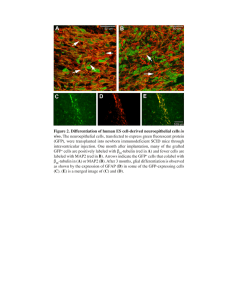
HIV A Biotechnology Tool HIV - a Retrovirus The HIV Genome and Function gag • core nucleocapsid proteins p55, p40 • p24 (capsid, or "core" antigen) • p17 (matrix) • p7 (nucleocapsid); pol • the enzyme proteins p66 and p51 (reverse transcriptase) • p11 (protease) • p32 (integrase). env • The Envelope Glycoproteins: – outer envelope glycoprotein gp120 – transmembrane glycoprotein gp41 • derived from glycoprotein precursor gp160 Regulatory Genes • Lentiviruses differ from other classes of retroviruses – presence of regulatory genes in their genome – are additional to the structural genes gag, pro-pol and env – subdivided into two groups : • the essential genes tat and rev • the accessory or auxiliary genes vif, vpr, vpu and nef. The Essential Genes • Tat: – promotes annealing of tRNA primer to the viral RNA genome – suppresses RT activity at later stages in the viral life cycle. • Rev: – regulates the expression of HIV proteins by controlling the export rate of mRNAs Rev • The HIV mRNAs are produced from the primary transcript by three different splicings: – Unspliced – singly spliced – doubly spliced • Rev is a doubly spliced product – Inhibts second splicing step – => TS and TL of un-, singly spliced Products How Rev works The Accessory Genes • Vpr: – activates HIV at low concentrations – cell cycle arrest – apoptosis in dividing cells • Vif: – associated with the infectious activity of the virus – may also be involved in viral replication, • Vpu: is required for the efficient assembly and release of new HIV viruses • Nef: interacts with host cell signal transduction proteins – for long term survival of infected T cells – for destruction of non-infected T cells by inducing apoptosis Replication Developement of a selfinactivating Lentivirus Vector Based on the paper of: HIROYUKI MIYOSHI, ULRIKE BLO¨MER,† MASAYO TAKAHASHI,‡ FRED H. GAGE, AND INDER M. VERMA* Laboratory of Genetics, The Salk Institute for Biological Studies, La Jolla, California 92037 Why HIV? • retrovirus vectors derived from oncoretroviruses (like murine leukemia virus (MLV)) require proliferation of the target cells for integration – Cannot be used for gene transfer into nondividing cells such as hepatocytes, myoblasts, neurons, and hematopoietic stem cells • Lentiviruses such as human immunodeficiency virus type 1 (HIV-1) can infect nondividing cells HIV - Pseudotyped • HIV vectors were pseudotyped with the vesicular stomatitis virus G glycoprotein (VSV-G) – Are able to infect a variety of tissues Risks & Precautions • HIV-1 is the etiologic agent of AIDS • Possible generation of replicationcompetent virus during the production of vectors – => three-plasmid expression system : • packaging, • envelope • vector constructs Elimination of all accessory genes • Elimination of all accessory genes (vif, vpr, vpu, and nef) from a packaging construct is possible without losing the ability to transduce nondividing cells Risks & Precautions • possibility of insertional activation of cellular oncogenes by random integration of the vector provirus into the host genome – => construction of a self-inactivating (SIN) vector in which the viral enhancer and promoter sequences have been deleted. LTR Modification • The transcriptional inactivation of the long terminal repeat (LTR) in the SIN provirus – prevents the mobilization by replicationcompetent virus – enable the regulated expression of genes from internal promoters by eliminating any cis-acting effects of the LTR. Another Modification • Replacement of the U3 region of the 5’ LTR with the cytomegalovirus (CMV) promoter – Tat-independent transcription with no decreases in viral titer – Hybrid 5’LTR reduces possibilty of recombination (and thus the possible generation of replication competent virus) Immune Response • No cellular immune response could be detected at the site of injection • second injection of the HIV vector into the animals is possible – lack of any potent humoral immune response to the vector Germline Transmission and Tissue-Specific Expression of Transgenes Delivered by Lentiviral Vectors Carlos Lois,* Elizabeth J. Hong,* Shirley Pease, Eric J. Brown, David Baltimore† Science (VOL 295/1 FEBRUARY 2002) TG Animals • The ability to introduce and express exogenous genes of interest in animals has become an indispensable tool to modern biologists Pronuclear Injection • Transgenic mice are currently generated by pronuclear injection • this technique is – – – – relatively inefficient Technically demanding costly impractical in most other animal species. Retroviruses • Another approach to transgenesis: • retroviruses as gene delivery vehicles – Can stably integrate into the genome of cells. • Moloney murine leukemia virus (MoMLV): – impractical for creating transgenic animals • silencing of the provirus during development: • results in low to undetectable levels of transgene expression Lentiviruses • Lentiviruses are a class of retroviruses: – that cause chronic illnesses in the host organisms they infect. Among retroviruses, lentiviruses – are able to infect both dividing and nondividing cells – lentiviruses might be immune to developmental silencing ( in contrast to oncoretroviruses) • => development as gene delivery vehicles Lentiviral Backbone The Vector FUGW • 5‘LTR -CMV Enhancer/ Promotor The Vector FUGW • To increase the titer of the virus: – the human immunodeficiency virus–1 (HIV-1) flap element • inserted between the 5’ long terminal repeat (LTR) and the human ubiquitin-C internal promoter The Vector FUGW • internal promoter driving the GFP reporter gene – human ubiquitin-C promoter: • expression across different cell types The Vector FUGW • Enhanced GFP (EGFP) The Vector FUGW • Increase in Transcription: – the woodchuck hepatitis virus posttranscriptional regulatory element (WRE) downstream of GFP Two Infection Methods • A: Injection into the perivitelline space of single-cell mouse embryos • B: Removal of the zona pellucidae and incubation of denuded embryos with lentiviral suspension • Injection into perivitelline space Injection of virus into the perivitelline space of single-cell mouse embryos Injection into perivitelline space • 72 hours in culture – GFP expression was apparent in the blastula- or morula-stage embryos developing from the infected zygotes • Embryos were implanted into pseudopregnant females and were carried to term Results of Perivitelline Injection • 82% of founder animals carried at least one copy of the integrated transgene • 76% showed GFP fluorescence paws, tails, and face • All GFP-positive animals carried an integrated provirus • all animals with two or more copies of the provirus expressed the transgene at levels detectable by direct viewing of GFP fluorescence. Expression of GFP Expression of GFP Expression of GFP Discussion • The delivery of the virus by injection into the perivitelline space yielded transgenics with high efficiency; – however, the number of integrated proviruses in the genome varied, ranging from 0 to more than 20 • source of this variability – difficulty in controlling the volume of virus delivered into the perivitelline space during the injection. Incubation of denuded embryos • Removal of the zona pellucidae Incubation of denuded embryos • Incubation of denuded embryos for 3 days to the morula or blastocyst stage • Implantation into the uterus of timed pseudopregnant females – Denuded embryos • delayed in their development in vitro • the rate of implantation was lower than that of virusinjected embryos implanted with intact zona pellucidae Features of Incubation Method • there is still some nonlinearity and irreproducibility • But: – this method of virus delivery allows for better control of the number of proviral integrations per genome – requires no specialized equipment and may be easier for many laboratories that wish to use this technique The F1 Generation of TG Mice • Founders carrying transgene(s) transmitted most of them to a fraction of their progeny • Furthermore, ubiquitous GFP expression in transgenic F1 progeny – provirus is not inactivated through one round of gametogenesis and development GFP Expression in skeletal muscles • FMHGW vector: – histone 2B–GFP (H2BGFP) fusion gene • The H2B-GFP concentrates the fluorescence in the nuclei, making the signal more intense – Myogenin promtor • activity specific to skeletal muscle GFP Expression in skeletal muscles • 11.5 Embryos: • showed GFP fluorescence in – the paraxial and cephalic somites – limb buds – Extraocular muscles in the pattern expected for the myogenin protein muscle Tissue-specific GFP Expression • H2B-GFP is expressed in the somites and emerging musceles of the limb buds, eye and jaw (A) •(B) Higher magnification view of A showing the boundaries between somites Focus on nuclei • Immunofluorescence of frozen tissue sections (GFP Antiody): • Expression was limited to the nuclei of cells in the • skeletal muscle lineage • Negative were: cells of the – – – – – – Skin cartilage neural tube heart lung intestines Focus on the nuclei • Image (C) corresponds to a coronal section in the lumbar region showing the localization of GFP immunofluorescence to the somites and its exclusion from flanking skin and cartilage. •Image in (D) is a frontal section through the cervical region showing the localization of GFP immunofluorescence to the somites on either side of the neural tube (nt). GFP Expression in Thymus • GFP driven by the T lymphocyte– specific proximal lck • injection to mouse embryos • => mice expressing GFP exclusively in the thymus Lentiviral Vector System in other Mammals • FUGW lentivirus delivered to single-cell rat embryos by perivitelline injection • Showed expression throughout all tissues => Vectorsystem works also in other mammals Possibilities • overcomes many of the limitations of pronuclear injection: – more efficient – less invasive to the embryos – more cost-effective – technically less demanding – Incubation with denuded embryos obviates the need for micromanipulation and may be easier Possibilities • it has the potential to be extended to other animal species. • VSVG protein mediates viral entry: – finds receptors on the cells of all vertebrates, including primates – Also in birds, a class of animals for which no satisfactory method exists for creating transgenics Limitations • Use may be limited by – sequences that can decrease viral titers • splicing or polyadenylation signals inthe transgene • insertion of transgenes larger than 10 kilobases between the LTRs. • In cases where multiple proviral insertions are necessary for high levels of expression: – the establishment of pure breeding transgenic lines might be complicated by the independent segregation of the proviruses. Questions and Comments Greg@sglab.org http://www.sglab.org ->News ->Seminars