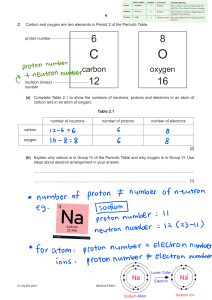
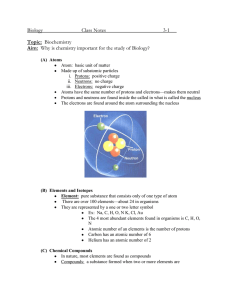
Name: __________________________ Date: ________________________ Grade 9 Chemistry Quiz True or False Indicate T or F to indicate if the statement is true or false. 1. ____ The proton is found in the nucleus of the atom. 2. ____ A lithium atom has more protons than an atom of helium. 3. ____ Molecular compounds are produced when metal and non-metal atoms bond. 4. ____ Noble gases form positive ions. 5. ____ Compounds are pure substances. 6. ____ The chemical formula for water is H2O2. 7. ____ Molecular compounds have high melting points. 8. ____ Ionic compounds form crystals and dissolve in water. 9. ____ Water is an ionic compound. 10. ____ Noble gases are very reactive elements that will react with water and air. 11. ____ Alkali metals are very hard shiny metals that form ions with a charge of 1-. 12. ____ Alkaline Earth Metals have a combining capacity of 2 (can bond with 2 other atoms). 13. ____ The least reactive families are the Halogens and Alkali metals. 14. ____ MgCl2 is a compound containing magnesium and chlorine. 15. ____ MgCl2 is a compound made up of 5 atoms. Fill in the Blanks Word Bank nucleus lustrous protons halogens neutrons gases neutrons electrons electricity unreactive Mendeleev element ductile isotopes heat protons noble compounds protons chemical Fill in the blanks with the term that best completes the statement. 1. An ____________________ is a substance that contains only one type of atom and cannot be broken down through chemical means. 2. The atom is composed of smaller pieces called __________________, __________________, and ______________________. 3. Most of the atom’s mass is found in the ___________________. 4. The atomic number is the number of __________________ an atom of the element has. 5. atomic mass = __________________________ + ________________________ 6. _____________________ have the same number of protons but a different number of neutrons. 7. A Russian scientist named ______________________ found that if you arranged the elements based on their _____________________ properties and atomic mass patterns emerged. 8. Metals are _________________, malleable, __________________, great conductors of ________________ and ___________, and are solids at room temperature (with the exception of mercury). 9. Most non-metals are ______________, some are solids, and one is a liquid at room temperature. 10. The _______________ gases are found on the far right of the periodic table. They are all _________________, and do not interact with all the other elements. 11. __________________ are the non-metals found right beside the noble gases. They are very reactive elements and are almost always found as _______________________. Matching Match the definition in column A with the term in column B. Write the letter of the response in the blank on the left. A B ____ 1. Atomic number of lead. ____ 2. The name for a column in the periodic table. ____ 3. A horizontal row in the periodic table. ____ 4. Malleable, ductile elements that are good conductors. ____ 5. Location of a neutron in an atom. ____ 6. Atoms of the same elements having a different mass. ____ 7. A positive, subatomic particle. ____ 8. Where electrons are found in the atom. ____ 9. The number of protons in a chlorine atom. ____ 10. The atomic mass of krypton. ____ 11. Formed by the attraction of opposite charges. ____ 12. A charged particle. ____ 13. Results from shared electrons. ____ 14. The outer electron shell ____ 15. Number of valance shell electrons in an oxygen atom. a b c d e f g h i j k l m n o p q r s energy shells isotope 83 molecular compound family neutron metalloid 17 period valence shell electron 82 6 ion nucleus metal ionic compound 84 proton Short Answer 1. Write the number of subatomic particles in copper: a. Protons: b. Neutrons: c. Electrons: 2. List three uses of copper. 3. Give two physical properties of copper. 4. What alloy does tin make when mixed with copper? 5. Why isn’t pure copper used for making bells? 6. How much would you have to zoom in on a map of Canada to replicate the power of an electron microscope? For each of the following atoms, draw a bohr diagram that shows the number of electrons in each energy shell. 7. Lithium 8. Flourine 9. Carbon 10. Sulfur What are the names of the following ionic compounds? 11. MgO _______________________________________ 12. NaCl _______________________________________ 13. Al2S3 _______________________________________ What is the chemical formula of the following ionic compounds? 14. Magnesium sulfide __________________________ 15. Beryllium phosphide __________________________ 16. Aluminum chloride __________________________ 17. Lithium chloride __________________________ What is the chemical formula of the following molecular compounds? 18. Dihydrogen dioxide _________________________ 19. Dihydrogen monoxide _________________________ 20. Carbon tetrahydride _________________________ 21. Dinitrogen hexachloride _________________________ *BONUS* What are the lyrics of the ASAP Science Periodic Table Song?