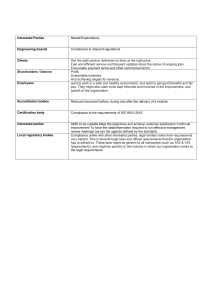
Principles behind ISO/IEC 17025 J.E.J. (Ned) Gravel, CD, PEng, CAE, CA-LS, IPL Principal, MOTIVA Training Inc. www.motiva-training.com Motivating Best Practice in Lab QMS 1 Outline • Brief history of development • What the accreditation process requires of assessors • How the use of principles benefits labs and assessors • The eight principles • Technical competence Motivating Best Practice in Lab QMS 2 About ISO/IEC 17025 “General requirements for the competence of testing and calibration laboratories” It sets specific requirements for laboratories to produce competent (valid) results. Motivating Best Practice in Lab QMS 3 Development of 17025 • Prepared by ISO/CASCO WG 10 and amended by WG25. Is now 5th version of the original standard developed within ISO (ISO/IEC Guide 25). • Groups of experts from around the world – some have 30 years of experience – national and international inputs: e.g., ANSI - NCSLI - SCC (Canada) - most of Europe – other national and international organizations • Competence is the issue • Also used for accreditation Motivating Best Practice in Lab QMS 4 Definition of Accreditation • ISO/IEC 17000: third-party attestation that a conformity assessment body fulfils specified requirements and is competent to carry out specific conformity assessment tasks = recognition of competence • Laboratory Accreditation: The formal recognition of the competence of a laboratory to carry out specific tests or specific types of tests Motivating Best Practice in Lab QMS 5 What does accreditation require of assessors? • A determination of competence • Assessment of technical competence by trained assessors • Assessor must be fully cognizant of each requirement which applies to the scientific discipline within the scope Motivating Best Practice in Lab QMS 6 17025 Assessor vs 9000 Auditor 17025 Assessor • Have you defined and validated your technical procedures? Are they documented in accordance with the test standard or method? Are you following them? • Do your procedures ensure valid results? • Do you understand the science behind the procedures? • Can you foresee and cope with any technical problems that may arise? • Do you have the correct equipment? Do you have adequate personnel? • Have you calculated your uncertainties or do you know the uncertainty inherent in your testing procedure? Motivating Best Practice in Lab QMS 9000 Auditor: • Have you defined your processes, policies and procedures? • Are they documented in accordance with the standard? • Are you following them? 7 A concern: Assessors may be required to defend a requirement to the applicant but may not be able to articulate why the requirement exists. Motivating Best Practice in Lab QMS 8 Principles: if no map, need compass! • Principles that underlie the requirements were developed and presented to ISO/CASCO/WG25 • Ned Gravel represented Canada on this Committee Motivating Best Practice in Lab QMS 9 How Principles Benefit Assessors and Labs • Principles replace blind adherence to the requirements of 17025 WITH • Understanding that instills confidence in a system that should produce competent (valid) results = VALUE ADDED Motivating Best Practice in Lab QMS 10 Value added, cont’d • Principles are not requirements and CANNOT be used to justify ANY assessment finding • Principles help us understand why a specific requirement exists. Motivating Best Practice in Lab QMS 11 • ISO 9000:2005: “MODEL FOR EXCELLENCE”…has great strength in its clear basis on principles • ISO/IEC 17025:2005 PRINCIPLES… give an understanding of the basis for requirements Motivating Best Practice in Lab QMS 12 Principles behind ISO/IEC 17025 • • • • • • • • Capacity Exercise of Responsibility Scientific Method Objectivity of Results Impartiality of Conduct Traceability of Measurement Repeatability of Test Transparency of Process Motivating Best Practice in Lab QMS 13 Capacity Concept that a laboratory has the resources (PEOPLE with the required skills and knowledge, the ENVIRONMENT with the required facilities and equipment, the QUALITY CONTROL, and the PROCEDURES) in order to undertake the work and produce competent results. Motivating Best Practice in Lab QMS 14 Exercise of Responsibility Concept that persons in the organisation have the authority to execute specific functions within the overall scope of work – and that the organisation can demonstrate accountability for the results of the work. Motivating Best Practice in Lab QMS 15 Scientific Method Concept that the work carried out by the organisation is based on accepted scientific approaches, preferably consensus-based, and that any deviations from accepted scientific approaches can be substantiated in a manner considered generally acceptable by experts in that field. Motivating Best Practice in Lab QMS 16 Objectivity of Results • Concept that the results produced within the scope of work of the organisation, are mainly based on measurable or derived quantities. • Concept that subjective test results are produced only by persons deemed qualified to do so and that such results are noted as being subjective or are known by experts in the field of testing to be mainly subjective. Motivating Best Practice in Lab QMS 17 Impartiality of Conduct Concept that the pursuit of competent results through the use of generally accepted scientific approaches is the primary and overriding influence on the work of persons executing tests - all other influences being considered secondary and not permitted to take precedence. Motivating Best Practice in Lab QMS 18 Traceability of Measurement • Concept that the results produced, within the scope of work of the laboratory, are based on a recognised system of measurement that derives from accepted, known quantities (SI system) or other intrinsic or wellcharacterised devices or quantities. • Concept that the chain of comparison of measurement between these accepted, known quantities or intrinsic devices or quantities, and the device providing the objective result, is unbroken for the transfer of measurement characteristics, including uncertainty, for the whole of the measurement chain. Motivating Best Practice in Lab QMS 19 Repeatability of Test Concept that the test which produced the objective results, will produce the same results, within accepted deviations during subsequent testing, and within the constraints of using the same procedures, equipment and persons used during a previous execution of the test. Motivating Best Practice in Lab QMS 20 Transparency of Process Concept that the processes existent within the laboratory producing the objective results, are open to internal and external scrutiny, so that factors which may adversely affect the laboratory's pursuit of objective results based on scientific method, can be readily identified and mitigated. Motivating Best Practice in Lab QMS 21 Tools to help you (http://www.motiva-training.com/index.php/tools-for-labs-qms/free-publications-for-labs) • Free sample Feedback Procedure and forms • Free sample Continual Improvement Procedure and forms • Free sample Internal Audit and Management Review Procedure and forms • Free implementation guide for 17025 Motivating Best Practice in Lab QMS 22 Want More Information? Drop us a line at info@motiva-training.com or call (613) 834-0712 J.E.J. (Ned) Gravel, CD, PEng, CA-LS, CAE, IPL Principal MOTIVA Training - Reaching People nedgravel@motiva-training.com See us on LinkedIn at http://ca.linkedin.com/in/nedgravel/ Motivating Best Practice in Lab QMS 23