Organic Chemistry Worksheet: Alkanes, Isomers, and Bonding
advertisement

Introducing Organic Chemistry
CARBON:
Carbon is an unusual element. Carbon atoms are able to covalently bond to other carbon atoms
to form chains that may vary from one to thousands of carbon atoms in length. This process of
the same element bonding together to form chains is termed ‘catenation’. Of all the elements,
only carbon forms truly stable long chain molecules. This allows carbon to form a vast number of
different compounds, ranging from the simplicity of carbon monoxide (CO) to the most
complicated carbon chains and rings that occur in biomolecules. Organic chemistry is the study
of carbon chemistry (ignoring CO2, CO, metal carbonates and metal hydrogencarbonates).
HYDROCARBONS:
Hydrocarbons are compounds composed of the elements hydrogen and carbon only. They are
simple molecular substances, each molecule held together with strong covalent bonds. Carbon
and hydrogen have similar electronegativities, the C−H bond is considered non-polar.
Consequently, weak instantaneous dipole - induced dipole intermolecular forces of attraction
(temporary van der Waal’s forces) exist between molecules.
Hydrogen is always monovalent, forming only one covalent bond.
Carbon is in group IV of the periodic table and is always tetravalent in its compounds. It
always forms a total of four covalent bonds e.g. four single covalent bonds (as in CH4) ; two
single covalent bonds and one double covalent bond (as in H2C=CH2) ; etc.
ALKANES:
The alkanes are the group made up of saturated hydrocarbons. A saturated hydrocarbon will
only contain single covalent bonds (no C=C double or C C triple covalent bonds exist).
The simplest alkane is methane (CH4(g))
(a) Draw a dot-and-cross diagram to show the valent electrons in methane.
(b) Draw a full displayed formula diagram to show the structural formula of methane.
(c) Using valent shell electron pair repulsion theory, deduce the shape of methane.
(d) Draw a 3D representation of methane, showing the bond angle that exists.
The next straight-chain alkane is ethane (C2H6(g))
(e) Draw a dot and cross diagram for the valent electronic structure of ethane.
(f) Draw a full displayed formula diagram to show the structural formula of ethane.
(g) Using molecular orbital building kits make a molecule of ethane.
(h) Draw a 3D representation of ethane, showing the bond angles that exist.
Introducing Organic Chemistry.doc - Page 1 - CMS Year12&13 - 30/10/02
The next straight-chain alkane in the series is propane (C3H8(g))
(i) Draw a full displayed formula diagram to show the structural formula of propane.
(j) Using molecular orbital building kits make a molecule of propane.
(k) Describe the bond angles that exist within propane.
The non-cyclic alkanes form a homologous series of compounds. A homologous series is a
group of chemically similar compounds in which the chemical formula of each member
differs from the previous one by the same ‘single unit’.
Chemical
Formula
CH4
C2H6
C3H8
C4H10
C5H12
C6H14
C7H16
C8H18
C9H20
C10H22
Name
Mr
Methane
Ethane
Propane
Butane
Pentane
Hexane
Heptane
Octane
Nonane
Decane
16
30
44
58
72
86
100
114
128
142
Tm
(°°C)
-183
-172
-188
-135
-130
-95
-91
-57
-54
-30
Tb
(°°C)
-162
-89
-42
0
36
-69
98
126
151
174
State
at r.t.p.
∆Hc
(kJ mol-1)
-890
-1560
-2220
-2877
-3509
-4195
-4853
-5512
-6124
-6778
Density
(g cm-3)
0.424
0.546
0.582
0.579
0.626
0.659
0.684
0.703
0.718
0.730
(l) What is the ‘single unit’ difference in the chemical formula between successive members of
the group?
(m) The series allows us to write a general formula for the non-cyclic alkanes. Determine a
general formula for the non-cyclic alkanes of the form: CnH? where n = the number of
carbon atoms present and ? is deduced in terms of n.
Introducing Organic Chemistry.doc - Page 2 - CMS Year12&13 - 30/10/02
The Boiling Points of the Alkanes versus their
Molecular Mass
200
Boiling Point ( C)
150
100
(n) Explain the trend found in the
boiling points of the alkanes.
50
0
-50
0
50
100
150
Molecular Mass
-100
-150
-200
Enthalpy of Combustion Versus the Molecular
Mass of the Alkanes
-8000
Enthalpy of Combustion (kJ/mol)
-7000
-6000
(o) Explain the trend found in the
enthalpy of combustion of the
alkanes.
-5000
-4000
-3000
-2000
-1000
0
20
40
60
80
100
120
140
160
0
Molecular Mass
1000
Introducing Organic Chemistry.doc - Page 3 - CMS Year12&13 - 30/10/02
ISOMERISM in the ALKANES:
Isomers are molecules with the same chemical formula, but different structural formula.
Non-cyclic alkanes can display structural isomerism, where there are differences in the order
in which the atoms are joined together. Chain isomerism is one form of structural isomerism
and occurs where differences exist in the arrangement of the carbon atoms.
*Take care* alkanes only possess single covalent bonds. Rotation can occur about these
single {or σ (sigma)} bonds leading to many possible conformations. Simple rotation about a
single bond does not constitute the formation of a new isomer. Each chain isomer will have the
carbon atoms physically bonded in a different order.
(p) The first alkane that displays isomerism is C4H10. Using molecular orbital building kits make
models of all the possible structural isomers of C4H10
(q) Draw a full displayed formula diagram to show the structural formula of each isomer.
(r) Using molecular orbital building kits make models of all the possible isomers of C5H12
(s) Draw a full displayed formula diagram to show the structural formula of each isomer.
(t) Using molecular orbital building kits make models of all the possible isomers of C6H14
(u) Draw a full displayed formula diagram to show the structural formula of each isomer.
(v) Draw a full displayed formula diagram to show the structural formula of each isomer of
C7H16
Alkanes, like all organic compounds, are named in a systematic manner. If we were to employ
‘trivial’ names for each compound it would require millions of unrelated terms, giving us no
indication of their actual composition. Systematic nomenclature enables us to name the vast
variety of organic compounds in such a way that it implies the particular structural formula of the
compounds concerned.
Introducing Organic Chemistry.doc - Page 4 - CMS Year12&13 - 30/10/02
NAMING ALKANES:
(i) The longest carbon chain must be identified. The name is based on this chain and depends
on the number of carbon atoms present.
Number of C
Atoms in Chain
Prefix used
1
2
3
4
5
6
7
8
9
10
meth
eth
prop
but
pent
hex
hept
oct
non
dec
Since they are alkanes, the name is terminated in ‘ane’. e.g. ethane & hexane
(ii) For branched alkanes, the name of each ‘branch’ and its position of attachment along the
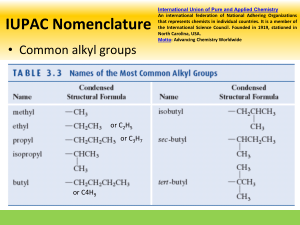
longest chain is indicated. The branches are termed ‘alkyl groups’ and their name depends
on the length of the branching chain and are terminated in ‘yl’ e.g. -CH3 = the methyl group ;
-C2H5 = an ethyl group
The position of the branch is always numbered from the end of the longest carbon chain to
give minimum values.
(iii) If the same ‘branch’ appears more than once:
• The repeated alkyl group is prefixed with: di- ; tri- ; tetra- ; penta- ; hex- ; etc.
• The position of each alkyl group along the longest chain is indicated, keeping the
numbers as small as possible.
Introducing Organic Chemistry.doc - Page 5 - CMS Year12&13 - 30/10/02
(iv) If a branched-alkane contains more than one
type of alkyl group, they are named in
alphabetical order (ignoring prefixes e.g. di- , trietc.)
(v) When naming cycloalkanes, the prefix ‘cyclo-‘ is used.
(w) Name all the isomers drawn in questions q, s, u, v
Introducing Organic Chemistry.doc - Page 6 - CMS Year12&13 - 30/10/02
CYCLOALKANES:
Carbon is able to form cyclic ring structures. Cycloalkanes are saturated cyclic hydrocarbons.
H
H
C
H
C
C
H
H
H
H
C
C
H
H
C
C
H
H
H
H
Cyclopropane
H
H
H
C
H
H
Cyclobutane
C
C
H
C
C
H
H
H
H
H
H
H
H
H
C
C
H
H
Cyclopentane
C
C
H
H
C
H
H
C
H
H
Cyclohexane
(x) Cycloalkanes do not possess the same general formula as the non-cyclic alkanes. Deduce a
general formula for the cyclic alkanes.
(y) What is the C-C-C bond angle in cyclopropane? Build a model of cyclopropane and use it to
explain why cyclopropane is relatively unstable w.r.t. non-cyclic alkanes.
(z) By building cyclobutane and cyclopentane, suggest why they are relatively unstable w.r.t.
non-cyclic alkanes.
(aa) Build a model of cyclohexane. Use it to demonstrate that there are two stable
conformations. These two conformations are often described as the ‘chair’ and ‘boat’ forms.
H
H
H
H
H
H
C
C
H
H
H
C
H
H
C
H
H
C
C
H
C
C
C
H
H
H
C
H
C
C
H
H
H
H
H
H
‘Boat’ Form
‘Chair’ Form
Use the models to help explain which conformation of cyclohexane is likely to be the more
stable.
Introducing Organic Chemistry.doc - Page 7 - CMS Year12&13 - 30/10/02
(ab) From the structural formulae shown below, identify;
(i) the structures that are equivalent;
(ii) the structures that are isomers of each other.
Introducing Organic Chemistry.doc - Page 8 - CMS Year12&13 - 30/10/02