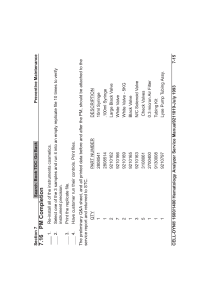
Transcatheter Heart Valve Engineering: Computational Methods & Design
advertisement

The Development of Computational Methods and Device Design Considerations Towards Improving Transcatheter Heart Valve Engineering Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Megan Heitkemper Graduate Program in Biomedical Engineering The Ohio State University 2020 Dissertation Committee: Christopher K. Breuer, Advisor Samir Ghadiali Scott M. Lilly i Copyright by Megan Heitkemper 2020 i Abstract The Development of Computational Methods and Device Design Considerations Towards Improving Transcatheter Heart Valve Engineering In the era of transcatheter aortic valve replacement (TAVR) and especially now with FDA approval for TAVR in low risk patient populations, the need for improved devices, device options, and patient specific pre-planning is especially important. This research uses both experimental and computational methods to study the mechanics and hemodynamics of transcatheter valve replacement with the overarching goal of improving the current technologies towards improved patient outcomes. In order to reduce the risk of fatal coronary obstruction during transcatheter valve replacement in an at-risk patient population, a patient specific 3D computational model to predict risk of coronary obstruction was developed using finite element analysis. The predictive index, DLC/d, was shown to have increased sensitivity and specificity of risk prediction as compared to the clinically used metrics. With the understanding that patient specific computational models are highly time consuming and impractical in a clinical setting, a 2D geometric model to predict risk of coronary obstruction was subsequently developed. Results suggest that while the 3D computational model is the most accurate at predicting risk of coronary obstruction, the 2D geometric model is still superior to the clinically used metrics. ii For transcatheter valve replacement expansion into lower risk and younger patient populations, durable transcatheter prostheses free from long term structural valve degeneration are needed. A potential solution was developed, in the form of a polymeric transcatheter aortic valve, called HA-TAV. Due to its unique material properties, geometry, and design, the HATAV showed reduced levels of blood damage related Reynolds shear stress and durability limiting pinwheeling of leaflets, while maintaining a comparable effective orifice area and regurgitant fraction to the leading commercially available transcatheter aortic valve. Another potential solution to the need for increased durability of transcatheter valves is a tissue-engineered heart valve, which is particularly exciting when considering their potential use in pediatric patients suffering from congenital valvular diseases. In this study, an in vitro methodology is developed that is capable of rapid and cost-effective analysis of the hemodynamic functionality of tissue-engineered prototypes. The method presented will move the field of heart valve tissue engineering further, allowing rapid development and design of prototypes. Combined, these studies provide concrete techniques, technologies, and methods to improve transcatheter heart valve engineering and thus transcatheter heart valve replacement. Optimization of the presented models, devices, and methodologies could result in improved transcatheter valve replacement options and eventually improved patient outcomes. iii Acknowledgements There are numerous people I would like to thank for their support and contributions to this research. First, I would like to express my gratitude to Dr. Christopher Breuer, who has guided me through the final and most challenging phases of completing this work. I hope that I will one day be able to advise students in the way that you have shown me is possible. I am also grateful to Dr. Scott Lilly, and Dr. Samir Ghadiali for helping to shape my research direction and passion for translational research as a part of my dissertation committee. I would like to thank Dr. Prasad Dasi and the Cardiovascular and Biofluid Mechanics Laboratory for my beginnings in heart valve research, and for the desire to improve upon heart valve replacements that remains as a goal and passion. Thank you especially to Dr. Hoda Hatoum for your patience in teaching lab techniques, your manuscript edits, and your invaluable friendship. Thanks also to Breandan Yates, Sri Krishna Sivakumar, Shelley Gooden, Atieh Yousefi, and Amirsepher Azimian for your contributions to this work and friendship throughout its duration. A special thanks goes to Dr. Susan James for your career and life advice along the way. iv Thank you to the Breuer lab in the Center for Regenerative Medicine, for taking me in in my last year and treating me as if I had been there all along. To Jake, Kevin, Roy, and Gabe; your friendship and support are greatly appreciated. I am also grateful to the Department of Biomedical Engineering for their financial and other constant support throughout my education. Thank you also to the Center for Clinical and Translational science at OSU and the TL1 training grant for the funding that made it possible to complete this work at OSU. I am grateful to Dr. Scott Lilly, and the entire structural heart team at Ohio State University Wexner Medical Center, for their commitment to research that has the potential to improve the lives of patients. The interdisciplinary collaboration with this team was one of the most impactful experiences in shaping my research and career interests. Last, but not least, I would like to thank my family for their endless support of my education. Thank you to my mom, Kris, for always having an ear to listen and a fierce belief in my ability, to my dad, Doug, for instilling in me a love of science and the determination to not give up, and lastly to my fiancé, Conor, for the constant encouragement, love, and never failingbelief in me throughout my graduate school journey. v Vita Bachelors in Physics and Mathematics, Wittenberg University, Springfield, OH …………....2016 Graduate Teaching Associate, Biomedical Engineering, The Ohio State University, Columbus, OH………………...…………………………….…...2017 Graduate Research Associate, Biomedical Engineering, The Ohio State University, Columbus, OH…………………………………………..2017 - 2019 Masters in Biomedical Engineering, The Ohio State University, Columbus, OH ………….. 2018 Graduate Fellow, Biomedical Engineering, The Ohio State University, Columbus, OH ………………………………………...2019 - Present Publications Heitkemper M, Sivakumar S, Hatoum H, Dollery J, Lilly SM, and Dasi LP. Simple Anatomical Model to Predict Risk of Coronary Obstruction During Transcatheter Aortic Valve Replacement. Journal of Thoracic and Cardiovascular Surgery, 2020. (In Press) Heitkemper M, Hatoum H, and Dasi LP. In Vitro Hemodynamic Assessment of a Novel Polymeric Transcatheter Aortic Valve. Journal of the Mechanical Behavior of Biomedical Materials, 2019. 98: p. 163-171. Heitkemper M, Hatoum H, Azimian A, Yeats B, Dollery J, Whitson B, Rushing G, Crestanello J, Lilly M, and Dasi LP. Modeling Risk of Coronary Obstruction During Transcatheter Aortic Valve Replacement. Journal of Thoracic and Cardiovascular Surgery, 2019. 159: (3) p. 829-838. Heikemper M, Dasi LP. (2019) ‘Polymeric Heart Valves’ in Kheradvar A Principles of Heart Valve Engineering. Academic Press. 343-359. Fields of Study Major Field: Biomedical Engineering vi Table of Contents Abstract ......................................................................................................................................................... ii Acknowledgements ....................................................................................................................................... iv Table of Contents ........................................................................................................................................ vii List of Tables ................................................................................................................................................ xi List of Figures ............................................................................................................................................. xii Chapter 1: Introduction ................................................................................................................................. 1 Chapter 2: Background ................................................................................................................................. 5 Native Heart Valves .................................................................................................................................. 5 Valvular Heart Valve Disease ................................................................................................................... 9 Acquired.............................................................................................................................................. 10 Mitral Regurgitation........................................................................................................................ 11 Aortic Stenosis ................................................................................................................................ 12 Mitral Stenosis ................................................................................................................................ 13 Aortic Regurgitation ....................................................................................................................... 13 Tricuspid Regurgitation .................................................................................................................. 14 Congenital ........................................................................................................................................... 14 Heart Valve Replacement ....................................................................................................................... 16 Delivery............................................................................................................................................... 16 Surgical ........................................................................................................................................... 16 Transcatheter ................................................................................................................................... 17 Prosthetic Valves ................................................................................................................................ 19 Bioprosthetic ................................................................................................................................... 19 Mechanical ...................................................................................................................................... 23 Tissue Engineered ........................................................................................................................... 26 Polymeric ........................................................................................................................................ 29 History of Polymeric Valves ....................................................................................................... 29 Design Considerations and Challenges ....................................................................................... 33 vii Material ................................................................................................................................... 33 Surface Modifications ............................................................................................................. 37 Geometry................................................................................................................................. 39 Manufacturing ......................................................................................................................... 41 Investigational Valves ................................................................................................................. 43 Summary and Conclusions.......................................................................................................... 47 Chapter 3: Specific Aim 1........................................................................................................................... 49 3.1 3D Modeling of CO .......................................................................................................................... 49 3.1.1 Introduction ........................................................................................................................ 49 3.1.2 Methods.............................................................................................................................. 50 Study Population ..................................................................................................................... 51 Three-dimensional (3D) Computational Model ...................................................................... 54 In Vitro Validation .................................................................................................................. 57 Statistical Analysis .................................................................................................................. 59 3.1.3 Results ................................................................................................................................ 60 Current guidelines (𝒉, 𝑺𝑶𝑽𝒅) ................................................................................................. 60 3D predictive model(𝑫𝑳𝑪/𝒅) ................................................................................................ 61 Comparison to current guidelines ........................................................................................... 62 3.1.4 Discussion .......................................................................................................................... 64 3.1.5 Limitation ........................................................................................................................... 67 3.1.6 Conclusion ......................................................................................................................... 67 3.2 2D Modeling of CO .......................................................................................................................... 69 3.2.1 Introduction ....................................................................................................................... 69 3.2.2 Methods.............................................................................................................................. 71 Study Population ..................................................................................................................... 71 2D Anatomical Models ........................................................................................................... 74 Statistical Analysis .................................................................................................................. 79 3.2.3 Comparison to current guidelines ...................................................................................... 82 3.2.4 Discussion .......................................................................................................................... 85 3.2.5 Limitations ......................................................................................................................... 88 3.2.6 Conclusion ......................................................................................................................... 89 viii Chapter 4: Specific Aim 2........................................................................................................................... 90 4.1 Development of a Polymeric Transcatheter Valve ........................................................................... 90 4.1.1 Introduction ........................................................................................................................ 90 4.1.2 Materials and Methods ....................................................................................................... 92 Valve Stent Design.................................................................................................................. 92 Leaflets’ materials ................................................................................................................... 94 Hemodynamic Parameters ...................................................................................................... 95 Effective Orifice Area (EOA) ................................................................................................. 97 Regurgitant Fraction (RF) ....................................................................................................... 98 Pinwheeling Index (PI) ........................................................................................................... 98 Particle Image Velocimetry (PIV) .......................................................................................... 98 Vorticity Calculations ............................................................................................................. 99 Principal Reynolds Shear Stress (RSS) ................................................................................... 99 Statistical Analysis ................................................................................................................ 100 4.1.3 Results .............................................................................................................................. 100 Hemodynamic Assessment ................................................................................................... 100 Pinwheeling........................................................................................................................... 101 Velocity Vector Field and Vorticity Contours ...................................................................... 102 Reynolds Shear Stress (RSS) ................................................................................................ 104 4.1.4 Discussion ........................................................................................................................ 106 Hemodynamic Assessment and Pinwheeling........................................................................ 106 Velocity and Vorticity........................................................................................................... 107 Reynolds Shear Stress (RSS) ................................................................................................ 108 Polymeric TAVs as an alternative for bioprosthetic TAVs .................................................. 109 4.1.5 Summary .......................................................................................................................... 109 4.1.6 Limitations ....................................................................................................................... 110 4.2 Effect of Leaflet Opening Geometry on Valve Performance and Turbulent Shear Stresses .......... 111 4.2.1 Introduction ...................................................................................................................... 111 4.2.2 Materials and Methods ..................................................................................................... 113 Hemodynamic Performance .................................................................................................. 113 Particle Image Velocimetry (PIV) ........................................................................................ 115 ix 4.2.3 Results .............................................................................................................................. 117 Hemodynamic Assessment ................................................................................................... 117 Velocity Vector Field and Vorticity Contours ...................................................................... 119 Reynolds Shear Stress (RSS) ................................................................................................ 121 4.2.4 Discussion ........................................................................................................................ 122 Chapter 5: Specific Aim 3......................................................................................................................... 124 5.1 Hemodynamic Evaluation of a Fetal Tissue-Engineered Pulmonary Valve ................................... 124 5.1.1 Introduction ...................................................................................................................... 124 5.1.1 Methods............................................................................................................................ 126 Hemodynamic Assessment ................................................................................................... 126 Geometric Orifice Area (GOA) ............................................................................................ 130 Regurgitant Fraction (RF) ..................................................................................................... 130 Pinwheeling Index (PI) ......................................................................................................... 131 5.1.2 Results .............................................................................................................................. 131 5.1.3 Discussion ........................................................................................................................ 134 5.1.4 Future Work ..................................................................................................................... 135 Chapter 6: Summary and Future Work ..................................................................................................... 136 References ................................................................................................................................................. 139 Appendix A: Supplemental Materials pertaining to 3D modeling of coronary obstruction in Section 3.1 .................................................................................................................................................................. 155 Appendix B: Supplemental Materials pertaining to 2D modeling of coronary obstruction in Section 3.2 .................................................................................................................................................................. 159 x List of Tables Table 2.1 Common polymers used in polymeric heart valve engineering (adapted from Polymeric Heart Valves [2]) ....................................................................................................... 31 Table 2.2 Comparison of surface modifications for heart valve leaflets (adapted from Polymeric Heart Valves [2]) ....................................................................................................... 39 Table 3.1 List of coronary obstruction predictive parameters, including currently used parameters namely coronary ostium height, sinus of Valsalva diameter, and newly proposed predictive parameters based on the 3-dimensional computational modeling for each patient [164] ........................................................................................................................ 52 Table 3.2 Coronary obstruction predictive parameters including currently used parameters (coronary ostium height, sinus of Valsalva diameter) and newly proposed predictive parameters based on 3D computational modeling[198] .......................................................... 73 Table 4.1 Measured hemodynamic parameters of each valve[227] ..................................... 101 Table 5.1 Suggested pulsatile test conditions for pediatric populations[303, 304].............. 127 Table A.1 List of material properties for aortic root geometry, including aortic wall, leaflets, and calcium nodules.................................................................................................... 156 Table B.1 Detailed calculations of DLC2D/d (2) for patients Z and AB, who demonstrated the highest possible predicted risk of coronary obstruction with DLC2D/d (2) = 0.0 ........ 159 xi List of Figures Figure 2.1 Schematic of human heart detailing anatomy of the four chambers and the atrioventricular valves (adapted from TheMitralValve.org[9]) ...................................................... 6 Figure 2.2 (A) Posterior view of the human pulmonary valve[11] (B) Prosterior View of the human aortic valve [12] .................................................................................................................. 7 Figure 2.3 schematic of healthy and calcified aortic valve cusp. Cross sectional representation of a heart highlighting the aortic valve ecm structure [15] ................................................................. 9 Figure 2.4 Mitral valve apparatus and etiologies for mitral regurgitation [29] ........................... 12 Figure 2.5 Drawing of Healthy and Stenosed Aortic valve in the closed and open configurations [30] ................................................................................................................................................ 13 Figure 2.6 Schematic showing delivery and expansion of a prosthetic transcatheter aortic valve [41] ................................................................................................................................................ 18 Figure 2.7 (A) The general classification of bioprosthetic valves. (B) The various types of surgical and transcatheter heart valves[49] ................................................................................... 22 Figure 2.8 Seven landmark caged ball valves[50] ....................................................................... 23 Figure 2.9 Photograph of the Bjork-Shirley heart valve[51] ........................................................ 24 Figure 2.10 Examples of bileaflet mechanical valves from a variety of major manufacturers [52] ....................................................................................................................................................... 25 Figure 2.11 Photograph of Xeltis Pulmonary Heart Valve[63] .................................................... 28 Figure 2.12 (A) Polyurethane bileaflet mitral valve by Braunwald et al. [68]; (B) aortic Trileaflet valve made from silicone material by Roe et. al. [69] (Adapted from Polymeric Heart Valves [2])................................................................................................................................................. 30 Figure 2.13 Detailed geometric features of a trileaflet prosthetic heart valves [2]....................... 41 Figure 2.14 A balloon-expandable HA-LLDPE transcatheter valve developed by Dasi Cardiovascular Biofluid Mechanics Lab [2] ................................................................................. 44 xii Figure 2.15 A self-expanding SIBS-Dacron-based transcatheter valve developed by Blustien Biofluids Research Group [2, 143] ............................................................................................... 45 Figure 2.16 Self-expanding TRISKELE valve in sizes 23(left), 26 (center), 29 (right) manufactured from POSS-PCU leaflets [145] [2] ........................................................................ 46 Figure 3.1 Study population characterized by conventional parameters (coronary height [h] < 14 mm and sinus of Valsalva diameter [SOVd] < 30 mm) used to predict left coronary artery obstruction before transcatheter aortic valve replacement (TAVR) with origin located at (12,30), representing a left coronary artery height (LCAh) of 12 mm and a left a sinus of Valsalva diameter (SOVd) of 30 mm . Blue squares represent the only patients who would have been approved for TAVR under these current guidelines. SOV, Sinus of Valsalva diameter; CO, coronary obstruction [164] ............................................................................................................ 54 Figure 3.2 Example of patient specific 3-dimensional modeled aortic root with left coronary artery (LCA), right coronary artery (RCA), and yellow calcific nodules. A. Side view; B. aortic view; C. ventricular view. D. The measured distance (DLC) from a point on cusp/or cusp calcium (Pc) to a point on the upper ostium of the coronary artery (Po) following a transcatheter valve replacement from the idealized root schematic from the side view. E. Example finite element simulated post-transcatheter aortic valve replacement aortic root with DLC from a top view. F. Side view[164] ................................................................................................................ 57 Figure 3.3 A. The 3-dimensional (3D) printed aortic root model was manufactured from TangoPlus (Stratasys, Farmington Hills, Mich) material and VeroWhite (Stratasys) material was used for calcium nodule, both printed using Connex 350 3D printer (Stratasys). B. Particle image velocimetry (PIV) experiments were performed to validate the 3D printed calcified aortic root model and compare with in vivo ultrasound Doppler jet velocity for the patient. Detailed methodology of the PIV experiments may be found in Hatoum and colleagues[168] and Hatoum and colleagues[169] C. Comparison of the temporal velocity profile corresponding to a point located at the exit of the systolic jet of the valve. D and E. Doppler data. As can be seen, the result shows good agreement between the in vivo and in vitro data. The maximum velocity in the PIV data was found to be 2.10 m/second, which compares well with 2.24 m/second obtained from the ultrasound. F. Expansion tool with increasing diameter, which mimics balloon expansion[164] .............................................................................................................................. 59 Figure 3.4 Study population characterized by 3-dimensional predictive model (DLC/d < 0.7) used to predict left coronary artery obstruction before transcatheter aortic valve replacement. Blue dots represent the patients who were be approved for transcatheter aortic valve replacement under these suggested guidelines, red triangles represent those who were not approved and received other treatment, and the green diamond represents the 1 patient in whom transcatheter aortic valve replacement resulted in coronary obstruction (the model was not computed prior). DLC/d, Cusp to coronary ostium distance indexed with coronary artery diameter; CO, Coronary obstruction[164] ............................................................................................................ 61 xiii Figure 3.5 The mean and standard deviations of the parameter values (A) DLC/d, (B) coronary artery height (h), and (C) sinus of Valsalva diameter (SOVd) for those high-risk patients who successfully received a transcatheter aortic valve replacement without coronary obstruction compared with those who did not receive a transcatheter aortic valve replacement successfully. A significant difference between the 2 groups was found for the DLC/d parameter at significance level .05. Neither h nor SOVd was significantly different between the groups. DLC/d, Cusp to coronary ostium distance indexed with coronary artery diameter; TAVR, transcatheter aortic valve replacement; LCAh, left coronary artery height; SOVd, sinus of Valsalva diameter[164] 62 Figure 3.6 A-C. Sensitivity and specificity curves generated for each of the three parameters, DLC/d, coronary artery height (h), and sinus of Valsalva diameter (SOVd), to predict whether transcatheter aortic valve replacement within this high-risk patient population would result in coronary obstruction. LCAh, Left coronary artery height; SOVd, sinus of Valsalva diameter; DLC/d, cusp to coronary ostium distance indexed with coronary artery diameter[164] ....................................................................................................................................................... 64 Figure 3.7 The study population divided by risk of coronary obstruction due to height (h) ≤ 12 (32%), sinus of Valsalva diameter (SOVd) ≤ 30 (14%), height (h) ≤ 12 and sinus of Valsalva diameter (SOVd) ≤ 30 (43%), or height (h) > 12 and sinus of Valsalva diameter (SOVd) > 30 (11%). CO, Coronary obstruction[198] ........................................................................................ 72 Figure 3.8 Idealized schematic representing the calculated minimum distance from a point on leaflet calcium (Pc) to a point on the upper ostium of the coronary artery (Po) following transcatheter aortic valve replacement (TAVR) for the DLC2D/d (1) (A), DLC2D/d (2) (B), and DLC2D/d (3) (C) [198] .......................................................................................................... 76 Figure 3.9 Idealized schematic representing essential aortic root measurements: aortic left chord length (L), left sinus width at coronary ostium (w), left coronary ostium diameter (d), calcium nodule thickness on the left coronary cusp (t), and height of the left coronary artery from the aortic annulus (h) [198] ................................................................................................................. 77 Figure 3.10 Sensitivity and specificity of DLC2D/d (1) (A) and DLC2D/d (2) (B) to predict coronary obstruction in high-risk patients with height (h) < 14 mm and/or sinus of Valsalva diameter (SOVd) < 30 mm[198] ................................................................................................... 81 Figure 3.11 Sensitivity and specificity of DLC2D/d (3) to predict coronary obstruction in highrisk patients with height (h) < 14 mm and/or sinus of Valsalva diameter (SOVd) < 30 mm for varying values of α: (A) α = 0.9; (B) α = 1; (C) α = 1.1; (D) α = 1.2; (E) α = 1.3; (F) α = 1.4[198] ....................................................................................................................................................... 81 Figure 3.12 Comparative box-and-whisker plots for those who underwent successful transcatheter aortic valve replacement (TAVR) and those who did not for DLC2D/d (2) (A) height (h) (B), sinus of Valsalva diameter (SOVd) (C), and DLC/d (D). Upper and lower borders of the box represent the upper and lower quartiles, the middle horizontal line represents the xiv median, and the upper and lower whiskers represent the maximum and minimum values of nonoutliers. Outliers are represented by single dots[198] ............................................................ 83 Figure 3.13 Sensitivity and specificity of DLC2D/d (2) (A), DLC2D/d (2) for the entire population considered for transcatheter aortic valve replacement (TAVR) (B), height h (C); sinus of Valsalva diameter, SOVd (D); and DLC/d (E) to predict coronary obstruction for high risk patients with h < 14 mm and/or SOVd < 30 mm [198] ................................................................ 85 Figure 3.14 Idealized schematic representing the simple 2D anatomic model used to predict the risk of coronary obstruction during transcatheter aortic valve replacement, DLC2D/d, the calculated minimum distance from a point on leaflet calcium, Pc, to a point on the upper ostium of the coronary artery, Po. The optimal percent sensitivity and specificity of the 2D model, DLC2D/d, is compared to with current guidelines, h and SOVd, and a previous computational study of DLC/d to predict coronary obstruction in high-risk patients with h < 14 mm and/or sinus of SOVd < 30 mm.[198] ................................................................ 88 Figure 4.1 3D CAD model of cobalt chromium transcatheter stent frame, detailing stent thickness (0.55 mm), profile (25 mm), and major frame angle (θ = 60°)[227] ............................. 93 Figure 4.2 A. HA-TAV profile of stent frame and semi-closed leaflet position B. HA-TAV profile so stent frame and open leaflet position[227] ................................................................... 95 Figure 4.3 Schematic of left heart flow simulator[227] ................................................................ 96 Figure 4.4 Aortic flow (blue) and pressure (green) conditions that the valves were subject to over one cardiac cycle [227] ................................................................................................................. 97 Figure 4.5 En-face views of each valve at peak systole and mid diastole[227] ......................... 102 Figure 4.6 Phase averaged velocity vectors and vorticity contours throughout the cardiac cycle[227] ................................................................................................................................... 103 Figure 4.7 Phase averaged Principle Reynolds shear stresses (RSS) throughout the cardiac cycle [227] ............................................................................................................................................ 104 Figure 4.8 Normalized frequency of Principal Reynolds shear stress at the defined phases in the cardiac cycle[227] ....................................................................................................................... 105 Figure 4.9 3D printed orifices of round and triskele-like geometries in both small and large size ..................................................................................................................................................... 113 Figure 4.10 Schematic diagram of experimental set up .............................................................. 114 Figure 4.11 Locations of 7 pressure measurements along centerline of idealized aortic root chamber ....................................................................................................................................... 115 xv Figure 4.12 Pressure gradient at peak flow rate for each large orifice geometry measured experimentally............................................................................................................................. 118 Figure 4.13 Pressure gradient at peak flow rate for each small orifice Geometry measured experimentally............................................................................................................................. 119 Figure 4.14 Phase averages velocity vectors and vorticity contours throughout the cardiac cycle ..................................................................................................................................................... 120 Figure 4.15 Phase averaged Principle Reynolds shear stresses (RSS) throughout the cardiac cycle ............................................................................................................................................ 121 Figure 5.1 Examples depicting infected Melody Valves at time of explant from Ref[299]. (A) Melody valve removed surgically 1 week after the onset of infective endocarditis. Complete valve obstruction by vegetations is visible. (B) Melody valve removed surgically 2 years after the onset of infective endocarditis. Cultures of the valve were negative. Severe deterioration of the valve is visible, with budding on the leaflets. ....................................................................... 125 Figure 5.2 Proof-of-concept fetal valve chamber compatible for transcatheter implantation .. 127 Figure 5.3 Custom Transcatheter Fetal Valve Chamber ............................................................ 128 Figure 5.4 Schematic of the Fetal right heart pulse duplicator .................................................. 129 Figure 5.5 Kinematic viscosity of varying molarities of NaOH as compared to 40% glycerol 130 Figure 5.6 Representative pulmonary flow curves for 150 beats/min and average cardiac output of 1.4 L/min ................................................................................................................................ 132 Figure 5.7 Tissue-engineered pulmonary valve opening across the cardiac cycle .................... 133 Figure 5.8 Cross sectional flow through Tissue-Engineered Pulmonary valve scaffold .......... 133 Figure A1. All the patient-specific tissues, including aortic wall, leaflets, and calcium nodules were discretized using an explicit 4-node linear tetrahedron element, whereas the cylindrical stent was discretized using hexahedron elements. The total number of mesh elements for each patient was 35,580, 2835, and 3240 elements for the wall, the leaflets, and the calcium nodule, respectively. A, Calcification on the leaflets. B, The 3 cusps. C, Aortic root, including coronary arteries[164] ................................................................................................................................ 155 Figure A2. A, Aortic and ventricular views of the reconstructed patient aortic roots. The aortic views are oriented with the commissure of non- and left coronary cusps at the top. In the ventricular views, the top commissure corresponds to the left and right coronary cusps. Calcific nodules (yellow) are highlighted for each patient and the morphology of the calcific lesions are noted to be highly patient specific. B, The cross-sectional views of finite element analysis xvi generated geometries after transcatheter aortic valve replacement for the respective patients shown in A. Cross-sectional views depict both left and right coronary ostia to illustrate the final position of leaflets and calcific lesion relative to the respective coronary ostia center. LCA, Left coronary artery; RCA, right coronary artery[164] ...................................................................... 157 Figure A3. Comparison between in vitro validation technique for stent size 26 mm and the finite element analysis simulation with simulated transcatheter aortic valve expanded to a diameter of 26 mm. The final measurements are plotted with the y-axis showing the measured distance between native leaflets to the left coronary ostium (DLC) [164] ............................................... 158 xvii Chapter 1: Introduction Heart disease is the leading cause of death in the United States, killing more than 600,000 Americans each year [1, 2]. Valve disease, one subset of heart disease, results in over 290,000 heart valve replacements annually worldwide, and that number is estimated to triple to over 850,000 by 2050[3, 4]. The intricate structure of heart valves allows them to beat an astonishing 2.5 billion times over an average lifetime, ensuring adequate blood flow inside the heart, to the lungs, and to the rest of the body. In addition to their durability in a highly dynamic environment over a lifetime, heart valves grow with us as we grow, adapting to the increased blood output required as adults. Heart valve diseases, whether acquired or congenital, can be fatal if left untreated. Diseased heart valves are unable to repair themselves, and because there are no drugs that exist to treat heart valve disease, all medical interventions are based on surgical or transcatheter repair or replacement of the valve with dedicated devices. Transcatheter heart valve replacement, a minimally invasive approach originally developed to treat patients with aortic valve disease for whom surgical replacement was high-risk, has recently gained approval for low-risk patients suffering from aortic stenosis[5]. This approval has the potential to fundamentally shift the standard of care for aortic valve disease away from surgical valve replacement and will likely have implications for all heart valve replacements. While short-term data suggests that transcatheter valve replacement has equivalent or superior patient outcomes compared with surgical counterparts[6-8], the limitations associated with 1 bioprosthetic fixed-tissue valves remain. Additionally, the widespread use of transcatheter valves presents new and unique challenges. The number of investigational and clinically used devices to diagnose, inform treatment of, and treat valvular diseases have multiplied in the last 20 years, offering significant advances to the field and multiple options for patients. Even so, severe complications still occur during repair and/or replacement, and every device option has its limitations. From an engineering perspective, there is significant room for improvement within the field of heart valve replacement therapies. The objective of this study was to engineer solutions towards the optimization of transcatheter valve replacement therapy. The overarching hypothesis of this study is that transcatheter valve replacement therapies can be improved to mitigate adverse outcomes associated with current devices in specific populations. The following specific aims test this hypothesis. Specific Aim 1: Develop novel mechanistic index to accurately assess risk of coronary artery obstruction during TAVR within intermediate to high surgical risk patients and compare to clinical guidelines • Presumed risk for coronary obstruction can exclude patients (often with no other treatment options) from TAVR • Coronary obstruction occurs despite clinical guidelines for risk assessment • Mechanistic understanding of coronary obstruction is needed to accurately identify at risk patients Specific Aim 2: Engineer, characterize and improve upon a novel transcatheter heart valve for adults suffering from aortic stenosis 2 • Durability is important for patients who need a replacement heart valve, particularly for those earlier in life • Current transcatheter valve prostheses have limited durability compared to mechanical surgical valves, limiting their use within younger patient populations • A novel transcatheter heart valve could be engineered to have an extended life time beyond that of the prostheses that are currently available Specific Aim 3: Develop a novel methodology to characterize and improve upon a tissueengineered replacement heart valve for children with life threatening congenital heart defects • Children with born with congenital heart defects have unique and complex valve replacement needs including high durability and growth capacity • Currently, no commercially available device meets these requirements • Assessing the function of tissue-engineered prototypes is costly and time consuming This dissertation will cover these aims and includes a brief background on native heart valve anatomy and physiology, common valve diseases, and the current clinical standards for valve replacement therapies. This background along with a literature review of investigational device research is included in Chapter 2. Specific Aim 1, which details the development of two computational methods to predict coronary obstruction, can be found in Chapter 3. Chapter 4 and 5 are devoted to the device development and characterization in specific aims 2 and 3 respectively. Chapter 6 includes a summary of the completed work and discusses possible avenues for future work. 3 4 Chapter 2: Background Native Heart Valves The heart’s main function is to circulate blood to the body and lungs through the circulatory system. A healthy human heart is made up of four chambers, with one atrium and one ventricle on each the left and right side of the heart. The atria receive blood from the surrounding circulatory system, while the right ventricle functions to pump blood to the lungs and the left functions to pump oxygenated blood to the rest of the body. In addition to the four chambers within the heart, there are four heart valves that are essential to ensuring the unidirectionality of blood flow. Two of the four valves, called atrioventricular valves, reside between atria and ventricle. The other two valves, called semilunar valves, regulate blood flow out of the ventricle. Atrioventricular valves have a tension apparatus that allows them to close under pressure. The tension apparatus consists of chordae tendineae and the papillary muscles. The chordae tendineae extend from the valve leaflets and connect to the papillary muscles on the ventricle wall. On the right side of the heart, the atrioventricular valve is called the tricuspid valve and on the left side, the atrioventricular valve is called the mitral valve. The tricuspid valve has three leaflets while the mitral valve has only two. During systole, the portion of the cardiac cycle where blood is ejected from the ventricles, both the tricuspid and mitral valves remain closed by tension of the chordae tendineae. A schematic showing a cross sectional view of the heart and details of the atrioventricular valves is shown in Figure 2.1. 5 FIGURE 2.1 SCHEMATIC OF HUMAN HEART DETAILING ANATOMY OF THE FOUR CHAMBERS AND THE ATRIOVENTRICULAR VALVES (ADAPTED FROM THEMITRALVALVE.ORG[9]) Unlike the atrioventricular valves, semilunar valves do not have chordae tendineae or papillary muscles. They each contain three pocket-like leaflets arranged in a way that allows competency without relying on tension [10]. The leaflets meet at three commissures, which aid in anchoring the valve leaflets to the wall. The unique geometry of the semilunar valves creates a bulbous space behind the leaflets known as Sinuses of Valsalva. On the right side of the heart, the semilunar valve is called the pulmonary valve (Figure 2.2A) and on the left side of the heart, the semilunar valve is called the aortic valve (Figure 2.2B). In the aortic valve, coronary arteries 6 protrude from two of the three sinuses, the left and right, and function to supply blood to the heart muscle. FIGURE 2.2 (A) POSTERIOR VIEW OF THE HUMAN PULMONARY VALVE[11] (B) PROSTERIOR VIEW OF THE HUMAN AORTIC VALVE [12] Heart valves are arguably the most mechanically active connective tissues within our bodies [13]. While primarily passive structures, with every heartbeat heart valves are subject to cyclic bending, laminar shear stress, oscillatory shear stress, and tensile stretch [14]. The microstructure within the valve tissues, called the extracellular matrix (ECM), is responsible for the heart valve response to its dynamic environment by transmitting the mechanical stresses and strains to the valve cells [13]. These interactions mediate the response of the valve cells, called mechanobiology, and have been shown to be relevant to healthy valves, diseased valves, and to the development of tissue engineered valves. 7 Atrioventricular and semilunar valve leaflets have similar ECM structures, with each composed of three layers: the elastic layer, spongiosa, and fibrosa. In the atrioventricular valves, the elastic layer is called the atrialis, and in the semilunar valves it is called the ventricularis. Within the atrialis and ventricularis, elastic fibers are oriented radially, from leaflet hinge to coapting free edge, allowing for the extension and recoil of this layer as the valve opens and closes[10]. The middle layer of, called the spongiosa, is largely composed of proteoglycans (PGs) and glycoaminoglycans (GAGs) and is thought to function like a cushion, providing resistance to compression and flexibility [13]. The final layer, which does not directly contact the main flow, is the fibrosa. The fibrosa consists of a dense connective tissue that contains circumferentially oriented Type 1 collagen, which adds stiffness and strength to the valve. Figure 2.3 shows a schematic of the ECM structure for a native healthy and calcified aortic valve leaflet. The valve leaflets contain two primary cell types: valvular endothelial cells (VECs) and valvular interstitial cells (VICs) [13]. VICs make up the majority of the cell population within the valve leaflets and are believed to be fundamental to the maintenance and function of the valves. Specifically, VICs maintain structural and compositional integrity through remodeling of the ECM [13]. VECs on the other hand, line the outer surfaces of the leaflets. While in many ways similar to vascular endothelial cells, valvular endothelial cells align perpendicular to the direction of shear stress rather than in parallel. 8 FIGURE 2.3 SCHEMATIC OF HEALTHY AND CALCIFIED AORTIC VALVE CUSP. CROSS SECTIONAL REPRESENTATION OF A HEART HIGHLIGHTING THE AORTIC VALVE ECM STRUCTURE [15] Valvular Heart Valve Disease Valvular heart disease is an important and growing public health concern. In developed countries, the rate of degenerative heart valve disease is growing with older populations. However, most valvular heart disease is due to rheumatic heart disease[16]. In either case, heart valve disease manifests in three basic ways: regurgitation, stenosis, and atresia [17]. Regurgitation occurs when a compromised valve is unable to close tightly and backflow into a chamber of the heart occurs. Stenosis is the thickening or stiffening of valve leaflets that prevents the valve from opening fully, and thus limits the amount of blood that can flow through the valve. Atresia occurs if a heart valve does not contain an opening for blood to flow through. Heart valve diseases can be acquired or congenital, meaning developed before birth. Acquired heart valve disease usually affects the aortic or mitral valve and congenital heart valve disease more often affects the pulmonary or aortic valves [17]. In any case, diseases of the heart valves 9 are linked to changes in cellular function and ECM integrity, though the exact mechanisms causing these changes are still being investigated [13]. Acquired The majority of deaths due to valvular heart disease worldwide are due to acquired rheumatic heart disease (RHD) [16], and it has been estimated that RHD affects about 33.4 million people [18]. RHD is preceded by acute rheumatic fever, which is caused by an infection of the tonsillopharynx, often in children [19]. Rheumatic fever leads to progressive valve inflammation and fibrosis [19], and most commonly affects the mitral valve. From early to midlife populations, mitral regurgitation is the primary ailment, while with age, mitral stenosis becomes predominant [16]. In approximately one third of all RHD cases, both the mitral valve and aortic valve are affected, and RHD is close to twice as likely to occur in females than in males [20, 21]. The reduction in RHD in developed countries can be explained by the association between rheumatic fever with poor living conditions and minimal access to antibiotics. Even with significantly reduced rates of RHD, VHD is still a great health burden in developed countries. In 2006, it was estimated that approximately 2.5 % of the US population had moderate or severe VHD and that number is expected to continue growing as the average life expectancy does [22]. The vast majority of deaths due to VHD in the US and other developed countries are due to degenerative valve disease, rather than RHD [16]. Degenerative valve disease often involves progressive thickening, fibrosis and/or calcification of valve leaflets and worsens with age [22-24]. Acquired VHD is more common in the valves of the left heart with 10 mitral regurgitation and aortic stenosis as the two most common valvular heart diseases in developed countries, with mitral stenosis and aortic regurgitation close behind. In the right heart, tricuspid regurgitation is the most frequent VHD. Mitral Regurgitation Mitral regurgitation (MR) is the most frequent VHD [16], with prevalence of moderate to severe MR at 1.7% in the United States [25]. Caused by degenerative disease 61.3% of the time, MR can also be caused by Rheumatic (14.2%), ischemic (7.3%) and congenital heart disease (4.8%) [25]. MR is categorized into primary MR and secondary MR, where primary refers to mitral leaflet dysfunction while secondary, or functional MR, is caused by abnormal function of the chordae tendinea and the papillary muscles, often caused by diseased left ventricle or enlarged left atrium[26]. Schematic representations of primary and functional MR etiologies are shown in Figure 2.4. Primary MR is most often due to mitral valve prolapse, a degenerative process that results in a fibroelastic deficiency [27, 28], though in rare cases can be associated with disorders affecting connective tissue such as Marfan or Ehlers-Danlos syndromes. Secondary MR, is usually due to cardiomyopothies that result in ventricular remodeling and enlargement that disrupts mitral leaflet coaptation [16]. Both primary and secondary MR result in a compromised valve that cannot prevent blood flow from re-entering the left atrium. 11 FIGURE 2.4 MITRAL VALVE APPARATUS AND ETIOLOGIES FOR MITRAL REGURGITATION [29] Aortic Stenosis Aortic stenosis (AS) is a degenerative disease in which calcification build up, in a process similar to atherosclerosis, inhibits the flexibility of the aortic valve leaflets to the point that outflow obstruction occurs [23, 24]. Figure 2.5 depicts the differences between normal and stenotic aortic valves. In 81.9% of AS cases, degenerative disease is the underlying cause, though RHD and congenital heart disease can also cause AS [25]. At 33.9 % of all native VHDs, AS is the most frequent valve disease seen in the hospital or clinic [25]. 12 FIGURE 2.5 DRAWING OF HEALTHY AND STENOSED AORTIC VALVE IN THE CLOSED AND OPEN CONFIGURATIONS [30] Mitral Stenosis Like aortic stenosis, mitral stenosis (MS) results in an inflexible valve that inhibits blood flow. Due to its primary cause being RHD, mitral stenosis is the least common VHD in the United States [22], though it can also occur due to calcification of the mitral annulus. The prevalence of MS increases with age and is more common in women[31]. Aortic Regurgitation Like mitral and aortic stenosis, the prevalence of aortic regurgitation (AR) increases with age. In the entire US population, prevalence of aortic regurgitation is 0.5% [22]. Degenerative 13 AR accounts for 50.3% of all AR presenting to a hospital or clinic, while 15.2% is caused by RHD, 15.2% is related to congenital heart disease, and 7.5% is due to infective endocarditis [25]. Similarly to MR, AR is categorized into primary AR and secondary AR. Examples of pure valve dysfunction include bicuspid valve, rheumatic disease, or infection, while examples of causes of secondary AR include aortic root dilation, aortitis, acute dissection, systemic hypertension, or other systemic disease including Marfan syndrome and Ehlers-Danlos [16]. In either case, the ability of the aortic valve to prevent backflow into the left ventricle is compromised. Tricuspid Regurgitation Tricuspid regurgitation (TR) is the most frequent valvular disease of the right heart, though the prevalence of moderate or severe TR is only 0.8% [16]. Like mitral and aortic regurgitation, TR is the inability for the valve to prevent backflow, in this case between the right atria and right ventricle. Tricuspid regurgitation can result from enlargement of the right ventricle, often secondary to pulmonary hypertension, which can inhibit the tension mechanism of the tricuspid valve and is often accompanied by MR[16]. Other causes of tricuspid valve regurgitation include pulmonary hypertension, RHD, and Marfan’s syndrome. Congenital Congenital valve diseases are diseases present before birth that occur during fetus development. In this section, only a few of the most common congenital heart diseases will be discussed. Some congenital heart defects, including bicuspid aortic valve, can go unnoticed entirely often only presenting with symptoms in mid-late adulthood if at all. Bicuspid aortic valve is one of the most common congenital defects and affects approximately 2% of the 14 population with a strong male predominance[32]. In this disease, the aortic valve develops with only two leaflets rather than three, which can lead to stenosis or regurgitation. Often, patients are unaware of their bicuspid disease until adulthood, where symptoms including shortness of breath and difficulty exercising can present. Pulmonary valve stenosis is also common and can occur from the fusion of one or two valve leaflets or an abnormally small valve structure that obstruct blood flow to the lungs. Hypoplastic left heart syndrome (HLHS) which represents 2% -9% of congenital heart disease cases, is one CHD that is fatal to a fetus without immediate and ongoing intervention. In this disease, the left ventricle is underdeveloped, and the valves of the left heart can be underdeveloped or even completely closed off. The ductus arteriosis (a hole between the aorta and the right atrium) remains open at birth allowing oxygenated and deoxygenated blood to mix, though the heart is unable to pump and supply adequate oxygenated blood to the body. Ongoing research suggests that fetal interventions, such as balloon valvuloplasties, can be performed to open the valves and restore blood flow to the aorta during development, aiding in the return of normal blood circulation. Still, surgical interventions at birth and twice more are required to keep up with the growing bodies demand on the heart. A full heart transplantation or a series of three surgical interventions is required for management of this disease. The three surgical interventions include the Norwood procedure within a few days of birth, the Glenn procedure between 3-6 months of age, and then a Fontan surgery at 1 and ½ to 5 years of age. More details regarding these procedures can be found elsewhere[33]. 15 Heart Valve Replacement With no medical therapy to reverse valvular disease currently available, heart valve replacement or repair are the only tools to restore basic and necessary function to the heart. While minimally invasive repair techniques are currently are being studied, few are commercially available. In this section we will focus on valve replacement only. Delivery Heart valves can be replaced with prosthetic valves through open heart surgery or less invasive catheter based delivery techniques. Heart valve replacements with open heart surgery have been performed by cardiothoracic surgeons since the 1960’s, while the minimally invasive techniques were more recently developed and accepted in the early 1980’s[34]. Surgical The surgical replacement of heart valves was introduced in the 1960’s with the introduction of the Ross procedure, where a patient’s own pulmonary valve would be used to replace a failing aortic valve and a mechanical prosthesis would replace the pulmonary valve. Surgical replacement of the aortic valve was first performed in 1961 [35]. The surgery requires a sternotomy and that the patient’s blood be filtered through a heart lung bypass machine throughout the duration of the surgery. The prosthetic valve is then hand sutured into its anatomical position, the blood is returned to the patient’s heart, and the chest is closed. Surgical valve replacements often have long recoveries, and associated risks including the use of full anesthesia. Some comorbidities including severe obesity, prior sternotomies, and old age can prohibit a patient from undergoing this surgery. 16 Transcatheter Transcatheter based therapies for valve replacement were born out of need for patients who were at too high of risk to undergo an open-heart surgery. For aortic valve replacement surgeries studied in the Euro Heart Survey in 2001, one-third of patients across 25 countries with symptomatic aortic stenosis were not referred for surgical replacement due to the associated risks[36]. Due to the many contradictions facing surgical valve replacement in the 1980’s, a pioneering group that would come to be recognized as Percutaneous Valve Technologies (PVT) in 1999, set out to create a less invasive therapy[37]. Originally, this therapy was balloon aortic valvuloplasty (BAV) which essentially expanded a balloon inserted through standard catheterization techniques in a calcified aortic valve to expand its opening. While initially enthusiastic, the medical community soon recognized the technology only provided temporary relief of symptoms and a modest survival benefit due to the high incidence of early valve restenosis[38]. BAV still remains as a palliative option in patients unable to undergo surgical or transcatheter valve replacement and as a bridge to replacement in some cases. Following the initial introduction of BAV, the same group modified the procedure and began placing a balloon expandable stent frame containing a valve structure inside of the native calcified valve. The initial results were promising, with the first ever stented-valve (a bovine jugular vein in a metallic stent) used to treat degenerative ventriculo-pulmonary conduits in children in 2000 [39]. This initial success gave momentum towards implanting a stented valve in a calcified aortic valve, though the aortic valve presented unique challenges. After many device iterations and animal trials in various anatomic locations, the first-in-human transcatheter valve 17 replacement was performed with success in 2002 on a 57-year-old patient with severe aortic stenosis and multiple comorbidities contraindicating surgical replacement[40]. Briefly, the transcatheter valve replacement procedure includes the crimping of a stent onto a balloon, insertion of the device into a catheter, and insertion of the catheter through a vein or artery. The stent and balloon are then navigated with imaging guidance up to the correct location (inside of the aortic valve), the balloon is expanded opening the stented valve, and lastly the balloon is deflated and removed with the catheter. This process is depicted in Figure 2.6. FIGURE 2.6 SCHEMATIC SHOWING DELIVERY AND EXPANSION OF A PROSTHETIC TRANSCATHETER AORTIC VALVE [41] 18 Prosthetic Valves Of course, for heart valve replacement to be possible at all, surgically or through a catheter, functional replacements must be available. Many researchers and interdisciplinary teams have set out to develop effective and long-lasting solutions for replacing damaged heart valves, though the task is fraught with significant technical issues[42]. In all prostheses, the goal is to create a functional valve, that remains functional without adverse outcomes over a lifetime. Arguably none of the commercially available or investigational devices can claim they have achieved this goal, which contributes to the multifaceted decision process surrounding which prosthesis to implant[43]. In this section, we will give a brief background and discuss the current status of the four categories of heart valve prostheses including bioprosthetic, mechanical, polymeric, and tissues engineered heart valves. Bioprosthetic Bioprosthetic heart valves are the most commonly implanted surgical valve prosthesis with approximately 80% of all patients receiving one [44] and the only type of commercially available transcatheter valve. Bioprosthetic heart valves can be broken up into three general categories: human tissue valves, xenografts and transcatheter heart valves. A schematic showing these categories and their subcategories is shown in Figure 2.7a. Xenografts and transcatheter heart valves are made from animal tissue, most often of porcine valve leaflets or bovine pericardium in a tri-leaflet configuration[43]. Originally, xenograft tissue was mounted on a metallic stent using many sutures, but other designs have developed more recently including 19 stentless bioprostheses (ex. Edwards Prima Plus[45]) and sutureless bioprostheses (ex. Sorin Perceval S[46]). Examples of commercially available stented and stentless bioprosthetic surgical and transcatheter heart valves can be found in Figure 2.7b. In order to eliminate rejection of foreign biological tissues, xenograft valves are chemically fixed using glutaraldehyde. The main advantage to chemically-fixed tissues is that these decellularized tissues most closely resemble the ECM structure and function of the native human heart valve, without causing the immunogenic rejection response that can occur with homograft or untreated xenograft valves [47]. Additionally, chemically-fixed tissues have reduced risk of thrombosis, eliminating the need for anticoagulation therapy that is necessary for mechanical surgical heart valves. The current fixed-tissue valve leaflet technology also employs anti-calcific treatments, which increase their lifetime free from structural valve degeneration. The excellent hemodynamic function of this material due to these properties is one reason bioprosthetic are the only type of commercially available transcatheter prostheses. While the anti-calcification treatments have limited the calcification response, calcific aortic valve disease and subsequent valve degeneration is still an issue with the long-term durability of fixed-tissue valves, which is especially important factor for implantation into younger patient populations. For this reason, bioprosthetic valves are favorable in many moderate- to high-risk patients of advanced age [43]. According to the 2017 AHA/ACC guidelines for the management of patients with valvular disease, bioprosthetic valves are recommended for use in patients over 70[48]. For younger patients, lifetime of the replacement valve is often of major concern. Additionally, the fixed sizes and shapes of the fixed tissue limits 20 the manufacturability of tissue valves. Difficulty in manufacturing and chemical fixation, in addition to storage in glutaraldehyde, also leads to increased costs. 21 FIGURE 2.7 (A) THE GENERAL CLASSIFICATION OF BIOPROSTHETIC VALVES. (B) THE VARIOUS TYPES OF SURGICAL AND TRANSCATHETER HEART VALVES[49] 22 Mechanical The first mechanical heart valves were developed by Dr. Charles Hufnagel, a professor of experimental surgery at Georgetown Medical Center in 1952 [50]. His design, which would come to be known as the ball valve or caged ball, is shown in Figure 2.8, along with the subsequent ball valves that were developed. The Hufnagel ball valve contained a methacrylate chamber and methacrylate ball that were inserted into the descending aorta for patients suffering from aortic sufficiency. Some of these valves functioned for 30 years without significant wear[50]. As shown in Figure 2.8, the ball valve went through significant iterations, across many different groups, with the Star-Edwards ball valve as the most highly recognized. One iteration FIGURE 2.8 SEVEN LANDMARK CAGED BALL VALVES[50] of the Starr-Edwards ball valve designed for mitral valve implantation introduced in 1966 is still in production today, and continues to be used in developing countries for its reasonable cost[50]. 23 While many other groups pursued ball in cage valve designs, the next major mechanical valve prostheses were tilting disc valves made of pyrolitic carbon. The most famous of these being the Bjork-Shirley valve, a convexo-concave tilting disc (shown in Figure 2.9) which was made of pyrolyte disk and was developed to provide a larger flow orifice than previously designed valves. First implanted in 1975, the valve production ceased in 1986 due to a significant amount of catastrophic mechanical failures that have since resulted in approximately 130 patients of the 86,000 patients implanted with this valve receiving financial compensation [50]. FIGURE 2.9 PHOTOGRAPH OF THE BJORK-SHIRLEY HEART VALVE[51] 24 Bileaflet mechanical heart valves ae the third and final category of mechanical prostheses. These valves create three outflow areas designed to create a more uniform central flow and better hemodynamics than the ball and cage or tilting disc valves and are the most common mechanical valve type used today [43]. The St. Jude Medical bileaflet mechanical valve (St. Jude Mechanical Regent in Figure 2.10) was the first bileaflet valve to show favorable results in aortic valve replacement and mitral valve replacement without mechanical failures[53] and remains the most communally used bileaflet valve, even without significant changed from its original 1970’s design [43]. Other commercially available bileaflet mechanical valves can be found in Figure 2.10. FIGURE 2.10 EXAMPLES OF BILEAFLET MECHANICAL VALVES FROM A VARIETY OF MAJOR MANUFACTURERS [52] The main advantage of mechanical heart valve prostheses are their durability and freedom from reoperation. For this reason, they are often the choice for younger adult patients and children. Unfortunately though, regardless of the type of mechanical valve, lifelong anticoagulant therapy is required following implantation of mechanical prostheses in order to 25 reduce the risk of thrombosis formation that often localizes in the valve hinges due to complex and unsteady flows[52]. The prescribed anticoagulation therapy, often warfarin, increases the risk of major bleeding complications[54] which can limit patients’ ability to participate in activities that have an increased risk of injury, and can be of high concern for elderly patients that have risk of falls. The need for this anticoagulation therapy becomes increasingly complex for pregnant women, as pregnancy raises the risk for thrombosis. Additionally, there are potentially adverse effects of anticoagulation therapy on fetus development [55] and increased risk of bleeding complications rise during labor and delivery. Therefore, the use of mechanical valves is limited in young female patients, as many hope to become pregnant in the future[43]. In addition to risks of thrombosis that require anticoagulation therapy, there remain concerns of associated noise and probable platelet activation and hemolysis due to the bileaflet valve geometry. While the current designs have significantly reduced the amount of associated noise with mechanical valves, some patients still can hear a clicking noise that can lead to trouble sleeping and social embarrassment[56, 57]. Regurgitant jets at the leaflet hinged have been shown to create regions of high velocity and viscous shearing, leading to platelet activation and hemolysis[58]. Geometry optimization and addition of surface coating are avenues being studied to reduce platelet activation, thrombus formation, and protein aggregation associated with mechanical valve prostheses[43]. Tissue Engineered Although the commercially available mechanical and bioprosthetic heart valve prostheses improve the quality of life for patients, neither is without limitations. The common limitation of 26 both of these prostheses is that as non-living tissues, they are incapable of adaptation and growth in response to environmental changes[59]. Native heart valve mechanical function is a passive process driven by transvalvular pressure gradients, though the valves are still living tissues with structure and function that vary with age[60]. Remarkably, heart valve growth is not restricted to prenatal and early postnatal stages, but instead grows continuously throughout life[59]. The concept of tissue engineered valves is particularly promising when considering children with congenital valve defects that at present, require multiple reoperations inherent to non-living prostheses. For adult patients, tissue engineered valves also pose significant improvements, especially for those patients that require redo surgeries or minimally invasive prosthetic replacements due to structural valve degeneration common to bioprosthetic prostheses. There exists three essential pillars of tissue engineering: the scaffold (which ultimately degrades), the cells which can be seeded in vitro or recruited in vivo, and the mechanical and inflammatory response signaling following implantation[61]. While promising, heart valve tissue engineering has yet to be established as a routine clinical option, as the complexity of the technology has made it difficult for potential in vitro engineered valves to progress to the stage of clinical trials[59]. For tissue engineered heart valves to become a clinical option all standard design criteria for traditional tissue valve (including biocompatibility and durability) must be met, and there must be a complete understanding of the active behavior of the cells in the regulation of tissue growth remodeling, and homeostasis[62]. While many investigational polymer- based (non-allograft, xenograft) tissue engineered heart valves have been successful in large animal models, to date only one has advanced to 27 clinical trials[61]. Xeltis Pulmonary Valve, a tissue engineered pulmonary heart valve prosthesis, recently demonstrated good functionality at one-year follow-up in a U.S. clinical trial and promising re-intervention rates compared to gold standard treatment[63]. Xeltis, shown in Figure 2.11, is made from a commercial polymer RestoreX, which is designed to enable the natural restoration of heart valve function, through endogenous tissue restoration (ETR). While the details of the polymer are not published, we know that ETR is enabled by bioabsorbable polymers. While the pulmonary valve version with this technology is used clinically, the aortic position valve is still pre-clinical. FIGURE 2.11 PHOTOGRAPH OF XELTIS PULMONARY HEART VALVE[63] 28 Polymeric The main focus of this section will be to introduce the field of polymeric heart valve (PHV) engineering and to provide a brief overview of the current investigational technology and the challenges facing further development of PHVs. The discussion will be limited to flexible leaflet polymeric heart valves, intended for use in the aortic and mitral positions. HISTORY OF POLYMERIC VALVES Flexible leaflet polymeric heart valves were first introduced in the late 1950’s, with contributions from Akutsu[64], Berge[65], Braunwald[66], and Roe[67]. The first known mitral valve implantation occurred in 1960 by Braunwald[68] in which plaster casts of explanted human mitral valves were used to make molds for liquid polyurethane (Figure 2.12 (A)). In 1969, Roe[69] reported the first known polymeric aortic valve implantation, made from a silicone material (Figure 2.12 (B)).). 29 FIGURE 2.12 (A) POLYURETHANE BILEAFLET MITRAL VALVE BY BRAUNWALD ET AL. [68]; (B) AORTIC TRILEAFLET VALVE MADE FROM SILICONE MATERIAL BY ROE ET. AL. [69] (ADAPTED FROM POLYMERIC HEART VALVES [2]) The progression of development of PHVs continued slowly following these trials, partially due to the success of the Starr-Edwards ball-and-cage valve[70] and partially to the evolution of percutaneous mitral valve repair technologies[71]. From their start in the 1960’s using silicone and polyurethane, various designs of flexible leaflet PHVs have been developed from polymeric materials including polytetrafluoroethelyne (PTFE), various polyurethanes including polycarbonate urethane (PCU), polyether urethane (PEU), polyvinyl alcohol (PVA), polydimethylsiloxane- polyhexamethylene oxide (PDMS-PHMO), polyhedral oligomeric silsequioxane-polycarbonate urethane (POSS-PCU) and poly(styrene-block-isobutylene-blockstyrene) (SIBS). A summary of the outcomes of these materials for use in PHVs, including the advantages and shortcomings of each, can be found in Table 2.1. 30 Materi Advantages al PU’s Viscoelasticity, resistance to tearing Disadvantages Silicon e Biocompatability, Elastic/flexural properties PTFEs Hydrophobic, low coefficient of friction, low surface tension PCU PEU Resistance to oxidation and hydrolysis Reduction in calcification and thromboembolic events, viscoelasticity PVA Non-toxic, biocompatible, bio-stable HALLDP E XSIBS Biocompatible, high tensile and tear strength, reduced thromboembolic potential Biostable, Resistance to hydrolysis, oxidation, enzymatic activity Thrombosis, Calcification, Hydrolysis Durability/tearing/stiff ening Thrombus formation Fluid absorption Calcification, Leaflet stiffening, Instances of thromboembolism Calcification Low resistance to oxidation and hydrolysis Potential for foreign body response None yet reported None yet reported Referenc es 35,19,4451 29,30,31, 32,33,34 26,30,35 51,52 46,49 56-59,62 63-66 25,64,67 Table 2.1 Common polymers used in polymeric heart valve engineering (adapted from Polymeric Heart Valves [2]) Each of these were attempts to produce a valve with significant improvement over mechanical and bioprosthetic options. In 1968, a list of “Nine Commandments” for the development of a prosthetic heart valve was issued by Edwards laboratories[72], an adaption from Dwight 31 Harken’s famous “Ten Commandments” in 1967[73]. The “Nine Commandments”, still of major importance in the engineering process today, are as follows: “ 1. Embolism Prevention 2. Durability 3. Ease and Security of Attachment 4. Preservation of Surrounding Tissue Function 5. Reduction of Turbulence 6. Reduction of Blood Trauma 7. Reduction of Noise 8. Use of Materials Compatible with Blood and Tissue 9. Development of Methods of Storage and Sterilization “. While the progress in PHV engineering that meets these many requirements has been slow, most often due to limited in vivo durability as a result of material degradation, thrombosis, and calcification[74], [75], [76, 77], the field is constantly expanding. Over the past two decades there has been remarkable progress in polymer synthesis methods resulting in improved material properties[75] which have restored hope to the PHV engineering community. 32 DESIGN CONSIDERATIONS AND CHALLENGES Since approval for use in humans is unlikely unless the next generation of PHVs meet or exceed the functional durability and hemocompatability of THVs currently on the market[74], the material, surface modifications, and geometric design of emerging technologies are extremely important. Design considerations for PHVs include the need for sufficient effective orifice area, jet velocities and pressure gradients within a normal physiological range, and minimal regurgitation, damage to blood cells, and thrombogenic potential[75, 78]. Additional design considerations include how the PHV will attach to the native environment, leaflet coaptation, commissure gap, leaflet thickness and geometry, biostability, and peak stresses on the valve components[75, 78]. The following sections will introduce the most popular materials, surface modifications, geometries, and manufacturing techniques that are considered for use in current experimental PHVs. MATERIAL Polysiloxanes The earliest known implanted aortic valve was made of polysiloxane[69], a polymer with a backbone consisting of silicone and oxygen atoms[79, 80]. The main advantages of this material are its elastic and flexural properties, as well as its good biocompatibility[81], while its greatest failure is limited durability and tearing. In 1973, Mohri et al. reported good hemodynamic performance of silicone rubber, but was concerned about fluid absorption and thrombus formation[82, 83]. Additional discussion around the importance of design and 33 manufacturing for the consistency in durability, in conjunction with the material properties of a polymer was addressed [82]. Chetta and Lloyd described a second mode of failure for silicone rubber, where the valve leaflets became stiff and thickened, eventually failing to open[83, 84]. Polytetrafluoroethylenes (PTFE) /Expanded PTFE (ePTFE) Polytetrafluoroethylene(PTFE) and expanded polytetrafluoroethylene (eTPFE) commercially known at Teflon® and Gore-Tex® respectively, are highly crystalline, hydrophobic, and highly stable polymers. Their main advantages for use in polymeric PHVs include good hemodynamic properties, mainly due to a low coefficient of friction, inertness, and low surface tension[75, 80, 85]. The use of PTFE and ePTFE for PHVs is limited by repetitive instances of calcification and leaflet stiffening, in addition to a low resistance to thromboembolism [75, 80, 85]. Quintessenza et al. has shown successful intermediate use of nonporous 0.1mm PTFE for prosthetic bicuspid pulmonary valve implantation in patients with pulmonary insufficiency, and/or pulmonary stenosis[86]. The use of nonporous 0.1mm PTFE was shown to limit cellular in-growth and thickening, improving the leaflet mobility and pliability[86]. Polyurethanes (PU) Polyurethanes(Pus) are among the oldest tested and most commonly used polymers chosen for use in investigational PHVs, as well as for all blood-contacting medical devices[85]. PUs are considered segmented block copolymers, containing soft and hard segments. A superior advantage of PUs is the capability to manipulate their mechanical and hemodynamic functionality by varying 34 the type and/or molecular weight of the soft segment and coupling agents[85]. Since the initial use of PUs for investigational mitral valves in the early 1960’s[68], there have been many varieties of PU materials, with soft segments containing polyester, polyether, polycarbonate, and polysiloxane [87, 88]. The chemical differences between the soft and hard chains of the polymer are what give PUs the exceptional mechanical properties and biocompatibility that make the material so desirable, especially for biomedical applications[89, 90]. Studies by Lyman et al.[91], Zia et al.[92], and Zu et al.[93], show the feasibility of enhancing the biocompatibility of PUs through minimal changes to the chemical structures. While earlier PUs have been primarily plagued by thrombosis and calcification,[94-100], more recent trials have found that different grades of polyurethanes, polyether urethanes (PEUs) and polyether urethane ureas (PEUUs) demonstrated no evidence of thrombogenic events or calcification[96, 99, 101]. Additional variations of PUs including polyhedral oligomeric silsesquioxanes (POSS-PCUs)[102, 103] thermoplastics (TPUs)[80] and polydimethylsiloxanes (PDMS-PUs)[104, 105] have shown biocompatible and hemodynamic promise. The remaining challenge to realizing PUs as ideal polymers for PHVs are their long-term in vivo biostability[85]. There are multiple modes of biodegradation of PUs, including hydrolysis, oxidative degradation, enzymatic degradation, surface cracking, environment stress cracking, and calcification[106]. As more advances in the understanding of these modes of degradation are made, the bio-resistant properties of PUs can be tailored for use in PHVs[85]. 35 Polyvinyl alcohol (PVA) Polyvinyl alcohol (PVA), a hydrophilic synthetic polymer, has been of interest in recent years as a potential material for both PHV and tissue engineered heart valve (THV) applications. The polymer is non-toxic, biocompatible, and biostable [107-110], and has exhibited good mechanical properties[111]. Jiang et al. has shown that polyvinyl alcohol cryogels (PVA-C) have behavior similar to soft tissue [110, 111], while Mohammadi has shown that polyvinyl alcoholbacterial cellulose (PVA- BC)- based hydrogels have similar mechanical properties and anisotropic behavior as native porcine aortic valve leaflets [110, 112]. One limitation to using PVA for PHV application is that cell adhesion is not possible due to the hydrophilic nature of the polymer, which can cause a foreign body response[113]. Surface modifications to minimize this response by promoting endothelial cell attachment as described by Nuttleman et al.[109] will be discussed in section 2.2. Linear Low Density Polyethylene Linear low-density polyethylene (LLDPE) is a hydrophobic polymer with a high tensile and tear strength and relatively low bending stiffness[114, 115]. An interpenetrating network (IPN) between Hyaluranon (HA) and LLDPE has been shown to increase the material strength and durability, while providing the added benefit of increased biocompatibility[115, 116], making HA-LLDPE a highly attractive candidate for PHVs. The results of a study by SimonWalker et al. demonstrated that IPNs of HA-LLDPE are non-toxic, and reduce the thrombogenic potential of the material as compared to untreated LLDPE[117]. 36 Poly(styrene-b-isobutylene-b-styrene) (SIBS) Poly(styrene-block-isobutylene-block-styrene (SIBS) by Boston Scientific[118], a thermoplastic elastomer, and the more recent polyolefin thermoset elastomer xSIBS by Innovia LLC64 have been of recent interest for use in PHVs mainly due to their superior biostability and resistance to in vivo degradation[78]. These desirable properties are results of the polymer containing no reactive pendant groups, virtually eliminating any possibility of degradation due to hydrolysis, oxidation, or enzymatic pathways[78]. SURFACE MODIFICATIONS As it has been described in the previous section, many polymers have been selected for use in an experimental PHV for one of their promising material properties, such as superior durability, and have failed due to another. While some of the failures have led researchers to discontinue pursuing a polymeric material, such was the case for the early polysiloxanes, it is becoming increasingly popular to engineer the surface of a biomaterial to enhance its biocompatibility. Since biomaterial hemocompatability relies heavily on the blood interaction with the surface, it is logical to move towards surface modifications that do not interfere with the bulk properties of a material[75, 119]. Platelet adhesion and activation in response to injury in a blood vessel is a mechanism to minimize bleeding, essentially by covering the damaged portion and recruiting more platelets, initiating fibrin formation, and eventually developing a thrombus[120]. While this mechanism is important in a diseased vessel, risk of thrombo-embolism in response to an implanted biomaterial 37 is of major concern especially for cardiac applications. Since platelet activation is contact and flow-induced, the design and optimization of the mechanical function as well as the surface properties of PHVs is of extreme importance[78]. Surface characteristics including hydrophobicity/hydrophilicity, surface charge and surface free energy, as well as the topography of the surface all influence initial blood material interactions including protein absorption and denaturation[75, 78]. Research on the effectiveness of surface modifications including plasma immersion ion implantation[119, 121], cholesterol modification[122], and peptide modification[113, 123] have been shown to enhance the affinity of surfaces to endothelial cells, which have been shown to protect the valve experiencing a foreign body response from the immune system. Scherman et. al.[101] have shown promising results for heparinization of polyurethane leaflets in short term animal studies. Additional surface modifications, including the creation of topographic features that attract specific cells as shown by de Mel et al.[124], Milner et. al.[125] , have been shown to increase the hemocompatability of a material[75]. In addition to reducing thrombo-embolic risk, some surface modifications aim to reduce the amount of calcification deposited on the valve leaflets, to improve the PHV durability and function. Among these, a study by Joshi et al. demonstrated that 2- hydroxyethane bisphosphoric acid (HEBP)-bound PEUs may serve as a valid calcification resistant material for use in PHVs[126]. Table 2.2 summarizes popular surface modifications used for the improvement of biocompatibility of common biomaterials. 38 Modification Advantages References PIII Converts hydrophobic polymers to hydrophilic, Improves biocompatibility CholesterolIncreases surface energy, modified PU Increased endothelial cell attachment and retention Nanotopographic Mimics natural extracellular matrix, Surface Stimulates cell adhesion and proliferization Promotes endothelialization HEPB- bound Reduces surface degradation, PU’s Does not affect material properties, Decreased calcium permeation RGD Promotes endothelialization incorporation 69, 71 72 26, 74, 75 51, 76 63,73 PIII - plama immersion ion implantation, peptide mod 73; HEPB - hydroxyethane biophosphoric acid; RGD - R: arginine; G: glycine; D: aspartic acid Table 2.2 Comparison of surface modifications for heart valve leaflets (adapted from Polymeric Heart Valves [2]) GEOMETRY Arguably one of the most challenging aspects of designing a viable PHV is optimizing the leaflet geometry. Added freedom in this phase of PHV design comes from the seemingly limitless potential geometric shapes and leaflet thicknesses that were not previously possible with bioprosthetic HVs, which can be both an exciting and daunting challenge for engineers. Since leaflet geometry is essential to the function of a PHV, including its durability, hemodynamic function, and biocompatibility, this is no small task. An idealized PHV geometry would allow for maximal effective orifice area throughout systole, sufficient flexibility to minimize resistance to forward flow[75], fully coapting leaflets that minimize backflow, and 39 minimized and equal distribution of leaflet stresses to minimize blood trauma and maximize valve durability[78]. While the relative ease of manufacturing polymeric leaflets has provided the opportunity to design more complex valve designs, it is still extremely complex to mimic the anatomy of natural valves. For this reason, many designs have been proposed and tested including single leaflet[127], bileaflet, including Braunwald et al.’s original bileaflet designs[68] and other more successful trials [128, 129], as well as quadrileaflet designs[130]. However, the majority of modern PHV research is focused on trileaflet valves[75], due to studies that have shown superior mechanical efficiency and greater opening area[131], as well as improved stress distribution[132]. Detailed geometric features of a modeled trileaflet heart valve can be seen in Figure 2.13. 40 FIGURE 2.13 DETAILED GEOMETRIC FEATURES OF A TRILEAFLET PROSTHETIC HEART VALVES [2] There has been much debate over the shape of the native aortic leaflets, with descriptions of semilunar, sigmoid, paraboloids of revolution, and elliptical paraboloid shapes suggested, among others[80]. While some groups have put forth great effort into modeling and recreating realistic geometries, others have tossed aside the idea and have solely focused on recreating or even improving natural valve function, rather than form. In addition to leaflet shape, other important considerations include which position the valve will be manufactured in (closed, open, partially open), the leaflet thickness, and how the leaflets will be assembled to a stent frame (adhesive, stentless, or sutures). A significant amount of research has gone into optimizing leaflet thickness alone[95, 98, 133]. In order to achieve good hemodynamic performance including transvalvular pressure gradients, energy losses, and sufficient durability free from tearing, it has been shown that for PCU valves, the durability for a thickness between 100 and 300 microns is between 600 million and 1 billion cycles[134]. MANUFACTURING Various methods to manufacture PHVs are commonly used, the choice of which most heavily depends on the material properties of the polymer. While the choice in manufacturing method is somewhat limited by whether the material is sufficiently soluble with heat or solvent processing, or if it is a thermosetting plastic[135], it should not be overlooked as a crucial factor to PHV performance[75, 136]. 41 Dip Casting Dip casting or dip coating is a manufacturing method commonly used for silicones and polyurethanes. The technique requires a custom shaped mandrel, in the shape of valve leaflets, to be dipped into a polymer solution. The desired thickness of the leaflets can be attained through subsequent dips, allowing the solution to air dry in between each. Dip casting allows for complex leaflet geometries, and more precision with less cost than in conventional molding methods. Additionally, dip casting can provide a continuous integration of the leaflets and supporting frame as shown by Leat et. al[136]. One disadvantage of this manufacturing method is that the distribution of leaflet thickness is not easily controlled, leading to difficulty with reproducibility[75]. Film Fabrication In film fabrication, the leaflets and frame are manufactured separately. Leaflets are cut from polymer films and bonded to the frame[75]. In some cases, heat treatments can be applied to further shape the valve leaflets once they are attached to the frame[136]. While this method allows for increased control of leaflet geometry and ease of prototyping, its main drawback is the potential for decreased durability due to the boundary between the leaflets and frame. Cavity and Injection Molding Cavity molding is the process where a liquefied polymer is poured into a static mold, and then the mold is sealed and undergoes freeze thaw cycles in a water bath until a thin polymer is 42 formed[111]. Similarly, an injection molding machine is used to fabricate valve leaflets through high pressure injection of molten polymer, followed by hot and cold water baths[75, 137]. Cavity and injection molding have increased precision and the ability to tailor the elastic properties of some polymers through the number and rate of freeze thaw cycles[138, 139], but not without increased cost[137]. 3D printing With the rapid advances in 3D printing technology making printing some polymeric materials cheaper and faster than ever, it is no surprise that the technology has begun to take root in polymeric valve design. The ease of printing from computer aided designs has the potential to move the manufacturing process from two dimensional leaflets to leaflets enhanced with 3D surface structures and eventually fully three dimensional leaflets mimicking the native structures. INVESTIGATIONAL VALVES The material, surface modifications, manufacturing methods, and geometries of three current promising investigational valves will be discussed in detail below. To begin, there have been promising material results from interpenetrating networks (IPNs) of Hyaluronan (HA) and Linear Low Density Polyethylene (LLDPE) by Prawel et al.[115]. The LLDPE films were blow molded by Flex-Pack Engineering Inc. (Union-town, OH) from Dowlex 2056 resin and had measured average thickness of 0.08mm[115]. A swelling method was used to introduce Hyaluronan into the LLDPE[115]. Characterization of the material showed that 43 Hyaluronan concentration effects clotting, significantly decreasing clotting as compared to plain LLDPE sheets. Additionally, the HA-LLDPE showed decreased platelet adhesion and activation as compared to traditional bioprosthetic and mechanical HV materials[115]. Yousefi et al. has manufactured trileaflet surgical aortic heart valves from HA-LLDPE sheets[140] and has described extensively the impact of arched leaflet geometries and stent profile on their hemodynamic performance and durability. Further work has been done to manufacture a trileaflet HA-LLDPE balloon-expandable transcatheter aortic valve (Figure 2.14), and animal trials as well as accelerated durability testing are currently underway. FIGURE 2.14 A BALLOON-EXPANDABLE HA-LLDPE TRANSCATHETER VALVE DEVELOPED BY DASI CARDIOVASCULAR BIOflUID MECHANICS LAB [2] Claiborne et al. describes novel trileaflet surgical valve made from xSIBS[141], manufactured by custom compression molding. They describe an iterative design process, which lead them to choose a hemispherical leaflet geometry, tapered leaflet thickness, and smooth stent 44 edges, that significantly reduced the stresses in the valve leaflets from their previous models, which was a primary concern. In-vitro evaluation of this valve showed promising hemodynamic performance, including low regurgitant fraction in comparison to commercial tissue valves, but a decreased effective orifice area and higher peak velocity were observed[141]. The group has since designed a transcatheter aortic valve using the same polymer, xSIBS[142] as shown in Figure 2.15. FIGURE 2.15 A SELF-EXPANDING SIBS-DACRON-BASED TRANSCATHETER VALVE DEVELOPED BY BLUSTIEN BIOflUIDS RESEARCH GROUP [2, 143] In 2016, Rahmani et al. introduced the TRISKELE, an investigational trileaflet transcatheter aortic valve[144]. The valve, shown in Figure 2.16, was manufactured by automated dip-coating of POSS-PCU, which had been previously validated in vitro for its hemocompatibility, antithrombogenecity, biostability, and resistance to calcification[144]. The frame design, made from self-expandable nitinol wire, was numerically optimized to incur minimal stress and improved anchoring at physiological pressure loads. Additionally, the group 45 aimed to attain a single curvature in the open and closed positions in order to minimize energy dissipation. The TRISKELE valve demonstrated significant reduction in paravalvular leak in comparison to two commercially available valves, and has shown comparable hemodynamic performance. The valve is undergoing preclinical studies to investigate its durability and in vivo function. FIGURE 2.16 SELF-EXPANDING TRISKELE VALVE IN SIZES 23(LEFT), 26 (CENTER), 29 (RIGHT) MANUFACTURED FROM POSS-PCU LEAflETS [145] [2] Sherman et. al.[101] introduced a novel, self-homing investigational transcatheter valve in 2018. The valve, developed with Strait Access Technologies, is one of the first to specifically target the need for trancatheter valve therapies intended for use in insufficient valves rather than stenotic valves. The leaflets are made from a heparinized polyurethane, have demonstrated impressive durability, surpassing 600 million cycles in in-vitro fatigue testing studies[101]. The balloon expandable valve, shown in Figure 2.17, has a stent design that allows anchoring to 46 compliant roots. Preclinical data from 8 week survival studies in sheep have demonstrated good leaflet hemocompatability. The valve is currently being evaluated in long term animal studies. SUMMARY AND CONCLUSIONS In this review we have aimed to recognize the major advances towards the goal of realizing a clinically successful PHV while also describing the challenges that have led many prototypes to fail in pre-clinical stages. These are namely degradation, calcification, and the risk of thrombo-embolism. Despite their origins dating back to the late 1950’s, there has not yet been any PHV that has proven to be better, or even as good as the currently available bioprosthetics or mechanical valves. As the current valves improve, the quality and durability standards required to bring a PHV to market are continually raised. Even so, research aimed at developing a PHV is ongoing and many researchers are hopeful that their designs will outperform the existing technology. The most promising avenue for the realization of PHV technology is within the transcatheter aortic valve replacement (TAVR) sector. Here, a prosthetic valve promising both superior durability and hemocompatability in comparison to the fixed tissue components currently used would allow TAVR to become a routine procedure for all patients suffering from aortic valve stenosis regardless of their eligibility for the traditional, and more invasive surgical aortic valve replacement. Additionally, polymeric transcatheter aortic valves have reduced costs associated with their manufacture and storage as compared to the bioprosthetic 47 ones, which could reduce the cost of heart valve replacement therapy as a whole and bring this technology to developing as well as industrialized nations. 48 Chapter 3: Specific Aim 1 3.1 3D Modeling of CO 3.1.1 INTRODUCTION Transcatheter aortic valve replacement (TAVR) represents a major advance for the treatment of patients with severe aortic stenosis, whom conventional open-heart surgery has been deemed high risk [146-149]. Despite the overall effectiveness of TAVR, complications can limit the realization of mortality and quality of life benefits [149-152]. Among these is coronary obstruction, which can occur upon transcatheter valve deployment, and most often affects the left coronary artery (LCA) [153-160]. Coronary obstruction, as defined by the 2011 ACCF/AHA/SCAI guidelines for percutaneous coronary intervention, is considered as a > 50% obstruction of the left main coronary artery, >70% in any other coronary artery, or both [161, 162]. While this complication is rare (reported in up to 1% of all TAVR procedures) the outcomes are often catastrophic. Although a serious and potentially preventable complication, there is no consensus to which features reliably predispose risk of coronary obstruction during TAVR. Most of the guidelines developed so far have originated from clinical trials designed to avoid as many adverse outcomes as possible and were not based on simulations or mechanistic insights into the precise mechanics of coronary obstruction. In doing so, these guidelines have potential to exclude a large number of potential TAVR patients, often those who have no other treatment options available. 49 Despite the existing predictive models, 1-3% of TAVR patients still suffer from coronary obstruction. However, it has been shown that restrictively applying the current guidelines could have excluded 26 - 33% of patients who successfully received TAVR with no reported instance of coronary obstruction [163]. This clearly demonstrates the importance of patient specific modeling and the critical need for individualization of valve replacement therapy. The objective of this study is to better understand the physical mechanism of coronary obstruction beyond the conventional parameters of coronary height (h) and sinus of Valsalva diameter (SOVd) alone and introduce a new more accurate mechanistic index that can predict which high risk patients (i.e. patients with h < 14 mm and/or SOVd < 30mm) are not actually at risk and are indeed candidates for TAVR pre-operatively, allowing for the most patients possible to safely undergo TAVR without coronary obstruction. 3.1.2 METHODS In order to better understand the mechanism of coronary obstruction and develop a mechanistic index that can predict which high risk patients (i.e. patients with h < 14 mm and/or SOVd < 30mm) are not actually at risk and are indeed candidates for TAVR pre-operatively, a three-dimensional computational model that utilizes the pre-TAVR CT angiogram imaging is presented and compared against the conventional guidelines. The three-dimensional model employs computer-aided methodologies that predict the closest distance between native aortic valve cusp and the corresponding coronary artery ostium following TAV deployment. In vitro validation of this novel computational model was performed using 3D printed flexible patient specific aortic root geometries. Informed consent was obtained from all patients and the study complied with the Institutional Review Board of The Ohio State University. 50 STUDY POPULATION The study population included all “moderate to high-risk” patients, defined by left coronary artery height (LCAh) < 14 mm and/or SOVd < 30 mm, flagged from 600 aortic stenosis patients considered for TAVR at The Ohio State University Wexner Medical Center between January 2014 and September 2018. This filtering resulted in 28 patients (labeled A-AB; see Table 3.1) being flagged as moderate to high risk for left coronary artery obstruction during TAVR and included 78.5% women; mean [± SD] age, 80 ± 9 years with symptomatic severe aortic stenosis. The individual LCAh and SOVd are shown in Figure 3.1 for the study population with quadrants representing LCAh < 12 mm and SOVd < 30 mm based on Ribeiro et. al’s analysis as current guidelines (the figure is discussed later). With respect to the outcomes for these 28 patients, 23 received TAVR successfully while 5 patients did not receive a successful TAVR. These five include 1 male who suffered coronary obstruction, 2 females who underwent surgical aortic valve replacement with visual confirmation of coronary obstruction by the operating surgeon, and 1 male and 1 female (patients H and V) who each had extremely low lying coronary ostium (9mm and 7.6mm respectively) and were deemed surgically inoperable due to age and received medical management. 51 Table 3.1 List of coronary obstruction predictive parameters, including currently used parameters namely coronary ostium height, sinus of Valsalva diameter, and newly proposed predictive parameters based on the 3-dimensional computational modeling for each patient [164] Patie nt A B C D E F G H I J K L M N O P Q Sex Ag e LCA diamet er (mm) Valve diamet er (mm) 86 70 LCA Sinus heigh of t valsava (mm) diamet er (mm) 13 32 12 27 DLC /d TAV R Succe ssful? 25 23 Simulate DL d TAV C expansio n diamete r (mm) 25 3.5 23 7.4 Male Femal e Male Femal e Male Femal e Femal e Male Femal e Femal e Male Femal e Femal e Femal e Femal e Femal e Femal e 3.6 5.4 1.0 1.4 Yes Yes 88 89 7 9 36 30 5.3 5.5 26 23 26 23 2.3 3.9 0.4 0.7 Yes Yes 93 79 13 10 30 30 2.8 3.3 23 23 23 23 3.0 3.3 1.1 1.0 Yes Yes 81 12 29 5.6 26 26 5.7 1.0 Yes 94 81 9 9 32 30 4.6 4.0 NA 29 26 29 0.9 2.8 0.2 0.7 No Yes 75 9 28 5.7 NA 23 1.2 0.2 No 80 68 12 8 37 26 5.4 4.6 29 23 29 23 1.8 3.6 0.3 0.8 No Yes 88 13 30 5.0 29 29 7.0 1.4 Yes 91 19 28 3.6 29 29 9.1 2.5 Yes 81 9 31 3.1 29 29 3.7 1.2 Yes 77 9 31 3.2 23 23 5.0 1.6 Yes 87 19 27 3.2 20 20 12.6 3.9 Yes 52 R Femal 74 12 33 4.6 23 23 4.2 0.9 Yes e S Femal 84 9 31 4.2 26 26 6.4 1.5 Yes e T Femal 62 11 29 4.4 NA 23 1.9 0.4 No e U Femal 77 9 26 4.2 26 26 4.0 1.0 Yes e V Femal 91 8 31 4.6 NA 23 1.8 0.4 No e W Femal 82 11 27 3.2 23 23 3.6 1.1 Yes e X Femal 72 13 29 4.9 26 26 7.9 1.6 Yes e Y Femal 76 12 33 3.4 23 23 5.0 1.5 Yes e Z Male 61 9 29 2.6 26 26 7.0 2.7 Yes AA Femal 83 10 30 2.8 23 23 3.9 1.4 Yes e AB Femal 77 13 28 2.7 23 23 6.3 2.3 Yes e LCA, Left coronary artery; TAV, transcatheter aortic valve; DLC, distance from cusp to coronary ostium; DLC/d, the fraction of distance between the aortic cusp and coronary ostium post-TAV deployment available for blood flow toward the coronary ostium; TAVR, transcatheter aortic valve replacement. 53 FIGURE 3.1 STUDY POPULATION CHARACTERIZED BY CONVENTIONAL PARAMETERS (CORONARY HEIGHT [H] < 14 MM AND SINUS OF VALSALVA DIAMETER [SOVD] < 30 MM) USED TO PREDICT LEFT CORONARY ARTERY OBSTRUCTION BEFORE TRANSCATHETER AORTIC VALVE REPLACEMENT (TAVR) WITH ORIGIN LOCATED AT (12,30), REPRESENTING A LEFT CORONARY ARTERY HEIGHT (LCAH) OF 12 MM AND A LEFT A SINUS OF VALSALVA DIAMETER (SOVD) OF 30 MM . BLUE SQUARES REPRESENT THE ONLY PATIENTS WHO WOULD HAVE BEEN APPROVED FOR TAVR UNDER THESE CURRENT GUIDELINES. SOV, SINUS OF VALSALVA DIAMETER; CO, CORONARY OBSTRUCTION [164] THREE-DIMENSIONAL (3D) COMPUTATIONAL MODEL A 3D computational model was developed to assess risk of coronary obstruction during TAVR. Note that this model is only for patients who already satisfy a conservative risk stratification given by h < 14 mm and/or SOVd < 30 mm and as is shown later that there would not be any benefit to perform 3D computational modeling for lower risk patients. The model works by simulating the implantation of an idealized and cylindrical TAV prosthesis into a patient’s pre-procedural aortic root anatomy (including the calcified native cusps). The risk for coronary obstruction is then assessed through quantifying the closest distance of the cusp and the 54 corresponding coronary ostium. This distance is indexed to the coronary artery diameter to obtain a representative measure of the fractional obstruction of the native cusp “eclipsing” the ostium. The pre-procedural patient specific aortic root, calcium nodules and cusps were segmented for each of the 28 patients from pre-TAVR CT images using Mimics Research 18.0 (Materialise, Belgium). The segmented aortic wall, cusps and calcium nodules were then discretized in 3-Matic Research 13.0 (Materialise, Belgium) using explicit 4-node linear tetrahedron elements (Appendix A Figure A1). An idealized TAV stent (represented as an expandable cylinder) was discretized using hexahedral elements. An example of the segmented aortic root (red) and cusp with calcification (yellow) anatomy previous to TAV implantation is depicted in Figure 3.2A,B,C. Finite element analysis (FEA) was performed on each patient-specific 3D anatomical model using Abaqus/Explicit 6.9 software (Simulia, Providence, RI, USA) to simulate the opening of a TAV device stent that pushes the native cusps open towards the coronary ostium. For each patient anatomy, the simulation expanded the TAV device stent to the diameter of the valve size that would be appropriate for that patient’s anatomy, as determined by the structural heart team at Ohio State University Wexner Medical Center. The simulated TAV expanded diameters and the valve sizes received are given in Table 3.1. Material properties of the pressurized aortic root were assigned using an isotropic neo-Hookean hyper-elastic model based on the studies by Bosmons et al. (2016), Auricchio et al. (2014), and Martin et al. (2012). The strain energy function is described below. 55 𝑊= 𝜇 𝜆 (𝐼1 − 3 − 0.5 𝐿𝑛 𝐽 ) + (𝐿𝑛 𝐽)2 2 2 Which 𝜇 and 𝜆 are shear and bulk modulus respectively and are shown in Supplementary Table A.1 for each part. Calcium nodules were approximated to be linear elastic. The Young’s modulus was based on the nonlinear elastic material properties introduced by Billiar and Sacks [165]. Figure 3.2D shows a schematic of the post simulation anatomy highlighting the closest distance between the left cusp (point PC in the figure) and corresponding coronary ostium (point PO) denoted as DLC. DLC represents the predicted gap in mm as would be seen in a long axis plane showing both the coronary ostium and the native cusp. Figure 3.2E and 3.2F show different three dimensional perspectives of the post simulation anatomy for the same distance (DLC) from a cross sectional and top view for the same patient. 𝐷𝐿𝐶 was then normalized with respect to the corresponding coronary artery diameter (d), to obtain 𝐷𝐿𝐶/𝑑, which represents the fraction of distance between the aortic cusp and coronary ostium post TAV deployment available for blood flow towards the coronary ostium. A fractional value greater than unity indicates that the gap available for blood flow is greater than the coronary artery diameter. A fractional value approaching zero indicates total occlusion. Supplementary Figure A2 (Appendix A) visualizes the same for four patients. 56 FIGURE 3.2 EXAMPLE OF PATIENT SPECIFIC 3-DIMENSIONAL MODELED AORTIC ROOT WITH LEFT CORONARY ARTERY (LCA), RIGHT CORONARY ARTERY (RCA), AND YELLOW CALCIFIC NODULES. A. SIDE VIEW; B. AORTIC VIEW; C. VENTRICULAR VIEW. D. THE MEASURED DISTANCE (DLC) FROM A POINT ON CUSP/OR CUSP CALCIUM (PC) TO A POINT ON THE UPPER OSTIUM OF THE CORONARY ARTERY (PO) FOLLOWING A TRANSCATHETER VALVE REPLACEMENT FROM THE IDEALIZED ROOT SCHEMATIC FROM THE SIDE VIEW. E. EXAMPLE FINITE ELEMENT SIMULATED POST-TRANSCATHETER AORTIC VALVE REPLACEMENT AORTIC ROOT WITH DLC FROM A TOP VIEW. F. SIDE VIEW[164] IN VITRO VALIDATION The computational model was validated in-vitro as well as in-vivo. Two flexible patientspecific 3D printed models of aortic root geometries, the first with 3D printed patient calcium nodules, and the second without calcium nodules, were used to evaluate the potential effects of 57 rigid calcium nodules on cusps’ deformation during TAVR. The 3D printed aortic root model was manufactured using Connex 350 3D printer (Stratasys, Eden Prairie, MN) from TangoPlus material for the aorta and cusps with VeroWhite material used for the calcium nodules (Figure 3.3). The flexible 3D printed model with the calcification nodules itself was also validated, through comparison of patient hemodynamics (peak gradients and velocities) with experimental values obtained from left heart flow simulator studies [166, 167]. An idealized tool (Figure 3.3F) was used to open the cusps to the appropriate stent size for the 3D printed models and was compared against the results of the FEA simulation of the same patient with and without inclusion of calcium nodules. As shown in Supplementary Figure A3 (Appendix A) there was excellent agreement between the computational prediction and experimental measurements of DLC. Further validation of the 3D computational approach was achieved with the observation of coronary occlusion to occur in two patients (J and T in Table 3.1) as predicted by the model and confirmed by the operating surgeon during surgical AVR. 58 FIGURE 3.3 A. THE 3-DIMENSIONAL (3D) PRINTED AORTIC ROOT MODEL WAS MANUFACTURED FROM TANGOPLUS (STRATASYS, FARMINGTON HILLS, MICH) MATERIAL AND VEROWHITE (STRATASYS) MATERIAL WAS USED FOR CALCIUM NODULE, BOTH PRINTED USING CONNEX 350 3D PRINTER (STRATASYS). B. PARTICLE IMAGE VELOCIMETRY (PIV) EXPERIMENTS WERE PERFORMED TO VALIDATE THE 3D PRINTED CALCIFIED AORTIC ROOT MODEL AND COMPARE WITH IN VIVO ULTRASOUND DOPPLER JET VELOCITY FOR THE PATIENT. DETAILED METHODOLOGY OF THE PIV EXPERIMENTS MAY BE FOUND IN HATOUM AND COLLEAGUES[168] AND HATOUM AND COLLEAGUES[169] C. COMPARISON OF THE TEMPORAL VELOCITY PROFILE CORRESPONDING TO A POINT LOCATED AT THE EXIT OF THE SYSTOLIC JET OF THE VALVE. D AND E. DOPPLER DATA. AS CAN BE SEEN, THE RESULT SHOWS GOOD AGREEMENT BETWEEN THE IN VIVO AND IN VITRO DATA. THE MAXIMUM VELOCITY IN THE PIV DATA WAS FOUND TO BE 2.10 M/SECOND, WHICH COMPARES WELL WITH 2.24 M/SECOND OBTAINED FROM THE ULTRASOUND. F. EXPANSION TOOL WITH INCREASING DIAMETER, WHICH MIMICS BALLOON EXPANSION[164] STATISTICAL ANALYSIS A Mann-Whitney non parametric comparison of means was performed for each of the three parameters (DLC/d, h, and SOVd) to compare the mean parameter value between the two groups; the 23 that received TAVR successfully and the 5 patients that did not receive a successful TAVR. A sensitivity and specificity analysis was performed for each test (DLC/d, h, 59 and SOVd) by identifying how many patients would identify as true positive, false positive, true negative or false negative for coronary obstruction under a range of cutoff values. Sensitivity is then calculated as: 𝑆𝑒𝑛𝑠𝑖𝑡𝑖𝑣𝑖𝑡𝑦 = 𝑇𝑟𝑢𝑒 𝑝𝑜𝑠𝑖𝑡𝑖𝑣𝑒𝑠 , 𝑇𝑟𝑢𝑒 𝑝𝑜𝑠𝑖𝑡𝑖𝑣𝑒𝑠 + 𝐹𝑎𝑙𝑠𝑒 𝑛𝑒𝑔𝑎𝑡𝑖𝑣𝑒𝑠 While specificity is calculated as: 𝑆𝑝𝑒𝑐𝑖𝑓𝑖𝑐𝑖𝑡𝑦 = 𝑇𝑟𝑢𝑒 𝑛𝑒𝑔𝑎𝑡𝑖𝑣𝑒𝑠 , 𝑇𝑟𝑢𝑒 𝑛𝑒𝑔𝑎𝑡𝑖𝑣𝑒𝑠 + 𝐹𝑎𝑙𝑠𝑒 𝑝𝑜𝑠𝑖𝑡𝑖𝑣𝑒𝑠 as described in Lalkhen and McCluckey (2008) [170]. 3.1.3 RESULTS Here we present results that compare the ability of solely conventional parameters such as h and SOVd and the new parameter DLC/d to differentiate which high risk patients are not actually at risk and are indeed candidates for TAVR. Routine anatomical measurements of h and SOVd along with measured values from the 3D computational model, DLC and DLC/d, for the high risk study population (28 patients) are presented in Table 3.1. CURRENT GUIDELINES (𝒉, 𝑺𝑶𝑽𝒅) Figure 3.1 shows the risk assessment for left coronary obstruction occurrence under the existing guidelines (i.e. h < 12 and SOVd < 30mm) based on Ribeiro et. al [171]. Obstruction of the right coronary artery was not evaluated due to lack of right coronary obstruction in our patient population. Accordingly left coronary obstruction was expected to occur for 22 of the 28 patients, with left 𝑆𝑂𝑉𝑑 in the range 26.0-36.2 (mm) and left coronary artery height (LCAh) in the range of 7.09-19.0 (mm). 60 3D PREDICTIVE MODEL(𝑫𝑳𝑪/𝒅) The distribution of 𝐷𝐿𝐶/𝑑 among the patient population is shown in Figure 3.4. The range of values for 𝐷𝐿𝐶/𝑑 for the patients is between 0.0203 and 3.89. The horizontal line between 0.5 and 0.7 approximately separates patients who successfully received TAVR (above the line) from those who did not. The one blue data point that lies just below the horizontal line successfully received TAVR but only with coronary protection using a stent. FIGURE 3.4 STUDY POPULATION CHARACTERIZED BY 3-DIMENSIONAL PREDICTIVE MODEL (DLC/D < 0.7) USED TO PREDICT LEFT CORONARY ARTERY OBSTRUCTION BEFORE TRANSCATHETER AORTIC VALVE REPLACEMENT. BLUE DOTS REPRESENT THE PATIENTS WHO WERE BE APPROVED FOR TRANSCATHETER AORTIC VALVE REPLACEMENT UNDER THESE SUGGESTED GUIDELINES, RED TRIANGLES REPRESENT THOSE WHO WERE NOT APPROVED AND RECEIVED OTHER TREATMENT, AND THE GREEN DIAMOND REPRESENTS THE 1 PATIENT IN WHOM TRANSCATHETER AORTIC VALVE REPLACEMENT RESULTED IN CORONARY OBSTRUCTION (THE MODEL WAS NOT COMPUTED PRIOR). DLC/D, CUSP TO CORONARY OSTIUM DISTANCE INDEXED WITH CORONARY ARTERY DIAMETER; CO, CORONARY OBSTRUCTION[164] 61 COMPARISON TO CURRENT GUIDELINES The mean and standard deviations of the parameter values for those high risk patients who successfully received TAVR without coronary obstruction are compared to those who did not receive TAVR successfully in Figure 3.5. The means for these two groups were compared using a Mann-Whitney non parametric test, and the only significant difference between the two groups was found for the DLC/d parameter, with p < 0.00078. Neither LCAh nor SOVd was significantly different between the two groups with p = 0.35238 and p = 0.32218 respectively. FIGURE 3.5 THE MEAN AND STANDARD DEVIATIONS OF THE PARAMETER VALUES (A) DLC/D, (B) CORONARY ARTERY HEIGHT (H), AND (C) SINUS OF VALSALVA DIAMETER (SOVD) FOR THOSE HIGH-RISK PATIENTS WHO SUCCESSFULLY RECEIVED A TRANSCATHETER AORTIC VALVE REPLACEMENT WITHOUT CORONARY OBSTRUCTION COMPARED WITH THOSE WHO DID NOT RECEIVE A TRANSCATHETER AORTIC VALVE REPLACEMENT SUCCESSFULLY. A SIGNIFICANT DIFFERENCE BETWEEN THE 2 GROUPS WAS FOUND FOR THE DLC/D PARAMETER AT SIGNIFICANCE LEVEL .05. NEITHER H NOR SOVD WAS SIGNIFICANTLY DIFFERENT BETWEEN THE GROUPS. DLC/D, CUSP TO CORONARY OSTIUM DISTANCE INDEXED WITH CORONARY ARTERY DIAMETER; TAVR, TRANSCATHETER AORTIC VALVE REPLACEMENT; LCAH, LEFT CORONARY ARTERY HEIGHT; SOVD, SINUS OF VALSALVA DIAMETER[164] Figure 3.6 shows sensitivity and specificity curves generated for each of the three parameters to accurately predict whether TAVR within this high risk patient population would 62 not be successful. Figure 3.6A shows that the sensitivity of LCAh increases steadily from 0% at an LCAh cutoff of 7 mm to a 100% sensitivity at LCAh cutoff at 12 mm. Specificity of LCAh on the other hand drops steadily from 100% at 7mm to 0% at a cutoff of 19 mm. The crossover point for sensitivity and specificity for LCAh as an optimal predictor of coronary obstruction was at LCAh = 10mm with approximately 60% sensitivity and specificity. The sensitivity and specificity of SOVd as an independent predictor of unsuccessful TAVR is shown in Figure 3.6B. The sensitivity increases from 0% at SOVd of 28mm to 100% at 38mm. Specificity drops from 100% at 26mm to 0% at 38mm. The optimal crossover point occurs approximately at 30.5mm with a sensitivity and specificity of 40%. With respect to sensitivity and specificity of the 3D computational parameter DLC/d (Figure 3.6C), the sensitivity is 0% at a value of 0.2 and increases to 100% at a value of 0.45. The specificity drops from 100% at a value of 0.4 to 66% at a value of 1.0. The optimal crossover point is slightly below 0.45 with a sensitivity and specificity of 96%. There is a range of DLC/d from 0.45 to 0.70 for which the sensitivity and specificity exceed 95%. 63 FIGURE 3.6 A-C. SENSITIVITY AND SPECIFICITY CURVES GENERATED FOR EACH OF THE THREE PARAMETERS, DLC/D, CORONARY ARTERY HEIGHT (H), AND SINUS OF VALSALVA DIAMETER (SOVD), TO PREDICT WHETHER TRANSCATHETER AORTIC VALVE REPLACEMENT WITHIN THIS HIGH-RISK PATIENT POPULATION WOULD RESULT IN CORONARY OBSTRUCTION. LCAH, LEFT CORONARY ARTERY HEIGHT; SOVD, SINUS OF VALSALVA DIAMETER; DLC/D, CUSP TO CORONARY OSTIUM DISTANCE INDEXED WITH CORONARY ARTERY DIAMETER[164] 3.1.4 DISCUSSION Although the prevalence of coronary obstruction during TAVR procedure is rather low (< 1%), it is a time-sensitive and life-threatening complication. In order to minimize cases of coronary obstruction after TAV deployment, studies have introduced safety guidelines that attempt to use geometrical factors of patient aortic root geometry prior to implantation to assess 64 patient risk [163, 172-176]. In one such study, Ribeiro et. al. reported that the average coronary artery height (h) and mean sinus of Valsalva diameter (SOVd) were smaller for patients that suffered coronary obstruction during TAVR[171]. These concepts were applied to a large population of patients that underwent TAVR (n = 6688, 44 with coronary obstruction) and standard parameters indicating the potential for coronary obstruction were suggested as follows: 1) h < 12 mm, 2) SOVd < 30 mm [171]. In a smaller observational study Yamamoto et al.[176] examined 666 cases of TAVR (10 with coronary obstruction), and created their own criteria for potential risk: 1) h < 10 mm, 2) valve cusp length greater than h, and 3) shallow SOV (SOVd = 28.5 mm) with massive calcification. Although both of these reports suggest h and SOVd are important, other features are likely overlooked. The effect of the calcific nodules, for example, was not considered as a main anatomic predictor of coronary obstruction [171]. Tops et al. (2008), Apfaltrer et al. (2011), and Binder et al. (2013) suggested noninvasive evaluation of aortic root using multi-slice CT (MSCT), aortoiliac CT, multidetector CT (MDCT), and threedimensional modeling to provide more precise information on how aortic root geometry could play a role in complications such as coronary obstruction and paravalvar leakage.[173-175] Additional studies have focused on computational modeling using finite element analysis (FEA) as a powerful tool to optimize pre-operative planning of TAVR and evaluate its adverse outcomes in patient-specific geometries [177-182]. Not only have we shown that these safety guidelines that attempt to use geometrical features of patient’s aortic roots prior to implantation are not always accurate in flagging patients with risk of coronary obstruction, but also that they significantly reduce the number of patients who could have safely undergone TAVR without coronary obstruction. 65 In this study, we evaluate the predictive capacity of existing methods and propose a novel method for the investigation of coronary obstruction risk in patients with severe aortic stenosis prior to TAVR who were flagged as at risk based on conventional predictive guidelines. The novel method utilizes 3D reconstructed patient geometry for simulation of TAV deployment using FEA. The current guidelines for high risk of coronary obstruction include SOVd less than 30 mm [171] and coronary ostium height (h) greater than 12 (mm) [183, 184], although these guidelines are not consistently recognized throughout US hospitals. Individual transcatheter valve manufacturers impose their own guidelines, for example, CoreValve Evolut R & PRO manufacturers suggest that SOVd of ≥ 27 mm and ≥ 29 mm should be included (for the 26-mm and 29-mm Evolut R & PRO respectively). Similar to the SOVd guideline, coronary height h ≥14 is recommended by the CoreValve Evolut R & PRO manufacturers. The latter guideline would exclude all but two of the patients in this study population, many of whom who safely underwent TAVR without coronary obstruction. On the other hand, the patient specific 3D predictive model captures a much more accurate representation of the TAVR procedure and would capture the final configuration of TAV stent along with native cusp and aortic wall precisely. Based on our findings, the parameter 𝐷𝐿𝐶/𝑑 > 0.7 when h < 14 mm and/or SOVd < 30 mm should be considered as patients who are not actually high risk for left coronary obstruction and patients who have 𝐷𝐿𝐶/𝑑 < 0.5 are at severe risk of coronary obstruction and TAVR should not be attempted in these patients. Further studies are needed to resolve the patients where 𝐷𝐿𝐶/𝑑 lies between 0.5 and 0.7. Until then, these patients should be approached with caution with potential coronary protection strategies. Further, for patients with h > 14 mm and SOVd > 30 mm, there does not seem to be any benefit to perform simulations to evaluate DLC/d because there is no 66 known case of coronary obstruction in this group and furthermore the objective of the new computational model is not to replace the current guidelines but to only augment the predictive power. 3.1.5 LIMITATION In this study we are not looking at coronary obstruction from conduit of TAV, which can occur due to mal-positioning (supra-annular) of TAV. Additionally, right coronary obstruction is not evaluated, since obstruction of right coronary is much less prevalent compared to left coronary [153-155]. However, the 3D model is likely applicable to right coronary artery for preoperative risk assessment of coronary obstruction. Another limitation of the study is the small number of cases evaluated for coronary obstruction, which is due to its relatively rare occurrence. 3.1.6 CONCLUSION We have successfully developed a highly accurate model to screen patients for possible coronary obstruction during TAVR based on criteria that can be readily calculated from current pre-TAVR CT angiographic imaging utilizing 3D FEA analysis. Neither h nor SOVd is predictive of coronary obstruction when considering high risk patients with h < 14 mm and/or SOVd < 30mm. However, the new parameter DLC/d is predictive of coronary obstruction for the same high risk group. The performance of DLC/d was validated in-vitro and clinically. Results indicate that a significantly high fraction of patients who have h < 14 mm and/or SOVd < 30mm can be safely treated with TAVR if assessed with DLC/d as compared to the current guidelines using SOVd and h alone. These findings shed light on a rare but significant potential 67 complication during TAVR, and can assist heart teams in the decision-making process prior to the TAVR procedure. 68 3.2 2D Modeling of CO 3.2.1 INTRODUCTION Coronary artery obstruction is a rare, yet potentially fatal, complication that can occur during transcatheter aortic valve (TAV) replacement (TAVR). Although TAVR represents a major advance in the treatment of patients with severe aortic stenosis, complications such as coronary obstruction can determine the candidacy for or mitigate the mortality and quality of life benefits [149-152]. As TAVR gains momentum towards Food and Drug Administration Approval (FDA) approval for the treatment of low surgical risk populations, the potential to fundamentally shift the standard of care away from surgical valve replacement is becoming realizable[185, 186]. In an effort to avoid coronary obstruction during TAVR, potential TAVR candidates are assessed according to restrictive anatomical guidelines that often originate from clinical trials and device instruction for use designed primarily to avoid adverse outcomes [187]. These guidelines typically consider only coronary artery height (h) and sinus of Valsalva diameter (SOVd) (i.e. patients with h < 14 mm and/or SOVd < 30mm), and do not necessarily reflect consensus across devices or institutions[188]. Even with these guidelines however, coronary obstruction still has been reported. Perhaps more importantly, as many as one-third of patients who undergo successful TAVR might have been excluded had these guidelines been applied [163]. Efforts towards eliminating the occurrence of coronary obstruction and other adverse outcomes during TAVR through procedural pre-planning with experimental studies and computational simulations are not in short supply. An experimental study by Hatoum et al[189] simulated coronary obstruction in a patient who had it occur during the procedure and showed that a different 69 type of TAV might have permitted avoidance of this event. Numerous computational studies have developed finite element simulations modeling the implantation of transcatheter valves into patient specific roots to study potential adverse effects [177, 182, 190-193]. In one previous study of 28 potential TAVR candidates, we [194] introduced a three-dimensional (3D) computational model that was 38% more effective (based on sensitivity and specificity analyses) at predicting coronary obstruction than coronary artery heightand 58% more effective than Sinus of Valsalva diameter. Although these studies have identified tools to improve patient selection criteria and finally the outcomes of TAVR, their application within the field is impractical owing to large volumes of patients and lengthy set-up and simulation times. Dvir and colleagues[195] introduced a simplistic model to evaluate the risk of coronary obstruction preprocedurally in the context of valve-in-valve therapy. The model measures the distance between a virtual transcatheter valve to each coronary ostium, termed the VTC, and requires only pre-TAVR computed tomography (CT) scan data. VTC was found to be highly predictive of coronary obstruction in a large population, although the measurement strongly relies on the ability to identify the bioprosthetic surgical valves, often from their radiopaque stent posts, which makes it difficult to clearly identify the limits in a pre-TAVR CT scan[195]. The objective of the present study was to introduce a simple, yet highly accurate mechanistic index that can predict which high-risk patients (ie, patients with h < 14 mm and/or SOVd < 30 mm) are not actually at risk for coronary obstruction during TAVR in a native valve annulus. Our proposed model allows the derived index to be readily calculated solely from pre-TAVR CT angiographic imaging and to quickly identify patients who are viable candidates 70 for TAVR, allowing for the greatest possible number of patients to safely undergo TAVR without coronary obstruction. 3.2.2 METHODS A two-dimensional (2D) geometric model that utilizes pre-TAVR CT angiogram imaging is presented in this study as a part of an institutional review board-approved studyand compared against the conventional guidelines. The model aims to develop a simple mechanistic index that can predict which high risk patients (i.e. patients with h < 14 mm and/or SOVd < 30 mm) are not actually at risk for coronary obstruction and are indeed candidates for TAVR pre-operatively. It should be highlighted that this model is only for patients who already satisfy a conservative risk stratification as previously described. STUDY POPULATION The study population included all “high-risk” patients, defined by left coronary artery height (h) < 14 mm and/or SOVd < 30 mm, flagged from 600 aortic stenosis patients considered for TAVR at The Ohio State University Wexner Medical Center between January 2014 and September 2018. After excluding patients with bicuspid valves, previous valve replacement surgery, and risk of coronary obstruction due to right coronary artery height or sinus of Valsalva diameter, 105 patients composed the study population. Further stratification by risk resulted in 28 patients (labeled A-AB; see Table 3.2) being flagged as high risk for left coronary artery obstruction during TAVR (78.5% female; mean age, 80 ± 9 years) with symptomatic severe aortic stenosis. In Figure 3.7, the patient population is divided by risk of coronary obstruction due to h ≤ 12, SOVd ≤ 30, h ≤ 12 and SOVd ≤ 30, or h >12, SOVd > 30 based on an analysis by Ribeiro and colleagues[171]. We elected to interrogate left coronary obstruction only, because it is more 71 common and consequential than obstruction of the right coronary artery[154, 196, 197]. As shown in the figure, left coronary obstruction was expected to occur for 89 % of the patients(25 of 28), with left 𝑆𝑂𝑉𝑑 in the range 26 to 36 (mm) and h in the range of 7 to 19 (mm) (Table 4). FIGURE 3.7 THE STUDY POPULATION DIVIDED BY RISK OF CORONARY OBSTRUCTION DUE TO HEIGHT (H) ≤ 12 (32%), SINUS OF VALSALVA DIAMETER (SOVD) ≤ 30 (14%), HEIGHT (H) ≤ 12 AND SINUS OF VALSALVA DIAMETER (SOVD) ≤ 30 (43%), OR HEIGHT (H) > 12 AND SINUS OF VALSALVA DIAMETER (SOVD) > 30 (11%). CO, CORONARY OBSTRUCTION[198] Table 3.2 Coronary obstruction predictive parameters including currently used parameters (coronary ostium height, sinus of Valsalva diameter) and newly proposed predictive parameters based on 3D computational modeling[198] 72 Pati ent S e x A ge ,y A B C D E F G H I J K L M N O P Q R S T U V W X M F F F F F F F M F F F F F F F M M F F F F F F 88 68 89 81 81 77 84 77 61 79 83 82 70 81 74 76 86 93 88 72 77 91 87 91 Y M 94 Left coro nary arter y heigh t, mm 7 8 9 9 9 9 9 9 9 10 10 11 12 12 12 12 13 13 13 13 13 19 19 8 Sinus Left of coron Valsa ary va arter diam y eter, diam mm eter, mm 36 5.3 26 4.6 30 5.5 30 4 31 3.1 31 3.2 31 4.2 26 4.2 29 2.6 30 3.3 30 2.8 27 3.2 27 5.4 29 5.6 33 4.6 33 3.4 32 3.6 30 2.8 30 5 29 4.9 28 2.7 28 3.6 27 3.2 31 4.6 Valve Simul diam ated eter, TAV mm expan ded diame ter, mm 26 26 23 23 23 23 29 29 29 29 23 23 26 26 26 26 26 26 23 23 23 23 23 23 23 23 26 26 23 23 23 23 25 25 23 23 29 29 26 26 23 23 29 29 20 20 NA 23 DLC2D /d (1) DLC2D /d (2) DLC2D /d (3) Succe ssful TAV R? 1.5 1.4 1.2 0.7 1.3 2.8 2.3 0.8 1.7 2.2 1.5 1.2 0.3 0.9 0.8 1.7 1.2 1.2 1.1 1.5 1.7 2.0 1.1 0.6 0.4 1.4 0.4 0.7 1.3 2.8 2.3 0.8 0.5 1.2 1.0 1.2 0.3 0.8 0.8 1.3 0.9 0.6 1.1 1.2 1.4 2.0 1.1 0.6 0.4 1.4 0.3 0.6 1.3 2.8 2.3 0.8 0.2 0.9 0.7 1.1 0.2 0.2 0.7 0.6 0.9 0.1 0.7 0.5 0.7 0.8 0.8 0.4 9 32 NA 0.7 0.3 0.3 Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Not done Not done 4.6 26 73 Z F 75 9 28 5.7 23 23 0.1 0.0 0.3 Not done AA F 62 11 29 4.4 23 23 0.9 0.2 0.2 Not done AB M 80 12 37 5.4 29 29 0.6 0.0 1.2 No TAV, Transcatheter aortic valve; TAVR, transcatheter aortic valve replacement; NA, not applicable. With respect to the outcomes for these twenty-eight patients, a 3D computational risk assessment was performed prior to TAVR as described by Heitkemper and colleagues [194]. Based on this risk assessment, it was found that only 5 of the 28 patients would likely suffer coronary obstruction. The remaining 23 patients underwent successful TAVR. Four of the 5 patients likely to experience coronary obstruction based on the modeling were not offered TAVR owing to the risk of obstruction. These 4 patients included 2 females who were referred to surgery with visual confirmation of averted coronary obstruction by the operating surgeon, and 1 female and 1 male (patients X and Y, who had extremely low- lying coronary ostium [8 mm and 9 mm, respectively]), who were deemed noncandidates for surgical aortic valve replacement owing to age and instead received medical management. The fifth patient considered likely to experience coronary obstruction did in fact do so, because the risk assessment was not done before TAVR. 2D ANATOMICAL MODELS To correctly identify the risk of coronary obstruction within the study population, we aimed to elucidate the closest possible distance of the cusp relative to the corresponding coronary ostium, DLC2D, following a TAV stent deployment. A sketch of the idealized configuration of fully expanded cusps after TAVR is shown in Figure 3.8A. Locations of two points in this 2D crosssection are noted; 𝑃𝑐 , located on the outer tip of the cusp, and 𝑃𝑜 , representing the upper edge of 74 the coronary ostium. With this, 𝐷𝐿𝐶2𝐷 can be calculated using the Pythagorean theorem Equation (1): 𝐷𝐿𝐶2𝐷 = √(∆𝑥)2 + (∆𝑦)2 (1) Where ∆𝑥 and ∆𝑦 are the horizontal (x-direction) and vertical distances (y-direction) between 𝑃𝑐 and 𝑃𝑜 respectively. If the cusp is longer than the coronary artery height, ∆𝑦 is set to 0. Three different methods of estimating or modeling ∆𝑥 and ∆𝑦 were explored. In Figure 3.8A, the first method (DLC2D (1)) does not take into account any calcium that may be present on the cusp and the chord length (L) is the length of a chord connecting the cusp at the annulus to the point at which it intersects another cusp in the closed position. In Figure 3.8B, the second method (DLC2D (2)), the chord length remains the same as the first, but calcium present on the cusp is included. In Figure 3.8C the third method is shown, DLC2D (3). In this method, calcium present on the cusp is included and the chord length is a multiple of L, noted (Ltrue), so that Ltrue = αL, to take into account the fact that the true length of the leaflet, Ltrue when the leaflets are pushed open by the TAV stent may not be exactly equal to the chord length L. These models are purely phenomenological as opposed to the 3D model recently published [194], but carry an advantage of the relatively simple calculation without the need for complex finite element analysis. 75 FIGURE 3.8 IDEALIZED SCHEMATIC REPRESENTING THE CALCULATED MINIMUM DISTANCE FROM A POINT ON LEAFLET CALCIUM (PC) TO A POINT ON THE UPPER OSTIUM OF THE CORONARY ARTERY (PO) FOLLOWING TRANSCATHETER AORTIC VALVE REPLACEMENT (TAVR) FOR THE DLC2D/D (1) (A), DLC2D/D (2) (B), AND DLC2D/D (3) (C) [198] Each of the three models require the major anatomical features of a patients aortic root, including aortic chord length denoted L, sinus width at coronary ostium, w , coronary artery diameter, d , calcium nodule thickness on the cusp, denoted t , and coronary artery height from the aortic annulus, h to be measured from the patient’s pre-procedural CT imaging. Calcium nodules (t) were only measured if they were located on the upper half (near cusp tip) of the cusp. All the measurements were taken in the diastolic phase of the cardiac cycle. In Figure 3.9, an idealized 2D geometric sketch is shown depicting all parameters in one 2D view. 76 FIGURE 3.9 IDEALIZED SCHEMATIC REPRESENTING ESSENTIAL AORTIC ROOT MEASUREMENTS: AORTIC LEFT CHORD LENGTH (L), LEFT SINUS WIDTH AT CORONARY OSTIUM (W), LEFT CORONARY OSTIUM DIAMETER (D), CALCIUM NODULE THICKNESS ON THE LEFT CORONARY CUSP (T), AND HEIGHT OF THE LEFT CORONARY ARTERY FROM THE AORTIC ANNULUS (H) [198] The following equations demonstrate how ∆𝑥 and ∆𝑦 relate to the major anatomical features measured. The first method, DLC2D (1), did not include the presence of calcium on the cusp using Equation (2) and (3), respectively, ∆𝑥 = 𝑤 (2) ∆𝑦 = ℎ + 𝑑 − 𝐿 (3) Here, if the aortic chord length, L, is greater than the point on the upper ostia (PO), ∆𝑦 is set to 0. The second method, DLC2D (2) was identical with the exception of including calcium nodule thickness as shown in Equation (4) and (5), 77 ∆𝑥 = 𝑤 − 𝑡 (4) ∆𝑦 = ℎ + 𝑑 − 𝐿 (5) Here, in addition to the possibility that aortic chord length, L, is greater than the point on the upper ostia (PO) causing ∆𝑦 to be set to 0, ∆𝑥 is set to 0 if the calcium thickness, t, is greater than the sinus width, w. The third method of relating ∆𝑥 and ∆𝑦 to the measured parameters, DLC2D (3), aims to identify possible chord lengths by approximating Lnew as some constant (α) multiplied by L. Values of α between 0.9 and 1.4 in increments of 0.1 were explored. The third method of relating ∆𝑥 and ∆𝑦 to the measured anatomical features is shown in Equation (6) and (7), ∆𝑥 = 𝑤 − 𝑡 (6) ∆𝑦 = ℎ + 𝑑 − 𝛼𝐿 (7) Both ∆𝑥 and ∆𝑦 can be set to 0 in this method as described in the previous methods. DLC2D was calculated for the left coronary ostium by each of the three methods for every patient, as the predicted gap in (mm) available for coronary bound blood flow. 𝐷𝐿𝐶2𝐷 (in mm) was normalized with respect to the corresponding coronary ostium diameter (d), to obtain DLC2D/d, which represents the dimensionless distance between the aortic cusp and coronary ostium post-TAV deployment. These indices show the available distance for blood flow towards the coronary ostium. A value greater than unity indicates that the gap available for blood flow is greater than the coronary artery diameter. A fractional value approaching zero indicates total occlusion. 78 STATISTICAL ANALYSIS A sensitivity and specificity analysis was performed for each α in DLC2D/d(3) to identify which chord length, αL, resulted in the most accurate sensitive and specific model. The analysis revealed how many patients would identify as true positive, false positive, true negative or false negative for coronary obstruction under a range of cutoff values. Sensitivity was calculated as: 𝑆𝑒𝑛𝑠𝑖𝑡𝑖𝑣𝑖𝑡𝑦 = 𝑇𝑟𝑢𝑒 𝑝𝑜𝑠𝑖𝑡𝑖𝑣𝑒𝑠 , 𝑇𝑟𝑢𝑒 𝑝𝑜𝑠𝑖𝑡𝑖𝑣𝑒𝑠 + 𝐹𝑎𝑙𝑠𝑒 𝑛𝑒𝑔𝑎𝑡𝑖𝑣𝑒𝑠 While specificity was calculated as: 𝑆𝑝𝑒𝑐𝑖𝑓𝑖𝑐𝑖𝑡𝑦 = 𝑇𝑟𝑢𝑒 𝑛𝑒𝑔𝑎𝑡𝑖𝑣𝑒𝑠 , 𝑇𝑟𝑢𝑒 𝑛𝑒𝑔𝑎𝑡𝑖𝑣𝑒𝑠 + 𝐹𝑎𝑙𝑠𝑒 𝑝𝑜𝑠𝑖𝑡𝑖𝑣𝑒𝑠 as described in Lalkhen and [170]. Following this analysis of DLC2D/d (3) at varying chord lengths, αL, a sensitivity and specificity analysis was also performed for DLC2D/d (1), DLC2D/d (2), h and SOVd. A Mann-Whitney non parametric comparison of means was performed for the most accurate model (based on sensitivity and specificity analyses) ,DLC2D/d (2), h, and SOVd to compare the mean parameter value between the two groups; the 23 who underwent successful TAVR successfully and the 5 patients that did not undergo successful TAVR. 3. Results: This section presents data comparing the capability of conventional parameters such as h and SOVd with the newly introduced parameter DLC2D/d to differentiate which high-risk patients are not actually at risk and are good candidates for TAVR. Routine anatomic measurements of h and SOVd, along with calculated values from the 2D anatomic models DLC2D/d (1), (2), and (3) for the high-risk study population (28 patients) are presented 79 in Table 3.2. The individual anatomic measurements used to calculate DLC2D/d (2) for 2 patients (Z and AB) with the most severe prediction of coronary obstruction—ie, DLC2D/d (2) = 0.0—are presented in Table B.1. Figure 3.10 shows sensitivity and specificity curves generated for DLC2D/d (1) and DLC2D/d (2). In model DLC2D/d (1), which does not include calcium on the cusp, the optimal crossover point is slightly above 0.80, with a sensitivity and specificity of 83%. In comparison, the optimal crossover point for model DLC2D/d (2), which does include calcium on the cusp, is at 0.45 and results in a sensitivity and specificity of 85%. Figure 3.11 shows sensitivity and specificity curves generated for each chord length, αL, as described in model DLC2D/d (3). With respect to the sensitivity and specificity of the 2D anatomic parameter DLC2D/d (3), the highest sensitivity and specificity is found for α = 1, which is identical to that in model DLC2D/d (2). Sensitivity is 29% at a value of 0.0 and increases to 100% at a value of 0.8. The specificity drops from 100% at a value of 0.2 to 57% at a value of 1.0. The optimal crossover point is slightly above 0.60, with a sensitivity and specificity of 85%. In comparison, the optimal crossover point for α = 0.9 results in a sensitivity of 70% and specificity of 65%, and the optimal crossover point for α = 1.1 results in a sensitivity and specificity of 78%. For α = 1, there is a range of DLC2D/d (3) from 0.40 to 0.60, for which the sensitivity and specificity exceed 80%. For a ratio of DLC2D/d (2) ≥ 0.65, no coronary obstruction is expected to occur during TAVR. 80 FIGURE 3.10 SENSITIVITY AND SPECIFICITY OF DLC2D/D (1) (A) AND DLC2D/D (2) (B) TO PREDICT CORONARY OBSTRUCTION IN HIGH-RISK PATIENTS WITH HEIGHT (H) < 14 MM AND/OR SINUS OF VALSALVA DIAMETER (SOVD) < 30 MM[198] FIGURE 3.11 SENSITIVITY AND SPECIFICITY OF DLC2D/D (3) TO PREDICT CORONARY OBSTRUCTION IN HIGH-RISK PATIENTS WITH HEIGHT (H) < 14 MM AND/OR SINUS OF VALSALVA DIAMETER (SOVD) < 30 MM FOR VARYING VALUES OF Α: (A) Α = 0.9; (B) Α = 1; (C) Α = 1.1; (D) Α = 1.2; (E) Α = 1.3; (F) Α = 1.4[198] 81 3.2.3 COMPARISON TO CURRENT GUIDELINES Box-and whisker plots of the parameter values for those high risk patients who successfully received TAVR without coronary obstruction are compared to those who did undergo successful TAVR are compared in Figure 3.12. The means for these two groups were compared using a Mann-Whitney non-parametric test, and a significant difference between the two groups was found for the DLC2D/d (2) parameter, with p < 0.0018. Neither h nor SOVd was significantly different between the two groups with p = 0.35238 and p = 0.32218 respectively. Figure 3.12D shows the parameter values computed from the 3D computational model presented in Heitkemper and colleagues[194], in which a significant difference between the two groups was also reported. 82 FIGURE 3.12 COMPARATIVE BOX-AND-WHISKER PLOTS FOR THOSE WHO UNDERWENT SUCCESSFUL TRANSCATHETER AORTIC VALVE REPLACEMENT (TAVR) AND THOSE WHO DID NOT FOR DLC2D/D (2) (A) HEIGHT (H) (B), SINUS OF VALSALVA DIAMETER (SOVD) (C), AND DLC/D (D). UPPER AND LOWER BORDERS OF THE BOX REPRESENT THE UPPER AND LOWER QUARTILES, THE MIDDLE HORIZONTAL LINE REPRESENTS THE MEDIAN, AND THE UPPER AND LOWER WHISKERS REPRESENT THE MAXIMUM AND MINIMUM VALUES OF NONOUTLIERS. OUTLIERS ARE REPRESENTED BY SINGLE DOTS[198] Figure 3.13 shows sensitivity and specificity curves generated for DLC2D/d (2), DLC2D/d (2) for the entire population considered for TAVR, h, SOVd, and DLC/d computed from the 3D computational model presented by Heitkemper and colleagues[164]. The optimal crossover point for DLC2D/d (2) in the high-risk study population was at 0.45 and resulted in a sensitivity and specificity of 85%. At a DLC2D/d (2) of 0.6, the parameter was 100% sensitive, which would result in an expectation that 10 of the 28 patients (36%) would have coronary obstruction. In the entire 83 population considered for TAVR, the same model had an optimal sensitivity and specificity of 72% at DLC2D/d (2) of 1. The sensitivity of h steadily increased from 0% at an h cutoff of 7 mm to 100% at an h cutoff at 12 mm. In contrast, the specificity of h dropped steadily from 100% at a cutoff of 7 mm to 0% at a cutoff of 19 mm. The crossover point for sensitivity and specificity for h as an optimal predictor of coronary obstruction was 10 mm, with approximately 60% sensitivity and specificity. At h = 12 mm, the parameter was 100% sensitive, which would result in an expectation that 21 of the 28 patients (75%) would have a coronary obstruction. The sensitivity and specificity of SOVd as an independent predictor of unsuccessful TAVR is shown in Figure 3.13C. The sensitivity increased from 0% at an SOVd of 28 mm to 100% at 38 mm, and specificity dropped from 100% at 26 mm to 0% at 38 mm. The optimal crossover point occurred at approximately 30.5 mm with a sensitivity and specificity of 40%. At an SOVd of 38 mm, the parameter was 100% sensitive, which would result in the expectation that all 28 patients (100%) would have coronary obstruction. With respect to the sensitivity and specificity of the 3D computational parameter DLC/d, the sensitivity was 0% at a value of 0.2 and increased to 100% at a value of 0.45 (Figure3.13D). The specificity dropped from 100% at a value of 0.4 to 66% at a value of 1.0. The optimal crossover point was slightly below 0.45, with a sensitivity and specificity of 96%[164]. At DLC/d = 0.45, the parameter was 100% sensitive, which would result in an expectation that only 6 of the 28 patients (21%) would have coronary obstruction. 84 FIGURE 3.13 SENSITIVITY AND SPECIFICITY OF DLC2D/D (2) (A), DLC2D/D (2) FOR THE ENTIRE POPULATION CONSIDERED FOR TRANSCATHETER AORTIC VALVE REPLACEMENT (TAVR) (B), HEIGHT H (C); SINUS OF VALSALVA DIAMETER, SOVD (D); AND DLC/D (E) TO PREDICT CORONARY OBSTRUCTION FOR HIGH RISK PATIENTS WITH H < 14 MM AND/OR SOVD < 30 MM [198] 3.2.4 DISCUSSION Assessment of the risk of coronary obstruction during TAVR by geometric factors of patient aortic root geometry, h and SOVd, continues to anatomically exclude some patients from receiving the life-saving procedure[171-176] This study shows that these geometric factors significantly reduce the number of patients who might have safely undergone TAVR without coronary obstruction and introduces a simple method for the investigation of coronary obstruction risk in patients with severe aortic stenosis before TAVR. With this novel method, which uses pre-TAVR CT imaging, coronary obstruction risk can be calculated in a matter of minutes. The current criteria for high-risk coronary obstruction include SOVd < 30 mm [171]and h < 12 mm[183, 199]; however, these guidelines are not 85 consistently recognized throughout US hospitals. Individual transcatheter aortic valve manufacturers impose their own guidelines; for example, Medtronic suggests an SOVd of ≥27 mm and ≥29 mm for the 26-mm and 29-mm Evolut R and PRO, respectively. Similar to the SOVd guideline, h ≥ 14 is recommended by Medtronic; however, this guideline would exclude all but 2 of the patients in our study population, many of whom safely underwent TAVR without coronary obstruction. In addition, the patient-specific 2D predictive model DLC2D/d (2) provides a much more accurate representation of the TAVR procedure and can precisely capture the final configuration of a TAV stent along with native cusp and aortic wall. Although the 3 models are similar, DLC2D/d (1) and DLC2D/d (3) at α ≠ 1 had lower sensitivity and specificity in predicting coronary obstruction. DLC2D/d (1)'s suboptimal prediction of coronary obstruction risk suggests that evaluation of the presence of calcification on the leaflet is necessary to accurately screen for coronary obstruction risk. Likewise, DLC2D/d (3)'s suboptimal predictive ability of coronary obstruction risk at all α ≠ 1 suggests that an accurate approximation of leaflet length is also necessary. Based on our findings, DLC2D/d (2) and DLC2D/d (3) at α = 1 is the only model that can accurately predict the risk of coronary obstruction in patients who have been flagged as higher risk for coronary obstruction (h < 14 mm and/or SOVd < 30 mm). Those patients with DLC2D/d (2) ≥ 0.7 should be considered not actually at high risk for left coronary obstruction. Patients with DLC2D/d (2) <0.7 are at severe risk of coronary obstruction, and TAVR should not be attempted in these patients. In patients who have not been flagged as at risk (h < 14 mm and/or SOVd < 30 mm), the sensitivity and specificity of DLC2D/d (2) drops to 72%, suggesting a reduced ability to predict obstruction risk alone, without the previous stratification 86 of risk. An idealized schematic representing the simple 2D anatomic model, DLC2D/d (2), is shown in Figure 3.14, along with the optimal percent sensitivity and specificity of the 2D model compared with the current guidelines, h and SOVd, and a previous computational study, DLC/d, to predict coronary obstruction for high-risk patients with h < 14 mm and/or SOVd < 30 mm. The proposed 2D predictive model, in conjunction with conventional risk stratification, allows for more patients to be accurately screened for coronary obstruction before the TAVR procedure and can be computed in a fraction of the time required for more complex computational models. However, due to the lower sensitivity and specificity of the 2D model compared with the 3D model, the 2D model should not be used independently, but rather can serve as a filter to determine which patients require the more accurate 3D model described by Heitkemper and colleagues[164] to predict the risk of coronary obstruction. 87 FIGURE 3.14 IDEALIZED SCHEMATIC REPRESENTING THE SIMPLE 2D ANATOMIC MODEL USED TO PREDICT THE RISK OF CORONARY OBSTRUCTION DURING TRANSCATHETER AORTIC VALVE REPLACEMENT, DLC2D/D, THE CALCULATED MINIMUM DISTANCE FROM A POINT ON LEAFLET CALCIUM, PC, TO A POINT ON THE UPPER OSTIUM OF THE CORONARY ARTERY, PO. THE OPTIMAL PERCENT SENSITIVITY AND SPECIFICITY OF THE 2D MODEL, DLC2D/D, IS COMPARED TO WITH CURRENT GUIDELINES, H AND SOVD, AND A PREVIOUS COMPUTATIONAL STUDY OF DLC/D TO PREDICT CORONARY OBSTRUCTION IN HIGH-RISK PATIENTS WITH H < 14 MM AND/OR SINUS OF SOVD < 30 MM.[198] 3.2.5 LIMITATIONS This study has several limitations. First, we did not account for the possibility of coronary obstruction as a consequence of valve malpositioning (supra-annular). In addition, right coronary obstruction was not evaluated, because obstruction of right coronary is much less prevalent compared to left coronary [153-155]. However, the 2D model is likely applicable to right coronary artery for pre-operative risk assessment of coronary obstruction. Another limitation of the study is the small number of cases evaluated for coronary obstruction, which is due to its relatively rare occurrence. Although we identified likely coronary obstruction among patients deemed at prohibitive risk and referred for surgical AVR, we did not routinely perform this maneuver among 88 patients at no risk for coronary obstruction. Accordingly, the specificity of this exercise for coronary obstruction has not been established. Finally, the 2D model does not account for potential expansion of the native annulus from the radial force exerted by TAV implantation. This could impact the width, w, in equation (4) by under-estimating the true width available for coronary flow post expansion. Addressing this within a simplified mathematical model is not straight forward and may be better accounted for with 3D modeling. 3.2.6 CONCLUSION We have derived a simple and accurate model to screen patients for possible coronary obstruction during TAVR. The parameters involved in evaluation of the index are obtained from the anatomic measurements readily calculated from current pre-TAVR CT angiographic imaging. Although neither h nor SOVd is predictive of coronary obstruction in high-risk patients, characterized as h < 14 mm and/or SOVd < 30 mm, a new parameter, DLC2D/d (2), is capable of predicting coronary obstruction for the same high-risk group with a superior sensitivity and specificity. However, we note that full 3D modeling provides the most sensitive and specific prediction of coronary obstruction. These findings shed light on a rare but significant potential complication during TAVR and may assist heart teams in the decision-making process before the TAVR procedure. 89 Chapter 4: Specific Aim 2 4.1 Development of a Polymeric Transcatheter Valve 4.1.1 INTRODUCTION Transcatheter aortic valve replacement (TAVR) has emerged as a life-saving treatment for patients that are excluded from traditional surgical valve replacement surgeries due to risk of procedural complications[200]. The percutaneous valve replacement procedure is less invasive, avoiding morbidity and a long recovery following an open heart procedure, which in turn reduces length of hospital stay [201, 202]. Despite the advantages of TAVR, clinical studies have identified features associated with poor outcomes, including residual paravalvular leakage (PVL), leaflet calcification, and subclinical leaflet thrombosis, which negatively impact valve function[203-207]. While the typical functional lifetime of a bioprosthetic surgical valve ranges from 10-15 years[208], that of a transcatheter bioprosthetic is reduced to 7-10 years following replacement[209, 210]. Even though the most current transcatheter valve designs have addressed some of these issues, for example implementing skirts to reduce PVL, reduced functional lifetime still remains as a major disadvantage of TAVR. The commonality between all commercially available transcatheter aortic valves in the U.S. and Europe that have been approved by the FDA and CE respectively is the leaflet material, which is always chemically fixed pericardium tissue. Many of the features that are associated with poor outcomes of TAVR are inherent to chemical fixation of tissue based leaflets, including subclinical leaflet thrombosis and calcification buildup[211, 212]. Additional concerns with 90 crimping stability of these tissue components have risen in more recent years[213, 214]. Efforts to substitute the pericardium-based prosthetic heart valves with polymeric materials date back to the 1960’s with the first silicone valve surgical aortic implant reported by Roe et. al. in 1969[69]. Until recent years, the material science and engineering behind polymer chemistry has not been able to simultaneously produce a biocompatible, durable, and anti-thrombogenic polymeric leaflet substitute[75, 215], and improved materials are still under investigation. One such material, hyaluronan (HA) enhanced linear low density polyethylene, has shown promise as a leaflet substitute due to its strength, flexible nature, and tunable surface properties, as well as its cytocompatability, resistance to platelet adhesion and activation, and reduced clotting as compared to conventional heart valve materials such as fixed tissue and pyloritic carbon [115, 216]. In addition to the thrombotic proclivity of a material, thrombogenic potential is also highly dependent on flow conditions, which are significantly influenced by valve design[217-219]. Increased turbulent stresses are associated with increased thrombogenic potential, and therefore it is important to investigate turbulent stresses to characterize prosthetic valve function. Turbulent stress levels, and especially Reynolds shear stress, are well known to be an indirect measure of the shear stresses experienced by blood cells and platelets in a turbulent flow environment[220]. In a healthy native aortic valve, maximum Reynolds shear stress values have been reported as < 3 Pa, where in stenotic valves, this number is an order of magnitude higher at 30 Pa [221]. Previous studies have associated non-physiological flow following transcatheter valve replacement with increasing levels of blood damage, ranging from platelet activation to hemolysis [217, 222-224]. Therefore, an ideal prosthetic valve design would yield the least turbulent effects and decreased 91 levels of Reynolds stress while exhibiting surface hemocompatiblity (i.e. resistance to platelet adhesion, fibrosis, and contact activation). In the new era of both balloon-expanding and self-expanding transcatheter valve approval for use in low risk patients, efforts towards the development of durable polymeric valves are numerous and escalating [184, 185]. Of those, the hemodynamic performance of investigational valves including the TRISKELE valve[144], Polynova valve [225], and the Strait Access Technologies valve have been studied and published with promising results. While basic hemodynamic data on these and investigational polymeric surgical valves[128, 141, 226] are promising, the respective studies do not report the turbulent characteristics of these valves and thus their turbulent flow induced thrombogenic potential is unknown. We aim to characterize in-vitro the hemodynamic function and turbulent flow characteristics of a hyaluronan (HA) enhanced polymeric transcatheter aortic valve (HA-TAV) with a novel stent design that aims to reduce flow turbulence and decrease thrombogenic potential. 4.1.2 MATERIALS AND METHODS VALVE STENT DESIGN The polymeric transcatheter aortic valve was manufactured in house, as an assembly of an interpenetrated network of Hyaluronan (HA) and linear low density polyethylene (LLDPE) for the valve leaflets and a cobalt chromium (CoCr- MP35N) stent. The stent was designed in 3D CAD software (Solidworks 2018), and laser cut (STI Laser Industries) to be balloon expandable. The balloon expandable stent has a valve diameter is 26mm, and a height of 25mm. A total of 9 nonload bearing polypropylene sutures were used to attach the polymer leaflets to the stent frame to fix their position during crimping. Major features of the CoCr stent design include two distinct 92 rows of diamond shaped structures, where the bottom row is comprised of 6 uniform diamond shaped structures and the top row is comprised of three larger diamond shaped structures with a 60 degree angle, and 3 “V” shaped structures connecting them as shown in Figure 4.1. The 3 tips of the larger diamond shaped structures are the stent posts, and the “V” shaped structures serve to keep the native aortic valve leaflets from interfering with the functionality of the polymeric leaflets once implanted in the native aortic root. Another unique feature of this stent design is that the polymeric leaflets are attached outside of the stent, rather than being sutured to the inside, and fold under the “V” shaped structures to form the leaflets. A leaflet arch length (h/D) of 0.115 as described in Yousefi et. al.[140] was used for this valve. FIGURE 4.1 3D CAD MODEL OF COBALT CHROMIUM TRANSCATHETER STENT FRAME, DETAILING STENT THICKNESS (0.55 MM), PROFILE (25 MM), AND MAJOR FRAME ANGLE (Θ = 60°)[227] 93 LEAFLETS’ MATERIALS The polymeric leaflets were cut from sheets of interpenetrated networks of HA and LLDPE. Hyaluronan is a highly hydrophilic and anionic molecule, essential to the extracellular matrix of human heart valves[228]. It has been shown to be non-toxic, biodegradable, and nonimmunogenic and is therefore highly suitable for blood contacting applications [115, 229, 230]. One advantage of HA is that the molecule has extraordinary potential to be chemically modified, as a way to control its degradation and mechanical properties, as in an interpenetrating network. 80 µm thick polymeric sheets were blow-molded by Flex-Pack Engineering, Inc. (Uniontown,OH) from LLDPE resin (Dowlex 2056; Dow Chemical Company, Edegem, Belgium) and then a swelling process was used to form an interpenetrated network (IPN), where two polymers are combined at the molecular level, with HA. This method of introducing HA to the LLDPE has been shown to improve the ability of the two polymers to remain intact, as the polymers in an IPN cannot be separated unless chemical bonds are broken[148] [231]. The HA IPN has shown to be much more durable than surface treatments (such as heparin) that reduce platelet adhesion and improve hemocompatability [216, 232, 233] making it a promising material for prosthetic heart valve leaflets as anticoagulation therapy will not be necessary. Additional details of the manufacturing process and details of the desirable material properties of HA-LLDPE including high yield tensile and tear strengths can be found in previous works[115, 234-236]. Photographs of the valve are shown in Figure 4.2. 94 FIGURE 4.2 A. HA-TAV PROFILE OF STENT FRAME AND SEMI-CLOSED LEAFLET POSITION B. HA-TAV PROFILE SO STENT FRAME AND OPEN LEAFLET POSITION[227] HEMODYNAMIC PARAMETERS The hemodynamic performance of a polymeric TAV was compared against two of the leading commercially available transcatheter valves of comparable sizes, a 26mm Medtronic Evolut (Minneapolis, Minnesota) and a 26mm Edwards SAPIEN 3 (Irvine, California). The three valves were inserted into an aortic root model of physiological size and connected to an experimental pulse duplicator left heart flow simulator, shown in Figure 4.3, that is capable of creating pulsatile flow conditions under physiological pressure (120/80 mmHg), heart rate (60 bpm), and cardiac output (5 L/min) as previously described [169, 237-242]. A working fluid of 60/40 water to glycerin (99% pure glycerin) was used to provide density and kinematic viscosity comparable to blood, at 1060 kg/m3 and 3.5 ·10-6 m2/s respectively. Aortic and ventricular pressure as well as flow rate were collected at a sampling frequency of 100 Hz for 60 consecutive cardiac cycles. The aortic flow and pressure that were imposed on the valve are shown in Figure 4.4, where 95 the flow and pressure have been ensemble averaged over 60 cardiac cycles. Valve leaflet motion was recorded with en-face high speed imaging collected at 1000 frames per second throughout the cardiac cycle. From these data, effective orifice area (EOA), regurgitant fraction (RF), and pinwheeling index (PI) were computed for each of the valve types. FIGURE 4.3 SCHEMATIC OF LEFT HEART FLOW SIMULATOR[227] 96 FIGURE 4.4 AORTIC FLOW (BLUE) AND PRESSURE (GREEN) CONDITIONS THAT THE VALVES WERE SUBJECT TO OVER ONE CARDIAC CYCLE [227] EFFECTIVE ORIFICE AREA (EOA) The effective orifice area (EOA) is a common parameter that assesses valve performance through the quantification of valve stenosis. It is a measurement of the effective jet area during the valve opening phase of the cardiac cycle[243]. EOA was computed from the Gorlin relation: 𝐸𝑂𝐴 = 𝑄𝑟𝑚𝑠 51.6√∆𝑃 (8) Where 𝑄𝑟𝑚𝑠 is the root mean square aortic valve flow rate (cm3/s) and ∆𝑃 is the mean pressure drop (mmHg) over the full cardiac cycle. 97 REGURGITANT FRACTION (RF) Regurgitant fraction (RF) is a second common parameter that is used to assess valve performance. It represents the ratio of the closing (CV) and leakage volume (LV) to the forward flow volume (FV). A higher performing valve would demonstrate a low regurgitant fraction (≤ 15%)[244]. 𝑅𝐹 = 𝐶𝑉 + 𝐿𝑉 (9) 𝐹𝑉 PINWHEELING INDEX (PI) The pinwheeling index (PI) measures the twisting extent of the leaflets upon closure[242]. High pinwheeling indices have been shown to be linked with decreased leaflet durability[245-247]. PI is computed from still frames of high-speed imaging during valve closing phase as follows: 𝑃𝐼 = 𝐿𝑎𝑐𝑡𝑢𝑎𝑙 −𝐿𝑖𝑑𝑒𝑎𝑙 𝐿𝑖𝑑𝑒𝑎𝑙 (10) where 𝐿𝑎𝑐𝑡𝑢𝑎𝑙 represents the actual length of the free edge of a leaflet, and 𝐿𝑖𝑑𝑒𝑎𝑙 represents the shortest distance between the post and central coaptation region, as previously described by Midha et al.[248]. PARTICLE IMAGE VELOCIMETRY (PIV) Particle image velocimetry (PIV) was performed to visualize and evaluate the flow velocity field through the valves and to identify turbulence characteristics. Briefly, the flow of interest was seeded with florescent PMMA-Rhodamine B particles (average diameter ~10 µm) and illuminated by a thin laser sheet created with a double pulsed neodymium-doped yitrium lithium fluoride (NdYLF) solid state laser coupled with spherical and cylindrical lenses. Time-resolved recordings were acquired at spatial and temporal resolutions of 0.037 mm/pixel and 1000 Hz respectively. 98 250 repetitions of phase locked measurements were recorded for acceleration, peak, deceleration, and diastolic phases of the cardiac cycle. DaVis PIV software (DaVis 7.2; Lavision, Gӧttingen, Germany) used for all image post processing. More details of PIV techniques can be found elsewhere[200, 238, 239, 249, 250]. VORTICITY CALCULATIONS Vorticity is the curl of the velocity field and therefore is useful to visualize both rotational blood shearing and turbulence. High vorticity regions along the axis perpendicular to the plane indicate shear and rotation of the fluid particles[242]. Vorticity was computed as follows: 𝑑𝑉𝑥 𝑑𝑉𝑦 𝜔𝑧 = − ( − ) (11) 𝑑𝑦 𝑑𝑥 Where 𝜔𝑧 is the vorticity component with units of s-1; Vx and Vy are the x and y components of the velocity with units of m/s. PRINCIPAL REYNOLDS SHEAR STRESS (RSS) Large Reynold’s shear stress (RSS) is an indicator of high turbulence and has been widely correlated with increasing likelihood of blood and endothelial damage after implantation of heart valve prostheses[222, 251]. Principal RSS is a statistical quantity that measures the shear stress between fluid layers when particles decelerate or accelerate while changing direction [252] and is calculated as: 2 𝑢′ 𝑢′ − 𝑣 ′ 𝑣 ′ 2 √ 𝑅𝑆𝑆 = 𝜌 ( ) + (𝑢′ 𝑣 ′ ) (12) 2 99 where 𝜌 is the density of the working fluid (kg/m3) and 𝑢′ and 𝑣 ′ are the instantaneous velocity fluctuations in the x and y directions respectively (m/s). Equation (12) implicitly assumes no outof-plane component of instantaneous velocity, w’, and can be considered as a lower bound for the principle RSS [253]. The principal RSS was calculated for each spatial location downstream the valve and binned by RSS value frequency. Each bin was normalized to the maximum number of counts in any one bin, and this normalized frequency of principal RSS values was plotted. STATISTICAL ANALYSIS Statistical analysis in this study was performed using JMP Pro version 13.0.0 (SAS Institute Inc, Cary, NC). All data are presented as mean ± standard error. A non-parametric comparison of means was performed to compare the mean hemodynamic parameters and a p-value of p < .05 was considered statistically significant. Analyses were performed over 60 replicates. 4.1.3 RESULTS HEMODYNAMIC ASSESSMENT Hemodynamic parameters obtained from the flow and pressure data for each valve were given in Table 4.1. The HA-TAV had an EOA of 2.08 ± 0.04 cm2, within one standard deviation of the leading valve, SAPIEN 3 at 2.1 ± 0.025 cm2, through their means were significantly different (P < 0.001). Likewise, the HA-TAV had an increased effective orifice area as compared to the Evolut 1.8 ± 0.036 cm2, with a significance of P < 0.001. The RF of the HA-TAV (11.23 ± 0.55 %) is lower in comparison to the Evolut (15.74 ± 0.73 %) (P < 0.05) and slightly higher than the SAPIEN 3 (10.92 ± 0.11 %) (P < 0.05), putting it well within the range of the two leading commercially available valves. 100 HA-TAV Evolut SAPIEN 3 EOA (cm2) 2.08 ± 0.04 1.80 ± 0.04 2.10 ± 0.03 RF (%) 11.23 ± 0.55 15.74 ± 0.73 10.92 ± 0.11 Pinwheeling Index 0.05± 0.03 0.12 ± 0.05 0.37 ± 0.07 Table 4.1 Measured hemodynamic parameters of each valve[227] PINWHEELING The En-face views of valve opening and closing at peak systole and mid-diastole are shown for each valve in Figure 4.5. At peak systole, the Evolut and SAPIEN 3 are maximally open, with symmetrical orifices, while the HA-TAV is non-symmetrical and non-circular. Visual inspection of the images shows that the SAPIEN 3 has the largest twisting in the coaptation region, followed by the Evolut and then the HA-TAV. This is in accordance with the values reported in Table 4.1, where the PI was significantly decreased (P < 0.001) for the HA-TAV (0.0456 ± 0.03) as compared to the Evolut (0.122 ± 0.045) and SAPIEN 3 (0.366 ± 0.067). 101 FIGURE 4.5 EN-FACE VIEWS OF EACH VALVE AT PEAK SYSTOLE AND MID DIASTOLE[227] VELOCITY VECTOR FIELD AND VORTICITY CONTOURS Phase averaged velocity vector fields and corresponding vorticity contours are shown in Figure 4.6 at four time points in the cardiac cycle, namely, acceleration, peak systole, deceleration and diastole, which are denoted by a red dot along the representative aortic flow curve. Bright red and blue contours represent the shear layers, which correspond to the jet boundaries. The distance between the shear layers represent the width of the jet through the valve. 102 FIGURE 4.6 PHASE AVERAGED VELOCITY VECTORS AND VORTICITY CONTOURS THROUGHOUT THE CARDIAC CYCLE[227] The maximum value of velocity for the HA-TAV was 1.56 m/s during acceleration, 1.94 at peak systole, and 1.03 at deceleration phase. In comparison, the Evolut’s maximum velocity at acceleration phase was decreased (1.00 m/s), increased to reach 2.45 m/s at peak systole, and then 1.37 m/s during deceleration. The SAPIEN 3 velocity increased from 0.86 m/s during acceleration, 2.10 m/s at peak systole, and reached 0.94 m/s during deceleration. The velocity during diastole was 0.17 m/s for the HA-TAV, and 0.19 m/s for both the Evolut and SAPIEN 3. Developed shear layers occur sooner during acceleration phase in the HA-TAV as compared to the Evolut and SAPIEN 3. At peak systole, the shear layers were thinner with the HA-TAV compared to the Evolut and SAPIEN, and were characterized by lower vorticity 103 magnitudes 5 mm downstream the valve with the HA-TAV approximately half the magnitude of the SAPIEN 3 and approximately 4/5th of the magnitude of the Evolut. At the deceleration phase, the distance between the shear layers was significantly reduced for the HA-TAV, and only very slightly for the Evolut and SAPIEN 3, showing that the jet narrows sooner in systole for the HATAV. REYNOLDS SHEAR STRESS (RSS) Figure 4.7 shows the principal Reynolds shear stress (RSS) at acceleration, peak, deceleration and diastolic phases of the cardiac cycle for each valve. RSS is an important indicator of platelet activation due to the turbulent fluctuations of the blood velocity[222-224, 242, 249, 251, 254]. FIGURE 4.7 PHASE AVERAGED PRINCIPLE REYNOLDS SHEAR STRESSES (RSS) THROUGHOUT THE CARDIAC CYCLE [227] 104 For each valve, the highest values of RSS were present at peak systole. In comparison to the Evolut and SAPIEN 3, the HA-TAV had a significantly smaller region in which higher RSS values (>10 Pa) were present, concentrated near the stent frame alone. While in the HA-TAV and SAPIEN 3 the majority of the RSS had dissipated by the deceleration time point, the Evolut demonstrated slower dissipation of these stresses. This observation is clearly demonstrated in the distribution plots of the principal Reynolds shear stresses at acceleration, peak and deceleration in Figure 4.8. During acceleration, there is a single peak of the normalized frequency curve for the HA-TAV and the two commercial valves. The Evolut has the widest peak, indicating that it holds the highest number of higher RSS values. During peak systole, the HA-TAV has a similar frequency profile as it did in acceleration. The Evolut does not reach 0 frequency as quickly, and the profile of the frequency curve is not smooth indicating regions of high values of increased RSS, while the SAPIEN 3 is somewhat smoother, reaching 0 frequency at a lower value of RSS than for the Evolut. At deceleration, the frequency profile for the HA-TAV is no longer smooth, but still reaches 0 at a lower RSS value than the two commercially available valves. The Evolut has a wide second peak at higher RSS values, and the SAPIEN 3 has a sharp second peak at lower RSS values. FIGURE 4.8 NORMALIZED FREQUENCY OF PRINCIPAL REYNOLDS SHEAR STRESS AT THE DEFINED PHASES IN THE CARDIAC CYCLE[227] 105 4.1.4 DISCUSSION The potential of the novel HA-TAV was investigated in this in vitro study through (1) evaluating hemodynamic parameters (2) assessing velocity and vorticity and (3) analyzing turbulence characteristics through calculating RSS. HEMODYNAMIC ASSESSMENT AND PINWHEELING The effective orifice area of the HA-TAV was comparable to the SAPIEN 3, and both were larger than the Evolut. One reason for this difference may be that the HA-TAV and SAPIEN 3 are balloon-expandable, while the Evolut is self-expanding. Balloon expandable valves are known to contribute to reduced pressure gradients in TAVs and larger orifice areas, due to the radial force of the balloon anchoring into the aortic root[181, 200, 255]. The improved effective orifice area could also likely be due to the polymeric material and difference in the HA-TAV stent design that allows the leaflets to fold out beyond the confinement of the inner-diameter of the stent producing a unique three dimensional leaflet surface geometry during the forward flow phase. This leaflet surface geometry likely produces some out of plane component of the main jet, which could induce rotational flows and increase effective orifice areas. A particle streak video (Supplementary Video 2) of the HA-TAV show evidence of these potential rotational flow+s as compared to the more linear flows shown for the Evolut and SAPIEN 3. Regurgitant fraction is of major importance in the development of novel transcatheter aortic valves, as a high RF puts additional load on the heart to pump adequate blood supply to the rest of the body[239]. Also, additional consideration should be given to the development of a nonphysiological backwards flow jet that can induce platelet activation and hemolysis[140, 256-259]. RF of the HA-TAV falls within the levels of the SAPIEN 3 and Evolut, marking it as comparable 106 to these two commercially available valves and trace with regards to the standards presented by Nishimura et. al.[260] The pinwheeling index of the HA-TAV is significantly decreased in comparison to the Evolut and SAPIEN 3. This is likely an effect of the leaflet design, with arched profiles modified from Yousefi et. al.[140] that allow for there to be a balance between optimal coaptation area and minimized PI to ensure central gap closure and enhance leaflet durability respectively. The regurgitant fractions obtained in this study for the HA-TAV were found to be lower than those obtained with TRISKELE-26 valve (19.3%) while the effective orifice area was found to be higher for the HA-TAV as compared to the TRISKELE-26 (1.9 cm2) [144]. VELOCITY AND VORTICITY The increased velocity for the HA-TAV during the acceleration phase as compared to Evolut and SAPIEN 3 is likely due to a combination of the delay in opening of the polymeric leaflets (Supplementary Video 1), and the geometry of the orifice that is present throughout the cardiac cycle. The flexural properties of material used for prosthetic heart valve leaflets are highly important to heart valve design, as it has been shown that leaflet bending plays a critical role in bioprosthetic heart valve and function[261]. The smaller orifice produces higher jet velocities at this stage. However, at peak systole, the HA-TAV has reached max opening and shows decreased max jet velocities than for the Evolut and SAPIEN 3. In the HA-TAV, the shear layers are significantly diminished in approximately half of the distance downstream of the valve outlet as they are for the Evolut and SAPIEN 3, with much lower magnitudes in as little as 5 mm from the outlet. This decrease in vorticity fluctuation is indicative 107 of a decrease in turbulence (and therefore energy loss). This decrease in in vorticity and in turbulence may be due to the leaflet geometry that could induce a slightly out of plane component of the main jet giving way to rotational flows. In the deceleration phase, it is clear that the HATAV begins to close sooner after reaching max opening than the two tissue valves, as seen by the sudden diminishing of distance between shear layers (jet width) at this time point. REYNOLDS SHEAR STRESS (RSS) RSS magnitudes have an important role in determining the biocompatibility of a valve prosthesis because they can indicate regions of probable platelet activation from turbulent fluctuations of the blood velocity[222-224, 254, 262]. Previous in vitro studies attempted to set some thresholds that mark the onset of platelet activation[238]. The critical shear stress levels for hemolysis and platelet lysis under physiological exposure times have been shown to be between 150-400 Pa [221]. However, thresholds are not yet well-established, and the characterization of turbulent stress is still controversial[238]. At peak systole, where RSS was highest for each valve, it is clear that the HA-TAV has the least frequency of high RSS values that increase the likelihood of platelet activation (>100 Pa)[220, 263, 264]. This is likely due to the stent frame design that is both low in profile, and has a decreased number of “grid” like structures that have been shown to increase turbulence, unsteadiness, and skewness of velocity fluctuation[242, 249, 265-267]. The stent design aspects therefore also explain the increase in RSS of the Evolut compared to the SAPIEN 3, as the Evolut has an increase profile (protrudes further into the aorta) which increases the meshed flow contacting areas[242]. The leaflet flutter seen in the two tissue valves are also known to cause high cycle-to-cycle variability in the flow, which could have contributed to the increased frequency of 108 high Reynolds shear stress[268]. While each valve demonstrated a max RSS value exceeding 100 Pa at peak systole, at every cardiac phase the HA-TAV had decreased frequency of high RSS values suggesting that platelet activation and therefore thrombus formation is least likely to occur in this valve. POLYMERIC TAVS AS AN ALTERNATIVE FOR BIOPROSTHETIC TAVS While current bioprosthetic TAVRs have made a less invasive heart valve replacement surgery possible, there are still major concerns about their long term durability, especially when the life expectancy of a patient exceeds the ‘proven’ good midterm durability of 5-7 years[269]. The biological tissue used in all commercially available TAVs is prone to structural valve degeneration, which has been estimated to occur in over 50% of patients receiving transcatheter aortic valve replacements within 8 years[270]. Factors associated with complications post-TAVR such as subclinical valve thrombosis have been detected in 10% to 15% of patients receiving bioprosthetic TAVs[271], and introducing anticoagulation therapy poses its own severe risks[272]. Now that TAVR is approved for low risk patients, it is therefore crucial and urgent to develop not only a substitute of a leaflet material but rather a novel valve as a whole presenting excellent hemodynamic performance from appropriate interaction between leaflet and stent, and leaflet-stent and blood. The data presented herein demonstrate the hemodynamic potential of a polymeric based TAVR device to change the future of TAV replacement therapy. In-vivo data are ongoing to validate these in-vitro data. 4.1.5 SUMMARY The hemodynamic performance and turbulence of a novel polymeric transcatheter valve, the HA-TAV, were compared against two same size leading commercially available transcatheter 109 valves, the Medtronic Evolut and Edwards SAPIEN 3 in-vitro. Resulting measurements of hemodynamic parameters including EOA, RF, and PI have demonstrated that the HA-TAV exceeds baseline hemodynamic requirements and is directly comparable to the leading valves, while the results of turbulent flow characterization in the HA-TAV show improvement over the leading commercially available valves. Ongoing accelerated fatigue testing and in-vivo studies strongly indicate the potential for a polymeric valve to be an alternative solution to the prosthetic valves currently used in TAVR procedures. 4.1.6 LIMITATIONS Though we do not expect valve to valve variability in either of the commercially available valves, limited availability of the TAVs that were used has limited the study to n=1 of each valve type. Further, valve hemodynamics performance and turbulence characteristics are not the only factors used to assess valve performance and readiness for in vivo use and in the current state additional studies would be needed to claim that this valve is an alternative to those that are commercially available. Of these additional studies, accelerated fatigue testing is necessary to evaluate the expected long term durability of the sutured polymer and novel stent frame. Further studies are needed to comprehensively assess the mechanism by which the HA-TAV demonstrated superior hemodynamics. 110 4.2 Effect of Leaflet Opening Geometry on Valve Performance and Turbulent Shear Stresses 4.2.1 INTRODUCTION Turbulent blood flow is prominent in cardiovascular pathophysiology, particularly in the ascending aorta, and there is strong evidence that turbulence impacts the environments of platelets and erythrocytes on a cellular level in addition to the known effects turbulence has on energy efficiency[273]. In the specific case of prosthetic heart valve replacements, increased fluctuations of shear stress in turbulent blood flow have been shown to lead to hemolysis and platelet activation [274, 275], phenomena that can be detrimental to the success longevity of the prostheses. In the functional design of prosthetic heart valves, as well as many other cardiovascular devices, reduction of turbulence is therefore a major factor to be considered. A recent in-vitro study compared the principal Reynolds shear stresses(RSS) , one indirect measurement of shear stresses experiences by red blood cells and platelets in turbulent flow[220], downstream of a novel polymeric transcatheter heart valve and two leading commercially available transcatheter valves. The study has identified that the novel valve (HA-TAV) had significantly reduced frequencies of large RSS values at every phase in the cardiac cycle [227]. While the differences in RSS were attributed to the reduced number of “grid” like structures in the frame design [169, 242, 265-267] and leaflet flutter that was not present in the polymeric valve [268], the differing opening geometries of the valves were highlighted but not thoroughly studied. The authors eluded that the leaflet surface geometry likely produced an out of plane component to the main jet, which could induce swirling flows that effectively decrease vorticity fluctuations and 111 turbulence, due to a higher critical transitional Reynolds number from laminar to turbulent, though this mechanism was not sufficiently studied. The authors noted that the mechanisms by which the HA-TAV demonstrated superior hemodynamics needed further investigation [227]. Previous studies have shown that fluid swirl can indeed suppress turbulence through many differing modes[276] including reducing turbulent diffusion in the radial direction[277, 278], lowering the coefficient of turbulent drag force in pipes[279, 280], and prolonging the laminarturbulent transition[281, 282]. It was also shown that considerable reduction in pressure loss occurred for turbulent flow regimes with pipe rotation [283], and studies by Reich and Beer reported that in rotating pipe flows, it was the centrifugal force that was generated by the rotation that led to drag reduction[280]. Numerous computational fluid dynamics simulations have added to the experimental conclusions, leading to a consensus in literature that pipe rotation generates a stabilizing effect downstream from the inlet, leading to the laminarization, or suppressed turbulence phenomena[284]. While Bourgouin et al. studied the effect of swirler geometry on swirling flows using Large Eddy Simulations[285] and Ariyarante et al. studied swirl-inducing pipe geometries[286], no group has studied non-rotating, heart valve opening geometries in the context of turbulence suppression. With momentum building towards the use of polymeric materials in prosthetic heart valve protheses, there is consequently a newfound flexibility to design and manufacture varying valve opening geometry and it is not clear that the current protheses are optimized for performance. In this work, we aim to investigate how valve opening geometry impacts hemodynamic performance and turbulence characteristics. 112 4.2.2 MATERIALS AND METHODS HEMODYNAMIC PERFORMANCE Leaflet opening geometries modeled after a commercially available transcatheter valve, Edwards SAPIEN 3 (Irvine California) (round), and the investigational transcatheter polymeric valve, hyaluronanan-TAV (triskele-like) were 3D printed from RGD720 (Stratasys Ltd.). For each opening geometry, one large geometric orifice area (3.4 cm2) and one small geometric orifice area (1 cm2) were printed to represent maximal leaflet opening at peak flow rates and minimal opening during acceleration and deceleration phases of the cardiac cycle respectively (Figure 4.9). FIGURE 4.9 3D PRINTED ORIFICES OF ROUND AND TRISKELE-LIKE GEOMETRIES IN BOTH SMALL AND LARGE SIZE 113 Each 3D printed orifice was placed into an aortic root model of physiological size in series with a Medtronic Hancock II bioprosthesis (Minneapolis, Minnesota) mounted in an idealized aortic root model in a left heart pulse duplicator as previously described[227, 287, 288]. Figure 4.10 shows a FIGURE 4.10 SCHEMATIC DIAGRAM OF EXPERIMENTAL SET UP schematic of the experimental setup including both chambers. A working fluid of 60/40 water to glycerin was used to provide density (1060 kg/m3) and kinematic viscosity (3.5 cSt) comparable to blood, and physiological waveforms of aortic flow (average cardiac output of 5L/min ± 0.2L/min) and pressure (systolic to diastolic pressure of 120/80 mmHg) were imposed. Sixty consecutive cycles of instantaneous pressures were measured at 7 locations across each leaflet opening geometry, including 2 locations upstream as shown in Figure 4.11. Pressure drop (mmHg) at each location was calculated by averaging the maximum pressure over sixty cycles and subtracting the averaged maximum pressure at the location most upstream the valve (position 1). 114 FIGURE 4.11 LOCATIONS OF 7 PRESSURE MEASUREMENTS ALONG CENTERLINE OF IDEALIZED AORTIC ROOT CHAMBER PARTICLE IMAGE VELOCIMETRY (PIV) Velocity field through each leaflet geometry opening was visualized with particle image velocimetry (PIV) and turbulence characteristics were evaluated. The flow was seeded with florescent PMMA-Rhodamine B particles (average diameter ~10 µm) and illuminated by a thin laser sheet created with a double pulsed neodymium-doped yitrium lithium fluoride (Nd-YLF) solid state laser coupled with spherical and cylindrical lenses. Time-resolved recordings were acquired at spatial and temporal resolutions of 0.036 mm/pixel and 500 Hz respectively. 250 repetitions of phase locked measurements were recorded for acceleration, peak, deceleration, and 115 diastolic phases of the cardiac cycle. DaVis PIV software (DaVis 7.2; Lavision, Gӧttingen, Germany) used for all image post processing. Additional details of PIV techniques can be found in previous publications [289]. Vorticity, the curl of the velocity field, was computed as follows: 𝑑𝑉𝑥 𝑑𝑉𝑦 𝜔𝑧 = − ( − ) (13) 𝑑𝑦 𝑑𝑥 Where 𝜔𝑧 is the vorticity component with units of s-1; Vx and Vy are the x and y components of the velocity with units of m/s. High vorticity regions along the axis perpendicular to the plane indicate shear and rotation of the fluid particles[242]. Principal Reynold’s shear stress (RSS), a statistical quantity that measures the shear stress between fluid layers when particles decelerate or accelerate while changing direction [252], was calculated as: 2 𝑢′ 𝑢′ − 𝑣 ′ 𝑣 ′ 2 √ 𝑅𝑆𝑆 = 𝜌 ( ) + (𝑢′ 𝑣 ′ ) (14) 2 where 𝜌 is the density of the working fluid (kg/m3) and 𝑢′ and 𝑣 ′ are the instantaneous velocity fluctuations in the x and y directions respectively (m/s). Large RSS is an indicator of high turbulence and has been widely correlated with increasing likelihood of blood and endothelial damage after implantation of heart valve prostheses[222, 251]. Equation (12) implicitly assumes no out-of-plane component of instantaneous velocity, w’, and can be considered as a lower bound for the principle RSS [253]. The principal RSS was calculated for each spatial location downstream the valve and binned by RSS value frequency. Each bin was normalized to the maximum number of counts in any one bin, and this normalized frequency of principal RSS values was plotted. 116 4.2.3 RESULTS HEMODYNAMIC ASSESSMENT Pressure gradient at peak flow rate obtained from the flow and pressure data for each large leaflet opening geometry are presented as a function of distance from the inlet in Figure 4.12. The pressure gradient waveforms are characterized by a minimum followed by a gradual increase. The minimum denotes the highest pressure gradient that is located at the vena contracta followed by a conversion of energy from kinetic back to potential energy[290]. The triskele-like geometry produced lower pressure gradients at every location measured upstream and downstream the valve. The peak pressure gradient occurred at the orifice outlet and was measured at -5.9 mmHg. At the end of the valve chamber, where the pressure can be assumed to be fully recovered, the pressure gradient was measured as -3.9 mmHg. For the round leaflet opening geometry of the same size, the peak pressure gradient occurred at the office inlet and was measured as -8.1 mmHg. At the point where pressures can be assumed to be fully recovered, the pressure gradient increased slightly, to -4.5 mmHg. 117 FIGURE 4.12 PRESSURE GRADIENT AT PEAK FLOW RATE FOR EACH LARGE ORIFICE GEOMETRY MEASURED EXPERIMENTALLY In Figure 4.13, pressure gradient at peak flow as a function of the distance from the inlet is shown for the small leaflet opening geometries, triskele-like and round. A similar pattern to the large sized geometries, with the triskele-like geometry having consistently smaller pressure gradients than the round geometry at every location. The peak pressure gradient for the small sized triskele-like geometry was -51.4 mmHg and occurred at the coronary port. The pressure gradient recovered slightly to -41.0 mmHg. For the small round geometry, the pressure gradient peaked at the outlet of the orifice and measured -74.7 mmHg. The pressure gradient recovered to -60.3 mmHg. Interestingly, in the small triskele-like geometry a slightly positive pressure gradient is found at the inlet of the orifice. 118 FIGURE 4.13 PRESSURE GRADIENT AT PEAK FLOW RATE FOR EACH SMALL ORIFICE GEOMETRY MEASURED EXPERIMENTALLY VELOCITY VECTOR FIELD AND VORTICITY CONTOURS Phase averaged velocity vector fields and corresponding vorticity contours for the large triskele-like and round 3D printed leaflet opening geometries are shown in Figure 4.14 at four time points in the cardiac cycle. Acceleration, peak systole, deceleration and diastole phases are denoted by a red dot along the representative aortic flow curve. At peak systole the triskele-like leaflet opening geometry produced lower velocities than the round leaflet opening geometry at the orifice outlet, the sinotubular junction (STJ), and one valve diameter downstream the STJ. For both geometries, peak velocity was approximately the same at the STJ as it was one valve diameter away from the STJ, reaching a peak of approximately 1.4 m/s for the triskele-like leaflet opening geometry and 1.6 m/s for the round leaflet opening geometry. 119 Shear layers corresponding to the jet boundaries are represented by the red and blue contours. At peak systole, the shear layers are less pronounced with the triskele-like leaflet opening geometry compared to the round leaflet opening geometry. The shear layer appears to first attach to the aortic chamber wall approximately 16 mm from the outlet of the orifice. Shear layers are stronger along the confinement of the aortic chamber, and seem to attach along the wall of the upper sinus of Valsalva. There is a significantly larger region of flow separation for the round leaflet opening geometry, and flow attachment does not occur until approximately 42 mm from the orifice outlet. At the deceleration phase, the presence of shear layers has almost completely diminished for the triskele-like configuration but not for the round configuration. In the triskelelike leaflet opening geometry, the shear layers are significantly diminished at the STJ of the aortic root chamber, a much shorter distance than for the round leaflet opening geometry. FIGURE 4.14 PHASE AVERAGES VELOCITY VECTORS AND VORTICITY CONTOURS THROUGHOUT THE CARDIAC CYCLE 120 REYNOLDS SHEAR STRESS (RSS) The principal Reynolds shear stress (RSS) at acceleration, peak, deceleration and diastolic phases of the cardiac cycle for each large leaflet opening geometry are shown in Figure 4.15. The higher the RSS the more the turbulence in the flow field. In each geometry, the highest values of RSS presented at peak systole, with 11.3 Pa reported for the triskele-like leaflet opening geometry and 30.9 Pa for the round leaflet opening geometry. In comparison to the round leaflet opening geometry, the triskele-like geometry has a significantly smaller region in which higher RSS values were present in both the peak and deceleration phases of the cardiac cycle. During deceleration, peak values of RSS are more dispersed throughout the captured region of flow, whereas for the round geometry, the peak RSS values are more compact along the same region as the shear layers were shown in Figure 4.14. For both geometries, little to no RSS was seen during acceleration or diastole. FIGURE 4.15 PHASE AVERAGED PRINCIPLE REYNOLDS SHEAR STRESSES (RSS) THROUGHOUT THE CARDIAC CYCLE 121 4.2.4 DISCUSSION The effect of valve opening geometry on pressure gradient and turbulence characteristics was evaluated in this study using 3D printed idealized orifices that represented the opening geometries of a novel polymeric transcatheter aortic valve and a representative commercially available bioprosthetic transcatheter aortic valve. Differences in hemodynamic performance and turbulence characteristics between the opening geometries were observed. For both the large and small opening geometries, representative of a valve at peak systole, decreased pressure gradients were found at every downstream position for the triskele-like geometry compared to the round geometry. Decreased pressure drops are indicative of a decrease in energy loss across and downstream the geometry. The decrease in vorticity fluctuation seen for the triskele-like leaflet opening geometry is also indicative of a decrease in turbulence (and therefore energy loss). In this controlled experiment where opening and closing dynamics of heart valves prostheses that can be influenced by material properties among other variables can be ignored, we can attribute the decreased velocity and vorticity fluctuation during peak systole and deceleration phases as compared to the round opening geometry to the 3D curved surfaces in the triskele-like geometry. We expect that the 3D curved surfaces induce a radial and azimuthal velocity component of the flow as it exits the valve opening, leading to sooner flow reattachment and swirling flows that reduce turbulence, although the exact velocity fields cannot be distinguished from 2D velocity planes that were feasible to capture. Nonetheless, the consistent decrease in pressure loss shown for the triskele-like geometry assures us that there is a net reduction of turbulence with all planes considered. 122 The RSS magnitudes have an important role in determining the biocompatibility of a valve prosthesis because they can indicate regions of probable platelet activation from turbulent fluctuations of the blood velocity[222-224, 254, 262]. All current valve designs studied to date have mean turbulent shear stresses in excess of 20 Pa [169, 222-224, 242, 254, 262, 287, 288, 291], which is comparable to the round leaflet opening geometry as expected. Therefore, while a round leaflet opening configuration for a valve prosthesis is often chosen or required by material manufacturability, the results presented here show that it may not be the most optimal choice to limit turbulence effects on the blood. In order to mitigate the durability issues that are caused by blood damage, an appropriate leaflet opening geometry should be considered during the design and development of novel valve prostheses. 123 Chapter 5: Specific Aim 3 5.1 Hemodynamic Evaluation of a Fetal Tissue-Engineered Pulmonary Valve 5.1.1 INTRODUCTION Congenital heart defects are the most common type of birth defect in the United States, and the leading cause of birth defect-associated infant death [292]. Patients born with congenital pulmonary valve and right ventricular outflow tract abnormalities(the most common type of defect) frequently require surgical repair early in life[293]. Multiple repeat surgical operations for valve repair are often necessary given the limited durability and growth capacity of currently available valve replacement technologies [294]. Tissue engineered prosthetic pulmonary valves are an attractive potential solution to the inadequacy of current valve prostheses used in cardiac reconstructions by providing a living valve with the ability to grow, repair, and remodel over time, thus reducing the number of invasive, repeat surgeries required of these patients. Moreover, implanting tissue engineered valves in-utero while the fetus is still developing and receptive to bioengineered tissue, could further improve treatment of (and potentially reverse) some congenital heart defects. The Melody valve (Medtronic Inc.), which consists of a bovine jugular vein graft, is the only transcatheter pulmonary valve currently approved by the US Food and Drug Administration (FDA), however, it is only approved for clinical usage for treatment of failed right ventricular outflow track conduits and failed bioprosthetic pulmonary valves [295]. Therefore, while this technology has revolutionized the treatment paradigm for children with congenital heart disease, it is far from an ideal solution. In addition to its use only after failed 124 interventions, there is increasing evidence that the bovine jugular vein graft is associated with a higher risk of endocarditis compared to other bioprosthetic valves[12, 296, 297], and infective endocarditis after the percutaneous implantation (an estimated 3% annual incidence) (see Figure 5.1) [298]. FIGURE 5.1 EXAMPLES DEPICTING INFECTED MELODY VALVES AT TIME OF EXPLANT FROM REF[298]. (A) MELODY VALVE REMOVED SURGICALLY 1 WEEK AFTER THE ONSET OF INFECTIVE ENDOCARDITIS. COMPLETE VALVE OBSTRUCTION BY VEGETATIONS IS VISIBLE. (B) MELODY VALVE REMOVED SURGICALLY 2 YEARS AFTER THE ONSET OF INFECTIVE ENDOCARDITIS. CULTURES OF THE VALVE WERE NEGATIVE. SEVERE DETERIORATION OF THE VALVE IS VISIBLE, WITH BUDDING ON THE LEAFLETS. Additionally, as individuals with surgically corrected congenital heart disease are living longer into adulthood, the need for subsequent pulmonary valve replacements is increasing, heightening the concern of long-term valve durability. While the long term durability (beyond 5 years) of the Melody valve has yet to be reported in published literature, the time fixed tissue based prosthetic valves are free from degeneration is limited to 7-15 years post implantation[299]. Fixed tissue valves in the pulmonary position, including glutaraldehyde fixed 125 bovine jugular vein implants, have exhibited significant mechanical durability problems, and are particularly prone to calcification, which was shown to be the main cause of prosthetic pulmonary valve dysfunction in two separate studies[300, 301]. The commonality of biologically derived valve leaflets suggests that the Melody may be prone to similar degeneration in the long term. Over the last few years, our team has developed a tissue-engineered pulmonary valves (TEPVs) constructed from poly(glycerol sebacate) (PGS) and polycaprolactone (PCL) scaffold using a tube-within-a-tube technique and a zinc bioresorbable stent. The group has successfully demonstrated the feasibility of implantation of this novel TEPV into two fetal lambs and have identified the large animal sheep model as an appropriate model for studying fetal cardiac interventions. While the feasibility of implantation of these TEPVs has been demonstrated, there are still gaps in our understanding of the function and durability of these valves. In order to improve the TEPVs for future animal studies, the current function and potential failure mechanisms must be well understood. The goal of specific aim 3 was to develop a system capable of quantifying valvehemodynamic performance in-vitro under the unique conditions of the fetal heart in order to aid in rapid development and rational design of the fetal TEPV. 5.1.1 METHODS HEMODYNAMIC ASSESSMENT A dedicated right heart pulse duplicator was developed to evaluate in vitro TEHV performance under physiological heart conditions according to ISO 5840 for the newborn pediatric subpopulation including heart rate and cardiac output. The values for all pediatric 126 subpopulations can be found in Table 5.1. To our knowledge, no other right-heart pulse duplicator customized for simulation of fetal or pediatric hemodynamic evaluation exists. Pediatric subpopulation Systolic duration, % Beat rate, beats/min Newborn 50 60, 150, 200 Infant Toddler Child Adolescent 50 45 40 35 60, 120, 200 60, 100, 160 60, 80, 140 45, 70, 120 Cardiac output, L/min 0.3, 0.5, 1, 1.5 0.5, 1, 2, 3 1.5, 3, 4.5 2, 3.5, 5 2, 5, 7 Left-sided heart MAP, mm Hg 45 Right-sided heart MAP, mm Hg 20 55 65 80 100 20 20 20 20 MAP, Mean arterial pressure. Table 5.1 Suggested pulsatile test conditions for pediatric populations[302, 303] Briefly, a customized fetal valve chamber of size 6 mm in luminal diameter (to simulate sheep fetus annulus diameter between 109 and 115 days of gestation) was machined to provide a clear en-face view of the valve. The proof-of-concept chamber design can be seen in Figure 5.2. FIGURE 5.2 PROOF-OF-CONCEPT FETAL VALVE CHAMBER COMPATIBLE FOR TRANSCATHETER IMPLANTATION Its notable features include a right-angle connection, flexible silicone gaskets to deform around 127 the stented valve during expansion, and a clear viewing window. Following initial proof-ofconcept hemodynamic evaluation, a more complex fetal valve chamber was designed and manufactured. Figure 5.3 shows the improved chamber design, which includes pressure taps for acquiring transvalvular pressure gradients, a three-part chamber to allow easier access to the valve, and an angled dog-leg connection allowing for closer to physiological flow patterns. FIGURE 5.3 CUSTOM TRANSCATHETER FETAL VALVE CHAMBER In addition to the TEPV and custom chamber, the dedicated right-heart pulse duplicator is composed of a reservoir to mimic atrial function, a St. Jude Medical pediatric mechanical valve functioning as the tricuspid valve, a bulb/bladder pump controlled by compressed air to mimic right ventricular function, a compliance chamber to simulate pulmonary vascular compliance, and a flow valve to set pulmonary capillary resistance. The pump mechanism in the pulse duplicator was controlled by a custom LabVIEW program, modeled after the left-heart pulse duplicator described by Forleo [304]. 128 A schematic of the setup is shown in Figure 5.4. Initial hemodynamic studies were completed with a 60/40 water/glycerin mixture by volume with density of 1090 Kg/m3 and a kinematic viscosity of 3.88 cSt was used as the working fluid. For subsequent hemodynamic studies under conditions of degradation, a viscosity matched solution of 6M NaOH (for degradation) was used. A viscometer was used to determine viscosity of the different molarities as shown in Figure 5.5. FIGURE 5.4 SCHEMATIC OF THE FETAL RIGHT HEART PULSE DUPLICATOR 129 Kinematic Viscosity (cSt) 6 5 4 3 2 1 0 40% 5M NaOH 4M NaOH 3M NaOH 6M NaOH 7M NaOH 8M NaOH Glycerol Potential Working Fluid FIGURE 5.5 KINEMATIC VISCOSITY OF VARYING MOLARITIES OF NAOH AS COMPARED TO 40% GLYCEROL Valve opening and leaflet kinematics were assessed through en-face imaging throughout the cardiac cycle with a highspeed camera at 1000fps. The average flow rate and the flow waveform were captured using an HXL Transonic Inc. flow probe (Ithaca, NY, USA) and pulmonary pressure and ventricular pressure were measured with Validyne Engineering Corp. pressure transducers (Northridge, CA, USA) at a sampling rate of 100 Hz. Mean transvalvular pressure gradient, mean effective orifice area, and mean regurgitant fraction were calculated from these data as follows. GEOMETRIC ORIFICE AREA (GOA) The geometric orifice area (GOA) is a common parameter that represents the geometric area of the valve orifice valve opening and can be measured from en face imaging[305]. REGURGITANT FRACTION (RF) Regurgitant fraction (RF) is a second common parameter that is used to assess valve performance. It represents the ratio of the closing (CV) and leakage volume (LV) to the forward 130 flow volume (FV). A higher performing valve would demonstrate a low regurgitant fraction [244]. 𝑅𝐹 = 𝐶𝑉 + 𝐿𝑉 (16) 𝐹𝑉 PINWHEELING INDEX (PI) The pinwheeling index (PI) measures the twisting extent of the leaflets upon closure[242]. High pinwheeling indices have been shown to be linked with decreased leaflet durability[245-247]. PI is computed from still frames of high-speed imaging during valve closing phase as follows: 𝑃𝐼 = 𝐿𝑎𝑐𝑡𝑢𝑎𝑙 −𝐿𝑖𝑑𝑒𝑎𝑙 𝐿𝑖𝑑𝑒𝑎𝑙 (17) where 𝐿𝑎𝑐𝑡𝑢𝑎𝑙 represents the actual length of the free edge of a leaflet, and 𝐿𝑖𝑑𝑒𝑎𝑙 represents the shortest distance between the post and central coaptation region, as previously described by Midha et al.[248]. 5.1.2 RESULTS Initial in-vitro hemodynamic evaluation of the TEPV were promising, though there was an indication of room for improvement in both scaffold and valve design. Figure 5.6 A shows the resulting flow curve for the TEPV at 150 bpm and an average cardiac output of 1.4L/min. 131 FIGURE 5.6 REPRESENTATIVE PULMONARY FLOW CURVES FOR 150 BEATS/MIN AND AVERAGE CARDIAC OUTPUT OF 1.4 L/MIN At this heart rate and flow rate, the GOA was calculated to be 19.016 mm2. The regurgitant fraction was found to be 1.241 % and the pinwheeling index of the valve was found to be 0.1239. Figure 5.7 shows the TEPV opening across the cardiac cycle at acceleration phase, peak systole, deceleration, and diastole and highlights the unique opening and closing of the scaffold leaflets. In Figure 5.8, a plane parallel to the direction of flow is shown and the fluid area is highlighted by seeding particles. In this figure, signs or very the early degradation and unravelling of the scaffold material can be seen. 132 FIGURE 5.7 TISSUE-ENGINEERED PULMONARY VALVE OPENING ACROSS THE CARDIAC CYCLE FIGURE 5.8 CROSS SECTIONAL FLOW THROUGH TISSUE-ENGINEERED PULMONARY VALVE SCAFFOLD 133 5.1.3 DISCUSSION A dedicated fetal right heart pulse duplicator was developed and allows for the in-vitro hemodynamic evaluation of investigational fetal heart valve prostheses. Additionally, this study demonstrates the concept and basic hemodynamic functionality of a tissue-engineered pulmonary valve scaffold. Compared to the maximal geometric orifice area that could have been possible with the stent inner diameter of 6mm, a GOA or 28.274 mm2, the TEPV GOA was only 19.016 mm2. This approximately 33% reduction in available area is likely due to the excess length of the scaffold leaflets that is shown clearly in Figure 5.7. This excess length also likely contributed to the favorable regurgitant fraction result of only 1.241 % which is consistent with no pulmonary regurgitation [306, 307]. This highlights the need for an optimized leaflet length and profile in transcatheter heart valve engineering, in order to provide maximal opening while still providing sufficient length for coaptation. The degree of pinwheeling of the TEPV (0.1239) was comparable to that obtained with adult-size balloon-expandable valves deployed in the aortic position (0.122-0.366) and therefor indicates that the degree of twisting in the leaflets will likely not be a source of damage or inhibit short-term durability [308]. While no true control valve of similar size is available for comparison, the in-vitro evaluation revealed a hemodynamically competent and non-stenotic valve with predicted shortterm durability similar to commercially available balloon-expandable fixed-tissue transcatheter heart valves. Though the initial hemodynamic evaluation results are promising, further 134 hemodynamic evaluation at a range of physiological cardiac outputs and heart rates will further inform the future rapid design and development of the TEHV. In summary, limiting the number of necessary operations young patients with congenital heart defects must undergo to a single, interventional procedure in-utero would dramatically improve their quality of life. As such, there is a very real need for an improved transcatheter pulmonary valve device with superior durability and resistance to endocarditis and calcification. The ability to quantify valve performance in-vitro is essential to the rapid development and rational design of a superior TEPV that has potential to improve outcomes for patients with congenital pulmonary valve abnormalities. 5.1.4 FUTURE WORK Further studies are needed to optimize the manufacturing, design, and implantation procedure of TEPVs and to define the degradation time both in vitro and in vivo. 135 Chapter 6: Summary and Future Work The research presented here sought to identify shortcomings of current heart valve replacement technologies, provide insight into of the mechanisms behind their shortcomings, and work towards the development of improved heart valve technologies. The specific aims led to (1) the development of two novel mechanistic indices to accurately assess risk of fatal coronary artery obstruction during TAVR within intermediate to high surgical risk patients, (2) a novel polymeric transcatheter heart valve with potential to have improved durability over commercially available bioprosthetic valves, and (3) a novel methodology to characterize and improve upon a tissue-engineered replacement fetal heart valves for children with life threatening congenital heart defects. Significant accomplishments have been made in the field of heart valve engineering since the first valve prosthesis, the Hufnaegel ball and cage valve, was implanted in 1952 [50]. Astonishingly though, aside from the development of transcatheter valves, the prostheses’ materials and designs have remained relatively stagnant over the years. In the era of TAVR approval for use in low risk patient populations, the need for improved devices, device options, and patient specific pre-planning is especially important. This research uses both experimental and computational methods to study the mechanics and hemodynamics of transcatheter valve replacement with the overarching goal of improving the current technologies towards improved patient outcomes. 136 Avenues for future work relevant to aim 1 would include expansion of the 3D computational and 2D geometric models for predicting risk of coronary obstruction to larger patient cohorts in order to obtain additional information of coronary obstruction mechanisms in a broader population. With access to additional patient data of confirmed cases of coronary obstruction, we could extend these generalized risk predictions to be highly predictive of coronary obstruction in patient specific cases. Additionally, moving beyond finite element analysis of coronary obstruction mechanisms into computational fluid dynamics (CFD) simulations would further improve our ability to predict patient specific risk of coronary obstruction. Future work relevant to aim 2 would include a more in-depth assessment of valve performance including in vitro assessment in compliant and/or patient specific anatomies, accelerated fatigue testing to compare mechanical durability to the commercially available bioprostheses, and in vivo evaluation in large animal models of short- and long-term durability and biocompatibility. As addressed in section 4.2, limitations of 2D PIV do not allow for RSS measurements to be made in out of plane directions, and therefore a 3D PIV system may provide additional insight into the mechanisms behind improved hemodynamics with the triskele-like leaflet geometries that are seen in the HA-TAV. In terms of commercial potential, the HA-TAV introduced in aim 2 would need significant attention towards a dedicated delivery system. A number of limitations are associated with aim 3, where a dedicated right heart pulse duplicator was developed to characterize and aid in the rapid design and development of a transcatheter tissue engineered fetal pulmonary valve. While the developed methodology allows 137 for visual inspection and initial hemodynamic evaluation that is cost-effective and can occur at a much faster pace than in vivo evaluation, the degradable nature of the tissue-engineered scaffold is difficult to mimic in vitro. Future work includes a hemodynamic analysis over an accelerated degradation time period using a working fluid with known ability to degrade the scaffold, such as 6M NaOH. While this proposed experiment will not inform tissue formation, it will provide a timeline for the scaffold to degrade under dynamic conditions and allow for the polymer chemistry and design to be tuned as necessary. 138 References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. Minino, A.M., et al., Deaths: final data for 2008. Natl Vital Stat Rep, 2011. 59(10): p. 1-126. Heitkemper, M. and L.P. Dasi, Chapter 13 - Polymeric heart valves, in Principles of Heart Valve Engineering, A. Kheradvar, Editor. 2019, Academic Press. p. 343-359. Yacoub, N. and J. Takkenberg, Will heart valve tissue engineering change the world? Nature Clinical Practice Cardiovascular Medicine, 2005. 2(2): p. 60-61. Black, M.M. and P.J. Drury, Mechanical and Other Problems of Artificial Valves, in Current Topics in Pathology. 1994, Springer-Verlag Berlin Heidelberg. Coylewright, M., et al., TAVR in Low-Risk Patients. Journal of the American College of Cardiology, 2020. 75(10): p. 1208. Reardon, M.J., et al., Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. New England Journal of Medicine, 2017. 376(14): p. 1321-1331. Popma, J.J., et al., Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in LowRisk Patients. New England Journal of Medicine, 2019. 380(18): p. 1706-1715. Pibarot, P., et al., Echocardiographic Results of Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. Circulation, 2020. 141(19): p. 1527-1537. Filsoufi, A.C.a.F. Mitral Valve. 2013; Available from: www.TheMitralValve.org. Goodwin, R.L. and S.V. Biechler, Chapter 1 - Clinical anatomy and embryology of heart valves, in Principles of Heart Valve Engineering, A. Kheradvar, Editor. 2019, Academic Press. p. 1-12. Wapcaplet, Heart_labelled_large, D.o.t.h. heart, Editor. 2005. Mery, C.M., et al., Risk factors for development of endocarditis and reintervention in patients undergoing right ventricle to pulmonary artery valved conduit placement. The Journal of Thoracic and Cardiovascular Surgery, 2016. 151(2): p. 432-441.e2. Monroe, M., A. Zhu, and K.J. Grande-Allen, Chapter 2 - Heart valves' mechanobiology, in Principles of Heart Valve Engineering, A. Kheradvar, Editor. 2019, Academic Press. p. 13-39. Balachandran, K., P. Sucosky, and A.P. Yoganathan, Hemodynamics and mechanobiology of aortic valve inflammation and calcification. International journal of inflammation, 2011. 2011: p. 263870-263870. Menon, V. and J. Lincoln, The Genetic Regulation of Aortic Valve Development and Calcific Disease. Frontiers in Cardiovascular Medicine, 2018. 5(162). Huntley, G.D., J.J. Thaden, and V.T. Nkomo, Chapter 3 - Epidemiology of heart valve disease, in Principles of Heart Valve Engineering, A. Kheradvar, Editor. 2019, Academic Press. p. 41-62. Heart Valve Disease. 2020; Available from: https://www.nhlbi.nih.gov/health-topics/heartvalve-disease. WHO, World health statistics 2016, W.H. Organization, Editor. 2016. Tandon, R., et al., Revisiting the pathogenesis of rheumatic fever and carditis. Nat Rev Cardiol, 2013. 10(3): p. 171-7. 139 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. Sliwa, K., et al., Incidence and characteristics of newly diagnosed rheumatic heart disease in Urban African adults: insights from the Heart of Soweto Study. European Heart Journal, 2009. 31(6): p. 719-727. Manjunath, C.N., et al., Incidence and patterns of valvular heart disease in a tertiary care highvolume cardiac center: a single center experience. Indian Heart J, 2014. 66(3): p. 320-6. Nkomo, V.T., et al., Burden of valvular heart diseases: a population-based study. The Lancet, 2006. 368(9540): p. 1005-1011. Pawade, T.A., D.E. Newby, and M.R. Dweck, Calcification in Aortic Stenosis: The Skeleton Key. J Am Coll Cardiol, 2015. 66(5): p. 561-77. Rajamannan, N.M., et al., Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation, 2011. 124(16): p. 1783-1791. Iung, B., et al., A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J, 2003. 24(13): p. 1231-43. Nishimura, R.A., et al., 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 2014. 129(23): p. 2440-92. Pellerin, D., S. Brecker, and C. Veyrat, Degenerative mitral valve disease with emphasis on mitral valve prolapse. Heart, 2002. 88(suppl 4): p. iv20. Delling, F.N. and R.S. Vasan, Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, and molecular basis. Circulation, 2014. 129(21): p. 2158-2170. Shah, M. and U. Jorde, Percutaneous Mitral Valve Interventions (Repair): Current Indications and Future Perspectives. Frontiers in Cardiovascular Medicine, 2019. 6. Pick, A., Aortic stenosis. 2019: HeartValveSurgery.com. Movahed, M.R., et al., Increased prevalence of mitral stenosis in women. J Am Soc Echocardiogr, 2006. 19(7): p. 911-3. Roberts, W.C., The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol, 1970. 26(1): p. 72-83. Fruitman, D.S., Hypoplastic left heart syndrome: Prognosis and management options. Paediatrics & child health, 2000. 5(4): p. 219-225. Oliveira, J.M.F.d. and M.J. Antunes, Mitral valve repair: better than replacement. Heart (British Cardiac Society), 2006. 92(2): p. 275-281. Effler, D.B., R. Favaloro, and L.K. Groves, Heart Valve Replacement: Clinical Experience. The Annals of Thoracic Surgery, 1965. 1(1): p. 4-24. Iung, B., et al., A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J, 2003. 24(13): p. 1231-43. Cribier, A., The development of transcatheter aortic valve replacement (TAVR). Global cardiology science & practice, 2016. 2016(4): p. e201632-e201632. Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation, 1991. 84(6): p. 2383-97. Bonhoeffer, P., et al., Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet, 2000. 356(9239): p. 1403-5. 140 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. Cribier, A., et al., Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation, 2002. 106(24): p. 3006-8. Smith, C.R., et al., Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. New England Journal of Medicine, 2011. 364(23): p. 2187-2198. Kheradvar, A., Preface, in Principles of Heart Valve Engineering, A. Kheradvar, Editor. 2019, Academic Press. p. xv-xvi. Phillips, E.H. and C.J. Goergen, Chapter 4 - Surgical heart valves, in Principles of Heart Valve Engineering, A. Kheradvar, Editor. 2019, Academic Press. p. 63-84. Antunes, M.J., Porcine or bovine: does it really matter? Eur J Cardiothorac Surg, 2015. 47(6): p. 1075-6. Auriemma, S., et al., Long-term results of aortic valve replacement with Edwards Prima Plus stentless bioprosthesis: eleven years' follow up. J Heart Valve Dis, 2006. 15(5): p. 691-5; discussion 695. Santarpino, G., et al., REDO aortic valve replacement: the sutureless approach. J Heart Valve Dis, 2013. 22(5): p. 615-20. Nazir, R., Collagen–hyaluronic acid based interpenetrating polymer networks as tissue engineered heart valve. Materials Science and Technology, 2016. 32(9): p. 871-882. Nishimura, R.A., et al., 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol, 2017. 70(2): p. 252-289. Puri, R., V. Auffret, and J. Rodés-Cabau, Bioprosthetic Valve Thrombosis. Journal of the American College of Cardiology, 2017. 69(17): p. 2193. Gott, V.L., D.E. Alejo, and D.E. Cameron, Mechanical heart valves: 50 years of evolution. The Annals of Thoracic Surgery, 2003. 76(6): p. S2230-S2239. Blot William, J., et al., Twenty-Five–Year Experience With the Björk-Shiley Convexoconcave Heart Valve. Circulation, 2005. 111(21): p. 2850-2857. Kheradvar, A., et al., Emerging trends in heart valve engineering: Part III. Novel technologies for mitral valve repair and replacement. Ann Biomed Eng, 2015. 43(4): p. 858-70. Nicoloff, D.M., et al., Clinical and hemodynamic results with the St. Jude Medical cardiac valve prosthesis. A three-year experience. J Thorac Cardiovasc Surg, 1981. 82(5): p. 674-83. Goldhaber, S.Z., “Bridging” and mechanical heart valves: perils, promises, and predictions. 2006, Am Heart Assoc. D'Souza, R., et al., Anticoagulation for pregnant women with mechanical heart valves: a systematic review and meta-analysis. Eur Heart J, 2017. 38(19): p. 1509-1516. Moritz, A., et al., Closing click of St Jude medical and Duromedics Edwards bileaflet valves: complaints created by valve noise and their relation to sound pressure and hearing level. European heart journal, 1991. 12(6): p. 673-679. Pedersen, T., et al., Are sounds from mechanical heart valves equal for different valve types. J Heart Valve Dis, 2008. 17(5): p. 579-82. Jun, B.H., N. Saikrishnan, and A.P. Yoganathan, Micro particle image velocimetry measurements of steady diastolic leakage flow in the hinge of a St. Jude Medical® regent™ mechanical heart valve. Annals of biomedical engineering, 2014. 42(3): p. 526-540. Sarraf, M., E.M. Groves, and A. Kheradvar, Chapter 5 - Transcatheter heart valves, in Principles of Heart Valve Engineering, A. Kheradvar, Editor. 2019, Academic Press. p. 85-122. 141 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. van Geemen, D., et al., Age-Dependent Changes in Geometry, Tissue Composition and Mechanical Properties of Fetal to Adult Cryopreserved Human Heart Valves. PLOS ONE, 2016. 11(2): p. e0149020. Blum, K.M., J.D. Drews, and C.K. Breuer, Tissue-Engineered Heart Valves: A Call for Mechanistic Studies. Tissue engineering. Part B, Reviews, 2018. 24(3): p. 240-253. Sacks, M.S., F.J. Schoen, and J.E. Mayer, Bioengineering Challenges for Heart Valve Tissue Engineering. Annual Review of Biomedical Engineering, 2009. 11(1): p. 289-313. Xeltis trials show promising function of living heart valves restored in children. in International Conference of Tissue-Engineered Heart Valves. 2020. Rosewood, Abu Dhabi. Tetsuzo Akutsu, B.D., Willem J. Kolff, Polyurethane artificial heart valves in animals. Journal of Applied Physiology, 1959. 14: p. 1045-1048. Berge, T.L., A flexible cardiac valve prosthesis; preliminary report on the development of an experimental valvular prosthesis. Archivum chirurgicum Neerlandicum, 1958. 10(1): p. 26-33. Braunwald, N.S., It will work: The first successful mitral valve replacement. The Annals of Thoracic Surgery. 48(3): p. S1-S3. Roe, B.B., Moore, David, Design and fabrication of prosthetic valves. Eperimental Medicine and Surgery, 1958. 16(2-3): p. 177-182. Braunwald NS. , C.T., Morrow AG., Complete replacement of the mitral valve. Successful clinical application of a flexible polyurethane prosthesis. Journal of Thoracic and Cardiovascular Surgery, 1960. 40: p. 1-11. Roe, B.B., Late follow-up studies on flexible leaflet prosthetic valves. Journal of Thoracic and Cardiovascular Surgery, 1969. 58: p. 59-61. Matthews, A.M., The development of the Starr-Edwards heart valve. Texas Heart Institute Journal, 1998. 25(4): p. 282-293. Maisano, F., et al., The Evolution From Surgery to Percutaneous Mitral Valve Interventions: The Role of the Edge-to-Edge Technique. Journal of the American College of Cardiology, 2011. 58(21): p. 2174-2182. Pierie, W.R., et al., MATERIALS AND HEART VALVE PROSTHESES. Annals of the New York Academy of Sciences, 1968. 146(1): p. 345-359. Harken, D.E. and L.E. Curtis, Heart surgery—Legend and a long look. The American Journal of Cardiology, 1967. 19(3): p. 393-400. Claiborne, T.E., Polymeric trileaflet prosthetic heart valves: evolution and path to clinical reality. Expert review of medical devices. 9(6): p. 577-594. Ghanbari, H., et al., Polymeric heart valves: new materials, emerging hopes. Trends in Biotechnology, 2009. 27(6): p. 359-367. Kütting, M., et al., Polyurethane heart valves: past, present and future. Expert Review of Medical Devices, 2011. 8(2): p. 227-233. Kidane, A.G., et al., Current developments and future prospects for heart valve replacement therapy. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2009. 88B(1): p. 290-303. Claiborne, T.E., Polymeric trileaflet prosthetic heart valves: evolution and path to clinical reality. Expert review of medical devices, 2012. 9(6): p. 577-594. Kheradvar, A., et al., Emerging trends in heart valve engineering: Part I. Solutions for future. Ann Biomed Eng, 2015. 43(4): p. 833-43. Bezuidenhout, D. and P. Zilla, Flexible Leaflet Polymeric Heart Valves. 2014. 142 81. 82. 83. 84. 85. 86. 87. 88. 89. 90. 91. 92. 93. 94. 95. 96. 97. 98. 99. 100. 101. Colas, A. and J. Curtis, Silicone biomaterials: History and chemistry. 2004. 80-86. Mori, H., et al., Design and durability test of Silastic trileaflet aortic valve prostheses. J Thorac Cardiovasc Surg, 1973. 65(4): p. 576-82. Kiraly, R., et al., Hexsyn trileaflet valve: application to temporary blood pumps. Artif Organs, 1982. 6(2): p. 190-7. Chetta, G.E. and J.R. Lloyd, The design, fabrication and evaluation of a trileaflet prosthetic heart valve. J Biomech Eng, 1980. 102(1): p. 34-41. He, W. and R. Benson, 8 - Polymeric Biomaterials A2 - Kutz, Myer, in Applied Plastics Engineering Handbook (Second Edition). 2017, William Andrew Publishing. p. 145-164. Quintessenza, J.A., et al., Polytetrafluoroethylene Bicuspid Pulmonary Valve Implantation: Experience With 126 Patients. World Journal for Pediatric and Congenital Heart Surgery, 2010. 1(1): p. 20-27. Coury, A., et al., Medical Application of Implantable Polyurethanes: Current Issues. Prog. Rubber Plast. Technol., 1987. 3(4): p. 24-37. Kidane, A.G., et al., A novel nanocomposite polymer for development of synthetic heart valve leaflets. Acta Biomaterialia, 2009. 5(7): p. 2409-2417. Blackwell, J. and K.H. Gardner, Structure of the hard segments in polyurethane elastomers. Polymer, 1979. 20(1): p. 13-17. Blackwell, J. and C.D. Lee, Hard-segment polymorphism in MDI/diol-based polyurethane elastomers. Journal of Polymer Science: Polymer Physics Edition, 1984. 22(4): p. 759-772. Lyman, D.J., et al., The effect of chemical structure and surface properties of synthetic polymers on the coagulation of blood. II. Protein and platelet interaction with polymer surfaces. Trans Am Soc Artif Intern Organs, 1968. 14: p. 250-5. Zia, K.M., et al., Synthesis and characterization of novel, biodegradable, thermally stable chitinbased polyurethane elastomers. Journal of Applied Polymer Science, 2008. 110(2): p. 769-776. Xu, D., et al., Novel blood-compatible waterborne polyurethane using chitosan as an extender. Journal of Applied Polymer Science, 2008. 109(1): p. 240-246. Bernacca, G.M., et al., Calcification and fatigue failure in a polyurethane heart valve. Biomaterials, 1995. 16(4): p. 279-285. Bernacca, G.M., et al., Hydrodynamic function of polyurethane prosthetic heart valves: influences of Young's modulus and leaflet thickness. Biomaterials, 2002. 23(1): p. 45-50. Bernacca, G.M., I. Straub, and D.J. Wheatley, Mechanical and morphological study of biostable polyurethane heart valve leaflets explanted from sheep. Journal of Biomedical Materials Research, 2002. 61(1): p. 138-145. Akutsu, T., B. Dreyer, and W.J. Kolff, Polyurethane artificial heart valves in animals. J Appl Physiol, 1959. 14: p. 1045-8. Mackay, T.G., et al., New polyurethane heart valve prosthesis: design, manufacture and evaluation. Biomaterials, 1996. 17(19): p. 1857-63. Wheatley, D.J., et al., Hydrodynamic function of a biostable polyurethane flexible heart valve after six months in sheep. Int J Artif Organs, 2001. 24(2): p. 95-101. Wheatley, D.J., et al., Polyurethane: material for the next generation of heart valve prostheses? Eur J Cardiothorac Surg, 2000. 17(4): p. 440-8. Scherman, J., et al., Transcatheter valve with a hollow balloon for aortic valve insufficiency. Multimed Man Cardiothorac Surg, 2018. 2018. 143 102. 103. 104. 105. 106. 107. 108. 109. 110. 111. 112. 113. 114. 115. 116. 117. 118. 119. 120. Kidane, A.G., et al., A novel nanocomposite polymer for development of synthetic heart valve leaflets. Acta Biomater, 2009. 5(7): p. 2409-17. Kannan, R.Y., et al., Polyhedral Oligomeric Silsesquioxane Nanocomposites: The Next Generation Material for Biomedical Applications. Accounts of Chemical Research, 2005. 38(11): p. 879-884. Dabagh, M., M.J. Abdekhodaie, and M.T. Khorasani, Effects of polydimethylsiloxane grafting on the calcification, physical properties, and biocompatibility of polyurethane in a heart valve. Journal of Applied Polymer Science, 2005. 98(2): p. 758-766. Simmons, A., et al., Long-term in vivo biostability of poly(dimethylsiloxane)/poly(hexamethylene oxide) mixed macrodiol-based polyurethane elastomers. Biomaterials, 2004. 25(20): p. 4887-900. Santerre, J.P., et al., Understanding the biodegradation of polyurethanes: from classical implants to tissue engineering materials. Biomaterials, 2005. 26(35): p. 7457-70. Nuttelman, C.R., S.M. Henry, and K.S. Anseth, Synthesis and characterization of photocrosslinkable, degradable poly(vinyl alcohol)-based tissue engineering scaffolds. Biomaterials, 2002. 23(17): p. 3617-3626. Alves, M.H., et al., Poly(vinyl alcohol) physical hydrogels: new vista on a long serving biomaterial. Macromol Biosci, 2011. 11(10): p. 1293-313. Nuttelman, C.R., et al., Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J Biomed Mater Res, 2001. 57(2): p. 217-23. Nachlas, A.L.Y., S. Li, and M.E. Davis, Developing a Clinically Relevant Tissue Engineered Heart Valve—A Review of Current Approaches. Advanced Healthcare Materials, 2017. 6(24): p. 1700918-n/a. Jiang, H., et al., Design and manufacture of a polyvinyl alcohol (PVA) cryogel tri-leaflet heart valve prosthesis. Medical Engineering and Physics. 26(4): p. 269-277. Mohammadi, H., Nanocomposite biomaterial mimicking aortic heart valve leaflet mechanical behaviour. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine, 2011. 225(7): p. 718-722. Hersel, U., C. Dahmen, and H. Kessler, RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials, 2003. 24(24): p. 4385-415. Mat Web. Material Property Data. Available from: http://www.matweb.com/. Prawel, D.A., et al., Hemocompatibility and Hemodynamics of Novel Hyaluronan–Polyethylene Materials for Flexible Heart Valve Leaflets. Cardiovascular Engineering and Technology, 2014. 5(1): p. 70-81. James, S.P., et al., Glycosaminoglycan and Synthetic Polymer Material for Blood-Contacting Applications. 2015, Google Patents. Simon-Walker, R., et al., Hemocompatibility of hyaluronan enhanced linear low density polyethylene for blood contacting applications. Journal of Biomedical Materials Research Part B: Applied Biomaterials: p. n/a-n/a. Pinchuk, L., et al., Medical applications of poly(styrene-block-isobutylene-block-styrene) ("SIBS"). Biomaterials, 2008. 29(4): p. 448-60. Kondyurin, A., E. Pecheva, and L. Pramatarova, Calcium phosphate formation on plasma immersion ion implanted low density polyethylene and polytetrafluorethylene surfaces. J Mater Sci Mater Med, 2008. 19(3): p. 1145-53. Andrews, R.K., J.A. Lopez, and M.C. Berndt, Molecular mechanisms of platelet adhesion and activation. Int J Biochem Cell Biol, 1997. 29(1): p. 91-105. 144 121. 122. 123. 124. 125. 126. 127. 128. 129. 130. 131. 132. 133. 134. 135. 136. 137. 138. 139. 140. Satriano, C., et al., Surface free energy and cell attachment onto ion-beam irradiated polymer surfaces. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 2003. 208: p. 287-293. Stachelek, S.J., et al., Cholesterol-derivatized polyurethane: characterization and endothelial cell adhesion. J Biomed Mater Res A, 2005. 72(2): p. 200-12. Pierschbacher, M.D. and E. Ruoslahti, Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature, 1984. 309: p. 30. de Mel, A., et al., Development of cardiovascular bypass grafts: endothelialization and applications of nanotechnology. Expert Rev Cardiovasc Ther, 2008. 6(9): p. 1259-77. Milner, K.R. and C.A. Siedlecki, Fibroblast response is enhanced by poly(L-lactic acid) nanotopography edge density and proximity. Int J Nanomedicine, 2007. 2(2): p. 201-11. Joshi, R.R., et al., Phosphonated polyurethanes that resist calcification. J Appl Biomater, 1994. 5(1): p. 65-77. Hufnagel, C.A., Reflections on the development of valvular prostheses. Med Instrum, 1977. 11(2): p. 74-6. Daebritz, S.H., et al., New flexible polymeric heart valve prostheses for the mitral and aortic positions. Heart Surg Forum, 2004. 7(5): p. E525-32. Daebritz, S.H., et al., Introduction of a flexible polymeric heart valve prosthesis with special design for aortic position. Eur J Cardiothorac Surg, 2004. 25(6): p. 946-52. Butany, J., et al., Biological replacement heart valves. Identification and evaluation. Cardiovasc Pathol, 2003. 12(3): p. 119-39. Ghista, D.N., Toward an optimum prosthetic trileaflet aortic-valve design. Med Biol Eng, 1976. 14(2): p. 122-9. Yee Han, K., et al., Recent Advances in Polymeric Heart Valves Research. International Journal of Biomaterials Research and Engineering (IJBRE), 2011. 1(1): p. 1-17. Bernacca, G.M., et al., Polyurethane heart valve durability: effects of leaflet thickness and material. Int J Artif Organs, 1997. 20(6): p. 327-31. Daebritz, S.H., et al., Introduction of a Flexible Polymeric Heart Valve Prosthesis With Special Design for Mitral Position. Circulation, 2003. 108(10 suppl 1): p. II-134-II-139. Bezuidenhout, D., D.F. Williams, and P. Zilla, Polymeric heart valves for surgical implantation, catheter-based technologies and heart assist devices. Biomaterials, 2015. 36: p. 6-25. Leat, M.E. and J. Fisher, The Influence of Manufacturing Methods on the Function and Performance of a Synthetic Leaflet Heart Valve. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine, 1995. 209(1): p. 65-69. Rottenberg, D., et. al., Method for producing heart valves, H.S. Ltd., Editor. 2000: US. Hui, A., A. Duncan, and W. Wan, Hydrogel based artificial heart valve stent. ASMEPUBLICATIONS-BED, 1997. 36: p. 53-54. Wan, W., et al., Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. Journal of Biomedical Materials Research Part A, 2002. 63(6): p. 854-861. Yousefi, A., D.L. Bark, and L.P. Dasi, Effect of Arched Leaflets and Stent Profile on the Hemodynamics of Tri-Leaflet Flexible Polymeric Heart Valves. Annals of Biomedical Engineering, 2017. 45(2): p. 464-475. 145 141. 142. 143. 144. 145. 146. 147. 148. 149. 150. 151. 152. 153. 154. 155. 156. 157. 158. 159. Claiborne, T.E., et al., In Vitro Evaluation of a Novel Hemodynamically Optimized Trileaflet Polymeric Prosthetic Heart Valve. Journal of Biomechanical Engineering, 2013. 135(2): p. 021021-021021-8. Rotman, O.M., et al., Novel Polymeric Valve for Transcatheter Aortic Valve Replacement Applications: In Vitro Hemodynamic Study. Annals of Biomedical Engineering, 2018. Claiborne, T.E., et al., Polymeric trileaflet prosthetic heart valves: evolution and path to clinical reality. Expert Rev Med Devices, 2012. 9(6): p. 577-94. Rahmani, B., et al., In Vitro Hydrodynamic Assessment of a New Transcatheter Heart Valve Concept (the TRISKELE). Journal of Cardiovascular Translational Research, 2017. 10(2): p. 104115. Benyamin, R., et al., A new transcatheter heart valve concept (the TRISKELE): feasibility in an acute preclinical model. EuroIntervention, 2016. 12(7): p. 901-908. Leon, M.B., et al., Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. New England Journal of Medicine, 2016. 374(17): p. 1609-1620. Masson, J.-B., et al., Transcatheter aortic valve implantation: review of the nature, management, and avoidance of procedural complications. JACC: Cardiovascular Interventions, 2009. 2(9): p. 811-820. Smith, C.R., et al., Transcatheter versus surgical aortic-valve replacement in high-risk patients. New England Journal of Medicine, 2011. 364(23): p. 2187-2198. Dasi, L.P., et al., On the Mechanics of Transcatheter Aortic Valve Replacement. Annals of Biomedical Engineering, 2016: p. 1-22. Rodés-Cabau, J., Transcatheter aortic valve implantation: current and future approaches. Nature Reviews Cardiology, 2012. 9(1): p. 15. Dvir, D., et al., Coronary Obstruction in Transcatheter Aortic Valve-in-Valve Implantation. Preprocedural Evaluation, Device Selection, Protection, and Treatment, 2015. 8(1). Gurvitch, R., et al., Transcatheter aortic valve implantation: lessons from the learning curve of the first 270 high-risk patients. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions, 2011. 78(7): p. 977-984. Mizote, I., L. Conradi, and U. Schäfer, A case of anomalous left coronary artery obstruction caused by lotus valve implantation. Catheterization and Cardiovascular Interventions, 2016. Gökdeniz, T., et al., Concomitant complete atrioventricular block and left main coronary artery occlusion during transcatheter aortic valve implantation. Heart, Lung and Circulation, 2013. 22(12): p. 1048-1050. Dağdelen, S., H. Karabulut, and C. Alhan, Acute left main coronary artery occlusion following TAVI and emergency solution. Anadolu kardiyoloji dergisi: AKD= the Anatolian journal of cardiology, 2011. 11(8): p. 747. Zierer, A., et al., The transapical approach for aortic valve implantation. The Journal of thoracic and cardiovascular surgery, 2008. 136(4): p. 948-953. Flecher, E.M., et al., Coronary flow obstruction in percutaneous aortic valve replacement. An in vitro study. European journal of cardio-thoracic surgery, 2007. 32(2): p. 291-295. Gurvitch, R., et al., Coronary obstruction following transcatheter aortic valve‐in‐valve implantation for failed surgical bioprostheses. Catheterization and Cardiovascular Interventions, 2011. 77(3): p. 439-444. Bagur, R., et al., Coronary ostia stenosis after transcatheter aortic valve implantation. JACC: Cardiovascular Interventions, 2010. 3(2): p. 253-255. 146 160. 161. 162. 163. 164. 165. 166. 167. 168. 169. 170. 171. 172. 173. 174. 175. Crimi, G., G. Passerone, and P. Rubartelli, Trans‐apical aortic valve implantation complicated by left main occlusion. Catheterization and Cardiovascular Interventions, 2011. 78(4): p. 656-659. Maddox, T.M., et al., Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA, 2014. 312(17): p. 1754-1763. Levine, G.N., et al., 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Journal of the American College of Cardiology, 2011. 58(24): p. e44-e122. Ribeiro, H.B., et al., Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol, 2013. 62(17): p. 1552-62. Heitkemper, M., et al., Modeling risk of coronary obstruction during transcatheter aortic valve replacement. The Journal of Thoracic and Cardiovascular Surgery, 2020. 159(3): p. 829-838.e3. Billiar, K.L. and M.S. Sacks, Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp—part I: experimental results. Journal of biomechanical engineering, 2000. 122(1): p. 23-30. Hatoum, H., et al., Impact of patient-specific morphologies on sinus flow stasis in transcatheter aortic valve replacement: An in&#xa0;vitro study. The Journal of Thoracic and Cardiovascular Surgery. Hatoum, H., et al., An in&#xa0;vitro evaluation of turbulence after transcatheter aortic valve implantation. The Journal of Thoracic and Cardiovascular Surgery, 2018. 156(5): p. 1837-1848. Hatoum, H., B.L. Moore, and L.P. Dasi, On the significance of systolic flow waveform on aortic valve energy loss. Annals of biomedical engineering, 2018. 46(12): p. 2102-2111. Hatoum, H., F. Heim, and L.P. Dasi, Stented valve dynamic behavior induced by polyester fiber leaflet material in transcatheter aortic valve devices. Journal of the mechanical behavior of biomedical materials, 2018. 86: p. 232-239. Lalkhen, A.G. and A. McCluskey, Clinical tests: sensitivity and specificity. Continuing Education in Anaesthesia Critical Care & Pain, 2008. 8(6): p. 221-223. Ribeiro, H.B., et al., Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. Journal of the American College of Cardiology, 2013. 62(17): p. 1552-1562. Akhtar, M., et al., Aortic root morphology in patients undergoing percutaneous aortic valve replacement: evidence of aortic root remodeling. The Journal of thoracic and cardiovascular surgery, 2009. 137(4): p. 950-956. Apfaltrer, P., et al., Aortoiliac CT angiography for planning transcutaneous aortic valve implantation: aortic root anatomy and frequency of clinically significant incidental findings. American Journal of Roentgenology, 2012. 198(4): p. 939-945. Binder, R.K., et al., The impact of integration of a multidetector computed tomography annulus area sizing algorithm on outcomes of transcatheter aortic valve replacement: a prospective, multicenter, controlled trial. Journal of the American College of Cardiology, 2013. 62(5): p. 431438. Tops, L.F., et al., Noninvasive evaluation of the aortic root with multislice computed tomography: implications for transcatheter aortic valve replacement. JACC: Cardiovascular Imaging, 2008. 1(3): p. 321-330. 147 176. 177. 178. 179. 180. 181. 182. 183. 184. 185. 186. 187. 188. 189. 190. 191. 192. 193. Yamamoto, M., et al., Impact of preparatory coronary protection in patients at high anatomical risk of acute coronary obstruction during transcatheter aortic valve implantation. International journal of cardiology, 2016. 217: p. 58-63. Auricchio, F., et al., Simulation of transcatheter aortic valve implantation: a patient-specific finite element approach. Computer methods in biomechanics and biomedical engineering, 2014. 17(12): p. 1347-1357. Bosmans, B., et al., A validated methodology for patient specific computational modeling of selfexpandable transcatheter aortic valve implantation. Journal of biomechanics, 2016. 49(13): p. 2824-2830. Capelli, C., et al., Patient-specific simulations of transcatheter aortic valve stent implantation. Medical & biological engineering & computing, 2012. 50(2): p. 183-192. Capelli, C., et al., Patient-specific reconstructed anatomies and computer simulations are fundamental for selecting medical device treatment: application to a new percutaneous pulmonary valve. Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences, 2010. 368(1921): p. 3027-3038. Morganti, S., et al., Simulation of transcatheter aortic valve implantation through patientspecific finite element analysis: two clinical cases. Journal of biomechanics, 2014. 47(11): p. 2547-2555. Wang, Q., E. Sirois, and W. Sun, Patient-specific modeling of biomechanical interaction in transcatheter aortic valve deployment. Journal of biomechanics, 2012. 45(11): p. 1965-1971. Achenbach, S., et al., SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). Journal of Cardiovascular Computed Tomography, 2012. 6(6): p. 366-380. Holmes Jr, D.R., et al., 2012 ACCF/AATS/SCAI/STS Expert Consensus Document on Transcatheter Aortic Valve Replacement. Journal of the American College of Cardiology, 2012. 59(13): p. 12001254. Popma, J.J., et al., Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in LowRisk Patients. New England Journal of Medicine. 0(0): p. null. Mack, M.J., et al., Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. New England Journal of Medicine. 0(0): p. null. Ribeiro, H.B., et al., Coronary Obstruction Following Transcatheter Aortic Valve Implantation: A Systematic Review. JACC: Cardiovascular Interventions, 2013. 6(5): p. 452-461. Ribeiro, H.B., et al., Coronary obstruction following transcatheter aortic valve implantation: a systematic review. JACC: Cardiovascular Interventions, 2013. 6(5): p. 452-461. Hatoum, H., et al., A Case Study on Implantation Strategies to Mitigate Coronary Obstruction in a Patient Receiving Transcatheter Aortic Valve Replacement. Journal of Biomechanics, 2019. Finotello, A., S. Morganti, and F. Auricchio, Finite element analysis of TAVI: Impact of native aortic root computational modeling strategies on simulation outcomes. Medical Engineering & Physics, 2017. 47: p. 2-12. Wang, Q., et al., Simulations of transcatheter aortic valve implantation: implications for aortic root rupture. Biomechanics and Modeling in Mechanobiology, 2015. 14(1): p. 29-38. Capelli, C., et al., Patient-specific simulations of transcatheter aortic valve stent implantation. Medical & Biological Engineering & Computing, 2012. 50(2): p. 183-192. Bianchi, M., et al., Effect of Balloon-Expandable Transcatheter Aortic Valve Replacement Positioning: A Patient-Specific Numerical Model. Artificial Organs, 2016. 40(12): p. E292-E304. 148 194. 195. 196. 197. 198. 199. 200. 201. 202. 203. 204. 205. 206. 207. 208. 209. 210. Heitkemper, M., et al., Modeling Risk of Coronary Obstruction during Transcatheter Aortic Valve Replacement The Journal of thoracic and cardiovascular surgery, 2019. Dvir, D., et al., Coronary obstruction in transcatheter aortic valve-in-valve implantation: preprocedural evaluation, device selection, protection, and treatment. Circulation: Cardiovascular Interventions, 2015. 8(1): p. e002079. Dagdelen, S., H. Karabulut, and C. Alhan, Acute left main coronary artery occlusion following TAVI and emergency solution/TAVI sonrasi akut sol ana koroner arter tikanmasi ve acil çözüm. Anadulu Kardiyoloji Dergisi: AKD, 2011. 11(8): p. 747. Mizote, I., L. Conradi, and U. Schäfer, A case of anomalous left coronary artery obstruction caused by lotus valve implantation. Catheterization and Cardiovascular Interventions, 2017. 90(7): p. 1227-1231. Heitkemper, M., et al., Simple 2-dimensional anatomic model to predict the risk of coronary obstruction during transcatheter aortic valve replacement. The Journal of Thoracic and Cardiovascular Surgery, 2020. Holmes, D.R., et al., 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. Journal of the American College of Cardiology, 2012. 59(13): p. 12001254. Dasi, L.P., et al., On the Mechanics of Transcatheter Aortic Valve Replacement. Annals of Biomedical Engineering, 2017. 45(2): p. 310-331. Tice, J.A., F.W. Sellke, and H.V. Schaff, Transcatheter aortic valve replacement in patients with severe aortic stenosis who are at high risk for surgical complications: Summary assessment of the California Technology Assessment Forum. Journal of Thoracic and Cardiovascular Surgery, 2014. 148(2): p. 482-491.e6. Kheradvar, A., et al., Emerging trends in heart valve engineering: Part II. Novel and standard technologies for aortic valve replacement. Annals of biomedical engineering, 2015. 43(4): p. 844857. Mylotte, D. and N. Piazza, Transcatheter aortic valve replacement failure: déjà vu ou jamais vu? 2015, Am Heart Assoc. Dasi, L.P., et al., On the mechanics of transcatheter aortic valve replacement. Annals of biomedical engineering, 2017. 45(2): p. 310-331. Makkar, R.R., et al., Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. New England Journal of Medicine, 2015. 373(21): p. 2015-2024. Mylotte, D. and N. Piazza, Transcatheter Aortic Valve Replacement Failure Deja vu ou Jamais vu? Circulation-Cardiovascular Interventions, 2015. 8(4): p. 4. Kheradvar, A., et al., Emerging trends in heart valve engineering: Part IV. Computational modeling and experimental studies. Annals of biomedical engineering, 2015. 43(10): p. 23142333. Foroutan, F., et al., Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: systematic review of observational studies. BMJ, 2016. 354. Daubert, M.A., et al., Long-Term Valve Performance of TAVR and SAVR: A Report From the PARTNER I Trial. JACC: Cardiovascular Imaging, 2017. 10(1): p. 15-25. Haussig, S., et al., TCT-790 Long-term follow-up after transcatheter aortic valve implantation and durability of transcatheter aortic valves - results of a prospective single-center registry. Journal of the American College of Cardiology, 2017. 70(18 Supplement): p. B269. 149 211. 212. 213. 214. 215. 216. 217. 218. 219. 220. 221. 222. 223. 224. 225. 226. 227. 228. 229. 230. Pathak, C.P., et al., Treatment of bioprosthetic heart valve tissue with long chain alcohol solution to lower calcification potential. J Biomed Mater Res A, 2004. 69(1): p. 140-4. Hetzer, R., et al., Thrombosis and Degeneration of Hancock Valves: Clinical and Pathological Findings. The Annals of Thoracic Surgery, 1978. 26(4): p. 317-322. Kiefer, P., et al., Crimping May Affect the Durability of Transcatheter Valves: An Experimental Analysis. The Annals of Thoracic Surgery, 2011. 92(1): p. 155-160. Alavi, S.H., E.M. Groves, and A. Kheradvar, The Effects of Transcatheter Valve Crimping on Pericardial Leaflets. The Annals of Thoracic Surgery, 2014. 97(4): p. 1260-1266. Kannan, R.Y., et al., Polyhedral oligomeric silsesquioxane nanocomposites: The next generation material for biomedical applications. Accounts of Chemical Research, 2005. 38(11): p. 879-884. Simon-Walker, R., et al., Hemocompatibility of hyaluronan enhanced linear low density polyethylene for blood contacting applications. J Biomed Mater Res B Appl Biomater, 2018. 106(5): p. 1964-1975. Hatoum, H., et al., An in vitro evaluation of turbulence after transcatheter aortic valve implantation. The Journal of Thoracic and Cardiovascular Surgery, 2018. 156(5): p. 1837-1848. Hatoum, H., P. Maureira, and L.P. Dasi, A turbulence in-vitro assessment of ON-X and St. Jude Medical prostheses. The Journal of Thoracic and Cardiovascular Surgery, 2019. Movafaghi, S., et al., Hemocompatibility of superhemophobic titania surfaces. Advanced healthcare materials, 2017. 6(4): p. 1600717. Dasi, L.P., et al., Fluid mechanics of artificial heart valves. Clin Exp Pharmacol Physiol, 2009. 36(2): p. 225-37. Quantitation of the turbulent stress distribution downstream of normal, diseased and artificial aortic valves in humans. European Journal of Cardio-Thoracic Surgery, 1992. 6(11): p. 609-617. Giersiepen, M., et al., Estimation of shear stress-related blood damage in heart valve prostheses - in vitro comparison of 25 aortic valves. International Journal of Artificial Organs, 1990. 13(5): p. 300-306. Hanle, D.D., et al., Turbulence downstream from the Ionescu-Shiley bioprosthesis in steady and pulsatile flow. Medical & Biological Engineering & Computing, 1987. 25(6): p. 645-649. Jones, S.A., A relationship between reynolds stresses and viscous dissipation: Implications to red cell damage. Annals of Biomedical Engineering, 1995. 23(1): p. 21-28. Rotman, O.M., et al., Novel Polymeric Valve for Transcatheter Aortic Valve Replacement Applications: In Vitro Hemodynamic Study. Annals of Biomedical Engineering, 2019. 47(1): p. 113-125. Wang, Q., et al., In-vivo assessment of a novel polymer (SIBS) trileaflet heart valve. J Heart Valve Dis, 2010. 19(4): p. 499-505. Heitkemper, M., H. Hatoum, and L.P. Dasi, In vitro hemodynamic assessment of a novel polymeric transcatheter aortic valve. Journal of the Mechanical Behavior of Biomedical Materials, 2019. 98: p. 163-171. Simon-Walker, R., et al., Hemocompatibility of hyaluronan enhanced linear low density polyethylene for blood contacting applications. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2017. 106(5): p. 1964-1975. Camci-Unal, G., et al., Surface-modified hyaluronic acid hydrogels to capture endothelial progenitor cells. Soft matter, 2010. 6(20): p. 5120-5126. Laurent, T.C., U.B. Laurent, and J.R.E. Fraser, The structure and function of hyaluronan: an overview. Immunology and cell biology, 1996. 74(2): p. A1. 150 231. 232. 233. 234. 235. 236. 237. 238. 239. 240. 241. 242. 243. 244. 245. 246. 247. 248. 249. 250. 251. Jenkins, A., et al., Glossary of basic terms in polymer science (IUPAC Recommendations 1996). Pure and applied chemistry, 1996. 68(12): p. 2287-2311. Lagergre.H, P. Olsson, and Swedenbo.J, INHIBITED PLATELET ADHESION - NON-THROMBOGENIC CHARACTERISTIC OF A HEPARIN-COATED SURFACE. Surgery, 1974. 75(5): p. 643-650. Olsson, P., et al., PREVENTION OF PLATELET-ADHESION AND AGGREGATION BY A GLUTARDIALDEHYDE-STABILIZED HEPARIN SURFACE. Thrombosis and Haemostasis, 1977. 37(2): p. 274-282. Dean, H., Development of a Biopoly™ Micro-composite For Use In Prosthetic Heart Valve Replacements. Fort Collins: Colorado State University, Master of Science, 2011. James, S.P., M. Zhang, and G. Beauregard, Outer layer having entanglement of hydrophobic polymer host and hydrophilic polymer guest. 2010, Google Patents. Zhang, M., S.P. James, and E. Rentfrow, The effect of IPN surface modification on the mechanical properties of UHMWPE. Biomed Sci Instrum, 2001. 37: p. 7-12. Hatoum, H., et al., Aortic sinus flow stasis likely in valve-in-valve transcatheter aortic valve implantation. Journal of Thoracic and Cardiovascular Surgery, 2017. 154(1): p. 32-43.e1. Hatoum, H. and L. Dasi, Sinus Hemodynamics in Representative Stenotic Native Bicuspid and Tricuspid Aortic Valves: An In-Vitro Study. Fluids, 2018. 3(3): p. 56. Hatoum, H., et al., Impact of patient-specific morphologies on sinus flow stasis in transcatheter aortic valve replacement: An in vitro study. The Journal of thoracic and cardiovascular surgery, 2018. Hatoum, H., et al., Implantation Depth and Rotational Orientation Effect on Valve-in-Valve Hemodynamics and Sinus Flow. The Annals of thoracic surgery, 2018. Hatoum, H., et al., Sinus hemodynamics variation with tilted transcatheter aortic valve deployments. Annals of biomedical engineering, 2018: p. 1-10. Hatoum, H., et al., An in vitro evaluation of turbulence after transcatheter aortic valve implantation. The Journal of Thoracic and Cardiovascular Surgery, 2018. Dasi, L.P., et al., FLUID MECHANICS OF ARTIFICIAL HEART VALVES. Clinical and experimental pharmacology & physiology, 2009. 36(2): p. 225-237. Standardization, I.O.f., Cardiovascular implants in Cardiac valve prostheses. 2016. Gunning, P.S., et al., Total ellipse of the heart valve: the impact of eccentric stent distortion on the regional dynamic deformation of pericardial tissue leaflets of a transcatheter aortic valve replacement. Journal of The Royal Society Interface, 2015. 12(113). Martin, C. and W. Sun, Simulation of long-term fatigue damage in bioprosthetic heart valves: effects of leaflet and stent elastic properties. Biomechanics and Modeling in Mechanobiology, 2014. 13(4): p. 759-770. Doose, C., et al., Valve-in-valve outcome: design impact of a pre-existing bioprosthesis on the hydrodynamics of an Edwards Sapien XT valve. Eur J Cardiothorac Surg, 2017. 51(3): p. 562-570. Midha, P.A., et al., Valve Type, Size, and Deployment Location Affect Hemodynamics in an In Vitro Valve-in-Valve Model. JACC: Cardiovascular Interventions, 2016. 9(15): p. 1618-1628. Hatoum, H., et al., Effect of severe bioprosthetic valve tissue ingrowth and inflow calcification on valve-in-valve performance. Journal of Biomechanics, 2018. 74: p. 171-179. Dasi, L.P., et al., Passive flow control of bileaflet mechanical heart valve leakage flow. Journal of Biomechanics, 2008. 41(6): p. 1166-1173. Nygaard, H., et al., Two-dimensional color-mapping of turbulent shear stress distribution downstream of two aortic bioprosthetic valves in vitro. J Biomech, 1992. 25(4): p. 429-40. 151 252. 253. 254. 255. 256. 257. 258. 259. 260. 261. 262. 263. 264. 265. 266. 267. 268. 269. Gunning, P.S., et al., An in vitro evaluation of the impact of eccentric deployment on transcatheter aortic valve hemodynamics. Annals of Biomedical Engineering, 2014. 42(6): p. 1195-1206. Hasler, D. and D. Obrist, Three-dimensional flow structures past a bio-prosthetic valve in an invitro model of the aortic root. PLOS ONE, 2018. 13(3): p. e0194384. Schoephoerster, R.T. and K.B. Chandran, Velocity and turbulence measurements past mitrial valve prostheses in a model left ventricle. Journal of Biomechanics, 1991. 24(7): p. 549-562. Bianchi, M., et al. Simulation of Transcatheter Aortic Valve Replacement in patient-specific aortic roots: Effect of crimping and positioning on device performance. in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS. 2015. Garcia, M.J., et al., Mechanisms of hemolysis with mitral prosthetic regurgitation study using transesophageal echocardiography and fluid dynamic simulation. Journal of the American College of Cardiology, 1996. 27(2): p. 399-406. Maraj, R., et al., Evaluation of hemolysis in patients with prosthetic heart valves. Clinical Cardiology, 1998. 21(6): p. 387-392. Minniti, C.P., et al., Elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease: association with hemolysis and hemoglobin oxygen desaturation. Haematologica, 2009. 94(3): p. 340-347. Wilson, J.H., et al., Severe hemolysis after incomplete mitral valve repair. The Annals of Thoracic Surgery, 1990. 50(1): p. 136-137. Nishimura, R.A., et al., 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, 2014. 63(22): p. e57-e185. Sacks, M.S. and A.P. Yoganathan, Heart valve function: A biomechanical perspective. Philosophical Transactions of the Royal Society B: Biological Sciences, 2007. 362(1484): p. 13691391. Nygaard, H., et al., Two-dimensional color-mapping of turbulent shear stress distribution downstream of two aortic bioprosthetic valves in vitro. Journal of Biomechanics, 1992. 25(4): p. 429-440. Ramstack, J.M., L. Zuckerman, and L.F. Mockros, Shear-induced activation of platelets. Journal of Biomechanics, 1979. 12(2): p. 113-125. Liu, J.S., P.C. Lu, and S.H. Chu, Turbulence characteristics downstream of bileaflet aortic valve prostheses. Journal of Biomechanical Engineering, 2000. 122(2): p. 118-124. Antohe, B.V. and J.L. Lage, A general two-equation macroscopic turbulence model for incompressible flow in porous media. International Journal of Heat and Mass Transfer, 1997. 40(13): p. 3013-3024. Mößner, M. and R. Radespiel, Flow simulations over porous media – Comparisons with experiments. Computers and Fluids, 2017. 154: p. 358-370. Yang, S.K. and M.K. Chung, Turbulent flow through spacer grids in rod bundles. Journal of Fluids Engineering, Transactions of the ASME, 1998. 120(4): p. 786-791. Peacock, J.A., An in vitro study of the onset of turbulence in the sinus of Valsalva. Circ Res, 1990. 67(2): p. 448-60. Salaun, E., et al., Bioprosthetic aortic valve durability in the era of transcatheter aortic valve implantation. Heart, 2018. 104(16): p. 1323-1332. 152 270. 271. 272. 273. 274. 275. 276. 277. 278. 279. 280. 281. 282. 283. 284. 285. 286. 287. 288. 289. 290. 291. 292. D., D., First look at long-term durability of transcatheter heart valves: assessment of valve function up to 10 years. Presented at: EuroPCR 2016. Paris, France. , 2016. Puri, R., V. Auffret, and J. Rodés-Cabau, Bioprosthetic valve thrombosis. Journal of the American College of Cardiology, 2017. 69(17): p. 2193-2211. Shoeb, M. and M.C. Fang, Assessing Bleeding Risk in Patients Taking Anticoagulants. Journal of thrombosis and thrombolysis, 2013. 35(3): p. 312-319. Morshed, K.N., et al., Theory to predict shear stress on cells in turbulent blood flow. PloS one, 2014. 9(8): p. e105357-e105357. Grigioni, M., et al., A discussion on the threshold limit for hemolysis related to Reynolds shear stress. J Biomech, 1999. 32(10): p. 1107-12. Antiga, L. and D.A. Steinman, Rethinking turbulence in blood. Biorheology, 2009. 46(2): p. 77-81. Nikulin, V., S. Savtchenko, and N. Ashgriz, A model for the turbulent suppression in swirling flows. Physics Letters A, 2017. 381(48): p. 3989-3995. Vladimirov, V., B. Lugovtsov, and V. Tarasov, Suppression of turbulence in the cores of concentrated vortices. Journal of Applied Mechanics and Technical Physics, 1980. 21(5): p. 632637. Ramos, J., Incompressible swirling flows. Engineering computations, 1986. Imao, S., M. Itoh, and T. Harada, Turbulent characteristics of the flow in an axially rotating pipe. International journal of heat and fluid flow, 1996. 17(5): p. 444-451. Reich, G. and H. Beer, Fluid flow and heat transfer in an axially rotating pipe—I. Effect of rotation on turbulent pipe flow. International Journal of heat and mass transfer, 1989. 32(3): p. 551-562. Taghavi, R. and S. Farokhi, Turbulent swirling jets with excitation. 1988. Brethouwer, G., Y. Duguet, and P. Schlatter, Turbulent–laminar coexistence in wall flows with Coriolis, buoyancy or Lorentz forces. Journal of Fluid Mechanics, 2012. 704: p. 137-172. White, A., Flow of a Fluid in an Axially Rotating Pipe. Journal of Mechanical Engineering Science, 1964. 6(1): p. 47-52. Zaman, E., et al., An integral criterion for turbulence suppression in swirling flows. The Canadian Journal of Chemical Engineering, 2018. 96(9): p. 2025-2034. Bourgouin, J.-F., et al., Sensitivity of swirling flows to small changes in the swirler geometry. Comptes Rendus Mécanique, 2013. 341(1): p. 211-219. Ariyaratne, C. and T. Jones, Design and optimization of swirl pipe geometry for particle‐laden liquids. AIChE Journal, 2007. 53: p. 757-768. Hatoum, H. and L.P.J.A.o.b.e. Dasi, Reduction of pressure gradient and turbulence using vortex generators in prosthetic heart valves. 2019. 47(1): p. 85-96. Hatoum, H., et al., A turbulence in vitro assessment of On-X and St Jude Medical prostheses. 2020. 159(1): p. 88-97. Moore, B.L. and L.P. Dasi, Coronary Flow Impacts Aortic Leaflet Mechanics and Aortic Sinus Hemodynamics. Annals of Biomedical Engineering, 2015. 43(9): p. 2231-2241. Hatoum, H., et al., Differences in Pressure Recovery Between Balloon Expandable and Selfexpandable Transcatheter Aortic Valves. 2020. 48(2): p. 860-867. Hatoum, H., et al., In-vitro characterization of self-expandable textile transcatheter aortic valves. 2020. 103: p. 103559. Oster, M.E., et al., Temporal trends in survival among infants with critical congenital heart defects. Pediatrics, 2013. 131(5): p. e1502-8. 153 293. 294. 295. 296. 297. 298. 299. 300. 301. 302. 303. 304. 305. 306. 307. 308. Jonas, R., Comprehensive Surgical Management of Congenital Heart Disease. 2004, London: CRC Press. Poynter, J.A., et al., Association of pulmonary conduit type and size with durability in infants and young children. Ann Thorac Surg, 2013. 96(5): p. 1695-701; discussion 1701-2. Sinha, S., J. Aboulhosn, and D.S. Levi, Transcatheter Pulmonary Valve Replacement in Congenital Heart Disease. Interventional Cardiology Clinics, 2019. 8(1): p. 59-71. Sharma, A., et al., A Systematic Review of Infective Endocarditis in Patients With Bovine Jugular Vein Valves Compared With Other Valve Types. JACC: Cardiovascular Interventions, 2017. 10(14): p. 1449-1458. McElhinney, D.B., et al., Infective endocarditis after transcatheter pulmonary valve replacement using the Melody valve: combined results of 3 prospective North American and European studies. Circulation: Cardiovascular Interventions, 2013: p. CIRCINTERVENTIONS. 112.000087. Hascoet, S., et al., Infective Endocarditis Risk After Percutaneous Pulmonary Valve Implantation With the Melody and Sapien Valves. JACC: Cardiovascular Interventions, 2017. 10(5): p. 510-517. D., D., First look at long-term durability of transcatheter heart valves: assessment of valve function up to 10 years. , in Presented at: EuroPCR 2016. 2016. Soor, G.S., et al., Pulmonary site bioprostheses: morphologic findings in 40 cases. Arch Pathol Lab Med, 2009. 133(5): p. 797-802. Voitov, A.V., et al., In search of the best xenogeneic material for a paediatric conduit: an analysis of clinical data†. Interactive CardioVascular and Thoracic Surgery, 2018. 27(1): p. 34-41. Standardization, I.O.f., Cardiovascular Implants - Cardiac valve prostheses, in Part 1: General Requirements. 2015. p. 24-25. Yoganathan, A.P., et al., A new paradigm for obtaining marketing approval for pediatric-sized prosthetic heart valves. The Journal of Thoracic and Cardiovascular Surgery, 2013. 146(4): p. 879-886. Forléo, M.H., Application of passive flow control to mitigate the thromboembolic potential of bileaflet mechanical heart valves: an in-vitro study. Colorado State University. Libraries. Garcia, D. and L. Kadem, What do you mean by aortic valve area: Geometric orifice area, effective orifice area, or Gorlin area? The Journal of heart valve disease, 2006. 15: p. 601-8. Nishimura, R.A., et al., 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology, 2014. 63(22): p. e57-e185. Mercer-Rosa, L., et al., The impact of pulmonary insufficiency on the right ventricle: a comparison of isolated valvar pulmonary stenosis and tetralogy of fallot. Pediatric cardiology, 2015. 36(4): p. 796-801. Hatoum, H., et al., An in vitro evaluation of turbulence after transcatheter aortic valve implantation. Journal of Thoracic and Cardiovascular Surgery, 2018. 156(5): p. 1837-1848. 154 Appendix A: Supplemental Materials pertaining to 3D modeling of coronary obstruction in Section 3.1 The following provides additional details of the methods used in the 3D computational model to predict coronary obstruction in section 3.1. The pre-procedural patient specific aortic root, calcium nodules and cusps were segmented from pre-TAVR CT images using Mimics Research 18.0 (Materialise, Belgium). The segmented aortic wall, cusps and calcium nodules were then discretized in 3-Matic Research 13.0 (Materialise, Belgium) using explicit 4-node linear tetrahedron elements (Appendix A Figure A1). An idealized TAV stent (represented as an expandable cylinder) was discretized using hexahedral elements. FIGURE A1. ALL THE PATIENT-SPECIFIC TISSUES, INCLUDING AORTIC WALL, LEAFLETS, AND CALCIUM NODULES WERE DISCRETIZED USING AN EXPLICIT 4-NODE LINEAR TETRAHEDRON ELEMENT, WHEREAS THE CYLINDRICAL STENT WAS DISCRETIZED USING HEXAHEDRON ELEMENTS. THE TOTAL NUMBER OF MESH ELEMENTS FOR EACH PATIENT WAS 35,580, 2835, AND 3240 ELEMENTS FOR THE WALL, THE LEAFLETS, AND THE CALCIUM NODULE, RESPECTIVELY. A, CALCIFICATION ON THE LEAFLETS. B, THE 3 CUSPS. C, AORTIC ROOT, INCLUDING CORONARY ARTERIES[164] 155 Finite element analysis simulation of the idealized TAV stent (cylinder) expansion was performed in Abaqus/Explicit 6.9 software (Simulia, Providence, RI, USA). The assigned material properties of the wall, leaflets, and calcifium nodules are given in Table A.1. The interaction property assigned to the cylinder was a contact interaction with tangential behavior and frictionless mechanical contact properties. In order to simulate the expansion of the TAV, the boundary condition for the simulation was mechanical displacement of the cylinder and the displacement was set to be the maximum diameter TAV that the patient would have received clinically. The valve diameter and the maximum TAV expansion diameter used for each patient is shown in Table 3.1. The time period for expansion was set to 0.01. Aortic root geometry Wall Leaflets Calcium nodules μ (PKa) 500 1000 – λ (PKa) 10,000 10,000 – ρ (kg/m3) 1000 1000 1200 E (Mpa) – – 80 ν (Poisson) – – 0.3 Table A.1 List of material properties for aortic root geometry, including aortic wall, leaflets, and calcium nodules Following the completion of the simulation, the distance available for coronary blood flow was visualized using cross-section cut planes that depict the coronary ostia to illustrate the final position of leaflets and calcific lesion relative to the respective coronary ostia center, as shown in Figure A2. 156 FIGURE A2. A, AORTIC AND VENTRICULAR VIEWS OF THE RECONSTRUCTED PATIENT AORTIC ROOTS. THE AORTIC VIEWS ARE ORIENTED WITH THE COMMISSURE OF NON- AND LEFT CORONARY CUSPS AT THE TOP. IN THE VENTRICULAR VIEWS, THE TOP COMMISSURE CORRESPONDS TO THE LEFT AND RIGHT CORONARY CUSPS. CALCIFIC NODULES (YELLOW) ARE HIGHLIGHTED FOR EACH PATIENT AND THE MORPHOLOGY OF THE CALCIFIC LESIONS ARE NOTED TO BE HIGHLY PATIENT SPECIFIC. B, THE CROSS-SECTIONAL VIEWS OF FINITE ELEMENT ANALYSIS GENERATED GEOMETRIES AFTER TRANSCATHETER AORTIC VALVE REPLACEMENT FOR THE RESPECTIVE PATIENTS SHOWN IN A. CROSS-SECTIONAL VIEWS DEPICT BOTH LEFT AND RIGHT CORONARY OSTIA TO ILLUSTRATE THE FINAL POSITION OF LEAFLETS AND CALCIFIC LESION RELATIVE TO THE RESPECTIVE CORONARY OSTIA CENTER. LCA, LEFT CORONARY ARTERY; RCA, RIGHT CORONARY ARTERY[164] 157 FIGURE A3. COMPARISON BETWEEN IN VITRO VALIDATION TECHNIQUE FOR STENT SIZE 26 MM AND THE FINITE ELEMENT ANALYSIS SIMULATION WITH SIMULATED TRANSCATHETER AORTIC VALVE EXPANDED TO A DIAMETER OF 26 MM. THE FINAL MEASUREMENTS ARE PLOTTED WITH THE Y-AXIS SHOWING THE MEASURED DISTANCE BETWEEN NATIVE LEAFLETS TO THE LEFT CORONARY OSTIUM (DLC) [164] 158 Appendix B: Supplemental Materials pertaining to 2D modeling of coronary obstruction in Section 3.2 Patie nt Z 9.49 h, m m L, m m t, m m w, m m D, m m 15. 1 2.1 5 0.5 0 4.9 8 Δx = w - t ( mm) if negative → make 0.00 −1.65→ 0.00 A 12.30 18. 8.7 3.0 4.7 −5.66→ B 9 0 4 2 0.00 TAVR, Transcatheter aortic valve replacement. Δy = h + d – L ( mm); if negative → make 0.00 −0.63→ 0.00 −1.88→ 0.00 DLC2D/d (2 Success ) ful = (Δx2+Δy TAVR? 2/d) 0.0 Not undertak en 0.0 No Table B.1 Detailed calculations of DLC2D/d (2) for patients Z and AB, who demonstrated the highest possible predicted risk of coronary obstruction with DLC2D/d (2) = 0.0 159