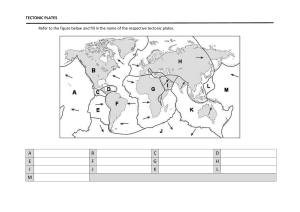
BATTERIES PROFILE Perfecting the purity of nickel Hpulcas GmbH introduces super pure nickel grade for high-performance electronic applications IT has long been known that the parts per million range quantity of trace elements has a tremendous influence on the properties of otherwise pure metals (AE van Ankel, Reine Metalle, Berlin 1939) which is also true for nickel. The norm for the purest nickel grade – Ni 270 – was established decades ago for vacuum tube parts. Since then it has become possible to reduce the content of trace elements in nickel drastically. For measurement and control devices, specific properties and their stability over time and different production lots is decisive. Both can be achieved by a strict limitation of trace elements. Current technologies for producing nickel Starting material Nickel is initially produced in the form of cathode plates or powder, both having a purity of up to 99.98%. As cathode plates have a number of undesirable properties, they are cut into squares and either dissolved (by a galvanising process) or melted, to be eventually used in the production of pure nickel, nickel alloys or stainless steel. Melt metallurgy It is extremely difficult to reach the highest purity of the starting material if nickel is processed by means of melt metallurgy. If nickel is melted under an ambient air, carbon, aluminium, silicon and titanium are added to deoxidise the melt as well as manganese and magnesium to globularise sulphur. These elements are supposed to be slagged, but remain partially in the melt and thus deteriorate the degree of purity. When melting by industrial vacuum metallurgy processes, the vacuum is not high enough, which is why the addition of deoxidisers cannot be completely avoided. Vacuum-melted slabs have either high oxygen or carbon content. To achieve high grades of purity, nickel made by melt metallurgy must be purified. This can be done by electroslag remelting (ESR) and zone refining (ZR). nickel production. These trials have always failed because the negative characteristics of cathode plates could not be completely overcome. These are: n The plates are covered with an orange peel effect, dotted with occasional warts. Direct rolling of cathode plates results in surface cracks; n As a result of the electrolytic winning process, cathode plates show a columnar grain structure, which resists deformation against the axis of the grains; n Cathode plates are produced by inserting a thin starter sheet into the electrolytic bath. Both sides of a sheet are receiving nickel layers built up by electrolytic deposition. The cathode plate therefore consists of three layers. Upon rolling, the surface of the plate, which is in contact with the rolls, flows faster than the material inside the plate. The strain thereby induced may split the plate horizontally during rolling, or at least loosen the cohesion of the layers, also resulting in splitting or the appearance of bubbles; n The starter sheet is usually corrugated to hinder bending in the hot bath. As a result, a cathode plate takes this form as well. Corrugation cannot completely hinder twisting, thus the plates get out of plane. To use them in an industrial process, plates must be flattened; n If the corrugation extends over the whole length of a plate, upon flattening, three-dimensional stresses are introduced into the plates, resulting in stress cracks upon hot rolling; n There could be substantial thickness differences of up to seven millimetres cross- and longwise throughout the plate. Depending on the distribution of thickness differences, upon rolling, plates can take different shapes, e.g. a wedge shape of a cathode plate results in a sabre shape of a rolled plate. Randomly dispersed thickness differences result in different elongation upon rolling, resulting at best in a fish-tail form of the end of a plate. Greater Powder metallurgy Powder can be produced by the Mond process, which allows one to achieve high degrees of purity. The Ni 270 norm presupposes that such qualities are to be produced by means of powder metallurgy. Strip can be directly rolled from powder or pressed into slabs, which are sintered and hot-rolled. Large slabs are difficult to produce by powder metallurgy, however, resulting in density variations. Products made by means of powder metallurgy are remaining porous to some extent; fully dense products require massive subsequent reduction. Production of high purity nickel directly from cathode plates Physical characteristics of cathode plates Because of the high degree of purity of cathode plates, it has frequently been tried to use them as starting materials for industrial 66 Pan European Networks: Government 22 www.paneuropeannetworks.com PROFILE BATTERIES PROPERTIES OF HIGH PURITY NICKEL AND THEIR APPLICATIONS The special properties of high purity nickel are used in the following applications: PROPERTY VALUE/ CHARACTERISTICS Ni 99.98 Degree of purity (Ni-content) SIGNIFICANCE APPLICATION Ni 99.2 Decreased amount of impurities drastically influences the mechanical properties of pure metal. 99.98% 99.2% Sputtering targets. Segregations of impurities facilitate stress corrosion fracture and intergranular corrosion. C-Content S-content <20 ppm 1,000 ppm Carbon results in hot shortness and increases hardness and electrical resistance. Surface segregation of sulfur results into a sulfur induced breakdown of the passive film on nickel facilitating corrosion. <2 ppm 1,000 ppm Segregation to boundaries results into a hot shortness and reduced mechanical stress. Si-content 0.2 ppm 1,000 ppm In the presence of silica (as well as Al and Ti oxides) thin products are exposed to breaking (wire) or developing holes (strip). Deep drawn products as electrode shells. Products relying on catalytically active surfaces. Foil, thin wire. Glass molds for optical quality glass. Oxides increase die wear. Expanded metal. Tensile strength, MPa 300-350 450 High purity nickel (HPN) can be deformed by up to 98% without intermediate annealing. Deep drawn parts (as CCFL-elektrodes); brazing spacer taking up compressive stress. Recrystallisation temperature, °C 350 690 Starting from about 500°C, nickel tends to sticking, when bell-annealed. HPN annealing temperatures are below the temperature, where sticking starts. HPN can be annealed after cladding to metals with low melting points as Al and Mg. Curie-point, °C 360±1 354 - 358 Consistent Curie temperature. HPN can be used for temperature sensing. Electrical resistance, μΩ*cm 7.1 9.0 HPN nickel component can be reduced in size. Less Joule heating during charging and discharging of batteries. Current collectors in batteries, battery tabs; Litz wire for use in aggressive climate and high temperature. +6,600 +4,700 to +5,800 The electrical temperature coefficient of resistance describes the change of resistance in dependence of a temperature change. Nickel shows one of the highest co-efficients of all metals. Temperature coefficient of electrical resistance 10-6K Sensors: Resistance thermometers, e.g. in e-cigarettes. Controls: Regulator coil in glow plugs for Diesel engines. Table 1: Comparison of the properties of high purity and less pure nickel, significance and use thickness differences may lead to stress cracks developing on the surface during rolling. In the worst cases, holes appear in the plate; and n Plates are loaded with hydrogen, which may create bubbles during hot processing and render the welding of plates into strip impossible. Technology to produce strip and wire directly from cathode plates Hpulcas has developed a range of technologies to overcome the deficiencies of cathode plates: n Plates are scanned and data are transformed into computer-aided design processable data. By means of CAD, a new surface lying 0.2mm below the current surface is created. Extra surface is then removed by milling, and orange skin effect and warts are also removed; n By the same technique, thickness differences surpassing one millimetre are also removed; n The declining edges of the plates are trimmed in order to hinder the edges tearing during hot rolling; n Hydrogen is driven out by heat treatment; n A columnar grain structure is converted into a globular structure by heat treatment; and n The three-layer structure is dissolved by promoting grain growth over the layer boundaries. Thereby the plates are prepared for processing. They are heated to the hot rolling temperature, hot rolled, levelled, brushed to remove the oxide scale, cut into rectangles of uniform width and welded into a strip. By a sequence of reducing and annealing steps, grain structure of the weld seam and the heat-affected zone are approximated with the grain structure of the plates. For the production of wire, the plates are cut into sticks, frontally welded and drawn to a desired size. Dieter Wittmann hpulcas GmbH +49 172 7492530 d.wittmann@hpulcas.com Reproduced by kind permission of Pan European Networks Ltd, www.paneuropeannetworks.com © Pan European Networks 2017 www.paneuropeannetworks.com Pan European Networks: Government 22 67