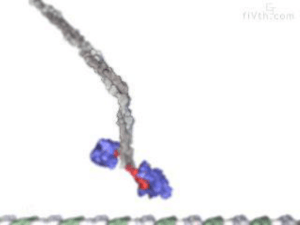
Non-Covalent Interactions and Its Importance Non-covalent interactions are the forces that hold molecules together without involving the sharing or transfer of electrons. They are essential for many biological processes, such as protein folding, DNA replication, enzyme catalysis, and cell signaling. In this post, I will explain what non-covalent interactions are, how they differ from covalent bonds, and why they are important for life. Non-covalent interactions can be classified into four main types: electrostatic interactions, hydrogen bonds, van der Waals forces, and hydrophobic interactions. Electrostatic interactions are the attraction or repulsion between charged groups or molecules. For example, salt dissolves in water because the positive sodium ions are attracted to the negative oxygen atoms of water, and the negative chloride ions are attracted to the positive hydrogen atoms of water. Hydrogen bonds are a special type of electrostatic interaction that occurs when a hydrogen atom is covalently bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom. For example, water molecules form hydrogen bonds with each other because the oxygen atom has a partial negative charge, and the hydrogen atoms have a partial positive charge. Van der Waals forces are the weak attraction between molecules that are close together due to fluctuations in their electron clouds. For example, geckos can cling to walls and ceilings because their feet have tiny hairs that interact with the surface molecules through van der Waals forces. Hydrophobic interactions are the tendency of non-polar molecules or groups to cluster together in water to minimize their contact with water molecules. For example, oil and water do not mix because oil is non-polar, and water is polar. Non-covalent interactions are weaker than covalent bonds, which involve the sharing or transfer of electrons between atoms. Covalent bonds are typically measured in hundreds of kilojoules per mole (kJ/mol), while non-covalent interactions are typically measured in tens of kilojoules per mole (kJ/mol) or less. However, non-covalent interactions can be additive and cooperative, meaning that many weak interactions can collectively produce a strong effect. For example, a single hydrogen bond between two water molecules is weak, but many hydrogen bonds between many water molecules create a strong network that gives water its unique properties. Non-covalent interactions are important for life because they allow molecules to interact with each other in specific and reversible ways. For example, proteins are made of amino acids that are linked by covalent bonds to form a long chain. However, the shape and function of a protein depend on how the chain folds into a three-dimensional structure, which is determined by the non-covalent interactions between different parts of the chain and the surrounding environment. Non-covalent interactions allow proteins to fold into different shapes depending on the conditions, such as temperature, pH, or the presence of other molecules. Non-covalent interactions also allow proteins to bind to other molecules, such as substrates, inhibitors, cofactors, or receptors, and regulate their activity. Similarly, DNA is made of nucleotides that are linked by covalent bonds to form a long chain. However, the information stored in DNA depends on how the chain forms a double helix structure, which is stabilized by hydrogen bonds between complementary base pairs. Non-covalent interactions allow DNA to unzip and replicate itself during cell division, or to transcribe itself into RNA during gene expression. In conclusion, non-covalent interactions are the forces that hold molecules together without involving the sharing or transfer of electrons. They are essential for many biological processes, such as protein folding, DNA replication, enzyme catalysis, and cell signaling. Non-covalent interactions can be classified into four main types: electrostatic interactions, hydrogen bonds, van der Waals forces, and hydrophobic interactions. Non-covalent interactions are weaker than covalent bonds, but they can be additive and cooperative to produce a strong effect. Non-covalent interactions allow molecules to interact with each other in specific and reversible ways.