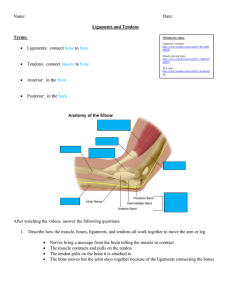
aarr 19 Blood lipids, diabetes, & obesity: clinical queries recklessly thrown at the Doc Who Lifts. Interview with Spencer Nadolsky Copyright © January 1st, 2018 by Alan Aragon Home: www.alanaragon.com Correspondence: support@alanaragon.com 2 Musculoskeletal optimization: Exploring the impact of nutrition and exercise strategies on tendon and ligament health. By Alexander Ketterer & Sten Van Aken 10 The three-month effects of a ketogenic diet on body composition, blood parameters, and performance metrics in crossfit trainees: a pilot study. Kephart WC, et al. Sports 2018, 6(1), 1; doi:10.3390/sports6010001 [MDPI - Sports] 12 Nutritional strategies of high level natural bodybuilders during competition preparation. Chappel AJ, et al. Journal of the International Society of Sports Nutrition (2018) 15:4 DOI 10.1186/s12970-0180209-z [JISSN] 14 Induced and controlled dietary ketosis as a regulator of obesity and metabolic syndrome pathologies. Gibas MK, Gibas KJ. Diabetes Metab Syndr. 2017 Nov;11 Suppl 1:S385-S390. [PubMed] 17 10 years and counting: inception and legacy of AARR – and a big thanks to the contributors. By Alan Aragon Alan Aragon’s Research Review – January 2018 [Back to Contents] Page 1 Musculoskeletal optimization: Exploring the impact of nutrition and exercise strategies on tendon and ligament health. By Alexander Ketterer & Sten Van Aken _________________________________________________ Introduction For decades, exercise biologists have focused on the mechanisms in which our musculoskeletal system, specifically the skeletal muscle, functions, acts and responds both inside and out, all the way to the bottom layer that addresses the very specific individual muscle cells. This foundation of knowledge has provided us with many clues as to what‘s best for ensuring optimal health and performance. As a result, we‘ve come to understand that there‘s a range of hypotheses and theories that benefit the skeletal muscle, such as a set amount of protein to support optimal growth and repair and a sufficient training stimulus. The same thing applies to our bone tissue, also part of the musculoskeletal system, where scientific research has led to recommendations such as opting for a sufficient amount of Vitamin D and calcium from our diet, as well as sufficient exercise, all favoring the optimization of bone health. There is no doubt that both nutrition and exercise play a pivotal role in supporting these parts of the musculoskeletal system. Yet there is something in the musculoskeletal system that hasn‘t received the proper attention that might be warranted. It is mostly associated with sports injuries and is known to take quite the effort to recover from. You‘ve also possibly encountered it on a piece of bone like chicken and had trouble tearing and cutting it with your teeth. I‘m talking of course about the tendons and ligaments, something we often refer to as our being connective tissue. But before we jump into what tendons and ligaments are, what do we mean if we refer to our ligaments and tendons as connective tissue? Fundamentals of connective tissue Connective tissue is a type of tissue that serves a structural purpose, like keeping your organs in place and preventing your eyes from popping out during a 1RM record attempt squat. It‘s the difference being a structure that is both strong, flexible and that can withstand an impact, compared to being a loose blob of curry on the ground. To answer why we refer to our tendons and ligaments as our connective tissue and what prevents us from being a loose blob on the ground in the first place is to also understand what makes connective tissue unique. Alan Aragon’s Research Review – January 2018 First, there are three things that all connective tissues have in common that set them apart from other tissues in our body. The first is that they all originate from the mesenchyme, a loose fluid of embryonic tissue that can transform into many different tissues. Think of this tissue fluid like the layers of paint being applied to a skeleton in a Sci-Fi movie or TV show (like Westworld) where eventually the layers add up to transforming the skeleton into a real human being. The second difference is that these cells originating from the mesenchyme, called the mesenchymal cells, can be situated any-which-way and are able to move from place to place. The third and final thing that separates connective tissue from other tissues is that literally all of it is composed of nonliving material, called the extracellular matrix (ECM). In this particular case, the cells that reside in the matrix are actually LESS important than the matrix itself. You can think of the matrix as representing a piece of dessert jello with flowing pieces of goodies inside that are protected. To expand on the analogy of the jello, the jello can be considered to be buildup of two components: The first is the ground structure, which fills up the space between cells and acts as a form of protection. It is flexible because it consists of tons of starchy protein molecules mixed with water. The anchors of this framework are made up of proteins called proteoglycans. These anchor points have long starchy strands connected to them like ropes called glycosaminoglycans, or GAGs, that clump together in water like the gluten in flour that makes it firm and stretchy. The second part and running throughout the jelly are the fibers, which provide support and structure to an otherwise shapeless ground structure. Elastin is one of those fibers and as the name slightly implies, has the property of being elastic. It allows tissues to ''snap back'' to their original shape after being stretched or contracted like your skin or pulling on your ear. Another good example of where to find elastin is silverskin, that is the white fibrous tissue that you sometimes find on the surface of a muscle. Elastin can mostly found in the artery walls, lungs and intestines, not forgetting the skin. Another class of fibers are the reticular fibers. Reticular fibers are sponge-like fibers that form delicate sponge-like networks that cradle and support your organs. Lastly, collagen is by far the strongest and most abundant type of fiber in our body making up for at least 30% of our total body protein [1]. Collagen differs from elastin in a way that it is slightly less stretchy and can actually be softened and melted away if it‘s cooked in say, the muscle of an animal - which makes the muscle taste moist. Taking these different fibers into consideration, the distribution of fibers determines if they‘re considered a form [Back to Contents] Page 2 of loose connective tissue that is characterized with having less fibers, more cells and more ground substance, or dense connective tissue, which is characterized by being either more tight, irregular and/or flexible tissue. Our focus in this article is going to be on the dense connective tissue, that is the regular type, which is characterized by tight bundles of collagen running parallel and relate to our central question about tendon and ligament health. The composition of tendons and ligaments Now that we‘ve got a general understanding of connective tissue and what characterizes the different tissues, we can start taking a closer look at the composition of tendons and ligaments. Although they differ in functionality, they share a basic framework and molecular composition, making them suitable for concerted discussion [2]. As discussed earlier, what makes up the ECM largely characterizes their structure and thus their function. Embedded in the ECM are the fibroblasts that are responsible for synthesizing the ECM‘s components (collagen, elastin, proteoglycans) and by that, determining its composition. Furthermore, the fibroblasts are interconnected across the tendon/ligament via gap junctions [3], allowing the cells to communicate and respond in a uniform fashion to internal stimuli like growth factors and external stimuli like loading. Collagen is the major type of fiber found in the ECM, making up around 80% of dry weight of ligaments and tendons [3][4], and is responsible for most tensile strength in this particular connective tissue. Just like type I, IIa and IIb muscle fibers, collagen comes in different isotypes as well. The vast majority of collagen is composed of collagen isotype I molecules that are arranged in a parallel, wave-like fashion and form composites within the ECM, allowing the tendon/ligament to withstand tension [5]. Another similarity between muscles and tendons/ligaments is their hierarchical structure, starting at the smallest functional unit and forming increasingly large composites all the way to the complete muscle/tendon/ligament. Other components of the ECM synthesized by the fibroblasts include elastin (2%) which we‘ve addressed earlier and proteoglycans (1-5%)[6], which are proteins with attached sugar groups, that are responsible for the crosslinking of collagen fibrils [7]. Now that we‘ve come to understand what makes up tendons and ligaments and how these characterizations shape the ECM, we can now understand the crucial role collagen synthesis plays in providing structural integrity for tendons and ligaments. Collagen synthesis and recovery As discussed earlier, the fibroblast are responsible for synthesizing the ECM including collagen, the main component of tendons and ligaments. Collagen synthesis can be considered a classic protein biosynthesis mechanism and thus also being concordant in its basic sequence with muscle protein synthesis. When stimulated, peptide chains called ‗‘preprocollagens‘‘ are synthesized by ribosomes inside the fibroblast. Preprocollagen is mostly comprised of the repeated amino acid sequence glycine-X-Y, resulting in glycine being the predominant amino acid of collagen. This abundance of glycine makes collagen a notable exception to the rest of the proteins inside the body, which usually contain only small amounts of glycine. Inside the ribosome, proline, the second most common amino acid in collagen, and lysine are hydroxylated by enzymes that require vitamin C as a cofactor. Besides hydroxylation, further modifications like cleaving and glycosylation take place, allowing the peptide chains to form a triple helix (procollagen) that gets secreted into the extracellular matrix. Outside the fibroblast, procollagen gets cleaved another time, resulting in the finished triple helix collagen molecule (tropocollagen). Lastly, the lysine and the hydroxylysine parts of the collagen molecule are oxidized by lysyl oxidase, allowing the collagen molecules to aggregate to collagen fibrils. Now that we understand how collagen is formed, we can start addressing the way in which tendons adapt to internal and external stimuli - but in order to do so, we have to understand their function first. Tendon and ligament function Ligaments connect bone to bone and are key structures for joint stability by blocking certain displacements of the joint and restrict movements within their physiological range [8]. While the function and properties of ligaments are pretty basic in nature, the functionality of tendons is a little more intricate and requires thorough explanation. Alan Aragon’s Research Review – January 2018 [Back to Contents] Page 3 injury but also more effective in transmitting high forces from muscle to bone [9]. Tendon and ligament adaptation The main function of a tendon is to transmit forces exerted by the muscle to the skeleton and shows two distinct mechanical properties: non-linear elasticity and viscoelasticity [9]. The tendon‘s non-linear elasticity, which differs depending on the tendon, can be observed in its non-linear stress/strain curve as shown in the top image. The toe region represents the „straightening ―of the wave-like collagen structure, leading to a non-linear slope. With increasing stress, the tendon‘s collagen fibrils orient themselves in the direction of the mechanical load and stretch, thus leading to the linear part of the stress/strain curve. This linear part of the stress/strain curve can be considered a representation of the tendon‘s elasticity, which means that when the loading is discontinued, the tendon will revert back to its original length. When the applied stress on the tendon results in tendon strain that exceeds approximately 4%, plastic deformation of the tendon starts to occur, and the tendon is lengthened irreversibly due to failure of crosslinks between collagen fibrils, consequently damaging the tendon. This irreversible lengthening of the tendon continues with increasing stress until the applied stress causes a strain of 8-10%, the point at which the tendon ruptures [9]. For a long time, tendons and ligaments were considered static tissues that show no adaptation to loading and act merely as structural components of the musculoskeletal framework. This widely held assumption among scientists and practitioners alike lead to the disregard of tendon strengthening protocols as a reasonable approach to injury prevention and rehabilitation. As more research on tendon health and development was performed, it turned out that these connective tissues are in fact highly dynamic and adaptable to training stimuli [10]. While the optimal training stimuli to elicit tendon adaptation are still not convincingly specified, the adaptations themselves and the adaptation process have been well established [10]. The tendon‘s second distinctive mechanical property is its viscoelastic behavior, which means the strain of the tendon is not exclusively dependent on the amount of stress that is applied to it but also on the rate at which the strain occurs, resulting in a non-constant relationship between stress and strain of a tendon. This behavior can be illustrated by comparing a slow, near maximum squat to a depth jump. In general, tendons at low strain rates, like those during a slow squat, are more deformable and less likely to rupture but absorb more mechanical energy and thus are less effective in carrying mechanical loads. In contrast, depth jumping exposes the tendons to a tremendously high rate of strain, resulting in the tendon being less deformable, more prone to Research has currently established two possible mechanisms that allow a tendon to adapt to increasing mechanical demands: the first is an increase of the tendon‘s crosssectional area (i.e. hypertrophy), with the second being a change of the tendon‘s mechanical properties (i.e. an increase in tendon stiffness). The first postulated mechanism is tendon hypertrophy induced by load-induced strain of the extracellular matrix [10]. This load-dependent strain is transmitted to the cytoskeleton of the embedded fibroblast, activating a signaling cascade that ultimately leads to an upregulation of collagen and matrix protein synthesis, allowing the tendon to increase in size. Furthermore, research demonstrated a second adaptive mechanism: mechanical loading increased the production of enzymes that are responsible for collagen crosslinking, thus leading to an increase in tendon stiffness associated with a lower electromechanical delay, greater rate of force development and jump height [10]. It is noteworthy that although tendons can adapt to mechanical loading via an increase in stiffness, healthy tendons are variable in their mechanical properties along their length. This makes sense, considering that the connection of two mechanically different tissues, like muscle and tendon or bone and tendon, can cause high stresses at the interface, possibly leading to injury. In order to align the mechanical properties of the connecting tissues, collagen crosslinking along the length of the tendon from bone to muscle decreases, leading to a stiffer tendon towards the bone and a more pliable tendon towards the muscle [11]. What happens when this mechanical variability is lost can be observed as a result of immobilization: preventing a joint to move causes an increase of tendon stiffness in the pliable region and an increase in ultimate tensile strength, likely due to increased collagen crosslinking [12]. Although ultimate Alan Aragon’s Research Review – January 2018 [Back to Contents] Page 4 tensile strength of the tendon increases, this reduction of pliability near the muscle increases the probability of damaging lengthening contractions. These damaging contractions are a result of the tendon‘s stiffness exceeding the isometric strength of the muscle, thus increasing injury risk post-immobilization [11]. Although an adaptation of tendon stiffness can be considered a good thing for dealing with higher mechanical loads, it is of vital importance that the tendon remains mechanically variable. Now that we have established the mechanisms by which tendons adapt, we can start taking a look at the temporal dynamics involving tendon adaptation compared to muscle tissue. Temporal dynamic of tendon adaptation Tendon tissue is characterized by a lower cell to overall dry mass ratio, vascularization, and metabolism compared to muscle tissue and research shows that the half-life of tendon collagen is almost tenfold higher compared to actin and myosin part of muscle tissue [10]. Furthermore, it has been postulated that tendon remodeling almost exclusively occurs in the outside regions of the tendon with the core of the tendon showing no significant collagen turnover [10]. These tendon properties combined with findings of heavy resistance training intervention studies, where changes of muscle morphology and architecture occurred as early as 3-4 weeks with no significant alterations of the tendons [10], can lead to the assumption that meaningful tendon adaptation occurs with a significant delay compared to muscle morphology and strength. That assumption is substantiated by exercise intervention studies that observed a 1-2 month delay in tendon adaptation in comparison to gains in muscle strength. What further underpins these findings is the fact that an increase in muscle strength can be achieved by neuronal adaptations that precede morphological changes of the muscle [10]. In contrast to that, adaptations in tendon resilience rely exclusively on the adaptation of the tissue structure, which tends to be a slower process due to the lesser rate of effective tissue renewal compared to muscle [10]. As a result of these hypotheses, it‘s possible that imbalances between muscle and tendon development might occur during the training process, which brings us to our next aspect of the tendon‘s adaptive process: which loading strategy is optimal for tendon health and development? How different loading patterns influence tendon development First off, this aspect of tendon adaptation has not been conclusively researched and needs further investigation. That being said, research and the aforementioned mechanism of fibroblast stimulation via deformation suggest that slow Alan Aragon’s Research Review – January 2018 repetitive high-magnitude (at about 90% isometric maximum voluntary muscle contraction) tendon strain might be the best approach to elicit the greatest adaptations in healthy tendons [10]. It is noteworthy that the specific muscle contraction type (isometric, concentric, eccentric) seems to be of little relevance for triggering the adaptation process. Research that incorporated other loading schemes like plyometric loading (i.e. a high-magnitude, high-frequency tendon strain loading protocol) failed to elicit significant adaptive changes in tendon structure, although improving muscle strength [10]. This finding might provide at least some insight concerning the high prevalence of tendon overuse injuries in sports with a plyometric loading profile like basketball or athletic jumping and warrants additional research. Furthermore, research suggests that fatiguing training with moderate loading, like classic muscle hypertrophy training, effectively triggers adaptations in muscle strength and size, yet doesn‘t provide sufficient stimulus for meaningful tendon adaptations to occur [10]. This lack of tendon adaptation could result in an increase in tendon strain as a result of increased muscle strength without the appropriate tendon adaptations, causing a possible risk for injury [10]. With that in mind, a compelling case might be made for implementing strength focused training phases from time to time, even for athletes that exclusively train for muscle size. While collectively these findings might indicate an appropriate strategy to promote tendon strength in healthy tendons, a different approach might be necessary for promoting recovery in injured tendons and ligaments. Research that used an in vitro tendon/ligament model, which properties more closely resemble developing/recovering tendons/ligaments (i.e. higher cell count, less matrix, higher expression of developmental collagen isotypes), provides findings that deviate from the findings concerning healthy tendons. Study of those engineered ligaments showed that the molecular response was independent of loading intensity and frequency [13]. Interestingly, it could be observed that the duration of the applied loading played a significant role in determining the molecular response. After about ten minutes of loading, the molecular response reached its maximum and additional time under load did not increase the molecular response any further. Moreover, it took six hours for the engineered ligaments to become responsive to loading again. These findings suggest that short bouts of lightly loaded exercise with potentially limited range of motion and extensive breaks between bouts might be the best way to aid tendon/ligament recovery. Hormonal influence on tendons and ligaments Lastly, although the specifics are not understood yet, hormonal status plays a role in influencing tendon and [Back to Contents] Page 5 ligament integrity. One hormonal effect that has been well established is estrogen‘s influence on ligament laxity [11]. Research showed that the degree of knee laxity and higher incidence of ACL rupture in female athletes compared to their male counterparts seems to be related to circulating estrogen levels [11]. In an attempt to confirm this, the researchers using the engineered ligaments exposed those ligaments to a physiologically high estrogen level that mimics the estrogen surge leading up to ovulation [14]. After 48 hours of exposure, they observed a decrease in ligament stiffness although the collagen content did not decrease, which hints at a decrease of collagen crosslinking. To test this hypothesis, the researchers measured the activity of the primary collagen crosslinking enzyme, lysyloxidase, under the aforementioned estrogen exposure of the ligaments. They observed that the activity of lysyl oxidase dropped as much as 80% after 48 hours of physiologically high estrogen exposure. Consequently, it seems reasonable to consider estrogen an influential factor for tendon and ligament integrity, especially in female athletes. In contrast to estrogen, little is known about the effects of testosterone on tendon and ligament properties. Research only suggest that the use of androgenic and anabolic steroids in supraphysiological doses stimulate collagen synthesis and tendon stiffness, yet reduces tissue remodeling ultimate stress and strain, which might warrant some implications for longterm drug users [10]. That being said, little is known about the effects of physiological levels of testosterone on tendon and ligament development. Another interesting observation was made when engineered ligaments were grown in media that was infused with isolated sera from human test subjects pre- and post-exercise. The ligaments that were grown in the media containing postexercise sera showed a significant increase in collagen content and mechanics compared to the ligaments grown in pre-exercise sera [15]. Furthermore, the same study showed that this stimulation of connective tissue was not mediated by growth hormone and IGF-1 but used the mTORC1 pathway, hinting at a global signal that improves connective tissue integrity in response to exercise. Finally, this study showed that the treatment of engineered ligaments with high doses of recombinant growth hormone had no effect on collagen content or ligament mechanics. Yet, IGF-1 dose-dependently stimulated collagen synthesis and affected ligament mechanics. This observation substantiates the idea that physiological levels of growth hormone have an indirect effect on tendons and ligaments via regulation of IGF-1. Now that we have briefly elaborated on the physiological and mechanical properties of tendons and ligaments and how they Alan Aragon’s Research Review – January 2018 respond to loading and hormonal status, it‗s time to discuss how we might improve tendon and ligament development via nutrition and supplementation. Nutrition and supplement considerations for optimizing tendon and ligament health Regarding everyday nutrition, consuming a leucine-rich diet seems to be beneficial for improving tendon and ligament health. Researchers observed that consumption of whey protein, which is rich in leucine, can increase tendon hypertrophy in response to strength training. This observation could possibly be explained by the fact that leucine activates the mTORC1 pathway, a pathway that was also activated in engineered ligaments after treating them with sera that were collected from human subjects post-exercise. In spite of this hypothetical connection, it is not clear whether the study‗s result is based on a direct effect of whey protein intake on tendons or a byproduct of increased muscle hypertrophy and strength gains caused by whey protein consumption [16]. With no conclusive evidence for a direct effect of whey protein on tendon health and the fact that the diet of most fitness enthusiasts is rich in leucine for muscle building purposes anyway, we can take a look at a more interesting aspect: intake of actual collagen and its processed derivatives. Research suggests that ingestion of collagen peptides has a plethora of effects on the human body. Besides their potentially beneficial effects on skin, osteoporosis, the immune system and precursor cell differentiation to only name a few [17] [18], research also suggest that ingestion of collagen peptides has beneficial effects on both collagen synthesis and tendon mechanics [19]. While measuring the plasma concentration of amino acids after oral collagen peptide ingestion, researchers observed high concentrations of oligopeptides, like proline-hydroxyproline and hydroxyproline-glycine, two hours after ingestion [18]. With collagen molecules heavily featuring the repeated amino acid sequence glycine-X-Y, with X and Y frequently occupied by proline and hydroxyproline, collagens high bio-availability and the fact that oligopeptides containing hydroxyproline are highly resistant to blood proteases [17], this observation seems comprehensible. Aside from collagen peptides providing the necessary amino acids for collagen synthesis, several studies also suggest that these stable hydroxyproline containing oligopeptides act as signaling molecules and thus being partly responsible for mediating the effects of collagen peptide ingestion [18]. Although the actual mechanism hasn‘t been established yet, this might make a compelling case for increasing collagen intake considering the fact that [Back to Contents] Page 6 hydroxyproline is almost exclusively found in collagen as a nutritional source . What further substantiates these hypotheses is a study on engineered ligaments [20]. During this double-blind crossover study, the researchers administered different doses(0g, 5g, 15g) of gelatin, a hydrolyzed form of collagen, to human test subjects along with a small dose (48 mg) of vitamin C, one hour prior to six minutes of jumping rope. The researchers collected blood samples of the participants at different time points and added the sera to the growth media of the engineered ligaments. They found a dose-dependent increase in collagen deposition with 15g of gelatin doubling the collagen synthesis rate in engineered ligaments. In another study performed on osteoarthritis patients, around 10 grams of collagen hydrolysate a day was significantly able to thicken the cartilage [21]. The same 10 grams of collagen hydrolysate in another study led to improvements in joint pain in athletes over a 24-week period [22]. Having these promising findings in mind, we have to take a look at actual collagen intake. Since collagen is the single most abundant class of proteins of the vertebrate body making up about one-third of an animals total protein content, it can‘t be found in non-animal products [17]. Despite this abundance of collagen in vertebrates, the average collagen intake is presumably pretty low among non-meat-eaters and meat-eaters alike. One of the reasons for low collagen intake might be that collagen is missing an essential amino acid, tryptophan, thus being considered a low-quality protein that doesn‘t get much attention. Further possible reasons include that cuts of meat high in collagen, like pork chitterlings or chicken gizzard [18], tend to be less desirable to the general population‘s palate or that the more chewy, fibrous parts of meats get trimmed off. Beef vs. fish and shellfish (adopted from [24] and modified from [18]) That being said, there are alternatives to eating fibrous meats to reap the beneficial effects of nutritional collagen. Collagen can be extracted from collagen-rich animal tissues like skin, bone and tendons. After extracting the collagen, it gets processed via hydrolysis. Depending on the degree of hydrolysis, you either get the partially hydrolyzed collagen product gelatin or the fully hydrolyzed collagen hydrolysate. While both being tasteless and available in powdered form, the main differences between the two products are price, availability, molecular weight and usage. Gelatin is higher in molecular weight, relatively cheap, found at any convenience store and can be dispersed in liquids or used as a gelling agent for jellos and such. Collagen hydrolysate on the other hand is more expensive, cannot be used as a gelling agent due to its lower molecular weight and is usually purchased at supplement stores. At this point in time, there are no studies demonstrating a meaningful difference in bio-availability although collagen hydrolysate is claimed to be easier to digest. Both products seem to be sufficiently absorbed by the intestines as free form amino acids and oligopeptides [17][18][23], making them equally suitable for increasing your collagen intake. Putting it all together – a proposed protocol for tendon and ligament prehab and rehab Preface: With research on influencing tendon and ligament development still in its infancy, these recommendations should be considered speculative and non-definitive. If you suffer from tendon or ligament injuries, please consult with your physician or physiotherapist first before implementing such a protocol. Protocol for strengthening healthy tendons Beef vs. fish and shellfish (adopted from [24] and modified from [18]) This protocol should be used for strengthening healthy tendons and ligaments that are particularly stressed during Alan Aragon’s Research Review – January 2018 [Back to Contents] Page 7 your preferred movements in order to reduce their chance of injury. First, pick a movement that targets the tendon or ligament you intend to strengthen. You can either choose an eccentric-concentric movement, like the squat for strengthening the patellar tendon, or opt for isometric exercise like knee extension against a non-elastic band. Research suggests that the chosen exercise should be performed for 5 sets of 4 repetitions with each repetition consisting of three seconds of high intensity contraction (8590% iMVC) followed by three seconds of relaxation and two minute inter-set rest intervals [10]. It is important that these exercises are performed at a joint angle that is close to optimal for force production, for example 60° knee flexion for patellar tendon training, in order for high tendon forces to occur. While performing isometric exercises at a certain angle is relatively easy to achieve, eccentric-concentric movements like the squat move through a wide range of angles during its full range of motion. To account for that and allow for sufficient time at which high tendon forces occur, the duration of a repetition is doubled from three to six seconds for dynamic movements [10]. This proposed set and rep scheme should be implemented three times per week along with your regular training for at least twelve weeks and can be applied to multiple exercises to strengthen different tendons and ligaments concurrently. To optimize training results, athletes are encouraged to ingest 15 g of gelatin or collagen hydrolysate with a small dose of vitamin C one hour prior to tendon training [11]. This can be done by dispersing the collagen product in a liquid that contains vitamin C (like juice) or by preparing a jello made from gelatin that also contains vitamin C. Moreover, it might be worth mentioning why the collagen product should be consumed one hour prior to training: while blood flow to inactive tendons is limited, suggesting limited nutrient uptake into the tendon post exercise, glucose uptake into active tendons is increased during exercise [25]. Thus, in concordance with the absorption rate of collagen peptides, taking the collagen product one hour prior to exercise seems reasonable to optimize nutrient uptake into the tendon. Lastly, leucine-rich protein should be consumed as part of training to reap the potential benefits of mTORC1 activation on tendon and ligament development [11]. rich protein remain the same, this protocol follows a different approach to training and is inferred from the observations regarding engineered ligaments, as they more closely resemble regenerating ligaments and tendons. Following injury, athletes should start their recovery training protocol as soon as possible by picking movements that target the injured tendon or ligament. Depending on the severity of injury, these movements can consist of weight supported exercises with limited range of motion if necessary. To maximally stimulate collagen synthesis, training bouts can be performed multiple times per day but should not exceed ten minutes of activity per single session. Furthermore, each training bout should be followed by at least six hours of rest to allow the connective tissue to become responsive to loading again and yield optimal results [11]. Training and supplementing for tendon and ligament health - the verdict Although research provides some insight regarding training and supplementing for tendon and ligament health, these observations are far from warranting definitive protocols and should be treated as such. To close in on best practices for tendon and ligament health, extensive research has to be conducted, including the role of hydroxyproline oligopeptides, overall training volume, rest intervals and in vivo studies of recovering tendons and ligaments. It also remains unclear to what degree the total amount of protein matters in a diet that already contains the required contains sufficient protein to stimulate maximum protein and if adding additional protein with specific amino acids might yield more optimal results on top of muscle growth. Despite this rudimentary understanding of training and supplementing for tendon and ligament health, the proposed protocols represent our ―best guesses‖ and might be worth a shot since they can be considered time- and cost-effective plus offering a desirable risk-reward ratio. References 1. 2. Protocol for accelerating the rehabilitation process after tendon and ligament injury This protocol can be implemented to potentially speed up the recovery process after tendon and ligament injury. While the recommendations for collagen supplementation and leucineAlan Aragon’s Research Review – January 2018 3. Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and diseaseassociated mutations on the most abundant protein in the human, type I collagen. J Biol Chem. 2002 Feb 8;277(6):4223-31. Epub 2001 Nov 9.[PubMed] Rumian AP, Wallace AL, Birch HL. Tendons and ligaments are anatomically distinct but overlap in molecular and morphological features--a comparative study in an ovine model. J Orthop Res. 2007 Apr;25(4):458-64. [PubMed] Massoud EI. Healing of subcutaneous tendons: Influence of the mechanical environment at the suture line on the healing process. World J Orthop. 2013 Oct 18;4(4):229-40. [PubMed] [Back to Contents] Page 8 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. Liu SH, Yang RS, al-Shaikh R, Lane JM. Collagen in tendon, ligament, and bone healing. A current review. ClinOrthopRelat Res. 1995 Sep;(318):265-78.[PubMed] Raspanti M, Congiu T, Guizzardi S. Structural aspects of the extracellular matrix of the tendon: an atomic force and scanning electron microscopy study. Arch Histol Cytol. 2002 Mar;65(1):37-43. [PubMed] Kleiner DM. Human Tendons: Anatomy, Physiology and Pathology. Journal of Athletic Training. 1998;33(2):185186. [PubMed] Scott JE. Elasticity in extracellular matrix 'shape modules' of tendon,cartilage, etc. A sliding proteoglycan-filament model. J Physiol. 2003 Dec1;553(Pt 2):335-43. Epub 2003 Aug 15. Review. [PubMed] Lorda-Diez CI, Canga-Villegas A, Cerezal L, Plaza S, Hurlé JM, García-Porrero JA, Montero JA. Comparative transcriptional analysis of three human ligaments with distinct biomechanical properties. J Anat. 2013 Dec;223(6):593-602.[PubMed] Wang JH, Guo Q, Li B. Tendon biomechanics and mechanobiology--a minireview of basic concepts and recent advancements. J Hand Ther. 2012 Apr-Jun;25(2):133-40. [PubMed] Mersmann F, Bohm S, Arampatzis A. Imbalances in the Development of Muscle and Tendon as Risk Factor for Tendinopathies in Youth Athletes: A Review of Current Evidence and Concepts of Prevention. Front Physiol. 2017 Dec 1;8:987. [PubMed] Baar K. Minimizing Injury and Maximizing Return to Play: Lessons from Engineered Ligaments. Sports Med. 2017 Mar;47(Suppl 1):5-11. [PubMed] Eliasson P, Fahlgren A, Pasternak B, Aspenberg P. Unloaded rat Achilles tendons continue to grow, but lose viscoelasticity. J ApplPhysiol (1985). 2007 Aug;103(2):459-63. [PubMed] Paxton JZ, Hagerty P, Andrick JJ, Baar K. Optimizing an intermittent stretch paradigm using ERK1/2 phosphorylation results in increased collagen synthesis in engineered ligaments. Tissue Eng Part A. 2012 Feb;18(34):277-84. [PubMed] Lee CA, Lee-Barthel A, Marquino L, Sandoval N, Marcotte GR, Baar K. Estrogen inhibits lysyl oxidase and decreases mechanical function in engineered ligaments. J Appl Physiol (1985). 2015 May 15;118(10):1250-7. [PubMed] West DW, Lee-Barthel A, McIntyre T, Shamim B, Lee CA, Baar K. The exercise-induced biochemical milieu enhances collagen content and tensile strength of engineered ligaments. J Physiol. 2015 Oct 15;593(20):4665-75. [PubMed] Farup J, Rahbek SK, Vendelbo MH, Matzon A, Hindhede J, Bejder A, RinggardS,Vissing K. Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Scand J Med Sci Sports. 2014 Oct;24(5):788-98. [PubMed] Wang L, Wang Q, Qian J, Liang Q, Wang Z, Xu J, He S, Ma H. Bioavailability and bioavailable forms of collagen after oral administration to rats. J Agric Food Chem. 2015 Apr 15;63(14):3752-6. [PubMed] Alan Aragon’s Research Review – January 2018 18. Koyama Y. Effects of Collagen Ingestion and their Biological Significance. J Nutr Food Sci 2016, 6:3. [PubMed] 19. Minaguchi J, Koyama Y, Meguri N, Hosaka Y, Ueda H, Kusubata M, Hirota A, Irie S, Mafune N, Takehana K. Effects of ingestion of collagen peptide on collagen fibrils and glycosaminoglycans in Achilles tendon. J Nutr Sci Vitaminol (Tokyo).2005 Jun;51(3):169-74. [PubMed] 20. Shaw G, Lee-Barthel A, Ross ML, Wang B, Baar K. Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am J ClinNutr. 2017 Jan;105(1):136-143. [PubMed] 21. Bohm S, Mersmann F, Arampatzis A. Human tendon adaptation in response to mechanical loading: a systematic review and meta-analysis of exercise intervention studies on healthy adults. Sports Med Open. 2015 Dec;1(1):7. [PubMed] 22. Clark KL, Sebastianelli W, Flechsenhar KR, Aukermann DF, Meza F, Millard RL,Deitch JR, Sherbondy PS, Albert A. 24-Week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain.Curr Med Res Opin. 2008 May;24(5):1485-96. [PubMed] 23. Watanabe-Kamiyama M, Shimizu M, Kamiyama S, Taguchi Y, Sone H, MorimatsuF,Shirakawa H, Furukawa Y, Komai M. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J Agric Food Chem. 2010 Jan 27;58(2):835-41. [PubMed] 24. Noguchi C, Kobayashi M, Koyama Y (2012) Amount of collagen ingested by Japanese adult women from their diet. Jpn J Nutr Diet 70:120-128. [PubMed] 25. Bojsen-Møller J, Kalliokoski KK, Seppänen M, Kjaer M, Magnusson SP.Low-intensity tensile loading increases intratendinous glucose uptake in the Achilles tendon. J ApplPhysiol (1985). 2006 Jul;101(1):196-201. [PubMed] ____________________________________________________ Dipl. pharm. Alexander Ketterer is a pharmaceutical scientist, powerlifting coach and personal trainer, operating his company Fortius Per Scientiam out of Constance, Germany ____________________________________________________ Sten van Aken is dietitian in training at the Hague University of Applied Sciences. He has a passion for research intertwined with nutrition and training and has previously written for Fit Zonder Fabels, a Dutch myth-busting lifestyle platform and worked as an associate researcher for Bayesian Bodybuilding. [Back to Contents] Page 9 Study strengths The three-month effects of a ketogenic diet on body composition, blood parameters, and performance metrics in crossfit trainees: a pilot study. Kephart WC, et al. Sports 2018, doi:10.3390/sports6010001 [MDPI - Sports] 6(1), 1; BACKGROUND: Adopting low carbohydrate, ketogenic diets remains a controversial issue for individuals who resistance train given that this form of dieting has been speculated to reduce skeletal muscle glycogen levels and stifle muscle anabolism. PURPOSE: We sought to characterize the effects of a 12-week ketogenic diet (KD) on body composition, metabolic, and performance parameters in participants who trained recreationally at a local CrossFit facility. DESIGN: Twelve participants (nine males and three females, 31 ± 2 years of age, 80.3 ± 5.1 kg body mass, 22.9 ± 2.3% body fat, 1.37 back squat: body mass ratio) were divided into a control group (CTL; n = 5) and a KD group (n = 7). KD participants were given dietary guidelines to follow over 12 weeks while CTL participants were instructed to continue their normal diet throughout the study, and all participants continued their CrossFit training routine for 12 weeks. Pre, 2.5-week, and 12-week anaerobic performance tests were conducted, and pre- and 12-week tests were performed for body composition using dual X-ray absorptiometry (DXA) and ultrasound, resting energy expenditure (REE), blood-serum health markers, and aerobic capacity. Additionally, blood beta hydroxybutyrate (BHB) levels were measured weekly. RESULTS: Blood BHB levels were 2.8- to 9.5-fold higher in KD versus CTL throughout confirming a state of nutritional ketosis. DXA fat mass decreased by 12.4% in KD (p = 0.053). DXA total lean body mass changes were not different between groups, although DXA dual-leg lean mass decreased in the KD group by 1.4% (p = 0.068), and vastus lateralis thickness values decreased in the KD group by ~8% (p = 0.065). Changes in fasting glucose, HDL cholesterol, and triglycerides were similar between groups, although LDL cholesterol increased ~35% in KD (p = 0.048). Between-group changes in REE, onerepetition maximum (1-RM) back squat, 400 m run times, and VO2peak were similar between groups. CONCLUSIONS: While our n-sizes were limited, these preliminary data suggest that adopting a ketogenic diet causes marked reductions in whole-body adiposity while not impacting performance measures in recreationally-trained CrossFit trainees. Whether decrements in dual-leg muscle mass and vastus lateralis thickness in KD participants were due to fluid shifts remain unresolved, and increased LDL-C in these individuals warrants further investigation. SPONSORSHIP: The only costs herein were the serum analyses, which were funded through residual funds provided to Michael D. Roberts through a prior grant awarded to him by the Applied Sports Performance Institute (ASPI). Alan Aragon’s Research Review – January 2018 With scant exceptions,1-3 the performance studies on ketogenic dieting are typically 4-6 weeks (or less). The present study was 12-weeks, which presumably is sufficient for accommodating an initial keto-adaptation period. Subjects were required to have a minimal strength-to-mass ratio of at least 1:1 (bodymass: back squat) and a minimum of 3 months of gym activity. This weeded out untrained/newbie trainees. Dual X-ray absorptiometry (DXA) was used to assess body composition. Blood ketone levels (beta-hydrodybutyrate; BHB) were measured weekly, this provided an objective marker of compliance to the ketogenic diet. Study limitations The authors acknowledged several limitations, the first being the small number of subjects. Instead of random allocation, the subjects were given volitional choice of whether they wanted to try the ketogenic diet (KD) or keep their habitual diet. This introduces the possibility of expectation bias for favorable outcomes in the keto group. In light of this, the authors diligently acknowledged the following: ―Additionally, there is the real chance that individuals in the KD group may have experienced a placebo effect in relation to performance outcomes. Alternatively stated, given that many of the KD participants experienced improvements in body composition and (anecdotally) many of these participants perceived themselves having more energy throughout the day, these phenomena could have motivated them to perform the exercise tests with more vigor relative to the CTL [control] group.‖ I would add to these limitations that there was no standardization of dietary intake prior to performance testing, which leaves open the confounding potential of variable levels of fuel availability. The control group was not required to track their dietary intake, which is a shame since this data would have been valuable in drawing inferences as to the results. Although the KD group was required to track intake, only 4 out of the 7 subjects submitted a food log at the 12-week point (food records for the other 3 subjects in KD were limited to baseline and 2.5 weeks). Comment/application The main findings were a lack of significant differences between groups in the performance tests. Performance was preserved in 1RM squat, 1RM power clean, and 400-meter run. The only performance parameter that showed improvement from baseline was push-ups to failure – but no between-group differences were seen. Individual performance data were reported (here), and it‘s always interesting to see the variability of responses. As for body composition [Back to Contents] Page 10 (complete graphical data here), KD lost a significant amount of bodyweight (3 kg) and body fat (2.5 kg) while the control group did not lose a significant amount of bodyweight (0.3 kg) or body fat (0.3 kg). Total lean body mass (LBM) was preserved and not significantly different between groups. However, when viewed segmentally, decreases in dual-leg lean mass and lateralis thickness approached statistical significance in KD, but the effects sizes for each decrease were large. Resting energy expenditure and respiratory quotient (an indicator of substrate utilization; the proportion of fat/carb utilization) were not significantly changed or different between groups. Expectedly, blood ketone levels were significantly higher in KD compared to control. I found it interesting that over time, blood BHB levels appeared to be on a downward trend, which suggests the potential for a decrease in adherence over time to the KD: An additional factor is a potential decrease in food reward via lower hedonic contribution to food intake.7 So, with the nearelimination of carbohydrate-containing foods, this lowers the probability of consuming hyperpalatable foods – many of which are comprised of combinations of fat and refined carbohydrate. But once again, the question is whether or not the KD is sustainable in the long-term, and what potential adverse effects might manifest as a result of long-term adherence. Definitive answers to these questions are still under investigation. The present study shows that for a 3-month period, going keto is fine for recreationally trained crossfitters seeking to decrease body fat without significant performance decreases (keep in mind that these were not elite athletes). However, the effects of keto dieting on highly trained endurance competitors has thus far been equivocal, with a mix of positive and negative results – not necessarily worth rolling the dice when the stakes are high. References 1. 2. Zinn C, Wood M, Williden M, Chatterton S, Maunder E. Ketogenic diet benefits body composition and well-being but not performance in a pilot case study of New Zealand endurance athletes. J Int Soc Sports Nutr. 2017 Jul 12;14:22. [PubMed] McSwiney FT, Wardrop B, Hyde PN, Lafountain RA, Volek JS, Doyle L Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism. 2017 Dec 5. pii: S00260495(17)30328-1. [PubMed] Wilson JM, Lowery RP, Roberts MD, Sharp MH, Joy JM, Shields KA, Partl J, Volek JS, D'Agostino D. The effects of ketogenic dieting on body composition, strength, power, and hormonal profiles in resistance training males. J Strength Cond Res. 2017 Apr 7. doi: 10.1519/JSC.0000000000001935. [PubMed] Aragon AA, Schoenfeld BJ, Wildman R, Kleiner S, VanDusseldorp T, Taylor L, Earnest CP, Arciero PJ, Wilborn C, Kalman DS, Stout JR, Willoughby DS, Campbell B, Arent SM, Bannock L, Smith-Ryan AE, Antonio J. International society of sports nutrition position stand: diets and body composition. J Int Soc Sports Nutr. 2017 Jun 14;14:16. [PubMed] Paoli A, Grimaldi K, D'Agostino D, Cenci L, Moro T, Bianco A, Palma A. Ketogenic diet does not affect strength performance in elite artistic gymnasts. J Int Soc Sports Nutr. 2012 Jul 26;9(1):34. [PubMed] Heatherly AJ, Killen LG, Smith AF, Waldman HS, Hollingsworth A, Seltmann CL, O‘Neal EK. Effects of ad libitum low carbohydrate high-fat dieting in middle-age male runners. Med Sci Sports Exerc. 2017 Nov 6. doi: 10.1249/MSS.0000000000001477 [Epub ahead of print] [PubMed] Alonso-Alonso M, Woods SC2, Pelchat M, Grigson PS2, Stice E, Farooqi S, Khoo CS, Mattes RD2, Beauchamp GK. Food reward system: current perspectives and future research needs. Nutr Rev. 2015 May;73(5):296-307. [PubMed] One of the most interesting aspects of this study was the reported decrease in protein intake from baseline in the KD group (remember, the control group was not required to report intake); 114 vs 89 g from baseline to the 12-week point. Again, this figure is based on only 4 out of the 7 subjects in the KD group who provided dietary intake reports at the final 12-week data collection point. Thus, it may or may not be an accurate representation of the mean intake. This is in contrast to Paoli et al,5 whose subjects‘ KD protein intake was 200.8 g versus their habitual intake of 83.5 g. 3. Another interesting result was that, despite an apparent decrease in protein intake, total energy intake in the KD group decreased by 551 kcal from baseline. This ‗spontaneous‘ decrease in energy intake as a result of going on a ketogenic diet has been seen repeatedly in the literature.5 The most dramatic case of this that I‘m aware of is a recent study by Heatherly et al,5 whose subjects had a rather massive 934 kcal decrease on a ketogenic diet without purposely restricting calories. The mechanistic basis of this phenomenon is not definitively understood. It could be either a function of the elevation of circulating ketones themselves, or it could be the increase in protein, or both. 5. Alan Aragon’s Research Review – January 2018 [Back to Contents] 4. 6. 7. Page 11 Nutritional strategies of high level natural bodybuilders during competition preparation. Chappel AJ, et al. Journal of the International Society of Sports Nutrition (2018) 15:4 DOI 10.1186/s12970-018-0209z [JISSN] BACKGROUND: Competitive bodybuilders employ a combination of resistance training, cardiovascular exercise, calorie reduction, supplementation regimes and peaking strategies in order to lose fat mass and maintain fat free mass. Although recommendations exist for contest preparation, applied research is limited and data on the contest preparation regimes of bodybuilders are restricted to case studies or small cohorts. Moreover, the influence of different nutritional strategies on competitive outcome is unknown METHODS: Fifty-one competitors (35 male and 16 female) volunteered to take part in this project. The British Natural Bodybuilding Federation (BNBF) runs an annual national competition for high level bodybuilders; competitors must qualify by winning at a qualifying events or may be invited at the judge‘s discretion. Competitors are subject to stringent drug testing and have to undergo a polygraph test. Study of this cohort provides an opportunity to examine the dietary practices of high level natural bodybuilders. We report the results of a cross-sectional study of bodybuilders competing at the BNBF finals. Volunteers completed a 34-item questionnaire assessing diet at three time points. At each time point participants recorded food intake over a 24-h period in grams and/or portions. Competitors were categorised according to contest placing. A ―placed‖ competitor finished in the top 5, and a ―Non-placed‖ (DNP) competitor finished outside the top 5. Nutrient analysis was performed using Nutritics software. Repeated measures ANOVA and effect sizes (Cohen‘s d) were used to test if nutrient intake changed over time and if placing was associated with intake. RESULTS: Mean preparation time for a competitor was 22 ± 9 weeks. Nutrient intake of bodybuilders reflected a high-protein, highcarbohydrate, low-fat diet. Total carbohydrate, protein and fat intakes decreased over time in both male and female cohorts (P < 0.05). Placed male competitors had a greater carbohydrate intake at the start of contest preparation (5.1 vs 3.7 g/kg BW) than DNP competitors (d = 1.02, 95% CI [0.22, 1.80]). CONCLUSIONS: Greater carbohydrate intake in the placed competitors could theoretically have contributed towards greater maintenance of muscle mass during competition preparation compared to DNP competitors. These findings require corroboration, but will likely be of interest to bodybuilders and coaches. SPONSORSHIP: No funding was received for this study. intakes of drug-free bodybuilding competitors during contest preparation while stratifying subjects according to having placed within the top-5, and those did not place within the top-5 (DNP). This was an important parameter to assess since it provides hints toward the relative effectiveness of the dietary strategies employed. The cohort studied here were competing ‗finals‘ type of contest where winners of regional contests were the overall winner earns professional status. As such, this sample is reliably representative of high-level competitive natural bodybuilders. It was pretty cool seeing the paper I did with Eric Helms and Peter Fitschen referenced throughout this manuscript.2 Furthermore, our work was put into context as such: “The strategies employed by the most successful natural bodybuilders can be compared to recommendations [11], which include protein intake of between 2.3 and 3.1 g/kg of LBM, fat intake of 15 to 30% of total calories, with the remaining calories from carbohydrate and a weekly weight loss of 0.5 to 1% of bodyweight (BW) [11]. Here we report the results of a recent cross-sectional study investigating the nutritional strategies of natural bodybuilding competitors at the BNBF finals.” The above excerpt accurately relays the recommendations in our paper, and I was eager to see how the results of the study lined up. Interesting odds & ends 35 men and 16 women volunteered to do the survey-based study, one male subject was excluded from the study due to failing a polygraph test, and ultimately, 32 men and 15 women were included in the analysis. The majority of the competitors used a coach for contest prep. Interestingly, there was a lack of difference in the use of a coach (versus not using a coach) in those who placed and those who did not. 100% of the women who placed used a coach, and 78% of the women who did not place used a coach. 60 & 59.1% of men who placed and did not place used a coach, respectively. Looking at Table 1, I also found it interesting that although this lacked statistical significance, men who placed had a higher mean age than those who did not place (36.1 vs 31.8 yrs), while this was the opposite for women who placed versus those who did not (33.7 vs 34.7 yrs). As an observational study, cause-and-effect cannot be established, but the findings are useful and thoughtprovoking. This was the first study to examine the dietary Another interesting finding was that among the 15 competitors who were able to provide body composition data (5M, 10F), fat-free mass index (FFMI) exceeded 25 in 2 men who placed. The relevance of this finding is that a FFMI is generally known (but not necessarily accepted) as the ―natty Alan Aragon’s Research Review – January 2018 [Back to Contents] This is an observational study, so… Page 12 The rate of weight loss in males who placed was 0.46% per week. This is really close to the lower end of our recommended weekly weight loss recommendations2 as well as a recent case study of a female physique competitor in contest prep.3 Slower rates of weight loss (0.7 vs 1.4%/wk) have also been seen to better preserve lean mass in an assortment of elite competitive athletes.4 limit‖ beyond which the likelihood of being drug-free drops precipitously. For an in-depth discussion of this topic, refer to Eric Helms‘ guest article in the August 2014 issue of AARR, which is accessible here. Dietary findings In Table 2, the mean macronutrient intakes at the start and end of contest preparation (22 weeks on average) in those who placed and did not place as follows: Here are the notable tidbits: Protein intake was higher in men who placed. Intake at the start & end of prep was 3.0 & 3.3 g/kg respectively. In men who didn‘t place, protein intake at the start and end of prep was 2.7 g/kg at both the start and end of prep. This difference was not seen among women. Carbohydrate intake was higher in men who placed. Intake at the start & end of prep was 5.1 & 4.6 g/kg respectively. In men who didn‘t place, intake at the start and end of prep was 3.7 & 3.6 g/kg at the start and end of prep. This difference was not seen among women, although it‘s interesting that in terms of absolute numbers, the slight opposite was seen. Differences in fat intake across groups and time points were less marked than differences in protein and carbohydrate. The latter differences were reflected in the differences in total caloric intake (more total kcal was consumed by men who placed). In Table 3 (here), absolute gram amounts of the macronutrients are listed. Total carbohydrate, fat and energy intakes significantly declined over time in both men and women, while there was a non-significant decrease in protein intake in men. The macronutrient recommendations in my paper with Helms & Fitschen2 encompassed the practices of the competitors in this study, with the exception of our protein recommendations (2.3-3.1 g/kg of lean body mass) being exceeded, most markedly by men who placed (consuming 3.0-3.3 g kg of total body mass). Alan Aragon’s Research Review – January 2018 Supplement use Here is the full table ranking the supplements used by the competitors. The top-5 supplements used collectively (starting from #1) were protein powder, multivitamin, BCAA (), creatine, and fat burners. Interestingly, pre-workout supplements just missed the top-5. Use of the top4 among these subjects is in line with previous research on bodybuilders.5 The authors of the present study noted that several competitors exceeded 400 mg supplemental caffeine per day, which is the limit of safety put forth by the European Food Safety Agency. In my observations, caffeine use (and abuse) is rampant among not just physique athletes, but athletes in general – both competitive and recreational. Concluding thoughts The authors acknowledge that misreporting (under-reporting carbohydrate & over-reporting protein) is a persistent confounder. Even with a meticulous population such as competitive bodybuilders, the accuracy of infrequent recall data is inherently challenged. Nevertheless, the findings of this study represent a thorough examination of the dietary habits of successfully competitive drug-free bodybuilders. References 1. 2. 3. 4. 5. Henselmans M, Schoenfeld BJ. The effect of inter-set rest intervals on resistance exercise-induced muscle hypertrophy. Sports Med. 2014 Dec;44(12):1635-43. [PubMed] Helms ER, Aragon AA, Fitschen PJ. Evidence-based recommendations for natural bodybuilding contest preparation: nutrition and supplementation. JISSN. 2014;11:20. [PubMed] Rohrig BJ, Pettitt RW, Pettitt CD, Kanzenbach TL. Psychophysiological tracking of a female physique competitor through competition preparation. Int J Exerc Sci. 2017 Mar 1;10(2):301-311. [PubMed] Garthe I, Raastad T, Refsnes PE, Koivisto A, Sundgot-Borgen J. Effect of two different weight-loss rates on body composition and strength and power-related performance in elite athletes. Int J Sport Nutr Exerc Metab. 2011 Apr;21(2):97-104. [PubMed] Hackett DA, Johnson NA, Chow CM. Training practices and ergogenic aids used by male bodybuilders. J Strength Cond Res. 2013;27:1609–17. [PubMed] [Back to Contents] Page 13 Study limitations Induced and controlled dietary ketosis as a regulator of obesity and metabolic syndrome pathologies. Gibas MK, Gibas KJ. Diabetes Metab Syndr. 2017 Nov;11 Suppl 1:S385-S390. [PubMed] BACKGROUND: A worsening epidemic of diabetes and its precursor, metabolic syndrome (MetS) is engulfing America. A healthy individual, with proper glucose regulation has an ability to switch between burning fat and carbohydrates. It has been suggested that signaling errors within this homeostatic system, characterized by impaired switching of substrate oxidation from glucose to fat in response to insulin, can contribute to the etiology of metabolic syndrome and occurs before the development of type II diabetes. Glucose regulation with restored insulin sensitivity facilitated through clinically regulated, benign dietary ketosis (BDK), may significantly reduce, regulate and reverse the adverse pathologies common to MetS and obesity. PURPOSE: The study assessed if prolonged maintenance of induced and controlled physiological, dietary ketosis, would reverse pathological processes induced by MetS including a reduction in fasting triglycerides, BMI (body mass index) and body fat mass (BFM), weight, a significant decrease and/or normalization of hemoglobin A1c (HgA1c) and an increase in resting metabolic rate (RMR) and blood ketones. DESIGN: A group of 30 adults, previously diagnosed with MetS by their primary care physician, were randomly prescribed to one of three groups: a sustained ketogenic diet with no exercise, standard American diet (SAD) with no exercise or SAD with 3-5 days per week of exercise (30 min.). RESULTS: The results demonstrated that the change over time from week 0 to week 10 was significant (p=0.001) in the ketogenic group for weight, body fat percentage, BMI, HgA1c and ketones. CONCLUSIONS: All variables for the ketogenic group out-performed those of the exercise and nonexercise groups, with five of the seven demonstrating statistical significance. SPONSORSHIP: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Study strengths A strength of this study was a 3rd group aside from the keto and control, which involved habitual diet plus exercise. In addition to the usual parameters, blood ketone levels & body fat were assessed at weeks 0, 3, 6, & 10. Note that there are issues with the chosen method of body composition assessment, as well as the exercise protocol, which I‘ll get to. Subjects met in small groups modeled after Clevelend Clinic‘s Share Medical Appointment (SMA) format, which presumably could have contributed to program troubleshooting and adherence. Alan Aragon’s Research Review – January 2018 There were a small number of subjects (10 per treatment – this is assumed since it was not explicitly stated in the manuscript, nor were any drop-outs reported). The reporting of important details was missing in this study, particularly the specifics of dietary intake. Energy and macronutritional intake were not specified for either the ketogenic or control groups. What was the nature of the subjects‘ habitual diet prior to undergoing the ketogenic diet? Who knows, but given that the subjects were diagnosed with the metabolic syndrome, there‘s a good chance that their dietary habits were crappy. But again, there‘s no way to know the nature of the total caloric decrease or the shift in macronutrition distribution from baseline/habitual diet to the ketogenic diet since none of this was reported in the manuscript. Adverse/side-effects? Nothing was reported. Furthermore, no details are reported about the group assigned 3-5 days per week of 30 minutes of exercise. What was the nature of the 30-minute bouts in terms of type and intensity? How well did they comply (how was adherence tracked)? The authors did not report this either. Body fat was assessed via hand-held bioelectrical impedance analysis (BIA). Although hand-held BIA is convenient and easy to use, it has repeatedly shown to have poor consilience with criterion (benchmark) methods. Esco et al4 found handto-hand BIA to significantly underestimate fat mass and overestimate lean mass in college-age female athletes when compared with the criterion method, duel X-ray absorptiometry (DXA). Varady et al5 found that hand-held BIA used on overweight women significantly underestimated fat mass and overestimated lean mass when compared to the criterion method, magnetic resonance imaging (MRI). It should be noted that the body composition assessment method used in the present study was single-frequency BIA, where the electrical current can pass through extracellular water (ECW), but cannot penetrate cell membranes. On the other hand, the more recently developed multi-frequency BIA (MF-BIA) divides the body into segments, and independently measures them at a range of frequencies, thus allowing the measurement of ECW, intracellular water (ICW), and total body water (TBW), which are then used to estimate body composition. MF-BIA has been shown to be more accurate than single-frequency BIA, having closer agreement with DXA.5,6 A little rant about the melodramatic tone of the paper I rarely encounter this in the peer reviewed literature (at least to this degree), but the tone of this manuscript is strongly indicative of bias. The manuscript oozes absolute fanfare for carbohydrate restriction. The unbridled fawning over an anticarbohydrate approach is palpable and cringe-worthy. I read it wondering how certain elements passed peer review. For [Back to Contents] Page 14 example, when discussing Feinman et al‘s review to tout the supremacy of aggressive carbohydrate restriction for diabetes,1 they went as far as appealing to the academic credentials of the paper: “Twelve points of clinical evidence were derived from the 26 researchers who are a combination of MD’s and/or PhD’s,” Furthermore, in the discussion section of their paper, two studies by Hall et al2,3 are falsely cited in attempt to bolster their case. The first one is a 2016 study2 where they reported Hall et al‘s finding of “increased EE, increased SEE and decreased RQ (Hall et al., 2016), which are correlates of glucose regulation with restored insulin sensitivity.” What they neglected to report was the most relevant finding of this study, which was that after switching to ketogenic diet (after 4 weeks on a conventional diet), body fat loss slowed, and this was accompanied by an increased loss of nitrogen, indicating a utilization/loss of bodily protein. The second by Hall et al they cited was a 2015 study3 where a 30% deficit (822 kcal) was imposed via either dietary fat or carbohydrate removal from the maintenance diet. The spotlight was put on the greater fat oxidation and lesser carbohydrate oxidation in the carb-restricted treatment. No mention was made of the primary finding, which was that the fat-restricted diet led to a significantly lower net fat balance throughout the study, leading to significantly greater body fat loss than the carbrestricted diet. Comment/application Oddly, it appears that bodyweight is reported in pounds instead of kilograms (judging by the high values in the range; 120-320 who-knows-whats), so who really knows what units of measurement were used to express the rest of the endpoints. The ketogenic diet showed statistically significant changes in all of those parameters except for two – TG and RMR – but the authors note that the changes in these markers were clinically meaningful despite not reaching statistical significance. The significant improvements seen in the ketogenic treatment are not surprising. As I discussed in my review of Kephart et al (earlier in this issue),7 a spontaneous decrease in energy intake as a result of going on a ketogenic diet has been seen repeatedly in the literature. The big question & challenge to ketogenic diet proponents is whether it‘s a sustainable long-term solution. Thus far, the literature collectively shows that adherence to a ketogenic diet (with a max of 50 g carbohydrate per day) diet diminishes over time. Non-ketogenic carbohydrate levels; roughly double the originally targeted intake or greater are consumed by the end of the most studies.8 An interesting finding was the lack of significant effect of exercise on any of the parameters. But to reiterate, no details about the nature of the exercise were reported. A target of 30 minutes 3-5 days per week could have been as insignificant as 3 moonlit walks, which could optimistically amount to about 300-500 kcal per week (easily negated by an extra few bites of food or an energy-dense snack in a single sitting). It‘s important to note that despite popular claims of exercise being useless for weight loss, a meta-analysis by Wu et al9 showed that that diet plus exercise caused significantly greater weight loss than diet alone. Importantly, this weight loss was greater in trials lasting a year or longer, indicating that exercise has a beneficial long-term effect on weight loss & weight loss maintenance. References As shown above as individual data points as well as the mean value as the thicker line (larger image here), the ketogenic diet treatment in red (compared to habitual diet and habitual diet plus exercise in blue and green, respectively) was the superior performer. Seven parameters were assessed: bodyweight, body fat, body mass index (BMI), glycated hemoglobin (HbA1c, a marker of glycemic control, in this study abbreviated as A1c), triglycerides (TG), resting metabolic rate (RMR), and ketones. Unfortunately, the units of measurement are not specified anywhere in the manuscript. 1. Feinman RD, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2015 Jan;31(1):113. [PubMed] 2. Hall KD, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. Am J Clin Nutr. 2016 Aug;104(2):324-33. [PubMed] 3. Hall KD, et al. Calorie for Calorie, Dietary Fat Restriction Results in More Body Fat Loss than Carbohydrate Restriction in People with Obesity. Cell Metab. 2015 Sep 1;22(3):427-36. [PubMed] 4. Esco MR, Olson MS, Williford HN, Lizana SN, Russell AR. The accuracy of hand-to-hand bioelectrical Alan Aragon’s Research Review – January 2018 [Back to Contents] Page 15 5. 6. 7. 8. 9. impedance analysis in predicting body composition in college-age female athletes. J Strength Cond Res. 2011 Apr;25(4):1040-5. [PubMed] Gába A, Kapuš O, Cuberek R, Botek M. Comparison of multi- and single-frequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of body composition in post-menopausal women: effects of body mass index and accelerometerdetermined physical activity. J Hum Nutr Diet. 2015 Aug;28(4):390-400. [PubMed] Thomson R, Brinkworth GD, Buckley JD, Noakes M, Clifton PM. Good agreement between bioelectrical impedance and dual-energy X-ray absorptiometry for estimating changes in body composition during weight loss in overweight young women. Clin Nutr. 2007 Dec;26(6):771-7. Epub 2007 Oct 23. [PubMed] Kephart WC, et al. The three-month effects of a ketogenic diet on body composition, blood parameters, and performance metrics in crossfit trainees: a pilot study. Sports 2018, 6(1), 1; doi:10.3390/sports6010001 [MDPI - Sports] Huntriss R, Campbell M, Bedwell C. The interpretation and effect of a low-carbohydrate diet in the management of type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Nutr. 2017 Dec 21. doi: 10.1038/s41430-017-0019-4. [PubMed] Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. dietonly interventions for weight loss: a meta-analysis. Obes Rev. 2009 May;10(3):313-23. [PubMed] Alan Aragon’s Research Review – January 2018 [Back to Contents] Page 16 Acknowledging the AARR contributors 10 years and counting: inception and legacy of AARR – and a big thanks to the contributors. By Alan Aragon _________________________________________________ The alpha and the omega In the beginning, AARR was an idea born from me spending roughly 3-6 hours a day answering questions and engaging in debates and discussions on the bodybuilding.com forums for approximately 5 years (2003-2008). Near the end of this period, in 2007, I gained the impetus to create a medium where I could better answer these questions and organize my ideas. There was no subscription-based research review that focused on nutrition and fitness, so I didn‘t have a solid sense of security about the idea. I chewed on the idea of doing AARR for a whole year before I actually launched it. This was a year of me worrying about whether or not it would succeed or fail, so I let fear keep me from going forward with releasing it. I eventually gained the courage to launch AARR at the start of 2008, and it has been going strong ever since then. In retrospect, it‘s pretty funny that I was afraid that the idea might flop, since at least 7 other research review subscription services were launched after AARR. At least 5 of these are still active, and all of them were directly inspired by AARR. Most of them cover research from the same allied fields in nutrition & exercise. All of this has profound implications in terms of AARR‘s impact and reach. AARR was instrumental in making research reviews a ―thing‖ – it started the domino effect of the brightest people in the allied fields starting their own research reviews. Did AARR make fitness-related research more appealing to practitioners and enthusiasts? Did AARR make science cool? I would like to think that it at least played a role in that, especially within the fitness industry. It‘s extremely gratifying that the impact has been positive, and it continues to grow through not just my work, but the work of others who have been inspired and influenced by AARR. While the other research reviews can be viewed as direct competitors, they can also be viewed as fraternal entities joined with me in the challenge of raising the educational bar of the industry. Collectively, we can empower practitioners and enthusiasts to achieve better results and better lives. Here‘s to the next 10 years of AARR. But before I forge ahead, I‘d like to thank the real heroes of of the publication. Alan Aragon’s Research Review – January 2018 The following list is comprised of all of the illustrious AARR guest authors since the beginning in 2008. The list is in alphabetical order of the author‘s last name, and the accompanying number is the number of times the person contributed (interviews, roundtables, and articles). Where possible, the name is linked to the person‘s website. A scant few of you are on ―stealth‖ mode from the rest of the world despite having written for my journal, and I can respect that. If your your name has no link, or a link that‘s not current, please contact me (support@alanaragon.com) with your updated connection to the planet, and I‘ll edit this article accordingly. Without further ado, here are the 109 heroes of AARR. Thank you all. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. Andrew Abbate (3) Joseph Agu (1) Ryan Andrew (1) Jay Ashman (1) Anoop Balachandran (1) Chris Bell (1) Miguel Blacutt (2) Adam Bornstein (1) Ian Capulet (2) Paul Carter (1) Andrew Chappell (1) Chi L. Chiu (2) James Clear (1) Sarah Conomacos (2) Bret Contreras (7) Christine Crumbley (1) JC Deen (2) Antony Dexmier (1) Brad Dieter (2) Aman Duggal (1) Steinar Ekren (3) Anya Ellerbroek (1) Sivan Fagan (1) Dell Farrell (1) Georgie Fear (1) James Fell (3) Peter Fitschen (2) Sergio Fontinhas (4) Kurtis Frank (2) Dan Garner (1) Evan Godbee (2) Chas Gonello (1) Jon Goodman (1) Molly Gregas (1) Louie Guarino (1) Jamie Hale (10) [Back to Contents] Page 17 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. 87. James Heathers (4) Eric Helms (10) Menno Henselmans (6) Joshua Hockett (2) Robert Hoenselaar (1) Anthony Howard-Crow (4) Mike Howard (2) Juma Iraki (1) Mike Israetel (2) Brian Jones (2) Alexander Ketterer (1) Kevin Klatt (2) Dylan Klein (2) Chelsea Knox (1) Evelyn Kocur (3) Justin Kompf (1) Bryan Krahn (5) James Krieger (6) Kedric Kwan (3) Adam Lawson (1) Roger Lawson II (1) Sohee Lee (2) Armi Legge (10) Sarah Lewis (4) Michael Limon (1) Martin MacDonald (1) Chris & Eric Martinez (2) Jason Maxwell (1) Lyle McDonald (8) John Meadows (2) Greg Mikolap (1) Joel Minden (2) Cameron Mochrie (2) Kasey Nadolsky (1) Spencer Nadolsky (4) Susy Natal (1) Mike T. Nelson (1) Pauline Nordin (1) Layne Norton (3) Greg Nuckols (1) Sol Orwell (1) Karen Pendergrass (1) Matt Perryman (3) Alex Ritson (1) Matthew Rizzo (1) Brandon Roberts (5) Chester Rockwell (1) Amber Evengeline Rogers (1) Tim Rowland (1) Jacob Schepis (1) Brad Schoenfeld (17) Alan Aragon’s Research Review – January 2018 88. Lou Schuler (2) 89. Sumi Singh (1) 90. Dean Somerset (1) 91. Brandon Stevens (1) 92. Jordan Syatt (1) 93. Dick Talens (1) 94. Russell Taylor (2) 95. Jason Tremblay (1) 96. Jorn Trommelen (1) 97. Nick Tumminello (2) 98. Adam Tzur (1) 99. Patrick Umphrey (2) 100. Tom Vachet (1) 101. Sten Van Aken (2) 102. Fredrik Tonstad Varvik (1) 103. Tom Venuto (2) 104. Jennifer Willett (1) 105. David A. Wiss (1) 106. Matt Woodward (1) 107. Ryan Zielonka (1) 108. Anastasia Zinchenko (1) 109. Mike Zourdos (1) [Back to Contents] Page 18 Blood lipids, diabetes, & obesity: clinical queries recklessly thrown at the Doc Who Lifts. Interview with Spencer Nadolsky _________________________________________________ There's a lot of talk amongst the low-carb/keto community about blood lipids - what's good, what's bad, what's illuminatti propaganda, etc. I'd like to discuss that. Let's begin with low-density lipoprotein (LDL) levels in the blood. LDL has traditionally be regarded as the "bad" cholesterol, while highdensity lipoprotein (HDL) has been called "good" cholesterol. Now, it seems that the LDL story has been complicated by claims that LDL particle size is what matters, since low-carb lore alleges that an increase in the "large, fluffy" LDL is not a threat to cardiovascular health. Another claim is along the lines of LDL particle number being what matters, regardless of type. What's your stance on this, and do you have any advice on what too look for in terms of lab results regarding HDL & LDL (in absolute amounts or ratio)? Hi Alan, the first thing we should do is get some nomenclature down. When we discuss LDL-C, we are talking about the cholesterol being carried by low density lipoproteins. When we discuss HDL-C, we are discussing the cholesterol being carried by high density lipoproteins. Cholesterol is carried by lipoproteins in the blood since it is not water soluble. Triglycerides are also carried this way. So for the smart AARR readers, just understanding this will give you the basics and understanding that most of the population doesn't have. Cholesterol is cholesterol. It is the lipoprotein carrying the cholesterol that makes the difference. Further, there are apolipoproteins that are bound that make up the lipoproteins. HDL particles have apolipoprotein A and the LDL particles and apolipoprotein B. Any particles that contain apolipoprotein B (also called apoB) are called apoB containing lipoproteins. All of these particles can cause atherosclerosis. So when someone says "bad" cholesterol when referring to LDL-Cholesterol, it's actually a misnomer. It is only "bad" due to being carried by a lipoprotein that can penetrate the artery lining (endothelium), become retained, and start the atherosclerosis process. Alan Aragon’s Research Review – January 2018 The story with HDL-Cholesterol being "good" is similar. The cholesterol contained in an HDL particle is the same as the LDL particle. It's just that the HDL particle does not cause atherosclerosis. Looking at simple pathophysiology would explain the story behind LDL particle size. Other much larger particles like chylomicron remnants up to 70 nm in diameter have been found to still penetrate the endothelium and be retained and cause atherosclerosis. The biggest fluffiest LDL particles are less than 40 nm. So why would particles that are about DOUBLE the size of the largest LDL particles be able to penetrate the endothelium and not the smaller LDL particles? That doesn't make too much sense. When controlling for size, the risk of cardiac events comes down to the particle number. It's possible that smaller and denser LDL particles are more readily retained and oxidized, but it's also true that those who have smaller LDL particles have insulin resistance which can accelerate atherosclerosis too. On a lipid panel that the doctor orders, you will see a total cholesterol, an HDL cholesterol, a triglyceride level, and usually a calculated LDL level. This standard test doesn't actually test the particle number or particle size. It's testing the cholesterol on each lipoprotein. The cholesterol amount usually correlates decently with the particle number but it can be discordant depending on the size of the particles. For instance, someone can have a lot of cholesterol on each lipoprotein (large but relatively fewer particles) and another person can have less cholesterol per particle but more particles with both of these people having the same cholesterol in total. If you did a particle test or checked their apoB levels (another more advanced test), you would see that the one person actually had more particles and is at a higher risk of atherosclerosis even though they technically had the same cholesterol levels. So when you look at the lipid panel your doctor ordered, you can actually calculate what is called their "non-HDLcholesterol". This is simply anything that is not HDL. Anything that isn't HDL is potentially atherogenic. You just take your total cholesterol and subtract the HDL cholesterol and you have your number. This cholesterol level has been found to be better than at predicting risk than just an LDL cholesterol level. This is of course if you can't check a particle count or apoB level. Thanks for going into detail with that. I must really love that question because I asked you something similar when I interviewed you in the July 2015 issue (I encourage the reader [Back to Contents] Page 19 to review that issue, Spencer discusses different aspects of this question). Let's shift gears for a minute and talk about beetus. I recently became aware of a less-common diagnosis of diabetes: type 3c. Can you give us the run-down on this - i.e., what are the most important aspects of it that academics, practitioners, and clinicians (basically the AARR readership, which includes enthusiasts) need to know about type 3c, as well as aspects that might have flown under the radar of articles in the lay press? Nowadays when most people think of diabetes (diabetes mellitus), they think of type 2 (excess weight, insulin resistance, etc.) since it accounts for 90-95% of cases. Type 1 diabetes mellitus (autoimmune disease, lack of insulin due to destruction of pancreas) makes up most of the rest of the cases. There are other genetic types out there that don't fit into those categories too. Then there is type 3c, which as you mentioned has been mentioned due to a relatively new report out saying it is often misdiagnosed. Type 3c, also known as pancreatogenic, diabetes occurs due to inflammation of the pancreas. In medicine this inflammation in the pancreas is known as pancreatitis to which there are many causes like alcohol, gallstones, really high triglycerides, etc. Sometimes those with multiple bouts of pancreatitis or chronic pancreatitis will have enough destruction of their pancreas to where they are not producing enough insulin. Most doctors will assume when they see these patients that it is type 2 diabetes since it occurs usually later in life (unless they have cystic fibrosis) and the insulin requirements may be low (at first). If the doctor starts thinking about autoimmune type 1 diabetes, the antibodies will be negative. When most people think of the pancreas, they think of insulin. However the pancreas makes digestive enzymes too! This is the exocrine role of the pancreas. Those with chronic pancreatitis may have develop some pancreatic insufficiency, which is where you don't produce enough digestive enzymes to help with food digestion. So a clue that someone may have type 3c (pancreatogenic) diabetes is if they have had chronic pancreatitis in the past and also have pancreatic insufficiency, which can be tested for if not diagnosed yet. So if you have someone who has had pancreatitis and doesn't seen to fit the picture of type 2 diabetes, keep this in mind! Thanks for the low-down on type 3c. Speaking of diabetes, type 2 is the most prevalent worldwide, and has been a growing problem. There is a mountain of literature on type 2 diabetes, but there's also a raging battle going on over how to treat and manage the disease. I'd like to hear your take on Alan Aragon’s Research Review – January 2018 how to pull diabetics (and prediabetics) back into normal glycemic control. One camp - which includes the major public health organizations - does not delineate a "diabetes diet" per se. It largely resembles traditional. mainstream recommendations. An opposing camp - the low-carb/keto proponents view carbohydrate restriction as a first line of defense, seeing it as not only the most effective solution, but also the most obvious and logical solution (to the point that they are collectively furious at the establishment for being either blind or in willful denial of this). I also see a third camp, I'll call it the Roy Tayor camp, whose approach involves an initial very-low-calorie formula diet intervention (~800 kcal for 3-5 months) with the goal of substantial weight reduction (~15 kg if possible), followed by a maintenance phase that gradually reintroduces solid foods. The interesting thing to me about Taylor's model is the remission of T2D occurring in a large percentage of the subjects despite the maintenance phase not being specifically focused on carbohydrate restriction. I'd love to hear your take on what's the optimal approach to take with pre-T2Ds and T2Ds. I never understand why these camps can't just look at the data objectively and use it to treat each patient as an individual. For example, I have had some patients come off insulin and other diabetes medicines gradually after slowly implementing a diet and exercise program. I have also had some with great success with a low carb high fat diet. I have also had amazing results with the really aggressive very low calorie diet (Roy Taylor style) getting patients off 100 units of insulin within a month. If the camps would take a more patient-centered approach, they would understand it comes down to patient preferences in conjunction with working with a doctor to guide them with evidence-based practice. Let's say a patient comes in on high doses of insulin and all you have to offer is a ketogenic diet. I do agree this is an option, but what if the patient simply hates that style of eating (most do by the way)? You just keep them on insulin and send them away with no other options? Maybe a more moderate approach with them would have been enough to get the ball rolling. The more aggressive approaches do work phenomenally, but our patients aren't robots that we just program to follow a certain diet. Having said that, I am going to tell you my approach. If a patient is willing, I go as aggressive as possible. I actually use a very low calorie protein-sparing modified fast diet [Back to Contents] Page 20 compared to the lower protein version in Dr. Taylor's protocol. This is because a bro will always be a bro and protein shakes are life. It's nothing short of amazing what happens with the patient's blood sugars and insulin requirements. As mentioned I have been able to quickly take people off high amounts of insulin. I also don't skimp on the exercise like they did in the Dr. Taylor protocol. This first option is extremely hard to follow though. It's not for everyone. But as you mentioned and from the Direct study, the patients may be able to back to eating more carbohydrate than if they just followed a ketogenic diet from the beginning. I think this is important when looking at the pathogenesis of type 2 diabetes. Keto proponents will say they "reverse" or "cure" type 2 diabetes with a ketogenic diet, but really what they are doing is managing it in a different way. If you gave these patients a glucose load, they would likely not do well with it. This is why I reserve a ketogenic diet for patients who have had type 2 diabetes for 10 or more years and are on high amounts of insulin. Their beta cell function of their pancreas may never recover even with a very low calorie diet, so in these instances a very low carb diet may do the trick. Again, if the patient doesn't want to follow it, it won't matter. These two approaches aren't usually needed for most patients though. If I can help a patient get into an energy deficit while also eating higher quality foods and adding a good exercise plan, they do very well. Not all patients are on high amounts of insulin and need an aggressive approach like the very low calorie or ketogenic diet. This especially goes for those with prediabetes. They just need some simple changes in their lifestyle that most of the readers of AARR could help them do. I really like the idea of a Taylor-esque protocol whose VLCD intervention phase is optimized with higher protein (and exercise). It just makes a ton of sense all the way around. Especially for patients who require aggressive treatment. Speaking of clinical interventions, in the November 2008 issue of AARR, I looked at tesofensine, since it seemed promising, and at the time, appeared to be the most effective weight loss drug. I'd like to know how you feel about treating obesity with such therapies. Are anti-obesity drugs ever necessary beyond proper diet & exercise -- in your personal observations with patients? How do you see the landscape looking for antiobesity drugs in general? Alan Aragon’s Research Review – January 2018 Tesofensine has yet to come to the market, but others have with decent results. As an obesity specialist physician, I believe these drugs do fill a gap between behavioral therapy and surgery for weight loss. The truth is, most people will fail a diet and exercise program. I know the readers of AARR may be angry at hearing that, but it's just a fact and clear in the data. What do we do then? Just keep telling these patients they need to try harder or keep doing the same things that have failed in the past? When you look at the pathophysiology in obesity, you will find it's not a simple dysfunction. Individuals may have excessive hunger when trying to lose weight compared to others. Some may have more reward center dysfunction, which leads to being drawn more towards those addictive-like foods (e.g. cookies, cakes, etc.). Do we tell them they just need to have more will power and eat more vegetables and lean protein? Sure, doing those things will help, but sometimes the patients need more. I have had patients who swear up and down that they are eating 1200 calories, yet have an RMR of about 2000 calories and absolutely no medical issue that would prevent them from losing weight (e.g. Cushing's disease etc.). When I put these individuals on an anti-obesity medicine, they often times lose at least 10% of their body weight. The medicines available only work on appetite and not peripherally like an uncoupler, which would boost metabolism. So clearly these patients were just eating much more than they knew or were willing to admit and this came from appetite. There are several medicines available for long-term obesity management. Each work at various receptors in the brain to increase satiety. While some believe you shouldn't need a medicine for weight loss, the paradigm is shifting to thinking about obesity as a chronic condition or disease just like hypertension or type 2 diabetes. You may need a medicine in those conditions for long-term so why not use medicines long-term in obesity? Of course there are responders and non-responders to each medicine as well. For example one patient may not lose any weight with one medication and then lose 30-40 pounds with another. We shouldn't keep patients on these medicines unless there is a real response, due to possible side effects (should be true for any medicine). [Back to Contents] Page 21 With this in mind, I don't want to belittle the behavioral component of obesity management either. I have had patients who had gastric bypass surgery (the most powerful tool we have in obesity management) and are on 3 different medicines for appetite, and they still say they can't help themselves from buying candy bars at the store. Clearly the physiology doesn't matter in these cases. In order to keep people active, it is very similar to keeping people on their nutrition. They really have to buy into the lifestyle. Almost obsessed, but without that negative connotation. See those initial changes of their body, strength, and energy and the patients start to love it. That initial barrier is tough though and getting them comfortable with a good support system is important. The medicines are simply a tool to help someone adhere to their lifestyle plan just like surgery would be too. ________________________________________________________________________________________________________ Agreed that appetite is the issue, along with underestimation (and of course under-reporting, willful or not) of intake. You have a unique spot in the fitness space being a doctor who has one foot in the clinic and another foot in the gym. Knowing the value of exercise, what's your approach to getting sedentary patients to become physically active (given that they have the capacity to do so)? This is a big stumbling block with a lot of folks, especially those who have no clue what to do, or are intimidated by gyms. Maytbe a more important question is, how to you get patients to become physically active, and STAY physically active? We all are familiar with the false-start exercise warrior. Dr. Spencer Nadolsky is a board certified family and obesity medicine physician. While earning a BA in exercise science In undergrad he wrestled heavyweight for the UNC Tar Heels and was ranked as high as 3rd in the nation at one point while also garnering Academic All-American status. He is the author of the fat loss prescription and now runs an online weight loss program called the fat loss prescription program More of Spencer’s stuff can be found at http://drspencer.com Since exercise is probably the best all-around "drug" or therapy available, I always ask my patients about how much and what type they do. As you mentioned, most are sedentary and the barrier to get started seems high for them. I always recommend walking, especially after meals to start if they can. Ideally my patient would all start lifting combined with some aerobic training. What I found universally is that most people just don't know what they are doing when it comes to weights. Not a clue. Not only that but the gym is a very intimidating place. I can relate because when I started swimming (really looked like drowning according to my wife), I was very self conscious at the pool. Everyone looked like they knew what they were doing and I was a bit lost. Despite looking great in my speedo, I was by far the worst swimmer in there. The 80 year old women were lapping me. Anyway, since many of my patients were scared to go to the gym and didn't know what they were doing, I just started going with them and showing them. I would show them that the weights weren't scary or too complicated and give them the simplest of plans. I was even fortunate enough to help set up a group exercise class at the hospital so that the patients would be even more comfortable working out with others in their same stage. Alan Aragon’s Research Review – January 2018 [Back to Contents] Page 22 ―Everyone has biases and everyone has various conflicts of interest. My biggest conflict of interest: I am paid to work with elite athletes to try and make them go faster in Olympic events within the rules of sport, with primary emphasis on middle to long distance Olympic events. Now if there was evidence that all I needed to do was change and shift an athlete‘s macronutrient intake to LCHF [low-carb, high-fat] to turn them into world beaters do you not think I would immediately do this? Am I an idiot? (Yes, opening up to cheap shots here). Do I not follow, read, conduct, publish, review and edit research? Do I not constantly have my ear to the elite athlete/coach ground? Am I just completely out of touch? A keto intervention is a relatively inexpensive (as one already needs to buy food) and easier intervention compared to money spent on various other performance interventions in technology, engineering, supplements, biomech, training camps, altitude etc etc etc. In this instance I would take any form of evidence for keto in elite athletes. Can someone please show me a definitive study in elite athletes in Olympic sports featuring sustained energy outputs >2min of duration where keto works (both training and competition)? Can someone please introduce me to a keto Olympic medalist in Olympic sports featuring sustained energy outputs >2min of duration? My athletes don‘t need to lose weight. My athletes don‘t need to have better insulin sensitivity. My athletes need to go faster. That is it. The evidence (literature, anecdotal or not) is seriously lacking at this point for the elite cohort I work with. If the evidence changes, I will be very happy to change and integrate this intervention in my toolkit.‖ — Trent Stellingwerff via Twitter If you have any questions, comments, suggestions, bones of contention, cheers, jeers, guest articles you‘d like to submit for consideration, send it over to support@alanaragon.com. Alan Aragon’s Research Review – January 2018 [Back to Contents] Page 23