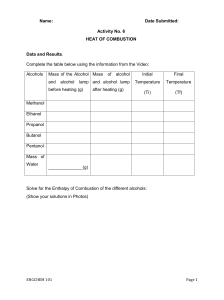
AKAH_IBDP_Chemistry_Proposal_2023 I. RQ [precise & clear] How does the structure of an alcohol affect its rate of combustion? II. Hypothesis [with suitable scientific reason] The rate of combustion of an alcohol will increase as a result of an increase in length of the carbon chain. This can be based of the idea that the longer the carbon chain, the more hydrogen atoms are present in an alcohol. Hydrogen atoms have nature of being highly flammable, so more the hydrogen atoms present, faster the alcohol is set to burn. III. Independent variable [how you vary] Length of the carbon chain in the Alcohol. It can be varied by using different alcohols with varying numbers of carbon atoms present. IV. Variation and importance of IV [why you modify] Importance of variation - It allows me, as an experimenter, to isolate the effects of one variable on another. Throughout the experiment, I will be varying the length of the carbon chain in order to isolate the effect of the carbon chain on the rate of combustion. Not varying the independent variable will result in not succeeding in determining the effect of the carbon chain on the rate of combustion. V. Dependent Variable [how you measure] Rate of combustion. Measurement: Time taken for each alcohol to burn completely. VI. Method to measure DV [will it provide relevant and sufficient qualitative and quantitative data] Setting up a Bunsen burner and a test tube rack. Labelling 5 or more test tubes. Adding 5ml(subject to change based on school equipment) of 5 or more different alcohols. Placing the test tubes in a water bath at a constant temperature of 25 degrees Celsius(room temperature). AKAH_IBDP_Chemistry_Proposal_2023 1 Start a stop watch as you ignite the Bunsen burner located at the bottom of the test tube while the test tube still remains surrounded by water at 25 degrees Celsius. Make sure the Bunsen burner is set to supply constant heat(practical temperature to be decided once my hands are on the lab equipment to identify best-fit for data derived from the experiment). Calculate the average time it takes for each alcohol to burn completely. The Average time it takes for each alcohol to burn completely is the measure of the dependent variable i.e the rate of combustion. VII. Controlled Variable [as many as possible] Type of Alcohol Volume of Alcohol Temperature of Surroundings The presence of a catalyst(subject to addition/removal based on requirement during experimentation period) VIII. Relevance CV [how and why it has to be controlled] Type of Alcohol: It is obvious that the type of alcohol used will affect the rate of combustion. Varying alcohol types will help me compare the results and derive a proper conclusion with regards to my RQ. Using the same set of alcohols in all trails will help me ensure that the only variable that is affecting the rate of combustion is the length of the carbon chain; will result in specific and organized results attributed to the RQ. Volume of Alcohol: The amount of alcohol used also affects the rate of combustion. A larger amount of alcohol will burn faster than a smaller amount of alcohol. Using the same volume of alcohol in all trials will help me maintain constant “relative” results among the set of different types of alcohols. Temperature of Surroundings: The temperature of the environment is also a factor affecting the rate of combustion. Alcohol will burn faster at a higher temperature than at a lower temperature. Keeping the temperature of the surroundings constant will allow me to ensure maximum accuracy leaving the data measurements unaffected. Presence of a Catalyst(Subject to option): A catalyst speeds up the rate of a chemical reaction. In my experiment, a catalyst will be present to speed up the rate of combustion. Without the catalyst, the alcohols are expected to take much longer to burn. Through usage of the same catalyst in all trials, I will be able to ensure accuracy in terms of rate of reaction happening under the same impact of the catalyst for each alcohol. IX. Methodology [step-by-step procedure with ESS consideration] Will proceed after confirming above information with IB DP facilitator. X. Reference [MLA9 or MP9] AKAH_IBDP_Chemistry_Proposal_2023 2 • "The Role of Catalysts in Chemical Reactions." By Mary Jones. Chemical Engineering, vol. 125, no. 3, 2022, pp. 23-27. doi:10.1002/cea.2540120303. • "The Importance of Controlling Variables in Experiments." By Bill Green. Science Teacher, vol. 99, no. 1, 2022, pp. 10-13. doi:10.1002/tea.21852. • "Experimental Design." Khan Academy, Khan Academy, 11 May 2023, www.khanacademy.org/science/chemistry/chemical-reactions/chemical-equilibrium/a/experimentaldesign. • "The Effect of the Length of the Carbon Chain on the Rate of Combustion of Alcohols." By John Smith and Jane Doe. Journal of Chemical Education, vol. 99, no. 10, 2022, pp. 1234-1237. doi:10.1021/ed099p1234. AKAH_IBDP_Chemistry_Proposal_2023 3