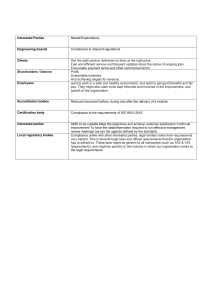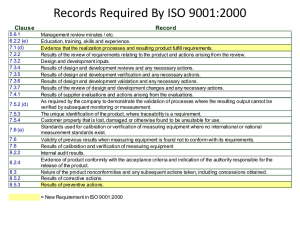
UNIT 6 CLAUSE WISE INTERPRETATION OF ISO 9001:2000 Clause Wise Interpretation of ISO 9001:2000 Structure 6.0 6.1 6.2 Objectives Introduction Clause-wise Explanation of ISO 9001:2000 6.2.1 6.2.2 6.2.3 6.2.4 6.2.5 6.2.6 6.2.7 6.2.8 6.3 6.4 6.5 6.6 Clause 1: Scope Clause 2: Normative Reference Clause 3: Terms and Definitions Clause 4: Quality Management System Clause 5: Management Responsibility Clause 6: Resource Management Clause 7: Product Realization Clause 8: Measurement, Analysis and Improvement Let Us Sum Up Key Words Answers to Check Your Progress Exercise Suggested Reading 6.0 OBJECTIVES After reading this unit, we shall be able to: • state the requirements of each clause of ISO 9001: 2000; • deduce the interrelationship between different clauses; • explain the mandatory procedures required to be maintained; and • specify the mandatory records required to be maintained. 6.1 INTRODUCTION The ISO 9001:2000 uses the process approach to implement the Quality Management System (QMS). The process approach includes: (i) identifying the processes necessary for the effective implementation of the quality management system, (ii) understanding the interactions between these processes and (iii) documenting the processes to the extent necessary to assure their effective operation and control documenting. The standard has eight clauses. The interpretation of each requirement manifested in different clauses is explained with the aim of suitably deploying the same in respective food safety related application areas. 6.2 CLAUSE-WISE EXPLANATION OF ISO 9001:2000 The ISO 9001:2000 outlines the requirements of the quality management systems. The Quality Management System. The eight clauses are as follows: 1. Scope 1.1 General 1.2 Application 47 ISO 9001:2000 2. Normative Reference 3. Terms and Definitions 4. Quality Management System 5. 6. 7. 48 4.1 General Requirements 4.2 Documentation Requirements 4.2.1 General 4.2.2 Quality Manual 4.2.3 Control of Documents 4.2.4 Control of Records Management Responsibility 5.1 Management Commitment 5.2 Customer Focus 5.3 Quality Policy 5.4 Planning 5.4.1 Quality objectives 5.4.2 Quality Management System Planning 5.5 Responsibility, Authority and Communication 5.5.1 Responsibility and Authority 5.5.2 Management Representative 5.5.3 Internal Communication 5.6 Management Review 5.6.1 General 5.6.2 Review input 5.6.3 Review output Resource Management 6.1 Provision of Resources 6.2 Human Resources 6.2.1 General 6.2.2 Competence, Awareness and Training 6.3 Infrastructure 6.4 Work Environment Product Realization 7.1 Planning of Product Realization 7.2 Customer-related Processes 7.2.1 Determination of Requirements Related to the Product 7.2.2 Review of Requirements Related to the Product 7.2.3 Customer Communication 7.3 Design and Development 7.3.1 Design and Development Planning 7.3.2 Design and Development Inputs 7.3.3 7.3.4 7.3.5 7.3.6 7.3.7 8. Design and Development Outputs Design and Development Review Design and Development Verification Design and Development Validation Control of Design and Development Changes 7.4 Purchasing 7.4.1 Purchasing Process 7.4.2 Purchasing Information 7.4.3 Verification of Purchased Product 7.5 Production and Service Provision 7.5.1 Control of Production and Service Provision 7.5.2 Validation of Processes for Production and Service provision 7.5.3 Identification and Traceability 7.5.4 Customer Property 7.5.5 Preservation of Product 7.6 Control of Monitoring and Measuring Devices Clause Wise Interpretation of ISO 9001:2000 Measurement, Analysis and Improvement 8.1 General 8.2 Monitoring and Measurement 8.2.1 Customer Satisfaction 8.2.2 Internal Audit 8.2.3 Monitoring and Measurement of Processes 8.2.4 Monitoring and Measurement of Product 8.3 Control of Non-conforming Product 8.4 Analysis of Data 8.5 Improvement 8.5.1 Continual Improvement 8.5.2 Corrective Action 8.5.3 Preventive Action 6.2.1 Clause 1: Scope The contents and interpretation are given here. 1.1 General ISO 9001:2000 International Standard specifies requirements for quality management system. The requirements covers the following: • • Demonstrating its ability to consistently provide product which meets customer and applicable regulatory requirements, and Aiming to enhance customer satisfaction through the effective application of the system, including processes for continual improvement of the system and the assurance of conformity to customer and applicable requirements. The term “product” means the product intended for, or required by, a customer. 49 ISO 9001:2000 1.2 Application All requirements of ISO 9001:2000 are generic and are intended to be applicable to all organisations regardless of type, size and product provided. Where any requirement of ISO 9001:2000 standard cannot be applied due to the nature of an organisation and its product, this can be excluded from the scope. However the requirements falling under clauses 4, 5, 6 and 8 cannot be excluded. The rationale for exclusion must be established. Such exclusions must not affect the organisation’s ability, or responsibility, to provide product that meets customer and applicable regulatory requirements. 6.2.2 Clause 2: Normative Reference The contents and interpretation are given here. ISO 9000:2000, Quality management systems – Fundamentals and Vocabulary is the reference document for ISO 9001:2000 standard. 6.2.3 Clause 3: Terms and Definitions The contents and interpretation are given here. For the purposes of this International Standard, the definitions given in ISO 9000:2000 apply. Throughout ISO 9001:2000 standard, whenever the term “product” occurs, it can also mean “service”. 6.2.4 Clause 4: Quality Management System The contents and interpretation are given here. 4.1 General Requirements The company shall establish, document, implement and maintain a quality management system. It shall also need to continually improve it. The company must: • Identify the processes needed by the system to enable them to meet customer requirements and identify their application toward this task. This includes broad business processes as well as work processes, 50 • Determine the sequence and any interaction between these processes (It needs to map out the processes), • Determine criteria and methods by which will ensure proper operation and control of these processes. For determining criteria the company can focus on outputs and objectives of the process concerned, • It must measure the performance of the processes as a tool to continuously improve them, • Ensure availability of resources of all kinds to support these processes to make sure that everything that is needed is available, otherwise this commitment to a quality system is futile, • Monitor, measure and analyze these key processes, and • Implement actions necessary to achieve planned results (objectives with respect to effectiveness, efficiency, cost etc.). Clause Wise Interpretation of ISO 9001:2000 If the company chooses to outsource any of the processes, it must ensure that it retains control over them. These controls should be identified within the company’s quality system. This may include how to control subcontract work, printing of material before delivery to client, installation of the goods to make sure that the customer is happy when they think the job is done. 4.2 Documentation Requirements 4.2.1 General: The quality management system documentations system must include: • Documented statements of quality policy and quality objectives. • A quality manual (which will explain how the company has planned to fulfill the requirements of ISO 9001:2000 standard.). • Documented procedures required by ISO 9001:2000. There are six of them. • Documents required by the company to ensure effective planning, operation and control of the processes. This consists of process profiles (which outlines the characterization of identified processes in terms of objectives, resources, roles, responsibilities, inputs, outputs, procedures, suppliers, customers, monitoring, documents and records pertaining to the process concerned) and associated documents. • Quality records required by ISO 9001:2000. 4.2.2 Quality manual: The organisation must establish a quality manual that includes: • The scope of the quality system including details of/justification for any exclusions. • The documented procedures or reference to them. • A description of the interaction between the processes. You may use any topological representation e.g. charts, tables, schematics, relation diagram etc., for the same. The generation, use and control of documentation should be evaluated with respect to the effectiveness and efficiency of the organisation against following criteria: • Functionality (e.g. speed of processing), • User friendliness, • Resources needed, • Policies and objectives, • Current and future requirements related to managing knowledge, • Benchmarking of documentation system, and • Interfaces used by organisation’s customers, suppliers and other interested parties. 4.2.3 • Control of documents: Documents must be controlled. A documented procedure must be established to define controls for: Approving documents (who approves which document). 51 ISO 9001:2000 • Reviewing and updating documents (frequency may be defined for the same). • Ensuring changes and current revision status of documents are identified (an amendment register may be maintained for the same). • Ensuring relevant versions of documents are available at point of use (controlled copies may be made available to the concerned). • Ensuring documents remain legible and identifiable (the documents need to be properly protected and maintained from getting damaged from heat, dust, fire, dirt etc.). • Ensuring documents of external origin are identified and distribution is controlled e.g. ISO 9001:2000, ISO 9000:2000 etc. • Preventing unintended use of obsolete documents (retain them in a separate file or folder if they are to be retained for any specific purpose. Otherwise destroy them. This is one of the mandatory documented procedures. 4.2.4 Control of records: Records must be established and maintained to provide evidence of conformity of the quality system. Records must remain legible, identifiable and retrievable. Procedures must exist to establish controls for identification, storage, protection, retrieval, retention time, and disposition of records. The formats used for records may be appropriately controlled e.g. by maintaining their master copies in a separate file and tracking the changes if any. This is one of the mandatory documented procedures. 6.2.5 Clause 5: Management Responsibility The contents and interpretation are given here. 5.1 Management Commitment Top management must provide evidence to support development and implementation of the QMS and its continual improvement by: • Communicating to the company, the importance of meeting customer, statutory and regulatory requirements. (this could be through meetings or documents) • Establishing the quality policy. • Ensuring quality objectives are established. • Conducting management reviews. • Ensuring the availability of resources. 5.2 Customer Focus Top management must ensure that customer requirements are determined and fulfilled to enhance customer satisfaction. A separate role may be defined for the same depending on the criticality of accuracy and speed required for the same. 5.3 Quality Policy Top management shall ensure that the quality policy:52 • Is appropriate to the purpose of the company (this need be in congruence with the vision, mission and values cherished by your company). • Includes a commitment to comply with requirements and continually improve of the QMS. • Provides a framework for establishment and review of quality objectives (there need be coherence between the two). • Is communicated and understood by the entire company (this could be accomplished through displays, screen-savers, printing on backside of identity documents etc). • Is reviewed for continuing stability (the periodicity may match with frequency of management reviews). This becomes one of the prime focuses of the standard and should not be undertaken lightly. The policy must link to the organisation’s objectives for commitment to quality goals. The policy must link to defined customer needs and expectations. 5.4 Clause Wise Interpretation of ISO 9001:2000 Planning 5.4.1 Quality objectives: Top management must ensure quality objectives are established at relevant functions and levels within the company. These objectives must be measurable and consistent with the quality policy. (Each process may take relevant objectives which if achieved help accomplish organisational objectives). 5.4.2 Quality management system planning: Top management must ensure that the planning of the quality system is carried out in order to meet the requirements. The integrity of the QMS when changes are planned and implemented. This need be done at both macro and micro level. At macro level it is achieved through preparation of quality plans for new project, product or contract and reviewing and revising the QMS based on the effectiveness of the quality plans. At micro level it is achieved by following the change control mechanism documented in control of document procedure. 5.5 Responsibility, Authority and Communication 5.5.1 Responsibility and authority: Top management must ensure responsibilities and authorities are defined and communicated to all concerned. The role specific activities in terms of providing information, participating, reviewing and signing off may be documented. 5.5.2 Management representative: Top management must appoint a representative (from own management) responsible and authorized for: • Ensuring processes needed are established implemented and maintained (assessed through internal audits). • Reporting to top management on the performance of the QMS and any need for improvement (e.g. in management review meetings). • Ensuring promotion of awareness of customer requirements throughout the company (e.g. through defining the customer order fulfillment process interfaces appropriately). This person does not necessarily have to report to the general manager or chief executive officer (although desirable), and can have other responsibilities. 53 ISO 9001:2000 5.5.3 Internal communication: Top management shall ensure appropriate communication processes are established and take place. This could consist of planned meeting on specified agenda w.r.t. intra and inter process participation. 5.6 Management Review 5.6.1 General: Top management must review the QMS at planned intervals to ensure continued suitability, adequacy and effectiveness. This includes assessing opportunity for improvement and changes including the quality policy and quality objectives. Records of these reviews must be maintained. 5.6.2 Review input: Inputs to management review must include: • Results of audits, • Customer feedback, • Process performance (effectiveness calculations) and product conformity, • Status of preventive and corrective actions, • Follow up of previous meetings, • Planned changes which could affect the QMS, • Recommendation for improvement, • Suitability of quality policy and objectives, and • Resource statement (current utilization level and new requirements). 5.6.3 Review output: Outputs from management review shall include decisions and actions related to: • Improvements to the QMS and processes, • Improvement of product related to customer requirements, and • Resource needs. 6.2.6 Clause 6: Resource Management Contents and Interpretation 6.1 Provision of resources The company must determine and provide resources needed to implement the QMS and continually improve effectiveness. The resources include money, machinery, manpower and material. 6.2 Human Resources 6.2.1 General: Personnel performing tasks affecting quality must be competent on the basis of appropriate education, training, skills and experience. The skills could be managerial, core-technical, behavioral. They could have current compatibility or future applicability. Competence, awareness and training: Identifying and providing resources will include trained personnel for: 1) Management activities. 2) Performance of work activities. 3) Verification activities (i.e.: inspection and test, audit and monitoring activities). 6.2.2 54 The company must: • Determine competence of personnel. • Provide training to satisfy their needs or take other actions like jobrotation etc. • Evaluate the effectiveness of training through operational results. • Ensure it's personnel are aware of the relevance of their activities and how they contribute to quality objectives. Clause Wise Interpretation of ISO 9001:2000 Records must be maintained to support the above. This points to an appraisal system, by which staff members set objectives and are developed through appropriate training plans developed as a result of these appraisals. This appraisal system should be aligned with your objectives and business plans. 6.3 Infrastructure The company must determine, provide and maintain infrastructure needed to achieve conformity to product requirements including: • Buildings (area, separation between activities, approach, safety etc.), workspace and associated utilities (water, power etc). • Process equipment, both hardware and software. • Supporting services such as transportation or communications. 6.4 Work Environment The company must determine and manage the work environment to achieve conformity to product requirements. The motivational aspects with regard to (w.r.t.) work also need attention to keep the job-satisfaction level above threshold. 6.2.7 Clause 7: Product Realization The contents and interpretation are given here. 7.1 Planning of Product Realization This requires your company to plan and develop your processes needed to realize (make/generate) the product in line with your QMS. In doing so you need to determine, where applicable: Your quality objectives and requirements (and possibly those of your customers) for each product. • The need to establish processes, documents to support those processes (You still need to use forms of some type be they paper or electronic, but no requirement here for documented procedures) and the need to provide adequate resources to fulfill these requirements. • The verification, validation, monitoring, inspection and test activities specific to the product and criteria for product acceptance (the final check before handing over to the customer). • Records to provide evidence that the above has taken place in the manner described in the QMS. 55 ISO 9001:2000 7.2 Customer – Related Processes Determination of requirements related to the product: The company must determine: Customer specified requirements. Requirements not stated by the customer, but necessary for intended use. Statutory and regulatory requirements. Any additional requirements. 7.2.1 • • • • Review of requirements related to the product: The company must review these requirements prior to commitment to supply and in doing so must also ensure that: Product requirements are defined. Contract/order requirements differing are resolved. The company can meet these requirements. 7.2.2 • • • Records of the above must be maintained. If the customer does not provide documentary evidence of requirements, these must be confirmed by your company before committing. The company must ensure all affected staff members are made aware of any relevant changes to requirements. Customer communication: The company must determine the need and implement systems in order to communicate with customers about: Product information Enquiries, contracts, order handling (including changes) Feedback and complaints 7.2.3 • • • 7.3 Design and Development 7.3.1 Design and development planning: The company must plan: • The stages of design and development. • Any review, verification or validation that may be appropriate for each stage. • The responsibilities for design and development and how interfaces are managed. Since any design is a reiterative process, the planning output must be updated, as appropriate, as the design and development progresses. The design plan need be updated to reflect the latest status of impacting parameters. Design and development inputs: These must be recorded and maintained and may include: Functional and performance specifications. Statutory/regulatory requirements (with regard to the geographic area where the product-application is planned). Experience with regard to (w.r.t.) previous (similar) designs. Other requirements which your company follows e.g. aesthetic, packaging, etc. 7.3.2 • • • • 7.3.3 56 Design and development outputs: These must be provided in a manner which allows verification against inputs and must: • • • • Meet input requirements. Provide information for other related activities (e.g. purchase and production) post development. Contain product acceptance criteria (test standards, methods, sampling plans etc.). Specify characteristics for safe and proper use (with regard to user, product and environment). 7.3.4 Clause Wise Interpretation of ISO 9001:2000 Design and development review: Review is carried out as per the plan in order to determine that the design will meet the requirements and to identify any perceived problems and propose actions for their rectification. Records of reviews must be maintained. 7.3.5 Design and development verification: This should occur in order to ensure design/developments have met the input requirements. Records of verification must be maintained. 7.3.6 Design and development validation: This must be in line with acceptance as guided by planning above and where practical should be completed prior to delivery or implementation. Validation must ensure that the development meets intended use of the outcome of development. Records validation must be maintained. 7.3.7 Control of design and development changes: The changes must be reviewed, verified and validated as appropriate, the documented review must include the effect of changes on constituent parts and delivered product. In short each change may be required to go through the entire design and development cycle. Records of changes must be maintained. 7.4 Purchasing 7.4.1 Purchasing process: The company must ensure purchased product/ service conform to requirements. Controls are dependent upon the effect of the purchased product/service on the final product. The company must select suppliers based upon their ability to supply in accordance with requirements and criteria for selection, evaluation and re-evaluation must be established. Records of evaluation must be maintained. 7.4.2 Purchasing Information: Purchasing information (e.g. purchase order) must be reviewed before release. Verbal purchase orders must be recorded. This must include in addition to product specifications where applicable: • Requirements for approval of product, procedures, processes and equipment. • Qualifications of personnel. • Quality management system requirements. 7.4.3 Verification of purchased product: The company must establish inspections to ensure purchases meet specified requirements. Where the company or its customer intend to visit the supplier these arrangements 57 need to be pre-intimated through the purchasing information. The company may enter into quality agreement with suppliers to avoid duplication of verification and also to have necessary transparency with regard to (w.r.t) inspection methodology. ISO 9001:2000 7.5 Production and Service Provision 7.5.1 Control of production and service provision: The company must carry out production and service under controlled conditions including where applicable: • Information describing specifications). • Work instructions (process sheets). • Suitable equipment (production environment configuration). • Monitoring and measuring devices (calibration and setting verification). • Implementation of monitoring and measurement activities (process capability tracking). • Implementation of release, delivery and post delivery activities (including necessary records to be maintained). 7.5.2 characteristics of the product (product Validation of processes for production and service provision: The company must validate any processes where resulting output cannot be verified by subsequent monitoring or measurement such as where deficiencies only come to light after delivery (e.g. soldering, welding, imaging etc.). Validation must demonstrate the ability of these processes to achieve planned results using: • Defined criteria for review and approval of processes (e.g. by conducting design of experiments studies), • Approval of equipment and qualification of personnel, • Use of specific methodology, • Requirements for records, and • Revalidation (at defined frequency). 7.5.3 Identification and traceability: The company must be able to suitably trace a product throughout product realization spectrum and identify product status with regard monitoring and measurement activities. Where traceability is a requirement, the company must control and record the unique identification of the product. 7.5.4 Customer property: The company must exercise care with customer’s property. This includes identifying, verifying, protecting and safeguarding. This can include intellectual property like customer supplied drawings, data, standards etc. Records must be maintained and the customer reported to if customer property is lost, damaged or unsuitable for purpose. 7.5.5 58 Preservation of product: The company must preserve product during processing and delivery including identification, handling, packaging, storage and protection. This applies to component parts of a product. This ensures that intentional and non-intentional damage to the stored material is prevented. 7.6 Control of Monitoring and Measuring Devices The company must determine where monitoring and measurement are required in order to meet determined requirements and establish processes to facilitate this. Measuring equipment must be (where applicable): • Calibrated/verified at specified intervals or prior to use against national/ International standards or where no standards exist, the means of verification shall be recorded. The external laboratories need be accredited ones. • Adjusted as necessary. • Identified to establish calibration status (through suitable means like color coding etc.). • Safeguarded from adjustments invalidating results. • Protected from damage/deterioration during handling/storage/ maintenance. Clause Wise Interpretation of ISO 9001:2000 The company must record and assess the validity of the previous results when the equipment is found not to conform to requirements and take appropriate actions on the products affected. Records of results of calibration and verification must be maintained. If the company uses computer software, its ability must be confirmed prior to initial use and reconfirmed as necessary. This may include anti-virus software. 6.2.8 Clause 8: Measurement, Analysis and Improvement The contents and interpretation are given here. 8.1 General Your company must plan and implement monitoring, measuring, analysis and improvement processes required to: • Demonstrate conformity of product. • Ensure conformity of the QMS. • Continually improve the effectiveness of the QMS. This must include determination of applicable methods including statistical techniques and the extent of their use. 8.2 Monitoring and Measurement 8.2.1 Customer satisfaction: As one of the measurements of the QMS, the company must measure customer perceptions to assess whether it has fulfilled requirements. The methods for collection and use of data must be determined. Some of the methods used could be customer surveys, visiting customers, noting key points in customer communication, customer suggestions, customer complaints, etc. 8.2.2 Internal audit: The company must conduct planned internal audits to ensure conformance with planned arrangements for product realization, the requirements of ISO 9001:2000 standard and the requirements of the QMS and also to ensure that the QMS is implemented and maintained. Audit must be planned with consideration to the status and importance of the processes and areas as well as the results of previous audits. The audit criteria, scope, frequency and methods must be defined. Auditors 59 should conduct audits objectively and impartially and not audit their own work. Audit principles like ethical behavior, fair presentation, due professional care etc., must be followed. ISO 9001:2000 Responsibilities and requirements for planning and conducting audits, reporting results and maintaining records must be defined in a documented procedure. Management responsible for the area under audit must ensure corrective actions are undertaken without delay. Follow up activities must include verification of actions taken and reporting verification results. Records must be maintained. This is one of the mandatory documented procedures. 8.2.3 Monitoring and measurement of processes: These methods must demonstrate the ability of processes to achieve planned results and where this is not the case corrective actions must be taken to ensure conformity. The process effectiveness measurement could be based on achievement of objectives as well as following the documented QMS. 8.2.4 Monitoring and measurement of product: The company must conduct this to ensure characteristics of product meet requirements. Evidence of such processes must be maintained indicating the person authorizing release. This must not occur until planned arrangements have been completed unless otherwise approved by a relevant authority, and where applicable the customer. Record must be maintained. 8.3 Control of Non-conforming Product The company must ensure that product not conforming to requirements is identified and controlled to prevent unintended use or delivery. Controls and responsibilities must be defined within a documented procedure. The company will deal with non-conforming product in one or more of the following ways: • Take action to eliminate the non-conformity. • Authorize its use by way of a concession by relevant authority and where applicable the customer. • Taking action to exclude its original intended use. Records of such non-conformances and subsequent actions and re-verification must be maintained. This is one of the mandatory documented procedures. 8.4 Analysis of data The company must collate suitable data to demonstrate the effectiveness of the quality system and to evaluate where continual improvement can be made. Analysis must provide information relating to: 60 • Customer satisfaction. • Conformance to product requirements. • Characteristics and trends of processes and products including opportunities for preventive actions. • Suppliers. Clause Wise Interpretation of ISO 9001:2000 8.5 Improvement 8.5.1 Continual improvement: The Company must continually improve the effectiveness of the implemented quality system through the use of quality policy, quality objectives, audit results, analysis of data, corrective and preventive actions and management review. This could be done periodically through decisions and actions noted in management review meeting output minutes. 8.5.2 Corrective action: The Company must eliminate the cause of nonconformities to prevent their recurrence. A documented procedure must be established to define the requirements for: • Reviewing non-conformities (including customer complaints). • Determining the causes of non-conformities. • Evaluating the need for action to prevent recurrence. • Determining and implementing actions required. • Recording the results of actions taken. • Reviewing corrective actions taken. This is one of the mandatory documented procedures. 8.5.3 Preventive action: The company must determine actions to eliminate potential non-conformities in order to prevent their occurrence. What this means is that when it plans a new product or service, it needs to think about what could go wrong and "mistake proof" it, so that the likelihood of its happening will be eliminated. These actions must be appropriate to the impact the potential problems may cause. A documented procedure must be established to define requirements for: • Determining potential non-conformities and their causes. • Evaluating the need for action to prevent their occurrence. • Determining and implementing action needed. • Recording the action taken. • Reviewing preventive action taken. This is one of the mandatory documented procedures. Check Your Progress Exercise 1 " Note: a) Use the space below for your answers. b) Compare your answers with those given at the end of the unit. 1) List six mandatory procedures required to be documented as per ISO 9001:2000. …………………………………………………………………………….. …………………………………………………………………………..… …………………………………………………………………………….. …………………………………………………………………………….. 61 ISO 9001:2000 2) List any five essential MRM inputs. …………………………………………………………………………….. …………………………………………………………………………….. …………………………………………………………………………….. …………………………………………………………………………….. 3) List any five mandatory records as per ISO 9001:2000. …………………………………………………………………………….. …………………………………………………………………………….. ………………………………………………..…………………………… …………………………………………………...………………………… 4) List any five (sub) clauses in ISO 9001:2000 which refer to customer related aspects. ……………………………………………………...……………………… ………………………………………………………..…………………… ………………………………………………………..…………………… ………………………………………………………..…………………… 5) Explain the main purpose of conducting internal audits. ………………………………………………………..…………………… ………………………………………………………..…………………… ……………………………………………………………………………. ..…………………………………………………………………………… 6.3 LET US SUM UP ISO 9001:2000 standard covers all aspects of quality management related applicable areas in an industry. The standard harmonizes its structure with ISO 14001 EMS standard. The standard consists of eight clauses further divided into twenty three sub-clauses. This structure follows PDCA (Plan-Do-CheckAct) approach in the organisation and sequence of the clauses. The intent of the clauses and the internal congruence between the clauses has been explained. The contents cover mandatory documents including plans, procedures and records. The overall focus is on customer satisfaction and continual improvement. 62 6.4 KEY WORDS Competence : Demonstrated ability to apply knowledge and skills. Corrective Action : Action to eliminate the cause of a detected non-conformity or other undesirable situation. Customer Satisfaction : Customer’s perception of the degree to which the customer’s requirements have been fulfilled. Document : Information and its supporting medium. Effectiveness : Extent to which planned activities are realized and planned results achieved. Preventive Action : Action to eliminate the cause of a potential non-conformity or other undesirable potential situation. Product : Result of a process. Quality : Degree to which a set of inherent characteristics fulfils requirements. Quality Management System : Management system to direct and control an organisation with respect to quality. Record : Document stating results achieved or providing evidence of activities performed. Requirement : Need or expectation that is stated, generally implied or obligatory. 6.5 ANSWERS TO CHECK YOUR PROGRESS EXERCISE Clause Wise Interpretation of ISO 9001:2000 " Your answer should include the following: Check Your Progress Exercise 1 1) ● Control of documents • Control of records • Internal audits • Control of non-conforming products • Corrective actions • Preventive actions 2) ● Result of audits • Status of corrective and preventive actions • Improvement suggestions • Customer feedback • Process performance and product conformity 63 ISO 9001:2000 3) ● Record of MRM (Clause 5.6) • Record of effectiveness of training (Clause 6.2) • Record of monitoring and measuring device (7.6) • Record of design verification (Clause 7.3.5) • Record of action taken on nonconformities (Clause 8.3) 4) ● Clause 5.2 (customer focus) • Clause 7.2.1 (customer requirements) • Clause 7.4.3 (customer communication) • Clause 8.2.1 (customer satisfaction) • Clause 8.5.2 (customer complaints) 5) ● Assessing adequacy of QMS documentation • Assessing level of consistency of implementation of QMS • Assessing effectiveness of implemented QMS • Assessing the level of understanding of QMS among the staff • Fulfilling the mandatory requirement of ISO 9001:2000 (Clause 8.2.2) 6.6 SUGGESTED READING http://www.iso.org ISO 19011:2002 – Guidelines for Quality and/or Environmental Management Systems Auditing. ISO 9000:2000 – Quality Management Systems - Fundamentals and Vocabulary. ISO 10012:2003 – Quality Assurance Requirements for Measuring Equipment. ISO 9004:2000 – Quality Management Systems - Guidelines for Performance Improvements. ISO 10013:1995 – Guidelines for Developing Quality Manuals. 64