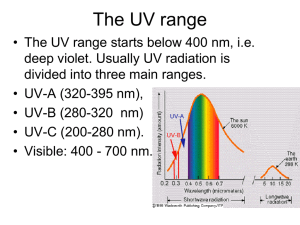
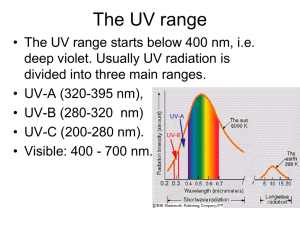
Chemistry Final Exam Study Guide Group 1: alkali metals Group 2: alkaline earth metals Group 15: pnictogens Group 16: chalcogens Group 17: halogens Group 18: noble gases Ionic compounds-composed of oppositely charged ions o Electrons are either added or subtracted from atoms to form ions Molecular compounds-composed of molecules, which are made of atoms Atomic number-number of protons Mass number-number of protons and neutrons Metal + nonmetal = ionic Nonmetal + nonmetal = covalent Troposphere-lowest region of the atmosphere in which we live o Contains 75% of our air by mass Air o 78% nitrogen o 21% oxygen o 1% other gases Air inversions-cooler air can be trapped beneath warmer air due to weather conditions o Pollutants often accumulate in the cooler air of an inversion layer Molecule-fixed number of atoms held together by chemical bonds in a certain spatial arrangement Chemical formula-symbolically represents the type and number of each element present Risk assessment-evaluating scientific data and making predictions in an organized manner about the probabilities of an occurrence Toxicity-intrinsic health hazard of a substance Exposure-the amount of the substance encountered Chemical reactions-characterized by the rearrangement of atoms when reactants are transformed into products Law of Conservation of Mass-number of atoms on each side of the arrow must be equal Balancing equations o If an element is present in just one compound on each side, balance it first o Balance free elements last o Balance polyatomic ions as a unit Incomplete combustion-if the amount of oxygen is altered, the hydrocarbon can burn incompletely Ozone-a secondary pollutant produced from one or more other pollutants Green chemistry-reduces pollution through design/redesign of chemical processes o Use less energy, create less waste, use fewer resources, use renewable resources Radiation o Short-range includes UV, X-rays, and gamma rays o Causes rupture of molecular bonds o Visible-ROYGBIV; used for photosynthesis and solar applications o Infrared-longest of the visible spectrum; causes molecules to bend and stretch o Microwaves-cause molecules to rotate Electromagnetic radiation o Shorter wavelength = higher frequency o Wavelength-distance traveled between successive peaks o Frequency-number of waves passing a fixed point in one second Of the sun’s radiation reaching Earth, approximately 53% is infrared, 39% is visible light, and 8% is UV Radiation may be described as having both wave-like and particle-like properties o The energy of radiation is quantized, with only certain values of energy being allowed, rather than an energy continuum Energy of radiation is related to its wavelength and frequency o Energy is proportional to frequency o Energy is inversely proportional to wavelength UV radiation-has sufficient energy to cause certain molecular bonds to break o UVA-lowest energy o UVB-medium energy o UVC-highest energy o Skin damage by UV When UV radiation is absorbed by skin, lower-energy UVA light removes electrons from molecules such as water, creating free radicals and other reactive oxygen species Higher-energy UVB light causes some chemical bonds to break DNA molecules are damaged by free radicals or UVB absorption This results in the release of melanin, the compound that gives skin its color o Biological effects of UV exposure UVC radiation is highly energetic and is absorbed completely by oxygen molecules (causes O=O double bonds to break) UVB is partially absorbed by ozone in the stratosphere (breaks O-O single bonds in ozone) Some UVB reaches Earth’s surface, where it is rapidly absorbed at the surface of the skin UVA is not absorbed by the atmosphere; since it is less energetic than UVB, it penetrates deeper into skin, causing more damage to underlying tissue o Skin cancer Most skin cancers are linked to exposure to sunlight Can appear at any age, but is more common in older people Can develop many years after repeated, excessive exposure has stopped The UVA in sunlight at Earth’s surface is most strongly linked with skin cancers; however, UVB may also play a role Cancer can arise in different types of skin cells; those in basal or squamous cells are common, but seldom fatal; cancers in melanocyte cells (melanoma) are most deadly Although some UV exposure is needed for production of vitamin D, too much exposure leads to cancers and eye disease o UV index Color-coded UV index scale is used to predict the risk of sunburn from overexposure to UV light from the sun Important factors considered by most UV index forecasts: The ozone concentration in the upper atmosphere Elevation Cloud cover Ozone layer-a region in the stratosphere with maximum ozone concentration o Concentration of 12,000 ppb in the stratosphere o Concentration of 20-100 ppb in the troposphere o The ozone layer provides a natural source of protection against harmful UVB radiation o Ozone can be naturally destroyed by dissociation of water molecules into free radicals o Data suggests that something beyond natural sources was responsible for destroying stratospheric ozone Chlorofluorocarbons (CFCs)-nontoxic, nonflammable, inexpensive, and widely available o Have revolutionized the air conditioning industry o These molecules are broken apart by UVC radiation in the upper atmosphere; the resulting atomic chlorine results in ozone destruction through a series of reactions o The lower stratosphere over the South Pole is the coldest spot on Earth o This creates stratospheric clouds (PSCs), which help support the chemical reactions that produce active chlorine that catalyzes ozone destruction Montreal Protocol o 1987 o Caused the production of CFCs to plummet o Recovery of ozone concentrations is slow, with atmospheric chlorine concentrations not expected to return to 1980 levels in Antarctica until the year 2070 Alternatives to CFCs o Hydrofluorocarbons (HFCs) replace chlorine with hydrogen to prevent the release of atomic chlorine, which catalyzes ozone destruction o HFCs are greenhouse gases, which play a role in climate change