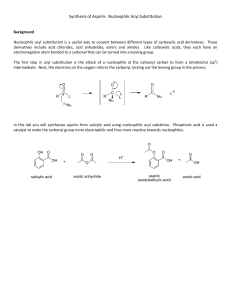
Prof. Zhang Chem 250 - W03L Kevin Liu Synthesis of Aspirin Abstract The main objective of this experiment was to synthesize acetylsalicylic acid (ASA), better known as aspirin, through the esterification reaction between salicylic acid (SA) and acetic anhydride (AA) using phosphoric acid as a catalyst. This was accomplished via usage of a boiling hot water bath in which the acetic anhydride, the salicylic acid, and a few drops of the phosphoric acid were mixed together. The presence of the hot water, catalyzed by the 85% phosphoric acid, provided the energy required for the esterification reaction to reach completion. The vacuum filtration process was then required to obtain the precipitate in a dry format, allowing for more streamlined weighing fashion. In analyzing the purity of the crude aspirin product obtained, an IR spectrum was collected and an iron (III) chloride colorimetric test was performed. The amount of crude aspirin synthesized was 2.343 grams, returning a percent yield of 89.9%. The melting point was experimentally determined to be 132.5-134.6°C. 1. Theoretical Introduction The drug Aspirin may very well be found in every household worldwide. Aspirin, also known as acetylsalicylic acid, was first brought to market by Bayer, a pharmaceutical company, in 18991 and has since grown to become one of the most well-known drugs throughout the world. ASA is formed through the esterification process of two precursor molecules known as salicylic acid (SA) and acetic anhydride (AA) (Figure 1). Figure 1. Molecular Structure of Salicylic Acid and Acetic Anhydride In order for the esterification reaction to proceed, a strong amount of catalytic strong acid must be incorporated in order for reaction completion. 2. Experimental Introduction The reactants used in this experiment for the esterification process were acetic anhydride as the carboxylic acid anhydride and salicylic acid as the OH-R group. Salicylic acid is a colorless solid that is soluble in most organic solvents (due to its highly polar nature) with a literature melting point of 158-161°C, a boiling point of 211°C, and a molecular weight of 138.121 g/mol2. Acetic anhydride is a vinegar-esk scented liquid that is slightly miscible in water with a literature melting point of -73°C, a boiling point of 139.5°C, and a molecular weight of 102.09g/mol3. In order for the esterification process to reach completion, a small amount of strong acid must be incorporated to catalyze the reaction. In this experiment, 5 drops of 85% phosphoric acid were added to the solution to allow the process to reach completion. After the product has cooled and crystals formed, vacuum filtration was utilized in order to collect the solidified product. The crude acetylsalicylic acid product obtained came in the form of powdery crystals with a literature molecular weight of 180.158 g/mol and accepted melting point range of 134-136°C4. Based on the assigned quantity of reactants, the theoretical yield of ASA was calculated to be 2.607 grams. The overall reaction mechanism between SA and AA, using 85% phosphoric acid catalytically, can be visualized in Figure 2. Figure 2. Reaction of Salicylic Acid and Acetic Anhydride to form Acetylsalicylic Acid 3. Results Table 1: Theoretical Yield + Percent Yield Calculations Yield from Experiment 2.343 g % Yield 89.9% SA: 2.00g/138.121 g/mol = 0.014476 mol AA: 1.08 g/cm3 = x g/5ml = 5.4 g/102.09 g/mol = 0.0528 mol ASA: 180.158 g/mol*0.014476 mol = 2.607g % Yield = Actual/Theoretical * 100% =2.373g/2.607g * 100% = 89.9% Melting Point (Range) 132.5-134.6oC Table 2: Percent Impurity (Including Purity Calculations) Percent Purity + Impurity % impurity = 0.00134/5.2 * 100% % impurity = 0.0258% % purity = 100% - % impurity % purity = 99.97% Table 3: Concentration Calculations of Std., Flask, and ASA Stock Calculations 40 mg/20 ml = 1.6 mg/ml 1.6 mg/ml * (10 ml/25ml) = 0.64 mg/ml Concentration (Flask 1) 0 mg SA/25 ml = 0mg/ml SA Concentration (Flask 2) 0.64 mg/ml * (1 ml/ 25 ml) = 0.0256 mg/ml SA Concentration (Flask 3) 0.64 mg/ml * (2 ml/ 25 ml) = 0.0512 mg/ml SA Concentration (Flask 4) 0.64 mg/ml *(3ml / 25 ml) = 0.0768 mg/ml SA ASA (Flask 5) 130 mg/25 ml = 5.2 mg/ml Table 4: Concentration and Absorbance of Std. Solutions Flask # Stock Concentration (mg/ml) Volume of SA (ml) Concentration Absorbance (SA) 1 0.64 0 0 0 2 0.64 1 0.0256 0.328 3 0.64 2 0.0512 0.758 4 0.64 3 0.0768 0.962 Table 5: Absorbance and Concentration of ASA Absorbance ASA Concentration of SA within ASA sample 0.032 (13x + 0.0146) = 0.032 x = 0.00134 Figure 3: 13C NMR of Acetylsalicylic Acid (ASA) Figure 4: 1H NMR of Acetylsalicylic Acid Figure 5: IR Spectra of Obtained ASA Product Figure 6: Absorbance vs. Concentration plot of Colorimetric Test Results 4. Discussion The amount of product obtained from the esterification process after vacuum filtration was 2.343 grams of crude acetylsalicylic acid. Given this value obtained, the percent yield of the experiment was determined to be 89.9% of the theoretical yield based on the salicylic acid acting as the limiting reagent (Table 1). Such a percent yield is highly acceptable as slight amounts of the product obtained may have been lost through the crystallization + filtration process as well as imperfect conduction of weighing methods and transfer techniques. Specifically, some of the ASA product may have been lost during the transfer of the crystallized product to the Buchner funnel as well as the transfer from filter paper to the scale. There was also the slight likelihood of unreacted reagents left in the boiling apparatus. The melting point of the obtained ASA product was determined to fall within the range of 132.5-136.4oC (Table 1). This is highly similar to the literature melting point of acetylsalicylic acid (134-136oC). Such a similarity suggests a relatively pure product obtained as displayed by the similar physical properties of our obtained product and the literature product. In order to further verify the purity of the product obtained, an IR spectra of the ASA sample was produced (Figure 5). As displayed in the IR spectra, the following functional groups can be identified at their corresponding displayed wavelengths: the carbonyl group at ~1700 cm-1, the ester (C-O bond) group at ~ 1200-1000 cm-1, and the carboxylic acid group present at ~2650 cm-1. While the ASA product did not return an IR spectra indicative of an impure product, theoretically if any reactant were still present within the product, they could have produced a broad stretch between 3200-3700 cm-1, indicating a hydroxyl group as a result of leftover salicylic acid. Using the provided 13C NMR and 1H NMR results, further analysis of the product can be ascertained. Moving from left-most to right-most within the 13C NMR result (Table 3): the left-most peak represents the carbon adjacent to the carboxyl group which is not part of the ester group; the second peak represents the carbon atom opposite of the carboxyl group (where a para group would attach); the third peak represents the carbon in between the first two assigned peaks; the fourth peak represents the carbon adjacent to the ester group; the right-most peak represents the carbon that belongs to the ester group itself. Based on the provided 1H NMR results, the peak at roughly 12 ppm can be attributed to the hydroxyl hydrogen, the peak at 2.35 ppm can be associated with the hydrogens bound to the carbon adjacent to the ester group on the benzene ring, and the remaining few peaks are attributed to the various hydrogens bound to the carbon atoms present within the benzene ring (Figure 4). The iron (III) chloride colorimetric test allotted for further verification of purity of the obtained substance. Through the colorimetric test, the ASA product was proved to be an extremely pure product, returning a percent purity of 99.97% (Table 2) based on the calculation of ASA concentration (Table 5). Using the dilution flasks containing varying concentrations of salicylic acid, the overall concentration of SA within the flasks were calculated as seen in Table 3. This was then used to create a calibration curve, plotting the concentration of SA within each individual flask against the absorbance level of the respective flask (Table 4). Based on the calibration curve displayed in Figure 6, the line of best fit according to the data points is 13x + 0.0146 = y. Within this equation, the x variable represents the concentration of SA whereas the y variable represents the absorbance value returned. The R2 value of this line of best fit returns a value of 0.984, meaning that the line is an accurate representation of the correlation between the data inputted. Based on this line of best fit, the amount of salicylic acid still present within the ASA product was determined to be 0.00134 (Table 5), or 0.0258% (Table 2). This minuscule amount of reactant remaining within the product achieved represents an extremely pure sample of acetylsalicylic acid obtained. References 1. Miner J, Hoffhines A. The discovery of aspirin's antithrombotic effects. Tex Heart Inst J. 2007;34(2):179-86. PMID: 17622365; PMCID: PMC1894700. 2. National Center for Biotechnology Information. PubChem Compound Summary for CID 338, Salicylic Acid. https://pubchem.ncbi.nlm.nih.gov/compound/Salicylic-Acid. Accessed May 1, 2023. 3. National Center for Biotechnology Information. PubChem Compound Summary for CID 7918, Acetic Anhydride. https://pubchem.ncbi.nlm.nih.gov/compound/Acetic-Anhydride. Accessed May 1, 2023. 4. National Center for Biotechnology Information. PubChem Compound Summary for CID 2244, Aspirin. https://pubchem.ncbi.nlm.nih.gov/compound/Aspirin. Accessed May 1, 2023.