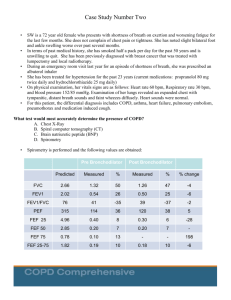
Official reprint from UpToDate® www.uptodate.com © 2023 UpToDate, Inc. and/or its affiliates. All Rights Reserved. Office spirometry AUTHOR: David A Kaminsky, MD SECTION EDITOR: Meredith C McCormack, MD, MHS DEPUTY EDITOR: Paul Dieffenbach, MD All topics are updated as new evidence becomes available and our peer review process is complete. Literature review current through: Jun 2023. This topic last updated: Mar 07, 2023. INTRODUCTION Spirometry is used to measure forced expiratory flow rates and volumes. It is the most commonly used pulmonary function test and is useful in the evaluation of patients with respiratory symptoms (eg, dyspnea, cough, wheeze), and the monitoring of lung function in patients with lung disease, being treated with drugs that can affect lung function, or at risk of lung disease (eg, smoking, occupational exposures, family history). In the office setting, spirometry is typically used to detect, confirm, and monitor obstructive airway diseases (eg, asthma, chronic obstructive pulmonary disease [COPD]) and monitor known restrictive lung disease [1-5]. In this setting, the clinician must be knowledgeable about issues related to equipment, performance of the forced expiratory maneuver, and interpretation of the data to obtain reliable and clinically useful information [6-8]. International guidelines for performance of office spirometry have been published [9,10]. Spirometry is not recommended as a screening test for asymptomatic adults [4,11], but rather facilitates diagnosis of respiratory diseases. The performance of spirometry in the office setting will be reviewed here. More general issues related to pulmonary function tests, the interpretation of flow volume loops, and the technique of bronchoprovocation testing are discussed separately. (See "Overview of pulmonary function testing in adults" and "Flow-volume loops" and "Bronchoprovocation testing".) ADVICE RELATED TO COVID-19 PANDEMIC Spirometry and other pulmonary function test (PFT) maneuvers can promote coughing and aerosol generation and could lead to spread of coronavirus disease 2019 (COVID-19; SARSCoV-2) by infected patients. It is difficult to screen patients for active COVID-19 infection, particularly those with underlying respiratory symptoms, and infected but asymptomatic patients can shed the virus. Thus, we agree with expert recommendations that spirometry and other PFTs be limited to patients in whom results are essential to immediate management decisions [12,13]. Use of nebulizers to administer bronchodilators or methacholine should be avoided. Measures to prevent spread of COVID-19 should include hand hygiene and personal protective equipment (PPE; glove, gown, face mask and shield) for staff and anyone else in the testing space (eg, interpreters). N95 masks or powered air purifying respirators (PAPR) are preferred over surgical masks. Patients should be brought to a testing room using an approach that avoids queuing or grouping individuals in a waiting area and that allows adequate time between patients for sufficient air exchange. Enhanced cleaning of the testing area should be performed between patients. EQUIPMENT Office spirometers should meet equipment specifications as described in international guidelines [5,10,14,15]. The majority of spirometers manufactured since 1990 are accurate, although some flow-sensing office spirometers can produce falsely high results [16-18]. Reference standards are discussed separately. (See "Selecting reference values for pulmonary function tests".) To avoid cross-contamination between patients when using permanent flow sensors, it is preferable to employ single use disposable flow sensors that practically eliminate the risk of inhalational cross contamination. Disposable one-way mouthpieces may also be used; otherwise, patients should be instructed not to inhale from the spirometer when only forced exhalation maneuvers are being obtained. Volume sensing spirometers maintain accuracy over many years but are more difficult to clean and are rarely used for office spirometry. QUALITY CONTROL Office spirometers should accurately measure the forced expiratory volume in one second (FEV1), forced expiratory volume in six seconds (FEV6), and forced vital capacity (FVC) and also provide quality checks and error messages. A survey of 17 spirometers used in primary care offices found only 1 of 17 met accuracy criteria; clearly manufacturers and practitioners need to be aware of potentially significant quality issues related to office spirometry [19]. In addition to internal calibration performed by the device, daily calibration checks with a three liter syringe are recommended [10]. When performing a calibration check, the threeliter syringe should be discharged into the spirometer three times. The volumes read by the machine should be within 3 percent of three liters. If the spirometer reading remains outside these limits after replacing the flow sensor, the device should be removed from use until checked by the manufacturer. It is also essential that the nurse or technician enter correct values for age, height, and sex at birth, as these values are used to generate the appropriate predicted values for the individual patient. Height should be measured with shoes off, preferably using a stadiometer, rather than relying on the patient's stated height. Percent predicted values that are unexpectedly higher or lower than expected are a clue that an incorrect age or height value may have been entered. Waist circumference, while not used to calculate predicted normal values, can also be measured because abdominal obesity is a common cause of mildly low values for FEV1 and FVC [20]. PROCEDURE Patients are usually seated during spirometry, unless otherwise noted. Nose clips or manual occlusion of the nares help to prevent air leakage through the nasal passages, although spirometry can be performed without nasal occlusion [21]. The deep inhalation should occur before the mouthpiece is placed in the mouth. Immediately after the deep inhalation, the mouthpiece is placed just inside the mouth between the teeth. The lips should be sealed tightly around the mouthpiece to prevent air leakage during maximal forced exhalation. The patient should then be instructed to blast out the air without hesitation (within two seconds of reaching full inflation). Exhalation should last until a plateau in exhaled volume is reached, or a maximum of 15 seconds. However, if measuring FEV6 as a surrogate for FVC, then exhalation need only last at least six seconds. To fully evaluate flow volume loops, it is necessary to perform a complete inspiratory maneuver at the end of the test. The maximal inspiration at the end of test requires vigorous coaching to achieve good quality results and patients should be informed that this aspect of the maneuver feels somewhat uncomfortable. (See "Flow-volume loops".) The patient is allowed to rest for several seconds and the procedure is repeated. Usually, three maneuvers are performed, although additional tests may be needed if one or more of the curves are unacceptable. COACHING THE PATIENT The most important task of the technician or person performing the test is to obtain maximal, reproducible efforts from the patient. Even with the use of accurate instruments, office spirometry results may be misleading if the patient's efforts are submaximal. Unlike most other medical tests in which the patient remains passive, accurate spirometry results require significant exertion on the part of the patient. The technician must instruct and encourage the patient to perform the breathing maneuvers in three phases ( figure 1): ● Phase 1: Coach the patient to take as deep a breath as possible ● Phase 2: Strongly prompt the patient to blast out the air into the spirometer without hesitation after reaching a full inspiration ● Phase 3: Encourage the patient to continue exhaling until a plateau in exhaled volume or 15 seconds is reached, unless just measuring FEV6 in which case the exhalation should last at least six seconds (three seconds for children) Patients in whom a flow volume loop is needed will need to perform an additional phase of deep and forceful inspiration to total lung capacity immediately after phase 3. ADEQUACY OF TEST An adequate test usually requires three acceptable and repeatable forced vital capacity (FVC) maneuvers [10]. The clinician and technician must learn to recognize the patterns of acceptable and unacceptable efforts, since poorly performed maneuvers often mimic disease patterns. Detection of poorly performed maneuvers requires direct inspection of both flow-volume curves and volume-time spirograms ( figure 2) [14,22]. An acceptable maneuver requires a sharp peak in the flow curve and an expiratory duration that reaches a plateau of exhaled volume or 15 seconds, or greater than six seconds if measuring FEV6 instead of FVC ( figure 3). At least three acceptable maneuvers should be available for analysis. Repeatability is determined by comparing the FVC and FEV1 values of the maneuvers. The two highest values for FVC and for FEV1 should be within 0.15 L of each other (for adults; the limit is 0.10 L for children) [10,23]. The grading system for the quality of spirometry factors in the number of acceptable maneuvers and the degree of repeatability for FEV1 and FVC separately ( table 1) [10]. INTERPRETATION All tracings from the forced expiratory maneuvers should be examined for acceptability and repeatability, according to the criteria mentioned above. The study should then be classified as normal or abnormal, the latter showing an obstructive, a possible restrictive, or a possible mixed pattern. Lung volumes are necessary to confirm whether or not the patient has a restrictive deficit. The severity of the ventilatory impairment is then assessed, according to the algorithm ( algorithm 1). (See 'Forced expiratory volume in one second' below and "Overview of pulmonary function testing in adults".) Forced vital capacity — The forced vital capacity (FVC) (also known as the forced expiratory volume) is the maximal volume of air exhaled with a maximally forced effort from a position of full inspiration and is expressed in liters [10]. The highest FVC from the three acceptable forced expiratory maneuvers is used for interpretation [10]. The FVC may be reduced by suboptimal patient effort, airflow limitation, restriction (eg, from lung parenchymal, pleural, or thoracic cage disease), or a combination of these ( algorithm 1). In general, a low FVC needs further evaluation with full pulmonary function tests to determine whether lung restriction is present [24]. Patients with obstruction and low FVC frequently demonstrate air trapping or failure to complete a full exhalation rather than an additional restrictive process. (See "Overview of pulmonary function testing in adults".) When FVC is low, restriction is not confirmed by lung volumes, and there is not obstruction (based on FEV1/FVC), this represents a "nonspecific" pattern. Nonspecific patterns are not clearly indicative of any lung disease subtype but may be associated with either ongoing or future obstructive or restrictive diseases [25]. Repeat testing to reassess lung function after several months to a year may be useful. Due to these potential problems with air trapping and incomplete exhalation, FVC is not used to grade restriction severity. We prefer to assess the severity of restrictive deficits by applying the z-score approach to total lung capacity measurements, when available. If lung volumes are not available, FEV1 may be used to assess the severity of previously established restriction, as was recommended in prior guidelines [23]. (See "Overview of pulmonary function testing in adults".) The slow vital capacity (SVC) is the maximal volume of air exhaled after a maximal inspiration, but without a forced effort. The SVC is rarely measured outside of hospital-based pulmonary function labs. For normal subjects, the slow and forced vital capacities are very close, whereas patients with airflow limitation tend to have a lower FVC than SVC. (See "Overview of pulmonary function testing in adults", section on 'Spirometry'.) Forced expiratory volume in six seconds — The forced expiratory volume in six seconds (FEV6) is sometimes used as a surrogate for FVC [21,26]. The FEV6 has the advantage of being more reproducible than the FVC and less physically demanding for the patient. Forced expiratory volume in one second — The forced expiratory volume in one second (FEV1) is the maximal volume of air exhaled in the first second of a forced exhalation that follows a full inspiration, expressed in liters [21]. The FEV1 reflects the average flow rate during the first second of the FVC maneuver. The FEV1 is the most important spirometric variable for assessment of the severity of airflow obstruction ( algorithm 1). The highest FEV1 from the three acceptable forced expiratory maneuvers is used for interpretation, even if it does not come from the maneuver with the highest FVC [10]. In patients with asthma, the FEV1 typically declines with clinical worsening of airways obstruction and increases with successful treatment of airways obstruction. The FEV1 should be used for determining the degree of obstruction (mild, moderate, or severe) and for serial comparisons when following patients with asthma or chronic obstructive pulmonary disease (COPD). FEV1 may also be used to grade the severity of restrictive or mixed obstructive/restrictive disorders once restriction has been confirmed by lung volumes; however, we prefer to grade pure restriction using total lung capacity when this measurement is available ( algorithm 1) [23]. (See "Overview of pulmonary function testing in adults".) The measured FEV1 should be reported based on z-score, instead of percent predicted. This measurement strategy helps to avoid age, sex, and height bias and is associated with important clinical outcomes [27,28]. The lower limit of normal (LLN) for FEV1 and other spirometry measures is defined by z-score <-1.645, which is equivalent to the fifth percentile in the distribution of healthy never-smokers. This replaces using a fixed percent of predicted value, which does not incorporate demographic features [27,29]. An FEV1 within the normal range may still represent a mild ventilatory impairment if obstruction or restriction is confirmed using other measures. Reference equations from the Global Lung Function Initiative, which assessed healthy individuals age 3 to 95 years across ethnic and geographic groups in 26 countries, are recommended for both pediatric and adult patients [27,30]. These reference equations are endorsed by multiple international expert groups and replace the older generation of equations, including those derived from the NHANES III study [23,31]. (See "Selecting reference values for pulmonary function tests".) As a rough guideline, the predicted FEV1 for a 50-year-old of average height is about 4 L for a male and 3 L for a female. When predicted values have not been calculated, patients with severe COPD generally have an FEV1 less than one liter, while those with moderate COPD have an FEV1 between 1 and 1.5 liters. Individuals with an FEV1 ≤75 percent of predicted are more likely to report dyspnea, wheezing, or cough, than those with an FEV1 >75 percent predicted [32]. (See "Selecting reference values for pulmonary function tests".) Ratio of FEV1/FVC — The FEV1/FVC ratio is the fraction of the forced vital capacity that can be exhaled in the first second. It is the most important parameter for detecting airflow obstruction in diseases like asthma and COPD ( algorithm 1). However, once it has been determined that a patient has airways obstruction, the FEV1/FVC ratio is not useful for gauging severity of disease, since the FVC often decreases with increasing obstruction due to air trapping or premature termination of exhalation. The FEV1, not the FEV1/FVC ratio, should be used to monitor patients with asthma or COPD. (See "Pulmonary function testing in asthma" and "Chronic obstructive pulmonary disease: Diagnosis and staging".) The use of z-score <-1.645 or the fifth percentile LLN for FEV1/FVC to detect airway obstruction reduces the misclassification associated with using a fixed threshold of 0.7 [29,33-36]. If FEV1/FVC is low, but FEV1 is normal, this is still considered indicative of mild airflow obstruction. In some cases, a low FEV1/FVC in the presence of a low FEV1 is classified as reflecting dysanapsis, or airways size being too small relative to lung volume size. While dysanapsis may be a physiologically normal variant, it has also been associated with COPD [37], bronchodilator responsiveness, and [38] severe asthma in children with obesity [39]. If FEV1/FVC is normal, but FEV1 is low, this may represent restriction, which should be evaluated by lung volumes. When lung volumes are not available, this pattern has also been labeled "Preserved Ratio Impaired Spirometry", or PRISm. It is unclear if PRISm is a distinct phenotype of lung disease, but multiple studies have demonstrated that PRISm is associated with increased cardiopulmonary disease and mortality [40-42]. When FVC maneuvers are routinely stopped after six seconds, the FEV1/FEV6 may replace the FEV1/FVC [43]. The advantages of the FEV1/FEV6 include less frustration for the patient and technician trying to achieve an end-of-test plateau, less chance of syncope, shorter testing time, and better repeatability, without loss of sensitivity or specificity [26,44-46]. The appropriate lower limit of normal for FEV1/FEV6 from NHANES III should be used [31,47]; unfortunately, the GLI equations do not include prediction equations for FEV6. Other flow measures — The transition from normal function to moderate airflow obstruction is generally gradual. Physiologists have searched for a test that is more sensitive than the FEV1 for detection of airflow obstruction in its early stages. None has proven to be as reliable as the index obtained by dividing the FEV1 by the FVC. The forced expiratory flow between 25 and 75 percent of the FVC (also known as FEF25-75 or maximal mid-expiratory flow rate) should not be used to detect "small airways disease" in adults, due to poor specificity and reproducibility [21]. Choosing the best values — Report the highest FVC and the highest FEV1 from three spirometric maneuvers, even if they are derived from different maneuvers [10]. Flow-volume loops — The flow-volume loop is a plot of inspiratory and expiratory flow (on the Y-axis) against volume (on the X-axis) during the performance of maximally forced inspiratory and expiratory maneuvers. Changes in the contour of the loop can detect upper airway obstruction. The analysis of flow-volume loops is discussed separately. (See "Flowvolume loops".) Post-bronchodilator spirometry — In patients who have evidence of airflow limitation on baseline spirometry, and no prior diagnosis of asthma or COPD, post-bronchodilator spirometry may be useful. If post-bronchodilator spirometry is normal, COPD is less likely. The assessment of bronchodilator responses in patients with asthma-like symptoms is described separately. Note, however, that a bronchodilator response alone does not distinguish asthma from COPD [48]. If the change in spirometry post-bronchodilator does not support a diagnosis of obstructive lung disease, referral for bronchial challenge testing (eg, with methacholine or exercise) may be helpful [49]. (See "Pulmonary function testing in asthma", section on 'Bronchodilator responses'.) LIMITATIONS Office spirometry has some important limitations, even when all of the above-described quality measures are employed. As examples: ● Abnormal spirometry results have little if any value in prompting smokers to quit [5054]. All patients who smoke should be advised to stop smoking and provided smoking cessation assistance. ● In a patient with asthma, which is characterized by variability in clinical symptoms and airflow obstruction, normal airflow at the time of office visits does not exclude airflow obstruction at other times. ● Patients with early interstitial lung disease may have normal spirometry and need further testing of gas transfer with a diffusing capacity of the lungs for carbon monoxide (DLCO) and/or exercise oximetry to identify the cause of dyspnea [55]. ● Misclassification rates due to suboptimal spirometry performance or interpretation are relatively high in the office setting [7,19,56]. Among eight general practices, rates for successfully meeting American Thoracic Society (ATS) quality standards were below 80 percent [7]. In 16 primary care offices involving 17 different spirometers, only 60 percent of patients had spirometry that met acceptability criteria [19]. Therefore, continuous quality review and feedback to nurses and technologists performing office spirometry are necessary and results should be verified by repeat testing in a Pulmonary Function Test (PFT) laboratory when important clinical decisions will be made based on the results [57,58]. ● There are multiple barriers to performing in-office spirometry. One comprehensive survey identified multiple barriers including lack of knowledge about how to interpret spirometry, lack of resources (equipment, personnel, skills, time), and lack of belief in importance of results [59]. Most of the barriers identified were also true for out-ofoffice (ie, hospital) spirometry, indicating that implementing spirometry in general faces many challenges. RISKS Spirometry is a low-risk procedure and has few side effects [14]. During the test, some patients may experience dizziness. The forced expiratory maneuver causes an increase in the pressure in the chest, abdomen, head, and eyes. In general, patients who have recently (eg, less than six weeks) had abdominal, intracranial, or eye surgery or a pneumothorax should not perform spirometry, although data are limited. Spirometry requires exertion and should be avoided in patients with unstable angina or a recent myocardial infarction. Rarely, performance of a forced expiratory maneuver will precipitate acute bronchoconstriction. This seems more likely to occur when a patient's asthma or COPD is poorly controlled. Treatment includes administering inhaled albuterol and supplemental oxygen. MONITORING Office spirometry is also useful for monitoring control of asthma The National Asthma Education and Prevention Program advises the following frequencies for spirometry testing when caring for patients with asthma [60]: ● At the time of initial assessment ● After treatment is initiated and symptoms and peak flow have stabilized ● During periods of progressive or prolonged loss of asthma control ● At least every one to two years For patients with chronic obstructive pulmonary disease (COPD), repeat spirometry is advised whenever there is a substantial increase in symptoms or decrease in exercise tolerance [4,61,62]. SOCIETY GUIDELINE LINKS Links to society and government-sponsored guidelines from selected countries and regions around the world are provided separately. (See "Society guideline links: Pulmonary function testing".) SUMMARY AND RECOMMENDATIONS ● Spirometry is used to detect and monitor obstructive airway disease in patients with respiratory symptoms and risk factors. (See 'Introduction' above.) ● Office spirometers should accurately measure the forced expiratory volume in one second (FEV1) and either the forced vital capacity (FVC) or the forced expiratory volume in six seconds (FEV6). Calibration checks should be performed daily with a three-liter syringe. (See 'Quality control' above.) ● Since poorly performed maneuvers often mimic disease patterns, the clinician and technician must learn to recognize the patterns of unacceptable efforts ( figure 2). (See 'Quality control' above.) ● An acceptable maneuver requires a sharp rise in the flow volume curve to the peak flow and an expiratory duration that reaches a plateau in expired volume or a duration of 15 seconds; however, if FEV6 is being measured instead of FVC, then only a duration of at least six seconds is required. At least three good quality maneuvers should be performed. (See 'Quality control' above.) ● Report the highest FVC and the highest FEV1 from three spirometric maneuvers, even if they are derived from different maneuvers. The fifth percentile lower limit of normal (LLN) for FEV1/FVC is preferred over the fixed threshold of 0.7 because it reduces the misclassification rate for detecting airway obstruction. (See 'Choosing the best values' above and 'Ratio of FEV1/FVC' above.) ● Reduction of the FEV1/FVC suggests airway obstruction ( algorithm 1) (see 'Interpretation' above). If FEV1/FVC is low but FEV1 is normal, this is still considered obstruction but may also represent dysanapsis, which can be a normal variant. ● A normal FEV1/FVC but reduced FVC suggests restriction, which must be confirmed by lung volume measurement. If restriction is not confirmed, this pattern has been termed "nonspecific". If lung volumes are not available, a low FEV1 and normal FEV1/FVC has also been referred to as Preserved Ratio Impaired Spirometry, or PRISm. ● The FEV1/FEV6 can be helpful instead of FEV1/FVC when the FVC maneuvers are routinely stopped after six seconds. (See 'Ratio of FEV1/FVC' above.) ● The FEV1 should be used for determination of the degree of impairment and for serial comparisons in obstructive and mixed obstructive-restrictive disorders. It may also be used in the absence of lung volumes for grading previously confirmed restriction. FEV1/FVC ratio is not useful for gauging severity of ventilatory impairment, since the FVC often decreases with increasing obstruction due to air trapping or premature termination of exhalation ( ● algorithm 1). (See 'Interpretation' above.) Office spirometry results should be verified by formal testing in a pulmonary function test (PFT) laboratory when important clinical decisions will be made based on the results. (See 'Interpretation' above.) ● Despite the potential benefits, ongoing barriers to office spirometry implementation include primary care provider uncertainty regarding the benefits of testing, lack of resources, and lack of confidence in spirometric interpretation. ACKNOWLEDGMENT The UpToDate editorial staff acknowledges Paul Enright, MD, who contributed to earlier versions of this topic review. Use of UpToDate is subject to the Terms of Use. REFERENCES 1. Enright P, Quanjer P. Don't diagnose mild COPD without confirming airway obstruction after an inhaled bronchodilator. COPD 2007; 4:89. 2. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest 2007; 132:403. 3. Lee TA, Bartle B, Weiss KB. Spirometry use in clinical practice following diagnosis of COPD. Chest 2006; 129:1509. 4. US Preventive Services Task Force (USPSTF), Siu AL, Bibbins-Domingo K, et al. Screening for Chronic Obstructive Pulmonary Disease: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315:1372. 5. Ruppel GL, Carlin BW, Hart M, Doherty DE. Office Spirometry in Primary Care for the Diagnosis and Management of COPD: National Lung Health Education Program Update. Respir Care 2018; 63:242. 6. Enright PL, Studnicka M, Zielinski J. Spirometry to detect and manage COPD and asthma in the primary care setting. Eur Respir Mon 2005; 31:1. 7. Walters JA, Hansen EC, Johns DP, et al. A mixed methods study to compare models of spirometry delivery in primary care for patients at risk of COPD. Thorax 2008; 63:408. 8. Lusuardi M, De Benedetto F, Paggiaro P, et al. A randomized controlled trial on office spirometry in asthma and COPD in standard general practice: data from spirometry in Asthma and COPD: a comparative evaluation Italian study. Chest 2006; 129:844. 9. Levy ML, Quanjer PH, Booker R, et al. Diagnostic spirometry in primary care: Proposed standards for general practice compliant with American Thoracic Society and European Respiratory Society recommendations: a General Practice Airways Group (GPIAG)1 document, in association with the Association for Respiratory Technology & Physiology (ARTP)2 and Education for Health3 1 www.gpiag.org 2 www.artp.org 3 www.educationforhealth.org.uk. Prim Care Respir J 2009; 18:130. 10. Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019; 200:e70. 11. United Kingdom National Screening Committee guidelines on COPD screening: https://v iew-health-screening-recommendations.service.gov.uk/document/329/download (Acces sed on February 24, 2023). 12. American Thoracic Society. Pulmonary Function Laboratories: Advice Regarding COVID-1 9. https://www.thoracic.org/professionals/clinical-resources/disease-related-resources/p ulmonary-function-laboratories.php (Accessed on March 31, 2020). 13. Wilson KC, Kaminsky DA, Michaud G, et al. Restoring Pulmonary and Sleep Services as the COVID-19 Pandemic Lessens. From an Association of Pulmonary, Critical Care, and Sleep Division Directors and American Thoracic Society-coordinated Task Force. Ann Am Thorac Soc 2020; 17:1343. 14. Ferguson GT, Enright PL, Buist AS, Higgins MW. Office spirometry for lung health assessment in adults: A consensus statement from the National Lung Health Education Program. Chest 2000; 117:1146. 15. Culver BH, Graham BL, Coates AL, et al. Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am J Respir Crit Care Med 2017; 196:1463. 16. Townsend MC, Hankinson JL, Lindesmith LA, et al. Is my lung function really that good? Flow-type spirometer problems that elevate test results. Chest 2004; 125:1902. 17. Liistro G, Vanwelde C, Vincken W, et al. Technical and functional assessment of 10 office spirometers: A multicenter comparative study. Chest 2006; 130:657. 18. Schermer TR, Verweij EH, Cretier R, et al. Accuracy and precision of desktop spirometers in general practices. Respiration 2012; 83:344. 19. Hegewald MJ, Gallo HM, Wilson EL. Accuracy and Quality of Spirometry in Primary Care Offices. Ann Am Thorac Soc 2016; 13:2119. 20. Leone N, Courbon D, Thomas F, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med 2009; 179:509. 21. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26:319. 22. Enright PL. Office spirometry. In: Atlas of office procedures, 1999. Vol 2, p.299. 23. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948. 24. Vandevoorde J, Verbanck S, Schuermans D, et al. Forced vital capacity and forced expiratory volume in six seconds as predictors of reduced total lung capacity. Eur Respir J 2008; 31:391. 25. Iyer VN, Schroeder DR, Parker KO, et al. The nonspecific pulmonary function test: longitudinal follow-up and outcomes. Chest 2011; 139:878. 26. Swanney MP, Jensen RL, Crichton DA, et al. FEV(6) is an acceptable surrogate for FVC in the spirometric diagnosis of airway obstruction and restriction. Am J Respir Crit Care Med 2000; 162:917. 27. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2022; 60. 28. Quanjer PH, Pretto JJ, Brazzale DJ, Boros PW. Grading the severity of airways obstruction: new wine in new bottles. Eur Respir J 2014; 43:505. 29. Miller MR, Quanjer PH, Swanney MP, et al. Interpreting lung function data using 80% predicted and fixed thresholds misclassifies more than 20% of patients. Chest 2011; 139:52. 30. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40:1324. 31. Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med 2008; 177:253. 32. Jakeways N, McKeever T, Lewis SA, et al. Relationship between FEV1 reduction and respiratory symptoms in the general population. Eur Respir J 2003; 21:658. 33. Hnizdo E, Glindmeyer HW, Petsonk EL, et al. Case definitions for chronic obstructive pulmonary disease. COPD 2006; 3:95. 34. Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax 2008; 63:1046. 35. García-Rio F, Soriano JB, Miravitlles M, et al. Overdiagnosing subjects with COPD using the 0.7 fixed ratio: correlation with a poor health-related quality of life. Chest 2011; 139:1072. 36. Çolak Y, Afzal S, Nordestgaard BG, et al. Young and middle-aged adults with airflow limitation according to lower limit of normal but not fixed ratio have high morbidity and poor survival: a population-based prospective cohort study. Eur Respir J 2018; 51. 37. Smith BM, Kirby M, Hoffman EA, et al. Association of Dysanapsis With Chronic Obstructive Pulmonary Disease Among Older Adults. JAMA 2020; 323:2268. 38. Vameghestahbanati M, Kirby M, Maltais F, et al. Dysanapsis and the Spirometric Response to Inhaled Bronchodilators. Am J Respir Crit Care Med 2021; 204:997. 39. Forno E, Weiner DJ, Mullen J, et al. Obesity and Airway Dysanapsis in Children with and without Asthma. Am J Respir Crit Care Med 2017; 195:314. 40. Wan ES, Balte P, Schwartz JE, et al. Association Between Preserved Ratio Impaired Spirometry and Clinical Outcomes in US Adults. JAMA 2021; 326:2287. 41. Marott JL, Ingebrigtsen TS, Çolak Y, et al. Trajectory of Preserved Ratio Impaired Spirometry: Natural History and Long-Term Prognosis. Am J Respir Crit Care Med 2021; 204:910. 42. Zheng J, Zhou R, Zhang Y, et al. Preserved Ratio Impaired Spirometry in Relationship to Cardiovascular Outcomes: A Large Prospective Cohort Study. Chest 2023; 163:610. 43. Jing JY, Huang TC, Cui W, et al. Should FEV1/FEV6 replace FEV1/FVC ratio to detect airway obstruction? A metaanalysis. Chest 2009; 135:991. 44. Jensen RL, Crapo RO, Enright P. A statistical rationale for the use of forced expired volume in 6 s. Chest 2006; 130:1650. 45. Bellia V, Sorino C, Catalano F, et al. Validation of FEV6 in the elderly: correlates of performance and repeatability. Thorax 2008; 63:60. 46. Vollmer WM, Gíslason T, Burney P, et al. Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J 2009; 34:588. 47. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159:179. 48. Janson C, Malinovschi A, Amaral AFS, et al. Bronchodilator reversibility in asthma and COPD: findings from three large population studies. Eur Respir J 2019; 54. 49. Coates AL, Wanger J, Cockcroft DW, et al. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. Eur Respir J 2017; 49. 50. Buffels J, Degryse J, Decramer M, Heyrman J. Spirometry and smoking cessation advice in general practice: a randomised clinical trial. Respir Med 2006; 100:2012. 51. Enright P. Does screening for COPD by primary care physicians have the potential to cause more harm than good? Chest 2006; 129:833. 52. Bize R, Burnand B, Mueller Y, et al. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Syst Rev 2012; 12:CD004705. 53. Wilt TJ, Niewoehner D, Kane RL, et al. Spirometry as a motivational tool to improve smoking cessation rates: a systematic review of the literature. Nicotine Tob Res 2007; 9:21. 54. Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ 2008; 336:598. 55. Boros PW, Enright PL, Quanjer PH, et al. Impaired lung compliance and DL,CO but no restrictive ventilatory defect in sarcoidosis. Eur Respir J 2010; 36:1315. 56. Joo MJ, Au DH, Fitzgibbon ML, et al. Determinants of spirometry use and accuracy of COPD diagnosis in primary care. J Gen Intern Med 2011; 26:1272. 57. Poels PJ, Schermer TR, Akkermans RP, et al. General practitioners' needs for ongoing support for the interpretation of spirometry tests. Eur J Gen Pract 2007; 13:16. 58. Schermer TR, Smeele IJ, Thoonen BP, et al. Current clinical guideline definitions of airflow obstruction and COPD overdiagnosis in primary care. Eur Respir J 2008; 32:945. 59. Yamada J, Lam Shin Cheung J, Gagne M, et al. Barriers and Enablers to Objective Testing for Asthma and COPD in Primary Care: A Systematic Review Using the Theoretical Domains Framework. Chest 2022; 161:888. 60. National Asthma Education and Prevention Program: Expert panel report III: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute, 2007. (NIH publication no. 08-4051). Full text available online: www.nhlb i.nih.gov/guidelines/asthma/asthgdln.htm (Accessed on August 12, 2009). 61. Qaseem A, Snow V, Shekelle P, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2007; 147:633. 62. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Di agnosis, Management and Prevention of chronic obstructive pulmonary disease: 2020 R eport. http://www.goldcopd.org (Accessed on December 16, 2020). Topic 6968 Version 29.0 GRAPHICS Technique for performing spirometry Unlike most other medical tests in which the patient remains passive, accurate spirometry requires a coordinated maximum effort. The technician should instruct and encourage the patient to perform the breathing maneuvers in three phases: Phase 1: coach the patient to take as deep a breath as possible; Phase 2: loudly prompt the patient to BLAST out the air into the spirometer; Phase 3: encourage the patient to continue exhaling for several more seconds. Graphic 55460 Version 1.0 Unacceptable spirometry Flow-volume curve patterns from unacceptable forced vital capacity maneuvers. Curve A (red) hesitating start; curve B (blue) submaximal blast (poor peak flow effort); curve C (green) excessive coughing at the beginning of the maneuver; curve D (orange) premature termination of effort. FVC: forced vital capacity. Graphic 57832 Version 2.0 Flow-volume curve variations Flow-volume curves from (A) a healthy person or from patients with (B) severe obstruction (emphysema), (C) severe restriction from interstitial disease (radiation fibrosis), (D) fixed central airway stenosis (or variable intrathoracic airway obstruction), and (E) poor effort. Graphic 65057 Version 2.0 Grading system for repeatability of FEV1 and FVC (graded separately) Grade Number of measurements Repeatability: Age >6 years Repeatability: Age ≤6 years* A ≥3 acceptable Within 0.150 L Within 0.100 L* B 2 acceptable Within 0.150 L Within 0.100 L* C ≥2 acceptable Within 0.200 L Within 0.150 L* D ≥2 acceptable Within 0.250 L Within 0.200 L* E ≥2 acceptable >0.250 L >0.200 L* or 1 acceptable N/A N/A U 0 acceptable and ≥1 usable N/A N/A F 0 acceptable and 0 usable N/A N/A The repeatability grade is determined for the set of prebronchodilator maneuvers and the set of post-bronchodilator maneuvers separately. The repeatability criteria are applied to the differences between the two largest FVC values and the two largest FEV1 values. Grade U indicates that only usable but not acceptable measurements were obtained. Although some maneuvers may be acceptable or usable at grading levels lower than A, the overriding goal of the operator must be to always achieve the best possible testing quality for each patient. FEV1: Forced expiratory volume in one second; FVC: forced vital capacity; N/A: not applicable. * Or 10% of the highest value, whichever is greater; applies for age 6 years or younger only. Reprinted with permission of the American Thoracic Society. Copyright © 2020 American Thoracic Society. All rights reserved. From: Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019; 200:e70. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society. Graphic 129852 Version 1.0 Classification and grading of ventilatory impairments based on spirometry [1 FEV1: forced expiratory volume in one second; FVC: forced vital capacity; LLN: lower limit of normal, the 5th percentile. * Low refers to levels below the 5th percentile, or a z-score <–1.645; absolute values are not used due to changes in spirometry with age and other factors. ¶ A reduced FVC does not prove a restrictive process. Confirmation of restriction requires evaluation of lung volumes in a pulmonary function laboratory (ie, total lung capacity z-score <–1.645 or below fifth percentile). Δ A reduced FVC with normal FEV1/FVC and lung-volumes is a "nonspecific" pattern that may be followed over time. One-third of patients with nonspecific patterns develop obstructive or restrictive disease in th next three years. ◊ Many patients with reduced FEV1/FVC and low FVC have simple obstruction with air-trapping or failure to complete exhalation. § The severity of obstructive and mixed obstructive/restrictive ventilatory impairments are physiologicall graded by decrement in FEV1. Patients with restriction should have restrictive impairment confirmed and graded based on total lung capacity, but may be monitored by changes in FEV1. FEV1 may also be used a an alternative method to grade severity of confirmed restriction when only spirometry or % predicted values are available. ¥ Z-score is the preferred method for grading severity based on 2022 European Respiratory Society/American Thoracic Society (ERS/ATS) guidelines because it reduces bias due to age, sex, and othe factors. Some spirometry software continues to report percent predicted, so we also include categorization based on this reporting method. The percent predicted severity classification has been adapted from earlier guidelines and modernized by reducing the number of distinct categories. References: 1. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2022; 60:2101499. 2. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948. Graphic 139645 Version 3.0 Contributor Disclosures David A Kaminsky, MD Other Financial Interest: MGC Diagnostics Inc [Speaker/faculty cardiorespiratory diagnostics course]. All of the relevant financial relationships listed have been mitigated. Meredith C McCormack, MD, MHS Consultant/Advisory Boards: Aridis [Pulmonary function testing]; Boehringer Ingelheim [COPD and asthma]; Celgene [Asthma]; GlaxoSmithKline [Chronic obstructive pulmonary disease]; MGC Diagnostics [Pulmonary function testing]; NDD Medical Technologies [Pulmonary function testing]. All of the relevant financial relationships listed have been mitigated. Paul Dieffenbach, MD No relevant financial relationship(s) with ineligible companies to disclose. Contributor disclosures are reviewed for conflicts of interest by the editorial group. When found, these are addressed by vetting through a multi-level review process, and through requirements for references to be provided to support the content. Appropriately referenced content is required of all authors and must conform to UpToDate standards of evidence. Conflict of interest policy