient’s skin color and temperature
Auscultate heart sounds every 2-4 hours
Monitor cardiac rhythms
Administer vasopressors and other medications as ordered
Monitor the patient’s pulmonary arterial pressures, neck vein distention, and
peripheral edema
Maintain IV and arterial access sites as well as central lines
Promote safety
Assess the patient’s medication regimen
Maintain adequate fluid intake
Assess the patient for overt and covert bleeding (refer to p. 1305 Patient
Teaching: PE)
Report abnormal values
Keep protamine sulfate and vitamin K available
Avoid invasive procedures
Maintain firm pressure on injection and venipuncture sites for 30 minutes.
Relieve anxiety
o Evaluation
Adequate tissue perfusion.
Pain management.
Effective airway clearance.