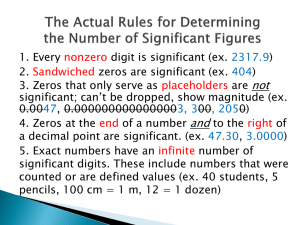
F QCF258 Science 2 QCF258 Science 2 Working Scientifically: Measurements P a g e |1 Significant figures and SI prefixes Significant figures in measurements Measurements are not exact. Any measurement involves an estimate and is therefore uncertain to some extent. We indicate the degree of certainty for a particular measurement by the number of significant figures (sf) that we use. How do we know which numbers are significant? All non-zero digits are significant. e.g. 345 has 3 sf Zeros before non-zero digits are not significant. e.g. 0.0045 has 2 sf 0.980 has 3 sf Zeros between non-zero digits are significant. e.g. 30 009 has ______________ 0.9005 has ______________ Zeros after the decimal point and before a non-zero digit are not significant. e.g. 0.00056 has ______________ 0.07 has ______________ Zeros after the decimal point and after a non-zero digit are significant. e.g. 0.9000 has ______________ 3.450 has ______________ When a number ends in zeros without a decimal point, the zeros may or may not be significant. e.g. 130 has 2 or 3 sf 1000 has ______________ 0.56 has 2 sf Scientific notation Scientific notation gives an unambiguous way to represent significant figures. When using scientific notation, any number given is significant. e.g. 2.000 x 105 has 4 significant figures 200 000 may have 1 to 6 significant figures Don't confuse scientific notation with exponents and powers! For example, one million is 1 000 000. This is 10 raised to the power of 6. It must be written as 1 x 106. You can’t write one million as 16 since this would be equal to 1x1x1x1x1x1 = 1! QUT International College CRICOS No. 00213J QCF258 Science 2 Working Scientifically: Measurements P a g e |2 Using significant figures in calculations In calculations, the accuracy of the answer is limited by the least accurate measurement. 1. For multiplication or division, the number of significant figures in the final answer is the same as the smallest number of significant figures provided. e.g. 5.18 x 0.02145 = ______________ 2. 7.900 x 0.041 = ______________ For addition or subtraction, the number of significant figures in the answer is based on the one with the smallest number of decimal places. e.g. 116.8 – 0.33 = _____________ 21 - 13.8 + 130.36 = _____________ Note! You can have errors in your answer if you round-off in the middle of a calculation. Don’t round until you get to the final answer! Learning Check How many moles of KCl in 37.273 g? Learning Check 1. Rewrite the following experimental readings to the stated number of significant figures: a) 0.001345 g to 3 sf b) 0.000375 g to 2 sf c) 13697 mL to 3 sf d) 1204 L to 3 sf e) 52146 g to 1 sf f) 21.02 mL to 3 sf 2. How many significant figures would be in the final answer for the following operations? Note that there is no need to work out the value! a) 3.246 x 12.5 ÷ 2.1 b) 51.234 + 3.65 – 1.4 c) (2.34 + 20.245 + 1.0) ÷ 2.4 d) (0.15 + 100.0 – 2.45) x 3.456 QUT International College CRICOS No. 00213J QCF258 Science 2 Working Scientifically: Measurements P a g e |3 SI prefixes There is an International System of Units. The International System is called SI, using the first two initials of its French name Système International d'Unités. The SI units were first chosen in 1875. Forty-eight countries have now signed this agreement, with the units reviewed every few years during an international conference. The metre is the standard (or SI) unit of length. We place a prefix in front of the metre to indicate units that are smaller or larger than a metre. For example: A kilometre is equal to 1000 metres. We write this as km. A nanometre is equal to 0.000000001 metres. We write this as 1 nm or 1 x 10-9 m. The most common SI prefixes are listed below: Prefix tera giga mega kilo hecto deci centi milli micro nano pico Symbol T G M k h d c m µ n p Multiplication Factor 10 12 10 9 10 6 10 3 10 2 10 -1 10 -2 10 -3 10 -6 10 -9 10 -12 Decimal Equivalent 1 000 000 000 000 1 000 000 000 1 000 000 1 000 100 0.1 0.01 0.001 0.000001 0.000000001 0.000000000001 Be careful with using capital letters in the prefix symbol – mL is not the same as ML !! We use prefixes in chemistry mostly for small measurements, like the following: Items that can be seen - macroscopic 3 mm to 5 mm 0.5 mm to 3 mm skin wrinkles, shapes of fingerprints, salt crystals, eyelashes sand grains, dust, tiny insects See Blackboard for worked solutions QUT International College Items that cannot be seen - microscopic 0.1 mm details of salt crystal shapes, details on a silicon chip 10 to 100 µm cells, bacteria 0.3 to 1 µm viruses, DNA 1 nm molecules, atoms 1 pm atomic nuclei 0.1 fm matter inside a proton Visit Blackboard for online revision activities CRICOS No. 00213J QCF258 Science 2 Working Scientifically: Measurements P a g e |4 Solutions Significant figures in measurements Measurements are not exact. Any measurement involves an estimate and is therefore uncertain to some extent. We indicate the degree of certainty for a particular measurement by the number of significant figures (sf) that we use. How do we know which numbers are significant? All non-zero digits are significant. e.g. 345 has 3 sf Zeros before non-zero digits are not significant. e.g. 0.0045 has 2 sf 0.980 has 3 sf Zeros between non-zero digits are significant. e.g. 30 009 has _____ 5 sf _________ Zeros after the decimal point and before a non-zero digit are not significant. e.g. 0.00056 has _____ 2 sf _________ 0.07 has _____ 1 sf _________ Zeros after the decimal point and after a non-zero digit are significant. e.g. 0.9000 has ______ 4 sf ________ 3.450 has ______ 4 sf ________ When a number ends in zeros without a decimal point, the zeros may or may not be significant. e.g. 130 has 2 or 3 sf 1000 has ____ 1, 2, 3 or 4 sf __________ 0.56 has 2 sf 0.9005 has _____ 4 sf _________ Scientific notation Scientific notation gives an unambiguous way to represent significant figures. When using scientific notation, any number given is significant. e.g. 2.000 x 105 has 4 significant figures 200 000 may have 1 to 6 significant figures Don't confuse scientific notation with exponents and powers! For example, one million is 1 000 000. This is 10 raised to the power of 6. It must be written as 1 x 106. You can’t write one million as 16 since this would be equal to 1x1x1x1x1x1 = 1! QUT International College CRICOS No. 00213J QCF258 Science 2 Working Scientifically: Measurements P a g e |5 Using significant figures in calculations In calculations, the accuracy of the answer is limited by the least accurate measurement. 3. For multiplication or division, the number of significant figures in the final answer is the same as the smallest number of significant figures provided. e.g. 5.18 x 0.02145 = 0.111111 = 0.111 3 sf 4 sf ∴ 3 sf 4. 7.900 x 0.041 = 0.3239 = 0.32 4 sf 2 sf ∴ 2 sf For addition or subtraction, the number of significant figures in the answer is based on the one with the smallest number of decimal places. e.g. 116.8 – 0.33 = 115.87 = 115.9 1 dp 2 dp ∴ 1 dp 21 - 13.8 + 130.36 = 137.56 = 138 no dp 1 dp 2 dp ∴ no dp Note! You can have errors in your answer if you round-off in the middle of a calculation. Don’t round until you get to the final answer! Learning Check How many moles of KCl in 37.273 g? 𝑀𝑀𝑀𝑀𝑀𝑀𝑀𝑀𝑀𝑀 𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚 𝑜𝑜𝑜𝑜 𝐾𝐾𝐾𝐾𝐾𝐾 = 39.10 + 35.45 = 𝟕𝟕𝟕𝟕. 𝟓𝟓𝟓𝟓 𝒈𝒈 𝒎𝒎𝒎𝒎𝒎𝒎−𝟏𝟏 2 dp 2 dp ∴ 2 dp 5 sf 𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚 37. 273 𝑀𝑀𝑀𝑀𝑀𝑀𝑀𝑀𝑀𝑀 𝑜𝑜𝑜𝑜 𝐾𝐾𝐾𝐾𝐾𝐾 = = = 0.4999731724 = 𝟎𝟎. 𝟓𝟓𝟓𝟓𝟓𝟓𝟓𝟓 𝒎𝒎𝒎𝒎𝒎𝒎 𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚 𝑚𝑚𝑚𝑚𝑚𝑚𝑚𝑚 74.55 4 sf ∴ 4 sf Learning Check Do not forget units for measurements! You will be penalised in assessment tasks for missing units. 1. Rewrite the following experimental readings to the stated number of significant figures: g) 0.001345 g to 3 sf 0.00135 g h) 0.000375 g to 2 sf 0.00038 g i) 13697 mL to 3 sf 13 700 mL j) 1204 L to 3 sf 1200 L k) 52146 g to 1 sf 50 000 g l) 21.02 mL to 3 sf 21.0 mL 2. How many significant figures would be in the final answer for the following operations? Note that there is no need to work out the value! e) 3.246 x 12.5 ÷ 2.1 2 sf f) 51.234 + 3.65 – 1.4 1 dp (∼56.x) ∴ 3 sf g) (2.34 + 20.245 + 1.0) ÷ 2.4 addition: 1 dp ∴ 3 sf (∼ 23.x) 2 sf h) (0.15 + 100.0 – 2.45) x 3.456 addition: 1 dp ∴ 3 sf (∼ 97.x) 3 sf QUT International College CRICOS No. 00213J