Chemical Engineering Exam Questions: Thermodynamics & Mass Transfer
advertisement

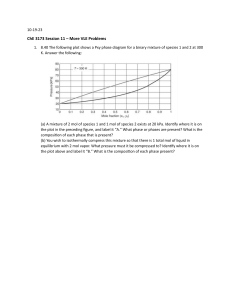
1) Consider a two-component mixture (A & B) in vapor-liquid equilibrium. Which of the following must be true? 1. μA, liq = μA, vap 2. xA = yA 3. vaporization & condensation have stopped A) Only 1 B) 1 and 2 C) 2 and 3 D) 1, 2, and 3 yA, yB Pvap, Tvap xA, xB Pliq, Tliq 2) The concentration of A is different in two immiscible liquids. What determines the direction of mass transfer of component A? A) B) C) D) E) concentration Pressure Solubility Energy other A in B A in C 3) What is the driving force for component A to move from liquid to vapor to reach equilibrium? A) pressure B) entropy C) enthalpy D) concentration E) Gibbs free energy vapor yA liquid xA 4) Phases a and b each contain component A. The two phases are not in equilibrium. The direction of mass transport of component A is ________. A) from a to b B) from b to a C) cannot tell from this information D) no mass transfer occurs a CA=0.001 mol/L b CA=0.020 mol/L 1) Air containing ammonia enters an absorption column. Water is used as the absorbent. What happens when these two fluids are first brought into contact? A) ammonia dissolves in H2O B) water transfers to air/NH3 C) no transfer D) both A & B Air + Ammonia Water Liquid Gas 2) 3) Two identical flasks are connected by a tube. Flask 1 contains water at 40°C. Flask 2 contains 50% more water at 35°C. As the system approaches equilibrium, _______________. A) water moves from 1 to 2 B) water moves from 2 to 1 C) no change occurs 1 2 H2O 40°C H2O 35°C 4) Two identical flasks are connected by a tube. Flask 1 contains water at 40°C. Flask 2 contains 50% more water at 40°C. As the system approaches equilibrium, _______________. A) water moves from 1 to 2 B) water moves from 2 to 1 C) no change occurs 1 2 H2O 40°C H2O 40°C 1) A liquid mixture containing species A and B is boiled by increasing the temperature at constant pressure. The saturation pressure is greater for A than for B. What happens over time? A) B) C) D) xA increases and yA increases xA increases and yA decreases xA decreases and yA decreases xA decreases and yA increases 2) An ideal liquid solution that is 30% A, 30% B, and 40% C is heated at a constant pressure until 80% of the original liquid has evaporated. Which component would you expect to have completely evaporated at that point? PAsat > PBsat > PCsat A) B) C) D) A B C none of them 3) A liquid mixture of 50 mol% n-pentane (Psat = 5.7 bar) and 50 mol% nheptane (Psat = 1 bar) is at high pressure. The mixture is partially vaporized by isothermally lowering the pressure to just below 3 bar. Which statement is correct? A) B) C) D) n-heptane is enriched in the gas phase n-pentane is enriched in the gas phase the gas phase has a 50/50 composition no vapor forms