2020
Juan A Ruiz, MD
[ACID-BASE DISORDERS]
PHYSIOLOGICAL APPROACH: “the simplest, most
rigorous, and most serviceable approach to
assessing acid–base disorders”.
ACID-BASE DISORDERS
Dr. JUAN A. RUIZ
juan.ruiz@uccaribe.edu
August, 2020
Pre-Test:
Match the clinical scenario with the most likely presenting acid base disturbance:
___66 y/o man taking high doses of loop diuretics
___24 y/o with opiate overdose, respiratory rate of 8/min.
___73 y/o with COPD and persistent vomiting
___22 y/o with anxiety, psychosomatic origin, respiratory rate 28/min.
___17 y/o Type 1 Diabetic who stopped insulin therapy 24 hours back
A. Respiratory alkalosis
B. Respiratory acidosis
C. Respiratory acidosis with concomitant metabolic alkalosis
D. Metabolic alkalosis
E. Metabolic acidosis
Appropriate interpretation of acid-base status requires simultaneous
measurement of serum electrolytes and arterial blood gases (ABGs), as well as an
appreciation by the clinician of the physiologic adaptations and compensatory responses
that occur with specific acid-base disturbances. In most circumstances, these
compensatory responses can be predicted through an analysis of the prevailing disorder in
a stepwise sequence.
In the determination of ABGs by the blood gas analyzer, both pH and paCO2 are
measured, but the reported HCO3- concentration is calculated from the HendersonHasselbalch equation. The calculated value for HCO3- reported with the arterial blood
gas panel should be compared with the measured total CO2 content (TCO2) obtained on
the serum electrolyte panel. This TCO2 content measures H2CO3, dissolved CO2 and the
HCO3- that exists in the serum. Because the amounts of H2CO3 and dissolved CO2 in the
blood are so small, TCO2 content is an indirect measure of HCO3-. Thus in clinical
practice the measured TCO2 in serum is taken as the serum HCO3-. Furthermore, most
acid–base disorders are first recognized by clinicians through abnormalities in venous
TCO2.
The terms “acidemia” and “alkalemia” refer to states in which the blood pH is
abnormally low (acidic) or abnormally high (alkaline). The process in which the
hydrogen-ion concentration is increased is called acidosis, and the process in which the
hydrogen-ion concentration is decreased is called alkalosis. However, in clinical practice
we tend to use the terms Acidosis and Alkalosis when referring to the “states” as well as
the “processes”.
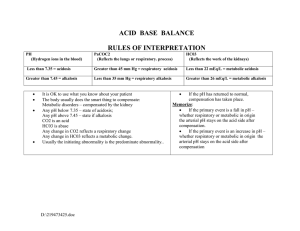
With the ABGs we should be able to identify the primary disturbance:
pH > 7.45 = Alkalemia
pH < 7.35 = Acidemia
Obviously, there is a range for “normal” but in order to evaluate these disorders the
values we take as standard for “normal” are:
TCO2~ HCO3- = 24
pH = 7.40
paCO2 = 40
Let us say that we have ABGs reporting a pH of 7.48, paCO2 23, paO2 88, and a serum
HCO3- (TCO2) of 10. First, we look at the pH and read this as a primary alkalemia; we
then look at the pCO2, which is abnormally low, indicating a: Respiratory Alkalosis.
The low HCO3- (TCO2) should be due to metabolic compensation of the Resp Alk, but
how much would that compensation be?
We can only figure this out by knowing the expected magnitude of the compensatory
response. Studies in dogs and humans have been done to obtain formulas to calculate
those changes. Empirical data have been used to construct confidence intervals that
define the limits of the secondary response to each acid-base disorder. In clinical practice,
these limits can be taken as +3 mEq/L for plasma [HCO3-] and +5 mmHg for paCO2.
Values falling outside these limits denote the presence of a mixed acid base disorder.
Metabolic compensation of Respiratory Alkalosis (Resp Alk):
Acute: Change in pCO2 X 0.2 = expected change in serum HCO3Chronic: Change in pCO2 X 0.4 = expected change in serum HCO3If we were dealing with a Chronic Resp Alk the expected decrease in serum HCO3(metabolic compensation of respiratory disturbance) would be given by the formula:
40-23=17 (change in pCO2 from a normal of 40)
17 X 0.4 = 6.8 (expected decrease in HCO3-)
Since compensation of Resp Alk would drive the serum HCO3- down, the HCO3should have been:
24 – 6.8= 17.
However, in the example the HCO3- is 10, so there is another process bringing the
HCO3- down beyond the expected compensation...
What is the final interpretation of the results in the example?
Respiratory Alkalosis and Metabolic Acidosis.
{The simultaneous presence of two or more simple acid–base disorders
defines a mixed acid–base disorder}.
What clinical entity could cause it?
Aspirin Intoxication
{Aspirin stimulates the respiratory center to cause tachypnea and RespAlk
and also causes metabolic acidosis due to increased production and
decreased renal elimination of organic acids}
Using the same example, if we change the pH value to 7.28 and keep the HCO3- (TCO2)
of 10, and the pCO2 of 23 we then read the ABGs as: Metabolic Acidosis . The
metabolic disturbance will then be compensated by the respiratory system and we would
now calculate the magnitude of change in pCO2 expected by using this formula:
Respiratory compensation of Met Acidosis:
Change (decline) in pCO2 =change (decline) in HCO3- X 1.2
If serum HCO3- is 10, the change or decline in HCO3- would be 24-10=14.
14 X 1.2 = 16.8 (change in HCO3- X 1.2)
Then, 16.8 would be the expected magnitude of the change in pCO2 from a normal of 40
required to compensate the metabolic acidosis by the respiratory system. The expected
pCO2 should then be:
40-16.8 = 23.2
Numerical relation between pCO2 and pH units:
A 24 y/o previously healthy man is rushed to the Emergency Room with shortness of
breath and chest tightness that began immediately after having an argument with his
girlfriend. On arrival his labs show:
ABGs: pH 7.56
pCO2 20 pO2 115
Basic Metabolic Panel: Glu 102
Na+ 140
TCO2 (HCO3-) 20
BUN 12
K+ 4.2.
Cr 0.6
Cl- 104
Is this a simple respiratory disturbance?
In order to determine if we are dealing with an acute simple respiratory disturbance
not yet compensated it will help to know that for each mm change in pCO2 the pH will
change (increase or decrease) in a magnitude of 0.008.
In the above example the change in pCO2 is:
40 - 20 = 20;
then 20 X .008 = 0.16
The change in pH expected would be added to the “normal” pH since lowering pCO2
“means” eliminating acid :
7.40 + 0.16 = 7.56
If this were a simple but chronic respiratory disturbance the pH will change in a
magnitude of 0.003:
40 – 20 = 20; then 20 X .003 = 0.06
7.40
+ 0.06 = 7.46
We added pH units because lowering the pCO2 would cause increase in pH; if by the
contrary we had increase in pCO2 this would signify hypoventilation and the pH will go
down.( In the respiratory system pCO2 “means” acid, if you have too much you are
acidemic, if you have too little you are alkalemic).
Metabolic Compensation of Resp Acidosis:
A 68 y/o 80 pack-year smoker with COPD presents with worsening productive cough,
fever, nausea and persistent vomiting for 3 days:
ABGs 7.39
pCO2 60
pO2 55
Basic Metabolic Panel (serum chemistry): Glu 130
HCO3- ( TCO2) 40
K+
3.2.
Na+ 130
BUN 32
Cl- 94
Cr 1.4
First, we check if this is a pure, simple respiratory disturbance, which one?
Since COPD points to Chronic Respiratory Acidosis as the probable disturbance we then
apply the formula using .003:
60 – 40 = 20 (change in pCO2)
20 X .003 = .06 (expected change in pH for a chronic simple respiratory
disturbance)
Since it is a respiratory acidosis the change in pH expected in a chronic simple
respiratory disturbance would be:
7.40 - .06 = 7.34
However, the patient has a pH of 7.39 indicating that there is another acid-base
disturbance: an added Metabolic Alkalosis due to his persistent vomiting. Now we use
the formula to check if serum HCO3- matches the expected compensation:
The metabolic compensation of Resp Acidosis is determined by:
Acute: Change in pCO2 X 0.1 = expected change in HCO3Chronic: Change in pCO2 X 0.35= expected change in HCO3To find the expected metabolic compensation for the Resp Acidosis we multiply the
change in pCO2 by 0.35 since we are dealing with Chronic Respiratory Acidosis (COPD):
60 – 40 = 20 (change in pCO2 )
20 X 0.35= 7
This will give us the expected change in serum HCO3- from a normal of 24 and since we
are compensating for a chronic Resp Acidosis the HCO3- compensation by the Kidneys
should stop at 31. However, the measured value is 40, confirming also the presence of
the Metabolic Alkalosis.
BTW: One pack-year is smoking a “pack” of 20 cigarettes a day for one year. If
someone has smoked 10 cigarettes a day for 6 years they would have a 3 pack-year
history. Someone who has smoked 40 cigarettes (2 packs) daily for 20 years has a 40
pack-year history and so on.
Respiratory Compensation of Metabolic Alkalosis:
The patient’s twin brother never smoked, never used neither illicit drugs nor
alcohol and always lived in celibacy. Six months ago he suffered a massive stroke and
became aphasic, incontinent, totally dependent and bedridden. He is brought for
evaluation after vomiting 14 times in less than 24 hours:
Basic Metabolic Panel: Glu 130
Na+ 130
HCO3- (TCO2) 39
His expected ABGs would look like:
BUN 43
K+ 2.8
Cr. 1.8
Cl- 96
a. ABGs 7.39; pCO2 58; pO2 55
b. ABGs 7.49; pCO2 50; pO2 71
c. ABGs 7.24; pCO2 92; pO2 40
Now the respiratory system has to compensate the metabolic acid-base disturbance:
Expected change in pCO2 = change in HCO3- X 0.7
39-24 = 15 (change in HCO3- )
15 x 0.7 = 10.5 (expected change in pCO2 from a “NORMAL” of 40,
thus the pCO2 of 50)
Anion Gap
The concept of the Anion Gap (AG) is based on the following assumptions: The
total concentration of anions and cations in plasma is equal, and some of the anions and
cations cannot be measured. The difference between the concentration of unmeasured
anions and cations can be estimated by calculating the AG representing the difference
between the primary measured cations and the primary measured anions. A normal AG
primarily reflects the concentration of non-bicarbonate buffers including albumin,
phosphate, sulfate, and other organic acids. In most clinical circumstances, a high AG
indicates that a metabolic acidosis is present.
AG = [Na+] - [Cl- + HCO3-]
Normal 10 ± 2
When the Metabolic Acidosis is high-anion gap (HAGMA) the differential diagnosis is:
K etoacidosis
U remia
S alicylates
M ethanol
A lcohols (ethanol, ethylene glycol)
U
L actic Acidosis
BTW: Kussmaul breathing is a deep and labored breathing pattern often associated with
severe metabolic acidosis. Kussmaul's sign is the paradoxical increase in JVP that
occurs during inspiration.
Other mnemonics for HAGMA:
"GOLD MARK"
G lycols (ethylene glycol & propylene glycol)
O xoproline, a metabolite of paracetamol / acetaminophen
L -lactate
D -lactate
M ethanol
A spirin
R enal failure
K etoacidosis
“MUDPILES”
M ethanol
U remia
D iabetic ketoacidosis
P aracetamol, Propylene
I nfection, Iron, Isoniazid
L actic acidosis
E thylene glycol
S alicylates
When the AG is “normal” (from 8 to 12) we then have a “Normal Gap Met Acidosis” and
the main differential diagnosis is:
HCO3- loss in GIT (Diarrhea)
HCO3- loss through the kidneys (Renal Tubular Acidosis)
In HAGMA the anion gap should change according to the change in HCO3-. If there is
just one acid-base abnormality, there should be a 1:1 correlation between the rise in the
anion gap and a corresponding drop in HCO3-. If the relation is not proportional the
possibility of another acid base disturbance can be evaluated using the formula for the
delta ratio also known as the delta/delta ( Δ / Δ):
This formula uses both the Δ gap and the Δ HCO3Δ gap
Δ HCO3
Pt gap - Normal gap (12)
Normal HCO3- (24) –Pt HCO3-
If the ratio is between 1 and 1.8 then we are dealing with a simple HAGMA.
If the ratio is <1.0 we are dealing with an added non-gap Acidosis (Diarrhea, RTA)
If the ratio is >1.8 we are dealing with an added Metabolic Alkalosis
A 16-year-old girl with type 1 diabetes is brought to the emergency room
complaining of abdominal pain after multiple episodes of vomiting. Unable to eat
anything she decided to stop using her insulin:
ABGs: pH 7.30
paCO2 36
Glucose 850 Na+ 138
paO2 110
K+ 2.0
HCO3- (CO2) 18
Cl- 90
AG = [Na+] - [Cl + HCO3]
AG = 138 - [90+ 18]
AG = 30
Using the delta ratio or delta/ delta ( Δ/ Δ )formula:
Δ gap
Δ HCO3-
Pt gap - Normal gap (12)
Normal HCO3- (24) –Pt HCO3-
30-12
24-18
= 18
6
=3
3 > 1.8
Since the ratio of 3 is >1.8 we are dealing with an added Metabolic Alkalosis, thus
besides having a HAGMA due to DKA, the patient also has a metabolic alkalosis due to
persistent vomiting.
Post Test
A 26-year-old man with type 1 diabetes mellitus is brought to the emergency department
with complaints of nausea and vomiting. He has also been experiencing drowsiness.He is
afebrile, has a pulse rate of 92 bpm, a blood pressure of 90/70 mmHg, and a respiratory
rate of 28 breaths/min. The physical examination does not reveal any abnormal findings,
except for mild generalized abdominal tenderness. His blood sugar levels come out to be
400 mg/dl, urinary ketones on a urine dipstick test are noted to be 4+. Serum electrolytes
include a sodium level of 138 mEq/L), a potassium of 3.5 mEq /L a serum HCO3- of 15
and a Cl- of 100 mEq /L
Which of the following would most likely be found on the arterial blood gas analysis in
this patient?
A. pH: 7.58
paCO2 54 mmHg HCO3- 44 mEq/L
B. pH: 7.44
paCO2 26 mmHg HCO3- 18 mEq/L
C. pH: 7.54
paCO2 23 mmHg HCO3- 21 mEq/L
D. pH: 7.33
paCO2 64 mmHg HCO3- 32 mEq/L
E. pH: 7.28
paCO2 29 mm/Hg HCO3- 17 mEq/L
A 50-year-old man with alcohol use disorder is brought to the emergency department
with confusion, epigastric abdominal pain and persistent vomiting for the last 3 days.
Physical examination findings are positive in the abdomen which shows purplish
periumbilical and flank hyperpigmentations and severe diffuse tenderness with guarding.
Blood pressure was 70/40 mmHg, respiratory rate 26/min, and heart rate 130/min. Labs
indicate a pH 7.36, paCO2 19, pO2 85, Na 132, K 4.2, Cl- 90, and HCO3- 20.
Which of the following is (are) this patient's acid-base disturbance?
A. HAGMA and respiratory acidosis
B. Compensated respiratory alkalosis
C. Chronic respiratory alkalosis
D. Normal gap acidosis and respiratory alkalosis
E. HAGMA, metabolic alkalosis and respiratory alkalosis
BTW: A given set of acid-base values obtained with these formulas are never diagnostic
of a specific acid-base disorder; clinical correlation is always required to establish the
correct diagnosis. These formulas should be used as tools to assist in diagnosis and to
inform management decisions. They are not perfect and above all: patients don’t always
read the books.
REFERENCES
1. Adrogué HJ, Gennari FJ, Galla JH, Madias NE. Assessing acid-base disorders
Kidney Int 2009; 76:1239-47 DOI: https://doi.org/10.1038/ki.2009.359
2. Berend K, de Vries APJ, Gans ROB. Physiological approach to assessment of acidbase disturbances. NEJM 2014; 371(15): 1434-45.
DOI: https://doi.org/10.1056/NEJMra1003327
3. Buche V (2014) Arterial Blood Gases: A Simplified Bedside Approach.
J Neonatal Biol. 3: 153. DOI: https://doi.org/10.4172/2167-0897.1000153
4. Rastegar A.Use of the ΔAG/ Δ HCO3- Ratio in the Diagnosis of Mixed Acid-Base
Disorders. J Am Soc Nephrol 18: 2429–2431, 2007
DOI: https://doi.org10.1681/ASN.2006121408
5. Internet Book of Critical Care (IBCC) https://emcrit.org/ibcc/ph/