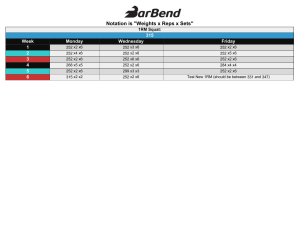
MASS ER I C H E L M S G REG N U C K O L S M IC HAEL Z O U R D O S ERIC T R E X L E R M O NTHLY A PP LICATIONS IN S TRENGTH S PO R T Can You Stay Shredded? Are there inevitable physiological consequences that make it difficult to stay really lean, or are people just doing it wrong? p.7 VOLU ME 6 , I SSU E 7 J U LY 2022 The Reviewers Eric Helms Eric Helms is a coach, athlete, author, and educator. He is a coach for drug-free strength and physique competitors at all levels as a part of team 3D Muscle Journey where he is also the Chief Science Officer. Eric regularly publishes peer-reviewed articles in exercise science and nutrition journals on physique and strength sport, in addition to contributing to the 3DMJ blog. He’s taught undergraduateand graduate-level nutrition and exercise science and speaks internationally at academic and commercial conferences. He has a B.S. in fitness and wellness, an M.S. in exercise science, a second Master’s in sports nutrition, a Ph.D. in strength and conditioning, and is a research fellow for the Sports Performance Research Institute New Zealand at Auckland University of Technology. Eric earned pro status as a natural bodybuilder with the PNBA in 2011 and competes in numerous strength sports. Greg Nuckols Greg Nuckols has over a decade of experience under the bar and a B.S. in exercise and sports science. Greg earned his M.A. in exercise and sport science from the University of North Carolina at Chapel Hill. He’s held three all-time world records in powerlifting in the 220lb and 242lb classes. He’s trained hundreds of athletes and regular folks, both online and in-person. He’s written for many of the major magazines and websites in the fitness industry, including Men’s Health, Men’s Fitness, Muscle & Fitness, Bodybuilding.com, T-Nation, and Schwarzenegger.com. Furthermore, he’s had the opportunity to work with and learn from numerous record holders, champion athletes, and collegiate and professional strength and conditioning coaches through his previous job as Chief Content Director for Juggernaut Training Systems and current full-time work on StrongerByScience.com. Michael C. Zourdos Michael (Mike) C. Zourdos, Ph.D., CSCS, has specializations in strength and conditioning and skeletal muscle physiology. He earned his Ph.D. in exercise physiology from The Florida State University (FSU) in 2012 under the guidance of Dr. Jeong-Su Kim. Prior to attending FSU, Mike received his B.S. in exercise science from Marietta College and M.S. in applied health physiology from Salisbury University. Mike served as the head powerlifting coach of FSU’s 2011 and 2012 state championship teams. He also competes as a powerlifter in the USAPL, and among his best competition lifts is a 230kg (507lbs) raw squat at a body weight of 76kg. Mike owns the company Training Revolution, LLC., where he has coached more than 100 lifters, including a USAPL open division national champion. Eric Trexler Eric Trexler is a pro natural bodybuilder and a sports nutrition researcher. Eric has a PhD in Human Movement Science from UNC Chapel Hill, and has published dozens of peer-reviewed research papers on various exercise and nutrition strategies for getting bigger, stronger, and leaner. In addition, Eric has several years of University-level teaching experience, and has been involved in coaching since 2009. Eric is the Director of Education at Stronger By Science. Table of Contents 7 BY ER I C HEL MS Can You Stay Shredded? In the fitness industry many claim that with their system, supplement, or coaching, you can have your lean, dream body 24/7. While some get really lean, few, including bodybuilders, maintain a shredded physique year round. Why is this? Are there inevitable physiological consequences that make it very difficult to stay really lean, or are people just doing it wrong? 23 BY MI CHAEL C. ZOUR DOS What's Worth Including in Your Warm-Up? Our previous forays into foam rolling determined that it acutely increases range of motion, but not performance. But does foam rolling enhance performance when combined with dynamic stretching? This article breaks down the new findings. 36 BY ER I C T R EXL ER Is Caffeine Tanking Your Testosterone? Many of us grab a cup of coffee before we start our day, or ingest a caffeinated supplement before a workout. A new observational study sought to investigate whether a man’s caffeine habit might be driving his testosterone levels downward. 52 BY MI CHAEL C. ZOUR DOS Accentuated Eccentrics are Overhyped Since you are stronger on the eccentric phase than the concentric phase, accentuated eccentric loading makes sense. However, the longitudinal data supporting this practice for enhancing strength gains is underwhelming. Does a new study turn the tides? 65 BY ER I C T R EXL ER Rye Versus Wheat: Evidence-Based Sandwich Guidelines A recent MASS article discussed the utility of fiber restriction for short-term weight cuts, but also cautioned against long-term adherence to low-fiber diets. A new study points to some potential mechanisms by which fiber might favorably impact body composition and health. 81 BY GR EG NUCKOL S & ER IC TREX LER Research Briefs In the Research Briefs section, Greg Nuckols and Eric Trexler share quick summaries of recent studies. Briefs are short and sweet, skimmable, and focused on the need-to-know information from each study. 125 BY MI CHAEL C. ZOUR DOS VIDEO: 1RM Prediction Part 2 Part 1 of this series suggested that reps performed equations have questionable efficacy for predicting 1RM. This installment breaks down the existing literature on submaximal velocity to predict 1RM. Does it fare better? Watch the video to find out. 127 BY ER I C HEL MS VIDEO: Periodization for Hypertrophy Part 2 Back in Volume 1 Dr. Helms noted in his intro to periodization videos that periodization for hypertrophy was a relatively unexplored topic. Five years later, we now have a number of meta-analyses on this topic as well as a broader understanding of how varying specific variables might impact hypertrophy. In part 2 of this video series, Dr. Helms covers the rationale specifically for periodizing exercises for maximizing hypertrophy. Letter From the Reviewers V olume 6, Issue 7 of MASS has arrived, and it’s one of our best yet. This month’s issue features one concept review, 12 study reviews, and 2 practical video lectures to help you take your training, nutrition, and coaching to the next level. In this month’s cover story, Dr. Helms explores a frequently asked question: can you stay shredded? Plenty of people aspire to get shredded at some point in their fitness journey, and many can achieve it. However, very, very few can maintain a very lean physique in perpetuity, or even for a period of several months. In this article, Dr. Helms dives into the scientific literature to explain the challenges we face en route to becoming shredded, the symptoms we might experience while attaining (and maintaining) a shredded physique, the physiological factors that directly promote those symptoms, the factors that make it easier or harder for certain individuals to stay lean, and some practical tips for maximizing your ability to get (and stay) shredded. On the training side, Dr. Zourdos has two fantastic articles this month. In the first, he covers a new paper investigating whether performance is improved by warmup routines involving a combination of foam rolling and dynamic stretching. In the second, he covers the popular concept of accentuated eccentrics. Some folks swear by training strategies that involve increased emphasis on heavy, controlled eccentrics, but this new study puts the idea to the test. On the nutrition side, Dr. Trexler reviews a new observational study suggesting that caffeine reduces testosterone levels. If that last sentence caused you to panic, take a few deep breaths and check the article out before you throw away your stash of coffee and pre-workout supplements. Dr. Trexler also covers an exploratory paper investigating how switching from refined wheat products to high-fiber rye products influences body composition, the gut microbiota, and the production of short-chain fatty acids. If you’re unfamiliar with short-chain fatty acids, you might be surprised to learn about their wide-ranging impacts throughout the body. Greg and Dr. Trexler teamed up on this month’s Research Briefs, collectively bringing you eight different study reviews on a diverse selection of training and nutrition topics. This month’s briefs cover squatting with bands, stretch-induced muscle hypertrophy, oral contraceptives, troubleshooting the weakest link of your split jerk, estimating the energy cost of various exercise modalities and intensities, habituation to caffeine’s ergogenic effects, krill oil’s effects on strength and hypertrophy, and how much cortisol levels matter for your gains. As for this month’s video content, Dr. Helms delivers part 2 of his video series on periodizing training for hypertrophy, with his newest installation focusing on exercise 5 selection. Dr. Zourdos is also contributing part 2 of an ongoing video series. His current series is all about practical strategies for estimating one-repetition maximum strength, and part 2 addresses the use of submaximal velocities. As always, be sure to check out the audio roundtables and join us in the Facebook group. Lastly, if you need some CEUs to maintain your current certifications, be sure to take advantage of our continuing education opportunities for NSCA, ACSM, NASM, and ACE. We hope you have a great month, and we thank you for being a part of MASS. Sincerely, The MASS Team Eric Helms, Greg Nuckols, Mike Zourdos, and Eric Trexler 6 COVER STORY Can You Stay Shredded? BY ERIC HELMS In the fitness industry many claim that with their system, supplement, or coaching, you can have your lean, dream body 24/7. While some get really lean, few, including bodybuilders, maintain a shredded physique year round. Why is this? Are there inevitable physiological consequences that make it very difficult to stay really lean, or are people just doing it wrong? 7 COVER STORY BA C KGRO UN D Getting really lean is a common goal, which a fair number of people regularly achieve, and it’s easy to find trainers and books to help you do so. However, getting lean and staying lean is often viewed as a holy grail, at least in bodybuilding-centric circles. People pursue this goal for many reasons, and it’s something many can relate to. As a bodybuilder and fan of bodybuilding, I’m awestruck by physiques lean enough to display all the anatomical muscular details of the human body. Thus, when I go through the grueling process of contest prep to get shredded, there’s always a part of me that wonders if maybe I could stay shredded, or at least stay something close to shredded. If you look around the fitness industry, and see what people buy, click on, and try, it’s apparent I’m not alone. The question is, why is it so hard for people to stay shredded once they get there? Speaking generally, regardless of the end-point body composition achieved, the difficulty of maintaining clinically meaningful, long term weight loss is well established (1). For those interested in learning how difficult it is (and why), I highly recommend reading Dr. Ben House’s excellent, in-depth guest article on this topic. However, while Dr. House’s review covers a related question, the present article isn’t about how hard it is to maintain weight loss. Rather, it specifically addresses the question of whether it’s sustainable to maintain a very low body fat. To discuss sustainability, however, I must acknowledge the subjectivity of the word. Everyone can technically sustain an extremely low level of body fat. Hypothetically, if you were locked in a room and only fed sufficient energy to lose weight until you got to essential levels of body fat, and then subsequently only fed enough to maintain those levels of body fat, you’d sustain a shredded physique. Whether or not you’d have full physiological functionality doing so, and whether you’d enjoy the experience enough for it to be worth it, however, are the more relevant questions. Indeed, if you’ve ever spoken to bodybuilders, they almost universally express sentiments of how difficult it is to get shredded for competition. At 3DMJ we’ve collectively prepped thousands of drug free physique competitors in the last decade, and we’ve been intimately involved in bodybuilding culture. When discussions of contest prep come up, we hear the same anecdotal reports time and time again of how it gets harder and harder as the weeks pass. Physique athletes report getting hungrier, more food focused, lethargic, tired, and irritable, and veterans notice they seem to get ill and injured more frequently as they get leaner. Indeed, many of these anecdotal experiences are mirrored in studies of physique 8 COVER STORY competitors during contest preparation and recovery. A collection of these findings are shown in Table 1, adapted from a review I led on the challenge of making physique sport a sustainable practice (2). However, it’s difficult to parse out whether these experiences and observations are caused by the state of being really lean, the process of getting really lean, or a combination of the two. Energy availability and RED-S To better understand the causes of the negative symptoms associated with getting really lean, we must discuss “relative energy deficiency in sport” (RED-S). RED-S describes the “impaired physiological functioning caused by 9 relative energy deficiency, and includes but is not limited to impairments of metabolic rate, menstrual function, bone health, immunity, protein synthesis, and cardiovascular health” (3). Importantly, research directly links RED-S to being in a chronic state of low energy availability, defined as the amount of calories consumed relative to lean body mass (LBM) when taking exercise activity into account. Mathematically, this is expressed as: (total energy intake - exercise expenditure) / LBM. If this value gets too low, athletes experience increased prevalence of RED-S symptoms. As reviewed by Anne Loucks (4, 5), a seminal researcher in this field, signs of metabolic and reproductive hormonal downregulation associated with RED-S are observed in diverse populations from lean, male Army Rangers during training, to exercising and sedentary normal weight women, to women with obesity undergoing rapid weight loss, when energy availability falls below ~30kcal/ kg of LBM/day through any combination of increased exercise energy expenditure and/or decreased energy intake. While 30kcal/kg/LBM/day is a decent rule of thumb to keep in mind, it should not be seen as a universal threshold that applies to all (6). Furthermore, most physique athletes in my experience simply won’t get into adequate contest shape without going lower than 30kcal/kg of LBM/day at a certain point, and even if you can stay above it, there is substantial individual variation as to when symptoms of RED-S crop up (in many cases, the threshold among athletes is higher, in the 30-45kcal/kg of LBM/day range). Differences in baseline non-exercise activity, one’s composition of LBM, and other individual physiological differences cause the appropriate energy availability for a given person to 10 vary (6). Regardless of where an individual’s threshold for low energy availability lies, you can view going below it as there not being enough “left over” energy for physiological function. When this continues chronically, adaptive downregulation across various aspects of physiology occur, which can impact performance and health (Figure 1). RED-S is relatively common among athletes with a high energy output, such as endurance athletes, or among athletes who are likely to restrict energy intake (3), such as physique athletes, weight class athletes, or athletes who benefit from a high power-to-weight ratio. When reflecting on the effects of RED-S and how energy availability is calculated, you might notice two things: 1) RED-S symptoms line up with the experiences of bodybuilders during contest prep, and 2) body fatness is not part of the energy availability equation. So, does this mean if a bodybuilder was to diet down to stage condition, then simply increase their calories or decrease their training energy expenditure to get out of a deficit, they’d be able to avoid all the RED-S symptoms and stay lean consequence free? Well, despite the current understanding that the singular cause of RED-S is low energy availability, independent of leanness, it is a little more complicated than that. Adaptive thermogenesis MASS readers are likely more familiar with the concept of metabolic adaptation, known more commonly in the literature as “adaptive thermogenesis,” than they are with RED-S and energy availability. Briefly, adaptive thermogenesis refers to a reduction in total energy expenditure following weight loss (or the increase following weight gain) beyond what would be predicted by changes in body composition (7, 8). For a deep dive, my colleague Dr. Trexler has a fantastic article that outlines its mechanisms and how to address it while dieting, and during maintenance post-diet. While the study of adaptive thermogenesis is distinct from the study of low energy availability, the two fields are interrelated and describe the same phenomena from different perspectives. The fitness industry focuses on adaptive thermogenesis because this research has been around longer and it attempts to understand how reductions in energy expenditure manifest, and how they impact efforts to lose weight and maintain weight loss. This lines up with the interests of the fitness industry, while low energy availability research doesn’t line up quite as well, as it addresses how to adequately fuel athletes for health and performance. Since adaptive thermogenesis is studied in relation to weight loss, the focus is on energy balance, rather than energy availability. People often have a difficult time conceptually integrating the two concepts, especially if they are new to the latter. The way to understand the link between the two is to consider the effects of adaptive thermogenesis beyond the simple quantitative reduction in energy expenditure. The causes of reduced energy expenditure are due to reduced sympathetic and increased parasympathetic nervous system tone and downregulation of the hypothalamic pituitary-thyroid and -gonadal axes, resulting in decreases in heart rate, thyroid hormone production, increases in skeletal 11 muscle work efficiency at low intensities, decreases in non-exercise activity expenditure, and reductions in sex hormone production (8). But these physiological changes don’t just reduce energy expenditure in a vacuum. Many of these changes also cause the symptoms associated with RED-S. Adaptive thermogenesis describes the degree to which the downregulation of physiological systems impacts energy expenditure, while RED-S describes how the downregulation impacts health and performance. Importantly, you can be at energy balance while being in a state of low energy availability and experiencing symptoms of RED-S. Unfortunately, adaptive thermogenesis doesn’t only occur during weight loss, but can persist during weight maintenance. In a classic study by Rosenbaum (7), seven trios of weight and sex matched participants spanning a range of bodyweights were compared. Each trio consisted of a participant who had lost at least 10% of their bodyweight and was maintaining that loss for 5-8 weeks, a participant who had lost at least 10% of their bodyweight and was maintaining it for at least a year, and a participant at their usual weight. Total energy expenditure was significantly lower among the weight-reduced participants compared to the participants at their usual weight, regardless of whether the weight loss had been maintained for 5-8 weeks, or a year or longer. Further, the reductions in energy expenditure were similar between the two weight-reduced groups. This seems to be a consistent trend when assessing the literature broadly (8), as 10% weight-reduced study participants display a ~15% lower total daily ADAPTIVE THERMOGENESIS DESCRIBES THE DEGREE TO WHICH THE DOWNREGULATION OF PHYSIOLOGICAL SYSTEMS IMPACTS ENERGY EXPENDITURE, WHILE RED-S DESCRIBES HOW THE DOWNREGULATION IMPACTS HEALTH AND PERFORMANCE energy expenditure on average compared to their non-weight-reduced counterparts. Considering the above, let’s do a little bit of math. Using this calculator (9), a 170cm (~5’6”), 70kg (~154lbs), 25 year old, very lean woman at 12% body fat, who performs moderate exercise 4-5 days per week has an estimated daily energy expenditure of 2491kcals. If she was sedentary, she would instead have an expenditure of 2041kcals; the difference between these two values can be used to represent her average exercise energy expenditure of 450kcals per day. If this woman was previously 77kg, and had lost 10% of her bodyweight to reach 70kg, we could reasonably expect a ~15% reduction in energy expenditure based on the literature. Thus, her daily energy expenditure of 2491kcals would instead be ~2117kcals. At 70kg and 12% body fat, she has 61.6kg of LBM. Thus, if she was eating at maintenance following weight loss, we could calculate her energy availabil- 12 ity using the previously mentioned equation ([total energy intake - exercise expenditure] / LBM) as follows: (2117kcals - 450kcals) / 61.6kg = 27.1kcal/kg of LBM/day. As you can see, this intake, despite being her maintenance calories, is below the ~30kcal/kg of LBM/day average threshold for low energy availability where we’d anticipate symptoms of RED-S would occur. Certainly, not everyone experiences a 15% reduction in total energy expenditure after weight loss; some experience less, some more. But, on average, if we accept the current understanding that energy availability is the sole cause of RED-S with no influence of body composition, it seems unlikely that the majority of individuals would be able to maintain a very low body fat after weight loss without experiencing some symptoms of RED-S. However, this begs the question: if it just comes down to energy availability, and body fat doesn’t enter the equation, why does physiological function remain downregulated in weight-reduced individuals in the first place? Body fat “set points” and leptin To answer the question I just posed, I don’t think it comes down to energy availability exclusively. I think body fat plays a role, and it’s hard to think otherwise when you understand the physiology at play. If you’ve observed discussions on dieting in the evidence-based fitness space, you might have heard the concept of a “body fat set point”. Generally, the idea is that people have a level of body fat that is “defended” (i.e., adap- tive thermogenesis occurs) when fat loss takes you below it, or when fat gain takes you above it. This concept originated from scientific research that’s been ongoing for the better part of 70 years. Indeed, the set point concept describes the original “lipostatic” model of body weight regulation proposed by Kennedy in 1953 (10). This model states that, like a thermostat, adipose tissue sends signals to the brain indicating whether body fat stores are below, at, or above a person’s body fat set point. In response, the brain sends signals to downregulate, maintain, or upregulate energy expenditure, and increase, maintain, or decrease energy intake, respectively, to get back to the body fat set point. This model was largely theoretical until the discovery of leptin in the 1990’s (11), a hormone that seemed to act as the proposed signal from the lipostatic model. Leptin is a hormone secreted by adipose tissue in proportion to the amount of adipose tissue present (12), and, in initial animal models, leptin would decrease with weight loss, increase with weight gain, and returned to baseline when animals compensatorily increased or decreased food intake following these states to return to a seeming “set point” (13). However, the pure lipostatic model has a lot of problems, and is not the current model used to understand body weight regulation. From an observational perspective, the lipostatic model fails to explain the obesity epidemic, and from a mechanistic perspective, leptin doesn’t behave exactly like the lipostatic model’s signal is supposed to. Specifically, it seems leptin release from adipose tissue is impacted by metabolic hormones, such 13 as insulin and others, that respond acutely to feeding and fasting (14). Leptin decreases precipitously upon the initiation of fasting, and this response precedes (and is disproportionate to) changes in body fat. Further, as research on leptin continued, it was discovered that, while leptin is primarily produced by adipose tissue (15), it is produced (and there are receptors for it) in other tissues as well, notably the stomach. Gastrically produced leptin is thought to be a signaller of short-term energy availability, while adipose tissue derived leptin may act as a long term signal of energy availability (16). Indeed, changes in macronutrients and energy intake can acutely change leptin (17). Also out of step with the lipostatic model is that leptin is much more effective at encouraging weight gain when levels are low, as opposed to encouraging weight loss when levels are high. Indeed, circulating leptin is quite high in those with common forms of obesity, but does not suppress excess energy consumption enough to cause weight loss (18). As reviewed by Speakman and colleagues (13; notably this is open access and very informative), to account for these observations and complexities, the “dual-intervention model” of body weight regulation was eventually proposed, which arguably is the best fit for the currently available data. It accounts for environmental factors that can overcome physiological set points, which lines up with the obesity epidemic and the nuances of leptin physiology. As shown in Figure 3, there are upper and lower points where physiological factors primarily influence energy intake and expenditure, modifying adiposity. Between these points, however, environmental factors dominate. These upper and lower points are thought to exist due to evolutionary predation and famine selection pressures (i.e., being too heavy and slow made you more likely to be eaten, being too lean made you more vulnerable to famine), respectively (13). Arguably, the latter was a greater threat to humans, resulting in a better defended lower intervention point, hence the struggles many have with weight gain and regain. This model provides hope for those interested in maintaining a lower body fat. Based on the model, if you can modify your environment to do the opposite of what the modern, obesogenic environment has done to our collective waist lines, you should be able to hang out closer to your lower, rather than your upper intervention point. In fact, by examining people living in a non-modern environment, we can see this is probably the case. One 14 such group, the Amish, live in traditionalist communities that typically don’t adopt most conveniences of modern technology. Bassett and colleagues (19) examined the physical activity and body composition of a sample of 98 Amish men and women from a community in Ontario that did not use electricity or gas power, and of whom the majority of men were farmers (78%) and the majority of women were homemakers (69%). The researchers gave the Amish participants pedometers to track their step count, and assessed their body composition via bioelectrical impedance measurements. In this agricultural community, they made their own food, and the requisite activity levels for day-to-day work were very high compared to modern standards. The men walked an average of 18,425 ± 4,685 steps per day, and the women an average of 14,196 ± 4,078. Interestingly, the men had an average body fat percentage of 9.4 ± 4.3%, and the women an average of 25.3 ± 6.7%. Importantly, these are bioimpedance measurements, so they aren’t as accurate or reliable, even at the group level, as alternative measurement options like DXA. However, with a sample of nearly 100 individuals, they are likely close to the true values. Notably, the more active men were maintaining, on average, a single digit body fat percentage. The women weren’t as lean relatively, even taking sex differences into account (the rough female equivalent to a male at ~9-10% body fat is ~17-18%), and also weren’t as active. While it’s tempting to isolate this difference in body fat percentage to the men being more active, it’s not as though the women weren’t reasonably active as well. Rather, other cultural or environmental aspects were likely at play, which led to the women being relatively higher in body fat (for example, the authors noted Amish women have an average of seven children, which can result in a higher average body fat). So, if we assume the men didn’t have RED-S - a reasonable assumption as the community had an ample food supply (earlier research on Amish men reports a daily energy intake of ~3600kcal/day [20]) and they weren’t athletes trying to stay lean - this suggests your environment plays a major role in how lean you stay. In support of this contention, decreases in sedentary activity (21) and ultra-processed food consumption (22) can lead to maintaining lower body fat levels. However, it’s important to point out that 9.4 ± 4.3% body fat is not 5 ± 1% body fat. These Amish dudes are lean, some more and some less than others, but on average they aren’t ready to don posing trunks to show off their striated glutes. Putting it all together If we put these models and observational data together, we can construct a relatively clear, albeit simplified (23) theoretical explanation of what determines the level of leanness you can sustainably maintain. Starting with the dual intervention model as the backdrop, when you are between your lower and upper intervention points of adiposity, you’ll likely feel fine. However, bringing in the RED-S model, this is only true until you reduce your energy intake or increase your energy expenditure to the point where you reach your threshold for low energy availability. When this happens, regardless of where your body fat level is between your intervention points, RED-S and 15 adaptive thermogenesis may occur. However, if you can manipulate your body fat gradually, so that you don’t reduce energy intake to the point where you reach a state of low energy availability, you can mitigate adaptive thermogenesis and symptoms of RED-S. That is, until you pass your lower intervention point, which is where body fat comes into the picture. As discussed, leptin transiently fluctuates in response to meals and acute changes in energy balance, and each time you eat you can get a nice bump in leptin. However, the largest contributor to your circulating leptin levels is far and away fat mass. To put a specific number to it, Considine and colleagues reported a strong correlation (r = 0.85, p < 0.001) between serum leptin and body fat percentage across a combined sample of 136 normal-weight participants and 139 participants with obesity (12). Meaning, in this large, diverse sample, body fat percentage explained ~72% of the variance in leptin. Thus, even if you’re eating at maintenance, when you’re between meals (which is most of the day), your leptin will fall to low levels when below your lower intervention point. As a consequence, total energy expenditure will remain suppressed, keeping you in a state of low energy availability, leading to symptoms of RED-S. Indeed, we can’t discount the important effect of chronic leptin levels; the only known intervention besides regaining lost body fat that alleviates adaptive thermogenesis (and likely RED-S symptoms for some) in weight-reduced individuals are multiple daily leptin injections (8). I know what some of you are thinking: “but Eric I know some people who walk around THE LARGEST CONTRIBUTOR TO YOUR CIRCULATING LEPTIN LEVELS IS FAR AND AWAY FAT MASS shredded who are just fine!” So do I, and this still lines up with the theoretical understanding I’ve proposed. Importantly, there is a ton of individual variation at play. Individual variation exists in where one’s lower intervention point is (some people have a leaner lower end point), the energy threshold for when RED-S symptoms crop up (some people do okay at lower values), and whether and how much a person experiences adaptive thermogenesis during and after weight loss (some people don’t experience much at all). Thus, you’ll see people who maintain a variety of different body fat levels, despite living in similar environments. For example, not everyone in our modern obesogenic environment has obesity. Likewise, the Amish men had a body fat standard deviation of 4.3%, meaning (if we trust the bioelectrical impedance measurements) some were walking around at 5% body fat, but just as many were walking around at 14% (maybe; 24). Also consider that when there are strong rewards at play, people might be okay with living with mild or even moderate RED-S symp- 16 toms. To harken back to a prior MASS article, I reviewed a paper on energy availability in a group of elite female sprinters who were maintaining reasonably lean (~20% body fat) physiques (article; 25). Interestingly, the sprinters with more indicators of low energy availability had higher fat mass (13.0 ± 2.3kg vs. 11.2 ± 1.6kg, p = 0.03) compared to the leaner sprinters with fewer indicators. While speculative, I guessed this was due to the selection pressures of being an elite sprinter, where having less fat mass means you can run faster. Thus, there were those with a lower intervention point at a lower body fat level who were able to stay leaner without issue, while the rest who weren’t so lucky had to stay in a perpetual weight-reduced, low energy availability state to stay lean (but not quite as lean). Simply put, athletes like to win, and they are often okay with some health and comfort trade-offs if being leaner will improve their performance (I would also note that influencers like your money and attention, and being leaner helps them get that too). This is why athletes in sports where a lower body fat improves performance tend to be leaner (26), and, while many of these athletes have the genetics to be naturally lean, not all of them do, which is why athletes in these sports are also more likely to experience RED-S (2). Testing the hypothesis that body fat matters We can assess the veracity of the theoretical explanation I’ve presented that it’s not just energy availability, but also your lower body fat intervention point that dictates how lean you can maintain. If body fat played no role, and it just came down to energy availability, you’d expect dieting to impact people in similar ways, regardless of their body fat level when starting the diet, but it doesn’t. For example, authors of a recently published meta-analysis reported that caloric restriction resulted in an increase in testosterone in the majority of studies on men with overweight or obesity, while the majority of studies on normal weight men reported a decrease (27). Likewise, muscle protein synthesis is blunted during an energy deficit in overweight dieters (28), but, in lean dieters, not only is protein synthesis blunted, but protein breakdown increases as well (29). Furthermore, lean individuals utilize two to three fold more energy from protein when fasting compared to individuals with obesity (30) and are more likely to lose lean mass while dieting (31). However, the most direct evidence we have to test my hypothesis that body fat matters, are observations of what happens when people get very lean, and then try to stay very lean. In a case series on physique athletes by Longstrom and colleagues, some of the competitors did just that, following conservative “reverse diets” to minimize fat gain post contest by slowly increasing calories and decreasing cardio (32). Longstrom measured body composition and metabolic hormones 1-2 weeks prior to competition, as well as 8-10 weeks post-contest once the competitors had carried out their post competition strategies. Generally, Longstrom reported that those who increased fat and body mass the most, experienced larger increases in leptin and resting metabolic rate, while smaller increases or no 17 changes occurred in those who gained very little fat and body mass. If you examine Figures 3 and 4 from this study, you can see that two of the male competitors (M1 and M2) only increased their body fat by ~2%, staying below 10% body fat even 8-10 weeks post competition. Likewise, one female competitor (F4) increased her body fat by just 2.7%, only getting up to ~15% body fat 8-10 weeks post show, which was the body fat that the other three females achieved at the end of their diets. Notably, these two male competitors experienced no appreciable change in leptin, and F2 had the lowest leptin value of the female competitors. Likewise, resting metabolic rate only slightly increased (M3), stayed the same (M2), or slightly decreased (F2) among these competitors. Finally, at the group level, the observations were also consistent with the hypothesis that fat mass does indeed play a role in hormonal and metabolic recovery. The change in fat mass was strongly associated (33) with the change in resting metabolic rate (τ = 0.90; p = 0.001) and the change in body fat percentage was strongly associated with changes in leptin (τ = 0.88; p = 0.003). Takeaways It’s difficult to piece together complex, distinct lines of research on how humans adapt to changes in short and long term energy availability. Different models tell a piece of the story, but not all of it. A pure focus on low energy availability can lead one to think that body fat plays no role in the symptoms we associate with RED-S, but effectively ignores ~70 years of research on body composition regulation. Similarly, a pure focus on adaptive thermogenesis can neglect the effect of these adaptations on health and performance, focusing only on how it changes energy expenditure. In totality, it’s likely that energy availability is the dominant variable impacting your physiology when you’re between your upper and lower body fat intervention points. However, when you go below your lower intervention point, you’ll 18 be persistently fought by your body and you probably won’t be able to get your calories high enough (without fat gain) to alleviate the negative effects you experience. With that said, some people can stay really lean, as they happen to have a leaner lower intervention point. For those of us that are not so lucky, that doesn’t mean all hope is lost. Rather, it just means that we have to respect wherever our lower intervention points might be. Further, you can do all the things we’ve talked about in MASS time and time again (like eating sufficient protein and lots of low energy density, high fiber fruits and vegetables, increasing activity and reducing sedentary time, reducing ultra-processed and highly palatable food intake, and of course, lifting lots of weights) to modify your environment so you can stay close to it. 19 References 1. Hall, K. D., & Kahan, S. (2018). Maintenance of Lost Weight and Long-Term Management of Obesity. The Medical clinics of North America, 102(1), 183–197. 2. Helms, E. R., Prnjak, K., & Linardon, J. (2019). Towards a Sustainable Nutrition Paradigm in Physique Sport: A Narrative Review. Sports (Basel, Switzerland), 7(7), 172. 3. Mountjoy, M., Sundgot-Borgen, J., Burke, L., Ackerman, K. E., Blauwet, C., Constantini, et al. (2018). International Olympic Committee (IOC) Consensus Statement on Relative Energy Deficiency in Sport (RED-S): 2018 Update. International Journal of Sport Nutrition and Exercise Metabolism, 28(4), 316–331. 4. Loucks A. B. (2004). Energy balance and body composition in sports and exercise. Journal of Sports Sciences, 22(1), 1–14. 5. Loucks A. B. (2003). Energy availability, not body fatness, regulates reproductive function in women. Exercise and sport sciences reviews, 31(3), 144–148. 6. Burke, L. M., Lundy, B., Fahrenholtz, I. L., & Melin, A. K. (2018). Pitfalls of Conducting and Interpreting Estimates of Energy Availability in Free-Living Athletes. International Journal of Sport Nutrition and Exercise Metabolism, 28(4), 350–363. 7. Rosenbaum, M., Hirsch, J., Gallagher, D. A., & Leibel, R. L. (2008). Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. The American Journal of Clinical Nutrition, 88(4), 906–912. 8. Rosenbaum, M., & Leibel, R. L. (2010). Adaptive thermogenesis in humans. International Journal of Obesity (2005), 34 Suppl 1(0 1), S47–S55. 9. Click the settings icon, then use the Katch-McArdle equation which takes body fat percentage into account to replicate. 10. Kennedy G. C. (1953). The role of depot fat in the hypothalamic control of food intake in the rat. Proceedings of the Royal Society of London. Series B, Biological Sciences, 140(901), 578–596. 11. Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L., & Friedman, J. M. (1994). Positional cloning of the mouse obese gene and its human homologue. Nature, 372(6505), 425–432. 12. Considine, R. V., Sinha, M. K., Heiman, M. L., Kriauciunas, A., Stephens, T. W., Nyce, et al. (1996). Serum immunoreactive-leptin concentrations in normal-weight and obese humans. The New England Journal of Medicine, 334(5), 292–295. 20 13. Speakman, J. R., Levitsky, D. A., Allison, D. B., Bray, M. S., de Castro, J. M., Clegg, D. J., et al. (2011). Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Disease Models & Mechanisms, 4(6), 733–745. 14. Ahima, R. S., & Flier, J. S. (2000). Leptin. Annual Review of Physiology, 62, 413–437. 15. Kasacka, I., Piotrowska, Ż., Niezgoda, M., & Łebkowski, W. (2019). Differences in leptin biosynthesis in the stomach and in serum leptin level between men and women. Journal of Gastroenterology and Hepatology, 34(11), 1922–1928. 16. Picó, C., Oliver, P., Sánchez, J., & Palou, A. (2003). Gastric leptin: a putative role in the short-term regulation of food intake. The British Journal of Nutrition, 90(4), 735–741. 17. Izadi, V., Saraf-Bank, S., & Azadbakht, L. (2014). Dietary intakes and leptin concentrations. ARYA Atherosclerosis, 10(5), 266–272. 18. Myers, M. G., Cowley, M. A., & Münzberg, H. (2008). Mechanisms of leptin action and leptin resistance. Annual Review of Physiology, 70, 537–556. 19. Bassett, D. R., Schneider, P. L., & Huntington, G. E. (2004). Physical activity in an Old Order Amish community. Medicine and Science in Sports and Exercise, 36(1), 79–85. 20. Weale, V.W., (1980). Eating patterns and food energy and nutrient intake of old order amish in Holmes county, Ohio (Doctoral dissertation, The Ohio State University). 21. Júdice, P. B., Hetherington-Rauth, M., Magalhães, J. P., Correia, I. R., & Sardinha, L. B. (2022). Sedentary behaviours and their relationship with body composition of athletes. European Journal of Sport Science, 22(3), 474–480. 22. Hall, K. D., Ayuketah, A., Brychta, R., Cai, H., Cassimatis, T., Chen, K. Y., et al. (2019). Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metabolism, 30(1), 67–77. e3. 23. I call this “simplified” because it holds up when conceptualizing what happens with normal weight individuals attempting to get lean and stay lean; however, it does not for individuals with obesity and/or metabolic disease. Large amounts of fat gain can change one’s intervention points, and leptin resistance, which is common in those with obesity, can impair the physiological responses which attempt to prevent further weight gain. 24. Standard deviations only accurately represent normally distributed data (i.e., shaped like a bell curve). It’s quite possible, given how the dual intervention model works, that body fat wasn’t normally distributed. There may have been just a few outlier men who were close to 5%, and then a lot clustering around 9-12% to produce the mean. 25. Sygo, J., Coates, A. M., Sesbreno, E., Mountjoy, M. L., & Burr, J. F. (2018). Prevalence 21 of Indicators of Low Energy Availability in Elite Female Sprinters. International Journal of Sport Nutrition and Exercise Metabolism, 28(5), 490–496. 26. Jeukendrup, A. and Gleeson, M., (2018). Sport Nutrition. Human Kinetics. 27. Smith, S. J., Teo, S., Lopresti, A. L., Heritage, B., & Fairchild, T. J. (2022). Examining the effects of calorie restriction on testosterone concentrations in men: a systematic review and meta-analysis. Nutrition Reviews, 80(5), 1222–1236. 28. Hector, A. J., McGlory, C., Damas, F., Mazara, N., Baker, S. K., & Phillips, S. M. (2018). Pronounced energy restriction with elevated protein intake results in no change in proteolysis and reductions in skeletal muscle protein synthesis that are mitigated by resistance exercise. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 32(1), 265–275. 29. Carbone, J. W., Pasiakos, S. M., Vislocky, L. M., Anderson, J. M., & Rodriguez, N. R. (2014). Effects of short-term energy deficit on muscle protein breakdown and intramuscular proteolysis in normal-weight young adults. Applied Physiology, Nutrition, and Metabolism, 39(8), 960–968. 30. Elia, M., Stubbs, R. J., & Henry, C. J. (1999). Differences in fat, carbohydrate, and protein metabolism between lean and obese subjects undergoing total starvation. Obesity Research, 7(6), 597–604. 31. Helms, E. R., Zinn, C., Rowlands, D. S., & Brown, S. R. (2014). A systematic review of dietary protein during caloric restriction in resistance trained lean athletes: a case for higher intakes. International Journal of Sport Nutrition and Exercise Metabolism, 24(2), 127–138. 32. Longstrom, J. M., Colenso-Semple, L. M., Waddell, B. J., Mastrofini, G., Trexler, E. T., & Campbell, B. I. (2020). Physiological, Psychological and Performance-Related Changes Following Physique Competition: A Case-Series. Journal of Functional Morphology and Kinesiology, 5(2), 27. 33. For those unfamiliar with the “τ” symbol, it represents Kendall’s tau, which is a nonparametric correlation coefficient, interpreted similarly to Pearson’s r. A value of zero reflects no correlation, and values closer to 1 or -1 represent stronger correlations, with the sign of the tau value (positive or negative) reflecting the direction of the association. █ 22 Study Reviewed: An Intense Warm-Up Does Not Potentiate Performance Before or After a Single Bout of Foam Rolling. Konrad et al. (2022) What’s Worth Including in Your Warm-Up? BY MICHAEL C. ZOURDOS Our previous forays into foam rolling determined that it acutely increases range of motion, but not performance. But does foam rolling enhance performance when combined with dynamic stretching? This article breaks down the new findings. 23 KEY POINTS 1. Researchers compared the acute effects of three warm-up conditions: 1) foam rolling only, 2) dynamic stretching followed by foam rolling, and 3) foam rolling followed by dynamic stretching. Outcomes assessed were sit-andreach test performance (hamstring range of motion), hamstring strength, and countermovement jump performance. 2. Findings showed that sit-and-reach performance improved from pre- to postwarm-up in all conditions, but with no difference between conditions. Strength and jump performance did not significantly change in any condition. Lastly, men increased their hamstring range of motion 7% more than women in the foam rolling first condition. 3. This study shows that combining dynamic stretching with foam rolling fails to acutely improve performance. However, previous literature indicates that dynamic stretching alone may improve performance. Therefore, it remains good practice to include dynamic stretching in a warm-up. Foam rolling can be included for range of motion based on personal preference and individual needs. T here’s no doubt that foam rolling acutely increases range of motion. However, its benefits on post-exercise recovery are small (2). Further, three different systematic reviews and meta-analyses (2, 3, 4) have concluded that pre-training foam rolling does not significantly improve acute strength performance. To be fair, foam rolling doesn’t seem to harm performance when completed as part of the warm-up (2, 3, 4, 5), and it comes with little downside. Research has also suggested that static stretching in conjunction with foam rolling does not enhance acute performance (6); however, research is mixed on the combination of dynamic stretching and foam rolling (7) to improve acute strength performance. The reviewed crossover design study from Konrad et al (1) assessed the sit-and-reach test (hamstring range of motion), dynamic and isometric hamstring strength, and coun- termovement jump height in men and women before and after three different warm-up protocols. In one condition, subjects performed dynamic stretching followed by foam rolling (foam rolling second). In another condition, the subjects foam rolled first (foam rolling first), then completed the dynamic stretching. Finally, in a third condition, subjects only foam rolled (i.e., no dynamic stretching). Findings showed that all conditions increased range of motion pre- to post-warm-up, with no significant differences between conditions. Dynamic strength, isometric strength, and countermovement jump height failed to significantly improve from pre- to post-warm-up. When comparing the sexes, researchers found that range of motion increased significantly more in men than in women (p < 0.001) in the foam rolling first condition. These findings suggest that pairing dynamic stretching and foam rolling in a warm-up does not improve ham- 24 string strength performance or range of motion more than foam rolling alone. Therefore, we can confidently state that foam rolling, on average, is unlikely to produce a meaningful benefit for acute strength performance. This article will aim to: in men and women. Further, the researchers compared the magnitude of change for each outcome measure between the sexes. 1. Review the present findings and discuss what we can and can’t infer from the study design. Subjects and Methods 2. Evaluate the research combining foam rolling and dynamic stretching in warm-ups. 27 “recreational to well-trained” soccer players (13 women and 14 men) completed the study. While the average age of the subjects was over 18 years old, some participants were under 18, as the authors stated, “Participants or (if under 18) their legal representatives, signed a written informed consent form.” Additional subject details are in Table 1. 3. Examine if foam rolling in conjunction with other warm-up strategies (i.e., static stretching) can benefit acute strength performance. 4. Provide recommendations for a well-structured lifting warm-up. Purpose and Hypotheses Purpose The presently reviewed study compared warm-ups consisting of foam rolling only, foam rolling + dynamic stretching (foam rolling first), and dynamic stretching + foam rolling (foam rolling second) for acute changes in hamstring range of motion, hamstring strength, and countermovement jump height Hypotheses The researchers did not provide hypotheses. Subjects Study Protocol The reviewed study was a counterbalanced crossover design with three conditions separated by at least 48 hours. In all three conditions, subjects completed low-intensity cycling for five minutes and then performed sit-and-reach (hamstring range of motion), dynamic and isometric torque, and countermovement jump height tests. Next, subjects underwent a condition-specific warm-up protocol and repeated the tests. The three condition-specific warm-up protocols were: 25 1. Foam rolling only. 2. Foam rolling followed by dynamic stretching (foam rolling first). 3. Dynamic stretching followed by foam rolling (foam rolling second). Foam Rolling Specifics Foam rollers consisted only of foam and not hard plastic. Each subject rolled the posterior thigh on each leg for two total minutes. Rolling was performed at a cadence of 2 seconds from distal to proximal and 2 seconds from proximal to distal (i.e., 2 seconds up the thigh and 2 seconds down). Each individual rolled with their body weight, and subjects were instructed to put pressure on the roller to the point of discomfort. The “point of discomfort” was intended as a 7 out of 10 on a 10-point visual analog scale. Dynamic Stretching Specifics The dynamic stretching protocol included three sets of 30 reps of butt kicks on each leg with 15 seconds between sets. Next, subjects laid face down while researchers placed a swiss ball on their backs, and then kicked the ball with their heel (leg curl motion), alternating each foot; however, the researchers did not specify the sets and reps for this exercise. An illustration of the swiss ball exercise can be seen in Figure 2. Findings The findings were simple. Range of motion significantly increased from pre- to postwarm-up in all conditions, but with no significant difference between conditions. Dynamic 26 and isometric torque and countermovement jump did not statistically change in any condition. Between-condition comparisons for all outcome measures are in Table 2. Sex-based comparisons revealed that women had a significantly greater range of motion at baseline than men in all conditions. Range of motion increased significantly more in men (+9.34%) than in women (+2.30%) in the foam rolling first condition. Men also experienced greater percentage increases in range of motion in the foam rolling second condition (men: +7.63%; women: +3.96%) and the foam rolling only condition (men: +6.34%; women: +3.97%); however, these differences were not statistically significant. As a disclaimer, I estimated all percentage changes from WebPlotDigitizer; thus, slight discrepancies may exist from the actual values. Changes in range of motion for both sexes can be seen in Figure 3. Interpretation The presently reviewed study from Konrad et al (1) showed that a warm-up consisting of foam rolling alone or foam rolling com- 27 bined with dynamic stretching increased acute range of motion, but not strength or jump performance. I’m not surprised that the foam rolling only condition failed to acutely improve performance. While a handful of studies show that foam rolling enhances acute strength performance (8, 9), two meta-analyses (2, 4) and a systematic review (3) have found that foam rolling does not significantly improve acute strength. Further, those same meta-analyses and systematic reviews show that foam rolling enhances acute range of motion; thus, the presently reviewed study’s findings agree with the majority of the literature examining a foam rolling only warm-up. The findings for the two dynamic stretching conditions are interesting for two reasons. First, foam rolling aside, dynamic stretching may improve acute strength performance. Mechanistically, dynamic stretching increases acute muscle blood flow and muscle fiber conduction velocity (7, 10). However, I say “may improve” because, although various reviews have determined a positive effect of dynamic stretching on strength (10, 11) some individual studies have shown no performance benefit with dynamic stretching (12, 13). Dynamic stretching may not improve performance if the protocol is too demanding such that it fatigues the athlete. However, dynamic stretching didn’t seem to be too fatiguing in this study since performance did not decline from pre- to post-warm-up in either dynamic stretching condition. It’s also possible that the foam rolling protocol was too fatiguing. The foam rolling protocol was a 7/10 discomfort, which is on the upper end of our recommendations (slide 12 here), so it wasn’t anything crazy. The second reason that the findings of the dynamic stretching condition are interesting is that a review (7) and a handful of recent studies (14, 15, 16, 17, 18, 19, 20, 21) have addressed the concept of warming up with foam rolling in conjunction with dynamic stretching and observed mixed results. Those studies are summarized in Table 3. When reviewing the eight studies in Table 3, there seems to be some efficacy for the combination of dynamic stretching and foam rolling. Seven of the eight studies that used a pre- to post-warm-up crossover design found that dynamic stretching + foam rolling improved some metrics (range of motion or performance) from pre- to post-warm-up. A warm-up consisting of dynamic stretching + foam rolling also improved jump performance more than walking (14) and improved jump performance and bench press strength more than jogging (15). Five studies (16, 17, 18, 19, 20) compared dynamic stretching + foam rolling to dynamic stretching alone; however, among the 12 comparisons in Table 3, dynamic stretching + foam rolling was more beneficial for only one performance outcome – hamstring muscle endurance (17). This finding was observed by Chen et al., and the study protocol utilized vibration foam rolling, which also has a mixed record on improving acute strength performance (22). Overall, the results on the efficacy of dynamic stretching + foam rolling, coupled with the fact that foam rolling alone doesn’t typically improve strength performance (2, 3, 4), seem to confirm that 28 a combination of both warm-up modalities doesn’t boost acute performance above dynamic stretching alone. However, the presently reviewed study by Konrad et al. (1) did not directly compare dynamic stretching only to dynamic stretching + foam rolling. The fact that foam rolling only and dynamic stretching + foam rolling result in similar increases in acute range of motion is unsurpris- ing, but useful for the lifter. If a lifter performs dynamic stretching, but skips foam rolling, they are unlikely to miss out on the range of motion benefits. Of course, we can only directly apply the findings from the presently reviewed study to the sit-and-reach test. Further, foam rolling provides similar acute range of motion benefits to static strength (5, 6), and dynamic stretching usually provides similar 29 range of motion benefits to static stretching (11). Therefore, when considering all of the range of motion literature, I suspect that dynamic stretching provides similar acute range of motion benefits to foam rolling. Still, if you want to incorporate foam rolling to increase range of motion, I don’t see the downside other than the extra time required. The last finding to discuss from the presently reviewed study is the greater increase in acute hamstring range of motion in men versus women in the foam rolling first condition. This difference could be attributed to the 5.27 cm shorter sit-and-reach at baseline in men (31.46 ± 5.15 cm) compared to women (36.73 ± 3.33 cm) in the foam rolling first condition (it was similarly different in all conditions). Therefore, as the researchers suggested, men seemingly had more potential to increase their range of motion. Notably, even with a 7.04% greater increase in the sit-and-reach test, men still had a shorter range of motion on the sit-and-reach test than women post-warm-up (men: 34.4 cm; women: 37.57 cm). Previous data also found that women have a greater range of motion than men in various joints (23, 24). Given the sexbased difference in baseline levels, men may require a greater absolute increase in acute range of motion pre-training to maximize performance; however, we currently lack the requisite data to substantiate this speculation. When considering how to structure a warmup, lifters usually consider four options before moving to an empty barbell and light weights. Those options are: 1) low-intensity walking/cycling, 2) static stretching, 3) foam rolling, and 4) dynamic stretching. Some may consider other warm-up techniques, such as massage, partner-assisted stretching, or even cooling or heating therapies; however, the aforementioned four are the most common and relevant to this article. If creating a hierarchy (dare I say a pyramid?) of these options, dynamic stretching would rank first for inclusion in a warm-up, as it’s the only one to at least somewhat consistently improve performance. Further, dynamic stretching probably results in similar increases in range of motion as both foam rolling and static stretching. Static stretching would rank last on the list unless a physical therapist suggests its inclusion for some reason for a specific individual. You may be gearing up to remind me that if static stretching is kept short and not too in- 30 tense, performance is unlikely to be harmed. I agree, and I’ve articulated that position in MASS on various occasions (one, two). However, static stretching also doesn’t offer any unique benefits and it is more likely to decrease acute performance compared to other warm-ups options. I’d rank foam rolling and low-intensity walking/cycling as two and three, respectively, without a strong opinion on the order. It’s really personal preference if someone wants to include foam rolling or low-intensity walking/cycling. I don’t see either option having a positive or negative effect on performance if performed appropriately, so if someone feels good doing them, then go for it, as long as time permits. Lastly, an important rule of warming up is to ensure that the warm-up doesn’t harm performance. So, even when considering dynamic stretching, I would keep the practice to about 5 minutes to avoid fatigue. Similarly, I would keep the duration and intensity of walking/cycling and foam rolling in check. Figure 4 represents a basic flow chart of warm-up prescriptions with optional items denoted with asterisks. shoulder joint) and lower body ranges of motion. Then, squat and bench press 1RM could be assessed before and after the warm-up period. It would also be nice to assess the combination of dynamic stretching and foam rolling on muscle endurance (reps performed). In that case, reps performed could be evaluated instead of 1RM or after 1RM. However, if researchers wanted to do both and avoid the potential fatigue of performing reps to failure after a 1RM test, they could use a within-subject design with a unilateral exercise such as the leg extension, and test 1RM on one leg and reps performed at a moderate load (i.e., 70% of 1RM) on the other leg. Next Steps While I found this study interesting, it would have been more valuable for MASS readers if it included range of motion in other joints and examined performance with free-weight exercises (e.g., squat, bench press, and deadlift). Therefore, I’d like to see this study replicated with a few changes. First, I would add a fourth condition of dynamic stretching only. Second, I would expand the foam rolling and dynamic stretching routines to target the upper body and then test upper body (e.g., 31 APPLICATION AND TAKEAWAYS 1. Konrad (1) found that combining dynamic stretching and foam rolling did not increase acute performance, nor did it improve acute hamstring range of motion more than foam rolling alone. 2. The warm-up literature as a whole suggests that dynamic stretching may improve performance, foam rolling is unlikely to increase performance, and there is probably a similar acute range of motion benefit between the two strategies. 3. It is good practice to include dynamic stretching as a part of your warm-up, but foam rolling may not provide additional benefits to dynamic stretching. Nonetheless, lifters should feel free to include it based upon personal preference or a physical therapist’s recommendation. 4. Most importantly, the first rule of a warm-up is to ensure that it’s not too fatiguing and does not harm performance. Therefore, a dynamic stretching routine should remain relatively short (~5 minutes) and should be adjusted by the individual lifter to best fit their needs. 32 References 1. Konrad A, Bernsteiner D, Reiner MM, Nakamura M, Tilp M. An Intense Warm-Up Does Not Potentiate Performance Before or After a Single Bout of Foam Rolling. Journal of Sports Science and Medicine. 2022 Mar 4;21(2):145-52. 2. Wiewelhove T, Döweling A, Schneider C, Hottenrott L, Meyer T, Kellmann M, Pfeiffer M, Ferrauti A. A meta-analysis of the effects of foam rolling on performance and recovery. Frontiers in physiology. 2019:376. 3. Cheatham SW, Kolber MJ, Cain M, Lee M. The effects of self‐myofascial release using a foam roll or roller massager on joint range of motion, muscle recovery, and performance: a systematic review. International journal of sports physical therapy. 2015 Nov;10(6):827. 4. Skinner B, Moss R, Hammond L. A systematic review and meta-analysis of the effects of foam rolling on range of motion, recovery and markers of athletic performance. Journal of Bodywork and Movement Therapies. 2020 Jul 1;24(3):105-22. 5. Konrad A, Nakamura M, Paternoster FK, Tilp M, Behm DG. A comparison of a single bout of stretching or foam rolling on range of motion in healthy adults. European Journal of Applied Physiology. 2022 Mar 17:1-3. 6. Konrad A, Nakamura M, Bernsteiner D, Tilp M. The accumulated effects of foam rolling combined with stretching on range of motion and physical performance: a systematic review and meta-analysis. Journal of Sports Science & Medicine. 2021 Sep;20(3):535. 7. Anderson BL, Harter RA, Farnsworth JL. The acute effects of foam rolling and dynamic stretching on athletic performance: a critically appraised topic. Journal of sport rehabilitation. 2020 Aug 13;30(3):501-6. 8. Su H, Chang NJ, Wu WL, Guo LY, Chu IH. Acute effects of foam rolling, static stretching, and dynamic stretching during warm-ups on muscular flexibility and strength in young adults. Journal of sport rehabilitation. 2017 Nov 1;26(6):469-77. 9. Morton RW, Oikawa S, Phillips SM, Devries MC, Mitchell CJ. Self-Myofascial Release: No Improvement of Functional Outcomes in” Tight” Hamstrings. International Journal of Sports Physiology & Performance. 2016 Jul 1;11(5). 10. Behm DG, Chaouachi A. A review of the acute effects of static and dynamic stretching on performance. European journal of applied physiology. 2011 Nov;111(11):2633-51. 11. Behm DG, Blazevich AJ, Kay AD, McHugh M. Acute effects of muscle stretching on physical performance, range of motion, and injury incidence in healthy active individuals: 33 a systematic review. Applied physiology, nutrition, and metabolism. 2016;41(1):1-1. 12. Curry B.S., Chengkalath D., Crouch G.J., Romance M., and Manns P.J. 2009. Acute effects of dynamic stretching, static stretching, and light aerobic activity on muscular performance in women. J. Strength Cond. Res. 23: 1811–1819. 13. Franco B.L., Signorelli G.R., Trajano G.S., Costa P.B., and de Oliveira C.G. 2012. Acute effects of three different stretching protocols on the Wingate test performance. J. Sports Sci. Med. 11: 1–7. 14. Richman ED, Tyo BM, Nicks CR. Combined effects of self-myofascial release and dynamic stretching on range of motion, jump, sprint, and agility performance. The Journal of Strength & Conditioning Research. 2019 Jul 1;33(7):1795-803. 15. Peacock CA, Krein DD, Silver TA, Sanders GJ, Von Carlowitz KP. An acute bout of self-myofascial release in the form of foam rolling improves performance testing. International journal of exercise science. 2014;7(3):202. 16. Smith JC, Pridgeon B, Hall MC. Acute effect of foam rolling and dynamic stretching on flexibility and jump height. The Journal of Strength & Conditioning Research. 2018 Aug 1;32(8):2209-15. 17. Chen CH, Chiu CH, Tseng WC, Wu CY, Su HH, Chang CK, Ye X. Acute effects of combining dynamic stretching and vibration foam rolling warm-up on lower-limb muscle performance and functions in female handball players. J. Strength Cond. Res. 2021 Mar 2. 18. Seçer E, Kaya DÖ. Comparison of Immediate Effects of Foam Rolling and Dynamic Stretching to Only Dynamic Stretching on Flexibility, Balance, and Agility in Male Soccer Players. Journal of Sport Rehabilitation. 2021 Sep 20;31(1):10-6. 19. de Cunha JC, Monteiro ER, Fiuza A, Neto VG, Araujo GS, Telles LG, de Meirelles AG, Serra R, Vianna JM, Novaes JS. Acute Effect of Foam Rolling Before Dynamic Stretching on the Active Hip Flexion Range-of-Motion in Healthy Subjects. Journal of Exercise Physiology Online. 2021 Apr 1;24(2):81-90. 20. Lin WC, Lee CL, Chang NJ. Acute effects of dynamic stretching followed by vibration foam rolling on sports performance of badminton athletes. Journal of sports science & medicine. 2020 Jun;19(2):420. 21. Hsu FY, Tsai KL, Lee CL, Chang WD, Chang NJ. Effects of dynamic stretching combined with static stretching, foam rolling, or vibration rolling as a warm-up exercise on athletic performance in elite table tennis players. Journal of Sport Rehabilitation. 2020 Apr 28;30(2):198-205. 22. Alonso-Calvete A, Lorenzo-Martínez M, Padrón-Cabo A, Pérez-Ferreirós A, Kalén 34 A, Abelairas-Gómez C, Rey E. Does Vibration Foam Roller Influence Performance and Recovery? A Systematic Review and Meta-analysis. Sports Medicine-Open. 2022 Dec;8(1):1-0. 23. Miyamoto N, Hirata K, Miyamoto-Mikami E, Yasuda O, Kanehisa H. Associations of passive muscle stiffness, muscle stretch tolerance, and muscle slack angle with range of motion: individual and sex differences. Scientific reports. 2018 May 29;8(1):1-0. 24. Hwang J, Jung MC. Age and sex differences in ranges of motion and motion patterns. International Journal of Occupational Safety and Ergonomics. 2015 Apr 3;21(2):173-86. █ 35 Study Reviewed: The Association Between Caffeine Intake And Testosterone: NHANES 2013-2014. Glover et al. (2022) Is Caffeine Tanking Your Testosterone? BY ERIC TREXLER Many of us grab a cup of coffee before we start our day, or ingest a caffeinated supplement before a workout. A new observational study sought to investigate whether a man’s caffeine habit might be driving his testosterone levels downward. 36 KEY POINTS 1. The presently reviewed study sought to determine whether urinary levels of caffeine (and 14 of its metabolites) were associated with blood testosterone levels among 372 men in the 2013-2014 NHANES cohort. As such, it was an observational, retrospective analysis of previously collected data. 2. Blood testosterone levels were negatively associated with urinary caffeine, along with 10 of its metabolites. However, 3 metabolites were positively associated with testosterone levels, and analyses looking at various quartiles of caffeine and two of its key metabolites (theobromine and theophylline) yielded very inconsistent results. 3. Due to some methodological shortcomings and inconsistent results within this study, along with a lack of compatibility with other studies on this topic, there is currently insufficient evidence to suggest that high caffeine intake will negatively impact testosterone levels in men. A lot of men are interested in optimizing their testosterone levels, and it’s not hard to figure out why. Testosterone levels can impact body composition, vitality, and libido, and also happen to decline as men age. Naturally, a lot of men are interested in the possibility of supporting optimal testosterone levels throughout all stages of adulthood, and keeping age-related testosterone reductions at bay (to the extent that such a feat is possible).The presently reviewed study (1) proposes a nightmarish suggestion: lay off the caffeine. To address this question, the researchers leaned on data from the National Health and Nutrition Examination Survey (NHANES). NHANES is a huge research program that’s been going on since the 1960s, and involves data collection in a variety of different forms, ranging from questionnaires to physiological measurements and blood tests. The NHANES researchers and staff currently aim to collect data from a representative sample of about 5,000 Americans per year, and epidemiologists publish findings from the NHANES data set extremely frequently. In the present study, the researchers gathered and retrospectively analyzed complete data, including demographic characteristics, blood testosterone levels, and urinary concentrations of caffeine and 14 of its metabolites, from 372 men that participated in the 2013-2014 NHANES data collection cycle. In their primary regression analysis, the researchers found that urinary caffeine levels (and the levels of 10 other caffeine metabolites) were negatively associated with blood testosterone levels. However, they also found that 3 caffeine metabolites were positively associated with testosterone levels. The researchers also used regression to compare quartiles stratified by urinary concentrations of caffeine and a couple of its key metabolites (theobromine and theophylline). These 37 models yielded very inconsistent results, and testosterone levels did not consistently drop when progressing from lower quartiles to higher quartiles of urinary caffeine or its primary metabolites. Finally, the researchers constructed a bunch of logistic regression models, which collectively found that urinary caffeine and its metabolites were not significantly predictive of an individual’s risk for having clinically low testosterone (<300 ng/ dL). The researchers concluded that caffeine was inversely associated with testosterone, and that “these effects of caffeine may serve as important risk factors in the etiology of low testosterone and reproductive dysfunction.” Read on to find out why I beg to differ. Purpose and Hypotheses Purpose The purpose of the presently reviewed study was “to quantify the strength and direction of the association between caffeine and testosterone.” Hypotheses The researchers hypothesized “that caffeine consumption is significantly associated with testosterone in men.” However, they did not commit to a particular direction or pattern by which varying levels of caffeine consumption would correlate with testosterone levels. 38 Subjects and Methods Subjects These researchers retrospectively analyzed data from the 2013-2014 NHANES cohort, which consisted of 10,175 participants. For this analysis, the researchers excluded all female participants and individuals under the age of 18, which left 2,958 adult males remaining. From this group of 2,958, only participants with complete data for the outcomes and covariates of interest (serum testosterone and sex hormone binding globulin, urinary creatinine and caffeine metabolites, demographic information, anthropometric data, and information about alcohol use, diabetes status, ethnicity, and smoking status) could be included. This resulted in a final sample of 372 men, whose data (stratified by quartiles of urinary caffeine levels) are presented in Table 1. Methods For the original data collection, participants reported to a lab after an overnight fast to complete all assessments. Participants provided a urine sample for determination of caffeine metabolites, a blood sample for determination of serum testosterone levels, went through a quick testing battery to collect anthropometric measurements, and provided all necessary information pertaining to their demographic characteristics and health-related information. As for statistical analysis, the researchers took a few different approaches. First, the sample was divided into four quartiles (stratified by urinary caffeine levels), which were compared to one another using ANOVAs (for continuous variables) and Chi-squared tests (for categorical variables). After that, the researchers constructed regression models to explore relationships between various caffeine metabolites and serum testosterone levels. Based on the methods, it sounds like these models were constructed one at a time (one for each caffeine metabolite), with each model adjusted for covariates including age, BMI, smoking, drinking, and urinary creatinine levels. The researchers also used regression to compare quartiles stratified by urinary concentrations of caffeine and a couple of its key metabolites (theobromine and theophylline). For each of the three metabolites, these models directly compared the mean predicted change in testosterone level when comparing the 2nd, 3rd, and 4th quartiles to the 1st quartile. The researchers also used logistic regression to determine if caffeine, theobromine, or theophylline were predictive of the likelihood of having clinically low testosterone levels, defined as serum levels <300 ng/dL. For these models, each quartile was compared to the first quartile for comparison purposes. For example, they constructed logistic regression models that answered the following questions: • Compared to individuals in the 1st quartile of urinary caffeine concentrations, were individuals in the 2nd quartile more likely to have low testosterone levels (<300 ng/dL)? • Compared to individuals in the 1st quartile of urinary caffeine concentrations, were individuals in the 3rd quartile more likely to have low testosterone levels? • Compared to individuals in the 1st quartile of urinary caffeine concentrations, were individuals in the 4th quartile more likely to have low testosterone levels? 39 This same sequence of questions was answered by building similar models to compare among the quartiles of urinary theobromine concentrations and theophylline concentrations. Findings Testosterone levels, stratified by urinary caffeine quartiles, are presented in Table 2. When I first saw these data, I noticed that the quartiles had pretty similar testosterone levels. For example, quartiles 1 through 3 were all in the range of 430-440 ng/dL; physiologically speaking, the difference between 430 and 440 is inconsequential. You could argue that the drop-off from quartile 3 (430 ng/ dL) to quartile 4 (398 ng/dL) is potentially approaching a physiologically relevant magnitude (not for hypertrophy or body composition, but perhaps for small impacts on things like perceived energy level or libido), but, generally speaking, raw testosterone values didn’t differ much across the four quartiles. I noticed that the reported p-value for the ANOVA comparing the four quartiles was p = 0.02, which is considered statistically significant. However, when I tried to replicate this ANOVA based on the reported means and standard errors, my results weren’t even close to being statistically significant. I think it’s possible that my inability to replicate the finding relates to something called “weighting.” The NHANES data are weighted, which involves using mathematical adjustments to make the sample data more representative of the population it was drawn from. You can see the impact of this weighting in Table 2; the four different quartiles have very different sample sizes, which is never the case for unweighted data. So, the raw data aren’t particularly interesting with regards to testosterone levels across quartiles, but the weighted analysis appears to tell a different story. I don’t personally have experience with weighted analyses of this nature, so I’m left to assume 40 consistency. For caffeine, the predicted mean testosterone values for each quartile were, from 1st to 4th: 427, 506, 405, and 400 ng/ dL. The 2nd caffeine quartile had significantly higher testosterone levels than the 1st quartile, but the 4th quartile had significantly lower testosterone levels than the 1st quartile. For theobromine, the predicted mean testosterone levels by quartile were 473, 398, 396, and 473 ng/dL. In other words, having moderate urinary theobromine levels, but not high urinary theobromine levels, was associated with statistically significant reductions in testosterone. For theophylline, the predicted mean testosterone levels by quartile were 463, 464, 410, and 414. The only statistically significant comparison was between the 1st and 4th quartile. that weighting is the cause for my inability to replicate the calculation. Next, the researchers constructed a series of regression models, adjusted for a bunch of covariates, to examine the relationships between individual caffeine metabolites and blood testosterone levels. The results are presented in Table 3. In short, caffeine itself was negatively correlated with testosterone levels, and the same was true for 10 other caffeine metabolites. However, 3 metabolites were positively correlated with testosterone levels. There was only one remaining metabolite that was not significantly correlated with testosterone levels in either direction. Looking at the regression models comparing quartiles grouped by caffeine, theobromine, and theophylline levels, there was a lot of in- As for the logistic regression models, none of them were statistically significant. In other words, quartiles grouped by urinary levels of caffeine, theobromine, and theophylline were not significantly predictive of an individual’s risk for having clinically low testosterone (<300 ng/dL). Nonetheless, the researchers concluded that they had “observed an inverse association between caffeine and serum testosterone,” and that “these effects of caffeine may serve as important risk factors in the etiology of low testosterone and reproductive dysfunction.” Criticisms and Statistical Musings When I read through this study the first time, I was surprised by a lack of clarity in the methods and results, and a lack of nuance and 41 specificity in the discussion section. Sometimes, if I am surprised enough by an apparent lack of clarity, I dig a little deeper to figure out why that might be the case. I looked into the other research from this lead author, and it ended up being a fruitful exercise. With a little bit of digging, I found out that this lead author has published only one other paper as the primary author, so I checked out the other one. Upon reading it, I noticed that the research team had submitted a paper about associations between 2,4-dichlorophenoxyacetic acid (2,4-D) and testosterone levels in July of 2021 (for context, the presently reviewed study was submitted about four months later, in November of 2021). Four months prior to submitting the presently reviewed paper on caffeine, they submitted a totally different paper that utilized data from the same exact NHANES cohort from 20132014, and looked at the same exact outcome variable. In many spots, the present caffeine paper seemed like a paraphrased version of the 2,4-D paper that happened to use a different predictor in the statistical models (urinary caffeine instead of 2,4-D). In some sections, the methods were hardly even paraphrased, with a few instances of entirely duplicated text. On the one hand, this duplicated text isn’t a huge deal; I don’t personally care if you find a different way to tell me how you did the same thing in your methods section. However, a comparison of these two papers provided a couple of important insights. First, this paper’s methods probably lacked important caffeine-specific considerations because the methods appear to be adapted from another project that had nothing to do with caf- feine. Second, this is the lead researcher’s first venture into caffeine research (according to PubMed, at least). These observations probably tell us a lot about why the methods seemed so non-specific to the research question about caffeine, and why the discussion left so much to the imagination. Now, let’s discuss some specific quibbles I had, in no particular order. First, the methods state that blood draws occurred in a fasted state, but do not elaborate on the specific fasting instructions. On the surface level, one might assume the most simplistic definition of fasted: no foods or beverages other than plain water. If adopting this assumption, then any caffeine identified in blood samples was from the day before, and higher urinary values could potentially reflect high intake from the day before (which probably correlates, to some extent, with high habitual intake), or could possibly reflect a general preference for evening or nighttime caffeine ingestion. To get to the bottom of this, I had to dig up the old NHANES handbooks and guidelines to figure out the standard procedure for fasted examinations. When NHANES study participants arrive, the research staff asks them some questions. The first question is: “When was the last time you ate or drank anything other than plain water? Do not include diet soda or black coffee or tea with artificial sweeteners like Sweet’N Low, NutraSweet, Equal, or Splenda.” Follow-up questions ask for details about the timing of various food, beverage, supplement, and medication intakes, but there is no mechanism to determine if a non-caloric, caffeinated beverage was recently consumed, 42 and the fasting instructions do not restrict the intake of such beverages. Black coffee, plain tea, diet soda, and even some diet energy drinks would all be on the table. That’s kind of a big deal. In this context, urinary caffeine biomarkers don’t specifically reflect how much caffeine a participant typically consumes; rather, they reflect when a participant happened to consume caffeine, and the dose of this particular caffeinated product, in temporal proximity to a one-time measurement. This also means that participants who happen to like their coffee black might have enjoyed some while traveling to the lab that morning, whereas those who prefer cream or sugar would have been more likely to abstain or delay consumption that morning. While some might interpret the results of this study to make inferences about “heavy” or “light” caffeine consumers, that’s not really what is being measured here. Urinary caffeine levels in this study aren’t directly indicative of how much or how frequently a participant consumes caffeine, as these urinary metabolites may be influenced by other caffeine-related preferences or tendencies, such as a participant’s preferred caffeine source, the way they flavor their coffee in the morning, or what beverage happened to pair well with their dinner the night before testing. Moving past the unknown timing of caffeine intake prior to the testing visit, there was also a lack of detail regarding typical sleep habits, sleep quality or quantity in the days leading up to testing, and habitual caffeine intake. In 2013-2014, the NHANES researchers definitely collected data pertaining to sleep and dietary intakes (including caffeine), and in- corporating this information would have been very valuable for the present study. Many nutritional epidemiologists are fond of using biomarkers for nutrient intakes rather than self-reported intake data, whenever it’s possible and feasible to do so. For several dietary outcomes, this makes a lot of sense. For example, very few people can give you a really good estimate of their folate intake, or even of their typical food choices and portion sizes from which an estimate of folate intake could be derived. If you can measure something in the blood or urine that accurately and objectively reflects a person’s habitual folate intake without relying on their memory or attention to detail, that’s a really nice way to quantify folate intake and observe its relationship to a variety of health-related outcomes. In contrast, it’s not particularly difficult to get a reasonably accurate estimate of a person’s approximate caffeine intake from a quick survey or interview. In addition, research suggests that concentrations of caffeine and its metabolites derived from “spot urine samples” (samples collected at a single time point, like those in the present study) only have weak to moderate correlations with caffeine intake (2). In summary, I’m fairly certain that pertinent details related to sleep and self-reported dietary habits are available within the NHANES data set, and this information would’ve been very informative. It’s possible that adding these variables and requiring “complete” data for all subjects would have further reduced their sample size to an untenable degree, but they also could’ve rectified that issue by expanding their sample to include more NHANES cohorts from different data collection cycles. 43 My final quibble is that, frankly, I’m not seeing a clear connection between the reported findings and the researchers’ conclusions. Some metabolites were positively correlated with testosterone levels, some were negatively correlated with testosterone levels, and there was no consistent pattern by which testosterone levels changed as you jump from one quartile to the next. Further, the discussion section focused largely on rodent data and prenatal exposure to caffeine, while failing to acknowledge a great deal of evidence that is more directly relevant to the research question at hand. I was also surprised to see the conclusion that “these effects of caffeine may serve as important risk factors in the etiology of low testosterone and reproductive dysfunction,” given that they used logistic regression to directly test whether urinary caffeine, theobromine, or theophylline concentrations were predictive of clinically low testosterone levels, and none of them returned a statistically significant result. If there’s a clear and internally consistent link between the reported findings and the stated conclusions, I’m unable to find it. Interpretation If you typically skip the “Criticisms and Statistical Musings” section, I encourage you to read it this time around – it’s not overly technical, but it provides a detailed justification for my skepticism of the present study’s findings. The short version is that the reported analysis isn’t very compelling, even when interpreted at the surface level. However, when you dive deeper, there’s even more reason for skepticism. For starters, there’s insufficient evidence to confidently identify a plausible mechanism by which commonly observed levels of caffeine intake would directly cause testosterone reductions in male adults. The most plausible mechanism, in my opinion, is a somewhat indirect relationship mediated by sleep disruption. Certain patterns of caffeine intake (but not all patterns of caffeine intake) could impair sleep, and impaired sleep can lead to reduced testosterone. However, as noted in the “Criticisms and Statistical Musings” section, we don’t know enough about these individuals’ caffeine habits or sleep habits to draw informed conclusions about this possibility. If the higher caffeine levels in the present study were mostly from people who ingested large doses of caffeine right before bed on the night prior to testing, and this is representative of their habitual caffeine intake and timing, then it’s very possible that caffeine is hindering their testosterone levels through chronic sleep impairment. However, that’s also an easy fix – restricting caffeine to the morning or early afternoon would probably rectify that situation. Similarly, it’s possible that some people ingest such large doses of caffeine in the morning and afternoon that it has yet to clear their system by bed time; once again, this could be rectified by simply lowering habitual caffeine intake to a more suitable dose. Unfortunately, spot assessments of urinary caffeine metabolites don’t give us much information about a person’s habitual caffeine intake, nor do they allow us to make inferences about the specific timing of caffeine intake. In other words, the present study simply doesn’t provide information 44 that would allow us to draw strong conclusions about the relationship between caffeine and testosterone. Nonetheless, if one wishes to link caffeine intake to low testosterone, they cannot rule out the possibility that high caffeine intake is more of an effect than a cause. For example, people with sleep issues might be consuming more caffeine because of their sleep impairment, rather than directly causing the sleep impairment by consuming large caffeine doses at inadvisable times of day. In this situation, poor sleep would be directly contributing to lower testosterone levels, and the caffeine could merely be a response to the sleep issue that has no direct impact on testosterone. For another example, it’s important to recognize that low levels of perceived energy, vitality, and vigor are among the most common symptoms of low testosterone in men. It’s very plausible to suggest that men experiencing some of these symptoms from low testosterone might be more inclined to reach for extra caffeine for an energy boost; in such a scenario, testosterone reductions lead to high caffeine intake rather than high caffeine intake leading to testosterone reductions. Finally, stress is a relevant confounding factor to consider. I personally tend to consume more caffeine when I’m quite busy, and these busy stretches of time are when I tend to experience heightened levels of chronic stress. Acute stress responses can be a bit variable, but chronic stress is associated with testosterone reductions in men (3), so it’s possible that stress could be an underlying factor that is simultaneously driving caffeine intake upward while driving testosterone levels downward. THE PRESENT STUDY SIMPLY DOESN’T PROVIDE INFORMATION THAT WOULD ALLOW US TO DRAW STRONG CONCLUSIONS ABOUT THE RELATIONSHIP BETWEEN CAFFEINE AND TESTOSTERONE While we’re on the topic of causation, it’s important to contextualize the present study within the broader research linking caffeine intake and testosterone levels. As I mentioned previously, the discussion section of the present study allocates a great deal of focus toward mechanistic studies in non-human research models, with some findings indicating that very high caffeine intake can lead to lower testosterone levels in rodents. However, we should be very wary of generalizing rodent testosterone responses to human testosterone responses. For example, consider the melatonin literature. I’ve heard very well-respected fitness influencers caution against melatonin supplementation purely because it has been shown to reduce testosterone levels in rodents. However, there is a robust body of literature showing that this effect is highly dependent on contextual factors of melatonin 45 administration and the specific species being studied (4), and there’s plenty of evidence showing that exogenous melatonin supplementation has inconsequential effects on human testosterone levels (5). Shifting focus away from rodent research, these researchers also acknowledged some observational studies indicating that prenatal caffeine exposure is associated with decreased testosterone levels later in life. I’m not an expert on fetal development, so I’ll sit that debate out for now, but it’s important to highlight that prenatal caffeine exposure has virtually nothing to do with the present study’s findings, which spe- cifically investigate the impact of recent caffeine intake in adult males. I found it odd that the researchers directed such minimal focus toward other observational studies that aimed to investigate the very same relationship between caffeine intake and testosterone among adult men. They did acknowledge that a study by Lopez and colleagues (6), which also used data from the NHANES study, found non-linear associations between caffeine intake and testosterone levels. However, they didn’t elaborate on the actual pattern of this association, probably because it looked like this: 46 As you can see in Figure 1, Lopez and colleagues didn’t find a single category of caffeine intake that was associated with meaningfully lower testosterone levels than consuming no caffeine at all. Of course, there are other observational studies exploring the relationship between the intake of caffeinated beverages and testosterone levels among adult males. For example, Svartberg and colleagues looked at the relationship between a variety of lifestyle factors and testosterone levels in 1,563 men (7). Results actually found that higher habitual coffee consumption (>4 cups/day) was associated with significantly higher levels of total testosterone and free testosterone when compared to lower consumption (1-4 cups per day). There are even some human trials that give us hints about how acute and chronic caffeine consumption might impact testosterone levels in men. Acutely, Beaven and colleagues (8) reported a dose-response relationship by which caffeine increased testosterone responses to exercise. While the results reported by Beaven et al might be related to achievement of a greater workload during the exercise bout, Wu also reported that caffeine increases the acute testosterone response to resistance exercise with a fixed workload (9). Ormsbee et al (10) studied six weeks of supplementation with multi-ingredient pre-workout supplementation (whey protein, casein protein, branched-chain amino acids, creatine, beta alanine, and caffeine) in resistance-trained men. While the study was confounded by a long list of ingredients, both the placebo group and supplement group experienced similar testosterone increases over time. On a related note, a study by MacKenzie and colleagues (11) investigated the effect of daily caffeine intake (200mg, twice per day for seven days) on two androgens that are related to testosterone (dehydroepiandrosterone [DHEA] and androstenedione). Neither androgen was significantly impacted by daily caffeine supplementation. A well-constructed epidemiological paper will take steps to summarize the existing knowledge related to the research topic, establish the biological plausibility of the research question, formulate a set of methods to directly address that question, describe those methods with a high level of clarity, report the observed results in conjunction with a nuanced discussion about any apparent inconsistencies or contradictions, and lean on a thorough understanding of the research topic to contextualize the newly reported findings within the broader literature. The presently reviewed study falls a bit short in each of these areas, and therefore fails to provide convincing support for the idea that men interested in optimizing their testosterone levels should necessarily restrict caffeine intake. As I noted in a recent Research Brief, men interested in supporting optimal testosterone levels should aim to be lean enough (but not too lean), and to achieve adequate total energy intake with a fairly moderate macronutrient distribution. Beyond that, a great article by the team at examine.com covers the rest of the current best practices for optimizing testosterone levels: adequate sleep, regular physical activity, and sufficient micronutrient status (with a specific focus on vitamin D, zinc, and magnesium) should have you covered. As long as your 47 caffeine intake isn’t messing up your sleep, I’m simply not seeing convincing evidence that negative effects on testosterone levels are likely. In many cases, I write MASS articles to inform readers about helpful, practical, evidence-based strategies that can improve their approach to training or nutrition. However, I have to play defense sometimes, which involves writing articles that proactively “get out ahead” of unreliable or poorly supported recommendations that readers might encounter. Given that the presently reviewed paper combines two hot topics with immense public interest (caffeine and testosterone), I wouldn’t be surprised if a number of influencers in the realm of health, fitness, or biohacking take the surface-level interpretation and run with it, leading to recommendations to limit caffeine intake in order to optimize testosterone levels. However, as a MASS reader, you’ve got the inside scoop, and you know that this study simply doesn’t provide strong evidence to support those recommendations. sponse relationship between caffeine intake and testosterone levels should become apparent. Personally, I am very skeptical that such a relationship exists, but it shouldn’t be hard to identify with a very simple and straightforward randomized controlled trial. AS LONG AS YOUR CAFFEINE INTAKE ISN’T MESSING UP YOUR SLEEP, I’M SIMPLY NOT SEEING CONVINCING EVIDENCE THAT NEGATIVE EFFECTS ON TESTOSTERONE LEVELS ARE LIKELY Next Steps It would be very easy to address this research question head-on, and would probably make for a nice master’s thesis project. All you’d need to do is recruit a group of men and randomly allocate them to some treatment arms. Personally, I’d opt for 4 different conditions, if possible: 0mg caffeine (placebo), 200mg caffeine, 400mg caffeine, and 600mg caffeine. After having the participants ingest their allocated supplement (or placebo) every morning for 2-4 weeks, any potential dose-re- 48 APPLICATION AND TAKEAWAYS If you want to support optimal testosterone levels, you’ll want to get to a bodyfat level that is compatible with testosterone optimization; obesity can reduce testosterone levels, but testosterone levels also tend to drop as we go from lean to shredded. In addition, you’ll want to make sure you’re eating enough total calories (that is, avoiding low energy availability) within a fairly balanced macronutrient distribution. Beyond that, try to get adequate sleep, regular physical activity, and sufficient micronutrient intake (especially vitamin D, zinc, and magnesium), and try to minimize chronic stress. If you heard about this paper and were worried about choosing between your caffeine and your testosterone levels, don’t sweat it – there is currently insufficient evidence to suggest that high caffeine intake will negatively impact testosterone levels in men. 49 References 1. Glover FE, Caudle WM, Del Giudice F, Belladelli F, Mulloy E, Lawal E, et al. The Association Between Caffeine Intake And Testosterone: Nhanes 2013-2014. Nutr J. 2022 May 17;21(1):33. 2. Rybak ME, Sternberg MR, Pao CI, Ahluwalia N, Pfeiffer CM. Urine Excretion Of Caffeine And Select Caffeine Metabolites Is Common In The Us Population And Associated With Caffeine Intake. J Nutr. 2015 Apr;145(4):766–74. 3. Smith GD, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P. Cortisol, Testosterone, And Coronary Heart Disease: Prospective Evidence From The Caerphilly Study. Circulation. 2005 Jul 19;112(3):332–40. 4. Yu K, Deng SL, Sun TC, Li YY, Liu YX. Melatonin Regulates the Synthesis of Steroid Hormones on Male Reproduction: A Review. Molecules. 2018 Feb 17;23(2):447. 5. Luboshitzky R, Levi M, Shen-Orr Z, Blumenfeld Z, Herer P, Lavie P. Long-Term Melatonin Administration Does Not Alter Pituitary-Gonadal Hormone Secretion In Normal Men. Hum Reprod. 2000 Jan;15(1):60–5. 6. Lopez DS, Advani S, Qiu X, Tsilidis KK, Khera M, Kim J, et al. Caffeine Intake Is Not Associated With Serum Testosterone Levels In Adult Men: Cross-Sectional Findings From The Nhanes 1999-2004 And 2011-2012. Aging Male. 2019 Mar;22(1):45–54. 7. Svartberg J, Midtby M, Bønaa KH, Sundsfjord J, Joakimsen RM, Jorde R. The Associations Of Age, Lifestyle Factors And Chronic Disease With Testosterone In Men: The Tromsø Study. Eur J Endocrinol. 2003 Aug;149(2):145–52. 8. Beaven CM, Hopkins WG, Hansen KT, Wood MR, Cronin JB, Lowe TE. Dose Effect Of Caffeine On Testosterone And Cortisol Responses To Resistance Exercise. Int J Sport Nutr Exerc Metab. 2008 Apr;18(2):131–41. 9. Wu BH. Dose Effects Of Caffeine Ingestion On Acute Hormonal Responses To Resistance Exercise. J Sports Med Phys Fitness. 2015 Oct;55(10):1242–51. 10. Ormsbee MJ, Mandler WK, Thomas DD, Ward EG, Kinsey AW, Simonavice E, et al. The Effects Of Six Weeks Of Supplementation With Multi-Ingredient Performance Supplements And Resistance Training On Anabolic Hormones, Body Composition, Strength, And Power In Resistance-Trained Men. J Int Soc Sports Nutr. 2012 Nov 15;9(1):49. 50 11. MacKenzie T, Comi R, Sluss P, Keisari R, Manwar S, Kim J, et al. Metabolic And Hormonal Effects Of Caffeine: Randomized, Double-Blind, Placebo-Controlled Crossover Trial. Metabolism. 2007 Dec;56(12):1694–8. █ 51 Study Reviewed: Short-Term Effects of Eccentric Overload Versus Traditional Back Squat Training on Strength and Power. Munger et al. (2022) Accentuated Eccentrics are Overhyped BY MICHAEL C. ZOURDOS Since you are stronger on the eccentric phase than the concentric phase, accentuated eccentric loading makes sense. However, the longitudinal data supporting this practice for enhancing strength gains is underwhelming. Does a new study turn the tides? 52 KEY POINTS 1. The presently reviewed study was a parallel-groups design that split 33 trained men into three groups for five weeks. The men performed: 1) squats with added load on the eccentric (accentuated eccentric group), 2) traditional squats (traditional group), or 3) their regular training (unsupervised group). 2. Squat 1RM and 20m sprint performance improved from pre- to post-study with no group differences. Eccentric squat 1RM and countermovement jump height increased significantly more in the accentuated eccentric and traditional groups than in the unsupervised group. 3. This study shows that accentuated eccentric loading does not enhance maximal squat strength more than regular squatting. Overall, the support in the literature for accentuated eccentric loading to enhance long-term strength is thin, and the practice is currently too cumbersome to recommend. W e’ve covered accentuated eccentrics on a few occasions (one, two, three, four), and the idea behind the practice is logical. People can handle more weight on the eccentric phase of a lift than on the concentric portion. Therefore, accentuated eccentrics typically employ weight releasers, which add load to the barbell on the eccentric then detach from the barbell at the bottom of the movement to lessen the load for the concentric phase. Some data suggest that accentuated eccentrics can increase acute one-repetition maximum (1RM) via an immediate potentiation effect (2). However, the current body of research on the efficacy of accentuated eccentrics for long-term strength is lukewarm, at best. Although a study from Douglas et al (3 - MASS Review) showed that accentuated eccentrics led to greater increases in squat strength than traditional training, the accentuated eccentric group trained at a much higher percentage of 1RM, which clouds the findings. Further, a meta-analysis from Buskard et al (4) found that accentuated eccentrics did not enhance 1RM strength more than traditional loading. So, does a new study suggest a more positive outlook for accentuated eccentric loading? The reviewed study from Munger et al (1) tested squat 1RM, eccentric squat 1RM, sprint performance, and countermovement jump height before and after five weeks of training in three groups of trained men. Two groups maintained their normal training, but replaced two lower body sessions per week with squat training under the supervision of the researchers. One of these groups squatted with accentuated eccentrics (accentuated eccentric group), while the other group performed traditional squats (traditional group). The third group (unsupervised group) simply maintained their normal training. Squat 1RM increased and sprint time decreased in all groups. Changes in eccentric 1RM and jump height were significantly greater in the accentuated eccentric 53 and traditional training groups than in the unsupervised group. These findings suggest that accentuated eccentrics do not further enhance squat 1RM when relative load (percentage of 1RM) is equated. At this point, I cannot confidently recommend accentuated eccentrics as a surefire strategy to boost strength more than traditional training. However, accentuated eccentrics are also unlikely to be harmful; thus, the strategy still warrants consideration. This article will aim to: 1. Review the present findings and evaluate the study design. 2. Evaluate the state of the literature on accentuated eccentrics for both acute and long-term strength. 3. Review the difference between accentuated eccentrics and eccentric overload training. 4. Discuss the implementation and practicality of accentuated eccentrics. Purpose and Hypotheses Purpose The purpose of the presently reviewed study was to compare changes in squat 1RM, eccentric squat 1RM, sprint performance, and jump performance in trained men after performing five weeks of supervised accentuated eccentric training, supervised traditional training, or their regular training on their own. Hypotheses The researchers hypothesized that subjects in the accentuated eccentric training group would improve performance in all outcome measures to a greater extent than the other two groups. Subjects and Methods Subjects 33 men who had at least one year of training experience and could squat at least their body weight participated in the study. Additional subject details are in Table 1. Study Overview This study was a parallel-groups design. Subjects were counterbalanced by 1RM squat strength into three groups: 1) accentuated eccentric loading group, 2) traditional group, and 3) unsupervised group. The accentuated eccentric and traditional groups maintained their normal training, but replaced two lower body sessions per week with either accentuated eccentric squat or traditional squat training under the supervision of the researchers. Each squat training session lasted ~30 minutes. The unsupervised group continued their normal lower body training on their own. They also performed 30 minutes of self-selected upper body training twice per week with the researchers. Squat 1RM, eccentric squat 1RM, countermovement jump height, and 20m sprint assessed before and after the five-week training program. The researchers analyzed the change in 20m sprint time during four distance windows (0-5 m, 5-10 m, 10-20 m, and 0-20 m). It’s worth briefly describing the eccentric testing protocol, since this is not a method we see often. For the test, the power rack’s safety bars were set at a height where the thigh was parallel to the ground. To successfully complete an eccentric squat 1RM attempt, the lifters had to lower the barbell to the safe- 54 ty bars over the course of at least three seconds. In other words, if the subjects descended too fast (i.e., <3 seconds), the attempt was deemed unsuccessful. Accentuated Eccentric and Traditional Groups The researchers split training into three blocks (weeks 1-2, weeks 3-4, and week 5) in the accentuated eccentric and traditional groups. During each block, the average percentage of 1RM (i.e., an average of concentric and eccentric phases) used in the accentuated eccentric group equaled the percentage of 1RM used in the traditional group. For example, in weeks 2-3, subjects in the traditional group used 80% of 1RM, while subjects in the accentuated eccentric group squatted 105% of 1RM on the eccentric and 55% of 1RM on the concentric [(105% + 55%) ÷ 2 = 80%]. The eccentric overload group performed all squats with a 3-second eccentric phase and maximal intended velocity during the concentric phase. The researchers did not provide details of the eccentric and concentric cadence for the traditional group. However, I suspect the traditional group trained with a self-selected eccentric and maximal intent on the concentric. The specific sets, reps, and relative loads in the accentuated eccentric and traditional groups are in Table 2. Findings Changes in 1RM squat, 1RM eccentric squat, and countermovement jump height was analyzed with a 3 (group) × 2 (time) repeated 55 measures analysis of variance (ANOVA). A “main time effect” indicates a significant change in the outcome measure for the full sample, ignoring the impact of group assignment. A “group × time interaction” indicates differences in the magnitude of change from pre- to post-study between groups. In the event of a group × time interaction, I’ll identify the group(s) in which the outcome measure changed significantly more than the other group(s). Changes in sprint performance were analyzed with a 3 (group) × 2 (time) × 4 (distance window) ANOVA; thus, I’ll report if there was a three-way interaction, and if there was a significant change in sprint time during the specific distance windows. Squat 1RM and Eccentric Squat 1RM There was a main time effect for squat 1RM (p < 0.001), indicating an increase in squat strength; however, there was not a significant group × time interaction (p = 0.093). There was a significant group × time interaction for eccentric squat 1RM (p = 0.001), which was driven by significantly greater increases in the accentuated eccentric (+16.9kg) and traditional groups (+12.7kg) than the unsupervised group (+2.0kg). Although the accentuated eccentric group gained 4.2kg more on their eccentric 1RM than the traditional group, this difference was only a 3.22% greater percentage increase, and a trivial between-group effect size (g = 0.10). The eccentric squat findings can be seen in Figure 1. Countermovement Jump Height and Sprint Performance There was a significant group × time interaction (p = 0.026) for countermovement jump height. This interaction was driven by significantly greater increases in the accentuated eccentric (+3.8cm) and traditional groups (+2.9cm) compared to the unsupervised group (+0.0cm) (Figure 2). The 0.9 cm 56 greater change in jump height in the accentuated eccentric than in the traditional group only equated to a 1.25% greater percentage increase, and a trivial between-group effect size (g = 0.10). There was no significant three-way interaction (p = 0.318) for sprint performance, indicating that sprint times did not change significantly more in one group than another. However, a significant time × distance window effect (p = 0.027) indicated a decrease in sprint time from pre- to post-study in all groups combined. The changes were -0.022 seconds (0-20 m), -0.018 seconds (5-10 m), +0.031 (10-20 m), and -0.035 seconds (0-5 m). Criticisms and Statistical Musings Squat 1RM increased from 120.8 ± 31.4kg to 129.3 ± 31.3kg in all groups combined; how- ever, the researchers did not report the changes in squat 1RM for each group. Even though there was no significant group × time interaction, knowing the changes in each group would have allowed me to calculate percentage changes and between-group effect sizes to see if findings leaned in favor of one training program or another. If the p-value for the group × time interaction was high, this wouldn’t matter, but in this case the p-value was 0.093 (i.e., close to 0.05). Based on the eccentric 1RM findings, I would imagine any meaningful differences in squat 1RM would be due to greater increases in the traditional and eccentric loading groups compared to the unsupervised group, but obviously I don’t know that for sure. Since subjects in the accentuated eccentric and traditional groups were “instructed to maintain habitual lower body training frequency, but to replace two of their leg train- 57 ing days with exercise prescribed during the study,” it’s likely that lifters trained with different lower body frequencies. Furthermore, the researchers did not state if they instructed the subjects to exclude squats from lower body sessions performed outside of the lab. I suspect most didn’t squat, because two days of high load squatting was probably enough, but I don’t know which lower body exercises the lifters performed in those sessions. Similarly, since subjects in the unsupervised group were “encouraged to maintain their lower body training regimen to prevent lower body strength loss,” we don’t know the overall lower body training frequency or specific squat training frequency of the subjects in the unsupervised group. Interpretation Accentuated eccentric loading can be supramaximal or submaximal. Supramaximal accentuated eccentrics use an eccentric load greater than the lifter’s concentric 1RM, while submaximal eccentrics use a load less than concentric 1RM. The reviewed study from Munger et al (1) used supramaximal eccentric loading. So, when interpreting the presently reviewed study, we’ll do so only in the context of other supramaximal eccentric studies. Before reviewing this study from Munger et al (1), I was already of the opinion that accentuated eccentrics didn’t boost long-term strength. Specifically, a meta-analysis from Buskard et al (4) found that accentuated eccentric loading did not significantly (p = 0.20) improve 1RM lower body strength compared to traditional training. Buskard did report an effect size of 0.33 in favor of accentuated eccentrics versus traditional loading, but this effect was driven by a single study by Cook et al (5). Further, Buskard’s meta-analysis only included four studies and seven total effects for maximal strength, and two of these studies did not actually compare accentuated eccentric loading to traditional training. One compared two eccentric only loading paradigms (6), and the other compared eccentric only versus concentric only training (7). Further, the Buskard meta did not analyze four studies (3 - MASS Review, 8, 9, 10) that compared accentuated eccentrics to traditional loading. Altogether, seven longitudinal studies have examined the effects of accentuated eccentrics on long-term strength gains. Those seven studies (1, 3, 5, 8, 9, 10, 11) are summarized in Table 3. After reviewing Table 3, you can probably see why I don’t hold accentuated eccentrics in high regard as a strategy to enhance strength. Three of the previous studies show no clear benefit of accentuated eccentrics; with the addition of the presently reviewed study, that total is now four of seven studies showing no benefit of accentuated eccentrics for long-term strength. Further, the three studies (1, 3, 5) that suggest a possible benefit come with significant caveats. First, Douglas et al (3), which Greg reviewed, did not equate for load between the traditional and accentuated eccentric groups. Therefore, the ~5% higher load in the accentuated eccentric group could account for their greater increase in squat strength. Second, the findings from Cook et al (5) were pretty remarkable. Cook found that rugby players who performed eccentric 58 only (i.e., not followed by a concentric) increased regular squat 1RM more than traditional training. Third, it’s not possible to fully interpret the results of English et al (11). English examined changes in leg press and calf raises strength in five groups, all of which performed the same concentric load on each rep (between 55-96% of 1RM). The five different groups lifted either 120%, 100%, 66%, 33%, or 0% of 1RM on the eccentric phase. The 120% group gained more leg press strength than the 0, 33, and 66% groups, but not more than the 100% group. Therefore, since most concentric loading was greater than 66% of 1RM, we can conclude the accentuated eccentric led to greater leg press 1RM gains 59 than submaximal eccentrics. Still, we cannot infer if accentuated eccentrics would have outperformed traditional training. Further, English also reported no difference between accentuated and submaximal eccentrics for calf strength gains. Therefore, on balance, four longitudinal studies show no benefit for accentuated eccentrics to enhance strength over traditional training, and the three other studies all come with significant caveats. I think the presently reviewed study from Munger et al (1) is one of the better designed longitudinal accentuated eccentric studies. First, this study equated load between the accentuated eccentric and traditional groups, and is one of only four in Table 3 to employ trained individuals. Further, Munger also tested eccentric 1RM, which is unique from other studies. Perhaps most importantly, Munger’s study is the only longitudinal study to date to train the free-weight squat with weight releasers. I’d wager that weight releasers are perhaps the most common method for lifters interested in max strength to apply an accentuated eccentric. So, in my book, the lack of benefit for accentuated eccentrics for strength in the presently reviewed study weighs pretty high compared to the other studies. Of course, this study is not without its faults, some of which were already mentioned in the “Criticisms and Statistical Musings” section, and others I’ll discuss in the “Next Steps” section. I do think it’s debatable if equating load between groups by decreasing the concentric load on the accentuated eccentric group is the best strategy. On one hand, if someone is doing repeated eccentrics, their concentric force production may be impaired, so concentric load may have to be decreased. On the other hand, one could argue that the accentuated eccentric group in this study was training in a mode less specific to a 1RM, as their concentric load never reached more than 65% of 1RM. The previous paragraph said, “On the one hand, if someone is doing repeated eccentrics, their concentric force production may be impaired, so concentric load may have to be decreased.” That statement is true, but, if someone only does one supramaximal eccentric, that may actually increase immediate concentric strength. In this way, lifters can also use accentuated eccentrics to potentiate acute performance. I’ll keep this discussion of accentuated eccentrics and acute strength brief since we’ve discussed it twice before (one, two). In short, similar to the body of literature on longitudinal studies, the data from studies examining a supramaximal eccentric to potentiate 1RM performance immediately are underwhelming. In general, these acute studies test if supramaximal eccentrics potentiate performance by loading a barbell to a lifter’s 1RM attempt. These studies typically add weight releasers to the barbell for the eccentric phase and see if this increases concentric 1RM or concentric velocity. For example, Doan et al. (2) found that an eccentric squat with a load of 105% of 1RM increased actual 1RM between 5-15kg for all subjects compared to a squat 1RM test without a supramaximal eccentric. Ojasto and Hakkinen (12) observed that eccentrics of 105, 110, and 120% of 1RM did not enhance actual 1RM performance; however, subjects did all of these conditions in the same session; thus, 60 it’s likely fatigue prevented any potential for increased performance. Lates et al (13) found that using 105% on the eccentric did not enhance concentric velocity at 80% of 1RM in the bench press. Similarly, Wagle et al (14) reported that accentuated eccentrics did not improve acute squat concentric kinematics at 80% of 1RM. Another study from Merrigan et al (15) found that a 120% of 1RM squat eccentric improved squat velocity at 65% of 1RM on the concentric, but not at 80% of 1RM. Thus, the Merrigan findings suggest that the benefits of accentuated eccentrics on acute squat performance are load-dependent. Additional Thoughts Some additional thoughts and bits of information as we finish up. First, although not the focus of this article, there are data on accentuated eccentric loading and hypertrophy. Of the longitudinal studies (9, 16, 17) comparing accentuated eccentrics to traditional training for hypertrophy, none have shown accentuated eccentrics to boost muscle growth further. Let’s briefly clarify some terminology. I have used the term accentuated eccentric loading throughout this article. Sometimes, you will see the term eccentric overload used. While both are technically correct, eccentric overload is often used when referring to flywheel training. Flywheel training is technically an accentuated eccentric. However, it would be submaximal and not supramaximal eccentric loading, so I didn’t discuss it in this article. However, we have a previous article evaluating the efficacy of eccentric overload with a flywheel to potentiate concentric performance. Lastly, perhaps the biggest hurdle to regularly implementing accentuated eccentrics is practicality. Many studies (see Table 3) implemented supramaximal eccentrics with a dynamometer or specific machines, which are absent in most gyms. The currently reviewed study and the aforementioned acute studies used weight releasers, which may be more well-known to MASS readers. However, weight releasers aren’t readily accessible or practical to use. You could buy weight releasers for about ~$100-200 USD (which isn’t cheap but isn’t an atmospheric price) or build some for about $50. It would be annoying to lug these to the gym, so you’d hope to convince the gym staff to let you leave them there. But the biggest hurdle is that, if you’re going to use them to apply a supramaximal eccentric load on every rep (such as in the longitudinal studies), you would need two training partners to attach the weight releasers to the barbell after each rep. Obviously, this is very cumbersome, and not practical if you train alone. Some gyms that primarily serve the strength athlete may have a community of people willing to help with weight releasers, since everybody tends to help each other out in those environments. However, if you train at a typical commercial gym, applying accentuated eccentrics all the time is probably not feasible. Aside from the practical limitations, the data supporting accentuated eccentrics is underwhelming; thus, I wouldn’t go to the trouble to implement them. Next Steps Despite covering seven different longitudinal accentuated eccentric loading studies, we are 61 APPLICATION AND TAKEAWAYS 1. The reviewed study from Munger et al (1) found that, when percentage of 1RM was equated between accentuated eccentric loading and traditional squatting, there was no difference in strength gains after five weeks of training. 2. Accentuated eccentric training makes sense on the surface. Since lifters can handle more weight on the eccentric, using a heavier load on that phase of a lift is logical. However, the longitudinal data do not consistently suggest that accentuated eccentric training enhances strength compared to traditional training. 3. Overall, I wouldn’t expect a huge boost in long-term strength with accentuated eccentrics. I also wouldn’t recommend somebody rush out and implement the practice due to cost and logistical concerns. However, there also doesn’t seem to be a downside to accentuated eccentrics. So, if you are at a gym with weight releasers and some friends (or even just acquaintances) are willing to help, then give it a shot. still in desperate need of more, since only one of them used weight releasers on a free-weight barbell exercise. I’d like to see this current study replicated, but with two changes. First, I would make the study at least eight weeks long to provide more time for any between-group differences to flesh out. I’d also replace the unsupervised group with a group performing accentuated eccentrics with a concentric load equated for velocity loss and/or repetitions in reserve to the traditional loading group. Equating concentric load for velocity loss or reps in reserve would take some pilot testing. For example, let’s say the lifters could perform an average of six reps to a 2RIR at 80% of 1RM on the squat. Then, if the loading on the supramaximal eccentric was 105% of 1RM, the researchers should determine the concentric load resulting in six reps to a 2RIR following the supramaximal eccentric. This would allow the study design to equate the level of effort (at least in terms of velocity loss and repetitions in reserve) on the set. 62 References 1. Munger CN, Jones BC, Halloran IJ, Eggleston GG, Post PG, Brown LE, Berning JM. Short-Term Effects of Eccentric Overload Versus Traditional Back Squat Training on Strength and Power. International Journal of Kinesiology and Sports Science. 2022 Jan 30;10(1):1-8. 2. Doan BK, Newton RU, Marist JL, Triplett-McBride NT, Koziris LP, Fry AC, Kraemer WJ. Effects of increased eccentric loading on bench press 1RM. The Journal of Strength & Conditioning Research. 2002 Feb 1;16(1):9-13. 3. Douglas J, Pearson S, Ross A, McGuigan M. Effects of accentuated eccentric loading on muscle properties, strength, power, and speed in resistance-trained rugby players. The Journal of Strength & Conditioning Research. 2018 Oct 1;32(10):2750-61. 4. Buskard AN, Gregg HR, Ahn S. Supramaximal eccentrics versus traditional loading in improving lower-body 1RM: A meta-analysis. Research Quarterly for Exercise and Sport. 2018 Jul 3;89(3):340-6. 5. Cook CJ, Beaven CM, Kilduff LP. Three weeks of eccentric training combined with overspeed exercises enhances power and running speed performance gains in trained athletes. The Journal of Strength & Conditioning Research. 2013 May 1;27(5):1280-6. 6. Schroeder ET, Hawkins SA, Jaque SV. Musculoskeletal adaptations to 16 weeks of eccentric progressive resistance training in young women. The Journal of Strength & Conditioning Research. 2004 May 1;18(2):227-35. 7. Johnson BL, Adamczyk JW, Tennoe KO, Stromme SB. A comparison of concentric and eccentric muscle training. Medicine and science in sports. 1976 Jan 1;8(1):35-8. 8. Yarrow JF, Borsa PA, Borst SE, Sitren HS, Stevens BR, White LJ. Early-phase neuroendocrine responses and strength adaptations following eccentric-enhanced resistance training. The Journal of Strength & Conditioning Research. 2008 Jul 1;22(4):1205-14. 9. Walker S, Häkkinen K, Haff GG, Blazevich AJ, Newton RU. Acute elevations in serum hormones are attenuated after chronic training with traditional isoinertial but not accentuated eccentric loads in strength‐trained men. Physiological Reports. 2017 Apr;5(7):e13241. 10. Godard MP, Wygand JW, Carpinelli RN, Catalano S, Otto RM. Effects of accentuated eccentric resistance training on concentric knee extensor strength. The Journal of Strength & Conditioning Research. 1998 Feb 1;12(1):26-9. 11. English KL, Loehr JA, Lee S, Smith SM. Early-phase musculoskeletal adaptations to 63 different levels of eccentric resistance after 8 weeks of lower body training. European journal of applied physiology. 2014 Nov;114(11):2263-80. 12. Ojasto T, Häkkinen K. Effects of different accentuated eccentric load levels in eccentricconcentric actions on acute neuromuscular, maximal force, and power responses. The Journal of Strength & Conditioning Research. 2009 May 1;23(3):996-1004. 13. Lates AD, Greer BK, Wagle JP, Taber CB. Accentuated eccentric loading and cluster set configurations in the bench press. The Journal of Strength & Conditioning Research. 2022 Jun 1;36(6):1485-9. 14. Wagle JP, Cunanan AJ, Carroll KM, Sams ML, Wetmore A, Bingham GE, Taber CB, DeWeese BH, Sato K, Stuart CA, Stone MH. Accentuated eccentric loading and cluster set configurations in the back squat: A kinetic and kinematic analysis. The Journal of Strength & Conditioning Research. 2021 Feb 1;35(2):420-7. 15. Merrigan JJ, Tufano JJ, Falzone M, Jones MT. Effectiveness of Accentuated Eccentric Loading: Contingent on Concentric Load. International Journal of Sports Physiology and Performance. 2020 Nov 12;1(aop):1-7. 16. Brandenburg, J.P.; Docherty, D. The effects of accentuated eccentric loading on strength, muscle hypertrophy, and neural adaptations in trained individuals. J Strength Cond Res 2002, 16, 25-32. 17. Friedmann-Bette, B.; Bauer, T.; Kinscherf, R.; Vorwald, S.; Klute, K.; Bischoff, D.; Muller, H.; Weber, M.A.; Metz, J.; Kauczor, H.U., et al. Effects of strength training with eccentric overload on muscle adaptation in male athletes. Eur J Appl Physiol 2010, 108, 821-836. █ 64 Study Reviewed: The Effects of High Fiber Rye, Compared to Refined Wheat, on Gut Microbiota Composition, Plasma Short Chain Fatty Acids, and Implications for Weight Loss and Metabolic Risk Factors (the RyeWeight Study). Iversen et al. (2022) Rye Versus Wheat: Evidence-Based Sandwich Guidelines BY ERIC TREXLER A recent MASS article discussed the utility of fiber restriction for short-term weight cuts, but also cautioned against longterm adherence to low-fiber diets. A new study points to some potential mechanisms by which fiber might favorably impact body composition and health. 65 KEY POINTS 1. A 2021 study indicated that a hypocaloric diet with heavy intake of rye products led to greater weight loss and reductions in C-reactive protein (an inflammation biomarker) than the same intervention with refined wheat products. The present study sought to determine if these effects were related to changes in the gut microbiota or circulating short-chain fatty acid levels. 2. Gut microbiota data are always a bit messy, but the results generally hint at the idea that rye and wheat led to divergent changes in the gut microbiota, which led to divergent changes in plasma short-chain fatty acid levels, which may have played a small role in the rye group’s more favorable body composition and C-reactive protein changes. 3. Preliminary studies suggest that short-chain fatty acids are associated with a wide range of positive physiological effects, and might be partially responsible for some of the health benefits linked to high fiber intake. Adequate consumption of fiber and other non-digestible carbohydrates is currently the best way to promote the production of short-chain fatty acids. B ack in Issue 5 of Volume 6, I wrote an in-depth article about short-term fiber restriction. The results of that study indicated that acute fiber restriction can facilitate short-term weight cuts lasting less than a week (2), but I also cautioned that fiber restriction is not an advisable long-term strategy for healthy lifters with no clinically relevant gastrointestinal symptoms or conditions. The reasoning for this recommendation is very straightforward: fiber does a ton of good stuff, through a number of potential mechanisms. In that article, I included a brief acknowledgement of one particularly fascinating mechanism by stating, “It’s possible that fiber is impacting these outcomes by promoting more favorable diversity of the gut microbiome and increasing the production of short-chain fatty acids and other metabolites with wide-ranging physiological effects.” That’s precisely what the presently reviewed study (1) investigated. This study was a secondary analysis from a 12-week weight loss study called the RyeWeight Study (3), in which 242 males and females with overweight or obesity were randomly assigned to two different intervention groups. One group was instructed to eat high-fiber rye products, while the other was instructed to eat refined wheat products, all within the context of a 12-week hypocaloric diet. 207 participants completed the full dietary intervention, with results indicating that rye consumption led to significantly larger reductions in weight, body-fat percentage, and C-reactive protein levels (a biomarker of inflammation) than refined wheat consumption. The present analysis included data from all 207 study completers to determine if the positive effects of the rye intervention might be related to 66 changes in gut microbiota composition (measured from fecal samples) or short-chain fatty acid levels (measured from plasma samples). In short, the results suggested that the rye intervention led to some small changes in gut microbiota composition and plasma shortchain fatty acid levels, which appeared to be related to changes in body composition and metabolic risk factors. Of course, the devil is in the details with any research involving the gut microbiota, so let’s dive in and see what we can learn from this study. Purpose and Hypotheses Purpose This was an exploratory analysis of a previously conducted weight loss trial. The primary purpose was to investigate “the effects of a dietary intervention on gut microbiota and plasma short-chain fatty acids and their potential roles as mediators of weight-loss induced by a hypocaloric diet rich in high fiber rye foods [versus] refined wheat in a 12-week weight-loss trial.” The secondary purpose was to investigate “if improvements in clinical risk markers caused by the intervention could be related to changes in gut microbiota and [short-chain fatty acids] in plasma and if baseline microbiota was associated with the response to the intervention.” Hypotheses The authors did not explicitly state hypotheses, which likely reflects the exploratory nature of the analysis (in other words, they were seeking to observe and learn rather than confirm or deny). Having said that, the introduction section of the paper clearly suggests that these researchers were at least interested in the possibility that high-fiber rye products might favorably impact body composition and metabolic risk factors by influencing gut microbiota composition and short-chain fatty acid production. Subjects and Methods Subjects The present study recruited males and females between the ages of 30-70 years old to participate. Study participants were required to have a BMI of 27-35 kg/m2 at the time of enrollment, along with sufficiently low values for serum thyroid stimulating hormone levels (≤4.00 mIU/L), plasma low-density lipoprotein (LDL) cholesterol levels (<5.3 mmol/L) levels, plasma triglyceride levels (≤1.8 mmol/L), and blood pressure (<160/105 mmHg), in addition to sufficiently high hemoglobin levels (≥120 g/L). Subjects were excluded from participation if they used nicotine products, did more than ten weekly hours of strenuous physical activity, implemented a weight loss program within the previous six months, used any weight loss medications or supplements within the previous six months, had any relevant cardiometabolic or gastrointestinal health issues, or were unable to consume any of the food products provided by the researchers. After screening 590 interested participants, 242 participants eventually began the dietary intervention; each diet group had 121 total participants, with roughly 61% of them being female. Both groups were around 56-57 years old, weighed 8889 kg, and had 39-41% body-fat and a BMI of about 30, with no significant differences 67 between groups. Of the 242 individuals who began the dietary intervention, 207 of them completed it (108 in the rye group, and 99 in the wheat group). The baseline characteristics for the 207 study completers were quite similar to the full sample of 242 study starters, and were very similar when comparing across groups. Methods After enrollment, 317 participants were provided with dietary guidance and wheat-based food products in order to complete a 2-week introductory period before the actual dietary intervention. This introductory period allowed for some degree of baseline standardization, and also allowed the researchers to exclude any participants who had insufficient adherence to instructions or were particularly resistant to weight loss in the context of this specific diet intervention. Participants were excluded from participation if they failed to meet a particular weight target during the 2-week introductory period (0.5kg of weight loss for non-menstruating participants, and weight stability for menstruating participants), but they were not made aware of this requirement ahead of time (which could have modified their behavior). Of the 317 participants who began the introductory period, 242 moved forward to the next step, which was to be randomly assigned to a group (wheat or rye) for the 12-week dietary intervention. During the intervention, participants were given dietary guidance that was intended to promote a daily energy deficit of around 500kcal, with a macronutrient breakdown of 45-60% carbohydrate, 10-20% protein, and 25-40% fat. In conjunction with this general dietary guidance, participants were provided an assortment of wheat or rye products (depending on their group assignment), including a variety of breakfast cereals, crip breads, and soft breads. They were instructed to consume enough of these products to reach approximately 650kcal/day, which was about 30-50% of their total energy intake for the day. The wheat and rye products were individually packaged in neutral and unlabeled containers, but the study wasn’t strictly “blinded” due to inherent differences in appearance and taste when comparing wheat products to rye products. The rye products provided around 30g/day of fiber, whereas the refined wheat products only provided around 8g/day of fiber. In the original paper from this study (3), the researchers primarily focused on outcomes related to body composition, appetite, and measures related to cardiometabolic risk factors (such as blood pressure, blood lipids, and biomarkers related to inflammation and insulin sensitivity). The presently reviewed study (1) expands upon the primary findings by measuring changes in the gut microbiota (via fecal samples) and changes in plasma levels of short-chain fatty acids. With these outcome measures, the researchers were specifically interested in observing how the two dietary interventions impacted the gut microbiota and circulating short-chain fatty acid levels, and exploring how any such changes might be related to the previously published changes in body composition and cardiometabolic risk factors. Outcomes were measured at weeks 0, 6, and 12 of the intervention. 68 Findings To fully describe the results of this study with a high level of detail would be an inefficient exercise for the purposes of MASS readers. To illustrate my point, the results section of this study spans from page 5 to page 15 in the published PDF version, which is about five times longer than the discussion section. In addition, that’s just referring to the results of the newest paper from this study; I also need to recap some results from the first paper in order to properly contextualize the findings from the new paper. As a result, my goal is to concisely highlight the most pertinent findings from this study. Both subjective and objective measures of compliance suggested that both groups ad- hered to their instructions to consume the assigned wheat or rye products. Furthermore, weighed food logs suggested that both groups reduced their calorie intake by around 100-200 kcal/day; not exactly the intended target of 500 kcal/day, but enough to promote weight loss, and a similar calorie reduction among both groups. Due to differences in the prescribed food products, the rye group increased daily fiber intake from 22g/day to around 37g/day, while the wheat group maintained a steady fiber intake of around 19-21g/ day. The rye intervention led to significantly greater weight loss than the wheat condition; at week 12, there was a 1.08kg difference between groups, after adjusting for baseline differences. As shown in Table 1, the rye group had significantly lower values for BMI, waist 69 circumference, hip circumference, fat mass, and android fat (after adjusting for baseline values) when compared to the wheat group. The rye intervention also led to statistically significant differences in C-reactive protein levels at week 6 and week 12; a drop was observed in the rye group, whereas values remained pretty stable in the wheat group. Even after removing some influential outliers, the rye group had CRP values that were 21% and 28% lower than the wheat group at weeks 6 and 12, and differences at both time points remained significant whether outliers were retained or removed from the analysis. There was a tendency to observe lower LDL levels in the rye group as well; the between-group difference was statistically significant at week 6 (difference = 0.14 mmol/L; p = 0.013), but not at week 12 (difference = 0.10 mmol/L; p = 0.095). One might assume that these differ- ences in C-reactive protein and LDL might relate to weight loss, but these analyses were adjusted for the observed change in body weight, and correlations between weight change and changes in C-reactive protein and LDL were not statistically significant. However, there were no other consistent and statistically significant between-group differences for the other cardiometabolic outcomes assessed or for subjective appetite outcomes. As for the gut microbiota results, the researchers analyzed changes in 110 different bacteria (grouped and compared at the genus level). With this many different statistical tests and comparisons occurring simultaneously, it’s critically important to adjust the analysis to minimize the likelihood of false positives. Of the 110 bacterial genera (the plural term for “genus”) tested, 45 differed significantly between the two groups. However, after adjust- 70 ing for multiple comparisons, between-group comparisons remained statistically significant for only 8 of these 45 genera at either the 6-week or 12-week mark. The relative abundance values of these 8 bacteria at week 0, 6, and 12 are presented in Table 2. Relative to the wheat group, the rye group experienced larger reductions in abundance of (Ruminococcus) torques group, (Eubacterium) ventriosum group, Anaerofilum, and Holdemania, and larger increases in Agathobacter, UCG-003, and Haemophilus. Both groups experienced decreases in Anaerotruncus (with a larger decrease observed in the rye group), and both experienced increases in Bifidobacterium. The researchers measured plasma levels of nine different short-chain fatty acids, which are presented in Table 3. Generally speaking, between-group comparisons were not statistically significant. Acetic acid levels were significantly higher in the rye group than the wheat group at week 6, but this was no longer the case at week 12. However, butyric acid levels increased within the rye group, and were significantly higher than the wheat group at weeks 6 and 12. Finally, the researchers tested a bunch of correlations to explore relationships among variables of interest (such as gut bacteria abundance, short-chain fatty acid levels, body composition, and cardiometabolic risk factors). It wouldn’t be productive to include results for all of the individual correlation tests, as there were dozens and dozens presented in 71 the paper, and many of them were quite weak and, frankly, likely to be spurious. However, the general results of the numerous correlation tests can be summarized as follows: • Baseline abundance of certain bacteria were correlated with changes in body composition, C-reactive protein, and other cardiometabolic risk factors, but not in a consistent manner. • Changes in the abundance of certain bacteria were correlated with changes in body composition, C-reactive protein, and other cardiometabolic risk factors, but not in a consistent manner. • In the rye group, changes in butyric acid and propionic acid were inversely correlated with body composition changes, and changes in succinic acid were inversely correlated with changes in C-reactive protein. In the wheat group, changes in succinic acid were inversely correlated with body composition changes. The researchers used nonparametric Spearman correlation tests (rather than parametric Pearson correlation tests), so the correlation coefficients are presented as Spearman’s rho values rather than Pearson’s r values. Nonetheless, both types of correlation coefficients refer to the relative strength of the correlation between two variables and range from -1 to 1, so they’re interpreted in a similar manner. In the vast majority of cases in the presently reviewed study, correlations were fairly weak (ranging from -0.25 to +0.25), and could possibly be confounded by changes in body weight over the course of the 12week weight loss intervention. Many of the observed correlations were also quite inconsistent, despite meeting the threshold for statistical significance (for example, abundance of was Barnesiella was positively correlated with C-reactive protein changes in the rye group, but negatively correlated with C-reactive protein changes in the wheat group). This inconsistency was particularly noteworthy for correlations involving bacterial abundance values. Criticisms and Statistical Musings If you look closely at Table 2, some of the p-values might catch you by surprise. For example, check out the p-values for Agathobacter – the corrected p-value comparing the groups was p = 0.01 at week 6 (well below the significance threshold), but p = 0.985 at week 12 (nearly as high and non-significant as a p-value can get). What gives? There are two important considerations to keep in mind when assessing these microbiota comparisons. First, it’s important to recognize that the gut microbiota changes a ton, even in the absence of any particular intervention. For example, a recent study found that for 78% of the microbial genera they tested, day-to-day variation (within the same individual) for absolute abundance was way larger than the variation between individuals, and up to 100-fold changes in abundance were observed during the study period (4). The presently reviewed study looked at relative abundance, which is a little more stable than measures of absolute abundance, but the point still holds: the gut microbiota is a 72 rapidly shifting landscape with considerable day-to-day variation, and any study using stool samples from an isolated point in time is looking at a single snapshot of a constantly moving target. The second consideration is statistical in nature. Due to the huge number of bacterial genera tested, the researchers needed to adjust their analysis to limit the risk of false positives. To accomplish this, they used the “false discovery rate” method, which is one of my favorites. This is a sequential adjustment method, and that the first step of the procedure is to complete the huge list of comparisons (in this case, comparing rye versus wheat values for the relative abundance of a long list of bacterial genera), then to rank-order their raw (unadjusted) p-values from smallest to largest. The next step is to apply a correction to each p-value, but the actual magnitude of adjustment varies as you progress from higher-ranked p-values to lower-ranked p-values. As a result, the degree to which a particular p-value is adjusted can change based on where it lands in this rank-ordered list of p-values. This means the adjusted p-value for a particular bacterial genus can change from week 6 to week 12, even if the unadjusted p-value was exactly the same; if it moved up or down the ranking list (due to changes in the p-values corresponding to other bacterial genera), the magnitude of adjustment would change. We can think of this like a bench press competition; you could bench 150kg and win your competition in June, but you could bench the exact same weight at another competition a few months later and come in 9th place, solely because the competitors at the second meet put up better numbers. To extend that metaphor, these adjusted p-values for bacterial abundance outcomes are like looking at the competition placings of a powerlifter whose strength levels vary wildly from day-to-day, and who happens to be competing against a huge field of lifters whose strength levels also vary wildly from day-to-day. As such, the variability observed in Table 2’s adjusted p-values is to be expected, and we should always interpret gut microbiota data with day-to-day and person-to-person variability at top of mind. Interpretation The results leave us with a lot to chew on, so a quick summary is warranted. When randomly assigned to weight loss interventions involving a bunch of high-fiber rye consumption or a bunch of low-fiber refined wheat consumption, the rye group lost more weight and had larger decreases in C-reactive protein, which is a biomarker for inflammation. The two different dietary interventions led to subtle, but in some cases statistically significant, divergences related to the gut microbiota. Bacteria in the gut feed on fiber and other digestion-resistant carbohydrates to create short-chain fatty acids, and the rye and wheat interventions also led to subtle divergences related to plasma short-chain fatty acid concentrations. A huge collection of fairly weak and inconsistent correlations were presented, which is to be expected for correlations involving bacteria abundance from single fecal samples. However, the results collectively hint at the idea that rye and wheat led 73 to divergent changes in the gut microbiota, which led to divergent changes in plasma short-chain fatty acid levels, which may have played a small role in the rye group’s more favorable body composition and C-reactive protein changes. It’s important to acknowledge that these data don’t offer rock-solid evidence for a causative chain of events by which carb source selection leads to gut bacteria changes, gut bacteria changes lead to circulating shortchain fatty acid changes, and short-chain fatty acids cause clinically relevant changes in body composition, inflammation, or cardiometabolic risk factors. These findings are generally, loosely compatible with such a hypothesis, but are far from offering convincing proof. Having said that, if you’re the type of person who requires rock-solid evidence that is intuitive and consistent, you might want to stay on the sidelines of the gut microbiota research for the next couple of decades; it’s very interesting, but it’s very messy. It’s very possible that all of this talk about short-chain fatty acids has taken you by surprise. In the fitness world, you hear all about plenty of other acids – essential amino acids, branched-chain amino acids, and even essential fatty acids make their way into all sorts of evidence-based content. You rarely hear about short-chain fatty acids, but I believe they deserve more attention than they get. Over the last few decades, there has been a considerable amount of research effort dedicated to understanding how short-chain fatty acids are formed, and what they actually do in the body. For starters, the short-chain fatty acids circulating in our blood are primarily created by the bacterial fermentation of non-digestible carbohydrates in the large intestine. As a result, production of short-chain fatty acids depends on having the right bacteria present in the colon, and feeding them the substrates they like. These substrates include nonstarch polysaccharides, resistant starch, oligosaccharides, disaccharides, and certain sugar alcohols (5). It might sound like meal planning has just become a complex puzzle to solve, but the reality is much simpler; you’re very likely to provide a large and diverse supply of these substrates if you’re consuming a well-rounded diet with plenty of fruits, vegetables, whole-grain products, or legumes (5). The presently reviewed study measured nine different short-chain fatty acids, but the main ones are acetate (acetic acid), propionate (propionic acid), and butyrate (butyric acid). These three short-chain fatty acids represent up to 90-95% of the short-chain fatty acid content in the colon (6), which are typically produced in a ratio of approximately 3:1:1 (7). Our understanding of the physiological activities of short-chain fatty acids is preliminary, and largely fueled by mechanistic studies in non-human research models, or observational studies identifying correlations between certain outcomes and short-chain fatty acid concentrations in plasma or in stool samples. However, the preliminary evidence seems to suggest that short-chain fatty acids have wide-ranging positive impacts throughout the body. Given that short-chain fatty acids are produced in the gastrointestinal tract, their 74 localized effects are a straightforward place to start. Butyrate is a key energy source for cells of the gastrointestinal tract, and shortchain fatty acids (and butyrate in particular) have been associated with beneficial effects on constipation (5), irritable bowel syndrome (8), colon cancer (9), and other medical conditions of the GI tract. The short-chain fatty acids that are not locally metabolized within the GI tract are then transported to the liver via the portal vein. Research suggests that short-chain fatty acids can reduce hepatic inflammation, hepatic cholesterol synthesis, and liver fat deposition, which has spurred interest in investigating the clinical potential of short-chain fatty acids in the context of nonalcoholic fatty liver disease (10). Moving on to more directly MASS-relevant topics, a recent review paper by Byrne and colleagues (7) detailed the apparent positive (but modest) effects of short-chain fatty acids on appetite regulation (e.g., increased satiety), energy homeostasis (e.g., increased energy expenditure), and blood lipids. In addition, a separate review by Canfora and colleagues explored the apparent positive (but modest) effects of short-chain fatty acids on glycemic control and insulin sensitivity (11). Shortchain fatty acids have even been linked to the nervous system, with recent reviews by Silva et al (12) and Mirzaei et al (13) discussing preliminary findings related to anxiety, depression, cognition, and a number of clinical conditions impacting the brain and other tissues of the nervous system. When first hearing about the potential beneficial effects of short-chain fatty acids, one might wonder why they haven’t seen a lot of research I WOULDN’T BE SURPRISED IF SHORT-CHAIN FATTY ACIDS PLAY AN IMPORTANT ROLE IN MEDIATING SOME OF THE POSITIVE EFFECTS ASSOCIATED WITH THE INTAKE OF VARIOUS HIGH-FIBER FOODS OR CERTAIN PATTERNS OF GUT BACTERIAL ABUNDANCE on “real-world” outcomes stemming from this long list of proposed mechanisms. I don’t want to oversell these preliminary observations or overstate their importance or generalizability, but there is one striking observation that leads me to view these findings with more optimism than your typical mechanistic or observational findings: in many cases, the preliminary research on short-chain fatty acids points toward mechanisms that seem to be very compatible with the less-understood benefits of dietary fiber. For example, research has suggested (14) that high-fiber diets might favorably impact inflammation, depressive symptoms, and the immune system, but the mechanistic links are very unclear. I am speculating here, but I wouldn’t be surprised if short-chain fatty acids play an important role in mediating some of the positive effects associated with the intake of various high-fiber foods or certain patterns of gut bacterial abundance. In other words, the 75 real-world evidence related to short-chain fatty acids could be right under our noses, buried within interventions that focus on dietary fiber intake or the gut microbiota. You might be wondering why we’re several pages into an article that seeks to explain a 1kg difference in weight loss from switching from rye bread to wheat bread. As I see it, many consumers of evidence-based fitness content have collectively gathered at two opposite ends of a spectrum. On one end, you’ll find the simplifiers. They might argue that you should eat the right number of calories, consume plenty of protein, and lift a few times per week, with any attention devoted to other details representing the futile exercise of “majoring in the minors” (I’m being a bit hyperbolic, but you get the idea). On the other end of the spectrum, you’ll find the optimizers; they’re eager to act upon even the most speculative and physiologically inconsequential ideas, as long as there’s a semi-plausible mechanism by which the idea may lead to some type of miniscule improvement that represents a tiny step toward optimization. When it comes to carbohydrate sources, dietary fiber, and downstream effects on the gut microbiota and short-chain fatty acid production, I encourage both the simplifiers and the optimizers to move toward the middle of the spectrum. For the simplifiers, it’s important to recognize that simplifying is good, but oversimplifying can be counterproductive. Different foods and beverages provide unique combinations of nutrients within unique food matrices, and the physiological effects of a particular food or beverage are more nuanced than their calorie or macronutrient content. While the “major” factors like calorie and protein intake are certainly where most of our focus should be placed, we can’t be overly reductive in the way we view foods and beverages. The presently reviewed RyeWeight Study doesn’t suggest that we need to lose sleep over every little detail of our diet, but it does provide an example of how a seemingly minor food swap can have a measurable impact on tangible physiological outcomes. When combined with the growing body of evidence pertaining to shortchain fatty acids, these findings suggest that we can use food source selection as a tool to optimally support our health and wellness. If your sole focus is on weight change and body composition, there’s no question that calorie intake, protein intake, and resistance training are your biggest priorities. However, if you’re also interested in feeling your best throughout the process, and potentially making small swaps and substitutions that could impact hunger, satiety, gastrointestinal comfort, and cardiometabolic risk factors, it would be advisable to seek out a diverse selection of food sources that provide some non-digestible carbohydrate, and to aim for a minimum daily fiber target. As mentioned in a previous MASS article, a good starting point for daily fiber intake is about 14g of fiber per 1,000kcals in the diet, but dieters can individualize from there based on gastrointestinal comfort, satiety, stool frequency and consistency, and personal preference. For the optimizers, it’s important to understand that we are currently working with a fairly incomplete understanding of the gut 76 microbiota and short-chain fatty acids. As a result, we shouldn’t overestimate the degree to which we can intentionally induce very specific alterations in gut bacterial abundance or short-chain fatty acid production. For many optimizers, the knee-jerk response to hearing about promising short-chain fatty acid findings might be to order a targeted probiotic supplement, or even to supplement with a short-chain fatty acid like butyrate directly. However, that’s probably not the best route to take at this point in time. As noted in a previous MASS article, the probiotic supplementation research lacks clarity and consistency, and there are very few guarantees when it comes to targeted, standalone probiotic supplementation. There are also logistical hurdles related to the ingestion of short-chain fatty acids. If we use butyrate as an example, we see that amounts consumed from conventional food products are relatively low (5), and supplement formulation is made challenging by butyrate’s poor oral bioavailability (15) and its pungent smell and taste (5). For now, the optimizers should resist their biohacking tendencies; rather than opting to directly supplement with short-chain fatty acids or very specific probiotic bacterial strains, a more advisable (and very boring) strategy is to simply consume a well-balanced diet with plenty of fruits, vegetables, whole-grain products, or legumes. Some researchers have suggested that incorporating some fermented foods or beverages into one’s diet might also be helpful for increasing short-chain fatty acid levels (16); it certainly wouldn’t hurt, but I’m personally a bit skeptical that this would move the needle to a physiologically meaningful degree. It’s also possible that there may be some beneficial impact of a combined supplement with prebiotics plus a thoughtfully constructed blend of probiotic bacterial strains, but when it comes to promoting short-chain fatty acid production, a food-focused approach is certainly the simplest (and potentially most effective) option. In summary, the dietary recommendations to support short-chain fatty acid production are virtually identical to best-practice recommendations for supporting “gut health” and microbial diversity, and that’s not coincidental. The results from the first paper from the RyeWeight Study (3) suggest that we can leverage fiber intake and food selection as a tool to modestly (but favorably) impact changes in weight and body composition. The presently reviewed follow-up paper (1) suggests that the beneficial effects induced by opting for rye products rather than refined wheat products might have been mediated, at least in part, by differential effects on the gut microbiota and subsequent production of short-chain fatty acids. This is compatible with preliminary mechanistic and observational evidence indicating that short-chain fatty acids can have small but physiologically relevant impacts on a variety of different tissues, organ systems, and physiological processes. For now, there doesn’t seem to be a particularly specific way to “biohack” your way to these positive outcomes, but the classic advice to consume a well-rounded diet with plenty of fruits, vegetables, whole-grain products, or legumes should go a long way. There is no question that calorie intake, protein intake, and resistance training are the most important and impactful factors driving changes in body 77 APPLICATION AND TAKEAWAYS When we make food choices, we aren’t just feeding ourselves – we’re feeding our gut bacteria as well. By consuming more nonstarch polysaccharides, resistant starch, oligosaccharides, disaccharides, and certain sugar alcohols, it’s very possible that we can influence the production of short-chain fatty acids by our gut microbiota, which appear to have wide-ranging effects across several different tissues, organ systems, and physiological processes. A modest shift in short-chain fatty acid production probably won’t be a huge “game changer” for any singular outcome, but might confer small advantages for a broad selection of outcomes, including body composition and appetite regulation. We still have a lot to learn about short-chain fatty acids, but for now it seems advisable to aim for adequate daily fiber intake (starting with a target of 14g of fiber per 1,000kcals in the diet and individualizing from there), and to eat a well-balanced diet with plenty of fruits, vegetables, wholegrain products, or legumes. composition. However, adequate total fiber intake and consumption of a diverse selection of non-digestible carbohydrate sources is associated with numerous benefits. There’s no need to sweat over every little food choice, but strategic food source selection offers an opportunity to modestly improve a wide range of diet-related outcomes within the framework of flexible dieting. ty acid production, and studies clearly documenting the physiological consequences of successfully altering short-chain fatty acid levels. When those types of studies become available, we’ll have a much more thorough understanding of the true potential of shortchain fatty acids, and the feasibility of actually acting upon that information with practical dietary strategies. Next Steps The research related to short-chain fatty acids is very promising, but very preliminary. At this point, we have applied studies exploring the positive effects of fiber intake, mechanistic and observational studies indicating what short-chain fatty acids appear to do in the body, and observational studies hinting at a link between the two areas of research. Moving forward, we’ll need some longitudinal human trials in which targeted dietary interventions are implemented with the specific intention of influencing short-chain fat- STRATEGIC FOOD SOURCE SELECTION OFFERS AN OPPORTUNITY TO MODESTLY IMPROVE A WIDE RANGE OF DIET-RELATED OUTCOMES WITHIN THE FRAMEWORK OF FLEXIBLE DIETING. 78 References 1. Iversen KN, Dicksved J, Zoki C, Fristedt R, Pelve EA, Langton M, et al. The Effects of High Fiber Rye, Compared to Refined Wheat, on Gut Microbiota Composition, Plasma Short Chain Fatty Acids, and Implications for Weight Loss and Metabolic Risk Factors (the RyeWeight Study). Nutrients. 2022 Apr 17;14(8):1669. 2. Foo WL, Harrison JD, Mhizha FT, Langan-Evans C, Morton JP, Pugh JN, et al. A ShortTerm Low-Fiber Diet Reduces Body Mass in Healthy Young Men: Implications for Weight-Sensitive Sports. Int J Sport Nutr Exerc Metab. 2022 Mar 21;1–9; ePub ahead of print. 3. Iversen KN, Carlsson F, Andersson A, Michaëlsson K, Langton M, Risérus U, et al. A Hypocaloric Diet Rich In High Fiber Rye Foods Causes Greater Reduction In Body Weight And Body Fat Than A Diet Rich In Refined Wheat: A Parallel Randomized Controlled Trial In Adults With Overweight And Obesity (The Ryeweight Study). Clin Nutr ESPEN. 2021 Oct;45:155–69. 4. Vandeputte D, De Commer L, Tito RY, Kathagen G, Sabino J, Vermeire S, et al. Temporal Variability In Quantitative Human Gut Microbiome Profiles And Implications For Clinical Research. Nat Commun. 2021 Nov 18;12(1):6740. 5. Pituch A, Walkowiak J, Banaszkiewicz A. Butyric Acid In Functional Constipation. Prz Gastroenterol. 2013;8(5):295–8. 6. Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG, Salazar N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front Microbiol. 2016;7:185. 7. Byrne CS, Chambers ES, Morrison DJ, Frost G. The Role Of Short Chain Fatty Acids In Appetite Regulation And Energy Homeostasis. Int J Obes. 2015 Sep;39(9):1331–8. 8. Załęski A, Banaszkiewicz A, Walkowiak J. Butyric Acid In Irritable Bowel Syndrome. Prz Gastroenterol. 2013;8(6):350–3. 9. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review Article: The Role Of Butyrate On Colonic Function. Aliment Pharmacol Ther. 2008 Jan 15;27(2):104–19. 10. Juárez-Hernández E, Chávez-Tapia NC, Uribe M, Barbero-Becerra VJ. Role Of Bioactive Fatty Acids In Nonalcoholic Fatty Liver Disease. Nutr J. 2016 Aug 2;15(1):72. 11. Canfora EE, Jocken JW, Blaak EE. Short-Chain Fatty Acids In Control Of Body Weight And Insulin Sensitivity. Nat Rev Endocrinol. 2015 Oct;11(10):577–91. 79 12. Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol. 2020 Jan 31;11:25. 13. Mirzaei R, Bouzari B, Hosseini-Fard SR, Mazaheri M, Ahmadyousefi Y, Abdi M, et al. Role Of Microbiota-Derived Short-Chain Fatty Acids In Nervous System Disorders. Biomed Pharmacother. 2021 Jul;139:111661. 14. Barber TM, Kabisch S, Pfeiffer AFH, Weickert MO. The Health Benefits of Dietary Fibre. Nutrients. 2020 Oct 21;12(10):E3209. 15. Steliou K, Boosalis MS, Perrine SP, Sangerman J, Faller DV. Butyrate Histone Deacetylase Inhibitors. BioResearch Open Access. 2012 Aug;1(4):192–8. 16. Annunziata G, Arnone A, Ciampaglia R, Tenore GC, Novellino E. Fermentation of Foods and Beverages as a Tool for Increasing Availability of Bioactive Compounds. Focus on Short-Chain Fatty Acids. Foods. 2020 Jul 25;9(8):999. █ 80 Research Briefs BY GREG NUCKOLS & ERIC TREXLER In the Research Briefs section, Greg Nuckols and Eric Trexler shares quick summaries of recent studies. Briefs are short and sweet, skimmable, and focused on the needto-know information from each study. 82 88 94 102 107 110 116 119 Squatting with Bands May be Ideal for Improving Jump Performance Estimating the Energy Cost of Exercise to Inform Dietary Adjustments Can Stretching Directly Cause Muscle Growth? An Update on Caffeine Habituation and Sensitivity to Ergogenic Effects Oral Contraceptives Still Don’t Impact Muscle Growth and Strength Gains How Much Do Cortisol Levels Matter For Training Adaptations? Is Your Split Jerk Limited by Upper Body or Lower Body Strength? Should You Take a Krill Pill To Enhance Strength Or Hypertrophy? 81 Study Reviewed: Effects of Variable Resistance Training Within Complex Training on Neuromuscular Adaptations in Collegiate Basketball Players. Shi et al. (2022) Squatting with Bands May be Ideal for Improving Jumping Performance BY GREG NUCKOLS Looking through the MASS archives, I was surprised to see that we haven’t directly reviewed a study investigating the impact of barbell training with bands since Volume 1 (2). In fact, the one article we wrote about training with bands can’t even be found in the archives. It was part of a mini bonus issue we released to incentivize sign-ups during the pre-sale period of our launch sale. However, there’s a reason why we haven’t written much about training with bands, in spite of the fact that training with bands is still somewhat popular: there just hasn’t been much longitudinal research on the topic lately. There have been a few studies looking at acute effects (things like bar velocity, power output, post-activation potentiation, and electromyographic measures within a single training session), but I think a 2018 meta-analysis by dos Santos and colleagues really put the brakes on longitudinal studies (3). That meta-analysis found that variable resistance training (training with bands or chains) didn’t lead to larger strength gains than training with plain old metal plates, and I suspect that many sports scientists didn’t want to invest a ton of time into studying an intervention that was likely to end with null results. With that said, there’s a major application of training with bands that’s (weirdly) under-researched: training for power output and explosiveness. Training with bands or chains seems like it would be ideal for improving jumping performance. When you’re training for maximal strength, you’re ultimately limited by your ability to produce force through a specific (rather small) “sticking region” of a particular lift. Both bands and straight weight can present a near-maximal challenge to the lifter through the sticking region, so it makes sense that they’re similarly effective for promoting maximal strength (assessed via 1RMs of barbell exercises). However, jump performance is dictated by your ability to produce force rapidly through a much longer range of motion: you start accelerating your body at the start of the concentric phase of a jump, and you need to rapidly apply force until the moment your feet leave the ground. With traditional barbell exercises, the top part of the range of motion is typically pretty easy – you can ease up after you 82 make it through the sticking region, and still complete the lift. With bands, on the other hand, the effective load on the bar increases as you progress through the concentric phase of the squat, requiring more effort through a larger portion of the concentric phase than you’d experience with straight weight. Thus, squatting with bands seems like it should be a more specific stimulus than squatting with straight weight for the purpose of improving jump performance. With that in mind, the present study by Shi and colleagues aimed to compare the effects of squatting with straight weight versus squatting with bands for improving maximal strength and explosive performance (1). 21 collegiate basketball players completed this study. Lack of resistance training experience wasn’t an exclusion criterion, but most collegiate basketball players have spent at least a bit of time in the weight room, and the subjects squatted approximately 125kg (275lbs) pre-training, so I suspect that all of the subjects had some prior resistance training experience. Subjects were randomized into two groups that each completed an eight-week training program, with two training sessions per week. Pre- and post-training, researchers assessed subjects’ maximal squat strength (1RM), countermovement jump height, squat jump height, standing broad jump distance, and 10-20m sprint times. The training sessions focused exclusively on lower body strength and power development, and consisted of complex training (one set of a strength exercise, followed by one set of a plyometric or high-velocity exercise). Subjects rested 3 minutes after each set of squats, and 4 minutes after each set of jumps. Details of the training program can be seen in Table 1. One group of subjects completed all of their squats with straight weight: just a barbell and metal plates. The other group of subjects replaced a portion of the load with resistance from elastic bands that were anchored to the floor and looped over the barbell. The resistance provided by the bands was approximately 35% of 1RM at the top of the lift, and 83 approximately nil at the bottom of the lift. Since the resistance provided by the bands (averaged over the entire range of motion) was about 17% of 1RM, the load provided by metal plates was reduced by approximately 17% of 1RM to equalize the total loading between the groups. For example, if a subject had a 200kg squat 1RM, and they were training with 80% of 1RM, they’d just use 160kg if they were in the straight weight group. If they were in the bands group, the bands on the bar would provide approximately 70kg of resistance at the top of the lift (200 × 35%), and virtually no resistance at the bottom of the lift. The average resistance provided would be 35kg, so the load on the bar would be reduced by 35kg. So, they’d complete their set of squats with 125kg of straight weight (from the barbell and metal plates), plus the bands. The total resistance would be approximately 195kg at the top of the lift, and 125kg at the bottom of the lift, for an average resistance of 160kg. The results of the study were pretty straightforward: squatting with bands improved vertical jumping performance more than squatting with straight weight. The difference was statistically significant for squat jump improvements (+21.4% vs. +12.9%; p = 0.008), but not quite statistically significant for countermovement jump improvements (+12.9 % vs. +5.6%; p = 0.056). Changes in all other outcomes were similar between groups: large increases in squat 1RM, small increases in broad jump distance, and minimal changes in 10m and 20m sprint times. You can see these results in Figure 1 and Table 2. The results of this study comport well with studies by Katushabe and Kramer (4) and by Joy and colleagues (5). Katushabe and Kramer studied male soccer players over 6 weeks, and found that squatting with bands tended to improve squat jump height to a greater extent than squatting with straight weight (+2.67cm vs. +1.38cm; p = 0.055). Like the present study, other measures of explosive performance didn’t differ between groups – chang- 84 es in 40m sprint times and performance in an agility drill were similar between groups. Joy and colleagues studied collegiate basketball players with at least one year of resistance training experience over 5 weeks. They also found that training with bands tended to result in larger increases in vertical jump height, maximal power (assessed during vertical jumping), and rate of power development than squatting with straight weight. Furthermore, improvements in 40-yard dash times didn’t differ between groups. However, a study by Andersen and colleagues attained different results that were nonetheless illuminating (6). Trained women completed 10 weeks of training, and completed countermovement jump tests at three dif- ferent depths: descending until they achieved approximately 60°, 90°, and 120° of knee flexion. Improvements in countermovement jump height didn’t significantly differ between groups, but the nominal increase was larger in the band group at 60° of knee flexion (+3cm vs. +2.6cm), and larger in the straight weight group at 90° and 120° of knee flexion (+1.9cm vs. +2.9cm, and +1.5 vs. 2.4cm). I suspect that the study by Andersen and colleagues obtained different results, primarily due to differences in the band resistance used in these four studies. In the present study by Shi and colleagues (1), band tension was 35% of 1RM at the top of the lift, and ~0% of 1RM at the bottom. In the study by Joy and colleagues (5), band tension was 30% of 85 1RM at the top of the lift, and ~0% of 1RM at the bottom. In the study by Katushabe and Kraemer (4), they report there was “20% of load coming from the power bands, and the difference coming from the weight plates” (which is somewhat vague, but appears to be within the same general ballpark as the Shi and Joy studies). However, band resistance accounted for more than 40% of the total resistance in the Andersen study (6): 58% of the resistance at the top of the lift and 44% of the resistance at the bottom of the lift at the start of the study, and 38% of the resistance at the top of the lift and 27% of the resistance at the bottom of the lift by the end of the study. When you squat with a lot of band tension, you can completely transform the typical resistance curve of a squat. When band tension is dialed in just right, you wind up with a smooth resistance curve that matches your natural strength curve pretty well, resulting in a consistent, high level of effort throughout the lift. With excessive band tension, on the other hand, the feel of the lift is completely inverted: instead of being hard at the bottom and easy at the top (as it would be with straight weight), the lift becomes easy at the bottom and very hard at the top. With heavy enough band tension, you may hit your sticking point when you’re in a quarter squat position. Through that lens, the results of the Andersen study make a lot of sense – they comport well with the principle of specificity, and don’t actually conflict with the results of Shi, Joy, and Katushabe. The subjects in the band group in the Andersen study experienced pretty large increases in countermovement jump performance at 60° of knee flexion, because that matches the range of motion where they were actually being challenged by their squat training. However, they experienced smaller improvements at 90° and 120° of knee flexion because their squat training wasn’t providing quite as much of a challenge at deeper knee flexion angles. In the other three studies, band tension was high enough to still provide more of a challenge than straight weight through the top portion of the concentric phase, but not so high that the bottom portion of the concentric became meaningfully easier. Overall, we can take three things away from the research on squatting with bands: 1. Squatting with bands doesn’t increase your 1RM squat to a greater extent than squatting with straight weight, on average. 2. Squatting with bands probably does increase your jumping ability to a greater extent than squatting with straight weight. However, using bands probably doesn’t increase your performance in less specific tests of power output and explosive performance (sprinting, broad jumps, or change of direction) to a greater extent than squatting with straight weight. Both of these findings comport well with the principle of specificity. 3. Excessive band tension can be counterproductive. Using band resistance equal to ~30-40% of 1RM at the top of the lift (and virtually no resistance at the bottom of the lift) to replace an amount of plates equal to 15-20% of 1RM seems to get the job done. More band tension beyond that point is unlikely to further improve results, and may actually result in smaller improvements in jump performance. 86 To be clear, these takeaways are somewhat tentative. Four studies isn’t a huge body of literature, and while the outcomes related to jump performance certainly lean in favor of squatting with bands, only a handful of the differences between groups achieved statistical significance, and we’re also not dealing with enormous effect sizes. However, I’m a bit more willing to interpret these results liberally because they fit well within the firm conceptual framework of the principle of specificity. With that said, a more conservative wait-and-see approach to interpreting these results is certainly still very justifiable. References 1. Shi L, Lyons M, Duncan M, Chen S, Chen Z-X, Guo W, Han D. (2022). Effects of variable resistance training within complex training on neuromuscular adaptations in collegiate basketball players. Journal of Human Kinetics. 2022 Training on Sprint Speed, Agility, Vertical Jump Height, and Strength in Collegiate Soccer Players. Int J Exerc Sci. 2020 Aug 1;13(4):950-963. PMID: 32922637; PMCID: PMC7449328. 5. Joy JM, Lowery RP, Oliveira de Souza E, Wilson JM. Elastic Bands as a Component of Periodized Resistance Training. J Strength Cond Res. 2016 Aug;30(8):21006. doi: 10.1519/JSC.0b013e3182986bef. PMID: 23669815. 6. Andersen V, Fimland MS, Kolnes MK, Saeterbakken AH. Elastic Bands in Combination With Free Weights in Strength Training: Neuromuscular Effects. J Strength Cond Res. 2015 Oct;29(10):2932-40. doi: 10.1519/ JSC.0000000000000950. PMID: 25807031. 2. Rivière M, Louit L, Strokosch A, Seitz LB. Variable Resistance Training Promotes Greater Strength and Power Adaptations Than Traditional Resistance Training in Elite Youth Rugby League Players. J Strength Cond Res. 2017 Apr;31(4):947955. doi: 10.1519/JSC.0000000000001574. PMID: 27465633. 3. Nilo Dos Santos WD, Gentil P, Lima de Araújo Ribeiro A, Vieira CA, Martins WR. Effects of Variable Resistance Training on Maximal Strength: A Metaanalysis. J Strength Cond Res. 2018 Nov;32(11):e52-e55. doi: 10.1519/ JSC.0000000000002836. PMID: 30540285. 4. Katushabe ET, Kramer M. Effects of Combined Power Band Resistance 87 Study Reviewed: Estimating Energy Cost of Body Weight Resistance Exercise Using a Multistage Exercise Test. Nakagata et al. (2022) Estimating the Energy Cost of Exercise to Inform Dietary Adjustments BY ERIC TREXLER This is going to be a rather atypical Research Brief, because I plan to discuss the reviewed study extremely briefly. The justification for this decision is two-fold: the findings largely reinforce the conclusions of a previous MASS article (while adding a tiny bit of nuance), and I want to provide some very practical recommendations that are described with a high degree of detail and clarity. As such, the vast majority of this particular Research Brief will focus on how to practically apply the information. You might recall a recent MASS article about a study investigating the energy cost of resistance exercise. The researchers found that average (group-level) energy expenditure during low-, moderate-, and high-load exercise was approximately 6kcal/min, with individual values consistently falling within the 4-8 kcal/min range (2). Greg even created a very helpful calculator you can use to determine your added energy cost from resistance exercise (that is, the additional energy expenditure induced from resistance exercise, above and beyond your typical resting level of energy expenditure). The presently reviewed study (1) also investigated the energy cost of resistance-type exercise, using a very different approach. Their study investigated bodyweight exercise, which can be operationally viewed as a unique type of low-load resistance training, and their study design lacked ecological validity (that is, it’s not particularly representative of a typical training protocol), but allows for some helpful observations. Participants (15 men) completed three different bodyweight exercises (calf raise, squat, and push-up) with very slow repetition cadences. Each repetition lasted six seconds (with a 3-second concentric phase and a 3-second eccentric phase), and repetitions were performed with a variety of different frequencies (1, 2, 3, 4, 5, or 6 reps per minute, equally spaced throughout the minute). For example, participants completed a repetition every 30 seconds in the 2 reps per minute condition, and completed a rep every 10 seconds in the 6 reps per minute condition. This means that the work:rest ratio ranged from 6:54 (in the 1 rep/min condition) to 36:24 (in the 6 rep/min condition). 88 The energy expenditure for each condition is plotted in Figure 1. As we can see, energy expenditure varies from exercise to exercise, and is particularly low when dealing with small muscle groups (in this case, the calves). Expenditure is also influenced by relative intensity; bodyweight squats activate a large amount of muscle mass, but are also considerably easier, rep-for-rep, when compared to push-ups. Easier exercises (calf raise and squat) stayed well below the 6kcal/min heuristic value associated with traditional resistance training, but as push-ups started to get more challenging, it looks like they were starting to level off as they approached ~6kcal/min (although more data would be needed at higher rep frequencies to determine if this was a legitimate trend or a couple of noisy data points). Generally speaking, these findings seem to be fairly compatible with the general heuristic that challenging resistance-type exercise is likely to fall within the range of 4-8kcal/min, and that you’re more likely to end up near the higher end of that range if you’re activating large muscle groups, working at a high relative intensity, or training with a relatively high work:rest ratio. exercise energy expenditure, which has multiple practical use cases. For example, you might want a good estimate of exercise energy expenditure because you’re adding some extra exercise to your weekly routine during a cut, and you’d like to know approximately how large of an energy deficit you’re creating. Conversely, you might be adding some extra exercise for health purposes during a bulk, and you’d like to make sure you aren’t unintentionally shrinking your energy surplus. Alternatively, you might be doing a particular bout of exercise that is a departure from your normal routine, and you’d like to make sure you’re fueling your body adequately for the energy demands of the activity. If you’re primarily interested in maintaining a particular level of energy balance (whether it’s a deficit, surplus, or maintenance) and you’re tracking your body weight and calorie intake regularly, one option is to skip the estimation process altogether and simply adjust I’m well aware that this study falls short of delivering any earth-shattering revelations that fundamentally change the way we view resistance exercise. However, I thought it was valuable to cover this study for two reasons. First, it lends some additional support to Greg’s proposed heuristic for resistance-type exercise from Issue 3 of Volume 6, while adding a little bit of nuance and expanding the scope of the conversation to include bodyweight exercises. Second, it gives us an opportunity to revisit the topic of estimating 89 your calorie intake as you go. Your weight changes observed after implementing the new exercise activity will tell you whether your energy balance is more positive or more negative than you intended. For example, if you were losing weight at your intended rate, but started losing weight faster than intended after adding some exercise to your weekly routine, then an increase in calorie intake would be warranted. In this scenario, you’d increase calorie intake incrementally until you were back on track with your intended rate of weight loss. This strategy (explained in more detail here) is a fantastic option that relies on the fewest assumptions possible, but it’s hard to implement without the help of good software that smoothes out some of the “noise” in your weight data and provides quantitative analytics to guide changes in calorie intake. It also doesn’t address the specific use case of prospectively planning a fueling strategy for an arduous exercise bout that isn’t typically part of your habitual exercise routine. Another option is to use simple heuristics rather than customized or individualized estimation methods. If you’re doing any kind of ambulatory exercise like walking, jogging, or running, then a decent heuristic is to assume that you’ll burn 100kcal per mile (3). For most forms of resistance exercise, it’s safe to assume that you’re burning around 6kcal/ min, although it could range from roughly 4-8 kcal/min depending on your body mass and the characteristics of the exercise bout (2). Heuristics are great when you’re looking for a simple strategy (which is made even simpler by Greg’s calculator), but they are a bit oversimplified and lack flexibility for in- dividualized estimates that can be adjusted to accommodate a wide range of exercise modalities and intensities. In this research brief, I’d like to propose a third option, which is individualized MET-based calculations. A MET, or metabolic equivalent, is a standardized unit of measurement that quantifies the rate of oxygen consumption observed in humans at rest. Well, kind of – the original MET was calculated by measuring the resting oxygen consumption of one 40-yearold guy (4), so I guess it actually quantifies the resting oxygen consumption of one human. Nonetheless, one MET equals 3.5 milliliters of oxygen consumption per kilogram of body mass per minute (3.5mLO2/kg/min). This metric is very useful for two reasons. First, it gives us a standardized unit for quantifying the metabolic demand of exercise, scaled to a resting level. If a particular exercise increases oxygen consumption to 7mLO2/kg/min, this can also be quantified as 2 METs, which indicates that oxygen consumption is 2x the typical resting level. Likewise, if a particular exercise increases oxygen consumption to 14mLO2/kg/min, this can also be quantified as 4 METs, which indicates that oxygen consumption is 4x the typical resting level. That might not seem like a huge deal, but it has allowed for researchers to compile enormous lists assigning MET values to a wide range of exercise modalities and intensities. For example, the 2011 Compendium of Physical Activities compiled by Ainsworth and colleagues (5) provides MET values for over 820 distinct forms of exercise and non-exercise physical activity. Second, and most importantly, oxygen consumption (and by extension, METs) can be 90 directly converted to an estimated energy cost, in kilocalories. A detailed justification of this conversion process is beyond the scope of a research brief, but the simplified version is that oxygen is consumed (and carbon dioxide is produced) when we metabolize energy substrates to create ATP. This relationship directly links energy oxygen consumption to energy expenditure, and allows us to estimate the energy cost of various activities based on the amount of oxygen consumed. In fact, just about every energy expenditure value we’ve ever mentioned in MASS that was obtained from a lab-based measurement, whether at rest or during exercise, is based on this relationship. These measures are often reported as kilocalories (a unit of energy), but the measurement technique most commonly used is called indirect calorimetry, which actually functions by measuring the oxygen and carbon dioxide concentrations of inhaled and exhaled breath rather than directly measuring energy production (hence the name, indirect calorimetry). It’s often said that 1 MET is equivalent to 3.5mLO2/kg/min, which converts to an energy expenditure rate of 1 kcal/kg/hour. This is actually an oversimplification; if you crunch the numbers, you’ll find that 3.5mLO2/kg/ min actually converts to an energy expenditure rate of 1.05 kcal/kg/hour. Surprisingly, the minimal amount of information we’ve covered gives us everything we need to calculate a decent energy cost estimate for a huge range of exercise modalities and intensities. First, you need to estimate the MET value of your exercise bout; the 2011 Compendium of Physical Activities is the list I prefer to use, but you can certainly find accurate MET values elsewhere. Second, you need to calculate your estimated resting energy expenditure (REE). You may be stunned to hear this, but the resting metabolic characteristics of that one 40-year-old guy aren’t perfectly representative of the entire human population. As a result, researchers have demonstrated that you can enhance the accuracy of exercise energy cost calculations by adjusting for your own resting energy expenditure (4). I’d personally recommend using the Cunningham equation (6) for this: REE = 500 + fatfree mass (in kg) × 22. This gives you REE in kcal/day, which you can divide by your weight (in kg) and 24 (hours per day) to convert your REE to kcal/kg/hour (REE [kcal/ kg/hour] = REE [kcal/day] ÷ weight (kg) ÷ 24). To estimate the energy cost of your exercise bout, enter this information (along with the duration of the bout, in minutes) into the following equation: However, we aren’t quite done yet. As Greg pointed out in his recent research brief, we’re actually interested in the added energy expenditure (beyond the resting level), not total energy expenditure. So, we should convert our REE (kcal/day) to kcals per minute (REE [kcal/min] = REE [kcal/day] ÷ 1440), then multiply that by the duration of our exercise bout to figure out how much energy we would have burned if we rested instead of exercising. We’ll subtract that value from the energy cost of exercise, but there’s one final step: we need to deal with energy com- 91 pensation, which has been covered in previous MASS articles (one, two, three). In short, when we increase our exercise energy expenditure, we often experience compensatory reductions in other areas of energy expenditure. Our current “best estimate” (7) is to anticipate an average of 30% compensation (e.g., adding 100 extra kilocalories of exercise would only increase total daily energy expenditure by 70 kilocalories because you compensated for 30 of them), but a typical range probably spans from ~10-50%. If you’re pretty shredded, in an aggressive calorie deficit, and very active, you’re likely to end up closer to 50%; if you’re not shredded, in neutral-to-positive energy balance, and have a low level of daily energy expenditure from exercise and other physical activity, you’re probably closer to 10%. In order to calculate a “compensation adjustment,” you’ll need to make an educated guess about your most likely magnitude of energy compensation, then calculate the correction factor as follows: Compensation Adjustment (CA) = (100 - % compensation) ÷ 100. So, if you anticipate a 30% magnitude of energy compensation, your compensation adjustment would be 0.7, and if you anticipate 40% compensation, the adjustment value would be 0.6. The final step is to subtract resting energy expenditure from the energy cost of exercise, then to multiply the result by the compensation adjustment (CA): I know, that’s a lot of steps. However, this approach to energy cost estimation is valu- able because it can be individualized based on your body size and estimated energy expenditure, and can be applied to any exercise modality and intensity for which you can find (or estimate) a reasonably accurate MET value. For example, the 2011 Compendium of Physical Activities provides estimates for light calisthenics, vigorous calisthenics, circuit training, various intensities of resistance training, and over 800 other types of exercise. In addition, I made this spreadsheet to take care of the actual calculations for you. So, between the 2011 Compendium of Physical Activities and the online calculator, you can estimate the added energy cost of just about any exercise or physical activity you complete. The estimate won’t be perfect, as it can be impacted by extraneous factors like genetics, cardiorespiratory fitness level, mechanical efficiency, and environmental conditions, but it’s probably our best equation-based option for prospective energy cost predictions. References 1. Nakagata T, Yamada Y, Naito H. Estimating Energy Cost of Body Weight Resistance Exercise Using a Multistage Exercise Test. J Strength Cond Res. 2022 May 1;36(5):1290–6. 2. João GA, Almeida GPL, Tavares LD, Kalva-Filho CA, Carvas Junior N, Pontes FL, et al. Acute Behavior of Oxygen Consumption, Lactate Concentrations, and Energy Expenditure During Resistance Training: Comparisons Among Three Intensities. Front Sports Act Living. 2021;3:797604. 92 3. Loftin M, Waddell DE, Robinson JH, Owens SG. Comparison Of Energy Expenditure To Walk Or Run A Mile In Adult Normal Weight And Overweight Men And Women. J Strength Cond Res. 2010 Oct;24(10):2794–8. 4. Byrne NM, Hills AP, Hunter GR, Weinsier RL, Schutz Y. Metabolic Equivalent: One Size Does Not Fit All. J Appl Physiol. 2005 Sep;99(3):1112–9. 5. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, et al. 2011 Compendium Of Physical Activities: A Second Update Of Codes And Met Values. Med Sci Sports Exerc. 2011 Aug;43(8):1575–81. 6. Cunningham JJ. A Reanalysis Of The Factors Influencing Basal Metabolic Rate In Normal Adults. Am J Clin Nutr. 1980 Nov;33(11):2372–4. 7. Careau V, Halsey LG, Pontzer H, Ainslie PN, Andersen LF, Anderson LJ, et al. Energy Compensation And Adiposity In Humans. Curr Biol. 2021 Oct 25;31(20):4659-4666.e2. 93 Study Reviewed: Influence of Long-Lasting Static Stretching on Maximal Strength, Muscle Thickness and Flexibility. Warneke et al. (2022) Can Stretching Directly Cause Muscle Growth? BY GREG NUCKOLS Stretching is one of a handful of topics where I consistently find myself at odds with other “evidence-based” fitness folks. The popular narrative is that stretching is useless at best (“It doesn’t actually increase range of motion long-term!” and “It doesn’t reduce injury risk!”) and counterproductive at worst (“It hinders performance!” and “It reduces muscle growth!”). However, when you actually dig into the research on stretching, an interesting, nuanced picture emerges. For example, intense stretching immediately before exercise might reduce muscle growth (2), but light stretching between sets may actually increase muscle growth (3). Similarly, intense, long-duration stretching right before an exercise test may reduce force and power output (4), but longitudinal stretching interventions may actually increase strength over time (5). In short, stretching isn’t all good or all bad – whether it helps or hinders you largely depends on the timing, intensity, and duration of your stretching sessions. Lately, there’s been more interest in stretch-mediated hypertrophy. We pretty consistently observe that training through a full range of motion results in more muscle growth than training through the top part of a range of motion (i.e., deep squats cause more quad growth than half squats; 6). There are two key differences between training through a full range of motion and training through the top half of a range of motion: 1) the total range of motion is different, and 2) training through the top part of a range of motion generally involves not training your prime movers at long muscle lengths. So, which of these differences explains why training through a full range of motion results in more muscle growth? Recent research suggests that the second factor – training at long muscle lengths – is far more important than the total range of motion you train through. If that weren’t the case, only training the top half of a lift would result in just as much muscle growth as only training the bottom half of a lift, and both would result in less muscle growth than training through a full range of motion. However, that’s now what the research shows. Partial range of motion training at long muscle lengths (for example, just doing the bottom 94 half of a squat) causes at least as much muscle growth as training through a full range of motion, and considerably more muscle growth than partial range of motion training at short muscle lengths (7, 8). This research suggests that there’s something special about training at long muscle lengths. At the moment, the leading hypothesis to explain these findings is the existence of “stretch-mediated hypertrophy.” In other words, there’s something about tension on a muscle in a stretched position that more effectively promotes hypertrophy than tension on a muscle in a shortened position. And, while I personally think that the existence of stretch-mediated hypertrophy provides us with a plausible, elegant idea that ties this entire line of research together, there’s one problem with it: there’s not a ton of evidence that stretching can directly cause hypertrophy. We do know that stretching can put a lot of tension on a muscle – sufficiently intense stretching can lead to muscle damage and DOMS, much like resistance training (14) – so sufficiently intense stretching should directly result in muscle hypertrophy if the notion of stretch-mediated hypertrophy is correct. If it doesn’t, then we need to find some other explanation for why training at long muscle lengths results in more muscle growth than training at short muscle lengths. en’t complete slam dunks. For example, Panidi and colleagues found that a stretching intervention increased gains in gastrocnemius cross-sectional area in adolescent volleyball players (9), but a skeptic might note that while gains in cross-sectional area differed between conditions, increases in muscle thickness didn’t differ between the stretching and non-stretching conditions. Furthermore, Simpson and colleagues found that a six-week stretching intervention increased gastrocnemius thickness in a sample of 11 males (10), but this finding also has a slight asterisk: when comparing stretched versus nonstretched legs, the increase in muscle thickness was slightly greater in the stretched legs (p = 0.04 for the time-by-condition interaction effect), but you can see the results for yourself in Figure 1. It’s certainly not a night-and-day difference. So, the stretch-mediated hypertrophy hypothesis finds itself in a weird spot. It would At this point, there have been dozens of studies on stretching, but hypertrophy following stretching interventions has only been observed a handful of times, so a skeptic could easily argue that these findings were false positives, swimming in a sea of “true” null results. Furthermore, the positive findings ar- 95 explain the results of studies examining the effect of range of motion on hypertrophy. It would explain why isometrics at long muscle lengths may result in more muscle growth than isometrics at short muscle lengths (11). It also has a lot of support from animal studies (on birds, rodents, and cats), finding that intense stretching interventions result in a ton of muscle growth (both hypertrophy and fiber hyperplasia; 12). However, there’s not much human evidence supporting the idea that a stretch stimulus effectively and independently promotes muscle growth. In situations like this, it’s nice to have a proof-of-concept study to fall back on. In proof-of-concept studies, you stack the deck in favor of the effect you’d like to observe. We’ve discussed this concept previously in the context of concurrent training. The first concurrent training study by Hickson was a great proof-of-concept study (13). It found that when you put subjects on a really intense training program and a really intense endurance training program, subjects gain less strength than they would when following a program without any endurance training. After Hickson established the existence of this “interference effect,” subsequent research was able to flesh out the details: how much endurance training is required to result in significant interference? What populations are most likely to experience the interference effect? How does the timing of endurance and resistance training affect the interference effect? Until recently, however, there wasn’t a great proof-of-concept study investigating the direct impact of stretching on muscle growth. The ideal proof-of-concept study would use an intervention that would likely exceed anything that would ever be used in the “real world,” to simply establish that stretching can independently cause hypertrophy. If such a study failed to find that stretching directly causes hypertrophy, that would put the concept of stretch-mediated hypertrophy on shakier footing. However, if such a study did find that stretching can cause hypertrophy in humans, it would put the idea of stretch-mediated hypertrophy on firmer evidentiary grounds, and open the door for subsequent studies to flesh out the details. As you might suspect, the study I’m reviewing in this research brief is the exact sort of proof-of-concept study I’ve been waiting on (1). 52 subjects were randomized into two groups: a stretching group and a non-stretching control group. Furthermore, the legs of the subjects in the stretching groups were randomly divided within-subject: one leg underwent the stretching intervention, and the other leg served as a non-stretching control leg. All subjects were “athletically active,” having “performed two or more training sessions per week in a gym or a team sport continuously for the previous six months.” The stretching intervention was quite intense. Each subject used an orthotic device that locked the foot in place while pulling the ankle into dorsiflexion. The orthotic is illustrated in Figure 2. The amount of stretch provided by this device could be manually adjusted, and subjects were instructed to cinch the stretching mechanism to the point 96 that the stretch resulted in pretty significant discomfort (an 8 on a subjective 1-10 pain scale). From there, they sat upright in a chair, propped their leg up on another chair of the same height, and stretched their calf for a full hour. This setup can be seen in Figure 2. The stretching intervention lasted for six weeks, and subjects stretched their calf for a full hour every day. As their range of motion improved, they were instructed to pull their ankle into more and more dorsiflexion using the orthotic device, to maintain the same discomfort rating throughout the intervention. Subjects were also instructed to keep a stretching diary, noting their daily stretching duration and intensity (the dorsiflexion angle of the orthotic device). For our purposes, the most important outcome was the change in gastrocnemius thickness. However, changes in dorsiflexion range of motion were also assessed, as were changes in dynamic and isometric plantarflexion strength. Hypertrophy was assessed via ultrasound. Flexibility was assessed via the knee-to-wall test, and by measuring the maximal dorsiflexion angle that could be achieved on the orthotic device used in the stretching intervention. Strength was assessed unilaterally on a leg press (subjects performed maximal isometric contractions and calf raise 1RM tests). Isometric strength increased significantly more in the stretching leg of the stretching group (+16.8%) than in the non-stretching leg of the strength group (+1.4%), whereas the control group experienced small reductions in strength (reductions of 1.4-1.6%). Dynamic strength followed a similar pattern, 97 though the non-stretching legs in the stretching group also experienced a notable increase in strength, suggesting that some amount of cross-education occurred: calf raise 1RMs increased by 25.1% in the stretching leg of the stretching group and 11.4% in the non-stretching leg of the stretching group, whereas the control group experienced small reductions in strength (reductions of 1.2-3.6%). These results can be seen in Table 1. Changes in flexibility followed a similar pattern. Knee-to-wall test performance increased substantially in the stretching leg of the stretching group (+13.2%), while all other groups and conditions experienced small reductions in performance (reductions of 0.8-2.4%). Maximum dorsiflexion angle on the orthotic device increased by 27.3% in the stretching leg of the stretching group and 7.5% in the non-stretching leg of the stretching group (suggesting that 98 some cross-education occurred), whereas the control group experienced minimal changes (increases of 0-0.7%). These results can be seen in Table 2. Finally, and most importantly, gastrocnemius thickness increased substantially in the stretching legs of the stretching group (+15.3%), while the non-stretching legs experienced a much smaller increase (+2.1%). This was a large (ŋ2 = 0.406; an eta squared of 0.406 is comparable to a Cohen’s d effect size of about 1.65), statistically significant (p = 0.015) difference. These results can be seen in Table 3. This study demonstrates that static stretching with sufficient intensity and volume can directly cause hypertrophy in humans. While this isn’t a completely novel finding (Simpson and Panidi previously observed similar effects; 9, 10), the results of this study are stronger and more conclusive than those observed in prior research. This is a pretty important finding, because it places the idea of stretch-mediated hypertrophy on firmer evidentiary grounds. Furthermore, this study confirms that longitudinal stretching interventions can directly increase dynamic strength and isometric force output (5). At first, I was tempted to write that, like most proof-of-concept studies, the results of the present study likely can’t be directly translated into “real world” practice. However, upon further reflection, I actually think that someone could directly apply the intervention used in the present study. If you don’t mind shelling out some money for an ankle stretching orthosis, and you spend at least an hour per day sitting around (watching TV, playing video games, working on your computer, etc.), you could conceivably try this intervention out for yourself, with minimal disruption to your day-to-day life. It’s just a question of how much discomfort you’re willing to endure to grow your calves. Realistically, though, this study is just a first step. Future research should examine other muscles and manipulate stretching duration and intensity to see just how much stretching is required to provide an adequate stimulus for muscle growth. Finally, we’re still a long way from fully understanding stretch-mediated hypertrophy. We can observe its effects, and we’ve now established its underlying assumption (stretch per se can independent- 99 ly contribute to hypertrophy in humans), but there’s a lot of work left to do before we understand the mechanistic underpinnings of this phenomenon. References 1. Warneke K, Brinkmann A, Hillebrecht M and Schiemann S. Influence of LongLasting Static Stretching on Maximal Strength, Muscle Thickness and Flexibility. Front. Physiol. 2022, 13:878955. doi: 10.3389/fphys.2022.878955 2. Evangelista AL, De Souza EO, Moreira DCB, Alonso AC, Teixeira CVS, Wadhi T, Rauch J, Bocalini DS, Pereira PEA, Greve JMD. Interset Stretching vs. Traditional Strength Training: Effects on Muscle Strength and Size in Untrained Individuals. J Strength Cond Res. 2019 Jul;33 Suppl 1:S159-S166. doi: 10.1519/ JSC.0000000000003036. PMID: 30688865. 3. Junior RM, Berton R, de Souza TM, Chacon-Mikahil MP, Cavaglieri CR. Effect of the flexibility training performed immediately before resistance training on muscle hypertrophy, maximum strength and flexibility. Eur J Appl Physiol. 2017 Apr;117(4):767-774. doi: 10.1007/s00421016-3527-3. Epub 2017 Mar 1. PMID: 28251401. 4. Chaabene H, Behm DG, Negra Y, Granacher U. Acute Effects of Static Stretching on Muscle Strength and Power: An Attempt to Clarify Previous Caveats. Front Physiol. 2019 Nov 29;10:1468. doi: 10.3389/fphys.2019.01468. PMID: 31849713; PMCID: PMC6895680. 5. Medeiros DM, Lima CS. Influence of chronic stretching on muscle performance: Systematic review. Hum Mov Sci. 2017 Aug;54:220-229. doi: 10.1016/j. humov.2017.05.006. Epub 2017 May 18. PMID: 28527424. 6. Schoenfeld BJ, Grgic J. Effects of range of motion on muscle development during resistance training interventions: A systematic review. SAGE Open Med. 2020 Jan 21;8:2050312120901559. doi: 10.1177/2050312120901559. PMID: 32030125; PMCID: PMC6977096. 7. Pedrosa GF, Lima FV, Schoenfeld BJ, Lacerda LT, Simões MG, Pereira MR, Diniz RCR, Chagas MH. Partial range of motion training elicits favorable improvements in muscular adaptations when carried out at long muscle lengths. Eur J Sport Sci. 2021 May 23:1-11. doi: 10.1080/17461391.2021.1927199. Epub ahead of print. PMID: 33977835. 8. Sato S, Yoshida R, Kiyono R, Yahata K, Yasaka K, Nunes JP, Nosaka K, Nakamura M. Elbow Joint Angles in Elbow Flexor Unilateral Resistance Exercise Training Determine Its Effects on Muscle Strength and Thickness of Trained and Non-trained Arms. Front Physiol. 2021 Sep 16;12:734509. doi: 10.3389/ fphys.2021.734509. PMID: 34616309; PMCID: PMC8489980. 9. Panidi I, Bogdanis GC, Terzis G, Donti A, Konrad A, Gaspari V, Donti O. Muscle Architectural and Functional Adaptations Following 12-Weeks of Stretching in Adolescent Female Athletes. Front Physiol. 2021 Jul 16;12:701338. doi: 10.3389/ fphys.2021.701338. PMID: 34335307; PMCID: PMC8322691. 10. Simpson CL, Kim BDH, Bourcet MR, Jones GR, Jakobi JM. Stretch training induces unequal adaptation in muscle fascicles and thickness in medial and lateral gastrocnemii. Scand J Med Sci Sports. 100 2017 Dec;27(12):1597-1604. doi: 10.1111/ sms.12822. Epub 2017 Jan 30. PMID: 28138986. 11. Oranchuk DJ, Storey AG, Nelson AR, Cronin JB. Isometric training and longterm adaptations: Effects of muscle length, intensity, and intent: A systematic review. Scand J Med Sci Sports. 2019 Apr;29(4):484-503. doi: 10.1111/ sms.13375. Epub 2019 Jan 13. PMID: 30580468. 12. Antonio J, Gonyea WJ. Skeletal muscle fiber hyperplasia. Med Sci Sports Exerc. 1993 Dec;25(12):1333-45. PMID: 8107539. 13. Hickson RC. Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol Occup Physiol. 1980;45(2-3):255-63. doi: 10.1007/BF00421333. PMID: 7193134. 14. Apostolopoulos N, Metsios GS, Flouris AD, Koutedakis Y, Wyon MA. The relevance of stretch intensity and position-a systematic review. Front Psychol. 2015 Aug 18;6:1128. doi: 10.3389/ fpsyg.2015.01128. PMID: 26347668; PMCID: PMC4540085. 101 Study Reviewed: Can I Have My Coffee and Drink It? A Systematic Review and Meta-analysis to Determine Whether Habitual Caffeine Consumption Affects the Ergogenic Effect of Caffeine. Carvalho et al. (2022) An Update on Caffeine Habituation and Sensitivity to Ergogenic Effects BY ERIC TREXLER Caffeine is a very popular ergogenic aid. It’s been a staple in multi-ingredient pre-workout formulas since they hit the market, and it’s been shown to improve many different types of exercise performance, including endurance, strength, and power (2). However, caffeine is also very popular for non-exercise applications, with some research suggesting that up to 85% of the US population consumes at least one caffeinated beverage per day (3). Any regular caffeine user can tell you that some of caffeine’s effects diminish over time as we become habituated to regular consumption, which leads to important questions: do performance improvements fade away over time? Do we need to cycle on and off caffeine in order to resensitize our receptors and restore caffeine’s ergogenic effects? The answers to these questions are surprisingly unclear, despite the large body of performance-related caffeine research, and they are questions we’ve repeatedly revisited in MASS (one, two, three, four). The presently reviewed meta-analysis (1) sought to determine if habitual caffeine consumption influences the acute ergogenic ef- fect of caffeine. The researchers systematically searched the literature for studies that met the following criteria: • Included healthy males or females, with no restrictions on age or training status, within a randomized single-blind or double-blind study design. • Quantified habitual caffeine consumption of participants in mg/kg/day, or provided sufficient data for the meta-analysts to calculate habitual intakes in these units. • Assessed the acute ergogenic effect of caffeine (at any dose and in any form) consumed before an exercise task, with a direct comparison to a placebo group or placebo condition. Once the literature search was complete and the researchers excluded all of the studies that failed to meet their criteria, they were left with 60 studies. Collectively, these studies included 1,137 total participants; 958 were males and 179 were females; 718 were “trained,” 400 were “untrained,” and 19 were “elite.” At that point, they went through to extract a ton of data for comparison purposes. 102 For example, they went through each study and noted the age, weight, sex, training status, and habitual caffeine consumption of participants, the characteristics of the exercise task, the supplementation protocol, and the primary exercise outcome tested. The researchers used meta-regression to determine if habitual caffeine intake was predictive of caffeine’s effect size for performance outcomes. The primary model indicated that caffeine had a statistically significant ergogenic effect (effect size [ES], expressed as Hedges’ g, = 0.25) Figure 1 shows the results of their comparisons. In short, the results indicated that caffeine had a small but positive effect on endurance, power, and strength outcomes. However, habitual caffeine intake (in mg/kg/day) did not significantly influence the observed effect size (p = 0.59). The researchers dug deeper to explore a number of other comparisons, which are presented in Figure 1. In short, caffeine was ergogenic across different exercise types (endurance, strength, and power), training statuses (trained or untrained), and sexes (male or female), with fairly similar effect sizes across these categories, and no significant influence of habitual caffeine intake within these comparisons. Acute caffeine was effective whether the experimental dose was higher or lower than the habitual intake of study participants, and the ergogenic effect was not meaningfully influenced by the duration of the caffeine withdrawal periods prior to the caffeine intervention. The only thing that seemed to threaten the ergogenic effect of caffeine was the dose ingested; doses below 3mg/kg and between 3-6 mg/kg were similarly effective, but doses above 6mg/kg led to a smaller pooled effect size (which was not statistically significant) and a wider confidence interval. This lines up pretty well with experimental evidence showing that higher caffeine doses (>6mg/kg) are more likely to induce uncomfortable side effects which may impair average performance at the group level and increase the inter-individual variability of performance responses (4). Since we’ve visited this topic several times in previous MASS issues (one, two, three, four), I’ll focus on the highlights and conclusions from this literature instead of retreading the study-by-study trajectory of this research topic. I think this is a really useful meta-analysis, because it confirms something that’s been observed many times in isolated studies: when you evaluate the acute ergogenic effect of caffeine, it tends to work for self-reported habitual caffeine consumers and non-consumers alike, and the effect sizes tend to be pretty similar when compared directly. This meta-analysis also provides several additional comparisons that have a ton of practical utility – it’s very helpful to see head-to-head comparisons of effect sizes for different sexes, exercise types, training statuses, dosing ranges, and so on. At this point, I think we can make a few conclusions with a reasonable degree of confidence: caffeine still works for habitual caffeine users, caffeine works across a broad range of exercises, caffeine works across different sexes and training statuses, and your “best bet” for dosing is probably in the range of 3-6mg/kg. Having said that, I do want to highlight a few considerations to prevent overconfidence in some of these conclusions. A meta-analysis 103 can only enable inferences based on the experimental data available, and there are some shortcomings in the caffeine literature. First, the literature skews heavily toward male subjects (in this meta-analysis, 84% of the data came from male participants), which isn’t rare for supplement research. All signs point to similar effects between males and females, but we should remember that we’re working with a limited set of female data. Speaking of limited data, the presently reviewed meta-analysis noted that only 24% of the studies in their search reported the mean habitual caffeine consumption of their participants. I still feel pretty confident that 60 studies give us plenty of data to draw from, but we should acknowledge that these findings come from less than a quarter of the relevant caffeine research to date, and may not be perfectly representative of the literature as a whole. Finally, and most importantly (by far): the randomized controlled trial is a very powerful tool, and we should use it whenever possible. Most of our knowledge about this caffeine habituation question comes from research that is observational in nature. Sure, they are employing randomized, placebo-controlled methods when determining the acute effect of caffeine intake, but the determination of habitual caffeine intake is based on self-reported habits rather than experimental manipulation of caffeine habituation status. The presently 104 reviewed meta-analysis provides our current “best guess” about the impact of habituation on the ergogenic effect of caffeine, but there are two major limitations of the underlying literature. First, we’re looking at habitual caffeine intake from each study at the group level, not the individual level. This meta-analysis is not strictly comparing the effects of caffeine among individuals who habitually consume large amounts versus small amounts of caffeine; rather, it’s comparing average, group-level effects of caffeine among groups of people with high versus low average daily intakes of caffeine. This approach should generally point us in the right direction, but there’s absolutely no question that there are individuals who consume very minimal caffeine who have been grouped into samples that are categorized, on average, as high caffeine consumers. Second, the time course of caffeine habituation (and re-sensitization) isn’t fully understood with a tremendous amount of detail, but appears to occur over periods of days rather than months. Let’s say you’re participating in a caffeine study and the research team asks how much caffeine you typically consume. You typically consume about 5 cups of coffee per day, but you haven’t been consuming much over the last 10-14 days due to changes in your schedule (because of work, social events, family obligations, and so on). So, how does a researcher classify your habitual intake? You’re generally consuming a pretty high level of daily caffeine intake, but you might not be particularly habituated at the time of testing. Individual studies don’t typically go into a lot of detail about this consideration, but it’s a pretty big deal when it comes to nuanced interpretation. The reason I focus so much on the observational nature of categorizing habitual caffeine consumption is because Greg reviewed one of the only studies (to my knowledge) that actually addressed the question of caffeine habituation and exercise performance from a truly experimental perspective. In the study (5), Lara and colleagues studied the ergogenic effect of pre-exercise caffeine supplementation (3mg/kg) over a 20-day period. The results generally suggested that the magnitude of caffeine’s ergogenic effect decreased with repeated use. However, the relative degree of effect size reduction varied among the different performance outcomes measured, and an effect size getting smaller is not the same as an effect size disappearing entirely. It’s also unclear if the effect sizes were on a trajectory involving continuous decreases over time, or if the effect size reductions had effectively plateaued at a lower (but still non-zero) magnitude of performance enhancement. Looking at the broader literature, I have no hesitation when stating that habitual caffeine users can still enjoy an ergogenic effect from acute caffeine use. It also seems that effect sizes are pretty similar when comparing self-reported heavy caffeine users to self-reported light caffeine users. However, the limited experimental evidence available causes me to hesitate a little bit when suggesting that there is absolutely no attenuation of caffeine’s ergogenic effects when it is consumed habitually. I wish there were more randomized controlled trials to sort out the discrepancy, but I find it difficult to unequivocally accept the conclusions from observational findings while entirely ignoring the small amount of experimental data available. We never want 105 to place a disproportionate amount of confidence in a single study, but we also have to reconcile experimental findings with observational findings when both are available, and experimental data generally tend to warrant a heightened level of consideration when compared to observational data. In summary, I’m quite confident that habitual caffeine consumers are still able to attain ergogenic benefits from acute caffeine consumption. However, in order to develop a more nuanced understanding of the time course by which caffeine habituation develops and reverses, and how performance varies across these processes, we probably need to lean on the good old-fashioned experiment. I’d love to see more randomized controlled trials that explore this question directly, and they are feasible studies to complete. Caffeine has an excellent safety profile within ergogenic dosing ranges and is an extremely affordable study ingredient. The biggest logistical challenge would be finding participants who are willing to come into the lab for very frequent performance testing, and selecting a performance outcome that is simultaneously sensitive enough to capture small changes in neuromuscular performance, easy enough to avoid soreness or performance decrements when completed frequently, but familiarized well enough to avoid time-related performance improvements over the course of the study. I think there’s a very intuitive series of studies on caffeine tolerance, habituation, and withdrawal time courses that would make for a great PhD dissertation or thesis project, and if I were entering a PhD program today (a daunting prospect that I’d prefer not to entertain, even hypothetically), it’s definitely the route I would pursue. Until more randomized controlled trials with experimental manipulation of caffeine habituation status become available, the current evidence suggests that it probably isn’t a huge deal for performance outcomes, and that caffeine is still ergogenic (to a fairly similar degree) when comparing heavy caffeine users to people who consume minimal caffeine. References 1. Carvalho A, Marticorena FM, Grecco BH, Barreto G, Saunders B. Can I Have My Coffee and Drink It? A Systematic Review and Meta-analysis to Determine Whether Habitual Caffeine Consumption Affects the Ergogenic Effect of Caffeine. Sports Med. 2022 May 10; ePub ahead of print. 2. Grgic J, Grgic I, Pickering C, Schoenfeld BJ, Bishop DJ, Pedisic Z. Wake Up And Smell The Coffee: Caffeine Supplementation And Exercise Performance-An Umbrella Review Of 21 Published Meta-Analyses. Br J Sports Med. 2020 Jun;54(11):681-688. 3. Mitchell DC, Knight CA, Hockenberry J, Teplansky R, Hartman TJ. Beverage Caffeine Intakes In The U.S. Food Chem Toxicol. 2014 Jan;63:136–42. 4. Guest NS, VanDusseldorp TA, Nelson MT, Grgic J, Schoenfeld BJ, Jenkins NDM, et al. International Society Of Sports Nutrition Position Stand: Caffeine And Exercise Performance. J Int Soc Sports Nutr. 2021 Jan 2;18(1):1. 5. Lara B, Ruiz-Moreno C, Salinero JJ, Del Coso J. Time Course Of Tolerance To The Performance Benefits Of Caffeine. PloS One. 2019;14(1):e0210275. 106 Study Reviewed: Effects of Oral Contraceptive Use on Muscle Strength, Muscle Thickness, and Fiber Size and Composition in Young Women Undergoing 12 Weeks of Strength Training: A Cohort Study. Sung et al. (2022) Oral Contraceptives Still Don’t Impact Muscle Growth and Strength Gains BY GREG NUCKOLS Near the end of Volume 4, I reviewed a study investigating the impact of oral contraceptives on strength and hypertrophy outcomes (2). In the interpretation section, I provided a thorough review of the state of the literature on the topic. If you’re interested in doing a deep dive, you should check out that article. But, to briefly summarize the state of the literature at the end of 2020: second- and third-generation combined oral contraceptives don’t seem to meaningfully impact either strength gains or hypertrophy outcomes. Since then, I’m only aware of two new papers on the topic. The first was a paper by Reichman and Lee (3), which actually wasn’t entirely new. In my prior review of the literature, I included a conference abstract by Lee et al from 2009, which hadn’t been formally published in a peer-reviewed journal (4). The recent Reichman paper is the formal, published document based on the same data Lee and colleagues presented in their conference abstract; as such, the findings in the Reichman paper findings are the same as those reported in the Lee abstract. I’m glad the study was finally published, but it doesn’t provide net new information for MASS readers. The second new study on the topic is the subject of this research brief (1). Fair warning: this will be a research brief. It just adds a few more data points to the conversation, but it doesn’t change the overall weight of the evidence on the topic. If anything, it just further solidifies the takeaways of my prior article on oral contraceptives. 74 subjects completed this study by Sung and colleagues, including 40 subjects who didn’t use oral contraceptives (and who hadn’t used oral contraceptives within the past year), and 34 who were presently using second-generation oral contraceptives (and who had been using oral contraceptives for at least one year). Subjects were not resistance-trained, but were generally active and healthy. Subjects in both groups completed 12 weeks of resistance training. The subjects performed three leg press sessions per week, following a simple linear progression. Each session consisted of three sets of leg press to failure, with two minutes of rest between 107 sets. When a subject completed more than 12 reps in a set, their training loads increased by 10%. Subjects also completed one recovery session of bodyweight squats per week, consisting of three sets of 15-20 reps, with 3-5 minutes of rest between sets. Before and after the 12-week training pro- gram, researchers assessed the subjects’ strength (via an isometric leg press test at 90° of knee flexion) and quadriceps size (via ultrasound measurements of rectus femoris, vastus intermedius, and vastus lateralis cross-sectional area). They also took vastus lateralis biopsies to assess changes in muscle fiber cross-sectional and myonuclear density. 108 No outcomes significantly differed between groups. The oral contraceptive group tended to gain a bit more strength (+28.02kg vs. +23.30kg), but the difference wasn’t statistically significant (p = 0.073). No other outcome was even within spitting distance of statistical significance (all p > 0.25). You can see the results in Tables 1 and 2. Overall, this study reinforces the overall theme of my previous article on the topic: oral contraceptives don’t seem to meaningfully affect strength or hypertrophy outcomes. However, this study is actually a pretty important addition to the literature. It’s the biggest study on the topic (in terms of sample size), and the researchers did a really good job of controlling for menstrual cycle phase, just to ensure that performance fluctuations throughout the menstrual cycle wouldn’t add noise to their results. The researchers monitored the subjects for two full cycles before the start of the training intervention to ensure that all subjects had a consistent cycle, and to ensure that strength testing took place at the same point in the menstrual cycle for all subjects. New studies confirming prior findings are always nice to see, but large, methodologically rigorous studies confirming prior findings are even nicer to see. PMC9092708. 2. Oxfeldt M, Dalgaard LB, Jørgensen EB, Johansen FT, Dalgaard EB, Ørtenblad N, Hansen M. Molecular markers of skeletal muscle hypertrophy following 10 wk of resistance training in oral contraceptive users and nonusers. J Appl Physiol (1985). 2020 Dec 1;129(6):1355-1364. doi: 10.1152/japplphysiol.00562.2020. Epub 2020 Oct 15. PMID: 33054662. 3. Riechman SE, Lee CW. Oral Contraceptive Use Impairs Muscle Gains in Young Women. J Strength Cond Res. 2021 May 14. doi: 10.1519/JSC.0000000000004059. Epub ahead of print. PMID: 33993156. 4. Lee CW, Newman MA, Riechman SE. Oral Contraceptive Use Impairs Muscle Gains in Young Women. FASEB. 2009 Apr;23:51. doi: 10.1096/fasebj.23.1_ supplement.955.25. References 1. Sung ES, Han A, Hinrichs T, Vorgerd M, Platen P. Effects of oral contraceptive use on muscle strength, muscle thickness, and fiber size and composition in young women undergoing 12 weeks of strength training: a cohort study. BMC Womens Health. 2022 May 10;22(1):150. doi: 10.1186/s12905022-01740-y. PMID: 35538569; PMCID: 109 Study Reviewed: Influence of Training-induced Testosterone and Cortisol Changes on Skeletal Muscle and Performance in Elite Junior Athletes. Bailey et al. (2022) How Much Do Cortisol Levels Matter For Training Adaptations? BY ERIC TREXLER There is absolutely no question that high levels of cortisol, a glucocorticoid hormone, can impact body composition when they get into high enough ranges. When people experience clinically high glucocorticoid levels for a prolonged period of time, due to medical scenarios involving Cushing’s syndrome or pharmacological treatment with exogenous glucocorticoids, they commonly gain weight and develop abdominal obesity and metabolic syndrome (2). In addition, muscle loss can be experimentally induced by administering exogenous glucocorticoids at high enough doses (3). While it might seem tempting (if not intuitive) to demonize cortisol, our relationship with cortisol is a bit too complicated for that. As reviewed by Hackney and Walz (4), cortisol is necessary and helps the human body liberate energy substrates during exercise, prioritize and orchestrate substrate utilization, and remodel proteins. In summary, as they put it: “cortisol and the other glucocorticoids are not the ‘bad guys’ of exercise endocrinology as some have made them out to be.” Nonetheless, the fitness industry has generally labeled glucocorticoids as “bad guys” that threaten recovery while simultaneously promoting fat gain and impairing strength and muscularity. Despite the overwhelmingly bad reputation of cortisol among lifting enthusiasts, it’s interesting to note that most of the fitness industry’s collective opinion about the relationship between cortisol and long-term changes in strength and body composition is informed by research with minimal applicability. Some of this evidence comes from unique clinical scenarios, such as studies on Cushing’s syndrome or exogenous glucocorticoid administration, which involve substantially higher cortisol levels than a typical lifter would experience. Some comes from studies assessing overtraining syndrome (often characterized by a low testosterone-to-cortisol ratio), which is rarely experienced by lifters, and often a secondary effect of low energy availability. Some of it comes from research exploring acute cortisol responses to certain stressors rather than chronic elevations, and some of that research even implies that larger cortisol responses to training might be predictive of better gains (5). Some comes from observation research linking cortisol to weight gain (6), but we know that there’s a 110 lot more to the psychological determinants of eating behavior and physical activity than the cortisol response alone. A much better and more applicable type of evidence would assess resting cortisol levels at different time points throughout a training program, while aiming to observe concurrent fluctuations in muscle thickness and strength. As you probably inferred, that’s exactly what the presently reviewed study did (1). Before we get into the details, I want to acknowledge a major caveat, because I’m well aware that this study fails to perfectly replicate the circumstances and training habits of the typical MASS reader. This study was conducted on elite junior sprinters, with a mean age of around 15-16 years. That’s certainly a big limitation, but we can still draw some useful inferences from it. Now, on to the details. The researchers enrolled 28 sprinters, in addition to 13 “non-athletic” controls, with an approximately even split of males and females within each group. They measured salivary hormone levels (testosterone and cortisol), muscle thickness, and muscle strength at several different time points throughout the training year. Saliva samples were taken between 2:30-3:00pm (after school, but before training) in order to control for diurnal variations throughout the day. To quantify muscle thickness, the researchers used A-mode ultrasound to take panoramic scans of the knee extensors and flexors, and used cumulative muscle thickness values from both scans. To quantify muscle strength, they measured maximal isometric knee flexion and extension using a handheld dynamometer, and once again used a cumu- lative value (incorporating both flexion and extension) for statistical analysis. Measurements were taken at four different time points across a 7-month training schedule. The first (T1) was during the general preparation period (at baseline), the second (T2) was during the specific preparation period (around month 3-4), the third (T3) was during the pre-competition period (around month 5-6), and the fourth (T4) was taken during the competition period (month 7). The researchers referred to T1-T2 as the “preparation phase” and T3T4 as the “competition phase.” Training involved a combination of resistance training and running, and generally transitioned from high-volume/low-intensity training in the preparation phase to low-volume/high-intensity training in the competition phase. As this is a Research Brief, we’ll focus on the outcomes most relevant to MASS readers: longitudinal changes in hormones, muscle thickness, and strength, and the correlations between them. The researchers also incorporated sprint performance and some more nuanced hierarchical regression modeling, but I think that would add a ton of complexity (and minimal value) to this brief. Starting with the basic information (longitudinal changes within each group), Table 1 shows values for testosterone, cortisol, the testosterone-to-cortisol ratio, muscle thickness, and muscle strength for each group at each time point. Now, moving on to the relationships between variables. Table 2A reports the correlations between changes in each variable during the preparatory phase (that is, from T1 to T2). Table 2B reports the correlations between changes in each variable during the competi- 111 112 tion phase (that is, from T3 to T4). Both portions of Table 2 are presented as correlation matrices in which each variable is numbered (one through five). If, for example, you wanted to see the correlation coefficient (r value) for the correlation between cortisol (numbered as variable #2) and muscle thickness (numbered as variable #4), you’d find the intersection between the 2nd column (since cortisol is #2) and the fourth row (since muscle thickness is #4). In other words, you identify each individual correlation coefficient by finding the “intersection” of the two variables you’re interested in, which makes the correlation matrix a very efficient way to communicate information about numerous correlations among several different pairings of variables. The researchers concluded that “this study demonstrated that [cortisol] levels increased in response to sprint training, to levels where it induced negative implications on both performance and skeletal muscle adaptation during the competition season. Such a catabolic physiological status resulted in significant losses in muscular force and [muscle thickness].” When I first read this paper, I came across this set of conclusions and immediately grew concerned that people would point to this paper to sell a bunch of unnecessary “cortisol blocker” supplements, to fuel even more catastrophizing about cortisol, or to promote an unwarranted narrative discouraging effortful training. Looking at the tables presented within this Research Brief, I really don’t see justification for this line of thinking. The quoted conclusion made by the authors isn’t strictly false or baseless, but it doesn’t really add up from my perspective. First and foremost, the cortisol increase from training simply wasn’t that big. I could get into all sorts of convoluted explanations about population-specific reference ranges and the trajectory of cortisol peaks and valleys throughout the day, but this one’s pretty simple: at no point in the 7-month training period did the athletes have higher cortisol levels than the healthy, non-athletic controls. The idea that training put these athletes into a catabolic state by driving cortisol levels through the roof appears, from my perspective, to lack face validity. There was only one time point where training did appear to drive athletes’ cortisol levels near, but still below, the levels of non-athletic controls. Whatever the athletes were doing from T1 to T2 (presumably pretty high-volume training) certainly seemed to move the needle, with cortisol increasing from 1.97 to 4.15 nmol/L (Table 1). During this time period, cortisol levels changed substantially, and there was also a generally higher level of variance in cortisol levels (the standard deviation at T2 was, by far, the largest of any time point for the athletes). If meaningful correlations between cortisol levels and muscle-related outcomes were to be observed, the preparatory phase (T1 to T2) would be the time for them to stand out – there were clearly some folks in the sample experiencing large increases in cortisol, and others experiencing much smaller increases, so any causative effect by which cortisol impaired gains would have been most pronounced in this time window. However, as we can see in Table 2A, these cortisol changes were weak- 113 ly (but positively) correlated with changes in muscle thickness and strength, with r values of r = 0.37 and r = 0.31. It was only during the competition period that cortisol levels were negatively correlated with muscle thickness (r = -0.30), and totally unrelated to muscle strength (r = 0.04). I suspect that the inverse correlation with muscle thickness was probably spurious, mostly because there wasn’t a ton of meaningful cortisol variance to model in the first place. Cortisol levels from T3 to T4 were virtually unchanged (2.89 to 2.84 nmol/L), and the standard deviations were pretty low (1.62 to 1.47). If cortisol levels were really driving muscle adaptations at the individual level, it should be most pronounced in the time period where cortisol levels actually increased meaningfully and had a substantial amount of variance (T1 to T2), and this time window provided virtually no evidence pointing toward a negative impact from cortisol. In fact, the correlation coefficients, while non-significant, point the opposite direction. I don’t want to oversimplify my stance and sound like a cortisol denier, because I’m not. It’s very clear that supraphysiological cortisol levels have unfavorable effects on body composition, in terms of both abdominal obesity and muscle atrophy. There’s also some experimental evidence suggesting that exogenous cortisol, within physiological ranges, may induce muscle breakdown (7). However, I suspect that regular resistance training might attenuate this effect, as research has suggested that inactivity markedly exacerbates the catabolic effects of exogenous glucocorticoids (3). This observation, combined with the fact that it’s exceedingly difficult to intentionally induce overtraining syndrome by resistance training, lead me to believe that the typical MASS reader shouldn’t spend a single moment worrying about training-induced increases in cortisol. Even in the present study, which included a combination of resistance training and sprint training, resting cortisol levels of athletes never even exceeded those of non-athlete controls. We’ll certainly experience some acute cortisol increases from hard training, but they’ll virtually never approach the cortisol levels observed with Cushing’s syndrome or pharmacological glucocorticoid interventions, and are likely offset by nocturnal suppression of cortisol levels (4). So, you probably aren’t training yourself into a state of chronically high, catabolic levels of cortisol. However, you might have high resting cortisol levels for different reasons altogether, such as a medical condition, insufficient sleep, or chronic psychogenic stress. While I’ve downplayed the importance of training-induced changes in cortisol, you’ll definitely want to address high cortisol levels if they’re resulting from any of these other causative factors. Certainly any symptomatic medical condition warrants a visit to the doctor, who can give you information about various treatment options. When it comes to sleep, there are a million reasons to correct sleep insufficiencies; it will most likely improve your gains, but will also dramatically enhance quality of life. Similarly, it’s quite possible that making an intentional effort to alleviate chronic psychogenic stress could enhance your gym-related progress, but it would most likely have a substantial posi- 114 tive impact on quality of life. In other words, there are cortisol-elevating things that are worth addressing (and might even impair your gains), but cortisol is merely an indicator of the underlying problem, not the root of the problem itself. If your cortisol is high because you aren’t sleeping enough, you have a sleep problem; if your cortisol is high because you’re chronically stressed, you have a stress problem. Your best bet is to address the sleep and the stress, not the cortisol itself. In summary, I wouldn’t worry about training-related cortisol fluctuations, and I certainly wouldn’t hold back on training effort to avoid cortisol elevations. If I had chronically elevated cortisol levels for other reasons, I’d make an effort to sort that out – not by purchasing a questionably efficacious “cortisol-blocker” supplement, but by getting to the root cause, and consulting with a qualified healthcare professional if necessary. 4. Hackney AC, Walz EA. Hormonal Adaptation And The Stress Of Exercise Training: The Role Of Glucocorticoids. Trends Sport Sci. 2013;20(4):165–71. 5. West DWD, Phillips SM. Associations Of Exercise-Induced Hormone Profiles And Gains In Strength And Hypertrophy In A Large Cohort After Weight Training. Eur J Appl Physiol. 2012 Jul;112(7):2693–702. 6. Chao AM, Jastreboff AM, White MA, Grilo CM, Sinha R. Stress, Cortisol, And Other Appetite-Related Hormones: Prospective Prediction Of 6-Month Changes In Food Cravings And Weight. Obes. 2017 Apr;25(4):713–20. 7. Simmons PS, Miles JM, Gerich JE, Haymond MW. Increased Proteolysis. An Effect Of Increases In Plasma Cortisol Within The Physiologic Range. J Clin Invest. 1984 Feb;73(2):412–20. References 1. Bailey J, Irving R, Dawson P, Brown DR, Campbell E. Influence of Training-induced Testosterone and Cortisol Changes on Skeletal Muscle and Performance in Elite Junior Athletes. Am J Sports Sci Med. 2021 Dec 16;9(1):13–23. 2. van der Valk ES, Savas M, van Rossum EFC. Stress and Obesity: Are There More Susceptible Individuals? Curr Obes Rep. 2018;7(2):193–203. 3. Ferrando AA, Stuart CA, Sheffield-Moore M, Wolfe RR. Inactivity Amplifies the Catabolic Response of Skeletal Muscle to Cortisol. J Clin Endocrinol Metab. 1999 Oct 1;84(10):3515–21. 115 Study Reviewed: How Does Lower-Body and Upper-Body Strength Relate to Maximum Split Jerk Performance? Soriano et al. (2022) Is Your Split Jerk Limited by Upper Body or Lower Body Strength? BY GREG NUCKOLS MASS may stand for “Monthly Applications in Strength Sport,” but we certainly don’t write about all strength sports with similar frequency. While I love all strength sports equally, there’s just a lot of juicy research that’s directly related to powerlifting performance, quite a bit less research directly related to weightlifting performance, and even less research directly related to strongman performance (and virtually no research related to more niche strength sports, like the Highland Games or Basque stone lifting). In addition, the research that does exist for weightlifting is valuable, but probably wouldn’t make for a great MASS article. Most of the research related to weightlifting focuses on applications of weightlifting training for improving the performance of team sport athletes – very little research focuses on improving weightlifting performance for its own sake. However, we certainly haven’t forgotten about the long-suffering weightlifters who read MASS, and this research brief may be pretty valuable if you struggle with split jerk performance. A recent study allowed me to develop a little tool that may help you diagnose the weak link in your split jerk (1). In a study by Soriano and colleagues, researchers assessed 1RM split jerk, strict overhead press, and back squat strength in 33 competitive weightlifters (20 males and 13 females). Back squats were performed weightlifting-style: with a high bar position and ass-to-grass depth. From there, the researchers assessed the independent relationships between overhead press strength and split jerk strength, and between squat strength and split jerk strength via linear regression. Furthermore, they assessed the relationship between split jerk strength and a combination of both squat and overhead press strength via multiple linear regression. Unsurprisingly, they found that overhead press strength, squat strength, and a combination of squat and overhead press strength were all strongly predictive of split jerk performance (r > 0.9). You can see these associations in Figures 1-3. The researchers also provided regression equations for all of these 116 Then, fill in your 1RM strict overhead press, squat, and split jerk numbers in cells B1-3. From there, the spreadsheet will take care of all of the necessary calculations. In cells B57, it will calculate a) your predicted split jerk 1RM based solely on overhead press performance, b) your predicted split jerk 1RM based solely on squat performance, and c) your predicted split jerk 1RM based on a combination of squat and overhead press performance. linear relationships, which allowed me to develop a little tool to help you assess the weak link in your split jerk performance. First, pull up this spreadsheet. Make a copy or download it. Don’t request editing access. Based on this data, cell B9 will tell you your likeliest weak link. If your actual split jerk 1RM is more than 15% lower than would be predicted based on your squat and overhead press strength, then the spreadsheet will identify some combination of speed, skill, and technique as the most likely factor limiting your split jerk performance. If your actual split jerk 1RM is within 15% of your predicted 1RM, the sheet will check whether lower body strength (squat 1RM) or upper body strength (overhead press 1RM) is most likely to be your limiting factor. If your predicted 117 split jerk 1RM based on overhead press performance is higher than your predicted split jerk 1RM based on squat performance, the spreadsheet will identify squat strength as your most likely limiting factor. If your predicted split jerk 1RM based on overhead press performance is lower than your predicted split jerk 1RM based on squat performance, the spreadsheet will identify overhead press strength as your most likely limiting factor. Furthermore, the spreadsheet will assign a confidence rating to its predictions. For example, if you “should” split jerk 120kg (based on your squat and overhead press strength), but your 1RM split jerk is only 90kg, the spreadsheet will identify speed/ skill/technique as your current limiting factor, and it will rate that as a high-confidence prediction, because there’s a huge gap between your predicted performance and actual performance. However, if your 1RM split jerk is 105 kg, the spreadsheet will still identify speed/skill/technique as your most likely limiter, but it will be a lower-confidence prediction, since the gap between your actual performance and predicted performance is considerably smaller. Similarly, if your predicted split jerk 1RM based on squat strength is 40kg higher than your predicted split jerk 1RM based on overhead press strength, the spreadsheet would identify overhead press strength as your most likely limiter, and it would have high confidence in that prediction. However, if the gap between those two predicted 1RMs was only 5kg, the spreadsheet would be less confident in predicting that overhead press strength is limiting your split jerk performance. To be clear, I don’t think this is a foolproof, 100% perfect tool for diagnosing weaknesses in the split jerk. However, if you’re struggling with your split jerk, this little tool may just help point you in the right direction. References 1. Soriano M, Jiménez-Ormeño E, Amaro-Gahete FJ, Haff GG, Comfort P. How Does Lower-Body and Upper-Body Strength Relate to Maximum Split Jerk Performance? Journal of Strength and Conditioning Research: June 1, 2022. doi: 10.1519/JSC.0000000000004289 118 Study Reviewed: The Effect Of Krill Oil Supplementation On Skeletal Muscle Function And Size In Older Adults: A Randomised Controlled Trial. Alkhedhairi et al. (2022) Should You Take a Krill Pill To Enhance Strength Or Hypertrophy? BY ERIC TREXLER Fish oil is a very popular dietary supplement among lifters and non-lifters alike, and we’ve covered it in MASS a few times now (one, two, three, four). As described in a previous article, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are the primary omega-3 fatty acids in fish oil, and they are important components of cell membranes and found in a variety of cell types, including blood cells, immune cells, cardiac tissue, skeletal muscle, eye tissue, brain cells, and other tissues of the nervous system. The general premise of fish oil supplementation is that chronically high intakes of EPA and DHA will increase their abundance in multiple body tissues, ultimately influencing a variety of different outcomes related to health and body function. Based on the specific tissues in which EPA and DHA are thought to be particularly impactful, researchers have studied the effects of fish oil supplementation on cognition, mental health, inflammation, immunity, muscle protein balance, neuromuscular function, and more. In the general population, fish oil is typically taken in hopes of reducing inflammation and oxidative stress while favorably impacting brain, eye, immune, or cardiovascular health. However, a lot of lifters have their eye on some promising (but very preliminary and context-dependent) findings linking fish oil to recovery, muscle protein synthesis, and body composition. The presently reviewed study (1) investigated the effects of krill oil supplementation on outcomes related to strength, hypertrophy, and physical function. Krill are tiny crustaceans who happen to have high content of omega-3 fatty acids, including EPA and DHA. After fish oil supplementation started getting popular, krill oil started to get marketed pretty heavily in the supplement world. While most fish oil supplements provide omega-3 fatty acids in triacylglycerol or ethyl ester form, a substantial portion of the omega-3 fatty acids in krill oil are in phospholipid form. Some studies have suggested that this phospholipid form might have enhanced bioavailability, and the authors of the present study point to other short-term trials suggesting that krill oil can increase plasma EPA+DHA levels and omega-3 index values more efficiently than a typical fish oil supplement (that is, they can achieve larger increases at an equated dose, 119 or similar increases at a smaller dose when compared to regular fish oil). Krill oil also contains a decent amount of choline and astaxanthin, which may have independent or synergistic effects of their own. The present study was a six-month trial, which began with a large, mixed-sex sample of 102 participants. Subjects were required to be at least 65 years old, have a BMI under 35, and complete less than an hour of weekly structured exercise. Participants were ineligible if they had any relevant medical conditions, used any medications or dietary supplements that might interfere with study outcomes, were allergic to seafood, or regularly consumed more than two servings of oily fish per week. After enrollment, participants were random- ly allocated to the krill oil group or placebo group for six months of supplementation in a double-blinded fashion. The krill oil group consumed 4g/day of krill oil, with 2g taken with lunch and 2g taken with dinner. This 4g daily dose provided a total of 1288mg of omega 3 fatty acids, 772mg of EPA, 384mg of DHA, 1156mg of combined EPA+DHA, and 316mg of choline. Throughout the 6-month supplementation period, participants were encouraged to maintain their normal diet and exercise habits, so there was no training or exercise program completed in conjunction with supplementation. While the study began with 102 participants, there were some individuals who were unable to finish the study, resulting in 49 study completers (26 female, 23 male) in the krill oil group and 45 study completers 120 (27 female, 18 male) in the placebo group. The researchers measured a number of outcomes related to strength, hypertrophy, and physical function, which were measured at baseline and re-measured after six weeks and six months of supplementation. After six months, krill oil effectively increased the EPA and DHA levels within red blood cells. The researchers observed statistically significant interaction effects, indicating that the krill oil group experienced more favorable changes over time, for maximal isometric knee extensor torque, grip strength, and muscle thickness of the vastus lateralis (Figure 1). However, between-group comparisons related to a physical performance test (designed to reflect activities of daily living), neuromuscular function, body composition (body-fat percentage and muscle mass), blood biomarkers (glucose, insulin, C-reactive protein, and blood lipids), and quality of life (measured via validated questionnaire) were all non-significant, with the exception of one neuromuscular function outcome (M-wave amplitude) with limited utility to MASS readers. If you follow the supplement literature related to strength and hypertrophy, you’re used to seeing null (non-significant) findings. Sure, there are always some “flash-in-the-pan” findings that spark some excitement here and there, but the list of dietary supplements that have stood the test of time and are believed to reliably enhance strength or hypertrophy outcomes are few and far between. As a result, when you open up a paper with some low p-values related to direct measures of muscle strength and muscle thickness, a nat- ural knee-jerk reaction might be to embrace the positive finding and expand your supplement stack without much additional thought or consideration. However, it’s important to resist the temptation to be swayed solely by statistical significance or a particularly low p-value. When critically appraising the potential utility of a supplement, you should consider a few important questions: • Which specific population(s) stand to benefit from this supplement? • What magnitude of improvement can realistically be anticipated? • When combined with resistance training, are these anticipated effects additive, synergistic, or redundant? As I work through these three bullet points, I will refer to fish oil and krill oil synonymously. From my read of the literature, there’s currently insufficient evidence to suggest that krill oil’s effects are truly distinct from those of fish oil. Rather, krill oil can be operationally viewed as a subtype of fish oil, which might have slightly better bioavailability that could allow for similar supplementation effects at lower relative doses. As for the first bullet point, there is good reason to believe that older adults may experience improvements in muscle mass or function from fish oil or krill oil supplements. As reviewed by Bird and colleagues (2), inflammation plays an important role in sarcopenia, or the progressive, age-related loss of skeletal muscle mass and function. As a result, risk factors for sarcopenia include age, physical inactivity, obesity, and certain chronic dis- 121 eases, which are all linked to increased levels of chronic inflammation and oxidative stress. While fish oil appears to have positive effects (2) related to muscle mass and function in people at high risk for sarcopenia (i.e., people with fairly elevated levels of chronic inflammation and oxidative stress), effects in young, healthy individuals are far less promising. The literature generally indicates that young people fail to experience the same degree of benefit related to strength or hypertrophy, outside of extreme physiological scenarios, such as muscular inactivity due to immobilization (3). These observations have a high degree of biological plausibility, as they’re quite consistent with the literature on antioxidant supplementation. As reviewed by Ismaeel and colleagues, certain antioxidant supplements have neutral to slightly negative effects on resistance training adaptations in young, healthy subjects, but neutral to slightly positive effects on older subjects with higher baseline levels of chronic inflammation and oxidative stress (4). For a much more detailed look at antioxidant supplementation and training adaptations, be sure to check out this Stronger By Science article. If you’re an avid exerciser in your 50s, 60s, 70s, or beyond, you might be reading the previous paragraph and thinking that fish oil is your key to success as you begin planning ahead for the potential impacts of sarcopenia. However, a recent review by Murphy and McGlory (5) casts a little bit of doubt on this idea. In the review, they note that the potential muscle-specific benefits of fish oil are quite inconsistent in the literature, with some of the more promising findings coming from studies with low quality or a high risk of bias. Ultimately, they conclude that “the available evidence does not indicate that ingestion of [long-chain omega-3 fatty acids] above current population recommendations (250–500 mg/day; 2 portions of oily fish per week) enhances exercise performance or recovery from exercise training in master athletes.” When discussing this particular topic, it’s also important to reinforce an important point from a recent MASS article by Dr. Helms: the sarcopenia-driving inflammation that we associate with age is heavily influenced by age-related drops in physical activity level. As he puts it, “if you’re a lifter aged 35-60, you’re more similar to a younger version of yourself than you are to a version of yourself at the same age who doesn’t lift.” As such, it’s very possible that avid exercisers and masters athletes experience training-related reductions in chronic inflammation that effectively nullify the muscle-specific impacts of fish oil, but more research is needed in this specific area. As for the second bullet point (regarding the magnitude of effects), check out Figure 1. We could approach this from a painfully detailed quantitative perspective, or we can let our eyeballs do the work. Look at, for example, the muscle thickness data, we can see that fish oil is no game changer when it comes to muscularity or body composition. The researchers reported a 3.5% increase in muscle thickness for the krill oil group, with a baseline value that appears to be around 33mm, give or take. In other words, we’re talking about an advantage that amounts to roughly one millimeter, with a measurement 122 tool that makes it exceedingly challenging to reliably identify a real change of 1mm. Furthermore, the present study found no significant effects in terms of whole-body indices of body composition. As seen in Figure 1, the observed effects for strength and hypertrophy are small, but you might still be interested in a tiny effect, as long as it’s additive (that is, extra gains that occur above and beyond the effects of training alone). But is it actually additive? That brings us to the third bullet point. A systematic review published by López-Seoane and colleagues in 2022 found that fish oil supplementation positively impacted outcomes related to muscle hypertrophy in the absence of exercise (6). That’s pretty cool, but here’s the bad news: the exact same researchers published a separate systematic review the year prior, and found that fish oil supplementation did not significantly impact outcomes related to muscle hypertrophy, strength, or muscle biomarkers of inflammation when combined with exercise (7). So, when we combine our observations related to these three bullet points, we’re likely to conclude that fish oil has the highest potential to improve muscle strength or hypertrophy outcomes in older, sedentary individuals who are not engaging in structured exercise. When an effective hypertrophy-promoting resistance training program is thrown into the mix, the muscular benefits may very well become redundant rather than additive. Furthermore, when you look at populations with a tendency to have lower baseline levels of chronic inflammation and oxidative stress (such as younger individuals, or older, healthy individuals who regularly exercise), the likelihood of strength or hypertrophy benefits appears to diminish. So, I don’t think it’s necessarily inadvisable to recommend that your sedentary family members take some fish oil, but I am skeptical that the typical MASS reader stands to meaningfully influence their strength or hypertrophy outcomes through fish oil or krill oil supplementation. Just to clarify, I’m certainly not suggesting that fish oil is entirely useless. In fact, I feel quite the opposite; EPA and DHA do important things in a wide range of tissues, and I believe it’s very important to seek out adequate intakes of essential fatty acids. As I’ve noted previously, getting at least 0.3-0.5g/day of combined EPA + DHA appears to be a positive thing for many different outcomes related to health and wellness, whether you’re achieving that intake from a few weekly servings of oily fish or a dietary supplement. Personally, I aim for around 0.5-1.0g/day of combined EPA + DHA, and I use an algae oil supplement (a plant-based fish oil alternative that’s rich in EPA and DHA) to facilitate that goal. There is also some evidence suggesting that fish oil can facilitate recovery from particularly arduous training sessions, even in healthy young people, but there’s also some directly contradictory evidence, so that particular question remains unsettled. In conclusion, fish oil might yield some small but detectable benefits for strength and hypertrophy in older and relatively sedentary adults, or in other individuals with elevated levels of chronic inflammation or oxidative stress. As a young-ish, healthyish, relatively active person, I’m not holding my breath when it comes to obtaining strength 123 or hypertrophy benefits from my algae oil supplementation. However, there are plenty of good reasons to intentionally seek out dietary sources of EPA and DHA, and to incorporate adequate amounts of them into your diet on a regular basis. References 1. Alkhedhairi SA, Aba Alkhayl FF, Ismail AD, Rozendaal A, German M, MacLean B, et al. The Effect Of Krill Oil Supplementation On Skeletal Muscle Function And Size In Older Adults: A Randomised Controlled Trial. Clin Nutr. 2022 Jun;41(6):1228–35. Induced By N-3 PUFA Supplementation In Absence Of Exercise: A Systematic Review Of Randomized Controlled Trials. Crit Rev Food Sci Nutr. 2022 Feb 3;1–11; ePub ahead of print. 7. López-Seoane J, Martinez-Ferran M, Romero-Morales C, Pareja-Galeano H. N-3 PUFA As An Ergogenic Supplement Modulating Muscle Hypertrophy And Strength: A Systematic Review. Crit Rev Food Sci Nutr. 2021 Jun 15;1–21; ePub ahead of print. 2. Bird JK, Troesch B, Warnke I, Calder PC. The Effect Of Long Chain Omega-3 Polyunsaturated Fatty Acids On Muscle Mass And Function In Sarcopenia: A Scoping Systematic Review And MetaAnalysis. Clin Nutr ESPEN. 2021 Dec 1;46:73–86. 3. Heileson JL, Funderburk LK. The Effect Of Fish Oil Supplementation On The Promotion And Preservation Of Lean Body Mass, Strength, And Recovery From Physiological Stress In Young, Healthy Adults: A Systematic Review. Nutr Rev. 2020 Dec 1;78(12):1001–14. 4. Ismaeel A, Holmes M, Papoutsi E, Panton L, Koutakis P. Resistance Training, Antioxidant Status, and Antioxidant Supplementation. Int J Sport Nutr Exerc Metab. 2019 Sep 1;29(5):539–47. 5. Murphy CH, McGlory C. Fish Oil for Healthy Aging: Potential Application to Master Athletes. Sports Med. 2021 Sep;51(Suppl 1):31–41. 6. López-Seoane J, Jiménez SL, Del Coso J, Pareja-Galeano H. Muscle Hypertrophy 124 VIDEO: 1RM Prediction Part 2 BY MICHAEL C. ZOURDOS Part 1 of this series suggested that reps performed equations have questionable efficacy for predicting 1RM. This installment breaks down the existing literature on submaximal velocity to predict 1RM. Does it fare better? Watch the video to find out. Click to watch Michael's presentation. 125 Relevant MASS Videos and Articles 1. Practical and Effective Ways to Predict Your 1RM. Volume 3 Issue 4. References 1. Banyard HG, Nosaka K, Haff GG. Reliability and validity of the load–velocity relationship to predict the 1RM back squat. The Journal of Strength & Conditioning Research. 2017 Jul 1;31(7):1897-904. 2. Pestaña-Melero FL, Haff GG, Rojas FJ, Pérez-Castilla A, García-Ramos A. Reliability of the load–velocity relationship obtained through linear and polynomial regression models to predict the 1-repetition maximum load. Journal of Applied Biomechanics. 2018 Jun 1;34(3):184-90. 3. García-Ramos A, Haff GG, Pestaña-Melero FL, Pérez-Castilla A, Rojas FJ, BalsalobreFernández C, Jaric S. Feasibility of the 2-point method for determining the 1-repetition maximum in the bench press exercise. International Journal of Sports Physiology and Performance. 2018 Apr 1;13(4):474-81. 4. Pérez-Castilla A, Suzovic D, Domanovic A, Fernandes JF, García-Ramos A. Validity of different velocity-based methods and repetitions-to-failure equations for predicting the 1 repetition maximum during 2 upper-body pulling exercises. The Journal of Strength & Conditioning Research. 2021 Jul 1;35(7):1800-8. 5. Pérez-Castilla A, Fernandes JF, Garcia-Ramos A. Validity of the bench press one-repetition maximum test predicted through individualized load-velocity relationship using different repetition criteria and minimal velocity thresholds. Isokinetics and Exercise Science. 2021 Jan 1;29(4):369-77. 6. Thompson SW, Rogerson D, Ruddock A, Greig L, Dorrell HF, Barnes A. A novel approach to 1RM prediction using the load-velocity profile: a comparison of models. Sports. 2021 Jun 22;9(7):88. 7. Jiménez-Alonso A, García-Ramos A, Cepero M, Miras-Moreno S, Rojas FJ, Pérez-Castilla A. Velocity performance feedback during the free-weight bench press testing procedure: an effective strategy to increase the reliability and one repetition maximum accuracy prediction. Journal of Strength and Conditioning Research. 2022 Apr 8;36(4):1077-83. 8. Macarilla CT, Sautter NM, Robinson ZP, Juber MC, Hickmott LM, Cerminaro RM, Benitez B, Carzoli JP, Bazyler CD, Zoeller RF, Whitehurst M. Accuracy of Predicting One-Repetition Maximum from Submaximal Velocity in the Barbell Back Squat and Bench Press. Journal of Human Kinetics. 2022 Apr 15;82(1):201-12. █ 126 VIDEO: Periodization for Hypertrophy Part 2 BY ERIC HELMS Back in Volume 1 Dr. Helms noted in his intro to periodization videos that periodization for hypertrophy was a relatively unexplored topic. Five years later, we now have a number of meta-analyses on this topic as well as a broader understanding of how varying specific variables might impact hypertrophy. In part 2 of this video series, Dr. Helms covers the rationale specifically for periodizing exercises for maximizing hypertrophy. Click to watch Eric's presentation. 127 Relevant MASS Videos and Articles 1. When it Comes to Hypertrophy, Not All Multi-joint Exercises are Created Equal. Volume 3, Issue 9. 2. Variety is the Spice of Life: If You Want Well-Rounded Triceps Growth, You Need Both Compound and Single-Joint Exercises. Volume 4, Issue 5. 3. Guiding Shoulder Hypertrophy Training with EMG. Volume 4, Issue 10. 4. Do You Need to Incline Press to Build your Upper Chest?. Volume 4, Issue 11. 5. Squats are Great, but Bodybuilders Need More. Volume 5, Issue 9. References 1. Physiopedia.com 2. Gentil, P., Soares, S., & Bottaro, M. (2015). Single vs. Multi-Joint Resistance Exercises: Effects on Muscle Strength and Hypertrophy. Asian Journal of Sports Medicine, 6(2), e24057. 3. Kubo, K., Ikebukuro, T., & Yata, H. (2019). Effects of squat training with different depths on lower limb muscle volumes. European Journal of Applied Physiology, 119(9), 1933–1942. █ 128 Just Missed the Cut Every month, we consider hundreds of new papers, and they can’t all be included in MASS. Therefore, we’re happy to share a few pieces of research that just missed the cut. It’s our hope that with the knowledge gained from reading MASS, along with our interpreting research guide, you’ll be able to tackle these on your own. If you want to peruse our full journal sweep, you can find it here, and you can find our historical archive here. 1. Gibbs et al. Does A Powerlifting Inspired Exercise Programme Better Compliment Pain Education Compared To Bodyweight Exercise For People With Chronic Low Back Pain? A Multicentre, Single-Blind, Randomised Controlled Trial 2. Proost et al. How to Tackle Mental Fatigue: A Systematic Review of Potential Countermeasures and Their Underlying Mechanisms 3. García et al. Movement Velocity As A Determinant Of Actual Intensity In Resistance Exercise 4. Boxman-Zeevi et al. Prescribing Intensity in Resistance Training Using Rating of Perceived Effort: A Randomized Controlled Trial 5. Wojdala et al. A Comparison Of Electromyographic Inter-Limb Asymmetry During A Standard Versus A Sling Shot Assisted Bench Press Exercise 6. Nicklas et al. A Meta-Analysis On Immediate Effects Of Attentional Focus On Motor Tasks Performance 7. Saeterbakken et al. Acute Effects of Barbell Bouncing and External Cueing on Power Output in Bench Press Throw in Resistance-Trained Men 8. Gantois et al. Analysis Of Velocity- And Power-Load Relationships Of The Free-Weight Back-Squat And Hexagonal Bar Deadlift Exercises 9. Fyksen et al. Cardiovascular Phenotype Of Long-Term Anabolic-Androgenic Steroid Abusers Compared With Strength-Trained Athletes 10. Lum et al. Comparing the Effects of Long-Term vs. Periodic Inclusion of Isometric Strength Training on Strength and Dynamic Performances 11. Liu et al. Effects of Exercise Training Intensity and Duration on Skeletal Muscle Capillarization in Healthy Subjects: A Meta-analysis 12. Vieira et al. Effects of Resistance Training to Muscle Failure on Acute Fatigue: A Systematic Review and Meta-Analysis 13. Bell et al. “I Want to Create So Much Stimulus That Adaptation Goes Through the Roof”: High-Performance Strength Coaches’ Perceptions of Planned Overreaching 14. Latella et al. Long-Term Adaptations in the Squat, Bench Press, and Deadlift: Assessing Strength Gain in Powerlifting Athletes 15. Steele et al. Long-Term Time-Course of Strength Adaptation to Minimal Dose Resistance 129 Training Through Retrospective Longitudinal Growth Modeling 16. Langer et al. Myofibrillar Protein Synthesis Rates Are Increased In Chronically Exercised Skeletal Muscle Despite Decreased Anabolic Signaling 17. Xie. Prevention Methods of Fitness and Bodybuilding Exercise Injury Based on Data Mining 18. van den Tillaar et al. The Acute Effects of Attaching Chains to the Barbell on Kinematics and Muscle Activation in Bench Press in Resistance-Trained Men 19. Wender et al. The Effect of Chronic Exercise on Energy and Fatigue States: A Systematic Review and Meta-Analysis of Randomized Trials 20. Čretnik et al. The Effect of Eccentric vs. Traditional Resistance Exercise on Muscle Strength, Body Composition, and Functional Performance in Older Adults: A Systematic Review With Meta-Analysis 21. Mansingh et al. Time to Train: The Involvement of the Molecular Clock in Exercise Adaptation of Skeletal Muscle 130 Thanks for reading MASS. The next issue will be released to subscribers on August 1, 2022. Copy editing by Lauren Colenso-Semple Graphics and layout by Kat Whitfield 131