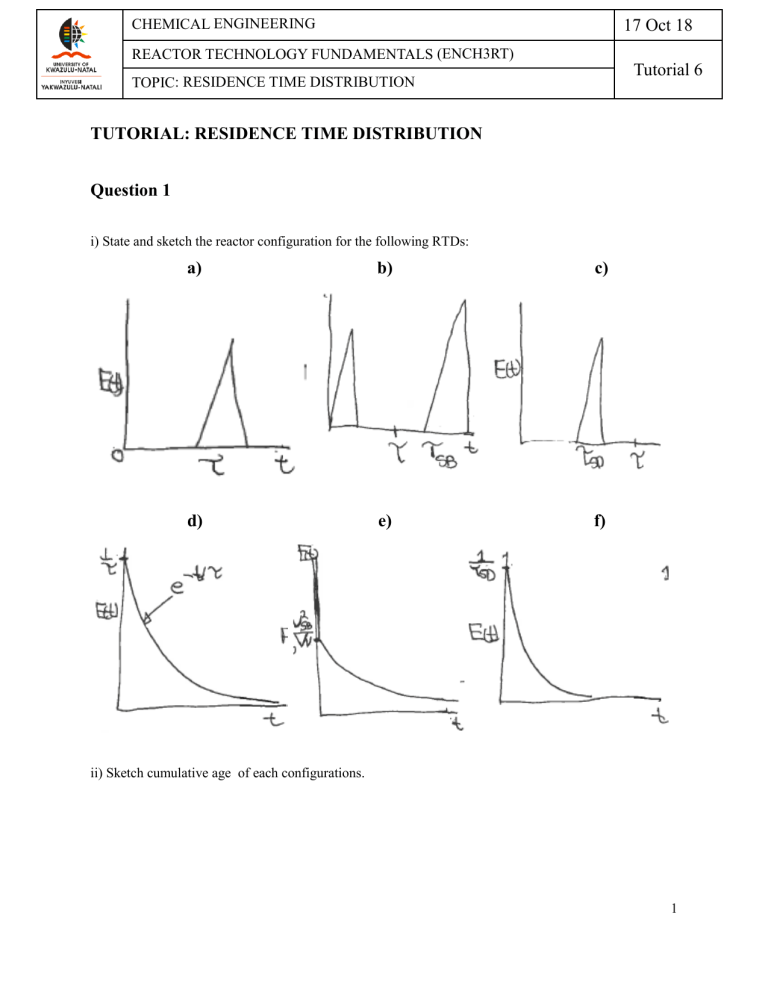
17 Oct 18 CHEMICAL ENGINEERING REACTOR TECHNOLOGY FUNDAMENTALS (ENCH3RT) Tutorial 6 TOPIC: RESIDENCE TIME DISTRIBUTION TUTORIAL: RESIDENCE TIME DISTRIBUTION Question 1 i) State and sketch the reactor configuration for the following RTDs: a) b) c) d) e) f) ii) Sketch cumulative age of each configurations. 1 17 Oct 18 CHEMICAL ENGINEERING REACTOR TECHNOLOGY FUNDAMENTALS (ENCH3RT) Tutorial 6 TOPIC: RESIDENCE TIME DISTRIBUTION Question 2 Consider the following pulse injection tracer data: t (min) 0.0 C (mol/dm3) 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 10.0 11.2 16.4 18.0 17.2 15.6 13.2 10.4 6.8 3.2 0.0 a) Plot E(t), stating all required assumptions b) Calculate the mean residence time and standard deviation c) Compare the calculated MRTD with the expectations that result from inspecting the E(t) curve Question 3 The first order-order reaction 𝐴 ⟶ 𝐵 -1 With k = 0.8 min is carried out in a real reactor with the following RTD function For 2𝜏 ≥ 𝑡 ≥ 0 then E(t) = √𝜏 2 − (𝑡 − 𝜏)2 min-1 (hemi circle) For 𝑡 > 2𝜏 then E(t) = 0 a) What is the mean residence time? b) What is the variance? 2 CHEMICAL ENGINEERING REACTOR TECHNOLOGY FUNDAMENTALS (ENCH3RT) TOPIC: RESIDENCE TIME DISTRIBUTION 17 Oct 18 Tutorial 6 c) What is the conversion predicted by the segregation model? d) What is the conversion predicted by the maximum mixedness model Question 4 The second-order reaction is to be carried out in a real reactor which gives the following outlet concentration for a step input. For 0 ≤ t ≤ 10 min then CT = 10(1 − e−0.1t ) For t ≥ 10 min then CT = 5 + 10(1 − e−0.1t ) a) Propose the model and calculate model parameters, α and β? b) What conversion can be expected in the real reactor? c) How would the model and conversion change if the outlet tracer concentration was For t ≤ 10 min, then CT = 0 For t ≥ 10 min, then CT = 5 + 10(1 − e2(t−10) ) 𝑑𝑚3 𝑑𝑚3 𝑚𝑜𝑙 𝜐0 = 1 , 𝑘 = 0.1 , 𝐶𝐴0 = 1.25 𝑚𝑖𝑛 𝑚𝑜𝑙. 𝑚𝑖𝑛 𝑑𝑚3 Question 5 A computational fluid dynamic study was conducted over a certain real reactor of volume 2.3 m3, a sketch of the results is shown below: 3 CHEMICAL ENGINEERING REACTOR TECHNOLOGY FUNDAMENTALS (ENCH3RT) TOPIC: RESIDENCE TIME DISTRIBUTION 17 Oct 18 Tutorial 6 From the figure, it would appear as if, due to the locations of the entrance and exit points, it is possible for a large fraction of the inlet stream to simply bypass the reactor (see the long vector near the top). The mixer is located near the bottom left of the reactor, and the fluid in this vicinity is fairly well mixed. However, past the mixer, the fluid appears to flow in plug mode to the exit point. The study was conducted at a flowrate of 0.5 m3/min; the same flowrate was used in an experiment during which tracer was pulsed into the reactor and the exist RTD measured; the results are given in the figure below, 4 CHEMICAL ENGINEERING REACTOR TECHNOLOGY FUNDAMENTALS (ENCH3RT) TOPIC: RESIDENCE TIME DISTRIBUTION a) 17 Oct 18 Tutorial 6 Propose an equivalent reactor network so as to compartmentalise the system. Based on this network, develop a model of the exit RTD E(θ) and use the experiment data to estimate any unspecified constants in your model. b) The fluid flowing through the reactor also contains solid particles of pure component B which reacts with component A from the liquid phase as it flows. Component A is at a high concentration of 300 mol/min3 and the conversion of A is low enough that the concentration of A can be considered constant and uniform everywhere inside the reactor. Component B, in the form of the particles, is disappearing due to the reaction. As B is consumed in each particle, an ash layer (component C) is left behind, as such, the particle total size does not change with time, but the unreacted core of B is disappearing with time. It is believed that the transport of component A from the bulk phase to the surface of the particle (external transfer) is limiting the reaction. i. Derive an expression to predict the rate of change of particle radius in terms of initial particle radius R, surface reaction rate (area-based) constant ks, external mass transfer coefficient kc, molar density of component B, viz. ρ and bulk concentration of A, viz. CAb. ii. If the particles are uniformly suspended in the fluid, what is the mean conversion? Apply the following numerical values: R = 1 cm; ks = 1.20 m/min; k = 4.5 m/min; ρ = 62 300 mol/m3; CAb = 35 mol/m3 iii. With regard to consuming component B, what would be the optimal flowrate for this system? In addition to this, can you suggest any simple/physical means of improving the operation of this reactor? 5





