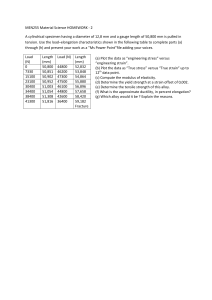
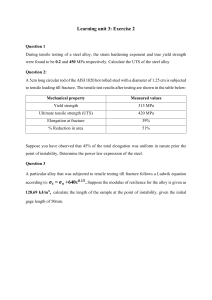
Overview of Mechanical Properties of Materials for Engineers Participant’s Workbook CONTACT INFORMATION ASME Headquarters 1-800-THE-ASME ASME Professional Development 1-800-THE-ASME Eastern Regional Office 8996 Burke Lake Road - Suite L102 Burke, VA 22015-1607 703-978-5000 800-221-5536 703-978-1157 (FAX) Midwest Regional Office 1117 S. Milwaukee Ave. Building B - Suite 13 Libertyville, IL 60048-5258 847-680-5493 800-628-6437 847-680-6012 (FAX) Northeast Regional Office 326 Clock Tower Commons Route 22 Brewster, NY 10509-9241 914-279-6200 800-628-5981 914-279-7765 (FAX) International Regional Office 1-800-THE-ASME Southern Regional Office 1950 Stemmons Freeway - Suite 5037C Dallas, TX 75207-3109 214-746-4900 800-445-2388 214-746-4902 (FAX) Western Regional Office 119-C Paul Drive San Rafael, CA 94903-2022 415-499-1148 800-624-9002 415-499-1338 (FAX) You can also find information on these courses and all of ASME, including ASME Professional Development, the Vice President of Professional Development, and other contacts at the ASME Web site... http://www.asme.org Overview of Mechanical Properties of Materials for Engineers by Professor Stephen Liu Colorado School of Mines Metallurgical and Materials Engineering Golden CO 80401 Copyright © 1999 by All Rights Reserved TABLE OF CONTENTS Part 1: PARTICIPANT NOTES……………………………………… 3 Part 2: BACKGROUND MATERIAL………………………………… 30 List of References Introduction to Metallurgical Principles Part 1 Participant Notes 3 Notes Overview of Mechanical Properties of Materials for Engineers Prepared by: Professor Stephen Liu Ph.D., CEng, MIM, FAWS Professor of Metallurgical Engineering Colorado School of Mines Golden, Colorado 80401 Notes Overview of Mechanical Properties of Materials for Engineers • Objectives and Outline – Elastic Behavior of Materials – Plastic Behavior of Materials – Impact and Fracture Toughness of Materials – Fatigue of Materials – Creep of Materials – Development of Mechanical Properties – Model Material Systems – Summary 4 Notes Elastic Behavior of Materials Chart Chartfor for Elastic Elastic Design Design •Contours •Contours show show Failure FailureStrength Strength •Selection •Selection of ofMaterials Materials for: for: ––Springs Springs ––Elastic ElasticHinges Hinges ––Pivots Pivots ––Elastic Bearings Elastic Bearings ––… … ––Yield-beforeYield-beforeBuckling BucklingDesign Design Ashby Ashbyand andJones Jones1980 1980 Notes Material Testing Smith Smith1993 1993 • Tensile Testing • Compression Testing • Fatigue Testing • Fracture Toughness Testing • Specimen Geometry • Extensometer 5 Notes Elastic Behavior of Materials • Stress-Strain Definition – Stress (Engineering Stress) S= F A e= ∆! !o – Strain (Engineering Strain) • Normal Tensile Strain • Normal Lateral Strain – Poisson’s Ratio ν =− Lateral Strain ; ν ≈ 0.33 Tensile Strain – True Stress σ= F A – True Strain dε = d! ε = Ln ! !o ! Notes Elastic Behavior of Materials • Stress-Strain Relationship Ashby Ashbyand andJones Jones1980 1980 6 Notes Elastic Behavior of Materials • Stress-Strain Relationship – Hooke’s Law • Normal Tensile σ = Eε n • Simple Compression τ = Eγ • Volumetric Shrinkage p = −κ∆ – E: Young’s Modulus – G: Shear Modulus – K: Bulk Modulus u (E ) = u (σ ) e.g . psi, ksi, Nm −2 κ ≈ E ; G ≈ 23 E Notes Problem Solving - Stress/Strain Definition • A low carbon structural steel sample of dimensions 0.5 in. x 0.5 in. x 6 in. was pulled along the long axis during tensile testing. The 2.00 in. gage marking originally notched on the specimen measured 2.5 in. apart after the test. Calculate the engineering strain and the percent elongation that the sample experienced. (Ans. ε = 0.25, % Elongation = 25) 7 Notes Problem Solving - Stress/Strain Definition • Calculate the engineering stress and strain, the true stress and stain using the tensile testing data reported in the following: – Load applied to specimen = 25,000 lb – Initial specimen diameter = 0.50 in. – Diameter of specimen under load = 0.45 in. (Ans. Eng. Stress = 127,600 psi, Eng. Strain = 0.23 True Stress = 157,000 psi, True Strain = 0.21) Notes Deformation of Materials • Stress-Strain Relationship Ashby Ashbyand andJones Jones1980 1980 8 Notes Deformation of Materials • Stress-Strain Relationship Ashby Ashbyand andJones Jones1980 1980 Notes Deformation of Materials • Stress-Strain Relationship True Stress σ= F A True Strain dε = d! ! ε = Ln ! !o Gladman Gladman1997 1997 9 Notes Plastic Behavior of Materials • Yield Strength – Lower and Upper Yield Point – Lüders Band (Hall 1970) (Hall 1970) Notes Problem Solving - Plastic Deformation • A sheet of thermomechanical controlled processing (TMCP) steel is cold rolled 20% to a thickness of 5.00 mm The sheet is then further cold rolled to 3.00 mm. What is the total percent cold deformation? (Ans. Total cold deformation = 52%) 10 Notes Problem Solving - Plastic Deformation • Calculate the percent cold reduction when an annealed eutectoid steel wire (with 0.8 wt. pct. carbon) is cold drawn from a diameter of 2.5 mm to a diameter of 1.25 mm. (Ans. Total cold reduction = 75%) Notes Problem Solving - Plastic Deformation • Calculate the percent cold reduction when an annealed eutectoid steel wire (with 0.8 wt. pct. carbon) is cold drawn from a diameter of 2.5 mm to a diameter of 1.25 mm. (Ans. Total cold reduction = 75%) 11 Notes Plastic Behavior of Materials • Dislocation-Grain Boundary Interaction • Strain Hardening τ = αGb ρ ( ) 1 2 Sub-Grain Boundary Dislocations pile-up against grain and sub-grain boundary Grain Boundary Notes Fracture Appearance • Ductile Fracture (Slow Crack Growth) – Cup and Cone Failure – Fibrous Zone – Shear Lip • Brittle Fracture (Fast Crack Growth) – Radial Zone MnS Carbides/ Silicates Shear Lip Metals MetalsHandbook HandbookV.10 V.10 Fibrous Zone Radial Zone 12 Notes Plastic Behavior of Materials • Hardness Testing – Not a Well-Defined Property • Tests use Different Combinations of the Elastic, Yielding and Work Hardening Characteristics – Relates Better to Tensile Strength than Yield Strength σ UTS ≈ 3.2 H V – Types of Measurement • Indentation - Brinell, Vickers, Rockwell • Height of rebound - Shore Notes Plastic Behavior of Materials • Hardness Testing – Measures Size of Indentation of Prescribed Geometry Under a Known Load • Brinell – – – – Indenter made of Hardened Steel Sphere 10 mm Diameter 3000 kg Load (Steel) Constant Load/(Diameter)2 Ratio Load Contact Surface Area of Indentation P HB = u (H B ) = kg f mm − 2 πD D − D2 − d 2 2 HB = ( ) 13 Notes Plastic Behavior of Materials • Hardness Testing – Measures Size of Indentation of Prescribed Geometry Under a Known Load • Vickers – – – – Diamond Square-Based Pyramid Indenter Indenter Included Angle - 136o Variable Load - 120 kg to 5 kg to grams Geometrically Similar Impressions Under Different Loads 136 2 P sin P 2 HV = HV = 1.8544 2 L2 L u (HV ) = kg f mm 2 Notes Plastic Behavior of Materials • Hardness Testing – Measures the Depth of the Indentation • Rockwell C, A, D – – – – Diamond Cone Indenter Indenter Included Agnle - 120o Pre-Load of 10 kgf Variable Load - 150 kg f (C), 60 kgf (A), 100 kgf (D) • Rockwell B, E, F, G, … Smith Smith1993 1993 – Indenters made of Hardened Steel Spheres – Variable Diameters - 1.59 mm (B, F, and G), 3.17 mm (E) – Variable Load - 100 kg f (B), 100 kg f (E), 60 kgf (F), 150 kgf (G) 14 Notes Plastic Behavior of Materials Hardness Hardness Testing Testing Hayden, Hayden,Moffatt, Moffatt, Wulff Wulff1965 1965 Notes Plastic Behavior of Materials • Hardness Testing – Measures the Height of Rebound of an Indenter • Shore – Diamond-Pointed Hammer Weighing 2.5 grams – Hammer Falls from Standard Height Down a Graduated Tube – Index of Hardness is the Height of the First Rebound 15 Effect of Deformation Rate Notes • Deformation Rate (ε" ) – Static (10-2 - 10-5 s-1) • Tensile Testing • Fracture Toughness Testing – Dynamic (100 - 10-2 s-1) • Charpy Impact Testing – Shock Loading (102 - 104 s-1) • Explosive Forming • Material Behavior at Different ε" – σ increases with increasing ε" – Toughness decreases with increasing ε" Toughness Toughnessisisaameasure measureofofthe theamount amountofofenergy energy that thataamaterial materialcan canabsorb absorbbefore beforefracturing. fracturing.ItIt isisrelated relatedtotocrack crackpropagation. propagation. Notes Charpy V-Notch Impact Testing Smith Smith1993 1993 16 Notes Charpy V-Notch Impact Testing Upper Shelf Energy Lower Shelf Energy Smith Smith1993 1993 Ductile DuctileBrittle BrittleTransition TransitionTemperature Temperature- -DBTT DBTT Fracture FractureAppearance AppearanceTransition TransitionTemperature Temperature- -FATT FATT Notes Fracture Toughness vs Strength Chart Chartfor for Elastic Elastic Design Design •Contours •Contours show show Process ProcessZone Zone(Plastic (Plastic Zone) Diameter Zone) Diameter •Selection •Selection of ofMaterials Materials for: for: –– Yield-before-Break Yield-before-Break Design Design –– Leak-before-Break Leak-before-Break Design Design Ashby Ashbyand andJones Jones1980 1980 17 Notes Fracture Toughness of Materials • Fracture Mechanics Approach – Linear Elastic Fracture Mechanics (LFEM) • Stress and Defect Dependent K = Y EG = Yσ πa • KI - Stress Intensity Factor (Mode 1) • KIC - Fracture Toughness K IC = Yσ f πa σσ--Applied AppliedNormal NormalStress Stress aa--Edge EdgeCrack CrackLength Lengthor orHalf Halfthe theLength Lengthofofan anInternal InternalThrough ThroughCrack Crack YY--Dimensionless DimensionlessGeometric GeometricConstant Constantofofthe theOrder Orderofof11 σσf --Fracture FractureStress Stress f Notes Problem Solving - LEFM • For a particular engineering application, a 2024 aluminum alloy plate must support 220 MPa in tension. Determine the largest internal flaw size that this material can support. – KIC=26.4 Mpa.m0.5 – Assume Y=1. (Ans. 2a = 9.2 mm) 18 Notes Fatigue of Materials •Repeated Loading Conditions – Cyclic Loading – S-N Curves (Fati gue Stren gth) Smith Smith1993 1993 Notes Fatigue of Materials σ + σ min •Mean Stress σ m = max 2 •Range of Stress σ r = σ max − σ min •Stress Amplitude σ a = •Stress Ratio R = σr 2 σ min σ max 19 Notes Fatigue of Materials • S-N Curves – Endurance Limit, Fatigue Strength Smith Smith1993 1993 Notes Fatigue of Materials • Micromechanism of Fatigue – Striations • Intrusion and Extrusion Metals MetalsHandbook HandbookV.10 V.10 20 Notes Fatigue of Materials Hardened Steel Connecting Rod Inclusion initiated Fatigue Fracture. Metals Handbook V. 10 Notes Fatigue of Materials Hardened Steel Valve Spring Inclusion initiated Fatigue Fracture Metals Handbook V. 10 21 Notes Fatigue of Materials • Fatigue Crack Propagation vs Stress and Crack Length da ∝ f (σ , a ) dn da = A∆K m dn log ( da = log A∆K m dn ) da = fatigue crack growth rate (mm / cycle , in / cycle ) dn ∆K = K max − K min = Stress Intensity Factor Range MPa m , ksi in A, m = Constants f (material , enviornmen t , frequency , T , R ) ( ) Notes Fatigue of Materials log ( da = log A∆K m dn ) Smith Smith1993 1993 22 Notes Fatigue/Fracture Toughness of Materials Crack CrackMonitoring MonitoringSystem System Smith Smith1993 1993 Notes Problem Solving - Fatigue • An alloy steel plate is subjected to repeated tensile and compressive loading with constant amplitude. The uniaxial fatigue cyclic stresses have magnitudes of 120 and 50 MPa, respectively. Given the static properties of the plate: yield strength of 1500 Mpa, fracture toughness K IC of 45 MPa.m0.5. If the plate contains a uniform through thickness edge crack of 1.0 mm, how many fatigue cycles are estimated to cause fracture? – Use da 3 −12 = 2.0 x10 ∆Κ dN y = 1 – Assume (Ans. Number of cycles to fracture = 2.80 x 106) 23 Notes Creep of Materials Creep CreepofofLead LeadPipes Pipes Ashby Ashbyand andJones Jones1980 1980 Notes Creep of Materials • Structures that operate at high temperature (e.g., reactors, steam plants, chemical plants, turbines) • Slow, continuous and permanent deformation with load ε = f (σ , t , T ) • Homologous temperature Th = T ; Th > 0.5Tm (K ) Tm Metals : Th > 0.3 ~ 0.4 Ceramics : Th > 0.4 ~ 0.5 24 Notes Creep of Materials • Mechanism of Creep – Dislocation Creep - Power Law Creep • At Th>0.5, atom (bulk) diffusion allowing dislocation to climb and glide away from obstacles under applied stress • At Th between 0.3 and 0.5, core diffusion predominates ε"ss = Aσ n e − Q RT – Diffusional Creep - Linear-Viscous Creep • At high Th, bulk diffusion predominates • At low Th, grain boundary diffusion and grain boundary sliding predominate Q − ε"ss = Cσ e RT d2 Notes Creep of Materials • Deformation Mechanism Maps (Summary of competitive creep mechanisms on normalized stress vs. homologous temperature space) bulk diff usion • Creep Damage Ashby Ashbyand andJones Jones1980 1980 25 Notes Creep of Materials • Creep Testing – Sample typically loaded in tension, at constant load, and at constant temperature – Steady-State Creep (Power Law Creep) – Creep-Rupture Diagram Ashby Ashbyand andJones Jones1980 1980 Notes Creep of Materials Cast CastNi-Alloy Ni-Alloy Turbine TurbineBlade Blade Creep CreepFailure Failure Metals Handbook V. 10 26 Notes Creep of Materials Cast CastNi-Alloy Ni-Alloy Turbine TurbineBlade Blade Stretching Stretchingand and Necking Neckingdue due to toCreep Creep Metals Handbook V. 10 Notes Problem Solving - Creep • Determine the time to stress rupture at 850oC for equiaxed MAR-M 247 alloy. The component is loaded to 207 MPa. (Ans. 16,627 hours) 27 Notes Mechanical Behavior of Materials • Development of Mechanical Properties – Solid-Solution Strengthening – Grain Refinement – Precipitation Strengthening – Microstructural Modification Notes Development of Mechanical Properties • Solid Solution Strengthening (Takeuchi 1969) ∆H El , Misfit = σ p 4πro2 (r − ro ) ∆H El , Misfit = 4 µbedgeη a ro3 sin θ r El , Misfit ∆H screw =0 Elastic Distortion = ∆a ⋅100 a NNa - -size misfit parameter a size misfit parameter bbedge - -edge component of a dislocation edge edge component of a dislocation σσp - -hydrostatic hydrostaticcomponent componentofofaastress stressfield field p 28 Notes Development of Mechanical Properties • Grain Refinement - Hall-Petch Relationship (Petch 1959) Hall-Petch Equation: σ y = σ i + κ yd − 12 Combined Solute and Grain Size Strengthening: σ y = σ i + ∑ κ iCi + κ y d − 12 i Stress σσi - -Friction i Friction Stress Coefficient κκy - -Strengthening y Strengthening Coefficient dd- -Grain GrainSize Size CCi - -Concentration ConcentrationofofSpecies Speciesi i i Notes Development of Mechanical Properties • Precipitation Strengthening Orowan Orowan Equation Equation ∆τ y = Gb L ∆τ c = Af 2 r 2 1 1 ∆τ ! = B ⋅ Gbf 2 r −1 1 (Takaheshi and Nagumo 1970) LL--Particle ParticleInterspacing Interspacing f f--Volume VolumeFraction FractionofofPrecipitates Precipitates rr--Mean Particle Mean ParticleSize Size 29 Part 2 Background Material 30 Background Materials As indicated in the preface, this 4-hour module has the objective of presenting introductory concepts in mechanical properties of materials to nonmetallurgical/materials engineers. Therefore, the content was selected and prepared based on the author’s own experience in the field of metallurgical engineering. The references consulted were basic, elementary texts typically used in undergraduate metallurgical engineering education. In fact, any solid textbook on mechanical metallurgy should contain the information presented in this module. The list below is, by no means, exhaustive and only represents some good titles that readers can refer to and further learn about mechanical properties of materials. 1. Engineering Materials 1 – Michael Ashby and David Jones, Pergamon Press, 1980. 2. Materials Selection in Mechanical Design – Michael Ashby, Butterworth Heinemann, 1992. 3. Materials Considerations in Design – Arnold Ruskin, Prentice Hall, 1967. 4. Foundations of Materials Science and Engineering – William Smith, McGraw Hill, 1993. 5. The Science and Design of Engineering Materials – James Schaffer, Ashok Saxena, Stephen Antolovich, Thomas Sanders, Jr., and Steven Warner, WCB-McGraw Hill, 1999. 6. The Structure and Properties of Materials – Vol. III – H. Hayden, W. Moffatt, and J. Wulff, John Wiley & Sons, 1965. 7. Deformation and Fracture Mechanics of Engineering Materials - Richard Hertzberg, John Wiley & Sons, 1976. 8. The Plastic Deformation of Metals – Robert Honeycombe, Edward Arnold, 1968. 9. Mechanical Metallurgy – George Dieter, McGraw Hill, 1986. 10. Mechanical Behavior of Materials - Thomas Courtney, McGraw Hill, 1990. 11. Materials for Engineering - John Martin, The Institute of Materials, 1996. 12. The Testing of Engineering Materials – Harmer Davis, George Troxell, and George Hauck, McGraw Hill, 1982. 13. Ensaios Mecânicos de Materiais Metálicos (Mechanical Testing of Metallic Materials) – Sergio A. de Souza, Edgard Blücher, 1974. 14. Metals Handbook – Vol. 8 - Metallography, Structures and Phase Diagrams – ASM Metals Handbook Series, 1973. 15. Metals Handbook – Vol. 10 – Failure Analysis and Prevention – ASM Metals Handbook Series, 1975. 16. Metals Handbook – Desk Edition, ASM International, 1984. 17. Damage Tolerance Design Handbook – MCIC-HB-01, Battelle Columbus Labs, 1975. 18. Advanced Materials and Processes – ASM Journal, 1990. Chapter III - Introduction to Metallurgical Principles STRUCTURE OF METALS Metals are crystalline solids whose atoms are arranged in regular patterns. Using highly sophisticated tools such as the transmission electron microscope and field-ion microscope, the individual atoms could be observed to form long range geometric patterns. This orderly atom arrangement is responsible for the crystalline structure of a metal and its many properties (physical, chemical and mechanical). The three most common crystalline structures found in metals are body-centered cubic, face-centered cubic and hexagonal close-packed. Their atom arrangements are shown schematically in Fig. 3-1. The black dots represent the location of individual atoms. The body-centered cubic (bcc) structure exhibits one atom at each corner of a cube and one in the center. Notice that the bcc structure is not close-packed. In fact, bcc crystals are only 68% packed. Thus, the empty space in a bcc crystal can accommodate atoms of other elements displaying a certain degree of solubility. The face-centered cubic (fcc) structure has an atom at each corner of the cube and one in the middle of each face. By comparison, this crystal structure is more closely-packed than bcc. Hexagonal close-packed structure exhibits a slightly different atomic arrangement than the fcc structure, with the atoms located along horizontal planes known as the basal planes. Figure 3-1. The three most common crystal structures in metals. left – bcc, center – bcc, and right - hcp. Different elements crystallize in different crystal structures. For example, iron crystallizes as bcc at room temperature, copper and aluminum crystallizes as fcc and magnesium as hcp. Table 3-1 provides additional information regarding crystal structure of different metals. Table 3-1. Crystal structures of common metals. Structure Body-centered cubic (bcc) Face-centered cubic (fcc) Metals with this structure Fe (at room temperature, α-ferrite, and near its melting point, δ -ferrite), Cr, Si, Nb, Mo, V, W Fe (at intermediate high temperature, γ -austenite), Ni, Cu, Al, Ag, Pb Hexagonal close-packed (hcp) Mg, Sn, Zn, FORMATION OF CRYSTALLINE SOLIDS Crystalline solids are usually produced by freezing or solidifying a liquid metal. In the liquid state, the molten metal loses its long-range ordering of atoms. When a melt starts to cool to its solidification temperature, atoms aggregate to form submicroscopic particles with specific atomic ordering, characteristic of the particular metal, called nuclei. These particles form at preferred sites such as the mold surface, second-phase particles, etc. As temperature continues to drop, more atoms will attach on these nuclei, promoting growth of the solid phase and at the expense of the liquid phase until the completion of solidification. Since each of the nuclei possesses a definite crystal orientation and atomic spacing, these will grow into larger solid particles with independent crystallography that are called grains. These grains meet along grain boundaries which are typically regions where the periodic and orderly arrangement of atoms is disrupted. Because of this atom disorder at grain boundaries, there are often differences in metal behavior at these locations. Most common engineering metals are alloys consisting of one major element and variable amounts of one or more alloying elements and whose properties are usually different from those of the pure metal. Alloys provide, in general, combinations of engineering properties that are superior, for specific applications, to those of the unalloyed metals. The crystal structure, the purity and the prior thermal and mechanical history of an alloy all have significant influence on the engineering properties of an alloy. In the making of an alloy, some of the parent metal atoms are replaced by new atoms, which take the positions previously occupied by the parent metal atoms. The replacement has a direct bearing on the properties of the alloy. The basic ways in which the new atoms are incorporated in a metal are listed below and illustrated in Figure 3-2: 1. Direct Substitution. If the new atom is similar in size and chemical behavior to those in the original unalloyed metal, it may directly replace one of the original lattice atoms. Thus, the new atoms are “dissolved” in the parent metal to form a solid solution. Examples of solid solutions are gold dissolved in silver, or copper in nickel. 2. Interstitial Solid Solution. When the new atom is small in relation to the parent atom, it can dissolve in the original structure, in the spaces between the parent metal atoms, without actually displacing any of them. In this case, an interstitial solid solution is formed. Small amounts of carbon and nitrogen, for example, can occur interstitially in iron. Figure 3-2. Schematic illustration of (a) substitutional and (b) interstitial solid solution. More often, the new atoms cannot completely dissolve in the parent metal, either interstitially or substitutionally. When the parent and alloying metal exhibit strong chemical affinity, the mutual solubility is generally lowered with increased probability of a chemical reaction. These circumstances usually result in the formation of intermetallic compounds of definite (or range of) chemical composition and crystal structure. Thus, it is entirely conceivable that the solid solutions coexist with the intermetallic phase resulting in a multiphase alloy. The individual phases may be seen and distinguished if the alloy is properly polished and etched and then examined under a microscope at magnifications from 50 to 2000 times. This procedure is known as metallography and results of metallographic preparations are microstructures such as those illustrated in Figs. 3-3. The microstructure of alloys usually consists of many randomly oriented grains of the different phases present. The overall arrangement of grains, boundaries and phases present in a metallic alloy is called its microstructure and is largely responsible for the properties of the alloy. The microstructure is affected by the composition or alloy content, and by other factors such as heating and cooling associated with forming and heat treating operations. The microstructure is greatly affected by the welding operation, which, in turn, influences the properties of the alloy. Figure 3-3. Typical microstructure of: (a) low-carbon pearlitic steel at 100X – white phase is ferrite and dark phase is pearlite; (b) commercial nickel-base superalloy, Udimet 700 at 1000X – multiphases present. PHYSICAL AND CHEMICAL PROPERTIES Metals exhibit certain properties that make them useful as engineering materials and allow them to be joined by welding processes. Some of these properties are electrical conductivity, thermal conductivity, ionization potential, work function, and ductility. Electrical conductivity and thermal conductivity of metals generally parallel each other because both involve electron in the transport of energy. Copper and aluminum are good examples of metals with high electrical and thermal conductivity. For the same reasons, they are often used as conductors and electrical wiring. While materials with high electrical conductivity are good candidates for electrical welding, high thermal conductivity may actually jeopardize the welding process because of the high rate of heat extraction from the weld region. Table 3-2 illustrates some of these characteristics. Table 3-2. Selected physical properties of metals and their weldability. Copper Thermal Conductivity W.m-1.oC-1 394 Electrical Conductivity (x108) Ω .m 1.72 Aluminum 222 2.83 Require high welding current Carbon Steel 46 16.0 Readily weldable Metal Welding Behavior Difficult to weld Arc welding stability depends on the number of charge carriers (e- and positive ions, mainly) in the plasma. Low ionization potential means easier removal of an electron from an atom. Elements such as potassium and sodium have low ionization potential, 4.3 and 5.12 eV, respectively, and are often added to electrode coatings to stabilize the welding arc. Aside from physical properties, the chemical reactivity of metals is also important. MECHANICAL AND FRACTURE PROPERTIES At grain boundaries, the arrangement of atoms is irregular and there are many holes (vacancies) or missing atoms (see again Fig. lid). The atom spacing may be larger than normal with the result that individual atoms can move about relatively easily in the grain boundaries. Because of this, the diffusion of elements— i.e., the movement of individual atoms through the solid structure— generally occurs more rapidly at grain boundaries than within the grains. Because of the disarray, it is easier for oddsized atoms to segregate at the boundaries. This segregation frequently leads to the formation of undesirable phases that adversely affect the properties of a material by reducing its ductility or making it susceptible to cracking when welded. Many unique things that profoundly affect the properties of an alloy occur at grain boundaries. Grain boundaries, for example, increase the strength of materials at room temperature by inhibiting the deformation of individual grains when the material is stressed. At elevated Temperatures, the atoms in the boundaries can move easily and slide past one another, thus reducing the material’s strength. As a result, fine-grained materials have better properties for room temperature service while coarse-grained materials are desirable for high temperature service. The structure of a metal could be characterized as having either few large grains (coarse-grained), or many small grains (fine-grained) or a mixture of both large and small grains (mixed grain size). The usefulness of a material or alloy is measured and described by its properties. Some of the more important physical and mechanical properties and tests used to determine them are listed below. HARDNESS Probably the most commonly and easily measured material property is hardness. The hardness of a material is basically its resistance to indention and has come to represent, in practice, an indication of a material’s strength. Most common hardness tests consist of using a fixed applied load to force an indentor into the material being tested. Typical hardness indentations are shown schematically in Fig. 16. Indentors are usually of a very hard material— in many cases diamond— and come in a variety of shapes. Typical shapes of indenters used for the various tests are also shown in Fig. 16. The applied load that produces the indention ranges from a gram to 3000 Kg. The factor indicative of the hardness level is either the depth of the penetration or the size of the impression. Thus the diagonal of an impression made with a pyramid or the diameter of an impression made with a sphere can be measured. Such a measurement is then converted into a hardness number by means of appropriate tables. The hardness number is always directly proportional to the ability of a material to resist indention. The specific hardness test used depends on the purpose for which the test is made as well as the size of the test material. Heavy applied loads make large, deep impressions while small light loads make small, shallow impressions, some so small that they can only be seen with a microscope. In order to measure the hardness of thin sheet material, a light load is used. To measure the hardness of a larger, but still restricted region such as a weld heat-affected zone, an intermediate load of perhaps 50 to 300 grams would be used. However, if the over-all hardness of a material is to be determined, a high load, such as the 3000 Kg Brinell test, would be used. The large impression produced by the high load and large indenter will not be influenced significantly by very local structure sensitive hardness fluctuations since they are averaged out. In such cases, more accurate average hardness measurement will be obtained. As noted above, the hardness of a material is roughly proportional to its strength. However, the strength of a material must be determined independently and then related to its hardness. Once this relationship is known, the hardness test, because of its simplicity, becomes a valuable tool. STRENGTH The strength of a material is the measure of its ability to sustain an applied load without failing, or deforming significantly. The greater a load the material can carry, the greater its strength. So that strength measurements can be universally used, they are described as pounds of load per square inch of cross-sectional area (psi), i.e., stress. The most frequently determined strength criterion is tensile strength even though it is often desirable to measure other kinds such as compressive or shear strengths. In a tensile test, a specimen of some fixed geometry is used. A typical test specimen is shown in Fig. ha. The sample is loaded (stressed) in the axial direction by means of an appropriate machine, usually hydraulic: a typical apparatus is shown in Fig. 17b. Initially, for every increment of applied load the bar elongates a proportional amount. This is represented in Fig. 17c by the line between points A and B. Material behavior of this type is called elastic. That is, the material is stretched somewhat like a rubber band. As the load is applied, the material stretches, but when the load is released, the sample returns to its original size. Thus in the elastic region, there is no permanent change in the size of the sample due to any applied load. During elastic extension, the spacing between individual atoms increases slightly in the tensile direction but there is no relative motion between the atoms which would cause atoms to slide past each other any great distance so that, as the load is removed these atoms move back to their original positions. In the elastic region, the proportionality between the applied stress and the resultant elongation, or strain, is called Young’s Modulus (E), When the load on the test sample is increased beyond that of point B in Fig. 17c, the proportionality between stress and strain no longer exists; that is, each increment of load produces a greater increment of strain than it did in the elastic region. Thus point B is called the elastic limit of the material, since beyond this point the material begins to behave plastically. Plastic deformation is permanent. When the load is removed from the sample, it does not return to its original size. On the atomic scale, when the elastic limit has been exceeded, the atoms move within the material and do not return to their original locations when the load is removed. From a practical standpoint, the elastic limit is a difficult point to determine with any precision. For most engineering work, a more practical measurement of the upper limit of elastic behavior is the yield strength. The yield strength is the stress required to produce a small, fixed arbitrarily chosen amount of permanent strain if the load were removed at that point. The most common amounts of permanent strain used to designate yield strengths are 0.02% and 0.2%. The 0.02% yield strength is illustrated as point C in Fig. 1.7c. It should also be noted that for some materials the yield strength is characterized by a particular kind of yielding rather than by an arbitrarily defined amount of permanent strain. In soft, low-carbon steel when the elastic limit is exceeded, an abrupt and substantial amount of strain or elongation occurs. This form of abrupt yielding is shown schematically in Fig. I 7d also. This kind of behavior is often described as a yield point. As the load is further increased beyond the yield point or yield strength, greater amounts of plastic strain occur. Point D on Fig. 1 7c indicates the maximum load that the sample will support. The stress associated with this load is the ultimate tensile strength. While the load increases up to this point the test sample not only gets longer, but also undergoes uniform reduction in diameter. At point D an instability in the sample occurs and the gage diameter starts to decrease more rapidly in one region than do other parts of the specimen. This behavior is called necking. Since the area of the sample decreases rapidly, the load required to continue elongating the sample also reduces until finally the sample breaks at point F. If the fracture stress, which is the fracture strength (or load) divided by the cross-sectional area of the sample at the fracture location, were determined, it would be greater than the ultimate tensile stress. As an engineering value, the ultimate tensile strength is considerably more useful than the fracture stress. The tensile test determines the strength of a material and its ductility. The ductility of a material is basically its ability to deform plastically without failing. Two measures of ductility are obtained from the tensile tests: the percentage of elongation and the percentage of area reduction. The manner in which the properties are determined is shown by the equation below. % Elongation = Final length— Original length Original length — X 100 (12) Original area— Final area >< 100 % Reduction in area =—— (13) Original area While yield strength and tensile strength measurements are used in designing, the ductility of a material is not. Nevertheless, ductility is an important property and generally the more ductile a metal is, the better it is. Minimum levels of ductility are customarily determined and specified by experience and empirical rules. The tensile strength of a material is most frequently determined at room temperature, but it can be determined at any temperature. In general, as the test temperature increases, the tensile and yield strength decreases while the ductility increases. The opposite of ductility is brittleness, a familiar but often confusing term. Brittle behavior is fracture without much prior ductility or deformation. Copper is ductile because it deforms a great deal before it breaks; glass is brittle because it breaks almost immediately without deforming. Both are strong materials. Although related, ductility is not a direct measure of strength, but to be useful a material must normally be both strong and ductile. A strong and ductile metal is often said to be a tough material. Fatigue Strength If a stress less than a materials tensile strength is applied, it will nor break; if the same load is removed and reapplied several times it may eventually break. Repeated bending of a paper clip to the point of failure is an example of a type of fatigue. The strength of a material to withstand repeated load applications is called fatigue strength. The fatigue strength is usually related to the number of cycles required to reach the point of failure. Fatigue strength is commonly determined by applying a stress first in tension and then at the same level in compression. That is the maximum limiting stress that is cycled above and below the zero stress level in Fig. 18. The closer the maximum stress to the tensile strength, the fewer the load cycle applications required before fracture occurs. As the stress level is reduced, a greater number of cycles are experienced before fracture occurs. In many materials the point at which the material will fracture is never reached, no matter how many cycles the load is applied. The stress associated with this point is called the endurance limit. The typical fatigue behavior of a material is shown in Fig. 18. The endurance strength of a material is frequently equal to roughly half of its tensile strength. Creep Strength If a load below a material’s tensile strength is applied at room temperature, the material initially elongates as the load is applied; sustaining the same load, however, causes no further measurable elongation. Yet if the same load were to be applied at an elevated temperature, the material would continue to gradually elongate as long as the load was maintained. This behavior is called creep. Maintaining the load long enough would eventually cause the material to rupture. Two factors other than stress are important in describing a material’s creep or rupture strength: time and temperature. The higher the temperature, the shorter the time needed to produce a given amount of creep or to produce rupture failure for a given applied load. At a given temperature, a higher load causes creep and rupture to occur sooner. Thus creep strength is designated as the stress required to produce a given amount of creep strain in a given period of time at a given temperature, e.g., 0.19~ creep strain in 100,000 hours at 10000F. Rupture strength is the stress to produce failure after a given period of time and at a given temperature. The general relationships between stress, time and temperature in the case of rupture are shown in Fig. 19. Similar curves could be drawn for creep deformation. FRACTURE TOUGHNESS A material with normal strength and ductility can behave quite differently if loaded under certain conditions, such as in a notched condition, at a low temperature or very abruptly. A combination of the above factors can frequently cause a metal to fail in a brittle manner at an effective strength much lower than its normal value. Such behavior is called notch sensitivity or brittle failure. It is, in a sense, the opposite of notch toughness. Notch tough materials are those that are relatively insensitive to notches and cold or impact loading. They usually fail in a reasonably ductile way in spite of the above-mentioned “embrittling” test conditions. A common test to measure fracture toughness is the Charpy Notched Bar Impact Test. The specimen used is shown in Fig. 20. In this test, the energy required to break the test bar over a range of temperatures determines the notched fracture roughness. In the test a pendulum hammer strikes the bar from a known height. As shown in Fig. 20, it is normal for the energy absorption to be lower at lower temperatures. In testing a material over a range of temperatures, the failure mode sometimes shifts rather abruptly from tough at high temperatures to brittle at low temperatures; the temperature at which it occurs is called the transition temperature. Toughness or brittleness is important characteristics in welded structures. Many factors affect fracture toughness, and not all materials are equally tough. Tougher materials have low transition temperatures and their fracture, at all temperatures, usually requires greater energy. TYPICAL PHASE TRANSFORMATIONS IN METAL SYSTEMS PHASE TRANSFORMATIONS (CRITICAL POINTS) Differences in temperature cause the atoms of many metals to vary in their crystallographic arrangements. For example the crystalline structure of iron at temperatures up to 16700F is body centered cubic; from 16700F to 25350F it is face centered cubic; and from 25350F to the melting temperature, 27950F, it is again body centered cubic. This change in crystalline structure is formally called an allotropic transformation or a phase transformation. Among other metals undergoing allotropic transformations at various temperatures are titanium, zirconium and cobalt. Many factors, including chemical composition, cooling rate and the presence of stress, influence this type of transformation. Another kind of transformation or change occurs when a metal melts or solidifies. When a metal melts, its orderly crystalline arrangement of atoms becomes a completely disorganized noncrystalline array. The changes in the crystalline structure of metals— from one solid arrangement to another, or from solid to liquid— are sometimes grouped together under the heading of phase changes. Pure metals solidify (and melt) at a single temperature. Most alloys solidify (and melt) over a range of temperatures. The exceptions to this will be mentioned later. Phase Diagrams Events such as phase changes and solidification are best shown by means of a drawing called a phase diagram (sometimes also referred to as equilibrium diagram or constitution diagram). A phase diagram has been called the metallurgist’s road map— from a diagram for a given alloy system, he can find for any given alloy composition at any specified temperature what phases and what percentages of each are present. He can also determine what phase changes tend to take place with either a change in composition, a change in temperature or both. It has at least one significant limitation. It is only an approximation of how alloys actually behave, however; for phase diagrams describe alloy system behavior under equilibrium conditions rarely encountered in practice. This is especially true under conditions found in welding because of the fast heating and cooling rates. (Equilibrium implies that a metal is stable in a desired state for a given environment— that is, extremely slow heating and cooling conditions and long hold times.) Most readily available phase diagrams describe alloy systems containing two elements (or components), while engineering alloys generally contain many elements. Phase diagrams for more than two element systems are quite complex and difficult to interpret. However, they are still the best way to study most alloy characteristics. The phase diagram shown in Fig. 14 is for the Cu-Ni alloy system. This is called an isomorphous binary system— i.e., a twoelement system in which both elements are completely soluble in each other in both the liquid and solid states. As shown in Fig. 14, the phase diagram is drawn with the alloy content plotted on the horizontal axis. The extreme left edge represents 100% of one element (Cu in Fig. 14), and the extreme right edge is 100% of the other element (Ni in Fig. 14). Temperature is plotted on the vertical axis. Figure 14 shows that at temperatures above the line labeled “liquidus,’ the only phase is liquid for all compositions. At temperatures below the line labeled solidus” the only phase is solid. Furthermore, all solid alloys formed are single phase because copper and nickel are completely soluble in each other in the solid state. Thus an alloy with 30% Cu and 70% Ni is a homogeneous solid solution, remaining solid up to 2425”F and melting completely at 24900F. The combination is the alloy Monel. In the region between the solidus and liquidus lines both solid and liquid phases coexist simultaneously. This illustrates the fact that most alloys solidify, or go from complete liquid to complete solid, over a range in temperature. As the diagram shows, only for pure Cu or pure Ni does complete solidification occur at a single temperature. Figure 13 shows a more complicated phase diagram for the silver-copper alloy system. The diagram is used extensively in the designing of brazing alloys. At temperatures above the line labeled “liquidus,” all alloys are entirely liquid. At temperatures below the line labeled ‘solidus” and within the regions labeled a and /3, the material present is all solid, but in two phases. The a region defines the temperature-alloy conditions where Cu is soluble in Ag and the phase is a solid solution. The same is true for the /3 region where Ag is in solid solution in Cu. In the regions between the solidus and liquidus lines on the left side of the diagram, the phases present are the liquid solution of Cu in Ag and solid solutions of Cu in Ag (a). For the same region on the right-hand side the phases present are the liquid and solid solutions of Ag in Cu (/3). Finally the area labeled (a + /3) is a mechanical mixture containing grains of both a and /3. The Ag-Cu alloy system is one in which each element has only a limited solubility in the other. The phase diagram depicts this and shows that as Cu is added to Ag it first dissolves, but as more is added so that the a phase boundary is exceeded, the alloy then contains the second solid phase /3, which is basically Cu with some Ag dissolved in it. The phase diagram illustrates another feature, i.e., an eutectic point. This is labeled on the diagram and represents an alloy composition which, like a pure metal, solidifies at one temperature. The eutectic composition solidifies somewhat uniquely: as the liquid cools, both the a and /3 phases are formed concurrently. Quite frequently they occur as alternating platelets and hence have a distinctive appearance. A final feature of the Cu-Ag phase diagram is that it shows solid solubility in relation to temperature. In principle, this is similar to the fact that more salt can be dissolved in hot water than in cold. In Fig. 15, the boundary line between the a ± /3 region and the a solid solution region shifts to lower alloy content as the temperature decreases. This means that if a given alloy containing copper is dissolved in silver with the copper content such that the alloy falls just within the a solid solution region at a high temperature, the solubility limit will be exceeded at lower temperatures and some /3 phase must be formed. Control of this behavior is important in all precipitation-hardened alloys (see Chapter 4) - FUNDAMENTALS OF WELD SOLIDIFICATION Figure 11 shows this in a mold (and a weld). Because of this, a fusion weld can be viewed as a tiny casting. It should be pointed out that the thermal conditions that exist during fusion welding produce cast structures with characteristics unique to welding; these are complex and to date not thoroughly understood. Therefore, they will not be discussed at length in this elementary text. SOLID-STATE TRANSFORMATIONS IN WELDMENTS EFFECT OF ALLOYING ELEMENTS CRACKING PHENOMENA ASSOCIATED WITH WELDING CORROSION OF WELDMENTS OXIDATION RESISTANCE When most metals are exposed to the air at elevated temperatures, there is a tendency for the oxygen in the air to unite with the metal and form an oxide. The ability of a material to resist oxide formation or to resist continuing and rapid oxidation is called its oxidation resistance. Metals such as gold, silver and platinum are very resistant to oxidation. Iron and copper oxidize rapidly. This is generally undesirable, particularly in the case of iron and steels, since many oxides, once formed, do not adhere to the metal. If exposure is continuous, the material gradually deteriorates. Metals such as aluminum and chromium also form oxides readily when exposed to air. However, in the case of these metals, the oxide is very tightly adherent to the metal and effectively seals it and prevents further oxidation. The chromium present in stainless steels serves this same function. Oxidation resistance decreases as the exposure temperature increases. CORROSION RESISTANCE Corrosion resistance of a material is broader property than oxidation resistance since it takes in the resistance of a metal to any kind of chemical or electrochemical attack, including oxidation. The presence of water or water solutions generally increases corrosive action. Corrosion resistance is enhanced by the formation of tight adherent oxide coatings as in the case of oxidation. PROPERTY CHANGES RELATED TO STRUCTURE CHANGES The particular microstructure of a metal determines its properties. The state of microstructure is determined by, among other things, heat treatment, alloy composition and fabrication history. Of particular interest is the fact that welding is an important determining factor in the nature of a metal’s microstructure. SUMMARY The crystalline nature of a metal— pure or alloyed— is reflected in its microstructure, the over-all arrangement of grains, boundaries and phases that determines its properties. The crystallographic arrangement of a given material is influenced by, among other things, heat treatment, alloy composition and fabrication history including, of course, welding. Certain definable, measurable mechanical and physical properties determine the usefulness of a metal or alloy. Hardness, oxidation resistance and corrosion resistance are self-explanatory. Hardness is roughly proportional to strength. Tensile strength, or the resistance of a material to a load in tension, is perhaps the most frequently used measurement of strength. The strength of a material cannot be completely assessed, however, without relating to ductility, the capacity of a material to deform plastically without breaking. Young’s Modulus (B — _stress strain) sets forth the relationship— in the elastic region— between the applied stress and the resultant elongation, or strain. Because the elastic limit, beyond which point plastic deformation is permanent, is difficult to determine precisely, engineers usually rely on a more practical measurement of the upper limit of elastic behavior: yield strength. To measure yield strength, a load is applied to the material being tested, and removed when an arbitrarily chosen amount of permanent strain, commonly 0.02% or 0.2% is produced. A test load not exceeding a material’s fracture strength may be reapplied in order to determine fatigue strength; reapplied at elevated temperatures, such a test load can be used to determine the amount of creep that will produce rupture failure. Another important property, fracture toughness, is the ability of a material to exhibit strength and ductility if loaded in a notched condition, at low temperature or very abruptly. Metals’ behaviors under various types of stress can be recorded on graphs. Phase diagrams are used to show phase changes and the solidification products characteristic of individual alloy systems, given certain conditions of composition and temperature. QUESTIONS ON METALLURGY 1. 1-low does the atomic arrangement of a metal differ in its solid and liquid states? Describe how a molten metal gains its crystalline structure, and explain where and why there may be differences in the solidified metal’s behavior. 3. Define ‘alloy” and state the basic ways in which such a metal can be created. 4. What constitutes an alloys microstructure? 5. Discuss the properties of alloys exhibiting the variations of grain size, mentioning in each case the behavior of the atoms at grain boundaries. 6. Name two types of temperature-induced transformations and tell how each affects the structure of the metal involved. 7. What is a phase diagram? What are its uses and limitations? 8. What relationship does a metal’s hardness have to its strength? How is hardness measured? 2 I-low does tensile strength differ from yield strength, fatigue strength and creep strength? In what ways are these properties interrelated? 10. Define ductility. Define brittleness. 11. What is fracture toughness and how is it measured? 12. Under what circumstances can oxidation be an important factor in corrosion resistance? 2. SUGGESTED READING A If/S Welding Handbook, Section 4, Fifth Edition, ‘Metals and Their Weldability” (1966) AWS Welding Handbook, Section 1, “Fundamentals of Welding,’Sixth Edition (1968), Fifth Edition (1962) Welding Metallurgy, Volume 1, G. F. Linnert, AWS (1965) Metals and How to Weld Them, Second Edition, T. B- Jefferson, G. Woods, J. F. Lincoln Arc Welding Foundation, Cleveland, Ohio (1962) Elements of Physical Metallurgy, A. S. Guy, Addison-Wesley Publishing Co, Inc., Reading, Mass. (1959) Physical Metallurgy, B. Chalmers, John Wiley & Sons, Inc., New York (1959) Welding for Engineers, H. Udin, F. Funk, J. Wulif, John Wiley & Sons, Inc., New York (1954) Structure and Properties of Alloys, R. M. Brick, A- Phillips, McGraw-Hill Book Company, Inc., New York (1949) YOUR PATH TO LIFELONG LEARNING ASME offers you exciting, rewarding ways to sharpen your technical skills, enhance personal development and prepare for advancement. *Short Courses - More than 200 short courses offered each year keep you up to speed in the technology fast lane--or, help you fill in any gaps in your technical background. *Customized Training at your organization’s site - Do you have ten or more people at your site who could benefit from an ASME course? Most of our courses can be offered in-house and tailored to your latest engineering project. Bring a course to your company too. *Self-study materials meet the needs of individuals who demand substantive, practical information, yet require flexibility, quality and convenience. Return to each program again and again, as a refresher or as an invaluable addition to your reference library. *F.E. Exam Review - A panel of seasoned educators outline a wide range of required topics to provide a thorough review to help practicing engineers as well as engineering students prepare for this challenging examination. 24 hours of videotape and notes. *P.E. Exam Review - A comprehensive review of all the major exam topics that demonstrates the necessary math, logic and theory...and provides shortcuts that reduce the time and effort required to prepare yourself for this challenging exam. 15 hours of videotape and notes. FOR INFORMATION CALL 1-800-THE-ASME AND MENTION CODE CD. INFORMATION REQUEST FORM Please mail to ASME at 22 Law Drive, P.O. Box 2900, Fairfield, New Jersey, 07007-2900 or fax to 201-882-1717, call 1-800-THE-ASME, or email infocentral@asme.org Send me information on the following: _____Short Courses _____ In-House Training _____ Self-Study Programs _____F.E. Exam Review _____ P.E. Exam Review Name___________________________________________________________ Title____________________________________________________________ Organization_____________________________________________________ Business Address_________________________________________________ City____________________________State_______________Zip___________ Business Phone ( )____________________Fax ( )______________________ E-mail Address____________________________________________________