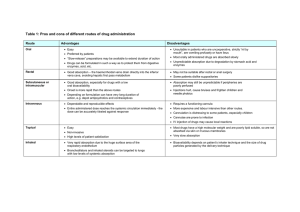
AfraTafreeh.com Last edited: 7/26/2022 PHARMACOKINETICS | DRUG ABSORPTION Pharmacokinetics | Drug Absorption Medical Editor: Sarah Abimhmed OUTLINE I) ROUTE OF ADMINISTRATION III) FACTORS AFFECTING II) MECHANISM OF ABSORPTION ABSORPTION (A) TRANSPORT MECHANISMS (A) PH (B) ABSORPTION IN OTHER ROUTES (B) BLOOD FLOW (C) TOTAL SURFACE AREA + CONTACT TIME (D) P-GLYCOPROTEIN IV) BIOAVAILABILITY (F) V) FACTORS AFFECTING BIOAVAILABILITY (A) SOLUBILITY OF THE DRUG (B) INSTABILITY OF THE VI) REVIEW QUESTIONS VII) REFERENCES ENVIRONMENT (C) FIRST-PASS EFFECT I) ROUTE OF ADMINISTRATION Absorption is the administration of a drug and the process of getting it into the circulation, therefore, understanding different routes of administration is important. o Enteral routes: p.o. (per os): oral or enteral route p.r. (per rectal): rectal route Buccal: between the lip and the teeth Sublingual: under the tongue o Parenteral routes (via injection): ID (Intradermal injection): in the dermis layer SC or SubQ (Subcutaneous injection): into the subcutaneous tissue IM (Intramuscular injection): into the muscle IV (Intravenous injection): into a vein Inhalation agent: into the lungs o Local routes: Topical route: on the skin The most common routes of administration are: o p.o. (oral) → in outpatient o IV (intravenous) → in the hospital Figure 1: Routes of Administration Figure 2 Dosage forms Pharmacokinetics | Drug Absorption PHARMACOLOGY: NOTE #1. 1 of 7 II) MECHANISM OF ABSORPTION The mechanism of absorption of the drugs is best explained with the oral route of drug intake: Figure 3: The route the drug takes when administered orally TRANSPORT MECHANISMS The transport mechanisms by which different drugs pass through the cell membrane depend on the characteristics of the drug, such as size, solubility, and charge. (1) Simple (Passive) Diffusion (3) Active Transport Drug characteristics: Small or Hydrophobic (lipophilic) Mechanism: Passive movement of molecules from areas of high concentration to areas of lower concentration Drug characteristics: Large or Hydrophilic Mechanism: Movement of molecules from areas of lower concentration to areas of higher concentration (against its concentration gradient), and this requires energy (ATP). Figure 4: Passive Diffusion (2) Facilitated Diffusion Drug characteristics: Large or Hydrophilic Mechanism: Passive movement of molecules from areas of high concentration to areas of lower concentration via specific transmembrane proteins (4) Endocytosis (Bulk Transport) Drug characteristics: Very large (e.g., Vitamin B-12) Mechanism: movement of macromolecules through the AfraTafreeh.com cell membrane. The drug binds onto receptors on the cell membrane, this triggers invagination of the drug in a process called ‘Endocytosis’. Then this drug is pushed out of the cell (to the vasculature) by ‘exocytosis’. Figure 5: Facilitated Diffusion Figure 7: Endocytosis Mechanism Drug Characteristics Passive Diffusion High → Low Hydrophobic. Small Facilitated Diffusion High → Low Needs protein carrier Hydrophilic Large Active Transport Low → High Needs ATP to be transported Hydrophilic Large Endocytosis Needs receptor-mediated endocytosis, then exocytosis to the vasculature Too Large (e.g., Vit. B12) Table 1: Summary of Transport Mechanisms Rectal route: the drug has to move across the gastrointestinal tract layers in there Intradermal, Subcutaneous, Intramuscular: the drug has to pass through different layers of the skin and tissues before it gets to the nearby capillaries Intravenous: the drug moves directly into the bloodstream (without having to pass through a membrane) 2 of 7 PHARMACOLOGY: NOTE #1. Pharmacokinetics | Drug Absorption AfraTafreeh.com III) FACTORS AFFECTING ABSORPTION (A) PH ●Drugs usually exists in 2 forms, a weak acid, HA (e.g. Aspirin) and a weak base, BH+ (e.g. amphetamines). (1) Weak Acids (2) Weak Bases HA dissociate into H+ and A●If there is a weak acid, A- is more difficult to be absorbed because it is charged (polar), the phosphate groups of the cell membrane are negatively charged so they will repel the charged molecule. BH+ dissociates into B + H+ ●Therefore, in order for the weak acid drug to be absorbed, it needs to exist in the HA form as it is not charged (nonpolar). The reaction needs to go in the opposite direction. Figure 8: Dissociation equation of weak acids ●For the reaction to go in the opposite direction, there needs to be more protons inside the environment of the drug. ●BH+ is polar, not easily absorbed. o B is non polar, easily absorbed. ▪ The reaction needs to shift to make ↑ B (to the right in the figure below) ●A way in which this can be achieved is by ↓ the protons in the environment so that the reaction can shift to make more protons (to reach equilibrium). ●To ↓ the protons, the environment needs to be more alkaline. o The reaction shifts to make ↑ B molecules that are in a non-polar, non-charged form which are more easily absorbed. ●The area in which weak base drugs are best absorbed in is the distal ileum. (i) Le Chatelier’s Principle: ●For example, if the drug is an acidic environment, the number of protons will increase and according to le chatelier’s principle when there is an increase in concentration on one side, the reaction will shift to establish equilibrium (to the left according to the equation in the figure above). ●The concentration of the non-polar weak acid will ↑. o Therefore, in an acidic environment, a weak acid will be in the HA form, this is will be easily absorbed across the phospholipid bilayer and into the blood stream. Figure 10: Absorption of weak basic drugs is enhanced in alkaline environments Figure 9: Absorption of weak acid drugs is enhanced in acidic environments ●This is important because when giving a weak acid drug, it needs to be in an acidic environment in order to enhance the absorption of the drug. o The most acidic area that weak acid are best absorbed in is the proximal duodenum. (B) BLOOD FLOW ●Adequate blood flow is needed to supply particular organs. o Absorption is the movement of the blood into the bloodstream, if there is ↓ blood flow to a particular organ which requires a specific drug to be absorbed, there will be ↓ absorption. There are certain situations in which there is ↓in blood flow: ●Shock state (e.g. septic, hypovolemic, neurogenic) o There is ↓ blood flow to the organs that absorb the drug (GI, Skin, etc.) Therefore, lower amounts of the drug will be absorbed into the bloodstream, as there is not enough perfusion to the GIT and Skin. o This occurs if the drug is taken orally, rectally or via the skin. ●The only way to ensure that the blood gets into the blood is via IV administration. o This is why patients in these state receive drugs via the IV route. Pharmacokinetics | Drug Absorption Figure 11: Decreased blood flow decreases perfusion to particular organs which then decreases absorption. PHARMACOLOGY: NOTE #1. 3 of 7 (C) TOTAL SURFACE AREA + CONTACT TIME (1) Contact Time ●If the patient has ↑ motility in the GI tract (e.g. diarrhea), the drug will pass by so fast that there will not be enough contact time for absorption to occur and enter the blood stream. o Therefore in case of diarrhea, absorption of the drug ↓ as there is ↓ contact with the cells of the GIT. (2) Total Surface Area ●The intestines have a large surface area, this is mainly due to the villi and microvilli. ●In case of diseases that destroy the villi and microvilli, surface area of the intestines ↓. o This occurs in IBD, celiac, gastroenteritis, etc. ●The absorption of the drug will consequently ↓. ●On the other hand, if the patient has slow transit time, as in the case of constipation, there is so much time for the drug to be absorbed (↑ contact time with the cells of the GIT) o Therefore there is ↑ absorption. Figure 13: Decreasing Total Surface Area (as in the case of diseases such as IBD, celiac, etc.) decreases absorption Figure 12: Contact time and absorption are directly proportional (D) P-GLYCOPROTEIN ●When a drug is ingested orally, it passes through the cell membrane via different mechanisms. REMEMBER: Mechanisms of drug transportation: • Passive Diffusion • Active Diffusion • Facilitated Diffusion • Endocytosis AfraTafreeh.com ●There are special transporters in the GIT can affect absorption, this is the case with P-Glycoproteins. o P-Glycoprotein are found in the apical surface of the cell. o They inhibit the process of drug absorption by pushing the drug back into the GIT instead of letting it pass to the blood stream. ▪ This leads to multi-drug resistance (MDR). Figure 14: P-Glycoproteins found in some cases inhibits absorption and leads to multi-drug resistance Table 2: Summary of factors affecting absorption Factors Affecting Absorption ↓ Absorption ↑ Absorption Weak acid drugs in alkaline environments Or weak base drugs in acidic environments. Weak acid drugs in acidic environments Or weak base drugs in alkaline environments. ↓ perfusion to organs that absorb drugs (e.g., skin, GIT) this can occur in shock states. ↑ perfusion to organs that absorb drugs ↓ contact time (e.g., diarrhea) ↑ contact time (e.g., constipation) Total Surface Area ↓ Total Surface Area, this occurs in diseases that destroy the brush border of the GIT cells. ↑ Total Surface Area P-Glycoprotein If P-Glycoproteins are found in apical surface of GIT cells. No P-Glycoproteins pH Blood Flow Contact Time 4 of 7 PHARMACOLOGY: NOTE #1. Pharmacokinetics | Drug Absorption AfraTafreeh.com IV) BIOAVAILABILITY (F) ●This is one of the most important components of absorption. (i) Definition: ●This is the fraction of the drug that enters the systemic circulation. (2) Bioavailability with IV vs Oral Administration ●When a drug is administered via the IV route, it does not pass through any membrane. o The amount of drug that is administered and that gets absorbed in the bloodstream is 100%, therefore bioavailability of IV drugs is 100%. ●However, if a drug is administered orally or through other routes that require passing through membranes, absorption is affected by various factors such as pH, blood flow patterns, surface area, contact time, solubility of the drug, size of the drug, etc. o All the blood that is administered will not enter the blood stream so bioavailability will not be 100%. (i) Calculating Bioavailability (F): (ii) Bioavailability is determined via this formula: ●If the drug is given IV, it will reach 100% and with time it will decrease until it is completely eliminated as it will be metabolized and excreted. o The area under the curve (AUC) will determine that. ●So, if 100 mg of the drug is given and only 50 mg is absorbed, therefore the bioavailability is 50%. Figure 15: Graph showing bioavailability of IV administered drugs (blue) and orally administered drug (brown) ●If the drug is given orally, the concentration will rise but it would not reach 100%. With time the concentration will fall as it becomes metabolized and excreted. Figure 16: Bioavailability with IV vs Oral Administration, orally administered drugs have lower bioavailability than IV administered drugs. V) FACTORS AFFECTING BIOAVAILABILITY (A) SOLUBILITY OF THE DRUG Hydrophobic (lipophilic), small, and nonpolar drugs can easily pass across the cell membranes (of the GIT) and get into the bloodstream and so they have (↑) Absorption and (↑) Bioavailability (F) Hydrophilic and large drugs cannot easily pass and so they have (↓) Absorption and (↓) Bioavailability (F) (B) INSTABILITY OF THE ENVIRONMENT (1) Stomach and Intestinal Metabolism On oral administration of the drug, e.g., penicillin G, as it reaches the stomach, it gets destroyed (metabolized) by protons [H+] from the HCl (hydrochloric acid) which are released by parietal cells, and this leaves almost none of the drug to be absorbed into the bloodstream, and ultimately this leads to (↓) Bioavailability (F) o This is why Penicillin G is given by IM or IV injections Figure 18: Metabolism by the Stomach and Intestines (2) Enzymatic Metabolism On oral administration of Insulin, it is broken down by proteases which are released from the pancreas, and this leaves the insulin molecule ineffective and decreases the amount of insulin that passes into the bloodstream → (↓) Bioavailability (F) o This is why Insulin is given by IV or SC injections Figure 19: Enzymatic Metabolism Figure 17: Effect of drug solubility on its bioavailability Pharmacokinetics | Drug Absorption PHARMACOLOGY: NOTE #1. 5 of 7 (C) FIRST-PASS EFFECT (1) First-pass metabolism in the Liver (2) Other routes of administration On oral administration of the drug, after passing through the GIT layers and environments that have been mentioned, it then passes through the portal circulation to the liver. o By the time it reaches the liver, only 90% of the active drug is remaining. Generally, drugs that are not taken orally, do not go through the first-pass effect Rectally-administered drugs: small portion of the drug may get into the portal system and go through the firstpass effect The liver is considered the site of first-pass metabolism, where the liver metabolizes the drug to such an extent that the bioavailability is drastically reduced. o Only about 30% of the drug gets to pass to the bloodstream and be distributed to the tissues. (Note: the bioavailability values are approximate and not accurate) E.g., Nitroglycerin, a drug that is used for anginal chest pain patients. AfraTafreeh.com 6 of 7 PHARMACOLOGY: NOTE #1. Pharmacokinetics | Drug Absorption AfraTafreeh.com VI) REVIEW QUESTIONS 1) 18-year-old female brought to ED due to drug overdose. Which route is most desirable for administering the antidote? a) IM b) IV c) Oral d) SC VII) REFERENCES ● UpToDate 2022 2) Drug A is a weakly basic drug with a pKA of 7.8. If administered orally, at which of the following sites of absorption will the drug be able to readily pass through the membrane? a) Mouth (pH 7.0) b) Stomach (pH 2.5) c) Duodenum (pH 6.1) d) Jejunum (pH 8.0) e) Ileum (pH 7.0) 3) Which mechanism of transport requires ATP to move the molecule across the cell membrane? a) Passive diffusion b) Facilitated diffusion c) Active transport d) Endocytosis 4) Which of the following drugs would have the highest bioavailability based on its administration route? a) Penicillin G taken orally b) Nitroglycerin taken orally c) Insulin taken intravenously d) Vitamin B12 taken orally 5) Which of the following routes has a 100% bioavailability? a) Intramuscular b) Subcutaneous c) Oral d) Intravenous e) Sublingual 6) Which is the best place for the absorption of weak acid drugs? a) Stomach b) Proximal duodenum c) Distal Duodenum d) Distal ileum 7) How can weak bases be absorbed in the GI tract? e) ↓ protons to ↑ B f) ↓ protons to ↑ BH+ g) ↑ protons to ↑ B h) ↑ protons ↓ BH+ 8) Why does absorption of drugs decrease in shock states? i) There is a reduced surface area of the GIT j) There is reduced blood perfusion to the organs that absorb the drugs k) Contact time of the drug to the GIT cells is reduced l) Shock patients have P-glycoproteins in the cell membrane which inhibit absorption of drugs. 9) What is the result of P-Glycoprotein action in the GI system? m) Multidrug resistance n) Inflammatory Bowel Disease o) Decreased Total Surface Area p) Decrease contact time 10) What is the formula to determine bioavailability of a drug? q) F = AUC IV / AUC oral r) F = AUC IV x AUC oral s) F = AUC oral / AUC IV t) F = [Oral Dose] / AUC IV Pharmacokinetics | Drug Absorption PHARMACOLOGY: NOTE #1. 7 of 7