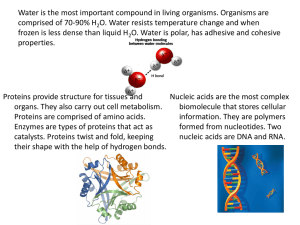
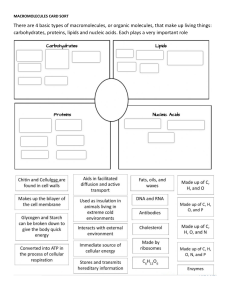
CHNOPS Mr. Defreitas 6 Elements of Life ● The six elements of life are Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, and Sulfur. ● These elements are the six most common elements found in biomolecules or biological macromolecules. Biomolecules ● Biomolecules are large, organic molecules that are critical to living things. ● They fulfill functions inside of organisms that are necessary to sustain life. ● There are four main categories of biomolecules: carbohydrates, lipids, proteins, and nucleic acids. Biomolecules 2 ● Carbohydrates are a necessary source of energy in organisms. ● Lipids are a major component of cell membranes and also provide organisms with energy. ● Proteins are structural molecules that make up muscles and bones; they also act as chemical messengers within the body. ● Nucleic acids contain organisms' genetic codes. Carbon ● Carbon can be found in all four of the biomolecules. Carbon is usually the most abundant element. ● In carbohydrates & nucleic acids, carbon atoms form ring-like structures that are central to the molecule. In lipids, carbohydrates form long chains. Proteins contain carbon arranged in a branched orientation. In nucleic acids, carbon is present in a ring-like structure because nucleic acids contain carbohydrates. Hydrogen ● Hydrogen is found in carbohydrates, lipids, proteins, and nucleic acids. Together with carbon, hydrogen can form hydrocarbons. Hydrocarbons can form long chains or ringed structures. ● Hydrogen is necessary for many aspects of life. During digestion, hydrogen and chlorine bond together to form the digestive acid hydrochloric acid (HCl). Hydrogen is also important to the process of ATP production called cellular respiration. ● Hydrogen is also critical for life because it is a large component of water. Nitrogen ● Nitrogen plays an important role in the structure of DNA. DNA contains nitrogenous bases that enable organisms to pass their genetic information to subsequent generations. ● In plants, nitrogen is a part of the molecule chlorophyll. Chlorophyll is a green pigment that absorbs sunlight to fuel photosynthesis. Photosynthesis provides the plant with glucose which can then be broken down into energy. Oxygen ● Oxygen is a colourless, odourless, tasteless gas essential to living organisms, being taken up by animals, which convert it to carbon dioxide; plants, in turn, utilize carbon dioxide as a source of carbon and return the oxygen to the atmosphere. ● Oxygen forms compounds by reaction with practically any other element. Oxygen is able to displace elements from their combinations with each other. Its most important compound is water. Phosphorus ● Non-metallic chemical element of the nitrogen family that at room temperature is a colourless, semitransparent, soft, waxy solid that glows in the dark. ● Highly combustible in many states. Used in matches, fireworks, smokescreen, fertilizers and rodenticides. ● In many compounds phosphorus is poisonous. Sulfur ● Non-metallic chemical element belonging to the oxygen group, one of the most reactive of the elements. Pure sulfur is a tasteless, odourless, brittle solid that is pale yellow in colour, a poor conductor of electricity, and insoluble in water. ● It reacts with all metals except gold and platinum, forming sulfides; it also forms compounds with several nonmetallic elements. Millions of tons of sulfur are produced each year, mostly for the manufacture of sulfuric acid, which is widely used in industry. fertilizers, pigments, dyes, drugs, explosives, detergents