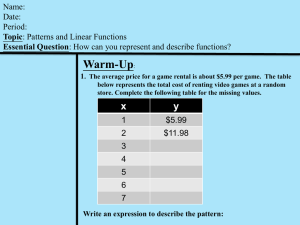
1. Physical Quantities and Units Learning Objectives: 1.1 Physical Quantities 1. understand that all physical quantities consist of a numerical magnitude and a unit 2. make reasonable estimates of physical quantities included within the syllabus 1.2 SI Units 1. recall the following SI base quantities and their units: mass (kg), length (m), time (s), current (A), temperature (K) 2. express derived units as products or quotients of the SI base units and use the derived units for quantities listed in this syllabus as appropriate 3. use SI base units to check the homogeneity of physical equations 4. recall and use the following prefixes and their symbols to indicate decimal submultiples or multiples of both base and derived units: pico (p), nano (n), micro (μ), milli (m), centi (c), deci (d), kilo (k), mega (M), giga (G), tera (T) 1.1 Physical Quantities: ▪ ▪ A physical quantity is a quantity that can be measured. It has a magnitude and a unit. ▪ Mass of trolley: 7.5 kg ▪ Magnitude: 7.5 ▪ Unit: kg You cannot have one or the other when defining a physical quantity. Direct and Indirect Measurements ▪ Measured directly using equipment – Direct Measurement – e.g., Weight of human Body etc. ▪ Measured indirectly – e.g., Measuring mass of earth, mass of electron etc. Estimation: ▪ A rough calculation of a value, number, quantity or extent of something is called estimation. ▪ Many estimates are based on formulas in which the input quantities are known only to a limited precision. Strategies ▪ Get big lengths from smaller lengths. ▪ Get areas and volumes from lengths ▪ Get masses from volumes and densities. Speed – walk two meters (imagine a meter rule to understand the distance) and time yourself. That is your speed. Convert it for km. See how many kilometers are there from your house to your school. See how quickly you reach your school from a vehicle and so on. Think how many apples are there in 1 kg. Now, estimate what will be the mass of 1 apple out of those? Measure the papers in front of you with a scale. Count the number and find the thickness of one. Some quantities need to be memorized in here as they are too unfathomable to estimate, such as – diameter of nucleus of an atom. Wavelengths of EM waves. Which Estimates are realistic? Option Explanation A The kinetic energy of a bus travelling on an expressway is 30 000 J. A bus of mass m travelling on an expressway will travel between 50 to 80 km h-1 , which is 13.8 to 22.2 m s-1 . Thus, its KE will be approximately ½ m(182 ) = 162m. Thus, for its KE to be 30 000J: 162m = 30 000. Thus, m = 185kg, which is an absurd weight for a bus; i.e. This is not a realistic estimate. B The power of a domestic light is 300 W. A single light bulb in the house usually runs at about 20 W to 60 W. Thus, a domestic light is unlikely to run at more than 200W; this estimate is rather high. C The temperature of a hot oven is 300 K. 300K = 27 0C. Not very hot D The volume of air in a car tire is 0.03 m3 . Estimating the width of a tire, t, is 15 cm or 0.15 m, and estimating R to be 40 cm and r to be 30 cm, volume of air in a car tire is = π(R 2 – r 2 )t = π(0.42 – 0.32 )(0.15) = 0.033 m3 ≈ 0.03 m3 (to one sig. fig.) 1.2 SI Base Units: Base units are basic sets from which all units are derived. Quantity Unit Symbol Mass kilogram kg Length metre m Time second s Temperature kelvin K Current ampere A Amount of substance mole mol Luminous intensity candela cd Derived Units: All the remaining units apart from SI-base units that are derived using those base units. ▪ E.g., Speed = distance/time → m/s ▪ They may be named after the scientists who discovered or derived those quantities but they can be represented by their base units. ▪ Example: ▪ Express the Newton N in terms of base SI units: ▪ Force = mass x acceleration ▪ F = kg m s -2 Recall rules for dealing with power: ▪ ▪ Example, time = s If in the equation it is written as ▪ 𝟏 𝒔 ▪ 𝟏 𝒔𝟐 → 𝒔−𝟐 ▪ 𝟏 𝒔−𝟐 → 𝒔𝟐 ▪ 𝟏 √𝒔 → 𝒔−𝟏 → 𝟏 𝟏 𝒔𝟐 𝟏 → 𝒔−𝟐 Show the SI base units of the following physical quantities: ▪ ▪ ▪ Force → Force = mass x acceleration = 𝒌𝒈 𝒎 𝒔−𝟐 Energy → Energy = Work done = Force x distance = 𝒌𝒈 𝒎 𝒔−𝟐 𝒎 = 𝒌𝒈 𝒎𝟐 𝒔−𝟐 Power → Power = Energy/time = 𝒌𝒈 𝒎𝟐 𝒔−𝟐 . 𝒔−𝟏 = 𝒌𝒈 𝒎𝟐 𝒔−𝟑 ▪ Density → Density = mass/Volume = 𝒎𝟑 = 𝒌𝒈 𝒎−𝟑 ▪ ▪ ▪ 𝒌𝒈 Kinetic Energy → Kinetic energy = ½ mass x velocity2 = kg. (m/s)2 = 𝒌𝒈 𝒎𝟐 𝒔−𝟐 Frequency → Frequency = 1 / Time Period = 1 / s = 𝒔−𝟏 Pressure → Pressure = Force/Area = 𝒌𝒈 𝒎 𝒔−𝟐 / 𝒎𝟐 = 𝒌𝒈 𝒎 𝒔−𝟐 𝒎−𝟐 = 𝒌𝒈 𝒎−𝟏 𝒔−𝟐 Homogeneity of equations: An equation is homogeneous if quantities on BOTH sides of the equation has the same SI units. Some constants (π) have no units, Numbers has no unit (Number of electrons). A homogeneous equation may not be physically/scientifically correct but a correct equation needs to be homogeneous.