Midterm exam paper
advertisement

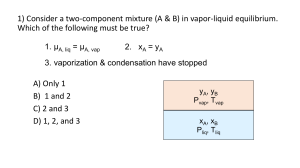
GE 105 Question 1. Multiple Choice Questions: (5 marks) (i) Enthalpy can be denoted as: (a) H (b) T (c) E (d) S (ii) A process in which no temperature can flow from system to surroundings is called (a) Isothermal process (b) Adiabatic process (c) Isochoric process (d) Isobaric process (iii) A system is said to be a close system if it can exchange with the surroundings (a) Both matter and energy (b) Neither matter nor energy (c) Only matter but not energy (d) Only energy but not matter (iv) In which reaction entropy is increasing: (a) H2O (l) (c) CaCO3(s) H2O (g) (b) N2O4 (g) CaO (s) + CO2 (g) (d) Fe3+ (l) + SCN- (l) (v) Consider the reversible reaction N2+ 3H2 expressed as: (a) [NH3]2 [N2] [H2]3 (b) [NH3] [N2]3 [H2] 2NO2 (g) (c) [NH3] [N2] [H2] FeSCN2+ (l) 2 NH3, its equilibrium constant ‘Kc’ is (d) [N2]2 [H2]3 Question 2. True or False questions (5 marks) (i) Chemical equilibrium is established when the concentration of reactants equals the concentration of products. (ii) Boyle’s law can be written as P1V1 = P2V2. (iii) Temperature is the example of extensive property. (iv) The units of work are same as those of energy, Joules or erg. (v) Chemical reactions that take place in one direction only are known as irreversible reactions. Question 3. Short Questions (i) What is first law of thermodynamics? Give example (5 marks) (ii) Classify polymers on the basis of its structure. (iii) Explain Charle’s law. (iv) Using partial pressure, write the equilibrium law (Kp) for the following reaction SbF5(g) + 4Cl2(g) SbCl3(g) (v) What is spontaneous and non spontaneous process. Question 4. Numerical questions (5 marks) (i) Calculate the maximum work obtained when 9.75 mol of an ideal gas expands isothermally and reversibly at 270C from a volume of 5 L to 25 L. (ii) According to Le Chatelier’s principle, what will be the equilibrium shift in the reaction 2C2H2 (g) + 5 O2 (g) when (a) Remove O2 (b) Add CO2 (c) Increase Pressure (d) Add heat 4 CO2 (g) + 2 H2O (g) + heat (iii) At mixture of 0.10 mol of NO, 0.050 mol of H2 and 0.10 mol of H2O is placed in a 1.0 L flask and allowed to reach equilibrium as shown below: 2 NO (g) + 2 H2 (g) N2 (g) + 2 H2O (g) At equilibrium [NO] = 0.062 M, [H2O] = 0.138 M, [N2] = 0.019 M, [H2] = 0.012 M Calculate the equilibrium constant, Kc, for this reaction. (iv) Calculate the internal energy change in each of the following cases:(i) (ii) 15 kJ of work is done on the system and 10 kJ of heat is given out by the system. A system absorbs 17 kJ of heat and does 13 kJ of work. (v) At what temperature would 12.10 moles of N2 gas have a pressure of 10.25 atm and in a 25.0 L tank?