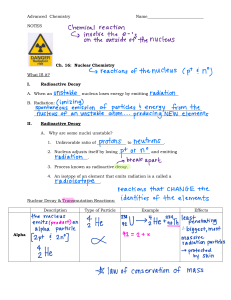
Lesson 2 Nuclear Decay and Reactions Focus Question How can nuclear reactions be useful? New Vocabulary radioactive alpha decay beta decay gamma decay nuclear reaction half-life activity fission chain reaction nuclear fusion Review Vocabulary isotope: each of the differing forms of the same atom that have different masses but have the same chemical properties; atoms with the same number of protons, but different numbers of neutrons Radioactive Decay • Nuclei that emit particles and energy are said to be radioactive. • A material that emits particles is also said to decay. • Nuclei decay from a less stable form to a more stable form. • Radioactivity is a natural process. Types of Radiation • The emission of an α particle from a nucleus is a process called alpha decay. • The mass number (A) of the decaying nucleus is reduced by 4. A→A−4 • The atomic number of the nucleus (Z) is reduced by 2. Z→Z−2 • The element changes, or transmutes, into a different element. Types of Radiation • Beta decay occurs when a neutron is changed to a proton or a proton changes to a neutron within the nucleus. • In beta decay, when a neutron (charge 0) changes to a proton (charge +1), an electron (charge −1) also appears. Types of Radiation • In beta decay, a nucleus with N neutrons and Z protons ends up as a nucleus of N−1 neutrons and Z+1 protons. • Another particle, an antineutrino, is also emitted in beta decay. • The symbol for the antineutrino is the Greek letter nu with a bar over it. (𝜈). Types of Radiation • When a proton changes to a neutron, the process is called positron emission. • This process releases a positron and a neutrino. A positron has the same mass as an electron but a charge of +1. • A redistribution of the energy within the nucleus results in gamma decay. The ray is a high-energy photon. • Neither the mass number nor the atomic number is changed in gamma decay. Types of Radiation • Gamma radiation often accompanies alpha and beta decay. • Radioactive elements often go through a series of successive decays, until they form a stable nucleus. • The next slide summarizes the three types of radiation. Types of Radiation Three Types of Radiation Alpha Decay Beta Decay Gamma Decay particle () particle () particle (photon) charge +2 charge 1 no charge least penetration medium penetration highest penetration transmutes nucleus: AA4 ZZ2 NN2 transmutes nucleus: AA ZZ+1 NN1 changes only energy: AA ZZ NN Nuclear Reactions and Equations • A nuclear reaction occurs whenever the energy or number of neutrons or protons in a nucleus changes. • Just as in chemical reactions, some nuclear reactions occur with a release of energy, while others occur only when energy is added to a nucleus. • Nuclear reactions can be described by words, diagrams, or equations. The following equation is for the alpha decay of uranium-238. 238U → 234Th + 4He 90 2 92 Nuclear Reactions and Equations • The symbols used for the nuclei in nuclear equations make the calculation of atomic number and mass number in nuclear reactions simpler. • The total number of nuclear particles (protons and neutrons) stays the same during the reaction, so the sum of the superscripts on each side of the equation must be equal. (For example: 238 = 234 + 4) • The total charge also is conserved during the reaction, so the sum of the subscripts on each side must be equal. (For example: 92 = 90 + 2) Nuclear Reactions and Equations • One form of nuclear reaction is the emission of particles by radioactive nuclei. • The reaction releases excess energy in the form of the kinetic energy of the emitted particles. • Examples of this are alpha and beta decay. Nuclear Reactions and Equations • Another example of transmutation occurs when a particle collides with the nucleus, often resulting in the emission of other particles, as in: 12C + 1H → 13N. 1 7 6 Nuclear Reactions and Equations Use with Example Problem 2. Problem A SOLVE FOR THE UNKNOWN • Write the equation. Write the nuclear equation for the decay of 87 radioactive 87 37Rb to 38Sr by the emission of a beta particle and an antineutrino. 87 37 Rb 87 38 Sr 0 1 e 00 EVALUATE THE ANSWER • The number of nucleons is conserved: 87 = 37 + 0 + 0 Response SKETCH AND ANALYZE THE PROBLEM • List the knowns and unknowns. KNOWN Initial: 87 37 Rb Final: 87 38 e, antineutrino Sr, 0 1 UNKOWN What is the nuclear equation? • The charge is conserved: 37 = 38 + (−1) + 0 Nuclear Reactions and Equations Use with Example Problem 2. Problem B Write the nuclear equation for the decay of 218 radioactive 222 86Rn to 84Po by the emission of an alpha particle. SOLVE FOR THE UNKNOWN • Write the equation. 222 86 Rb 218 84 Sr 42 He EVALUATE THE ANSWER • The number of nucleons is conserved: 222 = 218 + 4 Response • SKETCH AND ANALYZE THE PROBLEM • List the knowns and unknowns. KNOWN Initial: 222 86 Rn Final: 218 84 Po, 42 He UNKOWN What is the nuclear equation? The charge is conserved: 86 = 84 + 2 Nuclear Reactions and Equations KNOWN Use with Example Problem 3. carbon Response SKETCH AND ANALYZE THE PROBLEM • List the knowns and unknowns. 13 6 1 0 C What isotope is formed? neutron n Problem One type of neutron source used for research is known as a neutron howitzer. When beryllium target nuclei absorb energetic alpha particles from a plutonium source inside the neutron howitzer, they are transmuted into an excited form of 13 6C. When some of the excited 13 6C atoms relax to a stable state, they transmute again through emission of a neutron. What new isotope is created? UNKOWN SOLVE FOR THE UNKNOWN • Write the equation for the nuclear process. 13 6 • C A Z X 10 n Solve for A and Z. A 13 1 12 • Z 66 0 The element with Z = 6 is carbon, so the isotope is 12 6C. EVALUATE THE ANSWER • Releasing a neutron changes A, not Z, so the isotope changes while the element remains the same. Half-life • The time required for half of the atoms in any given quantity of a radioactive isotope to decay is the half-life of that element. • If you know the original amount of a radioactive substance and its half-life, you can calculate the amount remaining after a given number of half-lives. Half-Life 1 remaining original 2 t Half-life • Each particular isotope has its own half-life. • Half-lives of radioactive isotopes are used to date objects. • The decay rate, or number of decays per second, of a radioactive substance is called its activity. • Activity is proportional to the number of radioactive atoms present. Therefore, the activity of a particular sample is also reduced by one-half in one half-life. • The activity of a sample is also related to its half-life. The shorter the half-life, the higher the activity. • The SI unit for decays per second is a Becquerel (Bq). Artificial Radioactivity • Radioactive isotopes can be formed from stable isotopes by bombardment with α particles, protons, neutrons, electrons, or gamma rays. • The resulting unstable nuclei emit radiation until they are converted into stable isotopes. • Artificially produced radioactive isotopes are often used in medicine and medical research. For example: • PET scan • Destroying cancer cells Nuclear Fission • The division of a nucleus into two or more fragments is called fission. • For example, uranium-235 undergoes fission when it is bombarded with neutrons, resulting in the elements barium and krypton, along with three neutrons and an energy release. 1 0 n 235 92 U 92 36 Kr U 3 10 n 173 MeV 141 56 Nuclear Fission • Note that each fission releases three neutrons which can collide with additional uranium-235 nuclei, resulting in more fissions. • A chain reaction is a continual process of repeated fission reactions caused by the release of neutrons from previous reactions. Nuclear Fission • To create a controlled and useful chain reaction, the neutrons need to interact with the fissionable uranium at the correct rate. • Most neutrons released by the fission of U-235 atoms have high speeds and are called fast neutrons. • Naturally occurring uranium consists of less than 1 percent U-235 and more than 99 percent U-238. Therefore, many samples are enriched with more U-235. Nuclear Fission • U-238 absorbs most of the fast neutrons but does not undergo fission. Few neutrons from the original fission do cause another fission. • To compensate for this, the uranium is broken into small pieces and placed in a moderator that slows down the fast neutrons. • These slower neutrons are absorbed more easily by U-235 than by U-238, increasing the chances of another fission occurring. Nuclear Fission • The type of nuclear reactor used in the United States, the pressurized water reactor, contains about 200 metric tons of uranium sealed in hundreds of metal rods. • The rods are immersed in water, which serves as a moderator. • Rods of cadmium metal, which easily absorb neutrons, are placed between the uranium rods. Nuclear Fission • The cadmium rods are moved in and out of the reactor to control the rate of the chain reaction. Thus, the rods are called control rods. • About once a year, some of fuel rods must be replaced. The old rods are still extremely radioactive and must be stored in a location that can be secured. • Methods of permanently storing these radioactive waste products are currently being developed. Nuclear Fission • Water is a moderator, and it also transfers thermal energy away from the fission of uranium. • Energy released by the fission heats the water surrounding the uranium rods. Nuclear Fission • The water itself doesn’t boil because it is under high pressure, which increases its boiling point. • This water is pumped to a heat exchanger, where it causes other water to boil, producing steam that turns turbines. Nuclear Fusion • In nuclear fusion, nuclei with small masses combine to form a nucleus with a larger mass. • In the process of nuclear fusion, energy is released. This corresponds to a loss of mass. • An example of fusion is the process that occurs in the Sun, where four protons fuse in several steps to form one helium nucleus. Quiz 1. Which is the emission of 42He from a nucleus? A alpha decay C beta decay D positron emission CORRECT B gamma decay Quiz 2. In which process does a nucleus with N neutrons and Z protons end up as a nucleus with N−1 neutrons and Z+1 protons? A alpha decay C beta decay B gamma decay D positron emission CORRECT Quiz 3. Neither the mass number nor the atomic number is changed in which process? A alpha decay C gamma decay CORRECT B beta decay D positron emission Quiz 4. Which occurs whenever the energy or number of neutrons or protons in a nucleus changes? A chemical reaction C physical change B nuclear reaction D redox reaction CORRECT Quiz 5. Which is the division of a nucleus into two or more fragments? A fusion C activity B chain reaction D fission CORRECT