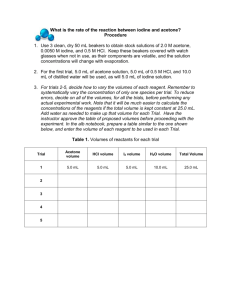
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/268278075 Reaction Kinetics of the Iodination of Acetone Conference Paper · October 2014 DOI: 10.13140/2.1.4981.7288 CITATIONS READS 0 104,732 3 authors, including: Savannah Brooke Carroll University of Tennessee 3 PUBLICATIONS 1 CITATION SEE PROFILE All content following this page was uploaded by Savannah Brooke Carroll on 15 November 2014. The user has requested enhancement of the downloaded file. Chemistry 479 Experiment 4 Reaction Kinetics of the Iodination of Acetone Savannah Carroll Lab Partner: Alexis Hughes Department of Chemistry University of Tennessee, Knoxville, TN November 5, 2014 1 Abstract Reaction kinetics for the iodination of acetone, a color changing reaction, in the presence of an acid catalyst were studied using spectrophotometer constructed in the lab. These results were equivalent to what may be recorded using a commercial spectrophotometer. The purpose of this experiment is to determine if the iodination reaction is a zero, first, or second order reaction. By systematically varying the concentrations of the reactants, the rate law is determined. The order of the reaction was also determined by creating an absorbance versus time plot with a linear fit for the rate law. Introduction The purpose of this experiment is to determine if the iodination of acetone is a first, second, or third order reaction. The time of the reaction is the time it takes for the brown color of the iodine to disappear meaning the concentration of iodine has become zero—this is measured by the spectrophotometer. The rate at which a chemical reaction occurs is dependent of the nature of the reaction, concentration of the reactants, temperature, and the presence of catalysts (in this case HCl). In this experiment the kinetics of the reaction between iodine and acetone in acid solution were studied, and the reaction proceeds as follows: (Meyer) The rate of this reaction is dependent upon concentration of the hydrogen ion and concentration of the reactants (Slowinski, Wayne, Masterton 1973). The rate law is therefore: Rate = k 2 (acetone) m (I2) n (H+) p where m, n, and p are orders of the reaction and k is the rate constant for the reaction. The rate law is useful in the description of substances that can influence the rate of the reaction. These influences can be grouped into two categories: those whose concentrations change with time, and those whose concentrations do not necessarily change with time. These influences can be changed between experiments to show how they influence the rate of a reaction (Tinoco 1978). The order of the reaction, that describes the way the rate of the reaction depends on concentration, may be determined by plotting absorbance data from each reaction versus time and utilizing the most linear function. Also from these plots, if the reaction is zero order with respect to iodine as the literature suggests, one can determine the rate constant from the negative slop of the graph. Since this experimental data recorded by the spectrophotometer is proportional to what can be recorded from the commercial instrument, these conclusions can be confirmed. The rate may also be expressed by the following equation, which says that the change in concentration of the iodine divided by the time required for that change determines the rate: Rate= -∆ (I2) / ∆t (Slowinski 1973). The spectrophotometer is able to measure this change in concentration by the color loss due to the presence then the loss of iodine. It is important to note that here, iodine is the limiting reactant in excess of acetone and HCl. Since the constant rate is being varied by the change in concentrations, we can use the rate of two reactions to solve for the order, this will be discussed in the “Results” section of this experiment. A Zero Order Reaction A zero order reaction has a constant rate, therefore the rate law is rate=k where k has units of Ms-1, and the sum of the exponents in the rate law is equal to zero. A change in 3 temperature is the only thing can affect the rate of a zero-order reaction, and if concentration from a zero order reaction is plotted against time then the result is a straight line with the slope being the negative of the rate constant, -k. This experiment utilizes the relationship between concentration of a reactant and time. The integrated rate law, in which the equation expresses the concentration of a reactant as a function of time. The integrated rate law of a zero-order reaction is given as: [A]t = -kt + [A] 0 A First Order Reaction A first-order reaction has a rate proportional to the concentration of one reactant and can be expressed as rate=k [A] with units of sec-1. The integrated form of a first order reaction can be expressed as ln [A]t = -kt + ln [A] 0 or ln [A]t [A] 0 = -kt or [A] = [A] 0 e-kt. A first order reaction can be determined by plotting a graph of ln [A] vs. time and a straight line with a slope –k should result (Slowinski 1973). A Second Order Reaction A second-order reaction has a rate proportional to the product of the concentrations of two reactants or to the square of the concentration of one reactant. For example, rate = [A] 2 or rate =k [A][B] (in other words, the sum of the exponents is two). The integrated rate law of a second order reaction is: 1 1 = + 𝑘𝑡 [𝐴] [𝐴]0 With the laboratory data, plots produced for [A] versus time, ln [A] versus time, and 1/[A] versus time, in order to find the function with the linear fit. The iodination of acetone is convenient because the color of the iodine reactant is readily observable and the change in concentration of 4 the iodine can be easily monitored by our spectrophotometer and the reaction proceeds quickly enough for multiple trials but slowly enough to obtain measurements. Also, the reaction is zeroorder with respect to the iodine so the change in concentration is linear with time. This also allows the initial rate of the reaction to be calculated as an average rate of the change in iodine concentration over relatively long periods of time. Experimental Procedure A spectrophotometer that was built in the previous laboratory was assembled with a blue diode as a light source for the initial step. LABview software was installed on a PC in order to record data continuously throughout the reaction. Next, a dilute of iodine was prepared by setting the voltmeter to read 3.5 Volts with about 4 mL of water, then Iodine was added drop wise until the voltage was about 1.0 Volts. Next, the commercial spectrophotometer was used to record a spectrum of the dilute from wavelengths 300nm to 800nm. On the commercial spectrum, markers were placed at the location of the wavelengths of the blue diode. The blue diode was the best choice because its wavelength is about 470nm, and the commercial iodine spectrum is expected to produce highest absorbance at these wavelengths. Four trials were taken to obtain data, and throughout these trials the volume of the mixture of iodine, acetone, HCl, and water was 3.5 mL for each trial and the amount of each reactant was varied. A table of the concentrations during each trial is shown below. Iodine Acetone HCl H2 0 Total Volume Trial 1 1.0 1.0 1.0 0.5 3.5 Table 1: Reactant Concentrations Trial 2 Trial 3 1.5 1.0 0.5 1.5 0.5 0.5 1.0 0.5 3.5 3.5 Trial 4 1.5 0.5 1.0 0.5 3.5 5 The reaction in this experiment can proceed very quickly. For this reason, a pipette was used to transfer each reactant into a small beaker before each trial so that they could be combined in the test tube and inserted into the spectrophotometer as quickly as possible. Multiple trials were taken for each concentration combination. The Beer-Lambert law employed in the last 𝐼 laboratory (A= -log (𝐼 )) was used a second time to express the data in terms of absorbance. 0 Results and Discussion Absorbance Figure 1: Absorbance vs Wavelength Iodine 2 1.8 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 0 Blue: 470 nm 300 400 Yellow: 595 nm 500 600 700 800 Wavelength (nm) The commercial spectrum suggests iodine will have the highest absorption at wavelengths from about 540-580 nm. This wavelength range is closest to the wavelength omitted by the yellow diode, suggesting this would have been a better choice. However, the dilution used in the commercial spectrum was modified following some test reactions. In the future, another commercial spectrum should be produced for the second dilution because the use of the blue diode gave desirable results for the purpose of this experiment. 6 As mentioned in the introduction, a reaction is a zero order if when the concentration is plotted versus the time the result is a straight line representing a constant rate. Therefore, the iodination of acetone is a zero order reaction and this is demonstrated in experimental plots for all concentration as well as the commercial spectrum. The slope of this line is the negative of the rate law constant (-k). The slope and therefore the rate law constant for each reaction as well as the commercial spectrum are shown in Table 1. Table 2 shows the orders of the reactants calculated using the equation: Rate x/Rate y = (k [acetone]a[iodine]I[HCl]h)x ÷ (k [acetone]a[iodine]I[HCl]h)y, an example is shown in Sample Calculation IV. Solving for a resulted in a value of 0.69, which rounds to one. Solving for h resulted in a value of 0.71 which also rounds to one. Adding the three exponents result in a pseudo-order of two. k (1/Ms) Trial 1 0.0011 Table 2. Rate Law Constants (k) Trial 2 Trial 3 Trial 4 0.0008 0.0017 0.0018 Average 0.00135 Data from individual plots of absorbance versus time was extrapolated to plot absorbance versus time for each reaction on one graph for the start and finish of each. The combined data is shown below, and an example plot can be found in the appendix that shows the absorbance before the start of the reaction as well as stabilization after the concentration of iodine reached zero. As can be seen in Figure 2, a linear function with a negative slope resulted from each reaction all of which varied in concentration of the reactants. Therefore, the reaction can be considered zero order with respect to iodine from this visual representation. It can also be said from this data that each reaction lasted between 100-120 seconds. Table 1 shows the rate constant from each reaction taken from the negative of the slope; these values indicate the rate is constant. Mathematically, the reaction is determined to be zero order with respect to iodine by 7 finding the rates of two reactions, the corrected concentrations of iodine, and solving for the coefficient; this is shown in Appendix A. Sample calculations. In “Reaction 2” acetone and HCl were not present in excess. For this reason, the reaction took about 180 seconds longer and values for k and the rate was not useful for this experiment. In the reactions where acetone and HCl are in excess, their concentrations remain mostly constant throughout the reaction. Figure 2: Absorbance Vs. Time 0.3 Absorbance 0.25 0.2 0.15 0.1 0.05 0 0 20 40 60 80 100 120 Time (s) Reaction 1 Reaction 2 Reaction 3 Reaction 4 Figure 3 shows the natural log of the absorbance for each of the four reaction versus time. The non-linearity of the graph further confirms that the reaction is zero order with respect to iodine. If the reaction were first order, there would be a linear trend for this plot. 8 Figure 3: ln(Absorbance) Vs. Time 6 5 ln(A) 4 3 2 1 0 0 20 40 60 80 100 Time (s) ln(A): T1 ln(A): T2 ln(A):T3 ln(A): T4 A plot of the inverse of the absorbance versus time also failed to show a linear trend. This plot, Figure 4, represents what would be a second order reaction if the trend wasn’t exponential. This graph tells us that during the timed reaction the inverse of the absorbance increases exponentially therefore the absorbance must be decreasing. Figure 4: 1/Absorbance Vs. Time 700 600 1/A 500 400 300 200 100 0 0 20 40 60 80 100 120 140 Time (s) 1/a: T1 1/A: T3 1/A: T4 Error 9 A potentially significant source of error in this experiment is room temperature. As mentioned in the introduction, temperature is known to affect the rate of the reaction. Since this experiment occurred over multiple laboratory periods, the room temperature and temperature of the solutions were likely different each time. In addition to temperature consistency, it would be beneficial to run more trials and utilize the concentration ratio that produces accurate and precise results. Other sources of error to be consider include the spectrometer’s voltage readings which may cause noise in the recorded data. Conclusion This reaction is experimentally beneficial for two reasons. First, the color of the iodine allows the concentration change to be readily followed; second, the reaction turns out to be zero order. Therefore, the rate of the reaction is independent of the iodine and the rate can be studied by making the iodine the limiting reactant in an excess of acetone and HCl. The experimental data supports these conclusions by showing a linear trend for an absorbance versus time plot as shown in Figure 2, and also by producing similar results for each reaction with varied concentrations of each reactant. The deductions from these graphical representations of the reactions can be verified by the kinetics equations. Through these methods, the overall order of the reaction was found to be zero, and the orders of acetone, HCl, and iodine were found to be one, one, and zero. Acetone and HCl may be omitted to give the rate law, rate = k [I2]I or rate = k. The average value for the rate constant which was found for each trial was found to be 0.00135 1/Ms. 10 Appendix A. Sample Calculations I) Calculation of the corrected concentration (Acetone, Trial 4 shown) M1V1=M2V2 Where M1 is the molarity of the given substance, V1 is the volume used of that substance, M2 and V2 are the total volume and molarity used 0.5 mL (V1) of a 6 M acetone solution (M1). M2 is the variable in question and V2 is 3.5 mL (total volume used) M2= [(6M)*(0.5mL)] / 3.5mL II) Calculation of The Rate (Trial 4) Using the equation: Rate= [I2]/s x 105 The concentration of Iodine in the sample (0.05 M) and the time it took for the reaction to go to completion (t = 116s) Rate= [(0.05)/(120)] = 0.00041 III) Determination of the Order Rate 4/Rate 3 = (k [acetone]a[iodine]I[HCl]h)2 ÷ (k [acetone]a[iodine]I[HCl]h)3 Where Rate 2 = 1.84729E-5 and Rate 4 = 1.23153E-5 and [Iodine]2= 0.002142857 and [Iodine]3= 0.001428571 (1.84729E-5) ÷ (1.23153E-5) = [0.0021] I / [0.0014] I 1.50= 1.4997I I= log (1.5) / log (1.4997) I=1 11 B. Experimental Data I) Corrected Concentrations of Reactants and Reaction time T1 (M1) Iodine 0.001429 Acetone 1.714286 HCl 0.285714 Time (s) 116 II) Corrected Concentrations T2 (M1) T3 (M1) T4 (M1) 0.002143 0.001429 0.002143 0.857143 2.571429 0.857143 0.142857 0.142857 0.285714 280 103 116 Rates of the Reactions Rates (M/s) Rate T1 Rate T2 Rate T3 Rate T4 Average(M/s) 1.23153E-05 7.65306E-06 1.387E-05 1.8473E-05 9.9989E-06 III) Intensity vs Time: Trial 4 Intensity Vs. Time: Trial 4 3 2.5 Intensity (V) 2 1.5 1 0.5 0 0 20 40 60 80 100 120 140 160 Time (s) IV) Absorbance vs Time: Trial 4 12 Absorbance Absorbance vs. Time: Trial 4 0.5 0.45 0.4 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 0 50 100 150 200 Time Absorbance vs Time: Trial 2 Absorbance vs Time: Trial 2 0.35 0.3 0.25 Absorbance V) 0.2 0.15 0.1 0.05 0 0 50 100 150 200 250 300 350 Time (s) 13 References I. "The Rate Law." - Chemwiki. N.p., n.d. Web. 29 Oct. 2014. II. Szwarc, M. "The Dissociation Energy of the C-N Bond in Benzylamine."Proceedings of the Royal Society of London. Series A Mathematical and Physical Sciences.Vol. 198, No. 1053 (1949): 285-92. Web. 29 Oct. 2014. III. Meyer, Earl N., and Flourence F. Lask. "Rate and Activation Energy of the Iodination of Acetone." Journal of the Chemical Society (2010): 1-5.Minnesota State University Moorhead. Web. 2 Nov. 2014. IV. Slowinski, Emil J., Wayne C. Wolsey, and William L. Masterton. "Experiment 20: Rate of Chemical Reactions." Chemical Principles in the Laboratory. Philadelphia: Saunders, 1973. 149, 150-52. Print. V. Tinoco, Ignacio, Kenneth Sauer, and James C. Wang. Physical Chemistry: Principles and Applications in Biological Sciences. Englewood Cliffs, NJ: Prentice-Hall, 1978. Print. Fifth. 14 View publication stats