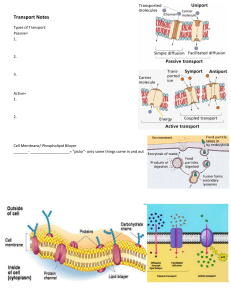
Online Homework for Exam 3 I . (8 points each; 24 total points) Give the IUPAC name for the following compounds. H OH A. B. C C C H H HO HO HO C. N H H H OH OH 1-Name of A: A. 3,4-octadiene B. 4,5-octadiene C. 3,5-octadiene D. 1-ethyl-3-propyldiene E. 1-propyl3-ethyldiene 2-Name of B: A. hexylamine B. cyclohexylamine C. pyridine D. cyclohexenamine E. nitrogencyclothing 3-Name of B: A. sucrose B. -glucose C. -galactose D. -glucose E. sugar II. (5 points each; 50 total points) As indicated by the boxes, provide the reagents, starting materials or major product(s) of the following reaction. Show stereochemistry/regiochemistry where appropriate. OH OH OH OH NaBH4 HO 4-A.HHO H H OH OH OH OH H H OH OH H A. H OH H HO H H H B. OH OH H OH H HO HH H HO CH3OH OH OH H OH H H H H H D. OH OH H OH H HO H OH OH H HO HO C. H OH H E. H OH H HO OH CH 2OH OH H H OH CH 2OH H H OH OH OH F. O O CH 3 O 5-B. A. B. C. D heat + O O O E. F. 6-C. G. H I. O O O O 1. EtNH2 Cl CH 3 O Cl 2. LAH, THF NHEt NHEt A. O B. C. NHEt D. 1 AlH E. Online Homework for Exam 3 2 Online Homework for Exam 3 III. (20 points) Give the mechanisms and label the thermodynamic and kinetic products for the following reaction (use electron flow arrows). Explain your choice for both products. Br Br H-Br 1 eq A. B. C. Br D. E. B. C. Br E. F. Br D. F. 16-Which intermediate(s) should be drawn?__ Br Br A. Br 17-Which is the thermodynamic product?__ 18-Which is the kinetic product?__ IV. A. (12 points) (i) Circle the more stable structure of D-glucose. (ii) Give the mechanism for the interconversion of the two forms of D-glucose (use electron flow arrows). (iii) Explain why acid enhances the reaction rate. OH HO HO OH H+ O H2O OH O HO HO OH A. 19-Which is more stable?___ B. OH OH O HO HO OH H2O OH HO HO O HO HO OH OH OH OH B. OH 2+ OH O HO HO OH O H A. OH OH C. HO HO OH 2+ O HO HO OH D. O OH OH E. F. 20-Which structure(s) could be drawn for the conversion mechanism(s)?___ B. (5 points) Explain why the isomerization of -OH is much slower for t-butylcyclohexanol than for glucose. H+ OH H H OH H H H OH2 O A. B. C. OH D. 21-Which structure(s) should be drawn for the conversion mechanism(s)?____ 3 O E. H Online Homework for Exam 3 V. (10 total points) Draw a typical phospholipid. Draw the structure it spontaneously forms in water (you can use the simple depiction of a phospholipid as I did in class). Indicate where glucose and a steroid would be positioned if they were in water when the structure formed. _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ A. B. _ C. D E 22-Which structure best represents a phospholipid?____ HOH HO H H _ HOH H OH OH HOH _ _ HO HO HO H H HO HO HO H OH OH _ HO HO H H H OH OH HOH HO HO HO H H A. B. C. H OH OH D 23-Which structure best represents the position of the compounds in a phospholipid?____ VI. (8 points) (i) The circles represent the core and surface of a protein. Place were you expect to find the following amino acid side chains for a protein that folded in water. O O NH3+ O- ONH3+ O O NH3+ - O - core surface core surface core O surface core surface NH3+ A. B. C. 24-Which structure best represents the position of the side chains in a protein?____ 4 D Online Homework for Exam 3 VII. (7 total points) (i) Draw the H-bonds (straight lines) that hold these two peptides together. What is the name of this secondary structure? R N H H N O R N H O O N H R H N R O R H N O R N H N H R H N O R O H N O N H R H N O O R A. R N H R R O O H N O R N H O N H R H N O R O H N O R N H R B. O O N H R H N R O R H N O R N H O R H N O O R C. 25-Which is the best structure?____ 26-What is the structure’s name A. -sheet B. anti parallel -sheet C. -helix D. parallel -sheet?___ (ii) (7 points) For which side chain, would the secondary structure be more stable: H-atom (glycine) or isobutyl group (leucine) in water (circle one). Explain your answer. O N H H N O O N H H N O 27-The more stable would be A. glycine or B. leucine?___ 28-What term(s) should you use in your discussion A. grease B. H-bond C. hydrophilic or D. hydrophobic?____ 5 Online Homework for Exam 3 VIII. (16 points) Each pair of molecules interact differently when placed in water. Show, label, and explain the interactions and structures that form between the molecules (if any) and between the molecules and water. CH 3NH 3+ (i) H O H CH 3CO 2- H O - H + O2CCH 3 CH 3NH 3 A. CH 3NH 3+ B. - O2CCH 3 C. 29-Which arrangement is most likely?___ CO 2- NH 3+ (ii) A. NH 3+ H O - NH 3+ O2C O2C B. C. CO 2- + H O H H - H3N O2C - NH 3+ O2C D. 30-Which arrangement is most likely?___ E. NH 3+ (iii) CO 2- A. NH 3+ H O H O H B. - H -O2C O2C CO 2NH 3+ NH 3+ C. D. + CO 2- H3N NH 3+ - 31-Which arrangement is most likely?___ 6 O2C E. NH 3+ Online Homework for Exam 3 IX. As indicated by the boxes, provide the reagents, starting materials or major product(s) of the following reaction. Show stereochemistry/regiochemistry where appropriate. X. ( 35 points) (i) Give the major product formed in each reaction and the mechanisms for their formation. (ii) Circle the aromatic ring that is more reactive for electrophilic addition, and explain your choice. (iii) Explain the regiocontrol for addition (ortho, meta, or para), showing key resonance structures. Make sure to explain why the other isomers are minor products. 7 Online Homework for Exam 3 36-Which structure is the key resonance structure _______? 37-Which structure(s) is the major product _______? : CH 3 O O : O CH 3 O: CH 3 O O O CH 3 O : HNO3 NO 2 NO 2 A. B. C. 38-Which structure is a key resonance structure _______? O O CH 3 O CH 3 O O O2N NO 2 O CH 3 O O2N H2SO4 CH 3 O O CH 3 O NO 2 A. B. C. 39-Which structure(s) is the major product _______? 8 NO 2 NO 2 D. E. Online Homework for Exam 3 9 : 40- Which molecule is more reactive for electrophilic addition? A. O CH 3 O: : O : O B.