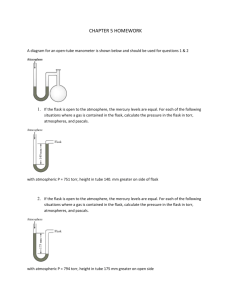
CHAPTER 5 HOMEWORK A diagram for an open-tube manometer is shown below and should be used for questions 1 & 2 1. If the flask is open to the atmosphere, the mercury levels are equal. For each of the following situations where a gas is contained in the flask, calculate the pressure in the flask in torr, atmospheres, and pascals. with atmospheric P = 751 torr, height in tube 140. mm greater on side of flask 2. If the flask is open to the atmosphere, the mercury levels are equal. For each of the following situations where a gas is contained in the flask, calculate the pressure in the flask in torr, atmospheres, and pascals. with atmospheric P = 794 torr, height in tube 175 mm greater on open side 3. Complete the following table for an ideal gas. P(atm) 6.03 0.250 5.65 V(L) 1.62 23.0 3.11 n(mol) 3 3.12 12.4 Temp 132oC 182 K o C o 25 C 4. A steel cylinder contains 160.0 mol argon gas at a temperature of 27 C and a pressure of 8.92 MPa. After some argon has been used, the pressure is 2.00 MPa at a temperature of 21 C. What mass (g) of argon remains in the cylinder? 5. A certain flexible weather balloon contains helium gas at a volume of 912 L. Initially, the balloon is at sea level, where the temperature is 27 C and the barometric pressure is 716 torr. The balloon then rises to an altitude of 7000 ft, where the pressure is 595 torr and the temperature is 14 C. What is the change in volume of the balloon (in L) as it ascends from sea level to 7000 ft? 6. A 18.0-L nickel container was charged with 0.530 atm of xenon gas and 1.70 atm of fluorine gas at 390 C. The xenon and fluorine react to form xenon tetrafluoride. What mass (g) of xenon tetrafluoride can be produced assuming 100 % yield? 7. Ethene is converted to ethane by the reaction C2H4(g) + H2(g) C2H6(g) C2H4 flows into a catalytic reactor at 26.0 atm and 310. C with a flow rate of 969.1 L/min. Hydrogen at 26.0 atm and 290. C flows into the reactor at a flow rate of 1400. L/min. If 10.9 kg of C2H6 are collected per minute, what is the percent yield of the reaction? 8. A sample of oxygen gas is collected over water at 25 C and a total pressure of 658 torr. The volume of the gas collected is 600.0 mL. What mass of oxygen is collected? The vapor pressure of water at 25 C is 23.8 torr. 9. Consider separate 1.0-L gaseous samples of Ne, Ar, and N2, all at STP and all acting ideally. Rank the gases in order of increasing average kinetic energy. 10. Consider separate 1.0-L gaseous samples of He, CH4, and Ne, all at STP and all acting ideally. Rank the gases in order of increasing average kinetic energy. 11. A glass vessel contains 38 g of fluorine gas. Assuming ideal behavior, which of the processes listed below would double the pressure exerted on the walls of the vessel? Check all that apply. A. Adding enough mercury to fill one-half the container. B. Raising the temperature of the container from 30. C to 60. C C. Raising the temperature of the container from -73 C to 127 C. D. Adding 28 g of nitrogen gas. E. Adding 32 g of nitrogen gas. 12. The method used by Joseph Priestly (credited with the discovery of oxygen) to produce oxygen made use of the thermal decomposition of mercury(II) oxide: HgO(s) Hg(l) + O2(g) (unbalanced) Calculate the volume of oxygen gas, measured at 25.0 C and 710 torr that can be produced from complete decomposition of 1.45 grams of mercury(II) oxide.