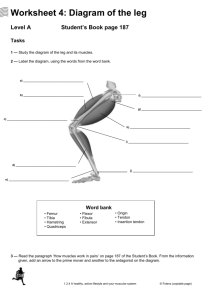
Contents MEDICAL RADIOLOGY Diagnostic Imaging Editors: A. L. Baert, Leuven M. Knauth, Göttingen K. Sartor, Heidelberg I Contents Stefano Bianchi · Carlo Martinoli Ultrasound of the Musculoskeletal System With Contributions by L. E. Derchi · G. Rizzatto · M. Valle · M. P. Zamorani Foreword by A. L. Baert Introduction by I. F. Abdelwahab With 1111 Figures in 3669 Separate Illustrations, 286 in Color 123 III IV Contents Stefano Bianchi, MD Privat-docent Université de Genève Consultant Radiologist Fondation et Clinique des Grangettes 7, ch. des Grangettes 1224 Genève Switzerland Carlo Martinoli, MD Associate Professor of Radiology Cattedra “R” di Radiologia - DICMI Università di Genova Largo Rosanna Benzi, 8 16132 Genova Italy Medical Radiology · Diagnostic Imaging and Radiation Oncology Series Editors: A. L. Baert · L. W. Brady · H.-P. Heilmann · M. Knauth · M. Molls · C. Nieder · K. Sartor Continuation of Handbuch der medizinischen Radiologie Encyclopedia of Medical Radiology Library of Congress Control Number: 2003057335 ISBN 978-3-540-42267-9 Springer Berlin Heidelberg New York This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitations, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer-Verlag. Violations are liable for prosecution under the German Copyright Law. Springer is part of Springer Science+Business Media http//www.springer.com ¤ Springer-Verlag Berlin Heidelberg 2007 Printed in Germany The use of general descriptive names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Product liability: The publishers cannot guarantee the accuracy of any information about dosage and application contained in this book. In every case the user must check such information by consulting the relevant literature. Medical Editor: Dr. Ute Heilmann, Heidelberg Desk Editor: Ursula N. Davis, Heidelberg Production Editor: Kurt Teichmann, Mauer Cover-Design and Typesetting: Verlagsservice Teichmann, Mauer Printed on acid-free paper – 21/3151xq – 5 4 3 2 1 0 Contents D e dicat ion To Maria Pia, Elena and Eugenio, the loves of my life – S.B. To Maura and Roberto, for their love, support and forbearance – C.M. V Contents Series Editor’s Foreword Modern ultrasound has now acquired a very important role in the spectrum of imaging modalities available for the study of the musculoskeletal system. This technique has become an indispensable tool in the clinical management of sports injuries, degenerative and traumatic lesions of the articulations and periarticular soft tissues, as well as – in certain circumstances – clinical management of the bones. Stefano Bianchi and Carlo Martinoli are internationally renowned leaders in their field who, as a long-standing and remarkable team, have acquired an exceptional expertise. This is amply demonstrated by their numerous and outstanding contributions to the literature, as well as by their worldwide lecturing and participation in teaching seminars on musculoskeletal ultrasound. Although some additional chapters have been authored by other well-known ultrasound specialists, most of the chapters have been prepared and written by Stefano Bianchi and Carlo Martinoli. This feature is a guarantee for uniformity and homogeneity of style, concept and presentation throughout the whole volume. An update of our knowledge and the latest insights into this subject are provided for each anatomic area of the musculoskeletal system. I would like to congratulate the authors most sincerely for their superb efforts in preparing this remarkable volume, which comprehensively covers the extensive and varied spectrum of musculoskeletal diseases, in the management of which ultrasound can make an important, if not essential, contribution to better clinical diagnosis and better guidance of therapy. Moreover, this work is superbly and abundantly illustrated by numerous anatomical drawings, photographs and ultrasound images, all realized with state-of-the-art and high-end equipment. These well chosen illustrations strongly enhance the didactic and educational value of this book. Without doubt, this outstanding volume will be of great value to certified general and musculoskeletal radiologists, radiologists in training, as well as orthopedic surgeons and rheumatologists in their daily clinical practice. I am confident that it will meet with the same success among readers as the previous volumes published in this series. Leuven Albert L. Baert VII Contents Foreword Over the last 15 years, musculoskeletal ultrasonography has become an important imaging modality used in sports medicine, joint disorders, and rheumatology. With the rapid development and sophistication of this modality, essential information for a better understanding of the pathophysiologic assessment of many disorders has been established. This, in turn, has aided both in making crucial decisions regarding surgical intervention and in monitoring the effects of therapy. Equally important is the ready availability, affordability, speed, and diagnostic accuracy of ultrasonography. Ultrasound of the Musculoskeletal System is an invaluable text comprising 19 chapters and approximately one thousand pages and figures. The authors have designed unique schematic drawings which aid in better understanding the anatomy of the body part in terms of its sonographic characteristics discussed in each chapter. Correlations of ultrasonography with CT and MRI findings are applied throughout the text, demonstrating not only the exact indications for its use, but also highlighting its limitations. Technical advances continue to improve the utility of ultrasonography as a diagnostic technique in musculoskeletal imaging. Drs. Bianchi and Martinoli have successfully capitalized on the collaboration between radiologists, orthopedists, and rheumatologists as exemplified by their representative images and correlative discussions. Many of the techniques described in the text have been pioneered or improved by Dr. Bianchi and Dr. Martinoli. This text should become a key library reference source for radiologists, orthopedists, and rheumatologists. It is extremely readable and its illustrations help in the clarification of points made in the text. Ultrasound of the Musculoskeletal System is the most comprehensive work of its kind to date. It establishes a higher standard in musculoskeletal imaging and should remain a classic for years to come. Ibrahim Fikry Abdelwahab, MD Formerly Professor of Radiology The Mount Sinai School of Medicine, Weill Medical College, Cornell University, and New York Medical College IX Contents Preface The use of ultrasound in the assessment of the musculoskeletal system started many years ago. Nevertheless, the continuing innovations in instrumentation and the advances in clinical applications suggest that we have only just started to “peel the onion” in this field. This fact has also been reflected in the length of time needed to prepare this book. The project started some five years ago, with an approximate estimation of 300 pages to cover the whole field. As our personal experience and the literature expanded as a result of new technological improvements, more and more information was added, resulting in a final book size of over 1000 pages. This textbook can be considered the result of a continuing cooperation of two friends and colleagues who started their common practice many years ago publishing scientific papers and teaching at courses and congresses, and then decided to put their experience into a monograph with the aim of sharing their own knowledge and, most importantly, their enthusiasm for this wonderful imaging technique. Given these considerations, this book aims to cover the whole of this field, thus providing both help to those who are already expert in ultrasound and want to acquire further knowledge and skills in this special area, as well as an introduction to beginners, irrespective of whether they are musculoskeletal radiologists, rheumatologists, orthopaedic surgeons, or in-training residents, among others. Since many of the difficulties encountered while learning musculoskeletal ultrasound result from an inability to correctly interpret the images, many figure captions, references for probe placement, oneto-one correlations with clinical photographs, anatomical and operative specimens, as well as images obtained with other modalities were systematically added to the ultrasound illustrations. Schematic drawings have also been extensively used throughout the chapters to emphasize depiction of anatomy, pathomechanisms and biomechanics underlying the disease processes. It was our deliberate intention to compile the book with a uniform style throughout. This is the reason why most of the chapters have been written by the two editors and by a relatively small numbers of authors who have worked or continue to work with the editors. The book begins with an introductory section on the instrumentation and general aspects of musculoskeletal ultrasound, followed by a systematic overview of the applications of this technique in the different areas of the upper and lower extremities. An additional final section devoted to both interventional and pediatric applications has been included. With regard to certain clinical applications, there is still considerable difference of opinion on the role of musculoskeletal ultrasound as compared to that of other imaging modalities, such as magnetic resonance imaging. Obviously, there is a “bias” towards the use of ultrasound in this text. However, every effort has been made to provide accurate accounts of present knowledge and experience, as well as to indicate the most advanced references of emerging applications. XI XII Preface A new textbook of this size inevitably contains errors and weaknesses -- we welcome corrections and suggestions for future editions. Meanwhile, happy reading! “Nulla res me delectabit, licet sit eximia et salutaris, quam mihi uni sciturus sum”. (Seneca, Epist. 6,4) “I might not be delighted with anything, even eminent and beneficial, if I am the only one to know it”. (Seneca, Epist. 6,4) Genève Genova Stefano Bianchi Carlo Martinoli Acknowledgments We are deeply indebted to the many colleagues who have provided information and illustrations of rare pathology, operative and anatomical views, as well as to the models who helped us to obtain correlative photos of anatomical landmarks. These colleagues are listed below. Special thanks go to Alberto Tagliafico (Genova, Italy) for the task of checking the entire book for errors, to the „Subject Index team“, including Enrico Capaccio, Maria Beatrice Damasio, Nunzia Pignataro, Nicola Stagnaro, Alberto Tagliafico and Simona Tosto, and to Jane Farrell for copyediting the manuscript and correcting language errors. Finally, it is a pleasure to acknowledge the skillful help, pleasant cooperation, and patience of the publisher’s staff during the five years of intense work it has taken to prepare this textbook. Elena and Eugenio Bianchi (Geneva, Switzerland) Silvio Boero (Genova, Italy) Gianni Cicio (Genova, Italy) Giovanni Crespi (Genova, Italy) Marino Delmi (Geneva, Switzerland) Jean H Fasel (Geneva, Switzerland) Sergio Gennaro (Genova, Italy) Maurizio Giunchedi (Lavagna, Italy) Claudio Guido Mazzola (Genova, Italy) Vincenzo Migaleddu (Sassari, Italy) Roberto Pesce (Genova, Italy) Nicolò Prato (Genova, Italy) Fabio Pretolesi (Genova, Italy) Maurizio Rubino (Genova, Italy) Federico Santolini (Genova, Italy) Giovanni Serafini (Pietra Ligure, Italy) Stefano Simonetti (Genova, Italy) Enrico Talenti (Padova, Italy) Paolo Tomà (Genova, Italy) Bruno Valle (Rapallo, Italy) Marzia Venturini (Genova, Italy) The Staff of the Institut de Radiologie, Clinique des Grangettes, (Geneva, Switzerland) and the Cattedra di Radiologia “R” – DICMI, Università di Genova (Genova, Italy). Contents Contents Intrumentation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 1 Technical Requirements Lorenzo E. Derchi and Giorgio Rizzatto . . . . . . . . . . . . . . . . . . . . . . . 3 General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17 2 Skin and Subcutaneous Tissue Maura Valle and Maria Pia Zamorani. . . . . . . . . . . . . . . . . . . . . . . . . 19 3 Muscle and Tendon Maura Valle and Maria Pia Zamorani. . . . . . . . . . . . . . . . . . . . . . . . . 45 4 Nerve and Blood Vessels Maura Valle and Maria Pia Zamorani. . . . . . . . . . . . . . . . . . . . . . . . . 97 5 Bone and Joint Maura Valle and Maria Pia Zamorani. . . . . . . . . . . . . . . . . . . . . . . . . 137 Individual Anatomic Sites . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 187 Upper Limb . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 187 6 Shoulder Stefano Bianchi and Carlo Martinoli . . . . . . . . . . . . . . . . . . . . . . . . 189 7 Arm Carlo Martinoli and Stefano Bianchi . . . . . . . . . . . . . . . . . . . . . . . . 333 8 Elbow Stefano Bianchi and Carlo Martinoli . . . . . . . . . . . . . . . . . . . . . . . . 349 9 Forearm Carlo Martinoli and Stefano Bianchi . . . . . . . . . . . . . . . . . . . . . . . . 409 10 Wrist Stefano Bianchi and Carlo Martinoli . . . . . . . . . . . . . . . . . . . . . . . . 425 11 Hand Carlo Martinoli and Stefano Bianchi . . . . . . . . . . . . . . . . . . . . . . . . 495 XIII XIV Contents Lower Limb . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 549 12 Hip Carlo Martinoli and Stefano Bianchi . . . . . . . . . . . . . . . . . . . . . . . . 551 13 Thigh Stefano Bianchi and Carlo Martinoli) . . . . . . . . . . . . . . . . . . . . . . . . 611 14 Knee Carlo Martinoli and Stefano Bianchi) . . . . . . . . . . . . . . . . . . . . . . . . 637 15 Leg Stefano Bianchi and Carlo Martinoli) . . . . . . . . . . . . . . . . . . . . . . . . 745 16 Ankle Carlo Martinoli and Stefano Bianchi) . . . . . . . . . . . . . . . . . . . . . . . . 773 17 Foot Stefano Bianchi and Carlo Martinoli) . . . . . . . . . . . . . . . . . . . . . . . . 835 Interventional Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 889 18 US-Guided Interventional Procedures Stefano BianchI and Maria Pia Zamorani . . . . . . . . . . . . . . . . . . . . . . 891 Pediatric Applications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 919 19 Pediatric Musculoskeletal Ultrasound Carlo Martinoli and Maura Valle . . . . . . . . . . . . . . . . . . . . . . . . . . . 921 Subject Index . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 961 List of Contributors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 975 Technical Requirements Instrumentation 1 Technical Requirements Technical Requirements Lorenzo E. Derchi and Giorgio Rizzatto 1.1.1 Transducers CONTENTS 1.1 1.1.1 1.1.1.1 1.1.1.2 1.1.1.3 1.1.2 1.1.2.1 1.1.2.2 1.1.2.3 1.1.2.4 1.1.2.5 1.1.2.6 1.1.3 Advances in US Technology 3 Transducers 3 Broadband Transducers 3 Focusing 6 Transducer Selection and Handling 6 Imaging Algorithms 7 Advances in Doppler Imaging 8 Compound Imaging 8 Extended Field-of-View Imaging 9 Steering-Based Imaging 11 Three-Dimensional Imaging 13 Elastographic Imaging 14 Ultrasound Contrast Media 14 References 15 1.1 Advances in US Technology US technology is rapidly advancing and being refined, and is aimed at both increasing image quality and opening new fields of applications. This chapter will review the main advances in US technology and address the clinical impact they have had or are likely to have in the future in the field of the musculoskeletal system. New developments in transducer technology and advances in the quality and presentation of US images will be discussed. L. E. Derchi, MD Professor of Radiology, Cattedra di Radiologia “R” - DICMI – Università di Genova, Largo Rosanna Benzi 8, 16132 Genova, Italy G. Rizzatto, MD Head of Department of Radiology, Ospedale di Gorizia, 34170 Gorizia, Italy The transducer is an essential element of US equipment, responsible for the generation of a US beam and the detection of returning echoes. It greatly influences spatial resolution, penetration and signal-to-noise ratio. In recent years, research in transducer technology has been focused on the development of piezoelectric crystals with lower acoustic impedances and greater electromechanical coupling coefficients, as well as on improving the characteristics of absorbing backing layers and quarter-wave impedance matching layers (Claudon et al. 2002). Currently, transducer arrays formed by ceramic polymer composite elements of variable shape and thickness and multilayered technology are used, leading to a more accurate shaping of US pulses in terms of frequency, amplitude, phase and length (Whittingham 1999a; Rizzatto 1999). These refinements led to the use of very short pulses and an increased bandwidth (Fig. 1.1). 1.1.1.1 Broadband Transducers One of the original objectives in designing broadband transducers was to improve axial resolution without changing the emission frequency. This is related to the fact that the shorter transmission pulses used in a broadband emission generate shorter echo pulses which can be faithfully converted into electric signals (Whittingham 1999b). Because short pulses suffer attenuation to a greater extent and are characterized by less penetration than long pulses, some specific techniques have been introduced by different manufacturers to compensate for these drawbacks, including single-pulse and multi-pulse techniques (Claudon et al. 2002). Among single-pulse techniques, the emission of a long, peculiarly shaped transmission pulse, which varies in frequency and 3 1 4 L. E. Derchi and G. Rizzatto a c b d Fig. 1.1a–d. Relationship between spatial pulse length and frequency spectrum. a,b Intensity versus time diagrams illustrate different pulse lengths (λ). Two sine wave pulses are shown lasting 2 µs (four-cycle) and 1 µs (two-cycle) respectively. c,d Corresponding Fourier power (intensity versus frequency) diagrams show the spectrum of frequencies present in the pulses shown in a and b. The bandwidth is measured between the 6 dB points on each side of the spectrum. The longer pulse in a generates a narrower bandwidth (1 MHz) than the shorter pulse (2 MHz) in b amplitude within the duration of the pulse itself, has been used instead of a simple sinusoidal pulse (Fig. 1.2). When the signal is received, a filter analyzes the signal frequencies as a short pulse, erasing the components introduced to make it long (chirp): the result is increased image penetration with an improved signal-to-noise ratio, without compromising axial resolution. Other multi-pulse techniques make use of a coded-emission mode consisting of transmission of an integrated sequence of many short, high-frequency transmission pulses which vary in terms of phase and are modulated in a code sequence. When the signal is received, the signal frequencies are compared with the transmission pulses by a matching decoding filter working at a high sampling rate. The subtraction process results in increased image penetration without loss of axial resolution or an increase in emission peak pulses (Claudon et al. 2002). Apart from advances in emission pulse technology, broadband transducers use a spectrum of frequency distribution (i.e., 12–5 MHz) instead of a single fundamental frequency (i.e., 10 MHz): the high-frequency components tend to increase the intensity maximum in the focal zone but cause a prompt decrease in intensity with depth, whereas the low-frequency components extend the penetration depth (Whittingham 1999b). In multiple-frequency imaging, the available broad bandwidth is subdivided into multiple frequency steps for transmission and reception of sound waves: these transducers enable selection of the optimal frequency range in a given scanning plane as though two or more independent transducers – each with a different center frequency – were available (Fig. 1.3). Other systems use the total transducer bandwidth for the transmitted pulse and then adjust the receiver bandwidth to lower frequencies as deeper depths are Technical Requirements a b Fig. 1.2a,b. US pulse shaping. a Intensity versus time diagram illustrates a short pulse wave (arrow) characterized by a few oscillations rapidly dampened by the backing material of the transducer. This short-duration pulse is associated with a broad bandwidth but, when transmitted through tissues, it is rapidly attenuated and absorbed resulting in a poor penetration of the US beam. b Intensity versus time diagram illustrates a chirp pulse. This pulse has a longer duration to increase the penetration of the US beam. It is not a simple sine wave: it is modulated in terms of phase and frequency to include a central component (arrow) – that a receive filter reads as a short pulse to obtain high axial resolution – and two sine queues (arrowheads) on each side of the central component to give penetration capabilities. Example of Chirped Emission (Siemens) * a b c d Fig. 1.3a–d. Multiple-frequency transducers. a,b Longitudinal US images obtained over the palmar aspect of the hand with a 18–6 MHz multiple frequency transducer by setting the center frequency at a 8 MHz and b 16 MHz respectively. Shifting on the lower frequencies of the bandwidth, penetration (large open arrows) of the field-of-view is achieved; on the other hand, the small superficial cyst (arrowheads) overlying metacarpal bone (thin white arrows) does not appear completely anechoic, subcutaneous tissue echoes are coarse and reverberation artifacts (asterisk) appear deep to the bone. Shifting the frequency band upward, a more defined echotexture is appreciated in the superficial part of the image as a result of an increased resolution. In contrast, a strong attenuation affects the deep part of the US image, which loses intensity. c,d Corresponding intensity versus frequency diagrams illustrate how the frequency band is modulated in multiple-frequency transducers. Example of “eXtreme High-Frequencies imaging” technology (Esaote) 5 6 L. E. Derchi and G. Rizzatto sampled. These systems give increased flexibility to the US examination, enabling the same transducer to change the image acquisition parameters during scanning based on the desired clinical information. In musculoskeletal imaging, this is particularly important when the study focuses on both superficial (i.e., subcutaneous tissue planes) and deep (i.e., muscle tissue layers) tissues in the same study and body area to be explored. 1.1.1.2 Focusing Reducing the width and thickness of the US beam has definite advantages in terms of contrast and spatial resolution. In modern linear-array transducers, focusing is currently not obtained by means of a fixed lens as in the old mechanical sector probes in which degrading of the image quality occurred at a short distance from the focal zone (Fig. 1.4a). Focusing is now produced electronically by activating a series of elements in the array with appropriate delays, so that the trigger pulses to the inner elements are delayed with respect to the pulses to the outer ones. In this way a curved wavefront results from constructive interference bringing the US beam toward a focus. By adjusting the values of the delays applied to the trigger pulses, the curvature of the wavefront and, therefore, the focal depth can be changed dynamically. As the resulting wavefront has the characteristics of a short excitation pulse, the axial resolution is preserved. When the pulses are received, the US machine continuously refocuses them according to the position from which the echoes come, thus giving real-time focal tracking along the depth axis: synchronization of the received signals is essential to minimize out-of-axis echo interference. An important factor influencing the lateral resolving power of the system is the dynamic aperture: this is achieved by activating variable numbers of elements dynamically to optimize focusing at many depths. As a rule, the higher the number of channels (electric pathways) involved in this process to activate the elements in a combined mode and with appropriate delays, the higher the complexity and the cost of the equipment, but the more accurately the beam can be focused. Recently, the introduction and refinement of matrix (1.5D probes) transducers led to further progress. In these transducers, the single row of long piezoelectric elements found in a conventional probe is replaced by more layers (three to seven) incorporated into a single thin layer to produce parallel rows of short elements. The slice thickness of the US image is improved by performing dynamic focusing in the elevation plane (Fig. 1.4b). This leads to better spatial and contrast resolution and reduction of partial-volume averaging artifacts (Rizzatto 1999). A less expensive alternative to 1.5D probes is the use of peculiar acoustic lenses –Hanafy lenses –placed in front of the piezoelectric elements. The Hanafy lens has non-uniform thickness and resonance properties: it produces a narrow and uniform image slice thickness and, simultaneously, a very broad bandwidth pulse. The inner portion of the lens is thinner, resonates at higher frequencies and focuses in the near field, whereas its outer portions resonate at lower frequency and are focused in both transmission and reception at the deepest part of the image providing better penetration (Claudon et al. 2002). 1.1.1.3 Transducer Selection and Handling A variety of linear-array transducers, including large (>40 mm), medium-sized (<40 mm) and small-FOV (hockey-stick-shaped) probes, are currently available in the frequency range used for musculoskeletal examinations. Selection of the most appropriate transducer primarily depends on the frequency but is also related to other factors. Hockey-stick probes are the best choice for imaging small superficial structures at sites in which the skin surface does not allow adequate contact with larger probes (i.e., soft tissues adjacent to bony prominences) or while performing dynamic maneuvers: they are, however, characterized by a restricted field-of-view which often allows only an incomplete evaluation of the structure of interest and surrounding anatomy. Compared with small transducers, high-frequency large-diameter transducers tend to have a large near-field beam width leading to a poor lateral resolution at shallow depths. Because they maintain beam shape to greater depths with less divergence of the US beam, they have the best potential for imaging deep-seated structures. During evaluation of the musculoskeletal system, probe handling has need of maximum stability over the region of interest; compression is never required, and the mobility of the probe to cover wide body areas is considerably less than in abdominal studies. Because pathologic findings may be very small in size and are often evaluated by placing the probe over curvilinear (i.e., humeral head) and irregular surfaces (i.e., cubital tunnel), stability of the transducer is a main factor Technical Requirements Fig. 1.4a,b. Elevation focusing. a Schematic drawing shows mechanical focusing of an electronic linear-array transducer (in gray) with a single row of elements (arrows) by an acoustic lens (in black). Note that focusing is applied uniformly to each crystal of the array. As shown on the right, a side view of the transducer illustrates the resulting slice thickness of the US beam. Using mechanical focusing, the beam has non uniform thickness throughout the scanning plane: it is narrow at a given depth but soon diverges away from the focal zone. b Schematic drawing shows a 1.5D array transducer made of three rows of elements (arrows) instead of a single row. Beam width reduction is achieved by electronic focusing control in the z-plane by introducing appropriate delays of crystal activation. The resultant slice thickness is uniformly narrow throughout the scanning plane a b required for high-quality examinations. In our experience, the best grip to obtain probe stability can be obtained by placing the ulnar fingers (long, ring, little) directly on the patient’s skin while holding the probe with the radial fingers (so that the probe hangs between the thumb and the index finger). This grip allows easy translation of the probe along its short axis at a given angle minimizing rotational changes. When possible, the examiner being in a lower position than the patient (i.e., the examiner seated on a chair and the patient supine on the bed at the level of the examiner’s shoulder) may also help to achieve probe stability. 1.1.2 Imaging Algorithms Recent technologic innovations in US have resulted in improved diagnostic performance for the evaluation of the musculoskeletal system, including wideband Doppler imaging, spatial compound imaging, extended field-of-view imaging, steering-based gray-scale imaging, elastography and 3D imaging. Because these new imaging procedures are many and characterized by different names depending on the manufacturer – so that considerable confusion may exist regarding how they work and how they 7 Technical Requirements Different from conventional B-mode in which the US images are obtained from a single angle of insonation (perpendicular to the transducers array), in compounding mode the digital beam-former steers the US beam at several (up to nine) steering angles during real-time acquisition rates (Claudon et al. 2002). When the signal is received, the lines of sight are rendered according to the rectangular geometry of the field-of-view of the US image. The advantages of compound mode are many, including reduction of image artifacts (e.g., speckle, clutter, noise, angle-generated artifacts), sharper delineation of tissue interfaces and better discrimination of lesions over the background as well as improvement in detail resolution and image contrast. In the musculoskeletal system, compound imaging leads to an improved delineation of structures composed of specular echoes, such as tendons and muscles (Lin et al. 2002). This derives from the fact that, when these structures are imaged, the highest echo amplitude is obtained at the point at which the US beam is perpendicular to them as a result of anisotropy (i.e., fibrillar echotexture of tendons, curved surfaces). With spatial compounding, images are generated from different view angles: therefore, the likelihood is greater that one of these angles will be perpendicular to the tendon or the muscle fibers to generate a higher echo amplitude even at insonation angles that cause anisotropy on conventional mode (Fig. 1.6a) (Lin et al. 2002). Edge shadows resulting from the boundaries of subcutaneous fat lobules, tendons, muscles, nerve fascicles, fascial planes and vessel walls are also erased because they reflect only weakly at oblique angles (Fig. 1.6b–e) (Claudon et al. 2002). Another advantage of compound imaging is reduction of speckle noise, a random artifact causing a grainy appearance of the US images as a result of scattering from tissue reflectors (Lin et al. 2002). Speckle reduction obtained by averaging frames from different angles of insonation leads to improved image definition and better signal-to-noise ratio. The resulting image appears smoother with better tissue-plane definition. Compounding with a high number of averaged frames worsens temporal resolution (Lin et al. 2002): this does not seem to be a problem in musculoskeletal US as the examination is free from respiratory and cardiac motion and, in most cases, static. In general, dynamic maneuvers during passive tendon or joint movement are not significantly affected by frame averaging. Recently, some compound mode systems have been developed using simultaneous emission of two different frequencies instead of one to improve contrast resolution (transmit frequency compound). Adaptive algo- rithms which perform real-time analysis of patterns at pixel level and refine the image by emphasizing patterns within the tissue texture and de-emphasizing artifacts and noise, can be combined with spatial compound imaging to further sharpen borders and tissue interfaces. Similarly, color B-mode imaging systems with contrast optimization (photopic imaging) can be applied to improve overall image contrast and definition of deep soft-tissue boundaries (Sofka et al. 2005). 1.1.2.3 Extended Field-of-View Imaging One of the main drawbacks of linear-array transducers to image the musculoskeletal system is the limited extension of the field-of-view (often < 4 cm wide). With these probes, displaying the full extent of an abnormality and showing its relationship with adjacent structures on a single image may be problematic: this creates inadequate reproduction of the full lesion on prints and difficulties for colleagues and the referring physician when reading the US images. Somewhat similar to the compound systems produced in the middle and late 1970s, extended field-of-view technology uses specific image registration analysis to track probe motion and reconstruct a large composite image during real-time scanning over long distances and curved body surfaces without using external positional devices. After selecting a scanning plane of interest, the examiner slides a standard probe along the skin surface in the direction of the scan plane while monitoring the image on the screen. During lateral probe motion, there is an advancing real-time portion of the image and a static portion which displays what has been scanned (Fig. 1.7). The reconstruction process is based on the fact that image features of a given frame and the next frame are very similar, except that the second image is slightly shifted or rotated relative to the first one (Weng et al. 1997). Successive frames are registered and blended with the previous ones based on an autocorrelation algorithm and an advanced parallel processing architecture requiring intense digital work. As determined on phantoms, geometric measurement of extended field-of-view US is accurate to within < 5% (Weng et al. 1997; Fornage et al. 2000). Particularly in the examination of the musculoskeletal system, this technique seems able to provide accurate data because of the absence of respiratory movements or pulsatility of large vessels (Weng et al. 1997; Barberie et al. 1998; Lin et al. 1999). Extended field-of-view imag- 9 10 L. E. Derchi and G. Rizzatto a b c d e Fig. 1.6a–e. Spatial compound imaging. a Schematic drawing illustrates the image acquisition process in compound mode. The US beam is steered out of axis providing multiple lines of sight at several angles during real-time acquisition. Signal processing renders the steered frames into a final image in real time as each new frame is acquired. With this system, a clearer delineation of borders (in black) and interfaces is obtained even when they are oriented at unfavorable angles. The acoustic shadow posterior to calcifications is usually thinner and less delineated than in the conventional mode. b,c Conventional cross-sectional b 12–5 MHz and c 17–5 MHz images of the median nerve at the mid-forearm. Deep to the flexor carpi radialis tendon (arrowheads), the nerve (arrow) appears as a rounded structure composed of many small hypoechoic dots related to the fascicles. Note how the fascicles are more clearly depicted as the frequency increases. The muscle tissue of the flexor digitorum profundus (dashed square) appears coarse and grainy. d,e Corresponding d 12–5 MHz and e 17–5 MHz compound images. The fascicles are better delineated compared with the images acquired in conventional mode. The best result is obtained with the combined use of spatial compounding and the 17–5 MHz US probe. Muscles (dashed square) exhibit a more homogeneous echotexture as a result of better suppression of speckle artifact and an increased signal-to-noise ratio. Example of SonoCT Imaging (Philips) Technical Requirements Fig. 1.7a–c. Extended field-ofview imaging. a–c Formation of a panoramic extended fieldof-view image over the gluteus minimus muscle (arrowheads). During real-time scanning, the probe is moved caudally (arrows). The box indicates where the current frame is obtained. Image frames are translated and rotated according to the estimated probe motion by means of image registration. The final panoramic image shows the whole length of the gluteus minimus from the iliac crest (Ic) to its insertion into the great trochanter (Gt). The relationships of the gluteus medius tendon (open arrows) with the gluteus minimus tendon (white arrow) are shown. The photographs at the upper left side of the figures indicate probe positioning. Example of Extended-FOV Imaging (Siemens) a b c ing can show the abnormality (most often large fluid collections, muscle injuries, tumors, etc.) in association with the appropriate landmarks, such as joints, tendons and muscles, which may even be remote from the structure of interest. Although training is important to obtain accurate images, the extended field-of-view technique contributes to an improved presentation of the US information for the referring physician (Weng et al. 1997; Barberie et al. 1998; Lin et al. 1999; Sauerbrei 1999). 1.1.2.4 Steering-Based Imaging In addition to spatial compound and Doppler systems, the beam steering function has recently been applied to B-mode imaging to obtain a parallelogram format with lateral sides parallel but oblique instead of a rectangular field-of-view. This function is obtained by activating consecutive ele- ments in the array with increasing delays so that a wavefront resulting from constructive interference sends oblique lines-of-sight along the depth axis. In musculoskeletal US, this function seems to be useful when anisotropic structures, such as tendons or ligaments, are examined with an incidence angle far from 90° due to their oblique course from surface to depth (distal biceps tendon, Achilles and supraspinatus tendon insertion, etc.). Beam steering may optimize depiction of the fibrillar echotexture in an otherwise hypoechoic tendon area, thus helping to avoid confusion between artifact and disease (Fig. 1.8). Given that many pathologies of the musculoskeletal system are larger than the small field-of-view of linear-array transducers, a steering technology (wide field-of-view) able to increase the lateral size of the image in the far field has been developed recently. The resultant trapezoid shape of the field-of-view leads to reproduction of large lesions in their full extent without the requirement for extended field-of-view algorithms (Fig. 1.9). 11 12 L. E. Derchi and G. Rizzatto Fig. 1.8a–d. Beam steering for gray-scale imaging. a,b Long-axis 14–7 MHz US images over the insertion of the Achilles tendon (white arrowheads) on the calcaneus (Ca) acquired a on conventional mode and b by steering the beam (void arrowheads) to produce an oblique wavefront. In b, note suppression (open arrow) of the artifactual hypoechoic intratendinous area (white arrow) due to steering the beam perpendicularly to the tendon insertion. c,d Correlative schematic drawings. Example of B-mode steering function (Toshiba) Ca c a Ca d b a c b d VL VM e Fig. 1.9a–e. Wide fieldof-view technology. a,b Transverse 12–5 MHz US images over the anterior thigh with c,d schematic drawing correlation in a 25-year-old patient who suffered a strain injury of the distal aponeurosis of the rectus femoris muscle resulting in an extensive peripheral hematoma (white arrows). a Using a conventional rectangular field-of-view, the hematoma cannot be displayed in its full extent: part of it (arrowheads) is out of the field-of-view of the US image. b With a trapezoidal field-of-view, the full width of the hematoma is depicted, including its more lateral portion (open arrows). Curved arrow, central aponeurosis. e Corresponding extended field-of-view imaging obtained on a transverse plane over the anterior thigh. In the panoramic view, the relationships of the muscle injury with adjacent anatomic landmarks, including the vastus lateralis (VL) and the vastus medialis (VM), are shown. Example of Wide-FOV (Philips) Technical Requirements 1.1.2.5 Three-Dimensional Imaging The improvement in fast digital computer processing and memory storage capacity has recently improved the possibility of applying 3D technology to US (Brandl et al. 1999; Wallny et al. 2000; Claudon et al. 2002). Three-dimensional acquisition can be achieved with US using either 2D conventional transducers equipped with a small electromagnetic positional sensor or dedicated “3D-volume transducers,” which are larger than standard probes and more difficult to handle but have the advantage of providing more exact assessment of each scanning plane (Fig. 1.10). These latter transducers sweep the US beam throughout the tissue volume by tilting the scan-head with a mechanized drive along the z-axis. During this procedure, serial slices are recorded resulting in a pyramid-shaped volume scan: for each slice, the angle between slices is known, minimizing distortion in the final image. Following volume scan acquisition, the monitor displays reconstructed slices according to longitudinal, transverse and coronal planes. Each plane can be oriented within the volume block for detailed analysis by parallel or rotational shifting around any of the three spatial axes (Brandl et al. 1999). Data can also be displayed as true 3D images using various rendering algorithms, including maximum intensity projection, transparent, surface and Doppler renderings (Brandl et al. 1999). Recently, volume transducers in the frequency range suitable for analysis of the musculoskeletal tissue have been introduced, opening new interesting perspectives for evaluation of a variety of disorders, including rotator cuff tears, infant hip, congenital clubfoot and bone lesions (Gerscovitch 1997; Wallny et al. 2000; Hünerbein et al. 2001). As well as dedicated systems, software programs for 3D rendering of power Doppler images are now available in many scanners, involving capture of a series of sequential images while the transducer is translated manually without the necessity for specific hardware. Although some inaccuracies occur if the motion is not uniform, the available technology seems able to produce vascular images of acceptable quality in the musculoskeletal system (Doria et al. 2000). M M M * M * a M b c * M * d Fig. 1.10a–d. Three-dimensional imaging. a Schematic drawing of a coronal view through the metatarsal bones demonstrates a conventional 2D scanning plane obtained along the x-axis (coronal) by placing the probe over the dorsal forefoot. b Corresponding drawing shows a reconstructed plane oriented over the z-axis (axial) by means of 3D technology. c,d Three-dimensional volume acquisitions over the forefoot using a high-frequency dedicated probe. Conventional US scans (upper images) reveal the metatarsal bones (M) as hyperechoic images with posterior acoustic shadowing. With 3D imaging, two reconstructed axial planes (lower images) have been obtained at the level of c the subcutaneous tissue and d the metatarsal bones according to the white bars shown in the upper images as reference. In c, the fat globules appear as confluent hypoechoic areas embedded in a homogeneous hyperechoic background; in d, the metatarsals (M) and the interosseous muscles (asterisks) are displayed in their long axis. Example of 3D-Voluson Technology (General Electric) 13 14 L. E. Derchi and G. Rizzatto 1.1.2.6 Elastographic Imaging In many clinical settings, physical examination provides essential information in detecting abnormalities and monitoring changes related to worsening or healing of disease. Manual palpation is part of the physical examination, with the aim of providing qualitative assessment of changes in tissue softness/ stiffness that often accompany pathologic states. Generally speaking, findings at palpation depend on the difference in stiffness between normal and pathologic tissues based on their histologic composition and supramicroscopic architecture. In many instances, however, the lesion may lie too deep or be too small to be detected by palpation despite a large difference in stiffness with the surrounding tissues. For these reasons interest is growing in developing methods for recognizing abnormal tissues based on shear elastic properties (Bamber 1999). US-based elastography measures tissue displacement (strains) responses to an external force on the assumption that the strain is smaller in harder than in softer tissues. The method is based on comparison of US radiofrequency waveforms obtained before and after light tissue compression with a conventional probe using a free-hand technique (Itoh et al. 2006). Analysis of strain is based on automated segmentation of continuous US images obtained during tissue compression. Color pixels are assigned to the elastographic image depending on the magnitude of strain, with a scale range from red (soft components) to blue (stiff components). In the musculoskeletal system, preliminary experience indicates that elasticity assessment may be promising to separate structures (i.e., degenerated from partially torn tendons) that are indistinguishable on gray-scale US imaging, as well as to disclose occult disease in otherwise normalappearing tissue, such as compartment syndromes (Fig. 1.11). It is obvious that lesions containing fat, fluid or synovium will be softer than fibrotic and collagen-containing disease processes. With future improvements in technology and experience, we expect that elastography will become an important tool for the diagnosis of musculoskeletal disorders in selected clinical settings. 1.1.3 Ultrasound Contrast Media The ability of US to enhance detection of blood flow with echo reflectors after the injection of a vari- ety of fluids was first described approximately 40 years ago (Gramiak and Shah 1968). Once it was found that the source of the additional intravascular echoes was related to microbubbles developing during the injection process, the pharmaceutical industry started to develop stabilized microbubble preparations to be injected into the venous system in a safe way that would cross the pulmonary capillary bed and provide vascular enhancement for the whole duration of the clinical study. The technology used has been that of encapsulated bubbles of gas, smaller in size than the red blood cells: several gases have been used, ranging from air to less diffusible drugs, such as sulfur hexafluoride or perfluorocarbons. The gas was appropriately encapsulated in phospholipid shells of different thickness and stiffness to obtain stability and duration over scanning. US contrast agents serve as an active source of sound reflectors creating an echogenic pattern in the flowing blood. In pharmacologic terms, microbubble-based contrast agents are considered “blood pool agents” until metabolized, as they are neither filtered by the kidney nor able to enter the interstitial spaces: some have recently been shown to exhibit specific uptake in the liver and spleen after their loss from the blood pool. When microbubbles are contacted by a high-intensity high-pressure US beam, they collapse producing a transient strong broadband signal; on the other hand, when the intensity of the US beam is low, microbubbles oscillate in the US field and undergo a process of resonation, rapidly contracting and expanding in response to pressure changes of the US wave, emitting a spectrum of harmonic signals. Specific US techniques have been developed to detect signals from microbubbles, including multipulse coded-emission modes and the so-called pulse or phase inversion in which consecutive pulses of opposite phase are transmitted along the same line: the signal subtraction leads to a relative increase in the nonlinear response from tissues by deleting the response from static structures which intrinsically have minor nonlinear components. In practice, two main imaging strategies are followed to optimize the microbubble response. With “destructive modes” (high mechanical index imaging), the signal derives from microbubble destruction produced by high-intensity US peaks: time intervals are needed for contrast replenishment between scans; with “non-destructive modes” (low mechanical index imaging), the harmonic response is collected from microbubble insonation at low-intensity US emission providing continuous imaging of microvessel 15 Technical Requirements Gt Gt a b Gt Gt c d Fig. 1.11a–d. Elastographic imaging. Two different patients with shoulder impingement syndrome presenting with a,b cuff tendinosis and c,d supraspinatus tendon tear. a Long-axis gray-scale 13–6 MHz US image over the supraspinatus demonstrates a slightly swollen but intact tendon (arrows) associated with thickened bursal walls (arrowheads). Both structures are hypoechoic and cannot be clearly separated. Gt, greater tuberosity. b Corresponding elastographic image helps to distinguish the bursa from the underlying tendon on the basis of its greater compressibility. c Long-axis gray-scale 13–6 MHz US image over a torn and retracted supraspinatus shows residual hypoechoic bursal tissue and fluid (arrowheads) over the humeral head. d On the elasticity image, this tissue is compressible (imaged in red): this finding may help to distinguish it from residual intact tendon fibers. Gt, greater tuberosity. Example of Real-time Tissue Elastography (Hitachi) perfusion (Claudon et al. 2002). Based on the latest advances, both techniques make use of gray-scale (and not Doppler) imaging to optimize detection of contrast enhancement. At present, the clinical use of US contrast agents is expanding but the experience is referred, in most cases, to abdominal applications. This is related to the fact that imaging of superficial tissues requires too high a transducer frequency band to induce a discrete harmonic response from the microbubbles. Recently, dedicated probes for use in contrast studies in superficial tissues and organs have overcome this limitation, leading to encouraging results in imaging arthritis and other rheumatologic conditions (see Chapter 5) (Klauser et al. 2005). References Bamber JC (1999) Ultrasound elasticity imaging: definition and technology. Eur Radiol 9:327–330 Barberie JE, Wong ADW, Cooperberg PL et al (1998) Extended field-of-view sonography in musculoskeletal disorders. AJR Am J Roentgenol 171:751–757 Brandl H, Gritzky A, Haizinger M (1999) 3D ultrasound: a dedicated system. Eur Radiol 9:331–333 Claudon M, Tranquart F, Evans DH et al (2002) Advances in ultrasound. Eur Radiol 12:7–18 Doria AS, Guarniero R, Molnar LJ et al (2000) Three-dimensional (3D) contrast-enhanced power Doppler imaging in Legg-Calvè-Perthes disease. Pediatr Radiol 30:871–874 Entrekin RR, Porter BA, Sillesen HH et al (2001) Real-time spatial compound imaging: application to breast, vascular and musculoskeletal ultrasound. Semin Ultrasound CT MR 22:50–64 16 L. E. Derchi and G. Rizzatto Fornage BD, Atkinson EN, Nock LF et al (2000) US with extended field of view: phantom-tested accuracy of distance measurements. Radiology 214:579–584 Gerscovich EO (1997) A radiologist’s guide to the imaging in the diagnosis and treatment of developmental dysplasia of the hip. II. Ultrasonography: anatomy, technique, acetabular angle measurements, acetabular coverage of femoral head, acetabular cartilage thickness, three-dimensional technique, screening of newborns, study of older children. Skeletal Radiol 26:447–456 Gramiak R, Shah PM (1968) Echocardiography of the aortic root. Invest Radiol 3:356–366 Hünerbein M, Raschke M, Khodadayan C et al (2001) Threedimensional ultrasonography of bone and soft-tissue lesions. Eur J Ultrasound 13:17–23 Itoh A, Ueno E, Tohno E et al (2006) Breast disease: clinical application of US elastography for diagnosis. Radiology 239:341–350 Klauser A, Demharter J, De Marchi A et al (2005) Contrast enhanced gray-scale sonography in assessment of joint vascularity in rheumatoid arthritis: results from the IACUS study group. Eur Radiol 15:2404–2410 Lin CD, Nazarian LN, O’Kane PL et al (2002) Advantages of real-time spatial compound sonography of the musculoskeletal system versus conventional sonography. AJR Am J Roentgenol 171:1629–1631 Lin EC, Middleton WD, Teefey SA (1999) Extended field of view sonography in musculoskeletal imaging. J Ultrasound Med 18:147–152 Rizzatto G (1999) Evolution of US transducers: 1.5 and 2D arrays. Eur Radiol 9:304–306 Sauerbrei EE (1999) Extended field-of-view sonography: utility in clinical practice. J Ultrasound Med 18:335–341 Sofka CM, Lin D, Adler RS (2005) Advantages of color B-mode imaging with contrast optimization in sonography of lowcontrast musculoskeletal lesions and structures in the foot and ankle. J Ultrasound Med 24:215–218 Wallny TA, Theuerkauf I, Schild RL et al (2000) The threedimensional ultrasound evaluation of the rotator cuff: an experimental study. Eur J Ultrasound 11:135–141 Weng L, Tirumalai AP, Lowery CM et al (1997) US extendedfield-of-view imaging technology. Radiology 203:877–880 Whittingham TA (1999a) An overview of digital technology in ultrasonic imaging. Eur Radiol 9:307–311 Whittingham TA (1999b) Broadband transducers. Eur Radiol 9:298–303 Skin and Subcutaneous Tissue General 17 Skin and Subcutaneous Tissue Skin and Subcutaneous Tissue Maura Valle and Maria Pia Zamorani CONTENTS 2.1 Histologic Considerations 19 2.2 Normal US Findings 20 2.3 2.3.1 2.3.2 2.3.2.1 2.3.2.2 2.3.2.3 2.3.2.4 2.3.2.5 2.3.3 2.3.3.1 2.3.3.2 Pathologic Findings 21 Skin Abnormalities 21 Subcutaneous Tissue Abnormalities 22 Edema 22 Cellulitis, Abscess and Necrotizing Fasciitis 23 Fatty Atrophyy 25 Traumatic Injuries 25 Foreign Bodies 27 Tumors and Tumor-Like Conditions 31 Lipomas 33 Pilomatricoma and Epidermal Inclusion (Sebaceous) Cysts 35 2.3.3.3 Hemangiomas and Vascular Malformations 36 2.3.3.4 Metastases and Lymphomas 38 References 41 2.1 Histologic Considerations From the histologic point of view, the skin varies in thickness from 1.5 to 4.0 mm and is composed of a superficial layer and a deep layer – the epidermis and the dermis, respectively (Fig. 2.1a). The epidermis is made of stratified epithelium, and can be divided into two main layers: the superficial stratum corneum, which is made of closely packed flattened dead cells, and the deep germinative zone (consisting of the stratum basale, stratum spinosum and stratum granulosum). In regions that are not subject to pressure, the epidermis is thin and hairy, whereas M. Valle, MD Staff Radiologist, Reparto di Radiologia, Istituto Scientifico fi “Giannina Gaslini”, Largo Gaslini 5, 16148 Genova, Italy M. P. Zamorani, MD Unité de Recherche et Dévelopement, Clinique des Grangettes, 7, ch. des Grangettes, 1224 Genève, Switzerland in areas undergoing attrition and local shocks (i.e., palms of the hands and soles of the feet), the skin is hairless and may thicken to an even greater extent as a result of a hypertrophied stratum corneum. Deep to the epidermis, the dermis is a thick layer containing large amounts of collagen and a rich network of vessels, lymphatics and nerve endings. It can be divided into a deep reticular layer, which is composed of bulky connective tissue, and a superficial papillary layer, which interdigitates with the base of the epidermis and provides an important mechanical and metabolic support to the overlying epidermis. Additional structures housed within the dermis are sebaceous and sweat glands, hair follicles and erector pili muscles. Deep to the dermis, the subcutaneous tissue lies between the skin and the fascia (Fig. 2.1a). It acts as a gliding plane between these structures, thus protecting deeper areas from acute and chronic trauma; it also stores fat and participates in temperature control. The subcutaneous tissue is formed by a network of connective tissue septa and fat lobules. The overall size and extent of these septa vary at different sites of the body: they may be tiny in “loose” skin or compact when the skin is firmly attached to the underlying fascia. In normal conditions, the thickness of the subcutaneous tissue varies greatly depending on the amount of fat contained within. In some areas of the body, such as the dorsal aspect of the hand, the fat is sparse, while in other regions, such as the thighs and the buttocks, it is abundant. The amount and distribution of subcutaneous fat is also related to the individual body habitus, sex and the meteorologic environment. Discrete vessels, lymphatics, sensory nerve endings and hair follicles are contained in the subcutaneous tissue. In areas where moving structures are tightly apposed, superficial “attritional” bursae separate the skin from the underlying tissues, and especially from the bone. These bursae are synovial-lined sacs tethered by dermis and periosteum. In the fingers and toes, the nails include the nail plate, the nail folds, the epidermis, the germinative matrix and the 19 2 20 M. Valle and M. P. Zamorani a * * c b Fig. 2.1a–c. Normal skin and subcutaneous tissue. a Photograph of a cadaveric cross-section of the anterior thigh demonstrates a superficial fi layer refl flecting the epidermis and dermis (black arrow), an intermediate thick layer representing fat contained fi to the quadriceps muscle, due to the in the subcutaneous tissue (double arrow) and a deep thin layer, located just superficial juxtaposed superficial fi and deep fascia (white arrow). b Corresponding transverse 17–5 MHz US image obtained in a healthy subject demonstrates the three tissue layers shown in a: the epidermis and dermis (black arrow) are homogeneously hyperechoic; flecting fat lobules (asterisks) and hyperechoic the subcutaneous tissue (double arrow) includes a hypoechoic background refl strands (arrowheads) due to connective septa; the apposed superfi ficial and quadriceps fasciae appear hyperechoic (white arrow). ficial tissues. From surface downward, note the epidermis and c Schematic drawing shows the normal architecture of the superfi dermis (1, 2); the subcutaneous tissue (3) containing fat lobules (asterisks) separated by connective tissue strands (arrowheads); the superficial fi and deep (muscle) fascia (4-5); and the muscles (6) dermis. The nail plate is similar to the stratum corneum of the skin. The proximal nail plate and the lateral folds overlie its sides. The undersurface of the nail plate is lined by squamous epithelium, which is continuous with that of the proximal nail fold and thickens at the nail root to form the germinative matrix. 2.2 Normal US Findings US of the skin is almost exclusively performed by dermatologists, who make use of dedicated equipment with ultra-high-frequency transducers working at 20–100 MHz. Although the in-plane resolution of these transducer is as high as <50 μm, the depth of field is markedly limited at such high frequencies, and is reported to be 1 mm or less (Erickson 1997). Therefore, these transducers are not suitable for a combined evaluation of the subcutaneous tissue in its full thickness. At 20 MHz, the echogenic dermis can be distinguished from the hypoechoic subcuta- neous fat and pilosebaceous units are recognizable (Fornage et al. 1993). The thick epidermis of the palm and sole can be recognized as well. In sites covered by thin hairy skin, the epidermis can be appreciated as an individual structure by means of 40 MHz frequency transducers. In aged skin, a subepidermal low-echogenic band is often appreciated as a result of increased water content. Normal skin thickness ranges have been established with US at different body sites (Fornage and Deshayes, 1986; Fornage et al. 1993). Further details on the US examination of the skin are beyond the scope of this chapter. An adequate assessment of the subcutaneous tissue can be efficiently performed be means of “less specialized” high-resolution transducers characterized by the same frequency range (5–15 MHz) appropriate for other musculoskeletal examinations. The type and frequency of the selected transducer vary depending on the region of the body to be examined. For the thin subcutaneous tissue of the dorsum of the hand and wrist, linear-array transducers working at a center frequency >7.5–10 MHz are the most appropriate. Superficial focusing capabilities and a thin 21 Skin and Subcutaneous Tissue stand-off pad are additional requirements. On the other hand, if the thick subcutaneous fat of the lateral part of the proximal thigh is the target of examination, US should be performed at lower frequencies, even as low as 5 MHz if needed, to obtain sufficient penetration for a reliable assessment. A large amount of gel that produces a homogeneous, uniform contact between probe and skin may be required to avoid formation of small air bubbles. Examination of certain body areas, such as the plantar aspect of the calcaneus, can be difficult to perform because the thickened stratum corneum can cause considerable US beam attenuation, leading to a decreased signal-to-noise ratio of the US image. The subcutaneous tissue appears at US as a discrete hypoechoic layer characterized by a hypoechoic background of fat and hyperechoic linear echoes corresponding to a web of connective septa (Fig. 2.1b). These septa run, for the most part, parallel or slightly obliquely to the skin surface. Subcutaneous veins are displayed as elongated or rounded echo-free structures that run inside the larger septa. Owing to their low blood pressure, normal veins collapse if pressure is applied over them with the probe. In selected cases, color Doppler imaging can be used to demonstrate blood flow signals within the vessels. Small sensory nerves can be appreciated as very tiny fascicular structures coursing alongside the largest superficial veins (Fig. 2.2). Both veins and sensitive nerves usually run in the deep part of the subcutaneous tissue. Knowledge of the close relationship of nerves with adjacent veins makes their detection easier: the sural nerve, for instance, can be easily detected at the posterior distal leg because it is satellite to the adjacent small saphenous vein. Lymphatics housed within the connective septa cannot be visualized with US, unless distended by fluid as in the case of subcutaneous edema. Dynamic US examination while applying either different degrees of pressure with the probe or finger palpation or manual mobilization of the skin is essential for evaluating masses, fluid collections and fibrosis of the subcutaneous tissue. 2.3 Pathologic Findings 2.3.1 Skin Abnormalities A detailed description of the US findings observed in the wide range of pathologic conditions affecting the skin is beyond the scope of this chapter. Briefly, the use of specialized 20–50 MHz transducers has been mainly proposed in the following settings: measurement of the thickness and depth of skin tumors prior to cryosurgery, laser surgery or radiotherapy; and monitoring the effects of therapy in chronic inflammatory processes, such as psoriasis (Schmid-Wendtner and Burgdorf 2005). Skin tumors appear as focal hypoechoic nodules, clearly distinguishable from the surrounding normal dermis because of the higher echogenicity of the latter. In most cases, including melanomas, the lateral boundaries of the tumor are ill defined, whereas there is a clear-cut basal demarcation. It has been reported that in the assessment of melanoma thickness the accuracy of US is comparable to that of histology. In tumor staging, the main limitations of US are related to overestimation of the tumor size due to either surrounding inflammatory infiltration Muscle a b Fig. 2.2a,b. Subcutaneous veins and nerves. a Transverse 12–5 MHz US image obtained over the posterior calf demonstrates the small saphenous vein (white arrowhead) and the adjacent sural nerve (black arrowhead) running in the deep subcutaneous tissue. Detection of the larger vein is a useful landmark for recognition of the smaller nerve. Arrows indicate the fascial plane. b Schematic drawing correlation shows subcutaneous veins (white arrowheads) and the nerve (black arrowhead) coursing in the connective spaces which separate fat lobules 22 M. Valle and M. P. Zamorani which, being hypoechoic, cannot be discriminated from neoplastic tissue, or inclusion of other structures (i.e., hair follicles and sweat glands) as part of the lesion itself. These errors are less frequent in the evaluation of advanced-stage tumors, when peritumoral inflammatory infiltration is generally less conspicuous (Fig. 2.3a). In addition, a precise demarcation of the tumor from the subcutaneous fat is often unfeasible in tumors extending deep to the dermis-subcutaneous separation plane due to their similar hypoechoic echotextures. Overall, the diagnostic value of US for staging skin tumors has been significantly downgraded in recent years and relegated to restricted use in a few specialized dermatologic centers. In contrast, in the postoperative follow-up of patients with melanoma, US has proved helpful in guiding the management strategy of the referring physician by facilitating detection of nonpalpable metastases occurring in the area of the original scar or skin graft or along the pathway of lymphatic drainage. In addition, US may add as well as in differentiating benign from malignant palpable masses by guiding definitive biopsy, and in the assessment of pharmacodynamic response to chemotherapy (Nazarian et al. 1996). Among non-neoplastic conditions, cutaneous scars appear as ill-defined focal hypoechoic bands with posterior acoustic shadowing usually extending into the subcutaneous tissue with a definite straight course (Fig. 2.3b). The examiner should be aware of the appearance of superficial scars because they * * may indicate the site and path of previous surgery or penetrating wounds. In scleroderma, the measurement of skin thickness by high-resolution US in clinically involved and non-involved areas can support an early diagnosis. In this setting, US may allow detection of the different stages of disease (Akesson et al. 1986; Scheja and Akesson 1997; Brocks et al. 2000; Clements et al. 2000). 2.3.2 Subcutaneous Tissue Abnormalities 2.3.2.1 Edema US demonstrates subcutaneous edema as a hyperechoic appearance of fat lobules. In the early stages, oedematous changes tend to involve the deep layer of the subcutaneous tissue, which becomes hypoanechoic due to fluid accumulation related to dilation of lymphatics, whereas the most superficial layers of the subcutaneous tissue retain a normal appearance (Fig. 2.4a,b). With progressive accumulation of fluid, the connective septa enlarge and become anechoic strands as a result of distension of the superficial network of lymphatic channels, until the fat lobules become individualized structures separated from one another by anechoic fluid (Fig. 2.4c-e). One should realize that the fluid that surrounds the lobules is not free but contained within dilated lymphatic * * Muscle a b Fig. 2.3a,b. Skin abnormalities. a Mycosis fungoides/Sézary syndrome. A 15-10-MHz US image over a skin papula in the anterior abdominal wall demonstrates a superfi ficial ill-defi fined hypoechoic tumor (asterisks) with signs of infiltration fi of the subcutaneous fat (arrowheads). Arrows, fascial plane. b Postoperative scar. Transverse 12–5 MHz US image over the lateral thigh in a patient who underwent previous resection for a liposarcoma shows a hypoechoic straight band (black arrow) extending with flecting a postoperative scar. Note that the hypoechoic band is surrounded by a a vertical course from the skin downward, refl peripheral hyperechoic halo (arrowheads) refl flecting fibrotic changes in the adjacent subcutaneous (asterisks) and underlying muscle (white arrow) Skin and Subcutaneous Tissue * * b * * d * c * e Fig. 2.4a–e. Subcutaneous tissue edema. a Schematic drawing illustrates the arrangement of fl fluid-fi filled dilated lymphatic channels (in black) within the subcutaneous tissue in cases of noninfl flammatory edema. Lymphatic vessels travel in the hyperechoic connective tissue septa (arrowhead) among fat lobules (asterisks). Once these vessels are distended, they make these septa thickened and hypoechoic. b Mild subcutaneous edema. Transverse 12-5-MHz US image over the pretibial region shows an increased echogenicity of fat lobules (asterisks) and fluid distention of the lymphatics running in the deep connective septa (black arrowhead). Note the normal appearance of the more superfi ficial connective septa (white arrowhead). c Transverse 17-5-MHz US extended-fi field-of-view image of the anteromedial knee with correlative d T1-weighted and e T2-weighted MR images in a patient with severe local subcutaneous tissue edema demonstrates striking enlargement and fluid distension of all septa (open arrowheads) of the subcutaneous tissue, reflecting fl overt dilation of lymphatic channels. Note the fat lobules (asterisks), which appear as individual structures separated by the intervening fl fluid. Arrow w indicates a patent superfi ficial vein channels. These findings are typically encountered in deep venous thrombosis or in local fluid collections. Graded pressure applied with the probe does not cause collapse of the anechoic strands. In selected cases, Doppler imaging can differentiate edema within the lymphatics from the adjacent enlarged subcutaneous veins. 2.3.2.2 Cellulitis, Abscess and Necrotizing Fasciitis Subcutaneous infections, which are referred to as cellulitis or panniculitis, are commonly encountered in clinical practice and properly assessed at physical examination. In most instances, the causative agents of cellulitis are group A Streptococcus pyogenes or Staphylococcus aureus. In these cases, US may have an important diagnostic value, especially for differentiating cellulitis from an abscess and distinguishing the latter from other softtissue masses (Chau and Griffith 2005). US can stage local spread of infection to deep tissue layers (involvement of muscles, bursae, tendon sheaths and joints), and can identify possible causative factors (e.g., foreign bodies, retained gauzes). In addition, it provides accurate guidance for diagnostic or therapeutic aspiration procedures (Chau and Griffith 2005). In cellulitis, US demonstrates an irregular ill-defined hyperechoic appearance of fat with blurring of tissue planes, progressing to hypoechoic strands reflecting edema (Nessi et al. 1990; Robben 2004). This appearance is nonspecific and cannot be distinguished from noninfectious causes of soft-tissue edema on the basis of echotextural findings alone (Struk et al. 2001; Robben 2004). 23 24 M. Valle and M. P. Zamorani Color and power Doppler imaging may help the clinical diagnosis by depicting a hypervascular pattern in cellulitis (Cardinal et al. 2001) (Fig. 2.5a,b). Phlebitis and occlusion of superficial veins may also be observed as associated findings. If untreated, infectious cellulitis can progress to abscess formation (Fig. 2.5c,d). In most cases, a subcutaneous abscess is demonstrated as an irregular fluid-filled hypoechoic area with posterior acoustic enhancement, containing variable amount of echogenic debris (pus) (Fig. 2.5c,d). Fluid-fluid levels within the collection with dependent layering of the more echogenic particulate material can be noted. In highly echogenic collections, a slight pressure with the probe or the fingers may help to confirm the liquid nature of the mass by causing fluctuation of the particles (Loyer et al. 1996). Doppler imaging modalities typically show hyperemic blood flow within the abscess wall and the surrounding tissues (Arslan et al. 1998). Cellulitis being essentially a clinical diagnosis, the main diagnostic role of US is to rule out deep venous thrombosis and * * * a * b * * * * * c d Fig. 2.5a–d. Subcutaneous tissue infection. a,b Cellulitis and c,d abscess. Images are from different patients. a Color Doppler fi 12–5 MHz US image reveals a diffusely increased echogenicity of the subcutaneous fat (asterisks) with blurring of the definition of connective septa and fatty lobules, and an increased vasculature. b Color Doppler 12–5 MHz US image shows signs of initial progression of cellulitis into abscess. There is diffuse subcutaneous edema with hyperechoic fatty lobules (asterisks) alternating with irregular hypoechoic areas (arrowhead) filled with Doppler signals. An intense hypervascular pattern is seen. c Gray-scale 12–5 MHz US image over the gluteal region in patient with tuberculosis demonstrates coalescence of hypoechoic serpiginous areas into a large hypoechoic abscess (asterisk) with loss of Doppler signals. d Power Doppler 12–5 MHz US image of a forearm abscess in an HIV-positive patient shows a large cavity fi filled with echogenic particulate material (asterisk) inside the subcutaneous tissue, displacing the fat lobules. Fluctuation of the echogenic material filling the abscess could be obtained on compression. The abscess dislocates and stretches the connective septa and the small vessels (arrowheads) contained within them. 25 Skin and Subcutaneous Tissue to identify an underlying abscess. Even if an abscess is not found but infection-related symptoms persist, US examination should be repeated because liquefaction may manifest with time (Robben 2004). In addition, if the abscess lies in proximity to the bone, US may reveal the osseous origin of the infection by depicting hypoechoic subperiosteal fluid (Robben 2004). Often associated with a previous trauma (e.g., open wound, insect bite), necrotizing fasciitis is a rare, rapidly progressive, life-threatening infection involving the subcutaneous tissue, fascia and surrounding soft-tissue structures, including muscles. A variety of aerobic and anaerobic bacteria may be involved as causative agents of necrotizing fasciitis, group A Streptococcus being the most common. In most cases, the patient is diabetic, immunocompromised or severely ill with profound toxicity. Although US is not rewarding at the early stages of infection when soft-tissue abnormalities may mimic cellulitis, it may be helpful for demonstrating the extent of fascial thickening and accumulation of cloudy fluid along the deep fascial layer (Fig. 2.6a). An amount of fluid >4 mm in depth has been regarded as highly sensitive and specific for the diagnosis of necrotizing fasciitis (Yen et al. 2002). In addition, US can reveal loculated abscesses in the fascial plane – allowing US-guided diagnostic aspiration – and gas formation in soft tissues in advanced disease (Robben 2004; Wilson 2004). Gas gangrene, which is produced by organisms of bowel origin or by Clostridium, is an ominous sign (Fig. 2.6b). Aggressive surgical debridement and a course of broad-spectrum antibiotics are critical for the patient’s survival. 2.3.2.3 Fatty Atrophy Focal reabsorption of the subcutaneous tissue and depigmentation of the overlying skin can be observed following local inadvertent injection of long-acting corticosteroids (Canturk et al. 2004). This “sideeffect” is somewhat related to the catabolic effect of the drug: thinning of the subcutaneous fat is dose-related, may be appreciated up to complete reabsorption of the fatty tissue layer and shows a maximal decrease 4–8 weeks after a single injection of steroids (Gomez et al. 1982). US is a reliable means to confirm the presence of focal shrinkage of the subcutaneous fat by comparing the affected side with either the contralateral healthy side or an adjacent normal area. In clinical practice, focal areas of subcutaneous atrophy may occur around the radial head following steroid injection for treatment of tennis elbow and at the buttock secondary to intramuscular injections. Although the US appearance of subcutaneous atrophy is rather specific, awareness of the clinical history is essential to correlate the US findings with a specific causative factor. 2.3.2.4 Traumatic Injuries In a traumatic setting, and especially in contusion traumas, changes of the subcutaneous tissue are commonly encountered. Depending on the strength and duration of the insult and the patient’s state a b Fig. 2.6a,b. Necrotizing fasciitis. Transverse 12–5 MHz US images over the lower anterolateral leg in a severely compromised diabetic patient with necrotizing fasciitis demonstrate accumulation of fluid along fascial planes (arrows) and scattered bright foci in the soft-tissues refl flecting initial gas formation (arrowheads) 26 M. Valle and M. P. Zamorani (anticoagulation therapy, steroids, etc.), soft-tissue abnormalities may range from simple hemorrhagic infiltration of fat lobules, to fat necrosis, hematomas and abscesses. US reveals bloody fat infiltration as an increased echogenicity of fatty lobules that can make the separation from the hyperechoic skin and the connective tissue strands of the subcutaneous tissue undefined (Fig. 2.7a). Hemorrhagic fat infiltration can be readily distinguished from simple edema because of the absence of anechoic fluid distending the connective septa. The differential diagnosis with a superficial hyperechoic lipoma is based on the clinical history and the oval, well-circumscribed appearance of the soft-tissue mass. Following a contusion trauma, subcutaneous fat necrosis may arise with edema, hemorrhage and fibrosis with lack of a discrete soft-tissue mass and volume loss of the subcutaneous tissue (Tsai et al. 1997; Ehara 1998). Fat necrosis appears as a hyperechoic focus containing hypoechoic spaces related to infarcted fat (Fernando et al. 2003) (Fig. 2.7b). In hematomas, the US appearance of the bloody collection varies over time. Soon after the blood leakage, fresh fluid may appear highly reflective up to a pseudosolid appearance because of fibrin and erythrocytes forming multiple acoustic interfaces. With time, the hematoma tends to become completely anechoic as a result of liquefaction of the clot and increases in size (Fig. 2.8a). A network of thin strands may often be seen resulting from fibrin organization (Fig. 2.8b). Fluid levels reflecting separation between serum (anechoic) and cellular com- a * ponents (echogenic) of blood can also be observed. Over a period of months, the hematoma eventually resolves, but a residual fibrous scar and focal retraction of the overlying skin may persist (Fig. 2.8c). As described in Chapter 12, a hematoma that has a peculiar disposition related to the subcutaneous tissue is the Morel-Lavallée lesion. This condition indicates a post-traumatic seroma which derives from local trauma usually located over the lateral aspect of the proximal thigh. The collection typically intervenes between the deep layer of the subcutaneous tissue and the fascia as a result of a shear strain mechanism causing disruption of the rich vascular plexus that pierces the fascia lata (Morel-Lavallée 1863). US depicts a Morel-Lavallée lesion as an elongated fluid collection overlying the straight echogenic appearance of the fascia (Parra et al. 1997; Mellado et al. 2004). In cases of an abscess secondary to trauma, the examiner should attempt to recognize any possible foreign body within it as the causative factor (Fig. 2.9). This is valid even if the patient denies previous open wounds, because the presence of foreign bodies requires surgical removal. In an effort to exclude a more extensive spread of infection that may deserve different treatment, the examiner should check the status of underlying regional muscles, tendon sheaths and joint spaces. Finally, a contusion trauma on the skin by a pointed, sharp object can be transmitted to the subcutaneous tissue causing laceration and focal discontinuity of fat lobules. This category of lesions results in “fat fractures” * Fig. 2.7a,b. Subcutaneous tissue contusion trauma and fat necrosis. a Transverse extended-fi field-of-view 12–5 MHz US image of the trochanteric region in a patient with local contusion trauma after a fall demonstrates an undefi fined increased echogenicity of fatty lobules (arrowheads) refl flecting hemorrhagic fat infi filtration. Note that the abnormal area is located just superfi ficial to the osseous prominence of the greater trochanter (asterisk). b Longitudinal 12–5 MHz US image over the anterolateral thigh in another patient with previous local contusion caused by a sharp object. US shows three well-circumscribed hypoechoic areas (arrows) surrounded by ill-defi fined hyperechoic halo (arrowheads) within the subcutaneous tissue (asterisk) representing fat necrosis b 27 Skin and Subcutaneous Tissue * * a T b c Fig. 2.8a–c. Superfi ficial hematoma: spectrum of 12–5 MHz US appearances. a Hematoma of the subcutaneous tissue examined a few days after blunt trauma. US demonstrates an echo-free fl fluid collection (asterisks) reflecting fl the phase of clot liquefaction. b Pretibial hematoma (arrowheads) examined 15 days after trauma reveals closely packed fibrous stranding within the collection refl flecting fibrin organization. T T, tibia. c Residual fibrous scar following a large hematoma in the buttock. US shows the scar as a hyperechoic reflection fl (arrows) with posterior acoustic shadowing (open arrowheads) causing distorsion of the adjacent subcutaneous fat (white arrowheads) that may mimic a tendon gap at physical examination. US can determine whether the discontinuity is limited to the subcutaneous fat or involves the deeper structures too (Thomas et al. 2001) (Fig. 2.10). Subcutaneous scars are easily depicted with US as vertically -oriented thin linear stripes surrounded by hyperechoic halo that interrupt the normal tissue layers. The abnormal tissue can extend deeply across the fascia into the muscles or the ligaments. Scars may eventually calcify (see Fig. 2.8c). * 2.3.2.5 Foreign Bodies Foreign bodies can be found in the subcutaneous tissues as the result of traumatic injuries or therapeutic procedures. In a post-traumatic setting, foreign bodies derive from open or penetrating wounds. Most are composed of plant fragments (wood splinters, thorns, etc.), metal or glass. In terms of prevalence, wood fragments are the most frequently found, fol- * a b Fig. 2.9a,b. Foreign-body-related abscess. a Longitudinal and b transverse 12–5 MHz US images over the dorsum of the hand in a patient with signs of local inflammation fl and a recent open wound. US demonstrates a subcutaneous collection (asterisk) with posterior acoustic enhancement (black arrowheads) and fl fluid-debris levels (open arrowheads). A small highly refl flective foreign body (white arrowhead) is contained within the collection. Surgery revealed an abscess containing a small wood splinter 28 M. Valle and M. P. Zamorani * * * * * a b Fig. 2.10a,b. Subcutaneous fat fracture. a Transverse and b longitudinal 12–5 MHz US images of the gluteal region in a patient with previous local blunt trauma reveal a wide fl fluid-fi filled gap (arrowheads) representing a subcutaneous fat fracture. Note the disrupted appearance of fatty lobules (asterisks) and the alignment of the fracture plane with the edge (white arrow) of the iliac bone a d b c e Fig. 2.11a–e. Foreign bodies: US appearance in two patients presenting with a–c wood and d,e glass fragments. a Long-axis and b short-axis 12–5 MHz US images of a carpenter who injured his left hand during manual work show an elongated hyperechoic foreign-body (arrow) inside the subcutaneous tissue. The fragment is surrounded by a hypoechoic rim (arrowheads) representing reactive edema and granulation tissue. c At surgery, a wood splinter 1 cm long was removed. d Sagittal 12–5 MHz US image of the distal forearm with e radiographic correlation in a patient who had an accident during which he broke a glass with his left hand. Initially, physical exploration was negative for foreign bodies and the wound was sutured. At 3 weeks after trauma, US demonstrated two bright linear images (arrows) with posterior reverberation (arrowheads) refl flecting retained glass fragments in the subcutaneous tissue, just superficial fi to the ulnar nerve (arrowheads). e Radiographic correlation Skin and Subcutaneous Tissue lowed by glass and metal fragments (Anderson et al. 1982). Part of them may remain at the site and unrecognized even after apparent successful removal by the patient at the time of the injury (Peterson et al. 2002). If missed, foreign bodies can results in granuloma formation, secondary soft-tissue infection with formation of an abscess, fistula, purulent tenosynovitis and septic arthritis. Bone destructive changes and damage to adjacent nerves may also occur (Choudhari et al. 2001; Peterson et al. 2002). An early diagnosis and prompt removal of foreign bodies is required to prevent complications. Physical examination has intrinsic limitations for detecting and localizing small foreign bodies due to the associated local soft-tissue swelling and pain. It has been reported that approximately 38% of foreign bodies can be overlooked at the initial clinical investigation (Anderson et al. 1982). The deep position of a fragment makes palpation more difficult and less successful. Plain radiography is the initial imaging modality to identify and localize foreign bodies but it can only show radio-opaque fragments: even if very small, metallic fragments are readily detected on plain films. Detection of glass fragments depends on their size and, less importantly, on their lead content, as even if lead-free, almost all glass material is radio-opaque to some degree on radiographs (Felman and Fisher 1969). Radiolucent fragments, such as wood splinters, plant thorns and plastic fragments, cannot be detected by X-rays. Although radiographs allow an estimate of the fragment’s location and its relationships with adjacent bones and joints, in relation with tendons, vessels and nerves cannot be investigated. In addition, local complications are not recognized. Xeroradiography and low-kilovoltage radiography have been proposed to increase the detection rate of foreign bodies, but these techniques are currently obsolete. US is an excellent means of detecting and evaluating post-traumatic foreign bodies (Dean et al. 2003; Soudack et al. 2003; Friedman et al. 2005; Jacobson 2005). In cases of suspected foreign bodies, the examiner should extend the study to a larger area than that closely surrounding the skin wound, as fragments may migrate far away from the entrance point as a result of repeated muscle contraction (Choudhari et al. 2001). As an example, it is not unrealistic to hypothesize that a retained fragment entered the soft tissues on the volar aspect of the wrist may dislocate proximally to reach the anterior distal forearm. As assessed in cadaveric and in vivo studies, the US appearance of foreign bodies varies to a great extent depending on the composi- tion (metal, glass, wood, etc.), shape and site of the fragment (Blyme et al. 1990; Horton et al. 2001). Either radio-opaque or radiolucent fragments can be identified with US as reflective structures with posterior acoustic shadowing or reverberation artifact, depending on the surface characteristics and composition of the foreign body (Boyse et al. 2001; Horton et al. 2001). In general, wood fragments are characterized by posterior acoustic shadowing, whereas glass and metal exhibit reverberations and comet tail artifact (Fig. 2.11). Although these findings lack specificity, they can help to identify foreign bodies as such. Detection of posterior acoustic artifact is particularly helpful for locating tiny fragments that, because of their small size, can go unnoticed. Similarly, a hypoechoic halo surrounding the fragments is of the utmost importance to distinguish them from adjacent soft-tissue structures, such as fat strands or muscles. As assessed in a comparative US-pathologic study, the halo correlates with fibrin, granulation tissue and collagenous capsule formation, whereas the hypervascular pattern seen at color Doppler imaging reflects neovasculature (Davae et al. 2003). The examiner should be aware that US is not accurate for evaluating the fragment’s size, as the technique is able only to delineate its surface. On the other hand, the relationship of foreign bodies with adjacent vessels, tendons, muscles and nerves can be precisely assessed. US can recognize a variety of complications, including abscess, granuloma, infectious tenosynovitis and septic arthritis (Fig. 2.12). Generally speaking, the main limitations of this technique occur in the acute phases of trauma, when open wounds or soft-tissue emphysema may make the examination difficult. In an acute setting, care should be taken to avoid contamination of the open wound with gel. In these circumstances, the use of sterile gel and a lateral approach to the skin wound can be recommended to image the fragment. If the foreign body is retained in the distal arm or in the distal leg, US examination can be better performed by placing the affected extremity in a water bath (Blaivas et al. 2004). As determined in an in vitro study, air bubbling can decrease the visibility of foreign bodies, leading to attenuation of the US beam deep to the gas (Lyon et al. 2004). In a preoperative setting, US can identify the foreign body, place a skin mark over it and measure the depth of the fragment relative to the skin. As described in Chapter 18, US can guide the removal of superficial foreign bodies during real-time scanning (Shiels et al. 1990). In summary, when a foreign body is suspected on clinical grounds, the examiner should briefly 29 30 M. Valle and M. P. Zamorani * * a * T T * T b T * T c Fig. 2.12a–c. Tenosynovial foreign body. a Short-axis and b long-axis 15–7 MHz US images over the palm show an elongated wood fragment (curved arrow) that has penetrated within the synovial sheath of the flexor tendons (T). A thin hypoechoic effusion (asterisks) in the tendon sheath allows the fragment to be precisely located in the synovial space. c Short-axis color Doppler 15–7 MHz US image reveals a hypervascular flow fl pattern in the flexor tendon sheath as an expression of reactive hyperemia discuss the context of trauma with the patient to hear about the nature of possible fragments (glass, wood, metal, etc.). Radiographs should be always performed before US examination. Then, US scanning should cover a wide tissue area around the wound, as foreign bodies may migrate far away from the penetration site. The examiner should seek for bright echoes in the soft tissues but, even more, for structures with posterior acoustic attenuation. Once detected, the fragment should be measured as regards its size, orientation, distance from the skin, and relationships with adjacent tendons, nerves and vessels. Signs of possible infectious complications, such as fluid collections and tenosynovitis, should be annotated as well. Instead of writing a long descriptive report, we prefer to mark the skin overlying the fragment reproducing its size and orientation and to measure the depth of the foreign body: these are important pieces of information for the surgeon before removal. For foreign bodies in deep locations, we recommend appending a drawing to the written report in an effort to better explain the relationship of the foreign body with the adjacent structures. Orthopaedic implants (screws, pins, etc.) can be found in the soft tissues as a consequence of loosening of orthopaedic devices. Metallic devices appear as bright hyperechoic structures with posterior reverberation artifact (Fig. 2.13). Although they are easily detected on plain films, US allows an excellent analysis of the relationship of loosened implants with adjacent structures, thus helping to plan their removal (Grechenig et al. 1999). Implantable subcutaneous devices are used as long-acting and effective methods of contraception. They consist of a single rod implanted in the subcutaneous tissue of the medial aspect of the arm to release levonorgestrol into the systemic circulation. Based of physical findings, identification of the rod can be difficult if it has inadvertently been inserted too deep or it has migrated away from the insertion point. If removal is required, US is an efficient modality to precisely localize nonpalpable rods, thus allowing their easy removal (Amman et al. 2003; Piessens et al. 2005). Rods appear as a small, elongated, hyperechoic structures with well-defined definite posterior acoustic shadowing, an appearance that correlate well with in vitro findings (Fig. 2.14) (Amman et al. 2003). MR imaging should be used only if US is unrewarding (Merki-Feld et al. 2001). Tissue expanders are widely used in plastic and reconstructive surgery (Neumann 1957). US can assess twisting of injection ports that are surgically inserted into the subcutaneous tissue (Kohler et al. 2005). Twisting is associated with failure of the injection procedure and fluid accumulation in the subcutaneous tissue. US easily demonstrates the upside-down position of the port by showing the linear hyperechoic appearance of the metallic base tilted toward the skin replacing the normal concave superior face of the soft silicone component (Kohler et al. 2005). Suture granulomas may occur after a surgical intervention in which nonabsorbable stitches are used. These tumor-like lesions usually develop slowly and may cause only vague symptoms or remain asymptomatic for many years. US is an accurate way to identify and characterize them by depicting suture material within (Fig. 2.15). As assessed in an in vitro study, the US appearance of surgical sutures is independent of their chemical composition. Monofilament sutures appear as straight bright double lines (like railway 31 Skin and Subcutaneous Tissue a b Cor c Fig. 2.13a–c. Loosened surgical screw. a Anteroposterior radiograph of the shoulder with correlative b transverse and c splitscreen sagittal 12–5 MHz US images over the pectoralis region in a patient with a loosened screw (curved arrow) following previous surgery on the shoulder. a Radiograph reveals the loosened screw projecting over the right chest but it does not indicate its precise location. b At US examination, the screw (curved arrow) appears as a hyperechoic structure with posterior reverberation artifact (straight arrows) presenting a head (white arrowhead) and multiple hyperechoic teeth (open arrowheads) at its anterior aspect corresponding to screw spirals. In c, the screw appears as a small hyperechoic dot (curved arrow) surrounded by fluid collection (arrowhead) due to local inflammatory fl reaction. US allows accurate assessment of the relationship of the screw with the short head of the biceps and the coracobrachialis muscles (open arrows) arising from the coracoid (Cor) lines) due to high-amplitude reflection of the US beam at the superficial and deep interface of the suture with the surrounding tissue; braided sutures most often produce a single echo (Rettenbacher et al. 2001). Both patterns show posterior reverberation artifacts. In general, the surrounding granuloma appears as an ill-defined hypoechoic mass, containing a liquefied center where the stitch lies. The main differential diagnoses are granulomas containing other foreign bodies and inflamed epidermoid cysts containing a hair. 2.3.3 Tumors and Tumor-Like Conditions Soft tissue masses of the subcutaneous tissue include a variety of lesions, such as calcifications, tophaceous gout or rheumatoid nodules, sebaceous cysts and tumors, ranging from the common lipomas and hemangiomas to the rare metastasis and primary malignant masses. Scattered calcifications in the subcutaneous tissue are observed in scleroderma and systemic lupus erythematosus. They appear as mottled hyperechoic lesions with posterior acoustic shadowing. US has little value in their assessment as they are manifest on plain films. Subcutaneous calcifications are often the result of drug injections. For the most part, they are encountered in the buttock and appear as well-delimited hyperechoic structures with strong posterior acoustic shadowing (Fig. 2.16a). In rheumatologic patients, subcutaneous nodules are mainly due to tophaceous gout or rheumatoid nodules (Tiliakos et al. 1982; Benson et al. 1983; Nalbant et al. 2003). Tophi are softtissue agglomerates of uric acid crystals that can develop in different areas of the body: the hand, the foot and the elbow the most commonly involved. 32 M. Valle and M. P. Zamorani a b c Fig. 2.14a–c. Subdermal contraceptive device (Implanon). a Short-axis and b long-axis 12–5 MHz US images over a flexible fl subdermal plastic implant (arrows) for long-acting release of synthetic hormones. In selected cases, US can assist in the localization and minimally invasive removal of the implant. c Photograph of an Implanon rod after surgical removal a b Fig. 2.15a,b. Suture granuloma. a Long-axis and b short-axis 12–5 MHz US images show a suture granuloma located in the lower abdominal wall after inguinal herniorrhaphy. Within the hypoechoic granuloma (arrows), the surgical suture appears as a hyperechoic rail-like line (arrowheads) when imaged in its long-axis. On the short-axis image, the suture assumes the appearance of a double dot (arrowhead) At US examination, tophi appear as heterogeneous masses containing hypoechoic areas related to chalky liquid material surrounded by hyperechoic tissue (Nalbant et al. 2003). Rarely, calcific deposits can be detected within the tophaceous mass in the form of hyperechoic spots with or without posterior acoustic attenuation (Fig. 2.16b) (Gerster et al. 2002). Rheumatoid nodules occur in 20–30% of rheumatoid patients who have a high serum level of rheumatoid factor and active articular disease (McGrath and Fleisher, 1989). They seem to derive from an immune complex process between rheumatoid factor and immunoglobulin G initiating small vessel abnormalities and then progressing to necrosis and granulation tissue. Gross examination of these nodules reveals a semifluid center sur- 33 Skin and Subcutaneous Tissue * * a b A * A * A c d Fig. 2.16a–d. Non-neoplastic subcutaneous masses. a Elaioma. Transverse 12–5 MHz US image demonstrates dystrophic calcification (arrows) in the subcutaneous tissue of the buttock, at the site of previous injection therapy. b Tophaceous gout. Longitudinal 12–5 MHz US image over the forefoot reveals tophi as para-articular ill-defi fined hypoechoic masses (asterisks) with posterior acoustic shadowing (open arrowheads) and hyperechoic surrounding halo (arrows), adjacent to the MIP joint. Note the osteoarthritic changes (white arrowheads) in the underlying joint. c,d Rheumatoid nodules. c Transverse and d longitudinal 12-5 MHz US images over the Achilles tendon (A) in an HIV-positive patient affected by longstanding rheumatoid arthritis show a rheumatoid nodule as a hypoechoic mass (arrows) arising from the paratenon and growing into the subcutaneous tissue. The nodule has a mixed echotexture with solid (asterisk) and fl fluid (arrowheads) components rounded by dense connective tissue. Rheumatoid nodules are usually found at pressure sites, such as the extensor aspect of the elbow, the fingers and the calcaneus, and correlate with a bad prognosis. US displays hypoechoic masses with a central sharply demarcated hypoechoic area reflecting necrosis (Fig. 2.16c,d) (Nalbant et al. 2003). 2.3.3.1 Lipomas Superficial lipomas typically appear as compressible, palpable soft-tissue masses in the subcutaneous tissue not adherent with the overlying skin. Lipomas have a male and familial predominance and tend to grow in the back, shoulder and upper arms with a predilection for the extensor surface. They are more common in the fifth and sixth decades. Although lipomas most often present as a solitary oval or rounded mass, they may be multiple (5%– 15%) (Murphey et al. 2004). At US examination, lipomas have a wide range of appearances. Typically, they present as elliptical compressible masses containing short linear reflective striations that run parallel to the skin (Fig. 2.17a). However, their internal echogenicity may vary from hyperechoic to hypoechoic or mixed relative to muscle depending on the degree of connective tissue and other reflective interfaces – such as cellularity, fat and water – within the mass (Fornage and Tassin 1991; Ahuja et al. 1998). At least theoretically, it has been postulated that lipomas composed of pure fat should be echofree lesions due to a low number of tissue acoustic interfaces (Behan and Kazam 1978). Based on different series, the incidence of hyperechoic lipomas, reflecting the so-called fibrolipomas, varies from 20% to 76% (Fornage and Tassin 1991; Ahuja et al. 1998; Inampudi et al. 2004). In a recent retrospective review of 39 US-diagnosed superficial and 34 M. Valle and M. P. Zamorani c b a * Muscle d e Fig. 2.17a–e. Subcutaneous lipoma: spectrum of typical US appearances. a Long-axis extended-fi field-of-view 12–5 MHz US image of a lipoma of the back shows an elongated well-defi fined compressible mass with its greatest diameter parallel to the skin. The mass has well-defi fined margins and appears slightly hyperechoic relative to adjacent fat. Its echotexture consists of short thin linear striations that run parallel to the skin. b Long-axis 12–5 MHz US image at the border of a nonencapsulated lipoma (arrows) in a patient with a palpable mass at the medial aspect of the left thigh with c correlative contralateral image. d Long-axis 12–5 MHz US image of an intrafascial lipoma shows a lenticular fatty mass (asterisk) contained in a split of the muscle fascia (arrows). Note the fascia dividing into two hyperechoic sheets (arrowheads) to envelop the lipoma. e Transverse 12–5 MHz US image of the left forearm in patient with pathologically-proven angiolipoma demonstrates a hyperechoic rounded mass (arrows) with small internal hypoechoic dots 25 lipomas and 14 nonlipomas, including other benign and malignant histotypes (Inampudi et al. 2004). This indicates that the variable echotexture of lipomas may make their differentiation from other masses subjectively difficult. Although many lipomas have a well-circumscribed appearance with an identifiable thin capsule, a significant proportion (12%–60%) have ill-defined borders blending imperceptibly with the surrounding subcutaneous fat (Fig. 2.17b,c) (Fornage and Tassin 1991; Ahuja et al. 1998; Inampudi et al. 2004). This may lead to difficulties in identifying them with US even if the mass is apparent clinically. Nonencapsulated lipomas may require comparison with the contralateral side to detect significant asymmetry of fat tissue. They should be referred to as “probable lipomas” in the report as long as there are corroborative clinical findings of a discrete mass (Roberts et al. 2003). In daily practice, the occurrence of a superficial palpable lump suggesting a lipoma in the absence of a definite nodule detectable with US is not uncommon. Graded compression with the probe or combined imaging and palpation may be helpful for detecting these “occult” lipomas. Both maneuvers can increase the detection rate of the mass, which is less compressible than the adjacent subcutaneous tissue. Most superficial lipomas do not present substantial internal vasculature at color and power Doppler imaging, a finding that may enhance the confidence of the examiner that a benign mass is present (Ahuja et al. 1998). Some lipomas grow in the deep subcutaneous tissue, in close contact with the fascia. Care should be taken when reporting on these masses not to lead the surgeon to believe that the lesion can be easily excised, because deep subcutaneous lipomas may adhere to the fascia. A well-delimited mass does not always mean an easily Skin and Subcutaneous Tissue removable lesion. Lipomas growing inside the deep fascia may also occur. The clinical diagnosis of these lesions may be difficult because they are firm and tethered to the deep plane and may mimic more aggressive tumors. At US examination, intrafascial lipomas appear as lenticular lesions growing into a split of the fascia, which retains a normal hyperechoic appearance (Fig. 2.17d). In these cases, US can rule out abnormalities of the underlying muscles and aggressive growth patterns suggestive of a malignant tumor. Lipomas containing other mesenchymal elements, such as fibrous tissue (fibrous lipomas), cartilage (chondroid lipomas), mucoid component (myxolipoma) and vessels (angiolipoma), may be encountered. In these cases, the presence of nonlipomatous elements may make the US appearance of the lesion less specific. Among these variants, angiolipomas account for 5%–17% of all lipomas (Lin and Lin 1974). They are well-defined hyperechoic subcutaneous masses containing small patchy hypoechoic areas and sparse internal vasculature (Fig. 2.17e) (Choong 2004). Relative to lipomas, angiolipomas have a greater angiomatous component composed of thin-walled capillaries which account for up to 90% or more of the lesion, and occur at an earlier age (early adulthood). Hibernomas (fetal lipomas) are rare benign tumors composed of brown fat. Brown fat is histologically distinct from white adipose tissue and plays a role in nonshivering thermogenesis of hibernating animals and newborn humans. In humans, brown adipose tissue progressively decreases through adulthood. Usual locations of tumors arising from brown fat are the parascapular and interscapular spaces, the mediastinum, the upper thorax and the thighs. US demonstrates a solid well-marginated hyperechoic mass somewhat resembling a lipomatous tumor and Doppler imaging may show a hypervascular pattern reflecting the presence of vascular structures and the increased cellular metabolism of hibernomas. Other rare forms of lipomas, including lipomatosis of nerves (see Chap. 4) and lipoma arborescens (see Chap. 14) are described elsewhere. Other space-occupying nonlipomatous masses containing fat may mimic the US appearance of lipomas. Among them, hemangiomas contain a variable amount of adipose tissue interspersed between abnormal vessels. However, in most cases their typical US appearance made of serpentine or tubular hypoechoic structures contained within the mass, scattered phleboliths and prominent blood flow at color and power Doppler imaging, allows the correct diagnosis to be made. Lipomatosis represents a diffuse overgrowth of mature adipose tissue histologically similar to simple lipomas. The fatty tissue extensively infiltrates the subcutaneous and muscular tissue and is not associated with nerve involvement. Many entities of superficial lipomatosis are described (Murphey et al. 2004). In multiple symmetric lipomatosis, which is commonly referred to as Madelung or Launois-Bensaude lipomatosis, multiple symmetric lipomas are found in the neck and the shoulder in association with alcoholism, hepatic disease and metabolic disorders (Uglesic et al. 2004). Dercum disease, which is also referred to as lipomatosis dolorosa or adiposis dolorosa, is a rare disorder occurring in middle-aged women, often obese, in which multiple painful subcutaneous lipomas occur (Wortham and Tomlinson 2005). 2.3.3.2 Pilomatricoma and Epidermal Inclusion (Sebaceous) Cysts Pilomatricoma (pilomatrixoma), also called calcifying epithelioma of Malherbe, is a benign superficial tumor of the hair follicle arising from the hair cortex cells in the deep dermis and extending into subcutaneous tissue as it grows (Malherbe and Chemantais, 1880). Most lesions arise in children less of 10 years of age and appear as small masses (<3 cm in diameter) with a rock-hard consistency and an irregular surface, which causes skin stretching over the mass (Hwang et al. 2005). Although the overall incidence of pilomatricoma is low, it is one of the most commonly excised superficial masses in children with epidermoid cysts. Preferential anatomic sites of pilomatricomas are the neck, the cheek, the preauricular area and the extremities, including arm and leg. US demonstrates pilomatricomas as hyperechoic masses relative to the muscle with posterior acoustic shadowing reflecting internal calcification or ossification (Fig. 2.18a) (Hwang et al. 2005). The amount and shape of calcification may vary from few scattered echogenic foci to gross clumped deposits within the mass or a completely calcified nodule. In most cases, a peripheral hypoechoic rim surrounding the calcific deposits is observed (Hwang et al. 2005). Peripheral color Doppler flow is often found in the peripheral region of the mass. 35 36 M. Valle and M. P. Zamorani a b Fig. 2.18a,b. Epidermal-related masses. a Pilomatricoma. Transverse 10–5 MHz US image in a child with a stiff superficial fi lump in the preauricular area reveals a mass (arrows) characterized by a peripheral hypoechoic rim and a hyperechoic center with fi foci causing posterior acoustic attenuation. b Epidermal inclusion cyst. US demonstrates a rounded hypoescattered calcified fi extension into the dermis choic mass (arrow) with posterior acoustic enhancement (white arrowheads) and a small superficial (open arrowhead) Epidermal inclusion cysts, which are also referred to as sebaceous, epidermoid, epidermal, infundibular or keratin cysts, derive from the focal proliferation of epidermal cells within the subcutaneous tissue. The theory that these cysts may develop from the subcutaneous implantation of keratinizing epithelial elements during embryogenesis or a previous trauma or surgery is widely accepted. Sebaceous cysts most often arise from swollen sebaceous glands or hair follicles and are limited to the skin surfaces in which sebaceous glands are present (i.e., the dorsal but not the ventral aspect of the hand). Epidermal inclusion cysts are lined with epithelial cells and filled with a white, cheesy material reflecting layers of keratin and cholesterol-rich debris. Clinically, epidermal inclusion cysts present as slow-growing, freely movable lumps beneath the skin. They usually remain asymptomatic unless they become infected, grow large enough to interfere with normal function, or rupture into the adjacent soft tissues. US shows epidermal cysts as ovoid or spherical sharply bordered hypoechoic masses with scattered echoes presenting posterior acoustic enhancement and a small extension into the dermis corresponding to their small opening that communicates with the skin (Fig. 2.18b) (Lee et al. 2001). However, the internal echogenicity of epidermoid inclusion cysts may vary depending on the hydration of the keratin, protein composition and microcalcifications (Fig. 2.19) (Vincent et al. 1985; Lee et al. 2001). The typical “onion-ring” (bull’s-eye) appearance described in the testis as a result of multiple layers of keratin debris is usually not observed in epider- mal cysts arising from soft tissues (Brenner et al. 1989; Maxwell and Mamtora 1990). Ruptured cysts may assume a lobulated or irregular contour as a result of intense granulomatous reaction and show color Doppler signals, possibly mimicking a neoplasm (Lee et al. 2001). 2.3.3.3 Hemangiomas and Vascular Malformations Even though the term “hemangioma” is often used in a general way to encompass both hemangiomas and vascular malformations, hemangiomas represent endothelial-lined neoplasms that mainly occur in childhood, growing to reach a maximum volume and then regress, whereas vascular malformations are composed of dysplastic vessels which show no cellular proliferation or regression. Hemangiomas can be categorized into capillary and cavernous types, whereas vascular malformations may be divided in high-flow, slow-flow and capillary lesions. Hemangiomas may be hypoechoic or hyperechoic relative to surrounding tissue and may have a homogeneous or complex appearance (Fig. 2.20a). High vessel density and high peak arterial Doppler shifts (>2 kHz) are typically observed and help in distinguishing hemangiomas from other soft-tissue masses (Fig. 2.20b–f) (Dubois et al. 1998, 2002). High-flow malformations are typified by an abnormal network of vascular channels (the nidus), interposed between a prominent feeding artery and a dilated draining Skin and Subcutaneous Tissue T b a c d Fig. 2.19a–d. Epidermal inclusion cyst. a Lateral radiograph of the middle finger in a patient with a palpable mass on the ventral aspect of the proximal phalanx reveals a superfi ficcial oval soft-tissue mass (arrows). b Transverse 12–5 MHz color Doppler US image of the affected finger demonstrates a wellcircumscribed hypovascular mass (arrows) characterized by a homogeneous texture of medium-level echoes in close relationship with the flexor tendons (T). Correlative c fat-suppressed T2-weighted and d gadolinium-enhanced fatsuppressed T1-weighted MR images show a homogeneous lesion (arrow) of high signal intensity on T2-weighted images, central non-enhancement and peripheral thin rim enhancement. Surgery revealed an epidermal inclusion cyst d T T a b e c f Fig. 2.20a–f. Hemangioma. Transverse a gray-scale and b color Doppler 15–7 MHz US images of the index finger fi in a patient with an indolent palpable mass demonstrate a well-circumscribed solid hypoechoic nodule (arrows) located just superficial fi to the fl flexor tendons (T). The mass reveals several intratumoral vessels. c Coronal fat-suppressed T2-weighted and transverse d T1-weighted, e fat-suppressed T2-weighted and f gadolinium-enhanced T1-weighted MR imaging correlation 37 38 M. Valle and M. P. Zamorani vein. Spectral Doppler analysis demonstrates high systolic arterial flow and arterialization of the veins (Fig. 2.21) (Dubois et al. 1999). Slow-flow (venous) malformations are characterized by abnormally dilated venous spaces and a normal arterial component. Often, they may be suspected on the basis of a subcutaneous bluish or reddish stain. In approximately 15% of cases they contain phleboliths (calcifications in venous thrombosis), which can be seen as hyperechoic foci with posterior acoustic shadowing (Fig. 2.22). Due to slow blood flow, color Doppler imaging may detect only sparse monophasic flow or no blood flow signals at all (Trop et al. 1999). Distinguishing between a slow-flow malformation and an involuted hemangioma may be problematic. In general, vascular malformations are distinguished from hemangiomas owing to the absence of solid tissue (Paltiel et al. 2000). In addition, hemangiomas have similar vessel density and peak systolic velocities but lower venous velocity (Paltiel et al. 2000). Finally, there are capillary malformations limited to the dermis. For the most part, US is unable to display such superficial abnormalities that typically present with a port-wine like stain. In some instances, however, an increased thickness of the subcutaneous tissue and some prominent veins may be demonstrated. 2.3.3.4 Metastases and Lymphomas Superficial metastases involving the skin and subcutaneous tissue account for approximately 0.5%–9% of tumors. They usually result from seeding of deep tumors during interventional (i.e., needle and surgical biopsy) or surgical procedures or represent a manifestation of end-stage cancer (Galarza and Sosa 2003). In some cases, however, skin metastases can be the first manifestation of an occult cancer, therefore requiring an accurate and early diagnosis (Giovagnorio et al. 2003). Histopathologically, metastases of the skin and subcutaneous tissue can develop from almost any kind of malignancy, but nearly half of them derive from melanoma, lung cancer and breast carcinoma (White 1985). In most cases, metastases appear as well-circumscribed solid hypoechoic masses (Nazarian et al. 1998). A lobulated shape and multiple peripheral vascular pedicles feeding internal irregular vessels seem the most important gray-scale and color Doppler US imaging findings for differentiating them from other benign soft-tissue masses (Fig. 2.23) (Giovagnorio et al. 1999, 2003). In follow-up studies, color Doppler imaging has been proposed as a mean to assess the pharmacodynamic response to chemotherapy a b c Fig. 2.21a–c. Arteriovenous malformation. a Transverse gray-scale 15–7-MHz US image of a 6-month-old infant born with a markedly swollen cheek and upper lip reveals marked thickening of the subcutaneous tissue of the lip (arrows). b Corresponding color Doppler 15–7 MHz US image demonstrates numerous enlarged vessels coursing through the thickened subcutaneous tissue. c Spectral Doppler analysis demonstrates high-velocity arterial waveforms within the vessels 39 Skin and Subcutaneous Tissue a c b Fig. 2.22a–c. Venous malformation. a Longitudinal 12–5 MHz US image of the middle forearm show an ill-defined fi sponge-like subcutaneous mass (arrowheads) containing a network of anechoic channels and a hyperechoic dot (arrow) with posterior acoustic shadowing, likely reflecting fl a phlebolith. b Corresponding 12–5 MHz color Doppler US image reveals only a few, weak signals of flow within the soft-tissue mass (arrowheads). c Radiographic correlation confi firms the presence of a few rounded phleboliths (arrow) within the lesion * a c * b Fig. 2.23a–c. Subcutaneous tissue metastases. a,b Gray-scale and c,d color Doppler 12–5 MHz US images in two patients with previously diagnosed malignancies demonstrate well-defi fined homogeneous hypoechoic nodules (asterisk) located within the subcutaneous tissue. In both nodules, correlative color Doppler imaging shows a hypervascular pattern with peripheral and internal vessels. Postsurgical histologic examination revealed metastases from a,c gut carcinoma and b,d colon adenocarcinoma d 40 M. Valle and M. P. Zamorani by depicting reduction of intratumoral blood flow (Fig. 2.24) (Nazarian et al. 1996). In patients operated on for melanoma, detection of any nonpalpable mass in the subcutaneous tissue or any suspected regional lymphadenopathy should be ascertained by means of US-guided biopsy (Fornage and Lorigan 1989). The subcutaneous tissue can be the primary site of involvement of peripheral T-cell (non-Hodgkin) lymphoma (Lee et al. 2003; Fujii et al. 2004; Giovagnorio 1997). This kind of lymphoma involves the skin and the subcutaneous tissue in two main forms: the cutaneous T-cell lymphoma, which is also known as mycosis fungoides or Sézary syndrome, and the subcutaneous panniculitis-like T-cell lymphoma (Lee et a b al. 2003). Mycosis fungoides is an indolent disorder presenting with cutaneous patches, plaques or erythroderma. With time, the skin lesions may progress to cutaneous tumors, peripheral lymphadenopathies and widespread extracutaneous involvement, with a corresponding drop in patient survival rate. At the stage of tumor formation, US is able to demonstrate diffuse or focal hypoechoic thickening of the skin; the imaging features of this lymphoma are, however, nonspecific (see Fig. 2.3a) (Fornage et al. 1993). The subcutaneous panniculitis-like T-cell lymphoma is a rare condition which may be a diagnostic challenge as it mimics inflammatory cellulitis associated with connective tissue disease (Lee et al. 2003; Sy et al. 2005). This disorder usually presents with multiple c Fig. 2.24a–c. Subcutaneous regional metastasis from melanoma. a Gray-scale and b,c color Doppler 15–7 MHz US images in a patient who had a melanoma in his left foot and some regional relapses reveal a small solid homogeneously hypoechoic nodule (arrow) with spiculated margins in the subcutaneous tissue of the left lower leg. The nodule is hypervascular at color Doppler imaging. c After a course of systemic chemotherapy and immunotherapy, the subcutaneous metastasis appears unchanged in size and echotexture but assumes a hypovascular pattern reflecting fl therapy-related change in tumor perfusion a b Fig. 2.25a,b. Subcutaneous panniculitis-like T-cell lymphoma. a Gray-scale and b color Doppler 12–5 MHz US images over an hardened ill-defi fined area in the back show diffuse pseudonodular thickening of the subcutaneous tissue (arrows) with a generalized decrease in echogenicity of the fat lobules and a diffuse hypervascular pattern mimicking cellulitis Skin and Subcutaneous Tissue palpable subcutaneous nodules, and may undergo rapid deterioration secondary to the onset of the hemophagocytic syndrome (marked anemia due to phagocytosis of red blood cells from monocytes and macrophages). US reveals marked increased echogenicity with swelling of the fat lobules and blurry differentiation between the skin and the subcutaneous tissue, an appearance resembling a diffuse inflammatory infiltrate with edema (Fig. 2.25) (Sy et al. 2005). Hypoechoic nodules surrounded by a hyperechoic rim can also be observed (Fujii et al. 2004). Given the similarity with inflammatory cellulitis, regional enlarged lymph nodes could possibly be misinterpreted as reactive in nature (Sy et al. 2005). References Ahuja AT, King AD, Kew J et al (1998) Head and neck lipomas: sonographic appearances. AJNR Am J Neuroradiol 19: 505–508 Akesson A, Forsberg L, Hederstrom E (1986) Ultrasound examination of skin thickness in patients with progressive systemic sclerosis (scleroderma). Acta Radiol Diagn 27: 91–94 Amann P, Botta U, Montet X et al (2003) Sonographic detection and localization of a clinically nondetectable subcutaneous contraceptive implant. J Ultrasound Med 22: 855–859 Anderson MA, Newmeyer WL 3rd, Kilgore ES Jr (1982) Diagnosis and treatment of retained foreign bodies in the hand. Am J Surg 144: 63–67 Arslan H, Sakarya ME, Bozkurt M et al (1998) The role of power Doppler sonography in the evaluation of superficial soft tissue abscesses. Eur J Ultrasound 8: 101–106 Behan M, Kazam E (1978) The echographic characteristics of fatty tissues and tumors. Radiology 129: 143–151 Benson CH, Gibson JY, Harisdangkul V (1983) Ultrasound diagnosis of tophaceous and rheumatoid nodules. Arthritis Rheum 26: 696 Blaivas M, Lyon M, Brannam L et al (2004) Water bath evaluation technique for emergency ultrasound of painful superficial structures. Am J Emerg Med 22: 589–593 Blyme PJ, Lind T, Schantz K et al (1990). Ultrasonographic detection of foreign bodies in soft tissue: a human cadaver study. Arch Orthop Trauma Surg 110: 24–25 Boyse TD, Fessell DP, Jacobson JA et al (2001) US of soft-tissue foreign bodies and associated complications with surgical correlation. RadioGraphics 21: 1251–1256 Brenner JS, Cumming WA, Ros PR (1989) Testicular epidermoid cyst: Sonographic and MR findings. AJR Am J Roentgenol 152: 1344 Brocks K, Stender I, Karlsmark T et al (2000) Ultrasonic measurement of skin thickness in patients with systemic sclerosis. Acta Derm Venereol 80: 59–60 Canturk F, Canturk T, Aydin F et al (2004) Cutaneous linear atrophy following intralesional corticosteroid injection in the treatment of tendonitis. Cutis 73: 197–198 Cardinal E, Bureau N, Aubin B et al (2001) Role of ultrasound in musculoskeletal infection. Radiol Clin North Am 39: 191–201 Chau CL, Griffith JF (2005) Musculoskeletal infections: ultrasound appearances. Clin Radiol 60: 49–59 Choong KKL (2004) Sonographic appearance of subcutaneous angiolipomas. J Ultrasound Med 23: 715–717 Choudhari KA, Muthu T, Tan MH (2001) Progressive ulnar neuropathy caused by delayed migration of a foreign body. Br J Neurosurg 15: 263–265 Clements PJ, Hurwitz EL, Wong WK et al (2000) Skin thickness score as a predictor and correlate of outcome in systemic sclerosis. Arthritis Rheum 43: 2445–2454 Davae KC, Sofka CM, DiCarlo E et al (2003) Value of power Doppler imaging and the hypoechoic halo in the sonographic detection of foreign bodies: correlation with histopathologic findings. J Ultrasound Med 22: 1309–1313 Dean AJ, Gronczewski CA, Costantino TG (2003) Technique for emergency medicine bedside ultrasound identification of a radiolucent foreign body. J Emerg Med 24: 303–308 Dubois J, Garel L (1999) Imaging and therapeutic approach of hemangiomas and vascular malformations in the pediatric age group. Pediatr Radiol 29: 879–893 Dubois J, Patriquin HB, Garel L et al (1998) Soft-tissue hemangiomas in children and infants: diagnosis using Doppler ultrasonography. AJR Am J Roentgenol 171: 247–252 Dubois J, Garel L. David M et al (2002) Vascular soft-tissue tumors in infancy: distinguishing features on Doppler sonography. AJR Am J Roentgenol 178: 1541–1545 Ehara S (1998) MR imaging of fat necrosis. AJR Am J Roentgenol 171: 889 Erickson SJ (1997) High-resolution imaging of the musculoskeletal system. Radiology 205: 593–618 Felman AH, Fisher MS (1969) The radiographic detection of glass in soft tissue. Radiology 92: 1529–1531 Fernando RA, Somers S, Edmonson RD et al (2003) Subcutaneous fat necrosis: hypoechoic appearance on sonography. J Ultrasound Med 22: 1387–1390 Fornage BD, Deshayes JL (1986) Ultrasound of normal skin. J Clin Ultrasound 14: 619–622 Fornage BD, Lorigan JG (1989) Sonographic detection and fine-needle aspiration biopsy of nonpalpable recurrent or metastatic melanoma in subcutaneous tissues. J Ultrasound Med 8: 421–424 Fornage BD, Tassin GB (1991) Sonographic appearances of superficial soft tissue lipomas. J Clin Ultrasound 19: 215–220 Fornage BD, McGavran MH, Duvic M et al (1993) Imaging of the skin with 20-MHz US. Radiology 189: 69–76 Friedman DI, Forti RJ, Wall SP et al (2005) The utility of bedside ultrasound and patient perception in detecting soft tissue foreign bodies in children. Pediatr Emerg Care 21: 487–492 Fujii Y, Shinozaki T, Koibuchi H et al (2004) Primary peripheral T-cell lymphoma in subcutaneous tissue: sonographic findings. J Clin Ultrasound 32: 361–364 Galarza M, Sosa FP (2003) Pure subcutaneous seeding from medulloblastoma. Pediatr Neurol 29: 245–249 Gerster JC, Landry M, Dufresne L et al (2002) Imaging of tophaceous gout: computed tomography provides specific images compared with magnetic resonance imaging and ultrasonography. Ann Rheum Dis 61: 52–54 Giovagnorio F (1997) Sonography of cutaneous non-Hodgkin’s lymphomas. Clin Radiol 52: 301–303 41 42 M. Valle and M. P. Zamorani Giovagnorio F, Andreoli C, DeCicco ML (1999) Color Doppler sonography of focal lesions of the skin and subcutaneous tissue. J Ultrasound Med 18: 89–93 Giovagnorio F, Valentini C, Paonessa A (2003) High-resolution and color Doppler sonography in the evaluation of skin metastases. J Ultrasound Med 22: 1017–1022 Gomez EC, Berman B, Miller DL (1982) Ultrasonic assessment of cutaneous atrophy caused by intradermal corticosteroids. J Dermatol Surg Oncol 8: 1071–1074 Grechenig W, Peicha G, Clement HG et al (1999) Ultrasonographic localization of a displaced screw in the carpal canal. A case report. Acta Radiol 40: 625–627 Horton LK, Jacobson JA, Powell A et al (2001) Sonography and radiography of soft-tissue foreign bodies. AJR Am J Roentgenol 176: 1155–1159 Hwang JY, Lee SW, Lee SM (2005) The common ultrasonographic features of pilomatricoma. J Ultrasound Med 24: 1397–1402 Inampudi P, Jacobson JA, Fessell DP et al (2004) Soft-tissue lipomas: accuracy of sonography in diagnosis with pathologic correlation. Radiology 233: 763–767 Jacobson JA (2005) Musculoskeletal ultrasound and MRI: which do I choose? Semin Musculoskelet Radiol 9:135–149 Kohler R, Kritikos N, Poletti PA et al (2005) Sonographic detection of a subcutaneous twisted expander injection port. J Ultrasound Med 24: 1441–1444 Lee HS, Joo KB, Song HT et al (2001) Relationship between sonographic and pathologic findings in epidermal inclusion cysts. J Clin Ultrasound 29: 374–383 Lee HJ, Im JG, Goo JM et al (2003) Peripheral T-cell lymphoma: spectrum of imaging findings with clinical and pathologic features. RadioGraphics 23: 7–28 Lin JJ, Lin F (1974) Two entities in angiolipoma. A study of 459 cases of lipomas with review of literature on infiltrating angiolipoma. Cancer 34: 720–727 Loyer EM, DuBrow RA, David CL et al (1996) Imaging of superficial soft-tissue infections: sonographic findings in cases of cellulitis and abscess. AJR Am J Roentgenol 166: 149–152 Lyon M, Brannam L, Johnson D et al (2004) Detection of soft tissue foreign bodies in the presence of soft tissue gas. J Ultrasound Med 23: 677–681 Malherbe A, Chemantais J (1880) Note sur l’épithélioma calcifie des glandes sebacées. Prog Med 8: 826–837 Maxwell AJ, Mamtora H (1990) Sonographic appearance of epidermoid cyst of the testis. J Clin Ultrasound 18: 188– 190 McGrath MH, Fleisher A (1989) The subcutaneous rheumatoid nodule (1989) Hand Clin 2: 127–135 Mellado JM, Pérez del Palomar L, Diaz L et al (2004) Long standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol 182: 1289–1294 Merki-Feld GS, Brekenfeld C, Migge B, et al (2001) Nonpalpable ultrasonographically not detectable Implanon rods can be localized by magnetic resonance imaging. Contraception 63: 325–328 Morel-Lavallée M (1863) Décollements traumatiques de la peau et des couches sous-jacentes. Arch Gen Med 1: 20–38; 172–200; 300–332 Murphey MD, Carroll JF, Flemming DJ et al (2004) Benign musculoskeletal lipomatous lesions. RadioGraphics 24: 1433–1466 Nalbant S, Corominas H, Hsu B et al (2003) Ultrasonography for assessment of subcutaneous nodules. J Rheumatol 30:1191–1195 Nazarian LN, Alexander AA, Rawool NM et al (1996) Malignant melanoma: impact of superficial US on management. Radiology 199: 273–277 Nazarian LN, Alexander AA, Kurtz AB et al (1998) Superficial melanoma metastases: appearances on gray-scale and color Doppler sonography. AJR Am J Roentgenol 170: 459–463 Nessi R, Betti R, Bencini PL et al (1990) Ultrasonography of nodular and infiltrative lesions of the skin and subcutaneous tissues. J Clin Ultrasound 18: 103–109 Neumann CG (1957). The expansion of an area of skin by progressive distension of a subcutaneous balloon: use of the method for securing skin for subtotal reconstruction of the ear. Plast Reconstr Surg 19: 124–130 Paltiel HJ, Burrow PE, Kozakewich HPW et al (2000) Soft-tissue vascular anomalies: utilities of US for diagnosis. Radiology 214: 747–754 Parra JA, Fernández MA, Encinas B et al (1997) Morel-Lavallée effusions in the thigh. Skeletal Radiol 26: 239–241 Peterson JJ, Bancroft LW, Kransdorf MJ (2002) Wooden foreign bodies: imaging appearance. AJR Am J Roentgenol 178: 557–562 Piessens SG, Palmer DC, Sampson AJ (2005) Ultrasound localization of non-palpable implanon. Aust N Z J Obstet Gynaecol 45:112–116 Rettenbacher T, Macheiner P, Hollerweger A et al (2001) Suture granulomas: sonography enables a correct preoperative diagnosis. Ultrasound Med Biol 27: 343–350 Robben SGF (2004) Ultrasonography of musculoskeletal infections in children. Eur Radiol 14: L65–L67 Roberts CC, Liu PT, Colby TV (2003) Encapsulated versus nonencapsulated superficial fatty masses: a proposed MR imaging classification. AJR Am J Roentgenol 180: 1419–1422 Scheja A, Akesson A (1997) Comparison of high frequency (20 MHz) ultrasound and palpation for the assessment of skin involvement in systemic sclerosis (scleroderma). Clin Exp Rheumatol 15: 283–288 Schmid-Wendtner MH, Burgdorf W (2005) Ultrasound scanning in dermatology. Arch Dermatol 141: 217–224 Shiels WE, Babcock DS, Wilson JL et al (1990) Localization and guided removal of soft-tissue foreign bodies with sonography. AJR Am J Roentgenol 155: 1277–1281 Soudack M, Nachtigal A, Gaitini D (2003) Clinically unsuspected foreign bodies: the importance of sonography. J Ultrasound Med 22: 1381–1385 Struk DW, Munk PL, Lee MJ et al (2001) Imaging of soft-tissue infections. Radiol Clin North Am 39: 277–303 Sy ANL, Lam TPW, Khoo US (2005) Subcutaneous panniculitislike T-cell lymphoma appearing as a breast mass: a difficult and challenging case appearing at an unusual site. J Ultrasound Med 24: 1453–1460 Thomas RH, Holt MD, James SH et al (2001) “Fat fracture”: a physical sign mimicking tendon rupture. J Bone Joint Surg Br 83: 204–205 Tiliakos N, Morales AR, Wilson CH Jr (1982) Use of ultrasound in identifying tophaceous versus rheumatoid nodules. Arthritis Rheum 25: 478–479 Trop I, Dubois J, Guibaud L et al (1999) Soft-tissue venous malformations in pediatric and young adult patients; diagnosis with Doppler US. Radiology 212: 841–845 Skin and Subcutaneous Tissue Tsai TS, Evans HA, Donnelly LF et al (1997) Fat necrosis after trauma: a benign cause of palpable lumps in children. AJR Am J Roentgenol 169: 1623–1626 Uglesic V, Knezevic P, Milic M et al (2004) Madelung syndrome (benign lipomatosis): clinical course and treatment. Scand J Plast Reconstr Surg Hand Surg 38: 240–243 Vincent LM, Parker LA, Mittelstaedt CA (1985) Sonographic appearance of an epidermal inclusion cyst. J Ultrasound Med 4: 609–611 Wilson DJ (2004) Soft-tissue and joint infection. Eur Radiol 14(Suppl 3): 64–71 White JW (1985) Evaluating cancer metastatic to the skin. Geriatrics 40: 67–72 Wortham NC, Tomlinson IP (2005). Dercum’s disease. Skinmed 4: 157–162 Yen ZS, Wang HP, Ma HM et al (2002) Ultrasonographic screening of clinically-suspected necrotizing fasciitis. Acad Emerg Med 9: 1448–1451 43 Muscle and Tendon 3 Muscle and Tendon Maria Pia Zamorani and Maura Valle CONTENTS 3.1 3.1.1 3.1.2 3.1.3 3.1.3.1 3.1.3.2 3.1.4 3.1.4.1 3.1.4.2 3.1.4.3 3.1.5 3.1.5.1 3.1.5.2 3.1.5.3 3.1.6 3.1.6.1 3.1.6.2 3.1.6.3 3.1.6.4 3.1.6.5 3.2 3.2.1 3.2.2 3.2.3 3.2.4 3.2.4.1 3.2.4.2 3.2.5 3.2.5.1 3.2.5.2 3.2.5.3 3.2.6 3.2.6.1 3.2.6.2 Muscle 45 Histologic Considerations 45 Normal US Anatomy and Scanning Technique 46 Anatomical Variants and Heritable Disorders 50 Muscle Agenesis, Anomalous and Accessory Muscles 50 Neuromuscular Disorders 52 Traumatic Lesions 54 Myotendinous Strains 55 Contusion and Laceration 56 Myositis Ossificans 57 Inflammatory and Ischemic Conditions 59 Idiopathic Inflammatory Myopathies 59 Pyomyositis, Abscess, and Hydatid Disease 61 Diabetic Muscle Infarction and Rhabdomyolysis 62 Tumors 64 Intramuscular Hemangioma 64 Deep-Seated Lipoma and Liposarcoma 66 Intramuscular Myxoma 67 Desmoid 69 Rhabdomyosarcoma and Metastases 71 Tendon 71 Histologic Considerations 71 Normal US Anatomy and Scanning Technique 72 Tendon Instability 75 Degenerative Changes and Tendon Tears Tendinosis and Partial Tears 76 Complete Tears and Postoperative Findings Inflammatory Conditions 83 Paratendinitis and Attrition Bursitis 84 Tenosynovitis 85 Enthesopathy 87 Tumors and Tumor-Like Conditions 88 Intratendinous and Tendon Sheath Ganglia Giant Cell Tumor of the Tendon Sheath References 45 76 79 88 89 91 M. P. Zamorani, MD Unité de Recherche et Dévelopement, Clinique des Grangettes, 7, ch. des Grangettes, 1224 Genève, Switzerland M. Valle, MD Staff Radiologist, Reparto di Radiologia, Istituto Scientifico “Giannina Gaslini”, Largo Gaslini 5, 16148 Genova, Italy 3.1 Muscle 3.1.1 Histologic Considerations On the whole, skeletal muscles can be regarded as the largest organ of the human body, accounting for approximately 25–35% of the total body weight in women and 40–50% in men (Hollman and Hettiger 1990). They are made up of two components: the muscle fibers, which are long and cylindrical in structure, representing the cellular unit of muscle, and stromal connective tissue. Individual muscle fibers are grouped together in bundles, which are commonly known as fascicles, and several fascicles join together to form an individual muscle (Fig. 3.1a). Thin connective tissue strands – the endomysium – separate the individual muscle fibers; a more substantial connective sheath with small vessels and nerve endings, the perimysium (also referred to as fibroadipose septa), envelops individual fascicles; a thick fibrous layer, the epimysium, surrounds the entire muscle (Fig. 3.1a). Muscle fibers vary in length and cross-sectional diameter depending on the individual muscle. Fascicles may be either coarse, as in the case of large muscles, or very fine, as in the case of small muscles that coordinate precise movement (Erickson 1997). They insert into the different connective tissue components of the muscle, including the peripheral epimysium and central major septa formed by converging fibroadipose septa. At their distal end, intramuscular septa join into large tendinous layers – commonly referred to as aponeuroses – or directly to tendons. The internal arrangement of the muscle varies according on the fascicular orientation, which reflects gross muscle shape and function. A parallel arrangement is found in strap-like (e.g., sartorius) and quadrilateral (e.g., thyrohyoid) muscles, in which fibers course nearly the full length of the long axis of the muscle; the rectus abdominis shows 49 Muscle and Tendon T a b Fig. 3.4a,b. Intramuscular aponeuroses. a Long-axis and b short-axis 12–5 MHz US images of the normal tibialis anterior muscle (arrowheads) demonstrate the feather-like arrangement of a circumpennate muscle created by the convergence of the fibroadipose septa upon the internal aponeurosis. The aponeurosis (straight arrows) appears as a highly reflective linear echo within the muscle that is thicker than the fibroadipose septa (curved arrow). T, tibia a b Fig. 3.5a,b. Muscle anisotropy. Short-axis 17–5 MHz US images of the biceps brachii muscle (arrows) examined with a perpendicular angle between the transducer face and the orientation of the muscle fibers and b an angle that deviates slightly from the perpendicular. In a, the muscle appears diffusely hyperechoic owing to the highest specular reflectivity from the perimysium interfaces. In b, the overall muscle becomes more hypoechoic with decreased intensity of echoes from the perimysium. On the other hand, the larger fibroadipose septa (arrowhead) are more visible. Tilting the probe over the muscle may be useful to distinguish artifactual hypoechoic patterns from mild strains fibroadipose septa, US is able to recognize the internal architecture of pennate muscles as semipennate, unipennate, bipennate, or multipennate (Fig. 3.6). Intramuscular vessels coursing within the hyperechoic septa are visible on color and power Doppler imaging. The outer muscle fascia (epimysium) appears as a well-delineated echogenic envelope circumscribing the hypoechoic muscle. Large hyperechoic septa (aponeuroses) directed within the muscle belly can be seen arising from it. In complex muscles, an individual hyperechoic fascial sheath surrounds each muscle belly thus helping the examiner to recognize the different heads. The interstice between juxtaposed fasciae of two adjacent muscles appears as a hypoechoic band and corresponds to loose connective tissue that allows some sliding of the muscles during contraction. Focal interruptions of the muscle fascia are found at the points where nerves, veins, and arteries (perforating vessels) enter the muscles. When the muscle fascia lies under the subcutaneous tissue, it adheres to the superficial fascia and cannot be distinguished from it. Dynamic US scanning performed during muscle contraction can show changes in size and relationship of fascicles and fibroadipose septa. On short-axis planes, contracted muscles usually appear thicker and more hypoechoic. Intramuscular septa change their appearance and orientation as a result of the action of 50 M. P. Zamorani and M. Valle a b Fig. 3.6a,b. Internal architecture of skeletal muscles. a Fusiform muscle. Long-axis 12–5 MHz US image over the deltoid muscle (arrows) demonstrates the fibroadipose septa (arrowheads) as hyperechoic lines separating the hypoechoic muscle bundles. These septa have a parallel arrangement along the muscle belly. b Pennate muscle. Long-axis 125 MHz US image over the tibialis anterior muscle (arrows) demonstrates the fibroadipose septa (arrowheads) as they converge on the highly reflective aponeurosis (curved arrow), giving the appearance of a feather the muscle fibers that attach into these structures. In the medial head of gastrocnemius, for instance, pennation angle increases from 15.5° to 33.6° when examined during isometric contraction (Fig. 3.7) (Narici et al. 1996). Shortening of muscles is well appreciated on long-axis images during concentric contraction. Recently, a method to measure muscle tissue perfusion by means of contrast-enhanced power Doppler US has been developed with quantification of intramuscular blood flow performed at rest and after exercise (Krix et al. 2005). 3.1.3 Anatomical Variants and Heritable Disorders 3.1.3.1 Muscle Agenesis, Anomalous and Accessory Muscles Muscle agenesis indicates the absence of one muscle or one head of a complex muscle as a result of incomplete or imperfect development. In general, the diagnosis is already evident at physical examination. US α A a β A b Fig. 3.7a,b. Pennation angle. Long-axis 12–5 MHz US images of the medial head of gastrocnemius obtained a at rest and b during isometric contraction demonstrate an increased pennation angle during muscle activation. The pennation angle is given by the incidence of the muscle fibers (dashed line) relative to the aponeurosis (A), which represents the direction of force generation (double arrow). Note that this angle is greater during contraction (β) than at rest (α) Muscle and Tendon causes profound US changes in muscle architecture with increased echogenicity, loss of heterogeneity, and shadowing (Fig. 3.10). The increased echogenicity of muscle reflects an increased number of acoustic interfaces related to fat accumulation, fibrosis, and inflammation. In neuromuscular disorders, the increased reflectivity of muscles is associated with a decreased ability of the US beam to penetrate deeper structures, leading to loss of bone edge definition and bone shadowing (Fischer et al. 1998; Walker et al. 2004). In addition, the disease process blurs the distinction between fibroadipose septa and muscle fascicles, making the image more homogeneously echogenic (Fig. 3.10a). Similarly, peripheral neuropathies are often associated with selective atrophy of the innervated muscles. US is able to evaluate the size and echotexture of the affected muscles by comparing the two extremities (Scholten et al. 2003). A definite loss in bulk of the affected muscle would suggest atrophy. This can be appreciated by simple pattern recognition analysis (concave or straight muscle boundaries instead of the normal convex surface). Because side-to-side differences in muscle thickness rarely exceed 20%, measuring the muscle diameters or cross-sectional area with the electronic calipers of the equipment seems to be a more reliable means to assess volume changes in a given group of muscles than subjective evaluation (Bargfrede et al. 1999). The ratio of muscle thickness to subcutaneous fat thickness was found to be helpful in specific neuromuscular disorders (decreased ratio in spinal muscle atrophy). In neuromuscular disorders, however, US has shown some limitations compared with MR imaging. The complex distribution of muscle involvement in some dystrophies seems more reliably mapped with MR imaging because of its better anatomic rendering and panoramic view. Based on echotextural pattern analysis, US is not as accurate as MR imaging in distinguishing early neurogenic atrophy (in which changes are mainly related to extracellular edema) from late atrophy (in which muscle tissue is gradually replaced by fat). Unlike MR imaging, in which early denervation is appreciated by a homogeneous hyperintense pattern on T2-weighted and STIR sequences (increase in free-water content) and late denervation by a hyperintense pattern on T1-weighted images (fatty replacement), at US the two processes have a similar hyperechoic pattern and can be hardly differentiated (Fig. 3.11) (Kullmer et al. 1998). Quantification of muscle echotexture to estimate the severity of atrophy would reduce the observer variability but is strongly influenced by the scanner and the MHG T soleus F ∗ a ∗ b c Fig. 3.10a–c. Neuromuscular disorders. a,b Transverse 12-5 MHz US images obtained over the a posteromedial and b posterolateral aspect of the middle third of the leg in a 12-year-old child with Duchenne dystrophy. The affected medial head of the gastrocnemius (MHG) and soleus exhibits a diffusely hyperechoic pattern with strong US beam attenuation (asterisks) and blurred distinction of fibroadipose septa. The acoustic shadowing leads to inability of the US beam to penetrate deep structures. In b, there is loss of bone edge definition of the fibula (F) caused by the abnormal muscle reflectivity (arrows). T, tibia. c Photograph showing calf muscle pseudohypertrophy. The patient had progressive symmetric muscle weakness associated with elevated serum CK levels, myalgia, cramps, and stiffness after exercise 53 54 M. P. Zamorani and M. Valle T T a b T T c d Fig. 3.11a–d. Neurogenic atrophy of muscles in two different patients with a,b recent-onset and c,d long-standing peroneal neuropathy. a Transverse 12–5 MHz US image over the tibialis anterior muscle with b fat-suppressed T2-weighted MR imaging correlation demonstrates normal volume and diffusely hyperechoic appearance of the muscle (arrowheads). The abnormal echotexture is related to intramuscular edema (curved arrow). c Transverse 12-5 MHz US image over the tibialis anterior muscle with d T1-weighted MR imaging correlation reveals decreased volume and hyperechoic appearance of the muscle (arrowheads). Although similar to that seen in a, the abnormal echotexture reflects fatty atrophy (curved arrow). T, tibia equipment settings (Bargfrede et al. 1999; Pillen et al. 2003; Scholten et al. 2003). Apart from the above limitations, US can be considered a useful tool complementary to electrophysiology to provide information on muscle morphology, which is beyond the scope of electrodiagnosis. In patients with unilateral disorders, US images of the affected muscle can be compared with those of the unaffected side. In these cases, careful positioning of the transducer by surface landmarks is needed to ensure symmetric imaging. Transverse images are best suited for muscle measurements. In patients with bilateral disorders, comparative US evaluation should be conducted by selecting a control muscle in a healthy area, possibly with similar degrees of overlying subcutaneous tissue. Finally, when examining an atrophied fatty-infiltrated muscle, the examiner must be aware that changes may occur not only as a result of a denervation process but also following disuse or a complete tendon tear (Yao and Metha 2003). Then the integrity of the tendon belonging to the affected muscle must be carefully assessed. 3.1.4 Traumatic Lesions Based on their pathomechanism, muscle injuries can be grouped into two main classes: extrinsic and intrinsic. Extrinsic injuries result from external trauma, either a contusion or a penetrating injury (laceration), whereas intrinsic injuries are most often the result of contraction and simultaneous elongation of a given muscle. In the first class, the location of the tear strictly matches the site of the trauma. These lesions typically occur in areas where the muscle is compressed between the applied outer force (direct blow) and an underlying hard bony surface (e.g., quadriceps muscles against the femoral shaft). On the other hand, intrinsic ruptures almost invariably lead to a disruption of muscle fibers near the myotendinous junction, which is considered the weakest ring of the muscle-tendonbone unit because it has less capacity for energy absorption than the other structures (Palmer et al. 1999). The myotendinous junction is the most common site of partial or complete muscle injury 56 M. P. Zamorani and M. Valle Clinically, muscle strain injuries can be classified into a four-step grading system: grade 1 indicates a tear affecting a small number of muscle fibers with an intact fascia; grade 2 refers to a moderate tear with the fascia remaining intact; grade 3 injury is a tear of many fibers with partial tearing of the fascia; grade 4 injury indicates a complete tear of the muscle and the fascia (Ryan 1969). Healing and recovery of function takes longer with a high-grade injury, and the long-term outcome is generally worse (Noonan and Garrett 1999). Initially, treatment of a muscle strain injury includes rest, application of ice, and compression for relieve of pain and swelling; nonsteroidal inflammatory drugs may also be administered for pain relief in the first days after trauma. After resolution of the acute pain and swelling, physical therapy performed avoiding excessive fatigue and with adequate warm-up before exercise may contribute to the restoration of muscle strength and flexibility (Noonan and Garrett 1999). The long-term outcome after muscle strain injury is usually good and complications are rare. Muscle strain injuries appear at US as avulsion and retraction of muscle fibers from the tendon or aponeurosis in which they attach (Fig. 3.12b,c) (Bianchi et al. 1998). The examiner must be aware that some muscles (e.g., rectus femoris) have a complex structure with internal tendons: in these cases, the injury may occur in the mid-portion of the muscle belly and not at its distal portion as may be expected (Bianchi et al. 2002). US signs of muscle tear include avulsion and proximal retraction of the fibroadipose septa. In low-grade injuries, the space between the retracted septa and the aponeurosis is filled with a hyperechoic area reflecting extravasation of blood and clots. These small lesions may go unnoticed if an accurate scanning technique with careful and systematic examination of the distal portion of the fibroadipose septa is not employed. On the other hand, larger muscle tears are characterized by a more substantial blood collection which makes them easily detectable. This does not occur immediately after the trauma, but 1–2 days later, when the collection tends to become more hypoechoic. A widely accepted classification of muscle injuries is based on a four-grade scale (Peetrons 2002). Grade 0 injury corresponds to a normal US appearance in spite of the presence of local clinical findings; in grade 1 injury, subtle US findings may be observed, including ill-defined hyperechoic or hypoechoic intramuscular areas or a swollen aponeurosis (Fig. 3.13); grade 2 and grade 3 correspond to partial and complete muscle tears, in which incomplete or full discontinuity of the muscle occurs. In mild trauma, an early assessment with US can lead to false negative results because the hematoma is diffuse and manifests as scattered blurred hyperechoic areas within muscle rather than as a focal well-defined hypoechoic collection: fat-suppressed T2-weighted MR imaging is superior to US in depicting mild strains soon after the trauma. During healing, the hemorrhagic cavity shrinks and its walls progressively thicken and collapse. The time at which the lesion is filled in can be considered an indicator for restarting low-level activity with care. However, this should be only decided in the absence of clinical symptoms and when a sufficient delay has occurred between the injury and the resumption of sports activities (never less than 4–6 weeks after the end of symptoms) (Peetrons 2002). In late phases, fibrous scars are seen as blurred hyperechoic zones within muscle: they are often observed in significant trauma or when the sporting activity was resumed too early (Fig. 3.14) (Peetrons 2002). Usually, scars are weakly symptomatic, but the risk of recurrent injury seems to be proportional to their extent in the muscle. 3.1.4.2 Contusion and Laceration Direct external trauma may result in local hematoma, contusion, and partial and complete muscle laceration. Although virtually all muscles can be involved during sporting or recreational activities, the most frequently injured are the vastus intermedius and the vastus lateralis. These anterior thigh muscles are particularly predisposed to injury in athletes whose sports require direct hard contact (e.g., soccer, football, rugby, and hockey). The mechanism of injury often consists of crushing of the muscle against the femoral shaft by the knee of another player. Contusion injuries following extrinsic trauma are depicted with US as muscle swelling with focal irregularities and echotextural changes. The muscle architecture is no longer recognized as it is altered by disruption of the muscle fibers and hematoma (Fig. 3.15a). Depending on the overall strength of the applied force, partial or complete tears can occur. Abnormalities are typically located at the actual site of trauma and not at the myotendinous junction: this helps in distinguishing a contusion injury from a muscle strain. If a large fluid collection is present, Muscle and Tendon a b ∗ c d e Fig. 3.13a–e. Myotendinous strains. Two different cases of central aponeurosis strain of the rectus femoris muscle following minimal trauma. a,b Case 1. a Short-axis and b long-axis 12–5 MHz US images over the middle third of the rectus femoris muscle demonstrate an ill-defined hyperechoic area (arrowheads) surrounding the aponeurosis related to edema and hemorrhagic changes. Note the normal-appearing external portion of the muscle (arrows). c–e Case 2. Short-axis 12–5 MHz US images obtained from c proximal to e distal over the rectus femoris reveal progressive swelling and hypoechoic appearance (arrowheads) of the central aponeurosis (straight arrows) and adjacent muscle fibers (curved arrow) with a small hematoma (asterisk) reflecting a myotendinous strain the muscle ends can be seen floating within the hematoma. Closed muscle trauma by a sharp object may be associated with laceration of the subcutaneous tissue. In these cases, the hematoma expands vertically through the subcutaneous layer and the muscle (Fig. 3.15b). A direct shock injury may also result in disruption of the muscle fascia causing a muscle hernia (Bianchi et al. 1995a; Beggs 2003). In these patients, US demonstrates interruption of the hyperechoic fascial layer and focal extrusion of muscle tissue within the subcutaneous fat (see Chapter 15). Muscle lacerations are much less common and are more often encountered after trauma than after sports accidents. In these instances, irrigation and debridement followed by suture repair of the fascia is indicated. 3.1.4.3 Myositis Ossificans There are three main complications of muscle tear: cysts and myositis ossificans and, more rarely, calcific myonecrosis (Peetrons 2002). Intermuscular and intramuscular cysts may be encountered after muscle trauma as well-defined echo-free masses with posterior acoustic enhancement. These cysts have an elongated shape and represent the residue of a local hematoma. Their most common location is the calf (see Chapter 15). In selected cases, they may require percutaneous needle evacuation. Calcific myonecrosis is a space-occupying calcified mass that typically develops in the anterior compartment of the leg late after a closed lower extremity 57 58 M. P. Zamorani and M. Valle a b c Fig. 3.14a–c. Healing rectus femoris strain. a Long-axis extended field-of-view and b short-axis 17–5 MHz US images of the rectus femoris muscle in a patient with prior myotendinous strain reveal an intramuscular echogenic area (arrows) in proximity to the central aponeurosis (arrowheads) representing residual scar tissue. c Correlative axial gradient-echo T2*-weighted MR image s s m m m a b Fig. 3.15a,b. Closed contusion trauma. Two different cases of thigh muscle injuries following blunt trauma by sharp objects. a Transverse 12–5 MHz US image over the vastus lateralis (m) reveals an extensive laceration of muscle tissue filled in with hypoechoic hematoma (arrowheads). Note the intact subcutaneous tissue (s). b Transverse 12-5 MHz US image over the medial thigh demonstrates combined laceration of the subcutaneous tissue (s) and the gracilis muscle (m) with interruption of the fascia (arrows). The defect is filled in with hypoechoic hematoma (arrowheads) Muscle and Tendon trauma, and is often seen in association with vascular injury or a compartment syndrome (Dhillon et al. 2004). In this condition, the injured muscle may be replaced with a complex mass consisting of a central cystic core containing necrotic muscle, fibrin, cholesterol, and organizing thrombus, together with a peripheral calcified rim. US demonstrates calcified myonecrosis as an intramuscular extensive calcified mass with posterior acoustic shadowing and may help to guide the aspiration of the fluid component as an aid in management (Batz et al. 2006). The main differential diagnosis of calcific myonecrosis is the more common myositis ossificans, given the fact that the extensive calcified shell may mask the internal fluid component at US examination. Myositis ossificans is a benign self-limiting condition presenting as an intramuscular mass with predominant involvement of the large muscles of the extremities, the large muscles of the thigh and the anterior muscles of the arm being the most commonly affected (Thomas et al. 1991). The term “myositis” is a misnomer because this condition is not inflammatory. It usually results from a severe contusion trauma or chronic microtrauma, but may also be seen in patients with other disease or may develop spontaneously. There is, however, debate as to whether unrecollected trauma is present in these cases. From the histologic point of view, this condition exhibits a typical maturation pattern that allows a proliferative mesenchymal response (early pseudosarcomatous phase) to evolve toward formation of heterotopic mature bone. During maturation of the lesion, a zonal pattern develops with three concentric zones: the inner zone is characterized by areas of hemorrhage and necrotic muscle with proliferating fibroblasts; the middle zone consists of immature osteoid formation and islets of cartilage preceding bone formation; and the outer zone is formed by mature bone (Gindele et al. 2000). Peripheral bone formation usually starts 6–8 weeks after the trauma, but it can occur earlier. In the late phase, the lesion can ossify as a whole with formation of a cortex and marrow spaces (Ackermann 1958). As it matures the lesion regresses in size, disappearing spontaneously in approximately 30% of cases (Schulte et al. 1995). Development of peripheral calcifications is a peculiar feature of myositis ossificans and makes this condition more easily diagnosed with X-ray modalities, including plain films and CT, than with US and MR imaging. In the early stages of disease (before the sixth week of evolution), when formation of calcifications has not yet occurred, the imaging diagnosis is not straightforward: it can be difficult to distinguish lesions at this stage from a soft-tissue malignancy. The US findings of myositis ossificans change with the lesion’s age, reflecting the evolving histology (Fornage and Eftekhari, 1989; Peck and Metreweli, 1988). Initially, the US appearance of myositis ossificans has been described as that of an intramuscular hypoechoic ovoid mass with an echogenic center, and even a so-called zone phenomenon matching the maturation process has been reported (Kramer et al. 1979; Thomas et al. 1991; Gindele et al. 2000). In more detail, early lesions are characterized by a peripheral thin hypoechoic zone enveloping a broader highly reflective zone within which a third central hypoechoic zone is found (Fig. 3.16a) (Thomas et al. 1991). With progressive maturation, the peripheral hypoechoic rim may become hyperechoic as a result of increasing ossification: a sheet-like or eggshell-like calcified rim is considered very suggestive of myositis ossificans (Peck and Metreweli, 1988). Then, visualization of the lesion center and the separation of the lesion from the underlying bony cortex may become more difficult because of the acoustic shadowing from peripheral calcifications (Gindele et al. 2000). The process of ossification is apparent with US approximately 2 weeks earlier than with plain radiographs (Peetrons 2002). Although the typical pattern of calcifications is characteristic, we believe that a standard radiograph must always be obtained to confirm the diagnosis and to exclude more aggressive calcified lesions, including paraosteal and soft-tissue sarcomas (Fig. 3.16b,c). After surgical resection, US has proved able to detect recurrence of myositis ossificans and to differentiate this condition from extraosseous sarcomas (Okayama et al. 2003). 3.1.5 Inflammatory and Ischemic Conditions Inflammatory myopathies include a heterogeneous group of acquired and potentially treatable disorders caused by an autoimmune process (idiopathic inflammatory myosites) or infectious agents (pyomyositis). Among ischemic conditions, we focus here mainly on diabetic muscle infarction and rhabdomyolysis. As previously stated, compartment syndromes are addressed in Chapter 15. 3.1.5.1 Idiopathic Inflammatory Myopathies Based on their unique clinical, histopathologic, immunologic, and demographic features, idio- 59 61 Muscle and Tendon a b Fig. 3.17a,b. Polymyositis and associated scleroderma. a Long-axis and b short-axis 12–5 MHz US images over the medial head of gastrocnemius reveal an intramuscular ill-defined hypoechoic area (arrows) with loss of the fibroadipose pattern, reflecting edema and fatty tissue infiltration. The subcutaneous tissue appears normal fications (Mulier et al. 1999; Wlachovska et al. 2004). This lesion has been described as having a “scaffolding” pattern between continuous muscle bundles on long-axis scans and a “checkerboard” pattern on short-axis images (Sarteschi et al. 1997). Longitudinal US images may also demonstrate muscle swelling with preservation of the normal fibrillar pattern, disrupted by hypoechoic lines in a geometric shape, somewhat resembling “dry cracked mud” (Fig. 3.18) (Pagonidis et al. 2005). Although imaging studies may suggest such an inflammatory process (very rapidly growing mass in a muscle compartment), incisional biopsy is usually needed to rule out soft-tissue malignancy and to avoid radical excision. Sarcoidosis, a systemic granulomatous disease, may occasionally involve the skeletal muscles, leading to either palpable nodules or chronic progressive wasting and muscle atrophy or acute myositis (Otake 1994; Tohme-Noun et al. 2003). The muscles of the proximal portions of the extremities are predominantly involved. In nodular-type sarcoidosis, US is able to display well-defined hypoechoic nodules elongated along the muscle fibers and to guide percutaneous biopsy to the appropriate site (Levine et al. 1996; Tohme-Noun et al. 2003). Histologic detection of noncaseating granulomas surrounded by normal muscle tissue allows a definitive diagnosis. In large sarcoid nodules, a hyperechoic center can be depicted with US (Otake 1994). In patients with pulmonary sarcoidosis and painful leg muscles, the possibility of muscular sarcoidosis should be taken into account by the examiner. Color Doppler imaging may be helpful to rule out phlebitis. 3.1.5.2 Pyomyositis, Abscess, and Hydatid Disease Pyomyositis is a suppurative bacterial infection of muscle, most commonly affecting the larger muscles of the lower limb (Chau and Griffith 2005). This condition most often occurs in immunocompromised patients with HIV-AIDS or diabetes and has a higher prevalence in tropical countries, where it is responsible for 3–5% of all hospital admissions (Canoso and Barza 1993; Trusen et al. 2003). However, it may follow even minor blunt trauma and local hematoma. The major causative agent is Staphylococcus aureus followed by Mycobacterium tuberculosis (psoas muscle infection following tuberculous spondylodiscitis), and Streptococcus pyogenes (Bickels et al. 2002). From the clinical point of view, pyomyositis presents with or without fever, dull cramping pain for 10–21 days, and localized muscle tenderness (Trusen et al. 2003). The US appearance of infection of the muscles has been described both in adults (Chau and Griffith 2005) and in children (Trusen et al. 2003). Initially (inflammatory phase), US reveals muscle swelling, a diffuse hyperechoic appearance reflecting edema, and hyperemia (Fig. 3.19) (Bureau et al. 1999; Chau and Griffith 2005). Small hypoechoic foci within the abnormal muscle related to early necrosis and small abscesses may be noted. At this stage, pyomyositis usually responds well to antibiotic therapy. Later in the course of the disease, an overt muscle abscess develops (suppurative phase). Muscle abscesses appear as fluid collections with well-defined posterior enhancement and variable echotexture, ranging from hypoechoic to hyper- Muscle and Tendon ∗ a b c d Fig. 3.19a-d. Pyomyositis in a 65-year-old man with fever and left thigh pain after sustaining blunt trauma to this area. a,b Transverse a gray-scale and b color Doppler 12–5 MHz US images reveal a swollen vastus lateralis muscle with heterogeneous echotexture consisting of increased echogenicity (arrows) as well as hypoechoic areas (asterisk) in which fibroadipose echoes are lost or spaced out. Posterior to this abnormal area, muscle tissue retains a normal appearance (arrowheads). Diffuse intramuscular hyperemia is detected at color Doppler imaging. c,d Correlative axial c T1-weighted and d T2-weighted MR images demonstrate marked hyperintense T2 signal and swelling of the vastus lateralis with irregular borders and diffuse fascial involvement (arrows) ∗ ∗ a F b c Fig. 3.20a–c. Muscle abscess. a Transverse 12–5 MHz US image over the anterior thigh in a middle-aged immunocompromised patient with fever, pain, and local signs of infection with b T2-weighted and c Gd-enhanced T1-weighted MR imaging correlation shows a swollen heterogeneous vastus intermedius muscle (arrows) with internal fluid-filled areas (asterisks) and debris, consistent with local abscess formation. F, femoral shaft. US-guided aspiration yielded purulent fluid that grew Staphylococcus aureus up. Symptoms resolved with percutaneous drainage and antibiotic therapy 63 Muscle and Tendon b ∗ a c d Fig. 3.22a–d. Diabetic infarction. a Anteroposterior plain film of the right leg in a 60-year-old patient with diabetic infarction of the distal lower extremity shows discrete soft-tissue swelling (arrows) in the anterolateral compartment musculature. b Longitudinal 12–5 MHz US image reveals a hypoechoic intramuscular area with deranged echotexture (arrows), which is limited to the tibialis anterior muscle. c,d Axial fat-suppressed c gradient-echo T2* and d gadolinium-enhanced T1-weighted MR images show diffuse edema of the tibialis anterior muscle (arrow) and a ring of high signal intensity after gadolinium administration surrounding an unenhanced central core (asterisk) the muscle fibers. As already described in Chapter 2, the term “hemangioma” encompasses a wide spectrum of lesions from capillary forms to vascular malformations – including capillary, cavernous, arteriovenous, venous, and mixed types – based on the predominant type of vascular channel involved (Olsen et al. 2004). In addition to their vascular components, hemangiomas can contain thrombus, calcification, hemosiderin, fat, smooth muscle, and fibrous tissue, reactive fat being the most common association. The variety of tissues found in muscular hemangiomas explains their heterogeneous appearance. US demonstrates a complex ill-defined mass within the affected muscle, characterized by a mixture of hypoanechoic and hyperechoic (reactive fat overgrowth) components (Fig. 3.23) (Derchi et al. 1989). Prominent vascular channels can be identified on gray-scale and Doppler imaging as well. One-toone correlation between US and MR images shows good correspondence between intratumor hyper- echoic areas and fat (high T1 signal), and hypoechoic components and blood-filled cavities (high T2 signal). Phleboliths within the mass are present in approximately 50% of cases and are best identified on plain films (Fig. 3.23f) (Murphey et al. 1995). At US, they appear as bright dots with posterior acoustic shadowing that are usually located within the hypoechoic component of the hemangioma. Doppler imaging characteristics of hemangiomas are described in Chapter 2. Overall, US can diagnose hemangiomas, especially when phleboliths are detected within the mass. During prolonged observation, very slow blood motion in the hypoechoic cavities of the mass can be appreciated on gray-scale imaging, like a “swarming mass”. In some instances, however, the assessment of hemangiomas may be difficult: in particular, the boundaries of the lesion are usually undefined, especially in large masses infiltrating more than one muscle or blending imperceptibly with the intermuscular fatty planes. 65 67 Muscle and Tendon ∗ b ∗ ∗ ∗ a c Fig. 3.24a–c. Intramuscular lipoma: infiltrative type. a Transverse 12–5 MHz US image over the anterior shoulder in a patient with a painless slowly growing mass with b,c axial T1-weighted MR imaging correlation demonstrates a large mass within the deltoid muscle characterized by a hyperechoic background (asterisks) and a striated pattern (arrowheads) due to intermingled muscle fibers with fat. The lipoma is delimited by a thin hypoechoic rim (arrows) reflecting peripherally displaced muscle tissue fat, in our experience MR imaging is much superior to US for the confident identification of adipose tissue in infiltrative lipomas. After fibrous and fibrohistocytic malignancies, liposarcoma represents the second most common type of soft-tissue sarcoma, accounting for approximately 10–25% of all soft-tissue sarcomas (Murphey et al. 2005). It is predominant in men around the fifth and sixth decades of life and does not represent the result of malignant transformation of a lipoma. Histopathologically, liposarcomas are grouped in five subtypes: well-differentiated, myxoid, round cell, pleomorphic, and dedifferentiated. Well-differentiated liposarcoma is the most common type (50%); it lacks metastatic potential but tends to recur locally. US shows large, multilobulated, well-defined masses which, in most cases, are indistinguishable from mature lipomas (Fig. 3.25) (Futani et al. 2003; Murphey et al. 2005). Based on gray-scale US findings, lipoma-like lesions with a complex appearance (containing thick septa and nodular or globular foci with echotexture other than that of fat) always merit further investigation with contrast-enhanced MR imaging (Fig. 3.26). Finding blood flow signals in a lipoma-like mass with color and power Doppler imaging should also alert the examiner (Bodner et al. 2002; Futani et al. 2003). Unlike well-differentiated liposarcoma, myxoid liposarcoma presents as a well-circumscribed multinodular mass whose gross pathologic appearance includes a smaller volume of fat (often <10% of the total) and a variable mixture of myxoid and round cell components. US demonstrates a complex hypoechoic mass with posterior acoustic enhancement, a nonspecific appearance quite different from that of typical lipomas (Sung et al. 2000). Based on the nonenhanced MR imaging findings (high T2 signal related to the myxoid component), myxoid liposarcoma may often resemble a cyst. This pitfall seems particularly likely in the popliteal fossa, where the cyst-like mass may mimic a Baker cyst. In these cases, US may be helpful in revealing that the mass does not meet the criteria for a cyst (Sung et al. 2000). Finally, the round cell, pleomorphic and dedifferentiated forms are locally aggressive tumors with high metastatic potential. They show nonlipomatous components which may be predominant with little or no fat (round cell and pleomorphic). Accordingly, the US and MR imaging diagnosis of these latter masses may be very difficult due to their nonspecific appearance. 3.1.6.3 Intramuscular Myxoma Intramuscular myxoma is a slowly growing benign tumor composed of abundant myxoid deposits and fibroblasts (Murphey et al. 2002; Luna et al. 2005). Intramuscular myxomas primarily affect patients 40–70 years old with a female predominance, and 70 M. P. Zamorani and M. Valle arise in the lower limb along the course of the sciatic nerve, in the limb girdle, and in the shoulder area. The term “desmoid” means a “tendon-like” lesion: in fact, the lesion is composed of arrays of fibroblasts and varying amounts of dense collagen. After their initial growth, most desmoids evolve into a progressive shrinkage of the mass, with a decrease in cellularity and volume of the extracellular spaces until they become an irregularly shaped mass of dense collagen tissue (Vandervenne et al. 1997). Accordingly, the MR imaging signal intensity pattern of desmoids varies with time, likely reflecting the different proportions of cellular tissue, myxoid tissue, and collagen (Vandervenne et al. 1997). Scant experience is reported in literature on the US features of extra-abdominal desmoids: these masses usually extend along the fascia and engulf muscle fibres, have variable echogenicity (depending on the degree of cellularity, water content and a d collagen), and ill-defined or sharp boundaries (Fig. 3.28a–c) (Mantello et al. 1989; Casillas et al. 1991). A faint fibrillar echotexture and posterior acoustic attenuation reflecting dense collagen tissue is often detected. Doppler imaging may demonstrate both a hypervascular and a hypovascular pattern: in general, lesions with abundant collagen are hypovascular. Although strikingly similar to other types of extra-abdominal desmoids in terms of both their histopathologic and imaging features, desmoid tumors of the abdominal wall are considered a distinct entity due to their definite relationship with women taking birth control pills (estrogen-sensitive tumor), pregnancy (during or following), abdominal surgery, and trauma. In addition, they may be part of the familial Gardner syndrome. These tumors most commonly arise in the rectus abdominis and oblique external muscles (Fig. 3.28d,e) (Robbin et al. 2001). b c e Fig. 3.28a–e. Intramuscular desmoids: spectrum of US appearances. a–c Extra-abdominal desmoid of the popliteal fossa. a Longitudinal 12–5 MHz US image over the medial head of gastrocnemius with b transverse fat-suppressed T2-weighted and c transverse gadolinium-enhanced fat-suppressed T1-weighted MR images demonstrates an intramuscular solid hypoechoic mass (arrowheads) with some faint fibrillar pattern elongated along the major axis of the gastrocnemius. At MR imaging, the mass (arrow) has heterogeneous high signal intensity on the T2-weighted image, which corresponds to increased cellularity, and is characterized by prominent bands of low signal intensity, likely related to the dense areas of collagen. d,e Desmoids of the abdominal wall. Two different cases observed in young women d taking birth control pills and e in the postpuerperal period. d Transverse 12–5 MHz US image over the left rectus abdominis muscle reveals an intramuscular ill-defined heterogeneously hypoechoic mass (arrowheads). e Longitudinal extended field-of-view 12–5 MHz US image demonstrates a large hypoechoic mass (arrowheads) with irregular margins, infiltrative growth, and aggressive behaviour arising from the right rectus abdominis muscle (arrows) Muscle and Tendon 3.1.6.5 Rhabdomyosarcoma and Metastases Rhabdomyosarcoma is the leading primary malignant tumor of striated muscle. It is extremely aggressive and has high potential for local invasion, early recurrence, and metastases. The tumor occurs throughout childhood and adolescence with two peaks of incidence between 2 and 6 years and 14 and 18 years. There are two main histotypes: embryonic (botryoid) and alveolar (anaplastic), the latter more commonly arising from the muscles of the extremities and characterized by a worse prognosis (Cohen et al. 1996). The diagnostic imaging investigation of rhabdomyosarcoma is essentially based on MR imaging. With this technique, the tumor is usually isointense to muscle on T1-weighted images and has high signal intensity on T2-weighted images (McCarville et al. 1999). After gadolinium administration, heterogeneous enhancement is usually observed related to necrotic areas. The US imaging features of rhabdomyosarcoma lack specificity; in these patients, the role of US is limited to guiding percutaneous biopsy. In clinical experience, the incidence of intramuscular metastasis from malignant tumors is low. Many factors (e.g., contractile activity, local changes in pH and oxygenation, accumulation of lactic acid, intramuscular blood flow volume and pressure, local temperature) are claimed to possibly interfere with the intramuscular growth of secondary tumors (Williams et al. 1997). However, the real prevalence of striated muscle metastases in autopsy series of patients who harbored malignancy at the time of death is much higher (16%) than expected (Pearson 1959). The reason for such a discrepancy is probably related to the fact that these lesions are painless and may not be detected when they are small (Chen et al. 2005). Primary tumors that most often spread to skeletal muscles are carcinomas of the breast, colon, and lung (Chen et al. 2005). Although the diagnosis may be suspected based on the clinical history, US imaging lacks specificity to show the histologic origin of the tumor, which can be established only by means of needle biopsy (Fig. 3.29) (Rubens et al. 1997; Yang et al. 1999; Ahuja et al. 2000; Chen et al. 2005). Finally, one should remember that USguided percutaneous procedures for biopsy and thermal ablation of abdominal tumors may cause needle-tract seeding of tumor cells in the subcutaneous tissue and the muscles of the abdominal wall (Kanematsu et al. 1997; Kim et al. 2000). This complication is not negligible and seems related to the number of needle passes and the needle size (Kim et al. 2000). 3.2 Tendon 3.2.1 Histologic Considerations Tendons are a critical link in the musculoskeletal system, acting to connect muscle to bone. They are made of type 1 collagen (approximately 70% of dry weight) embedded in an extracellular matrix and are characterized by high tensile strength, similar to that of bone (Erickson 1997). It has been estimated that a cross-sectional area of 1 cm2 of tendon tissue is able to support a load of up to 1 tonne (Erickson 1997). In tendons, collagen has a complex arrangement, made up of highly ordered bundles of fibers grouped into fascicles. Most fibers have a course longitudinal to the tendon axis; some, however, may assume transverse and spiral arrangements (Sharma and Maffulli 2005). This configuration of collagen leads to the higher tensile strength and reinforcement of the attachment sites of tendon. Endotendineum and peritendineum – composed of loose connective tissue, elastic fibers, and small vessels – envelop the collagen bundles and are in continuity with the epitendineum, a dense connective tissue layer tightly bound to the outer tendon surface. Tendons can be divided into two main classes: tendons with a straight path (type 1) and tendons that redirect their course across synovial joints before reaching their insertion (type 2). These two types have different envelopes in order to reduce friction during movements. Type 1 tendons are surrounded by the paratenon, a loose areolar and adipose tissue envelope that provides vessel pedicles entering the tendon substance at intervals along the tendon length and distributing longitudinally within the endotendineum (Fig. 3.30a). The paratenon blends with the epitendineum to form the peritendon. Type 2 tendons are covered by a synovial sheath with a cell lining identical to the synovium (Fig. 3.30b). This sheath is a complex structure composed of two interconnecting layers: an internal, visceral one covering the epitendineum and an external, parietal layer in continuity with the adjacent connective spaces (Erickson 1997). The sheath is infolded by the tendon, so that both visceral and parietal layers 71 Muscle and Tendon T ∗ ∗ a b ∗ Bone c T d T T ∗ T e ∗ T f Fig. 3.30a–f. Tendon envelopes and retinacula. a Schematic drawing and d corresponding short-axis 12–5 MHz US image of a type 1 tendon (T) invested by paratenon. In the US image, the peritendon (arrowheads) is demonstrated as a thin hyperechoic envelope that can be distinguished from the surrounding fat (asterisk). b Schematic drawing and e corresponding short-axis 12–5 MHz US image of a type 2 tendon (T) invested by synovial sheath in patient with serous tenosynovitis. The presence of anechoic synovial effusion (asterisks) allows accurate depiction of the synovial sheath, which consists of a combination of visceral (white arrowheads) and parietal (open arrowheads) layers, and the mesotendon (curved arrow). c Schematic drawing and f corresponding short-axis 12–5 MHz US image of the flexor digitorum tendons (T) of the fingers shows the normal appearance of an annular pulley (arrows), a retinaculum which prevents bowstringing of the underlying tendons during flexion of the fingers. Note that the superficial portion of the pulleys has a hyperechoic, fibrillar appearance due to the perpendicular incidence of the US beam, whereas its lateral portions are hypoechoic as a result of anisotropy. Arrowhead, phalangeal bone US beam and become more clearly visible and better separated one from another as the transducer frequency increases. At higher frequencies, fibrils have a higher reflectivity and become thinner and more numerous (Martinoli et al. 1993). On short-axis planes, US demonstrates the normal tendon echotexture made up of bright stippled clustered dots instead of the linear fibrillar echoes (Fig. 3.31b). Histologic correlation has demonstrated that the linear echoes visible within tendons depend on the acoustic interfaces at the boundaries of collagen bundles and endotendineum septa, based on their different histologic composition (Fig. 3.31c) (Martinoli et al. 1993). Given the highly ordered structure of superimposed planes of collagen and septa, tendons are strongly anisotropic structures at US examination, and the fibrillar echoes can be demonstrated with efficiency only when the US beam is perpendicular to them (Fornage et al. 1987; Fornage and Rifkin 1988). Even a slight obliquity of the angle of incidence can result in an artifactual decreased echogenicity which may obscure textural details and even mimic tendinous disease (Fig. 3.31d,e). In practice, this occurs if a curved rather than linear-array transducer is used, or when tendons with a curvilinear shape (i.e., rotator cuff) or oblique orientation to the skin surface (i.e., distal biceps tendon) are examined. The epitendineum can be seen as a reflective line surrounding the tendon. Tendons which derive from one muscle have a uniform fibrillar pattern. Additional intratendinous details may be appreciated in tendons which originate from one or more muscles. In the Achilles tendon, for instance, the convergent contributions from the two heads of the gastrocnemius and the interface between them (posterior) and the soleus (anterior) can be visualized as central thickened echoes due to the union of respective peritendinous envelopes (Bertolotto et al. 1995); in the quadriceps tendon, a trilaminar complex made up of distinct superficial (from rectus femoris), intermediate (from vastus medialis and lateralis), and deep (from vastus intermedius) layers 73 75 Muscle and Tendon of the muscle. Flexible stand-off pads or generous amounts of gel must be used for assessment of tendons that wrap around curvilinear joint surfaces. However, when bony prominences or narrow spaces preclude an adequate alignment of the probe on the long axis of tendon, US evaluation may be performed on short-axis planes. Although these planes do not allow depiction of the linear fibrillar echoes, tendons may reliably be identified as well, based on the critical variation in their echogenicity that can be induced by rocking the probe back and forth. Systematic scanning on short-axis planes has the further advantage of better depicting the relationships of tendons with adjacent structures, as well as confirming the presence and extent of pathologic findings. In addition, when multiple tendons run adjacent one to the other through a restricted space or across a joint, it is easier to distinguish each individual tendon on short-axis planes. Dynamics of tendon motion during joint activity or muscle contraction can be evaluated with US in real time, and this may be essential to rule out tendon abnormalities, to differentiate partial from complete tears, as well as to assess the status of a postoperative tendon. 3.2.3 Tendon Instability Dislocation occurs in tendons invested by synovial sheath, following the injury of one or more restraining structures of an osteofibrous tunnel, such as ligaments, retinacula, and annular pulleys. A wide spectrum of mechanical injures, ranging from violent acute trauma to repetitive minor damage may lead to instability of certain tendons as they reflect within osteofibrous tunnels: congenital hypoplasia of the bony groove or laxity of the fibrous roof of the tunnel may predispose to tendon instability (Fig. 3.32a). US evaluation may reveal the degree of tendon instability (i.e., intermittent subluxation, permanent subluxation, dislocation) as well as a number of associated findings, such as peritendinous effusion related to tenosynovitis or lesions in the tendon substance due to abnormal friction of the displaced tendon against bony edges (Fig. 3.32b,c). Dynamic examination may enhance detection of intermittent subluxation and assess whether spontaneous reduction is possible. The dynamic imaging afforded by US appears to be well suited for such T a ∗ MH d b T MH c e Fig. 3.32a–e. Tendon instability. a Schematic drawings illustrate congenital causes of tendon instability. On the left, the tendon (T) is stabilized within its bony groove by a retinaculum (arrowheads). Tendon instability can be observed in cases of flat bony groove (center) or laxity of the retinaculum (right). b Schematic drawings illustrate the different grades of instability of a tendon relative to its bony groove (asterisk): intermittent subluxation (left), permanent subluxation (center) or dislocation (right). The boundary of the groove is indicated by the vertical dashed line. c Schematic drawings illustrate the main abnormalities occurring in tendons (T) invested by a synovial sheath (arrowheads) during subluxation, including reactive tenosynovitis (center) and partial tear (right). d,e Intermittent subluxation of finger extensor tendon. Transverse 12–5 MHz US images of the knuckle of the middle finger in a patient with injury to the metacarpophalangeal joint and rupture of the sagittal band, acquired d while the hand was extended and e in the clenched fist position. With the finger extended, the extensor tendon (arrow) lies in a normal position with respect to the dorsal metacarpal head (MH); with the fist clenched, there is anterior dislocation of the tendon on the ulnar side of the bone (dashed-line) 76 M. P. Zamorani and M. Valle evaluation, especially in cases of subluxation, where this technique is more efficient and easier than MR imaging obtained with varied positioning. Permanent dislocations may readily be identified on static scans, whereas intermittent subluxation requires dynamic examination to assess whether spontaneous reduction is possible. Systematic scanning on short-axis planes is preferred for assessing the instability of tendons, because both the empty tunnel and the displaced tendon can be demonstrated in a single image. In the appropriate chapters, peculiar US features are described in detail for the instability of the long head of the biceps tendon (Farin et al. 1995; Ptasznik and Hennessy 1995; Prato et al. 1996; Martinoli et al. 2003), the peroneal tendons (Fessell et al. 1998; Magnano et al. 1998; Neustadter et al. 2004), the posterior tibial tendon (Prato et al. 2004), and the flexor (Klauser et al. 1999; Hauger et al. 2000; Martinoli et al. 2000; Klauser et al. 2002; Martinoli et al. 2005) and extensor (Lopez-Ben et al. 2003) tendons of the fingers (Fig. 3.32d,e). behind a retinaculum as well as excessive frictional forces against bony surfaces or adjacent accessory tendons can contribute to tendon damage at other levels also (Fessell and van Holsbeeck 1999). In addition, abuse or local injection of corticosteroids and systemic disorders, such as systemic lupus erythematosus, gout, rheumatoid arthritis, diabetes, hyperparathyroidism, and chronic renal failure, can cause a detrimental effect on the strength of tendons and predispose them to rupture. The quadriceps and patellar tendons, the extensor and flexor digitorum tendons and the posterior tibial tendon are primarily involved by systemic disorders. Most likely, some combination of trauma and predisposing factors, either mechanical or biochemical, is the initial cause of the degenerative process in tendons (Campbell and Grainger 2001). Then, a continuum exists with degenerative changes and minor intrasubstance tear leading to partial and complete rupture (Jacobson and van Holsbeeck 1998). 3.2.4.1 Tendinosis and Partial Tears 3.2.4 Degenerative Changes and Tendon Tears In an intact musculotendinous system, tendon ruptures are infrequent and typically develop at the insertion of tendon into bone, with or without avulsion of a small osseous fragment, or at the myotendinous junction, as a result of significant trauma or an excessive rate of loading. On the contrary, given the tough, fibrous nature of tendons, intrasubstance ruptures rarely occur outside the setting of predisposing degenerative changes that weaken the strength of the tendinous structure (Kainberger et al. 1997). Several theories have attempted to explain the degenerative process in tendons. Intrasubstance degeneration may derive from overuse injures, such as occur in certain sports (i.e., swimming, golf, tennis, running, basketball, and ballet dancing) in which repetitive submaximal loading and/or eccentric mechanical forces create recurrent microtrauma with microfailure of collagen bundles that do not heal completely, especially in the vulnerable areas where tendons exhibit reduced blood flow (Herring and Nilsson 1987). The supraspinatus, long head and distal biceps, extensor and flexor tendons about the elbow, patellar and Achilles tendons, tibialis posterior and flexor hallucis longus tendons are more frequently involved by overuse damage. However, constriction or compression Although painful tendinopathy is often called “tendinitis,” this term is actually a misnomer. In fact, these tendons are characterized by a degenerative noninflammatory process that is best termed “tendinosis” (Khan et al. 1996; Campbell and Grainger 2001). From the histopathologic point of view, the damage to collagen which occurs in tendinosis derives mainly from hypoxic and myxoid degeneration and leads to deposition of interfibrillar glucosaminoglycans (Khan et al. 1996; Movin et al. 1997; Schweitzer and Karasick 2000). The first process is likely caused by ischemia because of critical zones of hypovasculature in tendons; the second reflects deposition of large mucoid patches and vacuoles between the thinned degenerated fibers. In most patients, the two processes coexist and are associated with spontaneous tendon rupture (Schweitzer and Karasick 2000). As regards the location of the degenerative areas, the increased risk of certain anatomic sites for development of tendinosis has led to the concept of critical zones, in which several factors, such as aging, hypovascularization, and biomechanical effects in combination with repetitive trauma, play a specific causative role (Kainberger et al. 1997; Schepsis et al. 2002). In the Achilles tendon, for example, the predominant involvement of its middle third has been attributed to the fact that this is an area of low vas- 77 Muscle and Tendon culature at the watershed between two separate vascular networks which converge toward the middle from the myotendinous and the teno-osseous junction (Kainberger et al. 1997). Other biomechanical factors may be implicated in the involvement of the middle third of the Achilles tendon, such as the difference in tension between the gastrocnemius and soleus fibers, which fuse to become one tendon only about 5–6cm from the calcanear insertion (Kainberger et al. 1997). Then, the fibers around the medial portion of the tendon are specifically involved in patients with hyperpronation of the foot, such as subjects with marked forefoot varus, due to eccentric shear forces across medial tendon fibers (Fig. 3.33a) (Gibbon et al. 2000). On the other hand, in distal third Achilles tendinopathy, abnormalities most often involve the deep tendon surface as a probable result of the biomechanical conflict between these fibers and the posterosuperior angle of the calcaneus in full dorsiflexion (Fig. 3.33b) (Gibbon et al. 2000). In “jumper’s knee,” the deep central portion of the proximal insertion of Lat the patellar tendon is most often involved: somewhat similar to insertional Achilles tendinopathy, microtrauma occurring between the undersurface of the patellar insertion and a prominent patellar tip has been assumed to be a causative factor for chronic impingement and development of secondary degenerative changes (Khan et al. 1996). At the elbow, in the common extensor tendon, the fibers of the extensor carpi radialis brevis lie deep, just over the lateral ulnar collateral ligament, whereas the contribution from the extensor digitorum communis is superficial. Based on such anatomic consideration, some authors have speculated that the most common abnormalities of lateral epicondylitis involve the origin of the extensor carpi radialis brevis and, to a lesser extent, the anterior aspect of the extensor digitorum tendon (Connell et al. 2001). From the clinical point of view, tendinosis typically presents with tendon swelling, tenderness, and absent or moderate pain aggravated by activities and the coexistence of tenosynovitis (Fornage and ∗ Med a LE Calcaneus b LE RH RH c d Fig. 3.33a–d. Tendinosis: spectrum of US appearances. a Short-axis 12–5 MHz US image over the middle third of the Achilles tendon (arrows) in a 35-year-old man with marked hyperpronation of the foot demonstrates selective involvement of the medial tendon fibers (arrowheads). b Long-axis 12–5 MHz US image of the distal third of the Achilles tendon reveals selective involvement the deep tendon fibers (arrowheads) and retrocalcaneal bursitis (asterisk) probably as a result of chronic conflict with the posterosuperior angle (arrow) of the calcaneus during dorsiflexion. c,d Long-axis c gray-scale and d color Doppler 17–5 MHz US images of the common extensor tendon attachment on the lateral epicondyle (LE) in a 40-year-old tennis player demonstrate focal hypoechoic intratendinous changes and disappearance of the fibrillar echoes (arrowheads), signs that are typical of lateral epicondylitis. Color Doppler imaging reveals a hypervascular pattern in the intratendinous hypoechoic area. RH, radial head 78 M. P. Zamorani and M. Valle Rifkin 1988; Schepsis et al. 2002; Premkumar et al. 2002). US demonstrates degenerative changes as focal (nodular) or diffuse tendon thickening, and intratendinous hypoechoic areas with loss of the fibrillar echoes, reflecting a disorganized structure of the collagen bundles (Fig. 3.33a–c) (Fornage et al. 1984; Maffulli et al. 1990b; Martinoli et al. 1993; Åström et al. 1996; Grassi et al. 2000). As US technology progresses, new developments in signalprocessing software, such as compounding technology, are leading to a continuing improvement of image contrast and detail resolution. The result is an improved delineation of fine pathologic findings within degenerated tendons. This contributes to a more confident differentiation between normal and pathologic states and to a more reliable use of this technique. Care must be taken, however, in determining the true pathologic meaning of these subtle changes in the tendon substance, since minor abnormalities can be found also in asymptomatic tendons of aged individuals (Martinoli et al. 1999). Abnormally thickened tendons with altered echotexture (focal hypoechoic areas) may exhibit a hypervascular pattern at color and power Doppler imaging (Fig. 3.33d) (Weinberg et al. 1998; Öhberg et al. 2001; Terslev et al. 2001; Richards et al. 2001, 2005; Silvestri et al. 2003). Tendinosisrelated neovasculature is typically appreciated in relation to the thickened part of the tendon, both inside and outside it (Öhberg et al. 2001; Peers et al. 2003). It appears significantly enhanced by activity of the tendon before imaging (Cook et al. 2004). As regards the significance of increased blood flow within tendons, a positive correlation seems to exist between the overall number of vessels detectable and the tendon size; painful tendinosis typically appears more hypervascular than asymptomatic tendinosis, whereas no correlation has been found between microvascular response and duration of symptoms (Öhberg et al. 2001; Richards et al. 2005). In any case the meaning of the hypervascular pattern – whether it is causative of tendon abnormalities or secondary to attempts at healing – has yet to be defined (Richards et al. 2005). In terms of patient outcome, a hypervascular pattern detected at color and power Doppler imaging is not an unfavorable sign (Zanetti et al. 2003); as seen on gray-scale US, tendon heterogeneity seems more likely related to a worse clinical outcome after conservative treatment (Nehrer et al. 1997; Archambault et al. 1998). US-guided interventional procedures to treat chronic painful tendinosis are detailed in Chapter 18. Tendon heterogeneity on gray-scale US images does not necessarily mean tendinosis-related changes, but it may also reflect a partial tendon tear (Martinoli et al. 1999; Jacobson et al. 1999). An abrupt demarcation between degeneration, microtears, and interstitial tears is misleading, because these forms reflect, in themselves, a continuum of the same disease process and, in many cases, coexist and are treated identically (Campbell and Grainger 2001). With progress in US technology, interstitial tears can be identified in areas of tendon degeneration as thin hypoechoic clefts oriented along the long axis of the tendon and possibly reaching the tendon surface (Figs. 3.34, 3.35) (Connell et al. 2001; Campbell and Grainger 2001; Premkumar et al. 2002). Progressive tearing leads to contour irregularities or focal thinning (Chen and Liang 1997). Partial tears occur in the longitudinal orientation, parallel to the course of the tendon (longitudinal splits, fissurations) or in the transverse direction, perpendicular to the tendon fibers (Diaz et al. 1998; Bianchi et al. 2005, 2006). On a more macroscopic level, partial tendon tear may involve macroscopic amounts of fibers, and even produce discontinuities in individual portions of complex tendons (Bianchi et al. 1994a). US evaluation demonstrates both the intact and the retracted ruptured portions of tendon in association with a hematoma (Kalebo et al. 1990, 1992). The longitudinal fibrillar pattern is lost in the fractured part of the involved tendon, but it remains unaffected in the intact part (Fig. 3.35a,b) (Martinoli et al. 1993). Lack of tendon retraction is the most important feature for distinguishing partial from complete rupture. In specific clinical settings, tendons may be involved by metabolic disorders. In gout, deposition of urate tophi in the Achilles tendons may result in intratendinous nodules or diffuse thickening of the tendon, while in heterozygous familial hypercholesterolemia, US can depict bilateral intratendinous xanthomas in grossly enlarged Achilles tendons as focal or diffuse hypoechoic areas before they become apparent at physical examination (Kainberger et al. 1993; Bude et al. 1993, 1994, 1998; Bureau et al. 1998). This condition closely mimics highgrade hypoxic tendinosis. Calcifications may infrequently be encountered in tendons, although their relationship with tendon degeneration is unclear. Calcific deposits appears as linear echoes located preferentially at the insertion of tendon into bone (enthesis), reflecting a process of calcium hydroxyapatite or calcium pyrophosphate dihydrate crystal deposition. In predisposed subjects, extensive 82 M. P. Zamorani and M. Valle ∗ ∗ B B b ∗ a c Fig. 3.38a–c. Complete tendon tear. a Long-axis 12–5 MHz US image over the anterior elbow with b schematic drawing and c sagittal fat-suppressed T2-weighted MR imaging correlation shows a fluid-filled defect (asterisk) and the retracted proximal tendon end (curved arrow) of a ruptured distal biceps tendon. The tendon is shrunk and characterized by posterior acoustic attenuation (straight arrows), findings that are consistent with a complete tear. Note the retracted myotendinous junction of the biceps muscle (B) chronic tearing, the absence of fresh hemorrhagic fluid and the organized hematoma which fills the defect with echogenic material can be misleading, mimicking tendon integrity (Fig. 3.39). In synovial sheath tendons, an accurate scanning technique may be required to visualize the tendon ends, which can be retracted at a variable distance from the site of the tear, and to measure the amount of tendon retraction on longitudinal scans. If there is no retraction and the torn tendon ends are curled up, or if fluid does not fill the space created by the tear, gentle passive assisted movements can be helpful to enhance the separation of the tendon ends during stretching (Kainberger et al. 1997). In a degenerative setting, intense muscle contraction or abnormal stress forces exerted on healthy tendons may lead to avulsions at their sites of insertion into bone. These tears often lead to detachment and retraction of a bony fragment which remains embedded in the tendon. Avulsion injuries typically involve the supraspinatus tendon, causing retraction of a fleck of bone from the greater tuberosity, the peroneus brevis, leading to avulsion of the base of the fifth metatarsal, the flexor and extensor digi- ∗ C PM ∗ Talus a b Fig. 3.39a,b. Chronic nonoperated Achilles tendon tear. a Long-axis extended field-of-view 17–5 MHz US image over the posterior ankle in a patient who suffered an acute Achilles tendon rupture 1 year previously as a result of a bicycling injury with b sagittal T1-weighted MR imaging correlation demonstrates a gap of approximately 6 cm between the proximal (star) and distal (asterisk) blunt tendon ends. There is debris between the torn tendon ends and posterior herniation of the pre-Achilles fat (curved arrow) within the defect created by the tear. Note the fusiform, blunt contraction of the distal tendon segment. PM, posterior malleolus; C, calcaneus 83 Muscle and Tendon torum tendons, which may detach a small cortical fragment from the base of distal phalanx, and the triceps tendon, leading to an olecranon fracture (Fig. 3.40). The size and degree of displacement of the avulsed bony fragment is variable. As avulsion fractures are most commonly encountered in children and adolescents, tendon avulsion injuries are addressed in detail in Chapter 19. After surgical repair for a tendon tear, US imaging can help to monitor the healing process as well as to rule out recurrent tears (Campbell and Grainger 2001; Müller et al. 2002). Common postoperative findings include altered tendon size with areas of thickening or thinning, regardless of the time of follow-up, and permanent abnormal echotexture at the site of the tear. Although echotextural changes may partially regress with time, they persist for many years after surgery (Rupp et al. 1995). Heterotopic ossification may occasionally occur. In type 1 tendons (i.e., Achilles tendon), the paratenon becomes thickened and cannot be differentiated from the tendon tissue, thus leading to contour irregularities; the tendon gliding mechanism is often impaired or limited (Rupp et al. 1995). Dynamic scanning is essential in the postoperative evaluation of type 2 tendons as a means to assess tendon gliding function within the sheath and to exclude possible adhesions. Intratendinous sutures appear as bright double linear echoes (rail-like lines) due to highamplitude reflection of the US beam with posterior reverberation artifact (Maffulli et al. 1990a; Rupp et al. 1995). After repair of avulsion injuries, suture anchors can be depicted as small defects of the cortical bone filled with bright material with posterior reverberation artifact. During the healing process, however, these signs seem to have limited value with regard to evaluation of the functional results, such as muscle strength, endurance, and range of motion (Rupp et al. 1995; Müller et al. 2002). Recurrent rupture may manifest with partial disruption of the tendon fibers up to complete breakdown of the anastomosis (Fig. 3.41). In these cases, tendon thickening with intratendinous hematoma or tendon thinning with abundant fluid in the tendon sheath may indicate a small recurrent tear. In complete recurrent tears, tendon discontinuity may be associated with sutures floating freely within the hematoma. 3.2.5 Inflammatory Conditions Although less common than degenerative processes, some pathologic conditions in tendons are actually inflammatory in nature, and distinguishing these forms from simple tendinosis is important because the clinical management may differ. Although degenerative and inflammatory conditions may be treated with the same conservative measures (i.e., rest, ice, and nonsteroidal anti-inflammatory drugs), inflammatory lesions that fail to regress require more aggressive therapy with corticosteroids or even surgical procedures. The spectrum of US findings depends on the type of tendon involved, ∗ O Humerus a b Fig. 3.40a,b. Acute avulsion-type injury of the medial head of the triceps tendon. a Long-axis 12–5 MHz US image over the posterior elbow with b plain film correlation demonstrates a ruptured retracted medial head of the triceps tendon (arrowheads) containing a fragment of bone (arrow) within, as a result of avulsion trauma from the olecranon process (O). The retracted, thickened proximal tendon is seen surrounded by mild hypoechoic fluid (asterisk) 84 M. P. Zamorani and M. Valle 2 1 a b 1 ∗ ∗ ∗ 1 ∗ ∗ ∗ 2 2 c Fig. 3.41a–c. Postsurgical tendon retear. Two different cases. a,b Achilles tendon retear. a Long-axis gray-scale and b color Doppler 12–5 MHz US images show complete retear of a previously sutured Achilles tendon. The swollen tendon ends (1, 2) are separated by a wide gap filled with debris and fluid. Note the surgical stitches (arrowheads in a) lying free within the gap. Local blood flow signals (arrowheads in b) surround the tendon ends reflecting local hyperemia. c Extensor pollicis longus tendon retear. Long-axis 12–5 MHz US image over the dorsoradial aspect of the hand with schematic drawing correlation (insert)reveals a fluid-filled gap (asterisks) between the two retracted ends (1, 2) of the extensor pollicis longus tendon. Note the disconnected surgical stitches (arrowheads) attached to the tendon ends that float freely within the empty tendon sheath. A split-screen image was used, with the two screens aligned for an extended field of view as well as on the associated changes occurring in the tendon envelopes and associated synovial bursae. The inflammatory process mainly results in “peritendinitis” for tendons invested by paratenon (type 1) and “tenosynovitis” for tendons invested by synovial sheath (type 2). For both processes, the functional meaning is somewhat equivalent. Peculiar conditions, such as calcifying tendinitis, are addressed elsewhere (see Chapters 6, 18). 3.2.5.1 Paratendinitis and Attrition Bursitis Paratendinitis (peritendinitis) most often occurs in the Achilles tendon in association with high-grade hypoxic tendinosis during phases of exacerbation of disease (Fig. 3.42a) (Schweitzer and Karasick 2000). Clinically, paratendinitis is accompanied by diffuse discomfort and peritendinous swelling and tenderness (Schepsis et al. 2002). Fluid within and patchy thickening of the paratenon, irregularities of tendon margins, and adhesions are typically found (Gibbon et al. 2000; Martinoli et al. 1999). In the less common isolated paratendinitis (without tendinosis), the Achilles tendon itself is normal while the peritendinous tissues show edematous changes and tender nodules related to localized connective tissue hypertrophy (Fig. 3.42b,c). Acutely, this condition is encountered in long-distance (marathon) runners as a result of abnormal biomechanics; it may also follow training errors, including a sudden increase of exercise and change of terrain, particularly hillrunning. Paratendinous bursae (e.g., the retrocalcaneal bursa at the heel, the deep infrapatellar bursa at the knee, and the bicipitoradial bursa at the elbow) primarily act as shock absorbers by reacting to increased compression and frictional forces exerted by the overlying tendons (Gibbon and Wakefield 1999). In most cases, intrabursal fluid or synovial hypertrophy denote an inflammatory process that is mechanical in origin and occurs in proximity to the preinsertional portion of the tendon (Fig. 3.42d,e). If mild, fluid does not have clinical importance and reflects irritation due to local overload and work Muscle and Tendon At At At ∗ a b c At At ∗ ∗ Calcaneus d e Fig. 3.42a-e. Peritendinitis and bursitis. Three different cases. a Peritendinitis of the Achilles tendon in a patient with high-grade tendinosis and severe posterior ankle pain. Short-axis 12–5 MHz US image over the middle third of the Achilles tendon shows an abnormal hypoechoic tissue layer (arrows) on the posterior surface of the tendon (At), a finding characteristic of peritendinitis. b,c Isolated Achilles peritendinitis in a 30-year-old long-distance runner with heel pain of recent onset. b Short-axis 17–5 MHz US image over the mid-distal Achilles tendon with c sagittal fat-suppressed T2-weighted MR imaging correlation reveals a crescentic-shaped fluid collection (arrowheads) around the medial aspect of an otherwise normal Achilles tendon (At). Note the small amount of hypoechoic fluid (asterisk) distending the retrocalcanear bursa. d,e Heel bursitis in a 53-year-old female tennis-player who suffered from chronic heel pain. d Long-axis and e short-axis 17–5 MHz US images over the distal Achilles tendon (At) demonstrate discrete hypoechoic distention of the retrocalcanear bursa (asterisk) by fluid and hypertrophied synovium. Some fluid is also seen in the retro-Achilles bursa (arrowheads) and the subcutaneous tissue (arrows) around the Achilles tendon insertion stress. Bursitis may cause local discomfort only when mechanical synovitis is excessive or when the process is combined with a joint effusion in patients with primary inflammatory arthropathies such as rheumatoid arthritis and seronegative spondyloarthropathies, or metabolic disorders such as gout (Gibbon and Wakefield 1999; Ho et al. 2003). 3.2.5.2 Tenosynovitis In tendons with a synovial sheath, inflammation is mostly secondary to repetitive microtrauma, due to overuse or osseous friction, foreign bodies, or infection and arthritis. Acute serous tenosynovitis is diagnosed by identifying an increased amount of fluid within the tendon sheath (Gooding 1988; Stephenson 1990). The fluid typically encircles the tendon forming a “halo” around it: this sign helps to distinguish the tenosynovial nature of the effusion from other paratendinous cystic lesions, such as bursae or ganglion cysts, in which fluid is located eccentrically to the tendon. In subacute or chronic disease, the effusion is often associated with sheath thickening (Martinoli et al. 1999). Careful scanning technique is needed to demonstrate small but significant synovial effusions which, for instance, can be easily unrecognized if excessive pressure by the transducer causes collapse of the sheath. 85 86 M. P. Zamorani and M. Valle 1 2 T 3 T bone a b f 3 2 1 s c T s d e Ph g Fig. 3.43a–g. Stenosing tenosynovitis. a,b Schematic drawings illustrate the pathomechanism leading to conflict of a type 2 tendon under a thickened retinaculum in an osteofibrous tunnel. a Long-axis and b short-axis views over the affected tendon demonstrate increased thickness and narrowing of the retinaculum (black) which constrains the underlying tendon (intermediate gray), increased tendon thickness (1) proximal to the retinaculum with sheath fluid distension (light gray), abrupt narrowing of the tendon size (2) underneath the retinaculum, and mild tendon and sheath abnormalities (3) distal to level of the retinaculum. c–g Trigger thumb. c–e Short-axis 15–7 MHz US images obtained from c proximal to e distal over the first metacarpophalangeal joint in a 54-year-old woman with chronic painful extension of the thumb corresponding to the levels shown in a. Proximal to the A1 pulley, the flexor pollicis longus tendon shows progressive increase of its cross-sectional area (1); at the level of the sesamoids (s), it sudden decreases in size (2) under the thickened pulley (arrowheads); after exiting the pulley, the tendon returns to a normal size (3) over the proximal phalanx (Ph). f,g Long-axis 15–7 MHz US images of the flexor pollicis longus tendon (T) obtained over the thickened A1 pulley (arrowheads) during f flexion and g extension of the distal phalanx. Dynamic scanning demonstrates the difficult gliding (curved arrow) of the tendon as it passes deep to the thickened pulley during thumb extension Depending on its cellular content, the fluid in the synovial sheath may be anechoic or may contain subtle echoes in suspension. The entrapment and chronic conflict of tendons beneath a ligament or a pulley may cause a stenosing tenosynovitis, i.e., de Quervain disease, trigger finger (Fig. 3.43a,b). The involved tendons are diffusely swollen with textural disarrangement and focal or diffuse thickening of the synovial sheath. Simple synovial effusion is observed in acute cases, whereas chronic cases present with thickening of the retinacula, which usually represents an indication for operative management (Fig. 3.43c–e). Dynamic US examination with passive assisted movements may demonstrate the entrapment of the synovial sheath at the entrance of the narrowed tunnel (Fig. 3.43f,g). In infectious tenosynovitis, the effusion tends to be more echogenic and the overlying subcutaneous tissue may appear thickened and hyperechoic due to cellulitis (Brooke Jeffrey et al. 1987; Schechter et al. 1989). However, it must be emphasized that these characteristics are too subtle to allow a definitive diagnosis based on US findings alone. Needle aspiration of fluid, possibly obtained under US guid- ance, is necessary to confirm the infectious nature of tenosynovitis as well as to identify the causative bacteria and choose the appropriate antibiotic therapy. As an exception to this rule, the diagnosis may be straightforward on US findings when a foreign body is recognized within the synovial sheath (Fig. 3.44) (Howden 1994). In the setting of infection, color and power Doppler imaging may show increased flow but is unable to reliably differentiate an infected from a noninfected state (Newman and Adler 1998). Furthermore, in some instances there may be no increased blood flow in the sheath, (Craig et al. 2003). In tuberculous tenosynovitis, the tendon sheaths appear markedly thickened as a result of granulomatous changes (Riehl et al. 1997). Color and power Doppler imaging can detect the hyperemic state as increased flow signals within the inflamed sheaths (Breidhal et al. 1998). In rheumatologic disorders, such as rheumatoid arthritis and psoriatic arthritis, hypoechoic villous projections of the synovium (pannus) can develop inside the effusion and may even fill the synovial space (Fig. 3.45a-c) (Milosavljevic et al. 2005). Multiple tendons can be simultaneously involved, Muscle and Tendon a b c Fig. 3.44a–c. Infectious tenosynovitis. a Short axis 12-5 MHz gray-scale and b color Doppler US over the right palm in a 35 yearold patient who complained of increasing pain and local inflammatory symptoms one week after a minor penetrating trauma demonstrate a distended sheath of the flexor tendons (ft) of the long finger by echogenic effusion and hypechoic changes in the surrounding tissues related to cellulitis (arrows). Note the undefined outer boundaries of the tendon sheath and the intense superficial hyperemia (arrowheads). m, third metacarpal. c Photograph of the patient’s hand reveals reddish skin (arrow) due to intense local inflammation. especially the extensor carpi ulnaris along with other flexor and extensor digitorum tendons at the wrist and the posterior tibial tendon. In persistent synovitis, the biochemical and compressive damage related to the invading pannus and the mechanical stress caused by chafing of the tendon against the bone, as in the case of a dorsally subluxated ulna or Lister tubercle, increases the incidence of tendon ruptures (Swen et al. 2000). The propensity for tendon rupture is considered to be independent of the extent of the tenosynovitis and seems to correlate with aggressive rheumatoid arthritis with high titers of rheumatoid factor and bone erosions in an early stage of the disease (Swen et al. 2000). In these patients with loss of finger function, US can be used to differentiate the functional impairment due to joint disease from tendons tears as well as to detect partial tendon tears that cannot be identified at physical examination (Swen et al. 2000). Probe compression can be helpful to differentiate complex effusion from synovial thickening because fluid may be squeezed away, in contrast to noncompressible synovium. Similarly, Doppler imaging has a value in distinguishing the hypoechoic pannus from the effusion based on the presence or absence of flow signals, as well as in differentiating highly vascular, active pannus from hypovascular fibrous pannus (Fig. 3.45d) (Newman et al. 1996). Especially in patients refractory to drug treatment, an early diagnosis of tendon involvement with US is essential to both plan and improve the outcome after prophylactic tenosynovectomy (Brumfield et al. 1990). Future possibilities include follow-up of disease progression and quantification of response to ther- apy (Newman et al. 1996). Because Doppler imaging techniques are limited in their ability to detect slowly flowing blood and small vessels, microbubble-based US contrast seems promising to increase the US sensitivity to detect hyperemic states in tendons. In the tibialis posterior, US and MR imaging have similar accuracy in revealing signs of paratendinopathy, including peritendinous sheath effusion and hyperemia (Premkumar et al. 2002). 3.2.5.3 Enthesopathy An enthesis is the point of union between bone and a tendon, a ligament, an aponeurosis, or a capsule. Inflammation typically occurs at the enthesis in seronegative spondyloarthropathy and, to a lesser extent, in rheumatoid arthritis and gout, leading to soft-tissue thickening, cortical bone breakage, and new bone proliferation (Gibbon and Wakefield 1999; Balint et al. 2002). Bursitis and synovitis often occur in the surrounding tissues. In spondyloarthropathy, the most common sites of involvement are the knee (superior and inferior poles of the patella, tibial tuberosity), the heel (posterosuperior and posteroinferior poles of the calcaneus), and the ischial tuberosity, leading to local pain and stiffness (Balint et al. 2002). Pain films are normal in early disease, but they can reveal specific radiographic features, such as bony erosion, hyperostosis, fragmentation, and crystal deposition as late manifestation of enthesitis (Barozzi et al. 1998; Gnenc et al. 2005). Even better than radiography, US has proved 87 88 M. P. Zamorani and M. Valle T T ∗ a ∗ b ∗ T ∗ 4 c d Fig. 3.45a–d. Hypertrophied tenosynovitis in a 63-year-old woman with long-standing rheumatoid arthritis. a Photograph demonstrates considerable soft-tissue swelling (arrowheads) over the dorsoulnar aspect of the patient’s wrist. b Long-axis and c short-axis 17–5 MHz US images over the ventral aspect of the ulnar wrist reveal marked irregular thickening of the synovial sheath (arrowheads) of the extensor carpi ulnaris tendon (T) with intrasheath fluid (asterisks). d Correlative color Doppler image shows hyperemic synovial walls and intratendinous flow signal, suggestive of active disease. In this particular case, there was concomitant involvement of the distal radioulnar joint and bone erosions on the ulnar head to be a sensitive means of detecting bone erosions as cortical breakages with a step-down contour defect, and enthesophytes as step-up bony prominences at the end of the normal bony contour (Barozzi et al. 1998; Balint et al. 2002; Kamel et al. 2003; Gnenc et al. 2005). However, the advantages of US rely on its ability to depict associated soft-tissue changes, including periosseous edema, focal tendon thickening, abnormal tendon echotexture with calcifications, and bursal fluid distension at the site of tenderness (Lehtinen et al. 1994; Barozzi et al. 1998; Kamel et al. 2003). 3.2.6 Tumors and Tumor-Like Conditions Primary tumors (i.e., fibroma of the tendon sheath, clear cell sarcoma) arising from the tendon and its sheath are exceptional (Gandolfo et al. 2000; Fox et al. 2003). On the contrary, non-neoplastic spaceoccupying lesions, such as ganglion cysts and the localized giant cell tumor of the tendon sheath, are much more common. Although most of these lesions present as slowly growing painless soft-tissue swellings in the extremities, their mass effect may lead to compression of adjacent structures and transient arrest of tendon gliding in a finger, especially if the mass develops in proximity to retinacula or within an osteofibrous tunnel (Bertolotto et al. 1996). 3.2.6.1 Intratendinous and Tendon Sheath Ganglia Ganglion cysts usually arise from para-articular tissues but those originating within a tendon are rare (Bianchi et al. 1993). The etiology of intratendinous ganglia is not completely understood: recurrent injury to the tendon with subsequent cystic degeneration seems to be a possible cause (Kannus and Jozsa, 1991). The clinical relevance of intratendinous ganglia is based on the fact that they weaken the structure of tendons and predispose them to Muscle and Tendon features of the giant cell tumor reflect the underlying histologic composition and its hemosiderin content (low T2 signal) (Fig. 3.48d–f) (De Beuckeleer et al. 1997; Kitagawa et al. 2003). Further details on this tumor are reported in Chapter 11. References Ackermann LV (1958) Extra-osseous localized non-neoplastic bone and cartilage formation (so-called myositis ossificans). J Bone Joint Surg Am 40:279–298 Ahuja AT, King AD, Bradley MJ, et al (2000) Sonographic findings in masseter-muscle metastases. J Clin Ultrasound 28:299–302 al-Qattan MM (1996) Gantzer’s muscle: an anatomical study of the accessory head of the flexor pollicis longus muscle. J Hand Surg [Br] 21:269–270 Archambault JM, Wiley JP, Bray RC et al (1998) Can sonography predict the outcome in patients with achillodynia? J Clin Ultrasound 26:335–339 Arslan H, Sakarya ME, Bozkurt M et al (1998) The role of power Doppler sonography in the evaluation of superficial soft tissue abscesses. Eur J Ultrasound 8:101–106 ström M, Gentz CF, Nilsson P et al (1996) Imaging in chronic Achilles tendinopathy: a comparison of ultrasonography, magnetic resonance imaging and surgical findings in 27 histologically verified cases. Skeletal Radiol 25:615–620 Balint PV, Kane D, Wilson H (2002) Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis 61:905–910 Bancroft LW, Kransdorf MJ, Menke DM et al (2002) Intramuscular myxoma: characteristic MR imaging features. AJR Am J Roentgenol 178:1255–1259 Barberie JE, Wong AD, Cooperberg PL et al (1998) Extended field-of-view sonography in musculoskeletal disorders. AJR Am J Roentgenol 171:751–757 Bargfrede M, Schwennicke A, Tumani H et al (1999) Quantitative ultrasonography in focal neuropathies as compared to clinical and EMG findings. Eur J Ultrasound 10:21–29 Barozzi L, Olivieri I, De Matteis M et al (1998) Seronegative spondylarthropathies: imaging of spondylitis, enthesitis and dactylitis. Eur J Radiol 1:12–17 Batz R, Sofka CM, Adler RS et al (2006) Dermatomyositis and calcific myonecrosis in the leg: ultrasound as an aid in management. Skeletal Radiol 35:113–116 Beggs I (2003). Sonography of muscle hernias. AJR Am J Roentgenol 180:395–399 Bertolotto M, Perrone R, Martinoli C et al (1995) High resolution ultrasound anatomy of normal Achilles tendon. Br J Radiol 68:986–991 Bertolotto M, Rosenberg I, Parodi RC et al (1996) Case report: fibroma of tendon sheath in the distal forearm with associated median nerve neuropathy: US, CT and MR imaging appearances. Clin Radiol 51:370–372 Bianchi S, Abdelwahab IF, Zwass A et al (1993) Sonographic findings in examination of digital ganglia: retrospective study. Clin Radiol 48:45–47 Bianchi S, Zwass A, Abdelwahab IF et al (1994a) Diagnosis of tears of the quadriceps tendon of the knee: value of sonography. AJR Am J Roentgenol 162:1137–1140 Bianchi S, Zwass A, Abdelwahab IF et al (1994b) Evaluation of tibialis anterior tendon rupture by ultrasonography. J Clin Ultrasound 22:564–566 Bianchi S, Abdelwahab IF, Mazzola CG et al (1995a). Sonographic examination of muscle herniation. J Ultrasound Med 14:357–360 Bianchi S, Abdelwahab IF, Oliveri M (1995b) Sonographic diagnosis of accessory soleus muscle mimicking a soft tissue tumor. J Ultrasound Med 14:707–709 Bianchi S, Martinoli C, Abdelwahab IF et al (1998) Sonographic evaluation of tears of the gastrocnemius medial head (“tennis leg”). J Ultrasound Med 17:157–162 Bianchi S, Martinoli C, Waser NP et al (2002) Central aponeurosis tears of the rectus femoris: sonographic findings. Skeletal Radiol 31:581–586 Bianchi S, Martinoli C, Abdelwahab IF (2005) Ultrasound of tendon tears. 1. General considerations and upper extremity. Skeletal Radiol 34:500–512 Bianchi S, Poletti PA, Martinoli C et al (2006) Ultrasound appearance of tendon tears. 2. Lower extremity and myotendinous tears. Skeletal Radiol 35:63–77 Bickels J, Ben-Sira L et al (2002) Current concept review: primary pyomyositis. J Bone Joint Surg 84:2277–2286 Binzoni T, Bianchi S, Hanquinet S et al (2001) Human gastrocnemius medialis pennation angle as a function of age: from newborn to the elderly. J Physiol Anthropol 20:293–298 Bodner G, Schocke MFH, Rachbauer F et al (2002) Differentiation of malignant and benign musculoskeletal tumors: combined color and power Doppler US and spectral wave analysis. Radiology 223:410–416 Breidhal WH, Stafford Johson DB, Newman JS et al (1998) Power Doppler sonography in tenosynovitis: significance of the peritendinous hypoechoic rim. J Ultrasound Med 17:103–107 Brigido MK, Fessell DP, Jacobson JA et al (2005) Radiography and US of os peroneum fractures and associated peroneal tendon injuries: initial experience. Radiology 237:235–241 Brooke-Jeffrey Rjr., Laing FC, Schechter W et al (1987) Acute suppurative tenosynovitis of the hand: diagnosis with US. Radiology 162:741–742 Brumfield Rjr., Kuschner SH, Gellman H et al (1990) Results of dorsal wrist synovectomies in the rheumatoid hand. J Hand Surg 15:733–735 Bude RO, Adler RS, Bassett DR et al (1993) Heterozygous familial hypercholesterolemia: detection of xanthomas in the Achilles tendon with US. Radiology 188:567–571 Bude RO, Adler RS, Bassett DR (1994) Diagnosis of Achilles tendon xanthoma in patients with heterozygous familial hypercholesterolemia: MR vs. sonography. AJR Am J Roentgenol 162:913–917 Bude RO, Nesbitt SD, Adler RO et al (1998) Sonographic detection of xanthomas in normal-sized Achilles tendons of individuals with heterozygous familial hypercholesterolemia. AJR Am J Roentgenol 170:621–625 Bureau NJ, Roederer G (1998) Sonography of Achilles tendon xanthomas in patients with heterozygous familial hypercholesterolemia. AJR Am J Roentgenol 171:745–749 Bureau NJ, Chhem RK, Cardinal E (1999) Musculoskeletal infections: US manifestations. RadioGraphics 19:1585–1592 Campbell RSD, Grainger AJ (2001) Current concepts in imaging of tendinopathy. Clin Radiol 56:253–267 Canoso JJ, Barza M (1993) Soft tissue infections. Rheum Dis Clin North Am 19:293–309 91 92 M. P. Zamorani and M. Valle Casillas J, Sais GJ, Greve JL (1991) Imaging of intra- and extraabdominal desmoid tumors. RadioGraphics 11:959–968 Chason DP, Fleckenstein JL, Burns DK et al (1996) Diabetic muscle infarction: radiologic evaluation. Skeletal Radiol 25:127–132 Chau CLF, Griffith JF (2005) Musculoskeletal infections: ultrasound appearances Clin Radiol 60:149–159 Chen CK, Chiou HJ, Chou YH et al (2005) Sonographic findings in skeletal muscle metastasis from renal cell carcinoma. J Ultrasound Med 24:1419–1423 Chen YJ, Liang SC (1997) Diagnostic efficacy of ultrasound in stage I posterior tibial tendon dysfunction: sonographicsurgical correlation. J Ultrasound Med 16:417–423 Chepuri NB, Jacobson JA, Fessell DP et al (2001) Sonographic appearance of the peroneus quartus muscle: correlation with MR imaging appearance in seven patients. Radiology 218:415–419 Cheung YY, Rosenberg ZS, Colon E (1999) MR imaging of flexor digitorum accessorius longus. Skeletal Radiol 28:130–137 Chi-Fishman G, Hicks JE, Cintas HM et al (2004) Ultrasound imaging distinguishes between normal and weak muscle Arch Phys Med Rehabil 85:980–986 Cohen MD, Bugaieski EM, Haliloglu M et al (1996) Visual presentation of the staging of pediatric solid tumors. RadioGraphics 16:523–545 Connell D, Burke F, Coombes P et al (2001) Sonographic examination of lateral epicondylitis. AJR Am J Roentgenol 176:777–782 Cook JL, Kiss ZS, Ptasznik R et al (2004) Is vascularity more evident after exercise? Implications for tendon imaging. AJR Am J Roentgenol 185:1138–1140 Costa CR, Morrison WB, Carrino JA et al (2003) MRI of an intratendinous ganglion cyst of the peroneus brevis tendon. AJR Am J Roentgenol 181:890–891 Craig JG, van Holsbeeck M, Alca M (2003) Gonococcal arthritis of the shoulder and septic extensor tenosynovitis of the wrist: sonographic appearances. J Ultrasound Med 22:221–224 Dalakas MC, Hohlfeld R (2003) Polymyositis and dermatomyositis. Lancet 362:971–982 De Beuckeleer L, De Schepper A, De Belder F et al (1997) Magnetic resonance imaging of localized giant cell tumour of the tendon sheath. Eur Radiol 7:198–201 Delaney-Sathy LO, Fessell DP, Jacobson JA et al (2000) Sonography of diabetic muscle infarction with MR imaging, CT and pathologic correlation. AJR Am J Roentgenol 174:165–169 Derchi LE, Balconi G, de Flaviis L et al (1989) Sonographic appearance of hemangioma of skeletal muscle. J Ultrasound Med 8:263–267 Dhillon M, Davies AM, Benham J et al (2004) Calcific myonecrosis: a report of ten new cases with an emphasis on MR imaging. Eur Radiol 14:1974–1979 Diaz GC, van Holsbeeck MT, Jacobson JA (1998) Longitudinal split of the peroneus longus and peroneus brevis tendons with disruption of the superior peroneal retinaculum. J Ultrasound Med 17:525–529 Erickson SJ (1997) High-resolution imaging of the musculoskeletal system. Radiology 205:593–618 Farin PU, Jaroma H, Harju A et al (1995) Medial displacement of the biceps brachii tendon: evaluation with dynamic sonography during maximal external shoulder rotation. Radiology 195: 845–848 Fessell DP, van Holsbeeck MT (1999) Foot and ankle sonography. Radiol Clin North Am 37:831–858 Fessell DP, Vanderschueren GM, Jacobson JA et al (1998) US of the ankle: technique, anatomy, and diagnosis of pathologic conditions. RadioGraphics 18:325–340 Fischer AQ, Carpanter DW, Hartlage PL et al (1998) Muscle imaging in neuromuscular disease using computerized real-time sonography. Muscle Nerve 11:270–275 Fletcher CD, Martin-Bates E (1988) Intramuscular and intermuscular lipomas: neglected diagnoses. Histopathology 12:275–287 Fornage BD (1986) Achilles tendon: US examination. Radiology 159:759–764 Fornage BD (1987) The hypoechoic normal tendon: a pitfall. J Ultrasound Med 6:19–22 Fornage BD, Eftekhari F (1989) Sonographic diagnosis of myositis ossificans. J Ultrasound Med 8:463–466 Fornage BD, Rifkin MD (1988) Ultrasound examination of tendons. Radiol Clin North Am 6:87–107 Fornage BD, Romsdahl MM (1994) Intramuscular myxoma: sonographic appearance and sonographically guided needle biopsy. J Ultrasound Med 13:91–94 Fornage BD, Rifkin MD, Touche DH et al (1984) Sonography of the patellar tendon: preliminary observations. AJR Am J Roentgenol 143:179–182 Fox MG, Kransdorf MJ, Bancroft LW et al (2002) MR imaging of fibroma of the tendon sheath. AJR Am J Roentgenol 180:1449–1453 Futani H, Yamagiwa T, YasoJimat H et al (2003) Distinction between well-differentiated liposarcoma and intramuscular lipoma by power Doppler ultrasonography. Anticancer Res 23:1713–1318 Gandolfo N, Martinoli C, Cafiero F et al (2000) Malignant melanoma of soft-tissue (clear cell sarcoma) of the foot. Is MRI able to perform a specific diagnosis? Report of one case and review of the literature. Anticancer Res 20:3993–3998 Garcia J (2000) MRI in inflammatory myopathies. Skeletal Radiol 29:425–438 García-Díez AI, Ros Mendoza LH, Villacampa VM (2000) MRI evaluation of soft-tissue hydatid disease. Eur Radiol 10:462–466 Garrett WE Jr (1990) Muscle strain injuries: clinical and basic aspects. Med Sci Sports Exerc 22:436–443 Gibbon WW, Wakefield RJ (1999) Ultrasound in inflammatory disease. Radiol Clin North Am 37:633–651 Gibbon WW, Cooper JR, Radcliffe (2000) Distribution of sonographically detected tendoin abnormalities in patients with a clinical diagnosis of chronic Achilles tendinosis. J Clin Ultrasound 28:61–66 Gindele A, Schwamborn D, Tsironis K et al (2000) Myositis ossificans traumatica in young children: report of three cases and review of the literature. Pediatr Radiol 30:451–459 Gnenc H, Cakit BD, Tuncbilek I et al (2005) Ultrasonographic evaluation of tendons and entheseal sites in rheumatoid arthritis: comparison with ankylosing spondylitis and healthy subjects. Clin Rheumatol 24:272–277 Gooding GAW (1988) Tenosynovitis of the wrist. A sonographic demonstration. J Ultrasound Med 7:225–226 Gottlieb RH, Meyers SP, Hall C et al (1995) Pyomyositis: diagnostic value of color Doppler sonography. Pediatr Radiol 1:109–111 Grassi W, Filippucci E, Farina A et al (2000) Sonographic imaging of tendons. Arthritis Rheum 43:969–976 Hartgerink P, Fessell DP, Jacobson JA et al (2001) Full- versus partial-thickness Achilles tendon tears: sonographic accu- Muscle and Tendon racy and characterization in 26 cases with surgical correlation. Radiology 220:406–412 Harvie P, Patel N, Ostlere SJ (2003) Ulnar nerve compression at Guyon’s canal by an anomalous abductor digiti minimi muscle: the role of ultrasound in clinical diagnosis. Hand Surg 8:271–275 Harvie P, Patel N, Ostlere SJ (2004) Prevalence and epidemiological variation of anomalous muscles at Guyon’s canal. J Hand Surg [Br] 29:26–29 Hatori M, Matsuda M, Kokubun S (2002) Ossification of Achilles tendon: report of three cases. Arch Orthop Trauma Surg 122:414–417 Hauger O, Chung CB, Lektrakul N et al (2000) Pulley system in the fingers: normal anatomy and simulated lesions in cadavers at MR imaging, CT and US with and without contrast material distension of the tendon sheath. Radiology 217:201–212 Herring S, Nilsson K (1987) Introduction to overuse injuries. Clin Sports Med 6:225–239 Ho CF, Chiou HJ, Chou YH et al (2003) Peritendinous lesions: the role of high-resolution ultrasonography. J Clin Imaging 27:239–250 Hollman W, Hettiger T (1990) Sportmedizin Arbeits und Trainingsgrundlagen, 3rd edn. Schattauer, Stuttgart, p 30 Horcajadas AB, Lafuente JL, Burgos R (2003) Ultrasound and MR findings in tumor and tumor-like lesions of the fingers. Eur Radiol 13:672–685 Howden MD (1994) Foreign bodies within finger tendon sheaths demonstrated by ultrasound: two cases. Clin Radiol 49:419–420 Jacobson JA (1999) Musculoskeletal sonography and MR imaging: a role for both imaging methods. Radiol Clin North Am 37:713–735 Jacobson JA, van Holsbeeck MT (1998) Musculoskeletal ultrasonography. Orthop Clin North Am 29:135–167 Jelinek JS, Murphey MD, Aboulafia AJ et al (1999) Muscle infarction in patients with diabetes mellitus: MR imaging findings. Radiology 211:241–247 Kainberger F, Engel A, Barton P et al (1990) Injury of the Achilles tendon: diagnosis with sonography. AJR Am J Roentgenol. 155:1031–1036 Kainberger F, Seidl G, Traindl O et al (1993) Ultrasonography of the Achilles tendon in hypercholesterolemia. Acta Radiol 34:408–412 Kainberger F, Mittermaier F, Seidl G et al (1997) Imaging of tendons: adaptation, degeneration, rupture. Eur J Radiol 25:209–222 Kalebo P, Goksor LA, Sward L et al (1990) Soft-tissue radiography, computed tomography, and ultrasonography of partial Achilles tendon ruptures. Acta Radiol 31:565–570 Kalebo P, Allenmark C, Peterson L et al (1992) Diagnostic value of ultrasonography in partial ruptures of the Achilles tendon. Am J Sports Med 20:378–381 Kamel M, Eid H, Mansour R (2003) Ultrasound detection of heel enthesitis: a comparison with magnetic resonance imaging. J Rheumatol 30:774–778 Kanematsu M, Hoshi H, Takao H et al (1997) Abdominal wall tumor seeding at sonographically guided needle-core aspiration biopsy of hepatocellular carcinoma. AJR Am J Roentgenol. 169:1198–1199 Kannus P, Jozsa L (1991) Histopathological changes preceding spontaneous rupture of a tendon. J Bone Joint Surg Am 73:1507–1525 Khan KM, Bonar F, Desmond PM et al (1996) Patellar tendinosis (jumper’s knee): findings at histopathologic examination, US and MR imaging. Victorian Institute of Sport Tendon Study Group. Radiology 200:821–827 Kim SH, Lim HK, Lee WJ (2000) Needle-tract implantation in hepatocellular carcinoma: frequency and CT findings after biopsy with a 19.5-gauge automated biopsy gun. Abdom Imaging 25:246–250 Kitagawa Y, Ito H, Amano Y et al (2003) MR imaging for preoperative diagnosis and assessment of local tumor extent on localized giant cell tumor of tendon sheath. Skeletal Radiol 32:633–638 Klauser A, Bodner G, Frauscher F et al (1999) Finger injuries in extreme rock climbers: assessment of high-resolution ultrasonography. Am J Sports Med 27:733–737 Klauser A, Frauscher F, Bodner G et al (2002) Finger pulley injuries in extreme rock climbers: depiction with dynamic US. Radiology 222:755–761 Kouvalchouk JF, Fisher M (1998) Les muscles accessoires au niveau de la cheville. Mise au point. J Traumatol Sport 15:101–106 Kramer FL, Kurtz AB, Rubin C et al (1979) Ultrasound appearance of myositis ossificans. Skeletal Radiol 10:19–20 Kransdorf MJ, Jelinek JS, Moser RP (1993) Imaging of softtissue tumors. Radiol Clin North Am 31:359–372 Krix M, Weber MA, Krakowski-Roosen H et al (2005) Assessment of skeletal muscle perfusion using contrast-enhanced ultrasonography. J Ultrasound Med 24:431–441 Kullmer K, Sievers KW, Reimers CD et al (1998) Changes of sonographic, magnetic resonance tomographic, electromyographic, and histopathologic findings within a 2-month denervation period of examinations after experimental muscle denervation. Arch Orthop Trauma Surg 117:228–234 Lamminen AE, Hekali PE, Tiula E et al (1989) Acute rhabdomyolysis: evaluation with magnetic resonance imaging compared with computed tomography and ultrasonography. Br J Radiol 62:326–330 Lehtinen A, Taavitsainen M, Leirisalo-Repo M (1994) Sonographic analysis of enthesopathy in the lower extremities of patients with spondyloarthropathy. Clin Exp Rheumatol 12:143–148 Levine CD, Miller JJ, Stanislau G et al (1997) Sarcoid myopathy: imaging findings. J Clin Ultrasound 25:515–517 Lopez-Ben R, Lee DH, Nicolodi DJ (2003) Boxer knuckle (injury of the extensor hood with extensor tendon subluxation): diagnosis with dynamic US – report of three cases. Radiology 228:642–646 Luna A, Martinez S, Bossen E (2005) Magnetic resonance imaging of intramuscular myxoma with histologic comparison and a review of the literature. Skeletal Radiol 34:19–28 Maffulli N, Dymond NP, Regine R (1990a) Surgical repair of ruptured Achilles tendon in sportsmen and sedentary patients: a longitudinal ultrasound assessment. Int J Sports Med 11:78–84 Maffulli N, Regine R, Carrillo F et al (1990b) Tennis elbow: an ultrasonographic study in tennis players. Br J Sports Med 24:151–155 Magnano GM, Occhi M, Di Stadio M et al (1998) High-resolution US of non-traumatic recurrent dislocation of the peroneal tendons: a case report. Pediatr Radiol 28:476–477 Mani NB, Kalra N, Jain M et al (2001) Sonographic diagnosis of a solitary intramuscular cysticercal cyst. J Clin Ultrasound 29:472–475 93 94 M. P. Zamorani and M. Valle Mantello MT, Haller JO, Marquis JR (1989) Sonography of abdominal desmoid tumors in adolescents. J Ultrasound Med 8:467–470 Martinoli C, Derchi LE, Pastorino C et al (1993) Analysis of echotexture of tendons with US. Radiology 186:839–843 Martinoli C, Bianchi S, Derchi LE (1999). Tendon and nerve sonography. Radiol Clin North Am 37:691–711 Martinoli C, Bianchi S, Gandolfo N et al (2000a) US of nerve entrapments in osteofibrous tunnels of the upper and lower limbs. RadioGraphics 20:199–217 Martinoli C, Bianchi S, Nebiolo M et al (2000b) Sonographic evaluation of digital annular pulley tears. Skeletal Radiol 29:387–391 Martinoli C, Bianchi S, Prato N et al (2003) US of the shoulder: non-rotator cuff disorders. RadioGraphics 23:381–401 Martinoli C, Bianchi S, Cotten A (2005) Imaging of rock climbing injuries. Semin Musculoskelet Radiol 9:334–345 Masear VR, Hill JJ, Cohen SM (1988) Ulnar compression neuropathy secondary to the anconeus epitrochlearis muscle. J Hand Surg [Am] 13:720–724 Mastaglia FL, Garlepp MJ, Phillips BA et al (2003) Inflammatory myopathies: clinical, diagnostic and therapeutic aspects. Muscle Nerve 27:407–425 Matsumoto K, Hukuda S, Ishizawa M et al (1999) MRI findings in intramuscular lipomas. Skeletal Radiol 28:145–152 May DA, Disler DG, Jones EA et al (2000) Abnormal signal intensity in skeletal muscle at MR imaging: patterns, pearls and pitfalls. RadioGraphics 20:295–315 McCarville MB, Kaste SC, Pappo AS (1999) Soft-tissue malignancies in infancy. AJR Am J Roentgenol 173:973–977 Melis M, Marongiu L, Scintu F et al (2002) Primary hydatid cysts of psoas muscle. ANZ J Surg 72:443–445 Meng C, Adler R, Peterson M et al (2001) Combined use of power Doppler and gray-scale sonography: a new technique for the assessment of inflammatory myopathy. J Rheumatol 28:1271–1282 Middleton WD, Patel V, Teefey SA et al (2004) Giant cell tumors of the tendon sheath: analysis of sonographic findings. AJR Am J Roentgenol 183:337–339 Milosavljevic J, Lindqvist U, Elvin A (2005) Ultrasound and power Doppler evaluation of the hand and wrist in patients with psoriatic arthritis. Acta Radiol 46:374–385 Montet X, Mauget D, Martinoli C et al (2002) Tensor fasciae suralis: US and MR imaging. Skeletal Radiol 31:536–538 Movin T, Gad A, Reinholt FP et al (1997) Tendon pathology in long-standing achillodynia: biopsy findings in 40 patients. Acta Orthop Scand 68:170–175 Mulier S, Stas M, Delabie J et al (1999) Proliferative myositis in a child. Skeletal Radiol 28:703–709 Müller M, Kalebo P, Tidebrant G (2002) The ultrasonographic appearance of the ruptured Achilles tendon during healing: a longitudinal evaluation of surgical and nonsurgical treatment, with comparisons to MRI appearance. Knee Surg Sports Traumatol Arthrosc 10:49–56 Muren C, Lee M, Juhlin L (1994) CT-diagnosis of deep-seated lipomas with alarming symptoms. Acta Radiol 35:169–171 Murphey MD, Fairbairn KJ, Parman LM et al (1995) Musculoskeletal angiomatous lesions: radiologic-pathologic correlation. RadioGraphics 15:893–917 Murphey MD, McRae GA, Fanburg-Smith JC et al (2002) Imaging of soft-tissue myxoma with emphasis on CT and MR and comparison of radiologic and pathologic findings. Radiology 225:215–224 Murphey MD, Carroll JF, Flemming DJ et al (2004) Benign musculoskeletal lipomatous lesions. RadioGraphics 24:1344–1466 Murphey MD, Arcara LK, Fanburg-Smith J (2005) Imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. RadioGraphics 25:1371–1395 Narici MV, Binzoni T, Hiltbrand E et al (1996) In-vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J Physiol (Lond) 496:287–297 Nehrer S, Breitenseher M, Brodner W et al (1997) Clinical and sonographic evaluation of the risk of rupture in the Achilles tendon. Arch Orthop Trauma Surg 116:14–18 Neustadter J, Raikin SM, Nazarian LN (2004) Dynamic sonographic evaluation of peroneal tendon subluxation. AJR Am J Roentgenol 183:985–988 Newman JS, Adler RS (1998) Power Doppler sonography: applications in musculoskeletal imaging. Semin Musculoskeletal Radiol 2:331–3340 Newman JS, Laing TJ, McCarthy CJ et al (1996) Power Doppler sonography of synovitis: assessment of therapeutic response: preliminary observations. Radiology 198:582–584 Noonan TJ, Garrett WE Jr (1999) Muscle strain injury: diagnosis and treatment. J Am Acad Orthop Surg 7:262–269 Öhberg L, Lorentzon R, Alfredson H (2001) Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: an ultrasonographic investigation. Knee Surg Sports Traumatol Arthrosc 9:233–238 Okayama A, Futani H, Fumiyasu K et al (2003) Usefulness of ultrasonography for early recurrent myositis ossificans. J Orthop Sci 8:239–242 Olsen KI, Stacy S, Montag A (2004) Soft-tissue cavernous hemangioma. RadioGraphics 24:849–854 Otake S (1994) Sarcoidosis involving skeletal muscle: imaging findings and relative value of imaging procedures. AJR Am J Roentgenol 162:369–375 Pagonidis K, Raissaki M, Gourtsoyiannis N (2005) Proliferative myositis: value of imaging. J Comput Assist Tomogr 29:108–111 Palmer WE, Kuong SJ, Elmadbouh HM (1999) MR imaging of myotendinous strain. AJR Am J Roentgenol 173:703–709 Paul MA, Imanse J, Golding RP (1991) Accessory soleus muscle mimicking a soft tissue tumor: a report of 2 patients. Acta Orthop Scand 62:609–611 Pearson CM (1959) Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology 9:757–766 Peck RJ, Metreweli C (1988) Early myositis ossificans: a new echographic sign. Clin Radiol 39:586–588 Peers KHE, Brys PPM, Lysens RJJ (2003) Correlation between power Doppler ultrasonography and clinical severity in Achilles tendinopathy. Int Orthop 27:180–183 Peetrons P (2002) Ultrasound of muscles. Eur Radiol 12:35– 43 Peterson DA, Stinson W, Carter J (1993) Bilateral accessory soleus: a report on four patients with partial fasciectomy. Foot Ankle 14:284–288 Pillen S, Scholten RR, Zwarts MJ et al (2003) Quantitative skeletal muscle ultrasonography in children with suspected neuromuscular disease. Muscle Nerve 27:699–705 Pla ME, Dillingham TR, Spellman NT et al (1996) Painful legs and moving toes associated with tarsal tunnel syndrome and accessory soleus muscle. Mov Disord 11:82–86 Muscle and Tendon Prato N, Derchi LE, Martinoli C (1996) Sonographic diagnosis of biceps tendon dislocation. Clin Radiol 51:737–739 Prato N, Abello E, Martinoli C et al (2004) Sonography of posterior tibialis tendon dislocation. J Ultrasound Med 23:701–705 Premkumar A, Perry MB, Dwyer AJ et al (2002) Sonography and MR imaging of posterior tibial tendinopathy. AJR Am J Roentgenol 178:223–232 Ptasznik R, Hennessy O (1995) Abnormalities of the biceps tendon of the shoulder: sonographic findings. AJR Am J Roentgenol 164:409–414 Reeves ND, Maganaris CN, Narici MV (2004) Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol 91:116–118 Reimers CD, Finkenstaedt M (1997) Muscle imaging in inflammatory myopathies. Curr Opin Rheumatol 9:475– 485 Reimers CD, Finkenstaedt M, Witt TN et al (1993) Muscular ultrasound in idiopathic inflammatory myopathies of adults. J Neurol Sci 116:82–92 Richards PJ, Dheer AK, McCall IM (2001) Achilles tendon (TA) size and power Doppler ultrasound (PD) changes compared to MRI: a preliminary observational study. Clin Radiol 56:843–850 Richards PJ, Win T, Jones PW (2005) The distribution of microvascular response in Achilles tendinopathy assessed by color and power Doppler. Skeletal Radiol 34:336–342 Riehl J, Schmitt H, Bergmann D et al (1997) Tuberculous tenosynovitis of the hand: evaluation with B-mode ultrasonography. J Ultrasound Med 16:369–372 Robbin MR, Murphey MD, Temple T (2001) Imaging of musculoskeletal fibromatosis. RadioGraphics 21:585–600 Rodriguez-Niedenfuhr M, Vazquez T, Golano P et al (2002) Extensor digitorum brevis manus: anatomical, radiological and clinical relevance. A review. Clin Anat 15:286– 292 Rubens DJ, Fultz PJ, Gottlieb RH et al (1997) Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med 16:831–842 Rupp S, Tempelhof S, Fritsch E (1995) Ultrasound of the Achilles tendon after surgical repair: morphology and function. Br J Radiol 68:454–458 Ryan AJ (1969) Quadriceps strain, rupture, and Charlie horse. Med Sci Sports 1:106–111 Saltzman CL, Tearse DS (1998) Achilles tendon injuries. J Am Acad Orthop Surg 6:316–325 Sammarco GJ, Stephens MM (1990) Tarsal tunnel syndrome caused by flexor digitorum accessorius longus J Bone Joint Surg Am 72:453–454 Sarteschi M, Ciatti S, Sabò C et al (1997) Proliferative myositis: rare pseudotumorous lesion. J Ultrasound Med 16:771– 773 Schechter WP, Markison RE, Jeffrey REjr (1989) Use of sonography in the early detection of suppurative flexor tenosynovitis. J Hand Surg [Am] 14:307–310 Schepsis AA, Jones H, Haas AL (2002) Achilles tendon disorders in athletes. Am J Sports Med 30:287–304 Scholten RR, Pillen S, Verrips A et al (2003) Quantitative ultrasonography of skeletal muscles in children: normal values. Muscle Nerve 27:693–698 Schulte M, Mutschler W, Reiche W et al (1995) Diagnosis and spontaneous course of non-traumatic localized myositis ossificans. Unfallchirurgie 98:576–579 Schuurman AH, van Gils APG (2000) Reversed palmaris longus muscle on MRI: report of four cases. Eur Radiol 10:1242–1244 Schweitzer ME, Karasick D (2000) MR imaging of disorders of the Achilles tendon. AJR Am J Roentgenol 175:613–625 Sharma P, Maffulli N (2005) Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am 87:187–202 Silvestri E, Biggi E, Molfetta L et al (2003) Power Doppler analysis of tendon vascularization. Int J Tissue React 25:149–158 Steeds RP, Alexander PJ, Muthusamy R et al (1999) Sonography in the diagnosis of rhabdomyolysis. J Clin Ultrasound 27:531–533 Stephenson CA (1990) Sonographic diagnosis of tenosynovitis of the posterior tibial tendon. J Clin Ultrasound 18:114–116 Sung MS, Kang HS, Suh JS et al (2000) Myxoid liposarcoma: appearance at MR imaging with histologic correlation. RadioGraphics 20:1007–1019 Swen WAA, Jacobs JWG, Hubach PCG et al (2000) Comparison of sonography and magnetic resonance imaging for the diagnosis of partial tears of finger extensor tendons in rheumatoid arthritis. Rheumatology 39:55–62 Teefey SA, Middleton WD, Yamaguchi K (1999) Shoulder sonography. Radiol Clin North Am 37:767–785 Terslev L, Qvistgaard E, Torp-Pedersen S et al (2001) Ultrasound and power Doppler findings in jumper’s knee: preliminary observations. Eur J Ultrasound 13:183–189 Thomas EA, Cassar-Pullicino VN, McCall IW (1991) The role of ultrasound in the early diagnosis and management of heterotopic bone formation. Clin Radiol 43:190–196 Timins ME (1999) Muscular anatomic variants of the wrist and hand: findings on MR imaging. AJR Am J Roentgenol 172:1397–1401 Tohme-Nooun C, Le Breton C, Sobotka A et al (2003) Imaging findings in three cases of the nodular type of muscular sarcoidosis. AJR Am J Roentgenol 183:995–999 Trusen A, Beissert M, Schultz G et al (2003) Ultrasound and MRI features of pyomyositis in children. Eur Radiol 13:1050–1055 Vande Berg B, Malghem J, Puttemans T et al (1996) Idiopathic muscular infarction in a diabetic patient. Skeletal Radiol 25:183–185 Vandervenne JE, De Schepper AM, De Beuckeleer L et al (1997) New concepts in understanding evolution of desmoids tumors: MR imaging of 30 lesions. Eur Radiol 7:1013–1019 Walker FO, Cartwright MS, Wiesler ER et al (2004) Ultrasound of the nerve and muscle. Clin Neurophysiol 115:495–507 Weber MA, Krix M, Jappe U et al (2005) Pathologic skeletal muscle perfusion in patients with myositis: detection with quantitative contrast-enhanced US. Initial results. Radiology 238:640–649 Weinberg EP, Adams MJ, Hollenberg GM (1998). Color Doppler sonography of patellar tendinosis. AJR Am J Roentgenol 171:743–744 Williams JB, Youngberg RA, Bui-Mansfield LT et al (1997) MR imaging of skeletal metastasis. AJR Am J Roentgenol 168:555–557 Wlachovska B, Abraham B, Deux JF et al (2004) Proliferative myositis in a patient with AIDS. Skeletal Radiol 33:237– 240 Yang WT, Yeo W, Metreweli C. (1999) Imaging of iliopsoas metastasis. Clin Radiol 54:85–89 95 96 M. P. Zamorani and M. Valle Yao L, Metha U (2003) Infraspinatus atrophy: implications? Radiology 226:161–164 Yu JS, Resnick D (1994) MR imaging of the accessory soleus muscle appearance in six patients and review of the literature. Skeletal Radiol 23:525–538 Yu JS, Witte D, Resnick D et al (1994) Ossification of the Achilles tendon: imaging abnormalities in 12 patients. Skeletal Radiol 23:127–131 Zanetti M, Metzdorf A, Kundert AP et al (2003) Achilles tendons: clinical relevance of neovascularisation diagnosed with power Doppler US. Radiology 227:556–560 Zarins B, Ciullo JV (1983) Acute muscle and tendon injuries in athletes. Clin Sports Med 2:167–182 Zeiss J, Guilluiam-Hadet L (1996) MR demonstration of anomalous muscles about the volar aspect of the wrist and forearm. Clin Imaging 20:219-221 Nerve and Blood Vessels Nerve and Blood Vessels Maura Valle and Maria Pia Zamorani CONTENTS 4.1 4.1.1 4.1.2 4.1.3 4.1.3.1 4.1.3.2 4.1.3.3 4.1.4 4.1.5 4.1.5.1 4.1.6 4.1.6.1 4.1.6.2 4.1.6.3 4.1.6.4 4.1.7 4.1.7.1 4.1.8 4.1.8.1 4.1.8.2 4.1.8.3 4.1.8.4 Nerve 97 Histologic Considerations 97 Normal US Anatomy and Scanning Technique 98 Anatomic Variants, Inherited and Developmental Anomalies 101 Fibrolipomatous Hamartoma 101 Charcot-Marie-Tooth Disease 102 Hereditary Neuropathy with Liability to Pressure Palsies 103 Nerve Instability 104 Compressive Syndromes 104 Nerve Entrapment Syndromes 105 Traumatic Injuries 108 Stretching Injuries 108 Contusion Trauma 108 Penetrating Wounds 109 Postoperative Features 110 Rheumatologic and Infectious Disorders 112 Leprosy 112 Tumors and Tumor-Like Conditions 114 Peripheral Nerve Sheath Tumors 115 Hemangioma and Non-Hodgkin Lymphoma 119 Intraneural Ganglia 121 Nerve Encasement by Extrinsic Neoplasms 121 4.2 4.2.1 4.2.2 Blood Vessels 123 Histologic Considerations 123 Normal US Anatomy and Scanning Technique 125 4.2.3 Musculoskeletal-Related Vascular Disorders 126 4.2.3.1 Arterial Disorders 127 4.2.3.2 Venous Disorders 129 4.2.4 Vascular Tumors 133 References 133 M. Valle, MD Staff Radiologist, Reparto di Radiologia, Istituto Scientifico “Giannina Gaslini”, Largo Gaslini 5, 16148 Genova, Italy M. P. Zamorani, MD Unité de Recherche et Dévelopement, Clinique des Grangettes, 7, ch. des Grangettes, 1224 Genève, Switzerland 4.1 Nerve 4.1.1 Histologic Considerations From the histologic point of view, nerves are round or flattened cords, with a complex internal structure made of myelinated and unmyelinated nerve fibers, containing axons and Schwann cells grouped in fascicles (Fig. 4.1a) (Erickson 1997). Along the course of the nerve, fibers can traverse from one fascicle to another and fascicles can split and merge. Based on the fascicular arrangement, two theories have been hypothesized to explain the internal architecture of a nerve: the “cable” and the “plexiform” models (Stewart 2003). The first states that nerves are cable-like structures, in which fascicles run separately throughout the entire nerve length (Fig. 4.1b). The second asserts that fascicles alternate splitting, branching, and rejoining along the course of the nerve trunk (Fig. 4.1c). In fact, nerves have both cable and plexiform arrangement of the fascicles depending on the level of examination. In their more proximal portion (e.g., brachial plexus), a plexiform organization of the fascicles predominates. More distally (e.g., median nerve), nerves present a cable-like structure with high degree of somatic organization (e.g., sensory and motor fibers for a specific area of the skin or muscle contained in the same fascicle) (Stewart 2003). The nerve tissue is embedded in a series of connective tissue layers. A closer look at the nerve sheaths demonstrates an external sheath – the outer epineurium – which surrounds the nerve fascicles. Each fascicle is invested in turn by a proper connective sheath – the perineurium – which encloses a variable number of nerve fibers and is responsible for the “blood-nerve” barrier. Then, the individual nerve fibers are invested by the endoneurium. The connective tissue intervening between the outer nerve sheath and the fascicles is commonly referred to as the interfascicular 97 4 98 M. Valle and M. P. Zamorani * * a d b c Fig. 4.1a–d. Nerve histology. a Histologic cross-sectional view of the human sural nerve (black arrows) reveals some nerve fascicles (asterisks) of different size containing nerve tissue (violet) with collections of axons, myelin sheaths, and Schwann cells. Individual fascicles are invested by a thin sheath – the perineurium (arrowheads) – and are separated from each other by a loose connective tissue envelope – the epineurium (green) – containing small intraneural vessels (white arrows). Specimen stained using the van Gieson procedure (original magnification ×150). b,c Schematic drawings of a long-axis view through the nerve trunk illustrate the models of fascicular organization. Arrows indicate the axonal path. b In the “cable model,” the fascicles run parallel to the nerve axis without axonal exchange. c In the “plexiform model,” fascicles split and rejoin in various combinations with axons intermingling from one to another. d Schematic drawing of a cross-sectional view of the monofascicular (1), oligofascicular (2), and polyfascicular (3) nerve models. In complex motor and sensory nerves (3), fascicles (asterisks) are of different size and may be grouped in function-related areas within the nerve. This drawing (3) recalls the structure of the sciatic nerve, in which the nerves fibers for the tibial nerve (light gray) and for the peroneal nerve (dark gray) remain grouped tightly throughout the course of the nerve, even proximally epineurium (internal epineurium), as opposed to the outer epineurium which surrounds the entire nerve trunk. Generally speaking, the amount of connective tissue of the epineurium is more abundant in large multifascicular nerves and in regions in which the nerve is mobile across joints (Delfiner 1996). This thickening of the connective tissue seems to provide more cushioning for the nerve and, therefore, more resistance to compression injury (Delfiner 1996). Externally, the outer (external) epineurium is continuous with the mesoneurium, which is made up of loose areolar tissue. This latter structure is credited with not only supplying the framework for the blood supply entering the nerve, but also making the excursion of the nerve in its bed easier without traction on its blood supply during joint motion (George and Smith 1996). Nerves have a prominent vascular supply to ensure their continuous supply of local energy required for impulse transmission and axonal transport. The vascular supply is formed by an interconnected system of perineural vessels that course longitudinally in the external epineurium and branch among the fascicles (endoneural vessels). 4.1.2 Normal US Anatomy and Scanning Technique Thanks to the latest generation of high-frequency “small parts” transducers and compound technology, US has become a well-accepted and widespread imaging modality for evaluation of peripheral nerves. The improved performance of these transducers has made it possible to recognize subtle anatomic details at least equal to or even smaller than those depicted with surface-coil MR imaging and to depict a wide range of pathologic 100 M. Valle and M. P. Zamorani a b Fig. 4.3a,b. Normal nerve echotexture. a Short-axis and b long-axis 15–7 MHz US images over the median nerve (open arrows) at the mid-forearm. In a, the nerve fascicles (white arrow) are depicted as well-circumscribed individual structures of different size separated by echogenic epineurium. In this segment, 11 fascicles are distinguished in the cross-sectional area of the median nerve. In b, the nerve fascicles appear as elongated hypoechoic bands (white arrows) that run parallel to each other. The internal epineurium (white arrowheads) separates them more clearly, while the external epineurium (open arrowheads) helps to define the outer boundaries of the nerve color and power Doppler systems are, for the most part, unable to recognize the weak and small blood flow signals from the perineural plexus and the intraneural branches. Generally speaking, nerves are compressible and alter their shape depending on the volume of the anatomic spaces within which they run as well as on the bulk and conformation of the perineural structures (Fig. 4.4a,b). Even with slight pressure applied with the probe, they may be seen sliding over the surface of an artery or a muscle. As a general rule, each individual fascicle in a nerve runs independently of the others. Across synovial joints, they pass through narrow anatomic passageways – the osteofibrous tunnels – that redirect their course. The floor of these tunnels consists of bone, whereas the roof is made of focal thickenings of the fascia – the retinacula – that prevent dislocation and traumatic damage of the structures contained in the tunnel during joint activity (Martinoli et al. 2000b). When nerves cross tight passages, such as neural foramina and osteofibrous tunnels, subtle echotextural changes can be seen, with a more homogeneous hypoechoic appearance caused by tighter packing of the fascicles and local reduction in the volume of the epineurium (Sheppard et al. 1998). A careful scanning technique based on the precise knowledge of their position and analysis of their anatomic relationships with surrounding structures is essential for recognizing peripheral nerves with US. Unlike other structures of the musculoskeletal system, nerves do not show anisotropic properties. Therefore, appropriate probe orientation during scanning is not needed to image them; however, systematic scanning in the short-axis plane is preferred for following the nerves contiguously throughout the limbs (Martinoli et al. 1999). Long-axis scans are less effective for this purpose because the elongated fascicles may be easily confused with echoes from muscles and tendons coursing along the same plane. Once detected, the nerve is kept in the center of the US image in its short axis and then followed proximally and distally, shifting the transducer up or down according to the nerve’s course. With this technique – which we can call the “lift technique” – the examiner is able to explore long segments of a nerve in a few seconds throughout the limbs and extremities (Fig. 4.4c). If intrinsic or extrinsic nerve abnormalities are encountered during scanning, the US examination is then appropriately focused on the region of interest using oblique and longitudinal US scanning planes. Although all main nerves can be readily displayed in the extremities due to their superficial position and absence of intervening bone, depiction of the peripheral nervous system is not possible everywhere with US. In fact, most cranial nerves – except for the vagus – and the spinal accessory nerve (Giovagnorio and Martinoli, 2001; Bodner et al. 2002a), the nerve roots exiting the dorsal, lumbar and sacral spine, the sympathetic chains, and the splanchnic nerves in the abdomen cannot be visualized due to their course being to deep or interposition of bony structures. In addition, the perineural structures greatly influence nerve detection in the limbs and extremities. When nerves course deeply, as in obese patients, their evaluation can be difficult. As a general rule, nerves of the lower extremity run deeper than those of the upper extremity and are more difficult to visualize. Nerves coursing among hypoechoic muscles are Nerve and Blood Vessels a b c Fig. 4.4a–c. Response to compression and nerve scanning technique. a,b Schematic drawings of the cross-sectional view of a nerve lying over a stiff surface (bone) a at rest and b during external compression (arrow). Due to the flexibility of the epineurial sheath, the nerve flattens, whereas the fascicles – which are noncompressible structures – redistribute according to the nerve shape changes. c Photograph shows the standard technique for examining nerves in the limbs. The short axis of the median nerve at the wrist is centered in the field of view of the US image. Then, the transducer is swept upward (dashed arrow) along the course of the nerve in the forearm. This technique, which we can call the “lift technique,” allow a simple and reliable evaluation of long nerve segments in a single sweep, excluding possible intrinsic and extrinsic abnormalities along the nerve path. The ability of US to follow the entire course of nerves in the limbs so quickly is a major advantage over MR imaging detected easier than those surrounded by hyperechoic fat. Similarly, a nerve of a young physically active subject is better depicted than the same nerve examined in a subject with atrophic muscles. 4.1.3 Anatomic Variants, Inherited and Developmental Anomalies Given the characteristic US appearance of normal nerves, some anatomic variants can be recognized with this technique. Among these, the proximal bifurcation of the median nerve at wrist has been extensively reported in the literature (see Chapter 10) (Propeck et al. 2000; Iannicelli et al. 2000; Gassner et al. 2002). Similarly, some inherited and developmental anomalies of the peripheral nervous system, such as the fusiform enlargement of the median nerve by fibrofatty tissue (so-called fibrolipomatous hamartoma), the hypertrophy of nerves in Charcot-Marie-Tooth syndrome (Martinoli et al. 2002), and the focal enlargement of nerves in hereditary neuropathy with liability to pressure palsies (Beekman and Visser 2002) can be recognized with US. In these disorders, US findings may contribute to the understanding of pathophysiology by noninvasively revealing some important morphologic information. Further work is, however, required to fully analyze the impact and reliability of US in this field. 4.1.3.1 Fibrolipomatous Hamartoma Fibrolipomatous hamartoma is a developmental tumor-like nerve disorder related to the hypertrophy of mature fat and fibroblasts in the epineurium that often presents during early childhood. This condition – which is also referred to as neural fibrolipoma, perineural lipoma, fatty infiltration of the nerve, lipofibroma, or neural lipoma – has a definite predilection for the median nerve and its branches, with lower extremity involvement (plantar nerve, sciatic nerve) reported as being rare (Marom and Helms 1999; Wong et al. 2006). Fibrolipomatous hamartoma may be associated with local gigantism of an extremity, usually the hand or foot, related to bony overgrowth, fat proliferation in the soft tissues, and nerve-territory-oriented macrodactyly, characteristic of the condition known as macrodystrophia lipomatosa (Amadio et al. 1988; Murphey et al. 1999). The US appearance of fibrolipomatous hamartoma is pathognomonic of this entity, reflecting the morphology of the lesion. US demonstrates a 101 103 Nerve and Blood Vessels 1cm a c * va v 1cm b d Fig. 4.6a–d. Charcot-Marie-Tooth disease in a 37-year-old woman with type 1A disease. a Histologic slice of a fascicle of the sural nerve demonstrates the abnormal “onion bulb” appearance (arrow) of the Schwann cells, a peculiar finding of Charcot-MarieTooth disease. Original magnification ×800. b Transverse T1-weighted MR image of the posterior ankle reveals an enlarged tibial nerve (arrows) characterized by swollen fascicles. The nerve is much larger than normal. Note the atrophic changes in the flexor hallucis longus muscle (asterisk) and the adjacent posterior tibial artery (a) and veins (v). c Short-axis 12–5MHz US image over the middle forearm reveals hypertrophy of the median nerve (open arrows) and its fascicles. d Corresponding 12–5 MHz US image of the median nerve obtained for comparison in a healthy woman at the same magnification shown in c demonstrates a smaller median nerve (open arrows) and fascicles. Note the equivalent size of the flexor carpi radialis (arrowheads) and palmaris longus (white arrow) tendons in the two images. The magnification scale is indicated on the right constantly changing (because not all the causative genes have yet been described), the most common forms include the autosomal dominant types 1A and 2 that are related to DNA duplication of a region on chromosome 17 which codes for a peripheral myelin protein, and the X-linked type that is related to a mutation in the gene which codes for connexin 32, which is a gap-junction protein (Schenone and Mancardi 1999). The degree of electrophysiologic alterations varies widely among patients with different forms of the disease, especially in the type 1A, as a result of phenotypic differences and the action of stochastic factors or environmental modulation of disease severity (Schenone and Mancardi 1999). Nerves appear larger than normal but retain a normal fascicular echotexture (Heinemeyer and Reimers 1999; Martinoli et al. 2002). In considering the main genetic types of Charcot-Marie-Tooth disease, such as the autosomal dominant types 1A and 2, and the X-linked type, patients with type 1A have markedly larger fascicles than patients with the other disease subtypes. In these patients, the diameter of the fascicles and the resulting nerve area are more than twice those seen in healthy subjects and in type 2 and the X-linked type (Fig. 4.6c,d) (Martinoli et al. 2002). There is no correlation between the maximum fascicular size of the nerve and electrophysiologic features, such as distal latencies, velocities, and amplitude (Martinoli et al. 2002). In this specific clinical setting, US can be used to help the neurologist identify unrecognized disease in patients with nonspecific symptoms, to differentiate the 1A genetic subtype, and to provide a useful screening tool for a first selection of the individuals in an affected kindred who are to undergo genetic assessments. 4.1.3.3 Hereditary Neuropathy with Liability to Pressure Palsies Hereditary neuropathy with liability to pressure palsies, also known as tomaculous neuropathy, is 104 M. Valle and M. P. Zamorani an autosomal dominant inherited disorder characterized by a tendency to develop focal neuropathies after trivial trauma that is related to a deletion in chromosome 17p11.2-12 producing reduced expression of peripheral myelin protein 22 (Verhagen et al. 1993). Histopathologically, a sausage-shaped myelin sheath swelling, the so-called tomacula, is responsible for multifocal nerve enlargement. Electrophysiologic studies demonstrate one or more entrapment neuropathies on a background of motor and sensory polyneuropathy. The more frequently involved nerves are: the peroneal nerve at the fibular tunnel, the ulnar nerve at the cubital tunnel, the radial nerve at the spiral groove, and the median nerve at the carpal tunnel (Verhagen et al. 1993; Beekman and Visser 2002). US is able to recognize focal nerve enlargement not only at the osteofibrous tunnels that are typically involved, but also along the course of nerves throughout the limbs (Fig. 4.7). It is conceivable that the “sausage-shaped” myelin swellings (tomacula) found at teased nerve fiber studies in patients with this disorder are responsible for nerve enlargement (Beekman and Visser 2002). 4.1.4 Nerve Instability Dynamic US of the elbow can be used to help demonstrate abnormal dislocation of the ulnar nerve, with or without snapping triceps syndrome. This finding typically occurs in the cubital tunnel, an osteofibrous tunnel formed by a groove between the olecranon and the medial epicondyle and bridged by the Osborn retinaculum. As described in Chapter 8, dynamic scanning during full elbow flexion can allow continual depiction of the intermittent dislo- a b cation of the ulnar nerve over the medial epicondyle if the retinaculum is loose or absent (Jacobson et al. 2001). Dislocation of the medial edge of the triceps can also occur in combination with dislocation of the ulnar nerve (Jacobson et al. 2001). In this syndrome, the ulnar nerve dislocation is secondary to the snapping triceps and dynamic scanning demonstrates the medial head of the triceps and the ulnar nerve remaining in close continuity as they dislocate over the medial epicondyle (see Chapter 8). 4.1.5 Compressive Syndromes From a general pathophysiologic point of view, nerve compression can occur acutely or develop chronically. Short periods of constriction result in slowing and failure of conduction across the constriction point, whereas the nerve portion distal to the region that was compressed retains a normal function. The conduction abnormalities, which are generally referred to by the term “neuroapraxia,” tend to resolve but there may be a prolonged latency until full recovery. This type of injury typically occurs in the radial nerve at the spiral groove of the humerus, the so-called “Saturday night radial palsy”, and in the peroneal nerve around the fibular head and neck, the so-called “crossed leg peroneal palsy”. If local compression is prolonged, ischemia induced by direct severe compression, mechanical distortion of the nerve architecture, may cause more significant damage in the myelin sheath and axonal degeneration (Wallerian degeneration) of the nerve fibers and persistent nerve deficit due to disruption of the axoplasm after the compression has been relieved (Delfiner 1996). In chronic nerve Fig. 4.7a,b. Hereditary neuropathy with liability to pressure palsies in a 42-year-old man with mild median and ulnar neuropathy. a Long-axis 12–5 MHz US image of the ulnar nerve (arrowheads) at the middle forearm with b schematic drawing correlation reveals mild fusiform thickening (arrows) of the nerve out of osteofibrous tunnels 106 M. Valle and M. P. Zamorani close proximity to the compression level, where the nerve abruptly flattens. Given these features, US is an accurate means of identifying the level of compression as located just ahead of the swollen nerve portion. Although nerve flattening should be regarded as the main sign of nerve compression, quantitative analysis of nerve thickening by means of the ellipse formula [(maximum AP diameter) × (maximum LL diameter) × (π/4)] has proved to be the most consistent criterion for the diagnosis at various entrapment sites (Chiou et al. 1998; Duncan et al. 1999; Bargfrede et al. 1999). As an ancillary finding, dynamic scanning may show a reduced mobility of the nerve over the mass or beneath the retinaculum, but this latter sign is too subjective and hard to quantify with US (Nakamichi and Tachibana 1995). At least at the carpal tunnel level, the cross-sectional area of the median nerve has also been regarded as an index for selecting patients with severe disease for which surgical decompression is indicated (Lee et al. 1999). It is conceivable that loss of axons may be associated with nerve enlargement as an expression of an increased amount of endoneural edema (Beekman et al. 2004b). In entrapment neuropathies, the nerve echotexture may become uniformly hypoechoic with loss of the fascicular pattern at the level of the com- pression site and proximal to it (Fig. 4.9). In general, the hypoechoic changes occur gradually and become more severe as the nerve nears the site of compression (Martinoli et al. 2000b). They derive from swelling of the individual fascicles and decreased echogenicity of the epineurium. The outer lining of the nerve, which is normally undefined and part of a continuum with the epineurium and surrounding fat, becomes sharp and well delineated. Depiction of such changes may increase confidence in the diagnosis and in determining the exact level of the lesion. In cases of entrapment by scar tissue, diagnostic difficulties may arise in distinguishing echotextural changes related to the compressed nerve from the scar itself, because of a similar hypoechoic appearance. Then, an enhanced depiction of intraneural blood flow signals can be appreciated with color and power Doppler techniques as a sign of local disturbances in the nerve microvasculature that occur in a compressive context (Martinoli et al. 2000b). The hypervascular pattern is more clearly appreciated in swollen hypoechoic nerves of patients with chronic, longstanding disease. Intranervous flow signals are made up of many vessel pedicles that enter the nerve from the superficial epineurium to run perpendicular to the fascicles (Fig. 4.10) (Martinoli et al. 1999, 2000b). a c b d Fig. 4.9a–d. Entrapment neuropathies: echotextural changes. a,b Short-axis 17–5 MHz US images of the right median nerve obtained a at the distal radius and b just ahead of the compression point in a patient with longstanding carpal tunnel syndrome. As the nerve (arrows) approaches the site of compression, increasing hypoechoic changes are detected due to crowding of edematous fascicles and reduced echogenicity of the epineurium. This leads to a complete loss of the fascicular echotexture. c,d Schematic drawing correlation Nerve and Blood Vessels 1 3 b * * * 2 * a c d Fig. 4.10a–d. Entrapment neuropathies: microvascular changes. a Schematic drawing illustrates the nerve vascular system, made up of perineural vessels (1) coursing alongside the nerve. These vessels give off intraneural branches that pierce the outer epineurium (2) and distribute longitudinally (3) among the fascicles. b,c Long-axis 12–5 MHz color Doppler US images of the median nerve (asterisks) in a 56-year-old patient with carpal tunnel syndrome demonstrate subtle flow signals from the longitudinal perineural plexus (arrows) and a series of intranervous branches (arrowheads) running among the fascicles. d Corresponding transverse Gd-enhanced fat-suppressed T1-weighted MR image obtained just deep to the flexor retinaculum (arrowheads) reveals marked uptake of contrast material in the median nerve (arrow) Based on the US assessment, nerve entrapment syndromes can be divided into three main classes. The first includes large nerves (i.e., the median, the ulnar, the radial, the sciatic, the tibial, etc.) which are easily depicted with US at the site of compression. In these cases, US evaluation can be effectively performed with conventional (mid-range) equipment and the diagnosis is based on pattern recognition analysis and quantitative measurements. The second includes small nerves (i.e., the posterior and anterior interosseous, the musculocutaneous, the peroneal, the sural, the plantars, etc.) the depiction of which requires high-end equipment and high-performance transducers. In these cases, quantitative measurements are usually not applied. The third class includes nerves which are not detectable with US because they are either too small (i.e., most part of the saphenous, etc.), or have too deep a course and are hidden by intervening bone (i.e., the suprascapular nerve, the intrapelvic course of the sciatic and the femoral nerve, etc.). In these cases, the US diagnosis is based only on the indirect evaluation of the innervated muscles to identify denervation signs (see Chapter 3). In the first two classes, there are many sites of nerve entrapment that are amenable to US examination in the upper and lower limb, and whatever the site and the nerve involved, the US signs described previously are virtually pathognomonic of compressive neuropathy. They include: the spinoglenoid-supraspinous notch area in the posterior shoulder for the suprascapular nerve (see Chapter 6) (Martinoli et al. 2003); the quadrilateral space for the axillary nerve (see Chapter 6) (Martinoli et al. 2003; the spiral groove of the humerus for the radial nerve (see Chapter 7) (Peer et al. 2001; Bodner et al. 1999, 2001; RosseyMarec et al. 2004; Martinoli et al. 2004); the supinator area at the elbow for the posterior interosseous nerve (see Chapter 8) (Bodner et al. 2002b; Chien et al. 2003; Martinoli et al. 2004) and the wrist for the superficial branch of the radial nerve (see Chapter 10); the cubital and Guyon tunnels for the ulnar nerve (see Chapters 8, 10) (Chiou et al. 1998; Puig et al. 1999; Okamoto et al. 2000; Martinoli et al. 2000b, 2004; Nakamichi et al. 2000; Bianchi et al. 2004; Beekman et al. 2004a; Beekman and Visser 2004); the middle forearm for the anterior interosseous nerve (see Chapter 9) (Hide et al. 1999) and the carpal tunnel for the median nerve (see Chapter 10) (Altinok et al. 2004; Buchberger et al. 1991, 1992; Nakamichi and Tachibana 1995; Bertolotto et al. 1996; Lee et al. 1999; Chen et al. 107 108 M. Valle and M. P. Zamorani 1997; Duncan et al. 1999; Martinoli et al. 2002b; Kele et al. 2003; Bianchi et al. 2004; El Miedany et al. 2004; Yesildag et al. 2004; Wilson 2004; Wong et al. 2004; Kotevoglu and GülbahceSaglam 2005; Koyuncuoglu et al. 2005; Ziswiler et al. 2005); the posterior hip or proximal thigh for the sciatic nerve (see Chapter 12) (Graif et al. 1991); the fibular head and neck for the common peroneal nerve (see Chapter 14) (Martinoli et al. 2000b); the tarsal tunnel for the tibial nerve (see Chapter 16) (Martinoli et al. 2000b) and the intermetatarsal spaces for the interdigital nerves (see Chapter 17) (Redd et al. 1989; Read et al. 1999; Sobiesk et al. 1997; Quinn et al. 2001). A detailed overview of these syndromes is reported later in the chapters on the individual anatomic sites. With respect to the electrophysiologic findings, a positive correlation between the nerve cross-sectional area and the severity of electromyographic findings has been found, whereas only a modest negative correlation seems to exist between electrodiagnostic parameters, such as motor velocity, CMAP amplitude, distal SNAP, and the nerve cross-sectional area (Kele et al. 2003; Beekman et al. 2004; El Miedany et al. 2004; Ziswiler et al. 2005). Generally speaking, US can complement nerve conduction studies in the evaluation of nerve entrapment syndromes. It can be informative in patients with absent motor or sensory responses, when it is difficult to localize the site of compression. A positive US study can reduce the uncertainty of nerve conduction studies and, therefore, reduces the need for further exclusionary studies. In addition, US can identify abnormal findings in the nerve surroundings, such as synovitis, spaceoccupying masses, or anomalous muscles, providing important information in the preoperative setting. After surgical decompression, the US appearance and mobility of the affected nerves may improve, and it is possible to visualize the altered morphology of the osteofibrous tunnel after release of the retinaculum (Martinoli et al. 2000b; El-Karabaty et al. 2005). 4.1.6 Traumatic Injuries Traumatic nerve injuries derive from traction, contusion, and penetrating trauma. Here we attempt a brief overview of nerve trauma according to the different mechanisms involved. In many cases, however, multiple mechanisms may coexist and, therefore, an exact differentiation among them is not always feasible in clinical practice. 4.1.6.1 Stretching Injuries Nerve stretching injuries typically occur as a result of repetitive sprain or strain lesions, as well as with overuse. A characteristic injury is the avulsion of the nerve roots that occurs in brachial plexus trauma during motor vehicle accidents (see Chapter 6) (Shafighi et al. 2003; Graif et al. 2004). Another typical site of nerve traction is the popliteal fossa, where the peroneal nerve may be stretched during high-grade sprains, knee dislocation or fractures (see Chapter 12) (Gruber et al. 2005). In complete nerve lacerations, US reveals disruption of the fascicles with retraction and a wavy course of the nerve ends (Shafighi et al. 2003; Graif et al. 2004; Gruber et al. 2005). The outer nerve sheath may be intact. If traction injury causes partial nerve tear, a spindle neuroma (traction neuroma) can develop as an irregular swelling of hypoechoic tissue along the course of the severed nerve without evidence of nerve discontinuity (Fig. 4.11; see also Chapters 6, 12) (Bodner et al. 2001; Graif et al. 2004). In mild cases, the neuroma may involve only one or a few fascicles while the cross-sectional area of the nerve appears fairly normal or slightly enlarged. 4.1.6.2 Contusion Trauma Contusion trauma most often occurs where nerves run closely apposed to bony surfaces at sites of low mobility and, are therefore, more vulnerable to external injuries. In most cases, such trauma is self-resolving and does not cause morphologic changes detectable with US (Fig. 4.12). Repeated minor contusion trauma is usually required to cause abnormalities within the nerve substance that can be detected with US. A typical contusion trauma is that involving the radial nerve where it pierces the lateral intermuscular septum, or the deep peroneal nerve against the midfoot bones in soccer players who receive repeated blows over the dorsum of the foot (see Chapter 17) (Schon 1994; Quinn et al. 2001). These lesions lead to development of a segmental fusiform thickening of the nerve at the site of trauma. A peculiar kind of contusion trauma is that involving unstable ulnar nerves at the cubital tunnel in patients with absence of the Osborne retinaculum. In predisposed subjects, the repeated friction of the nerve against the epicondyle during elbow flexion may cause chronic damage and functional deficit, so-called “friction neuritis”. In these cases, the nerve appears swollen and hypoechoic as a result 109 Nerve and Blood Vessels sm/sp sa a b Fig. 4.11a,b. Stretching injury (burner/stinger syndrome) of the brachial plexus nerves in a 25-year-old rugby player with persistent tingling radiating from the left shoulder to the hand and progressive weakness of the limb muscles after a significant contact injury. a Short-axis and b long-axis 12–5 MHz US images over the interscalene area demonstrate segmental thickening of the C5 (open arrows), C6 (white arrows), and C7 (arrowheads) components of the plexus (upper and middle trunks), reflecting fusiform neuromas related to stretching trauma. sa, scalenus anterior; sm/sp, scalenus medius/scalenus posterior muscles. In the burner/stinger syndrome, MR imaging of the cervical spine should always be performed to rule out nerve damage inside the spinal canal as well as herniated disks, ligament injuries, facet injuries, and undisplaced fractures a b c d Fig. 4.12a–d. Nerve contusion trauma. Peroneal neuropathy in a 15-year-old boy with onset of foot-drop after receiving a blow on the lateral knee. a Lateral plain film demonstrates an exostosis (arrow) at the fibular metaphysis. b Transverse 12–5 MHz US image reveals impingement of the peroneal nerve (arrow) against the cartilaginous component (arrowheads) of the exostosis. The nerve is swollen and hypoechoic. c,d Correlative transverse fat-suppressed GRE T2* MR images demonstrate a hyperintense nerve (arrow) crossing the exostosis (arrowhead). Note the T2-hyerintense cap of the exostosis of fibrotic changes and shows a thickened external epineurium (see Chapter 8) (Jacobson et al. 2001). 4.1.6.3 Penetrating Wounds In penetrating wounds (glass fragments are often involved!), there may be a partial or complete inter- ruption of the nerve fascicles. Regenerating Schwann cells and axons grow randomly at the lesion site in an attempt to restore the continuity of the nerve. Generally, the gap between the separated fascicles is wide, and new axonal sprouts develop in many directions. A hypoechoic fibrous mass is the result of such a disorganized repair process. In complete tears, stump neuromas (terminal neuromas) appear as small hypoechoic masses in continuity 110 M. Valle and M. P. Zamorani with the opposite edges of the severed nerve (see Chapters 9, 10) (Provost et al. 1997; Graif et al. 1991; Simonetti et al. 1999). Usually, their size is slightly larger than the axial diameter of the nerve. Most have well-defined margins; however, when they are attached to the surrounding tissues by adhesions and encasing scar tissue, their borders may be irregular or poorly defined (Bodner et al. 2001). US depiction of terminal neuromas may map the location of the nerve ends, which may be displaced and retracted from the site of the injury (Fig. 4.13a–c). When the nerve ends are close together, the bulk of neuroma may encase them mimicking a partial tear. In some way, this seems to suggest that US is unable to quantify the grade of nerve damage within a spindle neuroma. When the nerve is partially torn, the hypoechoic neuroma may encase resected and preserved fascicles giving rise to a homogeneous fusiform swelling of the nerve or can be seen arising specifically from the resected fascicles, while the unaffected fascicles can be appreciated continuing their course alongside the fibrous mass (Fig. 4.14). In this latter instance, US is able to estimate the amount (percentage) of fascicles involved in the neuroma (Fig. 4.14d). Overall, US may help the clinical b 1 4.1.6.4 Postoperative Features In patients with partial nerve tear, a delicate procedure of internal neurolysis of the nerve and its sheath is mainly used for either repairing the interrupted nerve fascicles or removing intraneural scar tissue. With this procedure, the main risk consists of inadvertent damage to preserved fascicles and formation of a new postoperative scar close to the nerve surface. With complete transection of the nerve, a more complex surgical procedure is required. The appropriate selection of an adequate reconstruction technique depends on the length of the gap intervening between the nerve ends after removal of irreversibly damaged tissue and terminal neuromas. Where the gap is short, an “end-to-end” anastomosis is preferred given that substantial tension on the sutured 2 1 a examination and nerve conduction studies to provide information about the condition of the injured nerve, and especially in deciding whether early surgical treatment is required. This is particularly true for minor nerve lesions without axonal damage. 2 1 c 2 2 1 d e Fig. 4.13a–e. Complete nerve tear in a 12-year-old girl with loss of function of the median nerve after receiving a penetrating injury to the arm by a glass fragment. In the acute setting, the patient was operated on for laceration of the brachial artery. a,b Long-axis 15-7 MHz US images at the level of injury demonstrate discontinuity of the median nerve. Note the proximal and distal stumps (arrowheads) of the severed nerve ending in a hypoechoic terminal neuroma (1, 2). c Gross surgical view shows discrete retraction (4 cm gap) of the nerve ends. d After reconstructive surgery, a long-axis 12–5 MHz US image demonstrates the sural nerve graft (curved arrow) interposed between the nerve ends (arrowheads). A fusiform hypoechoic thickening is observed at the proximal (1) and distal (2) site of anastomosis: it should be regarded as a normal finding. A split-screen image was used, with the two screens aligned for an extended field of view. e Surgical correlation 112 M. Valle and M. P. Zamorani lections. A mild and fusiform increase in the nerve size at the sutures level is a normal finding. In contrast, marked irregular bulging of hypoechoic tissue at the anastomosis, possibly involving one side of the nerve, should be regarded as a pathologic sign, indicating inadequate fusion of the nerve edges and postsurgical neuroma formation (Graif et al. 1991; Peer et al. 2001). Excessive tension on the nerve edges and infection are possible causes of defective anastomosis. In this clinical setting, US may compensate for the limitations of electrodiagnosis and clinical examination by yielding reliable information on the size, extent, and localization of postsurgical scarring and neuromas with respect to further surgical intervention (Peer et al. 2003). In addition to primary (trauma-related, neurolysis-related) causes, scar formation may occur following surgery that has not been primarily directed to the nerve (i.e., fracture repair, vascular surgery, etc.). Scar tissue may encase the nerve as a whole or may lie adherent to its surface (Fig. 4.16). The nerve appears flattened and indistinguishable within the scar or may be distorted at its periphery with reactive focal swelling related to edema and venous congestion (Fig. 4.16c,h). Under these circumstances, nerve scarring may lead to persistent pain and delayed recovery of nerve function because of constant traction on the nerve and limited capability for longitudinal translation during joint movements. In the postoperative setting, US findings of incidental iatrogenic injuries to peripheral nerves have been reported in the radial, femoral, accessory, and sciatic nerves (Graif et al. 1991; Peer et al. 2001; Bodner et al. 2002a; Gruber et al. 2003). 4.1.7 Rheumatologic and Infectious Disorders In several rheumatologic disorders, such as rheumatoid arthritis, polyarteritis nodosa, Wegener’s granulomatosis, and Churg-Strauss and Sjögren syndromes, one of the clinical landmarks of vasculitis is the appearance of neurologic findings (Lanzillo et al. 1998; Rosenbaum 2001). From the pathophysiologic point of view, vasculitis-related neuropathy affects large nerve trunks producing a multifocal degeneration of fibers as a result of necrotizing angiopathy of small nerve arteries, so-called multiple mononeuropathy (Said and Lacroix 2005). In these patients, the neuropathy does not correlate with disease parameters, such as disease activity, rheuma- toid factor, and functional and radiologic scores, and there is sequential involvement of individual nerves both in time and anatomically (Nadkar et al. 2001). Nerve conduction velocities are usually not markedly reduced from normal, provided that the compound nerve or muscle action potential is not severely reduced in amplitude (Sivri and GulerUysal 1998). Although multiple mononeuropathy is the most common manifestation, nerve entrapment syndromes may also occur at sites where nerves pass in close proximity to either a synovial joint (i.e., cubital tunnel, tarsal tunnel, Guyon tunnel) or one or more synovial-sheathed tendons (i.e., flexor tendons at the carpal tunnel, flexor hallucis longus at the tarsal tunnel) or para-articular bursae (i.e., iliopsoas bursa at the hip). Because the clinical evaluation of nerves is often limited in these patients by simultaneous symptoms resulting from joint involvement, US imaging can contribute to distinguishing entrapment neuropathies related to derangement of joints, effusions, and synovial pannus from non-entrapment neuropathy. This is based on the fact that multiple mononeuropathy does not lead to an altered morphology of the affected nerve, whereas entrapment neuropathies do. 4.1.7.1 Leprosy Leprosy (Hansen disease) is a chronic infectious disease caused by Mycobacterium leprae, which, in its many and various clinical forms, involves the skin and nerves (Fig. 4.17a). Although in the Western world leprosy is almost only seen in immigrants, it is endemic in developing countries (tropics and subtropics) with 12 million people affected; it therefore represents the most diffuse neuropathy in the world. Leprosy is probably spread by droplet infection, but prolonged household contact is needed and most people are not susceptible to the disease. From the clinical point of view, leprosy can be grouped into two polar forms – tuberculoid and lepromatous – between which borderline forms show an intermediate spectrum of phenotypes (Ridley and Jopling 1966). In tuberculoid leprosy, there is an intense immune response: aggressive infiltration of epithelioid and lymphoid cells into the nerve causes thickening of the epineurium and perineurium and destruction of fascicles. In the lepromatous type, the immune response is indolent and active proliferation of bacilli occurs: this form shows better preservation of the nerve architecture. Transition Nerve and Blood Vessels humerus a humerus b d c e f g h Fig. 4.16a–h. Postoperative encasement of nerves by scar tissue. Two different cases. a,b Radial nerve buried in a fibrous callus after repair of humeral shaft fracture. a Long-axis and b short-axis 17–5 MHz US images show focal encasement of the radial nerve (open arrowheads) by an ill-defined hypoechoic mass (white arrowheads) developing over the fracture site (arrow) reflecting the formation of fibrous callus. The nerve’s fascicular echotexture is retained within the callus. The patient underwent a second surgical look to free the nerve from the callus. c–h Peroneal nerve encased in a scar after surgical stripping of the saphenous vein. c Photograph shows the surgical access. d Long-axis 12–5 MHz US image of the peroneal nerve (arrows) with e schematic drawing correlation reveals distortion and pinching of the nerve fascicles (open arrowheads) by hypoechoic scar tissue (white arrowheads). f–h Short-axis 12–5 MHz US images obtained from f proximal to h distal show the normal peroneal nerve (open arrow) which becomes indistinguishable (open arrowheads) within the scar (white arrowheads) and then as it (white arrow) exits the scar to return to a normal appearance toward a higher resistance form of leprosy may produce episodes of acute neuritis, such as the socalled “reversal reaction” and “erythema nodosum leprosum”. During these phases, a nerve segment may become intensely painful and tender. As the disease progresses, subsequent episodes of neuritis add to the deficit until the affected nerve may be completely destroyed. Sensory abnormalities usu- ally precede paralysis. The initial symptom of nerve involvement is sensory loss, which increases the frequency of minor trauma, leading to infections and eventually to mutilating injuries, and blindness. The preferred sites of nerve swelling in leprosy are similar to those of entrapment neuropathies (i.e., the cubital tunnel for the ulnar nerve, the carpal tunnel for the median nerve, the fibular neck for 113 114 M. Valle and M. P. Zamorani ME a b f g h ME d c e Fig. 4.17a–h. Reversal reaction in leprosy. a Microscopic view of the sural nerve in a 25-year-old patient with leprosy reveals the presence of Mycobacteria (purple) among the nerve tissue. Ziehl-Nielsen staining; original magnification ×800. b–h Right ulnar nerve of a 22-year-old man with borderline tuberculoid leprosy examined at the elbow during the course of a reversal reaction. b Long-axis and c short-axis gray-scale 12–5 MHz US images demonstrate high-grade swelling of the nerve (arrows) with smooth fusiform enlargement of individual fascicles. ME, medial epicondyle. Corresponding d long-axis and e short-axis color Doppler 12–5 MHz US images show dramatically increased blood flow within endoneural vessels. f–h Cranial to caudal sequence of Gd-enhanced fat-suppressed MR images through the medial elbow show marked contrast enhancement into the nerve (arrow) the common peroneal nerve, the tarsal tunnel for the tibial nerve). Compared with a chronically compressed nerve, however, the nerve enlargement is more extensive and less circumscribed. In leprosy patients, US is able to reveal nerve abnormalities including nerve swelling, hypoechoic changes in the epineurium, and loss of the fascicular echotexture (Fig. 4.17b,c) (Martinoli et al. 2000c). These changes require multiple episodes of lepromatous reactions and a cumulative effect with time to become apparent at US. In fact, nerve enlargement correlates well with patients who previously underwent reversal reactions (Martinoli et al. 2000c). During the course of a reversal reaction, the affected nerve segment is markedly thickened, intensely painful and tender (Fornage and Nerot 1987; Martinoli et al. 2000c). The onset of these reactions can be indicated by an intraneural hyperemic pattern at color and power Doppler imaging (Fig. 4.17d-h) (Martinoli et al. 2000c). These signs suggest rapid progression of nerve damage and a poor prognosis unless antireaction treatment is started (Martinoli et al. 2000c). More rarely, “cold” soft-tissue abscesses may be seen arising from the affected nerve and spreading through the fascial planes of the limbs and extremities (Fig. 4.18). 4.1.8 Tumors and Tumor-Like Conditions Peripheral nerve tumors include two main benign forms – the schwannoma (also referred to as neurinoma or neurilemmoma) and the neurofibroma – and the malignant peripheral nerve sheath tumor, which most often derives from the malignant (sarcomatous) transformation of a neurofibroma (Murphey et al. 1999). In addition, other masses, such as hemangiomas, lymphomas, and ganglion cysts, may occasionally develop within the nerve dissecting the fascicles and expanding inside the neural tissue. The occurrence of these masses is rare but they may cause nerve dysfunction and local symptoms and should not be mistaken for the more common nerve sheath tumors. Finally, a variety of extrinsic soft-tissue neoplasms, both benign with aggressive behavior and malignant, may involve a nerve during their local spread. 116 M. Valle and M. P. Zamorani a b c d Fig. 4.19a–d. Peripheral nerve sheath tumors. a,b Schwannoma of the tibial nerve at the posterior leg. a Long-axis gray-scale 12–5 MHz US image with b schematic drawing correlation depicts the tumor as a globoid hypoechoic mass (arrows) which develops eccentrically at the periphery of the nerve (arrowheads). c,d Neurofibroma of the median nerve at the distal forearm. c Long-axis gray-scale 12-5 MHz US image with d schematic drawing correlation shows the tumor as a hypoechoic spindleshaped mass (arrows) expanding within the nerve and involving the fascicles (arrowheads). Focal enlargement of the nerve and disappearance of the fascicular pattern is observed unpublished data). Such abnormalities are usually not seen in the distal end of the affected nerve. In addition, schwannomas may be seen developing from an individual fascicle, which appears diffusely thickened even at a distance from the mass, whereas the other fibers of the same nerve are displaced by the bulk of the tumor but remain unaffected with regard to size and echotexture (Fig. 4.21a). This can explain why some schwannomas seems to have central continuity with the long axis of the nerve. Neurofibromas, on the other hand, are intimately associated with the parent nerve, developing in a fusiform (not globoid) fashion, with the nerve entering and exiting from the extremities of the lesion (Fig. 4.19c,d) (King et al. 1997; Lin and Martel 2001). Histopathologically, they are composed of a mixture of cell types, the predominant one of which has characteristics of the perineurial cells. As the proliferative cells of a neurofibroma grow, they spread through the epineurium into the surrounding soft tissue. Neurofibromas can be categorized into three forms: localized, diffuse, and plexiform (associated with type 1 neurofibromatosis). The localized variety is the most common, accounting for approximately 90% of cases (Murphey et al. 1999). Often, a target sign formed by a subtle central hyperechoic region within the hypoechoic mass can be found in these tumors, reflecting a central fibrotic focus surrounded by peripheral myxomatous tissue (Fig. 4.21b–d) (Lin et al. 1999). Neurofibromas are less hypervascular than schwannomas at color and power Doppler imaging. Unlike localized neurofibromas, diffuse neurofibromas primarily involve the skin and the subcutaneous tissue and presents as a plaque-like elevation of the skin with thickening of the subcutaneous tissue (Fig. 4.22a,b) (Murphey et al. 1999). As regards the malignant peripheral nerve sheath tumor, the only findings which may make the exam- Nerve and Blood Vessels Fig. 4.20a–c. Cystic schwannoma. a,b Long-axis a gray-scale and b color Doppler 12–5 MHz US images over the radial nerve (arrowheads) at the arm with c MR-neurographic correlation show a rounded mass (arrows) with intratumoral cystic changes, related to accumulation of myxoid matrix, in continuity with the parent nerve (arrowheads). The tumor exhibits a hypervascular pattern made up of peripheral and central color Doppler signals a b c * * * T a 1 23 b c d Fig. 4.21a–d. Peripheral nerve sheath tumors: peculiar US findings. Two different cases. a Schwannoma of the median nerve at the bicipital sulcus. Long-axis 12–5 MHz US image depicts the tumor (T) as an eccentric hypoechoic mass in continuity with the nerve (open arrowheads). At its proximal and distal ends, the tumor is connected with a swollen fascicle (asterisks), whereas the other fascicles (white arrowheads) remain unaffected and displaced at the periphery of the mass. A split-screen image was used, with the two screens aligned for an extended field of view. b–d Neurofibroma. b Long-axis and c short-axis 12–5 MHz US images of a small neurofibroma in the thigh with d fat-suppressed T2-weighted MR imaging correlation reveal a well-delineated oval mass (arrows) in continuity with the posterior femorocutaneous nerve (arrowheads). The tumor is characterized by concentric hypoechoic and hyperechoic layers (1, 2, 3) consistent with the sonographic target sign 117 118 M. Valle and M. P. Zamorani a b c d * * * * * * * e f Fig. 4.22a–f. Neurofibroma: spectrum of US appearances. Three different cases. a,b Diffuse neurofibroma. a Transverse 12–5 MHz US and b T1-weighted MR images of the suprapatellar region in a 10-year-old child without neurofibromatosis show an illdefined infiltrative mass (arrowheads) extending along the subcutaneous tissue of the anterior knee. c,d Sessile neurofibroma. c Photograph of the right forearm of a 43-year-old man with neurofibromatosis shows a sessile cutaneous neurofibroma (arrow) associated with café-au-lait spots (arrowheads). d The 17–5 MHz US image demonstrates the sessile neurofibroma as a superficial solid hypoechoic mass (straight arrows) arising from the dermis (curved arrow). e–f Plexiform neurofibromas. e Long-axis 12–5 MHz US image over the sciatic nerve in an 8-year-old child with intra- and extra-abdominal neurofibromatosis demonstrates multiple neurofibromas (asterisks) arising from individual fascicles of the sciatic nerve (arrows). f Coronal T2weighted MR image of the pelvis and the proximal thigh shows innumerable neurofibromas along the course of a thickened and hyperintense sciatic nerve (arrows) iner suspect that a nerve tumor is malignant are a sudden increase in size of a previously stable nodule and the presence of indistinct margins and adhesions of the mass with surrounding tissues. Especially in patients with type 1 neurofibromatosis, a rapidly enlarging nodule indicates the need for immediate biopsy. Despite these differences, US cannot distinguish among schwannoma, neurofibroma, and malignant peripheral nerve sheath tumor (Lin and Martel 2001; Reynolds et al. 2004). US can contribute to the preoperative assessment of the extent of disease, by defining the relationship of the tumor to adjacent neurovascular structures and surrounding muscles and also by assisting surgical planning. After imaging assessment, fine needle aspiration biopsy of the mass can be confidently performed under US guidance. During biopsy, excruciating pain is frequently Nerve and Blood Vessels triggered by the needle insertion. From the surgical point of view, schwannomas may be shelled out preserving nerve continuity and function (Murphey et al. 1999). In the postoperative setting, residual hypoechoic thickening of the nerve at the site of tumor resection is almost invariably seen with US: this should be regarded as a normal finding (Fig. 4.23). Recurrence is unusual. In contrast, surgical resection of neurofibromas requires sacrificing the parent nerve because the mass cannot be separated from the nerve fascicles, and subsequent nerve grafting is needed to preserve and restore function. Although surgical management may be acceptable in cutaneous neurofibromas, deep-seated lesions are usually managed conservatively to avoid functional deficit. Type 1 neurofibromatosis (von Recklinghausen disease), a relatively common (1:2500–3000 births) inherited autosomal dominant disease related to an alteration of a gene on chromosome-17, presents with the typical clinical triad of cutaneous lesions (café-au-lait spots), skeletal deformities (scoliosis), and mental deficiency. Widespread involvement by neurofibromas of the localized, diffuse, and plexiform variety occurs with tumors arising from small dermal nerves and large deep-seated nerves. In neurofibromatosis, localized neurofibromas often involve the dermis and the subcutaneous tissue: when pedunculated, they are referred to as the “fibroma molluscum” (Fig. 4.22c,d) (Murphey et al. 1999). In plexiform (multinodular) neurofibromatosis – the pathognomonic form of the disease – innumerable neurofibromas are generated from the fascicles of a large nerve trunk, which is typically involved for a long segment together with its branches, leading to the so-called “bag-of-worms” appearance of the affected nerve at gross inspection and US imaging that results from the diffuse tortuous nerve thickening (Figs. 4.22e,f, 4.24) (Murphey et al. 1999). A disfiguring giant enlargement of the extremities may be associated, so-called elephantiasis neuromatosa (Murphey et al. 1999). Plexiform neurofibromas are indistinguishable from the more rare plexiform schwannomas which sporadically occur in children and young adults: the latter are not associated with type 1 neurofibromatosis and do not undergo malignant transformation (Fig. 4.25) (Ikushima et al. 1999; Katsumi et al. 2003). 4.1.8.2 Hemangioma and Non-Hodgkin Lymphoma Nerve hemangiomas are extremely rare tumors arising from the endothelial lining of the endoneurium from which new vessels arise or infold within nerves from the perineural tissue. Most are recognized in children and young patients; there is no gender prevalence. The tumors tend to enlarge with age, or because of stimulating factors such as and trauma (Bilge et al. 1989). Nerve hemangiomas have a predilection for the median nerve; a persistent median artery has been advocated to explain this prevalence (Prosser and Burke 1987). Clinical findings include palpable nerve swelling at the distal forearm with or without symptoms of carpal tunnel syndrome. US reveals a markedly swollen median nerve containing large intraneural fluid-filled spaces separating the fascicles (Fig. 4.26). Typically, these anechoic spaces are oriented according to the long-axis of the nerve and compressible with the transducer. Color and power Doppler imaging show slow-flowing blood within them (Fig. 4.26c). Venous waveforms are pre- Fig. 4.23. Peripheral nerve sheath tumors: postsurgical findings. Long-axis 12–5MHz US image over the tibial nerve (arrowheads) at the mid-posterior leg in a 48-year-old woman who was previously operated on for schwannoma shows residual hypoechoic thickening (arrows) of the nerve at the site of tumor resection with loss of the fascicular echotexture. This finding was stable at 3 year follow-up. A split-screen image was used, with the two screens aligned for an extended field of view 119 120 M. Valle and M. P. Zamorani * * * * * * * * * * Fig. 4.24. Plexiform neurofibromatosis. Long-axis extended-field-of-view 17–5 MHz US image over the median nerve (arrows) at the forearm in a patient with neurofibromatosis shows multiple plexiform neurofibromas (asterisks), some of which have a central hyperechoic area representing the target sign. The median nerve is markedly enlarged and shows a convoluted multinodular appearance a b c d Fig. 4.25a–d. Plexiform schwannoma. a Photograph of the right hand of a 4-year-old child with an elongated palpable lump (arrows) on the palm, growing in between the third and fourth metacarpals. b Extended-field-of-view 17–5 MHz US image oriented along the long axis of the lump with fat-suppressed c T2-weighted and d postcontrast GRE T1-weighted MR imaging correlation demonstrates a multinodular hypoechoic mass (arrows) made up of swollen convoluted fascicles arising from the median nerve (arrowheads) and branching distally. The tumor appears hyperintense in T2 and after gadolinium administration dominant at spectral Doppler analysis. Surgical neurolysis of nerve hemangiomas is not recommended because intraneural vessels are part of the “vasa nervorum” system and due to the intermingled distribution of vessels with fascicles. In symptomatic patients, carpal tunnel release may be performed to improve the clinical symptoms. Primary non-Hodgkin lymphomas affecting peripheral nerves are very rare. Most involve the sciatic nerve and are the result of direct spread from adjacent tumors (Roncaroli et al. 1997). Peripheral neuropathy may also be appreciated in the absence of direct involvement of the nerve as a paraneoplastic manifestation of lymphoproliferative disorders. From the histopathologic point of view, the affected nerves show extensive neoplastic infiltration of the endoneurium and perineurium. The nerve fascicles are separated by diffuse infiltrates of neoplastic lymphoid cells contained within a thickened epineurium (Eusebi et al. 1990). US reveals a heterogeneous nerve mass with distortion and swelling of the individual fascicles (Fig. 4.27). The treatment usually consists of chemo- and radiotherapy (Pillay et al. 1988). Nerve and Blood Vessels * * * * d a * * b c e f Fig. 4.26a–f. Hemangioma of the median nerve in a 40-year-old woman with carpal tunnel syndrome and a large intramuscular hemangioma extending through the flexor muscles of the forearm down to the carpal tunnel. The patient underwent release of the retinaculum and partial resection of the mass with removal of the flexor digitorum superficialis muscle. a Long-axis and b short-axis 17–5 MHz US images obtained at the distal radius show an enlarged median nerve (arrows) with intranervous abnormal fluid-filled spaces (asterisks) running alongside the fascicles (arrowheads). c Longitudinal color Doppler 12–5 MHz US image reveals slow-flowing blood within the intraneural spaces indicating a hemangioma. Vessels are compressible and exhibits venous waveforms. d,e Correlative transverse d T1-weighted and e T2-weighted MR images show increased T2 signal intensity in the epineurium surrounding the fascicles of the median nerve (arrows), due to the presence of abnormal vessels within the nerve substance. f Digital subtraction angiography confirms the presence of a venous network in the median nerve 4.1.8.3 Intraneural Ganglia The incidence of intraneural ganglia is relatively low, affecting most frequently the common peroneal nerve (Yamazaki et al. 1999). This nerve originates at the apex of the popliteal fossa from the sciatic nerve and moves downward to the fibular head, where it divides into its two terminal branches: the deep and the superficial peroneal nerve. Around the fibular neck, the deep peroneal nerve gives off a small recurrent articular branch to supply the capsule of the superior tibiofibular joint. The capsular ending of this small branch may lead to the development of intraneural ganglia (Spinner et al. 2003, 2005). In fact, this branch serves as a conduit for cyst fluid to pass from the joint space into the nerve (Spinner et al. 2003). The joint fluid dissects the epineurium among the fascicles and moves toward the deep peroneal nerve, the common peroneal nerve and even the sciatic nerve, forming an elongated intraneural cyst. As described in Chapter 14, intraneural ganglia do not have a fibrous capsule or a synovial lining and must be differentiated from the more common extraneural ganglia. As an extension of the superior tibiofibular joint, they appear as spindle-shaped cystic masses contained within the nerve sheath that grow in the space between the epineurium and the nerve fascicles (Martinoli et al. 2000b). 4.1.8.4 Nerve Encasement by Extrinsic Neoplasms Extrinsic soft-tissue tumors may involve normal nerves by contiguity. They may either displace and compress the nerve at the periphery of the mass without infiltrative signs or can incorporate the nerve. In the first instance, the integrity of the nerve can be preserved at surgery after removal or debulking of the mass. In the latter, the surgical procedure is obliged to sacrifice the encased nerve together with the tumor (Fig. 4.28). US may aid in assessing the extent of tumor preoperatively, and in defining the exact relationship of the mass with the nerve and its divisional branches (Fig. 4.29). 121 Nerve and Blood Vessels * * * a ** b Fig. 4.29a,b. Nerve involvement by extrinsic tumors. a Oblique longitudinal 12-5 MHz US image over the popliteal fossa demonstrates a large lipomatous mass (asterisks) encasing the peroneal nerve (arrowheads). A split-screen image was used, with the two screens aligned for an extended field of view. b Gross surgical view shows the bifurcation of the sciatic nerve (arrow) into the tibial (open arrowheads) and the peroneal (white arrowheads) nerves. This latter nerve can be seen infolding within the mass (asterisks). Pathologic examination revealed a liposarcoma 4.2 Blood Vessels An in-depth complete treatise on the arteries and veins running in the limbs and extremities and related-pathology is beyond the scope of a book on the musculoskeletal system. Here, we will focus on general aspects of vascular pathology related to musculoskeletal diseases. Some concepts are specifically addressed in other chapters of the book. The analysis of the proper vascular pathology of limb arteries and veins, such as atherosclerotic disease and venous insufficiency, is more appropriately dealt with in other textbooks and specific literature (Polak et al. 1989; Edwards and Zierler 1992; Foley et al. 1989; Fraser and Anderson 2004). 4.2.1 Histologic Considerations Based on their histologic architecture, the arteries can be divided into four different groups: elastic arteries, medium-sized muscular arteries, small arteries, and arterioles (i.e. the radial and ulnar arteries belong to the medium-sized muscular group). Elastic arteries are the largest in the body; they expand when the heart contracts and return to a normal caliber in diastole. Muscular arteries are small and middle-sized vessels with a relatively narrow lumen and thick walls consisting of circumferentially arranged smooth muscle fibers which restrict the lumen when they contract. The tonus of the smooth muscle component depends on the autonomic nervous system and is responsible for the round cross-sectional shape of the arteries, for blood pressure levels, and for regulatory functions of blood flow (i.e. increased flow volume in the skeletal muscles during exercise). The arterial wall is composed of three concentric layers: the intima (inner tunica) containing the endothelial lining; the media (intermediate tunica) housing smooth muscle tissue; and the adventitia (outer tunica) characterized by fibrous tissue merging with the loose connective space around the vessel (Fig. 4.30a). In a muscular artery, the lamina elastica interna lies between the intima and the media, whereas the lamina elastica externa separates the media from the adventitia. Compared with the arteries, veins have thinner walls and larger lumens. One of their main function is to store blood, and they need muscle to push the blood back to the heart. Because the venous walls may collapse, the vessel shape varies depending on the surrounding tissue conditions, including the subject’s positioning and gravity. In contrast to the arteries, the layering of the venous wall is not so distinct: the intima is very thin (only the largest veins contain discrete amount of subendothelial connective tissue); the media is thinner than the adventitia, and the two layers blend into each other. Peripheral veins may be double or multiple when accompany a medium-sized artery and are, in general, more variable than the arteries themselves, with anastomoses very often occurring between them. Many small to medium-sized veins contain valves (Fig. 4.31a). 123 Nerve and Blood Vessels These are loose, pocket-shaped folds of the intima, which extend into the lumen of the vein. The opening of the cusps prevents backflow of blood and encourages flow toward the heart. Blood flowing toward heart passes the pockets (Fig. 4.31a,b); if the flow reverses, blood fills the pockets thus occluding the lumen of the vein and preventing the pooling of blood (Fig. 4.31c,d). When a subject is standing, the venous return from the legs depends mainly on the activity of calf muscles, the so-called calf pump. 4.2.2 Normal US Anatomy and Scanning Technique Because the limb arteries are relatively superficial, very good quality US images are usually obtained with the transducers used for musculoskeletal applications. Similar to other applications, selection of the appropriate transducer frequency depends on the patient’s build and the depth of the vessel to be examined. Changing the machine settings from a musculoskeletal application to a vascular-specific setting and lowering the gain may help to reduce artifactual speckles within the vessel lumen that may generate confusion with thrombus. In normal states, limb arteries appear as pulsatile structures: pulsatility is better appreciated on short-axis planes during prolonged observation. This sign is usually sufficient to assess limb arteries during a conventional study of the musculoskeletal system for unrelated purposes. a Based on correlative microdissection studies and the use of high-resolution intravascular probes, US demonstrates the normal wall of a small to mediumsized muscular artery as a three-layered structure. An inner bright acoustic linear echo derives from the interface of blood with the intima and the lamina elastica interna, and an outer echogenic layer is produced by reflection at the interface between the lamina elastica externa and the adventitia (Fig. 4.30b) (Chong et al. 1993; Siegel et al. 1993). Being primarily composed of smooth muscle, the tunica media appears as a mid-hypoechoic band intervening between the two echogenic layers (Chong et al. 1993; Siegel et al. 1993). In contrast, the wall of elastic arteries, whose media have a high elastin content, appears uniformly echogenic (Chong et al. 1993; Siegel et al. 1993; Martin et al. 1997). When vascular disease is suspected, color Doppler imaging and spectral Doppler analysis can complement gray-scale findings to determine patency and vessel narrowing. As a rule, Doppler examination should be performed along the longitudinal axis of the vessels with a Doppler angle of 60° or less (Fig. 4.32a). Because most vessels of the extremities course parallel to the skin, beam steering should be used as a default setting to obtain adequate Doppler angles. At rest, spectral Doppler analysis of flow waveforms and color Doppler imaging of the upper and lower limb arteries demonstrate a characteristic pattern of high distal resistance (Fig. 4.32b). In response to exercise and muscle activation, vasodilation usually produces a higher forward flow b c Fig. 4.32a–c. Normal Doppler imaging findings in the brachial artery. a Pulsed Doppler analysis shows normal high-resistance pulsatile flow with oscillations in diastole. A steered color box is used to obtain an adequate Doppler angle and clear-cut Doppler tracings. b Color Doppler imaging appearance of flow in the high-resistance brachial artery (a). There is evidence of color flow signal in systole (1) but not in the early diastolic phase (2). Continuous blood flow is detected in the adjacent brachial vein (v) with complete filling of the vessel lumen, indicative of patency. c Following repeated fist clenching, the resulting vasodilation produces a higher forward flow throughout the cardiac cycle, particularly in diastole 125 Nerve and Blood Vessels which must be known by the sonologist when performing a US study of the musculoskeletal system. Other vascular pathology, even if relevant, has been omitted as outside the aims of this book. 4.2.3.1 Arterial Disorders Because of their superficial location and close apposition to the bones, the arteries of the limbs and extremities are particularly vulnerable to traumatic injuries. Based on its pathomechanism, arterial trauma can be arbitrarily subdivided into three main types: acute direct injuries following a penetrating wound by a sharp object or blunt arterial lacerations related to major stretching or contusion trauma (including high-grade sprains, bruising, dislocated joints and fracture-dislocations near arteries – such as supracondylar humeral fractures for the brachial artery, glenohumeral dislocations for the axillary artery, supracondylar femoral fractures for the popliteal artery, and knee dislocation for the posterior tibial artery); chronic repeated microtrauma causing progressive damage to the vessel wall that may lead to pseudoaneurysms, aneurysms, and vessel occlusion; and iatrogenic injuries resulting in either thrombosis or local hemorrhage. When major arterial trunks of the limbs are involved, direct traumatic injuries are clinical emergencies and, in most cases, require immediate surgical repair to avoid acute limb ischemia, hypotensive shock, and death related to blood loss (Davison and Polak 2004). Doppler US has been advocated for the diagnosis of acute arterial trauma to the extremities in an emergency setting, but its sensitivity is lower than that of CT angiography (Fry et al. 1993; Knudson et al. 1993; Miller-Thomas et al. 2005; Rieger et al. 2006). Similar to MR imaging, color Doppler US has substantial limitations in this field, related to the considerable amount of time needed to make the diagnosis. In addition, color Doppler imaging is operator-dependent, may be inadequate in the evaluation of arterial flow distal to an arterial injury, is susceptible to confusion created by collateral vessels, and may be unsuitable in patients with open wounds (Rieger et al. 2006). Color Doppler imaging seems more useful for identifying and monitoring minor arterial injuries occurring during trauma that do not require specific immediate operative management – such as intimal lesions, pseudoaneurysms, and minor vessel occlusions – in order to assess whether they resolve or progress (Schwartz et al. 1993). Similarly, gray-scale US and Doppler imaging techniques seem more relevant for identifying incidental arterial damage secondary to chronic microtrauma and overuse syndromes. These lesions typically occur in the hand, where the branches of the ulnar artery can be pinched between the skin and the underlying hamate as the result of repeated external trauma against the palm (Fig. 4.34). This condition, which is commonly referred to as “hypothenar hammer syndrome,” results in intimal injury, thrombosis or aneurysm with subsequent digital ischemia, pain or a palpable mass in the hand (see also Chapter 10) (Okereke et al. 1999; Liskutin et al. 2000; Velling et al. 2001). Similar vascular abnormalities may occur at the level of the dorsalis pedis artery following repeated blunt trauma over the dorsum of the ankle and midfoot (Yamaguchi et al. 2002; Ozdemir et al. 2003). In these cases, US and Doppler techniques should be the first-line imaging modality. Digital subtraction angiography or contrast-enhanced MR-angiography may still be required by the vascular surgeon for precise preoperative planning. Other uncommon causes of closed arterial damage associated with abnormalities of the musculoskeletal system are related to anatomic variants, such as: injury to the popliteal artery due to osseous abnormalities in patients with hereditary multiple exostoses (see Chapter 14) (Chamlou et al. 2002); popliteal artery entrapment syndrome, produced by anomalous proximal insertion of the medial head of the gastrocnemius (Fig. 4.35) (see Chapter 14) (Wright et al. 2004); and brachial artery entrapment in the arm secondary to the presence of a supracondylar process and the Struthers ligament, so-called supracondylar process syndrome (see Chapter 7) (Talha et al. 1987). As regards iatrogenic injuries, procedures of arterial catheterization may be responsible for vascular dissections, soft-tissue hematomas, pseudoaneurysms, and arteriovenous fistula formation (Clevert et al. 2005; Schwartz et al. 1991). These lesions are typically located at the puncture site, including the groin for the femoral artery and its divisional branches (see Chapter 12) (Roubidoux et al. 1990; Helvie et al. 1988) and the medial arm for the brachial artery (see Chapter 7) (Chuang et al. 2002). Compression-based femoral and median neuropathy is a well-established complication of hematomas and pseudoaneurysms following arterial catheterization (see Chapter 7) (Jacobs et al. 1992; Chuang et al. 2002). Color Doppler imaging is useful in differentiating complications of femoral 127 128 M. Valle and M. P. Zamorani a b e Carpal tunnel Hamate c f d Fig. 4.34a–f. Hypothenar hammer syndrome. Two different cases of manual workers – one a car mechanic, the other a weightlifter – who had occupational hammering in their right hands. a–d Occlusion of the ulnar artery. a Transverse and b longitudinal 15–7 MHz US images over the hypothenar eminence with c schematic drawing and d gross surgical view correlation show complete occlusion of the ulnar artery (arrows) at the point where it courses adjacent to the hamate hook. Note the adjacent uninjured sensory branch of the ulnar nerve (arrowhead). Operative view demonstrates a thickened pale artery coursing adjacent to the ulnar nerve. The patient had ischemic symptoms in the fourth and fifth fingers. e,f Aneurysm of the ulnar artery. e Transverse and f longitudinal 12–5 MHz US images over the hypothenar eminence demonstrate an aneurysm (white arrows) of the ulnar artery (large open arrow). There are no echoes evident in the lumen, indicating absence of thrombus. The patient complained of only mild pain over the aneurysm. Arrowheads, sensory branch of the ulnar nerve * a a b a d a a c a e * g a f h i Fig. 4.35a–i. Popliteal artery entrapment syndrome. a–c Series of transverse 17–5 MHz US images of the popliteal fossa with d–f T1-weighted MR imaging correlation in a 45-year-old man with exercise-induced claudication of his right lower extremity demonstrate an anomalous medial course of the popliteal artery (a), which passes from medial to lateral across an abnormal muscle band (dashed line in a-c, arrows in d-f) lying in the popliteal fossa. At color Doppler imaging (not shown), the vessel occlusion initiated at the point where the artery is closely apposed to the posteromedial corner of the medial femoral condyle (asterisk) and is, therefore, vulnerable to compression by the anomalous muscle. MR imaging allowed this muscle abnormality to be classified as type 2. g,h Spectral Doppler analysis performed g at the upper-popliteal artery level and h downstream in the tibial artery at the proximal leg reveals g normal pulsatile flow cranial to the occlusion and h dampening of the distal flow waveforms. i Anteroposterior digital subtraction angiography shows segmental occlusion (arrowheads) of the popliteal artery with collateral filling through the geniculate arteries Nerve and Blood Vessels artery catheterization, such as hematoma, pseudoaneurysm, and arteriovenous fistula. It demonstrates arterial pseudoaneurysm as a perivascular sac with thickened echogenic walls (mural thrombus) containing swirling flow with alternating red and blue colors (Fig. 4.36a–c). In general, the neck connecting the artery with the pseudoaneurysm is better recognized on color Doppler imaging than on gray-scale US (Schwartz et al. 1991). At Doppler spectral analysis, blood flow in the neck exhibits bidirectional high velocities as blood enters the cavity from the damaged artery in systole (flow displayed above the baseline) and exits in diastole (flow displayed below the baseline), the so-called “to-andfro” signal (Fig. 4.36d) (Sacks et al. 1989). On the other hand, characteristic findings of arteriovenous fistulas include: visible connection between artery and vein, multicolored (mosaic pattern) speckled mass at the fistula site, spreading of color pixels into the perivascular soft tissues, high diastolic flow in the arterial waveform proximal to the fistula site, decreased flow in the artery caudal to the fistula, and high-velocity turbulent flow, sometimes with a pulsatile component, in the efferent vein (Helvie and Rubin 1989; Roubidoux et al. 1990). US-guided procedures to treat pseudoaneurysms with direct probe compression and thrombin injection are described elsewhere (see Chapter 12). In the postoperative setting, US and Doppler techniques have proved valuable in evaluating by pass grafts to detect the onset of early failure, including stenoses, thrombosis, and infectious collections (Fig. 4.37). 4.2.3.2 Venous Disorders Direct trauma to the deep and superficial venous system only occasionally produces a vascular lesion, such as an aneurysm or a vein occlusion. Although rare, the possibility of a venous aneurysm should, however, be taken into account so as not to confuse an aneurysm with either a ganglion cyst (when patent) or a solid soft-tissue mass (when thrombosed). Demonstration of the continuity of the dilated venous segment with a superficial, even small, vein and blood flow detected within the mass may help the diagnosis (Fig. 4.38a–d). When thrombosed, venous aneurysms may be a diagnostic challenge because they appear as nonspecific solid avascular masses (Fig. 4.38e). Post-traumatic vein thrombosis may occasionally be encountered following muscle strains as a result of stretching of the vessel walls. This kind of trauma typically occurs in the infrapopliteal veins (the gemellary veins are the most commonly involved) of patients with tennis leg lesion (see Chapter 15) (Delgado et al. 2002). Post-traumatic muscle edema and hematoma may also produce compression and then occlusion of low-pressure intramuscular veins. Similarly, pro- * a b c d Fig. 4.36a–d. Iatrogenic pseudoaneurysm of the brachial artery. a Photograph of the anterior right elbow of a 72-year-old woman presenting with an enlarging pulsatile soft-tissue lump (arrows) that developed after a vein cannulation procedure. Transverse b gray-scale and c color Doppler 17–5 MHz US images over the lump reveal a large complex mass (straight arrows) with thickened walls and a central cavity filled with whirling flow (asterisk) consistent with a pseudoaneurysm of the brachial artery (arrowheads). The slow flow in the pseudoaneurysm makes blood echogenic at gray-scale imaging. Color Doppler imaging demonstrates continuity of the pseudoaneurysm cavity with a displaced brachial artery by means of a thin neck (curved arrow). d Spectral Doppler analysis obtained in the communicating tract displays bidirectional velocities as the forward flow (1) in systole is ejected (2) in diastole 129 130 M. Valle and M. P. Zamorani * * * * a b * * * * c Fig. 4.37a–c. Abscess around a bypass graft. a Long-axis and b short-axis 17–5 MHz US images over an occluded aorto-femoral bypass graft (arrows) in a 70-year-old diabetic patient with amputated lower leg and clinical signs of sepsis. Note the shrunken appearance of the graft surrounded by a fluid collection (asterisks). c Preoperative percutaneous drainage of the collection. The catheter (curved arrows) is seen inside the almost empty abscess (asterisks). As shown in the insert, aspiration resulted in purulent material * c a * b d e Fig. 4.38a–e. Venous aneurysms. Two different cases. Long-axis a,b gray-scale and c,d color Doppler 12–5 MHz US images obtained a,c,d without and b with probe compression over a compressible soft-tissue mass of the dorsum of the mid-foot in a patient with healed mid-tarsal fractures reveal a fluid-filled oval lesion (asterisks) connected at its opposite ends to a small superficial vein (arrowheads). As shown in b, the mass is fully compressible without internal thrombus. This sign, together with demonstration of the venous ends and of internal blood flow at color Doppler imaging, may avoid confusion with ganglion cysts. While releasing probe compression, blood flow (arrow) can be seen entering the aneurysm from the parent vein (arrowhead) to completely fill its cavity. e Thrombosed superficial varicose vein. Sagittal 12–5 MHz US image over the posteromedial aspect of the leg shows a polycyclic hypoechoic mass (arrows) in continuity with a thin pedicle (arrowheads) directed toward depth, an appearance nonspecific at US examination. After surgical resection, this mass proved to be a thrombosed varix longed absence of contracture of the calf muscles as a result of local pain and post-traumatic immobilization may be implicated as a possible cause of venous thrombosis. In the lower limb, compression US and color Doppler imaging can easily diagnose deep venous thrombosis and distinguish a vascular problem from other musculoskeletal conditions that may mimic it, including a ruptured Baker cyst (see Chapter 14) or a post-traumatic hematoma (see Chapter 15). The classic description of venous thrombosis is that of an enlarged vein with thickened walls containing echogenic material with multiple sur- rounding collateral vessels (Murphy and Cronan 1990). Based on the imaging findings, US can distinguish complete occlusive (Fig. 4.39a,b) from partial non-occlusive thrombosis (Fig. 4.39c,d). Non-occlusive thrombus may not alter the spectral Doppler flow pattern. In some instances, the head of the thrombus may float freely within the vessel lumen (Fig. 4.39e,f). This finding should be indicated in the report as it relates to an increased risk of embolism. Although many have tried to date the thrombus on the basis of its reflectivity, such attempts have been ineffective (Murphy and Cronan 1990). In chronic vein thrombosis, recanalization of the thrombus Nerve and Blood Vessels a b 1 2 c d * * e f * * g i a h A j Fig. 4.39a–j. Vein thrombosis: spectrum of US appearances in different patients. a,b Complete vein thrombosis. a Short-axis and b long-axis 12–5 MHz US images of an intramuscular vein (arrowheads) of the soleus containing highly echogenic material consistent with chronic thrombus. This vein was noncompressible at US. c,d Partial vein thrombosis. c Short-axis gray-scale 12–5 MHz US image of the popliteal vein shows a distended lumen containing reflective material (1) suggestive of thrombosis. d Correlative transverse color Doppler 12–5 MHz US image confirms the presence of an area of non-occlusive thrombus with blood flow surrounding the periphery of the clot with a crescentic appearance. Note that part of the non-echogenic lumen (2) was also thrombosed indicating successive phases of thrombus apposition with time. e,f Floating thrombus. e Short-axis and f long-axis 12–5 MHz US images of the greater saphenous vein demonstrate the proximal head of the thrombus (arrow) floating freely in the patent vessel lumen (asterisk). During real-time observation, the thrombus could be seen knocking against the vessel wall. This kind of thrombus correlates with the highest risk of embolism. g,h Recanalized thrombus. Long-axis g grayscale and h color Doppler 12–5 MHz US images over the posterior knee show an enlarged popliteal vein (arrowheads) containing heterogeneous thrombus. Tiny longitudinal hypoechoic channels with flow (curved arrow) are seen inside the thrombus reflecting a process of partial recanalization. Observe the popliteal artery (a) and superficial venous collaterals (asterisks). i,j Chronic vein occlusion. i Short-axis and j long-axis 12–5 MHz US images of the small saphenous vein demonstrate an occluded vessel (arrowheads) which appears markedly narrowed. The schematic drawings on the right side of the US images report the cross-sectional profile of a vein with disposition of thrombus (gray) and patent lumen (white) in relation to the different types of vein thrombosis described 131 132 M. Valle and M. P. Zamorani * * a v a b c d Fig. 4.40a–d. Common vascular and soft-tissue abnormalities associated with vein thrombosis. Three different cases. a Opening of collateral vessels. Long-axis color Doppler 12–5 MHz US image over a thrombosed greater saphenous vein (asterisks) demonstrates a process of partial recanalization (curved arrow) by a collateral vessel (arrowhead). b Muscle edema related to venous stasis. Short-axis gray-scale 12–5 MHz US image over the bicipital fossa shows a thrombosed cephalic vein (arrows) that lies superficial to the brachial artery (a), the median nerve (curved arrow), and a patent brachial vein (v). Note the subfascial edema (arrowheads) involving the biceps brachii muscle as a result of venous stasis. c,d Superficial thrombophlebitis of the lower leg. c Short-axis and d long-axis 12–5 MHz US images over a thrombosed lesser saphenous vein (arrows) demonstrate ill-defined vessel walls and a wide hyperechoic halo (arrowheads) surrounding the thrombosed vein consistent with reactive inflamed subcutaneous fat a b c d e f Fig. 4.41a–f. Angioleiomyoma. a,b Sagittal a gray-scale and b color Doppler US images of the palm demonstrates a subcutaneous sharply delineated solid hypoechoic mass (arrows) with a large arterial pedicle branching within (arrowhead). c Spectral Doppler analysis of intratumoral vessels shows high-resistance arterial waveforms. d–f Sagittal d T1-weighted, e postcontrast T1-weighted and f fat-suppressed T2-weighted MR imaging correlation reveal a predominantly hyperintense mass (arrows) in T2-signal intensity and after gadolinium administration. In the postcontrast image, small hypointense foci are visible within the tumor Nerve and Blood Vessels may present as a network of thin hypoechoic channels of flow within the echogenic thrombus, eventually causing obvious clot resorption and reopening of the vessel (Fig. 4.39g,h). Both reduction in spontaneous flow and incomplete vein compressibility accompany these stages in the post-phlebitic limb. Collateral vessels are often seen restoring venous patency (Fig. 4.40a). If recanalization does not occur, chronically thrombosed veins are characterized by narrowed size, thickened and irregular walls and collateral vessel formation (Fig. 4.39i,j). Soft-tissue or muscle edema may be ancillary findings with vein thrombosis as a consequence of venous stasis (Fig. 4.40b). As detailed in Chapter 15, US may easily diagnose thrombophlebitis, which requires treatment with anti-inflammatory drugs and not, at least routinely, anticoagulation therapy (Fig. 4.40c,d). 4.2.4 Vascular Tumors The most frequent vascular tumor, soft-tissue hemangioma, is not dealt with in this chapter as it has already been reported in its various forms: in Chapter 2 as part of skin and subcutaneous tissue masses, in Chapter 3 as regard its intramuscular location, and in Chapter 5 in relation to its synovial type. Similarly, the glomus tumor will be described in Chapter 11. A vascular-related tumor that has received specific attention in the US literature is angioleiomyoma (vascular leiomyoma), a rare benign histotype arising from the tunica media of the veins, composed of a conglomerate of thick-walled vessels associated with smooth muscle (Sardanelli et al. 1996; Hwang et al. 1998). It is most often found in an extremity, particularly the lower leg and the foot (50–70% of cases) (Hwang et al. 1998). Angioleiomyoma causes pain that is often related to problems with footwear and may be triggered by even light trauma. US demonstrates a sharply demarcated solid hypoechoic rounded nodule, usually less than 2 cm in diameter, with one or more arterial pedicles branching within (Fig. 4.41). Spectral Doppler analysis shows a highresistance flow pattern (Sardanelli et al. 1996). In clinical practice, angioleiomyoma should be considered in the differential diagnosis of painful nodular lesions of the extremity. Other vascular tumors includes rare aggressive histotypes, such as hemangioendothelioma, hemangiopericytoma, and angiosarcoma, all of which are characterized by a nonspecific US appearance. References Altinok T, Baysal O, Karakas HM et al (2004) Ultrasonographic assessment of mild and moderate idiopathic carpal tunnel syndrome. Clin Radiol 59:916–925 Amadio PC, Reiman HM, Dobijins JH (1988) Lipofibromatous hamartoma of nerve. J Hand Surg [Am] 13:67–76 Bargfrede M, Schwennicke A, Tumani H et al (1999) Quantitative ultrasonography in focal neuropathies as compared to clinical and EMG findings. Eur J Ultrasound 10:21–29 Beekman R, Visser LH (2002) Sonographic detection of diffuse peripheral nerve enlargement in hereditary neuropathy with liability to pressure palsies. J Clin Ultrasound 30:433–436 Beekman R, Visser LH (2004) High-resolution sonography of the peripheral nervous system: a review of the literature. Eur J Neurol 11:305–314 Beekman R, Schoemaker MC, van der Plas et al (2004a) Diagnostic value of high-resolution sonography in ulnar neuropathy at the elbow. Neurology 62:767–773 Beekman R, Van del Plas JPL, Uitdehaag BMJ (2004b) Clinical, electrodiagnostic and sonographic studies in ulnar neuropathy at the elbow. Muscle Nerve 30:202–208 Beggs I (1999) Sonographic appearances of nerve tumors. J Clin Ultrasound 27:363-368 Bertolotto M, Rosenberg I, Parodi RC et al (1996) Case report: fibroma of tendon sheath in the distal forearm with associated median nerve neuropathy: US, CT and MR appearance. Clin Radiol 51:370–372 Bianchi S, Montet X, Martinoli C et al (2004) High-resolution sonography of compressive neuropathies of the wrist. J Clin Ultrasound 32:451–461 Bilge T, Kaya A, Alatli M et al (1989) Hemangioma of the peroneal nerve: case report and review of the literature. Neurosurgery 25:649–652 Bodner G, Huber B, Schwabegger A, Lutz M et al (1999) Sonographic detection of radial nerve entrapment within a humerus fracture. J Ultrasound Med 20:131–136 Bodner G, Buchberger W, Schocke M et al (2001) Radial nerve palsy associated with humeral shaft fracture: evaluation with US: initial experience. Radiology 219:811–816 Bodner G, Harpf C, Gardetto A et al (2002a) Ultrasonography of the accessory nerve: normal and pathologic findings in cadavers and patients with iatrogenic accessory nerve palsy. J Ultrasound Med 21:1159–1163 Bodner G, Harpf C, Meirer R et al (2002b) Ultrasonographic appearance of supinator syndrome. J Ultrasound Med 21:1289–1293 Buchberger W, Schon G, Strasser K et al (1991) High-resolution ultrasonography of the carpal tunnel. J Ultrasound Med 10:531–537 Buchberger W, Judmaier W, Birbamer G et al (1992) Carpal tunnel syndrome: diagnosis with high-resolution sonography. AJR Am J Roentgenol 159:793–798 Chamlou R, Stefanidis C, Lambert T et al (2002) Popliteal artery pseudoaneurysm and hereditary multiple exostoses. Acta Chir Belg 102:467–469 Chen P, Massengill A, Maklad N et al (1996) Nerve territoryoriented macrodactyly: unusual cause of carpal tunnel syndrome. J Ultrasound Med 15:661–664 Chen P, Maklad N, Redwine M et al (1997) Dynamic high-resolution sonography of the carpal tunnel. AJR Am J Roentgenol 168:533–537 133 134 M. Valle and M. P. Zamorani Chien AJ, Jamadar DA, Jacobson JA et al (2003) Sonography and MR imaging of posterior interosseous nerve syndrome with surgical correlation. AJR Am J Roentgenol 181:219– 221 Chin EE, Zimmerman PT, Grant EG (2005) Sonographic evaluation of upper extremity deep venous thrombosis. J Ultrasound Med 24:829–838 Chiou HJ, Chou YH, Cheng SP et al (1998) Cubital tunnel syndrome: diagnosis by high-resolution ultrasonography. J Ultrasound Med 17:643–648 Chong WK, Lawrence R, Gardener J (1993) The appearance of normal and abnormal arterial morphology on intravascular ultrasound. Clin Radiol 48:301–306 Chuang YM, Luo CB, Chou YH et al (2002) Sonographic diagnosis and treatment of a median nerve epineurial hematoma caused by brachial artery catheterization. J Ultrasound Med 21:705–708 Clevert DA, Rupp N, Reiser M et al (2005) Improved diagnosis of vascular dissection by ultrasound B-flow: a comparison with color-coded Doppler and power Doppler sonography. Eur Radiol 15:342–347 Cronan JJ, Dorfman GS, Scola FH et al (1987) Deep venous thrombosis: US assessment using vein compression. Radiology 162:191–194 Davison BD, Polak JF (2004) Arterial injuries: a sonographic approach. Radiol Clin North Am 42:383–396 Delfiner JS (1996) Dynamic and pathophysiology of nerve compression in the upper extremity. Orthop Clin North Am 27:219–226 Delgado GJ, Chung CB, Lektrakul N et al (2002) Tennis leg: clinical US study of 141 patients and anatomic investigation of four cadavers with MR imaging and US. Radiology 224:112–119 Duncan I, Sullivan P, Lomas F (1999) Sonography in the diagnosis of carpal tunnel syndrome. AJR Am J Roentgenol 173:681–683 Edwards JM, Zierler RE (1992) Duplex ultrasound assessment of upper extremity arteries. In: Zwiebel WJ (ed) Introduction to vascular ultrasonography, 3rd edn. WB Saunders, Philadelphia, pp 223–235 El-Karabaty H, Hetzel A, Galla TJ et al (2005) The effect of carpal tunnel release on median nerve flattening and nerve conduction. Electromyogr Clin Neurophysiol 45:223–227 El Miedany YM, Aty SA, Ashour S (2004) Ultrasonography versus nerve conduction study in patients with carpal tunnel syndrome: substantive or complementary tests? Rheumatology 43:887–895 Erickson SJ (1997) High-resolution imaging of the musculoskeletal system. Radiology 205:593–618 Eusebi V, Bondi A, Cancellieri A et al (1990) Primary malignant lymphoma of sciatic nerve: report of a case. Am J Surg Pathol 14:881–885 Foley WD, Middleton WD, Lawson TL et al (1989) Color Doppler ultrasound imaging of lower-extremity venous disease. AJR Am J Roentgenol 152:371–376 Fornage BD (1988) Peripheral nerves of the extremities: imaging with US. Radiology 167:179–182 Fornage BD, Nerot C (1987) Sonographic diagnosis of tuberculoid leprosy. J Ultrasound Med 6:105–107 Fraser JD, Anderson DR (2004) Venous protocols, techniques and interpretations of the upper and lower extremities. Radiol Clin North Am 42:279–296 Fry WR, Smith RS, Sayers DV et al (1993) The success of duplex ultrasonographic scanning in diagnosis of extremity vascular proximity trauma. Arch Surg 128:1368–1372 Gassner EM, Schocke M, Peer S et al (2002) Persistent median artery in the carpal tunnel: color Doppler ultrasonographic findings. J Ultrasound Med 21:455–461 George V, Smith AG (1996) Anatomic considerations of the peripheral nerve in compressive neuropathies of the upper extremity. Orthop Clin North Am 27:211–218 Giovagnorio F, Martinoli C (2001) Sonography of the cervical vagus nerve: normal appearance and abnormal findings. AJR Am J Roentgenol 176:745–749 Graif M, Seton A, Nerubali J et al (1991) Sciatic nerve: sonographic evaluation and anatomic-pathologic considerations. Radiology 18:405–408 Graif M, Martinoli C, Rockind S et al (2004) Sonographic evaluation of brachial plexus pathology. Eur Radiol 14:193–200 Gruber H, Peer S, Kovacs P et al (2003) The ultrasonographic appearance of the femoral nerve and cases of iatrogenic impairment. J Ultrasound Med 22:163–172 Gruber H, Peer S, Meirer R et al (2005) Peroneal nerve palsy associated with knee luxation: evaluation by sonography – initial experience. AJR Am J Roentgenol 185:1119–1125 Heinemeyer O, Reimers CD (1999) Ultrasound of radial, ulnar, median and sciatic nerves in healthy subjects and patients with hereditary motor and sensory neuropathies. Ultrasound Med Biol 25:481–485 Helvie MA, Rubin JM (1989) Evaluation of traumatic groin arteriovenous fistulas with duplex Doppler sonography. J Ultrasound Med 16:177–181 Helvie MA, Rubin JM, Silver TM et al (1988) The distinction between femoral artery pseudoaneurysms and other causes of groin masses: value of duplex Doppler sonography. AJR Am J Roentgenol 150:1177–1180 Hide IG, Grainger AJ, Naisby GP et al (1999) Sonographic findings in the anterior interosseous nerve syndrome. J Clin Ultrasound 27:459–464 Hwang JW, Ahn JM, Kang HS et al (1998) Vascular leiomyoma of an extremity: MR imaging – pathology correlation. AJR Am J Roentgenol 171:981–985 Iannicelli E, Chianta GA, Salvini V et al (2000) Evaluation of bifid median nerve with sonography and MR imaging. J Ultrasound Med 19:481–485 Ikushima K, Ueda T, Kudawara I et al (1999) Plexiform schwannoma of the foot. Eur Radiol 9:1653–1655 Isobe K, Shimizu T, Akahane T et al (2004) Imaging of ancient schwannoma. AJR Am J Roentgenol 183:331–336 Jacobs MJ, Gregoric ID, Reul GJ (1992) Profunda femoral artery pseudoaneurysm after percutaneous transluminal procedures manifested by neuropathy. J Cardiovasc Surg 33:729–731 Jacobson JA, Jebson PJL, Jeffers AW et al (2001) Ulnar nerve dislocation and snapping triceps syndrome: diagnosis with dynamic sonography – report of three cases. Radiology 220:601–605 Katsumi K, Ogose A, Hotta T et al (2003) Plexiform schwannoma of the forearm. Skeletal Radiol 32:719–723 Keberle M, Jennett M, Kenn W et al (2000) Technical advances in ultrasound and MR imaging of carpal tunnel syndrome. Eur Radiol 10:1043–1050 Kele H, Verheggen R, Bittermann HJ et al (2002) The potential value of ultrasonography in the evaluation of carpal tunnel syndrome. Neurology 61:389–391 King AD, Ahuja AT, King W et al (1997) Sonography of periph- Nerve and Blood Vessels eral nerve tumors of the neck. AJR Am J Roentgenol 169:1695–1698 Knudson MM, Lewis FR, Atkinson K, Neuhaus A (1993) The role of duplex ultrasound arterial imaging in patients with penetrating extremity trauma. Arch Surg 128:1033–1037 Kotevoglu N, Gülbahce-Saglam S (2005) Ultrasound imaging in the diagnosis of carpal tunnel syndrome and its relevance to clinical evaluation. Joint Bone Spine 72:142–145 Koyuncuoglu HR, Kutluhan S, Yesildag A et al (2005) The value of ultrasonographic measurement in carpal tunnel syndrome in patients with negative electrodiagnostic tests. Eur J Radiology 56:365–369 Lanzillo B, Pappone N, Crisci C et al (1998) Subclinical peripheral nerve involvement in patients with rheumatoid arthritis. Arthritis Rheum 41:1196–1202 Lee D, van Holsbeeck MT, Janevski PK et al (1999) Diagnosis of carpal tunnel syndrome: ultrasound versus electromyography. Radiol Clin North Am 37:859–872 Lin J, Martel W (2001) Cross-sectional imaging of peripheral nerve sheath tumors: characteristic signs on CT, MR imaging, and sonography. AJR Am J Roentgenol 176:75–82 Lin J, Jacobson JA, Hayes CW (1999) Sonographic target sign in neurofibromas. J Ultrasound Med 18:513–517 Liskutin J, Dorffner R, Resinger M et al (2000) Hypothenar hammer syndrome. Eur Radiol 10:542 Marom EM, Helms CA (1999) Fibrolipomatous hamartoma: pathognomonic on MR imaging. Skeletal Radiol 28:260– 264 Martin AJ, Ryan LK, Gotlieb AI et al (1997) Arterial imaging: comparison of high-resolution US and MR imaging with histologic correlation. RadioGraphics 17:189–202 Martinoli C, Bianchi S, Derchi LE (1999) Tendon and nerve sonography. Radiol Clin North Am 37:691–711 Martinoli C, Bianchi S, Derchi LE (2000a) Ultrasonography of peripheral nerves. Semin US CT MR 21:205–213 Martinoli C, Bianchi S, Gandolfo N et al (2000b) US of nerve entrapments in osteofibrous tunnels of the upper and lower limbs. RadioGraphics 20:199–217 Martinoli C, Derchi LE, Bertolotto M et al (2000c) US and MR imaging of peripheral nerves in leprosy. Skeletal Radiol 29:142–150 Martinoli C, Schenone A, Bianchi S et al (2002) Sonography of median nerve in Charcot-Marie-Tooth disease. AJR Am J Roentgenol 178:1553–1556 Martinoli C, Bianchi S, Prato N et al (2003) US of the shoulder: non-rotator cuff disorders. RadioGraphics 23:381–401 Martinoli C, Bianchi S, Pugliese F et al (2004) Sonography of entrapment neuropathies in the upper limb (wrist excluded). J Clin Ultrasound 32:438–450 Miller-Thomas MM, West OC, Cohen AM (2005) Diagnosing traumatic arterial injury in the extremities with CT angiography: pearls and pitfalls. RadioGraphics 25:133–142 Murphey MD, Smith WS, Smith SE et al (1999) Imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlation. RadioGraphics 19:1253–1280 Murphy TP, Cronan JJ (1990) Evolution of deep venous thrombosis: a prospective eevaluation with US. Radiology 177:543–548 Nadkar MY, Agarwal R, Samant RS et al (2001) Neuropathy in rheumatoid arthritis. J Assoc Physicians India 49:217–220 Nakamichi K, Tachibana S (1995) Restricted motion of the median nerve in carpal tunnel syndrome. J Hand Surg [Br] 20:460–464 Nakamichi K, Tachibana S, Kitajima I (2000) Ultrasonography in the diagnosis of ulnar tunnel syndrome caused by an occult ganglion. J Hand Surg [Br] 25:503–504 Okamoto M, Abe M, Shirai H et al (2000) Diagnostic ultrasonography of the ulnar nerve in cubital tunnel syndrome. J Hand Surg [Br] 5:499–502 Okereke CD, Knight S, McGowan A et al (1999) Hypothenar hammer syndrome diagnosed by ultrasound. Injury 30:448–449 Ozdemir H, Mahmutyazicioglu K, Ozkokeli M et al (2003) Pseudoaneurysm of the dorsalis pedis artery: color Doppler Sonographic and angiographic findings. J Clin Ultrasound 31:283–287 Peer S, Bodner G, Mairer R et al (2001) Examination of postoperative peripheral nerve lesions with high-resolution sonography. AJR Am J Roentgenol 177:415–419 Peer S, Harpf C, Willeit J et al (2003) Sonographic evaluation of primary peripheral nerve repair. J Ultrasound Med 22:1317–1322 Pillay PK, Hardy RW Jr, Wilbourn AJ et al (1988) Solitary primary lymphoma of the sciatic nerve: case report. Neurosurgery 23:370–371 Polak JF, Culter SS, O’Leary DH (1989) Deep veins of the calf: assessment with color Doppler flow imaging. Radiology 171:81–485 Propeck T, Quinn TJ, Jacobson JA et al (2000) Sonography and MR imaging of bifid median nerve with anatomic and histologic correlation. AJR Am J Roentgenol 175:1721–1725 Prosser AJ, Burke FD (1987) Haemangioma of the median nerve associated with Raynaud’s phenomenon. J Hand Surg 12:227–228 Provost N, Bonaldi VM, Sarazin L (1997) Amputation stump neuroma: ultrasound features. J Clin Ultrasound 25:85–89 Puig S, Turkof E, Sedivy R et al (1999) Sonographic diagnosis of recurrent ulnar nerve compression by ganglion cysts. J Ultrasound Med 18:433–436 Quinn TJ, Jacobson JA, Craig JG, van Holsbeeck MT (2000) Sonography of Morton’s neuromas. AJR Am J Roentgenol 174:1723–1728 Read JW, Noakes JB, Kerr D et al (1999) Morton’s metatarsalgia: sonographic findings and correlated histopathology. Foot Ankle Int 20:153–161 Redd RA, Peters VJ, Emery SF et al (1989) Morton neuroma: sonographic evaluation. Radiology 171:415–417 Reynolds DL Jr, Jacobson JA, Inampudi P et al (2004) Sonographic characteristics of peripheral nerve sheath tumors. AJR Am J Roentgenol 182:741–744 Ridley DS, Jopling WH (1966) Classification of leprosy according to immunity: a five group system. Int J Leprosy 34:255– 273 Rieger M, Mallouhi A, Tauscher T et al (2006) Traumatic arterial injuries of the extremities: initial evaluation with MDCT angiography. AJR Am J Roentgenol 186:656–664 Roncaroli F, Poppi M, Riccioni L et al (1997) Primary nonHodgkin’s lymphoma of the sciatic nerve followed by localization in the central nervous system: case report and review of the literature. Neurosurgery 40:618–621 Rosenbaum R (2001) Neuromuscular complications of connective tissue diseases. Muscle Nerve 24:154–169 Rossey-Marec D, Simonet J, Beccari R et al (2004) Ultrasonographic appearance of idiopathic radial nerve constriction proximal to the elbow. J Ultrasound Med 23:1003–1007 Roubidoux MA, Hertzberg BS, Carroll BA et al (1990) Color 135 136 M. Valle and M. P. Zamorani flow and image-directed Doppler ultrasound evaluation of iatrogenic arteriovenous fistulas in the groin. J Clin Ultrasound 18:463–469 Sacks D, Robinson ML, Perlmutter GS (1989) Femoral arterial injury following catheterization: duplex evaluation. J Ultrasound Med 8:241–246 Said G, Lacroix C (2005) Primary and secondary vasculitis neuropathy. J Neurol 252:633–641 Sardanelli F, Renzetti P, Nardi F et al (1996) Imaging of angioleiomyoma. J Clin Ultrasound 24:268–271 Schenone A, Mancardi GL (1999) Molecular basis of inherited neuropathies. Curr Opin Neurol 12:603–616 Schon LC (1994) Nerve entrapment, neuropathy and nerve dysfunction in athletes. Orthop Clin North Am 25:47–59 Schwartz M, Weaver F, Yellin A et al (1993) The utility of color Doppler examination in penetrating extremity arterial trauma. Am Surg 59:375–378 Schwartz RA, Kerns DB, Mitchell DG (1991) Color Doppler ultrasound imaging in iatrogenic arterial injuries. Am J Surg 162:4–8 Shafighi M, Gurunluoglu R, Ninkovic M et al (2002) Ultrasonography for depiction of brachial plexus injury. J Ultrasound Med 22:631–634 Sheppard DG, Iyer RB, Fenstermacher MJ (1998) Brachial plexus: demonstration at US. Radiology 208:402–406 Siegel RJ, Chae JS, Maurer G et al (1993) Histopathologic correlation of the three-layered intravascular ultrasound appearance of normal adult human muscular arteries. Am Heart J 126:872–878 Silvestri E, Martinoli C, Derchi LE et al (1995) Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology 197:291–296 Simonetti S, Bianchi S, Martinoli C (1999) Neurophysiological and ultrasound findings in sural nerve lesions following stripping of the small saphenous vein. Muscle Nerve 22:1724–1726 Sivri A, Guler-Uysal F (1998) The electroneurophysiological evaluation of rheumatoid arthritis patients. Clin Rheumatol 17:416–418 Sobiesk GA, Wertheimer SJ, Schulz R et al (1997) Sonographic evaluation of interdigital neuromas. J Foot Ankle Surg 36:364–366 Spinner RJ, Atkinson JL, Tiel RL (2003) Peroneal intraneural ganglia: the importance of the articular branch: a unifying theory. J Neurosurg 99:330–343 Spinner RJ, Amrami KK, Rock MG (2005) The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol 35:172–179 Stewart JD (2003) Peripheral nerve fascicles: anatomy and clinical relevance. Muscle Nerve 28:525–541 Talha H, Enon B, Chevalier JM et al (1987) Brachial artery entrapment: compression by the supracondylar process. Ann Vasc Surg 1:479–482 Velling TE, Brennan FJ, Hall LD et al (2001) Sonographic diagnosis of ulnar artery aneurysm in hypothenar Hammer syndrome: report of 2 cases. J Ultrasound Med 20:921–924 Verhagen WIM, Gabreels-Festen AA, van Wensen PJ et al (1993) Hereditary neuropathy with liability to pressure palsies: a clinical, electroneurophysiological and morphological study. J Neurol Sci 116:176–184 Wilson D (2004) Ultrasound assessment of carpal tunnel syndrome. Clin Radiol 59:909 Wong BZY, Amrami KK, Wenger DE et al (2006) Lipomatosis of the sciatic nerve: typical and atypical MRI features. Skeletal Radiol 35:180–184 Wong SM, Griffith JF, Hui ACF (2004) Carpal tunnel syndrome: diagnostic usefulness of sonography. Radiology 232:93–99 Woodruff JM (1993) The pathology and treatment of peripheral nerve tumors and tumor-like conditions. Cancer J Clin 43:290–308 Wright LB, Matchett WJ, Cruz CP et al (2004) Popliteal artery disease: diagnosis and treatment. RadioGraphics 24:467–469 Yamaguchi S, Mn S, Yonemitsu Y et al (2002) A traumatic pseudoaneurysm of the dorsalis pedis artery: report of a case. Surg Today 32:756–757 Yamazaki H, Saitoh S, Seki H, et al (1999) Peroneal nerve palsy caused by intraneural ganglia. Skeletal Radiol 28:52–56 Yesildag A, Kutluhan S, Sengul N et al (2004) The role of ultrasonographic measurements of the median nerve in the diagnosis of carpal tunnel syndrome. Clin Radiol 59:910–915 Ziswiler HR, Reichenbach S, Vögelin E et al (2005) Diagnostic value of sonography in patients with suspected carpal tunnel syndrome: a prospective study. Arthritis Rheum 52:304–311 Bone and Joint 5 Bone and Joint Maria Pia Zamorani and Maura Valle CONTENTS 5.1 5.1.1 5.1.2 5.1.3 5.1.3.1 5.1.3.2 5.1.4 5.1.5 5.1.5.1 5.1.5.2 5.1.5.3 5.1.5.4 5.1.6 5.2 5.2.1 5.2.2 Bone 137 Histologic Considerations 137 Normal US Anatomy and Scanning Technique 138 Outgrowths 142 Anatomic Variants 142 Bone Exostoses 142 Defects 142 Irregularities of the Cortical Outline Acute Fractures 143 Stress Fractures 145 Fracture Healing 146 Erosions 148 Osteomyelitis 149 5.1 Bone 5.1.1 Histologic Considerations 143 5.2.3.6 5.2.3.7 Joint 150 Histologic Considerations 150 Normal US Anatomy and Scanning Technique 153 Pathologic Changes 156 Joint Effusion 156 Rheumatoid Arthritis and Other Inflammatory Arthropathies Septic Arthritis 162 Traumatic Injuries 163 Degenerative Joint Disease (Osteoarthritis) 166 Deposition Diseases 169 Postoperative Complications 173 5.3 5.3.1 5.3.2 5.3.3 5.3.4 5.3.5 Space-Occupying Masses 173 Bone Tumors 173 Pigmented Villonodular Synovitis 176 Lipoma Arborescens 178 Synovial Osteochondromatosis 178 Synovial Hemangioma 179 5.2.3 5.2.3.1 5.2.3.2 5.2.3.3 5.2.3.4 5.2.3.5 References 137 158 180 M. P. Zamorani, MD Unité de Recherche et Dévelopement, Clinique des Grangettes, 7, ch. des Grangettes, 1224 Genève, Switzerland M. Valle, MD Staff Radiologist, Reparto di Radiologia, Istituto Scientifico “Giannina Gaslini”, Largo Gaslini 5, 16148 Genova, Italy All bones consist of peripheral cortical (compact) bone and central medullary (trabecular or cancellous) bone. In long bones, there is an inverse relationship between the amount of cortical and cancellous bone at any given site: in the diaphysis, the cortical bone is thick whereas the trabecular bone is sparse; conversely, metaphyseal and epiphyseal regions are characterized by thin cortical bone and prominent cancellous bone. In addition to bone trabeculae, the medullary cavity contains bone marrow, including yellow marrow (housing fat and connective tissue) and red marrow (consisting of hematopoietic cells, fat and connective tissue). The distribution of hematopoietic and fatty marrow is dependent on age and metabolic state (Ricci et al. 1990). The outer surface of cortical bone is invested by the periosteum—a dense fibrous connective tissue layer that is anchored to the cortical bone by means of perforating Sharpey fibers—which plays a role in allowing rapid healing of fractures. The periosteum thickness varies depending on age: it is thicker and more active in children. Nutrient arteries and emissary veins cross the cortical bone through the nutrient foramina. In mature long bones, they are most often observed at the diaphysis level. In terms of histogenesis, the bone develops from two distinct processes referred to as intramembranous and endochondral ossification (Erickson 1997). Intramembranous ossification occurs through direct mineralization of vascular connective tissue and is responsible of the growth of flat bones; it also contributes to the width of the shaft of long bones. Endochondral ossification arises within a cartilage model and is responsible for the longitudinal growth of long bones and the formation of the axial skeleton (Fig. 5.1). 138 M. P. Zamorani and M. Valle * * Cartilage Bone c a Cuboid Bone d b Fig. 5.1a–d. Endochondral ossification. a,b Coronal 12–5 MHz US images over the lateral midfoot with c,d schematic drawing correlation show the growing cuboid at a,c 1 year of age and b,d at the end of development. The cuboid is a square bone with right angles (arrowheads). Initially, the cartilage (asterisks) forms a square model reflecting the definitive appearance of bone. The primary center of ossification is visible in the center of the future bone as a hyperechoic rounded image (arrows). During growth, endochondral ossification advances toward each end of the cartilaginous model. At the end of this process, the primary center has reached the ends of the cartilaginous model and assumes the definitive square shape 5.1.2 Normal US Anatomy and Scanning Technique There is no doubt that radiography is the first-line imaging modality for assessment of bone disorders: it allows a panoramic, low-cost and reproducible evaluation of bone. More accurate analysis can be obtained by means of CT, especially if complex anatomic areas must be examined. While CT allows an optimal assessment of the bone cortex, MR imaging is the technique of choice to evaluate the bone marrow. US has intrinsic limitations in the assessment of bone. In some applications, however, it can be useful to assess selected bone disorders, especially if performed as a complement to standard radiographs (Cho et al. 2004). With US, the interface between soft tissue and cortical bone is highly echogenic because of an inherent high acoustic impedance mismatch (Erickson 1997). The bone cortex appears as a regu- lar continuous bright hyperechoic line with strong posterior acoustic shadowing and some reverberation artifact (Fig. 5.2). Deeper structures, such as the internal cortical architecture, the endosteum and the underlying trabecular bone, remain inaccessible with US, except for rare pathologic conditions in which the cortex is extremely thinned or destroyed in its full thickness. In normal adults, the periosteum cannot be detected as a separate structure with US. Using very high frequency probes, it may appear as a thin hypoechoic line apposed to the bone cortex at certain sites in children. Given the straight and continuous appearance of the bright echo of the bony cortex, subtle surface irregularities and sites of penetration of nutrient vessels can be visualized (Fig. 5.3). A careful scanning technique and Doppler imaging allow easy depiction of the vessels entering the bone. The posterior acoustic shadowing of sesamoids or calcifications 139 Bone and Joint a b Fig. 5.2a,b. US appearance of normal bone: surface echotexture. a Longitudinal 12–5 MHz US image obtained over the diaphysis of the radius with b radiographic correlation demonstrates the bone surface as a continuous straight hyperechoic line (arrows) produced by a strong reflection of sound due to the marked difference in acoustic impedance of the soft tissues and bone. Reverberation artifact (arrowheads) projecting in the shadow beyond the bone can be seen Fig. 5.3a–c. US appearance of normal bone: nutrient vessels. a,b Longitudinal a gray-scale and b color Doppler 12–5 MHz US images over the diaphysis of the ulna reveal a small break (arrowhead) in the bone surface crossed by nutrient vessels (arrow). This finding should not be mistaken for fractures or erosions. c Radiographic correlation demonstrates a nutrient channel (arrowheads) piercing the ulnar shaft obliquely a b c located in close relationship with the bone surface can mimic cortical breaks. Growth plates in the immature skeleton may also resemble a focal discontinuity of the bone surface: they can be distinguished from fractures due to their peculiar anatomic location (Fig. 5.4). Marginal osteophytes or bone spurs can project over the cortex mimicking focal breaks. Previous surgery may also affect the continuity of the cortex. Focal interruptions of the hyperechoic corti- cal line are seen after construction of bone tunnels, such as in ligament reconstruction surgery or following ablation of screws and pins. Close correlation with standard radiographs allows a definitive diagnosis in nearly all the circumstances described above. A variety of focal projections (tuberosities, ridges, etc.) and defects (fossae, sulci) of bone modulate the cortical surface; they are often associated with tendon or ligament insertion (tuberosities, ridges) 141 Bone and Joint * Scaphoid * a T T b * * d c Fig. 5.5a–d. US appearance of normal bone: surface details. a Coronal 12–5 MHz US image obtained over the lateral aspect of the scaphoid with b correlative anteroposterior radiograph of the radial wrist demonstrates a blunt focal projection of bone (arrowhead) at its waist emerging from underneath the radial styloid (curved arrow) and separating the proximal articular surface covered by hyaline cartilage (arrow) from the extra-articular portion of bone. Distally, note the scaphoid tubercle (asterisk) in a deeper location. T, trapezius. The field-of-view of the US image is indicated by a dashed rectangle in b. c Coronal reformatted CT-arthrographic image and d anteroposterior conventional arthrogram obtained after intra-articular injection of contrast material within the radiocarpal joint show the relationship of the landmarks described above with the intra- and extra-articular portions of the scaphoid surface Soft-tissues Bone a b c d Fig. 5.6a–d. Bone surface abnormalities that are detectable with US. a Normal bone: a straight regular interface separates the bone from the soft-tissues. b Outgrowths or “plus images”: a focal projection of bone (arrows) is observed in the soft tissues. c Irregularities of the cortical outline: the bone–soft tissue interface is rough (arrowheads); focal breaks (white arrow) or step-off deformities (black arrow) can be seen. d Defects or “minus images”: a focal loss of bone (arrows) is observed. Soft tissues intervene within the defect 142 M. P. Zamorani and M. Valle lesions may be obscured by the curvature of bone and overlying structures. These are the main reasons why bone should invariably be checked during a standard US examination of the musculoskeletal system. Bone abnormalities seen at US can easily be correlated with clinical findings and can suggest the requirement for additional radiographic views or other imaging studies if further evaluation is warranted. 5.1.3 Outgrowths 5.1.3.1 Anatomic Variants Plus lesions can be related to normal anatomic variants that may become symptomatic because of compression exerted on the adjacent soft-tissue structures. The role of US in the assessment of bone variants is twofold: to detect them and to reveal associated pathologic changes in the adjacent soft tissues. US is not only able to demonstrate the relationship between the abnormal bony outgrowth and the surrounding soft tissues, but can also evaluate tendon or nerve impingement during dynamic scanning. Among possible examples of bone outgrowths that represent anatomic variants, the supracondylar process is a rare bony outgrowth that arises from the medial aspect of the distal humeral shaft (Sener et al. 1998; Subasi et al. 2002). It can give rise to a thick fibrous band (Struthers ligament) inserting into the distal humeral epiphysis. Due to the close relationship with the median nerve, the process and the adjacent ligament can cause a nerve entrapment syndrome (see Chapter 7). As described in Chapter 17, the peroneal tubercle of the calcaneus is a small bone ridge that gives insertion to the inferior peroneal retinaculum and separates the peroneus brevis from the peroneus longus tendons. Congenital enlargement of the tubercle appears at physical examination as a firm mass located just inferior to the tip of the lateral malleolus. Chronic friction of a hypertrophied tubercle with the adjacent tendons can cause stenosing tenosynovitis or tendon rupture (Bruce et al. 1999; Wang et al. 2005). 5.1.3.2 Bone Exostoses Bone exostoses (osteochondromas) are benign tumors arising, in most instances, from the metaphysis of long bones. They consist of a bony spur whose cap is covered by hyaline cartilage. Exostosis can be solitary or multiple, the latter condition being known as multiple hereditary exostosis (Murphey et al. 2000; Stieber and Dormans 2005). Most solitary osteochondromas occur in the distal femur, proximal tibia and proximal humerus. They may become symptomatic because of impingement on the adjacent softtissue structures, such as nerves, tendons and vessels (see Chapter 14) or, more rarely, because of neoplastic changes (chondrosarcoma) occurring in the cartilaginous cap. In other instances, exostoses may lead to formation of an inflamed synovial bursa as a result of chronic friction. US demonstrates exostoses as outgrowths of hyperechoic bone covered by hypoechoic cartilage (Fig. 5.7). The bone component of the exostosis appears as a continuous hyperechoic line, whereas the cartilaginous cap consists of a hypoechoic layer that may contain some hyperechoic foci with posterior acoustic shadowing related to cartilage calcifications (Murphey et al. 2000). US has been shown to allow accurate measurement of the cartilaginous cap thickness, a factor related to the risk of sarcomatous degeneration (Malghem et al. 1992). The main limitations of US are its inability to evaluate deep lesions inaccessible to the probe and the analysis of the osseous component of the lesion (Murphey et al. 2000). Local compression exerted on the adjacent soft tissues can be diagnosed with US. Deep venous thrombosis, arterial insufficiency and synovial bursa formation and bursitis (bursa exostosica) are associated findings detectable with gray-scale US and Doppler imaging (Fig. 5.8) (El-Khoury and Bassett 1979; Keeling et al. 1993; de Matos et al. 1983). 5.1.4 Defects US can detect a variety of “minus” lesions ranging from small para-articular erosions caused by chronic synovitis to large post-traumatic defects. One of the most common bone defects is the HillSachs lesion, a compressive fracture of the humeral head that follows anterior shoulder dislocation (see Chapter 6). The lesion derives from the traumatic action of the sharp anterior glenoid border against the posterolateral aspect of the dislocated humeral head. US has proved to be an efficient modality to detect a Hill-Sachs lesion and assess 147 Bone and Joint implants can be followed by complications, such as infection, impingement and mechanical failure. In infections, US can identify soft-tissue abscesses and sinus tracts, and assess their relationship with implants and vital structures (Fig. 5.12a,b) (Gibbon et al. 2002). In addition, US can be used to guide needle aspiration of fluid collections for cultural purposes. Recently, extensor pollicis longus tendon tethering following K-wire insertion to treat unstable distal radius fractures has been described with US (Harrison et al. 2004). After volar plate osteotomy for Colles fracture, tenosynovitis and tears of this tendon following impingement on the screw can be demonstrated with US as well (see Chapter 10). Ankle tendon impingement due to orthopaedic hardware has also been reported (Fig. 5.12c–e) (Shetty et al. 2002). In children with percutaneous cross-pin fixation for displaced supracondylar humeral fractures, dynamic US can evaluate altered gliding and impingement of the ulnar nerve in the cubital tunnel (Karakurt et al. 2005). Some authors have suggested that the process of fracture healing can be followed with color Doppler imaging and spectral analysis (Caruso et al. 2000). The rationale is based on the fact that, at the time of trauma, the blood supply to the fracture site is inter- * * a b * ta * tibia c ta * tibia d Fig. 5.12a–e. Complications of orthopaedic treatment of fractures. Two different cases. a,b Transverse and b longitudinal 12– 5 MHz US images of the left femur in a patient who was previously treated for a femoral shaft fracture with placement of a metal implant (open arrowheads) demonstrate a hypoechoic fluid collection (asterisks) surrounding the compression plate. Subsequent surgery disclosed an abscess. Note the posterior reverberation artifact (white arrowhead) of the plate compared with the femoral cortical bone (arrow). c Long- and d short-axis 17–5 MHz US images over the anterior cortex of the distal tibia with e radiographic correlation in a patient previously operated on for a tibial fracture reveal the surface contours of an interlocking screw head (arrow) impinging on the tibialis anterior tendon (ta). Reverberation (arrowhead) is shown deep relative to the screw head. Note the associated tenosynovitis (asterisks) of the tibialis anterior tendon e 148 M. P. Zamorani and M. Valle rupted; then, blood vessels reach the periosteal portion of the callus from adjacent soft tissues forming a new circulation to the callus (Postacchini et al. 1995). US is able to follow the formation of new vessels at the fracture site and to assess flow characteristics in them during development of fracture callus (Fig. 5.13a) (Caruso et al. 2000). In patients with normal callus development, Doppler spectral analysis reveals an initial decrease in the resistive index as a result of the neoangiogenetic process occurring during the early weeks after fracture (Fig. 5.13b). Over time, the arterial resistance progressively increases, reflecting a physiologic decrease in the degree of local vasculature that accompanies the mature phase of the callus. On the other hand, patients with non-union and delayed healing have higher resistances early, related to a poor formation of neovasculature. Although these features need further experience in larger series, Doppler imaging seems a promising modality for predicting normal or delayed fracture healing based on defective vasculature at the fracture site about 1 month after trauma (Caruso et al. 2000). However, standard radiographs remain the primary imaging technique for evaluating callus formation. 5.1.5.4 Erosions In patients who have rheumatoid arthritis, US has proved to be an excellent modality for detection of a early bone erosions, with a sensitivity superior even to plain films (Wakefield et al. 2000). Erosions typically occur in the hand, the capitate being the bone most commonly affected, followed by the triquetrum, hamate, scaphoid and trapezoid; the second and third metacarpal heads are also a common location (Cimmino et al. 2000). US demonstrates erosions as oval or rounded well-defined cortical breaks with an irregular floor visible in longitudinal and transverse planes (Fig. 5.14a,b). They initially affect the bare areas of the joint surface and share a common appearance in rheumatoid arthritis and other seronegative arthropathies. Hypoechoic synovial pannus and Doppler signals of flow are often detectable within them. Loss of definition of the articular cartilage and widening of the joint spaces are associated findings. Compared with standard radiographs, US can be considered a more sensitive, effective and reliable means for detecting erosions in rheumatoid arthritis (Wakefield et al. 2000; Alarcon et al. 2002; Weidekamm et al. 2003). In early disease, it has been shown able to detect 6.5fold more erosions than did radiography in 7.5-fold the number of patients. In advanced disease, these differences were 3.4-fold and 2.7-fold, respectively (Wakefield et al. 2000). Depending on their location, US has proved to be superior to radiography for depiction of erosions in the first, second and fifth metacarpophalangeal joints, but inferior in the fourth metacarpophalangeal joint due to the problem of access (Schmidt 2001). Erosions being most commonly found along the radial and ulnar sides of b Fig. 5.13a,b. Early callus formation following fracture of the distal tibia. a Color Doppler 12–5 MHz US image obtained 12 days after treatment shows a bone defect (arrowheads) related to the fracture site and multiple blood flow signals (arrow) in the periosseous soft tissues superficial to the fracture. b Spectral analysis reveals low-resistance (RI <0.50) arterial flow in the vessels surrounding the fracture. These features indicate initial normal development of fracture callus 150 M. P. Zamorani and M. Valle US cannot assess bone marrow and trabecular bone involvement, but is an excellent means of identifying abscess formation and adjacent soft-tissue involvement (Mah et al. 1994; Davidson et al. 2003). In the pediatric age group, deep soft-tissue swelling has been described as the earliest sign of disease followed by periosteal elevation and formation of a thin layer of subperiosteal fluid (see Chapter 19) (Mah et al. 1994). At US, periosteal elevation can be appreciated as single or multiple linear echoes surrounding the cortical bone, whereas subperiosteal fluid appears as an anechoic or hypoechoic collection separating the periosteum from the cortical bone as the result of superficial extension of the intraosseous process (Fig. 5.15a) (Steiner and Sprigg 1992; Sammak et al. 1999). Detection of blood flow within or around the infected periosteum demonstrated by Doppler imaging can be useful in distinguishing early from advanced acute osteomyelitis (Chao et al. 1999). Doppler US has also been found valuable in assessing the efficacy of antibiotic therapy (Chao et al. 1999). One should be aware, however, that a normal US examination does not exclude bone infection (Bureau et al. 1999). Later stages of disease are characterized by cortical irregularities and erosions, which are typically found in patients with symptoms lasting for more than 1 week (Fig. 5.15b–e). Then, subperiosteal collections may expand and form abscesses that can be drained under US guidance when medical therapy alone is inadequate (Abiri et al. 1989; Bureau et al. 1999; Craig 1999). US guidance contributes to reducing complications related to the procedure, such as the inadvertent contamination of uninvolved compartments and traumatic damage to vessels and nerves along the needle path (Bureau et al. 1999; Craig 1999). An opening (cloaca) connecting the infected bone with the abscess or a channel between the infected bone and the skin (sinus tract) can be seen as a defect of the cortical layer in continuity with the hypoechoic collection. Generally speaking, the value of US appears even more relevant in the postoperative phase when the use of MR imaging may be hampered by the presence of orthopaedic metallic implants. In this instance, US can reveal the fluid collection apposed to the implant, which appears as a bright linear structure with posterior reverberation artifact surrounded by hypoechoic fluid. Finally, it is important to point out that evaluation of osseous involvement requires composite imaging algorithms for specific clinical scenarios, with combined use of plain films, nuclear medicine, CT and MR imaging (Sammak et al. 1999). 5.2 Joint 5.2.1 Histologic Considerations Joint anatomy is variable depending on specific functional requirements. Based on their anatomic structure, joints can be divided in three main groups: fibrous, cartilaginous and synovial (Erickson 1997). In fibrous joints, the bone ends are linked by intervening solid connective tissue, including a sutural ligament (sutures), a collagenous interosseous ligament or membrane (syndesmoses) or cartilaginous periodontium (gomphoses). Cartilaginous joints are divided into symphyses—which contain a fibrocartilaginous disk—and synchondroses—which are formed by bony ends covered by cartilage but lacking synovium. Synovial joints are formed by adjacent bones connected by a cavity lined by synovial membrane. The above types of joints allow different degree of motion, which is minimal in the first group (fibrous) and maximal in the latter (synovial). Because synovial joints are the most commonly examined with US, we will specifically discuss their normal anatomy. Synovial joints are formed by articulating bone surfaces, fibrous capsule and ligaments, synovium and other intra-articular structures (menisci, labra, ligaments, fat pads, etc.) (Fig. 5.17). The subchondral bone plate is a thin layer of dense bone linked to the cancellous and cortical bone of the metaphysis that acts as a support for the articular cartilage. The main function of bone plates is to adsorb part of the load from the cartilage and transfer it to the cortical bone through the metaphysis. The microstructure of subchondral bone, with its peculiar orientation of trabeculae, reflects this function. The articular surfaces of bone are covered with hyaline cartilage (Fig. 5.16a). The cartilage thickness varies among joints: thicker cartilage is found in larger joints subjected to considerable loading, such as the weight-bearing joints of the lower limb. The cartilage thickness also varies in different sites of the same joint as an expression of local differences in load. The hyaline cartilage is formed by cells—the chondrocytes, which account for 0.1% of cartilage volume—and chondroid matrix consisting of collagen and proteoglycans. From the histologic point of view, four cartilage layers can be recognized from superficial to depth, based on a different architecture and orientation of collagen fibers. In the superficial layer, the collagen fibers run tangential 153 Bone and Joint intra-articular fibrocartilage structures (Fig. 5.17d). It also invests some transitional zones extending from the peripheral boundaries of the hyaline cartilage and the fibrous capsule, the so-called bare areas. At these sites, the bone is covered by synovium without the protective layer of cartilage: this makes it particularly vulnerable to synovitis-induced bone destruction (Sommer et al. 2005). Different fibrocartilage structures can be found inside the joint space or related to the articular capsule: their main function is to increase the congruence of the articular surfaces by filling the space between them and to act as shock absorbers thus preventing damage to the hyaline cartilage (Fig. 5.17d). Some joints contain fat pads, which are adipose struc- tures filling the space between the synovial membrane and the peripheral capsule (Fig. 5.17d). Intra-articular fat pads adapt their shape to joint movements and the amount of intra-articular synovial fluid; they absorb forces generated during joint motion. 5.2.2 Normal US Anatomy and Scanning Technique The indications for joint US are rapidly expanding due to the refinement of high-resolution transducers and to the fact that both radiologists and clinicians are increasingly aware of the potential of US a b Fig. 5.17a–d. General anatomy of synovial joints. Schematic drawings of a cross-sectional view of a synovial joint. a Joint capsule and articular cartilage. The joint capsule (straight arrows) is a fibrous sac that inserts beyond the articular surfaces of articulating bones. The thickness of the articular cartilage (asterisks) may vary among parts of the same joint depending on the different demands of loading and weight-bearing (arrowheads). The cartilage transmits loading to the subchondral bone plate (1) which, in turn, transfers part of it (curved arrows) to the cortical bone (3) through the metaphyseal region (2). b Synovial recesses and sesamoids. The synovial recesses arise from focal discontinuities of the capsule, allowing the synovium to extrude into the surrounding soft tissues. Synovial herniation may form communicating synovial pouches (1) or may link the joint cavity with adjacent synovial tendon sheaths (2). Sesamoids (asterisk) are small ossicles embedded in the fibrous capsule or the plantar plate. They can or cannot articulate with the joint surfaces. c Ligaments. These are fibrous bands formed by focal thickening of the capsule (1) or lying at a certain distance from it (2). The strongest ligaments insert into para-articular bone ridges or tubercles (3); these are appropriately oriented to counteract joint instability. d Synovium, fibrocartilages and fat pads. The synovial membrane (thin arrow) invests the joint cavity with the exception of fibrocartilaginous structures (asterisk) and intra-articular extrasynovial fat pads (thick arrow). Between the peripheral boundaries of the hyaline cartilage and the capsule, the synovium invests the bone directly. These zones are called “bare areas” (curved arrow) c d 156 M. P. Zamorani and M. Valle a b LM c * Talus d Fig. 5.20a–d. Normal ligaments. a–c Schematic drawings illustrate the relationship of a ligament (large straight arrows) with the underlying joint structures, including the hyaline cartilage (thin straight arrow), the joint cavity (asterisk) and the synovial membrane (s). The position of the fibrous capsule relative to the ligament may be variable: a internal (between the ligament and the synovium, i.e., the lateral collateral ligament of the knee); b bending to it (i.e., the glenohumeral ligaments of the shoulder, the anterior talofibular ligament of the ankle, the medial collateral ligament of the knee); c external (outside the ligament and the synovium, i.e., the anterior cruciate ligament of the knee). d Long-axis 17–5 MHz US image over the lateral ankle demonstrates the normal anterior talofibular ligament as a thick fibrillar band (arrowheads) joining the lateral malleolus (LM) and the talus. The deep surface of the anterior talofibular ligament is merged with the ankle joint capsule. Note the joint fluid (asterisk) in close contact with the ligament. Thin arrow, articular cartilage Boutry et al. 2005). Somewhat similar to tendons, ligaments are anisotropic structures. Therefore, care should be taken to place the probe as parallel as possible to them to avoid artifactual hypoechoic patterns that can mimic pathology. Often, changing the position of the joint improves ligament visualization. Small probes that can better hug the curves and bulges of the bony landmarks are preferred for imaging ligaments. Some ligaments located in the central portion of joints (i.e., the interosseous tarsal sinus ligaments and the cruciate ligaments of the knee) cannot be visualized with US because of the overlying osseous structures. Complex ligaments (i.e., the medial collateral ligament of the knee, the deltoid ligament of the ankle) are made up of individual components that can be distinguished with US as individual structures. In general, ligaments that stabilize a joint are best evaluated while stretched. For example, in the relaxed state, the calcaneofibular ligament of the ankle has a concave course which makes the evaluation of its cranial insertion difficult; with the ankle in dorsiflexion the ligament tightens, pushing the peroneals superficially, and is better depicted (Peetrons et al. 2004). Intra-articular fat pads appear at US as fat-like hyperechoic structures (Fig. 5.18c). The most important are rec- ognized in the knee (Hoffa pad) and the elbow (anterior and posterior fat pads) (Miles and Lamont 1989; Ferrara and Marcelis 1997). In most joints, small amounts of normal intra-articular fluid can be detected in the articular cavity by means of highresolution US. 5.2.3 Pathologic Changes 5.2.3.1 Joint Effusion Demonstration of an intra-articular effusion is a major step in the investigation of musculoskeletal disorders, as it points the clinician’s attention toward a joint problem and excludes other extraarticular sources of pain and disability. A joint effusion can derive from traumatic or mechanical causes as well as from inflammatory or infectious synovitis; more rarely, it can be related to neoplastic conditions. At physical examination, detection of synovial effusion depends on the overall amount of fluid and the type of joint is involved. Accurate palpation allows detection of medium to large effu- 158 M. P. Zamorani and M. Valle definite indication for sampling, analysis and cultural procedures in order to rule out microcrystal arthritis and infection. For injecting small joints, US guidance allows significantly greater accuracy than a blind approach (Raza et al. 2003). In our practice, US-guided aspiration of joint fluid significantly reduces the pain associated with needle puncture. In addition, real-time monitoring of the needle reduces the risk of potential damage to adjacent structures, including arteries and nerves. In the traumatic setting, hemorrhagic joint effusions may appear highly echogenic in the first few hours after the trauma (Fig. 5.21c). Lipohemarthrosis is a condition in which blood and bone marrow fat are found inside the synovial cavity. While blood usually derives from tears of the synovial membrane, fat comes from yellow bone marrow as a result of bone fracture or, more rarely, from periligamentous fat. In most cases, lipohemarthrosis can be considered a confident indicator of an intra-articular fracture: it is characterized by a multilayered appearance made up of a superficial hyperechoic layer reflecting fat and a deep hypoechoic layer due to sedimentation of the red blood cells. After 10–15 minutes of joint immobilization, a thin intermediate band due to the serum can be noted between the fat and the red blood cells (Fig. 5.21d,e) (Bianchi et al. 1995). 5.2.3.2 Rheumatoid Arthritis and Other Inflammatory Arthropathies US has been proposed for the early detection and follow-up of several chronic inflammatory disorders of joints, including rheumatoid arthritis (Wakefield et al. 2000; Keen et al. 2005; Scheel et al. 2006; Gibbon 2004) and seronegative arthropathies (Gibbon 2004; Milosavljevic et al. 2005; Kane 2005). Rheumatoid arthritis is a chronic systemic disease that affects approximately 0.5–1% of the population and has a definite prevalence (2:1 to 3:1) in women. The etiology of rheumatoid arthritis is unknown but it seems to be multifactorial, with any genetic susceptibility, expression of HLADR4 and environmental factors believed to play a role (Sommer et al. 2005). The diagnosis requires a spectrum of disease manifestations and can be made according to established clinical criteria, the description of which is, however, beyond the scope of this chapter (Arnett et al. 1988; Sommer et al. 2005). From the pathophysiologic point of view, synovial hyperemia is the first step of the inflam- matory process in rheumatoid arthritis that can be identified with diagnostic imaging modalities, including power Doppler contrast-enhanced US (Sommer et al. 2005). Then, the immune response mediated by cytokines (TNFα and IL-1) and the subsequent infiltration by inflammatory cells lead to edema and swelling of the synovium. This causes widening of the joint space, which may be further expanded by effusion (Fig. 5.22a). It is assumed that the above stages of the disease may be fully reversible. Later, the inflammatory response leads to hypertrophy of the synovial membrane by invasive granulation tissue with proliferation of synoviocytes, macrophages, lymphocytes, plasma cells and mast cells. As synovial hypertrophy continues, the hypertrophied synovium—usually referred to as “pannus” (the Latin for “cloth”)—undergoes villous transformation and expands concentrically into the joint space leading to damage of the central portion of the articular cartilage and the subchondral bone (formation of subchondral cysts and erosions) (Fig. 5.22b). Tenosynovial sheath involvement coexists in many instances (see Chapter 3). From the clinical point of view, the above abnormalities are encountered not only in rheumatoid arthritis but also in other forms of chronic arthritis. The hallmark of rheumatoid arthritis is bilateral symmetrical involvement of more than three joints. Early in its course, the disease usually affects the small hand joints, the second and third metacarpophalangeal and the third proximal interphalangeal joints being the more typically affected (a characteristic finding of rheumatoid arthritis is sparing of the distal interphalangeal joints, which are commonly involved in osteoarthritis and psoriatic arthritis). In more advanced disease, synovitis involves the larger joints of the limbs and extremities. The destructive action of the pannus is responsible for progressive joint surface damage, ligament and capsule tearing and, finally, joint instability and deformities (Fig. 5.22c,d). When imaging rheumatoid arthritis, one should consider that the disease progresses in a nonlinear fashion and that joint involvement is nonuniform, particularly in the early stages. Although a consensus has not been reached on which joints must be systematically checked, the symptomatic ones and those typically involved in rheumatoid arthritis (i.e., wrist and hand joints) should be examined (Sommer et al. 2005). For follow-up purposes, wrist and hand joints are the preferred sites for assessing the efficacy of therapy (Sommer et al. 2005). In terms of treatment, among drugs that have an influence on the course of disease are Bone and Joint a b c d Fig. 5.22a–d. Rheumatoid arthritis. Schematic drawings showing progression of joint damage during the course of disease. a Early involvement is characterized by joint effusion and pannus formation (1) associated with marginal erosions (2), cartilage thinning (3) and loosening of the capsuloligamentous structures (4). b As the disease progresses, the erosions increase in size, subchondral cysts become evident (5) and the hyaline cartilage appears increasingly thinned (6). Partial tears of the paraarticular structures (7) may occur leading to joint instability. c Later on, fibrous ankylosis (9) of the joint can take place with more evident destruction of the bone ends (10). d In some joints (carpal and tarsal joints) bone ankylosis (11) is the end stage. Inactive fibrous pannus (12) may replace active erosive pannus in the chronic phase the so-called biologic response modifiers (i.e., antiTNFα drugs) that inhibit certain cytokines, thus reducing the inflammatory activity. These drugs are expensive, have important side effects and must be used in patients with erosive aggressive arthritis in whom conventional drugs (NSAIDs, steroids, analgesics, etc.) do not produce a positive response. Early diagnosis of synovitis is, therefore, required to start adequate aggressive therapy before occurrence of structural damage to the joint (Herburn 1988). Because early changes in rheumatoid arthritis are nonosseous in nature, US has proved superior to conventional radiography in terms of disease detection (Gibbon 2004; Clement et al. 2005: Keen et al. 2005). In patients with rheumatoid arthritis and other seronegative arthropathies, US is an effective means for detecting early signs of synovitis, thus allowing prompt institution of an appropriate treatment (Grassi et al. 1993, 2001; Brown et al. 2004). As stated before, US is able to detect joint effusion –which accompanies acute inflammatory phases or exacerbation of disease – even in small synovial joints and can distinguish affected from adjacent normal joints. It can define synovial changes, allowing evaluation of pannus as hypoechoic vegetations protruding inside the synovial fluid or completely filling the articular space (Fig. 5.23a,b). Using MR imaging as the reference method, US has proved to have higher sensitivity and accuracy in detecting signs of inflammation in finger joints than do clinical and radiographic examinations, without loss of specificity (Szkudlarek et al. 2006). In other series, it was even more sensitive than MR imaging in detecting synovitis (Backhaus et al. 1999). The integrated use of Doppler imaging can help to distinguish hypervascular (active) from hypovascular (inactive) pannus, to monitor the response to therapy based on a decreased hyperemia (reflecting improvement in terms of symptoms and disease activity variables) and to differentiate active pannus from echogenic effusion (Fig. 5.23c–f) (Spiegel et al. 1987; Newman et al. 1996; Hau et al. 1999, 2002; Backhaus et al. 1999; Stone et al. 2001; Szkudlarek et al. 2001; Klauser et al. 2002; Fiocco et al. 2005; Kiris et al. 2006). In addition, Doppler US may have value in distinguishing noninflammatory synovial proliferation in osteoarthritis from inflammatory arthritis (Breidahl et al. 1996). Similar to gadolinium-enhanced MR imaging, some attempts have been made with US to obtain a quantitative estimate of the synovial volume. Although a correlation among the histologic findings, clinical markers of disease activity and synovial volume seems to exist, such measurements are time-consuming and, therefore, not currently applicable in routine practice. More recently, microbubble-based US contrast agents seem to be a promising adjunct to assess the activity of the disease process (Magarelli et al. 2001; Klauser et al. 2002). There have been many reports in the literature on power Doppler rather than on color Doppler imaging to detect synovial hyperemia. Current US technology indicates, however, that the sensitivity of color Doppler systems to detect slow and low blood flow signals is now at least equal to or even superior to that of power Doppler imaging. The main limitations of both techniques are essentially related to the lack of standardized examination technique, reproducibility, operator 159 161 Bone and Joint experience and choice of the equipment (Cardinal et al. 1996; Koski et al. 2006). In advanced disease, the inflammatory process may lead to massive erosions and extensive bone damage with disintegration of structures of and around joints, fibrosis, subluxation or dislocation and, at the end stage, bone ankylosis. Based on cartilage thickness measurements, US has proved able to estimate the amount of cartilage destroyed (Grassi et al. 1993; Grassi et al. 1999). US can also depict joint space abnormalities at an earlier stage than conventional radiography. A characteristic feature of rheumatoid arthritis is the release of a subset of loose bodies, called “rice bodies,” within the joint cavity (see Chapter 6). These particles are considered to be the result of sloughed fibrinogen-coated infarcted synovial tissue or aggregates of fibrin, fibronectin or multinuclear cells (McCarthy and Cheung 1982; Popert 1985). US may demonstrate rice bodies as hypoanechoic spherules measuring a few millimeters in size (Martini et al. 2003). In many instances, however, distinguishing them from hypertrophied synovium with US may be difficult (Fig. 5.24). Among the seronegative (rheumatoid factor negative) arthropathies, US has proved useful to examine patients with psoriatic arthritis (Kane et al. 1999; Fiocco et al. 2005; Ory et al. 2005; Kane 2005). Like rheumatoid arthritis, psoriatic arthritis is a chronic disorder with significant joint damage at an early stage of the disease process (Husted et al. 2001). The distal interphalangeal joints are typically affected in an asymmetric pattern. Characteristic radiographic features include joint erosions, joint space narrowing, bony proliferation including periarticular and shaft periostitis, osteolysis with “pencil-in-cup” deformity and acro-osteolysis, ankylosis, spur formation and spondylitis. In psoriatic arthritis, synovitis, enthesitis and tenosynovitis can be reliably assessed with US (Barozzi et al. 1998; Kane et al. 1999; Fiocco et al. 2005; Ory et al. 2005; Kane 2005; Falsetti et al. 2003). In general, the US findings are nonspecific as they may also occur in patients with rheumatoid arthritis and osteoarthritis (Fiocco et al. 2005; Ory et al. 2005). In psoriatic dactylitis (sausage digit), US may show subcutaneous soft-tissue enlargement and, to a lesser extent, tenosynovitis and joint synovitis, the latter sign correlating with joint space narrowing and periostitis on plain films (Fig. 5.25) (Barozzi et al. 1998; Kane et al. 1999). In seronegative arthropathies, unenhanced and contrast-enhanced color Doppler imaging are able to demonstrate active sacroiliitis by showing a hypervascular pattern around a b Fig. 5.24a,b. Rice bodies in rheumatoid arthritis. a Longitudinal 12–5 MHz US image over the anterior knee shows a distended suprapatellar recess (arrows) filled with heterogeneous solid tissue resembling synovial pannus. b Corresponding sagittal T2weighted MR image reveals multiple hypoechoic dots (arrowheads) in the fluid related to rice bodies. The US appearance of rice bodies may be virtually indistinguishable from synovial pannus 162 M. P. Zamorani and M. Valle * MPh * PPh Fig. 5.25. Psoriatic dactylitis. Longitudinal 17–5 MHz US image over the proximal interphalangeal joint of the middle finger in a 45-year-old woman with psoriatic arthritis shows extensive destruction of the articular surface (arrowheads) of the middle phalanx (MPh). Coexisting deformity (arrow) of the head of the proximal phalanx (PPh) and heterogeneous appearance of para-articular soft-tissues (asterisks) is found. The findings correspond to the radiographic sign referred to as the “pencil-incup” deformity the posterior aspect of the sacroiliac joints (Arslan et al. 1999; Klauser et al. 2005). In ankylosing spondylitis, there is predominant involvement of large joints, such as the knee, the shoulder and the hip, with uniform joint space narrowing and low-grade subchondral sclerosis and synovitis. Reiter arthritis is characterized by prevalent distal lower extremity involvement and conspicuous new bone deposition. Arthritis in inflammatory bowel disease is, for the most part, transitory and not destructive; the most commonly involved joints are the knee and the ankle. The features of juvenile idiopathic arthritis are discussed in detail elsewhere (see Chapter 19). 5.2.3.3 Septic Arthritis Septic arthritis is a serious condition leading to rapidly destructive joint disease (Goldenberg 1998; Mohana-Borges et al. 2004). This condition is most commonly caused by Staphylococcus aureus (in adults and children older than 2 years) and Neisseria gonorrheae (in young adults), which have a definite tropism for the synovium (Craig et al. 2003; Mohana-Borges et al. 2004). A variety of streptococci, including S. viridans and S. pneumoniae, group B, aerobic Gram-negative rods, viruses, mycobacteria and fungi may also produce joint infection in isolation or as a result of polymicrobial association (Jbara et al. 2006). Possible pathomechanisms of infection are: hematogenous seeding of the synovium from a distant focus or an adjacent area of osteomyelitis; spread from a contiguous infected site, such as the soft tissues in the diabetic foot; and inadvertent implantation during arthrocentesis or secondary to penetrating wounds and postoperative infection (Mohana-Borges et al. 2004). The most common pattern of presentation of septic arthritis is monoarticular. The most commonly involved joints are the hip, the knee, the shoulder, the elbow and the ankle (MohanaBorges et al. 2004; Chau and Griffith 2005). Infection causes lysis of the articular cartilage, joint space narrowing and periarticular osteopenia. Late complications of arthritis include joint subluxation, premature osteoarthritis, osteonecrosis, fibrous or bony ankylosis, and limb shortening. In the acute setting, US is a reliable way to detect early septic arthritis before the occurrence of substantial cartilage lysis and when radiographs are still noncontributory (Bureau et al. 1999). The main US sign of septic arthritis is detection of a joint effusion in a patient with clinical signs of joint infection (pain, redness, heat, soft-tissue swelling about the involved joint). As regards fluid echotexture, septic effusions often contain a diffuse pattern of low-level echoes and are clearly demarcated from the thickened synovial walls (Chau and Griffith 2005). Highly hyperechoic effusions with debris and septations are often encountered. This appearance might confuse the inexperienced examiner as the collection appears to be solid on static scans; however, dynamic examination and probe com- 163 Bone and Joint pression may show swirling of echoes indicating fluid (Fig. 5.26). Gas bubbles may also be found in joint infection. On the other hand, completely anechoic collections of infected joint fluid are rare. However, these characteristics are too subtle to allow a definitive diagnosis and needle aspiration of fluid, possibly obtained under US guidance, is needed to confirm the infectious nature of the effusion (Bureau et al. 1999; Widman et al. 2001). Power Doppler imaging almost invariably shows a synovial hyperemic flow pattern of hypertrophied synovium and para-articular tissues. Even though the absence of fluid in a joint does not exclude adjacent osteomyelitis, a negative US and Doppler imaging examination makes diagnosis of septic arthritis unlikely (Zawin et al. 1993). 5.2.3.4 Traumatic Injuries When affecting joint portions that are amenable to US examination, osteochondrosis and osteochondral fractures can be detected as surface irregularities of the cartilage or nidus formation involving the cartilage and the subchondral bone (Takahara et al. 1988). In degenerative osteoarthritis, the cartilage may appear progressively thinner and irregular, or even completely disintegrated, whereas the hyperechoic line of the subchondral bone shows irregularities. At US, osteophytes are usually depicted at the joint margins as beak-shaped hyperechoic bone projections covered by cartilage. Following joint surface fractures or other conditions leading to progressive Patella F e T b Fem e a c Fig. 5.26a–c. Septic arthritis. a Lateral radiograph of the knee in a newborn with fever and painful swollen knee suggesting an infection reveals diffuse soft-tissue swelling and an enlarged knee joint space. b,c Longitudinal 12–5 MHz US images obtained over b the patella and c the suprapatellar recess demonstrate the joint cavity filled with highly echogenic dense fluid (arrows) related to purulent material and debris. Note the hypoechoic appearance of the unossified patella and the epiphyseal cartilages of the femur (F) and tibia (T). e, ossification center of the distal femoral epiphysis. Aspiration of the joint fluid revealed Staphylococcus aureus infection 164 M. P. Zamorani and M. Valle derangement of joints (i.e., osteoarthritis, osteochondromatosis, neuropathic joint disease), loose bodies can be released into the joint cavity, possibly leading to intermittent locking of the joint and early degenerative changes. Intra-articular loose bodies are osseous, chondral or osteochondral fragments. They often have a three-layered structure composed of a superficial bright echo due to an artifact at the interface with fluid, an intermediate hypoechoic band due to the cartilage, and a deep hypoechoic surface with posterior acoustic shadowing due to the bone component (Bianchi and Martinoli 2000). In many instances, US can give a better delineation of loose bodies than can plain films and MR 1 2 * imaging (Fig. 5.27). On the other hand, MR imaging is superior in detecting the nidus of the fragment. A monolaminar appearance is observed in old extensively calcified fragments, which appear as hyperechoic images like gallstones without a detectable rim of hypoechoic cartilage (Bianchi and Martinoli 2000). During joint motion or while applying transducer pressure, loose bodies can be mobilized within joint recesses: this may be helpful for the differential diagnosis with either osteophytes or capsular and synovial calcifications. The diagnosis of joint instability basically relies on plain films. In some instances, however, the complex anatomy of joints and surrounding structures Qt 3 c a Qt * 1 2 3 * b d Fig. 5.27a–d. Osteochondral fracture. a Longitudinal and b transverse 17–5 MHz US images of the anterior knee in a patient with onset of painful swelling and locking of the knee following an episode of patellar dislocation show a distended suprapatellar recess (asterisks) containing an osteochondral loose body (arrowhead). The fragment is characterized by a trilayered structure composed of: a superficial bright echo (1) due to the acoustic impedance mismatch at the solid-fluid interface; an intermediate hypoechoic layer (2) due to the cartilage; and a deep bright echogenic surface (3) with slight posterior attenuation due to the detached subchondral bone. c Lateral radiograph is unable to reveal the loose body except for a subtle radio-opaque linear image (arrows) reflecting its bony component. Qt, quadriceps tendon. d Gross operative view demonstrates the loose body (arrowhead) fixed into the patellar nidus Bone and Joint may make detection of subluxation and dislocation of joints difficult on standard radiographs. If undetected, joint instability may lead to chronic local pain, secondary osteoarthritis and altered joint function. In specific clinical settings in which the physical examination may be inconclusive, US can contribute to the detection of occult positional joint abnormalities, including posterior shoulder dislocation and mild acromioclavicular joint instability (see Chapter 6) (Hunter et al. 1998; Bianchi et al. 1994; Bize et al. 2004; Borsa et al. 2005). Many joints contain fibrocartilaginous structures, including the meniscus in the knee, the labrum in the hip and the shoulder, the triangular fibrocartilage in the wrist, and the volar and plantar plates in the hand and foot. Because of their deep location and close contact with the bone, these structures can be evaluated with US only in part and not reliably. Although different authors have reported a high sensitivity and specificity of US in diagnosing knee meniscal and shoulder labral tears (Sohn et al. 1987a, b; Schydlowsky et al. 1998; Hammar et al. 2001), further evidence has demonstrated that US cannot be considered an accurate technique for diagnosing fibrocartilage tears (Azzoni and Cabitza 2002). In particular, distinguishing tears from degenerative states is problematic on the basis of the US findings due to a similar echotextural pattern. However, some conditions involving the superficial part of these structures, such as an extruded meniscus, a meniscocapsular detachment with fluid intervening between the capsule and the fibrocartilage or a meniscal ossicle can be inferred on US (see Chapter 14). Initial experience is also available in the literature on US investigation of the normal triangular fibrocartilage of the wrist and the volar plates (see Chapters 10, 11) (Boutry et al. 2004; Chiou et al. 1988; Keogh et al. 2004). However, further studies are needed to establish the ultimate value of US in imaging pathologic conditions affecting these structures. In contrast to the results of fibrocartilage evaluation, US has proved to be an effective modality for diagnosing parameniscal (see Chapter 14) and paralabral (see Chapters 6, 12) cysts (Peetrons et al. 1990; Rutten et al. 1998; Seymour and Lloyd 1998). These cysts are believed to derive from tangential or compressive forces that lead to trauma, degeneration and tearing of the fibrocartilage. Synovial fluid is extruded through the tear toward the peripheral margin of the fibrocartilage, expanding it and displacing the capsule outward into the surrounding tissues (McCarthy and McNally 2004). Because these cysts are almost invariably associated with a fibrocartilage tear, the US diagnosis of an associated meniscal or labral rupture is straightforward even in cases of unclear or doubtful findings (Fig. 5.28a,b). The cyst can track some distance from the fibrocartilage before becoming clinically palpable and US may show a narrow pedicle connecting it to the tear. Parameniscal and paralabral cysts appear as space-occupying masses with sharply defined borders. They often exhibit mixed internal echotexture as a result of mucoid degeneration or appear as undefined softening and swelling of the connective spaces in which they expand (Fig. 5.28c,d). Even if small in size, labral-related cysts may lead to neuropathy of adjacent nerves, such as the suprascapular nerve (posterior glenoid labrum cysts), the axillary nerve (inferior glenoid labrum cysts) and the femoral nerve (anterior acetabular labrum cysts) (see Chapters 6, 12) (Takagishi et al. 1991). Ligament tears can be demonstrated with US at different sites, including the ankle and foot (Campbell et al. 1994; Peetrons et al. 2004), the wrist and hand (Jones et al. 2000; Noszian et al. 1995; Finlay et al. 2004; Boutry et al. 2005), the knee (Ptasznik et al. 1995; Miller 2002; O’Reilly et al. 2003) and the elbow (Nazarian et al. 2003). The US features of a torn ligament vary depending on whether the lesion is acute or has healed. In acute phases, a partially torn ligament appears swollen and hypoechoic but continuous (Fig. 5.29a); an anechoic band over the superficial aspect of the ligament may be observed representing reactive soft-tissue edema (Peetrons et al. 2004). In complex ligaments, US can distinguish the abnormal hypoechoic portion of the ligament from the unaffected one retaining a normal appearance (Fig. 5.29b). In acute complete ruptures, a hypoechoic cleft reflecting the hematoma can be detected through the ligament substance and the free ends of the severed ligament may appear retracted and wavy (Fig. 5.30a,b). In doubtful cases, the ability to assess the ligament dynamically is a definite advantage of US: under stress, a normal ligament tightens preventing excessive widening of the joint space; if the ligament is torn, a paradoxical movement is obtained reflecting joint instability (Fig. 5.30) (De Smet et al. 2002; Brasseur et al. 2005). In chronic partial tears, the ligament always appears thicker than normal on US images. Calcifications within the ligament substance in old tears and irregularities of the bony insertions in avulsion injuries may be observed (Fig. 5.31) (Brasseur et al. 2005). A typical example is the Pellegrini-Stieda syndrome (calcification of the proximal end of the medial collateral ligament of the knee) (see Chapter 14). In liga- 165 167 Bone and Joint * LM Talus a MC * Tibia b Fig. 5.29a,b. Partial ligament tear. a Long-axis 17–5 MHz US image over the anterior talofibular ligament (arrowheads) in a patient following an inversion injury of the ankle demonstrates a markedly thickened and hypoechoic but straight ligament without signs of macroscopic discontinuity. A hypoechoic band of fluid (asterisk) underlines the superficial aspect of the ligament representing reactive soft-tissue edema. LM, lateral malleolus. Arrow, articular cartilage of the talus. b Coronal 17–5 MHz US image over the medial aspect of the medial femoral condyle (MC) reveals diffuse hypoechoic thickening of the superficial component (arrows) of the proximal medial collateral ligament, whereas the deep meniscofemoral component (arrowheads) is unaffected. Asterisk, medial meniscus underlying bone and at the joint margins (Felson 2004; Gupta et al. 2004). Osteoarthritis is the most widespread form of joint disease in the Western world and can be divided into idiopathic and secondary forms (Gupta et al. 2004). The causes of osteoarthritis include various combinations of systemic risk factors (aging, inheritance, estrogen deficiency), local joint vulnerabilities (previous injuries, bone malalignment) and extrinsic factors acting on the joint (obesity, muscle weakness, occupational and sports-related repetitive overuse but also chronic underuse) (Felson et al. 2004). The initial abnormality of osteoarthritis occurs in the articular cartilage with edema followed by fibrillation and superficial and deep clefts possi- bly evolving toward ulcerations and production of new cartilage and bone. In severe forms, complete cartilage loss associated with pathologic changes of the subchondral bone (i.e., sclerosis, cysts) and marginal osteophytes can occur. Intra-articular loose bodies may develop as a result of detachment of small cartilage pieces or bone fragments which may activate new chondral or bone formation. The diagnosis of osteoarthritis is essentially based on clinical and radiographic data. US is able to detect joint surface and hyaline cartilage abnormalities (Grassi et al. 2005). Changes include progressive thinning and irregularity of the cartilage layer up to its complete disintegration and irregularities of the underlying subchondral bone (Fig. 5.32) (Grassi et 168 M. P. Zamorani and M. Valle 1 LM * 1 2 Talus c a b d Lun e * 2 * Lun Scaph f Scaph g Fig. 5.30a–g. Complete ligament rupture. Spectrum of US appearances in a–d the anterior talofibular and e-g scapholunate ligaments. a Long-axis 17–5 MHz US image obtained at rest over the anterior talofibular ligament after an inversion injury with b schematic drawing correlation demonstrates the torn ends (1, 2) of the ligament in a wavy shape with hematoma insinuating between them. c,d While performing an anterior drawer test, there is opening (large arrows) of the joint space (asterisks) and the ligament ends are more clearly separated from each other. e Transverse 17–5 MHz US image over the dorsal aspect of the proximal carpal row shows a normal scapholunate ligament (arrows) joining the lunate (Lun) and the scaphoid (Scaph). f,g Transverse 17–5 MHz US images obtained in patient who underwent previous wrist injury. f In neutral position, there is absence of the scapholunate ligament with respect to the normal findings shown in e. The dashed lines demarcate the distance between the scaphoid and the lunate. g During ulnar deviation of the wrist, widening (arrows) of the scapholunate distance can be seen: this can be considered a sign of ligament tear al. 1999). One of the major limitations of US in evaluating osteoarthritis is the incomplete evaluation of the cartilage surface, which is, for the most part, masked by the ends of opposing bones. This is true for both tight and large joints. In the knee, for instance, articular cartilages that are vulnerable to tears and ulcerations are mainly located at the posteroinferior aspect of the femoral condyle and on the lateral facet of the patella: both surfaces are barely evaluated with US. Similarly, geodes (subchondral cysts) are not visible at US because they are completely surrounded by bone. On the other hand, osteophytes can be readily appreciated as beak-like bone projections covered by hypoechoic cartilage adjacent to the joint line (Fig. 5.33). They increase the surface area of the articular cartilage, thus lessening the stress and loading forces that are experienced by the joint and, at the same time, increasing its stability: typical locations of osteophytes are the posterior humeral head, the internal femorotibial and the anterior tibiotalar joints (Gupta et al. 2004). Finally, US can be useful in assessing para-articular soft-tissue abnormalities that can be responsible for pain in osteoarthritis and may help to guide intra-articular drug injection (Naredo et al. 2005; Migliore et al. 2005). Bone and Joint molecule – a step that would enhance amyloid formation – and in the generation of pain, dysfunction and even destructive arthropathy (Bancroft et al. 2004). In its articular form, amyloidosis most often involves the hip, the knee and the wrist leading to development of large amyloid-filled subchondral cysts in the juxtaarticular bone. The thickness of abnormal soft-tissue material measured with US in the anterior recess of the hip and the suprapatellar recess of the knee has been found to correlate positively with dialysis duration and radiological and histologic evidence of amyloidosis (Jadoul et al. 1993). Based on echotextural findings, amyloid deposits cannot be distinguished from other synovial pathology (Fig. 5.35a,b). MR imaging demonstrates amyloid with low to intermediate signal on T1- and T2-weighted sequences (Fig. 5.35c,d) (Cobby et al. 1991). 5.2.3.7 Postoperative Complications Detection and localization of postoperative infection may be a challenging task. Septic arthritis complicating hip and knee joint replacement procedures is an important risk factor reported to involve approximately 2% of cases (Goldenberg 1998). Based on the clinical and radiographic findings, it may be impossible to distinguish mechanical loosening of a prosthesis from septic loosening (Bureau et al. 1999). Nuclear medicine, CT and MR imaging can be impaired around orthopaedic hardware by metallic shielding and artifact. US is less hindered in many of these respects and can be considered the firstline diagnostic modality in this setting, provided that an adequate probe access is available (Chau and Griffith 2005). In the postoperative hip, infection can be suspected with US by the presence of a large joint effusion (mean bone-to-pseudocapsule distance: normal, 3.2 mm; infected, 10.2 mm) associated with an extracapsular noncommunicating soft-tissue fluid collection and local inflammatory changes (see Chapter 12) (van Holsbeeck et al. 1994). More recently, however, a retrospective study revealed some limitations of US as an indicator of adult hip joint effusion, even in the postoperative setting (Weybright et al. 2003). When a collection is present around a postoperative hip, US can effectively guide arthrocentesis to obtain material for Gram staining and bacterial culture (van Holsbeeck et al. 1994; Gibbon et al. 2002). Doppler US may also be used to rule out deep vein thrombosis after total hip or knee arthroplasty, especially if routine periopera- tive pharmacologic antithrombotic prophylaxis is not practised (Ko et al. 2003). Granulomatous synovitis can be seen as a result of reaction to small particles of silicone polymers or other synthetic components of prostheses secondary to shedding, so-called “silastic synovitis” or “detritic synovitis”. Often encountered in wrist implants, this condition derives from a kind of foreign-body response, in which activated macrophages provoke bone resorption and dissection around the implant (Bancroft et al. 2004). Standard radiographs show well-defined subchondral lucency and erosions usually associated with fracture and/or dislocation of the prosthesis (Rosenthal et al. 1983; Schneider et al. 1987). MR imaging confirms the radiographic findings and demonstrates multiple small hypointense particles that represent silicone fragments resulting from implant breakage and disintegration (Chan et al. 1998). US can demonstrate the implantderived debris as hyperechoic spots embedded within hypertrophied synovium: these fragments should be differentiated from calcifications based on the presence of posterior reverberation and ringdown artifact (Fig. 5.36). 5.3 Space-Occupying Masses 5.3.1 Bone Tumors The role of US for the detection and assessment of bone tumors is obviously poor, given its inability to define intraosseous processes. US can only detect lesions associated with considerable cortical thinning and/or large extraosseous spread, such as large tumors eroding the cortex (Saifuddin et al. 1998). While evaluating bone tumors, US can be useful in two main clinical situations: the detection of an otherwise unsuspected tumor, or as guidance for a percutaneous biopsy of a bone tumor already investigated with standard radiographs, CT and MR imaging. It is not uncommon for patients with a bone tumor, either primary or metastatic, to complain of nonspecific regional pain and to undergo US examination for a suspected soft-tissue abnormality before a radiographic study. Therefore, careful assessment of the bone surface must be part of a standard US examination of soft tissues and the US signs suggesting a possible abnormality of bone – even if minimal – must be known by the examiner. 173 175 Bone and Joint In osteolytic tumors with extraosseous extension, US shows a periosseous soft-tissue mass arising from a break or a deep defect in the hyperechoic bony cortex (Fig. 5.37). Direct continuity of the mass from the inner bone into adjacent soft tissues is a sign of the intraosseous origin of the tumor. Although US has limitations in assessing infiltration of periosseous tissue planes, the boundaries of the extraosseous component of the neoplasm are usually well circumscribed. In general, the tumor echotexture ranges from solid hypoechoic to a mixed heterogeneous appearance due to internal anechoic areas related to necrosis. In most cases, US is unable to suggest the histologic diagnosis as well as to differentiate malignant from locally aggressive benign tumors. Based on their fluid-filled appearance, US can diagnose intraosseous ganglia extruding into the paraosseous soft tissues (Bianchi et al. 1995). Occasionally, a presumptive diagnosis can be made, such as in the case of aneurysmal bone cysts presenting marked cortical thinning and multiple fluid-fluid levels (Fig. 5.38) (Haber et al. 1993; Gomez et al. 1998). In these instances, changing the patient’s positioning can show respective changes in the disposition of fluid layers within the tumor compartments (Fig. 5.38a-d). Recently, the US appearance of osteoid osteoma located in the proximal metaphysis of the right tibia and left femoral diaphysis of adolescents has been described (GilSanchez et al. 1999). Color Doppler imaging may be useful for detection of the hypervascular nidus and to guide percutaneous localization and biopsy. Doppler imaging should always be performed when evaluating soft-tissue tumors. It can show internal vasculature, thus helping to differentiate viable from necrotic tissue. When a bone tumor exhibits a large extraosseous component at MR imaging, US can be effective and reliable to guide percutaneous needle biopsy (Civardi et al. 1994; Saifuddin et al. 1998, 2000; Konermann et al. 2000; Yeow et al. 2000; GilSanchez et al. 2001; Torriani et al. 2002). Biopsy is a fundamental step in the investigation of suspected bone tumors and must be obtained in specialized centers to avoid inadequate tissue sampling, which is the most common reason for the inability to perform limb-salvage surgery (Skrzynski et al. 1996; Saifuddin et al. 2000). US-guided biopsy must be performed only in patients in whom the feasibility of the procedure has been previously checked on MR imaging or CT scan. The biopsy of a bone tumor must be performed in agreement with the referring orthopaedic surgeon in order to decide the most * b Tibia * a c Fig. 5.37a–c. Bone tumors. Two different cases. a Extended field-of-view 12–5 MHz US image over a slow-growing palpable pretibial mass in a patient with breast cancer shows a large well-circumscribed solid neoplasm causing osteolysis (straight arrows) and extending into the adjacent extraosseous spaces (white arrowheads). Dashed line indicates the level of destroyed tibial cortex. Residual small bone fragments (curved arrows) displaced by the growing neoplasm are seen. Biopsy revealed breast metastasis. b Longitudinal 12–5 MHz US image obtained over the shoulder in a patient with suspected rotator cuff disease reveals a large hypoechoic mass (asterisk) causing extensive osteolysis (white arrows) of the acromion (black arrow): some bone fragments are visible within the mass (arrowhead). c Anteroposterior radiograph confirms the osteolytic lesion (asterisk) and an associated pathologic fracture (black arrowhead). Biopsy revealed metastasis from lung cancer 176 M. P. Zamorani and M. Valle a c e b d g f Fig. 5.38a–g. Aneurysmal bone cyst in a 12-year-old boy with a stiff slow-growing painless swelling over the fibular head and neck. a,b Coronal 12–5 MHz US images obtained over the fibular head a with the patient supine and b standing reveal a complex mass (arrows) which creates a bulge on the surface of the bone with cortical thinning (arrowheads) sufficient to allow US beam penetration. The mass is characterized by multiple dual fluid levels, an appearance fairly specific for an aneurysmal bone cyst. When the patient’s position is changed, fluid levels rearrange parallel to the floor according to gravity. c,d Diagrams show the disposition of fluid levels as seen in a and b. e Photograph shows focal bulging (curved arrow) over the fibular head. f Anteroposterior radiograph and g transverse T2-weighted MR imaging confirm the US diagnosis appropriate needle path by consensus: this helps to avoid seeding of tumor cells out of the involved compartment. As described in Chapter 18, 14–18 gauge Tru-cut type automatic devices are recommended to achieve better diagnostic yield for histologic diagnosis. Fine-needle aspiration biopsy seems to have definite limitations in this field, even in detection of tumor recurrence or diagnosis of metastasis of a known primary tumor. Compared with CT guidance, the main advantages of US-guided biopsies of bone tumors rely on the ability of US: to recognize viable tumor and adjacent vessels with Doppler imaging; to allow continuous real-time visualization of the needle to avoid necrotic areas and the risk of injury to adjacent structures; and to perform the procedure more rapidly, reducing patient discomfort (Torriani et al. 2002). If the US biopsy is performed in experienced hands, complications are rare (Torriani et al. 2002). In rib lesions, local pain and hematoma, infection and pneumothorax have been reported as complications. 5.3.2 Pigmented Villonodular Synovitis Pigmented villonodular synovitis is an uncommon benign proliferative disease of the synovium that affects joints, bursae or tendon sheaths (Dorwart et al. 1984; Yang et al. 1998; Bianchi et al. 1998; Lin et al. 1999; Middleton et al. 2004). From the pathogenetic point of view, the etiology of pigmented villonodular synovitis remains controversial. Of the two most widely accepted theories, one implicates a chronic inflammatory process, the other a benign neoplasm (Byers et al. 1968; Mukhopadhyay et al. Bone and Joint 2006). The presence of histiocytes that contain fat or hemosiderin (a breakdown product of hemoglobin), multinucleated giant cells and plasma cells seems suggestive of inflammation, whereas the high cellularity, tendency for recurrence and recent cytogenetic studies revealing clonal cell proliferation favor a neoplastic process (Mukhopadhyay et al. 2006). The clinical presentation of articular pigmented villonodular synovitis depends on the morphologic form of disease, including a diffuse and nodular type. The more common diffuse pigmented villonodular synovitis grossly appears as a widespread proliferation of the synovium that shows fingerslike masses and villosities. It usually occurs in the third and fourth decades of life as a monoarticular arthritis affecting the knee (80% of cases) (see Chapter 14), the hip and the ankle. Patients’ complaints include insidious onset of progressive joint swelling, discomfort, pain, mechanical derangement and decreased range of motion. Focal pigmented villonodular synovitis more frequently occurs in the fifth and sixth decades, affecting joint, para-articular bursae and synovial tendon sheaths (giant cell tumor of tendon sheath), the latter being more common. It has a female predominance and affects the digits of the hand and foot, presenting as a slow-growing painless mass (Rao and Vigorita 1984). Plain radiographs can be normal or show intraarticular effusion, an ill-defined para-articular soft-tissue mass and, in longstanding disease, juxtaarticular bone erosions and subchondral cysts caused by pressure and hypertrophied synovium. CT shows hemosiderin and fat deposits, and is able to detect bone erosions that are not manifest on radiographs. MR imaging shows synovial effusion and hypertrophied synovitis containing scattered areas of hemosiderin that exhibits low signal intensity on T1- and T2-weighted sequences—darker on T2* gradient-echo sequences due to susceptibility artifact (Fig. 5.39). Although similar appearances can be observed in hemophilic and rheumatoid arthritis in the proper clinical setting, these findings are often considered to be diagnostic of pigmented villonodular synovitis (Hughes et al. 1995; Jelinek et al. 1989; Narváez et al. 2001). The US appearance of diffuse pigmented villonodular synovitis is nonspecific as it appears either as an intra-articular area of hypoechoic synovial thickening or as an irregular mass located within the joint cavity usually associated with a joint effusion (Fig. 5.39a). In some cases, US can detect pressure erosions on the bone cortex as defects of the joint surfaces filled with hypertrophied synovium. Doppler imaging can show a hypervascular pattern within the synovial mass (Yang et al. 1998; Lin et al. 1999). A pattern of relatively increased flow in the periphery of the synovial capsule may be present (Lin et al. 1999). Local recurrence after surgical or arthroscopic synovectomy takes place in almost 50% of cases (Sheldon et al. 2005). The nodular type of pigmented villonodular synovitis has been already described in Chapter 3 (Bianchi et al. 1998; Middleton et al. 2004). 5.3.3 Lipoma Arborescens Lipoma arborescens, also referred to as diffuse synovial lipoma or villous proliferation of synovium, is P Femur a b c Fig. 5.39a–c. Pigmented villonodular synovitis: diffuse type. a Longitudinal 12–5 MHz US image obtained over the suprapatellar recess demonstrates hypoechoic thickening of the synovium (arrowheads), a fairly nonspecific appearance. P, patella. b Sagittal T2-weighted and c fat-suppressed postcontrast T1-weighted MR images confirm the US finding (arrowhead) and reveal additional lesions (arrows) lying in the anterior and posterior joint recesses. The hypointense pattern of these lesions on T2-weighted sequences strongly suggests pigmented villonodular synovitis 177 Bone and Joint a b a c d b c Fig. 5.41a–d. Synovial chondromatosis. a Plain lateral radiograph of the index finger shows a small soft-tissue calcified mass on the volar (white arrow) aspect of the first phalanx. Some calcifications are also visible on the dorsal aspect of the proximal phalangeal joint (void arrow). Lack of bone erosion and of periosteal reaction suggests excluding bone involvement. b Longitudinal 17-5 MHz US images obtained at the level of the proximal interphalangeal joint reveal a conglomerate of calcifications (arrows) filling the ventral joint recess (arrowheads) between the bone and the flexor tendons (ft). MPh, middle phalanx; PPh, proximal phalanx. c Axial CT imaging correlation. d Surgical specimen of the same case shows the loose bodies tate or dense lobular calcifications, which occur in approximately two-thirds of cases. Treatment is surgical synovectomy; however, the recurrence rate is over 25% (Sheldon et al. 2005). 5.3.5 Synovial Hemangioma Synovial hemangioma is a rare intra-articular benign tumor arising from the synovium that is difficult to diagnose because of its nonspecific clinical presentation of nontraumatic recurrent swollen painful knee (Narváez et al. 2001; Okahashi et al. 2004). The knee of young adults and adolescents is usually affected. Clinical symptoms are related to the mass effect of the tumor, which leads to a decreased range of motion and repetitive episodes of bleeding causing joint pain and swelling and possibly mimicking other conditions, such as hemophilic arthropathy, arthritis or medial shelf syndrome. Hemangiomas arising from the synovium have a similar US appearance to other soft-tissue hemangiomas, but the presence of hypoechoic tissue with fluid-filled areas related to pooling of blood within vascular spaces may generate confusion with more common synovial conditions. Therefore, the examiner must be aware of this condition to avoid making a wrong diagnosis and wasting time for the patient. In the cavernous type of synovial hemangioma, slow-flowing blood can occasionally be appreciated within the anechoic spaces of the mass on gray-scale and color 179 180 M. P. Zamorani and M. Valle F F a b & c d e f Fig. 5.42a–f. Synovial hemangioma. a,b Transverse 12–5 MHz US images obtained over the suprapatellar region a without and b with compression with the probe demonstrate a fluid-filled suprapatellar recess (arrows) containing slow-flowing swirling echoes that change their echogenicity depending on the pressure exerted over them. F, femur. c Color Doppler imaging displays venous flow filling the fluid-filled cavities contained in the recess. d Sagittal T2-weighted and e fat-suppressed postcontrast T1-weighted MR imaging correlation show a markedly hyperintense lesion (arrow) containing linear low-signal structures (arrowhead) representing fibrofatty septations and intratumor vascular channels. f Lateral radiograph reveals fullness of the suprapatellar recess and scattered phleboliths (arrowheads), a feature of value for the diagnosis of synovial hemangioma Doppler US (Fig. 5.42a–c). MR imaging of synovial hemangioma is pathognomonic, with intermediate T1-signal intensity and marked hyperintensity on T2-weighted images and after gadolinium administration (Fig. 5.42d,e). Intratumor fat overgrowth, low T2-signal vascular channels and phleboliths can also be found (Fig. 5.42e,f). Circumscribed masses can be resected on arthroscopy, whereas diffuse lesions need open surgery (Sheldon et al. 2005). References Abiri MM, Kirpekar M, Ablow RC (1989) Osteomyelitis: detection with US. Radiology 172:509–511 Abreu M, Johnson K, Chung CB et al (2004) Calcification in calcium pyrophosphate dehydrate (CPPD) crystalline deposits in the knee: anatomic, radiographic, MR imaging, and histologic study in cadavers. Skeletal Radiol 33:392–398 Alarcon GS, Lopez-Ben R, Moreland LW (2002) High-resolution ultrasound for the study of target joints in rheumatoid arthritis. Arthritis Rheum 46:1969–1970 Al-Ismail K, Torreggiani WC, Al-Sheikh F et al (2002) Bilateral lipoma arborescens associated with early osteoarthritis. Eur Radiol 12:2799–2802 Arnett FC, Edworthy SM, Bloch DA et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324 Arslan H, Sakarya ME, Adak B et al (1999) Duplex and color Doppler sonographic findings in active sacroiliitis. AJR Am J Roentgenol 173:677–680 Azzoni R, Cabitza P (2002) Is there a role for sonography in the diagnosis of tears of the knee menisci? J Clin Ultrasound 30:472–476 Backhaus M, Kamradt T, Sandrock D et al (1999) Arthritis of the finger joints: a comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast-enhanced magnetic resonance imaging. Arthritis Rheum 42:1232–1245 Bancroft LW, Peterson JJ, Kransdorf MJ (2004) Cysts, geodes and erosions. Radiol Clin North Am 42:73–87 Barozzi L, Olivieri I, De Matteis M et al (1998) Seronegative spondylarthropathies: imaging of spondylitis, enthesitis and dactylitis. Eur J Radiol 27:12–17 Bejia I, Younes M, Moussa A et al (2005) Lipoma arborescens affecting multiple joints. Skeletal Radiol 34:536–538 Bianchi S, Martinoli C (2000) Detection of loose bodies in joints. Radiol Clin North Am 37:679–690 Bianchi S, Zwass A, Abdelwahab I (1994) Sonographic evaluation of posterior instability and dislocation of the shoulder: prospective study. J Ultrasound Med 13:389–393 Bianchi S, Zwass A, Abdelwahab IF et al (1995a) Sonographic evaluation of lipohemarthrosis: clinical and in vitro study. J Ultrasound Med 14:279–282 Bone and Joint Bianchi S, Abdelwahab IF, Rettagliata F et al (1995b) Expansible subchondral bone cyst of the left tibia secondary to osteoarthritis of the left knee. Can Assoc Radiol J 46:308–310 Bianchi S, Mazzola CG, Martinoli C et al (1998) Quiz case of the month. Intra-articular pigmented villonodular synovitis of the knee. Eur Radiol 8:1275–1276 Bize P, Pugliese F, Bacigalupo L et al (2004) Unrecognized bilateral posterior shoulder dislocation diagnosed by ultrasound. Eur Radiol 14:350–352 Bodner G, Huber B, Schwabegger A et al (1999). Sonographic detection of radial nerve entrapment within a humerus fracture. J Ultrasound Med 18:703–706 Bodner G, Buchberger W, Schocke M et al (2001) Radial nerve palsy associated with humeral shaft fracture: evaluation with US: initial experience. Radiology 219:811–816 Bodner G, Stockl B, Fierlinger A et al (2005) Sonographic findings in stress fractures of the lower limb: preliminary findings. Eur Radiol 15:356–359 Borsa PA, Jacobson JA, Scibek JS et al (2005) Comparison of dynamic sonography to stress radiography for assessing glenohumeral laxity in asymptomatic shoulders. Am J Sports Med 33:734–741 Boutry N, Larde A, Demondion X et al (2004) Metacarpophalangeal joints at US in asymptomatic volunteers and cadaveric specimens. Radiology 232:716–724 Boutry N, Lapegue F, Masi L et al (2005) Ultrasonographic evaluation of normal extrinsic and intrinsic carpal ligaments: preliminary experience. Skeletal Radiol 34:513–521 Boutry N, Vanderhofstadt A, Peetrons P (2006) Ultrasonography of anterosuperior calcaneal process fracture: report of 2 cases. J Ultrasound Med 25:381–385 Brasseur JL, Luzzati A, Lazennec JY et al (1994) Ultrasonoanatomy of the ankle ligaments. Surg Radiol Anat 16:87–91 Brasseur JL, Morvan G, Godoc B (2005) Dynamic ultrasonography. J Radiol 86:1904–1910 Breidahl WH, Newman JS, Tajanovic MS et al (1996) Power Doppler sonography in the assessment of musculoskeletal fluid collections. AJR Am J Roentgenol 166:1443–1446 Brigido MK, Fessell DP, Jacobson JA et al (2005) Radiography and US of os peroneum fractures and associated peroneal tendon injuries: initial experience. Radiology 237:235–241 Brown AK, Wakefield RJ, Conaghan PG et al (2004) New approaches to imaging early inflammatory arthritis. Clin Exp Rheumatol 22:18–25 Bruce WD, Christofersen MR, Phillips DL (1999) Stenosing tenosynovitis and impingement of the peroneal tendons associated with hypertrophy of the peroneal tubercle. Foot Ankle Int 20:464–467 Bureau NJ, Chhem RK, Cardinal E (1999) Musculoskeletal infections: US manifestations. RadioGraphics 19:1585–1592 Byers PD, Cotton RE, Deacon UW et al (1968) The diagnosis and treatment of pigmented villonodular synovitis. J Bone Joint Surg Am 50:290–305 Campbell DG, Menz A, Isaacs J (1994) Dynamic ankle ultrasonography. A new imaging technique for acute ankle ligament injuries. Am J Sports Med 22:855–858 Campeau NG, Lewis BD (1998) Ultrasound appearance of synovial osteochondromatosis of the shoulder. Mayo Clin Proc 73:1079–1081 Cardinal E, Lafortune M, Burns P (1996) Power Doppler US in synovitis: reality or artefact? Radiology 200:868–869 Caruso G, Lagalla R, Derchi L et al (2000) Monitoring of fracture calluses with color Doppler sonography. J Clin Ultrasound 28:20–27 Chan M, Chowchuen P, Workman T et al (1998) Silicone synovitis: MR imaging in five patients. Skeletal Radiol 27:13–17 Chao HC, Lin SJ, Huang YC et al (1999) Color Doppler ultrasonographic evaluation of osteomyelitis in children. J Ultrasound Med 18:729–734 Chau CLF, Griffith JF (2005) Musculoskeletal infections: ultrasound appearances. Clin Radiol 60:149–159 Chern TC, Jou IM, Lai KA et al (2002) Sonography for monitoring closed reduction of displaced extra-articular distal radial fractures. J Bone Joint Surg Am 84:194–203 Chiou HJ, Chang CY, Chou Y et al (1988) Triangular fibrocartilage of wrist: presentation on high resolution ultrasonography. J Ultrasound Med 17:41–48 Cho KH, Park BH, Yeon KM (2000) Ultrasound of the adult hip. Semin Ultrasound CT MR 21:214–230 Cho KH, Lee YH, Lee SM et al (2004) Sonography of bone and bone-related diseases of the extremities. J Clin Ultrasound 32:511–521 Cicak N, Bilic R, Delimar D (1998) Hill-Sachs lesion in recurrent shoulder dislocation: sonographic detection. J Ultrasound Med 17:557–560 Cimmino MA, Bountis C, Silvestri E et al (2000) An appraisal of magnetic resonance imaging of the wrist in rheumatoid arthritis. Semin Arthritis Rheum 30:180–195 Civardi G, Livraghi T, Colombo P et al (1994) Lytic bone lesions suspected for metastasis: ultrasonically guided fine-needle aspiration biopsy. J Clin Ultrasound 22:307–311 Clement JP-IV, Kassarjian A, Palmer WE (2005) Synovial inflammatory process in the hand. Eur J Radiol 56:307–318 Coari G, Iagnocco A, Zoppini A (1995) Chondrocalcinosis: sonographic study of the knee. Clin Rheumatol 14:511–514 Cobby MJ, Adler RS, Swartz R et al (1991) Dialysis-related amyloid arthropathy: MR findings in four patients. AJR Am J Roentgenol 157:1023–1027 Copercini M, Bonvin F, Martinoli C et al (2003) Sonographic diagnosis of talar lateral process fracture. J Ultrasound Med 22:635–640 Craig JG (1999) Infection: ultrasound-guided procedures. Radiol Clin North Am 37:669–678 Craig JG, Jacobson JA, Moed BR (1999) Ultrasound of fractures and bone healing. Radiol Clin North Am 37:737–751 Craig JG, van Holsbeeck M, Alva M (2003) Gonococcal arthritis of the shoulder and septic extensor tenosynovitis of the wrist: sonographic appearances. J Ultrasound Med 22:221–224 Davidson D, Letts M, Khoshhal K (2003) Pelvic osteomyelitis in children: comparison of decades from 1980–1989 with 1990–2001. J Pediatr Orthop 23:514–521 Davies AP, Blewitt N (2005) Lipoma arborescens of the knee. Knee 12:394–396 de Matos AN, Mendonca JM, Pereira MC (1983) Hereditary multiple exostoses. A rare cause of arterial insufficiency. Angiology 34:362–366 Delaunoy I, Feipel V, Appelboom T et al (2003) Sonography detection threshold for knee effusion. Clin Rheumatol 22:391–392 De Smet AA, Winter TC, Best TM et al (2002) Dynamic sonography with valgus stress to assess elbow ulnar collateral ligament injury in baseball pitchers. Skeletal Radiol 31:671–676 181 182 M. P. Zamorani and M. Valle Dorwart RH, Genant HK, Johnston WH et al (1984) Pigmented villonodular synovitis of synovial joints: clinical, pathologic, and radiologic features. AJR Am J Roentgenol 143:877–885 Doyle AJ, Miller MV, French JG (2002) Lipoma arborescens in the bicipital bursa of the elbow: MRI findings in two cases. Skeletal Radiol 31:656–660 Dzido G, Sprague SM (2003) Dialysis-related amyloidosis. Urol Nefrol 55:121–129 Ehrenstein T, Rikli DA, Peine R et al (1999) A new ultrasoundbased method for the assessment of torsional differences following closed intramedullary nailing of femoral fractures. Skeletal Radiol 28:336–341 El-Khoury GY, Bassett GS (1979) Symptomatic bursa formation with osteochondromas. AJR Am J Roentgenol 133:895–898 Enns P, Pvlidis T, Stahl JP et al (2004) Sonographic detection of an isolated cuboid bone fracture not visualized on plain radiographs. J Clin Ultrasound 32:154–157 Erickson SJ (1997) High-resolution imaging of the musculoskeletal system. Radiology 205:593–618 Falsetti P, Frediani B, Fioravanti A et al (2003) Sonographic study of calcanear entheses in erosive osteoarthritis, nodal osteoarthritis, rheumatoid arthritis and psoriatic arthritis. Scand J Rheumatol 32:229–234 Farin PU, Kaukanen E, Jaroma H et al (1996) Hill-Sachs lesion: sonographic detection. Skeletal Radiol 25:559–562 Farina A, Filippucci E, Grassi W (2002) Sonographic findings of the synovial fluid. Reumatismo 54:261–265 Felson DT (2004) An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am 42:1–9 Ferrara MA, Marcelis S (1997) Ultrasound of the elbow. J Belge Radiol 80:122–123 Filippucci E, Ciapetti A, Grassi W (2003) Sonographic monitoring of gout. Reumatismo 55:184–186 Finlay K, Lee R, Friedman L (2004) Ultrasound of intrinsic wrist ligament and triangular fibrocartilage injuries. Skeletal Radiol 33:85–90 Fiocco U, Ferro F, Vezzù M et al (2005) Rheumatoid and psoriatic knee synovitis: clinical, grey-scale and power Doppler ultrasound assessment of the response to etanercept. Ann Rheum Dis 64:899–905 Foldes K (2002) Knee chondrocalcinosis: an ultrasonographic study of the hyaline cartilage. Clin Imaging 26:194–196 Frediani B, Filippou G, Falsetti P et al (2005) Diagnosis of calcium pyrophosphate dihydrate crystal deposition disease: ultrasonographic criteria proposed. Ann Rheum Dis 64:638–640 Gerngross H, Sohn C (1992) Ultrasound scanning for the diagnosis of meniscal lesions of the knee joint. Arthroscopy 8:105–110 Gibbon WW (2004) Applications of ultrasound in arthritis. Semin Musculoskelet Radiol 8:313–328 Gibbon WW, Long G, Barron DA et al (2002) Complications of orthopedic implants: sonographic evaluation. J Clin Ultrasound 30:288–299 Gil-Sanchez S, Marco-Domenech SF, Arenas J et al (1999) Doppler duplex color localization of osteoid osteomas. Skeletal Radiol 8:107–110 Gil-Sanchez S, Marco-Domenech SF, Irurzun-Lopez J et al (2001) Ultrasound-guided skeletal biopsies. Skeletal Radiol 30:615–619 Goldenberg DL (1998) Septic arthritis. Lancet 351:197–202 Gomez J, Pinar A, Vallcanera A et al (1998) Sonographic findings in aneurysmal bone cyst in children: correlation with computer tomography findings. J Clin Ultrasound 26:59–64 Grassi W, Tittarelli E, Pirani O et al (1993). Ultrasound examination of metacarpophalangeal joints in rheumatoid arthritis. Scand J Rheumatol 22:243–247 Grassi W, Lamanna G, Farina A et al (1999) Sonographic imaging of normal and osteoarthritic cartilage. Semin Arthritis Rheum 28:398–403 Grassi W, Filippucci E, Farina A et al (2001) Ultrasonography in the evaluation of bone erosions. Ann Rheum Dis 60:98–103 Grassi W, Filippucci E, Farina A (2005) Ultrasonography in osteoarthritis. Semin Arthritis Rheum 34:19–23 Griffith JF, Rainer TH, Ching ASC et al (1999) Sonography compared with radiography in revealing acute rib fracture. AJR Am J Roentgenol 173:1603–1609 Gupta KB, Duryea J, Weissman BN (2004) Radiographic evaluation of osteoarthritis. Radiol Clin North Am 42:11–41 Haber HP, Drews K, Scheel-Walter H et al (1993) Aneurysmal bone cyst in early childhood: ultrasound findings. Pediatr Radiol 23:405–406 Hammar MV, Wintzell GB, Astrom KG et al (2001) Role of US in the preoperative evaluation of patients with anterior shoulder instability. Radiology 219:29–34 Harrison MR, Hamilton S, Johnstone AJ (2004) Pseudorupture of extensor pollicis longus following Kirschner wire fixation of distal radius fractures. Acta Orthop Belg 70:492–494 Hau M, Schultz H, Tony HP et al (1999) Evaluation of pannus and vascularization of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis by highresolution ultrasound (multidimensional linear array). Arthritis Rheum 42:2303–2308 Hau M, Kneitz C, Tony HP et al (2002) High-resolution ultrasound detect a decrease in pannus vascularisation of small finger joints in patients with rheumatoid arthritis receiving treatment with soluble tumour necrosis factor alpha receptor (etanercept). Ann Rheum Dis 61:55–58 Hauger O, Bonnefoy O, Moinard M et al (2002) Occult fractures of the waist of the scaphoid: early diagnosis by high spatial resolution sonography. AJR Am J Roentgenol 178:1239–1245 Herburn B (1988) What is a disease modifying antirheumatic drug? J Rheumatol 15:40–42 Herneth AM, Siegmeth A, Bader TR et al (2001) Scaphoid fractures: evaluation with high-spatial-resolution US initial results. Radiology 220:231–235 Herzenberg JE, Goldner JL, Martinez S et al (1986) Computerized tomography of talocalcaneal tarsal coalition. Foot Ankle 6:273–288 Hodgkinson DW, Nicholson DA, Stewart G et al (1993) Scaphoid fracture: a new method of assessment. Clin Radiol 48:398–401 Howard CB, Lieberman N, Mozes G et al (1992) Stress fracture detected sonographically. AJR Am J Roentgenol 159:1350– 1351 Hubner U, Shlicht W, Outzen S et al (2000) Ultrasound in the diagnosis of fractures in children. J Bone Joint Surg [Br] 82:1170–1173 Hughes TH, Sartoris DJ, Schweitzer ME et al (1995) Pigmented villonodular synovitis: MRI characteristics. Skeletal Radiol 24:7–12 Hunter JD, Franklin K, Hughes PM (1998) The ultrasound diagnosis of posterior shoulder dislocation associated with Erb’s palsy. Pediatr Radiol 28:510–511 Hurley ME, Keye GD, Hamilton S (2004) Is ultrasound really helpful in the detection of rib fractures? Injury 35:562– 566 Bone and Joint Husted JA, Gladman DD, Farewell VT et al (2001) Healthrelated quality of life of patients with psoriatic arthritis: a comparison with patients with rheumatoid arthritis. Arthritis Rheum 45:151–158 Iagnocco A, Coari G (2000) Usefulness of high resolution US in the evaluation of effusion in osteoarthritic first carpometacarpal joint. Scand J Rheumatol 29:170–173 Jacobson JA, Andresen R, Jaovisidha S et al (1998) Detection of ankle effusions: comparison study in cadavers using radiography, sonography, and MR imaging. AJR Am J Roentgenol 170:1231–1238 Jadoul M, Malghem J, Vande Berg B et al (1993) Ultrasonography of joint capsules and tendons in dialysis-related amyloidosis. Kidney Int 41:106–110 Jbara M, Patnana M, Kazmi F (2006) MR imaging: arthropathies and infectious conditions of the elbow, wrist and hand. Radiol Clin North Am 44:625–642 Jelinek JS, Kransdorf MJ, Utz JA et al (1989) Imaging of pigmented villonodular synovitis with emphasis on MR imaging. AJR Am J Roentgenol 152:337–342 Jones MH, England SJ, Muwanga CL et al (2000) The use of ultrasound in the diagnosis of injuries of the ulnar collateral ligament of the thumb. J Hand Surg [Br] 25:29–32 Kane D (2005) The role of ultrasound in the diagnosis and management of psoriatic arthritis. Curr Rheumatol Rep 7:319–324 Kane D, Greaney T, Bresnihan B et al (1999) Ultrasonography in the diagnosis and management of psoriatic dactylitis. J Rheumatol 26:1746-1751 Karakurt L, Ozdemir H, Yilmaz E et al (2005) Morphology and dynamics of the ulnar nerve in the cubital tunnel after percutaneous cross-pinning of supracondylar fractures in children’s elbows: an ultrasonographic study. J Pediatr Orthop B 14:189–193 Kayser R, Mahlfeld K, Heyde C et al (2003) Ultrasonographic imaging of fractures of the clavicle in newborn infants. J Bone Joint Surg [Br] 85:115–116 Keeling SL, Numa A, Wilde P et al (1993) Deep venous thrombosis caused by femoral exostosis. Med J Aust 158:50–51 Keen HI, Brown AK, Wakefield RJ et al (2005) MRI and musculoskeletal ultrasonography as diagnostic tools in early arthritis. Rheum Dis Clin North Am 31:699–714 Kellner H, Zoller W, Herzer P (1990) Ultrasound findings in chondrocalcinosis. Z Rheumatol 49:147–150 Keogh CF, Wong AD, Wells NJ et al (2004) High-resolution sonography of the triangular fibrocartilage: initial experience and correlation with MRI and arthroscopic findings. AJR Am J Roentgenol 182:333–336 Kim HK, Babyn PS, Harasiewicz KA et al (1995) Imaging of immature articular cartilage using ultrasound backscatter microcopy at 50 MHz. J Orthop Res 13:963–970 Kiris A, Ozgocmen S, Kokacoc E et al (2006) Power Doppler assessment of overall disease activity in patients with rheumatoid arthritis. J Clin Ultrasound 34:5–11 Klauser A, Frauscher F, Schirmer M et al (2002) The value of contrast-enhanced color Doppler ultrasound in the detection of vascularization of finger joints in patients with rheumatoid arthritis. Arthritis Rheum 46:647–653 Klauser A, Halpern EJ, Fraucher F et al (2005) Inflammatory low back pain: high negative predictive value of contrastenhanced color Doppler ultrasound in the detection of inflamed sacroiliac joints. Arthritis Rheum 53:440–444 Ko PS, Chan WF, Siu TH et al (2003) Duplex ultrasonography after total hip or knee arthroplasty. Int Orthop 27:168–171 Konermann W, Wuisman P, Ellermann A et al (2000) Ultrasonographically guided needle biopsy of benign and malignant soft tissue and bone tumors. J Ultrasound Med 19:465–471 Koski JM, Saarakkala S, Helle M et al (2006) Assessing the intra- and inter-reader reliability of dynamic ultrasound images in power Doppler ultrasonography. Ann Rheum Dis 65:1658-1660 Kumar V, Joseph B, Verghese J et al (1992) Measurement of femoral torsion with ultrasound: evaluation of a new technique using reference lines on the posterior surface of the femur. J Clin Ultrasound 20:111–114 Learch TJ, Braaton M (2000) Lipoma arborescens: high resolution ultrasonographic findings. J Ultrasound Med 19:385–389 Lee JI, Song IS, Jung YB et al (1996) Medial collateral ligament injuries of the knee: ultrasonographic findings. J Ultrasound Med 15:621–625 Lee MF, Chan PT, Chau LF et al (2002) Tarsal tunnel syndrome caused by talocalcaneal coalition. Clin Imaging 26:140–143 Leekam RN, Voorneveld CR (1996) Synovial osteochondromatosis of the distal radioulnar joint: ultrasound diagnosis. J Clin Ultrasound 24:207–208 Levinsohn EM, Santelli ED (1991) Bicipital groove dysplasia and medial dislocation of the biceps brachii tendon. Skeletal Radiol 20:419–423 Lewis D, Logan P (2006) Sonographic diagnosis of toddler’s fracture in the emergency department. J Clin Ultrasound 34:190–194 Lin J, Jacobson JA, Jamadar DA et al (1999) Pigmented villonodular synovitis and related lesions: the spectrum of imaging findings. AJR Am J Roentgenol 172:191–197 Maffulli N, Thorton A (1995) Ultrasonic appearance of external callus in long bone fracture. Injury 20:5–12 Magarelli N, Guglielmi G, Di Matteo L et al (2001) Diagnostic utility of an echo-contrast agent in patients with synovitis using power Doppler ultrasound: a preliminary study with comparison to contrast-enhanced MR imaging. Eur Radiol 11:1039–1946 Mah ET, LeQuesne GW, Gent RJ et al (1994) Ultrasonic features of acute osteomyelitis in children. J Bone Joint Surg Br 76:969–974 Malghem J, Vande Berg B, Noel H et al (1992) Benign osteochondromas and exostotic chondrosarcomas: evaluation of cartilage cap thickness by ultrasound. Skeletal Radiol 21:33–37 Markowitz RI, Davidson RS, Harty MP et al (1992) Sonography of the elbow in infants and children. AJR Am J Roentgenol 159:829–833 Martini G, Tregnaghi A, Bordin T et al (2003) Rice bodies imaging in juvenile idiopathic arthritis. J Rheumatol 30:2720–2721 Martino F, De Serio A, Macarini L et al (1998) Ultrasonography versus computed tomography in evaluation of the femoral trochlear groove morphology: a pilot study on healthy, young volunteers. Eur Radiol 8:244–247 Martinoli C, Bianchi S, Spadola L et al (2000) Multimodality imaging assessment of meniscal ossicle. Skeletal Radiol 29:481–484 Mathieu P, Wybier M, Busson J et al (1997) The medial collateral ligament of the knee. Ann Radiol 40:176–181 McCarthy CL, McNally EG (2004) The MRI appearance of cystic lesions around the knee. Skeletal Radiol 33:187–209 McCarthy DJ, Cheung HS (1982) Origin and significance of rice bodies in synovial fluid. Lancet II:715–716 183 184 M. P. Zamorani and M. Valle Middleton WD, Patel V, Teefey SA et al (2004) Giant cell tumors of the tendon sheath: analysis of sonographic findings. AJR Am J Roentgenol 183:337–339 Migliore A, Tormenta S, Martin Martin LS et al (2005) The symptomatic effects of intra-articular administration of hylan G-F 20 on osteoarthritis of the hip: clinical data of 6 months follow-up. Clin Rheumatol 25:1–5 Miles KA, Lamont AC (1989) Ultrasonic demonstration of the elbow fat pad. Clin Radiol 40:602–604 Milgram JW (1977) Synovial osteochondromatosis. A histopathological study of thirty cases. J Bone Joint Surg [Am] 59:792–801 Miller TT (2002) Sonography of injury of the posterior cruciate ligament of the knee. Skeletal Radiol 31:149–154 Miller TT, Adler RS, Friedman L (2004) Sonography of injury of the ulnar collateral ligament of the elbow: initial experience. Skeletal Radiol 33:386–391 Milosavljevic J, Lindqvist U, Elvin A (2005) Ultrasound and power Doppler evaluation of the hand and wrist in patients with psoriatic arthritis. Acta Radiol 46:374–385 Moed BR, Subramanian S, van Holsbeeck M et al (1998) Ultrasound for the early diagnosis of tibial fracture healing after static interlocked nailing without reaming: clinical results. J Orthop Trauma 12:206–213 Mohana-Borges AVR, Chung CB, Resnick D (2004) Monoarticular arthritis. Radiol Clin North Am 42:135–149 Monu JUV, Pope TL (2004) Gout: a clinical and radiologic review. Radiol Clin North Am 42:169–184 Morvan G, Mathieu P, Busson J et al (2000) Ultrasonography of tendons and ligaments of foot and ankle. J Radiol 81:361–380 Moss GD, Dishuk W (1984) Ultrasound diagnosis of osteochondromatosis of the popliteal fossa. J Clin Ultrasound 12:232–233 Moss SG, Schweitzer ME, Jacobson JA et al (1998) Hip joint fluid: detection and distribution at MR imaging and US with cadaveric correlation. Radiology 208:43–48 Mukhopadhyay K, Smith M, Hughes PM (2006) Multifocal PVNS in a child followed over 25 years. Skeletal Radiol 35:539–542 Munk B, Bolvig L, Kroner K et al (2000) Ultrasound for diagnosis of scaphoid fractures. J Hand Surg [Br] 25:369–371 Murphey MD, Choi JJ, Kransdorf MJ et al (2000) Imaging of osteochondroma: variants and complications with radiologic-pathologic correlation. RadioGraphics 20:1407–1434 Murphey MD, Carroll JF, Flemming DJ et al (2004) From the archives of the AFIP: benign musculoskeletal lipomatous lesions. RadioGraphics 24:1433–1466 Naredo E, Cabero F, Palop MJ et al (2005) Ultrasonographic findings in knee osteoarthritis: a comparative study with clinical and radiographic assessment. Osteoarthritis Cartilage 13:568–574 Narváez JA, Narváez J, Aguilera C (2001) MR imaging of synovial tumors and tumor-like lesions. Eur Radiol 11:2549–2560 Nazarian LN, McShane JM, Ciccotti MG et al (2003) Dynamic US of the anterior band of the ulnar collateral ligament of the elbow in asymptomatic major league baseball pitchers. Radiology 227:149–154 Newman JS, Laing TJ, McCarthy CJ et al (1996) Power Doppler sonography of synovitis: assessment of therapeutic response. Preliminary observation. Radiology 198:582–584 Nisolle JF, Blouard E, Baudrez V et al (1999) Subacromial-subdeltoid lipoma arborescens associated with a rotator cuff tear. Skeletal Radiol 28:283–285 Noszian IM, Dinkhauser LM, Orthner E et al (1995) Ulnar collateral ligament: differentiation of displaced and nondisplaced tears with US. Radiology 194:61–63 Okahashi K, Sugimoto K, Iwai M et al (2004) Intra-articular synovial hemangioma: a rare cause of knee pain and swelling. Arch Orthop Trauma Surg 124:571–573 O’Reilly MA, O’Reilly PM, Bell J (2003) Sonographic appearances of medial retinacular complex injury in transient patellar dislocation. Clin Radiol 58:636–641 Ory PA, Gladman DD, Mease PJ (2005) Psoriatic arthritis and imaging. Ann Rheum Dis 64:55–57 Pai VR, van Holsbeeck M (1995) Synovial osteochondromatosis of the hip: role of sonography. J Clin Ultrasound 23:199–203 Pancione L, Gatti G, Mecozzi B (1997) Diagnosis of Hill-Sachs lesion of the shoulder. Comparison between ultrasonography and arthro-CT. Acta Radiol 38:523–526 Pasciak M, Stoll TM, Hefti F (1996) Relation of femoral to tibial torsion in children measured by ultrasound. J Pediatr Orthop 5:268–272 Patten RM, Mack LA, Wang KY et al (1992) Nondisplaced fractures of the greater tuberosity of the humerus: sonographic detection. Radiology 182:201–204 Peetrons P, Allaer D, Jeanmart L (1990) Cysts of the semilunar cartilages of the knee: a new approach by ultrasound imaging. A study of six cases and review of the literature. J Ultrasound Med 9:333–337 Peetrons P, Creteur V, Bacq C (2004) Sonography of ankle ligaments. J Clin Ultrasound 32:491–499 Popert J (1985) Rice bodies, synovial debris, and joint lavage. Br J Rheumatol 24:1–2 Postacchini F, Gumina S, Perugia D et al (1995) Early fracture callus in the diaphysis of human long bones. Histologic and ultrastructural study. Clin Orthop 310:218–228 Ptasznik R, Feller J, Bartlett J et al (1995) The value of sonography in the diagnosis of traumatic rupture of the anterior cruciate ligament of the knee. AJR Am J Roentgenol 164:1461–1463 Rao AS, Vigorita VJ (1984) Pigmented villonodular synovitis (giant cell tumor of the tendon sheath and synovial membrane). A review of 81 cases. J Bone Joint Surg Am 66:76–94 Raza K, Lee CY, Pilling D et al (2003) Ultrasound guidance allows accurate needle placement and aspiration from small joints in patients with early inflammatory arthritis. Rheumatology 42:976–979 Ricci C, Cova M, Kang YS et al (1990) Normal age-related patterns of cellular and fatty bone marrow distribution in the axial skeleton: MR imaging study. Radiology 177:83–88 Roberts D, Miller TT, Erlanger SM (2004) Sonographic appearance of primary synovial chondromatosis of the knee. J Ultrasound Med 23:707–709 Rosenthal DI, Rosenberg AE, Schiller AL et al (1983) Destructive arthritis due to silicone: a foreign-body reaction. Radiology 149:69–72 Rutten MJ, Collins JM, van Kampen A et al (1998) Meniscal cysts: detection with high-resolution sonography. AJR Am J Roentgenol 171:491–496 Saifuddin A, Burnet SJD, Mitchell R (1998) Pictorial review: ultrasonography of primary bone tumors. Clin Radiol 53:239–246 Saifuddin A, Mitchell R, Burnett SJD et al (2000) Ultrasoundguided needle biopsy of primary bone tumours. J Bone Joint Surg Br 82:50–54 Sammak B, El Bagi A, Al Shahed M et al (1999) Osteomyelitis: a review of currently used imaging techniques. Eur Radiol 9:894–900 Bone and Joint Scheel AK, Hermann KG, Ohrndorf S et al (2006) Prospective 7-year follow up imaging study comparing radiography, ultrasonography, and magnetic resonance imaging in rheumatoid arthritis finger joints. Ann Rheum Dis 65:595–600 Schmidt WA (2001) Value of sonography in diagnosis of rheumatoid arthritis. Lancet 357:1056–1057 Schneider HJ, Weiss MA, Stern PJ (1987) Silicone-induced erosive arthritis: radiologic features in seven cases. AJR Am J Roentgenol 148:923–925 Schydlowsky P, Strandberg C, Galbo H et al (1998) The value of ultrasonography in the diagnosis of labral lesions in patients with anterior shoulder dislocation. Eur J Ultrasound 8:107–113 Senall JA, Failla JM, Bouffard JA et al (2004) Ultrasound for the early diagnosis of clinically suspected scaphoid fracture. J Hand Surg [Am] 29:400–405 Sener E, Takka S, Cila E (1998) Supracondylar process syndrome. Arch Orthop Trauma Surg 117:418–419 Seymour R, Lloyd DC (1998) Sonographic appearances of meniscal cysts. J Clin Ultrasound 26:15–20 Sheldon PJ, Forrester DM, Learch TJ (2005) Imaging of intraarticular masses. RadioGraphics 25:105–119 Shetty M, Fessell DP, Femino JE et al (2002) Sonography of ankle tendon impingement with surgical correlation. AJR Am J Roentgenol 179:949–953 Skrzynski MC, Biermann JS, Montag A et al (1996) Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumours. J Bone Joint Surg Am 78:644–649 Smith DN, Lee JR (1978) The radiological diagnosis of posttraumatic effusion of the elbow joint and its clinical significance: the ”displaced fat pad” sign. Injury 10:115–119 Sofka CM, Adler RS, Cordasco FA (2002) Ultrasound diagnosis of chondrocalcinosis in the knee. Skeletal Radiol 31:43–45 Sohn C, Gerngross H, Bahren W et al (1987a) Meniscus sonography: alternative to invasive meniscus diagnosis? Dtsch Med Wochenschr 112:581–584 Sohn C, Gerngross H, Griesbeck F (1987b) Value, technique and clinical use of meniscus sonography. Unfallchirurgie 90:173–179 Sommer OJ, Kladosek A, Weiler V et al (2005) Rheumatoid arthritis: a practical guide to state-of-the-art imaging, image interpretation and clinical implications. RadioGraphics 25:381–398 Spiegel T, King W, Weiner S et al (1987). Measuring disease activity: comparison of joint tenderness, swelling, and ultrasonography in rheumatoid arthritis. Arthritis Rheum 30:1283–1288 Steinbach LC (2004) Calcium pyrophosphate dehydrate and calcium hydroxyapatite crystal deposition diseases: imaging perspectives. Radiol Clin North Am 42:185–205 Steiner GM, Sprigg A (1992) The value of ultrasound in the assessment of bone. Br J Radiol 65:589–593 Stieber JR, Dormans JP (2005) Manifestations of hereditary multiple exostoses. J Am Acad Orthop Surg 13:110–120 Stone M, Bergin D, Whelan B et al (2001) Power Doppler ultrasound assessment of rheumatoid hand synovitis. J Rheumatol 28:1979–1982 Subasi M, Kesemenli C, Necmioglu S et al (2002) Supracondylar process of the humerus. Acta Orthop Belg 68:72–75 Szkudlarek M, Court-Payen M, Strandberg C et al (2001) Power Doppler ultrasonography for assessment of synovitis in the metacarpophalangeal joints of patients with rheumatoid arthritis: a comparison with dynamic magnetic resonance imaging. Arthritis Rheum 44:2018–2023 Szkudlarek M, Klarlund M, Narvestad E et al (2006) Ultrasonography of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis: a comparison with magnetic resonance imaging, conventional radiography and clinical examination. Arthritis Res Ther 8: [Epub ahead of print] Takagishi K, Maeda K, Ikeda T et al (1991) Ganglion causing paralysis of the suprascapular nerve: diagnosis by MRI and ultrasonography. Acta Orthop Scand 62:391–393 Takahara M, Shundo M, Kondo M et al (1988) Early detection of osteochondritis dissecans of the capitellum in young baseball players. Reports of three cases. J Bone Joint Surg Am 80:892–897 Torriani M, Etchebehere M, Amstalden EMI (2002) Sonographically guided core needle biopsy of bone and softtissue tumors. J Ultrasound Med 21:275–281 Tukenmez M, Percin S, Arslan M et al (2006) Use of ultrasound for diagnosis of interposition of soft tissue in bone fracture line. Ultrasound Med Biol 32:197–200 van Holsbeeck MT, Eyler WR, Sherman LS et al (1994) Detection of infection in loosened hip prostheses: efficacy of sonography. AJR Am J Roentgenol 163:381–384 Vilanova JC, Barcelo J, Villalon M et al (2003) Imaging of lipoma arborescens and the associated lesions. Skeletal Radiol 32:504–509 Wakefield RJ, Gibbon WW, Conaghan PG et al (2000) The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum 43:2762–2770 Wang CL, Shieh JY, Wang TG et al (1999) Sonographic detection of occult fractures in the foot and ankle. J Clin Ultrasound 27:421–425 Wang XT, Rosenberg ZS, Mechlin MB et al (2005) Normal variants and diseases of the peroneal tendons and superior peroneal retinaculum: MR imaging features. RadioGraphics 25:587–602 Ward SI, Teefey SA, Paletta GA Jr et al (2003) Sonography of the medial collateral ligament of the elbow: a study of cadavers and healthy adult male volunteers. AJR Am J Roentgenol 180:389–394 Weidekamm C, Köller M, Weber M et al (2003) Diagnostic value of high-resolution B-mode and Doppler sonography for imaging of hand and finger joints in rheumatoid arthritis. Arthritis Rheum 48:325–333 Weybright PN, Jacobson JA, Murry KH et al (2003) Limited effectiveness of sonography in revealing hip joint effusion: preliminary results in 21 adult patients with native and postoperative hips. AJR Am J Roentgenol 181:215–218 Widman DS, Craig JG, van Holsbeeck MT (2001) Sonographic detection, evaluation and aspiration of infected acromioclavicular joints. Skeletal Radiol 30:388–392 Wilson DJ (2004) Soft tissue and joint infection. Eur Radiol 14:64–71 Yang PY, Wang CL, Wu CT (1998) Sonography of pigmented villonodular synovitis in the ankle joint. J Clin Ultrasound 26:166–170 Yeow K-M, Tan C-F, Chen J-S et al (2000) Diagnostic sensitivity of ultrasound-guided needle biopsy in soft tissue masses about superficial bone lesions. J Ultrasound Med 19:849–855 Zawin JK, Hoffer FA, Rand FF et al (1993) Joint effusion in children with an irritable hip: US diagnosis and aspiration. Radiology 187:459–463 185 Shoulder Individual Anatomic Sites Upper Limb 187 Shoulder 6 Shoulder Stefano Bianchi and Carlo Martinoli CONTENTS 6.1 Introduction 190 6.2 6.2.1 6.2.1.1 6.2.1.2 6.2.1.3 6.2.1.4 6.2.2 6.2.2.1 6.2.2.2 6.2.2.3 Clinical Anatomy 190 Osseous and Articular Anatomy 190 Glenohumeral Joint 190 Acromioclavicular Joint 191 Sternoclavicular Joint 193 Scapulothoracic Plane 193 Muscles and Tendons 193 Rotator Cuff 193 Biceps and Rotator Cuff Interval 196 Deltoid and Extrinsic Muscles of the Shoulder 198 6.2.3 Bursae and Gliding Spaces 199 6.2.4 Neurovascular Structures 202 6.2.4.1 Suprascapular Nerve 202 6.2.4.2 Axillary Artery and Nerve 202 6.2.5 Thoracic Outlet Structures 202 6.2.5.1 Brachial Plexus Nerves and Vertebral Anatomy 204 6.3 6.3.1 6.3.2 6.4 6.4.1 6.4.1.1 6.4.1.2 6.4.1.3 6.4.1.4 6.4.1.5 6.4.2 6.4.2.1 6.4.2.2 Essentials of Clinical History and Physical Examination 205 Rotator Cuff Pathology 206 Thoracic Outlet and Brachial Plexus Pathology 209 Normal Ultrasound Findings and Scanning Technique 210 Biceps Tendon and Rotator Cuff 210 Long Head of the Biceps Tendon 210 Subscapularis Tendon 214 Supraspinatus Tendon 216 Infraspinatus and Teres Minor Tendons 223 Rotator Cuff Interval 226 Shoulder Beyond the Cuff 227 Glenohumeral Synovial Space 227 Subacromial Subdeltoid Bursa 229 S. Bianchi, MD Privat-docent, Université de Genève, Consultant Radiologist, Fondation et Clinique des Grangettes, 7, ch. des Grangettes, 1224 Genève, Switzerland C. Martinoli, MD Associate Professor of Radiology, Cattedra “R” di Radiologia – DICMI – Università di Genova, Largo Rosanna Benzi 8, 16132 Genova, Italy 189 6.4.2.3 6.4.2.4 6.4.2.5 6.4.2.6 Acromioclavicular Joint and Os Acromiale 232 Glenoid Labrum 234 Nerves around the Shoulder 235 Brachial Plexus and Other Nerves of the Neck 235 6.5 6.5.1 6.5.1.1 6.5.1.2 6.5.2 6.5.2.1 6.5.2.2 6.5.2.3 6.5.2.4 6.5.2.5 6.5.2.6 6.5.2.7 6.5.2.8 6.5.2.9 6.5.3 6.5.3.1 6.5.3.2 6.5.3.3 6.5.4 Shoulder Pathology 242 Pathophysiologic Overview 242 Impingement and Rotator Cuff Disease 242 Instability 245 Rotator Cuff Pathology 246 Cuff Tendinopathy 247 Partial-Thickness Tears 248 Full-Thickness Tears 251 Complete and Massive Tears 256 Intramuscular Cysts 259 Cuff Tear Arthropathy 262 Acromioclavicular Cysts 265 Postoperative Cuff 267 Calcifying Tendinitis 269 Biceps Tendon Pathology 275 Biceps Tendinopathy 275 Biceps Tendon Rupture 276 Biceps Tendon Instability 279 Shoulder Pathology Beyond the Rotator Cuff 283 6.5.4.1 Pectoralis and Deltoid Lesions 284 6.5.4.2 Adhesive Capsulitis (Frozen Shoulder) 287 6.5.4.3 Glenohumeral Joint Instability 289 6.5.4.4 Humeral Head Fractures 292 6.5.4.5 Degenerative Arthropathies and Loose Bodies 296 6.5.4.6 Inflammatory Arthropathies 300 6.5.4.7 Shoulder Arthroplasty 302 6.5.4.8 Septic Arthritis and Bursitis 303 6.5.4.9 Acromioclavicular Joint Trauma and Instability 304 6.5.4.10 Sternoclavicular and Costosternal Joint Pathology 307 6.5.4.11 Quadrilateral Space Syndrome 307 6.5.4.12 Suprascapular Nerve Syndrome 309 6.5.5 Thoracic Outlet and Brachial Plexus Pathology 313 6.5.5.1 Brachial Plexus Trauma 313 6.5.5.2 Neoplastic Involvement of the Brachial Plexus 315 6.5.5.3 Parsonage-Turner Syndrome 317 6.5.5.4 Thoracic Outlet Syndrome 318 6.5.6 Shoulder Masses 321 6.5.6.1 Elastofibroma Dorsi 322 References 324 190 S. Bianchi and C. Martinoli 6.1 Introduction 6.2 Clinical Anatomy The shoulder is one of the most common applications of musculoskeletal US due to the high incidence of rotator cuff disorders related to increasing aging and sporting activities. Many papers dealing with the US scanning technique of the rotator cuff tendons have already been published in the radiological, rheumatologic and orthopaedic literature and US is now widely recognized as an accurate means to evaluate rotator cuff disease (Ptasznik 2001; Bouffard et al. 2000; Brasseur et al. 2000; Thain and Adler 1999; Bretzke et al. 1985; Collins et al. 1987; Crass et al. 1985; Hall 1986; Middleton et al. 1984; Middleton et al. 1986b; Mack et al. 1988a; Middleton 1989; Seibold et al. 1999; Teefey et al. 2000; Naredo et al. 2002). With appropriate equipment and skilled hands, this technique provides assessment of rotator cuff pathology with high sensitivity and specificity in the diagnosis of both partial and fullthickness tears with some specific advantages over MR imaging, such as higher resolution capabilities and the ability to examine tissues in both static and dynamic states and with the patient in different positions. In addition to the rotator cuff, interest is also growing in the US evaluation of a variety of abnormalities of articular and para-articular structures located in and around the shoulder area (Martinoli et al. 2003). These conditions can mimic rotator cuff tears clinically and most commonly involve the glenohumeral and acromioclavicular joints and the soft-tissue structures around the shoulder, including the joint recesses, the bone and articular cartilage, the subacromial subdeltoid bursa, the labrum, the muscles and the suprascapular and axillary nerves. In these cases, US is able to redirect the diagnosis if a complete examination of the shoulder area is performed instead of a simple rotator cuff assessment. Furthermore, we include in this chapter a specific focus on the US assessment of brachial plexus nerves and the thoracic outlet syndrome as well as the US evaluation of local tumors leading to painful shoulder or snapping scapula syndrome. As for other sites in the body, a deep knowledge of anatomy, of the proper scanning technique and of the normal imaging findings is essential in order to perform an accurate shoulder examination with US. 6.2.1 Osseous and Articular Anatomy The shoulder girdle is composed of the scapula, the clavicle and the proximal humerus acting as a single biomechanical unit. Three joints – the glenohumeral, acromioclavicular and sternoclavicular joints – and two gliding planes – the subacromial and the scapulothoracic – allow a greater range of motion in the shoulder than is possible at any other joint in the body, reaching approximately 180° in almost all directions of movement. It is clear that such a wide range of shoulder mobility depends on these joints and gliding planes working together with synchronicity, in order to permit the arm and the hand to be positioned as required in space around the body. 6.2.1.1 Glenohumeral Joint The glenohumeral joint is a “ball-and-socket” joint made up of the relatively small and flat glenoid fossa and the large and round articular surface of the humeral head (Fig. 6.1a,b). Owing to a discrepancy in the size and curvature of the joint surfaces, the glenoid cavity covers only a small portion (about one-fourth) of the humeral head. This incongruity along with the relative laxity of the joint capsule provides wide mobility but makes the joint inherently unstable and, therefore, prone to subluxation and dislocation. The articular surfaces of the humeral head and the glenoid fossa are covered by a layer of hyaline cartilage, which is thicker in its center in the humerus and thinner at its outer edges in the glenoid (Fig. 6.1b). In the humerus, the articular cartilage reaches the anatomic neck, the site of attachment of the joint capsule. Closely attached at its base to the periphery of the glenoid, a concentric rim of fibrocartilage, the labrum, widens and deepens the shallow concavity of the bony glenoid, providing the joint with inherent stability and restricting anterior and posterior excursions of the humerus. Similar to the meniscus of the knee, the glenoid labrum has a triangular shape and is in direct continuity with the hyaline cartilage of the glenoid cavity (Fig. 6.1b). A loose fibrous capsule envelops the joint, extending from the base of the coracoid through the supraglenoid region cranially, onto the anatomic neck of the humerus laterally, and into the labrum posteriorly and inferiorly, Shoulder lar joint receives cranial-caudal shearing load due to muscle action. Because the articular surfaces of this joint are obliquely oriented, the applied tension leads the clavicle to slide and displace cranially. This tendency is resisted by the coracoclavicular ligaments, damage to which allows the typical superior prominence of the clavicle end. 6.2.1.3 Sternoclavicular Joint The sternoclavicular joint is the only articulation of the shoulder girdle with the thorax. It is a shallow saddle-shaped joint between the manubrium of the sternum and the first rib medially and the medial end of clavicle laterally. The articular surfaces of the manubrium and the clavicle are, at least in part, incongruent, that of the clavicle being wider than that of the manubrium. The sternoclavicular joint houses a fibrocartilaginous disk dividing the joint space into medial and lateral cavities, each of which lined with its own synovial membrane. The costoclavicular and interclavicular ligaments reinforce the joint and oppose to its tendency to anteroposterior instability. 6.2.1.4 Scapulothoracic Plane The scapulothoracic plane separates the body of the scapula and the subscapularis muscle from the thoracic surface, consisting of the superficial aspect of the serratus anterior muscle which overlies the ribs. This gliding plane allows the scapula and the glenoid cavity to tilt anteriorly and posteriorly around the rib cage during shoulder movements. In addition, the scapulothoracic articulation has an important role in shoulder abduction. 6.2.2 Muscles and Tendons From the anatomic point of view, the muscles of the shoulder may be subdivided into two main groups: intrinsic muscles (subscapularis, supraspinatus, infraspinatus, teres minor, teres major and deltoid), which originate and insert on the skeleton of the upper limb, and extrinsic muscles, which join the upper limb with either the spine (trapezius, latissimus dorsi, levator scapulae and rhomboid) or the thoracic wall (serratus anterior, pectoralis minor and pectoralis major). The clinical relevance is for the most part related to the intrinsic muscles and especially to the rotator cuff muscles and tendons. 6.2.2.1 Rotator Cuff There are four rotator cuff muscles: the subscapularis, which is located on the anterior aspect of the shoulder; the supraspinatus, which lies on its superior aspect; and the infraspinatus and teres minor, which are situated on the posterior shoulder (Fig. 6.3). They arise from the anterior and posterior aspects of the scapula. The subscapularis muscle takes its origin from the anterior aspect of the body of the scapula. The muscle belly gives rise to a series of two or three intramuscular tendons which direct laterally to join together to form the subscapularis tendon (Fig. 6.4). This tendon inserts onto the lesser tuberosity in a broad band and acts as an adductor and internal rotator of the arm. Its more cranial fibers are intra-articular in location and some of its superficial fibers overlay the bicipital sulcus and reach the greater tuberosity, merging with the coracohumeral and transverse humeral ligament. The supraspinatus muscle originates from the supraspinous fossa of the scapula and passes underneath the acromion and above the glenohumeral joint before inserting on the upper facet of the greater tuberosity (Fig. 6.5a). It is separated from the acromion, coracoacromial ligament and deltoid muscle by the subacromialsubdeltoid bursa. Anatomic studies indicate that the supraspinatus consists of two distinct portions: ventral and dorsal (Fig. 6.5b) (Vahlensieck et al. 1994). The ventral portion takes its origin from the anterior supraspinous fossa and inserts anteriorly onto the greater tuberosity to act as an internal rotator of the arm. This ventral portion may have an accessory site of insertion onto the lesser tuberosity. The dorsal portion of the supraspinatus lies more posteriorly, with muscle fibers arising from the posterior aspect of the supraspinous fossa and spine of the scapula, assuming a strap-like configuration made up of several small tendon slips that coalesce into a broad attachment inserting more posteriorly onto the greater tuberosity. This is the portion that acts primarily as a shoulder abductor. The individual layers of the supraspinatus tendon have different mechanical properties, leading to shearing between them, and can tense and slacken depending on shoulder movements. On the posterior shoulder, the infraspinatus muscle originates from the infraspinatus fossa and 193 194 S. Bianchi and C. Martinoli a b !CR c #L !CR !CR 3UPRA3 '4 3UPRA3 * 3UB3 '4 * # )NFRA3 )NFRA3 3UB3 '4 ,4 * 4M 4M !NTERIOR 0OSTERIOR Fig. 6.3a–c. Projectional images of rotator cuff muscles and tendons as seen in an anterior (a), lateral (b) and posterior (c) view of the shoulder. Note the relationship of the supraspinatus (SupraS), subscapularis (SubS), infraspinatus (InfraS), teres minor (Tm) and long head of the biceps tendon (asterisk) with the main palpable bony landmarks of the shoulder, including the acromion (Acr), the clavicle (Cl), the greater tuberosity (GT), the lesser tuberosity (LT) and the coracoid process (C). The coracoacromial ligament is shown as a blue strip covering the biceps and the supraspinatus !CR ,4 * * ,4 3UB3 * * #L ( 3UB3 Post a 0ECT-J Ant b Fig. 6.4a,b. Subscapularis anatomy. a Gross cadaveric view through the anterior aspect of the shoulder after removal of the deltoid muscle. The muscle belly of the subscapularis (SubS) has a broad origin from the anterior fossa of the scapula and converges into a flat and wide tendon (asterisks) which inserts onto the lesser tuberosity (LT). More caudally, another broad tendon, that of the pectoralis major (PectMj), parallels the course of the subscapularis inserting onto the lateral slip of the intertubercular sulcus. b Gross cadaveric view of the same specimen shown in a after removal of the myotendinous junction of the subscapularis displays part of the humeral head (H) covered by cartilage and the glenohumeral joint cavity (star). Note the tight acromioclavicular joint (arrowheads) delimited between the acromion (Acr) and the clavicle end (Cl). Drawing at the right side of the figure indicates the position of the subscapularis (in black) relative to the other cuff tendons and the biceps (in grey) as seen on a lateral view through the shoulder gives rise to a wide tendon that extends laterally to insert onto the greater tuberosity, just posterior and inferior to the supraspinatus tendon (Fig. 6.6). The teres minor muscle, the smallest muscle of the rotator cuff, has a more oblique course than that of the infraspinatus. This latter muscle arises from a narrow strip on the lateral border of the scapula and inserts just posterior and inferior to the infraspinatus into the most caudal segment of the greater tuberosity (Fig. 6.6). The posterior infraspinatus and teres minor muscles act as external rotators of the arm. Considered as a whole, the tendons of the rotator cuff muscles are broad and relatively flat, somewhat similar to belts, and converge toward the lesser and greater tuberosity to create a hood – commonly referred to as the “rotator cuff” – that covers the humeral head anteriorly, superiorly and posteriorly (Fig. 6.7). The subscapularis tendon is sepa- Shoulder Supsp !CR 3UPRA3 !CR # #L '4 (( a b * Post Ant * Fig. 6.5a,b. Supraspinatus anatomy. a Gross cadaveric view through the cranial aspect of the shoulder after removal of the trapezius and deltoid muscles. The origin of the supraspinatus muscle (SupraS) from the supraspinous fossa of the scapula is displayed. The supraspinatus muscle traverses the subacromial space passing underneath the acromioclavicular joint (arrowheads) to converge, over the humeral head (HH), in a strong tendon which inserts into the cranial aspect of the greater tuberosity. Observe the orientation of the acromion (Acr) and clavicle (Cl) compared with the long axis of the supraspinatus. b Gross cadaveric view through the lateral aspect of the shoulder after removal of the trapezius, the deltoid and the structures forming the acromioclavicular joint. The supraspinatus is shown in its long axis. The tendon consists of a smaller anterior portion (dashed arrows) and a larger posterior portion (large arrow). Both insert into the greater tuberosity (GT). Some fibers from the anterior portion of the supraspinatus may even insert into the lesser tuberosity after crossing the interval and the biceps tendon (asterisk). Note the acromion (Acr) and the coracoid (C) on each side of the supraspinatus. Drawing at the right side of the figure indicates the position of the supraspinatus (in black) relative to the other cuff tendons and the biceps (in grey) as seen on a lateral view through the shoulder )NFRA3 * '4 * )NFRA3 4M a 4M Post Ant b Fig. 6.6a,b. Infraspinatus and teres minor anatomy. a,b Gross cadaveric view through the posterior aspect of the shoulder after removal of the deltoid muscle illustrates the separate origin of the cranial infraspinatus (InfraS) and caudal teres minor (Tm) muscles from the infraspinous fossa of the scapula. These muscles converge to insert onto the posterior aspect of the greater tuberosity (GT) by means of two separate tendons (asterisk, infraspinatus; star, teres minor). Cranial to them, note the position of the scapular spine (arrows). Drawing at the right side of the figure indicates the position of the infraspinatus and teres minor (in black) relative to the other cuff tendons and the biceps (in grey) as seen on a lateral view through the shoulder rated from the other tendons of the rotator cuff by the ligamentous complex of the rotator interval and the long head of biceps tendon, which is positioned between it and the supraspinatus. The rotator cuff tendons have a constant relationship in the different positions of the humerus and, as a result of their combined activity, play an important role as stabilizers of the humeral head in the glenoid fossa during movements of the arm (for this reason, the rotator cuff tendons have also been referred to as “active ligaments”). The abduction of the arm when the humerus is kept close to the side of the body, for example, is mainly accomplished by contraction of the deltoid muscle, but the force of this muscle is also directed cranially, so that the humeral head would displace upward. The combined action of 195 Shoulder * 3UPRA3 * '# 3UB3 'L * ( a Post Ant c b Fig. 6.8a–c. Long head of the biceps tendon anatomy. a Gross cadaveric view through the glenohumeral joint cavity reveals the glenoid cavity (GC) covered by hyaline cartilage and surrounded by a thick fibrocartilaginous labrum (arrows). The biceps tendon (asterisk) arises from the top of the glenoid rim, in continuity with the superior glenoid labrum. b Arthroscopic view of the glenohumeral joint displays the origin of the long head of the biceps tendon (curved arrow) from the superior aspect of the glenoid (Gl). H, humeral head. c Gross cadaveric view through the proximal humerus demonstrates the curvilinear course of the biceps tendon (asterisks) as it reflects over the anterosuperior aspect of the humeral head, between the supraspinatus (SupraS) and subscapularis (SubS) tendons to reach the furrow between the greater and the lesser tuberosity, the intertubercular groove. Drawing at the right side of the figure indicates the position of the long head of the biceps tendon (in black) relative to the cuff tendons (in grey) as seen on a lateral view through the shoulder 3UPRA3 * * * 3UB3 * * * a b c Fig. 6.9a–c. Rotator cuff interval anatomy. Gross cadaveric views through the humeral head. a The long head of the biceps tendon (asterisks) is restrained between the supraspinatus (SupraS) and the subscapularis (SubS) tendons by a fibrous plate which courses above it and the joint capsule as a roof (arrows), reflecting the coracohumeral ligament and some crisscrossing fibers of the supraspinatus and subscapularis. b Fine anatomic dissection of the fibrous plate covering the biceps tendon (asterisks) reveals fibers of the coracohumeral ligament (curved arrow) overlying the joint capsule (arrowhead). Note the intra-articular location of the biceps tendon. c As the dissection progresses with more extensive removal of the joint capsule, the biceps tendon (asterisks) becomes visible up to its origin from the top of the glenoid rim. On the medial side of the biceps, a well-defined fibrous band reflects the superior glenohumeral ligament (arrows). Just cranially to the intertubercular groove, this ligament passes deep to the biceps tendon and joins the medial part of the coracohumeral ligament (not shown) to form the reflection pulley referred to as the “reflection pulley,” is more flexible than the fibrous plate described above (Weishaupt et al. 1999; Werner et al. 2000; Patton et al. 2001). It assumes a crescentic shape surrounding the anteromedial aspect of the biceps tendon(Fig. 6.9c). More distally, in the proximal bicipital groove, the biceps tendon lies in close contact with the subscapularis and is stabilized by fibrous bands arising from it. The superficial component of these fibers forms the transverse humeral ligament that, in distal continuity with the coracohumeral ligament, bridges the tuberosities transforming the biceps sulcus into an osteofibrous tunnel. The transverse humeral ligament is thin and weak and its role in stabilizing the biceps just distal to its exit from the rotator interval is not considered important unless the coracohumeral ligament is torn (Patton et al. 2001; Bennett 2001). 197 198 S. Bianchi and C. Martinoli The other belly of the biceps, the short head, takes its origin from the tip of the coracoid process of the scapula, in a more medial location than the long head, in close contact with the tendon of the coracobrachialis. The long and short bellies of the biceps continue down in two separate muscle bellies which join together just distal to the middle third of the arm to form a long fusiform muscle (see also Chapter 7). In contrast to the long head of the biceps, the tendon of the short head has a straight course and is not invested by a synovial sheath. In the rare cases when it is involved in shoulder pathology, this is usually injured as a result of to trauma (i.e., anteroinferior dislocation of the shoulder) or inflammatory states. 6.2.2.3 Deltoid and Extrinsic Muscles of the Shoulder In addition to the rotator cuff muscles and the biceps, the intrinsic muscles of the shoulder include the teres major and the deltoid. The teres major muscle arises from a raised oval area at the dorsal aspect of the inferior angle and the adjacent lateral border of the scapula and inserts into the medial lip of the intertubercular groove of the humeral shaft. This muscle acts as an adductor and medial rotator of the humerus and plays a role in stabilizing the proximal humerus during abduction. Together with the tendon of latissimus dorsi, the teres major forms part of the posterior wall of the axilla. The deltoid is a thick and powerful muscle supplied by the axillary nerve which forms something of a roof over the rotator cuff tendons and the glenohumeral joint. Its name derives from the fact that its shape is similar to an inverted Greek letter delta (∆). This muscle has a wide origin from the lateral third of the clavicle, the acromion and the spine of the scapula, and inserts on the anterolateral surface of the humerus at the middle third of the arm. The action of the deltoid muscle is multifaceted. In fact, it can be a flexor and medial rotator of the humerus with its anterior fibers (in that assisting the coracobrachialis, the subscapularis and the pectoralis major), an abductor of the humerus with its middle fibers (assisting the supraspinatus) and an extensor and lateral rotator of the humerus with its posterior fibers (assisting the infraspinatus and teres muscles). The primary function of the deltoid muscle, however, is to abduct the humerus. When the supraspinatus is torn, the abduction of the arm becomes the only result of a deltoid contraction, although the upward pull of the deltoid leads to superior subluxation of the humeral head. The extrinsic shoulder muscles which join the upper limb with the spine are the trapezius, the latissimus dorsi, the levator scapulae and the rhomboids. Among them, the trapezius is the most relevant during examination of the shoulder with US. This muscle is broad, flat and overlies the posterior neck and the superior half of the posterior trunk with a triangular shape (hypotenuse facing the spine). Its name derives from the fact that it becomes a trapezius when the muscles of the two sides are considered as a single muscle. The trapezius has a wide origin from the external occipital protuberance, the ligamentum nuchae and the spinous processes of C7 to T12 vertebrae and attaches to the lateral third of the clavicle, the acromion and the spine of the scapula. The trapezius receives supply from the accessory nerve and some cervical nerves (III–VII), and has its primary function in the elevation and rotation of the scapula.The extrinsic muscles which joint the shoulder with the thoracic wall are the pectoralis major, the pectoralis minor and the serratus anterior. The pectoralis major muscle is a strong fan-shaped muscle covering most of the upper part of the chest wall and forming, with its lateral part, the anterior wall of the axilla. This muscle is separated from the more cranial deltoid by a groove, the deltopectoral triangle, which is traversed by the cephalic vein (Fig. 6.10a). The pectoralis major has three heads arising respectively from the anterior aspect of the medial half of the clavicle (clavicular head), from the manubrium and body of the sternum and the costal cartilages from II to VI ribs (sternocostal head), and from the aponeurosis of the external oblique muscle (abdominal head). The muscle fibers converge laterally into a broad trilaminar tendon which crosses the myotendinous junction of the long head of the biceps and inserts on the lateral lip of the intertubercular groove of the humerus (Wolfe et al. 1992). The tendon layers fuse and twist 90° just before the tendon insertion at the lateral lip of the bicipital groove, where the posterior lamina inserts cranially and the anterior lamina comprises the most caudal part of the enthesis (Fig. 6.10a,b). Distal to the humeral tuberosities, the pectoralis tendon participates in retaining the long head of biceps tendon close against the anterior aspect of the humeral shaft. The main action of the pectoralis major is to adduct and internally rotate the humerus. Deep to the pectoralis major, the pectoralis minor is a smaller triangular muscle which takes its origin from the III, IV and V ribs and inserts onto the medial border of the coracoid process. It stabilizes the scapula against the tho- Shoulder Del 1 2 3 1 3 2 1 2 Tendon 3 Muscle a b Fig. 6.10a,b. Pectoralis major anatomy. a Frontal photograph of the thorax taken while the patient kept the arm abducted and b schematic drawing correlation of an anterior view through the shoulder show the distinct orientation of the clavicular head (1), the sternocostal head (2) and the abdominal head (3) of the pectoralis major muscle. They converge to form a broad tendon inserting into the lateral lip of the intertubercular groove. The separate contributions to this tendon twist on each other so that at the level of the axillary fold the tendon fibers of the clavicular head pass superficial to those arising from the sternal head and insert caudally, whereas the fibers from the abdominal head have the most cranial attachment onto the humeral shaft. Note the cephalic vein (arrowheads) as it traverses the space between the deltoid (Del) and the clavicular head of the pectoralis (1) – the deltopectoral triangle – where it deepens to reach the subclavian vein racic wall and is a useful landmark for the axillary vessels and nerves as it lies just superficial to them. Figure 6.11 illustrates the anatomic relationship among intrinsic and extrinsic muscles of the shoulder and the bones by means of one-to-one correlation between cadaveric specimens and CT images. 6.2.3 Bursae and Gliding Spaces Knowledge of the anatomy of synovial recesses and para-articular bursae is an essential prerequisite to avoid misdiagnoses and pitfalls in the interpretation of pathologic findings. Three main synovial spaces are found around the shoulder area: the glenohumeral joint cavity, the subacromial-subdeltoid bursa and the acromioclavicular cavity. In normal conditions, these spaces are separated from one other because the rotator cuff is interposed between the glenohumeral joint and the subacromial-subdeltoid bursa and the acromioclavicular capsule is found between the acromioclavicular joint and the subacromial-subdeltoid bursa. In some pathologic states, such as a defect in the rotator cuff or in the inferior capsule of the acromioclavicular joint, these spaces can communicate. The subacromial space, which is located between the coracoacromial arch and the humeral head, contains the rotator cuff tendons, the long head of the biceps tendon, the subacromial-subdeltoid bursa and a variable amount of connective tissue and fat (Fig. 6.12). The subacromial-subdeltoid bursa is a large synovium-lined structure located inferior to the acromion and the coracoacromial ligament that overlies the superior aspect of the supraspinatus tendon (Fig. 6.13). It also extends medially to the coracoid (subcoracoid bursa) and anteriorly to cover the bicipital groove, whereas its lateral and posterior boundaries are more variable and reach approximately 3 cm below the greater tuberosity (Bureau et al. 1996). From the functional point-of-view, the main role of the subacromial-subdeltoid bursa is to minimize the attrition of the cuff against the coracoacromial arch and the deltoid during movements of the arm. To facilitate gliding, the bursa is surrounded by a thin cleavage plane of peribursal fat. The subcoracoid bursa may be separated from the subacromial-subdeltoid bursa to form an individual cavity. In these cases, the bursa lies just inferiorly and medially to the coracoid and may simulate a cystic mass when distended by fluid if the examiner is not aware of its existence. In addition, care should be taken not to mistake it for the adjacent subscapularis recess of the glenohumeral joint. 199 200 S. Bianchi and C. Martinoli PMj Da Da PMj C V C Cl Cl HH V G LS G SubS LS Dm HH Dm SubS Tra InfraS Tra Dp Dp InfraS Da Da Cl Da PMj Da Acr PMj Pm CB SB Pm HH SB CB HH Dm Dm Cl Acr Dm Tm G SupraS SubS Tra InfraS Tm G SubS InfraS Dp Dp Tra Da PMj Da Da Pm CB HH HH SupraS HH Tm G Dp SB CB HH C Dm C Pm Dm Da PMj SB Dm Dp PMj Da Da * HH G InfraS Da Pm * * Dp Dm Da PMj CB SB Pm SB CB HH HH Dm Dp InfraS SupraS C Dm SubS InfraS C Tm G SubS HH Dm SubS G Dm G G Tm Tm SubS InfraS Dp Dp InfraS InfraS Dp Fig. 6.11. Sectional anatomy of the shoulder. Series of cadaveric sections (left) and corresponding CT images (right) displayed in sequence from cranial to caudal. Acr, acromion; Arrowheads, cleavage plane between infraspinatus and deltoid; asterisks, rotator cuff; C, coracoid process; CB, coracobrachialis; Cl, Clavicle; curved arrow, spinoglenoid notch; Da, deltoid, anterior part; Dm, deltoid, middle part; Dp, deltoid, posterior part; G, glenoid; LS, levator scapulae; HH, humeral head; InfraS, infraspinatus; open arrow, bicipital groove; Pm, pectoralis minor; PMj, pectoralis major; SB, short head of the biceps; stars, fibrocartilaginous glenoid labrum; SubS, subscapularis; SupraS, supraspinatus; Tm, teres minor; Tra, trapezius; V, axillary vessels; white arrow, anterior bundle of fibers of the supraspinatus tendon 202 S. Bianchi and C. Martinoli In addition to the subacromial gliding plane, the scapulothoracic plane facilitates movement of the scapula relative to the chest wall and rotation of the scapula during abduction and adduction of the arm. 6.2.4 Neurovascular Structures The rotator cuff muscles receive nerve supply from the suprascapular nerve (supraspinatus and infraspinatus), the subscapular nerve (subscapularis) and the axillary nerve (teres minor). The examiner should be aware of the anatomic course of the suprascapular and axillary nerves because these nerves are vulnerable to stretching injuries and trauma and may be involved by extrinsic compression (i.e., paralabral ganglia) leading to well-categorized entrapment syndromes: the suprascapular nerve syndrome (see Sect. 6.5.4.12) and the quadrilateral space syndrome (see Sect. 6.5.4.11). The musculocutaneous nerve will be described in Chapter 7. 6.2.4.1 Suprascapular Nerve The suprascapular nerve originates from the upper trunk of the brachial plexus (C5–C6 level) and descends through the suprascapular foramen formed by the supraspinous notch of the scapula and the superior transverse scapular ligament to reach the supraspinous fossa (Fig. 6.14). Then, the nerve continues inferiorly to the supraspinatus muscle passing through the tunnel formed by the inferior transverse scapular ligament and the spinoglenoid notch to distribute in the infraspinous fossa (Fig. 6.14). In the supraspinous fossa, the suprascapular nerve gives off motor branches to the supraspinatus muscle, whereas the innervation to the infraspinatus muscle is provided by distal branches arising in the infraspinous fossa. Along its entire course, the suprascapular nerve is accompanied by the suprascapular vessels. 6.2.4.2 Axillary Artery and Nerve The axillary artery continues the subclavian artery beyond the outer border of the first rib. It traverses deep to the pectoralis minor muscle and is accompanied by the cords and distal branches of the bra- chial plexus, and the axillary vein. The axillary artery can be palpable in the inferior part of the axilla, in proximity to the inferior glenohumeral joint capsule. Distal to the lateral border of the pectoralis minor, it sends three branches: subscapular, and anterior and posterior circumflex humeral arteries. The circumflex arteries have a horizontal course and anastomose to form a circle around the anterior and posterior aspect of the surgical neck of the humerus. The anterior circumflex humeral artery is smaller than the posterior and runs deep to the coracobrachialis and the biceps and in front of the surgical neck of humerus. It gives off an ascending branch, the arcuate artery, which accompanies the long head of the biceps tendon in the intertubercular groove. The posterior circumflex humeral artery is larger and crosses the posterior wall of the axilla through the quadrilateral space in association with the axillary nerve. It is a landmark for the US detection of the nerve. The axillary nerve arises from the posterior cord of the brachial plexus (C5–C6 level) near the coracoid process and proceeds along the inferolateral border of the subscapularis muscle to curve inferior to the glenohumeral joint capsule and pass into the posterior aspect of the arm. The nerve courses in association with the posterior circumflex artery through the quadrilateral space – a squared passageway bounded by the long head of the triceps muscle medially, the surgical neck of the humerus laterally, the teres minor muscle cranially and the teres major muscle caudally (Fig. 6.15) (Loomer and Graham 1989). It has two terminal branches: anterior and posterior. The anterior branch supplies the anterior deltoid muscle and overlying skin; the posterior branch innervates the teres minor and the posterior deltoid muscle and distributes to the skin overlying the distal deltoid and the proximal triceps muscle. 6.2.5 Thoracic Outlet Structures The thoracic outlet region includes the brachial plexus nerves and the subclavian artery and vein. These neurovascular structures traverse restricted spaces in which they can be compressed, the most important of which are the interscalene triangle, the costoclavicular space and the retropectoralis minor space (Fig. 6.16a) (Demondion et al. 2000). Both subclavian artery and brachial plexus nerves pass through the interscalene triangle, a space bordered by the anterior scalene muscle anteriorly, the middle scalene muscle posteriorly and the first rib inferiorly. 206 S. Bianchi and C. Martinoli C5 a b C6 * C7 * T1 short physical examination and a review of previous imaging studies. 6.3.1 Rotator Cuff Pathology First, the examiner should check whether previous shoulder accidents, including acute trauma, chronic microtrauma, sport-related injuries and episodes of shoulder instability, have occurred. Special attention should be given to the location, type, severity and circumstances of the referred pain. Patients with rotator cuff pathology typically complain of night pain and inability to sleep on the affected side. Generally speaking, the location and irradiation of shoulder pain is not related to involvement of a specific tendon. Most patients are fairly accurate in localizing pain. Often, patients with supraspinatus tendon tear complain of pain irradiated along the lateral aspect of the upper and middle third of the arm, in proximity to the insertion of the deltoid muscle. Pain distal to the elbow level in association with paresthesias is usually related to cervical or brachial plexus disorders rather than an isolated rotator cuff pathology. Next, the patient should be asked what kind of movement produces discomfort, or the examiner should attempt to produce pain with different maneuvers. In anterosuperior impingement syndrome, pain is reported during activities or maneuvers that require active abduction and forward elevation of the arm. Exacerbation Fig. 6.17a,b. Vertebral anatomy: neural foramina. a Schematic drawing of the cervical spine illustrates the anatomic correspondence between transverse processes and nerve roots. Each root (in yellow) leaves the intervertebral foramen sliding on the transverse process of its corresponding vertebral level. Because there are eight cervical nerves and only seven cervical vertebrae, the C8 root lies at the level of the T1 vertebra. The position f the vertebral artery (in red) relative to the bony tubercles b Photograph of the cervical spine shows the typical appearance of transverse processes, which exhibit prominent anterior (star) and posterior (asterisks) tubercles. Note the absence of the anterior tubercle at C7 level, whereas the lateral aspect of T1 is flat without any bony prominence of pain can also be noted during maximal elevation of the arm and internal rotation in posterosuperior impingement and during maximal internal rotation and adduction of the arm in anteromedial impingement. A basic physical examination of the affected shoulder for rotator cuff assessment is part of the routine US study (Moosikasuwan et al. 2005). The examination begins with observation on how the patient is undressing, because the act of slipping the shirt off is an indicator of the full range of movements that the patient is able to perform and is typically limited in rotator cuff disease. Then, the overall range of shoulder motion can be assessed by asking the patient to place the dorsal aspect of the hand behind the back as cranially as possible, between the scapulae (internal rotation and extension), and behind the neck (external rotation and abduction). With the patient seated, the affected shoulder is inspected and simultaneously palpated by the examiner. Swelling and tenderness around the shoulder, especially when located over the anterior aspect of the joint, more likely reflects an effusion in the subacromial subdeltoid bursa rather than an intra-articular effusion. In chronic cuff tears, palpable crepitus over the cranial aspect of the shoulder can be produced by rotation of the shoulder with the arm in 90° of elevation. A localized soft-tissue lump over the cranial aspect of the acromioclavicular joint is often related to a cyst arising from the acromioclavicular joint which develops following massive rotator cuff tear (Geyser Sign). Care should be taken Shoulder to correlate it with the tear because patients usually take medical advice for the lump and not for the underlying disorder (Fig. 6.18a). Ecchymosis over the anterior aspect of the shoulder and arm is typically correlated with an acute tear of the long head of biceps tendon but it may also be appreciated in cases of traumatic enlargement of a previous tear of the supraspinatus or subscapularis tendons. Except for the subscapularis, atrophy of rotator cuff muscles can be appreciated by inspection and palpation. Although the occurrence of a bilateral cuff rupture should be always kept in mind, comparative examination of the two shoulders for asymmetry may help the examiner to evaluate muscle atrophy. On the lateral shoulder, deltoid atrophy may reveal wasting from axillary neuropathy or from previous surgery with deltoid detachment for rotator cuff repair. On the posterior shoulder, wasting of the infraspinatus and teres minor muscles may derive from chronic rotator cuff tears, disuse, glenohumeral arthritis and suprascapular nerve palsy (Fig. 6.18b). In patients with biceps tendon tear, the retracted muscle can be palpated as a soft-tissue lump over the anterior aspect of the middle third of the arm, possibly mimicking a hypertrophied muscle, the so-called Popeye sign (Fig. 6.18c). Detection of the retracted biceps can be difficult in obese patients. Rotator cuff tendons may be palpated systematically for focal tenderness starting anteriorly with the subscapularis and the biceps and then moving posteriorly to evaluate the insertions of the supraspinatus and infraspinatus into the superior and posterior facets of the greater tuberosity. Finally, the acromioclavicular a b joint is assessed by applying a firm pressure over it with the thumb. If this pressure generates pain, the ache should be compared with that recalled by the patient to ensure matching of symptoms. A painful acromioclavicular joint may indicate an arthritic or traumatized joint. Acromioclavicular joint separation is noted by the painful prominence of the distal end of the clavicle associated with excessive mobility of the joint. The overall range of shoulder motion is frequently affected in patients with rotator cuff disorders. In these cases, examination of passive motion may be helpful to differentiate a real impingement syndrome with rotator cuff pathology from adhesive capsulitis (frozen shoulder). Whereas in rotator cuff disease without secondary adhesive capsulitis the range of motion is restricted during active but not passive motion, shoulder motion in adhesive capsulitis is always lost. In this disorder, the motion is for the most part restricted in external rotation tested in both 0° and 90° of abduction, although all directions are usually involved to some extent. Specific clinical tests to evaluate the strength of individual rotator cuff tendons have been described in the orthopaedic literature (Hawkins and Hobeika 1983). Supraspinatus function can be evaluated by testing the patient’s ability to resist a downward force applied to the humerus with the elbow extended and the arm in a position of internal rotation and 45° of forward flexion (Fig. 6.19a). If positive, the test generates pain, weakness or both symptoms. Then, two impingement maneuvers, which may be performed with the patient standing or supine, may help the c Fig. 6.18a–c. Physical findings around the shoulder. a Geyser sign. Photograph of the right shoulder shows a soft-tissue lump (arrow) over the cranial aspect of the acromioclavicular joint reflecting a cyst. This sign is pathognomonic of a complete tear of the supraspinatus tendon. b Wasting of the supraspinatus and infraspinatus resulting from suprascapular nerve palsy. Compared with the opposite side, note the loss in bulk of muscles contained in the supraspinous (arrowhead) and infraspinous (arrow) fossa of the right shoulder. c Popeye sign. Photograph shows a prominent lump (arrow) over the anterior aspect of the middle arm related to a ruptured long head of the biceps tendon. This sign results from the distal retraction of the muscle belly because of the tendon tear 207 208 S. Bianchi and C. Martinoli a b Fig. 6.19a,b. Clinical tests for assessing the strength of rotator cuff muscles. a Supraspinatus strength is tested with the patient’s arm in a position of 60° of forward elevation with the shoulder internally rotated and the elbow extended. A downward force (arrow) applied by the examiner is resisted by the patient. b A lift-off test is performed to evaluate subscapularis strength. The patient is asked to actively lift (arrow) the hand off of the lumbar region examiner to assess shoulder pain related to rotator cuff disease or biceps tendinitis. The first, which is referred to as Neer’s test, is obtained with maximal passive glenohumeral forward flexion with the shoulder in neutral rotation to obtain impingement of the supraspinatus and the biceps against the anterolateral margin of the acromion (Neer 1983). The second, Hawkins’ test, is performed with 90° forward flexion, slight horizontal adduction and internal rotation to compress the insertion of the supraspinatus and the subacromial bursa under the coracoacromial ligament (Hawkins and Hobeika 1983). The internal rotation of the shoulder reflects the action of the subscapularis tendon and can be assessed by means of the “lift-off test” (Gerber and Krushell 1991). To avoid the contribution of other muscles (i.e., pectoralis major, teres major) to internal rotation, this test measures the strength of the subscapularis in isolation by positioning the forearm behind the patient’s back. The patient is then asked to lift her or his hand off of the lumbar region, an action that requires the active contraction of the subscapularis (Fig. 6.19b). Inability to perform this maneuver indicates subscapularis tear. The combined action of the infraspinatus and teres minor cannot be differentiated during external shoulder rotation. The ability of these muscles considered as a whole can be estimated using the “horn-blower sign,” in which the patient’s arm is passively brought into 90° of abduction and full external rotation. The examiner holds the elbow while the patient is asked to maintain maximal external rotation. Any loss of active external rotation represents weakness of the posterior rotator cuff, whereas failure to maintain full external rotation of the abducted arm suggests a large posterior rotator cuff defect. Posterior deltoid contraction could give a false negative Hornblower‘s sign. Performing the test bilaterally is useful to avoid this potential pitfall (Hawkins and Hobeika 1983). Although strength tests are useful to support the clinical suspicion of rotator cuff disease, they have been found to have varying sensitivity and specificity in the diagnosis. Sonologists must at least be familiar with them because the orthopaedist can cite these maneuvers in the request for a US examination. In patients who have undergone previous surgery for rotator cuff tears, the examiner should spend some additional time reviewing the surgical report before starting the US examination, because surgical procedures can alter the local anatomy. One should also keep in mind that the surgical intervention may have consisted of acromioplasty and bursectomy without any suture of the torn tendons. In these cases, discontinuity of the rotator cuff must not be misinterpreted as a retear. Although conventional radiography is somewhat limited in evaluation of the rotator cuff and its findings become pathognomonic only in patients with chronic tear, previous imaging studies should be reviewed before starting the US examination. Advising the patient or the referring physician the day before the examination will usually ensure these studies available. Standard radiographs are the most common imaging studies performed before US examination. Pathologic changes associated with rotator cuff disorders include intratendinous or bursal cal- Shoulder cifications, acromial spurs, erosions and sclerosis of the tuberosities, a reduced subacromial space with superior subluxation of the humeral head and a lateral downsloping or low-flying acromion. Inferior humeral osteophytes, osteoarthritic changes and undersurface osteophytes of the acromioclavicular joint, and bony changes related to previous surgical procedures can be appreciated as well. In anterior shoulder dislocation, a compression fracture on the posterolateral aspect of the humeral head – commonly referred to as the Hill-Sachs fracture – is seen as the result of impaction of the displaced humeral head against the anterior aspect of the glenoid rim. Similarly, in the setting of posterior shoulder dislocation, a compression fracture on the anteromedial aspect of the humeral head, the so-called reversed Hill-Sachs or McLaughlin lesion, can be encountered due to the impaction of the humerus against the posterior glenoid rim. Both abnormalities can be detected on plain films and should redirect the US examination toward an instability problem. The examiner should be aware that the availability of standard radiographs is time-saving and essential for the adequate interpretation of troublesome US images related to disorders that can be more obvious on plain films. 6.3.2 Thoracic Outlet and Brachial Plexus Pathology Thoracic outlet pathology is conventionally divided into two main types – vascular and neurogenic – although vascular and nervous entrapment signs and symptoms, such as pain, numbness, tingling, weakness and other disturbances in the upper limb, often coexist as a single clinical picture. In general, brachial plexus nerves are more often involved than subclavian vessels. Brachial plexus syndromes often resemble more distal entrapment neuropathies and are often mistaken for a lower level (i.e., carpal tunnel, cubital tunnel) compression. To distinguish them from distal entrapment of individual nerves, one should consider that sensory and motor system abnormalities encountered in brachial plexus pathology are, in general, not clearly attributable to a single nerve. Patients with upper plexus involvement (C5–C7 level) complain of pain in the region of the trapezius and shoulder, with symptoms radiating along the lateral aspect of the extremity down to the territory of innervation of the median nerve. Motor symptoms include weakness of shoulder abduction (involvement of the deltoid and supraspinatus) and external rotation (involvement of the infraspinatus and teres minor). In overt cases, patients exhibit an extended and internally rotated arm, a pronated forearm and a flexed wrist. On the other hand, patients with lower plexus involvement (C8–T1 level) feel pain in the supraclavicular region, in the back of the neck and in the axilla down to the area of the hand innervated by the ulnar nerve, with sensory disturbances in the fourth and fifth fingers. In longstanding disease, muscle weakness may involve the ulnar-innervated muscles of the hand and forearm (flexor carpi ulnaris) resulting in a claw-hand deformity. Trauma to the neck, shoulder girdle and even the upper limb is often associated with the onset of a thoracic outlet syndrome related to brachial plexus involvement. Brachial neuritis (Parsonage Turner syndrome) may also be suspected when the onset of shoulder pain and disability follows a viral illness or unrelated previous surgical procedure. Apart from nerves, if the subclavian vein is selectively compressed, symptoms are mostly related to increased venous pressure in the upper extremity. Entrapment of the subclavian artery is rare and usually presents with arterial insufficiency and a cold extremity. When examining a patient with suspected thoracic outlet syndrome, objective findings are, in many cases, few. The physical examination should include general evaluation of the musculoskeletal and vascular systems of the upper extremity looking for temperature changes and areas of muscle atrophy. The supraclavicular and infraclavicular area should be palpated for tenderness and a radiating Tinel sign. Several provocative tests may be performed both before and during the US examination, including the Adson maneuver (Adson and Coffey 1927), the hyperabduction test or Wright maneuver (Wright 1945), the Eden maneuver, or military position (Eden 1939), and the Roos maneuver (Roos 1976). In particular, the Adson maneuver is performed by holding the patient’s arm down and checking the radial pulse while the patient inspires deeply and keeps the head extended and turned toward the involved extremity. The Wright maneuver is obtained with the patient seated or standing and the shoulder hyperabducted and rotated externally. If the test is positive, the patient complains of paresthesias in the extremity and any change in arterial pulse. The Roos test is performed by means of a 3-minute abduction with exercise (clenching fists). While performing these tests, the examiner must be aware that positive findings may also occur in normal subjects. 209 210 S. Bianchi and C. Martinoli 6.4 Normal Ultrasound Findings and Scanning Technique When examining the shoulder with US, appropriate positioning of the patient is essential to allow the examiner access to the patient’s shoulder and the US keyboard simultaneously. Positioning should be comfortable for the patient and the examiner, and allow examination of the shoulder in as short a time as possible. Different patient positions have been reported for the US examination of the shoulder, likely reflecting the preference and habit of the examiner. Many sonologists examine the shoulder using an anterior approach, standing in front of the patient while he or she is seated on the examination bed. Generally speaking, the anterior approach is easier to learn for the beginner and offers greater opportunity to correlate US images with probe positioning based on skin landmarks. At least in our opinion, this is particularly true while evaluating anterior structures, such as the biceps tendon and the rotator interval. Other sonologists prefer to perform the examination by a dorsal approach with the patient seated on the bed or on a revolving stool. This approach allows the examiner to perform a brief physical examination and prevents excessive backward curvature of the spine, thus improving the US assessment of the supraspinatus (Lyons and Tomlinson 1992). In addition, the dorsal approach makes guiding the patient to assume different arm positioning easier and increases stability during scanning (Allen and Wilson 2001). Depending on the examiner’s and patient’s height, an appropriate adjustment of the bed level allows a more comfortable examination, while a revolving stool makes the approach to the different aspects of the joint easier. An additional technique in which the patient is examined supine with the arm hanging down the side of the bed has been described for a better evaluation of the internal structure of the supraspinatus (Turrin et al. 1997; Turrin and Capello 1997). The US examination is well tolerated by patients and even preferred to MR imaging (Middleton et al. 2004A). The main reasons for this preference probably include a shorter examination time, the lack of discomfort related to positioning within the magnet, and a free environment with contact with the medical personnel and absence of the sense of isolation and anxiety which is typically produced during MR imaging examinations (Middleton et al. 2004a). Because most indications for shoulder US are concerned with the rotator cuff, most of this section will focus on the examination of these tendons. Before discussing the normal US anatomy and examination technique of the cuff, some important points should be taken into account. a. Rotator cuff US needs a rigorous standardized technique to obtain systematic and comprehensive assessment of the individual tendons within a short examination time. b. While examining the rotator cuff with US, it is essential to perform the assessment of each of the four tendon–muscle units and the biceps tendon by means of scanning planes oriented according to their long-axis and short-axis. Although this approach might seem boring and time-consuming, it is the only way that ensures subtle pathologic findings are not missed. This is true even for skilled examiners. c. Each tendon should be evaluated systematically from its myotendinous junction to the bony insertion and in the proper position during maximal tendon stretch so that the bony structures that limit US access, such as the acromion and the coracoid process, are moved away from it. 6.4.1 Biceps Tendon and Rotator Cuff Apart from the type of approach used, we perform a standard US examination of rotator cuff tendons starting with the long head of the biceps tendon as the initial key reference. The examination of the biceps is then followed by scanning the anterior (subscapularis), superior (supraspinatus) and posterior (infraspinatus and teres minor) aspects of the rotator cuff. To avoid confusion with the spatial planes of the body, we prefer to use the terms “long-axis” and “shortaxis” rather than “longitudinal” and “transverse” to indicate the orientation of the scanning plane according to the axis of the examined structure. 6.4.1.1 Long Head of the Biceps Tendon In most patients, the biceps tendon is assessed with the arm in neutral position. In most instances, a slight internal rotation of the arm can be helpful for a more accurate assessment. The first landmark to identify is osseous: the intertubercular sulcus, which is also referred to as the “bicipital groove.” Shoulder It lies between the lesser and the greater tuberosities and has a well-defined concave appearance (Fig. 6.20a,b). Once the groove is found, one should check its appearance, looking at its depth and presence of focal cortical erosions (Fig. 6.20c–e). The two tuberosities do not have the same appearance, the lesser having a more pointed and the greater a more rounded look. Care should be taken to examine the content of the bicipital groove. This cavity holds the long head of the biceps tendon invested by its proper synovial sheath, along with the ascending branch of the anterior circumflex artery, located on the lateral side of the tendon, and fatty tissue (Fig. 6.21). Visualization of the arcuate artery depends on its size and flow volume. In younger patients, it is almost invariably found. Awareness of its presence can avoid misdiagnosis of tendon sheath hyperemia. The transverse humeral ligament appears as a very thin hyperechoic band overlying the sulcus (Fig. 6.20b). GT LT GT a * LT b a GT b GT c Short-axis scans are the most useful planes for evaluating the biceps tendon. Because this tendon courses from cranial to caudal and from superficial to deep, a careful scanning technique is needed to distinguish it from both the adjacent fat (which is not affected by anisotropy and always appears hyperechoic) and the sheath fluid (Middleton et al. 1985). In fact, if the transducer is not maintained parallel to the tendon, this may appear artifactually hypoechoic mimicking fluid (Fig. 6.22a–c). Often, the transducer must be rocked slightly to ensure the best visualization of the fibrillar echotexture. In particular, a slight tilting of the probe (short-axis scans) or a slight pressure exerted with its caudal end on the skin (long-axis scans) may be helpful for this purpose (Fig. 6.22c,d,f). Once the tendon has reached maximum reflectivity, the orientation of the transducer should maintained constant while shifting the probe up and down. Cranially, at the intra-articular level, the biceps tendon assumes a GT LT LT LT d e Fig. 6.20a–e. Bicipital groove. a Anterior view of the proximal humerus from a caudal perspective reveals the bicipital groove (arrowhead) lying between the greater (GT) and the lesser (LT) tuberosities. b Corresponding 12–5 MHz transverse US image demonstrates the normal biceps tendon (asterisk) as a rounded echogenic structure contained within the bicipital groove (arrowheads). Over the tendon, a straight hypoechoic layer (curved arrow) related to the transverse humeral ligament bridges the greater (GT) and the lesser (LT) tuberosities transforming the bicipital sulcus into an osteofibrous tunnel. The US appearance of the intertubercular sulcus closely resembles the outline of bone visible in a. In this case, it has normal size and shape. c Congenital shallow intertubercular sulcus. The groove is wider and has flat walls. The depth of the groove can be measured on short-axis planes. A line (a) is first drawn tangential to the tuberosities; then, the distance (b) between this line and the deepest point of the groove is calculated: a distance ⱕ3 mm indicates a shallow sulcus and can be considered a predisposing cause for biceps tendon instability. d,e Bicipital groove osteophytes leading to an abnormally narrow sulcus and even to a true bicipital tunnel. Bony proliferation in this area may be associated with attrition of the tendon causing progressive narrowing of the biceps (arrow) and, perhaps, its rupture 211 Shoulder of the biceps should be always evaluated because a tear or calcification may occur at this level. In the evaluation of the long head of the biceps tendon, the importance of long-axis scans is limited to confirm the tendon integrity in doubtful cases based on visualization of its fibrillar echotexture. The pectoralis tendon is a broad flattened tendon which crosses anterior to the biceps to insert into the lateral lip of the intertubercular groove, receiving contributions from the three heads of the muscle: clavicular (superficial layer), sternal (intermediate layer) and abdominal (deep layer). When the arm is internally rotated, this tendon assumes an arcuate course over the biceps, whereas it becomes straight in external to the humeral tuberosities, the long head of the biceps tendon lies in front of the proximal humeral metaphysis. It is important to examine this level because even small effusions tend to fill the most dependent portion of the tendon sheath (Fig. 6.23). In this area, a small amount of intrasheath fluid, not sufficient to encircle the tendon, is a normal finding and should not to be indicated in the report. More caudally, the myotendinous junction of the biceps tendon can be appreciated as a gradual decrease in the size of the tendon and a parallel increase in the size of the muscle. It lies deep to the tendon of the pectoralis major and lateral to the short head of the biceps muscle (Fig. 6.24a). The distal portion SupraS Capsule InfraS GT * SubS ** bt * H Synovium Hs a Hs b bt * c * * bt * d Fig. 6.23a–d. Biceps tendon sheath. a Schematic drawing of a coronal view through the anterior shoulder demonstrates the relationships of the long head of the biceps tendon (bt) with the rotator cuff tendons, including the subscapularis (SubS), the supraspinatus (SupraS) and the infraspinatus (InfraS). In its intra-articular portion, the biceps tendon is overlaid by the capsule of the glenohumeral joint. More distally, the biceps enters the intertubercular sulcus, coursing in between the greater (GT) and the lesser (LT) tuberosities. At this level, it is invested by a sheath of synovial membrane which represents an anterior extension of the glenohumeral joint. b Short-axis 12–5 MHz US image over the biceps tendon (bt) obtained approximately 2 cm below the bicipital groove with c transverse T2-weighted MR imaging correlation reveals the sheath of the biceps tendon distended by fluid (asterisks). Note the mesotendon (arrowhead) connecting the visceral and parietal layers of the synovial envelope. Hs, humeral shaft. d Long-axis 12 –5 MHz US image over the extra-articular long head of the biceps tendon (bt) demonstrates a small amount of sheath effusion (asterisks). The overall longitudinal extension of the biceps tendon sheath is shown 213 214 S. Bianchi and C. Martinoli Hs b Hs SubS b PMj b Hs b a b c Fig. 6.24a–c. Pectoralis major tendon. a Schematic drawing of a coronal view through the anterior shoulder and the upper arm shows the humeral insertion of the pectoralis major (PMj) and the relationships of its tendon with the myotendinous junction of the long head of the biceps brachii muscle (b) and the more cranial subscapularis (SubS). Owing to its broad insertion, the pectoralis tendon is best examined by means of transverse planes shifting the probe up and down over it (arrows). The insert on the upper right side of the image illustrates the relationship of the pectoralis major tendon (arrowheads) with the underlying myotendinous junction of the biceps brachii (b). Hs, humeral shaft. b,c Transverse 12–5 MHz US images obtained on the long axis of the pectoralis major tendon (arrowheads) while the arm is kept b in external and c internal rotation. In external rotation, the tendon has a straight course pushing the myotendinous junction of the biceps toward depth. In internal rotation, the tendon is relaxed and tends to assume an arcuate course over the biceps. Note the more anterior position of the biceps relative to the pectoralis insertion on the humeral shaft (Hs) rotation (Fig. 6.24b,c). It is best evaluated with the arm abducted and externally rotated to stress the myotendinous region (Rehman and Robinson 2005). US is able to distinguish the three heads of the pectoralis major muscle but not the individual components of the tendon because the three tendon layers blend with no significant intervening connective tissue (Rehman and Robinson 2005). 6.4.1.2 Subscapularis Tendon After the biceps has been examined, the patient is asked to rotate the arm externally in order to evaluate the subscapularis tendon on the anterior aspect of the shoulder. This maneuver stretches the subscapularis and helps to move its tendon from underneath the coracoid process into a more superficial position for an adequate examination (Fig. 6.25). Dynamic scanning during passive internal and external rotation with the arm adducted may also be helpful to assess the integrity of the subscapularis. While the arm is in external rotation, the examiner must remember to neutralize the tendency for the patient to lift and abduct the elbow from the lateral chest wall. This can be easily avoided by keeping the hand in supination while rotating the arm externally. Conditions limiting external rotation, such as shoulder casting, may lead to a poor delineation of the anterior structures. Any of these constraints should be indicated in the report. When examined on its short axis, the multipennate structure of the normal subscapularis tendon creates a series of hypoechoic clefts among the fascicles that should not be confused with tendon tears (Fig. 6.26). In fact, these cleft are related to muscle fibers interposed with tendon fascicles. On short-axis scans, the lesser tuberosity has a flat appearance ending in a smooth downsloping contour located just caudal to the tendon insertion (Fig. 6.26). Such a bony landmark would be helpful when assessing partial tears 217 Shoulder Deltoid Deltoid SubS SubS Co HH HH a b Fig. 6.28a,b. Short head of the biceps tendon, coracobrachialis and pectoralis minor. a,b Transverse 12–5 MHz US images obtained a at the level of the coracoid process of the scapula and b approximately 2 cm caudal to it. In a, the relationship of the coracoid (Co) with the humeral head (HH), the subscapularis tendon (SubS) and the deltoid muscle are illustrated. The coracoid is easily identified with US owing to its medial position relative to the humeral head and the curvilinear hyperechoic appearance of its bony surface. In b, three individual structures are seen arising from the coracoid. From lateral to medial, they are: the hyperechoic tendon of the short head of the biceps (curved arrow), the hypoechoic myotendinous junction of the coracobrachialis (straight arrow) and that of the pectoralis minor (arrowheads) Del Acr GT a Del Acr GT b Fig. 6.29a,b. Appropriate positioning for visualization of the supraspinatus tendon. Long-axis 12–5 MHz US images obtained over the supraspinatus tendon (arrowheads) with the arm a in neutral position and b flexed at the elbow while keeping the hand placed over the posterior iliac crest and the elbow directed posteriorly (modified Crass or Middleton position). The supraspinatus tendon appears as a thick echogenic structure (arrowheads) emerging from underneath the acromion (Acr) to insert into the greater tuberosity (GT). In b, the acromion is moved away from the tendon and can be depicted in its full extent, even including visualization of its myotendinous junction. The gray vertical bars indicate the respective tendon extension as it appears in the US images. In this position, it is important to realize that the long axis of the tendon is oriented approximately 45° between the sagittal and coronal planes. Del, deltoid. The inserts at the upper left side of the figure indicate arm positioning 224 S. Bianchi and C. Martinoli bt a b Fig. 6.38a,b. Coracoacromial ligament. a Schematic drawing of a lateral view through the rotator cuff demonstrates the proper transducer positioning to examine the coracohumeral ligament. It has to be shifted proximally when oriented in the short axis over the anterior supraspinatus tendon (SupraS). Acr, acromion; C, coracoid; SubS, subscapularis tendon. b Corresponding 12–5 MHz US image demonstrates the coracohumeral ligament (arrowheads) as a slightly convex fibrillar band which overlies the myotendinous junction of the supraspinatus and the biceps tendon (bt). Note that the supraspinatus exhibits two discrete origins for the anterior cylindrical bundle (large arrow) and the posterior flat tendon component (thin arrows) forearm in a supination on the ipsilateral thigh or with the patient’s hand on the opposite shoulder. We believe the first approach works better as it avoids repositioning of the tendon too anteriorly, which may make it difficult to separate its fibers from the supraspinatus. Using such a posterior approach, the spine of the scapula may be a useful landmark to distinguish these tendons (Fig. 6.39). First, one should palpate the scapular spine and place the transducer over it, in a more medial position relative to the greater tuberosity (Fig. 6.39a): shifting the transducer up on the sagittal plane, the supraspinous fossa and the supraspinatus muscle can be found deep to the trapezius muscle (Fig. 6.39b). After that, the infraspinatus and teres minor muscle can be depicted as individual structures deep to the deltoid muscle by shifting the transducer down to the scapular spine (Fig. 6.39c). Each of these muscles is characterized by a central aponeurosis and should be evaluated and compared for size and echogenicity (Fig. 6.40a). The teres minor muscle is smaller than the infraspinatus and has a rounded cross-section while the infraspinatus is more oval in appearance. In some cases, these muscles are fused together and exhibit a common elongated central aponeurosis (Fig. 6.40b). Systematic scanning over these muscles may help to rule out echotextural changes related to tendon tears and nerve pathology. In fact, certain shoulder diseases, such as suprascapular neuropa- thy, can be recognized on the basis of muscle atrophy detected in these scans. After scanning the muscles, the transducer is swept toward the greater tuberosity on sagittal planes and the two tendons can be appreciated as individual hyperechoic structures arising from the respective muscles, the larger and more cranial being the infraspinatus, and the smaller and caudal, the teres minor (Fig. 6.41). Often, the profile of the posterior aspect of the greater tuberosity can demonstrate two separated facets at the insertion of these tendons (Fig. 6.41). On long-axis scans, the infraspinatus tendon appears as a thick beak-shaped structure coursing deep to the deltoid and superficial to the posterior aspect of the humeral head, the posterior labrum and the bony glenoid (Fig. 6.42a). The teres minor tendon, the smallest tendon of the cuff, has a more oblique course than that of the infraspinatus and arises eccentrically with respect to the muscle (Fig. 6.42b). Therefore, the probe should be oriented obliquely to image it in its long axis. Each tendon must be examined separately. Care should be taken to evaluate the infraspinatus tendon up to its insertion. In fact, at least when the arm is kept in internal rotation, the tendon may project over the lateral rather than the posterior aspect of the shoulder. Dynamic scanning during passive internal and external rotation with the arm adducted may help the examiner to assess the insertion level and the integrity of both tendons. 226 S. Bianchi and C. Martinoli 1 2 2 1 a b Fig. 6.41a,b. Normal infraspinatus and teres minor tendons. a Sagittal 12–5 MHz US image over the short axis of the infraspinatus and teres minor tendons. These tendons can be seen arising from, respectively, the larger infraspinatus muscle (open arrows) and the smaller teres minor muscle (white arrow). Note two separate facets (1, 2) in the posterior aspect of the greater tuberosity (dashed line) for the insertion of these tendons. b Photograph over the posterior aspect of the humeral head illustrates the shape of the greater tuberosity (dashed line) as well as the two facets (1, 2) for tendon insertion depicted with US. The insert at the upper left side of the figure indicates transducer positioning a Deltoid Deltoid b HH * a HH Gl b Fig. 6.42a,b. Normal infraspinatus and teres minor tendons. a,b Transverse 12–5 MHz US images over the long axis of a the infraspinatus and b the teres minor tendons. a The infraspinatus tendon arises within the muscle from a thick central aponeurosis (arrowhead) and appears as a thick beak-shaped structure (arrows) coursing deep to the deltoid muscle and superficial to the posterior aspect of the humeral head (HH), the posterior labrum (asterisk) and the bony glenoid (Gl). b Immediately caudal to it, the teres minor tendon (arrows) appears as a smaller fibrillar structure arising eccentrically relative to the muscle belly (arrowhead). The inserts at the upper left side of the figure indicate the respective transducer positioning. Note the slightly oblique orientation of the probe needed to image the teres minor tendon along its major axis 6.4.1.5 Rotator Cuff Interval Before entering the bicipital groove, the biceps tendon passes across the “rotator cuff interval”, a free space delimited by the subscapularis and supraspinatus tendons. In this space, the biceps tendon is retained in its proper location by the coracohumeral ligament, which courses above it as a roof, and by the superior glenohumeral ligament (Fig. 6.43a, 6.44a). At US, the coracohumeral ligament can be appreciated as a thick homogeneous echogenic band of tissue, tightened between the subscapularis and the supraspinatus and located just over the biceps (Fig. 6.43b). Often, a thin hypoechoic layer is seen arising from the deep edge of the supraspinatus tendon and intervening between the ligament and the biceps tendon, a finding that may represent the joint capsule (Fig. 6.43b). The coracohumeral ligament is best depicted on short-axis scans while the arm is kept in posterior flexion, because this position causes maximal opening of the rotator cuff interval, stretches the biceps against the humeral cartilage and tightens the ligament. Careful scan- 228 S. Bianchi and C. Martinoli CHL SupraS * SGHL GT SupraS LT * SubS SubS a b SupraS GT ** LT SubS GT * LT c SubS d Fig. 6.44a–d. Rotator cuff interval: intermediate and distal levels. a Schematic drawing with b corresponding transverse 12–5 MHz US image of the intermediate level of the rotator cuff interval. The medial cord of the coracohumeral ligament (CHL) and the superior glenohumeral ligament (SGHL) form an anterior sling (arrowheads) around the biceps tendon (asterisk), the so-called reflection pulley. In the US image, note that the biceps is elevated at this site relative to the bone and assumes an oblique orientation due to the pulley which surrounds it with a crescentic shape. The deep fibers of the pulley that infiltrates the undersurface of the biceps tendon are part of the superior glenohumeral ligament. SubS, subscapularis; SupraS, supraspinatus. c Schematic drawing with d corresponding transverse 12–5 MHz US image of the distal level of the rotator cuff interval. In the proximity of the bicipital groove, the biceps tendon (asterisk) lies in contact with the lesser tuberosity (LT) and the subscapularis tendon (SubS) and is stabilized by fibrous bands arising from it. Arrowheads indicate the insertion of the supraspinatus tendon into the greater tuberosity (GT) of movement of the arm. The large axillary recess arises, for instance, from a deep folding of the capsule that permits a complete elevation of the arm without stretching the inferior capsule. The same is true for the anterior and posterior recesses, which allow maximal external and internal rotation of the arm. In normal states, the small amount of synovial fluid contained in the joint space cannot be recognized with US. On the other hand, US has high sensitivity for appreciating even a minimal amount of pathologic fluid inside the main synovial recesses (i.e., the dependent axillary pouch, the posterior and anterior recesses and the sheath of the long head of the biceps tendon). Although a caudal approach through the axilla has been described to evaluate the axillary pouch, posterior transverse scans are usually preferred for better accessibility. Once the teres minor tendon is localized, the transducer is shifted more caudally to investigate the space intervening between the humeral metaphysis and the inferior neck of the scapula, where the axillary pouch lies. If distended by considerable effusion, this pouch is visible as a fluid-filled area. The posterior recess is best examined on transverse scans by placing the transducer over the infraspinatus tendon (Fig. 6.45). An effusion filling the posterior recess appears as a hypoanechoic crescent surrounding the tip of the posterior labrum. In larger effusions, the infraspinatus tendon can be seen displaced posteriorly by the fluid contained in the recess. In doubtful cases, the examiner can induce changes in the shape of the recess by passively moving the patient’s arm externally and internally, which results in reduced/increased tension of the posterior capsule and the overlying infraspinatus. Due to the lack of intervening vessels and easy accessibility, procedures of needle aspiration or injection in the posterior recess can be safely performed under US guidance while the patient is seated or prone (Fessell et al. 2000; Zwar et al. 2004). This recess can be selected for a safe USguided needle placement for shoulder arthrography (Cicak et al. 1992; Valls and Melloni 1997). Shoulder InfraS HH * Gl a * b Fig. 6.45a,b. Posterior recess of the glenohumeral joint. a Transverse 12–5 MHz US image over the posterior shoulder with b gradient-echo T2*-weighted MR imaging correlation demonstrates a hypoechoic effusion (asterisk) distending the posterior recess. This recess is located between the humeral head (HH) and the posterior aspect of the bony glenoid (Gl), deep to the infraspinatus (InfraS) tendon and muscle US evaluation of the anterior recess is more complex due to its deep location, and often requires a small curved-array transducer, lower frequencies and a careful scanning technique. When fluid is present in the anterior recess, it can be appreciated on transverse scans as a hypoechoic halo surrounding the anterior labrum. Similarly, the subscapularis recess (also known as the subscapularis bursa) is difficult to evaluate reliably with US because of its small size and problems of access related to its location deep to the coracoid tip. This is a small saddlebag-shaped recess located between the anterior neck of the scapula and the subscapularis tendon which may extend above the tendon to overlie its anterior aspect. Using transverse or sagittal scans, the main landmark to find is the coracoid: an effusion in the subscapularis recess can be demonstrated as a small hypoanechoic area located just caudally and posteriorly to the bone and adherent to the subscapularis tendon (Fig. 6.46). The subscapularis recess should not be confused with the larger subcoracoid bursa that extends more caudally and does not communicate with the glenohumeral joint as it is an extension of the subacromial subdeltoid bursa (Figs. 6.46b, 6.47) (Grainger et al. 2000). The subcoracoid bursa lies deep to the conjoined tendon of the short head of the biceps and the coracobrachialis, in a more medial location relative to the subscapularis tendon and the coracoid, and may contain abundant effusion in cases of anterior rotator cuff tears (Fig. 6.47c). It is best examined while keeping the patient’s arm adducted by scanning just inferiorly and medially to the coracoid. The distinction between the subscapularis recess and the subcoracoid bursa is relevant because the causes of a subscapularis recess effusion may be different from the causes of a subcoracoid bursa effusion (which is most often associated with rotator cuff tears, including tears of the rotator cuff interval) (Grainger et al. 2000). Finally, the synovial sheath of the long head of the biceps tendon is formed by an extrusion of the articular synovial membrane. As the sheath is merely an extension of the joint cavity, intra-articular effusion can lead to fluid in the sheath (see Fig. 6.23). Fluid secondary to an isolated biceps tendinitis is rare. 6.4.2.2 Subacromial Subdeltoid Bursa The subacromial subdeltoid bursa appears as a 2 mm thick complex comprised of an inner layer of hypoechoic fluid between two layers of hyperechoic peribursal fat (see Fig. 6.31) (van Holsbeeck and Strouse 1993). In normal states, the synovial membrane of the bursa cannot be depicted with US. Hypoechoic thickening of the bursal walls can be observed in a variety of shoulder disorders, among which anterosuperior impingement is the most important (Fig. 6.48a). In these instances, the bursa assumes a pseudosolid appearance and may be difficult to delineate from the underlying supraspinatus tendon, somewhat mimicking a degenerative tendinopathy. A notch sign in the upper profile of the bursa at the point where it passes deep to the coracoacromial ligament may help this differentiation (Fig. 6.48b,c). Because intrabursal fluid can migrate depending on gravity and arm positioning, 229 230 S. Bianchi and C. Martinoli Co Co * CjT SubS * SubS a c Acr Co * b a * Gl SubS HH b CjT * d Fig. 6.46a-d. Subscapularis recess of the glenohumeral joint. a Sagittal oblique T1-weighted MR image over the glenoid reveals the extension of the superior subscapularis recess (asterisk) in a patient with joint effusion. The subscapular recess is a small saddlebag-shaped recess lying anterior to the glenohumeral joint capsule, between the anterior neck of the scapula and the subscapularis (SubS) which extends above this muscle to overlie its anterior aspect. Note the anterior glenohumeral joint cavity (straight arrows) with the middle glenohumeral ligament (arrowhead) and the posterior synovial recess (curved arrow). b Schematic drawing of an oblique sagittal view through the glenoid (Gl) illustrates the relationships of the superior (asterisk) and inferior (star) glenohumeral recesses with the superior (in yellow), middle (in purple) and inferior (in green) glenohumeral ligaments. Observe that the superior subscapularis recess extends below the coracoid (Co) and above the subscapularis (a) up to reach the anterior aspect of the muscle. This recess should not be confused with the adjacent subcoracoid bursa (arrowhead) which intervenes between the subscapularis and the coracobrachialis and short head of the biceps tendon (b) and has a greater caudal extension (see Fig. 6.47). Note the axillary (arrows) and posterior (curved arrow) recesses of the glenohumeral joint. Acr, acromion. Unlike the superior subscapularis recess, the inferior recess (star) is smaller and lies deep to the subscapularis muscle. c Sagittal 12–5 MHz US image over the coracoid process (Co) demonstrates the superior subscapularis recess (asterisk), which is partially masked by the intervening bone and located between the conjoined tendon (CjT) of the short head of the biceps and the coracobrachialis and the tip of the subscapularis (SubS). d Transverse 12–5 MHz US image obtained just below the coracoid illustrates the relationships of the superior subscapularis recess (asterisks) with the conjoined tendon of the coracobrachialis and the short head of the biceps (CjT) and the subscapularis (SubS). HH, humeral head the various bursal portions should be systematically assessed. Care should also be taken not to apply excessive pressure with the probe over the bursa, so as not to overlook small effusions. When the patient is standing or seated, fluid tends to accumulate in the most dependent portions of the bursa and, more commonly, along the lateral edge of the greater tuberosity, producing a typical “teardrop” sign (Fig. 6.49a) (van Holsbeeck and Strouse 1993). When effusion is contemporarily present in the glenohumeral joint and the bursa, anterior transverse planes are the best suited to demonstrate fluid in both cavities. Using these planes, the intraarticular fluid can be appreciated as a hypoechoic halo surrounding the long head of the biceps tendon, while the bursal fluid appears as a crescent-shaped collection located just deep to the anterior deltoid muscle (Fig. 6.49b). The two effusions are separated by a thin hyperechoic structure which represents the bordering walls of the biceps tendon sheath and the bursa. More abundant collections tend to fill the bursal portion located posterior to the infraspinatus tendon. In these cases, detection of the infraspinatus may help to distinguish superficial bursal effusions 231 Shoulder sBT SubS HH Co * HH a * SubS CBr CjT * * SubS b c Fig. 6.47a–c. Subcoracoid bursitis. Transverse 12–5 MHz US images obtained inferiorly and medially to the coracoid in three patients with increasing distension of the subcoracoid bursa (asterisks). Similar to the subscapularis recess, the subcoracoid bursa extends deep to the conjoined tendon (CjT) of the short head of the biceps (sBT) and the coracobrachialis (CBr) and medial to the subscapularis tendon (SubS) to reduce friction among these structures. When distended by large effusion, this bursa extends more medially relative to the coracoid. A natural communication (arrowheads) between it and the larger subacromial subdeltoid bursa may exist in some people, thus helping the examiner to distinguish subcoracoid bursitis from joint fluid in the subscapularis recess. HH, humeral head SupraS * 2 a 1 GT 2 SupraS 3 1 b c Fig. 6.48a–c. Subacromial subdeltoid bursitis. a Short-axis and b long-axis 12–5 MHz US images over the supraspinatus tendon (SupraS) demonstrate hypoechoic thickening of the bursal walls (arrowheads) and a small amount of fluid (asterisk) within the bursal lumen. In a, the effusion tends to accumulate medially, in the dependent portion of the bursa. In b, the upper profile of the bursa shows a deep notch (arrow) at the level of the myotendinous junction of the supraspinatus (SupraS) reflecting the position of the coracoacromial ligament. c Schematic drawing of a coronal view through the shoulder illustrates the extension of the subacromial subdeltoid bursa (arrowheads). This bursa is composed of the subacromial (1) and the subdeltoid (2) bursae, which are in continuity and may extend laterally and inferiorly (3) even 3 cm below the greater tuberosity (GT). Similar to that seen in b, note the notch in the bursal profile produced by the coracoacromial ligament (arrow) 232 S. Bianchi and C. Martinoli * GT * SupraS bt * * InfraS HH HH Hs a * b c Fig. 6.49a–c. Dependent recesses of the subacromial subdeltoid bursa. a Coronal 12–5 MHz US image obtained at the proximal humeral diaphysis, just distal to the supraspinatus tendon (SupraS) and the greater tuberosity (GT), reveals the lateral pouch (asterisk) of the bursa distended by some fluid, the so-called teardrop sign. b Transverse 12–5 MHz US image over the anterior shoulder shows effusion distending the bursal lumen (asterisks) both medially and laterally to the biceps tendon (bt). A small amount of fluid (star) is also visible in the biceps tendon sheath. Note that the two spaces are separated by a hyperechoic cleavage plane (arrowhead): they may communicate when a full-thickness tear of the rotator cuff occurs. Hs, humeral shaft. c Transverse 12–5 MHz US image over the posterior shoulder demonstrates the infraspinatus tendon (InfraS) which separates the superficial posterior dependent portion of the bursa (asterisks) from the deep posterior synovial recess (star) of the glenohumeral joint. HH, humeral head from deep joint effusions (Fig. 6.49c). Demonstration of an effusion in both synovial spaces is, for the most, an indicator of full-thickness tear of the rotator cuff. Dynamic scanning performed with the probe placed over one cavity – either the bursa or a joint recess – while compressing the other with the hand can reveal communication between the two compartments as a result of rotator cuff tear. 6.4.2.3 Acromioclavicular Joint and Os Acromiale To examine the acromioclavicular joint, the transducer is placed over the top of the shoulder in a coronal plane. The width of the joint is measured and compared with that of the contralateral side. The evaluation of the acromioclavicular joint has to be included as part of the routine study of the shoulder, because its lesions can mimic rotator cuff disease. In fact, this joint is intimately related to the supraspinatus tendon, which runs directly underneath the joint. In spite of a similar echogenicity, the superior acromioclavicular ligament can be distinguished from the underlying joint cavity using high-frequency probes and dynamic scanning (Fig. 6.50a,b). This ligament forms an external inextensible band joining the mobile ends of the clavicle and the acromion, an appearance quite different from the content of the acromioclavicular joint which is limp and can change shape and width with shoulder movements. In young healthy subjects, the internal fibrocartilaginous disk can seldom be appreciated as a slightly hyperechoic structure, an appearance somewhat similar to the knee menisci or the glenoid labrum (Fig. 6.50c,d). The coracoclavicular ligaments are difficult to be detected with US due to the acoustic shadowing of the overlying clavicle. An os acromiale can occasionally be recognized as an incidental finding while scanning the acromioclavicular joint with US (Fig. 6.51). This accessory bone derives from the nonfused epiphysis of the anterior part of the acromion, has an overall frequency of approximately 8% of general population and is bilateral in one third of cases (Sammarco 2000). The os acromiale is triangular in shape and has a variable size (mean 22 mm). It can articulate with the acromion and the clavicle with a distinct articulation, a fibrocartilaginous union or a nearly complete union (Sammarco 2000). The deltoid muscle inserts on its anterolateral edge. The os acromiale is a potential source of anterosuperior impingement, either as a fragment mobilized by deltoid pulls or from osteophyte lipping. US is a sensitive means to identify or confirm this anomalous bone (Boehm et al. 2003). The diagnosis is based on detection of a well-defined cortical discontinuity on the superior aspect of the acromion, often mimicking a double acromioclavicular joint (Figs. 6.51, 6.52). At US, an os acromiale may exhibit flat bony margins (type I), marginal osteophytes (type II) or inverted bony margins (type III) (Boehm et al. 2003). A confident identification of the os acrominale from the adjacent 234 S. Bianchi and C. Martinoli Os Cl Acr Os a c b Os d f Cl g Acr Acr Os * e Os Cl Os Cl * Acr h Fig. 6.52a–h. Os acromiale. a,b Schematic drawings of a transverse view through the acromioclavicular joint showing adequate transducer positioning for US depiction of an os acromiale with c,d corresponding 12–5 MHz US images. Two individual articulations are demonstrated instead of one, the pseudo-articulation of the accessory ossicle (Os) with the acromion (Acr) being located in a more posterior site than expected for a true acromioclavicular joint. Cl, distal end of the clavicle. e,f Radiographic appearance of an os acromiale imaged by means of e acromioclavicular and f Bernageau views. g,h Oblique coronal T2-weighted MR images g over the pseudo-articulation of the os acromiale with the clavicle and h the acromion in a patient with rotator cuff tear and abundant fluid collection (asterisk) in the subacromial subdeltoid bursa acromioclavicular joint can easily be accomplished by shifting and rotating the probe over the acromion in order to identify two articulations instead of one. In case of an associated rotator cuff tear, the treatment is varied. In patients with impingement symptoms, a small mobile os acromiale can be resected, a large stable os acromiale treated by acromioplasty and a large unstable os acromiale by fusion to the acromion. The postoperative outcome is good. 6.4.2.4 Glenoid Labrum The fibrocartilaginous labrum can be demonstrated at US as a triangular homogeneously hyperechoic structure capping the bony rim of the glenoid (Schydlowsky et al. 1998a). The different portions of the labrum lie at various depths, the inferior being the most superficial and the anterior the deepest. Consequently, an adequate US scanning technique should first include a dynamic adjustment of the focal zone, based on the characteristics of each individual quadrant to be examined. The anterior labrum is best scanned with curved-array transducers and low frequencies (down to 5 MHz) using an anterior transverse approach (Fig. 6.53a). The patient’s arm is maintained adducted or abducted at 90° with the elbow flexed or with an axillary transverse approach placing the arm in the same position as before (Hammar et al. 2001). While evaluating the anteroinferior quadrant of the glenoid, difficulties may arise in patients who are obese or unable to put their arm in the proper position because of pain or apprehension. Contrary to the anterior labrum, the posterior labrum is more superficial in position and can be easily imaged at US using transverse planes while placing the patient‘s hand on the opposite shoulder (Fig. 6.53b). It appears as a triangular structure with the base directed medially and the apex pointing laterally. Changes in the shape of the labrum can be observed in different rotations of the arm. A more pointed appearance is noted when traction is applied on it by the capsule (during internal rotation for the posterior labrum). The superior labrum is very difficult to visualize due to problems of access related to the acoustic shadowing of the acromion. A tentative approach can be made in slender subjects by placing the probe just behind the head of the clavicle while abducting the arm to better differentiate the static glenoid from the moving humeral head (Fig. 6.53c). Even with appropriate technique, high-end equipment and skilled hands, US is unable to demonstrate superior labrum abnormalities, such as anterior to 236 S. Bianchi and C. Martinoli Acr SupraS * * HH Gl a InfraS Gl Gl b c Fig. 6.54a,b. Supraspinous and spinoglenoid notches. a Oblique coronal 12–5 MHz US image obtained medial to the acromion (Acr) reveals the supraspinous notch as a shallow groove located in the cranial aspect of the scapula just medial to the bony glenoid (Gl) and the superior labrum (asterisk). A couple of tiny hypoechoic dots (arrow) are appreciated in the supraspinous notch, deep to the supraspinatus muscle (SupraS), reflecting the suprascapular artery and the suprascapular nerve. b Transverse 10–5 MHz US image obtained over the posterior shoulder demonstrates the spinoglenoid notch (arrows) as a fat-filled concavity of the scapula located at the base of the glenoid (Gl) and deep to the infraspinatus muscle (InfraS). Note the posterior labrum (asterisk) and the humeral head (HH). c Transverse 12–5 MHz color Doppler US image helps to distinguish the suprascapular artery (arrowhead) from the adjacent suprascapular nerve (arrow) based on detection of blood flow signals in the artery HH HH Gl a TMj b Deltoid Tm Tm Hm c d Fig. 6.55a–d. Axillary nerve and posterior circumflex artery. a Oblique sagittal 12–5 MHz US image obtained over the axillary recess of the glenohumeral joint demonstrates the inferior fibrocartilaginous labrum (white arrows) between the humeral head (HH) and the bony glenoid (Gl). In proximity to these structures, the posterior circumflex artery (arrowhead) and the axillary nerve (curved arrow) are displayed. b Long-axis 12–5 MHz US image of the axillary nerve (curved arrows) along its course through the axilla. Note the relationship of the nerve with the posterior circumflex artery (arrowhead) and the deep teres major muscle (TMj). Sagittal c gray-scale and d color Doppler 12–5 MHz US images obtained over the posterior humeral metaphysis (Hm) demonstrate the axillary nerve (curved arrow) and the adjacent posterior circumflex artery (arrowhead) as they course superficial to the bone, below the teres minor muscle (Tm) and deep to the deltoid. The inserts at the upper left side of the figure indicate respective transducer positioning Shoulder supraclavicular, infraclavicular and axillary regions (Yang et al. 1998; Sheppard et al. 1998; Apan et al. 2001; Retzl et al. 2001; Martinoli et al. 2002; Demondion et al. 2003). The US examination of brachial plexus nerves is based on detection of some anatomic landmarks in the neck, including bones (roots), muscles (trunks) and vessels (divisions and cords). After exiting the neural foramina, the roots pass between two prominent apophyses of the transverse processes of the cervical vertebrae – the anterior and posterior tubercles – in close relationship with the vertebral artery and vein (Fig. 6.56). Each root emerges as an individual (monofascicular) hypoechoic structure, an appearance quite different from that of nerves in the extremities, which are composed of clusters of hypoechoic fascicles. Coronal planes are able to depict the nerve roots in the paravertebral area using the same longitudinal scan for the study of the vertebral artery and vein as a landmark (Fig. 6.57a,b). In these planes, the picture of the vertebral vessels is obscured at regular intervals by the acoustic shadowing from the anterior tubercles of the transverse processes. Moving the transducer slightly posteriorly, the vessels disappear and the roots appear as elongated hypoechoic images exiting the neural foramina, each of which is located over the costotransverse bar of the vertebra (Fig. 6.57c,d). Nevertheless, transverse planes are ideal to depict the relationship of the roots with the transverse processes at any given level. Based on the peculiar appearance of the transverse process of C7, in which the posterior tubercle is absent, US is able to assess the level of the nerve roots (Martinoli et al. 2002). For this purpose, scanning first reveals the C7 level and then moves either up or down on axial planes. The C7 root is detected in the same plane as the C7 vertebra is bordered by the posterior tubercle only (Fig. 6.58a–d). Shifting the transducer upward, the C6 vertebra is recognized due to the presence of prominent anterior and posterior tubercles: the C6 root appears as a hypoechoic structure held in between them (Fig. 6.58e–h). The transverse processes of C5 have basically the same shape as those of C6 and can be identified as successive steps cranial to the C6 level by taking into account the number of transverse processes encountered while sweeping the transducer cranially from C7. From the anatomic point of view, the higher the level, the smaller the space between the tubercles. Then, moving the transducer downward from C7, the lateral aspect of the T1 vertebra is flat without any tubercle; at this level, the C8 root can be appreciated near the foraminal outlet. More caudally, identification of the T1 root is not always feasible due to problems of access related to the too deep location of the intervertebral foramen between the T1 and T2 vertebrae. The T1 root shows a curving course below the first rib, and can be examined by using an axial oblique plane of approximately 45°. In addition to determining whether a lesion is preganglionic rather than postganglionic, or infraclavicular rather than supraclavicular, attributing SternoCl Thy IJV CA LC * Esoph 1 2 3 Fig. 6.56. Normal brachial plexus: paravertebral area. Transverse 12–5 MHz US image over the left anterolateral neck demonstrates the main landmarks for identification of the nerve roots. Note the position of the left lobe of the thyroid (Thy), the esophagus (Esoph), the common carotid artery (CA), the internal jugular vein (IJV) lying between the superficial sternocleidomastoideus (SternoCl) and the deep longus colli (LC) muscles. Deep to these structures, the lateral aspect of the C6 vertebra shows a wavy hyperechoic contour, which delineates the vertebral body (1), the pedicle (2) and the transverse process (3), which exhibits in turn two prominent anterior (asterisk) and posterior (star) tubercles. The C6 root (arrow) appears as a hypoechoic image contained between these tubercles. The insert at the upper left side of the figure indicates transducer positioning 237 238 S. Bianchi and C. Martinoli * * a v a b c d Fig. 6.57a–d. Normal brachial plexus: paravertebral region. a,c Oblique coronal 12–5 MHz US images over the lateral neck with corresponding b,d schematic drawings showing the position of the US transducer. a,b The vertebral artery (a) and vein (v) are demonstrated along their long axis. Note that these vessels are obscured at regular intervals by the intervening acoustic shadowing of the anterior tubercles (asterisks) of the transverse processes. c,d Shifting the transducer slightly posteriorly (black arrow in d), the vessels disappear and two nerve roots (open and white arrowheads) are appreciated as elongated hypoechoic images exiting the neural foramina (open and white arrows), each of which courses over a costotransverse bar (rhombi) a given level of nerve involvement is an important component of the imaging report since the list of possible clinical syndromes in a patient with brachial plexopathy is different according to the pattern of the injured roots and trunks (e.g., upper partial: C5, C6 [C7]; lower partial: C8, T1; complete: C5–T1) (Narakas 1993). Sweeping the transducer down to the interscalene region on short-axis planes, the nerve trunks are visualized as they pass in between the scalenus anterior and scalenus medius muscles (Yang et al. 1998). Visualization of the trunks in the interscalene space depends on the amount of fat between these muscles, and a careful scanning technique is needed because nerve fascicles can easily be confused with muscle fascicles. The upper and middle trunks are more readily identified with US (Fig. 6.59). They are arranged in series from superficial to deep and receive contributions from the C5 and C6 levels (upper trunk) and C7 level (middle trunk). One has to consider that the progression of the roots is anatomically constant down to the interscalene region, where they unite to form the three trunks: upper (C5 and C6), middle (C7) and lower (C8 and T1). Therefore, the ability of US to recognize the root levels in the paravertebral area also reflects on a confident identification of the trunks by simply following the nerves from where these latter arise. In the supraclavicular region, the nerves are visualized as a cluster of hypoechoic rounded images that represent the divisions (Yang et al. 1998). The divisions follow, for the most part, the posterior aspect of the subclavian artery, just over the straight hyperechoic appearance of the first rib and apical pleura (Fig. 6.60) (Sheppard et al. 1998; Yang et al. 1998). Crossing down the clavicle, in the infraclavicular area, the nerve cords continue their course along the axillary artery and behind the pectoralis minor muscle (Fig. 6.61). An individual identification of divisions and cords of the brachial plexus distal to the interscalene region is less feasible on US because these branches anastomose with each other in various combinations. Overall, we believe that the main advantage of US in brachial plexus imaging is its ability to follow up continuously each individual component of the plexus through the lateral neck by shifting the probe back and forth in short-axis plane. Similar to other sites in the body, anatomic variants of brachial plexus nerves and surrounding 240 S. Bianchi and C. Martinoli SA SA b First Rib L L a c Fig. 6.60a–c. Normal brachial plexus: supraclavicular region. a Oblique transverse 12–5 MHz US image over the supraclavicular region shows brachial plexus divisions and initial parts of the cords as clusters of round hypoechoic fascicles (arrows) located above and, for the most part, posterior to the subclavian artery (SA). Deep to these structures, the straight profile of the first rib and the lung (L), which appears as a bright hyperechoic interface due to its air content, are also demonstrated. b,c Oblique transverse b gray-scale and c color Doppler 12–5 MHz US images over the supraclavicular region. Doppler imaging may help to identify nerves (arrow) in this region based on detection of blood flow signals from the adjacent artery. The insert at the upper left side of the figure indicates transducer positioning PMj PMj Pm Pm AA a b Fig. 6.61a,b. Normal brachial plexus: infraclavicular region. Oblique transverse 12–5 MHz US images obtained under the clavicle a over the major axis of the axillary artery (AA) and b immediately behind it. The cords of the brachial plexus (open and white arrowheads) are visualized as elongated fascicular structures coursing around the axillary artery and deep to the pectoralis minor muscle (Pm). PMj, pectoralis major muscle. The insert at the upper left side of the figure indicates transducer positioning tissues possibly predisposing to compressive neuropathy can be demonstrated with US, including cervical rib, hypertrophied transverse process of C7 and variations in the scalene muscles (Fig. 6.62). Detection of a discrete arterial branch arising from the subclavian artery and encroaching on the brachial plexus nerves in the supraclavicular region is a normal finding. This blood vessel is the dorsal scapular artery (Fig. 6.63). In addition to brachial plexus nerves, US is also able to image other nerves running in the lateral cervical region, including the vagus nerve (Giovagnorio and Martinoli 2001), the recurrent laryngeal nerve (Solbiati et al. 1985) and the spinal accessory nerve (Bodner et al. 2002). The vagus nerve (CN X), the main parasympathetic nerve to the organs of the body, leaves the skull through the jugular foramen and passes inferiorly in the posterior part of the carotid sheath, in the angle between and posterior to the internal jugular vein and the carotid artery (Fig. 6.64a). In this location, it can be appreciated with US as a thin (<2 mm in cross-sectional diameter) vertically oriented cord-like structure containing three or four very small fascicles (Fig. 6.64b) (Giovagnorio et al. 2001). Its secondary branch, the recurrent laryngeal nerve, reaches the Shoulder SA First Rib a b c Fig. 6.62a–c. Accessory cervical rib. a Anteroposterior radiograph in an asymptomatic subject demonstrates a cervical rib (straight arrow) on the right and a hypertrophied transverse process (curved arrow) of the C7 vertebra on the left. The cervical rib articulates with a prominent posterior tubercle of C7 and the first rib. b Transverse 12–5 MHz US image obtained in the right paravertebral area demonstrates the close relationship between the C7 root (large arrow) and an abnormally prominent posterior tubercle (narrow arrow). c Oblique transverse 12–5 MHz US image obtained in the right supraclavicular region reveals the distal articulation (arrowheads) of the cervical rib as it bulges alongside the subclavian artery (SA) and the nerve divisions (large arrow) of the brachial plexus to connect with the first rib * SA * SA * a b Fig. 6.63a,b. Dorsal scapular artery. a,b Oblique transverse a gray-scale and b color Doppler 12–5 MHz US images obtained in the right supraclavicular region of an asymptomatic subject demonstrates an anomalous origin of the dorsal scapular artery (asterisks) from the subclavian artery (SA). Soon after its origin, the artery passes among the brachial plexus nerves (arrows) forming a cleavage plane between superficial (upper and middle trunks) and deep (lower trunks) clusters of nerve fascicles IJV Thy IJV CA T CA a b c Fig. 6.64a–c. Vagus and recurrent laryngeal nerves. a Schematic drawing shows the vagus nerve (arrow) inside the major neurovascular bundle, and behind the common carotid artery (CA) and the internal jugular vein (IJV). The recurrent laryngeal nerve (curved arrow) courses more medially, along the tracheoesophageal groove and immediately posterior to the thyroid lobes. b Transverse 12–5 MHz US image of the right neurovascular bundle shows the vagus nerve (arrow) as a very tiny structure characterized by a few hypoechoic fascicles surrounded by hyperechoic epineurium, between the common carotid artery (CA) and the internal jugular vein (IJV). c Transverse 12–5 MHz US image over the right lobe (Thy) of the thyroid gland demonstrates the small recurrent laryngeal nerve (curved arrow) as it ascends the neck alongside the trachea (T) 241 242 S. Bianchi and C. Martinoli posteromedial aspect of the lower pole of the thyroid after looping the subclavian artery (on the right) and the aortic arch (on the left). Then, it proceeds cranially in the tracheoesophageal groove to supply the intrinsic muscle of the larynx (Fig. 6.64a). Using a high-resolution transducers, small segments of this nerve may be recognized in a few patients with lean necks, deep to the thyroid (Fig. 6.64c) (Solbiati et al. 1985). The spinal accessory nerve (CN XI) is a motor nerve consisting of spinal and cranial roots which leaves the skull base through the jugular foramen and traverses the lateral cervical triangle, a space bordered by the sternocleidomastoideus muscle anteriorly, the trapezius posteriorly and the clavicle inferiorly, to supply the trapezius muscle. Its palsy causes limited shoulder elevation and retraction, the so-called drooping shoulder. The spinal accessory nerve passes underneath the sternocleidomastoideus muscle and, in the lateral cervical triangle, it becomes superficial, coursing immediately deep to the fascia and adjacent to superficial lymph nodes. At this site, it may be injured during lymph node biopsy or procedures of carotid surgery. US is able to depict the normal nerve as a small (1 mm in size) linear structure traversing the lateral cervical triangle and can reveal its traumatic damage in the appropriate clinical setting (Bodner et al. 2002). 6.5 Shoulder Pathology Knowledge of the complex pathophysiology and biomechanics underlying rotator cuff impingement and shoulder instability is an essential prerequisite for a correctly executed US examination and interpretation of the imaging findings. 6.5.1 Pathophysiologic Overview 6.5.1.1 Impingement and Rotator Cuff Disease Rotator cuff disease is the commonest cause of shoulder pain and dysfunction in adults. It derives from a wide range of pathologic conditions, including acute and chronic trauma, arthritis and shoulder instability (Laredo and Bard 1996). Most tears, however, occur in patients who lack a definite clinical history of trauma or systemic disease. In these cases, rota- tor cuff disease is believed to be secondary to local causes. From the pathophysiologic point of view, tendon ischemia was the first factor hypothesized to play a causative role in the pathogenesis of rotator cuff disease (Codman, 1934). This theory was supported by the histologic evidence of a relatively hypovascular area in the supraspinatus tendon, the so-called “critical zone”, which is located approximately 1 cm medial to the tendon attachment on the greater tuberosity (Fig. 6.65a). Microangiographic studies demonstrated that this zone is located at the limit between the tendon vasculature deriving from the myotendinous junction and that arising from the teno-osseous junction of the supraspinatus (Chansky and Iannotti 1991). The critical zone is, therefore, prone to ischemia and more susceptible to develop degenerative changes. More recently, tendon damage secondary to chronic contact of the supraspinatus tendon with the undersurface of the coracoacromial arch, the so-called “impingement syndrome”, was proposed as the main causative factor leading to rotator cuff tears (Neer, 1972). The clinical success of combined procedures of rotator cuff repair and anterior acromioplasty led to the widespread acceptance of this hypothesis. A consensus is now emerging that the causes of rotator cuff disease are manifold, including various combinations of extrinsic factors, such as morphology of the coracoacromial arch, tensile overload, repetitive overuse and kinematic abnormalities, and intrinsic factors, such as altered tendon vascular supply (Soslowsky et al. 1997). The degenerative process in the tendon substance may progress toward partial and complete tendon tears. As demonstrated on autopsy studies, rotator cuff pathology becomes more prevalent with increasing age. A disease prevalence of approximately 10% at 30 years, 50% at 60–70 years and 80% at 80 years has been reported and it is well known that asymptomatic rotator cuff lesions are not so uncommon, particularly in elderly subjects who do not realize the shoulder failure given their reduced demands (Leach and Schepsis 1983; Yamaguchi et al. 2001). Depending on the location of the contact, three main types of shoulder impingement have been described: anterosuperior (the most common), anteromedial and posterosuperior. As described above, the supraspinatus tendon lies in the subacromial space between the humeral head and the cover of the coracoacromial arch, which is formed (from posterior to anterior) by the anterior portion of the acromion and the acromioclavicular joint, the coracoacromial ligament and tip of the coracoid. In normal states, the tendon glides 244 S. Bianchi and C. Martinoli a b c d e f Fig. 6.66a–f. Types of acromial morphology. a–c Schematic drawings of a sagittal view through the shoulder with d–f corresponding outlet view radiographs demonstrate a,d type I or flat acromion, b,e type II or curved acromion and c,f type III or hooked acromion. Arrows indicate the different acromial shapes Spurs on the acromial attachment of the coracoacromial ligament are also considered signs of shoulder impingement. A poorly consolidated fracture of the greater tuberosity can lead to an abnormal upward displacement of the bony fragment and subsequent narrowing of the subacromial space. Anterosuperior impingement may occur in the absence of any evidence of anatomic abnormalities that may explain it. Unlike impingement syndrome related to alterations in the coracoacromial arch, these cases occur in athletes involved in sporting activities which require overhead motion of the arm (e.g., volleyball, throwing) and are somewhat related to glenohumeral joint instability (Jobe et al. 1989). During anterior instability, repetitive overload leads to some degree of anterior and superior translation of the humeral head with secondary restriction of the subacromial space and local attrition of the supraspinatus tendon against the anterior acromion and the coracoacromial ligament when the arm is abducted and externally rotated. In general, these patients have less advanced rotator cuff disease (i.e., tendinosis, partial-thickness tears) and benefit from therapy directed to the underlying instability, including strengthening of the rotator cuff. The same often occurs in slender young females who have weak scapular rotators. Once the impingement syndrome is established, chronic mechanical microtrauma induce progressive tendon degeneration and tearing as well as changes in the subacromial subdeltoid bursa. In the anterior impingement syndrome, three stages of increasing tendon damage have been described (Neer, 1972). Stage I is mainly appreciated in young adults in whom impingement leads to subacromial bursitis and absent or minimal tendon changes: this stage is usually reversible. Stage II is characterized by progressive thickening and an irregular appearance of the supraspinatus tendon and the subacromial subdeltoid bursa as a result of the degenerative process: surgery is usually considered (i.e., removal of the thickened bursa and release of the coracoacromial ligament) if conservative management fails. Stage III indicates progression of tendon damage to partial- and full-thickness tears: acromioplasty and cuff repair are often required. Far less common than anterosuperior impingement, anteromedial impingement (subcoracoid impingement) derives from encroachment of the superior portion of the subscapularis tendon and the long head of the biceps tendon against the tip of the coracoid during maximal internal rotation and forward flexion of the arm (Gerber et al. 1985). Laxity of the anterior capsule and ligaments and congenital anomalies of the coracoid process and the lesser tuberosity seem to be implicated as predisposing factors. Finally, a third type of shoulder impingement, the posterosuperior impingement (posterosubglenoid impingement) occurs as a result of pinching of the rotator cuff at the junction of the supraspinatus and infraspinatus tendons, between the greater tuberosity and the posterosuperior aspect of the glenoid rim, during maximal abduction and external Shoulder rotation of the arm (Walch et al. 1991). This kind of impingement causes degenerative changes and partial tears of the posterior supraspinatus tendon, typically involving its undersurface. 6.5.1.2 Instability Due to its peculiar anatomy, the shoulder joint is inherently unstable. Several shoulder structures may be involved in the pathogenesis of instability, including bony surfaces, joint capsule, ligaments and the fibrocartilaginous labrum (static restraints) and the rotator cuff tendons (dynamic constraints). In addition to anatomic factors, a combination of other predisposing factors related to both developmental and acquired diseases, often combined with one another, can be responsible for glenohumeral joint instability. The degree of glenohumeral joint instability ranges from subluxation to dislocation and indicates that the humeral head slips out of its socket during movements. In this setting, the clinician must realize whether a subluxation or dislocation has occurred and has to assess the state of the anatomic structures responsible for joint stability to establish a proper treatment. Based on its direction, shoulder instability can be defined as anterior, posterior or inferior to the glenoid, or multidirectional (Zarins and Rowe, 1984). Anterior instability accounts for approximately 96–98% of all shoulder dislocations. Although often encountered in sub- Acr jects with a loose anterior capsule and ligaments, anterior instability usually follows an acute injury that weakens the para-articular structures responsible for joint stability. The mechanism associated with anterior instability is abduction, extension and external rotation. Recurrent subluxations or dislocations may occur even after trivial trauma. The diagnosis of anterior instability is based on physical examination and plain films (Fig. 6.67). In most cases, anteroposterior views are sufficient to detect the anterior dislocation of the humeral head and no additional projections are needed. Unlike dislocation, a subluxation of the humeral head may be a subtle transient event that may be difficult to recognize. Posterior instability may be secondary to shoulder trauma and, when bilateral, is frequently observed in seizures as a result of the stronger convulsive contraction of the posterior muscles (infraspinatus and teres minor) relative to that of the subscapularis. The diagnosis is often missed because this condition is uncommon (4% of all shoulder dislocations) and may present with subtle clinical and radiographic findings. Standard radiographs, including anteroposterior and lateral views, may often be inadequate for detection of posterior dislocation and additional projections, such as the axillary view, may be required for this purpose. However, these projections are not easily obtained in the acutely injured patient. Approximately 50% of posterior shoulder dislocations go unrecognized and some authors have reported an average interval from the injury to the diagnosis of 1 year, particu- * HH a b Fig. 6.67a,b. Anterior shoulder instability. a Chronic anterior instability in an elderly patient. Note the anterior dislocation of the humeral head (HH) relative to the acromion (Acr) and the coracoid (asterisk). b Anterior glenohumeral dislocation, subcoracoid type. Anteroposterior radiograph demonstrates anterior displacement of the humeral head, which appears located inferior to the coracoid process. A Hill-Sachs deformity is present (arrow) 245 246 S. Bianchi and C. Martinoli larly in the case of a “locked” posterior dislocation which occurs when the posterior glenoid causes an impaction fracture on the humeral head preventing its repositioning (Hawkins et al. 1987). If not recognized early, posterior dislocation can lead to chronic joint stiffness, pain and reduced range of motion. Chronic longstanding dislocations are most often found in the elderly. In these cases, the prognosis is not good and the decision may often be to leave the shoulder dislocated and attempt to regain as much motion as possible with physical therapy or the insertion of a shoulder prosthesis. 6.5.2 Rotator Cuff Pathology Initially and for many years, investigators reported contradictory results, either enthusiastic or poor, in the ability of US to diagnose rotator cuff pathology (Mayer 1985; Mack et al. 1985; Middleton et al. 1985, 1986b; Hodler et al. 1988, 1991; Burk et al. 1989; Brandt et al. 1989; Soble et al. 1989; Hall 1989; Ahovuo et al. 1989a,b; Miller et al. 1989; Drakeford et al. 1990; Vick and Bell 1990; Misamore and Woodward, 1991; Nelson et al. 1991; Wulker et al. 1991; Wiener and Seitz, 1993; Guckel and Nidecker 1997). In many cases, the first studies made use of US criteria that nowadays have either been refined or are no longer accepted, examinations were performed with a scanning technique that has since been modified to improve visualization of the cuff, and old low-resolution equipments were employed. In the context of technological improvements, higher resolution capabilities and new criteria to diagnose rotator cuff tears, the current US technology is now increasingly able to reliably provide good accuracy in the assessment of rotator cuff tears (Teefey et al. 1999, 2004; Bouffard et al. 2000; Leotta et al. 2000; Roberts et al. 2001; Moosikasuwan et al. 2005). In addition, this technique allows the assessment of most of the stages of rotator cuff disease and the classification of rotator cuff tears based on the extent of tendon involvement, size and location of the tear. As already described, the supraspinatus is the rotator cuff tendon most commonly involved by either partial- or full-thickness tears as a result of subacromial impingement. In a large series of surgically proven rotator cuff ruptures, isolated tears of the supraspinatus tendon were found in 62% of cases, accounting for 18% of partial-thickness and 44% of full-thickness tears (Walch et al. 1999). Early degenerative changes and tears of the supraspinatus are typically observed in the anterior half of the tendon, just behind the long head of the biceps tendon (Fig. 6.68a). The smallest forms of rotator cuff tears are partial-thickness tears, which can in turn be located at either the articular (12%) or the bursal (5%) surface of the involved tendon. Intrasubstance tears occur more rarely (1%). If untreated, partial-thickness tears can enlarge to become full-thickness tears (Fig. 6.68b). Overall, one should consider that partial-thickness tears are more common than full-thickness tears and those involving the articular side of the rotator cuff are slightly more common than those of the bursal side. Beginning in the anterior third of the supraspinatus, most tears then propagate in a posterior direction to involve the middle and posterior tendon, eventually in some cases causing complete supraspinatus rupture (Fig. 6.68c). In more advanced disease, other rotator cuff tendons may additionally rupture as a result of excessive tensile forces due to the altered shoulder biomechanics related to the supraspinatus tear (Fig. 6.68d). The involvement of other tendons together with the supraspinatus has been reported in an additional 30% of patients (Walch et al. 1999). In these combined tears, the posterior extension of a supraspinatus tear to the infraspinatus occurs in approximately 20% of cases, whereas the anterior involvement of the subscapularis from a supraspinatus tendon tear is less common and accounts for approximately 10% of cases (Walch et al. 1999). As the lesion expands anteriorly into the subscapularis, disruption of the stabilizers of the biceps tendon (i.e., rotator cuff interval structures) occurs. Isolated rupture of the subscapularis tendon occurs in another 8% of cases: such ruptures are more common in sport traumas due to forceful stretching on an abducted and externally rotated arm. On the other hand, isolated rupture of the infraspinatus is rare and occurs in the spectrum of posterior posterosuperior subglenoid impingement. The classification of rotator cuff tears is somewhat confusing because different terms have been inappropriately used with the same meaning. In an effort to better understand the type of tendon tear and to standardize the observations of various examiners, the rotator cuff should be thought of in a three-dimensional view. A tear must be considered incomplete when it involves only a part of the tendon width on short-axis planes (Fig. 6.68a,b). Incomplete tears may be in turn subdivided into partial-thickness (Fig. 6.68a) or full-thickness (Fig. 6.68b) types depending on whether they result in an abnormal communication of the glenohumeral joint and the Shoulder a b c d Fig. 6.68a–d. Schematic drawings of a sagittal view through the shoulder illustrate the typical progression of a rotator cuff tear. a Initially, a partial-thickness tear (dark gray) of the anterior supraspinatus tendon (light gray) occurs. This tear tends to enlarge (arrows) in the vertical plane up to become b a full-thickness tear. Once established, a full-thickness tear expands in an anterior and posterior direction (arrows) up to cause c complete rupture of the supraspinatus tendon. d In advanced disease, the supraspinatus tendon tear may further expand either in a posterior direction (white arrow), involving the infraspinatus tendon, or in an anterior direction (black arrows), involving the biceps and the subscapularis tendon, to create a massive tear subacromial subdeltoid bursa. According to their depth, partial-thickness tears may involve the bursal side, the articular side or the midsubstance (intrasubstance) of the tendon (Ellman 1990). When a full-thickness tear involves the full width of a tendon, it becomes a complete tear (Fig. 6.68c). Then, it can become a massive tear as it spreads to involve more than one tendon with a total width of the affected cuff more than 3 cm (Fig. 6.68d). 6.5.2.1 Cuff Tendinopathy Rotator cuff tendinopathy is thought to be an early result of anterosuperior impingement (Neer stage II) and, at first, affects the supraspinatus tendon along with the subacromial bursa. The US appearance of tendinopathy includes swelling of the affected tendon and abnormal tendon echotexture with a heterogeneous hypoechoic appearance (Fig. 6.69). Tendon swelling can be appreciated with US as either a focal or – most often – a diffuse increase in tendon thickness (Farin et al. 1990). Because longaxis planes give a panoramic depiction of the tendon as a whole, they are the most adequate to recognize its thickening. Bilateral examination may occasionally be used to improve diagnostic confidence when only small changes in the tendon size occur. In these cases, care should be taken to evaluate the same level on both sides because the supraspinatus tapers toward the greater tuberosity and from anterior to posterior. Dynamic scanning obtained by placing the probe in the coronal plane over the lateral margin of the acromion while the patient abducts their arm in internal rotation may demonstrate difficult gliding of the thickened tendon and subacromial bursa underneath the acromion (Read and Perko 1998). Some thresholds in tendon size between the unaffected side and the affected supraspinatus (thickness difference ranging from 1.5 to 2.5 mm) or a tendon thickness greater than 8 mm have been proposed as indicators of tendinopathy (Crass et al. 1988a). Similar to other applications of musculoskeletal imaging, we believe US findings in rotator cuff pathology should be interpreted in the light of clinical data rather than on the basis of differences in measurements. In fact, measurements are not so reliable and their value is poor in the absence of clinical correlation. In addition, supraspinatus tendinopathy is often associated with diffuse wall thickening of the subacromial subdeltoid bursa and a small reactive bursal effusion. In many instances, a cleavage plane is lacking between these two structures and, therefore, it may be difficult to exclude the contribution of the bursa when measuring the tendon thickness. As 247 248 S. Bianchi and C. Martinoli GT a b Fig. 6.69a,b. Impingement syndrome with supraspinatus tendon abnormalities reflecting tendinosis. a Long-axis 12–5 MHz US image over the supraspinatus demonstrates a hypoechoic and swollen tendon (straight arrows). The tendon insertion (curved arrow) is more rounded and bulges over the greater tuberosity (GT). The subacromial subdeltoid bursa (arrowheads) can be distinguished from the underlying tendon on the basis of its more hypoechoic appearance. b Arthroscopic photograph reveals a reddish microvascular network (arrows) over the surface of the supraspinatus tendon reflecting intratendinous hyperemia regards the abnormal echotexture in tendinopathy, US findings seem to be related to subtle fibrillar tears and areas of mucoid degeneration intermixed with the reparative process occurring in the tendon substance. Nevertheless, a definite pathologic correlation of these abnormalities is lacking in the imaging literature because these patients are treated conservatively. Mild cortical changes in the greater tuberosity can also be observed. 6.5.2.2 Partial-Thickness Tears Partial-thickness tears account for approximately 13–18% of all rotator cuff tears and occur in a younger age group compared with full-thickness tears (Walch et al. 1999). US detection of these tears and their differentiation from focal tendinopathy is often challenging because the appearance of the two conditions may be similar. It must be noted, however, that the therapeutic approach is conservative for both, so their differentiation is clinically worthless. On the basis of the US findings, we believe that an accurate diagnosis of a partial-thickness tear should be made when a true defect or cleft within the tendon substance is clearly delineated on both long- and short-axis planes. As previously stated, partial tears most frequently affect the anterior third of the supraspinatus tendon. The main US finding is a localized hypoechoic area affecting only part of the tendon thickness. Because the echogenicity of the different tendon portions can vary depending on the incidence of the US beam, a reliable diagnosis of partial-thickness tears should be made only when the area does not change its hypoechoic appearance on short- and long-axis scans and while tilting the transducer over the tendon (van Holsbeeck et al. 1995). The size of the tear must be measured on long and short-axis planes and should be indicated in the report as a measurement (in mm) or a percentage of the tendon diameter (thirds of tendon thickness). In our opinion, the second option is more practical because it gives an estimate of the lesion with respect to the tendon size. With reference to partial-thickness tears may have either a bursal or articular or intratendinous extension. Bursal surface tears are better visible on US and typically appear as hypoechoic concave defects located at the bursal surface of the supraspinatus, in most cases close to the greater tuberosity (Figs. 6.70, 6.71). Focal herniation of hypoechoic bursal fluid or hyperechoic peribursal fat within the defect is often seen and represents a useful sign for detecting such tears (Fig. 6.72). Bursal effusion is usually moderate and needs accurate scanning technique for its detection: graded pressure with the probe can make fluid herniation into the tear more evident. In bursal tears, visualization of the integrity of the deep articular fibers is always required so as not to confuse these tears with full-thickness tears. Articular surface tears are more common than bursal ones, but are also more difficult to detect with US. They appear as a discontinuity of the articular line of the tendon filled with joint effusion and are associated with a normal insertion of the superficial bursal fibers (Fig. 6.73). Often, they appear as a deep mixed 249 Shoulder * * GT a b Fig. 6.70a,b. Bursal-side partial-thickness tear of the supraspinatus tendon. a Schematic drawing of a long-axis view through the supraspinatus tendon and b corresponding 12–5 MHz US image reveal a concave defect (dashed line) at the bursal surface of the supraspinatus tendon in close proximity to the greater tuberosity (GT). The defect is filled with hypoechoic bursal fluid (asterisks). Note the intact deep articular fibers (black curved arrow) of the tendon. 1, acromion; arrowhead, subacromial subdeltoid bursa; 2, supraspinatus tendon; straight arrow, glenohumeral joint cavity; white curved arrow, articular cartilage; 3, humeral head. Correlative MR imaging of the same case is provided in the insert at the left bottom side of the US image GT a b Fig. 6.71a,b. Bursal-side partial-thickness tear of the supraspinatus tendon. a Schematic drawing of a long-axis view through the supraspinatus tendon and b corresponding 12–5 MHz US image reveal detachment of the superficial tendon fibers (dashed line an a; arrowheads in b) from their insertion into the greater tuberosity (GT). Note a subtle hypoechoic cleft separating the ruptured bursal fibers from the intact deep articular fibers (black curved arrow) of the tendon. 1, acromion; arrowhead, subacromial subdeltoid bursa; 2, supraspinatus tendon; straight arrow, glenohumeral joint cavity; white curved arrow, articular cartilage; 3, humeral head. Correlative MR imaging of the same case is provided in the insert at the bottom left side of the US image hyperechoic and hypoechoic focus at the humeral neck, due to the separation of the retracted distal segment of the tendon from the surrounding intact tissue, resulting in a new acoustic interface within the tendon substance (Fig. 6.74) (van Holsbeeck et al. 1995; Teefey et al. 1999; Bouffard et al. 2000; Yao et al. 2004). Articular side tears are often accom- panied by bone irregularities in the greater tuberosity. Intrasubstance tears may be appreciated as subtle intratendinous longitudinal splits oriented from the bony insertion proximally without exiting onto either the bursal or the articular side of the tendon. They appear as thin fluid-filled intratendinous lines and must be assessed in their long and short axis to 250 S. Bianchi and C. Martinoli GT GT a b Fig. 6.72a,b. Bursal-side partial-thickness tears of the supraspinatus tendon. Two different cases. a Long-axis 12–5 MHz US image over the supraspinatus tendon shows focal herniation of hypoechoic bursal tissue within the defect (arrowheads). Note the thickened bursal walls (arrow) and the loss of the normal convexity of the peribursal fat at the site of the tear. GT, greater tuberosity. b Long-axis 12–5 MHz US image over the supraspinatus tendon reveals hyperechoic peribursal fat filling a small superficial defect (arrowheads) in the absence of local effusion. * GT a b Fig. 6.73a,b. Articular-side partial-thickness tear of the supraspinatus tendon. a Schematic drawing of a long-axis view through the supraspinatus tendon and b corresponding 12–5 MHz US image demonstrate detachment of the deep articular fibers (open arrow) of the tendon from their bone insertion. A small hypoechoic effusion (asterisk) is seen filling the tear. Note the intact bursal fibers (black curved arrow) of the tendon and the irregular cortical outline (arrowheads) of the greater tuberosity (GT). 1, acromion; arrowhead, subacromial subdeltoid bursa; 2, supraspinatus tendon; straight white arrow, glenohumeral joint cavity; white curved arrow, articular cartilage; 3, humeral head. Arthro-CT imaging correlation of the same case is provided in the insert at the bottom left side of the US image GT * GT * a b Fig. 6.74a,b. Articular-side partial-thickness tears of the supraspinatus tendon. Spectrum of US appearances. a,b Long-axis 12–5 MHz US images over the supraspinatus tendon show an intratendinous triangular fluid-filled defect (asterisk) with its base facing the cortical surface. The separation of the retracted distal segment of the tendon from the overlying intact tissue results in new acoustic interfaces (arrowheads) within the tendon substance. GT, greater tuberosity. Arthro-MR imaging correlation of the same cases is provided in the inserts at the upper right side of the US images 251 Shoulder avoid pitfalls related to anisotropy and confusion with focal tendinopathy (Fig. 6.75). In other cases, these tears may be characterized by a linear highlevel echo surrounded by a hypoechoic halo of fluid or edematous tendon, the so-called “rim rent” tears (Fig. 6.76) (Bouffard et al. 2000). In a series of 52 shoulders with arthroscopic correlation, US had 93% sensitivity, 94% specificity, 82% positive predictive value and 98% negative predictive value for detecting partial-thickness tears of the rotator cuff (van Holsbeeck et al. 1995). Another more recent study, performed with high-end equipment in which the US findings were controlled with arthroscopic findings, reported 67% sensitivity, 85% specificity, 77% positive predictive value, 77% negative predictive value and 77% accuracy in the diagnosis considering partial-thickness tears as true-positives and no tears as true-negatives (Teefey et al. 2000a). Compared with US, MR arthrography has a higher sensitivity for depicting small partial-thickness tears, particularly those occurring on the articular side of the cuff (Ferrari et al. 2002). 6.5.2.3 Full-Thickness Tears Full-thickness tears extend from the bursal to the articular surface of the tendon. As previously stated, the term “full-thickness” may refer to either a complete (full-width) or an incomplete (partial-width) tendon rupture (i.e., a tear located in the anterior third of the supraspinatus which allows communi- cation between the glenohumeral joint space and the bursa is a full-thickness tear but not a complete tear because the middle and posterior third of the tendon is unaffected). In general, full-thickness tears have a greater extension than partial tears and are, therefore, easier to be detected with US. A classification of full-thickness tears has been proposed in both the radiographic and clinical literature (Lyons and Tomlinson 1992). In small (<5 mm wide) fullthickness tears of the supraspinatus tendon, a thin hypoechoic cleft can be seen connecting the joint cavity and the bursa (Fig. 6.77). The identification of these tears may not be easy because of the lack of tendon retraction (the supraspinatus is maintained in the correct position by its intact portions) and the absence of changes in the inferior boundaries of the deltoid and subdeltoid fat. A focal bursal thickening or a small amount of fluid collected just over the lesion can increase the examiner’s confidence that a lesion is present. In this regard, some authors have even proposed performing the US examination after arthrography to obtain a better assessment of rotator cuff tears as a result of the induced bursal-joint distension (Fermand et al. 2000; Lee et al. 2002). Otherwise, small tears should always be confirmed on both long- and short-axis planes to avoid any confusion with the distal prolongation of supraspinatus muscle tissue. In some cases, a differential diagnosis between a partial-thickness tear and small full-thickness tear cannot be achieved even with high-resolution transducers. Larger fullthickness tears usually affect the anterior portion of the supraspinatus tendon at the level of the criti- b a GT a b Fig. 6.75a,b. Intrasubstance partial-thickness tear of the supraspinatus tendon. a Schematic drawing of a long-axis view through the supraspinatus tendon and b corresponding 12–5 MHz US image display a hypoechoic longitudinal split (void arrow) oriented from the tendon insertion into the greater tuberosity (GT) proximally with integrity of the more external bursal (b) and articular (a) fibers. 1, acromion; arrowhead, subacromial subdeltoid bursa; 2, supraspinatus tendon; white arrow, glenohumeral joint cavity; white curved arrow, articular cartilage; 3, humeral head 252 S. Bianchi and C. Martinoli a GT b Fig. 6.76a,b. Rim rent tear. a Long-axis 12–5 MHz US images of the supraspinatus tendon with b arthro-CT correlation demonstrate a small hypoechoic triangular defect (open arrowheads) with a central hyperechoic line (arrow) extending from the tendon insertion proximally. These tears relate to a minimal detachment of fibers from the greater tuberosity (GT) and should not be confused with linear intratendinous deposits in calcifying tendinitis. In most cases, they affect the articular fibers of the anterior supraspinatus tendon and are associated with irregularities (white arrowhead) in the underlying bone a b c d Fig. 6.77a–d. Small full-thickness tear of the supraspinatus tendon (perforation). a,c Schematic drawings of the supraspinatus tendon depicted in its a long-axis and c short-axis views with b,d corresponding 12–5 MHz US images demonstrate a thin funnel-like hypoechoic cleft (void arrows) connecting the deep glenohumeral joint cavity with the superficial bursa. In d, note slight focal thickening (open arrowheads) of the bursal walls in relation to the tear. In doubtful cases, this sign can enhance the diagnostic confidence of the examiner that a tear is present in the supraspinatus tendon. Arthro-CT imaging correlation of the same case is provided in the insert in d. 1, acromion; arrowhead, subacromial subdeltoid bursa; 2, supraspinatus tendon; GT, greater tuberosity; straight arrow, glenohumeral joint cavity; white curved arrow, articular cartilage; 3 and HH, humeral head Shoulder * GT * GT a b Fig. 6.78a,b. Non-retracted full-thickness tear of the supraspinatus tendon. a Long-axis 12–5 MHz US image over the posterior supraspinatus tendon with b T2-weighted MR imaging correlation displays a linear fluid-filled defect (arrowhead) with minimal proximal tendon retraction (arrow), leaving a small tendon remnant (asterisk) attached to the distal tip of the greater tuberosity (GT) cal area (Fig. 6.78). When the tear is localized in this area, the posterior supraspinatus can appear completely normal. In these cases, US proved to be accurate for predicting the size of the tear. Longaxis scans may be used to measure the amount of retraction of the torn tendon end from the greater tuberosity, whereas an estimate of the tear width can be obtained on short-axis scans from the distance between the torn tendon ends (Fig. 6.79) (Farin et al. 1996b). The accuracy of these measurements is worse in large-sized tears (Teefey et al. 2005), and may be somewhat related to shoulder positioning (Ferri et al. 2005). When scans using the Crass and Middleton positions were compared with the operative findings, the former appeared to reflect more accurately the true size of full-thickness tears in the long-axis plane, whereas both were equally accurate in evaluating the tear size in the short-axis plane (Ferri et al. 2005). Conversely, the Middleton position tended to overestimate, at any extent, the size of the tear. It is conceivable that the two positions can create a different tension across a cuff tear, thus affecting its measured size. In particular, the component of internal rotation in the Middleton position could contribute to increased tension along the tendon length and the subsequent overestimation of tear size (Ferri et al. 2005). The US appearance of full-thickness tears depends on the amount of joint effusion. When a large effusion is present, the tear appears as a focal hypoechoic area due to the fluid that fills in the tendon discontinuity (Figs. 6.80, 6.81). In these cases, graded pressure with the probe may be helpful to distinguish the hypoechoic fluid from the tendon. When the effusion is small, it tends to collect in the most dependent portions of the bursa and the joint cavity, thus not filling the tear. In these cases, the diagnosis is based on focal non-visualization of tendon fibers. In the absence of effusion or tendon retraction, tilting the probe and pressing it over the tendon can demonstrate the detachment of the fibers from their humeral insertion. Full thickness tear lead to a naked appearance of the greater tuberosity as the bone is no longer covered by the retracted tendon (Fig. 6.80). In these patients, care must be taken not to mistake the deltoid muscle for the supraspinatus. Among the indirect signs of supraspinatus tendon tears, the most important include focal herniation of the deltoid muscle and peribursal fat into the space created by the tear (Fig. 6.82a,b). This sign is more pronounced in full-thickness than in partialthickness tears and can be appreciated even better when pressure is applied with the probe. In addition, there may be prominent reflection of the US beam at the interface of fluid and the articular cartilage, a sign which is commonly referred to as the “uncovered cartilage sign” or the “cartilage interface sign” (Fig. 6.82c,d). Although this latter sign can be seen in large partial tears affecting the articular surface of the supraspinatus, it is most frequently encountered in full-thickness tears when there is anechoic fluid overlying the articular cartilage. One should be aware, however, that this latter sign is subjective and can also be appreciated in normal states (Jacobson et al. 2004). The occurrence of bone irregularities in the profile of the greater tuberosity is an important finding to be routinely sought because it is not simply related to aging but also sig- 253 254 S. Bianchi and C. Martinoli * * 1 a c bt 2 b d Fig. 6.79a–d. Size assessment of a supraspinatus tendon tear. a Long-axis and b short-axis 12–5 MHz US images of a full-thickness tear of the anterior supraspinatus tendon (asterisks) with c,d schematic drawing correlations showing transducer positioning demonstrate the amount of tendon retraction (1) and the width of the tear (2) as indicated by the distance (white lines) between the gray vertical bars. The ovoid intra-articular portion of the biceps tendon (bt) may help the examiner to establish that the affected portion of the tendon is the mid-anterior one a b Fig. 6.80a,b. Large full-thickness tear of the supraspinatus tendon. a Schematic drawing of a long-axis view through the supraspinatus tendon and b corresponding 12–5 MHz US image demonstrate a large fluid-filled defect (asterisk) in the region of the tear, where the tendon once inserted on the greater tuberosity (GT). Note the naked appearance of the greater tuberosity and the retracted rotator cuff tissue (open arrow) which overlies the humeral head. 1, acromion; arrowhead, subacromial subdeltoid bursa; 2, supraspinatus tendon; white straight arrow, glenohumeral joint cavity; white curved arrow, articular cartilage; 3, humeral head Shoulder Deltoid a b Fig. 6.81a,b. Full-thickness tear of the supraspinatus tendon. a Short-axis 12–5 MHz US image over the middle third of the supraspinatus tendon with b T2-weighted MR imaging correlation display a large fluid-filled full-thickness tear (arrow). Slight pressure applied with the transducer over the tear causes herniation (arrowheads) of hypertrophied bursal tissue into the defect with loss of the normal convexity of the cuff and peribursal fat GT a b GT c bt d Fig. 6.82a–d. Deltoid herniation and uncovered cartilage sign. As in Figure 6.81, a long-axis 12–5 MHz US image over the supraspinatus tendon with b schematic drawing correlation demonstrates herniation (large arrow) of the deltoid muscle and hypertrophied bursal tissue into the space created by a full-thickness tear. The small arrow indicates tendon retraction. Note the irregular cortical outline of the greater tuberosity (GT). c Long-axis and d short-axis 12–5 MHz US images reveal a small full-thickness tear of the anterior supraspinatus tendon. The acoustic interface between the fluid in the tear (arrowheads) and the surface of the articular cartilage produces a bright linear echo (curved arrow) which can be considered an indirect sign of a rotator cuff tear. bt, biceps tendon 255 256 S. Bianchi and C. Martinoli nificantly associated with rotator cuff tears, particularly with full-thickness supraspinatus tendon tears (Wohlwend et al. 1998; Huang et al. 1999; Jiang et al. 2002; Jacobson et al. 2004). This sign has been found to be very important, as it has the highest sensitivity and negative predictive value in the diagnosis of supraspinatus tendon tear (Jacobson et al. 2004). On the other hand, contradictory results are reported in literature as to whether the US finding of bursal fluid combined with a joint effusion may be considered a specific predictive sign for a rotator cuff tear (Hollister et al. 1995; Arslan et al. 1999). This could be explained by the fact that bursal or joint fluid is common in patients with shoulder impingement even in the absence of a rotator cuff tear (Jacobson et al. 2004). Considering full-thickness tears as true-positives and no tears as true-negatives, a recent study performed with high-end equipment in which the US findings were controlled with arthroscopic findings has reported 100% sensitivity, 85% specificity, 96% positive predictive value, 100% negative predictive value and 96% accuracy in the diagnosis (Teefey et al. 2000a). In terms of study reproducibility, a low level of interobserver variability was demonstrated in the US detection, characterization and localization of rotator cuff tears by comparing the results of two expert blinded observers in a group of 61 patients (Middleton et al. 2004). In the few discrepant cases, the disagreement concerned whether there was a full-thickness or a partial-thickness tear or whether a tear involved both the supraspinatus and infraspinatus tendons or one or the other of these tendons (Middleton et al. 2004b). Other diagnostic errors also occur in distinguishing tendinopathy from partial-thickness tears (Teefey et al. 2005). These data seem particularly important given that US is generally regarded as one of the more operatordependent imaging techniques. On the other hand, poor agreement is expected when there is marked disparity between the operators’ experience levels (O’Connor et al. 2005). Compared with MR imaging, US has been demonstrated to have a comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears if performed by an experienced examiner using high-end equipment (Jacobson 1999; Martin-Hervas et al. 2001; Teefey et al. 2004). When the examiner has comparable experience with both imaging tests, the decision regarding which test to perform for rotator cuff assessment does not need to be based on concerns about accuracy (Chang et al. 2002; Teefey et al. 2004). Instead, it can be based on other factors, such as the importance of ancillary clinical information (regarding lesions of the glenoid labrum, joint capsule, or surrounding muscle or bone), the presence of an implanted device, patient tolerance and cost (Teefey et al. 2004). 6.5.2.4 Complete and Massive Tears When a full-thickness tear spreads to involve the full width of the supraspinatus, the tendon retracts medially. The amount of tendon retraction depends mainly on the age of the tear. In acute lesions, the tendon is less retracted and its tip can still be detected with US (Fig. 6.83a–c). In the more common chronic ruptures, the tendon end disappears beneath the coracoacromial arch as a result of involutional processes in the tendon substance and upward displacement of the humeral head (Fig. 6.83d,e). This condition can be promptly recognized with US. The main US findings include nonvisualization of the tendon and herniation of the deltoid, which shows a rectilinear or convex inferior margin facing the humeral convexity. A broad area of the upper convexity of the humeral head appears uncovered by the supraspinatus, the so-called “naked head” sign. Joint and bursal fluid is often absent (Teefey et al. 2000b). Especially in cases of mild retraction of the torn tendon end, short-axis planes are essential to distinguish complete (full-thickness, full-width) from incomplete (full-thickness, partial-width) tears of the supraspinatus tendon (Fig. 6.84). A number of possible pitfalls may mask or simulate a complete tear of the supraspinatus tendon. Although most of these pitfalls are easy to recognize and, therefore, unlikely to present a diagnostic problem, others are potentially confusing. Among them, the continuous layer of hypoechoic humeral cartilage resting on a naked humeral head may create confusion with an intact tendon (Fig. 6.85a). Similarly, massive calcific deposits in the supraspinatus tendon related to calcifying tendinitis should not be mistaken for a naked humeral head (Fig. 6.85b) (Middleton et al. 1986a). Familiarity with these imaging findings, coupled with the knowledge of the normal US anatomy of the rotator cuff, can facilitate recognition of true disease and help avoid misdiagnosis. After assessing a complete rupture of the supraspinatus tendon, attention should always be directed to the infraspinatus and subscapularis tendons to detect any possible posterior or anterior extension 258 S. Bianchi and C. Martinoli * Acr HH HH a Acr * b Fig. 6.85a,b. Pitfalls in the diagnosis of complete supraspinatus tendon tear. a Long-axis 12–5 MHz US image over the cranial aspect of the humeral head (HH) in a patient with a non-fluid-filled retracted tear of the supraspinatus tendon demonstrates a thick hypoechoic layer (arrows) covering the cortical bone of the humerus. This is the articular humeral cartilage and should not be mistaken for residual fibers of the supraspinatus tendon. Acr, acromion. b Long-axis 12–5 MHz US image over the supraspinatus tendon in a patient with calcifying tendinitis. Massive calcific deposits (asterisks) with well-defined posterior acoustic shadowing occupy the supraspinatus tendon almost completely, giving it a hyperechoic appearance that possibly resembles a convex bony surface. The thin hypoechoic band (arrowheads) which overlies the hyperechoic calcifications reflects bursal tissue. A hypoechoic cleavage plane separates the humeral head (HH) from the intratendinous calcifications. This sign may be helpful in making a correct diagnosis. Acr, acromion of the lesion leading to a massive tear of the rotator cuff. Not uncommonly, a complete tear of the supraspinatus can be seen expanding in the posterior direction to involve the infraspinatus tendon. US findings of infraspinatus full-thickness tears are often similar to those already described for the supraspinatus (Fig. 6.86). Dynamic scanning during internal and external rotation of the arm can be helpful to demonstrate the torn infraspinatus tendon detached from its insertion on the humeral head. In these cases, atrophic changes in the infraspinatus muscle and a slight hypertrophy of the teres minor muscle can be appreciated on posterior sagittal scans (Fig. 6.87). The examiner should be aware, however, that infraspinatus muscle atrophy may also occur with either an intact tendon as a result of disuse in patients with full-thickness anterior cuff tendon tears or suprascapular neuropathy (Yao and Mehta 2002). Therefore, this finding does not imply that the infraspinatus tendon is ruptured. Due to the intrinsic interwoven structure of the supraspinatus and infraspinatus tendons, some fullthickness tears of the supraspinatus may progress at the posterior margin of the defect along a horizontal cleavage plane causing a complex pattern of delamination (Fig. 6.88a). These horizontal tears are probably related to shearing stress forces generated by the defect in the supraspinatus tendon. They consist of a fissuration parallel to the plane of the articular side of the tendon and appear as linear hypoechoic defects in the middle thickness of the tendon. Detection of hori- zontal tears has clinical relevance because it changes the surgical approach. Unlike arthro-CT or arthroMRI, US does not easily reveal these tears. Changes are usually subtle and experience is needed to correctly recognize this entity. When visible, horizontal tears appear as focal linear hypoechoic defects in the middle of the tendon (Fig. 6.89). In rare cases, insinuation of fluid into the tear can generate intramuscular cysts which appear as well-defined hypoanechoic masses inside the belly of the supraspinatus or infraspinatus muscle (Fig. 6.88b). Tears of the teres minor tendon are extremely rare and usually result from acute shoulder trauma rather than caudal progression of a tear from the infraspinatus. While tears of the infraspinatus tendon are almost invariably associated with rupture of the supraspinatus, subscapularis ruptures can also be encountered as an isolated problem. Subscapularis tendon tears are mainly related to acute traumatic lesions produced with the arm abducted and in external rotation. Similar to other rotator cuff tendons, complete tears of the subscapularis are revealed by the absence of tendon fibers and the concavity of the deltoid over the naked anterior surface of the humeral head. Incomplete tears of the subscapularis tendon often involve the cranial and preserve the caudal portion of the tendon (Fig. 6.90). This pattern should not be mistaken for complete tears. For this purpose, the morphology of the lesser tuberosity as seen on sagittal planes may help to establish the caudal limit of the tendon and avoid any confusion Shoulder HH * InfraS Gl a b Acr S * c d Fig. 6.86a–d. Complete (full-thickness, full-width) tear of the infraspinatus tendon. a Transverse 12–5 MHz US image over the posterior aspect of the glenohumeral joint demonstrates a fluid-filled rotator cuff tear (asterisk) producing herniation (open arrows) of the deltoid muscle and peribursal fat at the site of the tear. Note the naked appearance of the humeral head (HH) and the retracted end (arrowheads) of the infraspinatus tendon (InfraS) over the bony glenoid (Gl). b Corresponding schematic drawing shows transducer positioning over the long axis of the ruptured infraspinatus tendon (IS). Tm, teres minor. c Sagittal extended field-of-view 12–5 MHz US image over the posterior aspect of the glenohumeral joint with d T2-weighted MR imaging correlation reveals fluid (asterisk) filling the infraspinatus tendon tear and the deltoid muscle overhead (open arrows) falling into the tendon defect. At a more caudal level, note the ovoid appearance of the intact teres minor muscle (white arrows). S, scapular spine; Acr, acromion between incomplete and complete tendon ruptures (Fig. 6.91). In addition, because of the peculiar insertion of the subscapularis on the lesser tuberosity and relationships of this tendon with the long head of the biceps tendon, subscapularis tears usuallycause secondary instability of the biceps tendon. A more detailed explanation of the mechanism of involvement of the biceps tendon will be given later. Once a complete evaluation of rotator cuff tendons has been performed, the size and location of the tear has been determined and the degree of retraction of the torn tendon has been assessed, the status of the rotator cuff muscles should also be evaluated to rule out possible hypotrophy and fat degeneration (Sofka et al. 2004a). In fact, the orthopaedic literature has confirmed that recognition of muscle atro- phy may contribute to a more precise choice of either surgical or conservative treatment for patients with rotator cuff tears, and may be useful for proving that a post-traumatic lesion is not true but related to a pre-existing degenerative state. Furthermore, presence of muscle atrophy following surgical repair of a torn cuff may indicate that lack of functional recovery is due to the state of the muscles and is not related to unsuccessful surgery. 6.5.2.5 Intramuscular Cysts Cysts located within the rotator cuff muscles are essentially an imaging diagnosis as they are embed- 259 Shoulder b GT a c Fig. 6.89a–c. Delamination of rotator cuff tears. a Long-axis 12–5 MHz US image over the infraspinatus tendon in a patient with full-thickness tear of the posterior supraspinatus. A longitudinal hypoechoic cleft (arrows) in the middle thickness of the infraspinatus tendon is observed reflecting delamination of fibers extending posteriorly to a supraspinatus tendon tear. GT, greater tuberosity. b,c Oblique coronal arthro-CT images obtained over b the middle and c the posterior third of the supraspinatus tendon demonstrate an intratendinous horizontal cleavage plane (arrowheads) in continuity with the full-thickness tear (arrow) of the supraspinatus LT c a * LT b c Fig. 6.90a–c. Incomplete (full-thickness, partial-width) subscapularis tendon tear. a,b Transverse 12–5 MHz US images obtained over the anterior aspect of the humeral head (a upper level; b lower level) with c coronal T2-weighted MR imaging correlation reveals a full-thickness tear of the upper portion of the subscapularis tendon. At the cranial level, no appreciable cuff tissue is visible. Note the naked appearance of the lesser tuberosity (LT). A thin soft-tissue layer (void arrowheads) lies between the humeral head and the deltoid: this represents thickened bursal tissue and should not be mistaken for cuff remnants. Shifting the transducer slightly downward, some intact fibers (arrows) of the lower portion of the subscapularis are demonstrated. The residual tendon has normal thickness and attachment into the lesser tuberosity. This finding indicates a full-thickness tear of the subscapularis involving the cranial half of the tendon. In the MR image, note hyperintense fluid (asterisk) filling the wide gap left by the torn tendon fibers and the intact lower tendon third (arrows). C, coracoid. White arrowheads, long head of the biceps tendon 261 262 S. Bianchi and C. Martinoli * * * LT a b Fig. 6.91a,b. Incomplete (full-thickness, partial-width) subscapularis tendon tear. a Sagittal 12–5 MHz US image obtained over the short-axis of the subscapularis tendon with b sagittal arthro-CT correlation demonstrates a full-thickness tear of the upper two thirds of the subscapularis tendon. Note the flat appearance (dashed line) of the lesser tuberosity (LT), which curves toward depth just caudal to the tendon insertion. This can be a useful landmark to establish the caudal limit of the tendon. In this particular case, a wide fluid-filled cleft (asterisks) occupies the upper two thirds of the subscapularis insertion. Note some echogenic fibers (arrow) of the subscapularis tendon which remain inserted onto the lowest aspect of the lesser tuberosity. Compare Figure 6.90a with the normal short-axis appearance of the subscapularis shown in Figure 6.26a ded within the muscle and, therefore, cannot be recognized at either open or arthroscopic surgery. At US, intramuscular cysts appear as well-defined hypoanechoic masses with regular margins, located within the bellies of these muscles (Fig. 6.92). Color Doppler imaging does not usually reveal flow signals in the cystic wall. Once a cyst has been diagnosed, a careful search should be performed with US to identify possible full-thickness or partial undersurface (horizontal cleavage) tears of the rotator cuff tendons. In fact, a close association of these cysts with rotator cuff pathology has been described (Sanders et al. 2000). From the technical point of view, abduction and external rotation of the shoulder (ABER positioning) may be useful to better visualize these cysts, possibly because this position removes tension from the tendons and muscles of the cuff and thus makes entry of fluid into the tendon tear easier (Kassarjian et al. 2005). Although there are no studies in the radiological literature dealing with the pathologic findings of these lesions, their pathogenesis is still debated. Two main hypotheses seem possible. One theory is that they may represent synovial cysts due to progressive accumulation of articular fluid inside the muscle through a tendon tear (Fig. 6.88b). It has been noted that the tendon tear may not be located in the tendon of the muscle containing the cyst but in the tendon of an adjacent muscle, with the cyst developing as a result of a delamination process (Kassarjian et al. 2005). On the other hand, it has been proposed that these cysts are ganglia arising from the rotator cuff tendons as a result of a degenerative process. As with other cysts around the shoulder, needle aspiration of intramuscular cysts can be attempted under US guidance. 6.5.2.6 Cuff Tear Arthropathy In massive rotator cuff tears, the medial retraction of the torn thinned tendons and the contraction of the deltoid muscle cause upward displacement of the humeral head resulting in an increased conflict between the superior facet of the greater tuberosity and the inferior aspect of the acromion (Fig. 6.93a). Chronic local trauma leads to degenerative bony changes such as sclerosis, subchondral cysts, spurring and thinning of the acromion and cortical irregularities. In longstanding disease, subacromial changes are followed by a direct involvement of the glenohumeral space related to the incongruity between the articular surfaces. The resulting condition is referred to as eccentric (because of the upward displacement of the humeral head) osteoarthritis or “cuff tear arthropathy” (Neer et al. 1983b). This state can be considered an end-stage irreversible destructive arthropathy consisting of a reduced or absent subacromial space, thinning and loss of the articular cartilage at the lower third of the humeral head and the superior aspect of the glenoid cavity, inferior osteophytes of the humeral head, a rounded and irregular greater tuberosity due to abrasion during abduction of the arm with flat- Shoulder Acr * Acr * a c Cl Acr Acr * C * b d Fig. 6.92a–d. Intramuscular cyst. a Long- and b short-axis 12–5 MHz US images over the supraspinatus muscle (arrows) obtained by placing the transducer immediately posterior and medial to the acromioclavicular joint reveal an intramuscular cyst (asterisk). In this particular case, the cyst was associated with an articular-side partial-thickness tear of the supraspinatus tendon. Acr, acromion; Cl, clavicle. c Oblique coronal fat-suppressed Gd-enhanced T1-weighted and d sagittal proton density MR imaging correlations. In c, contrast enhancement is seen in the muscle tissue surrounding the cyst tening of the bicipital sulcus and a reduced thickness of the acromion. In chronic longstanding disease, the occurrence of a stress fracture of the acromion can occur as a result of local trauma induced by the humeral head (Hall and Calvert 1995). It has been suggested that rotator cuff arthropathy may derive from both mechanical factors and reduced cartilage nutrition due to the increased volume of the articular cavity and subsequent decrease in intra-articular pressure (Neer et al. 1983b). The diagnosis of rotator cuff arthropathy basically relies on its radiographic appearance. We believe standard radiographs are mandatory before a US study because examining a patient with rotator cuff arthropathy with US as a first examination may be a challenge, especially for the beginners. The main US findings include a massive tear of two or more rotator cuff tendons associated with a markedly reduced or absent subacromial space, loss of the articular cartilage, a rounded and irregular greater tuberosity due to abrasion during abduction of the arm with flattening of the bicipital sulcus, a reduced thickness of the acromion and marginal osteophytes on the inferior humeral head (Figs. 6.93, 6.94). Joint and bursal effusions may contain echogenic debris. The close contact of the humeral head with the undersurface of the acromion may make differentiation between these structures less immediate with US. The best way to separate these structures is by dynamic scanning on coronal planes (somewhat oriented along the long axis of the supraspinatus) over the tip of the acromion while abducting the patient’s arm in internal rotation. This maneuver may help to distinguish the moving humeral head from the stationary acromion and to appreciate the reduced distance between them. An additional problem may be related to the localization of the bicipital sulcus which is, at least in part, effaced by the abrasions in the greater tuberosity. This can lead to some technical problems even for the experienced examiner because the sulcus is a main landmark for rotator cuff evaluation. In the case of an intact subscapularis tendon, its identification may be helpful to localize the position of the flattened sulcus. A reduced thickness of the acromion may also be observed. These patients have a proximal migration of the humeral head such that it contacts the undersurface of the acromion. This contact point functions 263 Shoulder 6.5.2.8 Postoperative Cuff In the early stages, the impingement syndrome is treated conservatively with restriction of activities, physical therapy, anti-inflammatory drugs and, possibly, steroid injections in the subacromial subdeltoid bursa (Bokor et al. 1993). When conservative treatment fails, surgery is indicated. A basic knowledge of the type of surgical intervention performed and its extent is critical for the examiner to reach a correct interpretation of the US images. Before the examination, details of the surgical intervention should always be collected from the surgical reports or the patient’s records. Generally speaking, the main surgical techniques for impingement syndrome and rotator cuff disease involve subacromial decompression and rotator cuff repair or debridement. In patients with subacromial impingement but without rotator cuff tears, subacromial decompression may be performed with either an open procedure through an anterolateral deltoid splitting incision or arthroscopy (Fig. 6.97a,b). The open approach consists of excision of the anteroin- Del Del Del Del HH HH a b Acr c ferior aspect of the acromion, including the distal end of the clavicle, and resection or debridement of part of the coracoacromial ligament (Fig. 6.97c,d). If prominent osteophytes are present, the acromioclavicular joint and the distal 2.5 cm of the clavicle may be removed. On the other hand, arthroscopic subacromial decompression is carried out by resecting the anterior edge and inferior surface of the acromion along with the subacromial subdeltoid bursa and the subdeltoid fat. The coracoacromial ligament is released and the distal clavicle is resected as well. Combined open and arthroscopic approaches may be used in the event of large full-thickness tears of the rotator cuff. Although arthroscopy does not require deltoid incision (leading to secondary weakness of the muscle), this technique is more often associated with persistence or recurrence of pain (procedure failure reported in up to 3–11% of cases), as a result of insufficient excision of the acromion. Other complications include progression of rotator cuff tendinosis, residual or recurrent rotator cuff tears and postoperative adhesions. In patients with rotator cuff tear, the type of intervention mainly depends on the location, thickness and severity of Cl d Fig. 6.97a–d. Postoperative cuff: normal US findings. a,b Anterolateral deltoid splitting following open acromioplasty. Postsurgical coronal 10–5 MHz US images obtained lateral to the acromion while keeping the arm a abducted and b in neutral position. The deltoid (Del) tear produces a focal defect (arrows) in the normal convex muscle and causes herniation (arrowheads) of subcutaneous fat within the tear. Note that the gap in the muscle enlarges with the arm in neutral position. HH, humeral head. c,d Subacromial decompression including distal resection of the clavicle (Mumford procedure). c Postsurgical coronal 10-5 MHz US image over the acromioclavicular joint with d radiographic correlation demonstrate an increased distance (arrows) between the acromion (Acr) and the clavicle (Cl) 267 268 S. Bianchi and C. Martinoli the tear. In small partial-thickness tears, treatment ranges from debridement of frayed tendon tissue to a combined excision of the defect and repair of the adjacent healthy margins of the cuff. In fullthickness tears, repair may be performed with either a side-to-side suture (small tears) or tendonto-bone reattachment (large tears), both associated with acromioplasty. Usually, these procedures are carried out arthroscopically using three bursal portals (anterior, lateral and posterior) or with a mini open repair (least possible split in the deltoid, preserving the acromial origin of the muscle). In large full-thickness tears, a tendon-to-bone repair is usually performed, reattaching the tendon at a more proximal site (humeral neck) relative to the greater (supraspinatus) or the lesser (subscapularis) tuberosity (Figs. 6.98, 6.99). Nonabsorbable sutures or metallic anchors (arthroscopic repairs) are used for this procedure. Massive tears are, for the most part, treated with debridement alone. The diagnostic role of MR imaging of a shoulder that has undergone surgical treatment is controversial due to sutures, suture anchors and osseous changes that may alter signal intensities within the acromion, humeral head and rotator cuff tissue (Magee et al. 1997). US has the advantage that it is unaffected by the presence of intraosseous hardware. Nevertheless, postoperative shoulder US may be a challenge, especially if the operative details are not available. At US examination, a repaired supraspinatus usually appears much more heterogeneous than normal. The superficial tendon boundaries may assume a slightly concave profile a b when the supraspinatus is scarred and reduced in volume. In addition, the bursal surface of the tendon is often undefined as a result of bursal removal. Intratendinous nonabsorbable suture material and suture anchors may be seen as bright linear echoes with faint reverberation artifact (Figs. 6.98c, 6.100a). The examiner should be conscious that the retracted torn tendon is often implanted in the humeral neck rather than in the greater tuberosity. As a result, some bare bone in the region of the greater tuberosity should not necessarily be regarded as a recurrent tear. The most reliable US signs of a re-torn supraspinatus are: nonvisualization of the cuff because of complete tendon avulsion and retraction under the acromion, presence of a focal defect in the rotator cuff, a variable degree of tendon retraction from the surgical trough and detection of sutures floating freely in the fluid (Fig. 6.100b) (Crass et al. 1986; Hall 1986; Mack et al. 1988b; Prickett et al. 2003). In difficult cases, dynamic scanning may be helpful to distinguish the impairment related to a recurrent tear from adhesive capsulitis as well as to assess the functional result of acromioplasty. Overall, the diagnostic accuracy of US for detection of postoperative rotator cuff tears is similar to that for imaging of shoulders that have not been operated on (Mack et al. 1988; Furtschegger and Resch, 1988; Prickett et al. 2003). The most recent series based on newer equipment, current US criteria for tears and complete surgical validation of the results reported 91% sensitivity, 86% specificity and 89% accuracy for US identification of rotator cuff integrity postoperatively (Prickett et al. 2003). c Fig. 6.98a–c. Postoperative cuff: normal US findings. a Schematic drawing illustrates the modality of reattachment of the supraspinatus tendon to the greater tuberosity using a suture anchor (curved arrow) after a full-thickness cuff tear. b Anteroposterior shoulder radiograph demonstrates the metallic anchor (curved arrow) fixed at the level of the humeral neck. c Long-axis 12–5 MHz US image over the supraspinatus tendon reveals intratendinous sutures (arrowheads) and the drilled hole (arrows) in the bone containing the anchor to which they are connected. US displays an intact tendon repair 270 S. Bianchi and C. Martinoli sites. Although the pathogenesis of calcifying tendinitis is not completely understood, this condition seems to be related to hypoxic areas or metabolic factors in tendons and is typically associated with an intact rotator cuff. Local hypoxia is believed to lead to fibrocartilaginous metaplasia that is turn produces the calcifications (Flemming et al. 2003). Four stages of the disease can be recognized: precalcific, calcific, resorptive and postcalcific (Uhthoff and Sarkar 1989). In the resorption phase, the tendon develops increased vasculature and the calcium deposits are removed by phagocytes. There is significant correlation between acute pain attacks and histologic evidence of calcium resorption. At the time of diagnosis, patients may be asymptomatic or may present with either acute or chronic pain. Typical symptoms include either subacute low-grade shoulder pain increasing at night (formative phase) or a sharp acute pain limiting shoulder movements and seldom accompanied by fever due to rupture of the calcification in the adjacent structures (resorptive phase). The diagnosis of calcifying tendinitis is based on plain films (anteroposterior views in internal, neutral and external rotation, outlet view) which can accurately assess the size and location of the calcifications. Radiographs can also detect calcific deposits inside the bursa and the occurrence of focal erosions on the humeral head. Asymptomatic rotator cuff calcifications do not require treatment. In symptomatic cases, calcifying tendinitis can be managed conservatively with physical therapy and a short course of nonsteroidal anti-inflammatory drugs. Complications are best treated with more aggressive therapy including systemic steroids. Evacuation of the calcific material can be obtained by means of arthroscopy or US-guided puncture, lavage and aspiration as described in Chapter 18. At US, rotator cuff calcifications appear as intratendinous hyperechoic foci. Three main types of calcium deposits can be identified with US depending on the amount of calcium contained in the deposit. Type I calcifications appear as hyperechoic foci with well-defined acoustic shadowing, similar to gallstones (Fig. 6.101a). These calcifica- a b c d Fig. 6.101a–d. Calcifying tendinitis: types of calcification. a Type I calcification appears as an intratendinous hyperechoic focus (arrows) with well-defined posterior acoustic shadowing (arrowheads). This appearance correlates with the formative phase of calcium deposition. b Type II calcification presents as a hyperechoic focus (arrows) with faint shadowing (arrowheads). c,d Type III calcification may appear either as c a hyperechoic focus (arrows) with absent shadow or as d an undefined isoechoic or slight hyperechoic structure (arrows) with mobile internal echoes, reflecting a semiliquid content. Both type II and type III calcifications more likely correspond to the resorptive phase of calcifying tendinitis Shoulder tions correspond to the formative phase of calcium deposition and account for approximately 80% of cases. Type II and type III calcifications (“slurry” calcifications) look like hyperechoic foci with a faint (type II) or absent (type III) shadow and can be referred to the resorptive phase, in which the deposits are nearly liquid and can be successfully aspirated (Fig. 6.101b,c). In symptomatic patients, these deposits are more often associated with local hyperemia at color Doppler imaging (Chiou et al. 2002). Often, semiliquid deposits are difficult to diagnose because they appear nearly isoechoic with the tendon (Fig. 6.101d). An oval area of fibrillar loss and small hyperechoic dots within the affected tendon is the main criterion for detecting them. The shape of the calcification is quite variable, ranging from well-defined chunks of calcium to thin hyperechoic strands in the cuff (Fig. 6.102). Del * These stripe-like deposits are typically located at the preinsertional level (calcific enthesopathy) and should not be confused for intratendinous partialthickness tears, such as rim rent tears. Although standard radiographs can establish the tendon in which the calcific deposit is located, US examination is valuable to determine which portion of the tendon is affected, the distance of the calcification from an arthroscopic landmark such as the biceps tendon (particularly useful when the deposit does not bulge over the tendon surface) and, most importantly, whether the calcification cause impingement (Figs. 6.102a,d, 6.103). Dynamic examination can reveal the impingement of the calcification against the acromion while abducting the arm in internal rotation. In the case of semiliquid deposits, local compression and tilting the probe over the calcific focus can induce movements of the fluid calcium. * a d b e c f Fig. 6.102a–f. Calcifying tendinitis: shapes of calcification. a–c Series of 12–5 MHz US images with d–f corresponding radiographs demonstrate the range of appearances of intratendinous calcifications in patients with calcifying tendinitis. a,d Bulky ovoid calcification (asterisk) in the subscapularis tendon. Due to its large size, this deposit impinges on the deep surface of the deltoid muscle. b,e Diffuse slurry calcifications (arrows) in the supraspinatus tendon. c–f Preinsertional stripe-like deposits (large arrow) in the supraspinatus tendon 271 Shoulder 6.5.3 Biceps Tendon Pathology 6.5.3.1 Biceps Tendinopathy Tendinopathy of the long head of the biceps tendon, including tenosynovitis and tendinosis, derives from two main mechanisms: impingement and attrition. In the first, the intracapsular portion of the biceps is pinched between the humeral head and the coracoacromial arch during abduction and rotation of the arm. The mechanism is similar to that leading to supraspinatus impingement. In addition, if the supraspinatus is torn, the humeral head is displaced upward by the action of the deltoid so that the biceps tendon is pulled by the humeral head and becomes its main depressing structure (Fig. 6.108a). Chronic tension related to this overload may be contributory to tendon degeneration (Wallny et al. 1999). The second mechanism derives from chronic conflict between the intertubercular portion of the biceps and a narrowed bicipital groove caused by local periostitis, osteophytes and bony irregularities in the lesser tuberosity (Pfahler et al. 1999). The main signs of tendinopathy are biceps tendon hypertrophy related to edema and heterogeneous echotexture with fissurations (Fig. 6.108b,c). These abnormalities are maximal at the level of tendon reflection over the humeral head and at the proximal portion of the bicipital sulcus. Color flow signals may be recognized around the swollen tendon as well. In some cases, the extra-articular portion of the biceps may appear normal and this finding may be misleading if scanning does not systematically include its intra-articular portion. In biceps tendin- B Hs a b B * c d e Fig. 6.107a–e. Intraosseous penetration of calcifying tendinitis of the pectoralis major. a Transverse 12–5 MHz US image over the myotendinous junction of the long head of the biceps (B) demonstrates a swollen and hypoechoic pectoralis major tendon (arrowheads) and a cortical erosion (arrows) at the enthesis. Hs, humeral shaft. b Frontal image from a delayed bone scintigram shows a rounded focus (arrow) of marked increased radionuclide uptake at the level of the proximal right humeral shaft. c Anteroposterior radiograph obtained with internal rotation of the arm displays a faint calcification (arrow) adjacent to the humeral cortex. d CT scan demonstrates the typical “comet-tail” calcification (arrowheads) within the distal pectoralis major tendon and a well-defined cortical erosion of the enthesis (curved arrow), reflecting the intraosseous loculation of calcium from the pectoralis tendon. B, long head of the biceps brachii. e Oblique sagittal STIR sequence shows marked hyperintense signal within the soft tissues (arrowhead) and the medulla (asterisk) around the calcific focus (arrow). (Courtesy of Dr. Nicolò Prato, Italy) 275 278 S. Bianchi and C. Martinoli b c d e * a b * c * * d bm * * * e Fig. 6.110a–e. Recent biceps tendon rupture. a Photograph shows distal retraction of the muscle belly (arrows) of the long head of the biceps following tendon rupture, resulting in the characteristic Popeye appearance. b–e Series of transverse 12–5 MHz US images obtained from proximal to distal at the levels (horizontal white bars) indicated in a show an empty sheath (asterisk) just distal to the intertubercular sulcus. As the scanning plane proceeds distally, the retracted tendon end (arrow) is appreciated and surrounded by increasing amounts of sheath effusion (asterisks). In e, note the retracted muscle belly (bm) encircled by considerable intrafascial hematoma (asterisks) GT * LT SubS a b Fig. 6.111a,b. Indirect US signs of biceps tendon rupture. a,b Transverse 12–5 MHz US images obtained over a the rotator cuff interval and b the intertubercular groove. In a, the coracohumeral ligament (arrows) assumes a concave appearance following disruption of the intra-articular portion of the biceps tendon. Note the intact subscapularis (SubS) and small amount of fluid (asterisk) collected under the ligament instead of the biceps tendon. In b, the transverse humeral ligament (arrow) is seen folding inward the intertubercular sulcus, within the space left free by the retracted biceps. GT, greater tuberosity; LT, lesser tuberosity Shoulder Hs a Hs b B c Fig. 6.112a–c. Biceps tendon rupture and pectoralis major tendon. a Transverse 12–5 MHz US image obtained on the long axis of the pectoralis major tendon (arrowheads) in a patient with biceps tendon tear demonstrates a hypoechoic effusion (curved arrow) instead of the myotendinous junction of the long head of the biceps. b Normal contralateral side showing the myotendinous junction of the biceps (B) located just deep to the pectoralis insertion (arrowheads) Hs, humeral shaft. c Schematic drawing of a coronal view through the anterior shoulder and the upper arm shows a lower position of the retracted myotendinous junction of the biceps relative to the pectoralis major tendon. In doubtful cases, placing the probe on the long axis of the pectoralis tendon as a landmark may help the diagnosis of biceps tendon rupture and white” appearance is often noted on transverse scans (Fig. 6.113). Occasionally, there may be selfattachment of the ruptured tendon stump into the groove without retraction and care should be taken not to mistake it for a normal tendon. In these cases, the reattachment of the torn tendon in a more distal location may prevent muscle degeneration. The muscle may exhibit a globular appearance as a result of retraction but usually retains a normal internal echotexture (Fig. 6.114a,b). Finally, in rare instances biceps tendon tears may occur at the myotendinous junction with a normal-appearing tendon inside the groove (Fig. 6.114c–e). If the biceps tendon is examined without evaluating the muscle, such tears can be missed completely. Although the US findings of biceps tendon tears are multifaceted, the essential point is to establish whether the tendon is intact or torn: further information on position and echotexture of the tendon ends and the muscle does not affect the therapeutic decision (surgical vs. conservative), which is essentially based on clinical findings such as the patient’s age and activity. In general, biceps tendon ruptures are significantly associated with supraspinatus (96.2% of cases) or subscapularis (47.1% of cases) tendon tears as a result of the same impingement forces and tensile injuries (Beall et al. 2003). 6.5.3.3 Biceps Tendon Instability Due to its curvilinear course and reflection over the humeral head, the biceps is intrinsically predisposed to instability. As a rule, the biceps does not undergo medial subluxation or dislocation out of the bicipital groove when the coracohumeral ligament is intact. If the coracohumeral ligament is torn, as may occur in association with anterior supraspinatus tears, the biceps may dislocate over the intact subscapularis. In such cases, the ruptured lateral part of the coracohumeral ligament can be seen (Fig. 6.115a,b). Dynamic examination during rotational movements of the shoulder can reveal abnormally increased motion of the intraarticular portion of the biceps tendon, which is no longer stabilized by the pulley formed by the coracohumeral and superior glenohumeral ligaments. In these cases, abnormal stress forces can produce early local degeneration with biceps tendon thickening and fissurations. More caudally, the biceps may appear perched over the lesser tuberosity (Fig. 6.115c). Careful scanning technique is needed to image the subluxed long head of the biceps tendon because instability occurs at first cranially, at the intra-articular level. In addition, the slight medial 279 280 S. Bianchi and C. Martinoli SH * * SH LH LH a c * * *LH LH * b d Fig. 6.113a–d. Biceps tendon tear: spectrum of US appearances of the retracted muscle belly in relation to the age of the tear. a Short-axis and b long-axis 12–5 MHz US images over the long (LH) and short (SH) heads of the biceps muscle in a patient with a recent tear of the long head of the biceps tendon demonstrate hypoechoic fluid (asterisks) surrounding the belly of the long head. The muscle appears retracted but exhibits similar echotexture to the adjacent short head. c Short-axis and d long-axis 12–5 MHz US images over the long (LH) and short (SH) heads of the biceps muscle in a patient with chronic longstanding tear of the long head of the biceps tendon reveal marked echotextural differences between the two biceps heads with the long head being much more echogenic. This change reflects atrophy of muscle fibers and fatty muscle infiltration positioning that the tendon normally assumes as it enters the bicipital sulcus should not be mistaken for a pathologic finding. We believe that a proper diagnosis of biceps tendon subluxation can be made with US only when the tendon is seen overlying the lesser tuberosity on transverse scans in which the bicipital sulcus is clearly depicted. When the sulcus is not clearly seen, the apparent subluxation of the biceps tendon can be the result of either an incorrect scanning technique or anatomic variations. In the rare cases of intermittent instability, “to-and-fro” displacement of the tendon out of the groove can be seen. Dynamic scanning with the shoulder in maximal external and internal rotation may help the diagnosis (Farin et al. 1995). In these patients the biceps groove should be accurately imaged on transverse planes to assess its shape (Farin and Jaroma 1996). A congenital shallow intertubercular groove (<3 mm deep) with a flat medial wall predisposes the long head of the biceps tendon to instability (see Fig. 6.20c) (Levinshon and Santelli 1991). In rare instances, dislocation of the biceps tendon can be secondary to a combined tear of the lateral portion of the reflection pulley and the transverse ligament even if the subscapularis is normal. In these patients, the biceps can dislocate superficial to the subscapularis (Fig. 6.116) (Patton et al. 2001; Bennett 2001). When the biceps is subluxed, spurring in the lesser tuberosity may contribute to worsening the tendinopathy as a result of attrition. In these cases, the biceps may be markedly swollen and predisposed to longitudinal splits, as already described. The pathogenetic mechanism of this abnormality is similar to that occurring in the peroneus brevis at the ankle as a result of intermittent anterior subluxation over the lateral malleolus (see Chapter 16). Disruption of the cranial third of the subscapularis tendon, either in isolation or associated with supraspinatus tendon tear, is often associated with biceps instability (Bennett 2001). When the cranial third of the subscapularis is torn, the biceps tendon tends to sublux superficial to it on cranial transverse scans and to rest in a normal position on caudal transverse scans (Fig. 6.117). When the subscapularis tear becomes complete, the biceps slips medially within the glenohumeral joint (Ptasznik and Hennesy 1995; Farin et al. 1995; Farin 1996; Prato et al. 1996). The US diagnosis of biceps tendon dis- Shoulder PMj SH LH a Hs LH Hs d b c Fig. 6.120a–d. Pectoralis major tendon tear. a Photograph of a patient complaining of pain and a palpable defect (arrow) in the anterior wall of the axilla following an attempt to catch a heavy object. b Transverse 12–5 MHz US image over the defect reveals hypoechoic fluid filling the bed (arrowheads) of the ruptured pectoralis major tendon. The injury occurred at the enthesis with detachment of the tendon insertion into bone. Note the anterior displacement (arrow) of the myotendinous junction of the biceps (LH) which appears surrounded by fluid. SH, short head of the biceps. Hs, humeral shaft. c More medially, a transverse 12–5 MHz US image demonstrates a heterogeneous retracted muscle (PMj), especially at its myotendinous origin (arrows) fluid adjacent to the humeral cortex and along the tendinous bed related to the hematoma can help the diagnosis (Fig. 6.120b,c). The long head of the biceps tendon and its myotendinous junction are surrounded by fluid. As the tendon of the pectoralis major is a stabilizer of the long head of the biceps tendon distal to the humeral tuberosities, its rupture leads to elevation of the biceps from the humerus (Fig. 6.120d) (Martinoli et al. 2003). If the lesion occurs at the distal myotendinous junction, US demonstrates a normal tendon insertion on the humerus and swelling and a heterogeneous echotexture at the tendon-muscle junction related to disrupted muscle fibers and intervening hypoechoic hematoma, just deep to the deltoid muscle. In complete ruptures, the muscle belly is retracted medially and may exhibit atrophic changes. With time, adhesions may form a pseudotendon between the retracted muscle and actual tendon stump (Rehman and Robinson, 2005). When differentiation between partial and complete tears is doubtful with US, MR imaging is an accurate means to confirm the diagnosis (Connell et al. 1999; Lee et al. 2000; Carrino et al. 2000). Apart from traumatic injuries, the pectoralis major and minor muscles are the most common congenitally absent muscles (Fig. 6.121). Patients typically have a flattened chest wall with hypoplastic ribs and an elevated nipple. Agenesis of these muscles is often partial and may be part of a syndrome associated with other anomalies: the Poland syndrome (Demos et al. 1985). This syndrome is an autosomal recessive condition with an incidence of 1:30,000 live births, in which the absence of the pectoralis is unilateral and associated with syndactyly and hypoplasia of the ipsilateral upper extremity. US diagnosis of pectoralis agenesis is mainly based on the absence of a muscle belly and tendon. Transverse planes over the anterior chest wall and the myotendinous junction of the biceps are obtained on both sides for comparison. In pectoralis agenesis, a fibrous remnant of the tendon and muscle may occasionally be observed; this finding should not be mislead the examiner into thinking that a congenital absence of the muscle does not exist. There are few reports in literature dealing with spontaneous rupture of the deltoid muscle. In the reported cases, the injury occurred in patients with chronic, massive rotator cuff tears and was in some instances responsible for an acute onset of shoulder weakness. One of the possible causative factors claimed to explain rupture or detachment of the deltoid muscle is a history of repeated steroid injections for frozen shoulder and longstanding rotator cuff tears (Allen and Drakos 2002). Because, in patients with deltoid rupture and massive rotator cuff tear, contraction of the intact deltoid can lead 285 286 S. Bianchi and C. Martinoli B B * a b R * * c R R d * e * * R Fig. 6.121a–e. Pectoralis muscle agenesis. a Photograph of the thorax of a patient with congenital absence of the right pectoralis major shows a flattened chest wall (arrowheads), which causes the nipple to be elevated, and the lack of the anterior axillary fold (curved arrow). b,c Transverse 12–5 MHz US images over the b right and c left myotendinous junction of the biceps (B). On the affected side, there is absence of the pectoralis tendon (arrowheads) and the biceps is shifted forward relative to the humeral shaft (asterisk). d,e Sagittal 12–5 MHz US images over the d right and e left chest wall demonstrate complete absence of the right pectoralis muscle (arrows). In d, note the subcutaneous fat which reaches the costal plane, made up of a combination of ribs (R) and intercostal muscles (asterisks) the humeral head to protrude through the defect (a type of boutonnière) – most commonly in the anterior or middle third – humeral impingement on the undersurface of the deltoid could be regarded as another possible causative factor (Blazar et al. 1998; Bianchi et al. 2006). Upward displacement of the humeral head may lead to it causing attrition at different sites. If impingement acts on the anteromedial part of the acromioclavicular arch, it more likely generates acromioclavicular cysts (Tshering Vogel et al. 2005); if it affects the posterior part of the acromioclavicular arch, it may lead to stress fractures of the acromion (Dennis et al. 1986). It is conceivable that a more lateral location of impingement forces (possibly secondary to a small acromion size or to a large humeral head) may cause weakening and even tears of the deltoid attachment (Figs. 6.122, 6.123) (Bianchi et al. 2006). Detachment of the deltoid insertion from the anterolateral acromion is a frequent surgical practice that improves exposure during acromioplasty. Postoperative detachment of the deltoid is a potential complication after this procedure. US can identify this condition, which can be repaired surgically if recognized early. Intramuscular injection through the deltoid muscle is common practice to treat shoulder pain and infection. Repeated injection of drugs, however, can lead to fibrosis of the injection site, even evolving into contracture status of muscles (injection myopathy). Deltoid muscle contraction is an uncommon, often unrecognized, clinical entity which usually involves the intermediate portion of the muscle, this being the preferred site for intramuscular injection (Chen et al. 1998). Clinical findings include a palpable fibrous cord within the deltoid muscle, skin dimpling overlying the cord, wingling of the scapula and a restricted range of shoulder motion, in particular limited adduction of the glenohumeral joint. US is able to reveal multiple hypoechoic small-caliber fibrotic cords (diameter <1 cm) oriented along the long-axis of the muscle (pattern I), reflecting the initial stage of small focal fibrotic foci (Fig. 6.124) Shoulder a b Acr HH c d Fig. 6.122a-d. Disruption of the anterior two thirds of the deltoid muscle secondary to chronic humeral impingement in an elderly patient with massive rotator cuff tear. a Photograph shows the prominence of the humeral head (straight arrows) on the skin, which became increasingly visible during rotational movements of the arm. Curved arrow indicates the acromion. b Anteroposterior radiograph demonstrates marked superior translation of the humeral head with advanced signs of glenohumeral osteoarthritis and acromiohumeral osteoarthritis. c Oblique coronal 12–5 MHz US image demonstrates a nearly absent subacromial space and considerable bulging of the humeral head (HH) external to the lateral edge of the acromion (Acr). There is absence of the middle third of the deltoid muscle with the humerus approaching the superficial tissue planes of the superolateral aspect of the shoulder. d Arthro-CT correlation reveals a disrupted deltoid muscle (arrowheads) (Huang et al. 2005). As the injections continue or the abnormality evolves over time, the small-caliber cords may coalesce into larger hypoechoic areas (pattern II) or even develop into calcified masses (pattern III) (Huang et al. 2005). In advanced disease, treatment is based on distal release of the deltoid fibrous cords. 6.5.4.2 Adhesive Capsulitis (Frozen Shoulder) Adhesive capsulitis, also referred to as “frozen shoulder,” refers to an insidious syndrome of shoulder pain and restricted movement in the absence of shoulder impingement and rotator cuff injury. The patient generally complains of loss of the normal shoulder range of motion, particularly arm elevation and external rotation. This condition tends to occur in perimenopausal women and is associated with diabetes mellitus, some drug treatments (i.e., isoniazide and barbiturates), trauma and prolonged immobilization after reduction for shoulder dislocation. Although the pathophysiology of adhesive capsulitis is unknown, hypervascular synovial proliferation followed by deposition of collagen and formation of capsular adhesions is typically found in these patients, leading to a reduced articular volume and, as a consequence, to pain and severely restricted joint motion. Treatment includes physiotherapy, steroid injections and closed manipulation in the operating room. In refractory cases, hydrodilatation and anterior capsulotomy is indicated (Gam et al. 1998). 287 Shoulder Acr Acr HH HH a b c d Fig. 6.125a–d. Adhesive capsulitis. Dynamic 12–5 MHz US scanning over the long axis of the supraspinatus tendons in a patient with left adhesive capsulitis. US images are obtained with the arm a,b in a neutral position and c,d passively abducted while in internal rotation. a,c right side; b,d left side. With this maneuver, US allows direct visualization of the relationships among the acromion (Acr), humeral head (HH) and intervening supraspinatus tendon (open arrows) during active shoulder motion. On the healthy right side, the passage (curved white arrow) of the supraspinatus underneath the acromion was unobstructed during full shoulder abduction. Conversely, on the affected left side, the supraspinatus gliding showed a sudden block during abduction movement. Different from that seen in impingement syndrome, the left supraspinatus appeared normal and the tendon passage was abruptly and not gradually obstructed, with absence of subacromial soft-tissue abnormalities. After tendon blockage, the patient tended to elevate (straight white arrow) the shoulder rather than to abduct the arm. The inserts at the right side of the figure indicate transducer positioning the rotator cuff interval and increased vasculature depicted at color Doppler imaging around the intraarticular portion of the biceps tendon and the coracohumeral ligament (Fig. 6.126) (Lee et al. 2005). Mild fluid distension of the biceps tendon sheath and the subscapular recess are also seen. Nevertheless, these signs are operator- and equipment-dependent and, for the most part, difficult to quantify. In doubtful cases, MR imaging and MR arthrography are valuable to diagnose this condition (Mengiardi et al. 2004). 6.5.4.3 Glenohumeral Joint Instability Although the value of US in assessing glenohumeral joint instability is poor, this technique can incidentally detect a variety of instability injuries affecting the glenoid labrum and the bone (Rasmussen 2004). In anterior shoulder instability, the main criteria for anterior labral tear are an enlarged (>2 mm) hypoechoic zone at the base of the labrum, a hypoechoic cleft within an otherwise homogeneous labrum, a truncated, eroded, frayed, irregular shape or absence of the labrum and an abnormal motility of the labrum when dynamic scanning is performed; altered labral echogenicity seems to be an inaccurate finding (Fig. 6.127) (Loredo et al. 1995; Hammar et al. 2001; Schydlowsky et al. 1998b; Rasmussen 2004). On the other hand, a small altered labrum seems to indicate degenerative changes (Schydlowsky et al. 1998c; Hammar et al. 2001; Taljanovic et al. 2000). In patients with acute traumatic or recurrent anterior shoulder dislocations, US has a reported 88–95% sensitivity and 67–70% specificity for the diagnosis of labral tears (Schydlowsky et al. 1998b; Hammar et al. 2001; Rasmussen 2004). Nevertheless, even using highend transducers, the anterior capsular complex (capsule and inferior glenohumeral ligament) cannot be distinguished clearly from the anterior labrum. Although some attempts have been made to assess the capsular tightness during dynamic scanning, US seems unable to reliably identify the discontinuity of the anterior capsuloligamentous complex in cases of traumatic avulsion of the capsule from its glenoid insertion, so-called capsular stripping or shearing. In contrast, fragmentation of the anteroinferior rim of the glenoid, representing a Bankart lesion, may occasionally be identified with US as a V-shaped bony defect over the anterior aspect of the glenoid 289 290 S. Bianchi and C. Martinoli SupraS Bt C HH HH G Fig. 6.126. Adhesive capsulitis. Short-axis 12–5 MHz US image over the intra-articular portion of the biceps tendon (Bt) in a diabetic patient with adhesive capsulitis demonstrates homogeneous hypoechoic soft tissue (arrows) filling the space of the rotator cuff interval and making the ligament structures of the bicipital pulley undefined. Note the supraspinatus tendon (SupraS). HH, humeral head; C, coracoid G HH a c HH HH b G G d Fig. 6.127a–d. Fibrocartilaginous labrum tears: spectrum of US appearances. Transverse 12–5 MHz US images over the posterior aspect of the glenohumeral joint show different appearances of posterior labrum tears: a,b hypoechoic clefts (arrows) within a homogeneous labrum (arrowheads); c enlarged hypoechoic zone (straight arrows) at the base of the labrum (arrowhead); d complete absence of the labrum. HH, humeral head; G, bony glenoid Shoulder (Hammar et al. 2001). Overall, we believe that US has a intrinsic limitations in the evaluation of the fibrocartilaginous glenoid labrum. It may exclude labral tears when the labrum appears normal. In suspected abnormalities, MR and CT arthrography are the most reliable and specific technique to confirm a labrum tear by depicting contrast material extending into the labral defect. A scanning technique for documenting the presence, direction and extent of glenohumeral translation has been described in patients with voluntary posterior shoulder subluxation or dislocation (Bianchi et al. 1994). Although rare, this condition is often unrecognized clinically and may be misdiagnosed as a frozen shoulder. In this technique, the examiner stands behind the patient and acquires transverse images over the posterior glenohumeral joint. The distance between the dorsal bony glenoid and the tip of the humeral head is measured at rest and during subluxation. The patient is examined in different positions (neutral, 90° flexion, abduction and external rotation), including the one in which he/she perceives the shoulder has become subluxed. The measured distances are compared between the affected shoulder and the healthy one: distances between 12 and 18 mm are indicative of subluxation (Fig. 6.128). It is important, however, to point out that assessment of associated intra- articular lesions essentially depends on the use of contrast-based imaging modalities (CT arthrography and MR arthrography). In posterior shoulder dislocation, the relationship of the coracoid (anterior approach) or the posterior glenoid surface (posterior approach) with the dislocated humeral head can be assessed and the distances between these structures are measured without the need of painful rotation or abduction of the arm using both anterior and posterior approaches (Fig. 6.129) (Hunter et al. 1998; Bize et al. 2003). The distances measured in the affected shoulder are compared with those in the contralateral shoulder (care should be taken not to misdiagnose a bilateral dislocation) and a difference greater than 20 mm indicates dislocation (Bianchi et al. 1994). Quantitative measurements performed during dynamic US scanning have also been suggested for measuring increased laxity in patients with anterior and multidirectional shoulder instability (Jerosch et al. 1989; Krarup et al. 1999) as well as for assessing anterior and posterior glenohumeral translation in a selected series of swimmers (Borsa et al. 2005b) and professional baseball pitchers (Borsa et al. 2005c). Based on these studies, dynamic US seems to be a promising means for measuring glenohumeral joint laxity, replacing stress radiography for this purpose (Borsa et al. 2005a). Co InfraS HH G Fig. 6.128a–d. Posterior subluxation of the humeral head. a,b Axillary views and c,d corresponding 12–5 MHz US images over the posterior glenohumeral joint in a patient with voluntary shoulder instability. a,c During subluxation, the humeral head (HH) is more exposed and posteriorly positioned (arrow) with respect to the level of the bony glenoid (G) indicated by the dashed line. b,d Same images obtained after voluntary relocation of the shoulder show the exact apposition of the humeral head (HH) with respect to the glenoid (G). InfraS, infraspinatus tendon; Co, coracoid. US can help to confirm that the subluxation is in a posterior direction a c Co InfraS G b d HH 291 292 S. Bianchi and C. Martinoli Co Co * Bt Bt HH HH Gl a Gl b c d Fig. 6.129a–d. Posterior shoulder dislocation. a Transverse 10–5 MHz US image over the anterior aspect of the glenohumeral joint with b corresponding CT scan in a patient presenting with a clinical history of seizures and inability to move the arm shows posterior displacement (curved arrow) of the humeral head (HH), leaving the surface of the anterior half (arrowheads) of the glenoid (Gl) uncovered. There is a small effusion (asterisk) inside the subscapularis recess. Note the increased distance between the humeral head and the coracoid (Co). The biceps tendon (Bt) is normal. c Transverse 10–5 MHz US image over the posterior aspect of the glenohumeral joint reveals an abnormal backward prominence of the convex humeral head (HH) relative to the glenoid (Gl). d Correlative anteroposterior radiograph demonstrates a fixed posterior shoulder dislocation characterized by elevation of the humeral head, lack of visibility of the glenohumeral joint space and detection of two parallel lines of cortical bone visible on the medial aspect of the humeral head: the medial one (arrows) corresponding to the glenoid outline, the lateral one (arrowheads) to an anterior impaction fracture A variety of surgical procedures, both open and arthroscopic, can be used to repair the capsulolabral complex and to thicken and tighten the glenohumeral ligaments in patients with post-traumatic glenohumeral join instability (Mohana-Borges et al. 2004). Detailed description of these procedures is beyond the scope of this chapter. In the postoperative setting for glenohumeral instability, however, suture materials and anchors used for fixation along the capsuloligamentous complex can be visualized with US (Fig. 6.130). 6.5.4.4 Humeral Head Fractures Despite its limitations in assessing bones, US can accurately detect the humeral head injuries which accompany glenohumeral joint instability, including the Hill-Sachs and McLaughlin fractures and avulsions of the tuberosities. The Hill-Sachs lesion is a depressed intra-articular compression fracture located on the posterolateral aspect of the humeral head typically observed after episodes of anterior glenohumeral dislocations. It can be regarded as a hallmark of anterior glenohumeral joint dislocation because it occurs in up to 47% of patients after the first episode of dislocation and up to 100% in patients with recurrent disease (Resnick et al. 1997). The pathomechanism of Hill-Sachs fracture consists of a powerful contraction of the para-articular muscles that pull the humeral head against the anteroinferior glenoid rim (Calandra et al. 1989; Resnick et al. 1997). The size and location of the fracture must be evaluated because a large defect can facilitate new episodes of dislocation. US has a reported sensitivity of 91–100%, specificity of 89–100% and overall accuracy of 84–94% in detecting this lesion (Farin et al. 1996a; Pancione et al. 1997; Cicak et al. 1998). For this purpose, the posterolateral aspect of the shoulder is examined with the transducer in transverse planes. Deep to the infraspinatus tendon, the humeral head at this level should have a smooth, curvilinear surface. The Hill-Sachs lesion typically appears as a wedge-shaped shallow defect of the hyperechoic bony contour of the humeral head at the point where the anterior portion of the infraspinatus inserts into the greater tuberosity (Jerosch et al. 1990) (Fig. 6.131). Its size and shape can be accurately assessed with US. Dynamic examination with back and forth rotation makes it possible to judge whether the lesion reaches the glenoid cavity during movement and the extent to which the motion of the limb is hindered. It is important to avoid confusion between the smaller and 295 Shoulder normal radiographs. When undisplaced, these fractures appear as a double discontinuity of the cortical bone located at the notch between the humeral head and the greater tuberosity (humeral neck) and over the external slope of the greater tuberosity, often at the junction of the humeral shaft and the anatomic neck of the humerus, suggesting an elevated fragment (Fig. 6.133) (Patten 1992). In displaced fractures, the uplifted fragment may be angled or overlapping, and the supraspinatus tendon in continuity with it appears abnormally thickened and het- GT a SupraS erogeneous due to edema and contusion (Fig. 6.134). In these cases, visualization of a well-demarcated defect on the surface of the greater tuberosity can avoid misdiagnoses with calcifying tendinitis. Avulsion fractures of the lesser tuberosity can also be found in posterior shoulder dislocations as a result of subscapularis traction (Fig. 6.135) (Ross et al. 1989; Martinoli et al. 2003). Once a possible fracture of the tuberosities is found, additional radiographic views, particularly under fluoroscopic control, must be obtained to confirm the US findings. * b c Fig. 6.133a–c. Minimally displaced greater tuberosity fracture. a Long-axis 17–5 MHz US image over the supraspinatus tendon (SupraS) in a patient with anterior instability demonstrates a double discontinuity of the hyperechoic humeral surface at the notch between the humeral head and the greater tuberosity (straight arrow) and over the external slope (curved arrow) of the greater tuberosity (GT), suggesting an undisplaced greater tuberosity fracture. The initial plain film was negative. b Oblique coronal fat-suppressed T2-weighted MR imaging correlation shows hyperintense signal (asterisk) at the greater tuberosity reflecting post-traumatic marrow edema. c Follow-up radiograph performed 3 months later reveals subtle bony changes (arrowheads) around the greater tuberosity reflecting fracture healing SupraS SupraS a b Fig. 6.134a,b. Greater tuberosity fracture: spectrum of US appearances. a,b Long-axis 12–5 MHz US images over the supraspinatus tendon in two patients with a an undisplaced and b an angulated fracture of the greater tuberosity, respectively. In a, subtle elevation and fragmentation of the most superficial layer (open arrowheads) of the bony cortex (white arrowheads) of the greater tuberosity creates two hyperechoic parallel lines (white and open arrowheads) resulting from a recent acute traction trauma by the supraspinatus tendon (SupraS). In b, an avulsion fracture arising from the insertion of the supraspinatus tendon is observed, just distal to the humeral articular surface. Compare the discontinuity of the hyperechoic humeral surface at the humeral neck (straight arrow) and over the external slope (curved arrow) of the greater tuberosity with the undisplaced fracture shown in Figure 6.133a. The fracture fragment is tilted and rotated following traction by the intact supraspinatus tendon (SupraS) Shoulder SubS * * a HH b Fig. 6.136a,b. Glenohumeral joint osteoarthritis. a Anteroposterior view shows typical radiographic findings of advanced disease, including joint space narrowing, osteophytes (arrows) along the articular margins of the humeral head and the inferior margin of the glenoid, upward translation of the humeral head with reduced subacromial space (white arrowhead), diffuse subchondral sclerosis (black arrowheads) and multiple intra-articular osteochondral bodies (asterisks). b Transverse 12–5 MHz US image over the anteromedial shoulder demonstrates an osteophyte (curved arrow) projecting just deep to the subscapularis tendon (SubS). Note irregularities (straight arrow) in the cortical profile of the humeral cortex. HH, humeral head portions of the glenohumeral joint, including the axillary pouch, the biceps tendon sheath, the posterior glenohumeral recess and some bursal recesses (i.e., lateral, subcoracoid bursa) which communicate with the joint cavity as a result of a rotator cuff tear (Fig. 6.137). Most intra-articular loose bodies appear as hyperechoic images with posterior acoustic shadowing (Fig. 6.138). In some cases, however, a layer of hypoechoic cartilage may be identified over the echogenic interface corresponding to the subchondral bone (Bianchi and Martinoli 1999). The size and position of the fragments can be reliably determined with US. Their exact number, in contrast, cannot be established with certainty. Estimating the size of loose bodies is important before planning arthroscopic surgery because fragments that are too large cannot be removed arthroscopically and may make the procedure difficult and time-consuming. However, such an assessment may also be problematic using standard radiographs, because the unossified portion of the fragment leads to an underestimation of its actual size. Differentiation between loose bodies secondary to osteoarthritis, trauma and osteochondromatosis is mainly based on clinical and radiographic findings. In general, US detection of innumerable loose bodies of nearly equal size without joint space narrowing more likely reflects osteochondromatosis, whereas identification of a single fragment or a few fragments of different size and appearance is more likely associated with an osteoarthritis-related process or a post-traumatic nature (Campeau and Lewis 1998). In idiopathic synovial osteochondromatosis, the age range of the affected patients is wide but, in most cases, disease onset occurs in the fourth or fifth decades. Men are affected more frequently than women. At US, different patterns may be noted depending on whether the loose bodies contain cartilage alone, cartilage and bone or mature bone (Fig. 6.139a,b). When entirely cartilaginous (synovial chondromatosis), the intra-articular nodules are hypoanechoic and difficult to distinguish from surrounding effusion. Furthermore, cartilage-containing masses of synovial chondromatosis may be difficult to differentiate from “rice bodies,” which are seen in patients with chronic rheumatoid arthritis or tuberculosis (Mutlu et al. 2004). At US, rice bodies may appear as hypoanechoic spherules a few millimeters in size (Fig. 6.139c,d). They may fill the subdeltoid bursa and, in most cases, are distinguished with difficulty from the adjacent hypoechoic synovial pannus due to similar echogenicity. The pathogenesis of rice bodies is different from that of loose bodies. In the late stages of rheumatoid arthritis, rice bodies seem to derive from chronic articular inflammation leading to formation of elongated synovial villi which then become covered by fibrin and may snap off, producing fibrin grains similar to polished rice (Law et al. 1998; Reid et al. 1998). With increasing age, rice bodies undergo a degree of organization 297 Shoulder a b Humerus Humerus c d Fig. 6.139a–d. Intra-articular bodies: spectrum of US appearances. a,b Primary synovial osteochondromatosis. a Transverse 12–5 MHz US image over the subacromial subdeltoid bursa with b T2-weighted MR imaging correlation demonstrates multiple small hyperechoic low signal intensity nodules (arrowheads) filling a distended bursa (arrows), consistent with synovial osteochondromatosis. c,d Rice bodies in a patient with rheumatoid arthritis. c Coronal and d transverse 12–5 MHz US images respectively obtained over the lateral pouch and the anterior dependent portion of the subacromial subdeltoid bursa show multiple hypoechoic rounded filling defects (arrowheads) within an inflamed and enlarged bursa (white arrows) reflecting rice bodies. Mild effusion is observed into the sheath of the biceps tendon (open arrow) and may contain a core of mature collagen. Identification of rice bodies is clinically relevant as they are a persistent reason for continuing synovial inflammation. Their removal is usually associated with clinical improvement (Propert et al. 1982). Among the degenerative arthropathies that typically involve the shoulder, there are a variety of conditions related to crystal deposition diseases, including renal osteodystrophy, milk alkali syndrome, hypervitaminosis D and the so-called “Milwaukee shoulder syndrome”. This last condition, which is also known as apatite-associated destructive arthritis, hemorrhagic shoulder or rapid destructive arthritis of the shoulder, consists of massive rotator cuff tear, osteoarthritic changes, blood-stained noninflammatory joint effusion containing calcium hydroxyapatite and calcium pyrophosphate dihydrate crystals, synovial hyperplasia and extensive destruction of cartilage and subchondral bone (Llauger et al. 2000). Osteophytes are not characteristic of Milwaukee syndrome. This destructive arthropathy most commonly affects elderly patients, predominantly women, and manifests clinically as a rapid progressive and destructive arthritis of the shoulder with localized pain, swelling, variable limitation of joint motion and joint instability. Occasionally, there is rupture of the shoulder capsule with drainage of blood-stained fluid into the para-articular soft tissues lasting for weeks or months (Fig. 6.140). Radiographically, this condition resembles a neuropathy-like arthropathy with high-riding humeral head. Pseudoarthrosis between the humeral head, the coracoid and the acromion is commonly seen (Nguyen 1996). Although US is able to demonstrate a marked distension of the joint space by effusion and echogenic debris reflecting synovial prolifera- 299 300 S. Bianchi and C. Martinoli a b c Fig. 6.140a-c. Milwaukee shoulder. a Anteroposterior radiograph of the shoulder demonstrates advanced degenerative changes of the glenohumeral joint associated with upward migration of the humeral head (arrow) related to rotator cuff tear and pseudoarthrosis (arrowhead) between the humerus, the coracoid and the acromion with mild subchondral bone sclerosis and little osteophytosis. Note the calcific deposits in the lateral dependent portion of the bursa (curved arrow). b Coronal 12–5 MHz US image obtained lateral to the acromion reveals extensive subdeltoid bursitis with prominent synovial fronding and signs of rupture of the bursa into the subcutaneous tissue (arrows). c Photograph of the left shoulder demonstrates diffuse swelling and ecchymosis related to drainage of blood-stained fluid into the para-articular soft tissues tion and blood clots, calcified deposits, destruction of the cartilage and osteolysis of the subchondral bone, it is not reliable for differentiating this disorder from the more common osteoarthritis related to rotator cuff disease. Therapy includes analgesic drugs and repeated arthrocentesis followed by intra-articular steroid administration. In advanced disease, shoulder arthroplasty may be considered. In patients with chondrocalcinosis, US can depict deposition of pyrophosphate crystals in the cartilage of the humeral head (Peetrons et al. 2001). These deposits appear as a blurry hyperechoic line on the outer margin of the cartilage surface (Fig. 6.141). Grossly echogenic thickening of the synovium, especially prominent in the subacromial subdeltoid bursa, para-articular nodules within the soft tissues surrounding the cuff and deep bony erosions may be observed in dialysis-related shoulder arthropathy reflecting amyloid deposition of ß2-microglobulin, which is an amyloid protein that is not filtered by standard dialysis membranes (Kay et al. 1992; Sommer et al. 2002; Cardinal et al. 1996; Llauger et al. 2000; Slavotinek et al. 2000). US features of shoulder amyloidosis are varied and may include a heterogeneous and thickened rotator cuff, especially involving the supraspinatus and the subscapularis tendons (McMahon et al. 1991; Malghem et al. 1996). Based on these findings, US offers an early diagnosis and should be a useful tool to follow up the disease. In these patients, para-articular calcifications are often observed as a result of calciumphosphorus imbalance. 6.5.4.6 Inflammatory Arthropathies As a result of a widespread involvement of synovial tissues, rheumatoid arthritis usually affects the glenohumeral joint in association with the acromioclavicular joint and the synovial bursae around the shoulder. Radiographically, rheumatoid arthritis may cause uniform narrowing of the joint space, marginal erosions, erosions of the greater tuberosity, osteophytes, flattening of the glenoid cavity and sclerosis of apposing surfaces of the glenoid and humerus and pseudowidening of the acromioclavicular joint related to reabsorption of the distal end of the clavicle (Fig. 6.142a). US has proved able to reveal synovitis both at the early stages of disease, when no radiographic changes are yet evident (Alasaarela and Alasaarela 1994; Chhem 1994; Alasaarela et al. 1997; Gibbon and Wakefield 1999), and in an asymptomatic population with arthritic shoulder (Naranjo et al. 2002). This technique is used for the evaluation of shoulder girdle arthritis in an attempt to assess which synovial cavity is involved by the inflammatory process, to differentiate between effu- Shoulder HH a b Fig. 6.141a,b. Chondrocalcinosis. a Oblique coronal 12–5 MHz US image over the supraspinatus tendon with b radiographic correlation demonstrates a continuum of fine hyperechoic spots (arrows) located in series within the hypoechoic articular cartilage of the humeral head (HH), reflecting calcium pyrophosphate dihydrate crystal deposition disease * * HH Gl a b HH c HH d Fig. 6.142a–d. Rheumatoid arthritis. a Anteroposterior radiograph in a patient with longstanding disease shows confluent marginal erosions and subchondral cysts (arrows) in the humeral head. b Transverse 12–5 MHz US image over the posterior shoulder reveals a hypoechoic soft-tissue mass (asterisks) representing synovial pannus within the posterior recess. In addition to this finding, there are irregularities in the posterior aspect of the humeral head (HH), consistent with bone erosions (arrows). Gl, glenoid. c,d Transverse c gray-scale and d color Doppler 12–5 MHz US images over the anterior aspect of the humeral head (HH) demonstrate a rounded well-defined cortical erosion (arrowheads) filled with hypervascular synovial pannus (arrow) 301 302 S. Bianchi and C. Martinoli sion and synovial pannus and evaluate the extent of such involvement, as well as to detect subtle bone erosions that cannot be imaged on standard radiographs (Fig. 6.142b) (Speed and Hazleman 1999). In a selected group of patients with symptomatic disease, US assessment of synovitis has demonstrated subacromial subdeltoid bursitis as the most common finding, occurring in up to 69% of cases, followed by glenohumeral joint involvement in 58% and biceps tendinitis in 57% (Alasaarela et al. 1998a). Overall, no correlation exists between these findings and either the duration or stage of disease. A quantitative assessment of synovitis may be attempted by measuring the widest distance between the humeral head and the joint capsule in the axillary pouch and posterior recesses (Alasaarela and Alasaarela 1994; Alasaarela et al. 1998a; Koski 1989, 1991). Difficulties may arise with US when trying to distinguish effusion from the pannus in the posterior recess, because graded compression with the probe is not always able to squeeze the fluid away from this site. In addition, when pressure is applied over the pannus, this can be mobilized similarly to joint fluid. Doppler systems may be helpful to assess the activity of the inflammatory process by showing hyperemic blood flow within the synovial tissue (Alasaarela and Alasaarela 1994). In the biceps tendon sheath, hyperemic flow is detected to a greater extent in rheumatoid arthritis rather than in patients with degenerative disease (Strunk et al. 2003). The reliability of these findings seems, however, too limited for an objective assessment, particularly when Doppler imaging is used as an indicator of the response to therapy. It is possible that US contrast agent will have a role in this field (Wamser et al. 2003). Loss of definition and thinning of the articular cartilage can be demonstrated in advanced disease as well. As regards the bony surfaces, US is able to reveal erosions as well-defined cortical defects filled by hypoechoic pannus: they may be isolated, confluent or generalized (Fig. 6.142c,d) (Alasaarela et al. 1998b; Gibbon and Wakefield 1999; Hermann et al. 2003). As mentioned earlier, US is useful when obtaining a sample of fluid or synovium because it can identify the ideal puncture site (where the fluid accumulates more or the pannus is thicker) and can provide easy guidance for directing the needle. The intra-articular injection of corticosteroids or the lidocaine test can be performed under US guidance, thereby avoiding the risks of inadvertent intratendinous steroid injection or para-articular injections of anesthetic. In these circumstances, the procedure of needle placement is more accurate and less painful under US guidance than when performed blindly. The structures involved by the inflammatory process in polymyalgia rheumatica have also been investigated using US (Lange et al. 1998; Koski 1992; Cantini et al. 2001). Most studies report a frequency of bursitis (14–16%) lower than that of glenohumeral joint synovitis (57–66%) in this disease (Lange et al. 1998; Koski 1992). 6.5.4.7 Shoulder Arthroplasty Glenohumeral joint arthroplasty has become the procedure of choice to treat patients with pain and articular damage who do not respond to conservative therapy. Regardless of the underlying disease (e.g., osteoarthritis, rheumatoid arthritis, rotator cuff arthropathy, avascular necrosis, proximal humeral fractures), the procedure is performed to relieve pain and improve the range of shoulder motion. The prosthesis is composed of a metallic stem with a modular humeral head that articulates either with the native glenoid (shoulder hemiarthroplasty) or with a polyethylene or metal glenoid component (total shoulder arthroplasty) (Taljanovic et al. 2003). Reverse shoulder prostheses are also obtained by reversing the position of the ball (implanted on the glenoid) and the socket (implanted on the humeral head). Many types of device are available. Criteria for selection of a given type depend on the patient’s condition, the surgeon’s preference and the surgeon’s experience, and are beyond the scope of this chapter. The main complications with shoulder arthroplasty are loosening, superior migration, subluxation or dislocation of the humeral head and postoperative rotator cuff tear. After shoulder arthroplasty, MR imaging is of limited value owing to the artifact created by the metallic implant. US has proved able to provide information about the para-articular soft tissues and the rotator cuff after shoulder arthroplasty, especially in cases of poor postoperative outcome and absence of radiographic signs of loosening and migration (Westhoff et al. 2002; Sofka and Adler 2003). In this setting, the metallic hardware of the humeral component of the prosthesis is readily demonstrated, enabling one to recognize the following landmarks arranged in series: acromion, humeral component, greater tuberosity (Sofka and Adler 2003). The prosthesis itself does not hinder examination of the rotator cuff. Its metallic component appears as a linear echogenic interface with moderate posterior reverberation artifact. The examiner Shoulder should remember that moderate to severe regional muscle atrophy – often involving the deltoid and the teres minor – is frequently encountered in patients who have undergone shoulder replacement and that the subscapularis tendon (but not the supraspinatus) has often been taken off the lesser tuberosity to allow surgical access (deltopectoral approach). After placement of the prosthesis, the subscapularis tendon is usually reinserted more medially, at the site of humeral head resection rather than at the anatomic insertion site: however, this tendon may retear leading to an anteriorly unstable shoulder. In general, preservation of the rotator cuff tendons in these patients correlates with a good clinical outcome. In patients with loosening of the cup, dynamic examination can depict some degree of instability of the metallic hardware relative to the bony humerus (Fig. 6.143). 6.5.4.8 Septic Arthritis and Bursitis Septic arthritis of the glenohumeral joint has predilection for very young infants or elderly patients with chronic debilitating disorders, such as diabetes, cirrhosis and alcoholism. The intra-articular injection of corticosteroids greatly increases the likelihood of infectious disease because of steroid-induced * GT a b reduction in the host defences. In addition, septic arthritis may derive from accidental introduction of bacteria during nonsterile arthrocentesis procedures. Although US is a sensitive means for detection of even small glenohumeral joint effusions, US imaging findings usually do not allow the conclusive differentiation of a noninfected joint effusion from septic arthritis (Cardinal et al. 2001). Definitive diagnosis requires analysis of the fluid, possibly aspirated under US guidance, and must be performed in every patient in whom the likelihood of infection is present. As described in Chapter 18, large-bore (16–18 gauge) needles are ideal for this purpose, because purulent material can be too thick and viscous to be aspirated with a small needle. Although the most adequate puncture site may vary among patients, the posterior approach is usually prefered. Using this access, the needle should be inserted at mid-glenohumeral level and directed into the posterior recess through the infraspinatus. Septic arthritis is usually not associated with bursal infection unless a full-thickness tear of the rotator cuff is present and allows free communication between these two spaces. Nevertheless, the two entities may overlap and clinical differentiation may be difficult. At US examination, an infected subacromial subdeltoid bursa may appear distended by a complex effusion containing debris and septations (Fig. 6.144a) (Cardinal et al. 2001; Lombardi et al. 1992; Rutten et al. 1998). The bursal walls may be * Acr Acr GT c Fig. 6.143a–c. Shoulder arthroplasty. a Schematic drawing illustrates a conventional humeral stem for shoulder arthroplasty. b,c Oblique coronal 12–5 MHz US images obtained immediately lateral to the acromion (Acr) while keeping the arm b abducted and c in neutral position. A series of bright echogenic surfaces reflecting native bone and metallic wares are observed. From medial to lateral, they are: the polyethylene glenoid component (arrow) of the prosthesis, the cup of the humeral component (arrowhead) and the greater tuberosity (GT). There is mild reverberation artifact underneath the prosthesis materials, absence of the supraspinatus tendon and atrophy of the deltoid muscle (asterisks). Dynamic examination reveals some instability of the metallic hardware relative to the bony humerus with increased distance (double arrow) between the humeral ware and the greater tuberosity in neutral position 303 304 S. Bianchi and C. Martinoli thickened and peribursal hypoechoic strands reflecting edema in the surrounding soft tissues may be associated findings (Fig. 6.144b). Although color and power Doppler imaging may show hyperemic flow in the synovial walls and around the bursa, this is not regarded as a specific sign of infectious disease. When the joint recesses are free of fluid, US is a reliable means to obtain a correct diagnosis of isolated bursal involvement, thus avoiding arthrocentesis procedures with their potential complications (Lombardi et al. 1992). During aspiration of the infected bursa, US guidance may avoid inadvertent contamination of the underlying sterile joint by traversing the infected bursa with the needle. In sepsis of the acromioclavicular joint (Blankstein et al. 1985), US is a useful modality to exclude the involvement of the adjacent subacromial subdeltoid bursa and glenohumeral joint. Main US findings include superior bulging of the joint capsule, widening of the joint space with erosions of the bony edges and debris moving freely within the joint space (Widman et al. 2001). Although aspiration of the infected joint can be performed blindly, US allows this procedure to be carried out more confidently. 6.5.4.9 Acromioclavicular Joint Trauma and Instability Subluxation or dislocation of the acromioclavicular joint may be a source of shoulder pain which SupraS * HH is often mistaken for a post-traumatic rotator cuff lesion because of the close proximity of this joint with the rotator cuff tendons. US is more sensitive than standard radiographs in detecting low-grade sprains of the acromioclavicular joint. These lesions appear as widening of the joint cavity, distended by hematoma or effusion, and bulging of the superior capsule and ligament (Fig. 6.145). When the acromioclavicular joint is more severely injured with rupture of the coracoclavicular ligaments, an upward displacement of the distal end of the clavicle can be appreciated (Fig. 6.146). Although direct imaging of the coracoclavicular ligaments is not feasible with US because of the overlying clavicle, a hematoma in the soft tissues between the clavicle and the coracoid may be regarded as an indirect sign of injured ligaments. In addition, measurement of the coracoclavicular distance using anterior sagittal scans may increase confidence in the diagnosis (Sluming, 1995). In severe dislocations with gross displacement of the clavicle, disruption of the muscular insertion of the deltoid and/or the trapezius with a hematoma developing anteriorly (deltoid lesion) or posteriorly (trapezius lesion) to the cranial edge of the clavicle can also be demonstrated (Heers and Hedtmann 2005). Short-axis planes over the distal clavicle are useful to evaluate the common fascia of both muscles in order to avoid injuries being missed (Heers and Hedtmann 2005). These structures are important stabilizers of the acromioclavicular joint. Although US is not routinely used as the screen- * HH a b Fig. 6.144a,b. Septic bursitis. Two different cases. a Transverse 12–5 MHz US image over the short axis of the supraspinatus tendon (SupraS) shows irregular lining of the bursa with focal hypoechoic thickening of the synovium (asterisks). Aspiration revealed Staphylococcus aureus infection of the subacromial subdeltoid bursa. b Oblique sagittal 12–5 MHz US image obtained immediately lateral to the acromion in a diabetic patient with massive rotator cuff tear and recent onset of shoulder swelling, pain and fever demonstrates a heterogeneous bursal effusion containing material of mixed echogenicity (straight arrows). Small hyperechoic foci (curved arrows) within the synovial cavity suggest purulent material. Note the hypoechoic changes (arrowheads) in the soft-tissue layers surrounding the bursa reflecting peribursal reactive inflammation and edema. Aspiration revealed Streptococcus infection. HH, humeral head Shoulder 1 Cl 23 Acr Acr Cl Co a c Cl Acr Co b d Fig. 6.145a–d. Mild acromioclavicular joint sprain (type II injury). a Schematic drawing over the coracoacromial arch illustrates the normal relationships of the acromioclavicular joint (1) with the coracoacromial ligament (arrow) and the trapezoid (2) and conoid (3) components of the coracoclavicular ligament. Acr, acromion; Co, coracoid; Cl, clavicle. b Schematic drawing shows the alterations observed in a mild sprain of the acromioclavicular joint. The joint space is widened (curved arrow) without injury of the coracoclavicular ligament. c Coronal and d sagittal 12–5 MHz US images over the acromioclavicular joint in a patient with post-traumatic shoulder pain reveal a widened joint space (arrowheads) and hypoechoic fluid (arrows) distending the joint cavity. Acr, acromion; Cl, clavicle Cl Cl Acr Cl Acr Acr Co a c d Cl Acr b Acr e Acr Cl Cl f Fig. 6.146a–f. Acromioclavicular joint separation (type III injury). a Schematic drawing over the coracoacromial arch with b radiographic correlation demonstrates elevation (straight arrow) of the clavicle (Cl) relative to the acromion (Acr) with increased acromioclavicular joint and coracoclavicular distances (curved arrows) indicating rupture of the ligaments. Co, coracoid. c,d Coronal 12–5 MHz US images over the right acromioclavicular joint in a patient with post-traumatic joint dislocation. Note the upward displacement (arrow) of the distal end of the clavicle (Cl) relative to the acromion (Acr). The double-headed arrow between the dashed lines indicates measurement of the acromioclavicular joint width (c,e) and the superior displacement of the clavicle (d,f). e,f Coronal 12–5 MHz US images of the normal left acromioclavicular joint for comparison 305 306 S. Bianchi and C. Martinoli ing modality for acromioclavicular joint separation, some attempts have been made to correlate US findings in acute and chronic unstable acromioclavicular joints of varying severity with the radiographic scale described by Tossy (Tossy et al. 1963) and the Rockwood (Rockwood 1984) classification. At US, the width of the joint is measured using a coronal approach and compared with the contralateral side. Theoretically, measurements are best obtained with the patient’s arms hanging down and holding a 10 kg weight in each hand to increase stress on the capsuloligamentous structures and allow identification of subtle changes. Since variants can exist in the joint width among normal subjects, the measure must be related to the normal uninjured side. An index is then calculated by dividing the acromioclavicular joint width on the affected side by that on the normal side. In normal subjects, the acromioclavicular joint width should be no wider than 6 mm and the acromioclavicular index 1.0; patients with Tossy II instability have a mean acromioclavicular joint width of 10.2 mm on the injured side and an acromioclavicular index of 0.5; patients with Tossy III instability and indication for surgery have a mean acromioclavicular joint width of 22.3 mm on the injured side and an acromioclavicular index of less than 0.25 (Kock et al. 1996). As defined by Rockwood (Rockwood 1984), Tossy III type injury can be further subdivided depending on posterior displacement of the clavicle (type IV), marked increase in the coracoclavicular distance by 2 or 3 times and the scapula displaced inferiorly (type V) and dislocation of the * clavicle inferior to the acromion or the coracoid (type VI). Although coracoid process fractures may be secondary to anterior shoulder dislocation, they most frequently occur in association with type III acromioclavicular joint dislocations (Ogawa et al. 1997). The mechanism of these rare fractures seems related to the occurrence of direct trauma to the shoulder girdle and sudden strong pull of the short head of the biceps and the coracobrachialis inserting at the coracoid process, leading to an avulsion (Fig. 6.147). In most cases, conservative treatment is appropriate. In the case of large avulsed fragments or persistent pain, open reduction is advised with coracoid screw and acromioclavicular fixation. Post-traumatic osteolysis of the clavicle is a selflimiting disorder with gradual reparative changes over a period of 4–6 months that may occur several weeks up to several years after acromioclavicular trauma (Dardani et al. 2000). The key to diagnosis is the fact that changes occur only at the clavicular end while the acromion remains normal. Although the diagnosis is usually based on the patient’s history and radiographic findings, US is able to detect the same abnormalities seen on plain films. At US, the clavicular tip exhibits irregular cortical erosions associated with joint space widening, joint effusion and soft-tissue swelling, whereas the acromion remains intact (Fig. 6.148). Post-traumatic osteolysis of the clavicle should be considered in the differential diagnosis when a patient experiences chronic pain or soft-tissue swelling beyond the acute phase of the injury. Care must be taken not to confuse this Co * Co a b Fig. 6.147a,b. Coracoid fracture. a Sagittal split-screen 12–5 MHz US image over the coracoid (co) with b oblique sagittal CT reconstructed imaging correlation in a patient presenting with direct trauma to the shoulder girdle and acromioclavicular joint separation (type III injury) reveals detachment and caudal displacement of the coracoid tip (white arrows) resulting from traction by the short head of the biceps and the coracobrachialis (open arrows). Observe the nidus (arrowheads) of avulsion in the coracoid and the associated hematoma (asterisk) Shoulder Acr Acr a b Fig. 6.148a,b. Post-traumatic osteolysis of the clavicle. a Coronal 10–5 MHz US image with b radiographic correlation in a patient with painful tenderness over the acromioclavicular joint 6 months after a trauma demonstrates an irregular erosion (arrows) of the distal end of the clavicle. Acr, acromion. (Courtesy of Dr. Nicolò Prato, Italy) condition with shortening of the clavicle secondary to resection of the distal end of clavicle, which can be performed to treat acromioclavicular osteoarthritis with secondary impingement, rheumatoid arthritis, ankylosing spondylitis and infection. Both the patient’s history and local inspection allow a reliable differentiation between these conditions. 6.5.4.10 Sternoclavicular and Costosternal Joint Pathology Injuries to the sternoclavicular joint are uncommon given the strong ligamentous support of this joint. Traumatic sternoclavicular instability, including subluxation and dislocation, is always secondary to a well-defined traumatic event. In these patients, disability has a longer duration in cases of posterior dislocation than anterior dislocation, presumably because of coexistent injury to the mediastinal soft tissues posterior to the sternum. US has proved able to identify posterior sternoclavicular dislocation as well as to evaluate its reduction in the operating room (Benson et al. 1991; Pollock et al. 1996). In addition to traumatic injuries, other atraumatic conditions affecting the sternoclavicular joint are amenable to US examination, including degenerative osteoarthritis (Hiramuro-Shoji et al. 2003). Similar to that observed in the acromioclavicular joint, degenerative osteoarthritis of the sternoclavicular joint is characterized by narrowing of the joint space, osteophytes and para-articular cysts (Fig. 6.149a,b). This condition usually affects the dominant arm of women between the ages of 40 and 60 years. Previous neck surgery with spinal accessory nerve lesion is also claimed as a predis- posing factor, as it causes downward and forward drop of the shoulder leading to additional stress on the sternoclavicular joint (Hiramuro-Shoji et al. 2003). In rheumatoid arthritis, sternoclavicular joint involvement shows irregularities of the osseous surfaces with osteolysis of the medial end of the clavicle and synovial inflammation (Fig. 6.149c,d). Tietze’s syndrome, which is also referred to as costosternal syndrome or anterior chest wall syndrome, is a benign, self-limiting condition characterized by swelling of the costal cartilages and gradual onset of pain in the anterior chest wall exacerbated by coughing and sneezing. US is able to reveal an increased volume of the costal cartilages with irregular calcifications and perichondral soft-tissue swelling in clinically and radiographically apparently normal costochondral joints of patients with anterior chest pain (Choi et al. 1995; Kamel and Kotob 1997). In these patients, US has been proposed as a means to guide local steroid injection for treatment (Kamel and Kotob 1997). 6.5.4.11 Quadrilateral Space Syndrome In neuropathies around the shoulder, the small size of nerves, the complex regional anatomy and problems of access due to the acoustic shadowing from superficial bone structures, makes direct evaluation of nerves difficult with US. Axillary neuropathy may be caused by stretching injuries (anterior dislocation) or extrinsic compression in the quadrilateral space caused by fractures of the upper humerus, improper use of crutches, casts, fibrous bands and inferior (from the 9 to 7o’clock positions) paragle- 307 308 S. Bianchi and C. Martinoli Cl St St Cl a b Cl Cl St c St d Fig. 6.149a–d. Sternoclavicular joint abnormalities. a,b Degenerative osteoarthritis. a Transverse 12–5 MHz US image over the right stenoclavicular joint shows irregularities and osteophytes (arrowheads) of the articular surface of the clavicle (Cl) and sternum (St). b Normal contralateral joint for comparison. c,d Rheumatoid arthritis. c Transverse color Doppler 12–5 MHz US image over the right stenoclavicular joint with d coronal contrast-enhanced T1-weighted MR imaging correlation shows joint space widening, irregularities in the medial end of the clavicle (arrow) reflecting osseous erosions and synovial hyperemia (arrowheads) as seen at Doppler imaging and after gadolinium uptake noid cysts (Linker et al. 1993; Chautems et al. 2000; Tung et al. 2000). Static (fibrous bands, occlusion of the posterior circumflex artery, muscle hypertrophy) and dynamic (nerve stretching in some arm positions, such as occur in throwing athletes at the extremes of joint motion) traction and compression on the axillary nerve seem play a role in this syndrome (Perlmutter 1999). Iatrogenic damage during arthroscopic procedures around the coracoid or by posterior surgical arthroscopic portals has also been reported outside the quadrilateral space (Lo et al. 2004). When the entrapment of the axillary nerve occurs in the quadrilateral space, there is selective denervation of the teres minor muscle because the anterior branch of the nerve (supplying the deltoid) is spared. Clinically, axillary neuropathy is often found as an occasional finding during an examination of the shoulder for other symptomatic abnormalities. This would suggest that the disease may exist in an asymptomatic or subclinical entity (Sofka et al. 2004b; Cothran and Helms 2005). When symptomatic, it is associated with vague, often nonspecific, posterior shoulder pain, paresthesias over the external aspect of the shoulder and weakness exacerbated by abduction and external rotation of the arm. Because the teres minor is the only muscle involved, this condition can be difficult to recognize on the basis of clinical grounds alone, because the action of the teres minor cannot be clearly separated from the contribute of the infraspinatus. Even without any detectable softtissue abnormality along the course of the nerve, the diagnosis of axillary neuropathy is based on the evidence of volume loss and hyperechoic changes of the involved muscles in the absence of a tendon tear (Martinoli et al. 2003). These signs are particularly suggestive given that teres minor tendon disruptions are extremely rare, even in cases with massive rotator cuff tears. At US, the atrophy of the teres minor is best assessed by comparing the appearance of this muscle with that of the adjacent infraspinatus on sagittal scans (Fig. 6.150). On the other hand, atrophy of the deltoid can be revealed by a reduced thickness of this muscle relative to the contralateral 309 Shoulder Del SSp InfraS a b * * c d Fig. 6.150a–d. Axillary neuropathy with selective denervation of the teres minor muscle. a Sagittal extended field-of-view 12–5 MHz US image obtained over the right posterior fossa demonstrates loss in bulk and increased echogenicity of the teres minor muscle (arrowheads), a finding that is consistent with fatty atrophy, whereas the infraspinatus muscle (arrows) is preserved. SSp, spine of the scapula; Del, deltoid muscle. b Oblique coronal T1-weighted MR imaging correlation confirms chronic denervation in the form of fatty infiltration of the teres minor (arrowheads) related to axillary neuropathy. Note the normal infraspinatus muscle (InfraS) and some hypointense structures (curved arrow) crossing the quadrilateral space, consistent with the axillary nerve and the posterior circumflex artery. c,d Long-axis 12–5 MHz US images over c the right (arrowheads) and d the left (arrows) teres minor muscles demonstrate striking echotextural differences, with the right belly being reduced in bulk and much more echogenic than the left. On both sides, tendons are intact (asterisk) one on coronal scans (Fig. 6.151). In addition, US is able to demonstrate any possible space-occupying lesion in the quadrilateral space, such as paralabral cysts extending off the inferior aspect of the glenoid in association with a tear of the inferior labrum (Sanders and Tirman 1999; Robinson et al. 2000). The main differential diagnosis of quadrilateral space syndrome is the Parsonage-Turner syndrome, in which the involvement of muscles usually relates to more than one nerve distribution. 6.5.4.12 Suprascapular Nerve Syndrome Suprascapular neuropathy is an unusual syndrome leading to chronic shoulder pain and weakness (Fehrman et al. 1995). This condition may be secondary to a constriction of the suprascapular nerve at the suprascapular notch or at the spinoglenoid notch as a result of a variety of condition, including stretching injuries, ligament abnormalities, overuse or space-occupying lesions. From the pathophysiologic point of view, if the suprascapular nerve is entrapped at the supraspinous notch, both supraspinatus and infraspinatus muscles undergo denervation changes; if it is compressed at the spinoglenoid notch, denervation is limited to the infraspinatus muscle whereas the supraspinatus is spared. Because the suprascapular nerve is a purely motor nerve, there is no sensory loss. Paralabral cysts are the leading cause of suprascapular neuropathy (Takagishi et al. 1991; Bousquet et al. 1996; Bredella et al. 1999; Tung et al. 2000; Weiss and Imhoff 2000; Ludig et al. 2001; O’Connor et al. 2003). Two possible theories have been hypothesized to explain the origin of these cysts. The first assumes that they are secondary to tears of the glenoid labrum allowing the joint fluid to extrude into the periarticular tissues; the second suggests that they would arise 310 S. Bianchi and C. Martinoli a c d Acr GT b e Acr Acr GT Fig. 6.151a–e. Axillary nerve injury with deltoid denervation due to damaging of the anterior branch of the axillary nerve in a patient who had undergone previous shoulder dislocation and humeral fracture in a traffic accident. a Long-axis 12–5 MHz US image over the intact supraspinatus tendon shows marked atrophy of the deltoid muscle (arrows). b Corresponding contralateral normal side showing the normal deltoid (arrows). Acr, acromion; GT, greater tuberosity. c Oblique-coronal T1-weighted and d fat-suppressed T2-weighted MR images confirm marked thinning of the deltoid muscle (arrows), which exhibits slightly increased T2 signal related to the denervation process. e Photograph of the right shoulder shows prominence of the acromion (Acr) and the coracoid (arrowhead) on the skin due to the atrophy of the deltoid muscle from areas of myxoid degeneration of para-articular structures following labral tears, a pathogenesis somewhat similar to that of other ganglion cysts. In the shoulder, paralabral cysts are usually associated with tears of the superior and posterior glenoid labrum (from the 8 to 11 o’clock positions), as a result of a SLAP lesion or posterior instability, respectively. Only rarely they extend off the anterior and inferior aspect of the glenoid. Once developed, paralabral cysts can show a progressive enlargement due to a one-way valve mechanism leading to the passage of the joint fluid into the cyst through a thin pedicle (Fig. 6.152a,b). During their expansion, they spread into the spinoglenoid notch, the suprascapular notch, or both notches of the scapula lying deep to the myotendinous junction of either the supraspinatus or the infraspinatus, and may or may not cause nerve entrapment and muscle denervation (Fig. 6.152c) (Tung et al. 2000). US can easily recognize secondary changes of nerve damage, including loss in bulk and increased reflectivity of the innervated muscles due to edema and fatty replacement (Figs. 6.153, 6.154). A correlation was found between the size of paralabral cysts and the onset of denervation symptoms, significantly more muscle denervation occurring with larger cysts (volume 6.0 cm3; diameter 3.1 cm) compared with all other paralabral cysts (volume 2.2 cm3) (Tung et al. 2000). A careful scanning technique is recommended for imaging the posterior shoulder, starting with near-field settings to examine the rotator cuff tendons and then adjusting both the focal zone and image magnification to the far-field in order to explore the scapular notches (Martinoli et al. 2003). In fact, even large cysts could be easily missed while performing a standard US examination of the shoulder due to their deep location, far from the rotator cuff tendons. In many cases, spinoglenoid cysts develop in the most cranial portion of the notch, in close proximity to the scapular spine. Placing the hand on the opposite shoulder during scanning may be helpful to decrease the depth of the posterior fossa and to make this area more readily examined with US. US demonstrates paralabral cysts as rounded or 312 S. Bianchi and C. Martinoli Del InfraS SSp a SSp b * * c d Fig. 6.154a–d. Suprascapular neuropathy: spinoglenoid notch entrapment. a Sagittal extended field-of-view 12–5 MHz US image obtained over the right posterior fossa demonstrates loss in bulk and increased echogenicity of the infraspinatus muscle (InfraS), whereas the teres minor (arrows) retains a normal echotexture. SSp, spine of the scapula. Del, deltoid muscle. b Sagittal 12–5 MHz US image obtained over the spinoglenoid notch with c transverse and d oblique coronal MR imaging correlation using STIR sequences reveals a cystic lesion (asterisk) located inferior to the spine of the scapula (SSp) and deep to the infraspinatus muscle (arrows), consistent with a ganglion cyst. Note the diffuse high signal intensity of the infraspinatus muscle related to denervation edema and the preserved teres minor (arrowheads) oval hypoechoic lesions with well-defined margins, relatively fixed in location and shape during active and passive shoulder movements (Hashimoto et al. 1994; Bouffard et al. 2000; Martinoli et al. 2003). The continuity of the cyst with a defect in the posterior labrum can be revealed with US. A mass effect on the adjacent tendon and muscle is often demonstrated as well. Then, a careful evaluation of rotator cuff tendons should be carried out to exclude a possible tendon rupture as the cause of the muscle atrophy. The main differential diagnosis of paralabral cysts includes varicosities in the spinoglenoid notch (Carroll et al. 2003). Although enlarged spinoglenoid notch veins appear as hypoechoic round or oval images mimicking a cyst, they are not stationary and change shape and size during shoulder movements (an increased venous size is typically appreciated while the arm is in external rotation, whereas these vessels tend to collapse in internal rotation) (Fig. 6.155). On the other hand, Doppler imaging does not demonstrate flow signals within these veins because the flow velocities are too low. In recent papers, the association of vascular abnormalities in the spinoglenoid notch area with suprascapular neuropathy has been described (Bredella et al. 1999; Ludig et al. 2001; Carroll et al. 2003). In these cases, it is not still clear whether the dilated venous plexus and the compressed nerve are a separate expression of a narrow suprascapular tunnel or whether the varicosities themselves may lead to nerve impingement. In cases of suprascapular neuropathy caused by paralabral cysts, percutaneous needle aspiration of the cyst can be attempted under US guidance (Hashimoto et al. 1994; Chiou et al. 1999). As described in Chapter 18, the procedure has three main goals: to confirm the diagnosis by showing a viscous mucoid content; to drain the fluid as much as possible to reduce the internal pres- Shoulder * * SA a b c Fig. 6.157a–c. Brachial plexus trauma in a young patient following a motorcycle accident. a Long-axis 12–5 MHz US image obtained over the upper trunk nerves at the interscalene area shows a transected nerve (C7). Note the hypoechoic swollen appearance of the proximal and distal stumps (arrowheads), each of which ends in a terminal neuroma (asterisk). b Long-axis 12–5 MHz US image over the divisions and cords (open arrows) of the plexus at the supraclavicular area demonstrates ill-defined fusiform hypoechoic swelling (arrowheads) of three nerves bundles, superficial to the subclavian artery (SA). c Coronal T2-weighted MR imaging correlation demonstrates an increased signal (arrow) in the soft tissues of the interscalene area * * SA a b SA c d e Fig. 6.158a–e. Recent brachial plexus trauma in a patient who underwent a bicycle accident 15 days previously. a–c Series of short-axis 12–5 MHz US images obtained from a proximal to c distal over brachial plexus nerves (open arrows) demonstrate progressive swelling of some fascicles (arrowheads) as they course superficial to the subclavian artery (SA). In c, the abnormal fascicles are encased in a hypoechoic and irregular mass (arrowheads) reflecting a traumatic neuroma. Asterisks, scalene muscles. d Oblique sagittal T1-weighted and e fat-suppressed T2-weighted MR images demonstrate the neuroma as a well-defined mass (arrowheads) encasing the cords of the plexus. Due to its recent formation, the neuroma is hyperintense on T2-weighted sequences Overall, we believe US is more accurate than MR imaging for establishing the level of involvement, namely whether the upper or the lower plexus are injured, in patients with postganglionic injuries. On the other hand, MR imaging is more sensitive for detecting pseudomeningoceles and lesions occurring in the inner spine. In clinical practice, we suggest a combined approach with MR imaging and US to evaluate the traumatized patient, the first technique to evaluate the spine and the foramina, the second to assess the nerves outside the spine. Detection of nerve abnormalities with US may have clinical implications. It may provide an early assessment of the status of the plexus in the immediate phases after the trauma, when clinical findings are not yet conclusive as to whether or not brachial plexus damage requires intervention. In general, patients with total plexopathy have the largest neuromas, as these probably reflect the sum of more than one lesion. On the other hand, patients with small neuromas are usually managed conservatively and show the best functional recovery without surgery. 6.5.5.2 Neoplastic Involvement of the Brachial Plexus Imaging of brachial plexus tumors should consider two main classes of disorder: metastatic disease and radiation plexopathy, and neurogenic 315 316 S. Bianchi and C. Martinoli primary tumors. Although many histotypes have been reported to metastasize to the brachial plexus, including breast cancer, bronchogenic carcinoma, lymphoma and squamous cell carcinoma of the head and neck, the nerve involvement in patients with breast cancer is far more common – accounting for approximately 24% of all nontraumatic brachial plexopathies – because one of the major lymphatic drainage routes of the breast is through the apex of the axilla (Wittenberg and Adkins 2000). In some patients, the metastatic tumor appears as a welldefined solid mass usually located in the soft tissues of either the suprascapular or the infraclavicular area (pattern I). It may exhibit irregular margins and a hypoechoic echotexture and can be seen encasing the nerves with an abrupt nerve-to-tumor interface (Fig. 6.159a,b) (Graif et al. 2004). Most often, the neoplasm invades the brachial plexus leading to a segmental thickening and hypoechoic appearance of the involved nerves without causing a clear mass * T effect (pattern II). The infiltrative spreading of the tumor causes an abnormal fusiform thickening of the nerve (Fig. 6.159c–e). Satellite lymph nodes are often associated. Color Doppler imaging can depict intratumoral vessels and may help in depicting displacement and infiltration of the subclavian vessels and distinguishing the infiltrated cords from the adjacent blood vessels. Compared with MR imaging, US seems better able to delineate the tumor tissue and the nerve involvement in the interscalenic and supraclavicular area owing to its higher spatial resolution capabilities. On the other hand, MR imaging has the advantage of a panoramic view with the possibility of delineating both vertebral and pleural involvement. In patients who have received radiation therapy to the axillary region, radiationinduced damage to the brachial plexus nerves is a common cause of brachial plexopathy, accounting for approximately 30% of the cases of nontraumatic plexopathies (Wittenberg and Adkins 2000). The * * * a b SA Rib c d e Fig. 6.159a–e. Metastasis of breast carcinoma with brachial plexus involvement. Two different cases. a Long-axis 12–5 MHz US image over the divisions and cords of the plexus in the supraclavicular region with b coronal T1-weighted MR imaging correlation shows two individual nerve branches (open and white arrowheads) abruptly encased by a large hypoechoic solid mass (T) with spiculated margins and infiltrative spreading (asterisks) in the surrounding soft tissues. MR image demonstrates that the mass (asterisk) arises from the first rib and is associated with apical subpleural involvement. c Long-axis and d short-axis 12–5 MHz US images over the divisions and cords of the plexus with e correlative transverse T2-weighted MR image show an abnormal hypoechoic thickening of the involved nerves (arrows) over the interscalene and supraclavicular area, reflecting an infiltrative spreading of the tumor. The individual nerves have undefined margins and tend to coalesce in a thick cord-like structure coursing alongside the subclavian artery (SA). MR imaging demonstrates the cranial extension (arrows) of the metastatic tumor in the paravertebral area Shoulder distinction between recurrent or residual disease and radiation-induced neuropathy can be difficult both clinically and on imaging studies. Neurologic damage after radiation therapy may be observed several months to years after treatment. Common symptoms of radiation neuropathy include upper brachial plexus involvement, doses in excess of 60 Gy, a latency period of less than 1 year (peak at 10–20 months), absent pain and lymphedema in the upper limb. On the other hand, neoplastic plexopathy seems more typically associated with symptoms related to the lower plexus nerves, early and severe pain, hand weakness, a dose of less than 60 Gy and a period of latency after completion of radiation therapy of more than 1 year (Wittenberg and Adkins 2000). In radiation fibrosis, US demonstrates diffuse thickening of the nerve fascicles in the absence of a focal mass. Unlike tumor infiltration (see for comparison Fig. 6.159c,d), the nerve thickening is more uniform and some faint fascicular pattern is preserved (Fig. 6.160). However, this finding is far from being specific to radiation fibrosis and the differentiation between radiation damage and residual tumor or recurrence can be problematic as the two conditions may coexist (Graif et al. 2004). Postirradiation plexus lesions should be operated on as early as possible to stabilize the clinical course (as soon as paresthesias appear and before the onset of pain). US can provide a useful means to monitor changes in the cross-sectional volume of the affected nerve fascicles over time. Neurogenic primary tumors of the brachial plexus, including for the most neurofibromas and schwannomas, are far less common than metastatic disorders (Graif et al. 2004). The US characteristics of these tumors are the same as those already described in other locations of the body. The feature of value in distinguishing them from other soft-tissue masses – and especially from enlarged supraclavicular lymph nodes – is demonstration of the continuity between the tumor and the nerve of origin (Fig. 6.161) (Shafighi et al. 2003). 6.5.5.3 Parsonage-Turner Syndrome Parsonage-Turner Syndrome, which is also known as “acute brachial plexus neuritis,” is a rare clinical entity of unknown cause consisting of sudden severe shoulder pain followed by the onset of profound muscle weakness and flaccid paralysis of the shoulder girdle and upper arm. This disorder has a peak rate of incidence between the third and fifth decades and a slight male predominance. Although different factors, including viral infection, trauma, surgery and autoimmunity have been suspected to play a causative role, the etiology of the disease remains unknown. There is usually no loss of sensation associated with the weakness. Several patterns of weakness are reported, the involvement of the suprascapular nerve being the most common. The most frequent pattern relates to the multiple involvement of the axillary (deltoid and teres minor), suprascapular (supraspinatus and infraspinatus), long thoracic (serratus anterior) and musculocutaneous (coracobrachialis, biceps brachii, brachialis) nerves. Regarding the nerve root involvement pattern, C5 and C6 SA a b Fig. 6.160a,b. Radiation fibrosis. a Short-axis and b long-axis 12–5 MHz US images of the brachial plexus nerves (white arrows) in the supraclavicular region obtained 1 year after radiation therapy for breast carcinoma in a patient with reversible brachial plexopathy. There is mild homogeneous swelling of the nerve fascicles (arrowheads) which appear less defined. No focal mass is observed. SA, subclavian artery 317 318 S. Bianchi and C. Martinoli SA SA 1st * SA rib a b c * d e f Fig. 6.161a–f. Schwannoma of the brachial plexus. a Series of short-axis 12–5 MHz US images obtained from a proximal to c distal over brachial plexus nerves with d–f corresponding oblique sagittal T1-weighted MR images demonstrate a rounded mass (asterisk) with smooth contour and solid hypoechoic echotexture in the mid-portion of the supraclavicular area. The mass lies adjacent to the subclavian artery (SA) and is in proximal and distal continuity with one of the nerve cords (arrow). Note the spared fascicles (arrowheads) as they pass alongside the tumor. The continuity between the mass and the nerve of origin helps to rule out a supraclavicular lymph node are the most commonly affected. The prognosis is generally benign, with approximately 75% recovery within 2 years, and treatment is symptomatic (analgesic drug and physical therapy). Electrodiagnostic studies may indicate the complex pattern of muscle involvement. Imaging studies may be helpful to rule out any other local abnormalities, such as rotator cuff tears, shoulder impingement syndrome and calcific tendinitis, thus preventing unnecessary surgery in some patients owing to diagnosis failure (Helms et al. 1998; Helms 2002). At US examination, the affected muscles appear smaller in volume as a result of atrophy and diffusely hyperechoic in relation to denervation edema and fatty infiltration (Fig. 6.162). Although US is able to confirm the clinical diagnosis, MR imaging seems more reliable for depicting the overall extent of muscle atrophy around the shoulder (Bredella et al. 1999; Helms, 2002). 6.5.5.4 Thoracic Outlet Syndrome Thoracic outlet syndrome is a range of disorders arising from the passage of the subclavian artery and vein and brachial plexus nerves through the three anatomic spaces of the thoracic outlet – the interscalene triangle, the costoclavicular space and the retropectoralis minor space (subcoracoid tunnel) – the narrowing of which can lead to arterial, venous or nervous compression (Demondion et al. 2000). Compression of these neurovascular structures with related onset of symptoms may occur at rest or during dynamic maneuvers, such as during holding the arm overhead and backward (hyperabduction). Typical symptoms include upper limb ischemia, pallor, coolness, fatigability, pain, muscle cramp and pulselessness. In the arterial thoracic outlet syndrome, color Doppler imaging and waveform analysis must be obtained from both subclavian and axillary arteries. These techniques can demonstrate high peak systolic velocities in the subclavian artery at the compression site and diminished or absent blood flow in the axillary artery (or the distal arteries of the arm) with hyperabduction maneuvers (Fig. 6.163) (Longley et al. 1992). This latter sign actually seems to be more reliable because the vessel stenosis most often occurs at the level of the costoclavicular space as a result of fibro-osseous or fibromuscular abnormalities and, therefore, cannot be directly depicted with US due to Shoulder * a b * c Fig. 6.162a–c. Parsonage-Turner syndrome in a patient with recent onset of intense weakness of the shoulder muscles. a Sagittal 12–5 MHz US image over the posterior fossa with b oblique sagittal T1-weighted MR imaging correlation demonstrates marked hyperechoic echotexture of the infraspinatus (open arrow) and teres minor (white arrows) muscles. Note that the intramuscular tendons (arrowheads) appear hypoechoic due to anisotropy. c Oblique coronal and transverse (in the insert) fat-suppressed T2weighted MR images reveal marked high signal intensity throughout the supraspinatus (asterisks) and the infraspinatus (star) muscles. Due to a coexisting involvement of the teres minor, the overall denervation pattern is characteristic of a neurogenic deficit of both suprascapular and axillary nerves a c b d Fig. 6.163a–d. Arterial thoracic outlet syndrome. a,b Spectral Doppler waveform analysis obtained from the axillary artery while keeping the arm a in a neutral position and b during hyperabduction test. In neutral position, the axillary artery shows normal high-resistance pulsatile flow. During the stress maneuver, the normal arterial blood flow abruptly converts into a “tardus-parvus” poststenotic pattern, characterized by low-velocity systolic peaks (arrowheads). This abnormal pattern was transient and returned to normal as soon as the patient assumed a neutral position again. c,d Photographs showing the positioning of the patient and transducer, respectively 319 Shoulder 6.5.6 Shoulder Masses Depending on the age of presentation, up to 60% of benign soft-tissue tumors arising around the shoulder are lipomas (Kransdorf 1995). US detection of superficial lipomas arising in the subcutaneous tissue (Fig. 6.165a,b), within the fat planes of the axilla or deep in the anterior deltoid muscle (Fig. 6.165c,d) is generally not a diagnostic problem. In contrast, deepseated lesions within or among shoulder muscles may be difficult to recognize with US (Fig. 6.165e-g). In these cases, contact of the mass with the surrounda ing anatomic structures during certain movements of the arm may lead to symptoms that can mimic a true impingement syndrome. If arising in the region of the neurovascular bundles, lipomas may also cause nerve entrapment, resulting in weakness, pain and numbness. Apart from lipomas, the other soft-tissue tumors arise around the shoulder with a similar incidence as elsewhere in the body. A peculiar tumor-like condition which has a predilection for the shoulder area is elastofibroma dorsi, which is almost invariably located in the inferior part of the thoracoscapular space elevating the inferior angle of the scapula. It merits a separate discussion. b c d Del Del Del Del e f g S 1 InfraS * Scapula * 2 S * 3 4 InfraS Scapula Fig. 6.165a–g. Lipomas around the shoulder girdle: spectrum of US appearances. a,b Subcutaneous lipoma. a Photograph shows a prominent soft-tissue mass (arrow) lying on the posterior aspect of the shoulder. b Extended field-of-view 12–5 MHz US image demonstrates a superficial solid mass (arrows) within the subcutaneous tissue, characterized by thin and highly reflective linear echoes oriented parallel to the skin and embedded in a hypoechoic background, consistent with a lipoma. c,d Intramuscular lipoma. c Transverse 12–5 MHz US image with d T1-weighted MR imaging correlation demonstrates an ovoid compressible solid mass (arrows) with well-defined outlines inside the deltoid muscle (Del). Note the typical echotexture and the homogeneous high T1 signal intensity of the mass. e–g Deep-seated intermuscular lipoma. e Sagittal 12–5 MHz US image over the right infraspinous fossa with f oblique sagittal T1-weighted MR imaging correlation reveals a homogeneous hyperechoic lipoma (asterisk) causing superficial displacement of the infraspinatus muscle (InfraS). S, spina of the scapula; 1, supraspinatus; 2, subscapularis; 3, infraspinatus; 4, teres minor. The patient complained of right shoulder pain exacerbated by internal rotation of the arm and underwent US examination for a suspected impingement syndrome. g US appearance of the normal left posterior fossa for comparison 321 Shoulder the superficial muscles, since the tumor echotexture blends with that of skeletal muscle. Elastofibromas have a peculiar texture composed of an inhomogeneous echogenic background with interspersed linear or curvilinear hypoechoic strands, reflecting the histology of the tumor: these strands are typically arrayed in series with oblique orientation throughout the mass. One-to-one comparison with the CT, MR imaging and gross pathology findings revealed that the hypoechoic strands are compatible with areas of fat, whereas the echogenic background reflected the predominantly fibroelastic bulk of the mass (Fig. 6.167) (Bianchi et al. 1997). It is known that fat can assume a variable echogenicity at US, including an anechoic appearance (pure fat) or a hyperechoic appearance (fat interspersed with other tissues). It has been clearly shown that pure fat is anechoic, whereas fat interspersed with other tissues tends to become hyperechoic (Fornage et al. 1991). The fat within the stripes is relatively homogeneous. Therefore we can expect it can be hypoechoic. In contrast, the higher echogenicity of the fibroelastic background of the mass could result primarily from the amount of acoustic interfaces caused by fibrous tissue against interspersed fat or by intrinsic heterogeneity of the fibrous tissue itself, reflecting varying proportions and distribution of degenerated elastic fibers and collagen. Although elastofibromas have been shown to have increased a fluorodeoxyglucose (FDG) metabolism at positron emission tomography (PET) and PET-CT (Pierce and Henderson 2004), color Doppler imaging does not usually detect blood flow signals within them (Bianchi et al. 1997). The main differential diagnoses for parascapular masses are lipomas and metastases (Fig. 6.168a–c). However, elastofibroma has a typical US appearance which should allow it to be distinguished from these lesions on the basis of a well-defined multilayered pattern. Diagnostic pitfalls might be encountered with US in cases of parascapular hemangiomas. In fact, hemangiomas may exhibit a complex ill-defined appearance with prominent hyperechoic components reflecting fat and prominent hypoechoic vascular channels (Fig. 6.168d,e). The hypervascular appearance of these masses at color Doppler imaging and the detection of phleboliths (which occur in approximately 50% of cases) should help the differential diagnosis. The distinction between elastofibromas and extra-abdominal desmoids, which contain variable amounts of collagen and may also be found around the shoulder, is essentially based on the absence of well-defined striations at US. Overall, we believe that, in the appropriate clinical setting, the US-based diagnosis of elastofibroma can obviate unnecessary patient anxiety and the need of further imaging and unnecessary surgical procedure or biopsy. b Fig. 6.167a,b. Elastofibroma dorsi. a Transverse 12–5 MHz US image with b CT correlation reveal an ill-defined crescent-like mass (large arrows) with the typical striated appearance made of alternating hypoechoic planes of fat (small arrows) and fibroelastic tissue. The elastofibroma dorsi grows in the fat plane interposed between the extrinsic back muscles (arrowhead) and the costal plane (curved arrow) 323 Shoulder appearance and incidence of “hidden” rotator interval lesions. Arthroscopy 17:173–180 Benson LS, Donaldson JS, Carroll NC (1991) Use of ultrasound in management of posterior sternoclavicular dislocation. J Ultrasound Med 10:115–118 Bianchi S, Martinoli C (1999) Detection of loose bodies in joints. Radiol Clin North Am 37:679–690 Bianchi S, Martinoli C, Abdelwahab IF et al (1997) Elastofibroma dorsi: sonographic findings. AJR Am J Roentgenol 169:1113–1115 Bianchi S, Martinoli C, Abdelwahab IF et al (2006) Imaging findings of spontaneous detachment of the deltoid muscle as a complication of massive rotator cuff tear. Skeletal Radiol (35:410-415) Bianchi S, Zwass A, Abdelwahab IF (1994) Sonographic evaluation of posterior instability and dislocation of the shoulder: prospective study. J Ultrasound Med 13:389–393 Bigliani LU, Morrison D, April EW (1986) The morphology of the acromion and its relationship to rotator cuff tears. Orthop Trans 10:228–234 Bigliani LU, Ticker JB, Flatow El et al (1991) The relationship of acromial architecture to rotator cuff disease. Clin Sports Med 10:823–838 Bize P, Pugliese F, Bacigalupo L et al (2003) Unrecognized bilateral posterior shoulder dislocation diagnosed by ultrasound. Eur Radiol 14:350–352 Blankstein A, Amsallem JL, Rubinstein E et al (1985) Septic arthritis of the acromioclavicular joint. Arch Orthop Trauma Surg 103:417–418 Blazar PE, Williams GR, Iannotti JP (1998) Spontaneous detachment of the deltoid muscle origin. J Shoulder Elbow Surg 7:389–392 Bodner G, Harpf C, Gardetto A et al (2002) Ultrasonography of the accessory nerve: normal and pathologic findings in cadavers and patients with iatrogenic accessory nerve palsy. J Ultrasound Med 21:1159–1163 Boehm TD, Kenn W, Matzer M et al (2003) Ultrasonographic appearance of os acromiale. Ultrashall Med 24:180–183 Bokor DJ, Hawkins RJ, Huckell GH et al (1993) Results of nonoperative management of full-thickness tears of the rotator cuff. Clin Orthop 294:103–110 Borsa PA, Jacobson JA, Scibek et al (2005a) Comparison of dynamic sonography to stress radiography for assessing glenohumeral joint laxity in asymptomatic shoulders. Am J Sports Med 33:734–741 Borsa PA, Scibek JS, Jacobson JA et al (2005b) Sonographic stress measurement of glenohumeral joint laxity in collegiate swimmers and age-matched controls. Am J Sports Med 33:1077–1084 Borsa PA, Wilk KE, Jacobson JA et al (2005c) Correlation of range of motion and glenohumeral translation in professional baseball pitchers. Am J Sports Med (33:1392-1399) Bouffard JA, Lee SM, Dhanju J (2000) Ultrasonography of the shoulder. Semin Ultrasound CT MR 21:164–191 Bousquet JC, Denjean S, Faure C et al (1996) Synovial cyst involving isolated paralysis of the infraspinatus muscle. Ultrasonographic diagnosis and MRI. J Radiol 77:275– 277 Brandt TD, Cardone BW, Grant TH et al (1989) Rotator cuff sonography: a reassessment. Radiology 173:323–327 Brasseur JL, Montagnon D, Hacquard B et al (2000) Osteoarticular ultrasonography of the shoulder. J Radiol 81:343–345 Bredella MA, Tirman PFJ, Fritz RC et al (1999) Denervation syndromes of the shoulder girdle: MR imaging with electrophysiologic correlation. Skeletal Radiol 28:567–572 Bretzke CA, Crass JR, Craig EV et al (1985) Ultrasonography of the rotator cuff. Normal and pathologic anatomy. Invest Radiol 20:311–315 Bureau NJ, Dussault RG, Keats TE (1996) Imaging of bursae around the shoulder joint. Skeletal Radiol 25:513–517 Burk DLjr, Karasick D, Kurtz AB et al (1989) Rotator cuff tears: prospective comparison of MR imaging with arthrography, sonography, and surgery. AJR Am J Roentgenol 15:87–92 Cahir J, Saifuddin A (2005) Calcific tendonitis of pectoralis major: CT and MRI findings. Skeletal Radiol 34:234–238 Calandra JJ, Baker CL, Uribe J (1989) The incidence of HillSachs lesions in initial anterior shoulder dislocations. Arthroscopy 5:254–257 Campeau NG, Lewis BD (1998) Ultrasound appearance of synovial osteochondromatosis of the shoulder. Mayo Clin Proc 73:1079–1081 Cantini F, Salvarani C, Olivieri I (1999) Shoulder sonographic findings in polymyalgia rheumatica. J Rheumatol 26:668– 673 Cantini F, Salvarani C, Olivieri I et al (2001) Shoulder ultrasonography in the diagnosis of polymyalgia rheumatica: a case-control study. J Rheumatol 28:1049–1055 Cardinal E, Buckwalter KA, Braunstein EM et al (1996) Amyloidosis of the shoulder in patients on chronic hemodialysis: sonographic findings. AJR Am J Roentgenol 166:153–156 Cardinal E, Bureau NJ, Aubin B et al (2001) Role of ultrasound in musculoskeletal infections. Radiol Clin North Am 39:191–201 Carrino JA, Chandnanni VP, Mitchell DB et al (2000) Pectoralis major muscle and tendon tears: diagnosis and grading using magnetic resonance imaging. Skeletal Radiol 29:305–313 Carroll KW, Helms CA, Otte MT et al (2003) Enlarged spinoglenoid notch veins causing suprascapular nerve compression. Skeletal Radiol 32:72–77 Chan R, Kim DH, Millett PJ, et al (2004) Calcifying tendinitis of the rotator cuff with cortical bone erosion. Skeletal Radiol 33:596–599 Chang CY, Wang SF, Chiou HJ et al (2002) Comparison of shoulder ultrasound and MR imaging in diagnosing fullthickness rotator cuff tears. Clin Imaging 26:50–54 Chansky HA, Iannotti JP (1991) The vascularity of the rotator cuff. Clin Sports Med 10:807–822 Chautems RC, Glauser T, Waeber-Fey MC et al (2000) Quadrilateral space syndrome: case report and review of the literature. Ann Vasc Surg 14:673–676 Chen CKH, Yeh L, Chen CT et al (1998) Contracture of the deltoid muscle: imaging findings in 17 patients. AJR Am J Roentgenol 170:449–453 Chhem RK (1994) Advantages and limitations of ultrasound in the evaluation of the rheumatoid shoulder. J Rheumatol 21:1591–1592 Chiou HJ, Chou YH, Wu JJ et al (1999) Alternative and effective treatment of shoulder ganglion cyst: ultrasonographically guided aspiration. J Ultrasound Med 18:531–535 Chiou HJ, Chou YH, Wu JJ et al (2002) Evaluation of calcific tendonitis of the rotator cuff: role of color Doppler ultrasonography. J Ultrasound Med 21:289–295 Choi YW, Im JG, Song CS (1995) Sonography of the costal cartilage: normal anatomy and preliminary clinical application. J Clin Ultrasound 23:243–250 325 326 S. Bianchi and C. Martinoli Cicak N, Matasovic T, Bajraktarevic T (1992) Ultrasonographic guidance of needle placement for shoulder arthrography. J Ultrasound Med 11:135–137 Cicak N, Bilic R, Delimar D (1998) Hill-Sachs lesion in recurrent shoulder dislocation: sonographic detection. J Ultrasound Med 17:557–560 Codman EA (1934) The shoulder, 2nd edn. Thomas Todd, Boston, Mass Collins RA, Gristina AG, Carter RE et al (1987) Ultrasonography of the shoulder: static and dynamic imaging. Orthop Clin North Am 18:351–360 Cone RO, Danzig L, Resnick D et al (1983) The bicipital groove: radiographic, anatomic and pathologic study. AJR Am J Roentgenol 141:781–788 Connell DA, Potter HG, Sherman MF et al (1999) Injuries of the pectoralis major muscle: evaluation with MR imaging. Radiology 210:785–791 Cooper AM, Hayward C, Williams BD (1993) Calcium pyrophosphate deposition disease involvement of the acromioclavicular joint with pseudocyst formation. Br J Rheumatol 32:248–250 Cothran RL, Helms C (2005) Quadrilateral space syndrome: incidence of imaging findings in a population referred for MRI of the shoulder. AJR Am J Roentgenol 184:989–992 Craig EV (1984) The geyser sign and torn rotator cuff: clinical significance and pathomechanics. Clin Orthop 191:213– 215 Craig EV (1986) The acromioclavicular joint cyst: an unusual presentation of a rotator cuff tear. Clin Orthop 202:189– 192 Crass JR, Craig EV, Bretzke C et al (1985) Ultrasonography of the rotator cuff. Radiographics 5:941–953 Crass JR, Craig EV, Feinberg SB (1986) Sonography of the postoperative rotator cuff. AJR Am J Roentgenol 146:561–564 Crass JR, Craig EV, Feinberg SB (1987) The hyperextended internal rotation view in rotator cuff ultrasonography. J Clin Ultrasound 15:416–420 Crass JR, Craig EV, Feinberg SB (1988a) Clinical significance of sonographic findings in the abnormal but intact rotator cuff: a preliminary report. J Clin Ultrasound 16:625–634 Crass JR, Craig EV, Feinberg SB (1988b) Ultrasonography of rotator cuff tears: a review of 500 diagnostic studies. J Clin Ultrasound 16:313–327 Cvitanic O, Schimandle J, Cruse A et al (1999) The acromioclavicular joint cyst: glenohumeral joint communication revealed by MR arthrography. J Comput Assist Tomogr 23:141–143 Dalal A, Miller TT, Kenan S (2003) Sonographic detection of elastofibroma dorsi. J Clin Ultrasound 31:375–378 Dardani JSY, Fisher RA (2000) MR observations of posttraumatic osteolysis of the distal clavicle after traumatic separation of the acromioclavicular joint. J Comput Assist Tomogr 24:159–164 Demondion X, Boutry N, Drizenko A et al (2000) Thoracic outlet: anatomic correlation with MR imaging. AJR Am J Roentgenol 175:417–422 Demondion X, Bacqueville E, Paul C et al (2003a) Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology 227:461–468 Demondion X, Herbinet P, Boutry N et al (2003b) Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol 24:1303–1309 Demos TC, Johnson C, Love L et al (1985) Computed tomogra- phy of partial unilateral agenesis of the pectoralis muscles. J Comput Assist Tomogr 9:558–559 Dennis DA, Ferlic DC, Clayton ML (1986) Acromial stress fractures associated with cuff-tear arthropathy: a report of three cases. J Bone Joint Surg Am 68:937–940 De Santis D, Palazzi C, D’Amico E et al (2001) Acromioclavicular cyst and “porcupine shoulder” in gout. Rheumatology 40:1320–1321 Drakeford MK, Quinn MJ, Simpson SL et al (1990) A comparative study of ultrasonography and arthrography in evaluation of the rotator cuff. Clin Orthop 253:118–122 Durr HR, Lienemann A, Silbernagi H, et al (1997) Acute calcific tendinitis of the pectoralis major insertion associated with cortical bone erosion. Eur Radiol 7:1215–1217 Edelson JG, Taitz C (1992) Anatomy of the coracoacromial arch: relation to degeneration of the acromion. J Bone Joint Surg Br 74:589–594 Eden KC (1939) The vascular complications of cervical ribs and first thoracic rib abnormalities. Br J Surg 27:111–139 Ellman H (1990) Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop 254:64–74 Farin PU (1996) Sonography of the biceps tendon of the shoulder: normal and pathologic findings. J Clin Ultrasound 24:309–316 Farin PU, Jaroma H (1996) The bicipital groove of the humerus: sonographic and radiographic correlation. Skeletal Radiol 25:215–219 Farin PU, Jaroma H, Harju A et al (1990) Shoulder impingement syndrome: sonographic evaluation. Radiology 176:845–849 Farin PU, Jaroma H, Harju A et al (1995) Medial displacement of the biceps brachii tendon: evaluation with dynamic sonography during maximal external shoulder rotation. Radiology 195:845–848 Farin PU, Kaukanen E, Jaroma H et al (1996a) Hill-Sachs lesion: sonographic detection. Skeletal Radiol 25:559–562 Farin PU, Kaukanen E, Jaroma H et al (1996b) Site and size of rotator-cuff tear: findings at ultrasound, double-contrast arthrography, and computed tomography arthrography with surgical correlation. Invest Radiol 31:387–394 Fehrman DA, Orwin JF, Jennings RM (1995) Suprascapular nerve entrapment by ganglion cysts: a report of six cases with arthroscopic findings and review of literature. J Arthr Rel Surg 11:727–734 Fermand M, Hassen CS, Ariche L et al (2000) Ultrasound investigation of the rotator cuff after computed arthrotomography coupled to bursography. Joint Bone Spine 67:310–314 Ferrari FS, Governi S, Burresi F et al (2002) Supraspinatus tendon tears: comparison of US and MR arthrography with surgical correlation. Eur Radiol 12:1211–1217 Ferri M, Finlay K, Popowich T et al (2005) Sonography of full-thickness supraspinatus tears: comparison of patient positioning technique with surgical correlation. AJR Am J Roentgenol 184:180–184 Fessell DP, Jacobson JA, Craig J et al (2000) Using sonography to reveal and aspirate joint effusions. AJR Am J Roentgenol 174:1353–1362 Flemming DJ, Murphey MD, Shekitka KM et al (2003) Osseous involvement in calcific tendinitis: a retrospective review of 50 cases. AJR Am J Roentgenol 181:965–972 Fornage BD, Tassin GB (1991) Sonographic appearance of superficial soft tissue lipomas. J Clin Ultrasound 19:215– 220 Shoulder Furtschegger A, Resch H (1988) Value of ultrasonography in preoperative diagnosis of rotator cuff tears and postoperative follow-up. Eur J Radiol 8:69–75 Gam AN, Schydlowsky P, Rossel I et al (1998) Treatment of „frozen shoulder“ with distentson and glucocorticoid compared with glucocorticoid alone: a randomised controlled trial. Scand J Rheumatol 27:425–430 Gerber C, Krushell RJ (1991) Isolated rupture of the tendon of the subscapularis muscle. J Bone Joint Surg 73:389–394 Gerber C, Terrier F, Ganz R (1985) The role of the coracoid process in the chronic impingement syndrome. J Bone Joint Surg Br 67:703–708 Gibbon WW, Wakefield RJ (1999) Ultrasound in inflammatory disease. Radiol Clin North Am 37:633–690 Giovagnorio F, Martinoli C (2001) Sonography of the cervical vagus nerve: normal appearance and abnormal findings. AJR Am J Roentgenol 176:745–749 Goldman AB (1989) Calcific tendinitis of the long head of the biceps brachii distal to the glenohumeral joint: plain film radiographic findings. AJR Am J Roentgenol 153:1011–1016 Gotoh M, Higuchi F, Suzuki R, et al (2003) Progression from calcifying tendinitis to rotator cuff tear. Skeletal Radiol 32:86–89 Graif M, Martinoli C, Rochkind S et al (2004) Sonographic evaluation of brachial plexus pathology. Eur Radiol 14:193–200 Grainger AJ, Tirman PFJ, Elliott JM et al (2000) MR anatomy of the subcoracoid bursa and the association of subcoracoid effusion with tears of the anterior rotator cuff and the rotator interval. AJR Am J Roentgenol 174:1377–1380 Groh GI, Badwey TM, Rockwood CA Jr (1993) Treatment of cysts of the acromioclavicular joint with shoulder hemiarthroplasty. J Bone Joint Surg Am 75:1790–1794 Guckel C, Nidecker A (1997) Diagnosis of tears in rotator-cuffinjuries. Eur J Radiol 5:168–176 Guha A, Graham B, Kline DG, Hudson AR (1996) Brachial plexus injuries. In: Wilkins RH, Rengachary SS, eds. Neurosurgery, 2nd edn. McGraw-Hill, New York, pp 3245–3250 Hall F (1986) Ultrasonographic evaluation of the rotator cuff and biceps tendon. J Bone Joint Surg Am 68:950–951 Hall FM (1986) Sonographic and arthrographic assessment of the shoulder after operative repair of a torn rotator cuff. AJR Am J Roentgenol 147:646–647 Hall F (1989) Sonography of the shoulder. Radiology 73:310 Hall RJ, Calvert PT (1995) Stress fracture of the acromion: an unusual mechanism and review of the literature. J Bone Joint Surg Br 77:153–154 Hammar MV, Wintzell GB, Astrom KG et al (2001) Role of US in the preoperative evaluation of patients with anterior shoulder instability. Radiology 219:29–34 Hashimoto BE, Hayes AS, Ager JD (1994) Sonographic diagnosis and treatment of ganglion cysts causing suprascapular nerve entrapment. J Ultrasound Med 13:671–674 Hawkins RJ, Hobeika P (1983) Physical examination of the shoulder. Orthopedics 6:1270–1278 Hawkins RJ, Neer CS, Pianta RM et al (1987) Locked posterior dislocation of the shoulder. Bone Joint Surg Am 69:9–18 Heers G, Hedtmann A (2005) Correlation of ultrasonographic findings to Tossy’s and Rockwood’s classification of acromioclavicular joint injuries. Ultrasound Med Biol 31:725–732 Helms CA (2002) The impact of MR imaging in sports medicine. Radiology 224:631–635 Helms CA, Martinez Z, Speer KP (1998) Acute brachial neuritis (Parsonage Turner syndrome): MR imaging appearancereport of three cases. Radiology 207:255–259 Hermann KA, Backhaus M, Schneider U et al (2003) Rheumatoid arthritis of the shoulder joint: comparison of conventional radiography, ultrasound and dynamic contrastenhanced magnetic resonance imaging. Arthritis Rheum 48:3338–3349 Hiramuro-Shoji F, Wirth MA, Rockwood CA et al (2003) Atraumatic conditions of the sternoclavicular joint. J Shoulder Elbow Surg 12:79–88 Hodler J, Fretz CJ, Terrier F et al (1988) Rotator cuff tears: correlation of sonographic and surgical findings. Radiology 169:791–794 Hodler J, Terrier B, von Schulthess GK et al (1991) MRI and sonography of the shoulder. Clin Radiol 43:323–327 Hollister MS, Mack LA, Patten RM et al (1995) Association of sonographically detected subacromial/subdeltoid bursal effusion and intraarticular fluid with rotator cuff tear. AJR Am J Roentgenol 165:605–608 Huang CC, Ko SF, Ko JY et al (2005) Contracture of the deltoid muscle: sonographic evaluation with MRI correlation. AJR Am J Roentgenol 185:364–370 Huang LF, Rubin DA, Britton CA (1999) Greater tuberosity changes as revealed by radiography: lack of clinical usefulness in patients with rotator cuff disease. AJR Am J Roentgenol 172:1381–1388 Hunter JD, Franklin K, Hughes PM (1998) The ultrasound diagnosis of posterior shoulder dislocation associated with Erb‘s palsy. Pediatr Radiol 28:510–511 Jacobson JA (1999) Musculoskeletal sonography and MR imaging: a role for both imaging methods. Radiol Clin North Am 37:713–735 Jacobson JA, Lancaster S, Prasad A et al (2004) Full-thickness and partial-thickness supraspinatus tendon tears: value of US signs in diagnosis. Radiology 230:234–242 Jarvi OH, Lansimies PH (1975) Subclinical elastofibromas in the scapular region in an autopsy series. Acta Pathol Microbiol Scand 83:87–108 Jerosch J, Castro WH, Jantea C et al (1989) Possibilities of sonography in the diagnosis of instabilities of the shoulder joint. Ultraschall Med 10:202–205 Jerosch J, Marquardt M, Gortzen M (1990) Sonographic diagnosis of Hill-Sachs lesions in unstable shoulder joints. Ultraschall Med 1:251–253 Jiang Y, Zhao J, ven Holsbeeck MT et al (2002) Trabecular microstructure and surface changes in the greater tuberosity in rotator cuff tears. Skeletal Radiol 31:522–528 Jobe FW, Kvitne RS, Giangarra CE (1989) Shoulder pain in the overhand or throwing athlete: the relationship of anterior instability and rotator cuff impingement. Orthop Rev 18:963–975 Kamel M, Kotob H (1997) Ultrasonographic assessment of local steroid injection in Tietze’s syndrome. Br J Rheumatol 36:547–550 Kassarjian A, Torriani M, Ouellette H et al (2005) Intramuscular rotator cuff cysts: association with tendon tears on MRI and arthroscopy. AJR Am J Roentgenol 185:160–165 Katayose M, Magee DJ (2001) The cross-sectional area of supraspinatus as measured by diagnostic ultrasound. J Bone Joint Surg Br 83:565–568 Kay J, Benson CB, Lester S et al (1992) Utility of high-resolution ultrasound for the diagnosis of dialysis-related amyloidosis. Arthritis Rheum 35:926–932 327 328 S. Bianchi and C. Martinoli Kock HJ, Jürgens C, Hirche H et al (1996) Standardized ultrasound examination for evaluation of instability of the acromioclavicular joint. Arch Orthop Trauma Surg 115:136–140 Koski JM (1989) Axillary ultrasound of the glenohumeral joint. J Rheumatol 16:664–667 Koski JM (1991) Validity of axillary ultrasound scanning in detecting effusion of the glenohumeral joint. Scand J Rheumatol 20:49–51 Koski JM (1992) Ultrasonographic evidence of synovitis in axial joints in patients with polymyalgia rheumatica. Br J Rheumatol 31:201–203 Kransdorf MJ (1995) Benign soft-tissue tumors in a large referral population: distribution of diagnoses by age, sex and location. AJR Am J Roentgenol 164:395–402 Kransdorf MJ, Meis JM, Montgomery E (1992) Elastofibroma: MR and CT appearance with radiologic-pathologic correlation. AJR Am J Roentgenol 159:575–579 Krarup AL, Court-Payen M, Skjoldbye B et al (1999) Ultrasonic measurement of the anterior translation in the shoulder joint. J Shoulder Elbow Surg 8:136–141 Lange U, Teichmann J, Stracke H et al (1998) Elderly onset rheumatoid arthritis and polymyalgia rheumatica: ultrasonographic study of the glenohumeral joints. Rheumatol Int 17:229–232 Laredo JD, Bard H (1996) La coiffe des rotateurs et son environnement. Saurapans, Paris Law TC, Chong SF, Lu PP et al (1998) Bilateral subacromial bursitis with macroscopic rice bodies: ultrasound, CT and MR appearance. Australas Radiol 42:161–163 Leach RE, Schepsis AA (1983) Shoulder pain. Clin Sports Med 2:123–135 Lee HS, Joo KB, Park CK et al (2002) Sonography of the shoulder after arthrography (arthrosonography): preliminary results. J Clin Ultrasound 30:23–32 Lee J, Brookenthal KR, Ramsey ML et al (2000) MR imaging assessment of the pectoralis major myotendinous unit: an MR imaging-anatomic correlative study with surgical correlation. AJR Am J Roentgenol 174:1371–1375 Lee JC, Sykes C, Saifuddin A et al (2005) Adhesive capsulitis: sonographic changes in the rotator cuff interval with arthroscopic correlation. Skeletal Radiol 34:522–527 Leotta DF, Martin RW (2000) Three-dimensional ultrasound imaging of the rotator cuff: spatial compounding and tendon thickness measurement. Ultrasound Med Biol 26:509–525 Levinsohn EM, Santelli ED (1991) Bicipital groove dysplasia and medial dislocation of the biceps brachii tendon. Skeletal Radiol 20:419–423 Linker CS, Helms CA, Fritz RC (1993) Quadrilateral space syndrome: findings at MR imaging. Radiology 188:675–676 Llauger J, Palmer J, Roson N et al (2000) Nonseptic monoarthritis: imaging features with clinical and histopathologic correlation. RadioGraphics 20:263–278 Lo IKY, Burkhart SS, Parten PM (2004) Surgery about the coracoid: neurovascular structures at risk. Arthroscopy 20:591–595 Lombardi T, Sherman L, van Holsbeeck MT (1992) Sonographic detection of septic subdeltoid bursitis: a case report. J Ultrasound Med 11:159–160 Longley DG, Yedlicka JW, Molina EJ et al (1992) Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol 158:623–630 Loomer R, Graham B (1989) Anatomy of the axillary nerve and its relation to inferior capsular shift. Clin Orthop Rel Res 243:100–105 Loredo R, Longo C, Salonen D et al (1995) Glenoid labrum: MR imaging with histologic correlation. Radiology 196:33–41 Ludig T, Walter F, Chapuis D et al (2001) MR imaging evaluation of suprascapular nerve entrapment. Eur Radiol 11:2161–2169 Lyons AR, Tomlinson JE (1992) Clinical diagnosis of tears of the rotator cuff. J Bone Joint Surg Br 74:414–415 Mack LA, Matsen FA, Kilcoyne RF et al (1985) US evaluation of the rotator cuff. Radiology 157:205–209 Mack LA, Nyberg DA, Matsen FA (1988a) Sonographic evaluation of the rotator cuff. Radiol Clin North Am 26:161–177 Mack LA, Nyberg DA, Matsen FR et al (1988b) Sonography of the postoperative shoulder. AJR Am J Roentgenol 150:1089–1093 Malghem J, Vande Berg B, Jadoul M et al (1996) Sonographic findings in patients with dialysis-related amyloidosis. AJR Am J Roentgenol 166:153–156 Martin-Hervas C, Romero J, Navas-Acien A et al (2001) Ultrasonographic and magnetic resonance images of rotator cuff lesions compared with arthroscopy or open surgery findings. J Shoulder Elbow Surg 10:410–415 Martinoli C, Bianchi S, Santacroce E et al (2002) Brachial plexus sonography: a technique for assessing the root level. AJR Am J Roentgenol 179:699–702 Martinoli C, Bianchi S, Prato N et al (2003) US of the shoulder: non-rotator cuff disorders. RadioGraphics 23:381–401 Martinoli C, Bianchi S, Pugliese F et al (2004) Sonography of entrapment neuropathies in the upper limb (wrist excluded). J Clin Ultrasound 32:438–450 Mayer V (1985) Ultrasonography of the rotator cuff. J Ultrasound Med 4:607–608 Mayerhoefer ME, Breitenseher MJ, Roposch A et al (2005) Comparison of MRI and conventional radiography for assessment of acromial shape. AJR Am J Roentgenol 184:671–675 McMahon LP, Radford J, Dawborn JK (1991) Shoulder ultrasound in dialysis related amyloidosis. Clin Nephrol 35:227– 232 Mellado JM, Salvado E, Camins A et al (2002) Fluid collections and juxta-articular cystic lesions of the shoulder: spectrum of MRI findings. Eur Radiol 12:650–659 Mengiardi B, Pfirmann CWA, Gerber C et al (2004) Utility of MR arthrography in the diagnosis of adhesive capsulitis. Radiology 233:486–492 Middleton WD (1989) Status of rotator cuff sonography. Radiology 173:307–309 Middleton WD (1992) Ultrasonography of the shoulder. Radiol Clin North Am 30:927–940 Middleton WD, Edelstein G, Reinus WR et al (1984) Ultrasonography of the rotator cuff: technique and normal anatomy. J Ultrasound Med 3:549–551 Middleton WD, Edelstein G, Reinus WR et al (1985a) Sonographic detection of rotator cuff tears. AJR Am J Roentgenol 144:349–353 Middleton WD, Reinus WR, Totty WG et al (1985b) US of the biceps tendon apparatus. Radiology 157:211–215 Middleton WD, Reinus WR, Melson GL et al (1986a) Pitfalls of rotator cuff sonography. AJR Am J Roentgenol 146:555–560 Middleton WD, Reinus WR, Totty WG et al (1986b) Ultrasonographic evaluation of the rotator cuff and biceps tendon. J Bone Joint Surg Am 68:440–450 Shoulder Middleton WD, Payne WT, Teefey SA et al (2004a) Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol 183:1449–1452 Middleton WD, Teefey SA, Yamaguchi K (2004b) Sonography of the rotator cuff: analysis of interobserver variability. AJR Am J Roentgenol 183:1465–1468 Miller CL, Karasick D, Kurtz AB et al (1989) Limited sensitivity of ultrasound for the detection of rotator cuff tears. Skeletal Radiol 18:179–183 Misamore GW, Woodward C (1991) Evaluation of degenerative lesions of the rotator cuff: a comparison of arthrography and ultrasonography. J Bone Joint Surg Am 73:704–706 Mohana-Borges AVR, Chung CB, Resnick D (2004) MR imaging and MR arthrography of the postoperative shoulder: spectrum of normal and abnormal findings. RadioGraphics 24:69–85 Montet X, Zamorani-Bianchi MP, Mehdizade A et al (2004) Intramuscular ganglion arising from the acromioclavicular joint. Clin Imaging 28:109–112 Moosikasuwan JB, Miller T, Burke BJ (2005) Rotator cuff tears: clinical, radiographic, and US findings. RadioGraphics 25:1591-1607 Mudge MK, Wood VE, Frykman GK (1984) Rotator cuff tears associated with os acromiale. J Bone Joint Surg Am 66:427–429 Mutlu H, Silit E, Pekkafali Z et al (2004) Multiple rice body formation in the subacromial-subdeltoid bursa and knee joint. Skeletal Radiol 33:531–533 Narakas AO (1993) Lesions found when operating traction injuries of the brachial plexus. Clin Neurol Neuropathol 95:56–64 Naranjo A, Marrero-Pulido T, Ojeda S et al (2002) Abnormal sonographic findings in the asymptomatic arthritic shoulder. Scand J Rheumatol 31:17–21 Naredo E, Aguado P, De Miguel E et al (2002) Painful shoulder: comparison of physical examination and ultrasonographic findings. Ann Rheum Dis 61:132–136 Neer CS (1972) Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am 54:41–50 Neer CS (1983) Impingement lesions. Clin Orthop Rel Res 173:60–77 Neer CS, Craig EV, Fukuda H (1983) Cuff-tear arthropathy. J Bone Joint Surg Am 65:1232–1244 Nelson MC, Leather GP, Nirschl RP et al (1991) Evaluation of the painful shoulder: a prospective comparison of magnetic resonance imaging, computerized tomographic arthrography, ultrasonography, and operative findings. J Bone Joint Surg Am 73:707–716 Nguyen VD (1996) Rapid destructive arthritis of the shoulder. Skeletal Radiol 25:107–112 Ochi M, Ikuta Y, Watanabe M et al (1994) The diagnostic value of MRI in traumatic brachial plexus injury. J Hand Surg 19:55–59 O’Connor EE, Dixon LB, Peabody T et al (2003) MRI of cystic and soft-tissue masses of the shoulder joint. AJR Am J Roentgenol 183:39–47 O’Connor PJ, Rankine J, Gibbon WW et al (2005) Interobserver variation in sonography of the painful shoulder. J Clin Ultrasound 33:53–56 Ogawa K, Yoshida A, Takahashi M (1997) Fractures of the coracoid process. J Bone Joint Surg Br 79:17–19 Pancione L, Gatti G, Mecozzi B (1997) Diagnosis of Hill-Sachs lesion of the shoulder: comparison between ultrasonography and arthro-CT. Acta Radiol 38:523–526 Patten RM, Mack LA, Wang KY et al (1992) Nondisplaced fractures of the greater tuberosity of the humerus: sonographic detection. Radiology 182:201–204 Patton WC, McCluskey III (2001) Biceps tendinitis and subluxation. Clin Sports Med 20:505–529 Peetrons P, Rasmussen OS, Creteur V et al (2001) Ultrasound of the shoulder joint: non «rotator cuff» lesions. Eur J Ultrasound 14:11–19 Perlmutter GS (1999) Axillary nerve injury. Clin Orthop 368:28–36 Petersilge CA, Witte DH, Sewell BO et al (1993) Normal regional anatomy of the shoulder. Magn Res Imaging Clin North Am 1:1–18 Peterson CJ, Gentz CF (1983) Ruptures of the supraspinatus tendon: the significance of distally pointing acromioclavicular osteophytes. Clin Orthop 174:143–148 Pfahler M, Branner S, Refior HJ (1999) The role of the bicipital groove in tendinopathy of the long biceps tendon. J Shoulder Elbow Surg 8:419–424 Pierce JC III, Henderson R (2004) Hypermetabolism of elastofibroma dorsi on PET-CT. AJR Am J Roentgenol 183:35–37 Pollock RC, Banckes MJ, Emery RJ (1996) Diagnosis of retrosternal dislocation of the clavicle with ultrasound. Injury 27:670–671 Prato N, Derchi LE, Martinoli C (1996) Case report:sonographic diagnosis of biceps tendon dislocation. Clin Radiol 51:737–739 Prato N, Banderali A, Neumaier CE (2003) Calcific tendinitis of the rotator cuff as a cause of drooping shoulder. Skeletal Radiol 32:82–85 Prickett WD, Teefey SA, Galatz LM et al (2003) Accuracy of ultrasound imaging of the rotator cuff in shoulders that are painful postoperatively. J Bone Joint Surgery 17:1084–1089 Propert AJ, Scott DL, Wainwright AC et al (1982) Frequency of occurrence, mode of development, and significance of rice bodies in rheumatoid joints. Ann Rheum Dis 41:109–117 Ptasznik R (2001) Sonography of the shoulder. In: van Holsbeeck MT, Introcaso JH (eds) Musculoskeletal ultrasound. WB Saunders, Philadelphia, pp 463–516 Ptasznik R, Hennessy O (1995) Abnormalities of the biceps tendon of the shoulder: sonographic findings. AJR Am J Roentgenol 164:409–414 Rasmussen OS (2004) Anterior shoulder instability: sonographic evaluation. J Clin Ultrasound 32:430–437 Read JW, Perko M (1998) Shoulder ultrasound: diagnostic accuracy for impingement syndrome, rotator cuff tear, and biceps tendon pathology. J Shoulder Elbow Surg 7:264–271 Rehman A, Robinson P (2005) Sonographic evaluation of injuries to the pectoralis muscles. AJR Am J Roentgenol 184:1205–1211 Reid HS, McNally E, Carr A (1998) Soft-tissue mass around the shoulder. Ann Rheum Dis 57:6–8 Resnick D, Kang HS (1997) Shoulder. In: Resnick D, Kang HS (eds) Internal derangement of joints: emphasis on MR imaging. WB Saunders, Philadelphia, pp 163–333 Retzl G, Kapral S, Greher M, Mauritz W (2001) Ultrasonographic findings of the axillary part of the brachial plexus. Anesth Analg 92:1271–1275 Roberts CS, Walker JA, Seligson D (2001) Diagnostic capabilities of shoulder ultrasonography in the detection of complete and partial rotator cuff tears. Am J Orthop 30:159– 162 329 330 S. Bianchi and C. Martinoli Robinson P, White LM, Lax M et al (2000) Quadrilateral space syndrome caused by glenoid labral cyst. AJR Am J Roentgenol 175:1103–1105 Rockwood CA Jr (1984) Injuries to the acromioclavicular joint. In: Rockwood CA, Greene DP (eds) Fractures in adults, 2nd edn, vol 1. JB Lippincott, Philadelphia, pp 860–910 Roos DB (1976) Congenital anomalies associated with thoracic outlet syndrome. Am J Surg 132:771–778 Ross GJ, Love MB (1989) Isolated avulsion fracture of the lesser tuberosity of the humerus: report of two cases. Radiology 172:833–834 Rutten MJ, van den Berg JC, van den Hoogen FH et al (1998) Nontuberculous mycobacterial bursitis and arthritis of the shoulder. Skeletal Radiol 27:33–35 Ryu KN, Lee SW, Rhee YG et al (1993) Adhesive capsulitis of the shoulder joint: usefulness of dynamic sonography. J Ultrasound Med 12:445–449 Sammarco VJ (2000) Os acromiale: frequency, anatomy and clinical implications. J Bone Joint Surg Am 82:394–400 Sanders TG, Tirman PFJ (1999) Paralabral cyst: an unusual cause of quadrilateral space syndrome. Arthroscopy 15:632–637 Sanders TG, Tirman PF, Feller J et al (2000) Association of intramuscular cysts of the rotator cuff with tears of the rotator cuff: magnetic resonance imaging findings and clinical significance. Arthroscopy 16:230–235 Schydlowsky P, Strandberg C, Galatius S et al (1998a) Ultrasonographic examination of the glenoid labrum of healthy volunteers. Eur J Ultrasound 8:85–89 Schydlowsky P, Strandberg C, Galbo H et al (1998b) The value of ultrasonography in the diagnosis of labral lesions in patients with anterior shoulder dislocation. Eur J Ultrasound 8:107–113 Schydlowsky P, Strandberg C, Tranum-Jensen J et al (1998c) Post-mortem ultrasonographic assessment of the anterior glenoid labrum. Eur J Ultrasound 8:129–133 Seibold CJ, Mallisee TA, Erickson SJ et al (1999) Rotator cuff: evaluation with US and MR imaging. RadioGraphics 19:685–705 Shafighi M, Gurunluoglu R, Milomir N et al (2003) Ultrasonography for depiction of brachial plexus injury. J Ultrasound Med 22:631–634 Sheppard DG, Iyer RB, Fenstermacher MJ (1998) Brachial plexus: demonstration at US. Radiology 208:402–406 Slavotinek JP, Coates PTH, McDonald SP et al (2000) Shoulder appearances at MR imaging in long-term dialysis recipients. Radiology 217:539–543 Sluming VA (1995) Technical note: measuring the coracoclavicular distance with ultrasound: a new technique. Br J Radiol 68:189–193 Soble MG, Kaye AD, Guay RC (1989) Rotator cuff tear: clinical experience with sonographic detection. Radiology 173:319–321 Sofka CM, Adler RS (2003) Sonographic evaluation of shoulder arthroplasty. AJR Am J Roentgenol 180:1117–1120 Sofka CM, Haddad ZK, Adler RS (2004a) Detection of muscle atrophy on routine sonography of the shoulder. J Ultrasound Med 23:1031–1034 Sofka CM, Lin J, Feinberg J et al (2004b) Teres minor denervation on routine magnetic resonance imaging of the shoulder. Skeletal Radiol 33:514–518 Solbiati L, De Pra L, Ierace T et al (1985) High-resolution sonography of the recurrent laryngeal nerve: anatomic and pathologic considerations. AJR Am J Roentgenol 145:989–993 Sommer R, Valen GJ, Ori Y et al (2000) Sonographic features of dialysis-related amyloidosis of the shoulder. J Ultrasound Med 19:765–770 Soslowsky LJ, Carpenter JE, Buccheri et al (1997) Biomechanics of the rotator cuff. Orthop Clin North Am 28:17–30 Speed CA, Hazleman BL (1999) Sonographic assessment of shoulder complaints in rheumatic diseases. J Rheumatol 26:668–673 Steiner E, Steinbach LS, Schnarkowski P et al (1996) Ganglia and cysts around joints. Radiol Clin North Am 34:395– 425 Strunk J, Lange U, Kürten B et al (2003) Doppler sonographic findings in the long bicipital tendon sheath in patients with rheumatoid arthritis as compared with patients with degenerative diseases of the shoulder. Arthritis Rheum 48:1828–1832 Takagishi K, Maeda K, Ikeda T et al (1991) Ganglion causing paralysis of the suprascapular nerve: diagnosis by MRI and ultrasonography. Acta Orthop Scand 62:391–393 Taljanovic MS, Carlson KL, Kuhn JE et al (2000) Sonography of the glenoid labrum: a cadaveric study with arthroscopic correlation. AJR Am J Roentgenol 174:1717–1722 Taljanovic MS, Jones MD, Hunter TB et al (2003) Joint arthroplasties and prostheses. RadioGraphics 23:1295–1314 Teefey SA, Middleton WD, Yamaguchi K (1999) Shoulder sonography: state of the art. Radiol Clin North Am 37:767– 785 Teefey SA, Hasan SA, Middleton WD et al (2000a) Ultrasonography of the rotator cuff: a comparison of ultrasonographic and arthroscopic findings in one hundred consecutive cases. J Bone Joint Surg Am 82:498–504 Teefey SA, Middleton WD, Bauer GS et al (2000b) Sonographic differences in the appearance of acute and chronic fullthickness rotator cuff tears. J Ultrasound Med 19:377–378 Teefey SA, Rubin DA, Middleton WD et al (2004) Detection and quantification of rotator cuff tears: comparison of ultrasonographic, magnetic resonance imaging, and arthroscopic findings in seventy-one consecutive cases. J Bone Joint Surg Am 86:708–716 Teefey SA, Middleton WD, Payne WT et al (2005) Detection and measurement of rotator cuff tears with sonography: analysis of diagnostic errors. AJR Am J Roentgenol 184:1768–1773 Thain LM, Adler RS (1999) Sonography of the rotator cuff and biceps tendon: technique, normal anatomy, and pathology. J Clin Ultrasound 27:446–458 Tossy JD, Mead NC, Sigmond HM (1963) Acromioclavicular separations: useful and practical classification for treatment. Clin Orthop 28:111–119 Tshering Vogel DW, Steinbach LS, Hertel R et al (2005) Acromioclavicular joint cyst: nine cases of a pseudotumor of the shoulder. Skeletal Radiol 34:260–265 Tung GA, Entzian D, Stern JB et al (2000) MR imaging and MR arthrography of paraglenoid labral cysts. AJR Am J Roentgenol 174:1707–1715 Turrin A, Cappello AS (1997) Sonographic anatomy of the supraspinatus tendon and adjacent structures. Skeletal Radiol 26:89–93 Turrin A, Cappello A, Mauri M et al (1997) Echography of the shoulder with the patient supine in the diagnosis of rotator cuff rupture. Radiol Med 94:170–175 Shoulder Uhthoff HK, Sarkar K (1989) Calcifying tendinitis. Baillieres Clin Rheumatol 3:567–581 Vahlensieck M, van Haack K, Schmidt HM (1994) Two portions of the supraspinatus muscle: a new finding about the muscles macroscopy by dissection and magnetic resonance imaging. Surg Radiol Anat 16:101–104 Valls R, Melloni P (1997) Sonographic guidance of needle position for MR arthrography of the shoulder. AJR Am J Roentgenol 169:845–847 van Holsbeeck MT, Introcaso J (1989) Sonography of the postoperative shoulder. AJR Am J Roentgenol 152:202 van Holsbeeck MT, Strouse PJ (1993) Sonography of the shoulder: evaluation of the subacromial-subdeltoid bursa. AJR Am J Roentgenol 160:561–564 van Holsbeeck MT, Kolowich PA, Eyler WR et al (1995) US depiction of partial-thickness tear of the rotator cuff. Radiology 197:443–446 Vanarthos WJ, Monu JU (1995) Type 4 acromion: a new classification. Contemp Orthop 30:227–229 Vick CW, Bell SA (1990) Rotator cuff tears: diagnosis with sonography. AJR Am J Roentgenol 154:121–123 Wadhwani R, Chaubal R, Sukthankar et al (2001) Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med 20:795–801 Walch G (1999) 959 surgical-proved rotator cuff tears 1988– 1996. EULAR symposium épaule doloreuse 28:4–9 Walch G, Liotard JP, Boileau P et al (1991) Le conflit glenoidien postérieur: un autre conflit de l’epaule. Rev Chir Orthop 77:751–754 Wallny T, Wagner UA, Prange S et al (1999) Evaluation of chronic tears of the rotator cuff by ultrasound: a new index. J Bone Joint Surg Br 81:675–678 Wamser G, Bohndorf K, Vollert K et al (2003) Power Doppler sonography with and without echo-enhancing contrast agent and contrast-enhanced MRI for the evaluation of rheumatoid arthritis of the shoulder joint: differentiation between synovitis and joint effusion. Skeletal Radiol 32:351–359 Weishaupt D, Zanetti M, Tanner A et al (1999) Lesions of the reflection pulley of the long biceps tendon: MR arthrographic findings. Invest Radiol 34:463–469 Weiss C, Imhoff AB (2000) Sonographic imaging of a spinoglenoid cyst. Ultraschall Med 21:287–289 Werner A, Mueller T, Boehm D et al (2000) The stabilizing sling for the long head of the biceps tendon in the rotator cuff interval: a histoanatomic study. Am J Sports Med 28:28–31 Westhoff B, Wild A, Werner A et al (2002) The value of ultrasound after shoulder arthroplasty. Skeletal Radiol 31:695– 701 Widman DS, Craig JG, van Holsbeeck MT (2001) Sonographic detection, evaluation and aspiration of infected acromioclavicular joints. Skeletal Radiol 30:388–392 Wiener SN, Seitz WH Jr (1993) Sonography of the shoulder in patients with tears of the rotator cuff: accuracy and value for selecting surgical options. AJR Am J Roentgenol 160:103–107 Wittenberg KH, Adkins MC (2000) MR imaging of non-traumatic brachial plexopathies: frequency and spectrum of findings. RadioGraphics 20:1023–1032 Wohlwend JR, van Holsbeeck M, Craig J et al (1998) The association between irregular greater tuberosities and rotator cuff tears: a sonographic study. AJR Am J Roentgenol 171:229–233 Wolfe SW, Wickiewicz TL, Cavanaugh JT (1992) Ruptures of the pectoralis major muscle: an anatomic and clinical analysis. Am J Sports Med 20:587–593 Wright IS (1945) The neurovascular syndrome produced by hyperabduction of the arms: the immediate changes produced in 15 normal controls, and the effects on some persons of prolonged hyperabduction of the arms, as in sleeping, and certain occupations. Am Heart J 29:1–19 Wulker N, Melzer C, Wirth CJ (1991) Shoulder surgery for rotator cuff tears: ultrasonographic 3-year follow-up of 97 cases. Acta Orthop Scand 62:142–147 Yamaguchi K, Tetro AM, Blam O et al (2001) Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg 10:199–203 Yang WT, Chui PT, Metreweli C (1998) Anatomy of the normal brachial plexus revealed by sonography and the role of sonographic guidance in anesthesia of the brachial plexus. AJR Am J Roentgenol 171:1631–1636 Yao L, Metha U (2003) Infraspinatus muscle atrophy: implications? Radiology 226:161–164 Yen CH, Chiou HJ, Chou YH et al (2004) Six surgery correlated sonographic signs for rotator cuff tears: emphasis on partial-thickness tear. Clin Imaging 28:69–76 Zarins B, Rowe CR (1984) Current concepts in the diagnosis and treatment of shoulder instability in athletes. Med Sci Sports Exerc 16:444–448 Zwar RB, Read JW, Noakes JB (2004) Sonographically guided glenohumeral joint injection. AJR Am J Roentgenol 183:48– 50 331 Arm 7 Arm Carlo Martinoli and Stefano Bianchi CONTENTS 7.1 Introduction 333 7.2 7.2.1 7.2.2 7.2.3 Clinical and US Anatomy 333 Anterior Arm 333 Posterior Arm 336 Neurovascular Bundle 339 7.3 7.3.1 7.3.1.1 7.3.1.2 Arm Pathology 340 Anterior Arm 341 Bicipital Sulcus Pathology 341 Median Neuropathy Following Brachial Artery Catheterization 341 7.3.1.3 Supracondylar Process Syndrome 342 7.3.2 Posterior Arm 344 7.3.2.1 Spiral Groove Syndrome 344 References 333 347 7.1 Introduction Pathology of muscles and tendons of the arm is not very common and clinically relevant. On the other hand, compressive neuropathies affecting the main nerve trunks of the upper limb, and especially the median nerve and the radial nerve, may present with a spectrum of confusing and, sometimes, ambiguous clinical pictures for the physician. These neuropathies are often related to anatomic constraints, may be acute or chronic, and require a thorough understanding of the pathophysiology and clinical correlation. Current improvements in US technology have contributed significantly to the more accurate diagnosis of these conditions. C. Martinoli, MD Associate Professor of Radiology, Cattedra “R” di Radiologia – DICMI – Università di Genova, Largo Rosanna Benzi 8, 16132 Genova, Italy S. Bianchi, MD Privat-docent, Université de Genève, Consultant Radiologist, Fondation et Clinique des Grangettes, 7, ch. des Grangettes, 1224 Genève, Switzerland 7.2 Clinical and US Anatomy The arm extends from the shoulder to the elbow. It is formed by two main compartments – anterior and posterior – separated by a plane passing through the humerus and the lateral and medial intermuscular septa, which are thick fibrous extensions of the brachial fascia attached to the medial and lateral supracondylar ridge of the humerus (Fig. 7.1). The anterior compartment (flexor compartment) contains three muscles – the coracobrachialis, the biceps brachii and the brachialis – and the musculocutaneous nerve. The posterior compartment (extensor compartment) houses the large triceps brachii muscle, consisting of three heads – long, lateral and medial – and the radial nerve. At the upper medial aspect of the arm, the main neurovascular bundle, consisting of the brachial artery, some veins and three nerves – median, ulnar and radial – courses in the neurovascular compartment, a groove delimited by a division of the medial intermuscular septum and bounded by the biceps anteriorly and the triceps posteriorly. A basic description of the normal and US anatomy of the anterior and posterior compartments is included here. 7.2.1 Anterior Arm The anterior compartment of the arm houses three muscles: the coracobrachialis, the biceps brachii and the brachialis (Fig. 7.2). The coracobrachialis takes its origin from the tip of the coracoid process, medial to the insertion of the short head of the biceps, and continues down and laterally to insert onto the medial aspect of the middle third of the humeral shaft. The biceps brachii is formed by a combination of two muscle bellies: the long head and the short head. As already described in Chapter 6, the long head originates from a long tendon which Arm the two heads of the biceps unite to create a large muscle which is located superficial to the brachialis and ends in a long distal tendon which attaches into the radial tuberosity (see Chapter 8). The brachialis muscle is located between the distal biceps brachii and the humeral shaft (Fig. 7.2a). It originates from the distal half of the anterior face of the humerus and the intermuscular septa and descends more distally than the biceps brachii to continue in a short tendon which inserts into the coronoid process of the ulna and the ulnar tuberosis (see Chapter 8). From the biomechanical point of view, the coracobrachialis plays a role as an extensor and adductor of the arm, whereas the brachialis and the biceps brachii are powerful flexors of the forearm. Furthermore, the biceps brachii is a supinator of the forearm and a weak flexor of the arm. US examination of the anterior arm is best performed with the patient lying supine keeping the arm abducted (Fig. 7.3). Different degrees of internal and external rotation of the arm may be helpful in evaluating the anatomic structures placed more laterally and medially. Sweeping the probe down from the tip of the coracoid, trans- verse US images demonstrate the coracobrachialis muscle followed by the two heads of the biceps brachii (Fig. 7.3a–c). More distally, the biceps is seen overlying the deep brachialis muscle, which rests over the anterior humeral cortex (Fig. 7.3d,e). The lateral and medial intermuscular septa separate the anterior muscles from posterior lateral and medial heads of the triceps muscle. Among the four nerves of the arm (median, ulnar, radial and musculocutaneous), the musculocutaneous is the one crossing the anterior aspect of the arm (Fig. 7.4a). This nerve arises from the lateral cord of the brachial plexus (C5–C7 level). It pierces the coracobrachialis and then descends on the anterior aspect of the brachialis between this muscle and the biceps (Fig. 7.4b,c). On transverse US images, the musculocutaneous nerve can be recognized piercing the coracobrachialis (Fig. 7.3c). Its detection may be not straightforward in obese patients. After coursing between the brachialis and biceps brachii the nerve pierces the superficial fascia of the arm to enter the subcutaneous tissue and emerge above the elbow crease as the lateral cutaneous nerve of the SHB Deltoid * LHB CBr a BB HH SubS CBr H b c b BB c CBr Br CBr d Br H d e e Fig. 7.3a–e. Anterior muscles and musculocutaneous nerve. a–e Series of transverse 12–5 MHz US images obtained from cranial (a) to caudal (e) over the anterior aspect of the arm. a The coracoid process (asterisk) of the scapula, which is the origin of the conjoined tendon of the coracobrachialis and the short head of the biceps, appears as a rounded hyperechoic structure with well-defined posterior acoustic shadowing. b The coracobrachialis muscle (CBr) can be found between the deltoid and the subscapularis (SubS). The musculocutaneous nerve (arrowheads) is recognized as a thin hypoechoic elongated structure running just superficial to the medial aspect of the coracobrachialis. HH, humeral head. c At the proximal arm, the long head (LHB) and the short head (SHB) of the biceps become progressively visible. The smaller belly of the long head lies lateral to the short head. On these planes, the musculocutaneous nerve (arrowhead) can be seen running inside the coracobrachialis muscle CBr. H, humerus. d At the mid-arm, the two heads of the biceps muscle (BB) fuse together. The musculocutaneous nerve lies among the distal part of the coracobrachialis (CBr), the proximal part of the brachialis (Br) and the biceps brachii (BB) muscles. H, humerus. e At the distal arm, the biceps brachii overlies the large brachialis muscle. The musculocutaneous nerve is found in the hyperechoic cleavage plane separating these two muscles. The photograph at the bottom right of the figure indicates probe positioning 335 336 C. Martinoli and S. Bianchi 3 CBr 1 3 3 2 BB Br 4 a b c forearm. The nerve then divides in two small terminal branches, anterior and posterior. The musculocutaneous nerve supplies the coracobrachialis, the biceps brachii and the brachialis and then distributes to the skin of the forearm as the lateral (antebrachial) cutaneous nerve. On the anterolateral aspect of the arm, the cephalic vein courses over the superficial fascia and the biceps muscle. Some anatomic variants can be found at the anteromedial aspect of the arm. The most common vascular anomaly is the proximal division of the humeral artery in the radial and ulnar artery. Although this variant is not associated with clinical symptoms, it should be described in the US report as it can cause problems during attempted catheterization of the humeral artery. Another rare but potentially symptomatic variant is the supracondylar process of the humerus (Fig. 7.5a–c). This bone anomaly refers to a triangular spur-like process which arises 5–7 cm above the medial epicondyle and is typically oriented distally and medially ending with a beak-like apex (Sener et al. 1998). The supracondylar process is a primitive remnant present in climbing mammals encountered in approximately 1% of normal limbs. It is usually found in association with a ligament, commonly known as the ligament of Struthers, which joins its tip with the medial epicondyle. In these cases, the medial aspect of the humeral metaphysis and the ligament of Struthers form the boundaries of an osteofibrous tunnel which encircles the neurovascular bundle of the forearm (Fig. 7.5d). The radiographic appearance of Fig. 7.4a–c. Neurovascular structures of the anterior and medial aspect of the arm. a Schematic drawing illustrates the median (1) and ulnar (2) nerves as they descend the bicipital fossa, a longitudinal groove delimited by the medial head of the triceps posteriorly and the biceps and brachialis muscles anteriorly. At the proximal arm, the median nerve courses lateral to the brachial artery (4) whereas, at the middle third of the arm, it crosses the artery to descend medial to it down to the antecubital fossa. b,c The musculocutaneous nerve (3) is seen piercing (arrowhead) the coracobrachialis muscle (CBr) to enter the anterior compartment of the arm where it lies between the posterior brachialis (Br) and the superficial biceps brachii (BB). At the distal arm, this nerve becomes subcutaneous to divide into its terminal branches the supracondylar process is characteristic but MR imaging is the technique of choice to image the ligament (Pecina et al. 2002). At US, transverse planes are the most adequate to display the supracondylar process. However, because this bony process is thin, difficulties may arise when the US beam is perpendicular to it. Tilting the probe anteriorly and posteriorly may be helpful to visualize it based on its posterior acoustic shadowing. The ligament may be even more difficult to see with US than the bony process. Once detected, a careful scanning technique is needed to rule out possible signs of entrapment of the median nerve and the brachial artery which course just deep to the ligament. A possible proximal bifurcation of the artery and, occasionally, of the nerve can be encountered together with supracondylar process (Gunther et al. 1993). 7.2.2 Posterior Arm The posterior compartment of the arm contains the large triceps muscle (Fig. 7.6). As its name indicates, the triceps consists of three heads: long, lateral and medial. The proximal tendon of the long head arises from the infraglenoid tubercle of the scapula and continues down with a large muscle belly located at the medial aspect of the arm (Fig. 7.6a); the lateral head and the medial head take their origin from the posterior aspect of the humerus, the first superior, the second inferior to the spiral groove of the radial Arm Br T H a a MN H UN b c d Fig. 7.5a–d. Supracondylar process and ligament of Struthers. a,b Transverse 12–5 MHz US images obtained over the supracondylar region in a patient referring a firm deep-seated local mass. a The brachial artery (open arrowhead) and the median nerve (curved arrow) are located just superficial to the medial aspect of the humeral shaft (H), between the brachialis (Br) and the triceps brachii (T) muscles. A small bony spur (double arrow) with posterior acoustic attenuation is shown anterior to the nerve and the artery. b More accurate probe positioning obtained by tilting the transducer anteriorly reveals a well-defined hyperechoic bony structure (open arrow) in continuity with the medial cortex of the humerus (white arrow) reflecting the supracondylar process. Note the close relationship of the bone process with the median nerve (curved arrow), the brachial artery (open arrowhead) and veins (white arrowheads). c Anteroposterior radiograph of the arm confirms the US diagnosis showing a typical supracondylar process (arrow). d Schematic drawing demonstrates the supracondylar process (arrow) and the ligament of Struthers (arrowheads) connecting it with the medial epicondyle. The brachial artery (a) and the median nerve (MN) can be seen passing through the supracondylar foramen, while the ulnar nerve (UN) lies outside it RN RN LoHT LaHT dba MeHT * a * b c Fig. 7.6a–c. Schematic drawing of a coronal view of the posterior compartment of the arm illustrates the anatomy of the triceps brachii muscle and the radial nerve from surface (a) to depth (c). a–c The relationships among the three muscle bellies of the triceps – the long head (LoHT), the lateral head (LaHT) and the medial head (MeHT) – are shown. The bellies of the triceps muscle have separate origins and coalesce distally in a strong common tendon (asterisk) which inserts onto the posterior aspect of the olecranon. The long head arises from the infraglenoid tubercle through a strong tendon (black arrow), while the lateral and medial heads take their origin from the posterior aspect of the humeral shaft, the first above, the second below the spiral groove. Note the radial nerve (RN, black line) and the satellite deep brachial artery (dba, dashed line) which course from medial to lateral inside the spiral groove, between the two heads 337 338 C. Martinoli and S. Bianchi nerve (Fig. 7.6b,c). Distally, the long and the lateral heads of the triceps converge to insert into a flat tendon that attaches to the olecranon process (see Chapter 8); the medial head inserts, for the most part, directly into the olecranon, but also on the medial aspect of the distal triceps tendon. The triceps muscle is a powerful extensor of the forearm; because the long head crosses the shoulder joint, it also plays a role as an extensor and adductor of the arm. For an adequate evaluation of the posterior arm, the patient is asked to sit on the bed with the examiner behind him/her. A slight degree of elbow flexion may be useful to stretch the distal myotendinous junction and the triceps tendon. Alternatively, the patient can lie prone, but this position is less comfortable, particularly for elderly subjects. Transverse US images are first obtained over the lateral aspect of the arm to display the lateral head (Fig. 7.7a,b). Visualization of the superficial long head and the deep medial head is obtained by shifting the transducer more medially (Fig. 7.7c–e). The radial nerve originates from the posterior cord of the brachial plexus (C5–C8) and supplies the extensor muscles of the upper limb (i.e., the triceps, the lateral part of the brachialis, the brachioradialis, the forearm extensors) and the skin of the dorsal forearm and dorsolateral aspect of the hand. After leaving the axilla, this nerve enters the arm at the posterolateral aspect of the humeral shaft alongside the brachial artery, first between the coracobrachialis and the teres major and then between the bellies of the medial and lateral heads of the triceps. Then, it winds closely around the posterolateral aspect of the humeral shaft, passing in the spiral groove between the long and the lateral heads of the triceps accompanied by the deep brachial artery and vein (Fig. 7.6). More distally, the radial nerve pierces the lateral intermuscular septum and enters the anterior compartment of the arm coursing between the brachialis and brachioradialis muscles. Transverse US scans obtained with the patient seated in front of the examiner with the arm in internal rotation are the best to demonstrate the radial nerve, which courses adjacent to the bone along the posterolateral aspect of the humeral shaft (Fig. 7.8). The brachial artery, the coracobrachialis and the teres major muscles are LoHT LoHT LoHT LaHT LaHT MeHT H a MeHT bb H LaHT MeHT dd MeHT ME H * H c LaHT LoHT LaHT LE d e c a b e Fig. 7.7a–e. Triceps muscle. a–e Series of transverse 12–5 MHz US images obtained from cranial (a) to caudal (e) over the posterior aspect of the arm. a At the proximal arm, the long (LoHT) and the lateral head (LaHT) of the triceps can be demonstrated superficial to the humerus (H). The long head is located medial to the lateral head. b Sweeping the probe down from the level illustrated in a, the medial head (MeHT) can be seen arising from the posterior aspect of the humerus. It is the deepest component of the triceps. c At the middle arm, the three heads of the triceps dispose in two layers: superficial (including the long and lateral heads) and deep (consisting of the medial head). In the superficial layer, the eccentric distal aponeurosis of the long head is seen separating this muscle belly from the lateral head. d Moving the probe down toward the distal third of the arm, the conjoined tendon (arrowhead) of the long and the lateral heads is progressively appreciated. Observe the superficial position of this tendon relative to the deep medial head. e A more distal image over the olecranon fossa (asterisk) shows the distal triceps tendon (arrowhead) and the distal myotendinous junction of the medial and lateral heads. ME, medial epicondyle; LE, lateral epicondyle. The photograph at the bottom right of the figure indicates probe positioning Arm play a role as predisposing causes for nerve disease. In this clinical setting, US serves as an adjunct to electrodiagnostic testing and clinical evaluation for patient’s investigation. This technique also provides the surgeon with important information concerning surgical exploration and reconstruction. 7.3.1 Anterior Arm 7.3.1.1 Bicipital Sulcus Pathology Because the ulnar nerve is relatively unconstrained in the proximal arm, it is only exceptionally involved in entrapment syndromes at this site. In general, compression of this nerve in the upper arm relates to space-occupying lesions, such as large aneurysms of the brachial artery or anomalous muscles (e.g., chondroepitrochlearis muscle). On the other hand, the median nerve is subject to compression at different levels in the upper arm. Penetrating trauma during falls or glass wounds are most often responsible for nerve injury (Fig. 7.10). In these cases, the proximity of nerves and vessels in the bicipital sulcus leads to complex injuries with contemporary involvement of the median nerve, the brachial artery and veins, and possibly the ulnar nerve. Given the complexity of these traumas, it is not unusual to find patients sutured for vascular bleeding at the first surgical look and then submitted to US examination for a missed nerve transection. In the preoperative assessment of complete nerve tears, US is an accurate means to identify the level of the tear and to map the location of the nerve ends, that may be displaced and retracted from the site of the injury, based on the identification of hypoechoic stump neuromas. In this application, US has shown some advantages over MR imaging as a result of its higher spatial resolution capabilities for imaging a restricted area in which many nerves and vessels run close together. A peculiar type of iatrogenic median nerve injury can be observed at the midhumerus following brachial artery catheterization. In addition to traumas, compression of the median nerve in the bicipital sulcus may also occur at the distal humerus if a bony spur and ligament is present. When a mass is palpable over the bicipital sulcus, US is able to distinguish a neurogenic tumor from other soft tissue neoplasms based on the continuity of the mass with the parent nerve (Fig. 7.11). Furthermore, US may identify with certainty which is the nerve of origin (the median, the ulnar) of a neurogenic mass: an assessment not always easy on MR imaging, especially for large-sized tumors. 7.3.1.2 Median Neuropathy Following Brachial Artery Catheterization In routine outpatients or in cases in which the femoral approach is not appropriate, the percutaneous brachial approach is a well-established alternative. The brachial approach is safe with a low complication rate. Nevertheless, the close proximity of the * A a b Fig. 7.10a,b. Median nerve transection at the middle third of the arm in a 14-year-old girl following a glass wound. a Long-axis 15–7 MHz US image over the bicipital sulcus demonstrates the median nerve (arrowheads) ending abruptly (arrows) at the level of the penetrating trauma. Note the heterogeneous appearance (asterisk) of the overlying subcutaneous tissue. In this particular case, the distal stump of the nerve was identified approximately 4 cm below. b Gross surgical view confirms the complete transection of the median nerve and the gap intervening between the stumps (arrows) measured preoperatively with US. The brachial artery (a) was undamaged 341 342 C. Martinoli and S. Bianchi a a T a b * T c Fig. 7.11a–c. Schwannoma of the median nerve at the bicipital fossa. a,b Transverse 15–7 MHz US images obtained a just proximal to the tumor and b at the tumor level demonstrate an eccentric solid hypoechoic mass (T) in continuity with the fascicles of the median nerve (arrowheads). In b, note the brachial artery (a) and the ulnar nerve (curved arrow) displaced by the bulk of the tumor. c, Longitudinal 12–5 MHz US image depicts the tumor (T) connected at both ends with the median nerve (arrowheads). At its proximal end, the mass is in continuity with a swollen fascicle (asterisk), whereas the other fascicles (straight arrows) remain unaffected and appear displaced at the periphery of the mass. After surgical resection, pathologic examination revealed a schwannoma brachial artery to the median nerve, the mobility of the brachial artery in the arm, as well as the winding unpredictable course of the nerve, which lies at first lateral to the artery and then crosses to its medial side, allow the possibility of incidental median nerve injury during a catheterization procedure. This complication seems more likely in patients under anticoagulation therapy (Chuang et al. 2002). Clinically, the onset of a neuralgic tingling sensation and paresthesias radiating from the elbow to the first three fingers suggests nerve irritation and damage. Needle injury may result in epineurial hemorrhage leading to compression of the fascicles and impaired nerve function (Macon and Futrell 1973). US and Doppler imaging are useful to identify the hematoma enclosed in the epineurium and the displaced fascicles (Chuang et al. 2002). In this setting, US may have a role in distinguishing an epineurial hemorrhage from a traumatic neuroma, an extrinsic collection or a pseudoaneurysm of the brachial artery. In epineurial hemorrhage, the collection is typically aligned between the artery and the fascicles, which are eccentrically displaced (Fig. 7.12). On the contrary, traumatic neuromas appear as fusiform hypoechoic areas encasing most nerve fascicles but not displacing them. Extrinsic collections are usually larger in size and may cause major nerve displacement. Finally, pseudoaneurysms appear as pulsatile sacs in continuity with the injured artery by means of a neck. Color Doppler imaging can help the diagnosis by showing whirling blood flow within the sac and “to-and-fro” waveforms at the arterial neck indicating communication with the artery (Fig. 7.13). In patients with onset of neuralgic symptoms, US can successfully guide the percutaneous aspiration of the hematoma to obtain an early decompression of the fascicles (Chuang et al. 2002). 7.3.1.3 Supracondylar Process Syndrome In individuals with a supracondylar process, the median nerve and, in rare instances, the ulnar nerve can be compressed in an osteofibrous tunnel created by a firm fibrous band with a vertical course, commonly referred to as the “ligament of 344 C. Martinoli and S. Bianchi Struthers”, which joins the anomalous bony process and the medial epicondyle. Clinically, this condition typically affects young sportsmen as a result of intense muscular activity in the elbow and forearm and may start with pain and numbness in the first three fingers and weakness of forearm muscles innervated by the median nerve (Sener et al. 1998). US can demonstrate the relationship of the median nerve with the anomalous bone and ligament. Although not yet reported in the radiological literature, displacement of the nerve by these structures may represent an indicator of entrapment. Therapy includes excision of the ligament of Struthers and ablation of the supracondylar process. The brachial artery can also be compressed by an anomalous insertion of the pronator teres muscle into the supracondylar process (Talha et al. 1987). 7.3.2 Posterior Arm 7.3.2.1 Spiral Groove Syndrome Within the spiral groove, the close relationship of the radial nerve with the humeral cortex and its fixity as it penetrates the lateral intermuscular septum makes it vulnerable to extrinsic pressure. Clinically, radial nerve entrapment at the middle arm is characterized by combined features of both superficial radial nerve and posterior interosseous nerve palsy. Radial nerve palsy essentially results in wrist-drop due to denervation of the forearm extensors, whereas the triceps muscle (acting on forearm extension) is usually spared because its innervation arises above. Sensory loss over the dorsolateral forearm and hand maybe associated. The main causes of radial nerve compression in the spiral groove include axillary crutches, pressure on a wheelchair armrest and improper positioning of the arm such as occurs when an individual falls asleep leaning against a hard surface following drug- or alcohol-induced stupor, the so-called Saturday night palsy. Strenuous physical activity has also been implicated as a possible cause of radial nerve injury in patients with fibrous bands arising from either the lateral or long head of the triceps. Most of these cases recover fully within a few days or weeks. Recovery may be delayed by several months and occasionally may be incomplete. In a more severe traumatic setting, and especially in patients with closed traction injuries, usually associated with fractures of the midshaft of the humerus, there may be direct contusion and laceration of the nerve by fracture fragments. In general, the surgical outcome of radial nerves lacerated by tidy wounds or traction is better than that of nerves damaged by humeral fractures. A severe traction rupture of the radial nerve, with a gap between the stumps exceeding 10 cm, is best treated by musculotendinous flexor-to-extensor transfer. Furthermore, if the interval since injury exceeds 1 year, transfer is more likely to improve function (Shergill et al. 2001). Somewhat similar to other sites of nerve entrapment, the main signs of radial nerve impingement in the spiral groove are a swollen nerve with a uniformly hypoechoic appearance and loss of the fascicular pattern (Bodner et al. 1999, 2001). In entrapment syndromes due to fibrous bands arising from the adjacent bellies of the triceps, abrupt changes in the nerve cross-sectional area at the site of compression and direct visualization of the constricting fibrous band can be seen with US (Fig. 7.14). In contusion traumas, the nerve fascicles may appear focally swollen and hypoechoic and the fat space surrounding the nerve thickened and diffusely hyperechoic (Fig. 7.15). In malaligned or fragmented fractures of the midshaft of the humerus, the radial nerve can be seen displaced on the edge of fracture fragments or pinched in between them (Fig. 7.16) (Bodner et al. 1999, 2001; Peer et al. 2001; Martinoli et al. 2004). In addition, it may appear encased or displaced by a hypertrophied callus and scar tissue. In the postoperative setting, the radial nerve may be stretched over orthopedic hardware for osteosynthesis. In patients with onset of progressive radial nerve palsy after internal fixation of a humeral shaft fracture with a compression plate, the conflict of the nerve with the metallic plate can be nicely depicted and US may be helpful in deciding whether early surgical treatment has to be instituted (Peer et al. 2001; Martinoli et al. 2004). In these cases, US reveals the dislocation of the compression plate and the thinning or thickening of the nerve which rides on the detached proximal end of the plate (Fig. 7.17). These findings indicate the need for a second surgical look for recovery of the nerve function. Space-occupying masses arising in the spiral groove are rare and may be nonpalpable even if large due to their deep location. Similar to the bicipital fossa, neurogenic tumors involving the radial nerve can be encountered in the spiral groove area (Fig. 7.18). Arm T T H a H H b c T T d e f Fig. 7.18a–f. Schwannoma of the radial nerve at the spiral groove. a–c Transverse 12–5 MHz US images obtained a just proximal to the tumor, b at the tumor level and c just distal to the tumor with d–f T2-weighted MR imaging correlation demonstrate a hyperintense solid mass (T) in continuity with the fascicles of the radial nerve (arrows). Note the close proximity of the mass and the parent nerve with the humerus (H) References Bodner G, Huber B, Schwabegger A et al (1999) Sonographic detection of radial nerve entrapment within a humerus fracture. J Ultrasound Med 18:703–706 Bodner G, Buchberger W, Schocke M et al (2001) Radial nerve palsy associated with humeral shaft fracture: evaluation with US – initial experience. Radiology 219:811–816 Chuang YM, Luo CB, Chou YH et al (2002) Sonographic diagnosis and treatment of a median nerve epineurial hematoma caused by brachial artery catheterization. J Ultrasound Med 21:705–708 Gunther SF, DiPasquale D, Martin R (1993) Struthers’ ligament and associated median nerve variations in a cadaveric specimen. Yale J Biol Med 66:203–208 Macon WL IV, Futrell JW (1973) Median-nerve neuropathy after percutaneous puncture of the brachial artery in patients receiving anticoagulants. N Engl J Med 288:1396 Martinoli C, Bianchi S, Pugliese F et al (2004) Sonography of entrapment neuropathies in the upper limb (wrist excluded). J Clin Ultrasound 32:438–450 Pecina M, Boric I, Anticevic D (2002) Intraoperatively proven anomalous Struthers’ ligament diagnosed by MRI. Skeletal Radiol 31:532–535 Peer S, Bodner G, Meirer R et al (2001) Examination of postoperative peripheral nerve lesions with high-resolution sonography. AJR Am J Roentgenol 177:415–419 Sener E, Takka S, Cila E (1998) Supracondylar process syndrome. Arch Orthop Trauma Surg 117:418–419 Shergill G, Bonney G, Munshi P et al (2001) The radial and posterior interosseous nerves. J Bone Joint Surg Br 83:646– 649 Talha H, Enon B, Chevalier JM et al (1987) Brachial artery entrapment: compression by the supracondylar process. Ann Vasc Surg 1:479–482 347 Elbow 8 Elbow Stefano Bianchi and Carlo Martinoli CONTENTS 8.1 Introduction 349 8.2 8.2.1 8.2.1.1 8.2.1.2 8.2.1.3 8.2.2 8.2.2.1 8.2.2.2 8.2.2.3 8.2.2.4 8.2.3 8.2.3.1 8.2.3.2 Clinical Anatomy 349 Joint and Ligament Complexes 350 Elbow Joint 350 Medial Collateral Ligament 351 Lateral Collateral Ligament 351 Muscles and Tendons 352 Anterior Elbow 352 Medial Elbow 353 Lateral Elbow 354 Posterior Elbow 355 Neurovascular Structures 356 Median Nerve and Brachial Artery 356 Radial Nerve and Posterior Interosseous Nerve 357 8.2.3.3 Ulnar Nerve 357 8.2.4 Bursae 357 8.3 Essentials of Clinical History and Physical Examination 358 8.3.1.1 Tendon Abnormalities 358 8.3.1.2 Ligament Instability 358 8.3.1.3 Cubital Tunnel Syndrome 358 8.4 8.4.1 8.4.2 8.4.3 8.4.4 Ultrasound Anatomy and Scanning Technique 359 Anterior Elbow 360 Medial Elbow 363 Lateral Elbow 364 Posterior Elbow 367 8.5 8.5.1 8.5.1.1 8.5.1.2 8.5.2 8.5.2.1 8.5.2.2 8.5.2.3 8.5.3 8.5.3.1 Elbow Pathology 370 Anterior Elbow Pathology 371 Distal Biceps Tendon Tear 371 Bicipitoradial (Cubital) Bursitis 372 Medial Elbow Pathology 376 Medial Epicondylitis (Epitrochleitis) 376 Medial Collateral Ligament Injury 377 Epitrochlear Lymphadenopathies 377 Lateral Elbow Pathology 378 Lateral Epicondylitis 378 S. Bianchi, MD Privat-docent, Université de Genève, Consultant Radiologist, Fondation et Clinique des Grangettes, 7, ch. des Grangettes, 1224 Genève, Switzerland C. Martinoli, MD Associate Professor of Radiology, Cattedra “R” di Radiologia – DICMI – Università di Genova, Largo Rosanna Benzi 8, 16132 Genova, Italy 349 8.5.3.2 Lateral Collateral Ligament Injury 380 8.5.3.3 Supinator Syndrome (Posterior Interosseous Neuropathy) 383 8.5.4 Posterior Elbow Pathology 384 8.5.4.1 Distal Triceps Tendon Tear 384 8.5.4.2 Olecranon Bursitis 386 8.5.4.3 Cubital Tunnel Syndrome 390 8.5.4.4 Ulnar Nerve Instability 392 8.5.4.5 Snapping Triceps Syndrome 394 8.5.5 Bone and Joint Disorders 396 8.5.5.1 Synovitis 396 8.5.5.2 Osteoarthritis and Osteochondral Damage 400 8.5.5.3 Occult Fractures 401 8.5.5.4 Posterior Dislocation Injury and Instability 402 8.5.6 Elbow Masses 404 References 405 8.1 Introduction Being capable of a wide range of hinged and rotational motion, the elbow is intrinsically predisposed to acute injures and degenerative changes. Although clinical examination and routine radiography are essential to evaluate elbow disorders, US has become increasingly important in the diagnostic investigation of several abnormalities affecting tendons and muscles, joints, ligaments, nerves and other softtissue structures around the elbow joint. After US examination, CT and MR imaging may be required to further address the status of the joint cavity, the articular cartilage and the bone. 8.2 Clinical Anatomy A brief description of the complex anatomy of the elbow with emphasis given to the anatomic features amenable to US examination, including joints and ligament complexes, muscles and tendons, neurovascular structures and bursae, is included here. 354 S. Bianchi and C. Martinoli BT BRRAD PRT a b head) at the medial aspect of the coronoid process. Distally, the pronator teres inserts along the lateral surface of the radial shaft through a flat tendon (Fig. 8.5a). The median nerve passes in between the two bellies of the pronator teres and is separated from the ulnar artery by the ulnar head of this muscle (Fig. 8.5). During pronation of the forearm, the pronator teres works together with the pronator quadratus. There are four superficial flexor muscles of the hand and wrist that arise from the common flexor tendon, arranged from medial or lateral as the flexor carpi radialis, the palmaris longus, the flexor digitorum superficialis and the flexor carpi ulnaris. The flexor digitorum profundus has a separate more distal origin from the anteromedial aspect of the ulna, the coronoid process and the anterior surface of the interosseous membrane. The superficial and deep flexor muscles are primary flexors of the wrist and fingers. In addition, the common flexor tendon provides dynamic support to the underlying ulnar collateral ligament in resisting valgus stress. 8.2.2.3 Lateral Elbow The lateral compartment of the elbow includes the extensor muscles of the wrist and hand that arise from the lateral epicondyle as the “common extensor tendon”, the brachioradialis, the extensor carpi radialis longus and the supinator muscles. Fig. 8.5a,b. Brachial artery and median nerve. a Same schematic drawing as Fig. 8.4b after removal of the distal biceps tendon and the superficial humeral belly of the pronator teres muscle (1) reveals the course of the brachial artery (arrowheads) and the adjacent median nerve (straight arrows) in the pronator area and beneath the “sublimis bridge” (curved arrow) of the flexor digitorum superficialis muscle (fds). Br, brachialis muscle; BB, biceps muscle; 2, deep (ulnar) belly of the pronator teres muscle. b Gross dissection of the cubital fossa demonstrates the brachial artery (curved arrows) and the median nerve (straight arrows) as they infold in the space between the brachioradialis (brrad) and the pronator teres (prt) muscles. The distal biceps tendon (bt) has previously been removed The common extensor tendon is a flattened tendon which originates from the anterolateral surface of the lateral epicondyle (Fig. 8.3b). It receives contributions of fibers from four superficial extensor muscles: extensor carpi radialis brevis, extensor digitorum communis, extensor digiti minimi and extensor carpi ulnaris. The extensor carpi radialis brevis makes up most of the deep articular fibers, whereas the extensor digitorum contributes to the superficial portion of the common extensor tendon (Connell et al. 2001). The extensor digiti minimi and carpi ulnaris provide only minor components to the common extensor tendon. Overall, these muscles act as extensors of the wrist and/or fingers and also play a role in radial (extensor carpi radialis brevis) and ulnar (extensor carpi ulnaris) deviation of the wrist. The common extensor tendon origin is separated from the joint capsule by the lateral ulnar collateral ligament (Fig. 8.3b). Cranial to and separately from the common extensor tendon, the brachioradialis (anterior) and the extensor carpi radialis longus (posterior) muscles arise from the supracondylar ridge of the humerus and the lateral intermuscular septum. The supinator is the deepest of the lateral muscles. It has two heads between which the posterior interosseous nerve, motor branch of the radial nerve, passes to reach the posterior elbow (Fig. 8.6a) (see also Sect. 8.2.3.2). The superficial head arises from the lateral epicondyle, the lateral collateral and annular ligaments and from behind the supinator crest and fossa of the 356 S. Bianchi and C. Martinoli ME tm LE O TT T FCU FCU a fcu O ME b c d Fig. 8.7a–d. Ulnar nerve and cubital tunnel. a Photograph of the posteromedial aspect of the elbow illustrates the course of the ulnar nerve (dashed black line) between the bony prominences of the medial epicondyle (ME) and the olecranon (O) covered by the cubital tunnel retinaculum (dark gray) and, more caudally, by the aponeurosis and the belly of the flexor carpi ulnaris muscle (light gray). LE, lateral epicondyle. b Schematic drawing of the cubital tunnel on cross-section view reveals the relationships of the ulnar nerve (arrow) with the medial epicondyle (ME) and the olecranon (O). Note the Osborne retinaculum that covers the cubital tunnel as a roof. c Schematic drawing of the posterior aspect of an extended elbow demonstrates the ulnar nerve (arrows) as it passes through the cubital tunnel, beneath the Osborne retinaculum (dark gray) and the flexor carpi ulnaris muscle (fcu, light gray). tm, triceps muscle; T, distal triceps tendon. d Gross dissection of the cubital tunnel shows the triangular arcuate ligament that unites the humeral (fcu1) and ulnar (fcu2) heads of the flexor carpi ulnaris muscle. The forceps elevate the ligament making the course of the nerve (arrows) visible 8.2.3 Neurovascular Structures The elbow is traversed by the ulnar, median and radial nerves that cross through its posteromedial, anterior and lateral aspects respectively. In the elbow area, the median nerve is accompanied by the brachial artery, the radial nerve gives off a main motor branch, the posterior interosseous nerve, and the ulnar nerve travels across an osteofibrous tunnel, the cubital tunnel. 8.2.3.1 Median Nerve and Brachial Artery In the cubital fossa, the median nerve courses behind the lacertus fibrosus and superficial to the brachialis muscle. More distally, it progressively deepens to pass between the ulnar and humeral heads of the pronator teres muscle in more than 80% of individuals. At the elbow, the median nerve gives off small muscular branches to the pronator teres, palmaris longus, flexor carpi radialis and flexor carpi ulnaris. Then, it courses deep to the tendinous bridge connecting the humero-ulnar and radial heads of the flexor digitorum superficialis muscle, the so-called sublimis bridge (Fig. 8.5). At the elbow, the brachial artery is superficial and courses along the medial border of the biceps muscle and tendon overlying the brachialis (Figs. 8.4b,c, 8.5). Then, it passes between the median nerve (medial) and the biceps tendon (lateral) beneath the bicipital aponeurosis to divide, at the proximal forearm, into the radial and ulnar arteries. 358 S. Bianchi and C. Martinoli 8.3 Essentials of Clinical History and Physical Examination In the history of the patient complaining of elbow pain or dysfunction the examiner has to consider possible systemic articular diseases (rheumatoid arthritis and similar conditions), occupational disorders (drill diseases which can cause joint osteoarthritis) and traumas (missed radial head fractures may be a cause of long-lasting discomfort), even if sustained in the past. Sport activities are also a critical part of the history: tennis and golf practice can cause microtrauma and overuse injuries to the common extensor and flexor tendon origins with the onset of clearly defined clinical syndromes. With chronic symptoms, it is important to analyze as accurately as possible how the pain radiates and where it is localized, as well as its eliciting factors, because these characteristics can help to focus the US examination and suggest the correct diagnosis. At physical examination, the range of elbow motion and the end-point of motion must be investigated at the level of both the radio-capitellar and trochlea-ulnar joints (flexion/extension) as well as at the proximal radio-ulnar joint (pronation/supination). Then, previous standard radiographs, if any, must be reviewed before starting the US examination in order to exclude bone abnormalities that may be overlooked or misinterpreted at US, such as joint erosions, osteoarthritic changes and heterotopic calcifications. In a post-traumatic setting, a careful review of the radiographs should be obtained prior to the US examination to rule out subtle fractures, especially involving the radial head, that may be overlooked at first observation. 8.3.1.1 Tendon Abnormalities When a tendon lesion is suspected, specific resisted movements must be checked. Due to its superficial position, the distal biceps tendon can easily be palpated during resisted flexion while keeping the elbow 90° flexed and supinated. The rupture of this tendon is typically associated with retraction of the muscle into the arm, where it can be appreciated as a lump (see Sect. 8.5.1.1). Nevertheless, the retracted muscle belly can be difficult to detect in obese patients or when local swelling and pain limit proper physical examination. The distal triceps tendon can also be palpated without difficulty on the posterior elbow with the joint 90° flexed. Its integrity can be assessed by asking the patient to extend the elbow against resistance: a complete tear of the distal triceps tendon causes complete loss of extension power (see Sect. 8.5.4.1). In the case of a patient with suspected lateral epicondylitis, the examiner should immobilize the patient’s elbow with one hand while compressing the common extensor tendon origin with the fingers over the lateral epicondyle. In lateral epicondylitis, this maneuver elicits pain radiating from the epicondylar area down through the forearm. Pain is typically exacerbated by extending the wrist against resistance (see Sect. 8.5.3.1). In medial epicondylitis, pain can be elicited by firm pressure over the medial common tendon or by resisted wrist flexion (see Sect. 8.5.2.1). 8.3.1.2 Ligament Instability Specific clinical tests may be helpful in the setting of ligament instability. To evaluate the integrity of the lateral and medial collateral ligaments, the examiner may grasp the posterior aspect of the patient’s elbow with one hand and the patient’s wrist with the other. While locking the elbow, a valgus or varus stress is applied to assess the integrity of the medial and lateral collateral ligaments respectively. These clinical maneuvers are more reliably performed by placing the probe over the ligament in order to demonstrate even minor widening of the joint space during stressing (Fig. 8.9) (see Sects. 8.5.2.2, 8.5.3.2). 8.3.1.3 Cubital Tunnel Syndrome A useful clinical maneuver to assess the state of the ulnar nerve is the “Froment’s test”. The patient is asked to pinch a sheet of paper between thumb and index finger. In case of overt ulnar neuropathy, the patient grasps the paper by flexing the thumb (activation of the median-innervated flexor pollicis longus as a compensation for the weakness of dorsal interosseous muscles) (see Sect. 8.5.4.3). In patients with cubital tunnel syndrome, palpation of the ulnar nerve at the cubital tunnel may be painful and may reproduce symptoms. 360 S. Bianchi and C. Martinoli 8.4.1 Anterior Elbow US examination of the anterior elbow may be performed with the patient facing the examiner with the elbow extended resting on a table (Barr and Babcock 1991). A slight bending of the patient’s body towards the examined side makes full supination and assessment of some structures of the anterior compartment, such as the distal biceps tendon, easier. A full elbow extension can be obtained by placing a pillow under the joint. Raising the table can also be helpful and allows for a more comfortable examination for both the patient and the examiner. If the patient is unable to obtain a complete elbow extension, longitudinal scans can be difficult to perform, particularly when using large-sized probes. As an alternative in the elderly or for severely traumatized patients, the anterior aspect of the elbow can also be examined with the patient supine holding his or her arm along the body. The main anterior structures amenable to US examination are: the brachialis muscle, the distal biceps muscle and tendon, the brachial artery, the median and radial nerve, the anterior synovial recess with the anterior fat pad and the radio-capitellar and trochlea-ulna joints. Transverse US images are first obtained by sweeping the probe from approximately 5 cm above to 5 cm below the trochlea-ulna joint, perpendicular to the humeral shaft. Cranial US images of the supracondylar region reveal the two main muscles of the anterior aspect of the distal arm: the superficial biceps muscle and the deep brachialis muscle (Fig. 8.10a). The biceps lies just deep to the subcutaneous tissue surrounded by the brachial fascia. It has a bipennate appearance with a central hyperechoic layer reflecting the aponeurosis. The brachialis muscle is located between the biceps and the humeral bony cortex and is much larger than the biceps. The brachial artery and the median nerve course alongside these muscles: the artery typically lies lateral to the nerve (Fig. 8.10b). Shifting the transducer more distally, the distal biceps tendon appears as a hyperechoic structure that overlies the brachialis muscle (Fig. 8.10b,c). A careful scanning technique is required to image this tendon. The distal biceps tendon is best examined on longitudinal planes with the patient’s forearm in maximal supination to bring the tendon insertion on the radial tuberosity into view (Fig. 8.11) (Miller a br a a br b a br c R U Fig. 8.10a–c. Normal distal biceps tendon. Transverse 12−5 MHz US images obtained over the anterior elbow in a healthy subject demonstrate the distal biceps tendon: a at the myotendinous junction, b at the level of the humeral trochlea and c below the joint line, just before its insertion. In a, the distal biceps tendon takes its origin from a wide echogenic aponeurosis (arrowheads) that is located centrally within the muscle (arrows). Note the brachialis muscle (br) that lies deep to the biceps. a, brachial artery. In b and c, the distal biceps tendon (large arrow) appears as an oval hyperechoic structure that lies superficial to the brachialis (br). Close to its medial side, the brachial artery (a) and the median nerve (curved arrow) are seen, whereas the radial nerve (small arrow) lies more laterally between the brachialis and brachioradialis muscles. Note the aponeurosis (arrowheads) of the brachialis R, radius; u, ulna. The inserts at the upper left side of the figures indicate probe positioning Elbow S Br ∗ RH HC ? ★ a ? ★ Br RH HC b c Fig. 8.11a–c. Normal distal biceps tendon. a Long-axis 12−5 MHz US image of the anterior elbow with b sagittal T1-weighted (T1w) SE MR imaging correlation shows the curved appearance of the distal biceps tendon (arrows) that inserts on the bicipital tuberosity (asterisk) of the radius. The tendon has a fibrillar appearance and courses superficial to the brachialis (Br) and the supinator (S) muscles. Observe the squared appearance of the radial head (RH), the rounded humeral capitellum (HC) covered by a band of hypoechoic cartilage and the anterior fat pad (stars). c Photograph illustrating the scanning technique to image the distal portion of the biceps tendon. The patient’s forearm is kept in maximal supination (curved arrow) and the inferior edge of the transducer is pushed against the patient’s skin. and Adler 2000). Because of an oblique course from surface to depth, portions of this tendon may appear artifactually hypoechoic if the probe is not maintained parallel to it (Fig. 8.12a,c). Accordingly, the distal half of the probe must be gently pushed against the patient’s skin to ensure parallelism between the US beam and the distal biceps tendon, thus allowing a complete visualization of its echogenic fibrillar pattern (Fig. 8.12b,d). In thick large elbows, however, the distal portion of this tendon may be difficult to examine owing to its deep location. In general, transverse planes are less useful for examining the distal part of the biceps tendon because slight changes in transducer orientation may produce dramatic variation in tendon echogenicity and this create confusion between the tendon and the surrounding strucutres. In conditions of maximal anisotropy, the tendon and the artery may exhibit the same size and echogenic pattern on transverse scans (Fig. 8.12e,f). As stated earlier, the median nerve courses on the internal side of the brachial artery, whereas the radial nerve can be appreciated between the brachioradialis and the brachialis muscle (Figs. 8.10b,c; 8.13). The coronoid fossa appears as a concavity of the anterior surface of the humerus filled with hyperechoic tissue related to the anterior fat pad (Fig. 8.14). The fat pad has a triangular shape with its base located anteriorly, deep to the brachialis muscle. At this level, the anterior capsule is imaged inconsistently with US (Miles and Lamont 1989). In normal states, a small amount of fluid can be recognized between the fat pad and the humerus (Fig. 8.14). On transverse US images, the anterior aspect of the distal humeral epiphysis appears as a wavy hyperechoic line covered by a thin layer (2 mm thick) of hypoechoic articular cartilage (Fig. 8.15). Its lateral third corresponds to the humeral capitellum that shows a typical convex shape and articulates with the radial head. The medial two thirds of 361 362 S. Bianchi and C. Martinoli v a c e v b a d a f Fig. 8.12a–f. Normal distal biceps tendon and anisotropy. a,b Schematic drawings and c,d corresponding long-axis 12-5MHz US images of the biceps tendon obtained with oblique (a,c) or perpendicular (b,d) incidence of the US beam. e,f Respective short-axis scans. In c and e, an inadequate orientation of the US beam leads to a hypoechoic appearance of the tendon (arrows) relative to the surrounding fat due to anisotropy. When incorrectly imaged, the tendon can be distinguished from the adjacent brachial artery (a) and cubital vein (v) with difficulty because all look hypoechoic br br HC a BR BR b 2( (# Fig. 8.13a,b. Median nerve and brachial artery. Longitudinal gray-scale (a) and color Doppler (b) 12−5 MHz US images over the antecubital fossa demonstrate the normal appearance of the median nerve (white arrows in a) and the brachial artery (open arrows in b). Both lie superficial to the brachialis muscle (br). Note the humeral capitellum (HC) and the radial head (RH). The inserts at the upper left side of the figures indicate probe positioning 364 S. Bianchi and C. Martinoli ∗∗ br br Ulna Ulna ∗ a br br br br ∗ ∗ b The common flexor tendon is best examined in longitudinal planes. It appears shorter than the common extensor tendon origin and inserts onto the medial aspect of the epitrochlea (Fig. 8.17a). Deep to this tendon, the anterior bundle of the medial collateral ligament appears as a cord-like structure that joins the epitrochlea with the more cranial aspect of the ulna, the so-called sublimis tubercle (Fig. 8.17a,b). The proper positioning for examination of the anterior bundle of the medial collateral ligament is obtained with the patient supine keeping the shoulder abducted and externally rotated and the elbow in 90° of flexion (Ward et al. 2003). At US examination, the anterior component of the medial collateral ligament has a fibrillar pattern and a fanlike shape (Ward et al. 2003). It looks hyperechoic: however, the ligament echogenicity may vary depending on patient and probe positioning (Fig. 8.17a,b). With the patient’s elbow in the extension position lying on the examination table, it usually appear hypoechoic in comparison with the overlying flexor tendon. In a recent US study with cadaveric correlation, the ligament thickness was reported to range from approximately 2.6 to 4 mm, without significant differences in sidedness, stress application or hand dominance (Ward et al. 2003). The other components of this ligament, namely the posterior and transverse bundles, are not depicted as accurately as the anterior one on br br c Fig. 8.16a,c. Brachialis tendon. a Mid-sagittal 12−5MHz US image of the antecubital fossa with b T1w SE MR imaging and c diagram correlation shows the brachialis tendon (arrow) as a short and thick structure which inserts on the anterior ulna, just caudal to the apex (asterisk) of the coronoid process. br, brachialis muscle; arrowheads, distal biceps tendon. The insert at the upper left side of the figure indicates probe positioning US examination, even using high-resolution transducers. However, these latter portions are a less frequent source of morbidity and play a minor role in stabilizing the elbow against valgus stress. 8.4.3 Lateral Elbow The lateral aspect of the elbow is best examined with both elbows in extension, thumbs up, palms of the hands together (Barr and Babcock 1991). When examining the radial collateral ligament and the capsule, the elbow should be extended, keeping the hand pronated. Along the lateral elbow, high-resolution US can demonstrate the common extensor tendon, the lateral ulnar collateral ligament, the radial nerve with its superficial and deep (posterior interosseous nerve) branches, and the radio-capitellar joint. The common extensor tendon origin is best visualized in longitudinal planes as a beak-shaped hyperechoic structure located between the subcutaneous tissue and the lateral ulnar collateral ligament (Fig. 8.18). Deep to this tendon, the lateral epicondyle appears as a smooth down-sloping hyperechoic structure. The individual contributions from the extensor muscles to the common extensor tendon cannot be discriminated with US because they are 366 S. Bianchi and C. Martinoli interwoven with each other. The deep tendon fibers relative to the extensor carpi radialis brevis overlie the lateral ulnar collateral ligament and cannot easily be separated from it. In fact, these structures are intimately related and, although they run in a slightly different direction, they have the same fibrillar appearance (Connell et al. 2001). On transverse US images, the common extensor tendon origin has an oval cross-sectional shape and is located just superficial to the lateral epicondyle. Immediately distal to the myotendinous junction, the muscular bellies of the extensor carpi radialis brevis, extensor digitorum, extensor digiti minimi and extensor carpi ulnaris usually appear as a single bulk. Anterior to the lateral epicondyle, the main trunk of the radial nerve courses between the brachialis and the brachioradialis muscles. It is reliably exam- ined by means of transverse US images obtained between these muscles as a small rounded structure composed of a few scattered hypoechoic dots reflecting the fascicles (Fig. 8.19a) (Bodner et al. 2002). The recurrent radial artery can be seen adjacent to the nerve and should not be confused with one of its fascicles. Color Doppler imaging may be helpful to precisely identify it. High-resolution US is able to visualize the radial nerve as it divides into the superficial cutaneous sensory branch and the posterior interosseous nerve (Fig. 8.19b,c). The fascicles in these latter nerves are very small and a meticulous scanning technique based on tracking the nerve bundle according to its short axis is needed for their visualization. At the lateral elbow, US can visualize the posterior interosseous nerve as it pierces the supinator muscle and enters the arcade brrad br HC a br RH b s c RN Fig. 8.19a–c. Radial nerve. Transverse 12−5 MHz US images obtained over the anterolateral elbow demonstrate the normal radial nerve and its divisional branches at the level of humeral capitellum (a), radial head (b) and radial neck (c). In a, the main trunk of the nerve (arrow) lies in the hyperechoic space between the brachialis (br) and brachioradialis (brrad) muscles, superficial to the humeral capitellum (HC). In b, the radial nerve (arrow) passes over the radial head (RH) in close relation to the annular ligament (arrowheads). Typically, this ligament appears as a curved hyperechoic band that covers the radial head like a belt. In c, the cutaneous sensory branch (straight arrow) and the posterior interosseous nerve (curved arrow) can be appreciated over the supinator muscle (s) as a result of bifurcation of the main trunk of the nerve. RN, radial neck. The inserts at the upper left side of the figures indicate probe positioning Elbow of Frohse, passing between the superficial and deep parts of this muscle (Fig. 8.20). Across the supinator, the nerve moves toward the posterior compartment. Accordingly, an appropriate scanning technique should include repositioning of the patient with the elbow in semiflexion, placing the forearm forward and more transversely oriented over the examination table. During pronation, the nerve may assume an angulated course at the proximal edge of the arcade of Frohse. One should not mistake this appearance for a pathologic finding. Within or just after leaving the supinator muscle, the posterior interosseous nerve can be seen further subdividing into a few subtle branches directed to the muscles of the posterior forearm. These latter branches are difficult to examine because their size approximates the spatial resolution capability of current US equipment. Once given off anterior to the lateral epicondyle, the superficial cutaneous sensory branch of the radial nerve continues into the anterior forearm. At the proximal forearm, it joins the radial artery and can be demonstrated coursing between the extensor carpi radialis longus and the brachioradialis. The lateral aspect of the radio-capitellar joint can clearly be delineated with US (Fig. 8.18a). A triangular hyperechoic structure is usually seen filling the peripheral portion of the articular rim between the two bony surfaces. This structure corresponds to a synovial projection, somewhat similar to a meniscus (lateral synovial fringe) (Fig. 8.18a). The appearance of the radial head varies with different degrees of rotation of the forearm: in pronation, the radial head has a more squared appearance, whereas in supination it tends to assume a smoother contour. Dynamic US scanning may be helpful to assess the status of the radial head and to exclude possible occult nondisplaced fractures. Superficial to it, the annular ligament is visible as a belt-like homogeneous hyperechoic structure (Fig. 8.19b). It is best visualized by means of high-resolution transducers. With the probe placed over the radial head, passive supination and pronation movements of the forearm allow a better differentiation of the fixed annular ligament from the rotating radial head. At the radial metaphysis, the annular recess is visualized with US only if distended by fluid. 8.4.4 Posterior Elbow The posterior aspect of the elbow may be examined by keeping the joint flexed 90° with the palm resting on the table (Barr and Babcock 1991). This posi- brrad ss ss ds ds Radius a brrad ss ds ds b Fig.8.20a,b. Posterior interosseous nerve. a Long-axis and b short axis 12−5 MHz US images obtained at the proximal forearm over the brachioradialis muscle (brrad) demonstrate the normal posterior interosseous nerve as it crosses the supinator muscle. Within the bellies of the supinator (ss, superficial part of the supinator muscle; ds, deep part of the supinator muscle), the nerve appears as a thin hypoechoic structure composed of a few fascicles (arrows) embedded in a hyperechoic fatty plane. The inserts at the upper left side of the figures indicate probe positioning 367 368 S. Bianchi and C. Martinoli tion allow easy demonstration of the main structures of the posterior elbow: the cubital tunnel and the ulnar nerve, the triceps muscle and tendon, the posterior fossa with the posterior fat pad and the olecranon bursa. Cranial to the olecranon, US reveals the hypoechoic bellies of the triceps muscle and its tendon that is located eccentrically and slightly medial with respect to the midline (Fig. 8.21). The distal triceps tendon appears hyperechoic and typically exhibits striations as it fans out toward its insertion on the olecranon, a pattern somewhat similar to the quadriceps. These striations, with alternating hypo- and hyperechoic bands, are more likely due to interposition of fat between the tendon fibers and should not be misinterpreted as tendinosis or tear (Fig. 8.22). If examined in full elbow extension, the distal triceps tendon may also appear wavy, possibly mimicking a rupture. Tendon laxity is particularly evident in the elderly and represents a normal finding (Rosenberg et al. 1997). In addition, the preinsertional fibers of this tendon may appear hypoechoic owing to their oblique course (Fig. 8.22). Changes in orientation of the probe allow adequate correction of anisotropic effects in this area. The most distal portion of the triceps tendon should always be evaluated carefully to rule out enthesis calcifications. The olecranon fossa appears as a wide and deep concavity of the posterior aspect of the humeral shaft filled with the hyperechoic posterior fat pad (Fig. 8.21a) (Miles and Lamont 1989). At both sides of this fossa, the posterior aspect of the medial and lateral epicondyles can be seen on transverse images. While examining the joint at 45° flexion, intra-articular fluid tends to move from the anterior synovial space to the olecranon recess, thus making the identification of small intra-articular effusions easier. Gentle rocking motion of the patient’s elbow during scanning may be helpful to shift elbow joint fluid into the olecranon recess. More distally, the tm tm ✟ ★ O c b ∗ ∗ T a HS O T b c Fig. 8.21a–c. Normal distal triceps tendon and olecranon fossa. a Extended-field-of-view mid-sagittal 12−5 MHz US image obtained with the elbow flexed over the olecranon process (O) and the posterior aspect of the distal humerus. The distal triceps tendon (arrowheads) appears as a beak-shaped hyperechoic structure in continuity with the hypoechoic bellies of the triceps muscle (tm) that inserts approximately 1 cm distal to the apex (star) of the olecranon. Deep to the triceps, the olecranon fossa is delimited by the hyperechoic spoon-shaped contour of the humerus and the echogenic posterior fat pad (asterisks). Note the posterior rounded appearance of the trochlea (T) and the straight profile of the humeral shaft (HS) just above the posterior fossa. b,c Transverse 12−5 MHz US images obtained at the levels (vertical white bars) indicated in a. In b, the cross-sectional appearance of the distal myotendinous junction of the triceps is seen over the posterior trochlea (T). Observe that the tendon (curved arrow) arises slightly eccentrically relative to midline and the distal muscle (arrowheads). In c, the oval cross-sectional shape of the distal triceps tendon (arrows) is seen lying over the olecranon (O). The insert at the upper left side of the figure indicates probe positioning 370 S. Bianchi and C. Martinoli the inside slope of the medial epicondyle. It typically appears as an ovoid structure close to the hyperechoic bony cortex of the epicondyle (Fig. 8.24a,b). In the distal portion of the tunnel, the ulnar nerve is visible between the humeral and ulnar heads of the flexor ulnaris carpi muscle (Fig. 8.24c,d). In normal states, the cross-sectional area of the ulnar nerve is slightly greater at the level of the epicondyle (6.8 mm 2) than at the distal arm (5.7 mm 2) and the proximal forearm (6.2 mm2) (Chiou et al. 1998). One should be careful not to confuse this normal increase in nerve size inside the cubital tunnel for a sign of ulnar neuropathy. Some discrepancies exists in literature on as what the size of the ulnar nerve should be considered normal. A cross-sectional area of ⱖ7.5mm 2 was initially indicated as the threshold value for the cubital tunnel syndrome (Chiou et al. 1998). More recently, 7.9mm 2 has been found as the mean value for the normal ulnar nerve at the cubital tunnel level (Jacob et al. 2004). These discrepancies seem, at least in part, related to differences among races and in study design. In the cubital tunnel, the ulnar recurrent artery and veins can readily be distinguished from the adjacent nerve on color Doppler imaging. In cases of engorgement, these veins become dilated and could mimic swollen individual nerve fascicles. Doppler ME imaging can help to avoid this pitfall. The cubital tunnel retinaculum and the arcuate ligament consist of thin fascia and, at least in normal states, they are not visualized with US, even using very high frequency US transducers. Dynamic imaging of the cubital tunnel is performed throughout full elbow flexion to assess the position of the ulnar nerve and the medial head of the triceps muscle relative to the medial epicondyle (see Sects. 8.5.4.4, 8.5.4.5) (Fig. 8.25). For this purpose, the probe is placed in the transverse plane over the epicondyle while the patient is asked to slowly flex the elbow (Jacobson et al. 2001). During this maneuver, it should be emphasized that the application of firm pressure on the skin with the transducer must be avoided because it may prevent the dislocation of the nerve from the tunnel. 8.5 Elbow Pathology A variety of disorders can involve the soft tissues of the elbow. Multiple conditions related to specific anatomic sites may exhibit overlapping symptoms and are easily confused clinically. ∗ ME ∗ O a fcu11 fcu fcu22 fcu fcu Ulna Ulna c O b fcu Ulna d Fig. 8.24a–d. Normal cubital tunnel. a Transverse 12−5 MHz US image at the proximal cubital tunnel level (condylar groove) with b T1w SE MR imaging correlation show the normal relationship of the ulnar nerve (arrow) with the medial epicondyle (ME). Observe the distal triceps tendon (asterisks) over the olecranon (O). c Transverse 12−5 MHz US image at the distal cubital tunnel level (proper cubital tunnel) with d T1w SE MR imaging correlation demonstrates the nerve (arrow) beneath the arcuate ligament (arrowheads) that joins the humeral (fcu1) and ulnar (fcu2) heads of the flexor carpi ulnaris muscle. The inserts at the upper left side of the figures indicate probe positioning Elbow a c b d Fig. 8.25a–d. a,b Photographs illustrating the scanning technique to assess the position of the right ulnar nerve relative to the medial epicondyle with elbow extension (a) and during progressive degrees of elbow flexion (b). Note that the probe remains stabilized on transverse plane between the medial epicondyle and the olecranon during full elbow motion. c,d Schematic drawings of the medial elbow examined in c extension and d flexion illustrate the mechanism of ulnar nerve instability at the cubital tunnel. Note the absence of the Osborne retinaculum (see for comparison Fig. 8.7c). When the elbow is extended, the ulnar nerve (white arrow) is contained within the tunnel. Elbow flexion (black arrow) dislocates the ulnar nerve anteriorly to the medial epicondyle (ME). fcu, flexor carpi ulnaris muscle. Dashed line, appropriate probe positioning during scanning 8.5.1 Anterior Elbow Pathology 8.5.1.1 Distal Biceps Tendon Tear One of the most common causes of acute anterior elbow pain is rupture of the distal biceps tendon. These tears account for less than 5% of all biceps tendon lesions, proximal injuries being far more common (Agins et al. 1988). They typically occur after 40 years of age (mean 55 years) in manual laborers who attempt to lift a heavy object (or in weightlifters and body builders) or during vigorous eccentric contraction of the biceps against resistance. Distal biceps tendon tears may occur with either avulsion of the tendon by the radial tuberosity (more commonly) or midsubstance tear or injury at its myotendinous junction. Similar to other tendons, there is a relatively hypovascular zone within the distal biceps tendon, approximately 10 mm from its insertion on the radial tuberosity (Seiler et al. 1995). Repetitive impingement of this zone between the radius and the ulna during pronation movements seems to be a predisposing factor to start the degenerative process in the tendon sub- stance (Seiler et al. 1995). In most cases, the rupture of the distal biceps tendon is associated with tearing of the lacertus fibrosus, but this latter structure may also remain intact. Clinically, a complete tendon tear presents with pain and a palpable defect with a proximal lump in the anterior aspect of the arm related to the retracted muscle (Fig. 8.26). Although weakened, elbow flexion is preserved due to the strong action of the brachialis muscle; on the contrary, supination of the forearm is more severely compromised because of the limited strength of the small supinator muscle. In most cases, the clinical diagnosis is straightforward and does not require an additional imaging study. Nevertheless, occult ruptures are more common than once thought and, in daily practice, they are becoming increasingly diagnosed with US even some time after the trauma. A delayed clinical diagnosis occurs mainly in the absence of significant muscle retraction because of an intact lacertus fibrosus, or when the retracted muscle is hidden from palpation with surrounding edema and hemorrhage. An early diagnosis of distal biceps tendon rupture is important because surgical outcome is improved in patients treated in the first weeks after trauma before the occurrence of tendon adhesions, degenera- 371 372 S. Bianchi and C. Martinoli a b Fig. 8.26a,b. Distal biceps tendon tear: physical findings. Photographs of two different patients who underwent a subacute and b chronic complete rupture of the distal biceps tendon. In a, the patient injured his left tendon while attempting to lift a heavy object. He presented with hemorrhagic skin over the medial elbow and proximal forearm and with a proximal lump (arrowheads) in the anterior aspect of the arm related to the retracted muscle. In b, the patient was a competitive body-builder who refused surgical repair of the ruptured tendon. Note the defect (arrowhead) in the anterior left arm due to the retracted muscle in comparison with the right side tive changes and fatty muscle infiltration. The main US features of a complete tear of the distal biceps tendon include nonvisualization of the distal tendon, which appears proximally retracted (up to more than 10 cm from the radial tuberosity), and detection of hypoechoic fluid in the tendinous bed related to hematoma (Fig. 8.27) (Lozano and Alonso 1995; Miller and Adler 2000). The effusion is best recognized around the tendon stump (Fig. 8.28). With highresolution transducers, US is not sensitive enough to can depict the normal lacertus fibrosus as a very thin fibrillar band over the pronator teres. The status of the lacertus fibrosus is, however, not a critical issue as it is not routinely involved in surgical repair of a torn distal biceps tendon. In addition, there is no evidence that the degree of tendon retraction is in itself predictive of the status of the lacertus fibrosus (Fig. 8.29) (Miller and Adler 2000). In case of its rupture, however, US can recognize perifascial fluid around the anterior and lateral aspects of the flexor-pronator group of muscles and a more striking tendon retraction (Fig. 8.29b). The less common tendinitis and partial tears of the distal biceps tendon present with localized pain and tenderness over the antecubital fossa. These conditions usually follow repetitive microtrauma or forceful biceps activation. Pain can be exacerbated during resisted elbow flexion or supination of the hand and is worsened by direct palpation of the tendon. At US, partial tears appear as hypoechoic thickening or thinning of the tendon and as contour irregularities or waviness without tendon discontinuity (Fig. 8.30) (Miller and Adler 2000). The assessment of these tears may be difficult with US due to anisotropy related to the oblique course of the tendon and to its deep position. The US appearance of biceps tendinitis is very similar to that of partial tears and the diagnostic accuracy of US for differentiating these conditions relies on availability of a high-quality transducer as well as on the overall experience of the examiner. In doubtful cases, MR imaging is an accurate means to confirm the diagnosis of partial tears (Falchook et al. 1994). Surgical treatment in complete tendon tears includes repair and reattachment of the retracted tendon to the radial tuberosity or, alternatively, to the brachialis muscle or the ulnar tuberosity. The first technique gives better results in restoring supination but has a significantly higher risk of radial nerve injury. After surgery, the tendon appears thickened and hypoechoic with internal linear hyperechoic images related to sutures (Fig. 8.31). 8.5.1.2 Bicipitoradial (Cubital) Bursitis The distal biceps tendon is not invested by a synovial sheath but it is covered by a paratenon. Just proximal to the tendon insertion, it is in contact with the bicipitoradial (cubital) bursa. This bursa is located between the Elbow dbt ∗ ∗ ∗ ∗∗ br br b a ∗∗ dbt d c ∗ brrad brrad br br b ∗ ∗ br br c d Fig. 8.27a–d. Complete rupture of the distal biceps tendon. a Long-axis 12−5 MHz US image over the brachialis muscle (br) shows hypoechoic fluid (asterisks) filling the distal bed of the retracted distal biceps tendon (dbt) and surrounding its myotendinous junction. In this particular case, the tendon edge (arrowheads) lies distal to the joint line. b−d Short-axis 12−5 MHz US images obtained at the levels (vertical white bars) indicated in a demonstrate the torn and retracted tendon end (straight arrows) surrounded by hypoechoic hematoma (asterisks). The relationships of the torn tendon with the brachial artery (arrowhead), radial nerve (curved arrow), brachialis (br) and brachioradialis (brrad) muscles are shown ∗ ∗ dbt b b c ∗ a c Fig. 8.28a–c. Complete rupture of the distal biceps tendon. a Long-axis 12−5 MHz US image obtained proximal to the elbow joint with b,c correlative transverse T2w SE MR images acquired at the levels (vertical white bars) indicated in a show the retracted edge (arrows) of the distal biceps tendon (dbt) with hypoechoic fluid (asterisks) filling the gap 373 374 S. Bianchi and C. Martinoli dbt dbt br br ∗ ∗ br br b a ∗ fpg fpg v a v b c Fig. 8.29a–c. Acute complete rupture of the distal biceps tendon associated with a torn bicipital aponeurosis. a Long-axis 12−5 MHz US image over the brachialis muscle (br) demonstrates a markedly retracted tendon edge (dbt), the hematoma at the rupture site (asterisks) and the absence of the tendon (arrows). b Short axis 12−5 MHz US image obtained at the level (vertical white bar) indicated in a reveals fluid (arrowheads and curved arrow) in the soft tissues surrounding the flexor-pronator group (fpg) of muscles, suggestive of a coincident injury of the lacertus fibrosus. In this case, the injury of the bicipital aponeurosis was surgically confirmed. a, brachial artery; v, cubital veins. c Gross operative view of the same case ∗ a b ∗ c d Fig. 8.30a–d. Partial rupture of the distal biceps tendon. a Long-axis and b short-axis 12−5 MHz US images obtained at level distal to the elbow joint with c, d correlative transverse T1w SE MR images demonstrate a thickened and heterogeneous tendon (arrows) inserting on the radial tuberosity (asterisk) Elbow a Fig. 8.31a,b. Postoperative distal biceps tendon. After surgical repair, a long-axis and b shortaxis 12−5 MHz US images reveal a thickened and wavy distal biceps tendon (arrows). Adhesions and irregularities in the peritendinous tissues are also seen. Observe the sutures, which appear as bright echoes (arrowheads) within the tendon substance b distal biceps tendon and the radial tuberosity to reduce friction during pronation of the forearm (Skaf et al. 1999). Bicipitoradial bursitis is a rare condition that may result from several causes (infection, inflammatory arthropathy, amyloidosis, etc.) but it is most commonly secondary to repetitive mechanical trauma as well as to tendinosis and tearing of the distal biceps. On clinical grounds, swelling of the bicipitoradial bursa can be appreciated as a nonspecific mass in the antecubital fossa often associated with antecubital pain, especially upon elbow motion and forearm rotation. Because the clinical picture of bursitis is similar to tendinitis and the deep location of the bursa makes it difficult to palpate, a definite diagnosis of cubital bursitis relies mainly on imaging findings. When the bicipitoradial bursa is only mildly distended, US may have difficulty in distinguishing it from the adjacent distal biceps tendon that appears hypoechoic due to anisotropy (Miller and Adler 2000). Usually, transverse scans with the forearm supinated perform better in delineating the bursal shape. At US, bicipitoradial bursitis appears as a hypoechoic mass located in proximity to the distal biceps tendon (Liessi et al. 1996). It may have septa, thick walls and echogenic content. Rice bodies have been described in this bursa with US (Spence et al. 1998). When distended by a large amount of fluid, the bicipitoradial bursa can surround the distal portion of the distal biceps tendon completely, thus mimicking a tenosynovitis (Fig. 8.32). Bicipitoradial bursitis must be differentiated from synovial and ganglion cysts and other soft-tissue masses. Ganglia commonly arise from the anterior capsule and may expand at a variable distance from the joint, dissecting the soft tissues of the forearm (Steiner et al. 1996). Visualization of a pedicle that connects the cyst with the elbow joint cavity may help the diagnosis. Calcified bursitis may be encountered in patients with renal osteodystrophy (Fig. 8.33). For asymptomatic bursitis no treatment is necessary, whereas most symptomatic patients are successfully treated with rest, physiotherapy and anti-inflammatory drugs. T ∗ a T T ∗ ∗ ∗ ∗ b Fig. 8.32a,b. Bicipitoradial bursitis. a Longitudinal and b transverse 12−5 MHz US images over the antecubital fossa at level distal to the joint line show fluid distension of the bicipitoradial bursa (asterisks) which almost completely surrounds the adjacent normal distal biceps tendon (T), thus mimicking a tenosynovitis process 375 376 S. Bianchi and C. Martinoli T a b T T c d Fig. 8.33a–d. Calcified bicipitoradial bursitis in a woman with chronic renal failure who presented with a palpable mass in the antecubital fossa and difficulties in pronation. a Photograph shows focal soft-tissue swelling (arrowheads) over the anterior proximal forearm. b Transverse and c longitudinal 12−5 MHz US images reveal extensive hyperechoic deposits (arrows) with faint posterior acoustic shadowing related to calcifications with the bicipitoradial bursa. The bursa exhibits thickened walls and the distal portion of the biceps tendon (T) is completely surrounded by calcifications. d Correlative lateral radiograph shows the bulk of calcifications (arrows) in the antecubital fossa 8.5.2 Medial Elbow Pathology 8.5.2.1 Medial Epicondylitis (Epitrochleitis) Medial epicondylitis, commonly referred to as “golfer’s elbow”, “medial tennis elbow” or “pitcher’s elbow”, occurs far less commonly than lateral epicondylitis and usually presents with pain and tenderness over the anterior aspect of the medial epicondyle that is enhanced by grasping and by resisted pronation of the forearm. Some sporting activities requiring repetitive valgus stress to the elbow joint, such as golf, javelin throwing and squash, may predispose to this condition. Medial epicondylitis is produced by degeneration and tearing of the common flexor tendon relative to overuse of the flexor-pronator group of muscles. Enthesopathy is frequently observed instead of tendinopathy. In this condition, joint effusion is absent and the elbow retains a full range of movements. The US appearance of medial epicondylitis is similar to the appearance of the other degenerative tendinopathies that involve the attachment of tendons to bone and includes hypoechoic changes in the tendon substance secondary to tendinosis or to partial-thickness tears (Fig. 8.34) (Ferrara and Marcelis 1997). Complete tear of the common flexor tendon is rare. In this clinical setting, US can help to distinguish tendinopathy from a lesion of the underlying medial collateral ligament. Ulnar neuropathy may be associated with tendinosis of the common flexor tendon. Elbow T ∗ ∗ ME a ME b Fig. 8.34a,b. Medial epicondylitis. a Longitudinal and b transverse 12−5 MHz US images at the medial elbow in a golf player with chronic elbow pain reveal a swollen common flexor tendon (arrowheads) with a full-thickness hypoechoic area (asterisk) compatible with severe tendinosis. A normal-appearing medial collateral ligament (arrows) underlies the abnormal tendon origin. ME, medial epicondyle; T, proximal portion of the common flexor tendon 8.5.2.2 Medial Collateral Ligament Injury The medial collateral ligament is stronger than the lateral collateral ligament. Its degeneration and tearing with or without an injury of the adjacent common flexor tendon may be secondary to acute or chronically repeated overstretching in valgus stress during the acceleration phases of throwing or may result from a fall or from posterior dislocation of the elbow (see Sect. 8.5.5.4). Baseball pitching is the sporting activity most commonly associated with medial collateral ligament injuries and medial joint instability. When the anterior band is injured, high-resolution US reveals a thickened hypoechoic ligament with surrounding effusion slightly posterior and deep to the medial epicondyle (Vanderschueren et al. 1998; Jacobson and van Holsbeeck 1998; Ward et al. 2003). Calcifications can also be associated with ligamentous tears (Nazarian et al. 2003). In cases of complete rupture, US examination may show either a gap or focal hypoechoic areas in the proximal and distal portion of the ligament (Fig. 8.35) (de Smet et al. 2002). To improve the diagnostic confidence, high-frequency US examination can provide dynamic assessment of the degree of medial joint laxity in both neutral and valgus stressed positions (de Smet et al. 2002). In a series of asymptomatic baseball pitchers, the medial elbow joint space of the throwing arm was significantly wider during valgus stressing than the joint space in the elbow of the nonthrowing arm (Nazarian et al. 2003). In symptomatic patients, widening of the trochlea-ulna joint and soft tissue falling into the distracted joint space suggest a medial collateral ligament injury (de Smet et al. 2002). Dynamic US scanning may be particularly useful in the event of partial-thickness tears, in which the ligament is continuous but lax (Fig. 8.9). Examination of the noninjured elbow should be obtained to compare the amount of joint widening that occurs during valgus stressing. 8.5.2.3 Epitrochlear Lymphadenopathies Just proximal to the elbow and adjacent to the medial epicondyle and the medial neurovascular bundle, small lymph nodes may enlarge as a result of reactive or septic inflammation (Barr and Kirks 1993). One of the leading causes of medial epitrochlear regional fm fm ∗ ME Ulna Ulna Fig. 8.35. Medial collateral ligament injury. Longitudinal 12−5 MHz US image obtained with valgus stress over the anterior band of the medial collateral ligament (arrowheads) shows a focal hypoechoic area in the proximal ligament and mild widening of the elbow joint (arrows) compatible with a ligamentous injury. ME, medial epicondyle; fm, flexor muscles 377 378 S. Bianchi and C. Martinoli lymphadenopathy is “cat-scratch” disease, an infection caused by a gram-negative bacterium, Bartonella henselae, usually transmitted by scratches of the hand by an animal (most patients have a history of exposure to a cat!). However, enlarged lymph nodes in the epitrochlear area may also be involved by other disorders, including benign and malignant forms. US reveals the appearance of reactive lymph nodes consisting of oval hypoechoic masses with an echogenic hilum, often hypervascular at color Doppler imaging (Fig. 8.36). This appearance is typical and works well to rule out other soft-tissue masses, such as neurogenic tumors or sarcomas. The US examination should be extended up to the axillary region in order to rule out the possible coexistence of axillary lymphadenopathies. In cat-scratch disease, lymphadenopathies may be multiple and contiguous. Clinically, they are accompanied by painful soft-tissue swelling and systemic symptoms, such as fever and malaise. The involved nodes have a hypervascular pattern at color and power Doppler imaging and tend to develop central necrosis and liquefaction (Carcía et al. 2000; Gielen et al. 2003). Hyperechoic infiltration of the perinodal fat due to cellulitis is a characteristic finding (Fig. 8.37a). Enlarged lymph nodes most often regress over weeks to months. Whatever the cause of epitrochlear lymphadenopathies, US may exclude a local softtissue mass, thus obviating the need for biopsy or resection of this pseudotumor (Gielen et al. 2003). With time from the acute process, and especially in the elderly, the reactive nodes may undergo diffuse, massive adipose infiltration leading to a broad and hyperechoic medulla and progressive atrophy of the outer cortex (Fig. 8.37b). In these cases, the examiner should be careful not to mistake these atrophic nodes for lipomas or other hyperechoic soft-tissue masses. Detection of a thin continuous hypoechoic rim related to the atrophic cortex of the node may help the diagnosis (Fig. 8.37b). 8.5.3 Lateral Elbow Pathology 8.5.3.1 Lateral Epicondylitis The most common disorder involving the lateral elbow is lateral epicondylitis, also known as “tennis elbow”, caused by repetitive traction on the osteotendinous attachment of the common extensor tendon (Regan et al. 1992). This condition can be the result of a b c Fig. 8.36a–c. Epitrochlear lymphadenopathy. a Long-axis and b short-axis 12−5 MHz US images over the medial elbow in a patient with a painful palpable mass associated with the medial epicondyle. US identifies an oval hypoechoic mass with echogenic hilum consistent with a superficial lymph node (arrowheads). c Color Doppler imaging reveals a hypervascular pattern of the node with a vessel pedicle (arrow) that enters the hilum and branches through the hypoechoic cortex. This lymph node regressed 2 weeks after medical treatment chronic microtrauma secondary to repetitive overuse related to professional or recreational activities leading to progressive degeneration and/or partial tears of the common extensor tendon (tendinopathy) or to damage to the bone insertion (enthesopathy). The extensor carpi radialis brevis is the more commonly affected component of the common extensor tendon. Although lateral epicondylitis typically occurs in tennis players who injure this tendon–especially during the backhand stroke, in which the extensors Elbow a b Fig. 8.37a,b. Epitrochlear lymphadenopathies in two different individuals with a active cat-scratch disease and b without any signs of infectious or inflammatory abnormalities. In a, the inflamed node is completely hypoechoic (arrowheads) with loss of definition of the echogenic hilum and appears surrounded by abnormally hyperechoic fat (arrows) due to perinodal cellulitis. In b, massive adipose infiltration has occurred in an epitrochlear lymph node (arrowheads) leading to a broad hyperechoic medulla. The cortical portion is markedly reduced in thickness and appears as a thin peripheral hypoechoic rim (arrows) are subjected to a greater tensioning–this condition is seen far more commonly in nonathletes. In tendinopathy, patients report a localized pain over the common extensor tendon during or just after repetitive muscle activation, whereas in enthesopathy, pain is confined to the tendon’s insertional area. Physical examination reveals localized tenderness over the lateral aspect of the elbow radiating down to the proximal forearm or well localized over the lateral aspect of the epicondyle respectively. Intra-articular effusion is not an associated finding. In chronic longstanding disease, pain at rest and limitation in joint extension can be noted. The diagnosis is usually based on clinical findings and does not require imaging studies. US may be useful to confirm the clinical diagnosis in doubtful or refractory cases, to reveal the extent and severity of the disease and to monitor the response to therapy. The main US features of lateral epicondylitis are preinsertional hypoechoic swelling of the tendon with focal or diffuse areas of decreased reflectivity in the tendon substance and loss of the fibrillary pattern related to tendinosis, fluid adjacent to the common tendon and ill-defined tendon margins (Figs. 8.38, 8.39) (Maffulli et al. 1990; Connell et al. 2001; Miller et al. 2002d; Levin et al. 2005). In a recent series, the mean size of the focal hypoechoic areas was 8.7 mm (range 3–15 mm) (Connell et al. 2001). Although early tendon abnormalities may be confined to the superficial fibers (Fig. 8.38a,b), involvement of the deep fibers of the extensor carpi radialis brevis component is more common and may even extend down to the joint capsule (Fig. 8.38c,d). Similarly, the anterolateral and mid-portion of the common extensor tendon is more commonly involved, whereas the posterior portion usually remains unaffected (Fig. 8.38b) (Connell et al. 2001). In high-grade tendinosis, the angiofibroblastic infiltration based on migration of fibroblasts and vascular granulation tissue within the tendon substance causes a striking hypervascular pattern of the intratendinous hypoechoic areas at color and power Doppler imaging (Fig. 8.39b). Spurring at the common extensor tendon insertion and cortical irregularities at the anterolateral surface of the lateral epicondyle may also be recognized, although bony changes do not correlate with disease activity. Intratendinous calcifications may also be seen as part of crystal deposition diseases (Fig. 8.40). In partial tears, the common extensor tendon may appear thinned compared with the opposite side. In practice, discrimination of focal areas of tendinosis and partial tears can be difficult and US is reliable for recognizing a partial tear only when discrete anechoic cleavage planes with no fibers intact are visible in the tendon substance (Connell et al. 2001). These tears typically appear as longitudinal splits oriented from the bony insertion distally (Fig. 8.41). Thickening of peritendinous soft tissues and a thin layer of superficial fluid over the extensor tendon are also more often observed with partial tears. In complete tears, US identifies a fluid-filled gap separating the tendon from its bony attachment site (Fig. 8.42) (Jacobson and van Holsbeeck 1998; Connell et al. 2001). Overall, US 379 380 S. Bianchi and C. Martinoli ∗ ∗ RH ∗ ∗ LE LE a b RH ∗ ∗ ∗ ∗ LE c LE d Fig. 8.38a–d. Lateral epicondylitis: spectrum of US appearances in a weightlifter with bilateral lateral elbow pain. a Long-axis and b short-axis 12−5 MHz US images over the right common extensor tendon origin reveal a hypoechoic focus (asterisks) of tendinosis in the superficial fibers of an otherwise normal-appearing tendon (arrowheads). c Long-axis and d short-axis 12−5 MHz US images over the left common extensor tendon origin demonstrate a large hypoechoic area (asterisks) affecting both superficial and deep fibers of the tendon (arrowheads), indicating severe tendinopathy. On cross-section, the abnormal hypoechoic areas with loss of fibrillary echotexture (asterisks) are seen involving the full thickness of the anterior half of the tendon (arrowheads). In both elbows, observe the integrity of the deepest fibers in relation to the lateral collateral ligament. LE, lateral epicondyle; RH, radial head has proved to be as specific but not as sensitive as MR imaging for evaluating epicondylitis (Miller et al. 2002). On the other hand, US of the common extensor tendon has high sensitivity but low specificity in the detection of symptomatic cases (Levin et al. 2005). Conservative treatment with rest, anti-inflammatory drugs, physiotherapy and local steroid injections gives satisfactory results in most cases of lateral epicondylitis. Surgical intervention with excision of the degenerated tissue, resection of the common extensor tendon and debridement of the extensor tendon origin with release of the annular ligament may be advocated in refractory cases. Confirmation of the disease and exclusion of other causes of lateral elbow pain which may mimic or accompany lateral epicondylitis, such as posterior interosseous nerve entrapment or lateral collateral ligament inju- ries, should, however, be ascertained with imaging modalities, and possibly with US, before submitting the patient to surgery. 8.5.3.2 Lateral Collateral Ligament Injury In lateral epicondylitis, the lateral elbow ligamentous complex, and especially the lateral ulnar collateral ligament, should be routinely assessed because this ligament is commonly injured in association with tears of the common extensor tendon as a result of the same forces or overuse mechanisms on adjacent structures (Bredella et al. 1999). An unsuspected tear of this ligament may be the cause of conservative therapy failure in patient with lateral epicon- Elbow ∗ ∗ RH LE a 2( ,% b Fig. 8.39a,b. Lateral epicondylitis in a professional tennis player with a history of chronic right lateral elbow pain. a Long-axis gray-scale 12−5 MHz US image reveals a hypoechoic focus (asterisks) in the most superficial fibers of the common extensor tendon origin (arrowheads), whereas the deep fibers are preserved. b Color Doppler imaging demonstrates a striking hypervascular pattern composed of series of tiny vessels throughout the intratendinous hypoechoic areas, characteristic of tendinosis. LE, lateral epicondyle; RH, radial head RH a LE b Fig. 8.40a,b. Calcifying lateral epicondylitis. a Long-axis 12−5 MHz US image with b radiographic correlation in a patient with calcium pyrophosphate crystals deposition disease and recent onset of lateral elbow pain demonstrates large calcified foci (arrows) within the common extensor tendon origin. LE, lateral epicondyle; RH, radial head 381 382 S. Bianchi and C. Martinoli ∗ ✟ ★ ∗ ∗ RH LE Fig. 8.41. Partial-thickness tear of the common extensor tendon. Long-axis 12−5 MHz US image in a manual laborer who presented with acute onset of lateral elbow pain reveals a linear hypoechoic split (star) extending from the lateral epicondyle (LE) through the substance of the common extensor tendon origin. The torn deep fibers (arrowheads) are retracted just distal to the hypoechoic area. Note the integrity of the underlying lateral ulnar collateral ligament (asterisks). RH, radial head ∗ LE RH a LE LE ∗ ∗ RH RH b c Fig. 8.42a–c. Complete rupture of the common extensor tendon. a Long-axis 12−5 MHz US image in a golfer player who complained of longstanding elbow pain with coronal b T1w SE and c fatsuppressed T2w SE MR imaging correlation shows a retracted common extensor tendon. Note the gap (arrowheads) related to the tear that separates the avulsed tendon edge (asterisk) from the lateral epicondyle (LE). RH, radial head Elbow dylitis. In addition, when the torn lateral ulnar collateral ligament is not recognized preoperatively, the operative release of the common extensor tendon may be responsible for worsening of symptoms and onset of posterolateral rotatory instability of the elbow (see Sect. 8.5.5.4). When the more superficial extensor carpi radialis brevis is torn, the deep lateral ulnar collateral ligament becomes more clearly distinguishable with US as a cord-like fibrillary structure located over the joint space (Fig. 8.41). An isolated ligament tear appears as discontinuity of the deepest fibers of the extensor tendon origin, whereas tears involving both the ligament and the common extensor tendon cause a full-thickness interruption of fibers over the lateral aspect of the radiocapitellar joint and soft tissue hematoma around the proximal margin of the capitellum (Connell et al. 2001). Dynamic scanning during careful varus stressing can disclose lateral ulnar collateral ligament injury by depicting widening of the lateral elbow joint space compared with the opposite normal elbow (Fig. 8.43). In “pulled elbow”, a common injury among children due to slipping of the annular ligament over the radial head following forceful pronation, US is able to depict an increased distance between the radial head and the humeral capitellum probably due to the impingement of the annular ligament (Kosuwon et al. 1993) - see also chapter 19. 8.5.3.3 Supinator Syndrome (Posterior Interosseous Neuropathy) Supinator syndrome, also referred to as “posterior interosseous syndrome” or “radial tunnel syndrome”, is a rare compression neuropathy of the upper limb affecting the posterior interosseous nerve just near or behind the supinator muscle (Spinner 1968). This nerve is vulnerable to injury at the proximal edge of the superficial belly of the supinator muscle that forms a free, strong, fibrous arch, the “arcade of Frohse”. At this site, the posterior interosseous nerve may be tethered and entrapped by fibrous bands, ∗ ∗ RH LE a c ∗ RH b ∗ LE Fig. 8.43a–c. Complete tear of the common extensor tendon and the lateral ulnar collateral ligament. a, b Longitudinal 12−5 MHz US images obtained over the common extensor tendon origin a in neutral position and b with varus stressing. In a, US identifies a large horizontal hypoechoic cleft through the full thickness of the common extensor tendon origin (arrows) and the lateral ulnar collateral ligament (asterisks). In b, varus stress on the elbow shows widening of the radio-capitellar joint space (dashed lines). RH, radial head; LE, lateral epicondyle. c Correlative STIR MR image confirms the complete rupture of both structures (arrowheads) 383 384 S. Bianchi and C. Martinoli fan-shaped recurrent radial vessels or by tightness of the passage within the superficial and deep layers of the supinator. In addition, it may be compressed by a variety of soft-tissue masses, such as paraosteal lipomas and deep ganglia. Radial head and neck fractures, including Monteggia fracture-dislocations, may also displace and encase the posterior interosseous nerve by callus as it passes through the supinator tunnel. Clinically, the posterior interosseous neuropathy produces a clinical picture distinct from a lesion of the radial nerve in the arm. In fact, the patient has a “finger drop” rather than the characteristic “wrist drop” of a radial neuropathy, because muscle weakness spares the extensor carpi radialis (Fig. 8.44). Extension of the fingers at the metacarpophalangeal joints is impaired and there is deficit of abduction and extension of the thumb. In addition, posterior interosseous neuropathy may cause burning pain and tenderness over the lateral elbow, possibly mimicking a “resistant lateral epicondylitis”. High-resolution US is able to identify the impingement of the posterior interosseous nerve in the supinator area. The compressed nerve typically appears swollen and hypoechoic proximal to or inside the supinator muscle (Bodner et al. 2002). In post-traumatic settings, the nerve may appear displaced by a malaligned radial head and may exhibit alternate thickened and thinned segments between the superficial and deep bellies of the supinator muscle as a possible result of stretching injury (Fig. 8.45). In addition, the nerve may be seen encased by hypoechoic scar tissue following a radial fracture (Fig. 8.46). Decompressive surgery of the posterior interosseous nerve is indicated if there is continuous worsening or no recovery of function with a few months. 8.5.4 Posterior Elbow Pathology 8.5.4.1 Distal Triceps Tendon Tear Distal triceps tendon tear is an uncommon condition that mostly occurs at or close to the olecranon process of the ulna, often associated with a fleck of bone attached to the retracted tendon as a result of avulsion fracture (Fig. 8.47). The mechanism involves either forced flexion of the elbow against a contracting triceps, as occurs during a fall on an outstretched arm, or relates to a direct blow onto the olecranon process. Local steroid injection into the olecranon bursa, anabolic steroid abuse and pre-existing tendinosis may also have a role in the tendon rupture. As a rule, complete tears occur more U epl R c U a b d epb epl epb R Fig. 8.44a–d. Posterior interosseous nerve syndrome in a young woman who presented with a intense weakness in extending the right fingers, especially involving thumb movements, and b a longitudinal skin depression (arrow) over the dorsum of the forearm following a contusion to the lateral elbow. c Transverse 12−5 MHz US image at the middle dorsal forearm reveals loss in bulk and a hyperechoic appearance of the extensor pollicis longus (epl) and extensor pollicis brevis (epb) muscles relative to fatty atrophy. Surgery confirmed the traumatic injury of the posterior interosseous nerve. d Normal contralateral side. U, ulna; R, radius Elbow s s s s s s Radius a s s R R s s b R c s d e Fig. 8.45a–e. Posterior interosseous nerve syndrome in a patient with malaligned Monteggia fracture-dislocation (type IV). a Extended field-of-view 12−5 MHz US image reconstructed according to the longitudinal axis of the supinator tunnel demonstrates the posterior interosseous nerve (arrowheads) which alternates thickened and thinned portions as it traverses the supinator muscle (s). b–d Serial T2*GRE MR images reveal slight hyperintensity in the supinator muscle (s) due to denervation edema. The nerve (arrows) appears markedly hyperintense. R, radius. e Radiograph shows the malalignment of the radius, which appears subluxated anterolaterally s s a b Radius Fig. 8.46a,b. Posterior interosseous nerve syndrome. a Transverse 12−5 MHz US image obtained over the supinator area in patient with a previous radial head fracture and radial nerve deficit demonstrates the posterior interosseous nerve (arrowheads) entrapped within a hypoechoic scar (arrows) in the area of the supinator muscle (s). b Gross operative view shows the main trunk of the radial nerve (asterisks) as it splits into the superficial cutaneous sensory branch (arrowheads) and the deep posterior interosseous nerve (narrow arrows). This latter nerve is irregularly swollen as it passes over the bone (large arrows) as a result of the scar encasement visible in a 385 386 S. Bianchi and C. Martinoli 1 1 ∗ 2 ∗ 2 a b c Fig. 8.47a–c. Avulsion fracture of the olecranon in a boy following a bicycle accident. a Reconstructed midsagittal 12−5 MHz US image over the posterior elbow with b lateral radiographic correlation demonstrates the avulsion fracture of the olecranon process (1) from the ulnar shaft (2) due to a traction mechanism by the distal triceps tendon (arrows). Note the coexisting avulsion of the cartilaginous growth plate (asterisks). c Lateral radiograph of the opposite healthy side shows incomplete ossification (curved arrow) between the olecranon and the ulnar shaft frequently than partial tears, whereas disruption of either the muscle bellies or the myotendinous junction is rare. Complete rupture of the distal triceps tendon presents clinically with complete inability to extend the elbow, given the absence of other muscles that can assist in this movement. In the acute phase, however, the clinical diagnosis may be hampered by local soft-tissue swelling, inflammatory edema and pain that limit the physical examination. In such cases, US may be useful both to confirm the tendon injury and to differentiate between complete tears that require immediate surgery to avoid retraction of the tendon and partial tears that may be treated conservatively. In acute complete ruptures, US demonstrates the distal triceps tendon as wavy, retracted and surrounded by fluid (Fig. 8.48) (Kaempffe and Lerner 1996). US examination is also reliable to delineate the degree of tendon retraction and can help in the diagnosis of atypical ruptures, such as in cases of tears occurring at the myotendinous junction (Fig. 8.49). Due to the close anatomic relation of the distal triceps tendon with the medial epicondyle and the cubital tunnel, an acute ulnar nerve compression syndrome may occur secondary to a distal triceps tendon tear (Duchow et al. 2000). Degenerative tendinosis can be appreciated as a thickened hypoechoic tendon. 8.5.4.2 Olecranon Bursitis Olecranon bursitis, the most common superficial bursitis in the body, appears clinically as a lump overlying the olecranon process due to fluid distension or hypertrophy of the synovial membrane. The most common cause of olecranon bursitis is repetitive local contusion (student’s elbow, miner’s elbow) that leads to a painless local swelling covered by normal skin. Calcific enthesopathy of the distal triceps tendon is a predisposing factor. However, bursal distension can be appreciated in a variety of systemic disorders, such as rheumatoid arthritis, gout, hydroxyapatite and calcium pyrophosphate deposition diseases, as well as in septic conditions (e.g. Staphylococcus, tuberculosis); also patients under chronic hemodialysis treatment may occasionally have olecranon bursitis. When bursitis is secondary to infection or gout, bursal swelling is typically painful and associated with skin warmth and erythema due to local inflammatory changes. Because systemic findings are often absent in septic bursitis, the likelihood of an infected bursa must always be kept in mind. Similarly, when the patient has a history of tuberculous disease, a specific etiology of bursitis should first be suspected. 388 S. Bianchi and C. Martinoli ∗ ∗ O ∗ ∗ a b c Fig. 8.50a–c. Chronic traumatic olecranon bursitis in a manual laborer who had recently injured several times his posterior right elbow. a Midsagittal and b transverse 12−5 MHz US images over the olecranon process (O) show a markedly distended olecranon bursa (arrowheads) containing thick septa (curved arrows) and anechoic effusion (asterisks). Straight arrows, distal triceps tendon. c Photograph showing the bursal lump (arrows) on the posterior elbow ∗ ∗ ∗ O a ★ ∗ b ∗ ∗ O O c following bursal rupture. In such patients, subcutaneous nodules can be seen in the olecranon region and along the proximal ulna. These nodules should be considered in the differential diagnosis as they can mimic olecranon bursitis or a solid neoplasm Distal ∗ Fig. 8.51a–c. Hydroxyapatite olecranon bursitis. a Transverse and b,c longitudinal 12−5 MHz US images of a painful softtissue mass over the olecranon (O) show the olecranon bursa filled with homogeneous highly echogenic fluid (asterisks) that could be seen fluctuating during probe compression. The bursa exhibits thickened walls (arrowheads) and septa (arrows). In this case, needle aspiration of the bursal fluid revealed calcium milk solution to the olecranon bursa, an additional small subolecranon bursa can exist at the posterior aspect of the proximal ulnar shaft. In rare instances, this bursa can be involved by the same processes affecting the larger olecranon one (Fig. 8.54). Elbow ∗ ∗ O a b Fig. 8.52a,b. Tuberculous olecranon bursitis. a Longitudinal gray-scale and b color Doppler 12−5 MHz US images in a patient with painful soft-tissue swelling over the posterior elbow. The olecranon bursa (arrowheads) shows irregular wall thickening and ill-defined margins due to coexisting peribursal cellulitis. Only a small amount of intrabursal fluid is seen (asterisks). dt, distal triceps tendon; O, olecranon ∗ ∗ ∗ H ∗ ★ a b Fig. 8.53a,b. Calcific olecranon bursitis in a patient with renal osteodystrophy. a Posterior midsagittal 12−5 MHz US image with b lateral radiographic correlation demonstrates a large calcification (asterisks) that lies superficial to the insertion of the distal triceps tendon (arrowheads) reflecting an extensively calcified bursa. Note the relation of the mass with the posterior olecranon fossa (star) and the humeral shaft (H) a b Fig. 8.54a,b. Subolecranon bursitis nodule in a patient with severe rheumatoid arthritis. a Transverse 12−5 MHz US image reveals a painless heterogeneous soft-tissue mass (arrows) with mixed echotexture located in the subcutaneous tissue over the proximal posterior ulna, compatible with subolecranon bursitis. b Photograph of the same case shown in a 389 390 S. Bianchi and C. Martinoli 8.5.4.3 Cubital Tunnel Syndrome Ulnar nerve compression inside the cubital tunnel, the second most common entrapment syndrome of the upper limb after carpal tunnel syndrome, may occur either at the condylar groove or at the edge of the arcuate ligament (proper cubital tunnel). There are several causes of ulnar nerve damage, including direct extrinsic compression of the nerve against a shallow condylar groove, bone abnormalities (cubitus valgus, deformities from previous elbow fractures, osteoarthritis with medial osteophytes and loose bodies, heterotopic ossification) and a variety of space-occupying soft-tissue lesions, including thickening of the capsule and the medial collateral ligament, ganglia and accessory muscles (anconeus epitrochlearis muscle) (Stewart 1993). Clinically, the entrapment of the ulnar nerve at the elbow presents insidiously with medial elbow pain and a spectrum of complaints ranging from sensory symptoms in the ring and little fingers to weakness of the ulnarinnervated hand muscles. Wasting of hand muscles is best appreciated at the first interosseous space and hypothenar eminence and causes a typical semiflexion deformity of the ring and little fingers that is commonly referred to as “claw hand” (Fig. 8.55). In addition, the little finger may stay slightly abducted (Wartenberg sign). The diagnosis is essentially based on electrophysiologic studies. US typically demonstrates an abrupt narrowing and displacement of the nerve within the tunnel, possibly in association with a thickened retinaculum or a space-occupying lesion (Fig. 8.56) (Puig et al. 1999; Martinoli et al. 2000; Okamoto et al. 2000). Proximal to it, the compressed nerve appears swollen with loss of the fascicular pattern and, in some cases, hypervascularity at color Doppler imaging. As assessed by quantitative analysis with US, the nerve cross-sectional area at the epicondyle is significantly larger in patients with cubital tunnel syndrome than in healthy subjects or in the opposite normal elbow (Okamoto et al. 2000; Chiou et al. 1998). An ulnar nerve area ≥7.5 mm2 at the level of the epicondyle has been indicated as the threshold value for cubital tunnel syndrome (Chiou et al. 1998). These data are somewhat contradictory with a more recent paper which indicates 7.9mm2 as the mean cross-sectional area for the normal ulnar nerve at the elbow (Jacob et al. 2004). Besides assessing the ulnar nerve, a wide spectrum of extrinsic causes for nerve entrapment may be recognized with US as well, including congenital anomalies such as an accessory anconeus epitrochlearis muscle (Fig. 8.57), and acquired disease which IV III II ∗ ∗ c ∗ a II b ∗ III ∗ IV d Fig. 8.55a–d. Claw-like deformity in the right hand of a young patient with severe ulnar neuropathy at the cubital tunnel level. a Photograph of the dorsal aspect of the hand reveals loss in bulk of the dorsal interosseous muscles (arrows) that lie in the intermetacarpal spaces. The atrophy of the ulnar-innervated hand muscles is more obvious at the first intermetacarpal space (asterisk). b Photograph of the palmar aspect of the hand shows the fourth and fifth fingers extended at the metacarpophalangeal joint and flexed at the interphalangeal joints. c Transverse 12−5 MHz US image at the dorsal aspect of the hand demonstrates a hyperechoic appearance of the dorsal interosseous muscles (asterisks) related to neurogenic fatty atrophy. d Contralateral healthy side. II–III–IV, metacarpals 392 S. Bianchi and C. Martinoli a ME O b c ME O L L d e in turn leads to an increased content–i.e., lipoma (Fig. 8.58)–or a decreased size–i.e. fracture residuals (Fig. 8.59)–of the tunnel. Surgical decompression of the ulnar nerve at the elbow may include slitting the Osborne fascia and the aponeurosis of the flexor carpi ulnaris leaving the nerve inside the cubital tunnel. Alternatively, the nerve may be transposed out of the condylar groove and the cubital tunnel, anterior to the medial epicondyle and superficial to the flexor muscles (Fig. 8.60). This surgical option is preferred in cases of ulnar neuropathies caused by bone and joint disease. After surgical transposition, persistent symptoms are usually related to an excessive angling of the ulnar nerve as it passes deep to the arcuate ligament or to incomplete stabilization of the nerve in its new position. US is able to identify scar tissue along the course of the nerve in patients with recurrent symptoms or relapse of compressive causes (Fig. 8.61). Fig. 8.58a–e. Cubital tunnel syndrome in a patient presenting with a superficial soft-tissue mass on the posteromedial elbow. a Reconstructed longitudinal and b transverse 12−5 MHz US images obtained just proximal to the cubital tunnel demonstrate the ulnar nerve (curved arrow) that shows a bowing course over an oval solid hyperechoic lesion (arrowheads) with well-defined margins, consistent with a lipoma. Note the close relationship of the mass with the nerve. c Transverse 12−5 MHz US image obtained at the cubital tunnel level shows the lipoma (arrowheads) that infolds within the tunnel leading to compression of the ulnar nerve (curved arrow). ME, medial epicondyle; O, olecranon. d,e Correlative transverse T1w SE MR images obtained d at the distal arm and e at the cubital tunnel levels confirm the lipomatous nature of the space-occupying lesion (L). Curved arrow, ulnar nerve; ME, medial epicondyle; O, olecranon 8.5.4.4 Ulnar Nerve Instability In the congenital partial or complete absence of the cubital tunnel retinaculum, the ulnar nerve may subluxate over the tip of the epicondyle or dislocate anterior to it with a transient snapping sensation during flexion of the elbow, to return inside the tunnel when the joint is extended. Ulnar nerve instability at the cubital tunnel can be considered a normal variant, being reported in between 16% and 47% of asymptomatic healthy people, subluxation being the most common form (Childress 1975; Okamoto et al. 2000). The condition is bilateral in almost three quarters of cases and asymptomatic at both clinical examination and nerve conduction studies. Patients may occasionally complain of only mild discomfort with tingling and paresthesias when the flexed elbow hits a firm surface such as the edge of a desk. In rare instances, however, chronic 394 S. Bianchi and C. Martinoli ∗ a b ∗ ME c ∗ d microtrauma of the nerve over the medial epicondyle due to repetitive dislocation can cause friction neuritis with symptoms and signs of ulnar nerve impairment. In such cases, nerve instability should be treated with surgical transposition of the nerve in order to avoid more serious damage. Dynamic US scanning is an ideal means to depict the instability of the ulnar nerve during progressive elbow flexion, to recognize nerve abnormalities related to friction neuritis as well as to establish whether ulnar neuropathy is produced by compressive causes or instability because of the overlap in clinical findings (Jacobson and van Holsbeeck 1998; Jacobson et al. 2001). In subluxation, the nerve is seen moving over the apex of the medial epicondyle during full active elbow flexion but no further. In dislocation, the nerve may be seen snapping completely out of the cubital tunnel and migrating over the common flexor tendon origin (Fig. 8.62). During dynamic scanning, the snapping sensation may be felt by the examiner through the transducer. Careful scanning technique is needed to avoid excessive pressure with the probe over the epicondyle, which can prevent the nerve Fig. 8.61a–d. Postoperative patient with recurrence of symptoms after decompressive surgery of the ulnar nerve at the cubital tunnel for a ganglion cyst. a,b Transverse 12−5 MHz US images obtained a at the cubital tunnel level and b at the proximal forearm with c,d T1w SE MR imaging correlation show a relapsed cyst (asterisks) which constricts the transposed ulnar nerve (arrow). The patient underwent surgery again and the postoperative course was finally uneventful. ME, medial epicondyle from dislocating. In cases of symptomatic friction neuritis, the ulnar nerve appears markedly swollen and hypoechoic with loss of fascicular echotexture, probably reflecting localized intraneural edema and fibrotic changes (Fig. 8.63). However, these abnormalities may occasionally be encountered in healthy subjects too, without any implication of disease. 8.5.4.5 Snapping Triceps Syndrome With elbow flexion, anterior dislocation of the medial head of triceps muscle relative to the medial epicondyle can occur in combination with dislocation of the ulnar nerve. In this condition, referred to as “snapping triceps syndrome”, the dislocation of the muscle leads to concurrent dislocation of the adjacent ulnar nerve as these structures are contiguous (Fig. 8.64). Two palpable “snaps” are typically appreciated over the medial elbow, the first one reflecting dislocation of the ulnar nerve and the second, dislocation of the medial head of triceps muscle. The clinical presen- Elbow ME a d ME b e ME c f ME ME a b ME ME c Fig. 8.62a–f. Dynamic study of the cubital tunnel in ulnar nerve dislocation. a–c Schematic drawings and d–f respective series of transverse 12−5 MHz US images obtained a,d with extended elbow and during progressive degrees of elbow flexion (b,e and c,f). When the elbow is extended, the ulnar nerve (arrow) is contained within the tunnel. Elbow flexion gradually pushes the nerve over the medial epicondyle (ME) until it snaps completely out of the cubital tunnel to lie superficial to the common flexor tendon origin (ft). O, olecranon d Fig. 8.63a–d. Dynamic study of the cubital tunnel in a patient with recurrent ulnar nerve dislocation and clinical symptoms of ulnar neuropathy. a–d Series of transverse 13−8 MHz US images acquired a with extended elbow and b–d throughout elbow flexion show a markedly swollen and hypoechoic nerve (arrow) that flattens and dislocates over the medial epicondyle (ME) during elbow flexion. In the symptomatic patient, this finding is suggestive of ulnar neuropathy based on a friction mechanism 395 396 S. Bianchi and C. Martinoli a b tation of this syndrome is variable and may include medial elbow pain, snapping sensation, ulnar neuropathy or a combination of these szymptoms (Spinner and Goldner 1998). Somewhat similar to the isolated dislocation of the ulnar nerve, the snapping triceps may however remain asymptomatic and probably unrecognized in most cases. Although the cause of snapping triceps is still unknown, some possible congenital and acquired conditions have been advocated to explain this syndrome, such as a hypertrophied triceps muscle, an accessory triceps tendon and abnormal medial head of the triceps muscle, as well as post-traumatic osseous abnormalities. Differentiation between snapping triceps syndrome and isolated ulnar nerve dislocation as causes for medial elbow snapping is important in symptomatic subjects as the surgical treatments differ. For this purpose, dynamic US scanning is accurate in allowing direct visualization of transient dislocation of both structures during active flexion and extension of the elbow (Fig. 8.65) (Jacobson et al. 2001). 8.5.5 Bone and Joint Disorders 8.5.5.1 Synovitis A variety of inflammatory diseases can affect the elbow. The main pathologic findings are joint effusion, synovial hypertrophy and destructive bone Fig. 8.64a,b. Snapping triceps syndrome. Schematic drawings of the posterior aspect of the elbow in a extension and b 90° flexion demonstrate the ulnar nerve (arrows) as it passes through the cubital tunnel and a prominent medial head (mh) of the triceps muscle (tm). Note the absence of the Osborne retinaculum when compared with Fig. 8.7c. With elbow flexion, the medial edge of the triceps (arrowheads) and the ulnar nerve move anterior to the tip of the epicondyle. T, distal triceps tendon; fcu, flexor carpi ulnaris abnormalities. Physical examination reveals a swollen joint with local inflammatory changes and a reduced range of movements. Incomplete elbow extension can be due either to increased intra-articular fluid or to destructive changes of the articular surfaces. Clinically, joint effusion can be palpated as a localized swelling and tenderness over the anterolateral aspect of the joint, at the level of the radio-capitellar joint, or posteriorly at both sides of the triceps tendon. The accumulation of joint fluid can be reliably recognized with US by examining the distended recesses of the elbow joint, including the larger coronoid fossa and the smaller radial fossa anteriorly and the olecranon fossa posteriorly (Fig. 8.66) (DeMaeseneer et al. 1998). Small amounts of fluid initially collect in the olecranon recess and are best revealed with US while keeping the elbow flexed. In fact, the interposition of the olecranon in elbow extension may make visualization of a small amount of joint fluid more difficult in this recess (DeMaeseneer et al. 1998). With increasing quantities, synovial processes cause progressive elevation of the anterior and posterior fat pads giving them a crescent-shaped appearance of them (Figs. 8.67, 8.68) (Koski 1990; DeMaeseneer et al. 1998). With the elbow extended, the anterior fat pad is pushed by the brachialis against the bone and less fluid tends to collect in the coronoid and radial fossae compared with when the elbow is flexed (DeMaeseneer et al. 1998). The recesses located inferiorly to the anterior fat pad, including the annular one, fill with fluid only in cases with large amounts of joint effusion (Fig. 8.69). In the case of small effusions, and 398 S. Bianchi and C. Martinoli ∗ HC ∗ RH a HC c ∗ ∗ ∗ HC ∗ HC b d Fig. 8.67a–d. Synovitis of the elbow joint: anterior joint recess. Longitudinal 12−5 MHz US images over the anterior coronoid recess a in normal state and b,c in two cases of joint synovitis presenting with b mild and c marked distention of the anterior synovial spaces by fluid and hypertrophied synovium. In normal conditions, a thin layer of fluid (arrow) may be encountered in the anterior coronoid recess, deep to the anterior fat pad (asterisks). This is a normal finding. When joint fluid expands into the anterior joint spaces, the anterior fat pad (asterisks) becomes elevated to assume a typical crescentic or “sail-like” appearance. In markedly distended joints, the anterior bulging of the joint cavity is more conspicuous and may extend down to the joint level. HC, humeral capitellum; RH, radial head. d Corresponding T2w SE MR image of the case illustrated in b tm ∗ O ∗ ∗ tm ∗ TR a b Fig. 8.68a,b. Synovitis of the elbow joint: posterior joint recess. a Longitudinal 12−5 MHz US image over the posterior olecranon recess with b T2w SE MR imaging correlation in a patient with rheumatoid arthritis presenting with painful elbow and loss of extension. US shows a bulk of hypoechoic synovial pannus filling the recess (arrows). Deep to the triceps muscle (tm), the posterior fat pad (asterisks) is elevated by the pannus. Note the prominence of the tip of the olecranon (O) and the humeral trochlea (TR) bulging within the recess of the hyaline cartilage and subchondral bone on the joint surfaces (Fig. 8.70). In the olecranon fossa, care should be taken not to confuse the synovial pannus with the normal fat pad that may appear slightly hypoechoic (Fig. 8.71). In doubtful cases, graded compression with the probe can help to distinguish between them. When there are clinical concerns for septic arthritis, US-guided aspiration of the joint fluid can be performed (Jacobson and van Holsbeeck 1998; Lim-Dunham et al. 1995). Elbow ∗ ∗ ∗ RH ∗ ∗ HC a ∗ b Fig. 8.69a,b. Synovitis of the elbow joint: annular (periradial) recess. a Longitudinal 12−5 MHz US image over the anterior aspect of the radio-capitellar joint with b transverse T2w SE MR imaging correlation in a patient with rheumatoid arthritis reveals filling of the annular recess (white arrows) by hypoechoic synovial fluid (asterisks). The annular recess lies around the radial metaphysis and communicates with the joint cavity through a thin passageway (open arrow) deep to the annular ligament. Note the rounded profile of the humeral capitellum (HC) and the squared profile of the radial head (RH) ∗ ✟ ◆ ✟ ◆ ∗ ✟ ◆ RH HC a ∗ ∗ ∗ RH HC b Fig. 8.70a,b. Rheumatoid arthritis. a,b Longitudinal 12−5 MHz US images over the anterior aspect of the radio-capitellar joint a in a normal subject and b in a patient with severe longstanding rheumatoid arthritis. In a, note the articular cartilage (rhombi) and the regular profile of the subchondral bone of the humeral capitellum (HC) and radial head (RH). In b, there is complete loss of the cartilage layer and the surface of bones appears diffusely irregular, reflecting erosions. Synovial pannus (asterisks) can be seen within the joint space and distending the annular recess T LE ∗ ∗ ME T ME LE a b Fig. 8.71a,b. Synovitis of the elbow joint: pitfall. a,b Transverse 12−5 MHz US images over the posterior olecranon recess a in a normal subject and b in a patient with rheumatoid arthritis and an olecranon recess (arrows) appears markedly distended by fluid. In a, the normal hypoechoic fat contained. In b the olecranon fossa, between the lateral (LE) and medial (ME) epicondyles, should not be confused with the synovitis process shown in b. In doubtful cases, careful dynamic examination with elbow flexion and extension movements may be helpful for the diagnosis. Note the erosion (arrowhead) on the posteromedial aspect of the lateral epicondyle. T, distal triceps tendon 399 400 S. Bianchi and C. Martinoli 8.5.5.2 Osteoarthritis and Osteochondral Damage Osteoarthritis of the elbow is basically post-traumatic in nature. It is typically seen in male patients with a history of manual labor (vibration tools), sport-related overuse or fracture malalignment. The dominant extremity is more frequently involved. Given the physiologic attitude of the elbow joint to valgus posture, the external radio-capitellar compartment is most commonly affected. Clinical findings are related to the degenerative process itself (stiffness and loss of motion, usually extension, related to spurring, swelling due to synovitis, local pain), compression of the ulnar nerve inside the cubital tunnel (local pain and tenderness, tingling of the ring and little fingers and, in chronic compression, wasting of the ulnar-innervated intrinsic hand muscles) as well as intra-articular loose bodies (intermittent joint locking and effusion). Intra-articular loose bodies commonly migrate into the dependent portions of the joint and in the humeral depressions above the joint line, particularly the olecranon fossa, resulting in mechanical symptoms such as intermittent locking and loss of extension. In patients without a synovial effusion, the intra-articular location of a fragment can be established by demonstrating it between the articular cartilage and the anterior and posterior intracapsular fat pads. The small radial annular recess is rarely involved by loose bodies. Dynamic examination performed during flexion and extension of the elbow may be helpful in mobilizing the joint fluid and small loose bodies as well as for differentiating br ✟ ◆ them from local heterotopic ossification and spurring (Fig. 8.72) (Bianchi and Martinoli 2000). In primary synovial chondromatosis, multiple chondral or osteochondral loose bodies typically display nearly equal size and can vary in number from a few to hundreds (Fig. 8.73). Advanced disease may result in disintegration of the articular surfaces. As already described in other anatomic sites, in the initial phase of disease the treatment includes removal of the loose bodies and synovectomy to prevent recurrence. Similar to a loose body, the os supratrochleare dorsale is an intra-articular ossicle located in the olecranon fossa that may be associated with pain and progressive loss of elbow extension with locking symptoms (Obermann and Loose 1983). This accessory ossicle is generally believed to be the result of a congenital anomaly rather than the consequence of previous trauma and may cause deepening and remodeling of the olecranon fossa as it increases in size. Differentiation between an os supratrochleare dorsale and a loose body is clinically not relevant because both fragments are treated by surgical removal. In adolescents, US is also able to recognize deformities of the humeral capitellum in osteochondritis dissecans (Takahara et al. 1998, 2000a,b). This condition typically occurs in 13- to 16-year-olds, mainly as a result of chronic lateral impaction or repetitive valgus stress. The anterolateral articular surface of the capitellum is typically involved with localized subchondral bone flattening and subsequent fragmentation and loosening of bone fragments (Takahara et al. 2000a). US examination is best performed with an anterior approach while br ✟ ◆ ∗ HC a b Fig. 8.72a,b. Anterior spur mimicking a loose body. a Longitudinal 12−5 MHz US image with b lateral radiographic correlation demonstrates a prominent spur (arrow) over the coronoid fossa of the humerus. The spur is intracapsular in location and appears bordered by fluid (asterisk). During elbow movements, it remained still. Note the thin hypoechoic layer of articular cartilage (rhombi) that covers the humeral capitellum (HC). Br, brachialis muscle Elbow br br ∗ ✟ ◆ ∗ ✟ ◆ ◆ ✟ ✟ ◆ HC a b c d Fig. 8.73a−d. Primary synovial chondromatosis. a Longitudinal and b transverse 12−5 MHz US images over the anterior coronoid recess demonstrate multiple intra-articular loose bodies as hyperechoic fragments (arrowheads) of similar size with posterior acoustic shadowing lying inside the recess. Note the elevation of the anterior fat pad (asterisks) over the fragments and the thin hypoechoic layer of articular cartilage (rhombi) that overlies the humeral capitellum (HC). br, brachialis muscle. c Lateral radiograph and d transverse T1w GRE MR imaging of the same case showing the loose bodies (arrowheads) keeping the elbow extended (to view the proximal and middle parts of the anterior capitellum) and with a posterior approach while keeping the elbow flexed (to view the middle and distal parts of the anterior capitellum) (Takahara et al. 2000b). Detached bony fragments are depicted as echogenic foci in the osteochondral defect. US has also proved to be accurate in determining whether the lesion is stable or unstable (loosened fragments), with good (89%) agreement with surgical findings and MR imaging (Takahara et al. 2000b). In these patients, delay in the appropriate management can be avoided by an early US examination. Similar abnormalities may be encountered in Panner disease, a condition related to avascular necrosis of the ossification center of the capitellum that occurs in 5–11 years old children secondary to traumatic injures. Somewhat comparable to Legg-CalvéPerthes disease in the hip, this latter condition has a benign outcome with no residual deformity of the capitellum and absence of loose body formation (Vanderschueren et al. 1998). 8.5.5.3 Occult Fractures Due to the anatomic complexity of the elbow joint, some undisplaced fractures, such as those involving the radial head and neck and the coronoid process, may remain occult radiographically, even when additional projections are performed. When cast immobilization is not employed as a prophylactic measure to avoid overtreatment, persistent pain and disability may lead the referring physician to acquire a US examination to rule out any possible soft-tissue abnormality about the elbow. With careful scanning technique, high-resolution US is able to identify acute elbow fractures based on detection of a step-off deformity or focal discontinuity of the hyperechoic cortical line (Fig. 8.74). In these cases, however, additional radiographic or MR imaging studies should always be obtained to confirm the US diagnosis. Dynamic scanning during careful passive-assisted pronation and supination of the forearm with the probe placed 401 402 S. Bianchi and C. Martinoli br br C a HC b br br C c HC d Fig. 8.74a–d. Occult fracture of the right coronoid process in a woman following a ski accident. a The patient had a negative radiographic examination performed soon after the injury. b Two weeks later, she was submitted to US examination due to persistent elbow pain and loss of extension. US identified an interruption (curved arrow) of the hyperechoic cortical profile of the coronoid process (C), just cranial to the insertion of the brachialis (br). There was associated mild intra-articular effusion. HC, humeral capitellum. c Left healthy side for comparison. d Additional oblique view of the right elbow confirms the fracture in the transverse plane over the radial head may be useful to exclude any fracture at this site. In doubtful cases, associated US signs, such as joint effusion, can be easily detected in intra-articular fractures and may suggest a more detailed analysis of the bone contour (Major and Crawford 2002). On the other hand, the absence of effusion in elbow injuries with negative plain radiographs may make further bone investigation with MR imaging unnecessary (Kessler et al. 2002). More than in adults, US seems to have a potential role for the evaluation of elbow fractures in children. In fact, there are difficulties in assessing bony abnormalities about the elbow in skeletally immature patients using plain radiographs because of the absence of the secondary centers of ossification (Fig. 8.75). When a radiographic sign of joint effusion is present but a fracture is not visualized, US may help in distinguishing the separation of the distal humeral epiphysis (Dias et al. 1988; Ziv et al. 1996) from elbow dislocation in neonates, as well as in detecting or excluding radial head (Lazar et al. 1998) and supracondylar fractures (Davidson et al. 1994; Brown and Eustace 1997). 8.5.5.4 Posterior Dislocation Injury and Instability Elbow dislocation is most common in children less than 10 years old and accounts for 5−8% of all fractures and dislocations in adults, being second only to the shoulder. Usually, the ulna and radius dislocate posteriorly following a hyperextension mechanism, such as during a fall on the outstretched hand. The posterior translation can cause impaction fractures (i.e., coronoid process, humeral capitellum) and a variety of soft-tissue lesions, involving joint and ligaments, vessels and nerves. In such cases, US can occasionally be required to demonstrate soft-tissue complications, such as heterotopic ossification, contusion of the brachialis muscle and injuries to the brachial artery and the median and ulnar nerves (Figs. 8.76, 8.77). After a dislocation, instability of the elbow joint may result following progressive disruption of the lateral ulnar collateral ligament (posterolateral rotatory instability), tearing of the anterior and posterior joint capsule and then rupture of the medial collateral ligamentous complex (multidirectional instability). Elbow ∗ ∗ R HC a ∗ HC R b c Fig. 8.75a–c. Radial fracture in a 5-year-old child presenting with left lateral elbow pain and disability after a fall. a Longitudinal 12−5 MHz US image at the anterolateral elbow demonstrates increased distance between the humeral capitellum (HC) and the radial epiphysis related to an intervening hyperechoic joint effusion (asterisks). Note the hyperechoic dot (arrowheads) within the radial epiphysis representing the ossification center. At the radial metaphysis, US reveals a focal irregularity of the hyperechoic cortical line (arrow) suggesting a fracture. R, radius. b Contralateral healthy side for comparison. c Lateral radiograph of the left elbow confirms the diagnosis of radial fracture (arrow) a br br br br a ∗ b a a br br c d Fig. 8.76a–d. Partial tear of the brachialis muscle as a consequence of posterior dislocation injury. a,b Longitudinal and c transverse 12−5 MHz US images of the anterior elbow obtained in the supracondylar area demonstrate a wide hypoechoic defect (large arrows) in the substance of the brachialis muscle (br) related to hematoma. The torn muscle tissue is surrounded by anechoic spaces. Note the relationship of the injured brachialis with the normal distal biceps muscle (arrowheads), brachial artery (a) and median nerve (curved arrow). d Contralateral healthy side. Corresponding transverse 12−5 MHz US image shown in c reveals an intact brachialis 403 406 S. Bianchi and C. Martinoli Ferrara MA, Marcelis S (1997) Ultrasound of the elbow. J Belge Radiol 80:122−123 Fitzgerald SW, Curry DR, Erickson SJ et al (1994) Distal biceps tendon injury: MR imaging diagnosis. Radiology 191:203−206 Gelberman RH, Yamaguchi K, Hollstien SB et al (1998) Changes in interstitial pressure and cross-sectional area of the cubital tunnel and of the ulnar nerve with flexion of the elbow. J Bone Joint Surg Am 80:492−501 Gielen J, Wang XL, Vanhoenacker F et al (2003) Lymphadenopathy at the medial epitrochlear region in cat-scratch disease. Eur Radiol (13:363-1369) Jacob D, Creteur V, Courthaliac C et al (2004) Sonoanatomy of the ulnar nerve in the cubital tunnel: a multicentre study by the GEL. Eur Radiol 14:1770-1773 Jacobson JA, van Holsbeeck MT (1998) Musculoskeletal ultrasonography. Orthop Clin North Am 29:135−167 Jacobson JA, Jebson PJL, Jeffers AW et al (2001) Ulnar nerve dislocation and snapping triceps syndrome: diagnosis with dynamic sonography: report of three cases. Radiology 220:601−605 Kaempffe FA, Lerner RM (1996) Ultrasound diagnosis of triceps tendon rupture. A report of 2 cases. Clin Orthop 332:138−142 Kessler T, Winkler H, Weiss C et al (2002) Ultrasound diagnosis of the elbow joint in fracture of the head of the radius. Orthopade 31:268−270 Koski JM (1990) Ultrasonography of the elbow joint. Rheumatol Int 10:91–94 Kosuwon W, Mahaisavariya B, Saengnipanthkul S et al (1993) Ultrasonography of pulled elbow. J Bone Joint Surg Br 75:421−422 Lazar RD, Waters PM, Jaramillo D (1998) The use of ultrasonography in the diagnosis of occult fracture of the radial neck. J Bone Joint Surg Am 80:1361−1364 Levin D, Nazarian LN, Miller TT et al (2005) Lateral epicondylitis of the elbow: US findings. Radiology 237:230-234 Liessi G, Cesari S, Spaliviero B et al (1996) The US, CT and MR findings of cubital bursitis: a report of five cases. Skeletal Radiol 25:471−475 Lim-Dunham JE, Ben-Ami TE, Yousefzadeh DK (1995) Septic arthritis of the elbow in children: the role of sonography. Pediatr Radiol 25:556−559 Lin J, Jacobson JA, Fessell DP et al (2000) An illustrated tutorial of musculoskeletal sonography. II. Upper extremity. AJR Am J Roentgenol 175:1071−1079 Lozano V, Alonso P (1995) Sonographic detection of the distal biceps tendon rupture. J Ultrasound Med 14:389−391 Maffulli N, Regine R, Carrillo F et al (1990) Tennis elbow: an ultrasonographic study in tennis players. Br J Sport Med 24:151−154 Major NM, Crawford ST (2002) Elbow effusions in trauma in adults and children: is there an occult fracture? AJR Am J Roentgenol 178:413−418 Markowitz RI, Davidson RS, Harty MP et al (1992) Sonography of the elbow in infants and children. AJR Am J Roentgenol 159:829−833 Martinoli C, Bianchi S, Gandolfo N et al (2000) Ultrasound of nerve entrapments in osteofibrous tunnels. Radiographics 20:199−217 Martinoli C, Bianchi S, Giovagnorio F et al (2001) Ultrasound of the elbow. Skeletal Radiol 30:605−614 Miles KA, Lamont AC (1989) Ultrasonic demonstration of the elbow fat pad. Clin Radiol 40:602−604 Miller JH, Beggs I (2001) Detection of intra-articular bodies in the elbow with saline arthrosonography. Clin Radiol 56:231−234 Miller T, Adler RS (2000) Sonography of tears of the distal biceps tendon. AJR Am J Roentgenol 175:1081−1086 Miller TT, Shapiro MA, Schults E, Kalish PE (2002) Comparison of sonography and MRI for diagnosing epicondylitis. J Clin Ultrasound 30:193−202 Nazarian LN, McShane JM, Ciccotti MC et al (2003) Dynamic US of the anterior band of the ulnar collateral ligament of the elbow in asymptomatic major league baseball pitchers. Radiology 227:149−154 Obermann WR, Loose HW (1983) The os supratrochleare dorsale: a normal variant that may cause symptoms. AJR Am J Roentgenol 141:123−127 Okamoto M, Abe M, Shirai H et al (2000) Diagnostic ultrasonography of the ulnar nerve in cubital tunnel syndrome. J Hand Surg [Br] 25:499−502 Popovic N, Ferrara MA, Daenen B et al (2001) Imaging overuse injury of the elbow in professional team handball players: a bilateral comparison using plain films, stress radiography, ultrasound, and magnetic resonance imaging. Int J Sports Med 22:60−67 Puig S, Turkof E, Sedivy R et al (1999) Sonographic diagnosis of recurrent ulnar nerve compression by ganglion cysts. J Ultrasound Med 18:433−436 Regan W, Wold LE, Conrad R et al (1992) Microscopic histopathology of chronic refractory lateral epicondylitis. Am J Sports Med 20:746−749 Rosenberg ZS, Bencardino J, Beltran J (1997) MR imaging of normal variants and interpretation pitfalls of the elbow. MRI Clin North Am 5:481−499 Sasaki J, Takahara M, Ogino T et al (2002) Ultrasonographic assessment of the ulnar collateral ligament and medial elbow laxity in college baseball players. J Bone Joint Surg Am 84:525−531 Seiler JG, Parker LM, Chamberland PDC et al (1995) The distal biceps tendon: two potential mechanisms involved in its rupture. Arterial supply and mechanical impingement. J Shoulder Elbow Surg 4:149−156 Skaf AY, Boutin RD, Dantas RWM et al (1999) Bicipitoradial bursitis: MR imaging findings in eight patients and anatomic data from contrast material opacification of bursae followed by routine radiography and MR imaging in cadavers. Radiology 212:111−116 Spence LD, Adams J, Gibbons D et al (1998) Rice body formation in bicipitoradial bursitis: ultrasound, CT, and MRI findings. Skeletal Radiol 27:30−32 Spinner M (1968) The arcade of Frohse and its relationship to posterior interosseous nerve paralysis. J Bone Joint Surg Br 50:809−812 Spinner RJ, Goldner RO (1998) Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. J Bone Joint Surg Am 80:239−247 Steiner E, Steinbach LS, Schnarkowski P et al (1996) Ganglia and cysts around joints. Radiol Clin North Am 34:395−425 Stewart JD (1993) Compression and entrapment neuropathies. In: Dyck PJ, Thomas PK (eds) Peripheral neuropathy, 3rd edn. Saunders, Philadelphia, pp 1354−1379 Takahara M, Shundo M, Kondo M et al (1998) Early detection of osteochondritis dissecans of the capitellum in young Elbow baseball players. Reports of three cases. J Bone Joint Surg Am 80:892−897 Takahara M, Ogino T, Takagi M et al (2000a) Natural progression of osteochondritis dissecans of the humeral capitellum: initial observations. Radiology 216:207−212 Takahara M, Ogino T, Tsuchida H et al (2000b) Sonographic assessment of osteochondritis dissecans of the humeral capitellum. AJR Am J Roentgenol 174:411−415 Vanderschueren G, Prasad A, van Holsbeeck M (1998) Ultrasound of the elbow. Semin Musculoskel Radiol 2:223−235 Ward S, Teefey S, Paletta G et al (2003) Sonography of the medial collateral ligament of the elbow: a study of cadavers and healthy adult male volunteers. AJR Am J Roentgenol 180:389−394 Ziv M, Litwin A, Katz K et al (1996) Definitive diagnosis of fracture-separation of the distal humeral epiphysis in neonates by ultrasonography. Pediatr Radiol 26:493−496 407 Forearm 9 Forearm Carlo Martinoli and Stefano Bianchi CONTENTS 9.2 Clinical and US Anatomy 9.1 Introduction 409 9.2 9.2.1 9.2.2 9.2.3 Clinical and US Anatomy Volar Forearm 409 Dorsal Forearm 415 Mobile Wad 417 9.3 9.3.1 9.3.1.1 9.3.1.2 9.3.1.3 9.3.1.4 9.3.2 Forearm Pathology 417 Volar Forearm 419 Pronator Syndrome 419 Anterior Interosseous Nerve Syndrome 419 Other Compression Neuropathies 419 Penetrating Injuries 421 Dorsal Forearm and Mobile Wad 421 References 409 409 423 9.1 Introduction Although the soft tissue anatomy of the forearm is complex due to the high number of muscles involved in the spectrum of wrist and fingers movements, musculoskeletal pathology amenable to US examination is relatively uncommon in this area. Only a few specific conditions affecting the median nerve proximal to the carpal tunnel level merit separate consideration. C. Martinoli, MD Associate Professor of Radiology, Cattedra “R” di Radiologia – DICMI – Università di Genova, Largo Rosanna Benzi 8, 16132 Genova, Italy S. Bianchi, MD Privat-docent, Université de Genève, Consultant Radiologist, Fondation et Clinique des Grangettes, 7, ch. des Grangettes, 1224 Genève, Switzerland Strong septal attachments of the antebrachial fascia to the radius, the ulna and the interosseous membrane divide the forearm into three distinct compartments – volar, dorsal and the so-called mobile wad – each of which house several muscles (Fig. 9.1). The volar compartment (flexor compartment) contains eight muscles – the flexor pollicis longus, the flexor digitorum profundus, the flexor digitorum superficialis, the pronator teres, the palmaris longus, the flexor carpi radialis, the flexor carpi ulnaris and the pronator quadratus – and the most relevant neurovascular structures of the limb, including the median nerve along with its main divisional branch, the anterior interosseous nerve, the ulnar nerve and the ulnar artery. The dorsal compartment (extensor compartment) houses eight muscles: the supinator, the extensor pollicis brevis, the abductor pollicis longus, the extensor pollicis longus, the extensor indicis proprius, the extensor digitorum communis, the extensor digiti minimi and the extensor carpi ulnaris. At the radial aspect of the forearm, three other muscles – the extensor carpi radialis brevis and longus (extensors) and the brachioradialis (flexor) – form the so-called mobile wad. The superficial sensory branch of the radial nerve and the radial artery run between the mobile wad compartment and the volar compartment of the forearm. A basic review of the compartmental normal and US anatomy of the forearm with a description of the courses of the radial, median and ulnar nerves is included here. 9.2.1 Volar Forearm The volar (anterior) compartment of the forearm includes the flexor and pronator (antebrachial) muscles. It can be divided by a transverse septum into two layers: deep and superficial (Boles et al. 1999). Forearm 5 7 2 6 4 1 8 3 a b c Some anomalous muscles may be encountered in the forearm, the two more common of which are the anomalous palmaris and the Gantzer muscle. The palmaris longus is one of the most variable muscles in the human body, with an overall incidence of anomalies of 9% (Reimann et al. 1944). Occasionally, its muscle belly can be found in a central position between discrete proximal and distal tendons (digastric variant), or even distally. When located distally, the muscle has a long proximal tendon, an appearance resembling a “reversed” palmaris (Schuurman and van Gils 2000). A palmaris with double muscle bellies may also occur: in this latter configuration, the two bellies – one proximal and one distal – are separated by a central tendon lying in between (Reimann et al. 1944). The Gantzer muscle (found in approximately 52% of people) is an accessory slip of the flexor pollicis longus which arises from the medial epicondyle in 85% of cases and has a dual origin from the epicondyle and the coronoid process in the rest (Al-Quattan 1996). It inserts onto the ulnar side of the flexor pollicis longus and its tendon. Both anomalous palmaris and Gantzer muscle may contribute to median and anterior interosseous nerve compression. The major nerves and vessels of the forearm are located within or traverse the volar compartment (Fig. 9.3). The median nerve enters the volar compartment passing between the superficial and deep heads of the pronator teres muscle. It then crosses the ulnar artery and proceeds toward depth to pass Fig. 9.2a–c. Schematic drawings of a coronal view of the muscles of the volar compartment of the forearm from deep (a) to superficial (c). a The deep layer includes the flexor pollicis longus (1) and the flexor digitorum profundus (2), which have a wide origin from the interosseous membrane, the radius and the ulna. Their distal tendons pass superficial to the pronator quadratus (3) before entering the carpal tunnel. b Superficial to these muscles, the flexor digitorum superficialis (4) is a broad muscle which arises from the humerus, the ulna and the radius. Its distal tendons are disposed in series over those of the flexor digitorum profundus. c Over the flexor digitorum superficialis, the pronator teres (5), the palmaris longus (6), the flexor carpi radialis (7) and the flexor carpi ulnaris (8) originate from the medial epicondyle. While the pronator teres traverses the proximal forearm obliquely to insert into the radius, the other superficial muscles lie adjacent one to the other and descend the forearm to continue in long distal tendons down to the wrist below the fibrous arch formed by the flexor digitorum superficialis, the so-called “sublimis bridge”, where it is closely apposed to the deep surface of this muscle. At the middle forearm, the median nerve runs in the midline, as its name indicates, between the superficial flexor digitorum superficialis and the deep flexor digitorum profundus. More distally, at the distal forearm, it becomes more lateral and superficial to enter the wrist. Along its course through the forearm, the median nerve provides motor function to the pronator teres, the flexor carpi radialis, the flexor digitorum superficialis and the palmaris longus. It also sends branches to the proximal part of the flexor pollicis longus and the flexor digitorum profundus. Approximately 5–8 cm distal to the lateral epicondyle, the anterior interosseous nerve is a purely motor nerve which branches off the median nerve at the level of the deep head of the pronator teres. It travels along the anterior surface of the interosseous membrane with the anterior interosseous branch of the ulnar artery, between the muscle bellies of the flexor pollicis longus and flexor digitorum profundus, and then deep to the pronator quadratus. This nerve supplies the flexor pollicis longus, part of the flexor digitorum profundus (for the index and middle finger) and the pronator quadratus. After exiting the cubital tunnel, the ulnar nerve enters the volar compartment of the forearm passing on the anterior surface of the flexor digitorum profundus, under the flexor carpi ulnaris. At the middle of the forearm, it is reached by the ulnar 411 412 C. Martinoli and S. Bianchi e c 19 17 d 18 d b H c a U a 2 8 d 4 g 19 f c 1 9 h b 4 g8 R 1,2 d U a e 3 f b a 5 4 9 b e BT BA b c b f 7 c 3 2,4 g8 a R U f Fig. 9.3a–f. Schematic drawings of coronal (a–c) and transverse (d–f) views through the forearm showing the main nerves (in black) and arteries (in white) and their relationships with surrounding bones and muscles. a Basically, the forearm is crossed by three main neurovascular pedicles: ulnar, central and radial. The ulnar pedicle is formed of the ulnar nerve (a) and the ulnar artery (g); the central pedicle consists of the median nerve (b) and the anterior interosseous nerve (h), the latter arising from it at the middle third of the forearm; the radial pedicle includes the superficial branch of the radial nerve (c) and the radial artery (f). The course of the Martin–Gruber anastomosis is indicated by a dashed line. At the elbow level, note the position of the brachial artery (e) and the posterior interosseous nerve (d). b,c Main forearm muscles located b deep and c superficial to the neurovascular bundles illustrated in a. Note the relationship of the nerves and arteries with the supinator (9), the flexor pollicis longus (1), the flexor digitorum profundus (2), the pronator quadratus (3), the flexor digitorum superficialis (4) and the flexor carpi ulnaris (8) muscles. d–f The relationship of the nerves and arteries with the muscles of the forearm compartments is demonstrated at the level of the elbow (a), the middle (b) and the distal (c) forearm. The individual anatomic structures are indicated with the same numbers and letters used in Figs. 9.1 and 9.2. H, humerus; U, ulna; BA, brachialis; BT, biceps tendon; R, radius artery and its satellite veins. Thereafter, the nerve and vessels proceed distally together, emerging on the radial side of the flexor carpi ulnaris tendon, between this tendon and the tendon of the flexor digitorum superficialis for the little finger to enter the Guyon canal. In the forearm, the ulnar nerve supplies the flexor carpi ulnaris and the ulnar portion of the flexor digitorum profundus. In up to 30% of people, a crossover of fibers from the median nerve to the ulnar nerve – the Martin–Gruber anastomosis – occurs at the proximal forearm. This anastomosis can be responsible of anomalous innervation of intrinsic hand muscles and thus can lead to unclear clinical presentation of some nerve entrapment syndromes (Fig. 9.3a). The two main arteries in the forearm are the radial and the ulnar arteries, which are terminal divisions of the brachial artery (Fig. 9.3). The ulnar artery travels through the volar compartment with the ulnar nerve. It arises at the level of the neck of the radius, just medial to the distal biceps tendon, and courses deep to the “sublimis bridge” accompanied by the median nerve. At the middle third of the forearm, the ulnar artery traverses posterior to the median 414 C. Martinoli and S. Bianchi fcr pl fcu fds MN prt fpl UN fdp R U a pl fcr fds MN fcu fds UN fdp fpl R U b MN RN fcu fds fpl Fig. 9.5a,b. Volar compartment of the forearm. a,b Transverse 12–5 MHz US images obtained a just distal to the sublimis bridge and b, more caudally, at the middle third of the forearm demonstrate the relationships of the deep muscles – the flexor pollicis longus (fpl) and the flexor digitorum profundus (fdp) – with the superficial muscles – the pronator teres (prt), the flexor carpi radialis (fcr), the flexor digitorum superficialis (fds), the flexor carpi ulnaris (fcu) and the palmaris longus (pl) – of the volar forearm. The two layers of muscles are separated by a transverse hyperechoic cleavage plane (curved arrows) representing an extension of the antebrachial fascia within which the median nerve (MN), the ulnar nerve (UN) and the ulnar artery (straight arrow) are found. From proximal (a) to distal (b), observe the muscle belly of the palmaris longus which continues in a thin superficial tendon. R, radius; U, ulna. The photograph at the right of the figure indicates probe positioning fdp UN AIN b MN fds fpl R a c AIN fdp U Fig. 9.6a-c. Anterior interosseous nerve. a Schematic drawing of a coronal view of the elbow after removal of the distal tendon of the biceps brachii (bb) the distal part of the brachialis (ba) and the superficial belly of the pronator teres muscle (prt) reveals the course of the median nerve (arrow) in the pronator area and the origin of the anterior interosseous nerve (arrowheads) deep to the flexor digitorum superficialis muscle (fds). b Schematic drawing of a transverse view through the middle forearm illustrates the close relationship of the anterior interosseous nerve (AIN) with the anterior aspect of the interosseous membrane (arrow) and the bellies of the flexor pollicis longus (fpl) and flexor digitorum profundus (fdp). The anterior interosseous nerve runs in a deeper position compared with the median nerve (MN). Observe the ulnar nerve (UN) which courses between the flexor carpi ulnaris (fcu), the flexor digitorum profundus (fdp) and the flexor digitorum superficialis (fds) muscles. RN, superficial sensory branch of the radial nerve. c Transverse 12–5 MHz US images obtained over the volar compartment at the middle forearm reveal the respective position of the median (MN) and anterior interosseous (AIN) nerves relative to the flexor digitorum superficialis (fds), the flexor digitorum profundus (fdp), the flexor pollicis longus (fpl) and the interosseous membrane (arrows). R, radius; U, ulna Forearm MN MN UN UN a R U b R U MN UN Fig. 9.7a–c. Ulnar artery. a–c Transverse 12–5 MHz US images obtained from a proximal to c distal reveal the ulnar artery (straight arrow) which traverses the forearm leaving the median nerve (MN) to reach the ulnar nerve (UN). R, radius; U, ulna. The photograph at the upper right of the figure indicates probe positioning the best ways to identify the bellies of the superficial flexors (flexor carpi radialis, flexor carpi ulnaris and palmaris longus) and the flexor pollicis longus is to start scanning over their distal tendons and then sweep the probe proximally on transverse planes. The scanning technique to examine these tendons and the pronator quadratus will be addressed later (see Chapter 10). U R c a 9 14 b 16 11 9.2.2 Dorsal Forearm Similar to the volar compartment, the muscles of the dorsal (posterior) compartment of the forearm, can be arbitrarily divided in two layers: deep and superficial. The deep muscles include the supinator, the extensor pollicis brevis, the abductor pollicis longus, the extensor pollicis longus and the extensor indicis proprius (Fig. 9.8a). The anatomy of the supinator muscle and its relationships with the posterior interosseous nerve has already been described (see Chapter 8). The remaining four muscles take their origin from the posterior aspect of the radial and ulnar shaft and from the interosseous membrane distal to the position of the supinator muscle. They insert into the metacarpal (abductor pollicis longus), 15 12 13 a 10 b Fig. 9.8a,b. Schematic drawings of a coronal view of the a deep and b superficial muscles of the dorsal compartment of the forearm. a The deep layer of muscles includes the supinator (9), consisting of two heads – superficial (a) and deep (b) – and, more distally, the abductor pollicis longus (11), the extensor pollicis brevis (10), the extensor pollicis longus (12) and the extensor indicis proprius (13). These latter muscles originate from the posterior aspect of the radial and ulnar shaft and the interosseous membrane. b In a more superficial position, the extensor digitorum communis (14), the extensor digiti minimi (15) and the extensor carpi ulnaris (16) are found arising from the lateral epicondyle of the humerus 415 416 C. Martinoli and S. Bianchi the proximal (extensor pollicis brevis) and the distal phalanx (extensor pollicis longus) of the thumb, and the middle and distal phalanx of the index finger (extensor indicis proprius) respectively. From lateral to medial, the abductor pollicis longus is the largest and most superficial muscle of the group. Close to it, the extensor pollicis brevis lies in a more distal position and is partially covered by the abductor. The extensor pollicis longus is larger and its tendon is longer than the brevis. Finally, the extensor indicis proprius is narrow and elongated, and lies medial to and alongside the extensor pollicis longus. Apart from the abductor pollicis longus which abducts and extends the thumb, the other deep extensors act to extend the phalanges. From lateral to medial, the extensor muscles of the superficial layer include the extensor digitorum communis, the extensor digiti minimi and the extensor carpi ulnaris (Fig. 9.8b). In association with the extensor carpi radialis brevis, these muscles share a proximal strong tendon that originates from the lateral epicondyle of the humerus (see Chapter 8). The extensor digitorum longus and extensor digiti minimi insert onto the middle and distal phalanges of the four medial fingers (extensor digitorum longus) and the little finger (extensor digiti minimi). The extensor carpi ulnaris inserts distally into the base of the fifth metacarpal. On the whole, the superficial extensor muscles are innervated by distal branches of the radial nerve (posterior interosseous nerve). As a functional part of the long head of the triceps, the anconeus muscle has already been described in Chapter 8. As a rule, an accurate and systematic US examination of the dorsal muscles of the forearm should begin at the level of the wrist, where their individual tendons are easily distinguished within the six compartments. Then, US scanning should be performed by shifting the transducer upward to depict the myotendinous junction and the belly of the appropriate muscle to be evaluated. This “retrograde” technique is particularly helpful, even for the experienced examiner, to increase confidence on establishing the identity of the forearm muscles. At the middle third of the dorsal forearm, the muscle bellies of the superficial and deep layers are divided by a transverse hyperechoic septum (Fig. 9.9). More deeply, the hyperechoic straight appearance of the interosseous membrane and the profile of the radial and ulnar shafts separate the dorsal compartment from the volar compartment (Fig. 9.9). a b a Edc Edm Ecu Apl R b Epb Volar Epl U Fig. 9.9a,b. Dorsal compartment of the forearm. a Proximal and b distal transverse 12–5 MHz US images obtained at the middle third of the forearm reveal the two layers of extensor muscles located over the posterior aspect of the interosseous membrane (arrowheads) and seperated by a transverse hyperechoic septum (arrows). From lateral to medial, the superficial layer of muscles includes the extensor digitorum communis (Edc), the extensor digiti minimi (Edm) and the extensor carpi ulnaris (Ecu), whereas the deep layer houses the abductor pollicis longus (Apl), the extensor pollicis brevis (Epb) and the extensor pollicis longus (Epl). R, radius; U, ulna. The photograph at the upper right of the figure indicates probe positioning Forearm 9.2.3 Mobile Wad The mobile wad, which is also referred to as the radial group of forearm muscles, contains two wrist extensors (the extensor carpi radialis brevis and the extensor carpi radialis longus) and a forearm flexor (the brachioradialis). These muscles lie in a radial position compared with the ventral and the dorsal muscles of the forearm (Fig. 9.10). The extensor carpi radialis longus and the brachioradialis are the most superficial and lateral. Both arise from the supracondylar ridge of the humerus and the lateral intermuscular septum, more cranially than the extensor carpi radialis brevis. The brachioradialis is a large muscle forming the lateral boundary of the cubital fossa (Fig. 9.10a). Distally, it inserts onto the lateral surface of the distal end of radius, just proximal to the radial styloid. Although acting as a flexor of the elbow, the brachioradialis is innervated by the radial nerve, like an extensor muscle. Partially covered by the brachioradialis, the extensor carpi radialis longus lies between it and the extensor carpi radialis brevis (Fig. 9.10). The extensor carpi radialis brevis arises more distally than the longus and is partially overlapped by it. The tendons of the extensor carpi radialis muscles pass through the anatomic snuffbox to insert into the dorsal aspect of the base of the second (longus) and third (brevis) metacarpals. Both muscles extend and abduct the wrist joint. The US scanning technique to examine the muscles of the mobile wad does not differ significantly from that used for the dorsal compartment (Fig. 9.11). The superficial sensory branch of the radial nerve and the radial artery are located between the mobile wad compartment and the volar compartment of the forearm (Fig. 9.3a). After branching off the main trunk of the radial nerve, the superficial radial nerve initially travels with the radial artery deep to the brachioradialis. It then passes between that muscle and the extensor carpi radialis longus to emerge from under the lateral boundary of the brachioradialis (Fig. 9.12a). At the distal forearm, this nerve pierces the antebrachial fascia and becomes subcutaneous, providing sensory innervation for the dorsum of the hand, the first web space and the proximal phalanges of the three radial fingers (Fig. 9.12b,c). While crosing the fascia, the radial nerve can be compressed in the scissoring of the brachioradialis and the extensor carpi radialis longus during pronation and supination of the forearm. At this site, dynamic US can show transverse sliding of the nerve during pronation and supination movements. The radial artery is located 18 19 a 17 b Fig. 9.10a,b. Schematic drawings of a coronal view of the mobile wad compartment of the forearm illustrated a without and b with removal of the brachioradialis muscle. a The brachioradialis (19) is a large palpable muscle arising from the supracondylar ridge of the humerus and the lateral intermuscular septum which continues distally with a long and strong tendon. b More deeply, the extensor carpi radialis brevis (17) and the extensor carpi radialis longus (18), the first arising from the lateral epicondyle, the second from the supracondylar ridge of the humerus, descend in the forearm in association with the brachioradialis more lateral and superficial compared with the ulnar artery. Initially, it is covered by the brachioradialis and then becomes more superficial at the middle and distal thirds of the forearm, where it runs between the brachioradialis and the flexor carpi radialis tendons. 9.3 Forearm Pathology Similar to the arm, musculoskeletal pathology affecting muscles and tendons is uncommon in the forearm and, for the most part, should derive from open wounds, contusion or penetrating trauma. Although unusual, there are some peculiar pathologic conditions affecting the median nerve in the proximal forearm as well as its main divisional branch, the anterior interosseous nerve, which may give rise to pain in the volar aspect of the forearm and weakness of the innervated flexor muscles. These conditions include pronator syndrome and anterior interosseous nerve syndrome. To the best of our knowledge, the latter is the only one which has received attention in the imaging literature. 417 418 C. Martinoli and S. Bianchi Ecrb BrRad Ecrl a H Br a Ecrb BrRad Ecrl a R b SH Ecrb DH DH R U c Fig. 9.11a–c. Mobile wad compartment of the forearm. a–c Series of transverse 12–5 MHz US images obtained at the elbow and the proximal forearm from a proximal to c distal reveal the bulk of muscles of the mobile wad, consisting of the brachioradialis (BrRad), the extensor carpi radialis longus (Ecrl) and the extensor carpi radialis brevis (Ecrb). The relationships of these muscles with the posterior interosseous nerve (arrowhead), the superficial sensory branch of the radial nerve (arrow), the radial artery (a) and the superficial (SH) and deep (DH) heads of the supinator muscle are shown. Br, brachialis; H, humerus; R, radius; U, ulna. The photograph at the upper right of the figure indicates probe positioning * * BrRad ECRL R R R a b c Fig. 9.12a–c. Superficial branch of the radial nerve. a–c Series of transverse 15–7 MHz US images obtained at the distal forearm from a proximal to c distal. a The superficial radial nerve (arrow) courses just deep to the antebrachial fascia (arrowhead) between the brachioradialis muscle (BrRad) and tendon (asterisk) and the extensor carpi radialis longus (ECRL). b More distally, it crosses the fascia and c moves to the subcutaneous tissue. R, radius. The photograph at the right of the figure indicates probe positioning Forearm 9.3.1 Volar Forearm 9.3.1.1 Pronator Syndrome Pronator syndrome is an insidious entrapment neuropathy of the median nerve in the proximal volar forearm. In this syndrome, the compression may occur either in the area where the nerve traverses deep to the lacertus fibrosus of the biceps, or as it crosses between the two heads of the pronator teres, or as it passes under the fibrous arch (sublimis bridge) of the flexor digitorum superficialis. Hypertrophy of the pronator teres, aberrant fibrous bands connecting the pronator teres to the tendinous arch of the flexor digitorum superficialis or the flexor carpi radialis with the ulna, direct trauma and forearm–elbow fractures have been reported as the possible causes. The main clinical features of this uncommon and somewhat controversial clinical entity are aching in the proximal volar forearm or distal arm, typically exacerbated by repetitive pronation and supination movements paresthesias in one or more of the radial three and a half fingers and weakness of the flexor pollicis and abductor pollicis longus with intact forearm pronation. Nocturnal pain (so typical of carpal tunnel syndrome) is usually not seen in these patients. Diagnosis of pronator syndrome is essentially based on clinical signs and symptoms and should be considered seriously when median nerve disturbances are not relieved after carpal tunnel release. The role of diagnostic imaging has not yet been assessed in this neuropathy. US could reinforce the likelihood that a pronator syndrome is present, when asymmetry of the pronator teres (the belly of the affected side larger than the contralateral side) and local flattening, distortion and an abnormal course of the nerve between the heads of the pronator or beneath the arcade of the flexor digitorum superficialis are seen (Fig. 9.13). Initial treatment of pronator syndrome is conservative because many patients recover over the course of a few months. In the remaining patients, surgical decompression of the nerve below the elbow (possibly associated with carpal tunnel release) is successful in many cases. 9.3.1.2 Anterior Interosseous Nerve Syndrome The entrapment of the anterior interosseous nerve in the forearm, a condition also known as the Kiloh– Nevin syndrome (Kiloh and Nevin 1952), occurs where the nerve branches off the median nerve, in proximity to the pronator teres and the tendinous bridge connecting the heads of the flexor digitorum superficialis (Stern 1984). The anterior interosseous nerve may be compressed alone or together with the main trunk of the median nerve by a variety of conditions, such as fibrous bands arising from the pronator teres and the flexor digitorum superficialis, hypertrophied anomalous muscles (Gantzer muscle) and accessory tendons from the flexor digitorum superficialis to the flexor pollicis longus. Similar to pronator syndrome, an isolated anterior interosseous neuropathy leads to pain in the volar forearm and difficulty in performing pinching movements with the digits (formation of a triangle instead of a circle with the first two digits) and handwriting. The thenar muscles are spared and there is no sensory loss (Fig. 9.14a). Muscle weakness is typically limited to the flexor pollicis longus, the flexor digitorum profundus to the index finger (middle finger also involved in 50% of cases), and the pronator quadratus (Fig. 9.14a). Differential diagnosis includes brachial plexus lesion and selective injury to the fibers of the median nerve at the elbow or in the arm that are destined to become the anterior interosseous nerve. In general, US examination of the anterior interosseous nerve is inconclusive in the absence of a mass because this nerve is too small and located deeply in the forearm. In rare cases, however, the nerve and its fascicles may appear swollen compared with the contralateral side (Fig. 9.14c). Besides direct nerve assessment, US diagnosis of an overt anterior interosseous neuropathy may be suggested by loss in bulk and increased reflectivity of the innervated muscles: the flexor pollicis longus, the flexor digitorum profundus and the pronator quadratus (Fig. 9.14d) (Grainger et al. 1998; Hide et al. 1999; Martinoli et al. 2004). 9.3.1.3 Other Compression Neuropathies Because of their free, unconstricted course, the radial and ulnar nerves are rarely compressed in the forearm. A reported site of compression of the sensory branch of the radial nerve is its point of emergence between the tendons of the brachioradialis and the extensor carpi radialis longus in the distal forearm. Repeated pronation and supination of the forearm is believed to be contributory to positional impingement of the nerve in the scissoring of these two tendons. From the biomechanical 419 420 C. Martinoli and S. Bianchi a prt * d br * * e a a a prt prt prt prt b c f Fig. 9.13a–f. Pronator syndrome in a patient with persisting symptoms of median neuropathy irradiated to the volar forearm and wrist after carpal tunnel release. a Transverse 12–5 MHz US image obtained at the elbow level, over the medial edge of the humeral trochlea (asterisk) demonstrates a flattened median nerve (arrow) presenting with an abnormal medial course between the pronator teres (prt) and the brachialis (br). a, brachial artery. b More distally, in the pronator area, transverse 12–5 MHz US image shows the flattened median nerve (arrow) coursing between the two heads of the pronator teres (prt). The nerve lies more medially than expected and not so closely associated with the ulnar artery (a). This anomaly suggested positional entrapment of the median nerve in the pronator area. c Contralateral normal side. Note the rounded cross-sectional profile of the normal median nerve (arrow) which runs adjacent to the ulnar artery (a). prt, pronator teres. d,e Transverse d T1-weighted and e fat-suppressed T2-weighted MR images of the elbow confirm flattening of the median nerve (arrow) which appears slightly hyperintense in the T2-weighted sequence. Asterisk, medial edge of the humeral trochlea. f Schematic drawing of a coronal view of the elbow after removal of the distal tendon of the biceps brachii (bb), the brachialis muscle and the superficial belly of the pronator teres (prt) reveals the abnormal course of the median nerve (arrows) in the pronator area described in this particular case. Arrowheads, brachial artery point of view, the nerve is anchored by fascia at this site and cannot adjust its position as the adjacent tendons do. Patients complain of pain and burning sensation over the dorsoradial aspect of the forearm, which increase in intensity with palmar flexion and ulnar deviation of the wrist or quick repeated pronation and supination movements. More distally, the entrapment of the sensory branch of the radial nerve may occur around the radial aspect of the wrist, so-called Wartenberg syndrome (see Chapter 10). On the mid-distal forearm, ulnar nerve compression may occur from casts positioned for wrist fractures or may be related to direct injuries, including contusion trauma (from a direct blow) or penetrating wounds. In contusion trauma, there may be discrepancy between severity of clinical pic- ture and normal electrodiagnostic studies. Tinel’s sign is usually positive on the ulnar aspect of the forearm. US can assess whether a nerve abnormality (fusiform neuroma) exists at the lesion site and may help the clinician to decide which is the most appropriate treatment (conservative vs. operative) to be instituted. In the area between the pronator and the carpal tunnel, the median nerve may occasionally be compressed by space-occupying masses (i.e., lipomas, ganglion cysts) or anomalous muscles. Among them, a reversed palmaris can produce a mass effect on the flexor tendons and the median nerve at the distal forearm (Depuydt et al. 1998). In these cases, US is an ideal means to reveal dynamic impingement of the median nerve by the anomalous muscle at rest and during contraction (Fig. 9.15). 422 C. Martinoli and S. Bianchi fcr ft fds fpl fdp pq c pq R ft b fcr * a fds * ft d e fcr * * f g Fig. 9.15a–g. Reversed palmaris muscle and carpal tunnel syndrome. a Photograph of a woman presenting with a fusiform soft tissue lump (arrowheads) in the volar wrist and clinical symptoms of carpal tunnel disease. The lump increases in size and stiffness while clenching the fist. b Transverse and c longitudinal 12–5 MHz US images over the mass reveal an additional muscle belly (white arrows) over the flexor digitorum superficialis muscle (fds) and tendons (ft), reflecting a reversed palmaris. In a, observe the median nerve (open arrow) and other adjacent deep muscles, the flexor pollicis longus (fpl), the flexor digitorum profundus (fdp) and the pronator quadratus (pq). R, radius. d Transverse 12–5 MHz US image over the anomalous muscle obtained during contraction. Active contraction leads to an increased thickness of the muscle belly. This change can be easily palpated at physical examination and would lead to compression on the underlying median nerve (arrow). Note tenosynovial effusion (asterisks) in the sheath of the flexor tendons (ft) and the normal flexor carpi radialis tendon (fcr). e Transverse 12–5 MHz US image obtained at the proximal forearm demonstrates a long thin tendon (arrowheads) of the palmaris instead of the muscle belly. The anomalous tendon is located superficial to the flexor digitorum superficialis. f Axial T1-weighted and g sagittal fat-suppressed T2-weighted MR images reveal the anomalous reversed palmaris (arrows), a hyperintense appearance of the median nerve (arrowheads) in the T2-weighted sequence and fluid effusion (asterisks) in the flexor tendon sheath reflecting tenosynovitis c Radius a b d Fig. 9.16a–d. Flexor carpi radialis tendon tear. a Photograph of a boy complaining of weakness of wrist flexion and a soft tissue lump (white arrows) on the volar aspect of the wrist after receiving a penetrating wound (open arrow) in the middle forearm by a sharp object. b Longitudinal and c transverse 12–5 MHz US images over the distal lump reveal a retracted tendon end (arrows) of the flexor carpi radialis which appears swollen and diffusely hypoechoic. d At the level of the wound, transverse 12–5 MHz US image demonstrates an empty sheath (arrowheads) of the flexor carpi radialis tendon Forearm a R a a R b R c a d References Al-Quattan MM (1996) Gantzer’s muscle. An anatomical study of the accessory head of the flexor pollicis longus muscle. J Hand Surg [Br] 21:269–270 Boles CA, Kannan S, Cradwell AB (1999) The forearm: anatomy of muscles compartments and nerves. AJR Am J Roentgenol 174:151–159 Depuydt KH, Schuurman AH, Kon M (1998) Reversed palmaris longus muscle causing effort-related median nerve compression. J Hand Surg [Br] 23:117–119 Grainger AJ, Campbell RSD, Stothard J (1998) Anterior interosseous nerve syndrome: appearance at MR imaging in three cases. Radiology 208:381–384 Hide IG, Grainger AJ, Naisby GP et al (1999) Sonographic findings in the anterior interosseous nerve syndrome. J Clin Ultrasound 27:459–464 Fig. 9.17a–d. Complete tear of the superficial branch of the radial nerve by a glass wound. a–c Series of transverse 12–5 MHz US images of the middle third of the forearm obtained a proximal to, b at the level of and c distal to the cut line. In a, note the superficial course of the radial nerve (straight arrow) which runs closely associated with the radial artery (a). In b and c, two adjacent neuromas are found connected with the proximal (white arrowhead) and distal (open arrowhead) stumps of the severed nerve. R, radius. d Photograph shows the cut line (arrow) at the middle third of the forearm. Kiloh LG, Nevin S (1952) Isolated neuritis of the anterior interosseous nerve. Br Med J 1:850–851 Martinoli C, Bianchi S, Pugliese F et al (2004) Sonography of entrapment neuropathies in the upper limb (wrist excluded). J Clin Ultrasound 32:438–450 Reimann AF, Daeseler EH, Anson BJ et al (1944) The palmaris longus muscle and tendon: a study of 1600 extremities. Anat Rec 89:495–505 Sallomi D, Janzen DL, Munk PL et al (1998) Muscle denervation patterns in upper limb nerve injuries: MR imaging findings and anatomic basis. AJR Am J Roentgenol 171:779–784 Schuurman AH, van Gils APG (2000) Reversed palmaris longus muscle on MRI, report of four cases. Eur Radiol 10:1242–1244 Stern MB (1984) The anterior interosseous nerve syndrome (the Kiloh-Nevin Syndrome): report and follow-up study of three cases. Clin Orthop 187:223–227 423 Wrist 10 Wrist Stefano Bianchi and Carlo Martinoli CONTENTS 10.1 Introduction 425 10.2 10.2.1 10.2.2 10.2.3 Clinical Anatomy 425 Osseous and Articular Anatomy Tendons and Retinacula 427 Neurovascular Structures 430 10.3 Essentials of Clinical History and Physical Examination 433 De Quervain Disease 433 Carpal Tunnel Syndrome 433 10.3.1 10.3.2 10.4 10.4.1 10.4.2 US Scanning Technique and Normal US Anatomy 434 Dorsal Wrist 434 Volar Wrist 441 10.5 10.5.1 10.5.2 10.5.3 10.5.4 Wrist Pathology 449 Dorsal Wrist Pathology 449 Ventral Wrist Pathology 456 Bone and Joint Disorders 472 Wrist Masses 483 References 425 425 492 10.1 Introduction In recent years, substantial improvement in transducer technology has led to a growing interest in the US evaluation of the hand and wrist (Bianchi et al. 1999, 2001; Chiou et al. 2001; Creteur and Peetrons 2000; Ferrara and Marcelis 1997; Fornage and Rifkin 1988; Lee 1998; Milbrat et al. 1990; Read et al. 1996; Teefey et al. 2000; Lee and Healy 2005). US transducers with frequencies ranging from 10 to 15 MHz allow accurate assessment of tendons, joints, nerves and vessels of the extremities without requiring stand-off pads. The association of standard radiographs with high-resolution US works well in the S. Bianchi, MD Privat-docent, Université de Genève, Consultant Radiologist, Fondation et Clinique des Grangettes, 7, ch. des Grangettes, 1224 Genève, Switzerland C. Martinoli, MD Associate Professor of Radiology, Cattedra “R” di Radiologia – DICMI – Università di Genova, Largo Rosanna Benzi 8, 16132 Genova, Italy evaluation of wrist and hand disorders. Radiographs can recognize most bone and joint disorders and US can be used to assess a wide spectrum of pathologic conditions affecting soft-tissue structures. 10.2 Clinical Anatomy From the anatomic point of view, the wrist is complex. For this reason, we will take a little time here to review the basic anatomy of the wrist with emphasis on the structures that can be assessed with US. 10.2.1 Osseous and Articular Anatomy The wrist is composed of eight carpal bones arranged in two rows: proximal and distal. From lateral to medial, the proximal row includes the scaphoid, lunate, triquetrum and pisiform, whereas the distal row is formed by the trapezium, trapezoid, capitate and hamate. The arrangement of the carpal bones forms a ventral concavity which is transformed in an osteofibrous tunnel, the carpal tunnel, by the transverse carpal ligament. There are three joints in the wrist which, in normal conditions, do not communicate with one another: the distal radio-ulnar, radiocarpal and midcarpal joints (Fig. 10.1). Wrist movements are obtained by the concurrent action of the radiocarpal joint and midcarpal joint: wrist flexion and extension is produced half at the radiocarpal joint and half at the midcarpal joint, whereas radial and ulnar deviation of the wrist involves, at a higher extent (60%), the midcarpal joint. 10.2.1.1 Distal Radio-ulnar Joint The distal radio-ulnar joint articulates the rounded head of the ulna with the ulnar notch of the distal Wrist carpal space which increase stability to the ulnar side of the wrist and the distal radio-ulnar joint and absorb mechanical forces across the ulnar side of the wrist during axial loading. The complex includes the triangular fibrocartilage itself and other supporting structures which blend with it, such as the meniscus homologus, the ulnar collateral ligament, the volar and dorsal radio-ulnar ligament and the sheath of the extensor carpi ulnaris tendon. The triangular fibrocartilage is a biconcave disk positioned between the ulnar styloid and the radius. Its thickness is inversely proportional to the degree of ulnar variance. Even using high-resolution transducers, most wrist ligaments are not visible with US and their proper evaluation requires MR imaging, MR arthrography or thin collimation spiral CT arthrography. Clinically relevant structures that are amenable to US examination are the scapholunate ligament and the triangular fibrocartilage complex. 10.2.2 Tendons and Retinacula The wrist is crossed by flexor and extensor tendons which course along its ventral and dorsal aspects respectively. Among them, nine flexor tendons and nine extensor tendons move toward the fingers without any attachment to the carpal bones; two primary wrist flexors and three wrist extensors insert onto the distal carpal row and the metacarpals; and one tendon, the palmaris longus tendon, attaches to the transverse carpal ligament and to the palmar aponeurosis. 10.2.2.1 Extensor Tendons The extensor tendons course over the dorsal aspect of the wrist. They run within series of adjacent osteofibrous tunnels delimited by depressions of the surface of radius and ulna and by the extensor retinaculum, a 2 cm wide thickening of the dorsal fascia attached to the radial styloid laterally and to the pisiform and triquetrum medially. From the deep surface of the retinaculum, vertical fibrous bands insert into the cortical bones, at both sides of the tendons, dividing the extensor tunnel into six compartments numbered from radial (I) to ulnar (VI). In each compartment, a single synovial sheath formed by visceral and parietal layers surrounds one or more tendons (Fig. 10.2). A variable amount of fatty tissue fills the space between the synovial sheath and the bone surface. From the biomechanical point of view, these tunnels give lateral stabilization and avoid bowstringing of the extensor tendons during wrist and finger movements. A bony protuberance, the Lister tubercle, is found between the second and third tunnels, acting as a useful landmark in the US identification of these compartments (Fig. 10.2). The first compartment, the most radial, contains the abductor pollicis longus and extensor pollicis brevis tendons (Fig. 10.3). Medial to this, the second compartment houses the extensor carpi radialis longus and brevis which insert on the dorsal aspect of the base of the second and third metacarpals respectively. The third compartment contains the extensor pollicis longus. As already stated, this compartment is separated from the second one by the Lister tubercle of the radius (Fig. 10.3a). The fourth compartment is wide and encloses the tendons of the extensor digitorum for the second through the fifth fingers, and the tendon of the extensor indicis proprius, which is absent or rudimentary in approximately 40% of individuals (Fig. 10.4). The fifth compartment encloses the extensor digiti quinti proprius, whereas the sixth compartment, the most ulnar, includes the extensor carpi ulnaris tendon which courses along the dorsomedial aspect of the distal ulna to insert onto the base of the fifth metacarpal (Fig. 10.4). The tendons of the first compartment and the tendon of the extensor pollicis longus form the volar and dorsal boundaries of the anatomic snuff-box, a skin depression on the radial aspect of wrist crossed by the radial artery (Fig. 10.3a,b). To recall the exact name of the extensor tendons seems difficult but it is even harder to remember the exact position of them in each individual compartment, and especially in the first, second and fourth compartments. For an easier comprehension, one should keep in mind that: in the first compartment the extensor pollicis brevis tendon is more dorsal than the abductor pollicis longus; in the second compartment, the extensor carpi radialis brevis tendon is closer to the Lister tubercle than the extensor carpi radialis longus; in the fourth compartment, the extensor indicis proprius tendon is positioned on the ulnar side of the tendon for the index finger of the extensor digitorum; the tendon of the extensor pollicis longus crosses the tendons of the second compartment to reach the thumb (Fig. 10.3a,b). As a memo, the tendons from the first to the third compartment alternate as to longus and brevis as they proceed in an ulnar direction: abductor pollicis longus, extensor pollicis brevis, extensor carpi radialis longus, extensor carpi radialis brevis, extensor pollicis longus. 427 428 S. Bianchi and C. Martinoli III )) )) II ))) ))) IV (EPL) (EIP, EDC) (ECRB, ECRL) )6 )6 V VI (EDQ) (ECU) 6 6 ) 6) 6) Radius I Ulna (EPB, APL) b III II a V IV Ulna Radius c Fig. 10.2 a−c. Position of the extensor tendons relative to the bony surfaces of the dorsal radius and ulna. a Dorsal aspect of the wrist bones illustrates the relationships of the six compartments of the extensor tendons (I−VI) with the Lister tubercle (arrow). b Schematic drawing of a transverse view at the level of the distal radio-ulnar joint outlines the extensor tendons and their synovial sheath. The tendons are labeled with numbers that correlate with the dorsal compartments (I−VI). The first compartment contains the abductor pollicis longus (APL) and extensor pollicis brevis (EPB), the second the extensor carpi radialis longus (ECRL) and extensor carpi radialis brevis (ECRB), the third the extensor pollicis longus (EPL), the fourth the extensor indicis proprius (EIP) and extensor digitorum (EDC), the fifth the extensor digiti quinti (EDQ), the sixth the extensor carpi ulnaris (ECU). Observe the prominence of the Lister tubercle (arrow) which separates the second from the third compartment. c Transverse 15−8 MHz US image over the dorsal wrist illustrates the typical dorsal shape of the distal radius and ulna shown in the diagram in b. The depiction of the Lister tubercle (arrow) makes the identification of the overlying extensor tendons easier P ∗ IV R III I a b c Fig. 10.3 a−c. Anatomic snuff-box. a Schematic drawing of a coronal view of the wrist bones illustrates the relationship among the tendons of the first (I), second (II) and third (III) compartments. Note the course of the extensor pollicis longus tendon (III) which crosses the tendons of the second compartment to reach the thumb. The anatomic snuff-box (arrow) is a triangular space delimited by the tendons of the first and third compartments. b Photograph of the dorsolateral aspect of the wrist in a young woman showing the main surface features visible during contraction of the radial extensors. The abductor pollicis longus and extensor pollicis brevis (I) bound the hollow of the anatomic snuff-box (arrow) anteriorly, and the extensor pollicis longus (III) bounds it posteriorly. Observe the tendons of the fourth compartment (arrowheads) which diverge as they proceed distally over the dorsal hand. c Photograph of the ventral lateral aspect of the wrist shows the position of the abductor pollicis longus and extensor pollicis brevis tendons (open arrow) relative to the anatomic snuff-box (asterisk) and the radial styloid (R). The flexor carpi radialis (white arrow) and palmaris longus (arrowhead) tendons are also delineated on a more ventral location. Note the pisiform bone (P) Wrist EDC EPL U a b 10.2.2.2 Flexor Tendons At the volar aspect of the wrist, nine flexor tendons enter the carpal tunnel to reach the fingers. There are four tendons from the flexor digitorum superficialis for the second through fifth fingers, four from the flexor digitorum profundus for the same fingers and the flexor pollicis longus tendon. The flexor digitorum superficialis muscle gives rise to four tendons at the distal radius, just cranial to the proximal edge of the transverse carpal ligament. Then, these tendons pass within the carpal tunnel to diverge toward the fingers. During active finger movements, tendons of the flexor digitorum superficialis can be palpated at the wrist between the prominences of the flexor carpi radialis and ulnaris tendons. The four tendons of the flexor digitorum profundus traverse the wrist just deep to the respective tendons of the flexor digitorum superficialis. In the carpal tunnel, the tendon of the index finger is separate whereas the remaining tendons to the third through fifth fingers may become completely independent only in the palm. The lumbrical muscles arise in the palm from the tendons of the flexor digitorum profundus. The tendon of the flexor pollicis longus lies deep to the flexor carpi radialis in the distal forearm and passes on the radial side of the flexor digitorum tendons of the index finger in the carpal tunnel. On approaching the wrist, the tendons of the flexor digitorum superficialis and profundus become enveloped by a common synovial sheath. On transverse views, this sheath is “ε” shaped with a superficial extension which lies in front of the flexor digitorum superficialis, a middle extension lying between the flexor digitorum superfi- Fig. 10.4 a,b. Anatomy of the extensor tendons. a Schematic drawing of a coronal view of the dorsal wrist showing the relation among tendons of the fourth, fifth and sixth compartments. In the fourth compartment, the extensor indicis proprius (intermediate gray) runs together with the extensor digitorum (black). b Photograph of the dorsal wrist in a young woman during forced wrist dorsiflexion demonstrates the diverging tendons of the extensor digitorum (EDC) over the skin. Other surface landmarks include the skin depression of the anatomic snuff-box (arrow), the extensor pollicis longus tendon (EPL) and the head of the ulna (U) cialis and profundus and a deep extension behind the flexor profundus. Just radial to the common flexor tendon sheath, the flexor pollicis longus tendon is enveloped by a separate sheath. The primary flexors of the wrist, the flexor carpi radialis and the flexor carpi ulnaris, course outside the carpal tunnel and are readily palpable because they lie in a more superficial position than the flexor digitorum tendons (Fig. 10.5). The flexor carpi radialis tendon is a long flattened tendon which becomes oval in shape as it approaches the wrist. This tendon originates nearly midway between the elbow and wrist, is invested by an own synovial sheath and inserts on the palmar aspect of the base of the second metacarpal after coursing in a separate fibrous tunnel (vertical groove) made by an extension of the transverse carpal ligament. Its action allows flexion and concurrent radial deviation of the wrist. The flexor carpi ulnaris, the only tendon of the wrist not invested by a synovial sheath together with the palmaris longus tendon, is smaller in size and shorter relative to the flexor carpi radialis. This tendon courses on the ulnar side of the wrist housing the pisiform, which is considered a sesamoid bone in it, and inserts on the hook of the hamate (piso-hamate ligament) and on the fifth metacarpal (piso-metacarpal ligament). The flexor carpi ulnaris tendon is a landmark for the adjacent ulnar artery and nerve, both located just radial to them. Its action allows flexion and concurrent ulnar deviation of the wrist, an essential action in some tasks such as using a screwdriver or a mallet. The palmaris longus tendon is a long thin tendon which passes in the midline and superficial to the transverse carpal ligament (Fig. 10.5). Distally, it splits into diverging bundles which intermingle with 429 430 S. Bianchi and C. Martinoli P 0 A a -. b the transverse carpal ligament and the palmar aponeurosis. It is absent in approximately 20% of individuals. 10.2.3 Neurovascular Structures The wrist is crossed by the median nerve, the ulnar nerve and the superficial cutaneous branch of the radial nerve. In the wrist area, the ulnar nerve is accompanied by the ulnar artery and the median nerve gives off a sensory branch, the palmar cutaneous branch. Fig. 10.5 a,b. a Photograph of the anterior aspect of the wrist with b cadaveric correlation shows the flexor carpi radialis tendon (black arrow) which serves as a guide to the radial artery (a) which lies just lateral to it. The long lean tendon of the palmaris longus (arrowhead) is a landmark for the median nerve (MN) which is deep and frequently lateral to it. More medially, the flexor carpi ulnaris tendon (open arrow) is seen moving down to the pisiform (P). This tendon may be used as a key reference for the ulnar artery and nerve which lie lateral to it Throughout the carpal tunnel, the median nerve is covered by a strong fibrous band commonly referred to as the transverse carpal ligament or the flexor retinaculum (Fig. 10.6a,b). This is a localized thickening of the fascia that inserts on the tubercle of the scaphoid and trapezium (radial side) and on the pisiform and hook of the hamate (ulnar side) (Fig. 10.7). The median nerve provides sensory supply to the palmar aspect of the first three fingers and the radial half of the fourth, and motor supply for the muscles of the thenar eminence. Just proximal to the transverse carpal ligament, the median nerve sends a palmar cutaneous branch, which is a sensory nerve that supplies the radial half of the palm. This latter branch is very small and typically vulnerable to injury during carpal tunnel release. 10.2.3.1 Median Nerve At the distal forearm, the median nerve courses in the fascial plane intervening between the flexor digitorum profundus and the flexor digitorum superficialis muscles. As the nerve approaches the wrist, it shifts radially and then moves superficially along the lateral margin of the flexor digitorum superficialis to align itself with the midline before entering the carpal tunnel (Fig. 10.6). Inside the tunnel, the median nerve runs superficial to the tendons of the flexor pollicis longus and the flexor digitorum superficialis for the second finger although its position may vary somewhat depending on wrist position. The nerve has an oval cross-section at the proximal tunnel and tends to become more flattened as it progresses distally through the tunnel (level of the hamate hook). 10.2.3.2 Ulnar Nerve In the distal forearm, the ulnar nerve lies on the radial side of the flexor carpi ulnaris and on the ulnar side of the ulnar artery. Here, it gives off two small branches: the palmar and dorsal cutaneous branches. More distally, the ulnar nerve pierces the deep fascia to continue in the wrist superficial to the transverse carpal ligament throughout the Guyon tunnel (Fig. 10.8). This small tunnel lies in a more superficial and medial location relative to the carpal tunnel. It is bounded by the pisiform medially (proximal tunnel), the hook of the hamate laterally (distal tunnel), the transverse carpal ligament (floor) and the palmar carpal ligament (roof). The Guyon tunnel contains 432 S. Bianchi and C. Martinoli ( Pisiform Pisiform a ( Carpal Carpal Carpal tunnel Tunnel Tunnel a A DE b 0 d BC ✟ Ss d D Carpal Carpal tunnel tunnel ( s ∗ d Hamate Hamate FCU a c e Fig. 10.8 a−e. Guyon tunnel anatomy. a Ventral view of the wrist bones illustrates the course of the ulnar artery and the ulnar nerve in the Guyon tunnel relative to the flexor carpi ulnaris tendon (fcu), the pisiform (P) and the hook of the hamate (H). The transverse carpal ligament (arrowheads) forms the floor of the Guyon tunnel. In the distal portion of the tunnel, the ulnar nerve divides into a superficial sensory branch (straight arrow) and a deep motor branch (curved arrow). b,c Gross anatomic views with d,e corresponding diagrams of the proximal (b,d) and distal (c,e) Guyon tunnel obtained at the levels (horizontal white bars) indicated in a show the main trunk of the ulnar nerve (void arrow) and its divisions, deep (d) and superficial (s). Close to the nerve, the ulnar artery (a) bifurcates in the respective deep (asterisk) and superficial (star) branches. In d, observe the position of the ulnar nerve relative to the pisiform, the transverse carpal ligament (black arrow) and the palmar carpal ligament (arrowheads) the ulnar nerve (medial) and the ulnar artery (lateral) and veins embedded in fatty tissue. The ulnar nerve bifurcates within this tunnel into two terminal divisions – the superficial sensory branch and the deep motor branch – the latter supplying most of the intrinsic muscles of the hand, including the hypothenar muscles, the two medial lumbrical muscles, the adductor pollicis and the interosseous muscles. The ulnar nerve gives sensory supply to the medial aspect of the palm, the little finger and the medial half of the ring finger. Distal to the Guyon tunnel, the superficial branch has a straight course while the deep motor branch reflects across the palm to end at the first interosseous space (Fig. 10.8a). 10.2.3.3 Radial Nerve (Cutaneous Terminal Branch) At the distal radial aspect of the forearm, the superficial cutaneous branch of the radial nerve emerges between the tendons of the extensor carpi radialis longus and the brachioradialis to reach the subcutaneous tissue. At this point, the nerve is covered by a fascial band which connects the tendon and myotendinous junction of the brachioradialis muscle with the tendon of the extensor carpi radialis longus. More distally, the radial nerve pierces the fascia and overlies the anatomic snuff-box traversing the extensor tendons of the first compartment to provide sensory supply to the dorsum of the wrist, hand, thumb and proximal portion of the radial fingers. 10.2.3.4 Radial and Ulnar Arteries The brachial artery has two terminal branches: the radial artery and the ulnar artery. At the distal forearm, the radial artery courses superficially over the ventral aspect of the distal radius where its pulse can readily be felt. Then, it curves dorsally over the radial aspect of the wrist, passes deep to the extensor tendons of the first compartment and crosses the floor of the anatomic Wrist snuff-box. The ulnar artery enters the wrist on the lateral side of the ulnar nerve and runs together with the nerve throughout the Guyon tunnel, superficial to the transverse carpal ligament (Fig. 10.8). Somewhat similar to the nerve, the ulnar artery splits into a superficial palmar branch and a deep palmar branch. 10.3 Essentials of Clinical History and Physical Examination Before US examination, the patient’s history should be carefully investigated to rule out any possible systemic articular disorder (rheumatoid arthritis and similar conditions), sporting or occupational activities possibly related to tendinitis and overuse syndromes, as well as local trauma (occult fractures, tendon ruptures, ligament sprains). At physical examination, the range of wrist movements (flexion-extension, ulnar-radial deviation, pronation-supination) can readily be assessed. An accurate location of the site of pain may be helpful in the case of tendinitis. In addition, movements that cause pain should also be tested. Recent standard radiographs, if any, should be reviewed for signs of joint and bones disease (i.e., osteoporosis, marginal erosions, focal bone lesions), abnormal position of bones (reflecting ligaments tears) and soft-tissue thickening and calcifications. When a space-occupying mass is encountered over the dorsal or palmar aspects of the wrist, intermittent variations in its size with time can suggest the diagnosis of a ganglion cyst. When the mass is linked to an adjacent tendon and follows it during movements, an intratendinous ganglion should be suspected. a 10.3.1 De Quervain Disease In de Quervain disease, an inflammatory disorder affecting the first compartment of the extensor tendons, patients report tenderness and pain over the radial styloid. Typically, the wrist pain increases during grasping heavy objects. A useful diagnostic test is the Finkelstein test (Fig. 10.9). During this maneuver, the patient holds his or her thumb inside the clenched fist while the examiner tilts the patient’s hand in an ulnar direction to stretch the tendons of the first compartment. The Finkelstein test indicates de Quervain disease when it causes pain over the radial styloid that resemble the one described by the patient. Care should be taken, however, not to rely on this finding alone, because the Finkelstein test can be positive in normal subjects if the examiner applies excessive tension and in cases of rizarthrosis and radial styloiditis. As an alternative test, the examiner can maximally abduct the patient’s thumb while keeping the wrist in radial deviation. This latter maneuver is more specific because it pushes the tendons against the retinaculum and not toward the bone, thus recalling the same stress forces that generate symptoms in de Quervain disease. Both tests should be performed by the examiner because they help to direct the US examination to the first compartment. 10.3.2 Carpal Tunnel Syndrome Patients with carpal tunnel syndrome typically complain of night tingling and burning pain over the radial aspect of the hand and the first three fingers. b Fig. 10.9 a,b. Finkelstein test for evaluation of de Quervain disease. a Schematic drawing of a sagittal view through the wrist during ulnar deviation outlines tension of the first compartment tendons resulting from stretching over the radial styloid. b The Finkelstein sign is performed as follows: while the patient adducts the thumb into the palm making a fist, the examiner tilts the wrist in ulnar deviation (curved arrow) to stretch the tendons of the first compartment (arrowheads). A positive test causes localized excruciating pain over the radial styloid 433 434 S. Bianchi and C. Martinoli The same symptoms can be felt during the day when a fixed position of the hand grasping an object is required, such as holding a heavy book or the telephone receiver. Because of the tingling, it is not unusual for patients to refer findings of carpal tunnel syndrome to a vascular disorder. Two clinical tests can be helpful to establish the diagnosis: the Tinel test and the Phalen test. The Tinel test is performed by tapping the volar aspect of the carpal tunnel with a reflex hammer (Fig. 10.10a), while, in the Phalen test, a full flexed wrist position is maintained for 1 min (Fig. 10.10b).Both tests are positive if they reproduce the patient’s symptoms. The examiner should be aware, however, that false negatives may occur in cases of chronic entrapment disease. 10.4 US Scanning Technique and Normal US Anatomy The patient is asked to sit comfortably in front of the examiner with both wrists and elbows resting on the examination table. Aged or traumatized patients may lie supine with the arm resting at the side of the body, although examination of the opposite side may become problematic in this position. For dynamic scanning of the extensor tendons, the hand is best placed on a gel tube with the fingers hanging over its edge to make fingers movements easier. The routine US examination of the wrist begins with evaluation of its dorsal aspect, followed by the palmar one. Depending on the specific clinical presentation, US images can be obtained in different a positions of the wrist (flexion and extension, radial and ulnar deviation, pronation and supination). Evaluation of gliding of the flexor and extensor tendons must always be performed during passive and active movements of the fingers. 10.4.1 Dorsal Wrist Transverse US images are the best for detection and a proper identification of the extensor tendons. Assessment of the individual tendons is based on their anatomic position and behavior at dynamic examination (Lee anh Healy 2005). Detection of the extensor tendon for the third finger, for instance, is straightforward when transverse US scans are obtained during active flexion and extension of this finger while the others are maintained fixed by the examiner. On the other side, the extensor carpi radialis and the extensor carpi ulnaris are not affected by fingers movements and can be distinguished only on the basis of their anatomic position. US images are first obtained at the level of the distal epiphysis of the radius. The most useful landmark at this level is the Lister tubercle. This appears as a hyperechoic bony prominence over the dorsal surface of the radius. The tubercle separates the medial third compartment from the lateral second compartment (Fig. 10.2c). The extensor tendons appear as oval or rounded hyperechoic structures of different size. The extensor carpi radialis brevis and longus are the largest while the extensor pollicis longus and the extensor digiti quinti are the smallest. With high-resolution transducers, the extensor retinaculum appears as a thin transversely oriented b Fig. 10.10 a,b. Clinical tests for evaluation of carpal tunnel syndrome. a The Tinel sign elicits paresthesias by tapping the median nerve at the palmar crease. b The Phalen sign provokes paresthesias at the end of the range of flexion of the wrist Wrist fcr fcr a fds fds fpl fpl ∗ Radius Radius MN fdp fdp ∗ Ulna Ulna a fds fds fcu fcu fdp fdp ∗ ∗ fcu FCU UN FDS fds fcr FCR Ulna Ulna Radius Radius b c Fig. 10.23 a−c. Ventral wrist structures proximal to the carpal tunnel. Transverse 12−5 MHz US images obtained over the a radial and b ulnar sides of the proximal wrist (level of radial and ulnar metaphyses) demonstrate the relationship among ventral tendons, nerves and vessels proceeding toward the wrist over the pronator quadratus (asterisks). From lateral to medial, these structures are: the radial artery (a), the flexor carpi radialis (fcr) and flexor pollicis longus (fpl), the median nerve (MN), the flexor digitorum superficialis (fds) and flexor digitorum profundus (fdp), the ulnar artery (white arrow), the ulnar nerve (UN) and the flexor carpi ulnaris (fcu). In a, observe the palmaris longus tendon as a very superficial and thin hypoechoic band (open arrow) lying medial to the flexor carpi radialis. c Gross anatomic view of the ventral wrist shows the relationship of the palmaris longus (arrows) with the flexor carpi radialis (fcr), the flexor digitorum superficialis (fds) and the flexor carpi ulnaris (fcu) tendons. The inserts at the upper left side of the figure indicate probe positioning a more medial location. Anatomic variations in the number of wrist arteries can be found. The presence of a median artery of the forearm, close to the median nerve, can be readily assessed with US. When evaluating wrist vessels, care should be taken not to apply excessive pressure with the transducer on the artery to avoid its collapse and non-visualization. Proximal to the carpal and Guyon tunnels, the median and ulnar nerves are recognized based on their peculiar fascicular echotexture. Approaching the wrist, the median nerve becomes more superficial and lateral and then runs toward midline and in a deeper position to enter the carpal tunnel (Jamadar et al. 2001). The palmar cutaneous branch of the median nerve arises from its palmar-radial quadrant approximately 5 cm cranial to the proximal wrist crease (Taleisnik 1973). It remains bound at the main nerve trunk to leave it after approximately 2 cm (Fig. 10.25). After piercing the antebrachial fascia or the transverse carpal ligament and entering the palm, the palmar cutaneous branch of the median nerve supplies the skin of the thenar and midpalmar areas. Awareness of the palmar cutaneous branch is important from the surgical point of vies to avoid inadvertent resection during release of the transverse carpal ligament performed with a too radial approach. Injury of this branch is followed by postoperative sensory disturbances. On short axis planes high-resolution US transducers can image this small nerve division. The ulnar nerve is found at the medial aspect of the distal forearm between the tendon of the flexor carpi ulnaris and the ulnar artery. Because of its close relationship with the ulnar artery, the ulnar nerve can be easily identified by detecting the pulsatility or the presence of color flow signals in the adjacent artery. 443 444 S. Bianchi and C. Martinoli pq c a b Scaphoid Radius d a Radius b MN Pisiform ft MN ft c d Fig. 10.24 a−d. Longitudinal scanning planes over the ventral wrist obtained with a 12−5 MHz US transducer demonstrate from lateral (a) to medial (d) according to the reference diagram shown at the upper left side of the figure: a, the course of the radial artery (arrowheads), which is superficial between the skin and the pronator quadratus (pq) and then deepens to enter the anatomic snuff-box; b, the diverging course of the flexor carpi radialis (arrowhead) and flexor pollicis longus (arrow) over the scaphoid bone; c, the superficial course of the median nerve (MN) relative to the flexor tendons (ft) in the carpal tunnel and d, the flexor carpi ulnaris tendon (arrowhead) which courses superficial to the pisiform fcr b b a a a c fcr FCR b d Fig.10.25 a−d. Palmar cutaneous branch of the median nerve. a Schematic drawing of a coronal view through the lateral wrist and b corresponding gross anatomic specimen outline the course of the median nerve (arrows) and its palmar cutaneous branch (arrowheads) relative to the flexor carpi radialis tendon (fcr) and the transverse carpal ligament. c,d Transverse 15−7 MHz US images obtained c at the distal radius and d at the proximal carpal tunnel level reveal the palmar cutaneous branch as a small hypoechoic fascicle (straight arrow) which leaves the median nerve (curved arrow) and pierces the transverse carpal ligament (arrowheads) to run between it and the flexor carpi radialis tendon (fcr) Wrist verse carpal ligament which holds the flexor carpi radialis tendon. The nine flexor tendons (four from the flexor digitorum superficialis, four from the flexor digitorum profundus and the flexor pollicis longus) can be imaged inside the carpal tunnel as individual structures (Fig. 10.26). The identification of each of these tendons is easily accomplished based on their anatomic position (radial flexors rest on the radial side of the tunnel, ulnar flexors on the ulnar side) and by their action at dynamic US scanning. Compared with the round cross-sectional profile of the flexor digitorum tendons, the flexor pollicis longus is more oval in shape and its major axis is vertically oriented on transverse planes. At least in part, this may depend on the course of this tendon which diverges radially to reach the thumb. The median nerve courses superficial and parallel to the second and third flexor tendons and medial to the flexor pollicis longus tendon, just deep to the transverse carpal ligament (Fig. 10.26). Its cross-section is usually an ellipse, but its shape may change depending on wrist positions and varies among subjects (Kuo et al. 2001). In addition, even the size of the nerve seems to change relative to the wrist activity (MassyWestropp et al. 2001). During flexion of the fingers or fist clenching, transverse US images demonstrate passive shifting movements of the median nerve on the underlying gliding flexor tendons (Nakamichi and Takibana 1992). Some anatomic variants of clinical relevance in the intracanal structures can be identified with US. The presence of anomalous muscles coursing within the carpal tunnel has been reported, includ- 10.4.2.2 Proximal Carpal Tunnel The most useful bony landmarks to identify the proximal carpal tunnel are the pisiform at its ulnar side and the scaphoid at its radial side (Fig. 10.7). At US examination, these bones appear as round hyperechoic structures with posterior acoustic shadowing. Once these landmarks are demonstrated in a single image, the orientation of the probe should be adjusted to optimize the depiction of the soft tissues contained within the tunnel (Fig. 10.26). Tilting the probe back and forth may be helpful to distinguish the hypoechoic median nerve by the adjacent anisotropic tendons. Relative to the flexor carpi radialis, the flexor pollicis longus tendon runs in a deeper location, slightly closer to the midline. Oblique longitudinal US images can depict these tendons in the same plane (Fig. 10.24b). The proximal carpal tunnel is larger in size compared with the distal tunnel. In a comparative US-cadaveric study, US has proved to be accurate in evaluating the different diameters, the outline and the cross-sectional area of the carpal tunnel and the median nerve (Kamolz et al. 2001). The transverse carpal ligament appears as a thin slightly convex band of 1−1.5 mm thickness (Fig. 10.26). Its attachments to the pisiform and the scaphoid are readily detected with US. Because of its curvilinear shape, the anisotropic transverse carpal ligament may appear hypoechoic when the US beam is not perpendicular to it. This is particularly true at its attachments. Even with a careful scanning technique, high-resolution US is unable to depict the lateral division of the trans- a fcr fcr fpl Sca a s p s s s p p p Pis s fpl p Sca s s s p p Pis p b Fig. 10.26 a,b. Proximal carpal tunnel and Guyon tunnel. a Schematic drawing and b corresponding transverse 12−5 MHz US image show the proximal level of the carpal tunnel delimited by the scaphoid (Sca) and the pisiform (Pis). The transverse carpal ligament (arrowheads) forms the roof of the carpal tunnel and the floor of the Guyon tunnel. The palmar carpal ligament (light gray) forms the volar boundary of the Guyon tunnel. US image demonstrates the tendons of the flexor digitorum superficialis (s) and profundus (p), the tendons of the flexor pollicis longus (fpl) and flexor carpi radialis (fcr) and the median nerve (straight arrow) extending through the carpal tunnel, with the nerve lying palmar-radially. At the pisiform level, the ulnar nerve (curved arrow) courses medial to the ulnar artery (a) within the Guyon tunnel 445 Wrist fcr fpl Sca s s s s p p p p Pis a b d c e Fig. 10.28 a−e. Persistent median artery of the forearm and bifid median nerve. a Schematic drawing in the axial plane and b gross anatomic coronal view of the carpal tunnel outline the course of a persistent median artery (arrowheads) interposed between the two trunks (arrows) of a bifid median nerve. c Transverse 12−5 MHz US image obtained at the middle forearm show the relationship of the persistent median artery (arrowhead) with the median nerve (arrow). Observe that the median nerve is not yet divided at mid-forearm level. Transverse d gray-scale and e color Doppler 12−5 MHz US images obtained at the proximal carpal tunnel level of the same case shown in c demonstrate the median artery (arrowhead) located between the radial and ulnar trunks (arrows) of a bifid median nerve. Note that the two nerve trunks and the artery are enveloped by a common epineurium. The patient had mild intermittent symptoms related to carpal tunnel syndrome. Sca, scaphoid; Pis, pisiform; fcr, tendons of the flexor carpi radialis; fpl, flexor pollicis longus tendon; p and s, tendons of the flexor digitorum superficialis and profundus Fig. 10.29. Persistent median artery of the forearm. Transverse gray-scale 12−5 MHz US image of the proximal carpal tunnel in an asymptomatic subject reveals a persistent median artery (arrowhead) on the ulnar side of the median nerve (arrows). Note the anechoic appearance of the artery relative to the hypoechoic nerve fascicles. In the insert at the lower right side of the figure color Doppler imaging demonstrates flow signals inside the vessel 447 448 S. Bianchi and C. Martinoli a ✟ ★ fpl s s s s p p p Tra p ∗ ✟ ★ Ham fcr fpl p Tra a s s p s s p p ∗ Ham b Fig. 10.30 a,b. Distal carpal tunnel. a Schematic drawing and b corresponding transverse 12−5 MHz US image show the distal level of the carpal tunnel delimited by the trapezium (Tra) and the hamate (Ham). The transverse carpal ligament (open arrowheads) inserts on the tubercle (star) of trapezium and the hook (asterisk) of the hamate. US image demonstrates the tendons of the flexor digitorum superficialis (s) and profundus (p), the tendons of the flexor pollicis longus (fpl) and flexor carpi radialis (white arrowhead in a, fcr in b) and the median nerve (open arrow). At the hamate level, the transverse carpal ligament is thicker than at the proximal carpal tunnel (see for comparison Fig. 10.26b) and the ulnar nerve divides into two terminal branches: a deep motor (curved arrow) and a superficial sensory (straight white arrow) branch. a, ulnar artery 1 22 3 -. flexor tendons a b Fig. 10.31a,b. Median nerve beyond the carpal tunnel. a Transverse 12−5 MHz US image obtained beyond the distal edge of the transverse carpal ligament with b gross anatomic correlation reveals the division of the main trunk of the median nerve (MN) into three branches (1, 2, 3), the common palmar digital nerves 10.4.2.4 Guyon Tunnel The Guyon tunnel is located in a medial and superficial position relative to the carpal tunnel. It is delimited by the dorsal aspect of the transverse carpal ligament and the superficial palmar carpal ligament on the radial side, and by the lateral aspect of the pisiform on the ulnar side. The transverse carpal ligament and the pisiform are easily detected with US. On the contrary, the superficial palmar carpal ligament is very thin and difficult to visualize. Once the curvilinear shape of the pisiform is found, care should be taken to identify the ulnar artery as a round, pulsatile hypoechoic structure. The ulnar nerve lies in between these two structures and can be better depicted by means of subtle tilting movements of the probe. It appears as a small structure of 2−2.5 mm in size, containing a few internal hypoechoic fascicles (Fig. 10.32a,b). The most commonly encountered anomalous muscle in the tunnel is the accessory abductor digiti minimi (Timins 1999) (see Sect. 10.5.4.4). Distal to the pisiform, the distal Guyon tunnel can be imaged with very high-resolution transducers. At this level, the ulnar nerve can be seen dividing into two terminal branches: the superficial sensory branch continues to run close to the ulnar artery, whereas the deep 450 S. Bianchi and C. Martinoli E A C D G F B a b Fig. 10.33 a,b. Schematic drawings illustrate typical sites of overuse tendinopathies in the a dorsal and b ventral wrist, including: A, de Quervain tenosynovitis; B, intersection syndrome; C, extensor pollicis longus tenosynovitis, D, extensor carpi ulnaris tenosynovitis; E, flexor carpi radialis tenosynovitis; F, flexor digitorum superficialis and flexor digitorum profundus tenosynovitis; G, flexor carpi ulnaris tendinopathy abduction of the thumb against resistance, such as occur while holding the baby‘s head (Baby Wrist) (Anderson et al. 2004). Low grade chronic microtrauma at the level of the radial styloid can lead to localized thickening of the extensor retinaculum of the wrist, narrowing of the first compartment of the extensor tendons and subsequent impingement and inflammation of the extensor pollicis brevis and abductor pollicis longus tendons. Clinically, patients complain of tenderness and pain over the radial styloid exacerbated by wide movements of the thumb and forceful pinching of objects. As already described in Sect. 10.3.1, a useful diagnostic test, the Finkelstein test, is performed by applying passive ulnar deviation of the wrist with the thumb maximally flexed, a maneuver that aggravates the patient’s pain. Treatment of de Quervain disease relies on anti-inflammatory drugs and splinting. Resistant cases are treated with more invasive approaches such as local injections and surgical release of the retinaculum. A vertical septum splitting the first compartment seems to predispose to local tendon friction and is encountered more frequently in patients than in cadaver surveys (Bahm et al. 1995). Several authors have described the US appearance of de Quervain disease (Gooding 1988; Marini et al. 1994; Nagaoka et al. 2000; Trentanni et al. 1997; Giovagnorio et al. 1997). Both longitudinal and transverse US images are performed over the radial styloid. Although lon- gitudinal planes are more valuable during dynamic scanning, transverse images give a better view of the retinaculum, internal septa and accessory tendons. The affected tendons are typically swollen and, as a whole, they have a more rounded cross-section under the retinaculum than in normal subjects (Figs. 10.34, 10.35). In acute phases, a synovial sheath effusion surrounding the tendons can be demonstrated caudal to the distal edge of the retinaculum, whereas in chronic longstanding disease the extensor tendons may appear hypoechoic or may have a heterogeneous echotexture. A thickened and hypoechoic extensor retinaculum should be accurately searched for at US because its demonstration can indicate the need for surgical decompression. Accessory vertical septa appear as thin vertical hypoechoic bands intervening between the tendons (Nagaoka et al. 2000). Demonstration of a vertical septum has clinical implications because it acts as a barrier to diffusion of injected steroids and requires opening of both tunnels at surgery (Leslie et al. 1990). In some cases, the inflammatory process may selectively involve one tendon when a septum is present (Fig. 10.36). In a postoperative setting, high-resolution US can identify complications, such as the volar subluxation of tendons due to an excessive section of the retinaculum (Fig. 10.37). In conclusion, although the clinical diagnosis of de Quervain tenosynovitis is not difficult, US can help to confirm it, detect whether a vertical septum is Wrist APL APL EPB EPB Radius Radius a b c Fig. 10.38 a−c. Wartenberg syndrome. a,b Short-axis and c long-axis 15−7 MHz US images over the radial nerve at the wrist in a patient with symptoms of superficial radial neuropathy after intravenous infusion in the cephalic vein. a Proximal to the level of injury, a normal-appearing nerve (arrow) is seen adjacent to an occluded cephalic vein (arrowhead). b,c At the level of puncture, a fusiform hypoechoic thickening of the nerve (arrow) with loss of the fascicular echotexture can be appreciated as a result of trauma. Note the position of the nerve relative to the abductor pollicis longus (APL) and extensor pollicis brevis (EPB) tendons APL Radius radialis longus and the extensor carpi radialis brevis – at the level at which they are crossed by the abductor pollicis longus and extensor pollicis brevis. This condition is usually secondary to occupational repetitive flexions and extensions of the wrist, such as occur in rowers and weightlifters. The clinical diagnosis is not straightforward because intersection syndrome may be easily confused with the more distal de Quervain disease. Wrist splints and local steroid injections are curative in most patients. Intersection syndrome appears at US as an ill-defined hypoechoic area between the two tendon groups, probably corresponding to local soft-tissue edema and tenosynovial fluid, with loss of the hyperechoic cleavage plane between them (Fig. 10.39). A true synovial bursa filled by fluid is a rare finding. 10.5.1.4 Extensor Pollicis Longus Tenosynovitis As already stated, the extensor pollicis longus tendon (third compartment of the extensor tendons) is a thin tendon that reflects over the medial aspect of the Lister tubercle before reaching the dorsum of the hand. Because of mechanical friction and its small size, the extensor pollicis longus is frequently affected by tenosynovitis that presents with local pain over the Lister tubercle and, less commonly, with local crepitus during thumb movements. This condition can be associated with previous fractures of the distal radius (Denman 1979) and leads to considerable tendon weakness, partial and complete tears if untreated. In extensor pollicis longus tenosynovitis, the synovial sheath effusion is typically found just proximal to the Lister tubercle and after the tendon has crossed the extensor carpi radialis longus (Fig. 10.40). Due to the restricted space under the fascia, the synovial sheath of this tendon may be distended with fluid in the area of the Lister tubercle and over the radial wrist extensors only when the amount of effusion is remarkable. 10.5.1.5 Extensor Carpi Ulnaris Tenosynovitis Extensor carpi ulnaris tenosynovitis is mostly secondary to instability of the retinaculum of the sixth compartment, as a result of mechanical friction of this tendon against the ulna. The patient typically complains of a localized pain over the dorsum of the ulna. Clinical findings are nonspecific and can mimic disorders of the distal radio-ulnar joint, especially when a snapping sensation is present. Although highresolution US cannot accurately recognize distal radio-ulnar joint pathology, it can readily measure the tendon size, and is able to identify intrasubstance longitudinal splits related to recurrent tendon subluxation and to evaluate tendon sheath effusion and synovial hypertrophy (Figs. 10.41, 10.42). The relevance of a dynamic examination has to be emphasized in this setting. 453 454 S. Bianchi and C. Martinoli ∗ a ∗ ∗ c ∗ Radius Radius b ∗ Fig. 10.39 a−d. Intersection syndrome. a−c Serial sequence of transverse 12−5 MHz US images obtained from a cranial to c caudal over the distal dorsal forearm demonstrate tenosynovial effusion (asterisks) in the sheath of the extensor carpi radialis longus and brevis at the level in which these tendons are crossed (arrow) by the muscle bellies of the abductor pollicis longus and extensor pollicis brevis. Note the loss of the hyperechoic fat plane intervening between these two tendon groups. See Fig. 10.15a for comparison with normal findings. d Photograph of the forearm and wrist of the same patient shows soft-tissue swelling (arrows) at the radial aspect of the distal dorsal forearm Radius Radius ∗ Radius Radius d EPL ECRB EDC Radius b A ECRB EPL EDC ECRL B Radius c C a ∗ ∗ d EPL ECRL Scaphoid Fig. 10.40 a−d. Extensor pollicis longus tenosynovitis. a Dorsal aspect of the wrist bones illustrates the course of the extensor pollicis longus tendon (arrowheads) relative to the Lister tubercle (arrow) and the typical clepsydra-like distribution of sheath fluid (asterisks) in a case of tenosynovitis. The narrow tunnel of the third compartment intrinsically hinders the sheath distension of the extensor pollicis longus at the level of the Lister tubercle except in cases of abundant effusion. Most often, the fluid distributes just proximal to the Lister tubercle and after the tendon has crossed the extensor carpi radialis longus. b−d Transverse 15−7 MHz US images over the third compartment of the extensor tendons obtained at the levels (horizontal white bars) indicated in a show the typical distribution of fluid (asterisk) in the sheath of the extensor pollicis longus tendon (EPL) relative to the Lister tubercle (arrow) and the extensor carpi radialis brevis (ECRB) and longus (ECRL). EDC, extensor digitorum tendons 456 S. Bianchi and C. Martinoli head, and may lead to stripping of the retinaculum. Tear of the retinaculum related to rheumatoid arthritis will be discussed later (see Sect. 10.5.3.2), because this finding is closely related to the involvement of the distal radio-ulnar joint and the extensor carpi ulnaris tendon sheath. Regardless of the cause of retinaculum tear, the extensor carpi ulnaris tendon undergoes anterior (volar) dislocation. The instability of the extensor carpi ulnaris may result in either subluxation, when the flattened tendon moves over the medial aspect of the ulna, or intermittent dislocation, when there may be either spontaneous phases of dislocation and reduction, or permanent dislocations. Because of its highresolution capabilities and dynamic scanning, US is the ideal imaging tool to confirm the instability of the extensor carpi ulnaris tendon (Fig. 10.43). Permanent dislocation of the extensor carpi ulnaris tendon is uncommon and can be identified by means of transverse planes obtained over the posteromedial aspect of the ulna. The diagnosis of intermittent dislocation is difficult if this possibility is not kept in mind by the examiner. To avoid 10.5.2 Ventral Wrist Pathology Similar to the dorsal wrist, tendinopathies of the flexor tendons are commonly encountered, most often at the insertion of the flexor carpi radialis tendon and within the carpal tunnel for the flexor digitorum tendons (Fig. 10.33b) (Daenen et al. 2004). In addition to tendinopathies, compression neuropathy of the median nerve at the carpal tunnel is the leading pathology of the wrist as regards prevalence of disease and clinical relevance. The entrapment of the ulnar nerve at the Guyon tunnel is rare and, in many cases, secondary to other disorders. Ulna Ulna a false negative results, care should be taken not to limit the US examination to the static assessment of the tendon. On the contrary, transverse planes obtained during progressive pronation of the forearm can disclose the progressive displacement of the extensor carpi ulnaris tendon over the ulnar head. c e A B Ulna b d Ulna f Fig. 10.43 a−f. Extensor carpi ulnaris instability. a−d Dorsal transverse 12−5 MHz US images obtained over the distal epiphysis of the ulna during progressive pronation of the forearm. a When the wrist is supinated, US shows the groove on the ulnar cortex (open arrowheads) for the extensor carpi ulnaris tendon (arrow) and an irregular appearance of the retinaculum (white arrowhead). b,c During progressive pronation, the extensor carpi ulnaris tendon (open arrow) subluxes (curved arrow) over the internal wall of the groove. Note the flattened appearance of the tendon as a result of tensile forces applied on it. d In full pronation, the extensor carpi ulnaris (arrow) dislocates out of the groove and exhibits a more rounded appearance. e,f Schematic drawings of a transverse view through the ulnar head e in the normal state and f when the retinaculum is torn. In e, the intact retinaculum (1) maintains the extensor carpi ulnaris (2) within the osteofibrous tunnel. 3, styloid process of the ulna; 4, triangular fibrocartilage. In f, the retinaculum tear leads the extensor carpi ulnaris first to sublux (A) and then to dislocate (B) out of the groove Wrist 10.5.2.1 Flexor Carpi Radialis Tenosynovitis At the proximal wrist, the flexor carpi radialis tendon is held inside a splitting of the transverse carpal ligament bounded posteriorly by the scapho-trapezium-trapezoid joint. More distally, the tendon passes below the tubercle of the trapezium to become deep and insert onto the base of the second metacarpal. Although flexor carpi radialis tenosynovitis was only recently described (Fitton et al. 1968; Parellada et al. 2006), this condition is not widely recognized. Middle-aged women are most frequently affected. They report pain over the radial aspect of the volar wrist and a local lump, often misinterpreted as a volar ganglion. As an additional finding, tingling on the skin of the thenar eminence can be observed due to the close relationship of this tendon with the palmar branch of the median nerve (Kerboull and Le viet 1995). Pathogenesis includes friction inside the carpal tunnel where the tendon curves to reach its posterior insertion and osteoarthritis of the first carpometacarpal joint and scapho-trapezium joint, which are considered the leading causes (Le Viet 1995). In this latter circumstance, tendon inflammation is secondary to the presence of volar osteophytes that cause impingement over the posterior aspect of the tendon during flexion and extension movements of the wrist. Surgery is only indicated if conservative treatment fails. In most cases, US examination is a c 10.5.2.2 Flexor Carpi Ulnaris Tendinopathy With the exception of the palmaris longus, the flexor carpi ulnaris is the only wrist tendon without a synovial sheath because of its straight course from the forearm to the distal insertion into the pisiform. The term “tendinopathy” is the most appropriate to describe this condition, because fluid cannot be demonstrated surrounding the tendon even in acute clinical settings. The most common disorder affecting the flexor carpi ulnaris tendon is calcifying tendinitis. This disorder predominantly affects young to middle-aged women, presenting with pain located just proximal to the pisiform. In general, the onset of pain is acute and physical examination shows a tender pisiform covered by inflamed warm skin. Symptoms are related to the rupture of intratendinous calcified deposits into the surrounding tissues with secondary acute inflamma- b e fcr fcr Trapezium requested to rule out a volar ganglion because of a local swelling (see paragraph Sect. 10.5.4.1). The main US signs include a swollen and irregularly hypoechoic tendon (Fig. 10.44). A synovial effusion can often be found within the tendon sheath as an expression of tenosynovitis (Fig. 10.45). In some cases, longitudinal fissures can be encountered, especially arising from the deep surface of the tendon. Scaphoid d Fig. 10.44 a−e. Flexor carpi radialis tendinopathy. a Long-axis and b short-axis 12−5 MHz US images of the flexor carpi radialis tendon (fcr) show a swollen hypoechoic tendon (arrowheads) and bony irregularities (curved arrow) at the scapho-trapezium joint level suggestive of osteoarthritis. c Transverse Gd+T1w SE and d coronal T2w tSE MR images demonstrate hypervascular synovium and mild distention of the sheath (arrowheads) of flexor carpi radialis. e Photograph of the same patient shows a localized swelling (arrow) over the involved tendon, just proximal to the scaphoid. In this case, physical examination presumed that the lump was a volar ganglion 457 458 S. Bianchi and C. Martinoli ∗ ∗ fcr fcr ∗ a ∗ fcr fcr ∗ MN ft ft fpl fpl b ft ft c Fig. 10.45 a−c. Acute tenosynovitis of the flexor carpi radialis tendon. a Short-axis and (b) long-axis 12−5 MHz US images at the wrist demonstrate abnormal distension of the sheath of flexor carpi radialis tendon (fcr) by abundant hypoechoic fluid (asterisks), whereas the tendon echotexture is normal. Note the relationship of the flexor carpi radialis with the median nerve (MN), the flexor digitorum (ft) and the flexor pollicis longus (fpl) tendons. c Photograph of the same patient shows the mass effect (arrows) of tendon sheath effusion on the radial side of the ventral wrist tion. Therapy includes anti-inflammatory drugs, ice and immobilization. In cases of severe refractory pain, a brief (1−3 days) course of intramuscular steroids can be indicated. The diagnosis of flexor carpi ulnaris tendinopathy is based on clinical and radiological findings. Standard radiographs obtained in anteroposterior and lateral views can be negative, small calcifications being easily masked by the pisiform. If th