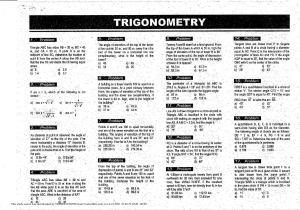
Manipal University Jaipur Department of Chemical Engineering CE 1731- TRANSPORT PHENOMENA LABORATORY-III IV YEAR, 7TH SEMESTER, 2019 Prelab Report EXPERIMENT #2 Batch Crystallizer INSTRUCTOR: Dr. Harsh Pandey GROUP#4 Tanima Sharma Akshay Srivastava Shounak Bhattacharya Prelab submitted on: 20th August 2019 Experiment to be performed on: 22nd August 2019 This study source was downloaded by 100000867410217 from CourseHero.com on106-10-2023 12:03:35 GMT -05:00 https://www.coursehero.com/file/45220137/Batch-Crystallizer-Tanimapdf/ Table of Contents Objective: ..............................................................................................................................3 Introduction: ..........................................................................................................................3 Experimental Setup:...............................................................................................................5 Procedure: .............................................................................................................................5 Observations: .........................................................................................................................5 Precautions: ...........................................................................................................................6 References: ............................................................................................................................6 This study source was downloaded by 100000867410217 from CourseHero.com on206-10-2023 12:03:35 GMT -05:00 https://www.coursehero.com/file/45220137/Batch-Crystallizer-Tanimapdf/ Objective: To study the performance of a batch crystallizer. Calculate the yield of MgSO4 crystals and its efficiency. Introduction: Crystallization is a separation process, widely applied in the chemical and pharmaceutical industry. The principle of crystallization is based on the limited solubility of a compound in a solvent at a certain temperature, pressure, etc. [1]. A change of these conditions to a state where the solubility is lower will lead to the formation of a crystalline solid. The general technique involves dissolving the material to be crystallized in a hot solvent and cooling the solution slowly. The dissolved material has a decreased solubility at lower temperatures and will separate from the solution as it is cooled. This phenomenon is called crystallization if the crystal growth is relatively slow and selective or precipitation if the process is rapid and nonselective [2]. Since the impurities are usually present in much smaller amounts than the compound being crystallized, most of the impurities will remain dissolved in the solvent even when it is cooled. There are two basic types of crystallization process [3]: 1. Solution Crystallization: When crystals are produced by cooling a solution, the process is called solution crystallization. 2. Melt Crystallization: When crystals are generated by cooling a molten solid in the absence of any solvent is called melt crystallization. All crystallization processes are aimed at creating a supersaturated solution or melt. The supersaturation is the driving force under whose influence new crystals are formed and present crystals grow. As shown in Figure 1, for most substances, the solubility increases with increasing temperature, with sodium chloride being a notable exception. In batch crystallization process, the solvent evaporates until the concentration has reached the desired value and cooling is the effected by transfer of sensible heat to the surrounding and evaporation at the free surface [1]. The yield of crystals produced by a given cooling maybe estimated from the concentration of the initial solution and the solubility at the final temperature allowing for evaporation, by making solvent and solute balances [4]. Figure 1: Solution concentration vs Temperature Graph [5] This study source was downloaded by 100000867410217 from CourseHero.com on306-10-2023 12:03:35 GMT -05:00 https://www.coursehero.com/file/45220137/Batch-Crystallizer-Tanimapdf/ Batch crystallization is characterized by the fact that the system is always in the unsteady state. The initial super saturation at which crystallization starts will drop quickly from relatively high value to the saturation value [3]. If crystal growth is to continue, the solution must be maintained in the metastable region. As a consequence, cooling must continue and the batch temperature must continue to drop during the growth period. In batch crystallization it is comparatively easy to penetrate the labile zone producing a fine mass of fine crystals. By using controlled seeding, the solution will not become labile thereby adding crystal growth [2]. The yields and efficiency of a crystallizer can be calculated as follows: 𝑐1 = 𝑐2 = 𝑐2 = 𝑀𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝑖𝑛 𝑓𝑒𝑒𝑑 𝑀𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 𝑀𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝑖𝑛 𝑡ℎ𝑒 𝑑𝑖𝑠𝑐ℎ𝑎𝑟𝑔𝑒𝑑 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 𝑀𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 𝑖𝑛 𝑡ℎ𝑒 𝑑𝑖𝑠𝑐ℎ𝑎𝑟𝑔𝑒𝑑 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 𝑀𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝑖𝑛 𝑓𝑒𝑒𝑑 − 𝑀𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝑜𝑏𝑡𝑎𝑖𝑛𝑒𝑑 𝑀𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 %𝑦𝑒𝑖𝑙𝑑 = 𝐹𝑠 𝑐1 − 𝑆𝑐2 𝐹𝑠 𝑐1 Where, Fs is the mass of solvent in feed and S is mass of solvent present at the end. % 𝑟𝑒𝑐𝑜𝑣𝑒𝑟𝑦 𝑜𝑟 𝑒𝑓𝑓𝑖𝑐𝑖𝑒𝑛𝑐𝑦 = 𝑀𝑎𝑠𝑠 𝑜𝑓 𝑐𝑟𝑦𝑠𝑡𝑎𝑙𝑠 𝑜𝑏𝑡𝑎𝑖𝑛𝑒𝑑 × 100 𝑀𝑎𝑠𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝑖𝑛 𝑓𝑒𝑒𝑑 This study source was downloaded by 100000867410217 from CourseHero.com on406-10-2023 12:03:35 GMT -05:00 https://www.coursehero.com/file/45220137/Batch-Crystallizer-Tanimapdf/ Experimental Setup: The apparatus shown in Figure 2 shows the experimental setup for batch crystallizer. It consists of a jacketed type crystallizer, which has a stirrer and a heater attached to it to stir the solution and to heat the solution respectively. A rotameter is connected to the crystallizer to measure the flow rate of the cooling water coming from the cryostat bath in LPH. Thermocouples (three) are used to measure the temperatures. A control panel is present which consists of switches for the heater, motor and pump and a speed regulator for the agitator is also provided. The thermocouples are there for monitoring the following temperatures: T1 Temperature of feed tank solution T2 Inlet Temperature of water T3 Outlet Temperature of water Figure 2 : Batch Crystallizer Apparatus Procedure: Start-up: 1. Ensure that all the manual valves are closed. 2. Solution of MgSO4 and water is made by measuring 150g of crystals in1500ml of water. 3. It is fed to the jacketed feed tank. 4. The main power supply is switched on. 5. The temperature is set to 60oC and the heater is switched on. 6. Agitator is set to its maximum speed for keeping constant temperature in the tank. 7. Once the temperature is reached steady state, the heater is switched off. 8. Cold water is supplied using rotameter. 9. T1, T2 and T3 temperatures are noted down after every minute (60sec). 10. Once crystallization is achieved, the water supply is stopped. 11. Final temperature is noted down. 12. Crystals are collected on a sieve mesh, dried and its weight is measured. This study source was downloaded by 100000867410217 from CourseHero.com on506-10-2023 12:03:35 GMT -05:00 https://www.coursehero.com/file/45220137/Batch-Crystallizer-Tanimapdf/ Shut-down: 1. The main power supply is switched off. 2. The tank is cleaned and remaining water is drained out using drain valve. Observations: Table 1 : Observation Table T (min) F (LPH) T1(˚C) T2(˚C) T3(˚C) Precautions: 1. 2. 3. 4. 5. 6. Always wear safety gears and lab coat while working in the lab. Make sure that the readings are taken carefully. The apparatus should be switched off while not in use. Ensure all the valves are closed while not performing the experiment. Ensure that the control panel switches are OFF before switching on the main power supply. Use RO water so as to avoid corrosion and scaling in the apparatus. References: 1. A. S. Foust, L. A. Wenzel, C. W. Clump, L. Maus, and L. B. Andersen, Principles of Unit Operations, 2nd edition. John Wiley and Sons. 2. Y. A. Cengel and A. J. Ghajar, Heat and Mass Transfer : Fundamentals and Applications, 5th edition. McGraw Hill Publications. This study source was downloaded by 100000867410217 from CourseHero.com on606-10-2023 12:03:35 GMT -05:00 https://www.coursehero.com/file/45220137/Batch-Crystallizer-Tanimapdf/ 3. C. J. Gaenkoplis, Transport Processes and Separation Process Principles, 4th edition. Pearson Education, 2015. 4. B. K. Dutta, Principles of Mass Transfer and Separation Processes, 2nd edition. PHI learning Pvt. Ltd., 2007. 5. R. H. Perry and D. W. Green., Perry’s Chemical Engineers’ Handbook. Mc-Graw Hill Publications, 2008. This study source was downloaded by 100000867410217 from CourseHero.com on706-10-2023 12:03:35 GMT -05:00 https://www.coursehero.com/file/45220137/Batch-Crystallizer-Tanimapdf/ Powered by TCPDF (www.tcpdf.org)