Physical Activity & Heart Failure Literature Review
advertisement

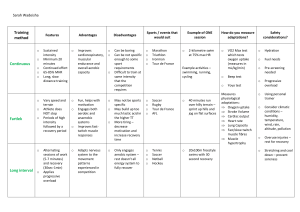
Literature Review 2.1 Introduction This section presents related literature on the topic under study. 2.2 Physical activity and heart failure It is well known that lifestyle plays a major role in the way the body functions. It could be detrimental or of immense benefit to whole body homeostasis. Important lifestyle behaviours include physical activity, sedentary lifestyle, sleep, and smoking. Physical activity is defined as any bodily movement produced by the contraction of skeletal muscles that require energy expenditure above resting level (Pelliccia et al., 2021). Physical activity constitutes most activities carried out as part of daily routine and is markedly different from exercise. Exercise is a sub-type of physical activity which is planned and consists of repetitive whole or partial body movements performed to maintain or improve physical fitness (Pelliccia et al., 2021). Although chronic HF is characterized by progressive exercise intolerance and exertional dyspnoea during minimal exercise (Conrad et al. 2018), available data suggests that exercise is beneficial for HF patients in terms of decreased mortality and morbidity, improved quality of life, functional capacity and cardiac and vascular function. However, exercise training should be considered as an adjunct in these groups of patients. The relative success of an exercise training programme is commonly determined by comparing changes between baseline and post training fitness markers. Maximal oxygen consumption is the most commonly reported measure of functional capacity in HF patients but is rarely achieved in activities of daily living. In carrying out exercise training, it is important to clearly establish the frequency, type, intensity and length of intervention. This perhaps is the reason why programme design is the most important and controversial issue regarding the role of exercise training in HF. Most exercise training programmes have followed the standard prescription of continuous aerobic exercise used in cardiac rehabilitation (Smart 2010), with resistance exercise sometimes added. However continuous aerobic exercise may not optimally stress the peripheral muscles, as they are often atrophied and have fewer fibres, oxidative enzymes, and capillaries in HF patients (Duscha et al 2008). Similarly, there has been some controversy on the effect of exercise dose on the efficacy of training. While Morris et al., (2002) argue that volume of exercise rather than method of delivery determine improvement in functional capacity, other researchers suggest that programme duration especially those designed to last 12 weeks and beyond, have more influence on functional capacity (Støylen et al 2012). 2.2.1 Exercise type and dose Aerobic training Aerobic exercise is generally recommended for stable patients on optimal medical treatment, in New York Heart Association (NYHA) class I - III, reflecting the inclusion criterion commonly used in previous studies. Study protocols have generally consisted of primarily aerobic exercise, usually performed on stationary bicycle or treadmill, but have also included aerobic circuit training, performed for periods of 30–60 min, 3–5 times per week for a duration of 3–6 months. The exertion level prescribed has been set to 60–80% of maximum aerobic capacity (VO2max) (Metra et al. 2017). Current guidelines recommend a moderate exercise intensity (40–80% of VO2max) initially, in stable individuals, as this has been most studied. For patients with more severe symptoms of NYHA class III, a lower starting intensity of <40% of VO2max is recommended. After a run-in period, intensity may be gradually progressed to a goal of 85–85% of maximum (McCoy et al. 2017). Interestingly, a meta-analysis on the dose-response relationship between PA and risk of heart failure, showed a higher optimal dose (intensity × frequency), compared to what is recommended for mortality reduction, while another review could not confirm that “the higher is the better”. High intensity interval training High intensity interval training (HIIT) typically involves short intervals of very high intensity activity (>90% of VO2max) alternating with recovery periods of either complete rest or low intensity activity. It has been suggested as an alternative approach in low-risk heart failure patients. A recent meta-analysis did show superior effects of a HIIT protocol with higher increases in VO2max as compared to a moderate intensity training protocol, although the effect disappeared in subgroup analysis of isocaloric protocols (Kinno et al. 2017). In addition, the Norwegian-German SMARTex study showed that HIIT training was not superior to medium intensity aerobic training for HFrEF. Thus, current guidelines recommend HIIT training, mainly as an alternative strategy for low-risk patients aiming for return to high intensity activities, or sports participation. Resistance training Resistance training has been found to complement aerobic training, as the combination can lead to greater increases in aerobic capacity when compared to aerobic exercise alone. Furthermore, it may prevent sarcopenia associated with advanced heart failure. For heart failure patients unable to perform aerobic exercise, resistance training has been shown to improve muscle strength, aerobic capacity, as well as measures of quality of life. Study protocols have typically included a series of 8–10 exercises performed at 40–80% of the 1 repetition maximum (1RM), for 1–3 sets of 10–15 repetitions per exercise. Current guidelines recommend strength training programs with a frequency of 2–3 days per week in addition to aerobic training. Resistance training may also be specifically considered in low-risk patients aiming to return to power-sports, such as weightlifting (Kingsley et al. 2016). Peripheral muscle training For patients with severe heart failure symptoms and pronounced reduction in exercise tolerance, peripheral muscle training may be used in the initial stages of rehabilitation in patients with muscle wasting and low exercise tolerance (Kingsley et al. 2016). The RPE of these exercises may be relatively high as the central circulation is engaged to a lesser extent. Techniques may include endurance training using various weights, pulleys or resistance bands. As adaptation to peripheral training increases, transition into central circulatory training, including aerobic and strength training protocols may be introduced. Aquatic exercise Water immersion has several immediate physiological effects. Hydrostatic pressure leads to increased venous return and cardiac preload, resulting in increased stroke volume and cardiac output via the Frank-Starling mechanism (Kingsley et al. 2017). Total peripheral resistance is lowered due to vasodilation of periphery as well as abdominal organs. As result, heart rate may be unchanged or somewhat lower relative to relative perceived exertion of the activity performed. There has been some concerns that the hemodynamic changes described could be potentially harmful for certain heart failure patient categories, such as those with mitral regurgitation or severe systolic impairment. However, recent meta-analyses have shown positive effects of aquatic training on aerobic capacity, muscle strength and quality of life comparable to that of land based aerobic exercise. Current guidelines conclude that larger trials are needed to confirm the safety of aquatic training in heart failure. Respiratory training Inspiratory muscle training involves progressive resistance to provide loading to the inspiratory muscles to achieve a strengthening effect. Typically, training protocols involve a high training frequency of six or seven times per week, with intensities from 30 to 60% of maximum for 10–12 weeks. Inspiratory muscle training has shown positive effects on maximal inspiratory pressure, walking distance, and dyspnea in heart failure patients and is recommended as an initial alternative to aerobic exercise in the most severely deconditioned patients, as a bridge to conventional exercise training. 2.3 Heart Failure Patients and Barriers to Exercise Adherence and Exercise Uptake Heart failure (HF) is known to lead to poor health-related quality of life as well as high morbidity and mortality rates. It is also the most common reason for hospitalization in older adults (Fleg et al. 2015). Physical activity in HF patients is known to have positive outcomes such as improved physical capacity and quality of life, and reduced health care utilization. However, adherence to physical activity is less than 50% in HF patients; it even seems more difficult to achieve than dietary modification and medication regimes. Inspite of the fact that published meta-analyses show the positive impact of exercise-based CR, critically low attendance and adherence rates still constitute a major problem in CR. Predictors of regular participation in physical activity in healthy adults have been well documented. Lower age positively correlates with physical activity as well as better self-efficacy, greater social support, better knowledge of perceived benefits, and a positive attitude toward physical activity (Warraich et al. 2018). Compared with men, women are less physically active. Engaging in regular physical activity or having an early history of physical activity has also been described as a predictor of future physical activity, as have higher education and income level, support from a health care provider, and support from surrounding people. Being single or having an inactive partner has been negatively correlated with physical activity levels in older adults. Depression has been identified as being negatively correlated with physical activity levels. Barriers and motivations to participation in regular physical activity have been well studied in the adult population. Barriers to physical activity include internal barriers, such as lack of time, fear of injury, lack of knowledge, lack of self-discipline or motivation, and ill health or changing health status (Yang et al., 2018). External barriers to physical activity include environmental considerations (eg, no facilities nearby), safety, cost, friends/partner not interested, and barriers related to the weather. Motivations toward physical activity in adults include advice by health care providers, family influences, improvement in physical or motor competence, health benefits, and psychosocial reasons such as enjoying group interaction and meeting with friends. Chronic health conditions have been identified both as a barrier and as motivations toward physical activity in the older adult population; individuals may exercise to prevent further physical decline, but their ability to participate in physical activity could be limited by the same conditions (Pandey, 2019). Data on rehabilitation in cardiac patients have shown that lower adherence to physical activity is associated with older age, lower social and economic status, lack of motivation, and financial and medical concerns. Data on HF-specific barriers to physical activity have shown that experiencing symptoms and lack of energy is associated with lower adherence to physical activity (Pandey, 2019). Patients with HF have been described as being less physically active in daily life compared with healthy adults, but a few studies have described the level of physical activity in HF patients living at home. To our knowledge, there has been only one study where the amount of physical activity in 68 HF patients has been examined, using an accelerometer. The authors found that 44% of the patients were sedentary, 35% were moderately physically active, and 15% were physically active on a low level (Kitzman et al, 2017). The variance in daily activity in that study could be partly explained by the patients’ symptoms and self-efficacy. In order to promote physical activity in HF patients, it is essential to know how physically active they are and to understand the barriers and motivation they experience for being more physically active. Motivation not only affects exercise participation, but is also a critical factor in exercise adherence. There are sex differences in physical activity that could be explained by a number of factors. Research has indicated that older women’s personal backgrounds are less favorable for physical activity than those of men (for instance, reported lower levels of education and income, fewer women were married, and a greater number lived alone) (Yang et al., 2018). In addition, women perceive their health as poorer, are more likely to experience barriers to physical activity, and indicate lower self-efficacy for physical activity than men. Motivation and barriers to exercise are important to consider when promoting physical activity in HF patients. Among older patients with acute heart failure, physical function is markedly impaired, and frailty rates and the burden of coexisting conditions are high. Even among older patients with stable and well-compensated heart failure, severe impairments in physical function are often present owing to the combined effects of aging, cardiovascular dysfunction, and skeletal-muscle dysfunction. As patients with chronic heart failure transition to acute decompensated heart failure, physical function worsens further, and this decline is exacerbated by hospitalization and bed rest. These deficits often persist. Many patients never recover baseline function, lose independence, and have high risks of re-hospitalization and death after discharge (sometimes referred to as “post-hospital syndrome”) (Pandey, et al., 2019). 2.4 Occurrence of Heart Failure In Relation To Physical Inactivity Previous studies have also yielded conflicting results regarding the benefits of exercise for patients’ health status. For example, Coats et al., (1990) reported an improvement in patient reported symptoms after 8 weeks of exercise training among 11 rigorously selected HF patients. Another randomized trial among 99 HF patients by Belardinelli et al., (1999) revealed improvements in quality of life as measured using the 21- item Minnesota Living With Heart Failure Questionnaire (MLHFQ), after 8 weeks of exercise training compared with usual care, and these improvements were sustained even at 12 months. In contrast, the Exercise Rehabilitation Trial (EXERT) among 181 patients randomly assigned to 3 months of supervised exercise training and followed by 9 months of home-based training or usual care showed no differences in MLHFQ scores (McCoy et al., 2017). Further emphasizing the confusion surrounding the association of training-induced improvement in exercise capacity and quality of life is the study by (Metra & Teerlink, 2017) in which 24 weeks of exercise training improved peak oxygen consumption but not quality of life (MLHFQ) scores. Most of these studies were conducted prior to current guideline recommendations for pharmacologic and device therapies, including beta-blockers, biventricular pacemakers, implantable cardioverter-defibrillators and left ventricular assist devices. Thus, critical questions remain about whether exercise training can improve patient-reported health status. However, the ‘HF ACTION’ trial (Flynn et al 2009), which is one of the largest trial (2331) to examine the effects of exercise training on clinical outcomes in stable HF patients on optimum medical therapy, showed modest but statistically significant improvements in selfreported health status compared with usual care patients without training. This improvement was reported to occur early and persisted over time. 2.5 Effects of exercise training in heart failure Exercise training has similar effects in heart failure patients and healthy individuals, eliciting positive effects on several of the underlying pathophysiological components, including improvement of risk factors such as diabetes and hypertension. Peak oxygen consumption has also been found to increase with exercise training in heart failure (Nordgren et al. 2015). The effect is thought to be mediated via several pathways, including reversal of concentric remodeling with subsequent improvements of myocardial contractility and compliance, as well as improved microvascular circulation, oxygen extraction and metabolic function of large muscle groups. Furthermore, a combination of aerobic and resistance training has been shown to elicit greater improvements in aerobic capacity as compared to aerobic exercise alone. Meta-analyses have shown that exercise protocols have a positive impact on exercise capacity and quality of life in patients with heart failure. Effects on mortality and re-hospitalization have also been found, albeit modest. Findings from the recent meta-analysis performed on individual level data in the EXTRAMATCH II study did show conflicting results, with no significant effect on mortality or heart failure hospitalization, although the authors conclude that uncertainty around effect estimates precludes from drawing any definitive conclusions (Lewinter et al. 2015). Overall, the recent ESC guidelines on Sports Cardiology, gave regular discussion about exercise participation and provision of an individual exercise prescription for all patients with heart failure, a class I, level of evidence A recommendation. Exercise-based cardiac rehabilitation (exCR) has been shown to improve exercise capacity (commonly estimated by 6-minute walking test or cycle ergometry) and reduce cardiovascular morbidity and mortality, post-myocardial infarction (Clark et al, 2015). Thus, exCR has a major role also in patients with heart failure, with the recent ESC-guidelines recommending exCR in all stable patients with heart failure, to improve exercise capacity, quality of life and to reduce the frequency of hospital admissions. References Belardinelli R, Georgiou D, Cianci G and Purcaro A.1999. Randomized, controlled trial of longterm moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome Circulation 99 1173–82 Chiarantini D, Volpato S, Sioulis F,2010. et al. Lower extremity performance measures predict long-term prognosis in older patients hospitalized for heart failure. J Card Fail;16:390-395. Clark RA, Conway A, Poulsen V, et al. 2015. Alternative models of cardiac rehabilitation: A systematic review. Eur J Prev Cardiol; 22: 35–74. Coats A J S, Adamopoulos S, Meyer T E, Conway J and Sleight P.1990. Effects of physical training in chronic heart failure Lancet 335 63–6 Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. 2018. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet;391:572–80. Duscha B D, Schulze P C, Robbins J L and Forman D E. 2008. Implications of chronic heart failure on peripheral vasculature and skeletal muscle before and after exercise training. Heart Fail. Rev. 13 21–37 Fleg JL, Cooper LS, Borlaug BA, et al. 2015. Exercise training as therapy for heart failure: current status and future directions. Circ Heart Fail;8:209-220. Flynn K E, Pina I L, Whellan D J, Lin L, Blumenthal J A, Ellis S J, Fine L J, Howlett J G, Keteyian S J, Kitzman D W, Kraus W E, Miller N H, Schulman K A, Spertus J A, O’Connor C M and Weinfurt K P. 2009. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial JAMA 301 1451–9 Hsu C-Y, Hsieh P-L, Hsiao S-F and Chien M-Y. 2015. Effects of Exercise Training on Autonomic Function in Chronic Heart Failure: Systematic Review Biomed Res. Int. 2015 1–8 Kingsley J D and Figueroa A. 2016. Acute and training effects of resistance exercise on heart rate variability Clin. Physiol. Funct. Imaging 36 179–87 Kinno M, Nagpal P, Horgan S and Waller A H. 2017. Comparison of Echocardiography, Cardiac Magnetic Resonance, and Computed Tomographic Imaging for the Evaluation of Left Ventricular Myocardial Function: Part 2 (Diastolic and Regional Assessment) Curr. Cardiol. Rep. 19 Kitzman DW, Haykowsky MJ, Tomczak CR. 2017. Making the case for skeletal muscle myopathy and its contribution to exercise intolerance in heart failure with preserved ejection fraction. Circ Heart Fail 10(7):e004281-e004281. Lewinter C, Doherty P, Gale CP, et al. 2014. Exercise-based cardiac rehabilitation in patients with heart failure: a meta-analysis of randomised controlled trials between 1999 and 2013. Eur J Prev Cardiol. Epub November 14, 2014. McCoy J, Bates M, Eggett C, Siervo M, Cassidy S, Newman J, Moore S A, Gorman G, Trenell M I, Velicki L, Seferovic P M, Cleland J G F, MacGowan G A, Turnbull D M and Jakovljevic D G. 2017. Pathophysiology of exercise intolerance in chronic diseases: the role of diminished cardiac performance in mitochondrial and heart failure patients Open Hear. 4 e000632 Metra M and Teerlink J R. 2017. Heart failure Lancet Nordgren L, Soderlund A.2015. Being on sick leave due to heart failure: encounters with social insurance officers and associations with sociodemographic factors and self-estimated ability to return to work. Eur J Cardiovasc Nurs. Epub February 3, 2015. Pandey A, Kitzman D, Whellan DJ, et al., 2019. Frailty among older decompensated heart failure patients: prevalence, association with patient-centered outcomes, and efficient detection methods. JACC Heart Fail;7:1079-1088. Pelliccia A, Sharma S, Gati S, Ba¨ck M, Bo¨ rjesson M, Caselli S, et al. 2020. ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease: The Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur Heart J 2021;42:17–96. Smart N. 2010. Exercise Training for Heart Failure Patients with and without Systolic Dysfunction: An Evidence-Based Analysis of How Patients Benefit. Cardiol. Res. Pract. 2011 1– 7 Støylen A, Conraads V, Halle M, Linke A, Prescott E and Ellingsen Ø. 2012. Controlled study of myocardial recovery after interval training in heart failure: SMARTEX-HF--rationale and design. Eur. J. Prev. Cardiol. 19 813–21 Warraich HJ, Kitzman DW, Whellan DJ, et al. 2018. Physical function, frailty, cognition, depression, and quality of life in hospitalized adults ≥60 years with acute decompensated heart failure with preserved versus reduced ejection fraction. Circ Heart Fail;11(11):e005254-e005254. Yang X, Lupón J, Vidán MT, et al. 2018. Impact of frailty on mortality and hospitalization in chronic heart failure: a systematic review and meta-analysis. J Am Heart Assoc 7(23):e008251-