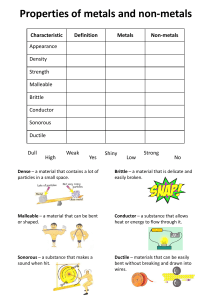
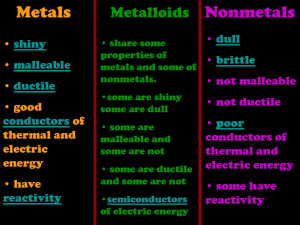
METALS AND NONMETALS THE PROPERTIES OF METALS AND NON-METALS THE PROPERTIES OF METALS STRONG AND HARD • Most metals are generally strong and hard. • They are difficult to break down into smaller pieces and changing their shape usually involves a lot of effort CONDUCTORS • They are very good conductors of heat and electricity. • This is why they are used for pots and pans and in electrical circuits to carry the current. DENSE • Most metals have high density. • This is why they feel heavy and sink when placed in water. SHINY AND SILVERY • Most metals are silvery in colour, except copper and gold. • Some go dull over time. • All metals are shiny when freshly cut. HIGH MELTING AND BOILING POINTS • Most metals have very high melting and boiling points. • They need to be heated to very high temperatures before they melt to become liquids. SOLIDS • All metals are solids at room temperature. • The only exception is mercury. • Mercury is the only liquid under normal conditions. MALLEABLE • This property means that metals can be hit without shattering. • They can be hammered into shape, even when cold and not break into lots of small pieces. DUCTILE • This property means that metals can stretched very thinly without them breaking. • This is why they can be drawn out into wires. THE PROPERTIES OF METALS ARE : • • • • • • Strong and hard Conductors Dense Shiny and silvery High melting and boiling points Solid • Malleable • Ductile THE PROPERTIES OF NONMETALS APPEARANCE • Non-metals have no luster, this means that they are dull in appearance. NON-CONDUCTORS/INSULATORS • Non-metals are poor conductors of heat and electricity • This is why they are used for pot and pan handles and in electrical circuits to carry the current. BRITTLE • Non-metals are brittle which means that they can and often do break easily. NOT DUCTILE • This means that non-metals can not be easily stretched very thinly , if at all without them breaking. NOT MALLEABLE • Not malleable - This property means that non-metals cannot be hit without shattering. • This means that non-metals would break if it was hit or fell. LOW DENSITY • Most non-metals have low density. • This is why they are likely light and float when placed in water. LOW MELTING POINT • Most non-metals have very low melting and boiling points THE PROPERTIES OF NON-METALS ARE : • No luster (dull appearance) • Poor conductor of heat and electricity • Brittle (breaks easily) • NOT ductile • NOT malleable • Low density • Low melting LETS COMPARE THE PROPERTIES BETWEEN METALS AND NON-METALS (Silver and Shiny) METALS NOT MALLEABLE BRITTLE SHINY & SILVERY NO LUSTER/DULL DUCTILE POOR CONDUCTOR NON METALS NOT MALLEABLE LOW DENISITY STRONG & HARD HIGH MELTING POINT CONDUCTOR HIGH BOILING POINT NOT DUCTILE LOW MELTING POINT MALLEABLE DENSE METAL OR NON METAL ? Properties of wood: • Poor conductor of heat • Poor conductor of electricity • Not Ductile • Dull ANSWER: NON-METAL METAL OR NON METAL ? Properties of gold: • Shiny • Dense • Good conductor of heat and electricity • Malleable • Ductile ANSWER: METAL METAL OR NON METAL ? Properties of iron: • Shiny • Silver • Ductile • Malleable ANSWER: METAL METAL OR NON METAL ? Properties of a plastic chair: • Poor conductor of electricity • Not Ductile • Low Density • Low Boiling Point ANSWER: NON-METAL METAL OR NON METAL ? Properties of aluminium: • Good conductor of heat • Good conductor of electricity • Malleable • Ductile ANSWER: METAL METAL OR NON METAL ? Properties of copper: • Good conductor of heat • Good conductor of electricity • Malleable • Ductile ANSWER: METAL METAL OR NON METAL ? Properties of a rubber band: • No luster/ Dull • Poor conductor of electricity • Not Ductile • Low Boiling Point ANSWER: NON-METAL METAL OR NON METAL ? Properties of a straw: • No luster/ Dull • Not malleable • Poor conductor of electricity • Not Ductile ANSWER: NON-METAL