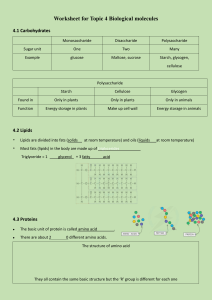
PYSO 1: Lab 1 LAB 2 CHEMISTRY OF LIFE: BIOLOGICAL MOLECULES READING in HUMAN PHYSIOLOGY, 7th Edition, Silverthorn. Biomolecules: pp. 29-37 Experimental controls: p. 19 OBJECTIVES 1. Perform chemical assays and interpret results for the presence of biological molecules (carbohydrates, proteins, lipids) in unknown test solutions. 2. Understand and explain the use of positive and negative controls. Exercise 1: Biological Molecules Ex. 1A: Structure – Function Living organisms are composed of molecules that come in many different shapes and forms. The largest classes of biological molecules are the 4 organic compounds known as macromolecules – carbohydrates, proteins, lipids and nucleic acids. These molecules are often found in long chain polymers made up of simpler building block monomers. Each macromolecule has a variety of diverse functions in the body due to its unique structure. Answer questions # 1-3 on the worksheet (p.4). Ex. 1B: Carbohydrates - Reducing Sugars and the Benedict’s Test Sugars are small carbohydrate molecules used as a source of energy by most organisms and produced in the chloroplasts of plants. There are many kinds of carbohydrate molecules, all of which are made up of carbon, hydrogen, and oxygen atoms. The simplest carbohydrates are simple sugars, or monosaccharides, which include glucose, the main source of energy in the body, and fructose, the sugar found in fruit. Disaccharides consist of two monosaccharides, and include lactose, the sugar contained in milk, and sucrose, common table sugar. Some sugars, known as reducing sugars, contain free aldehyde or ketone groups, and these can be detected using the Benedict's Test. Benedict's solution is blue and contains cupric ions. When heated, the cupric ions (Cu++) react with certain sugars and become reduced to (Cu+), forming an insoluble reddish precipitate of copper (I) oxide. Depending on the amount of sugar present, the solution changes from blue green reddish brown as more cuprous ions are formed. All monosaccharides and some disaccharides are reducing sugars. Some disaccharides do not react with Benedict's reagent because the reactive groups (free aldehydes or ketones) are not exposed. In this exercise, you will test for the presence of reducing sugars in unknown solutions. Follow the instructions below, then record data in Table 1 and answer questions # 4-6 on the worksheet (p.4). Procedure: 1. Make a boiling water bath using a hot plate and a beaker filled 1/3 full of water. 2. Obtain six test tubes and label them –, +, 1, 2, 3, 4. 3. Place 1ml of the negative control in the – tube, 1ml of positive control in + tube, 1ml of each of the 4 unknowns in the correct corresponding tubes. 4. Add 1 ml of Benedict’s reagent to each tube and mix. 5. Heat tubes in boiling water bath for 3 minutes, let cool, then record results in Table 1. Student Safety: Wear safety glasses as there is some risk of spitting when heating tubes. 1 PYSO 1: Lab 1 Ex. 1C: Carbohydrates - Starch and the IKI Test Organisms store simple sugars in the form of large polysaccharide molecules, which are often comprised of thousands of linked monosaccharides. Some of these polysaccharides are broken down as needed for energy, while others serve a structural function. Cellulose, the main component in plant cell walls, is a source of fiber in our diet, and the most abundant organic compound on earth. Starch, a polysaccharide produced by plants to store sugars for later energy needs, is a primary source of carbohydrate in our diets. In this exercise, you will use the iodine test to detect the presence of starch. Starch interacts with iodine to produce a bluish-black color (a yellowish-brown color indicates that no starch is present). Follow the instructions below, then record data in Table 2 and answer questions # 7-8 on the worksheet (p.5). Procedure: 1. Obtain six test tubes and label them –, +, 1, 2, 3, 4. 2. Place 1ml of the negative control in the – tube, 1ml of positive control in + tube, 1ml of each of the 4 unknowns in the correct corresponding tubes. 3. Add several drops of Lugol’s iodine (IKI) solution to each tube, mix. 4. Record the results in Table 2 below. Ex. 1D: Proteins - The Biuret Test A protein’s structure is determined by the sequence of amino acids in its polypeptide chain. Amino acids are connected to each other by peptide bonds. Each protein has a unique sequence that results in a unique shape. It is the 3D shape that gives a protein its function. Biuret reagent contains copper sulfate (CuSO4), which reacts with peptide bonds in the presence of sodium hydroxide (NaOH), resulting in a violet color. Only proteins with four to six linked amino acids will react in this way, thus free amino acids will not be detected. The intensity of the color is related to the number of amino acids linked together. We will use Biuret reagent to identify the presence of proteins if reagent reacts with peptide bonds turning violet/lavender. If solution remains blue, is negative. Procedure: 1. Obtain six test tubes and label them –, +, 1, 2, 3, 4. 2. Place 1ml of the negative control in the – tube, 1ml of positive control in + tube, 1ml of each of the 4 unknowns in the correct corresponding tubes. 3. Add 1 ml of biuret reagent to each tube, mix. Student Safety: Wear safety glasses and gloves as Biuret reagent contains NaOH. 4. Record the results in Table 3 below. Follow the instructions below, then record data in Table 3 and answer questions # 9-10 on the worksheet (p.5). 2 PYSO 1: Lab 1 Ex. 1E: Lipids - The Sudan Test The body stores lipids as reserve energy. Lipids are hydrophobic (“water-fearing”) and thus much harder to break down for energy than carbohydrates. Lipids, however, contain more energy per unit weight than carbohydrates. Therefore it is more efficient for the body to use lipids as stored energy. The body will use its carbohydrate source for initial fuel, but once this fast fuel runs out, the body will turn to breaking down lipids for a rich energy source. Lipids are fat molecules and there are many different kinds. In this lab, we will study triglyceride molecules, the lipids found in vegetable oils and animal fats for energy storage. Triglycerides are composed of three fatty acid molecules and one glycerol molecule bonded in an ester linkage. The base elements of these molecules are C, H and O. Like lipids, the chemical Sudan IV is not soluble in water; it is, however, soluble in lipids. Therefore to test for the presence of lipids in a solution you will use a Sudan IV Test. In this test, dark red Sudan which contains ethanol is added to a solution to dissolve any lipids. If lipids are present the Sudan reagent will stain them a reddish-orange color, giving a positive test result. Follow the instructions below, then record data in Table 4 and answer questions # 11-12 on the worksheet (p.6). Procedure: 1. Obtain six test tubes and label them –, +, 1, 2, 3, 4. 2. Place 1ml of the negative control in the – tube, 1ml of positive control in + tube, 1ml of each of the 4 unknowns in the correct corresponding tubes. 3. Add several drops of Sudan reagent to each tube, mix. Student Safety: Wear gloves. 4. Record the results in the table below. (Look for stained red lipids at the lipid/water interface.) Ex. 1F: Evaluate Unknowns As a group, review the data in Tables 1-4 and evaluate the results of your experiments to decide which biological molecules are present in each of your four unknown test solutions. They may each contain 0, 1, 2 or 3 of the following biological molecules (reducing sugar, starch, protein, lipid). Identify the biological molecules that were present in your four unknown test solutions and answer questions # 13-17 on the worksheet (pp.6-7). Before you leave: Turn off hot plates. Wash your tubes and remove all labels and markings (use green scrub pads). Invert tubes in test tube racks to dry at sink. Clean up your area and wipe down your lab bench. 3 PYSO 1: Lab 1 GROUP MEMBER NAMES: Sahar Bahar, Raymond Truong, Emely Estrada, Kevin Nguygen, Erika Lizarraga, LAB DAY & TIME: ___6-18__________ Lab 2 Worksheets – Results & Questions Chemistry of Life –Biological Molecules (to be turned in as a GROUP) Ex. 1A: Structure – Function(from p.1) 1. a) Name the basic building block(s) for each of the biological macromolecules listed below. Carbohydrates Building Block monoscharides Proteins Amino Acids Nucleic Acids Nucleotides b) Unlike the other biological macromolecules, all lipids do not share a common building block. Instead, what is the defining characteristic shared by all lipids? defined by water insolubility / hydrophobic 2. List several functions for each of the 4 biological molecules. Carbohydrates Functions Short term energy storage structure cell wall Proteins Lipids transport O2 Long term engery enzymes insulates body structural support cushions body Nucleic Acids Making protein, genetic information 3. Give an example of a specific biological molecule and explain how its structure determines its function. glucose is a simple sugar that can be easy taking up by cells. Ex. 1B: Carbohydrates - Reducing Sugars and the Benedict’s Test (from p.1) Fill-in Table 1 and answer the questions below: 4. Why is glucose a good choice for the positive control? Explain the purpose of a positive control. Because glucose is a simple sugar and can be easily detected by the regent 5. Are reducing sugars such as glucose donating electrons or receiving electrons? Is the sugar itself reduced or oxidized? Explain. Sugar itself is being reduced, so reduced sugar is donating electrons 6. From the available test solutions on the counter, what is the best choice for the negative control? Why? 4 PYSO 1: Lab 1 Explain the purpose of a negative control. Water is the best negative control because its neutral, and no response is expected Table 1: Reducing Sugars and the Benedict’s Test Neg. Reducing Sugar (+/-) blue Color and any other Observations Pos. + Unk 1 + Unk 2 Unk 3 Unk 4 - - - Orange Orange blue blue blue Ex. 1C: Carbohydrates - Starch and the IKI Test from p.2) Fill-in Table 2 and answer questions below: 7. What is the best choice for a positive control for this experiment? Why? Starch- 1% starch is a simple starch so its easily detected by the reagent. 8. Negative control? WAter Table 2: Starch and the IKI Test Neg. Starch (+/-) red Color and any other Observations orange Pos. Unk 1 Unk 2 Unk 3 Unk 4 + - + + - blueish black Orange red blueish black blueish black orange red Ex. 1D: Proteins - The Biuret Test from p.2) Fill-in Table 3 and answer questions below: 9. What did you use for your positive control? Albumin 10. Negative control? water 5 PYSO 1: Lab 1 Table 3: Proteins and the Biuret Test Neg. Protein (+/-) Color and any other Observations Pos. Unk 1 + blue Unk 2 - Unk 3 + lavender/violet blue - Unk 4 - violet/lavender blue blue Ex. 1E: Lipids - The Sudan Test from p.3) Fill-in Table 4 and answer questions below: 11. What did you use for a positive control? --1% OIL 12. Negative control? water Table 4: Lipids and the Sudan Test Neg. Lipids (+/-) - pink Color and any other Observations Pos. Unk 1 Unk 2 Unk 3 Unk 4 + - - + - redish layer pink pink red layer pink Ex. 1F: Unknowns from p.3) 13. Identify which biological molecules, if any, were present in each of the four Unknown test solutions. Unknown #1- only sugar Unknown #2- starch and protein Unknown #3- starch and lipid Unknown #4- none 6 PYSO 1: Lab 1 14. Comment on your overall results, thoughts, challenges, etc.. Overall the experiment was simple and results were easily determined. only challenge was one of the unknown was mixed poorly and hard to tell. having the positive and neg was very helpful in determining our results. 15. How would the experiment have been different if you didn’t have controls available? Would you be more or less confident in your results? Explain. Less confident in our results, we wouldnt have been able to compare our results to the positive and negative control. 16. If you tested chicken noodle soup, which biological molecules would you expect to find? All 4 is present 17. For each of your Unknowns identify a possible food source that would match your results (e.g. if your unknown tested positive for protein only, a possible matching food could be egg whites). Food Unknown #1 simple sugar/ table sugar Unknown #2 oatmeal Unknown #3 rice 7 PYSO 1: Lab 1 Unknown #4 water 8