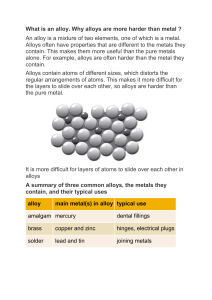
Heat Resistant Alloys: Properties, Selection & Applications
advertisement

HEAT RESISTANT ALLOYS James Kelly Director of Technology TABLE OF CONTENTS INTRODUCTION...............................................................................................1 DEFINITIONS What Are Heat Resistant Alloys? .........................................................2 Rolled Alloys Products Nominal Compositions ..................................3 Heat Resistant Alloy Specifications .....................................................4 EFECT OF ALLOYING ELEMENTS ............................................................5 RESISTANCE TO THE ENVIRONMENT .....................................................12 Oxidation ..................................................................................................13 Laboratory Oxidation Testing ..............................................................17 Carburization............................................................................................22 Metal Dusting/Catastrophic Carburization/Carbon Rot.....................26 Nitriding .....................................................................................................29 Sulphidation .............................................................................................30 Halogen Gas Hot Corrosion ...............................................................34 Molten Salts .............................................................................................38 Molten Metals ..........................................................................................40 Magnetism ................................................................................................45 STRENGTH AT TEMPERATURE ..................................................................48 Tensile Strength ......................................................................................48 Elastic Modulus.....................................................................................48 Yield Strength..........................................................................................49 Ductility.....................................................................................................49 Creep and Rupture .................................................................................50 Creep-Rupture Testing ..........................................................................52 10,000 hour Rupture Strength Data ....................................................54 0.0001% per hour Minimum Creep Rate Data ...................................55 THERMAL FATIGUE ........................................................................................56 WEAR .................................................................................................................58 Erosion .....................................................................................................58 Galling ......................................................................................................58 PHYSICAL METALLURGY .............................................................................60 Sigma .......................................................................................................60 Grain Growth ..........................................................................................63 HEAT RESIS TANT ALLOY GRADES ...........................................................65 Iron-Chromium Alloys ............................................................................65 Fe-Cr-Ni alloys, Ni under 20%.............................................................67 Fe-Ni-Cr alloys, Ni 30 to 40% ..............................................................69 Ni-Cr-Fe alloys, Ni 45 to 60% ..............................................................71 Ni-Cr-Fe alloys, Ni over 60%, 15 to 25%Cr.......................................72 Cast heat resistant grades.....................................................................74 DESIGN ..............................................................................................................78 Thermal Strain.........................................................................................79 Weldments ...............................................................................................81 Thermal Expansion ................................................................................82 Thermal Expansion Coefficients ..........................................................84 Section Size .............................................................................................85 i SELECTING THE ALLOY ...................................................................................86 Temperature..............................................................................................86 Atmosphere ...............................................................................................87 CUTTING AND FORMING..................................................................................88 Shearing ....................................................................................................90 Bending and Forming................................................................................90 Spinning and Deep Drawing .....................................................................93 Machining ..................................................................................................94 Forging ......................................................................................................95 WELDING ............................................................................................................96 Carbon Steel vs Stainless.........................................................................97 Shielding Gases ........................................................................................98 Cold Cracking versus Hot Cracking..........................................................99 Distortion ...................................................................................................100 Penetration ................................................................................................101 Fabrication Time........................................................................................101 Welding Austenitic Alloys ..........................................................................102 Alloys under 20% Nickel ...........................................................................103 Alloys over 20% Nickel..............................................................................104 Gas Metal Arc Welding .............................................................................105 Flux Cored Welding...................................................................................106 Shielded Metal Arc Welding......................................................................107 Gas Tungsten Arc Welding .......................................................................108 Plasma Arc Welding..................................................................................109 Submerged Arc Welding ...........................................................................111 Resistance Welding ..................................................................................111 Weld Filler Selection .................................................................................113 Dissimilar Metal Joints ..............................................................................114 Heat Resistant Alloy Weld Filler Metals ....................................................115 BRAZING AND SOLDERING..............................................................................116 APPLICATIONS Bolts...........................................................................................................119 Cast Link Belts ..........................................................................................120 Muffles .......................................................................................................123 Radiant tubes ............................................................................................125 Rotary Retorts & Calciners .......................................................................126 Salt Pots ....................................................................................................126 Springs ......................................................................................................133 FIELD FAILURES ................................................................................................134 The Melted Radiant Tube .........................................................................134 The Hole in the Box...................................................................................135 The Culprit Copper ....................................................................................136 THUMBNAIL BIOGRAPHIES OF RA ALLOYS ..................................................139 CHEMICAL SYMBOLS........................................................................................140 BIBLIOGRAPHY ..................................................................................................141 HISTORY.............................................................................................................143 TRADEMARKS....................................................................................................144 GERMAN STANDARDS COMPARED WITH AMERICAN TEMPERATURE CONVERSION CHART ii INTRODUCTION We cannot but marvel at the fact that fire is necessary for almost every operation. By fire minerals are disintegrated, and copper produced, in fire is iron born and by fire it is subdued, by fire gold is purified. Pliny the Elder, Natural History, Book XXXVI, 200 Rolled Alloys has specialized in supplying wrought heat and corrosion resistant alloys for a half century now. We have an experienced sales force, laboratory personnel and a more detailed inventory of heat and specialty corrosion resistant alloys than any other supplier. Our technical expertise, to which this paper is an introduction, includes documented field experience and laboratory studies back to 1952. Our current laboratory data, both oxidation testing and metallography, has been generated under the direction of Timothy J. Carney During these years, Rolled Alloys worked with the industrial furnace builders, and with those fabricators who also specialize in heat resistant alloy fabrication, to create the present market for RA330®, RA333®, RA 253 MA ®, RA 353 MA ®, and RA 602 CA TM alloys. We have modified the chemistry and mill processing of RA330 on three separate occasions to maximize its effectiveness in heat treat applications. Beginning in the 1970’s, Rolled Alloys initiated and drafted eleven separate ASTM specifications for our proprietary and semi-proprietary alloys. We, together with our suppliers, generated the data to obtain ASME Code coverage of RA330 to 1650°F (900°C). Although RA330 and RA333 are both sold to published ASTM, UNS or AMS chemistries, our internal purchasing specifications are designed for more rigorous quality control levels than required by these industry-wide specifications. We currently stock over a dozen different grades of heat resisting alloys, ten more aerospace grades used in gas turbine engines, specialty welding fillers and weld overlay wires, along with several alloys designed for corrosion applications. Several of these are proprietary to Rolled Alloys, and we ha ve significant market share in others. Bulletin 401 minor corrections May 13, 2005 ©Rolled Alloys 2005 Note: This document continues to be developed and expanded. If your copy is more than two years old, a newer edition may be available. Contact Rolled Alloys Technology & Marketing Services, +1-734-847-0561, FAX +1-734-847-3915. James Kelly -1- What are heat resistant alloys? Heat Resistant alloys, from our perspective, are those solid solution strengthened alloys (intended) for use at temperatures over 1400°F (760°C) and limited in the extreme to 2400°F (1316°C), which is near the melting point of these materials. These materials cannot be strengthened by heat treatment, as they are used over very broad temperature ranges, and above the temperatures where hardening mechanisms other than solid solution, carbides or nitrides are effective. There are two fundamental types of heat resistant alloys, the “ferritic” and the “austenitic”. Nearly all heat resistant alloys of interest to us are austenitic. The ferritic alloys, such as RA446, are simply iron with anywhere from 11% to about 26% chromium added. They have a body-centered cubic crystal structure, the same as does iron. These ferritic grades also have a little manganese, silicon, carbon and nitrogen, mostly included for their benefits in hot working the alloy at the steel mill. Ferritic grades are very weak and often brittle, and may be difficult to weld. They are magnetic. In spite of poor strength, ferritic heat resistant alloys may be used for their good resistance, at red heat, to sulphur bearing atmospheres, and to attack by low melting point metals, including molten copper. Note—the ferritic grades have some resistance to hot SO2 or H2S gas, but they are not resistant to aqueous corrosion by sulphuric acid. When enough nickel is added to the iron—chromium mix, the alloy becomes austenitic. One example is RA310, with 25% chromium, just like RA446. With the addition of 20% nickel to a 25%Cr-iron base, the alloy becomes austenitic, with roughly ten times the strength of RA446, and much greater ductility. Austenitic alloys of interest to us cover the range from about 8% to 80% nickel. The austenitic alloys all have much greater creep-rupture strength than the ferritics. At room temperature the austenitics are more ductile and generally easier to fabricate. They are non-magnetic as supplied, although after certain high temperature service conditions some may become magnetic. It is these austenitic alloyswhich are of primary interest to us. The straight chromium grades also divide into two more classes of stainless steel, the martensitic and the precipitation hardening. However, neither of these are of any use above 1200°F (~650°C). An addition of carbon permits the straight chromium grades to be hardened by heat treatment, and constitute the martensitic stainlesses. These include types 410, and the 440’s A, B and C. Martensitic stainlesses can be heat treated to maintain high strength through about 900°F (482°C), with a maximum use temperature of 1200°F (649°C). Like the ferritic stainlesses, all straight chromium grades embrittle severely after being held in the 700 to 1000°F (370 to 540°C) temperature range, the so-called “885°F” (475°C) embrittlement. There is a class of very low carbon martensitic stainlesses which obtain their high strength by an age-hardening, or precipitation hardening, process. An addition of copper (such as in 174PH®), molybednum or titanium is responsible for the precipitation hardening mechanism. -2- Rolled Alloys Products, Nominal Composition Cr RA333® RA330® RA330HC RA 253 MA ® Ni Si Heat Resistant 25 45 1.0 19 35 1.25 19 35 1.25 21 11 1.7 Mo Co W Al Ti Other 3 ---- 3 ---- 3 ---- ----- ----- 0.05C 18Fe 0.05C 43Fe 0.40C 43Fe 0.17N 0.08C 0.04Ce 65Fe 0.16N 0.05C 0.05Ce 36Fe 0.1Y 0.08Zr 9.5Fe 0.07C 45Fe 0.05C 62Fe 0.05C 52Fe 0.08C 8Fe 0.05C 14Fe 0.15N 73Fe RA 353 MA ® 25 35 1.2 -- -- -- -- -- RA 602 CA® RA800H/AT RA309 RA310 RA600 RA601 RA446 25 21 23 25 15.5 22.5 25 63 31 13 20 76 61.5 -- -0.4 0.8 0.5 0.2 0.2 0.5 -------- -------- -------- 2.2 0.4 --0.2 1.4 -- -0.6 --0.2 -0.08 RA410 RA410S RA17-4 Stainless 12 -12 -15.5 4.7 0.3 0.3 0.3 ---- ---- ---- ---- ---- RA321 RA347 17.3 17 0.7 0.7 --- --- --- --- 0.2 -- AL-6XN Corrosion Resistant 20.5 24 0.4 6.3 -- -- -- -- RA20 20 33 0.4 2.2 -- -- -- -- RA2205 22.1 5.6 0.45 3.1 -- -- -- -- RA X RA625 Aerospace 22 47 0.3 21.5 61 0.1 9 9 1.7 -- 0.6 -- -0.4 -0.4 3 5.8 9.4 4 --- -20 10.6 13 37.7 50 ----14 15 0.5 0.4 1.5 1.4 --- 0.9 2.2 3.2 3 --- ® RA718 C-263 René 41® 19 20 19.3 TM WASPALOY 19 RA188 22 L-605 20 9.3 9.5 52 51 52.7 57 22.5 10.5 0.1 -0.07 ---- -3- 0.14C 0.06C 3.3Cu 0.05C 0.01C 0.5Cb 70Fe 87Fe 87Fe 0.3Cb 75Fe 70Fe 0.04C 0.22N 0.02C 48Fe 3.3Cu 0.5Cb 0.02C 40Fe 0.16N 67Fe 0.08 19Fe 3.6 Cb 0.05C 5Fe 5Cb 19Fe 0.02Zr 0.001B 0.08C 3Fe 0.06C 2Fe 0.10C 2Fe 0.10C 2Fe Heat Resistant Alloy Specifications Alloy UNS Product Form (W.Nr.) RA333 N06333 Plate, sheet, strip (2.4608) Bar Smless pipe, tube Welded pipe Welded tube RA330 N08330 Plate, sheet, strip (1.4886) Bars & shapes Billets & bars Smless pipe, tube Welded pipe Welded tube Fusion weld pipe RA 253 MA S30815 Plate, sheet, strip (1.4893) Bars and shapes Pipe Welded tube RA 353 MA S35315 Plate, sheet, strip (1.4854) Bars and shapes Pipe RA 602 CA N06025 Plate, sheet, strip (2.4633) Rod, bar, wire RA800H/AT N08811 Plate, sheet, strip (N08810) Rod and bar Smlss pipe &tube RA309 S30908 Plate, sheet, strip (1.4833, Bars and shapes 1.4933) Pipe RA310 S31008 Plate, sheet, strip (1.4845) Bars and shapes Pipe RA446 S44600 Plate, sheet, strip (1.4763) RA600 N06600 Plate, sheet, strip (2.4816) Rod, bar, wire Smlss pipe & tube RA601 N06601 Plate,sheet, strip (2.4851) Rod, bar, wire Bar, forgings,rings Smlss pipe & tube ASME ASTM AMS W.Nr./EN -B 718 5593 2.4608 -B 719 5717 -B 722 -B 723 -B 726 SB-536 B 536 5592 1.4886 SB-511 B 511 5716 -B 512 SB-535 B 535 SB-710 B 710 -B 739 -B 546 SA-240 A 240 - 1.4893 SA-479 A 479 1.4835 SA-312 A 312 SA-249 A 249 ASME Code Case 2033-1 -A 240 - 1.4854 ---A 312 -B 168 2.4633 -B 166 SB-409 B 409 - -SB-408 B 408 - (1.4876) SB-407 B 407 SA-240 A 240 - 1.4833 SA-479 A 479 1.4833 SA-312 A 312 SA-240 A 240 5521 1.4845 SA-479 A 479 5651 1.4845 SA-312 A 312 -A 176 - 1.4763 SB-168 SB-166 SB-167 SB-168 SB-166 -SB-167 -4- B 168 B 166 B 167 B 168 B 166 -B 167 -2.4816 5665 5870 2.4851 5715 EFFECT OF ALLOYING ELEMENTS Starting with a base of iron, the most important alloying elements in heat resisting alloys are: chromium (Cr) for oxidation resistance and nickel (Ni) for strength and ductility. Other elements are added to improve these properties, but heat resistant alloys are primarily alloys of iron, chromium and nickel, and a few are mainly nickel and chromium. Silicon is one of the most effective elements in contributing carburization resistance. CHROMIUM (symbol Cr) Chromium is the one element present in all heat resisting alloys. Oxidation resistance comes mostly from the chromium content (the same is true of aqueous corrosion resistance). Chromium adds to high temperature strength, and to carburization resistance. Metallurgically speaking, chromium tends to make the atomic structure “ferritic”, that is, with a body centered cubic (BCC) crystal structure. High chromium also contributes to sigma formation. RA446, which is essentially 25% chromium, 75% iron, is a ferritic alloy. Both the tendency to form ferrite, and to form sigma, are counteracted by nickel. NICKEL (symbol Ni) Nickel is present, anywhere from 8% up to 80%, in all of the “austenitic” heat resistant alloys. When added to a mix of iron and chromium, nickel increases ductility, high temperature strength, and resistance to both carburization and nitriding. Nickel decreases the solubility of both carbon and nitrogen in austenite. High nickel is bad for sulphidation resistance. Again speaking metallurgically, nickel tends to make the atomic structure “austenitic”, that is, with a face centered cubic (FCC) crystal structure. Nickel counteracts, but doesn’t necessarily stop, the tendency for an alloy to form sigma. While RA446 with 25%Cr 75%Fe (iron) is a ferritic alloy, rather weak, if one substitutes 20% nickel for some of that iron one gets a 25% chromium, 20% nickel, 55% iron alloy called RA310. RA310 is much stronger and more ductile than RA446. IRON (symbol Fe) Heat resistant alloys may contain anywhere from 8 to 75% iron. In some proportions iron is a strengthening element, but it is easily oxidized and carburized unless protected by other elements. Metallurgically speaking, iron is a ferritizing element. Iron itself has a ferritic, or body centered cubic (BCC) crystal structure. Iron base alloys require a certain amount of nickel to be added before they become austenitic. -5- EFFECT OF ALLOYING ELEMENTS, continued THE NEXT GROUP of alloying elements present in all heat resisting alloys is silicon, carbon, nitrogen, sulphur and phosphorus. All may be considered impurities arising from the steel making process. They may either be tolerated at some level as undesirable impurities, or controlled for their effects on metal properties. Silicon, for example, affects the fluidity of the molten metal, which is an important variable in the steelmaking process. Carbon is controlled within certain limits in heat resisting alloys as a strengthening element, normally above 0.04%. In corrosion resistant grades carbon is considered an undesirable element, and is kept as low as practical, under 0.03%. Nitrogen may be controlled like carbon as a strengthening element in both the heat and the corrosion resistant grades. When not used deliberately, there may be about 0.05% or so N in austenitic stainless and nickel alloys. Sulphur is generally undesirable, but some sulphur is used to improve machinability. Phosphorus is quite harmful to weldability in nickel alloys. SILICON (symbol Si) Silicon improves both carburization and oxidation resistance, as well as resistance to absorbing nitrogen at high temperature. At high enough levels, silicon improves resistance to alkali metal hot corrosion.Silicon can decrease weldability in some, not all, alloys. In the U.S., Rolled Alloys has long been the only company to produce wrought heat resistant alloys containing silicon. RA330® has 1.2%Si, RA333® about 1% silicon. All the cast heat resistant alloys have silicon, in part because it increases fluidity of the molten metal. In Europe silicon is used to improve a number of heat resistant alloys, such as the AvestaPolarit inventions, RA 253 MA ® and RA 353 MA ®, and the German alloys 314 (1.4841) and 1.4828. The metallurgical effects of silicon are that it tends to make the alloy ferritic, or to form sigma. In RA330, the 35% nickel content is more than enough to prevent any embrittling sigma to form from the Si. Silicon decreases the solubility of carbon in the metal (technically it increases the chemical “activity” of carbon in the alloy). A silica (silicon oxide) layer, just under the chromium oxide scale on the alloy, is what helps the alloy resist carburization. CARBON (symbol C) Carbon, even a few hundredths of a percent, is a strengthening element. As the carbon level increases, the alloy becomes stronger, but it also becomes less ductile. Most wrought heat resisting alloys contain around 0.05 to 0.10% carbon, with RA 602 CA near 0.2%, and RA330HC at 0.40% C. The cast heat resisting alloys usually have from 0.35% up to 0.75% carbon. While strong, the cast alloys are not very ductile. Corrosion resistant grades, by contrast, have less than 0.03% carbon, and sometimes much less. -6- CARBON (symbol C), continued Carbon is an “austenitizing element”, and tends to retard or prevent formation of ferrite and sigma. Carbon may actually be dissolved in the alloy, or, more commonly, it is present as small, hard particles called carbides. These are chemical compounds of carbon with chromium, molybdenum, tungsten, titanium, ziconium or columbium (niobium). NITROGEN (symbol N) A small amount of nitrogen serves to strengthen austenitic heat resisting alloys. Too much nitrogen can embrittle them. Nitrogen is used to strengthen Outokumpu’s heat resistant grades 153 MA ®, 253 MA ® and 353 MA ®, likewise Haynes® HR-120®. Nitrogen is also an “austenitizing” element. It tends to retard or prevent ferrite and sigma formation. A small amount of nitrogen is specified in RA446. This causes a little austenite to form (in with the ferrite) while it is being hot worked. This in turn helps keep the grain size from getting too large. Nitrogen at 0.23% is used in the corrosion resistant alloy AL-6XN® to prevent sigma formation. It also raises the tensile and yield strengths of AL-6XN, and increases its resistance to chloride pitting corrosion. SULPHUR (symbol S) Sulphur is normally regarded as an impurity, and is commonly below 0.010% in most nickel alloys. It has the benefit of improving machinability, so for 304 and 316 bar it is kept up around 0.02%. Free machining stainless steels, such as 303, may have much higher sulphur, 0.3%. To improve hot workability, and therefor maximize yields, the steel mill normally refines the metal to a very low sulphur content. This is fairly easy to do with current melting processes such as the AOD (argon-oxygen decarburization) or ESR (electro-slag remelt) furnaces. AL-6XN is refined to extremely low sulphur, 0.001% being not uncommon. Sulphur is also detrimental to weldability. Along with simply removing the sulphur in the refining process, the harmful effects of S on hot working and welding may be reduced to a degree by the addition of some manganese. PHOSPHORUS (symbol P) Phosphorus is harmful to weldability. Phosphorus cannot be removed during the refining process. To produce alloys with low phosphorus, one must start with low phosphorus raw materials. Because phosphorus is so harmful to nickel alloy weldability, the nickel weld fillers themselves are normally specified to have no more than 0.015% phosphorus, and even lower P would be preferred. -7- EFFECT OF ALLOYING ELEMENTS, continued OTHER METALLIC ALLOYING ELEMENTS include cobalt, manganese, tungsten, molybdenum, titanium, aluminum, columbium (also called niobium), zirconium, the rare earth elements such as cerium, lanthanum and yttrium, and boron. Copper and vanadium are used in some corrosion resistant alloys but not in the heat resistant grades. Some of these elements are added for strength, others like aluminum and the rare earth elements are largely for oxidation resistance. COBALT (symbol Co) Cobalt at the 3% level in RA333 improves strength slightly and enhances oxidation resistance at extreme temperatures. Larger amounts are required for a significant strengthening, such as the 15%Co in cast Supertherm® or 12.5% in the LBGT combustor alloy 617. Cobalt base alloys L605 and 188 are very strong, but oxidation resistant only to 1800 or 2000°F (1000 or 1100°C). Cobalt is an austenitizing element, like nickel. High cost and variations in availability tend to limit the use of cobalt alloys to gas turbine engine applications. MANGANESE (symbol Mn) Manganese is used in steelmaking to improve hot workability. It is mildly detrimental to oxidation resistance, so is limited to 2% maximum in most heat resistant alloys, and restricted further, to 0.80% max, in RA 253 MA. Manganese improves weldability, and is added to many austenitic weld fillers. RA330-04 achieves its hot cracking resistance from about 5% manganese added to the 35%Ni 19%Cr base. Manganese is usually considered an austenitizing element. It increases solubility for nitrogen and has for decades been used in the Nitronic® series of stainless steels from AK Steel (formerly Armco), both as a partial substitute for nickel and to permit a substantial nitrogen addition. TUNGSTEN (symbol W) Tungsten is a large, heavy atom used as a strengthening addition, 3% in RA333, 5% in the cast alloy Supertherm and 14% in Haynes alloy 230®. Tungsten is a carbide forming element, that is, it reacts with the carbon in the alloy to form a hard particle, which may incorporate other carbide forming elements such as chromium. Tungsten also promotes formation of sigma, and of ferrite. Tungsten metal, with thoria or rare earth oxide additions, is used for the electrode in gas tungsten arc welding. It is tungsten’s very high melting point, 6170°F (3410°C), which is required for this application. Tungsten oxidizes in air readily above 950°F (510°C), but is prevented from doing so by the argon or helium weld shielding gas. -8- MOLYBDENUM (symbol Mo) Molybdenum is another large, heavy atom used to increase high temperature creep-rupture strength. 3% Mo is used in RA333. This is about as much Mo as can be tolerated in a heat resistant alloy without serious oxidation problems in heat treat furnace applications. Alloys X, 625 and 617 all contain 9% molybdenum, which is very good for strength but not so good for oxidation at extreme temperatures (2100°F/1150°C). Molybdenum promotes sigma formation, unless counterbalanced by austenitizing elements such as nickel, cobalt, etc., and is a ferritizer. Molybdenum is also a carbide forming element. Molybdenum helps weldability in austenitic alloys, both stainless and nickel base. Commercially pure molybdenum metal is used for vacuum furnace fixturing, because of its very high melting point, 4730°F (2610°C), and high temperature strength. However, molybdenum metal has no oxidation resistance above 800°F (427°C) and would literally disappear in a cloud of white smoke if exposed to air at red heat. TITANIUM (symbol Ti) Titanium is added in small amounts, about 0.3- -0.7%, for strength in austenitic alloys. Around 0.1—0.2%Ti is used, as part of steel mill melting practice, in deoxidation of nickel alloys. Ti is a strong carbide former, and it is the titanium carbides that strengthen RA800AT. Titanium also promotes sigma and ferrite, but it is normally used in such small amounts as to be inconsequential in this respect. In aqueous corrosion alloys titanium is referred to as a “stabilizing” element. Age hardening alloys used in aerospace, such as A-286, X750, C-263, the various Nimonic® alloys, Renè 41®, WASPALOYTM and 718, depend upon some larger amount of titanium, to 3% or so, for their age hardening properties. Titanium metal itself, although it has a very high melting point (3040°F/1671°C), is not really a heat resistant metal. Titanium alloys are used up to about 600°F (316°C) in aerospace applications. ALUMINUM (symbol Al) Aluminum is added at the 1 to 5% level for oxidation resistance. RA 602 CATM has 2.2% Al, alloy 601 contains 1.4% Al, and Haynes 214 4.5% aluminum. Aluminum is a ferritizing element, and promotes sigma formation. It is used in the age hardening (precipitation hardening) alloys. At around 0.1 to 0.4%, aluminum is added to most nickel alloys as a deoxidizing agent, in the last stages of AOD refining. -9- COLUMBIUM (symbol Cb) . . . also called NIOBIUM (symbol Nb) Columbium is added at the 0.4 to 0.8% level for strength in several heat resisting alloys, and to prevent corrosion after welding in 347 stainless and nickel corrosion resistant alloys 20Cb3®, G-3 and G-30. This low amount of Cb is harmful to weldability, while higher amounts are beneficial. About 2 to 2.7%Cb is used in various high nickel weld fillers (82, 182), while the 3.6% Cb in 625 is good for both strength and weldability. Columbium is very harmful to oxidation resistance, practically speaking around 1800°F/980°C and higher. For this reason we limit the amount of residual Cb that may be present in RA330, and in RA333. Columbium is a strong carbide former, a ferritizing element and promotes sigma formation. At the 5% level, it is the age hardening element in alloy 718. ZIRCONIUM (symbol Zr) Zirconium is a strong carbide former. It is added in very small amounts, less than 0.1%, to increase strength in RA 602 CATM, and in alloy 214. THE RARE EARTH ELEMENTS cerium, lanthanum and yttrium are used singly or in combination to increase oxidation resistance in austenitic alloys both wrought and cast, and in the newer ferritic heat resistant alloys. The technology has been known, but little used, since about 1940 in Germany. CERIUM (symbol Ce) Cerium is the major rare earth element responsible for the excellent oxidation resistance of RA 253 MA. The cerium is added as an alloy of several rare earths, called mischmetal. For chemistry control purposes, the mill analyzes only for Ce. Mischmetal is encountered in everyday life as the “flint” in a cigarette lighter. It oxidizes (burns) very readily. In RA 253 MA and RA 353 MA the Ce helps chromium form a thinner, tighter and more protective oxide scale. Residual cerium oxides in the metal may contribute to creep-rupture strength. LANTHANUM (symbol La) Used at the 0.02 to 0.05% range to for oxidation resistance in Haynes alloys 230, S, 556 and 188. YTTRIUM (symbol Y) Used at the 0.005 to 0.1% level for oxidation resistance in 214 and RA 602 CA. A larger amount, 0.5%, of yttria (Y2O3) is used as an oxide dispersion strengthening element in the ODS ferritic alloys such as Inconel® MA956. As yttria, it also increases oxidation resistance. - 10 - BORON (symbol B) Boron increases creep-rupture strength, and is used at rather low concentrations, 0.002% is typical. Boron is somewhat harmful to weldability of nickel alloys, so nickel alloy weld filler is often made without boron, even though the matching base metal alloy has a boron addition. This is specifically true of alloys such as RA333, X and 230. Boron is an interstitial element and tends to concentrate at the grain boundaries. It is used in high temperature braze alloys, specifically the Nickel-Silicon-Boron braze alloys developed by Dr. Robert Peaslee. - 11 - RESISTANCE TO THE ENVIRONMENT “Corrosion resistance” at high temperatures is a general term for resistance to a variety of hot gaseous or liquid environments that can eat holes through the metal, turn it completely into a pile of scale or seriously embrittle a formerly ductile alloy. It includes (but is not limited to) the effects of oxygen, nitrogen, chlorine, carbon, sulfur, phosphorus, various molten salts and low melting metals. In many high temperature environments one significant effect of corrosion is to continuously change the actual chemistry of the alloy throughout its life. With the exception of selective leaching (parting corrosion), the examples being dezincification of copper-zinc alloys 1, or denickelification2 of the 67Ni-31Cu alloy Monel® 400, this is rather uncommon in aqueous corrosion of nickel base alloys or stainless steels. Nevertheless in high temperature corrosion selective removal of one or more alloying elements through the life of the equipment is the norm. Or, the metal may increase in carbon and nitrogen content, with consequent major change in mechanical properties, and no loss in cross section. Heavily carburized metal actually increases in volume. The types of high temperature corrosion most commonly encountered are oxidation, carburization, sulfidation, hot salt corrosion, chlorination and attack by low melting metals. Nitriding may be important, but is less often considered, if not encountered. It is difficult to run high temperature corrosion tests in the laboratory and obtain results that can be used to predict metal behavior in service. Even two laboratories running the same type of test may not come up with numerical results that agree with one another, although the alloy rankings should be similar. Based on extensive experience and lab work, we have confidence that our oxidation data may be used to compare relative performance of one alloy with another, at that test temperature. Still, good performance of a new alloy in this test only indicates that the alloy MAY perfo rm well in service. As oxidation rates vary with thermal cycling, among other variables, the data are not directly useful for predicting metal wastage/corrosion rates of high temperature equipment in service. It is even more difficult to obtain data useful as an engineering tool to predict life of equipment in sulphidation, carburization, liquid metal environments and other types of high temperature corrosion. We must emphasize that laboratory data are a necessary first step, but the laboratory test itself must be validated by documented service experience for it to be a useful engineering tool. In the following pages we will present the results of both laboratory testing, controlled service experience and experience reported by others. Read these data, as well as those of other suppliers, with a critical eye. References 1. The Corrosion Handbook, page 69, edited by Herbert H. Uhlig, Ph.D., John Wiley & Sons, New York, 1948 2. D.R. Lenard and R.R. Welland, Corrosion Problems with Copper-Nickel Components in Sea Water Systems, NACE Corrosion 98, Paper Number 599, NACE Houston, Texas - 12 - OXIDATION For our purposes, this means the high temperature chemical reaction of a metal with the oxygen in the air. Simply put, most metals can burn when they get hot enough. Some, like magnesium (once used in flash bulbs) and titanium do burn in the conventional sense and cause serious industrial fires. Even iron burns. Of course, lighting a match to a nail does absolutely nothing. But if one takes very fine iron wire—specifically, 0000 steel wool—it may indeed be ignited by a match. There is no actual flame, but a red hot “coal” develops and enough sparks fly to endanger clothing. There are two basic ways in which a metal may be resistant to oxidation. First, it may be inert and simply not react chemically with oxygen in the air. Two examples come to mind, the precious metals gold (Au) and platinum (Pt). Because of its high melting point, 3217°F (1769°C), coupled with oxidation resistance, platinum is actually used for some laboratory ware and other items which must withstand extreme temperature. The second way a metal may resist oxidation, and the one of interest to us, is that the metal or alloy may form an adherent oxide film, which protects it from further oxidation. The element most often used to form such a protective oxide layer, or scale, is chromium. It forms the oxide Cr2O3, otherwise known as chromia. Although chromium itself oxidizes even more readily than iron, the oxide it forms is very thin, and adheres tightly to the metal. This oxide layer forms very quickly at high temperature, but once formed it protects the metal against further oxidation. The chromia scale also protects the alloy against carburization and sulfidation, to a degree. The protection is by no means perfect. The scale contains defects through which oxygen and other elements may pass, to continue to react with the alloy. It also cracks from both thermal and mechanical strains, and small pieces spall off each time the metal is cooled down. For a high temperature alloy to have useful oxidation resistance, the scale must be able to “heal” these defects, by more chromium diffusing to the surface to form a new protective film. Other elements are added to the alloy to improve the protective nature of this oxide film or scale. One of the most effective is silicon. Silicon oxidizes to SiO2, or silica. If enough silicon is present, the silica forms a sub-scale underneath the chromium oxide scale. This silica subscale is how silicon provides resistance to carburization, in alloys such as RA330. At the 1.2% Si level in RA330, silicon also contributes to oxidation resistance. In RA85H, which is no longer produced, the silicon was much higher, 3.5%. At this high level silicon appeared to offer resistance to molten alkali salt corrosion. The effectiveness of the chromium oxide scale may be improved by very small additions of rare earth elements, such as cerium. Cerium promotes a thinner scale, which is more protective against oxidation because it cracks and spalls off less than would a thicker scale. It is the 0.04% cerium in RA 253 MA that is responsible for the excellent oxidation resistance of this rather lean 21Cr, 11Ni alloy. - 13 - OXIDATION, continued Aluminum is also used to improve oxidation resistance. In order to actually develop an Al2O3 , or alumina, scale a rather high amount of aluminum is required. At 2.2% aluminum, RA 602 CA alloy will form an alumina subscale. This contributes to the oxidation resistance of RA 602 CA. RA 602 CA does not oxidize internally. The 1.7% Al typical in alloy 601 is not enough to form an alumina scale, but it is enough to enhance oxidation resistance of 601. Because of aluminum at this somewhat lower level, 601 oxidizes internally. This is not a problem in plate gauges, though perhaps it may be a consideration in thin sheet. The 4.5% aluminum in Haynes alloy 214 is enough to form an alumina scale, and 214 is extremely oxidation resistant (above 1800°F/982°C). The Protective Film While chromium is given credit for promoting oxidation resistance and is without question the most effective element in this respect, it is actually the most easily oxidized. This may sound like double talk, but it really isn’t. When pure chromium or a chromium-bearing alloy is exposed to oxygen, even at room temperature, it is oxidized and a layer of chromium oxide (and oxides of other elements as well) is formed. Even the chromium plate on automobiles, or the cutlery on our dinner tables, has a microscopically thin and transparent film of chromium oxide present. When formed at high temperatures, the oxide coating becomes green, black, blue or yellow, depending upon its thickness and which of the numerous chromium oxide compounds is formed. This in turn depends upon the temperature and availability of oxygen to combine with chromium. The oxide layer is dense, is inclined to be tightly adhering, and effectively seals out the air or oxygen from the metal underneath. So long as the oxide layer is intact, the metal is protected and further oxidation proceeds very slowly. Several things may tend to destroy our protective layer: Expansion and contraction, as the result of heating and cooling, will “pop” the oxide layer, because the base metal and the oxide expand and contract at different rates. The more rapid the rate of expanding and contracting, or the more quickly the metal is heated and cooled, the more hazard there is of the protective coating flaking off. Certain combinations of chromium, iron, nickel, silicon and other oxides are more tightly adhering than others at different temperatures. With some alloys it is possible to reach a temperature where the scale or oxide is no longer tightly adhering and will be loose, thereby offering little or no protection. Thus, an excessive temperature for the specific alloy can destroy the protection normally offered by the oxide layer. Some examples are 321, which is acceptable at 1600°F (870°C) but scales unacceptably at 1800°F (980°C), and 309, which isn’t very useful above 1900°F (1040°C). - 14 - The Protective Film, continued Composite radiant tube, RA333 for 4 feet (1.2metre) on the firing end, middle portion RA330 and exhaust end fabricated of RA309. Used at a nominal furnace operating temperature 1750°F ( 955°C) for annealing malleable iron castings. It is to be expected that the tube metal temperature would be perhaps 100—150°F (55—85°C) higher. A jam-up in the furnace broke the tube. Note the crater-like appearance of local oxidation, or warts, on the RA309. When an alloy is used at a temperature exceeding its capabilities the scale may breakdown locally, a condition sometimes called “warts”. We have observed this on 309 (above) and 310, occasionally on RA 253 MA, RA330 and 600 alloy. On one occasion we saw warts on an RA333 brazing muffle. Upon investigation we found that the alloy had been heated in service to the incipient melting temperature. The grains were sliding apart so as to leave voids at the triple points, resulting in apparent porosity of the 11gage (3mm) muffle wall. Mechanical deformation and creep, such as the stretch of a bar under load, may also destroy the protection. While the metal is ductile and yields in creep, the oxide coating is fragile and brittle and will spall off. In service, a given item may appear to have insufficient oxidation resistance, whereas that particular property would have been more than adequate had the strength been sufficient to avoid excessive creep. Laboratory data which do not duplicate cyclic conditions or stresses imposed in actual service can be misleading as a measurement of an alloy’s oxidation resistance. - 15 - The Protective Film, continued Of great concern are environments that promote the destruction of the protective layer by some chemical reaction. For example, we know of one case where minute amounts of potassium nitrate/nitrite austempering salts were present on fixturing used in a carburizing atmosphere. The salts attacked the protective oxide coating, so that a normally carburization resistant alloy carburized very quickly and uniformly. In years past, we knew of a few cases where parts being heat-treated were first coated with sal ammoniac (ammonium chloride). The presence of this chloride salt resulted in a chemical attack upon the protective oxide coating, so that the alloys normally selected for the strength, oxidation resistance and thermal shock resistance requirements were not suitable. Another form of chemical destruction that may be encountered is corrosion from welding fluxes. Fluoride-bearing fluxes from coated welding electrodes must be carefully and thoroughly removed. Otherwise they continue to function as a flux, damaging not only oxidation resistance, but also carburization resistance. Green rot might be considered one form of destruction of the protective oxide coating. To the best of our knowledge, green rot tends to be more prevalent in alloys containing about 65% or more nickel. Green rot is the result of the alloy being alternately exposed to oxidizing and reducing conditions. When the alloy is exposed to the oxidizing environment, a protective oxide coating is formed, as we previously discussed. When the alloy is exposed to highly reducing conditions, the nickel and other less stable oxides may be reduced to pure metal, which disappears as a powder; but the chromium oxide, being more stable, is not reduced. Upon exposure of the alloy to an oxidizing environment once more, the oxygen is free to penetrate to the metal and form another layer of oxide, since there are now voids in the coating where some of the oxides previously existed. With continuous exposure to the two conditions, a mass is eventually formed consisting only of porous chromium oxide, with or without other oxides that may have been sufficiently stable to resist reducing. This actually has little strength and no ductility. It has the characteristic greenish-black color of chromium oxide and, upon fracture, has the appearance of rotten wood. Hence the name, green rot. Catastrophic oxidation is, as its name implies, oxidation which proceeds so rapidly that complete failure of the material occurs in an extremely short time. Certain elements, such as molybdenum, columbium (niobium) and vanadium, form oxides that are volatile at relatively low temperatures. If these oxides are formed and retained in the scale, they act as fluxes and destroy the protective film1, 2. The effect of molybdenum is important enough that we would like to quote directly from the late Howard S. Avery’s classic work on heat resistant alloys, Cast Heat-Resistant Alloys for High-Temperature Weldments: “Where in fact the addition of molybdenum has conferred - 16 - The Protective Film, continued better hot strength, the chief problem may be surface stability, especially in the 1800— 2300°F (980—1260°C) range. This is most serious under those conditions that cause catastrophic oxidation which stems from the volatile nature of molybdenum oxide (MoO3). This oxide is likely to form in stagnant atmospheres, with a threshold for trouble around 1400—1500°F (760—816°C).” Catastrophic oxidation may be a serious problem under certain operating conditions. That is, a stagnant atmosphere, or solid deposits under which the atmosphere is of course stagnant, and extreme temperatures. Alloy X (N06002, W.Nr. 2.4665), containing 47% nickel, 22% chromium and 9% molybdenum, may completely disappear from catastrophic oxidation when heated for some months at 2200°F (1200°F). At lower temperatures in free-flowing atmospheres alloy X is highly oxidation resistant. It has, after all, served for decades as the primary alloy used in gas turbine flight engine combustors. However, alloy X may not well tolerate stagnant conditions or temperature extremes. Laboratory Oxidation Testing In order to evaluate new and competitive alloys we perform considerable laboratory oxidation testing at Rolled Alloys, at temperatures up to 2200°F (1204°C)3, 4 . We measure weight gain, that is, the total amount of oxygen (and nitrogen) that has reacted with the test specimens. Specimens are usually of plate gages, and the tests are cyclic. Samples are heated in porcelain crucibles, 4 to 6 in a tray, for about 160 hours (one week) at temperature. The tray is then removed from the furnace, lids are quickly placed on the crucibles to contain spalling oxide, and the assembly allowed to air cool to room temperature. The crucible, containing specimen and scale, is then weighed every cycle. Results are reported as weight gain, in milligram/centimeter2 . The numerical results are valid only for the specific conditions of the test. Which means they are not useful for predicting metal wastage of components in actual service. However they are of value when one compares the data from new alloys, with those of existing grades. For example, we have a great deal of experience with the good performance of RA333 and RA330. Likewise, 309 is about the only one of our heat resistant alloys that occasionally gives disappointing performance, generally around 1900°F (1040°C) or above. If an alloy performs well on test, that means it MAY perform well in service. As a simple coupon test for 3000 hours or so does not simulate all the things that can happen in service, it is possible for an alloy to look very good in the laboratory and not at all so good in production equipment. One thing the coupon test does not simulate is the effect of creep strain spalling off the scale. Another is the effect of stagnant atmospheres, which may occur in certain areas of electrically heated equipment, or underneath solid deposits. Alloys with high molybdenum contents are subject to catastrophic oxidation under these conditions. - 17 - OXIDATION, continued And, finally, the laboratory test does not properly simulate time. 3000 hours seems a reasonable length of time to run a test in our laboratory, but that is still only about 4 months. If one expects the equipment to last 1, 2 or 10 years, it would be hard to make a case that a 4 month test adequately represents service conditions. The specimen continually changes chemistry throughout the test (it loses chromium, silicon and aluminum by scaling). Thin samples, simply from having less total chromium, may show greater oxidation rates than thick specimens. In our considered view, significant extrapolations of oxidation, or other high temperature corrosion data, are not valid. Nevertheless the declining availability of experienced engineers in the U.S.A. has generated pressure to extrapolate such data, valid or not. Data shown on the following bar graphs is all for 3000 hour (~18 weeks) exposure, in order to compare all alloys for about the same exposure time. All but the RA309 tests were run for 3000 hours, that one being extrapolated from a 1600 hour run. As this is weight gain data, high numbers mean heavy oxidation, small numbers a relatively light degree of oxidation. One would expect to use these numbers, along with service experience, as a guide to an alloy’s usefulness. However, unlike what is assumed about aqueous corrosion rates, oxidation data ought in our opinion be viewed qualitatively. Numbers under 20 may give assurance that the alloy, in plate form, should not lose structural integrity due to metal loss. One might want a little actual service background when considering alloys with weight gains in the 100-300 mg/cm2 range. One example is 304 stainess, which gains 64 mg/cm2 at 1600°F (871°C), and in the neighborhood of 300 mg/cm2 at 1800°F (982°C). By experience, we know that 304 1/4” plate will simply disappear in 2-3 months when used in air around 1700-1800°F (930—980°C). We would look at the higher alloys, for more elevated temperatures, somewhat differently. Note 600 alloy which shows a 153 mg/cm2 weight gain at 2100°F (1149°C). Nevertheless, alloy 600 plate is a useful material for retorts and muffles operating in the 2100-2200°F (1150—1200°C) temperature range. Likewise RA 353 MA is used quite successfully at such temperatures. RA 602 CA is clearly the best by far in our test series. The good resistance to scaling of RA 602 CA in test has also been borne out by service experience in rotary calciners and CVD retorts. Bear in mind that these data still represent simple laboratory oxidation testing, which does not take into account many of the ways by which the protective oxide scale may be damaged. The alloys were cycled to room temperature once a week. More rapid thermal cycling would not only increase oxidation rates but might also change the relative performance of some alloys. In static 1000 hour oxidation testing, for example, 310 is somewhat superior to RA330. When thermal cycling is added, RA330 better retains its protective oxide. Another point to remember is that alloys high in molybdenum and columbium may be sensitive to catastrophic oxidation, particularly under stagnant atmospheres. - 18 - References 1. Leslie and Fontana, Transactions ASTM, Vol 41, pages 1213-1247, 1949, ASTM Philadelphia, Pennsylvania 2. H.S. Avery, Cast Heat-Resistant Alloys for High-Temperature Weldments, WRC Bulletin 143, August 1969, Welding Research Council, New York, New York 3. Gene Rundell and James McConnell, Oxidation Resistance of Eight Heat-Resistant Alloys at 870, 980 1095 and 1150C, Oxidation of Metals, Vol. 36, Nos. 3/4, 1991 4. J.C. Kelly and J.D. Wilson, Oxidation Rates of Some Heat Resistant Alloys, HeatResistant Materials II, Conference Proceedings of the 2 nd International Conference on Heat-Resistant Materials 11-14 September, 1995 Gatlinburg, Tennessee - 19 - Exposed for 3000 Hours Cycled Every 160 Hours 250 200 200 Weight Gain (mg/cm2) 1600F 1800F 2000F 2100F 154* 150 113 100 64 54 53 50 32 20 19 5 1 11* 1 4 0 RA304 RA309 RA310 Alloys - 20 - RA 253 MA RA330 Exposed For 3000 Hours Cycled Every 160 Hours 350 333 324 295 300 2 Weight Gain (mg/cm) 1800F 2000F 2100F 2200F 2250F 250 232 200 150 100 156 153 145 143 138 83 61 58 50 50 36 32 21 4 11 49 34 18 21 12 4 11 18 0 RA800H RA625 RA600 RA X RA 353 MA Alloys - 21 - RA333 RA601 RA 602 CA CARBURIZATION The femper of Iron for Files It must be made of the best Steel, and excellently tempered, that it may polish, and fit other iron as it should be: Take Ox hoofs, and put them into an Oven to dry, that they may be powdered fine: mingle well one part of this with as much common Salt, beaten Glas, and Chimney-soot, and beat them together, and lay them up for your use in a wooden Vessel hanging in the Smoak; for the Salt will melt with any moisture of the place or Air. The powder being prepared, make your iron like to a file: then cut it checquerwise, and crossways, with a sharp edged tool: having made the Iron tender and soft, as I said, then make an Iron chest to lay up your files in, and put them into it, strewing on the powders by course, that they may be covered all over: then put on the cover, and lute well the chinks with clay and straw, that the smoak of the powder may not breath out; and then lay a heap of burning coals all over it, that it may be red-hot about an hour: when you think the powder to be burnt and consumed, take the chest out from the coals with Iron pinchers, and plunge the files into very cold water, and so they will become extream hard. This is the usual temper for files; for we fear not if the files should be wrested by cold waters. But I shall teach you to temper them excellently G. B. Della Porta, 1589, Sources for the History of the Science of Steel 1532—1786, Ed. Cyril Stanley Smith Carburizing is one of the most commonly performed steel heat treatments. For perhaps three thousand years it was performed by packing the low carbon iron parts in charcoal, then raising the temperature of the pack to red heat for several hours. The entire pack, charcoal and all, was then dumped into water to quench it. The surface became very hard, while the interior or “core” of the part retained the toughness of low carbon steel. Pack hardening is uncommon today. Now, low carbon steel parts are heated in a prepared furnace atmosphere that provides the carbon which diffuses into the surface layers of the steel. Temperatures are usually around 1750°F (950°C). This atmosphere has traditionally been “endothermic”, made by partially burning natural gas. Typical composition1 of an endothermic gas (Class 302) is 39.8% nitrogen, 20.7% carbon monoxide, 38.7% hydrogen and 0.8% methane, with a dew point –5°F (–20°C). This carrier gas is subsequently enriched by a small, controlled addition of a hydrocarbon gas, such as propane, or an easily vaporized liquid, which is the source of carbon. 100% nitrogen, from bulk tanks, may also be used as a carrier gas, with propylene or other hydrocarbon injected to provide the necessary carbon. The end result is that low carbon steel parts acquire a high carbon steel surface. When the steel is quenched it combines the hardness and wear resistance of this high carbon steel “case” with the toughness of the low carbon steel interior (core). The alloy bar frame baskets2, radiant tubes and other fixturing in the furnace also pick up carbon through many, many heat treat cycles. The fixtures are made of carburization resistant alloys. Even though the atmosphere is reducing to iron, it is still oxidizing to the chromium and silicon which provide most of the alloy’s resistance to carburization. Even a carburization resistant alloy eventually carburizes. Austenitic alloys do not harden when quenched, like the steel work pieces. Nevertheless, once the heat resistant alloy has picked up sufficient carbon, its room temperature ductility will be greatly reduced. - 22 - Carburization embrittles high temperature alloys, so that they can not be straightened or weld repaired. The degree of embrittlement depends upon the amount of carbon absorbed3, and upon the microstructure. Generally speaking, once an alloy has absorbed about 1% carbon it will no longer have measurable room temperature ductility. We once examined a sample of 310 sheet which contained 4% carbon, and could readily be broken by hand. Enough ductility may remain while at red heat for the metal to perform its task. This, so long as it is not excessively strained at high temperature, or impacted at room temperature. Alloy 601 used in a powdered iron sinteri ng muffle, grain growth is from the operating temperature. Brittle fracture at room temperature comes from the large amount of carbon, 2.34%, absorbed during service. The nitrogen-hydrogen atmosphere is not supposed to be carburizing. Carbon enters the atmosphere from the organic compounds used when the green powder compact is pressed. Carburization resistance in an alloy is conferred almost entirely by the protective oxide scale 4, along with the nickel content. The oxide scale is primarily chromia, with silicon being a very potent assist5. Nickel lowers the solubility of carbon in the alloy, so that a very high nickel grade simply will not carburize to the same level as will a lower nickel material. RA330 usually does the best job for the money. RA333, RA600, RA 353 MA, RA601 and RA 602 CA are all more carburization resistant but also more expensive. 800H does not well tolerate the effects of carburization, in part because it lacks silicon but also, and more importantly, because it is invariably coarse grained. RA 253 MA has worked as furnace fixturing because it is strong, but RA 253 MA is not resistant to carburization. Even RA309 has somewhat better carburization resistance than RA 253 MA, which is of practical consequence in steel coil annealing covers. These lower alloys such as RA309 and RA310, and the common stainlesses do not possess adequate resistance to carburization for use as fixturing in commercial carburizing heat treat furnaces. Ferritic grades such as 446 are quite poor in carburization resistance. - 23 - Carburization, continued When nickel heat resisting alloys become carburized, it happens that many also become magnetic. A pocket magnet, then, becomes a handy tool to judge whether or not alloy fixturing has enough ductility remaining to be weld repaired or straightened. Not due to carburization, but a purely mechanical problem that may occur in a carburizing atmosphere is of some concern. Soot may deposit from the atmosphere and “coke” in any crevices, such as cracks in weld joints or surface defects on castings. The growth of this soot deposit acts like tree roots growing in rock. It literally pries open lack of fusion in the weld or turns small pin holes into large cavities. In the case of wrought alloys, which are free of surface defects, we emphasize the need to have designs and weldments that do not provide crevices in which carbon deposition may occur. This is one reason why full penetration welds of the return bend to straight leg are essential for maximum life in radiant tubes. On low fire soot may deposited in the root crevice (as well as in surface defects of cast return bends). On high fire this soot burns out, locally overheating and weakening the metal. Carburization testing Laboratory carburization testing must be carried out in some approximation of the industrial atmosphere of interest. The test temperature should be similar to that anticipated in service. In addition it would be a good idea to include thermal cycles about like the expected service conditions 6. Finally, test time is important. We have noted that carburization resistance depends upon the chromia scale, and, in some grades, the silica subscale. For this reason the test atmosphere must contain an oxygen partial pressure comparable to the expected service atmosphere, in order to form a similar protective scale 7 . One may also wish to consider the nitrogen level, as nitrogen from the atmosphere can react with alloying elements such as chromium, and may affect carburization. There have been laboratory carburization tests run in an atmosphere of hydrogen—2% methane, with no control of oxygen partial pressure. As the alloy will not develop much of a protective scale, such an atmosphere is an excellent way to achieve the objective of actually carburizing the alloy. As it is the oxide scale which is primarily responsible for carburization resistance, it is unlikely that such a test should rank alloys as they perform in actual service. Whether ethylene tubes or heat treat fixtures, some partial pressure of oxygen is almost always present. - 24 - Carburization testing, continued In the absence of dew point (oxygen partial pressure) control the results may not even be repeatable. Very small amounts of oxygen can form enough alumina or titania scale, for example, to inhibit brazing. Even alloy 800H contains enough titanium to turn light gray in a common vacuum heat treat furnace. In order to braze even stainless steel (with no Al or Ti) in hydrogen it is normally considered that the dew point should be –60°F (–51°C) or lower8 . This is necessary to dissociate the oxides of most alloying elements. Alumina and titania will not be dissociated by this atmosphere. One might expect that grades such as N06601, N06025 and N0811 would form aluminum and titanium oxide films in a nominal hydrogen— methane atmosphere. Such films may affect carburization. When the atmosphere simulates that of industrial interest, carburization testing may require long exposure. There is some period of time during which significant carbon absorption does not take place. Experience related to us from one furnace company indicated that the test had to be run for at least 1000 hours, before the results correlated with service experience. The following results, from that same company, are from tests conducted in an electrically heated industrial carburizing furnace. The higher temperature results, 1900°F (1038°C) are from a composite electric heating element made of the five alloys shown, and the 1750°F (954°C) results are from plate samples exposed to the actual furnace operating temperature. In both cases the total exposure hours were distributed as follows: 20% of the time in endothermic gas enriched with natural gas to carbon potential 1.0-1.2%C relative to iron, 70% of the hours in nitrogen, and 10% of the time reflected air burnout cycles at 100°F (56°C) reduced temperature. Various depth of cuts were machined in the samples and the carbon contents analyzed. Results here are reported at 0.045” (1.14mm) depth on the element and 0.20” (0.508mm) depth on the plate sample. 1900°F (1038°C) 2260 hour exposure 1750°F (954°C) 4300 hour exposure alloy %carbon %carbon RA333 RA330 617 601 600 310 1.53 3.03 2.86 2.98 1.56 -- 0.344 0.443 1.6 1.096 -3.92 - 25 – METAL DUSTING A somewhat aggravating problem in carburizing atmospheres is “metal dusting”, a.k.a. “catastrophic carburization”, or “carbon rot”. This occurs at lower temperatures, typically 800—1200°F (430—650°C) in heat treating furnaces. Such temperatures exist in a carburizing furnace (nominal 1750°F/950°C) where alloy tube hangers, atmosphere sampling tubes or electrical leads pass through furnace walls, and in some areas of Ipsen furnace chains. The exact mechanism may be disputed, but the effect is that the metal disappears. A bar may look just like a beaver had chewed away on it. In other cases, the metal literally appears worm-eaten on the surface. In the petrochemical industry, a small amount of sulphur (40—50 ppm H2S) is sometimes added to the process gas stream to “poison” the high temperature chemical reaction that is metal dusting. Alloys vary greatly in susceptibility to metal dusting. RA333, by experience and several years testing in the heat treat industry, is the best known choice. RA85H was also good, though not quite so resistant as is RA333. RA330 is so-so, 800H perhaps worse, and 600 alloy is the least resistant. Neither 310 nor 601 will solve metal dusting problems. One direct alloy comparison, below, shows two RA333 GMAW beads with only minor surface smoothing, while the 3/16” (4.8mm) RA310 plate between them suffered nearly complete loss of section9. This example is from a rotary retort used to carburize small parts at an operating temperature of about 1750°F (940°C). Spiral flights of RA310 welded to the inside served to transport work pieces through the retort. As the retort was externally fired, the 3/8” (7.9mm) 600 alloy shell was above the temperature range for metal dusting. Metal dusting was a serious problem with flights at the entry end of the retort. Here the cold work pieces chilled the RA310 flights down into the metal dusting temperature range. - 26 - METAL DUSTING, continued Furnace chain severely attacked by metal dusting. This is an application where RA333 has given the best service life in original equipment. - 27 – METAL DUSTING, continued RA330 carburizing furnace anchor bolt, 3/4” (19 mm) diameter. Failure by metal dusting. RA330 gave better life than 600 alloy in this application. Three possible solutions here, depending upon the cost of furnace down-time, are to: 1.) Simply keep replacing the part in RA330 2.) Alonize® the replacement RA330, or 3.) Replace the part in RA333. The following test results are from a direct comparison of alloys for 25,594 hours (3 years) at temperature, in the metal dusting zone of a Surface Combustion carburizing furnace. 1” (25.4mm) Sch 40 oxygen probes of various alloys with different surface treatments were inserted through the furnace roof. The atmosphere is endothermic enriched with 0.7—0.8% methane to a 1.20% carbon potential, operating temperature 1700°F (927°C). Metal dusting occurs in the region where temperatures are roughly 1100°F (600°C), as the pipe passes through the refractory. Alloy Condition Results RA333® As received Preoxidized Dark, no pits at 27,594 hours Some pits at 16,183 hours RA85H® As received Preoxidized Black, no pits at 8122 hours Black, no pits at 7549 hours RA330® As received Pitted, test stopped at 19,472 hours 214TM As received Preoxidized Many pits, test stopped at 19,472 hours Many pits, test stopped at 19,472 hours HR-120TM As received Pitted—removed from test at 11,264 hours HR-160TM As received Pitting started at 24,422 hours Preoxidizing treatments provided no benefits or were counter productive. Although aluminum diffusion coatings are usually considered to provide resistance to metal dusting, the 4.5% nominal aluminum content of alloy 214 was ineffective. - 28 - NITRIDING Nitrogen reduces alloy ductility in a manner similar to carbon. A great deal of attention is given to carbon-pickup in alloys at high temperature, but the nitrogen content is rarely analyzed. An increase in nitrogen content may occur during high temperature service in air, though not usually sufficient to cause of failure. RA446 plate exposed 3000 hours in air at 2100F (1150C). Initial nitrogen content 0.089%, after exposure nitrogen reached 1.15%. Chromium nitrides in this photomicrograph are the needles at 60 angles. Surface, at top, shows some internal oxidation. Absence of nitrides probably due to chromium depletion from oxidation. Mill certification, Jessop Steel Co. Heat 26445 0.18C 0.70Mn 0.47Si 0.26Ni 24.84Cr 0.03Mo 0.03Cu 0.089N Nitrogen has been associated with blistering and severe reduction of creep-rupture strength in carburized HL (30Cr 20Ni) steam-methane reformer tubes10. Carburization decreases nitrogen solubility in Ni-Cr-Fe alloys, by removing chromium from the matrix. Because of the reduced solubility, nitrogen then diffused ahead of the advancing carburized front. This locally concentrated the nitrogen, and contents as high as 0.462% were measured. It was postulated that this could result in high nitrogen gas pressure, which the authors associated with microvoids and cracks. A lamellar phase near the grain boundaries was apparently a nitride phase. Commercial nitriding, e.g. the Floe process, usually is done with RA600 alloy fixturing. Carbo-nitriding is a process carried out in an atmosphere containing both carbon and nitrogen. Temperatures are usually higher than for nitriding but lower than carburizing, roughly 1300—1650°F (705—900°C), and times are shorter. The life of alloy fixturing in a carbo-nitriding application cannot be expected to equal that in a straight carburizing environment, probably for two reasons. First, because the embrittling effect of carbon and nitrogen combined is more drastic. Second, because the cycles are much shorter. The hours or years of exposure are not the important things affecting an alloy’s (quenching fixture) life, but rather the number of cycles it receives. Thermal fatigue cracking gradually develops and grows with each cycle. A part in a carbo-nitriding environment will receive many more cycles in a given length of time than if it were in a carburizing application, and its life will be shortened accordingly. - 29 - NITRIDING, continued References 1. Furnace Atmospheres, Metals Handbook ® Ninth Edition, Volume 4 Heat Treating, ASM, Metals Park, Ohio 1981 2. G. R. Rundell, Evaluation of Heat Resistant Alloys in Composite Fixtures, Corrosion 86 Paper Number 377, National Association of Corrosion Engineers, Houston, Texas 1986 3. D. E. Wenschof and J. A. Harris, The Influence of Carburization on the Mechanical Properties of Wrought Nickel Alloys, Corrosion/77 Paper No. 9, National Association of Corrosion Engineers, Houston, Texas 1977 4. R. H. Kane, Carburization of Cast Heat-Resisting Alloys in Synthetic Petrochemical Environments, Corrosion/83 Paper Number 266, National Association of Corrosion Engineers, Houston, Texas 1983 5. D. B. Roach, Carburization of Cast Heat-Resistant Alloys, Corrosion/76 Paper No. 7, National Association of Corrosion Engineers, Houston, Texas 1976 6. D. J. Hall, M. K. Hossain, and J. J. Jones, Factors affecting carburization behavior of cast austenitic steels, Materials Performance, January 1985, Houston Texas 7. R. H. Kane, Effects of Silicon Content and Oxidation Potential on the Carburization of Centrifugally Cast HK-40, Corrosion/80 Paper Number 168, National Association of Corrosion Engineers, Houston, Texas 1980 8. Brazing of Heat-Resistant Alloys, Low-Alloy Steels, and Tool Steels, ASM Handbook ® Volume 6, Welding, Brazing and Soldering, ASM International, Metals Park, Ohio 1993 9. James Kelly, Metal Dusting in the Heat Treating Industry, Stainless Steel World 99 Comference, The Hague, Netherlands 1999 10. J. R. Schley and F. W. Bennett, Destructive Accumulation of Nitrogen in 30 Cr 20Ni Cast Furnace Tubes in Hydrocarbon Cracking Service at 1100C, Corrosion, September, 1967 National Association of Corrosion Engineers, Houston, Texas SULPHIDATION Environments containing sulphur may rapidly attack high nickel alloys. The problem is more severe under reducing, or low oxygen, environments. The higher the nickel the more sensitive the alloy is to sulphidation attack. If sulphur is a problem, we do not suggest using any alloy with more than 20% nickel. RA310, with 25% chromium and 20% nickel, is useful in many sulphur bearing environments. RA309, at 13% nickel, may be preferred for some applications. Under the most severe conditions an alloy completely free of nickel, such as RA446 may be required, in spite of other disadva ntages it has. When the environment is oxidizing the alloy is more likely to form a protective chromium oxide scale, rather than a chromium sulphide. Under reducing environments the alloy forms chromium sulphide, which is non-protective. - 30 - SULPHIDATION, continued An oxidizing environment is one in which sulphur is present as sulphur dioxide (SO2), and there is some excess oxygen (O2), or even carbon dioxide (CO2) and/or water vapor (H2O). In reducing environments sulphur is in the form of hydrogen sulphide (H2S), there may be hydrogen (H2 ), carbon monoxide (CO), methane (CH4) or other sources of carbon, and rather little CO2 or H2O. Sometimes the distinction isn’t obvious. For example, there may be solid deposits on metal in an oxidizing environment. Underneath those deposits, in contact with the metal the actual amount of oxygen available to form a scale may be very, very small. We have heard it stated that oxygen partial pressures are about 10-8 underneath calcium sulphate deposits on some fluidized bed components. If the deposit contains sulphur, then the metal may be heavily attacked under the deposit, regardless of how much oxygen is in the atmosphere above it. An example of under deposit attack is shown below. This is 1/4” (6.35mm) thick RA 253 MA from a kiln processing ferrous sulphate monohydrate to red iron oxide pigment. Atmosphere air plus the SO2 and SO3 driven off in the process, operating temperature 1840°F (1004°C). After about a year the RA 253 MA kiln shell had developed holes roughly 3/4” (20mm) across, some rather long. Previously used RA310 had failed by more uniform thinning, and lasted 2 to 2 1/2 years. From Rolled Alloys Report Number 94-72 - 31 - SULPHIDATION, continued Types of Scale Developed on Type 310 Stainless Steel as a Function of Oxygen and Sulfur Partial Pressures in the Gas Environment at Temperatures of 750, 875, and 1000C. Conversion factor: 1 atm = 0.101356 MPa. ANL Neg. No. 306-79-6251 Most sulphidation failures occur under highly reducing conditions. That is, where a source of carbon, such as methane (CH4) is present along with the hydrogen sulphide Even 1/2% of H2S can be quite destructive. One example is in carbon black manufacture. Low-grade oil is heated, with very little oxygen, to break it down into soot—which is carbon- or lamp black. The oil used as feed stock normally contains up to 3 percent sulphur. High nickel alloys are quite unsuited for high temperature service in the sulphidizing environments of carbon black plants. Specifically, RA330, RA333, alloys X, 800H, 600, 601, 617 and even some of the high cobalt alloys may fail by sulphidation. - 32 - SULPHIDATION, continued Nickel reacts chemically with sulphur very readily. Unlike metal oxides, which at least are solid, metal sulphides, or metal-metal sulphide eutectics, are often molten at operating temperature. If sufficient molten metal sulphide forms underneath the chromium oxide scale, it may literally wash that scale away. Valid laboratory corrosion testing for sulphidation resistance requires very long time exposure. In general, the corrosion rate in sulphidation may be more or less parabolic for some period of time. Eventually, for a variety of reasons, corrosion enters a “break-away” mode2 , where corrosion rates accelerate dramatically. It is the time to break-away corrosion, rather than the linear or parabolic corrosion rate, that is significant. Melting points of some metal-metal sulphide eutectics are3 : 1175°F (635°C) for Ni-Ni3S2, 1611°F (877°C) for Co-Co4S3 and 1810°F (988°C) for Fe-FeS. The Fe0-FeS eutectic melts at 940C4 . CrS-Cr2S3 doesn’t melt until 2462°F (1350°C)5. References 1. K. Natesan, CORROSION AND MECHANICAL BEHAVIOR OF MATERIALS FOR COAL GASIFICATION APPLICATIONS, ANL-80-5, Argonne National Laboratory, Argonne, Illinois U.S.A. 1980 2. Maurice A. H. Howes, High-Temperature Corrosion in Coal Gasification Systems, Final Report (1 October 1972-31 December 1985) as subcontractor to The Materials Properties Council, Inc., New York, New York. 3. Binary Alloy Phase Diagrams, Thaddeus B. Massalski, Editor, 1986, American Society for Metals, Metals Park, Ohio 4. Stanislaw Mrowec, Teodor Werber, Gas Corrosion of Metals, translation published by the Foreign Scientific Publications Department of the National Center forScientific, Technical and Economic Information, Warsaw, Poland 1978 5. Handbook of Chemistry and Physics, 65th Edition, CRC Press Inc., Boca Raton, Florida 1984—1985 - 33 - HALOGEN GAS HOT CORROSION Unlike oxides, metal halides are volatile. When halogens are present in high temperature environments any oxide scale present becomes porous and non-protective. In order to form a protective scale it is generally considered that the metal chloride vapor pressure must be below 10-4 atmosphere. For CrCl3 and NiCl2 that would be about 600°C, just slightly lower for CoCl2. For FeCl3 the limit is much lower, about 160°C, and lower yet for MoCl5, 50°C, and AlCl3, about 75°C. As a practical matter, the high nickel alloys 600 (UNS N06600) and 400 (N04400) are most commonly chosen for hot halogen gas resistance. Good discussions of this subject are given in the old INCO® Corrosion Engineering Bulletins CEB-3 for HCl and Cl2 , and CEB-5 for HF and F 2. Data for 100% Cl2, and for HCl follow. Corrosion in dry Chlorine Gas Metal Approximate temp, °F, at which given corrosion rate, mils/year, is exceeded in short time tests in dry Cl2 Corrosion in Dry Chlorine, 100% Nickel alloy 600 alloy 400 316 304 Platinum CopperA SteelB Gold Silver 30 60 120 600 1200 950 950 750 600 550 900 350 250 250 100 1000 1000 850 650 600 950 450 350 300 150 1100 1050 900 750 650 1000 500 400 350 250 1200 1200 1000 850 750 1050 500 450 400 450 1250 1250 1000 900 850 1050 550 450 400 500 A copper metal ignites in hot Cl2 at about 600°F carbon steel ignites in hot Cl2 at about 450-500°F B - 34 - Suggested upper temp limit for continuous service, °F 1000 1000 800 650 600 500 400 400 --- Corrosion in Dry Hydrogen Chloride, 100% Metal Approximate temperature, °F, at which given corrosion rate, mils/year is exceeded in short time tests in dry HCl limit for continuous service, °F Corrosion rate, mil/year, in dry HCl Nickel alloy 600 alloy 400 316 304 Platinum Copper Steel Gold Silver Suggested upper temp 30 60 120 600 1200 850 800 450 700 650 2300 200 500 1800 450 950 900 500 700 750 -300 600 -550 1050 1000 650 900 850 -400 750 -650 1250 1250 900 1100 1100 -600 1050 -850 1300 1350 1050 1200 1200 -700 1150 --- 950 900 450 800 750 2200 200 500 1600 450 Both of these tables were abstracted from INCO Bulletin CEB-3. The data were obtained from short-time laboratory tests and offer only a rough guide to maximum practical temperature limit of materials. The original data from which INCO developed their table was published in 1947, M.H. Brown, W.B. DeLong and J.R. Auld, “Corrosion by Chlorine and by Hydrogen Chloride at High Temperatures”, Ind. & Eng. Chemistry, Vol 39, No. 7 pp 839-844 At lower halogen concentrations alloys forming a chromia layer can tolerate higher temperatures. Data in Bender and Schütze, Paper 00239 Corrosion 2000, show that alloy 600 can form a protective oxide at 800°C in 0.1%Cl2, 100 hour test. At 2%Cl2, same temperature, the alloy does not develop a protective scale. Grain size has an effect, 600 with finer grains, 75µm (ASTM 4.5), being superior to 600 with coarser grain size, 125µm (ASTM 3). Fine grain size increases diffusion rate of chromium to the surface. - 35 - Longer time tests show lower corrosion rates. The following industrial data were obtained from 30 day test exposures. 100% Chlorine Gas, type 304/321 stainless, 30 day test Temp mils/yr mm/yr F C 572 300 6 0.15 617 325 7 0.18 662 350 9 0.23 707 375 15.5 0.39 752 400 33 0.84 797 425 115 2.9 100% Chlorine Gas, alloy 600, 30 day test Temp mils/yr mm/yr F C 977 525 8 0.2 1022 550 12 0.3 1067 575 15 0.38 1112 600 24 0.61 1157 625 47 1.2 Corrosion of nickel alloys by hot 100% F2 gas is given in Table 14, CEB-5. Most of that data is reproduced below. Corrosion by dry fluorine gas °F 80 400 700 1000 Temperature Material 400 200 nickel 304 304L 347 600 °C Exposure time, hours 5 24 24* 120 5 24 24* 120 5 24 120 5 5 27 204 370 Corrosion Rate, mils per year 538 2.4 0.5 -0.2 1.0 0.9 -0 1.7 0.6 0 2.7 1.1 29.8 11.3 21.3 7.2 24.5 16.1 44.5 13.8 ----3451 0.5 0.5 0.7 0.1 3.3 0.5 0.3 0.1 6.1 7.5 25.4 4.0 0.6 1.9 1.7 2.4 1.2 1.7 1.2 0.5 0.4 1565 6018 -4248 78.0 All tests were made in flowing fluorine gas, except * which were conducted in bombs at initial pressure of 250 psi. - 36 - The original source of this fluorine data was: R.B. Jackson, General Chemical Division, Allied Chemical Company, “Corrosion of Metals and Alloys by Fluorine,” Contract AF 04 (611)-3389 Corrosion Tests in Hydrogen Fluoride Gas Temperature: 930 to 1110F (500 to 600C). Test duration 36 hours. From Table 17, INCO CEB-5 Material Corrosion Rate mils/year mm/yr Comments Hastelloy® alloy C Inconel® alloy 600 Hastelloy alloy B Nickel 200 Nickel 201 Monel® alloy 400 Monel alloy K-500 70-30 Copper-Nickel 0.3 0.7 2 9 14 13 16 16 iridescent tarnish film “ black film “ “ adherent dark film “ “ 0.008 0.02 0.05 0.2 0.36 0.33 0.41 0.41 Hastelloy is a registered trademark of Haynes International Inconel and Monel are registered trademarks of Special Metals, Inc. In atmospheres containing a significant partial pressure of oxygen these laboratory data in pure halogens or halide gases have limited utility as the basis for alloy selection. The heat resistant alloy X (UNS N06002) has outperformed alloy 600 in oxidizing gases containing HCl. Alloy 59 (N06059) has performed satisfactorily in an oxidizing atmosphere with HF, where alloy 617 weld filler was inferior to alloy 600. Examination of the 600 alloy part, removed from service after many years life, showed some corrosion from sulphur and phosphorous as well. At this writing, August 2002, it is not clear to us whether it is the better oxidation resistance of the higher chromium alloys, or some molybdenum effect, that is responsible. If the customer intends to perform tests in his environment, we would suggest including a heat resistant alloy such as the 3%Mo alloy RA333® (N06333). RA333 has shown good resistance to hot corrosion by the fluoride flux used in aluminum salt bath brazing environments. - 37 - MOLTEN SALT CORROSION Hot chloride salts, and particularly salt fumes mixed with air, are very corrosive to heat resistant alloys. In general the higher nickel alloys, such as 600, are preferred, although we have seen tolerable results from the 1.7% silicon grade, RA 253 MA. Corrosion in Molten Chloride Heat Treat Salts, 1100-2200°F (600-1200°C) Depth of Intergranular Attack Grade Nickel, weight % RA85H 15 RA 253 MA 11 RA600 76 RA309 13 RA330 35 Silicon, weight % 3.5 1.7 0.2 0.8 1.2 mm 0.11 0.18 0.19 0.32 0.35 inch 0.0044 0.0069 0.0075 0.0125 0.0138 Plate samples were exposed in a commercial heat treat salt line. They saw 210 to 252 cycles in preheat salts 700°C (1290°F) and 815°C (1500°F), high heat salt 1200°C (2200°F), quench in 600°C (1100°F) nitrate/nitrite salt, air cool. Preheat and high heat salts were mixtures of potassium, sodium and barium chlorides. The alkali metals in the salt turn the protective chromium oxide scale into an alkali chromate, which is non-protective and water soluble. As fast as the scale is removed, more chromium diffusing to the surface reforms the scale. Eventually most of the chromium may be removed from the alloy, leaving primarily iron and nickel. A more detailed account of hot salt corrosion mechanisms is given on pages 126-132 under Salt Pots. Fluoride salts are more aggressive than are chloride salts. Molten fluorides are used to flux metals and alloys for brazing operations. Along with fluxing the oxide film on the workpiece, fluorides also attack the chromium oxide film on heat resistant alloy fixturing. A service trial of various alloy fixtures used in aluminum salt bath brazing at 1125°F (607°C) gave the following results: Alloy RA333 600 Nickel 200 C-276 601 Total Life, days 197 (end of test—no failure) 112 51 40 14 Other work has shown the 25% chromium ferritic grade, RA446, to be unsuitable for aluminum salt bath brazing operations. - 38 - Vanadium Pentoxide Equipment fired with residual fuel oil suffers corrosion wherever the fuel ash metal. Heavy oils such as No. 6 or “Bunker C” may contain both sulphur When this oil is burned, the vanadium forms vanadium pentoxide, V2O5. pentoxide, along with sodium sulfate, makes a molten compound which corrosive. It will eat away most heat resistant alloys in less than a year. deposits on hot and vanadium. This vanadium is aggressively A high level of sulphur in the oil might be 2 or 3%, while only 0.05% (or, 500 parts per million) of vanadium is “high” enough to be destructive. Venezuelan oil is particularly high in vanadium and is often used in the Northeastern U.S.A. The alloys with good resistance to fuel ash corrosion are usually cast compositions that are both weak and brittle. 50Cr-50Ni cast IN -657 (UNS R20501) is the best, while HE (28Cr 9.5Ni) is said to be reasonable. IN-657 is expensive and readily embrittled, and HE is particularly weak and brittle. Available wrought alloys are not at all as resistant to fuel ash corrosion but are used for their much better ductility. RA333, RA625, RA330, RA 253 MA and RA310 have all been used or are on trial. Frankly, we have no good comparative field data for these wrought alloys. Mostly based on rumor, we might suggest RA333 or RA 253 MA as worth trying, but they definitely will not be as good as 50%Cr-50%Ni cast. - 39 - MOLTEN METALS From time to time one or another heat resistant alloy is used in contact with a low melting point metal in its molten state. Depending upon which metals are involved, the temperature and the state of stress, that molten metal may dissolve, or may crack, the heat resistant alloy. General Nickel—with respect to nickel-chromium -iron or nickel-chromium alloys, the higher the nickel content, the more rapidly the solid metal dissolves in the molten. As a ROUGH rule of thumb, where contact with low melting metals is concerned the lower nickel alloys, or even the ferritic stainlesses, are preferred. High nickel alloys, such as RA600 (76%Ni) tend to be attacked more severely. Molybdenum—in resisting corrosion by molten zinc alloys, a molybdenum addition appears to benefit austenitic stainless or nickel alloys. One example is 316L, which at 2% Mo seems to work better than does 304, in contact with molten zinc for galvanizing or die casting operations. AL-6XN® alloy, 6.3% Mo, has performed better than 316L at 1000°F (538°C) in 26% aluminum, 7% lead, 67% zinc. Alloy C-276, 15% Mo, has been used in continuous zinc galvanizing at about 850°F (454°C). Dissimilar Metals—must not be used in contact with molten metals. A phenomenum known as mass transfer1 may dissolve the higher nickel alloy preferentially. One example known to us was a lead pot fabricated of heavy RA330 alloy plate. It had been welded with the 72% nickel 19% chromium 2.7% columbium (niobium) weld filler 82, ERNiCr-3. It failed when the weld bead separated from the base metal. Analysis of the weld bead showed that it was now a lead alloy, with about 5% columbium (niobium) and traces of chromium and nickel. Embrittlement—liquid metal embrittlement may occur just below the melting point of the low melting metal. The same molten metal may either dissolve or crack2 the heat resisting alloy, depending upon the stress level, and how much molten metal is present. Aluminum—molten aluminum dissolves any Fe, Fe-Cr, Ni-Cr-Fe or Ni-Cr alloy, likewise for the cobalt alloys. Nevertheless, bar of alloys such as RA446 (25% Cr, balance Fe) has been used for stoppers in bottom pour aluminum ladles. Life is erratic, depending upon how long it takes the aluminum to reduce and/or wash away the hot rolling scale from the bar. RA330 (35%Ni 19%Cr 1.2%Si balance Fe) 11 gage/3mm wall cooling tubes have been used in an aluminum melting furnace, right above the metal. Wherever molten aluminum splashes on the RA330 it goes right through it like hot water throug h snow. Titanium tubing has been used to siphon molten aluminum. That it has been successful at all is due entirely to the tenacious oxide film on the titanium. - 40 - MOLTEN METALS, continued Antimony—we have no definite experience. There are indications that lead baths which have been contaminated by antimony, from using scrap lead, are corrosive to Ni-Cr-Fe alloys. Bismuth—To satisfy OSHA, one American file manufacturer switched from molten lead to bismuth in its 1450°F (788°C) austenitizing baths. The lead, now bismuth, pots are fabricated of RA330 plate welded with RA330-04 (35%Ni 19%Cr %Si 5%Mn 0.25%C) weld filler. These pots have two loops of 2” Sch 40 RA330 pipe welded to the bottom. An induction coil fits through the loops and heats the bismuth. When too much heat is applied, the loops are attacked. When maintained at 1450°F (788°C) no problems have been reported to us. Cadmium—we have no experience. Calcium—molten calcium can crack RA330, and presumably higher nickel alloys as we ll. RA330 retorts are commonly used to process ferrites, for the electronics industry, at high temperatures under a hydrogen atmosphere. Calcium carbonate has been used as part of the mix. The hydrogen reduces it to calcium metal. The metallic calcium vapors haven’t been much problem but down toward the retort base it is cooler, and molten calcium condenses on the retort wall. RA330 retorts crack at this location. The fracture surface is very similar in appearance to Figure 12, page 60, Volume 10, 8th Edition, Metals Handbook (ASM). This figure illustrates 2024-T4 aluminum cracked by mercury. Copper—molten copper and copper base alloys penetrate the grain boundaries of any austenitic iron, nickel-chromium-iron or nickel-chromium alloy. Even carbon steel, austenitized by immersion in molten copper, can have the austenite grain boundaries neatly outlined by copper metal. Launders for handling molten copper are successfully made of the high chromium ferritic alloy RA446 (25%Cr, balance Fe). Siphons for handling molten copper have been 446 seamless tubing. E-Brite ® (26%Cr 1%Mo, balance Fe) works slightly better, when available. Skimmers for removing slag from ladles of molten brass or copper are mild steel, 430 stainless (16.5%Cr, balance Fe) and, more likely, RA446. All of the austenitic alloys will fail rapidly in contact with molten copper or copper alloys. The old Belgian alloy UMCo-50, 50%Co 28%Cr 22%Fe, was said to function well in contact with molten copper. We have no experience to confirm this. Haynes® International have made this alloy under their own trade name HS 150. - 41 - MOLTEN METALS, copper, continued Molten copper attack can be a problem in muffles used for copper brazing steel. Eventually some copper braze spills onto the bottom of the muffle. With an exothermic brazing atmosphere the Ni-Cr-Fe alloy (usually RA330) muffle develops a scale which may be protective enough to prevent small amounts of copper from actually wetting the muffle floor. With enough copper the scale may be penetrated and the muffle attacked. We have seen some 15 pounds of copper, with the appearance of cast bars, removed from the corrugated bottom of an 11 gage/3mm wall RA333 muffle. One shop reported longer life when the 15% Ni grade RA85H was used for muffle bottoms, rather than RA330 or RA601. One might consider the 11% nickel alloy RA 253 MA for muffle bottoms, bearing in mind that all austenitic alloys will eventually fail from molten copper attack. A dry hydrogen or hydrogen-nitrogen brazing atmosphere does not permit the muffle to develop any protective oxide film. Even small amounts of spilled copper will completely penetrate the nickel alloy floor along the grain boundaries. Hydrogen then escapes through the hole and burns like a to rch, locally overheating, and sometimes melting, the surrounding area. One practical solution is a sheet of 430 stainless or fibrous refractory on the muffle floor, to keep molten copper from contacting the austenitic alloy muffle. Lead—molten lead heat treating baths, or lead pans, are fabricated of mild steel, RA309, RA310, RA 253 MA and RA330. The lead itself isn’t terribly corrosive to these alloys, although the lower nickel grades may be preferable. Alloy 600 is another story, this high nickel allo y is dissolved by molten lead. With other alloys it is the lead oxide on the surface that attacks the metal sides severely at the lead-air interface. The molten lead is usually covered with so-called charcoal, more likely sulphur bearing coke of some sort, to reduce lead fumes and oxidation. The lead still oxidizes. Sulphidation and carburization also occur at the lead-air interface, caused by this protective covering. The most direct approach to this local corrosion is to make the metal wall twice as thick at the lead-air interface. Pure lead should be used. Antimony, brought in when scrap lead is used, increases attack from the molten metal itself. Lithium—a vessel fabricated in the 1970’s of RA333 for the US Navy liquid metal embrittled & cracked from residual stress in the formed head, when operated 1650°F (900°C) with molten lithium. Had we been asked, we would have suggested first annealing the head to remove forming stresses. Corrosion of RA333 occurs primarily by selective leaching of the nickel. Alloy X behaves in a similar manner. Based on laboratory tests, TZM molybdenum and pure iron (to 1000°C) are said to have good resistance to molten lithium corrosion. Ferritic stainlesses are said to be subject to chromium leaching. However E-Brite was found significantly more resistant to molten lithium than either nickel or cobalt base alloys. Note that these are laboratory test results, not necessarily confirmed in service. - 42 - MOLTEN METALS, continued Magnesium—used in reduction of TiCl4 is normally contained in mild steel pots, or steel pots lined with 430 stainless. Melting at 1202°F (650°C), magnesium tends to leach the nickel out of Ni-Cr-Fe alloys. Because carbon steel scales on the outside (fireside) of the melting pot, a few experimental clad pots have been tried. These have been either RA 253 MA or RA330 explosively clad to mild steel. The nickel-chromium-iron alloy outside provides high temperature strength and oxidation resistance while the carbon steel inside is more compatible with the molten magnesium. Rare Earths—the same manufacturer of ferrites who had problems with molten calcium cracking RA330 has also had both cast and fabricated Ni-Cr-Fe alloy grids crack. Deposits on the cast grid analyzed 34% samarium, 10% praseodymium and 1% neodymium. Apparently rare earth compounds used in the manufacture of ferrites were reduced to metallic form by the hydrogen atmosphere. They dripped on the cooler grid at the bottom end of the retort. A lower nickel alloy would probably have been more satisfactory for these grids. Selenium—In the 1970’s, RA330 was used as 1” (25mm) diameter fabricated tubular heating elements in five 9’s purity selenium and arsenic selenide at 500 and 600°F (260 and 316°C), respectively. No degradation of product purity was reported, nevertheless we urge anyone planning to use RA330 for such an application to run their own test program. Silver—silver braze alloys have long been known to crack or dissolve austenitic alloys. Cold worked 300 series stainless steels can not be silver brazed without danger of cracking. One reason is that silver braze, in contrast to copper braze metal, melts well below the annealing or even stress relieving temperature of the austenitic alloy to be brazed. In hydrogen atmosphere braze retorts, molten silver braze alloy dripping on the bottom of an RA330 retort will penetrate this austenitic alloy at the grain boundaries and cause hydrogen leaks. Solder (lead-tin)—no molten metal attack reported. Temperatures are low, and the chloride fluxes used are more of a corrosive problem than is the solder itself. RA333 alloy has been used in tin can soldering applications, again more to withstand the ammonium chloride flux than the Pb-Sn alloy. Tin—both RA446 3/16” (4.8mm) plate and RA 253 MA sheet have been used for side shields in the tin float process of plate glass manufacture. Tin at 600°C (1112°F) under hydrogen atmosphere is reported to have dissolved, then re-deposited, 304 stainless steel, and to have pock-marked carbon steel in the same bath, in the process of decontaminating soil. Zinc—molten zinc and zinc-aluminum alloys are used for galvanizing and die casting. Our observations have been that commercially pure iron, 316L stainless, RA85H, 309, AL-6XN and alloy C-276 have all been used in molten zinc/zinc alloy with some degree of success. RA330 is no good at all in molten zinc, and it seems reasonable to assume that the other nickel-chromium-iron alloys such as 800H or 600 are as bad or worse. - 43 - MOLTEN METALS, zinc, continued Zinc die casting pots have been heated by gas fired immersion tubes fabricated of RA309. The tubes are usually plasma sprayed with zirconia to enhance life, but this coating is subject to damage by mechanical abuse. The 309 weld bead is attacked to a greater degree than the base metal. One failed 309 tube, which had leaked full of zinc die casting alloy, was heated rapidly with an oxy-acetylene torch to melt out the zinc. The high thermal stress coupled with zinc wetting the 309 metal inside cracked the tube. The fracture surface was typical of liquid metal embrittlement, i.e., it looked like RA330 cracked by molten calcium or 2024-T4 aluminum cracked by mercury. When 1” (25mm) round bars of both RA330 and 316 stainless were both used in the same zinc die cast alloy scrap recovery project the 35% nickel alloy was severely eaten away and chromium was selectively leached out. The 316 bars merely developed a galvanized coating with no appreciable metal loss. Temperature was about 1000°F (540°C) We observed that one steel company involved in continuous hot-dip galvanizing of sheet made the 850°F (454°C) zinc pot and sink arms of low carbon, low manganese, low silicon nearly pure iron. At the zinc-atmosphere interface the pot was sheathed with 316 stainless steel. The iron sink roll was weld overlaid with 316 stainless, as were the journals. Sleeve bearings, to ride on these 316 overlaid journals, were fabricated of C-276 (UNS No. N10276) sheet. The chute through which the steel sheet passes into the zinc had a tip of C-276 where it entered the molten zinc bath. Recently we have found definite success with AL-6XN alloy for small sink rolls and bearings for galvanizing wire. Initially the company used 316, then Rolled Alloys convinced them to try RA85H, which was an improvement. On test, AL-6XN looked even better. This was confirmed in service, and for the past 3 or 4 years they have been using AL-6XN. They tried 316 for the trunnion sleeve, over AL-6XN trunnions, and the 316 did not last long. Now AL6XN is used for both the trunnion and the sleeve bearing, as well as for the sink roll itself. Rolled Alloys laboratory immersion testing in molten zinc ranked these alloys similar to how they behaved in service: 250 hour test in molten zinc, 850°F (454°C) alloy AL-6XN 556TM 1008 RA309 RA85H® RA446 316 original thickness average metal loss inch (mm) inch (mm) 0.120 (3.05) 0.0056 (0.142) 0.110 (2.79) 0.0034 (0.086) 0.1328 (3.37) 0.0104 (0.264) 0.118 (3.0) 0.017 (0.432) 0.1164 (2.96) 0.0226 (0.574) 0.2008 (5.10) 0.0234 (0.594) 0.1188 (3.02) 0.044 (1.12) - 44 - metal loss, ratio to AL-6XN® 1.00 0.6 1.9 3.0 4.0 4.2 7.9 MOLTEN METALS, continued References 1. David H. Gurinsky, The Behavior of Materials in Aggressive Liquid Metals, pages 5-20, Nuclear Metallurgy, A Symposium on Behavior of Materials in Reactor Environment, February 20, 1956, Institute of Metals Division, American Institute of Mining and Metallurgical Engineers, New York, New York, U.S.A. 2. J.E. Cantwell and R.E. Bryant, How to Avoid Alloy Failures in: 1. Piping by liquid metal attack, 2. Flare tips by severe cracking, pages 114-117, Hydrocarbon Processing, May, 1973 MAGNETISM Austenitic heat resistant alloys are non-magnetic as produced. After high temperature service they sometimes become rather strongly magnetic. Usually this indicates that for one reason or other the metal is no longer fit for service, nor is it capable of being weld repaired. Service conditions that cause this magnetism are often carburization, internal attack by molten salts or selective attack by some molten metal. There are three metallic elements which, in their pure state, are magnetic. Iron, of course, is magnetic and has a ferritic (body centered cubic) structure. Pure nickel is also magnetic, even though it has an austenitic (face centered cubic) structure. Likewise iron-nickel alloys, in all combinations, are magnetic, even though they may be austenitic. Cobalt is the third magnetic element, with a hexagonal close packed structure at temperatures below 783°F (417°C), and a face centered cubic structure at higher temperatures. It is easy to confuse ferritic and austenitic with magnetic and non-magnetic. Ferritic stainlesses are magnetic, the small amount of ferrite in an austenitic stainless weld bead (E308, E309, etc.) is also magnetic, and austenitic stainless and nickel alloys are usually non-magnetic. But, an austenitic alloy can also be magnetic. The 66% nickel 31% copper alloy 400 is usually non-magnetic, but depending upon the exact chemistry, a particular heat may be magnetic, at least on a cold day. And, of course, commercially pure nickel is an austenitic metal, and it is also a magnetic metal. Older Canadian coins are a high nickelcopper alloy, and are magnetic (the newer are not, to save on nickel), and many European coins are magnetic. Alloys of iron and nickel are magnetic, the magnetic properties sharply increasing at about 30% nickel and being highest in the range 50 to 80% nickel1. It is the addition of chromium that makes alloys based on iron and nickel (or cobalt) become non-magnetic. Even RA600, with 76% nickel and 8% iron, is non-magnetic, because of its 15.5% chromium. Consider going the other way, and removing chromium. If 10.5% of that chromium were removed and replaced by nickel (and the %ages recalculated), the new 85.3%Ni 8%Fe 5%Cr alloy would become a magnetic, austenitic alloy. Carburization makes nickel heat resisting alloys become magnetic because the chromium reacts chemically with carbon to form chromium carbides. Although the chromium is still present in the alloy, it is effectively - 45 - MAGNETISM, continued removed from the solid solution matrix of nickel, iron and chromium. Lower nickel grades such as RA310 or RA 253 MA do not so readily become magnetic when carburized. This is approximately illustrated by the following Fe-Ni-Cr ternary diagram of magnetism vs alloy content2. Chromium is physically removed from the alloy by the normal corrosion mode in neutral salt pots. RA330 depleted to 12-15% Cr is common, and we have observed metal which used to be RA330 but which had become a 1%Cr-Fe-Ni alloy. The iron oxide component of scale is also magnetic, so it is possible that a slight degree of magnetism felt on a used fixture is simply the scale, rather than carburization. Their will also be a slight chromium-depleted zone underneath the, largely chromium oxide, scale. There are a couple other times when austenitic stainless steels may be magnetic. The specified chemistry range of 309S stainless (UNS S30908) is broad enough that a small amount of ferrite may be present in the hot rolled annealed metal, just enough to feel with a magnet. This can be very upsetting to customers who think they have the wrong material, because austenitic heat resistant alloys are supposed to be non-magnetic. Also, when a leaner stainless such as 304 is cold worked it becomes magnetic because a small amount of the austenite actually transforms to martensite (a hard, magnetic phase). This is evident on sheared edges, and especially so in deep drawn sheet components. Cold working has little or no effect on the magnetism of higher alloys such as RA330 or RA333. RA310 is normally non-magnetic, right down to liquid nitrogen temperatures, and has been used for structural elements around the superconducting magnets in MRI equipment for hospitals. Reference 1. R. H. Krikke, J. Hoving and K. Smit, Monitoring the Carburization of Furnace Tubes in Ethylene Plants, Paper No. 10, Corrosion 76, National Association of Corrosion Engineers, Houston, Texas 1976 2. W.P. Rees, B.D. Burns, and A.J. Cook, Constitution of Iron-Nickel-Chromium Alloys at 650° to 800°C, July 1949 JISI - 46 - - 47 - STRENGTH AT TEMPERATURE The strength of a metal at high temperature is measured differently than at room temperature. For room temperature applications—steel guitar strings, automobile frames, claw hammers, etc.—the designer needs to know the tensile strength, yield strength or hardness. At cherry red heat, though, the only important mechanical property is creep or rupture strength. Above about 1000-1200°F (540-650°C), tensile or yield strength can NOT be used as a basis for design. This is important. Tensile Strength Tensile strength, or ultimate strength, is the stress required to pull a specimen until it breaks apart in two pieces. It is calculated by dividing the breaking load, in pounds (Newtons), by the specimen cross sectional area, in square inches (mm2), to give pounds/inch2 (Newtons/millimeter2). The strength of wire, both steel and nickel alloy, is usually reported only by its tensile strength in psi (N/mm2, or MPa). The tensile test is carried out by mounting a specimen in a machine which pulls on it with a slowly increasing load until it breaks. The load in pounds (Newtons) is measured and recorded throughout the test. Elastic Modulus In the early stages of the tensile test the specimen is stretching elastically, like a rubber band. Were the test to be stopped, when the load was removed the specimen would go back to its original length. This is the “elastic” portion of the tensile test, where a plot of stress versus strain would be a straight line. The slope of that line, stress divided by strain, is the Elastic Modulus, also called Young’s Modulus, with the symbol E. This is the measure of the stiffness of the metal, or how “springy” it is. At ordinary temperatures, for example, the modulus of steel is about 30,000,000 psi (207 GPa), while that of 6061-T6 aluminum is only about 10,000,000 psi (69 GPa). One may say that steel is three times stiffer than aluminum. This means that, in the elastic range (room temperature, stress less than the yield strength) for a given stress aluminum will stretch or bend three times as much as will steel. At room temperature the modulus of RA333 is 29,200,000 psi (201 GPa). The modulus decreases at higher temperatures. By about 1000°F (538°C) the material is no longer elastic. - 48 - Yield Strength At some point during the tensile test, usually well before the specimen breaks, it takes a permanent stretch. This is called the “Yield Strength” (or Proof Strength). For austenitic alloys it is usually recorded on the mill test report as either the 0.1% Offset Yield Strength, or, more commonly in the U.S., the 0.2% Offset Yield Strength. Tensile Strength Stess = (Load/Area) 0.2% Y.S. 0 Drop in stress due to thinning of specimen cross section. 0.02% Y.S. Strain, in./in. (mm/mm) = Change in length/Initial length Ductility Before the specimen breaks it has stretched out a great deal, and has necked down in the area where it breaks. The amount it had stretched when it broke is the “% Elongation”, and the amount it necked down is the “% Reduction of Area”. Both are measures of ductility. For example, at room temperature an RA333 tensile specimen might have 48% Elongation and 62% Reduction of Area. The Tensile Strength could be 107,000 psi (738 MPa, or N/mm2 ) and the 0.2% Offset Yield Strength 47,000 psi (324 MPa) When designing a machine part, obviously the design stress has to be below the tensile strength of the metal, or the thing would break in two. But the machine would also be useless if its parts bent, or yielded, so the designer must keep the stress somewhere below the yield strength of the metal. For heat resistant alloys, yield and tensile strength may be used for design up to about 1000°F (5380°C). Above this temperature, the life of the part will be limited by the metal’s creep-rupture properties, and not by its tensile properties. - 49 - Creep-Rupture Why creep and rupture strength? Metals behave much differently at high temperatures than they do near room temperature. If a metal bar is loaded to just below its yield strength at room temperature, that load can be left there practically forever. Nothing will happen, unless it corrodes away or stress-corrosion cracks. Now let us say that this metal bar is loaded, again keeping the stress below the yield strength—while it is glowing cherry red, 1500°F (816°C). A very small amount of deformation will occur at first (first stage creep). Then that metal bar will begin to stretch, but very, very slowly. It will keep on stretching for hours, weeks, maybe years, until it finally breaks in two. All this, when it wasn’t even loaded up to the yield strength (as measured by a short-time tensile test). Creep The rate, or speed, at which the metal is stretching, in % per hour, is called its “creep rate”. Creep rate is expressed as per cent deformation per hour. For some period of time the creep rate is more or less constant. This is the “minimum creep rate”, or “secondary creep rate”. The minimum creep rate (mcr) is used as one basis for design at high temperature. That is, at high temperature one must assume that the metal is going to creep, or deform, to some degree. This is true even for light loads. Theoretically, the designer might settle on an acceptable amount of creep deformation over the projected life of the equipment. He would then pick his design strength based on the speed of deformation, that is, creep rate, acceptable in his application. In practice, in the furnace industry one design criterion is the stress required for a minimum creep rate of 1% in 10,000 hours, or 0.0001% per hour. Design stress may be set at some fraction of this number. The ASME uses for one of its criteria 100% of the extrapolated stress for 1% in 100,000 hour mcr, or 0.00001%/hr. The other measure of creep, and the one used in Europe, is “total creep”. That is, the stress required for the specimen to actually stretch a total of, say, 1%. Minimum creep rate data and total creep rate data are not interchangeable. People tend to have rather strong feelings about one or the other creep measurement, so whenever possible we provide both minimum creep and total creep data. Rupture “Rupture Stress”, or “Creep-Rupture Strength”, is reported as both a stress, and a number of hours. It is the stress required to completely break a specimen within a given amount of time. In the furnace industry another common criterion for setting design stresses is to use some fraction of the stress that would result in rupture at 10,000 hours. ASME uses whichever is lower, 67% of the extrapolated 100,000 hour rupture stress, or 100% of the extrapolated 1% in 100,000 hour minimum creep rate. - 50 - Rupture Third-Stage Creep Elongation Second-Stage Creep First-Stage Creep Time The picture below shows a broken creep-rupture specimen of RA330, tested at 2000°F (1093°C). About full scale Creep strength is more important than rupture strength. For example, at 1800°F (982°C) the alloys RA330, RA309 and RA310 all have comparable 10,000 hour rupture strength, about 560--660 psi (3.9--4.6 N/mm2). However in service an RA330 muffle or retort can retain its shape for years, whereas one of RA309 or RA310 would collapse. The reason is, these stainless heat resisting grades have only 40—55% of the creep strength of RA330 at 1800°F (982°C). Normally we expect the strongest alloy to do the best job. This does depend on how that strength is achieved. For example, RA333, RA85H, RA 253 MA and RA 353 MA are strengthened by various alloy additions, with a medium-fine grain size. As a result, they all have good to excellent thermal fatigue resistance in quench applications. The least expensive way to obtain high creep-rupture strength is by giving the alloy a high temperature solution anneal. An aim of ASTM 5 or coarser grain size gives much better creep and rupture strength than does a finer grain size. However, coarse grained materials lose thermal fatigue resistance as they gain creep strength. In our experience, material with grain size coarser than ASTM 4 will be unsatisfactory in liquid quench applications. A quenching fixture, for example, made of 800H would resist creep deformation but quickly break up in pieces from thermal fatigue. The supposedly “weaker” RA330, with its finer grain, can give very good life in quenching service. - 51 - Creep-Rupture Testing Above 1800°F (982°C) oxidation affects the results of a creep-rupture test. As the creep voids oxidize the material undergoes an apparent strengthening. This can be seen by comparing the 2000°F (1093°C) results obtained using 0.252” (6.4mm) diameter test specimens with those from 0.505” (12.83mm) diameter specimens. For an alloy such as RA330 the results from the thinner specimen are so influenced by oxidation as to be unrealistically high. RA333 is considerably less affected at this temperature. By 2200°F (1204°C) even the largest available (0.505”/12.83mm dia.) test specimens in RA333 are probably affected. This makes it difficult to compare very high temperature creep rupture data from different sources, as the test specimen diameters are rarely recorded. For both RA330 and RA333 all currently published creep-rupture data was obtained using the la rger diameter specimens, at Joliet Metallurgical Laboratories, Joliet, Illinois, U.S.A.. - 52 - Cantilever Beam Creep Test RA85H RA601 RA330 RA310 RA309 For design purposes, creep and rupture data are usually plotted on log-log charts. A visual illustration of relative creep strengths is obtained by simply clamping alloy strips at one end and measuring how much they sag or droop from their own weight. These five alloys were held at 1600°F (871°C) for 500 hours. The maximum stress in each beam, caused by its own weight, was calculated to be 1890 psi (13 N/mm2). RA309 sagged 6 inches (152 mm) in the first six hours, and continued to bend in the opposite direction once the free end touched the furnace floor. RA310 sagged 6 inches (152mm) in about 48 hours. RA85H and RA601 sagged very little in 500 hours, with RA330 showing slightly more deformation. - 53 - Average 10,000 Hour Rupture Strength, psi TEMPERATURE °F ALLOY 900 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 2100 2200 COR-TEN B 22,000 12,500 -- 2,000 + -- -- -- -- -- -- -- -- -- -- RA446 -- -- 3,500 2,700 -- 1,100 -- 450 -- 230 -- -- -- -- 304L -- 25,000 15,600 9,700 6,000 3,700 2,300 1,400 -- -- -- -- -- -- 304, 304H -- 36,000 22,200 13,800 8,500 5,300 3,250 -- -- -- -- -- -- -- 316L -- 39,000 23,500 14,200 8,500 5,100 3,050 -- -- -- -- -- -- -- 321 -- -- 23,500 12,900 7,200 4,000 2,280 -- -- -- -- -- -- -- 321H -- -- 24,800 15,200 9,200 5,600 3,400 -- -- -- -- -- -- -- -- 48,000 27,500 15,600 9,000 5,100 2,900 -- -- -- -- -- -- -- RA 253 MA -- -- 22,000 14,000 8,500 5,200 3,750 2,500 1,650 1,150 860 680 -- -- RA309 -- -- -- 17,000 8,000 4,800 2,700 1,600 1,000 560 -- -- -- -- RA310 -- -- -- 14,400 7,400 4,500 2,800 1,500 940 660 -- -- -- -- -- 29,000 17,000 11,000 7,200 4,300 2,700 1,700 1,050 630 400 (280) -- -- -- -- -- 17,500 11,000 7,300 5,200 3,500 1,900 1,200 -- -- -- -- RA 353 MA -- -- 19,300 12,200 7,800 5,400 3,600 2,600 1,860 1,300 930 680 (450) (320) RA333® -- -- 25,000 16,500 12,000 9,200 5,700 3,100 1,800 1,050 630 360 -- 140 RA600 -- -- 21,500 13,500 9000 6200 3700 2350 1650 1150 -- -- -- -- -- 42,000 30,000 22,000 13,500 7,000 3600 1850 1200 820 -- (330) (200) -- RA 602 CA -- -- -- 31,200 -- 11,300 -- 3200 2180 1490 990 670 440 290 RA625 -- -- -- 42,500 22,500 12,000 -- -- -- -- -- -- -- -- RA718 -- 128,000 98,000 70,000 -- -- -- -- -- -- -- -- -- -- 617 -- -- -- ~45,000 ~32,000 ~13,000 ~8500 ~5000 ~3000 ~2000 ~1300 ~700 -- -- ® 347,347H ® ® RA330 RA800AT ® RA601 ® ® * COR-TEN B is a Registered Trademark of US Steel Corporation + One Heat Tested 617 data picked off log-log curves published by Special Metals Corp. - 54 - ( ) Extrapolated data Average Stress, psi, for 0.0001% Per Hour Minimum Creep Rate TEMPERATURE °F ALLOY 900 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 2100 2200 COR-TEN ® B 20,800 11,100 -- 1,700 + -- -- -- -- -- -- -- -- -- -- RA446 16,000 6,000 3,000 1,500 680 260 130 -- -- -- -- -- -- -- 304L -- -- 7,700 4,950 3,200 2,050 1,300 -- -- -- -- -- -- -- 304, 304H -- 25,500 16,500 10,800 7,000 4,600 2,950 -- -- -- -- -- -- -- 316L -- 23,500 14,000 8,300 4,900 2,900 1,750 -- -- -- -- -- -- -- 321 -- -- 20,000 8,800 3,850 1,700 750 -- -- -- -- -- -- -- 321H -- -- 20,300 12,000 7,100 4,200 2,500 -- -- -- -- -- -- -- 347, 347H -- 53,000 27,500 14,800 7,800 4,100 2,150 -- -- -- -- -- -- -- RA 253 MA® -- -- 18,000 11,600 7,700 5,000 3,350 2,300 1,500 890 490 (250) -- -- RA309 -- -- -- 16,000 8,800 3,400 2,400 1,400 600 220 -- -- -- -- RA310 -- -- -- 14,900 5,900 3,300 2,100 1,100 570 280 -- -- -- -- RA330® -- 21,000 10,500 7,600 5,300 3,600 2,700 2,100 1,000 500 -- -- -- -- RA800AT -- -- -- 17,000 9,100 6,000 -- 3,600 1,500 1,050 -- -- -- -- RA333® -- -- 22,000 9,800 7,700 6,400 4,200 2,700 1,650 880 -- -- -- -- RA601 -- 41,000 27,000 18,000 7,200 4,100 2,700 2,000 -- 760 -- 430 -- -- RA718 -- -- 100,000 74,000 43,000+ -- -- -- -- -- -- -- -- -- * COR-TEN® B A Registered trademark of US Steel Corporation + One Heat Tested ( ) Extrapolated - 55 - THERMAL FATIGUE Metal parts exposed to fluctuating temperatures for long periods eventually deteriorate. Experimental work and theoretical analysis indicate the cause to be plastic flow induced by expansion and contraction during heating and cooling. The effect can be minimized by proper design, selection of alloys that combine high hot strength with low thermal expansion coefficients, and by favorable operating conditions. H. S. Avery, The Mechanism of Thermal Fatigue, Metal Progress August 1959 Thermal fatigue is the cracking which happens after a metal is repeatedly heated and cooled rapidly. Heat resistant alloys all have high coefficients of thermal expansion. Most will expand at a rate of about 2/10 inch per foot (17mm per meter) when heated from room temperature to 1800°F (982°C). Heat resistant alloys also have low thermal conductivity, perhaps one fourth that of carbon steels. Uneven heating and cooling, not only with respect to different parts of the same fixture, but from surface to center of the metal itself, is the rule for heat resistant alloy service. Rapid cooling is usually thought of as oil or water quenching. Even a nitrogen gas quench is effectively a rapid cool if carried out from 2000°F (1100°C). This is common in vacuum heat treating of tool steels and some stainless grades. Individual round bars crack because the surface of the metal heats, or cools, before the center does. Since metal expands when heated, and then contracts the same amount when cooled again, this alternately strains the center and the outside surface. After some number of these strain cycles the metal cracks. In carburizing service, and in salt bath heat treating, cracks start at the surface and grow deeply. In neutral hardening operations the bar may begin to crack internally, and give no external sign that anything is wrong until it suddenly breaks. In fixtures or bar baskets, one area individually quenches faster than another. The bottom members of deep bar frame baskets cool and contract before the middle and top do. In a rigidly welded angle frame design the long bottom side pieces may crack while the shorter ends and the top frame remain sound. The most important items to consider regarding equipment which will be thermally cycled are: 1.) Design—basically flexible or loose. This may include corrugations, serpentine rather than straight flat bars and loose, pinned joints rather than rigidly welded. 2.) Light sections. Thinner metal heats and cools more uniformly than thick. 3.) Grain size & alloy choice. Material for thermal cycling service should have a grain size ASTM 4 or finer, if possible. No alloy will compensate for inadequate design where cracking from thermal cycling is concerned. - 56 - THERMAL FATIGUE, continued Some alloys are better than others, of course. Both strength and ductility are important. RA333 has been our best alloy in resisting thermal fatigue, because it is both strong and ductile. The strength, in turn, can permit additional life improvement. That is, if the designer makes use of RA333’s strength to use thinner plate and smaller diameter bars. Thinner sections heat and cool more unifo rmly, so the thermal strains are lower. The use of the lightest possible metal sections cannot be overemphasized. We had one customer who cut his life in half simply by going from 1/2” (12.7mm) dia. RA330 in his bar frame basket, up to 5/8” (15.9mm) dia. bars. He thought he was making the basket stronger, which he was where load carrying ability was concerned. But the thermal strains from quenching the larger bar are significantly greater. It is thermal stresses that cause more distortion and cracking in heat resistant alloy equipment than do the mechanical loads imposed on the part. Ductility alone is not enough. RA600 is ductile, but RA333 survives repeated quenching better because of its strength. And for that matter, the tensile ductility of RA333 at 1600°F (871°C) has been measured at 75% elongation. - 57 - WEAR Wear resistance is often related to hardness at room temperature. But even at room temperature, heat resistant alloys are rarely harder than Rockwell B100 (Brinell 240). These austenitic heat resistant alloys do not possess wear resistance in the conventional sense. There is some limited information available for erosion, and for galling resistance. Erosion Erosion resistance appears somewhat related to oxidation resistance. AvestaPolarit provide the following information for their “MA” grades: Coupons of three different MA grades were exposed in the cyclone of the Nässjö plant in Sweden, 4200 hours fired with wood waste and 1800 hrs with Polish coal. Normal temperatures 1580-1635ºF (860-890ºC), with peak bed temperatures of 1920ºF (1050ºC). Grade RA 153 MA ® RA 253 MA RA 353 MA Maximum Thickness Reduction inch mm 0.071 0.024 0.008 1.8 0.6 0.2 Until the development of the 25Cr 35Ni grade RA 353 MA, the 21Cr 11Ni alloy RA 253 MA had been considered one of the most erosion resistant materials for fluidized bed cyclone construction. During 1999, tube shields of RA 353 MA were installed in-bed in a number of coal fired fluidized bed boilers, in particularly erosive areas. Final results are still pending. Galling Austenitic stainless and nickel alloys are known to be susceptible to galling at room temperature. The situation does not improve at elevated temperatures. By far the best antigalling resistance at high temperature is possessed by the cobalt alloys, e.g., L605, 188, 556, X-40 (25.5Cr 54Co 10.5Ni 7.5W 0.50C). All of these are relatively soft, solid solution or carbide strengthened grades--NOT the hardfacing Stellite® alloys. The cobalt alloys in question form a relatively soft, lubricious oxide which prevents galling. A combination of a cobalt base against nickel or iron base alloy is a good, practical approach to minimizing galling problems. Due to the cost of cobalt alloys, their anti-galling properties are rarely used, or even known, outside of the gas turbine industry. In the 1960’s General Electric’s J79 engine, used to power military aircraft, used cast X-40 linkage in the afterburner system. The X-40 parts were regarded as “self lubricating”. - 58 - WEAR, Galling, continued In other industries it may be that a weld overlay of, for example, L605 (Haynes 25) on one of the nickel or stainless parts would function to prevent galling against the other side of the couple. Boron nitride spray is used for high temperature lubrication. - 59 - PHYSICAL METALLURGY An important property of alloys utilized for heat resistant service is the ability of the metal to retain its desirable characteristics throughout the range of probable operating temperatures. Some high alloy ferrous metals are subject to embrittlement at certain elevated temperatures as a result of the formation of a constituent called the “sigma phase.” If appreciable amounts of the extremely hard, brittle sigma phase can be formed an alloy steel may lose ductility to such an extent that its usefulness may be seriously impaired. Although the existence of this phase has been observed for a number of years, the possibility of the occurrence of phases other than the well-known alpha and gamma may sometimes be overlooked in the consideration of alloys suitable for high temperature applications. Francis B. Foley, The Sigma Phase, Alloy Casting Bulletin Number 5, July 1945, Alloy Casting Institute, New York, New York, U.S.A. Sigma Phase All of our nickel-bearing stainless and nickel base alloys have an austenitic structure, and are ductile and non-magnetic when they are placed in service. Ideally, a heat resistant alloy should retain these qualities throughout its service life. Some materials change after a few hundred or thousand hours in service, and become brittle instead of tough and ductile 1. This usually happens with high chromium, low nickel grades such as 309 and 310. The most common problem is that the alloy forms a hard, brittle nonmagnetic phase, called sigma. The overall chemical composition of the alloy remains the same. Sigma forms in the 1100-1600°F (600-870°C) temperature range. It happens more quickly, and embrittles more severely, when the alloy has been cold worked. Sigma may not seriously harm the alloy while it is operating at high temperature. But enough sigma can completely embrittle the alloy when it reaches room temperature. Chromium, silicon, molybdenum, columbium, aluminum and titanium promote sigma. Nickel, carbon and nitrogen retard its formation. The ASTM specifications for 310S (N31008) permit 1.5% silicon maximum, and AMS 5521 1.00% silicon. All RA310 plate, sheet and bar is made to restricted silicon, 0.75% maximum, to reduce sigma in RA310. One example of a failure due to sigma involved a long, heavy wall 310S muffle. It operated about 1200°F (650°C) with a vacuum inside, which tended to collapse it. After a few years the user inserted jacks and tried to jack up the roof which had fallen in. But, instead of straightening, the 310 roof cracked badly. And these cracks grew further when they tried to weld repair them. - 60 - Sigma phase, continued The solution would be not to use 310S or 309S at this low temperature. Indeed, below about 1400°F (760°C) these two grades have limited usefulness. Even 304H, which will form a certain amount of sigma, will not embrittle as badly as 310S. Although RA330 might normally be regarded as overkill for a 1200°F (650°C) application, RA330 does not form sigma or embrittle at any temperature range. Faced with an existing, brittle 310S muffle the only thing to do is to anneal it by heating 1900°F (1038°C) or higher. This will re-dissolve the sigma and restore ductility so that the metal can be straightened and weld repaired. Of course, after it goes back into service, sigma will again begin to form. We mentioned that silicon promotes sigma, but that RA330 does not embrittle from sigma. This is because RA330 has sufficient nickel, along with moderate chromium, that even silicon as high as 2% would be unlikely to result in sigma. There are no recorded instances, either in service or laboratory test, where RA330 has embrittled from sigma. The embrittlement due to sigma varies from alloy to alloy, can take a long time to occur and is less harmful at elevated temperature than at room temperature. The following is taken from work done for the ASME on superheater tube materials 2. Test Temp Condition 304 Charpy V-notch energy, foot-pounds (J) Alloy 321 347 316 310 800 68°F unexposed (20°C) 18 mo 1200°F 36 mo 1200°F 100 (136) 100 (136) 50 (68) 100 (136) 100 (136) 75 (102) 100(136) 50 (68) 35 (47) -65 (88) 40 (54) 100 (136) 25 (34) 10 (14) 100 (136) 50 (68) 55 (75) 68°F 18 mo 1350°F (20°C) 36 mo 1350°F 85 (115) 75 (102) 100 (136) 95 (129) 90 (122) 45 (61) 70 (95) 30 (41) 10 5 60 30 68°F 4 mo 1500°F (20°C) 6 mo 1500°F 18 mo 1500°F 30 mo 1500°F 34 mo 1500°F 36 mo 1500°F -100 (136) 70 (95) ---- -100 (136) 100 (136) 100 (136) --- 65 (88) ------ -65 (88) 35 (47) -25 (34) -- 20 (27) ------ -100 (136) ----- 1200°F unexposed (649°C) 18 mo 1200°F 36 mo 1200°F 100 (136) 100 (136) 100 (136) 100 (136) 100 (136) 100 (136) 100 (136) 85 (115) 85 (115) 100 (136) 100 (136) 100 (136) 100 (136) 85 (115) 60 (81) 100 (136) 75 (102) 80 (108) 1350°F unexposed (732°C) 18 mo 1350°F 36 mo 1350°F -100 (136) 100 (136) 100 (136) 100 (136) 100 (136) 100 (136) 100 (136) 100 (136) 100 (136) 95 (129) 85 (115) -35 (47) 40 (54) -85 (115) 70 (95) - 61 - (14) (7) (81) (41) Sigma Phase, continued 1500°F unexposed 100 (816°C) 4 mo 1500°F 6 mo 1500°F 12 mo 1500°F 18 mo 1500°F 30 mo 1500°F 34 mo 1500°F 100 (136) -100 100 100 --- 100 (136) -100 (136) 100 (136) 100 (136) 100 (136) -- 100 (136) 100 (136) ------ 100 (136) -100 (136) 100 (136) 100 (136) -40 (54) 100 (136) 30 (41)--- 100 (136) -- --- --- --- -- Chemical Compositon of Tube Materials Tested Above alloy UNS Cr Ni Mo Cb Ti C Fe 304A S30400 18.48 10.93 ---0.07 bal B 321 S32100 17.79 12.23 --0.45 0.05 bal 347 S34700 17.93 10.90 -0.56 -0.06 bal 316C S31600 16.77 13.20 1.96 --0.06 bal 310 S31008 24.56 21.42 ---0.07 bal 800D N08800 20.69 34.66 ---0.05 bal A B current production 304 averages 9% nickel 321 currently melted to typical 9.3% nickel C average nickel content of current production 316L is about 10.2% D titanium and aluminum not reported, specification is 0.15--0.60% each The Metal Properties Council3 performed studies on 310 and other materials after various elevated temperature exposures. The following are some test results: Test Temp ºF 80 80 80 80 1200 1500 1800 Condition AR PE 1200 PE 1500 PE 1800 PE 1200 PE 1500 PE 1800 Ultimate 0.2% Offset Tensile, Yield, psi psi 92,600 38,100 87,200 33,500 86,200 32,600 84,500 27,600 52,100 23,300 26,600 14,400 10,800 7,000 72.1 Elong % RA % 46.5 44.6 34.9 40.4 39.8 54.4 64.6 63.2 60.9 35.7 47.0 57.8 48.6 -- Charpy energy ft-lb 88.3 76.8 24.7 87.2 ---- Lateral Expansion, inches 0.070 0.065 0.019 0.060 --- 310 Heat No. 24659, 1 inch thick plate, from Jessop Steel Co., Washington, Pennsylvania AR –as received, mill annealed PE – pre-exposed 1000 hours at temperature, ºF All data is average of three tests Some reduction of Charpy V-notch energy is shown after exposure at 1500F. However the exposure time, 1000 hours, was too short for much sigma formation to occur. - 62 - Sigma Phase, continued Both RA 353 MA ® and RA 253 MA show a reduction in toughness after intermediate exposure. In this case chromium nitride precipitation is in part responsible 4. Exposed 5000 hours at ºF 1292 1472 1652 200 hours at ºF 1742 1832 1922 2012 Charpy V-notch Impact, foot-pounds RA 353 MA RA 253 MA 310S 8.9 7.4 5.9 6.6 3.7 34 4.4 3.7 16 7.4 25 72 204 ----- ----- RA330® shows retains high tensile ductility and Charpy V-notch energy after 1000 hour exposure to 1400ºF 5: Test Temp ºF 75 75 1400 1400 Condition Ultimate Tensile, 0.2% Offset Elong Yield, psi % RA % Charpy V -notch impact energy, foot-pounds AR PE 1400 AR PE 1400 85,000 88,500 35,000 -- 34,900 32,600 18,800 -- 70 60.5 59 -- 240 (test machine limit) 96 167 130 47.5 40.5 65 -- AR=as received, annealed PE = pre-exposed at 1400°F Grain Growth Most Rolled Alloys heat resisting alloys are produced to a medium-fine grain size, usually somewhere in the range ASTM 1-8 (250-11µm) for the smaller bar, sheet and light plate sizes. The 3/4” (19mm) diameter bar RA333SA, meant for furnace belt pins, and all forms of 800H/AT are definite exceptions, these grades being annealed 2150°F (1177°C) minimum to deliberately coarsen grain size. These aside, the grain size of a metal sample may be an aid to estimating what temperature the metal may been subject to in service. - 63 - Grain Growth, continued Grain size of RA330 versus time and temperature. The following is old data, laboratory annealing of 0.04% carbon alloy, arc furnace melted (no AOD remelt), hot rolled hand mill sheet, box annealed. Response to grain growth may vary from heat to heat, and may be influenced by prior mill processing: Temperature F C 1900 1038 1950 1066 2000 1093 2050 1121 2100 1149 2150 1177 2200 1204 5 5 5 4 4 4 3 3 ASTM Grain Size Number (µm) Time at temperature, minutes 10 15 30 60 120 (63.5) 4 (90) 4 (90) 3 (127) 3 (127) 2 (180) (63.5) 4 (90) 4 (90) 3 (127) 3 (127) 2 (180) (90) 4 (90) 4 (90) 3 (127) 2 (180) 2 (180) (90) 3 (127) 3 (127) 3 (127) 2 (180) 1 (254) (90) 3 (127) 2 (180) 2 (180) 2 (180) 1 (254) (127) 3 (127) 3 (127) 2 (180) 1 (254) 1 (254) (127) 3 (127) 2 (180) 1 (254) 1 (254) 00 (508) Light plate coupons were exposed6 in a vortex finder at an Eastern U.S.A. chemical company for 1862 hours at 1850°F (1010°C). Because of the long time exposure, as compared with the maximum 2 hours of the above table, RA309, RA310 and RA330 all experienced significant grain growth, while RA 253 MA and RA333 showed no measurable effect. Although the initial grain size was not recorded, these particular materials most likely were produced with ASTM 4-7 (88-31µm) initial grain size. Alloy RA333 RA 253 MA RA330 RA310 RA309 Sample thickness inch mm 0.259 6.58 0.242 6.15 0.243 6.17 0.190 4.83 0.192 4.88 Final Grain Size ASTM µm 5 62 4 88 00 508 4-00 88-508 00 and 508 and coarser coarser References 1. Symposium on the Nature, Occurrence and Effects of Sigma Phase, Special Technical Publication No. 110, ASTM, Philadelphia, Pennsylvania, U.S.A. June, 1950 2. George E. Lien, editior, Behavior of Superheater Alloys in High Temperature, High Pressure Steam, The American Society of Mechanical Engineers, New York, New York, U.S.A. 1968 3. Gene R. Rundell, Rolled Alloys Investigation 27-84, August, 1984 Temperance, Michigan, U.S.A. 4. Rolled Alloys Bulletin 1353, RA 353 MA® alloy 5. Private communications of January 10 and June 22, 1972, Crucible Inc., Materials Research Center. 6. Gene R. Rundell, Rolled Alloys Investigation 27-84, August, 1984 Temperance, Michigan, U.S.A. - 64 - HEAT RESISTANT ALLOY GRADES Now that we have reviewed the influence of the various alloying elements, and some of the environmental and mechanical requirements to be met in service, it is time to take a look at some of the available alloys on the market today. Iron-Chromium Alloys These range from simple ferritic or martensitic grades such as 409, RA446 and RA410, through enhanced oxidation resistant grades from AK Steel (formerly Armco) & Allegheny Ludlum to advanced oxide dispersion strengthened (ODS) alloys from Kanthal® and Special Metals ®. The iron-chromium alloys have low coefficients of thermal expansion, comparable to or slightly lower than that of carbon steel. These alloys have low ductility, and those with higher chromium contents, such as RA446, might even be called brittle. Because of their low strength at temperature (excepting the ODS versions), their use is limited to non-stressed parts. All ferritic or martensitic alloys with 12% or more chromium embrittle very severely when held in the 800-1000F (430-540°C) temperature range. This embrittlement is well known in the petrochemical field. It is called “885°F” (475°C) embrittlement, that being the temperature of most severe embrittlement. The metal can lose ductility to the point that it will crack in several pieces just from clamping it in a vise. 409, formerly called MF-1 by Allegheny Ludlum, is the lowest chromium alloy that qualifies as stainless. It has the advantage of being low enough in Cr to avoid 885°F/475°C embrittlement for some time, although it is reported to embrittle after some 50,000 hours service. 409 is processed in the mill to be a minimum cost grade. Automotive catalytic converter shells are made of 409, as are stainless exhaust systems. Being very low carbon and titanium stabilized, 409 is formable and weldable. A matching composition flux cored wire is available, as is a columbium (niobium) stabilized solid wire. 409 plate is sometimes welded with alloy 82 wire for better weld bead toughness. 409 has usable oxidation resistance up to about 1200°F (650°C). 410 is a martensitic grade, having enough carbon, about 0.14% C, that it can be hardened by heat treatment. It also hardens when welded, and requires both pre-heat and immediate post weld anneal to keep the weldment from cracking. 410S is a lower carbon, more weldable version of 410. 430 is the most broadly available ferritic stainless, used for both corrosion resistance and as a heat resistant grade. Commercial kitchens and bake ovens use quantities of 430. Very cheap, magnetic, “silverware” is 430. 430 sheet has been used to line the bottom half of RA330 brazing muffles, to protect the austenitic alloy muffle bottom from braze attack. - 65 - HEAT RESISTANT GRADES, continued 439 is about a percent higher in chromium, and titanium stabilized. It is not broadly available from distributors. AK Steel’s (formerly Armco) 18 SRTM uses both silicon and a critical ratio of titanium to aluminum to achieve oxidation resistance well in excess of what would be expected from its chromium level. Currently, 18SR sheet is available in full coil lots only. Allegheny Ludlum’s ALFA-IVTM uses aluminum and rare earths to achieve extremely good oxidation resistance with 20%Cr. This grade is made only in very light gage strip for automotive catalyst support systems. Oxide dispersion strengthened (ODS) grades achieve extreme temperature oxidation resistance in the same manner as ALFA-IV, that is, by about 5% aluminum with rare earths. These alloys are produced by mechanically incorporating the rare earth oxide, Y2 O3, into the Fe-Cr-Al matrix. As a result, the ODS alloys have very high creep rupture strength, quite unlike conventionally produced ferritic grades. The ODS ferritic grades available in the US are Inconel® MA956 and Kanthal® APM. Sandvik has in recent years begun extruding Kanthal APM into finished radiant tubes for industrial furnace use. At Rolled Alloys we have used Kanthal APM to 2100°F (1150°C) in our oxidation test tray (currently it is RA 602 CA). The disadvantages of the ODS materials at this time include cost in the neighborhood of $50/lb ($110/kg), limited availability and fabrication. Melting from arc welding destroys the oxide dispersion, leaving the weldment with only the (very low) strength of a conventional ferritic stainless. There has been some degree of success with laser seam welding 1/4” (6.35mm) MA956 plate. RA446, at 25% chromium, has the oxidation resistance needed for 2000°F (1100°C) service. This permits it to be used around molten copper or brass. The largest single use may be as electrodes for heating neutral salt baths. This high chromium, along with no nickel at all, gives RA446 the best resistance to sulphidation—usually—of the heat resistant alloys. No longer available in sheet gages (under 3/16 inch/4.8mm), RA446 is very weak at red heat, having at best less than 10% the creep strength of an austenitic nickel alloy. RA446 has a very high ductile-to-brittle impact transition temperature, at least 250°F (120°C). This means that at room temperature RA446 plate may crack whe n hit in a mechanical press break. In spite of its mechanical properties, RA446 is used for applications where nothing else will handle the corrosive environment. - 66 - HEAT RESISTANT GRADES, continued Nominal Chemistry, Ferritic and Martensitic Alloys alloy 409 410 430 439 18 SR ALFA IV Kanthal APM MA956 RA446 UNS S40900 S41000 S43000 S43035 ---S67956 S44600 EN -1.4006 -------- Cr 11 12 16.5 17.2 17.3 20 22 19.4 25 Si 0.4 0.3 0.5 0.5 0.6 0.4 0.3 0.05 0.5 Al ----1.7 5 5.8 4.5 -- Ti 0.4 --0.5 0.25 --0.4 -- C 0.015 0.14 0.08 0.015 0.015 0.02 0.05 0.02 0.05 Other -----0.03 Ce+La rare earths 0.5 Y2 O3 -- Iron-Chromium-Nickel Alloys, Nickel 20% and under These range from the high volume 304 and 321 up to a true heat resistant alloy, RA310. All of these grades can embrittle from sigma formation to some degree, only RA85H and 314 (W.Nr. 1.4841) have sufficient carburization resistance for heat treat service. Oxidation resistance and strength include some of the best available (RA 253 MA). This is also the group from which alloys with useful sulphidation resistance are chosen. 304 The basic “18-8” stainless is AISI type 304. Flat rolled products are usually either 304L, dual certified with 304, or 304H, likewise dual certified. Bar may actually be just plain 304, with 304L and 304H bar also available. Although the “L” grade is principally used for appearance or for aqueous corrosion resistance, the “H” version of this steel may be used to about 1500°F (815°C). 304 is limited to this temperature by oxidation resistance. Because 304 is austenitic, it retains strength at temperature. It has a fairly high coefficient of expansion. We would prefer some other material fo r an item that was to be heated and cooled rapidly. But for constant temperature or slow heating and cooling, at temperatures not above 1500°F/815°C, 304 can be considered and is used quite extensively. 316L Not really a heat resistant alloy but used as such anyway, particularly for fans. With 0.03% carbon maximum the design stresses at 1500°F (816°C) might be about 40% lower than for 304H. Nevertheless the 316L is chosen to better resist aqueous corrosion for fans which must operate part of the time at high temperature, and also near room temperature. 316H, even though it would be stronger at high temperatures, is rarely used because it is not broadly available. - 67 - Fe-Cr-Ni alloys, Nickel 20% and under, continued 321 This is a modification of the basic 18-8 grade with the addition of titanium to stabilize it against carbide precipitation in high temperature service or from the heat of welding. ASTM specifications require 321 to be annealed 1900°F (1038°C) minimum, which is too high for the grade to develop titanium carbides, i.e., be properly stabilized. For maximum resistance to carbide precipitation in service, hence resistance to polythionic acid stress corrosion cracking, it is suggested that welded fabrications of 321 be heat treated for 4 hours at 1600°F (871°C). 321 resists oxidation in high temperature service to about a 100°F (56°C) higher temperature than does 304, and is used up to 1600°F (871°C). RA 253 MA® achieves excellent strength and oxidation resistance through rare earths, a heavy calcium deoxidation, nitrogen and silicon. It was the first commercial NiCrFe alloy to use this technology, previously restricted to electrical resistance alloys (and the cobalt alloy 188). RA 253 MA is strong, with two to three times the creep strength of RA309. RA 253 MA is not particularly carburization resistant (RA309 is slightly better) nor has it performed well in REDUCING sulphidizing conditions (H2S). In oxidizing atmospheres RA253 MA has very good resistance to SO2 (sulphur dioxide), tolerating some 12% SO2 for extended periods at 1800F (982C). The maximum suggested continuous use temperature for RA 253 MA is 2000°F (1100°C). RA309 (really 309S, 0.08% max carbon) is one of the most widely used heat resistant alloys. Low cost, useful cyclic oxidation resistance to around 1850-1900°F (1010-1040°C) and fairly good sulphidation resistance characterize this grade. RA309 tolerates carburization well enough to be the grade of choice in carbon saggers. Fabrication is simple, and 309 weld fillers are often used for dissimilar metal welds. RA310 is one of the three alloys which should be considered where sulphidation is concerned, the other two being RA446 and RA309. The ferritic RA446 and the lower nickel RA309 might be preferred for very strongly reducing environments with sulphur present. In a more complex mix of chemicals RA310 is generally superior to RA309 in hot corrosion and is considered one of the standard materials of construction for coal gasifier and coal fired fluid bed combustor internals. RA310 has very good oxidation resistance, better than RA330 at constant temperature but not so good as RA330 when the temperature cycles. RA310 maintains useable oxidation resistance beyond 2100°F (1150°C). HR3C, also known as 310HCbN, is a nitrogen-columbium strengthened version of 310 with improved hot corrosion resistance up to perhaps 1600°F (870°C). Thousands of feet of steam boiler tubing are on test at TVA. The columbium (niobium) addition helps hot corrosion resistance at moderate temperature but is harmful to oxidation resistance around 1800°F (982°C) and upwards. Intermediate temperature embrittlement can be a problem. - 68 - 314 (W. Nr. 1.4841) is essentially 310 with 2% silicon. Rolled Alloys supplanted 314 with RA330 a generation ago in the U.S.A. 314 is widely used in Europe, and is often casually referred to as “310” there. For the most part, it is the German mills that make this grade. Silicon increases the already good oxidation resistance of 310 and adds both carburization and nitriding resistance.. However, coupled with the high chromium, silicon also increases the rate, and amount, of sigma formation. 314 may has lower creep strength than 310. Nominal Chemistry, Fe-Cr-Ni Alloys, Nickel 20% and under alloy 304 321 RA 253 MA RA309 RA85H RA310 HR3C 314 UNS S30400 S32100 S30815 S30908 S30615 S31008 -S31400 EN 1.4301 1.4541 1.4835 --1.4845 --- Cr 18.3 17.3 21 23 18.5 25 25 25 Ni 9 9.3 11 13 14.5 20 20 20 Si 0.5 0.7 1.7 0.8 3.5 0.5 0.5 2.0 C 0.05 0.01 0.08 0.05 0.20 0.05 0.06 0.10 N --0.17 ---0.25 -- Other 70Fe 0.2Ti 70Fe 0.04Ce 65Fe 62Fe 1Al 61Fe 52Fe 0.4Cb 52Fe 51Fe Iron-Nickel-Chromium alloys, Nickel 30-40% This nickel range covers some of the most successful heat resistant grades, such as RA330 and 800H, and the newest, RA 353 MA ® RA800AT TM , a.k.a. 800HT®, N08811, is a very strong alloy broadly used in the petrochemical and refining industries. Here 800AT is used as their basic structural material, much as RA330 is the basic heat treat alloy. 800AT gets its strength by a combination of: 1.) High temperature grain coarsening anneal, 2100°F (1149°C) minimum, commonly resulting in grain size ASTM 1-3. 2.) Carbon 0.06-0.10% 3.) Combined aluminum + titanium 0.851.20%. For the money, it is hard to beat the strength of 800AT. The alloy does have some drawbacks. First, the very coarse grains which are necessary for high creep-rupture strength are quite bad for thermal fatigue/ thermal shock resistance. This, coupled with mediocre oxidation resistance, largely keeps 800AT out of heat treat service. The second disadvantage is that the high combined aluminum + titanium content causes 800AT to form a very small amount of the age hardening constituent gamma prime at around 1100°F (600°C) or so. This has been suggested as the cause of cracking problems, and may be why the 800AT chemistry has been less well accepted in Europe. The cracking problem may be avoided by heat treating the welded fabrication 1625°F (885°C) for 1 1/2 hours, for thicknesses up to 1inch (25mm). Add one hour per inch (25mm) of thickness greater than 1” (25mm)1 . - 69 - Iron-Nickel-Chromium alloys, Nickel 30-40%, continued There are three versions of “800” alloy. The original “Incoloy®”, 0.10% max (no minimum) carbon, was announced in July, 1951, courtesy the Korean War nickel shortage. And perhaps influenced by the success of 35%Ni 15%Cr Misco Metal. Prior to this time the only heat resistant alloy Inco promoted was the 76%Ni alloy 600. Eventually Incoloy became Incoloy 800, and was available in two grades. Incoloy 800 Grade 1 was fine grained, annealed around 1800°F (980°C), and Grade 2 was solution annealed for greater creep-rupture strength. By applying a minimum carbon of 0.05%, Grade 2 became Incoloy 800H in the early 1970’s. During the 1980’s the ASME design stresses for 800H were challenged. In response the carbon was increased slightly to 0.06-0.10%, and the Al + Ti controlled, and raised from 0.7% typical to about 1% typical. RA330® is truly the workhorse alloy of the heat treating industry. The majority of all wrought alloy fixturing in use today is RA330, with some AISI 330 and a smaller amount of RA333®, and alloys 600 and 601. 35% nickel has been found to be the optimum level for carburization resistance and strength in the Fe-Ni-Cr alloy system. The 1.25% silicon addition enhances both carburization and oxidation resistance. RA330 has much better resistance to deformation (creep strength) than RA309 or RA310. At intermediate temperatures RA330 never embrittles from sigma like RA309 or RA310. A combination of fairly high melting point, 2450°F (1343°C) and good oxidation resistance permits RA330 to be used at more extreme temperatures than any other currently available grade. It is not uncommon for RA330 retorts to operate as high as 2250°F (1230°C) metal temperature. Our highest temperature well documented experience with RA330 was a palladium brazing muffle, 11 gage (3mm) operating 2300 to 2370°F (1260 to 1300°C), outlasting muffles of alloys 600 and 601. .RA330HC uses 0.4% carbon to provide high shear strength for use as pins in cast link belts, usually with cast HT links. RA 353 MA® may be regarded as an improved RA330 for use at 1830°F (1000°C) and higher, where it has twice the strength. Because of its oxidation resistance, weldability by GMAW, and 100°F (56°C) higher melting point, compared to 601, RA 353 MA is finding extensive use in retorts, kilns, brazing muffles, radiant tubes, utility coal burners and boiler tube shields. Oxidizing hot corrosion resistance is good, as are its hot erosion capabilities in cyclone applications. Welding is by either RA 353 MA DC lime type covered electrodes, or matching RA 353 MA GTAW and GMAW bare wire. - 70 - Nominal Chemistry, Fe-Ni-Cr alloys, Nickel 30-40% alloy RA800H/AT RA330 RA330HC RA 353 MA UNS N08811 N08330 -S35315 EN -1.4886 -1.4854 Cr 21 19 19 25 Ni 31 35 35 35 Si 0.4 1.2 1.2 1.2 C 0.06 0.05 0.40 0.05 Other 0.4Al 0.6Ti 45Fe 43Fe 43Fe 0.16N 0.05Ce 36Fe Nickel-Chromium-Iron alloys, Nickel 45-60% These alloys include RA333 and other superalloys developed for gas turbine use, as well as advanced grades for thermal processing. Costs are higher, and usage te nds to be in niche markets. RA X, developed in the early 1950’s by Haynes® as Hastelloy® alloy X, has been for years the standard alloy for gas turbine engine combustors. It is now slowly being replaced by alloys 188 and 230 in flight engines, and by 230 and 617 in land based gas turbines. Rather few people use X in heat treat service. It has excellent oxidation resistance in freeflowing atmospheres to rather high temperature, 2100°F (1150°C). Nevertheless, at more extreme temperatures or under stagnant atmospheres the 9% molybdenum content may render this alloy susceptible to catastrophic oxidation. For example, RA333 maintains oxidation resistance to 2200°F (1200°C), while alloy X plate at that temperature may completely disappear. This was a problem for us when some RA333 Mo reduction muffles were welded with alloy X covered electrodes. In service the weld beads disappeared, causing the whole muffle to fail. RA333® is one of the best performing wrought alloys for industrial heating applications, through 2200°F (1200°C). RA333 is strengthened with 3% each of cobalt, tungsten and molybdenum, and has a 1% silicon addition to enhance carburization resistance. RA333 is particularly good in resisting erosion from flame impingement, as in radiant tubes. RA333 permits a thinner tube, thus better heat transfer and energy efficiency, without danger of burning a hole through the tube. Direct service comparison with 601 in the same furnace has confirmed the superiority of RA333. Because of both strength and ductility, RA333 has excellent resistance to thermal shock. This includes water quenching applications, where RA333 has outperformed RA330, 600 (ductile, but weak) and alloy X. RA333 3/4” bar is used for cast link belt pins. RA333 pins tend to outlast the usual cast HT links. A higher alloy link, such as Supertherm®, may be suggested to maximize overall belt life when using RA333 pins. Rotary retorts of 3/16” (4.8 mm) RA333 plate, used to harden steel shot, have been giving 10 year life since the 1960’s. RA333 kilns 35 foot (1070 mm) long have been used to calcine zeolites for a decade now. - 71 - Nickel-Chromium-Iron alloys, Nickel 45-60%, continued RA333 is highly resistant to metal dusting, as shown by both years of experience and long term comparative testing. 617 alloy is strong, and has been used for land based gas turbine combustors, and in nitric acid catalyst support grids. It is carburization resistant at high temperatures, and oxidation resistant to reasonably high temperature. Because of its 9% molybdenum, it may be susceptible to catastrophic oxidation under stagnant conditions. 230 is a strong alloy, with good retention of ductility and excellent oxidation resistance. It has also been used in nitric acid catalyst support grids, and for parts of high temperature vacuum retorts. Nominal Chemistry, Ni-Cr-Fe alloys, Nickel 45-60% alloy RA333 RA X 617 230 UNS N06333 N06002 N06617 N06230 W/Nr 2.4608 2.4665 2.4663 -- Cr 25 22 22 22 Ni 45 47 54 60 Si 1 0.3 0.03 0.4 C 0.05 0.08 0.08 0.10 Other 3Co 3Mo 3W 18Fe 9Mo 1.7Co 0.6W 19Fe 12.5Co 9Mo 1Al 0.4Ti 1Fe 14W 1.5Mo 0.3Al 0.02La Nickel over 60%, 15 to 25% Chromium This group includes RA601, the Krupp VDM alloy 602CA, and RA600, often simply called by Inco’s tradename “Inconel®” RA601 is a strong, carburization resistant and very oxidation resistant alloy, developed by James Hosier. It was introduced in the 1960’s. It is used for retorts and muffles, bar product being relatively uncommon. Although 601 is very oxidation resistant, it is commonly welded with alloy 82 (ERNiCr-3), which is not. As a consequence 601 fabrications may require frequent rewelding, as the old 82 (columbium/niobium bearing) weld disappears. Using RA 602 CA weld fillers is suggested to address this problem. RA 602 CA® is a fairly recent development. It is very strong, extremely oxidation resistant, and resists grain growth in high temperature service. 602CA has been used in Germany for steel mill annealing furnace rolls. In the USA it has been used for kilns operating as high as 2100°F (1150°C), sintering muffles, CVD retorts and vacuum furnace fixtures. RA600 has moderate hot strength, good ductility and resistance to oxidation, and very good carburization resistance. Compared to RA330, 600 is nearly as oxidation resistant but somewhat lower in creep strength. RA600 has poor resistance to sulphidation, even in oxidizing atmospheres (sulphur present as SO2). - 72 - Nickel over 60%, 15 to 25% Chromium, continued RA600 has good resistance to corrosion by neutral heat treat salts and salt fumes. It is appropriate for automated salt pot fixturing. Economics generally favor RA309 or RA330 for the salt pot itself. RA600 has good resistance to dry chlorine and dry hydrogen chloride gas at temperatures up to 900-1000°F (480-540°C). As an aqueous corrosion alloy, RA600 is resistant to hot, concentrated caustic (sodium or potassium hydroxide) solutions. A bit of history. “Inconel” was originally sold for corrosion applications, not high temperature. In 1938 a salesman named Paul Goetcheus, working from the Chicago office of Steel Sales, sold the first Inconel sheet to Buick Motor Division, for carburizing boxes. Previously, Buick had used cast boxes weighing 200 pounds, to heat treat 50 pounds of work. Mr. Goetcheus moved on to head up the Rolled Products Division of Michigan Steel Casting Company, in 1944. There he worked to promote the use of a wrought (Rolled, in their terminology) 35Ni 15Cr alloy. In 1953 Mr. Goetcheus became the first president of Rolled Alloys, and the 35-15 alloy, Misco Metal, became RA330. Nominal Chemistry, Nickel over 60%, 15 to 25% Chromium alloy UNS W/Nr Cr RA601 N06601 2.4851 22.5 RA 602 CA N06025 2.4633 25 RA600 N06600 2.4816 15.5 Ni 61.5 63 76 Si 0.2 -0.2 C 0.05 0.2 0.08 Other 1.4Al 14Fe 2Al 0.1Y 0.08Zr 9.5Fe 0.2Ti 8Fe This concludes our general discussion of the various wrought materials that might be selected for a given application and those that we have selected to cover the range of temperatures, atmospheres, stresses and cyclic conditions. We think one of our alloys will perform to best advantage in almost every application. Therefore, we are fulfilling our slogan, “ALL THE BEST HEAT RESISTING ALLOYS, READY WHEN YOU NEED THEM”. - 73 - Cast Heat Resistant Alloys2 Heat resistant alloy castings are available in chemistries similar, although never identical, to those of the wrought alloys. In addition there are a number of chemistries that are only available as castings. Selection of cast versus wrought will depend, among other things, upon experience, economics and delivery time. Two aspects which influence whether one’s experience with either is good, or is bad, are: 1.) Design. Appropriate design may influence life more than the simple choice of wrought versus cast. A good design in either metal form may outlast a poor one in the other. This effect of design may or may not get factored into the user’s evaluation of his own experience. 2.) Quality. In fabrications this usually means the welds. With castings it is internal shrinkage, porosity and residual casting stresses. Cast grids, for example, may last only a few months, or for many years, depending on the foundry source. Advantages of Cast Alloy 1. Initial Cost. Since cast parts avoid all the forging, rolling, cutting and welding of a fabrication, the price per pound of fixture may be lower. 2. Creep Strength. Similar compositions are inherently stronger at high temperature in the cast form than in wrought. This is because of the microstructure, and because cast heat resistant alloys are usually much higher carbon than the wrought “equivalent”. 3. Shapes. Certain shapes can be cast that are not commonly available hot rolled, or that cannot be fabricated economically from available wrought product forms. 4. Compositions. Some alloys are available only as castings, because they lack sufficient ductility to be worked into wrought forms. This is particularly true of the very high chromium alloys. Disadvantages of Cast Alloy 1. Delivery. When equipment is down, fabrications can often be delivered in a couple of days to get back on stream. This is rarely true of castings. Weight. Cast parts are almost invariably thicker and heavier than the equivalent fabrication. This simply increases the dead weight that goes through each heat treat cycle. With radiant tubes and muffles thicker cast walls increase fuel costs for the same volume of work heat treated. 2. 3. Embrittlement. Many cast alloys quickly become very brittle in service. They are unable to withstand rough handling when cold, and weld repair is extremely difficult. - 74 - Cast Heat Resistant Alloys, continued 4. Soundness. Castings invariably have some degree of porosity, internal shrinkage cavities, internal oxides and cold shuts. When these defects are open to the surface they are subject to attack by carbon deposits or molten salts. 5. Pattern cost. A pattern must be made for each different part design. This is all right for production runs but quite uneconomical for one’s and two’s at a time. Advantages of Wrought Alloy 1. Section Size. Wrought alloys are available right down to nearly foil thickness. Thinner sections often permit weight reduction of 50% or more. With lighter sections the initial cost of a fabrication becomes competitive with, or less than, a casting. Handling the fixture is easier, and much less unproductive metal goes through each furnace cycle. 2. Thermal Fatigue. Thinner sections that reduce thermal stresses, and the inherently greater ductility of wrought metal, promote better resistance to thermal cycling and shock. 3. Surface Finish. The smooth surface of wrought alloy helps avoid focal points for accelerated corrosion by molten salts or carbon deposits. 4. Soundness. Wrought materials are normally free of the internal and external defects such as shrink, porosity, etc., found in castings. 5. Availability. Wrought heat resisting alloys are immediately available from stock in numerous product forms. Fabrications are quickly procured to minimize expensive down time. Disadvantages of Wrought Alloy 1. Creep strength. Few wrought alloys match the high strength of heat resistant castings. Where creep-rupture is truly important, this must be considered in product design. 2. Composition. Alloys such as 50Cr 50Ni, 28Cr 10Ni or 35Cr 46Ni, all with excellent hot corrosion and/or carburization resistance, are available only as castings. - 75 - Cast Heat Resistant Alloys, continued The effect of cast alloy surface and internal defects versus wrought alloy soundness on service performance is illustrated by our old case history, RA330-108. The application was a grid for suspending loads in a gantry furnace at a commercial heat treat shop. The work was neutral hardening from temperatures up to 1850°F (1010°C), quenched in either molten salt, oil or brine. When the cast HT (35%Ni 17%Cr) grid was practically new and had been exposed to only a few cycles, a particular job required increased working area for the grid. RA330 allo y plate was formed and welded to the outside of the existing cast grid. As can be seen in the photo, the cast alloy portion suffered surface attack from soot and the quenching salt, and failed from thermal fatigue. Note the cracks in the center of the cast ribs which occur along the plane of weakness of the dendritic structure. The wrought alloy RA330 exhibited very little surface attack and no fractures. - 76 - Cast Heat Resistant Alloys, continued Nominal Chemistry, Cast Heat Resistant Alloys3,4,5 alloy HC HD HE HH-2 Thermax® 40B HI HK HL HT HU HP Supertherm® HOM-3 22H® MO-RE® 40MA IN-657 HX UNS W/Nr J92605 -J93005 -J93403 1.4339 J93633 1.4837 --J94003 -J94204 1.4840 J94614 -J94605 -J95405 1.4865 J95705 1.4857 -----2.4879 --R20501 2.4813 N06006 - - Cr 28 29 28 25 25 28 25 30 17 18 26 26 26 28 35 50 17 Ni 2 5 9 13 13 16 20 20 35 38 35 35 46 48 46 47.5 66 Si 0.8 1.5 1.5 1 1 1 1.4 1.4 1.7 1.7 1.3 1.5 1 1 1 0.4 2 C 0.3 0.4 0.3 0.3 0.4 0.4 0.4 0.4 0.5 0.5 0.5 0.5 0.45 0.5 0.45 0.06 0.5 W ----0.5 ------5 3 5 ---- Co -----------15 3 ----- Other 67Fe 63Fe 61Fe 60Fe 59Fe 0.3Ti 54Fe 54Fe 47Fe 44Fe 40Fe 36Fe 13Fe 16Fe 3Mo 16Fe 14Fe 1.3Cb 1.5Cb 0.5Fe 13Fe Where metal dusting is a problem, one large captive shop has standardized on RA333 as their wrought alloy, and on Supertherm for cast fixturing, both grades being found extremely resistant to metal dusting (carbon rot). We have been told that nickel-aluminide alloy castings for heat treat service are quite strong. However, Midwestern experience has been that they contribute to heavy sooting in carburizing furnaces. References 1. 1998 ASME Boiler & Pressure Vessel Code, Section VIII, Division 1, paragraph UNF-56 (page 205), ASME, New York, New York. 2. Selecting the Alloy, Bulletin 113, Rolled Alloys, Temperance, Michigan 48182 U.S.A. 3. High Alloy Data Sheets, Heat Series, Steel Founders’ Society of America, 1973 4. Metals & Alloys in the Unified Numbering System , ASTM DS-56G, 8th Edition, ISBN 0-7680-0407-1 1999 Society of Automotive Engineers, Inc., Warrendale, Pennsylvania, U.S.A. 5. Stahlschlüssel, 18th Edition, 1998, Verlag Stahlschlüssel Wegst GmbH, D-71672 Marbach, German. - 77 - DESIGN Stresses (compressive, tensile, or shear) due to unequal temperature distribution and non-uniform temperature gradients, cause more failures in high—temperature equipment than all other influences combined amounting . . . to about 90 per cent of the total number of cases. And it is destructive chiefly because the engineer does not include in his design proper allowance for or provision against temperature inequalities or because the operator imposes temperature differentials which cause localized dimensional changes with accompanying stresses greater than the elastic strength of the alloy at the given temperature. F. A. Fahrenwald. Some Principals Underlying the Successful Use of Metals at High Temperatures, Proceedings of ASTM, 1924 V. 24 High temperature equipment design has certain unique features not commonly found, nor at least emphasized, in mechanical engineering texts. The first and most important is that metals expand in volume with heat. This simple statement is so obvious, yet often dismissed or given but slight consideration in design. If thermal expansion is somehow restrained, the resulting stresses will equal the yield strength of the metal at temperature. One must design to permit free expansion (and contraction) or the metal will bend, buckle or crack. A corollary to this is that most heat resistant alloys have rather poor thermal conductivity, less than 1/4 that of carbon steel and only 1/30 that of copper. Thermal gradients, hence thermal strains, are the rule and not the exception in high temperature equipment. Next, one should be aware of the significance, and the limitations, of creep-rupture data. These data are obtained under very closely controlled laboratory conditions of constant temperature and stress. Even so, there is considerable scatter, 15 to 20%, in rupture data, and possibly more in creep. When using published average creep-rupture data for design one must include a safety factor, and be clearly aware of the range over which temperature will be controlled in service. It can be surprising how rapidly mechanical strength drops off with temperature. For example, an increase in service temperature from 1700°F (927°C) to 1800°F (982°C) could drop the life of an RA330 component from 10 years down to only 15 months, under the same load. In practice, the furnace industry often designs to an allowable stress of one half the stress required for a minimum creep rate of 0.0001% per hour, at the service temperature. The ASME Boiler & Pressure Vessel Code is more conservative, designing to either 100% of the extrapolated 0.00001%/hour minimum creep rate, or 67% of the extrapolated 100,000 hour rupture stress, whichever is lower. - 78 - DESIGN, continued Rotating components, such as kilns, are often designed to much higher stresses than are static components. Kiln failures may be due to hot corrosion, more often to flite design, but rarely, if e ver, to fatigue from the rotation. An item of some minor confusion is elastic modulus. Although modulus data are published at elevated temperatures, the numbers are obtained by a means involving the speed of sound through the material. In practice, above about 1000°F (540°C) stress is no longer proportional to strain. In other words, at red heat these alloys are simply not elastic, and the modulus data has no real meaning. One cannot calculate a simple beam deflection at 1650°F (900°C) using anyone’s published modulus data. At such temperatures strain is proportional to both time and stress, and not simply to stress alone. Thermal Strain This point is such an important consideration for high temperature equipment design that it must be examined in some detail. A large portion of the many field failures reported to us happen because the designer or user did not appreciate the significance of thermal expansion. This expansion must be accommodated not only by design but by installation practice as well. Heat resistant alloys expand a great deal when heated. This expansion is roughly 3/16” to 1/4” for each foot of length (16 mm per meter), when heated from room temperature to 1800°F (982°C). If the metal is not free to expand, it will stretch, bend or warp permanently with each thermal cycle. Eventually, this repeated strain will fatigue the metal and the equipment will break. It is important to recognize just how large the total expansion can be, in typical heat treat service. A 48” (1220 mm) long RA330 heat treat basket, for example, oil quenched from 1550°F (843°C) will contract 0.692 (17.6 mm)—more than 11/16”—in overall length. Since the bottom of the basket enters the quench while the top frame is still red hot, the bottom members contract before the top does. A flexible bar frame design may tolerate this. But, a mechanically strong and rigid welded angle frame design may be inclined to crack or distort. This is because this “strong” design cannot accommodate the relative thermal contraction of the bottom versus the top of the frame. - 79 - Thermal strain, continued As temperature goes up the metal not only expands but diminishes rapidly in strength. The short-term yield strength of RA330, for example, averages about 37,200 psi (256 MPa) at room temperature, but only 40% of that figure, or 15,400 psi (106 MPa) at 1600F (871C). The short-term modulus, for whatever that is worth, drops from 28.5x106 psi (196 GPa) to 19.5x106 psi (134 GPa). The combination of differential thermal expansion/contraction and reduction in strength at heat is why quenched grids or large bar frame baskets tend to bow like a rocking chair, convex to the quench. In general any piece of metal which is hotter on one side will, when cooled, become concave on what was the hot side. As well as being the cause of distortion in service, this principle may be used to straighten metal parts1,2. The equation for calculating thermal stress in the elastic region is: S = aETK 1— ? a = coefficient of thermal expansion T = temperature difference ? = Poisson’s ratio E = elastic modulus K = restraint coefficient The formula may be found in S.Timoshenko, Theory of Elasticity, McGraw-Hill, New York, NY 1934 Apply this formula to RA330. Assume a plate 1000F on one side and 800F (538 to 427°C) on the other. a = 9.3 x 10-6 inch/inch°F E = 23.8 x 106 psi T = 200°F K = 1 ? = 0.297 The calculated stress = 62,970 psi. Average 0.2% offset yield strength of RA330 at 1000°F (538°C) is 25,000 psi. So, one may assume that a temperature differential of only 200°F (110°C) in this temperature range would cause permanent plastic deformation. The restraint coefficient in real structures will be some number less than one. Nevertheless, one rough, but good, rule of thumb is that a 200°F (110°C) temperature differential will yield most austenitic heat resistant alloys. - 80 - Weldments Weldments can fail from repeated thermal cycles. All welds, butt or fillet, must be completely fused. In thermal or mechanical cycling, the unwelded areas behave as large cracks or notches. Repeated thermal strains cause the “crack” to grow outward through the weld bead, a small step each cycle. Since this crack cannot be seen from the outside, there is no warning sign that the part is about to break. This fully welded joint can both thermal and and mechanical fatigue. The unfused void in this fillet resist weld acts as a stress riser may cause premature failure. Incompletely penetrated weld joints will not tolerate thermal strains and are the most common cause of weldment failure in high temperature service. A couple of examples: 1. Bar frame heat treating baskets. Incompletely fused welds crack a little more each time the basket is quenched. The weld may break in service, or when the basket is straightened. This happens even though the remaining weld metal is still ductile. 2. Furnace fans. Each time the fan starts up, it goes through one fatigue cycle. This is because centrifugal force, gas loading and the temperature differential between blade and hub all stress the blades. Eventually, just starting and stopping the fan will cause low cycle fatigue failure of incompletely penetrated welds. The blades may also flutter or vibrate - 81 - Weldments, continued during operation, which causes more fatigue crack growth. All welds of fan blades to the hub must be fully penetrated. A higher strength weld filler such as RA333 may be helpful in resisting mechanical loads. But no weld filler will compensate for inadequate weld joint design. Incidentally, it is more difficult to achieve weld penetration by the arc in nickel alloys than in stainless. A joint design that makes a good fan in 316L stainless (W.Nr. 1.4404) may well not allow adequate weld penetration in RA330. The result can be that the nickel alloy fan fails even though a stainless fan of same design performed well. More root gap may be required to achieve full penetration in a nickel alloy. Thermal Expansion A simple way to calculate the thermal expansion of a fixture is to use the chart below. Pick the alloy, read down the column to the operating temperature and read the number, which is how much (in inches) each foot of metal will expand. (Multiply by 83.33 to get how many millimeters each meter of metal will expand) Remember that thermal expansion occurs in all three dimensions. It is really a volume expansion, not just an expansion in one direction. So while the fixture is increasing in length, it is also increasing in width and height. A hole, incidentally, will expand at the same rate as the piece of solid metal that would just fill that hole. Example: An RA330 D-muffle 36 inches wide and 20 feet long operates at 1800°F. How far will the free end expand? Looking down the RA330 column we find a total expansion of 0.208 inches/foot at 1800°F (982°C). Multiply this figure by the length of the muffle, 0.208 in/ft X 20 ft = 4.16 inches total expansion. How wide will it be in the hottest zone? 36 inches + 0.208 in/ft X 3 ft = 36.624 inches. - 82 - Thermal Expansion, continued Temperature Range °F 70-200 -400 -600 -800 -1000 -1200 -1400 -1600 -1800 -2000 Total Thermal Expansion, inches/foot SA-387 RA446 RA321 RA309 RA 253 MA RA310 RA 353 MA RA330 RA333 RA601 RA600 RA 602 CA 0.0104 0.0281 0.0471 -0.0870 ------ 0.0141 0.0370 0.0610 0.0859 0.111 0.137 0.164 0.193 0.224 -- 0.00874 0.0225 -0.0526 0.0681 0.0854 0.102 0.123 0.152 -- 0.0145 0.0372 0.0604 0.0876 0.115 0.144 0.174 0.204 0.237 -- 0.0137 0.0356 0.0591 -0.108 --0.185 --- 0.0131 0.0348 0.0569 0.0806 0.106 0.133 0.160 0.186 0.214 0.245 0.0134 0.0345 0.0566 0.0796 0.104 0.129 0.154 0.181 0.209 -- 0.0129 0.0341 0.0566 0.0797 0.104 --0.180 0.208 -- 0.0109 ---0.0960 0.122 0.148 0.173 0.201 -- 0.0119 0.0317 0.0516 0.0727 0.0949 0.120 0.147 0.175 0.204 0.236 0.0115 0.0305 0.0502 0.0710 0.0937 0.117 0.142 0.167 0.193 -- 0.0103 0.0297 0.0496 0.0710 0.0915 0.115 0.144 0.174 0.201 0.227 The more general way to calculate thermal expansion is to use the mean coefficients of thermal expansion, such as those given on the next page. Multiply the length in inches, times the difference between room temperature and operating temperature, times the expansion coefficient. Note that these coefficients are all multiplied by 10-6, which is the same as dividing by one million. For that 20 ft long RA330 muffle operating 1800°F 982°C) this is: 20 ft X 12 inches/foot X (1800-70F) X 10.0x10-6 = 240 inch X 1730F X 10x10-6 = 4.152 inches. To convert these numbers to the metric system, multiply by 83.33 to get millimeters expansion per meter of length - 83 - MEAN COEFFICIENTS OF THERMAL EXPANSION ALLOY 200 300 400 500 600 700 800 900 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 304 9.6 -- -- -- 9.9 -- -- -- 10.2 -- 10.4 -- -- -- -- -- -- -- -- 316 8.9 -- -- -- 9.0 -- -- -- 9.7 -- 10.3 -- -- 11.1 -- -- -- -- -- 2205 7.2 7.3 7.5 7.7 -- -- -- -- -- -- -- -- -- -- -- -- -- -- -- RA321 9.3 -- 9.4 -- 9.5 -- 10.0 -- 10.3 10.5 10.6 -- 10.9 -- 11.1 -- 11.4 -- -- RA309 8.8 8.9 9.0 9.2 9.3 9.4 -- -- 9.7 -- -- -- -- 10.0 10.1 -- -- -- -- RA310 8.4 8.6 8.8 -- 8.95 -- 9.2 -- 9.5 -- 9.8 -- 10.05 -- 10.15 -- 10.3 -- 10.6 6.7 -- 7.1 -- 7.4 -- -- -- 7.8 -- -- -- -- -- -- -- -- -- -- RA 253 MA 9.06 -- 9.34 -- 9.59 -- 9.81 -- 9.97 -- 10.14 -- 10.3 -- 10.5 -- 10.8 -- -- 410 5.5 -- -- -- -- -- -- -- -- -- 6.5 -- -- -- -- -- -- -- -- 8.3 8.4 8.6 8.7 8.9 9.0 -- 9.2 9.3 9.4 9.6 -- -- 9.7 9.8 9.9 10.0 -- -- 7.95 -- 8.29 -- 8.56 -- 8.80 -- 8.98 -- 9.24 -- 9.52 -- 9.72 -- 9.87 -- -- RA 353 MA 8.48 -- 8.68 -- 8.88 -- 9.07 -- 9.27 -- 9.46 -- 9.66 -- 9.86 -- 10.05 -- -- RA800AT SA-387 ® ® RA330 ™ HR-120 ® 7.9 -- 8.8 -- 9.0 -- 9.2 -- 9.4 -- 9.6 -- 9.9 -- 10.2 -- -- -- -- ® RA446 5.6 -- 5.7 5.8 -- 5.9 6.0 -- 6.1 -- 6.3 -- 6.4 -- 6.7 6.9 7.3 -- -- RA600 7.4 -- 7.7 -- 7.9 -- 8.1 -- 8.4 -- 8.6 -- 8.9 -- 9.1 -- 9.3 -- -- RA601 7.6 -- 8.01 -- 8.11 -- 8.3 -- 8.5 -- 8.87 -- 9.19 -- 9.51 -- 9.82 -- 10.18 RA 602 CA 6.6 -- 7.5 -- 7.8 -- 8.1 -- 8.2 -- 8.5 -- 9.0 -- 9.5 -- 9.7 -- 9.8 RA333 7.0 -- -- 8.0 -- -- -- -- 8.6 -- 9.0 -- 9.3 9.3 9.4 9.5 9.7 -- -- HH -- -- -- -- -- -- -- -- 9.5 -- 9.7 -- 9.9 -- 10.2 -- 10.5 -- 10.7 HK -- -- -- -- -- -- -- -- 9.4 -- 9.6 -- 9.8 -- 10.0 -- 10.2 -- 10.4 HT 7.9 -- 8.14 -- 8.37 -- 8.61 -- 8.85 -- 9.09 -- 9.33 -- 9.56 -- 9.8 HP -- -- -- -- -- -- -- -- 9.2 -- 9.5 -- 9.8 -- 10.0 -- 10.3 -- 10.6 E-BRITE® 5.17 5.3 5.44 5.56 5.67 -- -- -- -- 6.09 6.22 6.4 6.57 6.72 6.85 6.88 7.1 -- -- 825 ® 10.04 7.8 -- 8.3 -- 8.5 -- 8.7 -- 8.8 -- 9.1 -- 9.5 -- 9.7 -- -- -- -- ® 20Cb-3 8.2 8.3 8.4 -- 8.65 -- -- 8.9 8.95 -- 9.15 -- 9.3 9.4 9.5 -- -- -- -- AL-6XN® 7.9 8.3 8.37 8.42 8.6 8.7 8.8 8.85 8.96 -- 9.3 -- -- -- -- -- -- -- -- TiGr 2 4.8 -- -- -- 5.1 -- -- -- 5.4 5.6 -- -- -- -- -- -- -- -- NOTE: All coefficients are reported as inch/inch °F x 10-6, room temp to indicated temp. Multiply by 1.8 for metric units. - 84 - Section Size Thin, rather than thick, sections reduce the thermal gradients inherent in heat resistant alloys used under conditions of rapid thermal cycling. Bear in mind that these alloys combine high thermal expansion coefficients with low thermal conductivity. In quenching service, the effects of repeated thermal shock can be as important as mechanical loading. The lightest possible section size should be used, to permit more uniform heating and cooling. We have seen baskets used for neutral hardening (which see many, many quench cycles) last twice as long when made of 1/2” (12.7 mm) diameter RA330 bar, as when they were constructed of 5/8” (15.9 mm) dia. bar. A dramatic example of the effect bar diameter has on quench cracking is shown below. About 4X About 4X 1/2” (12.7 mm) dia. RA330, basket top frame 5/8” (15.9 mm) dia. RA330 from same heat treat basket A 1/2” (12.7 mm) diameter bar, which shows essentially no cracking, was used for the basket’s top frame. The basket vertical members were 5/8” (15.9 mm) diameter. One of these is shown in cross-section, on the right. This 5/8” (15.9 mm) dia. bar has cracks extending in depth to one half its radius. Even though this heavier bar should be mechanically stronger, it is clearly weaker in resisting thermal shock. References 1. John P. Stewart, Flame Straightening Technology for Welders, 9773 LaSalle Boulevard, LaSalle, Quebec Canada H8R 2N9, 1981 2. John P. Stewart, Distortion Control, 9773 LaSalle Boulevard, LaSalle, Quebec Canada H8R 2N9, 1989 - 85 - SELECTING THE ALLOY Technical data illustrating the properties of heat resistant alloys are very helpful guides in selecting an alloy suitable for a given application. However the behavior of alloys during long exposure to the many environments and temperatures that may be encountered cannot be completely documented nor described by laboratory tests. Experience obtained from many actual installations is most helpful. One must develop the judgment needed to determine which of the many factors involved are the most important. A few points to consider. Temperature is often the first—and sometimes the only—data point given when we are asked for suggestions regarding alloy selection. One cannot successfully chose an alloy based on temperature alone. Nevertheless one simple first guide to alloy selection is knowing the maximum temperature at which a given alloy may have useful long term engineering properties. Picking oxidation in air, or strength, as a limiting factor one might rate alloys as follows, in plate form. Thin sheet will have a lower limiting temperature due to proportionally greater losses to oxidation. Carbon steel, such as ASTM A 387 Grade 22 (2¼ Cr, 1 Mo). Typically considered 950°F (510°C), above which 304H is stronger. 409 ferritic stainless (UNS S40900, Werkstoff Nr. and EN 1.4512) 1200°F (650°C), limited by oxidation. Subject to embrittlement after several years’ service above about 600°F (316°C). Formable, weldable. 410S low carbon martensitic stainless (UNS S41008, W.Nr. 1.4000) 1200°F (650°C), limited by oxidation. Subject to “885°F” embritlement after long service above about 600°F (326°C). 410 martensitic stainless (UNS S41000, W. Nr. 1.4024) 1200°F (649°C), limited by oxidation. Subject to embrittlement after several years’ service above about 600°F (316°C). Can be hardened by heat treatment, difficult to weld. 304/304H & 316 stainless (S30400/S30409, W.Nr. 1.4301 & S31600, 1.4401) 1500°F (816°C). If product contamination by scale particles is a consideration, consider a 1200°F (649°C) limitation, and move up to RA309 for 1500°F (816°C) service. 321 (S32100, W.Nr. & EN 1.4541) stainless has about a 100°F (55°C) advantage over 304, and is used to 1600°F (1202°C). In Europe 316Ti (W.Nr. & EN 1.4571) is used to 1650°F (899°C), whether because of technical advantage over 321 or difference in philosophy we do not know at this time. RA309 (S30908, W.Nr. & EN 1.4833) is useful to about 1850-1900°F (1010-1038°C) above which our customers seem dissatisfied with its oxidation performance. RA800H/AT (UNS N08811) is a little more oxidation resistant, still we’d suggest keeping it below 2000°F (1093°C) - 86 - SELECTING THE ALLOY, continued RA 253 MA ® (UNS S30815, W.Nr. 1.4893, EN 1.4835) has superior oxidation resistance to a fairly definite upper limit of 2000°F (1093°C). Above this temperature the oxidation resistance may be adequate but no longer exceptional. RA310 (S31008, W.Nr. & EN 1.4845) is reasonably oxidation resistant to about 2150°F (1177°C), although the strength is quite low. RA330® (N08330) combines useful oxidation resistance and fairly high melting point so that it will tolerate more extreme temperature abuse than any other fabricable austenitic grade with which we are familiar. RA330 muffles are regularly used at 2100-2150°F (1149-1177°C). In one exceptional case an 11 gage (3mm) wall RA330 muffle provided six months service brazing with 65% palladium 35% cobalt filler at 2370°F (1300°C). RA 353 MA ® (S35315, EN 1.4854 ) has a melting point similar to that of RA330, with better oxidation resistance in laboratory tests. Field experience at this time is with muffles and calciners. Based on its chemistry and test results we would expect it to tolerate extreme temperature at least as well as does RA330. RA333® (N06333, W.Nr. 2.4608) in open air use is limited more by its incipient melting point than by oxidation. Temperatures to 2200°F (1204°C) may be considered, though stagnant conditions might not be desirable. We have no experience with this grade at 2300°F (1260°C). RA600 (N06600, W.Nr. 2.4816) excellent carburization resistance. Oxidation resistance does not drop off rapidly with temperature. RA600 is used at the same high temperatures as RA330, although somewhat more creep deformation may occur in service. RA601 (N06601, W.Nr. 2.4851) has deformed more than RA333 in 2150F (1177C) applications and should have a somewhat lower maximum temperature use. RA X (N06002, W.Nr. 2.4665) is designed for gas turbine combustors where the hot gases continually sweep over the metal surface. Due to its 9% molybdenum content this grade may be subject to catastrophic oxidation under stagnant conditions, or in open air above roughly 2150°F (1177°C). Know the atmosphere which the alloy must resist—is it air, inert gas, reducing, etc.? Or vacuum? Vacuum—obviously, metal loss from oxidation doesn’t exist so rather lean alloys may be used to extreme temperature if mechanical properties suit. Occasionally chromium is a concern, as it can evaporate from the alloy fixture, then deposit on cooler areas of the furnace. Sometimes parts will diffusion bond themselves to the fixture at very high temperature. One cure for this is to paint braze stop-off on the parts or fixture. Nicrobraze ® Orange Stop-Off, from Wall Colmonoy Corp. is used for this purpose. Alloys commonly used as fixturing for vacuum heat treating tool or stainless steel include RA330, RA600 and RA601. - 87 - SELECTING THE ALLOY—atmosphere, continued Air—those alloys useful in just plain hot air are also suited for oxidizing products of combustion of natural gas and even coal. Generally oxidation and strength are the only issues. “Oxidation” usually refers to metal wastage, but concern about product contamination from scale is an occasional issue. For example, glass forming operations take place around 1100-1400°F (600-760°C) where 304 stainless might suit as a structural element. But because scale from this stainless gets onto the glass, RA330 is used for its much higher oxidation resistance. With respect to oxidizing products of combustion of coal, a major end use of RA 253 MA is for coal nozzles in powdered coal fired utility boilers. Oxidizing products of combustion from heavy fuel oils may be corrosive due mostly to small amounts of vanadium in the oil, particularly in oil from Venezuela. The vanadium pentoxide formed is very corrosive at red heat. The only alloy said to resist V2O5 hot corrosion is the cast 50Cr-48Ni alloy (“50-50”), UNS R20501. Short of that, one might consider RA333, although it is definitely not as resistant as the 50%Cr alloy. While oxidizing products of combustion of coal can readily be handled by alloys such as RA 253 MA, or higher nickel grades if one prefers, reducing products of coal combustion are another matter. In reducing atmospheres, which do occur in certain areas of the current generation of low-NOx burners, sulphidation from both coal and oil fuels can be a serious matter. We would not suggest use of any alloy with higher nickel than RA310. That is, limit nickel content of the alloy to about 20%, to minimize sulphidation attack. In all other atmospheres there may be some potential for carburization or hot corrosion. If the atmosphere really is hydrogen, argon or nitrogen then no reaction with the alloy should occur. But sometimes the atmosphere as it exists in the furnace is unintentionally different than the pure gas pumped into the furnace. A classic case is a coil annealing cover for carbon steel. The atmosphere is nominally nitrogen-hydrogen, which would be quite neutral. But residual palm oil from cold rolling steel sheet vaporizes and makes the atmosphere carburizing enough to deposit soot inside the cover. This “inert” atmosphere will also mildly carburize the cover itself, usually RA309 or RA330. When steel rod coils are annealed the carbon potential of the atmosphere is controlled to 0.4%C, to be neutral to the AISI 4140 or 1045 steel rod being annealed. This atmosphere is actually carburizing to Ni-Cr-Fe heat resistant alloy, and tends to embrittle RA 253 MA. A less common situation is sulphidation of alloy fixturing used to anneal copper cathodes, electrolytically refined copper. The cathodes have residual copper sulphate from the electrolyte used in this process. This is the source of sulphur, which will attack nickel alloy furnace fans, in particular, used for annealing cathodes in a reducing or neutral atmosphere. - 88 - SELECTING THE ALLOY—atmosphere, continued Intentionally carburizing atmospheres are commonly used in heat treatment of steel parts. Resistance to embrittlement from carbon absorbtion is largely conferred by the total chromium, nickel and silicon content of the heat resistant alloy. Wrought alloys commonly used to resist carburization include RA330, RA333, RA600 and RA601. Of the lower nickel grades only RA85H, at 3 1/2% silicon, has useful carburization resistance in heat treat furnaces. RA800H/AT is too coarse grained, and too low in silicon, for practical use in such applications. RA X has good carburization resistance and is occasionally used. In high chlorine, or fluorine, atmospheres high nickel alloys are preferred. RA600 is the usual choice. When some amount of oxygen is also present, with only moderate halogen levels, RA601 may be useful. Under oxidizing conditions chromium, molybdenum and tungsten form highly volatile oxychlorides. - 89 - CUTTING AND FORMING Mild steel and heat resisting alloys do handle differently, and it is well to know the product you are working with to get the most out of it. There are some rules to know, but for the most part designing and fabricating alloys is using common sense based on the properties of the alloys and what you expect them to accomplish. Shearing In the first place, the yield strengths of heat resisting alloys in the annealed condition are a little higher, and their tensile strengths a lot higher, than mild steel. Shear capacity, for example, has to be about 50% greater. On our shear rated 3/8” (9.5 mm) mild steel, we regularly shear 1/4” (6.35 mm) heat resisting alloy. The hydraulic shear, rated 3/4” (19 mm) mild steel, will handle 1/2” (12.7 mm) alloy plate. Good shearing practice cuts about 20% of the metal and fractures the remaining 80%. Heavier thickness plate is best cut by abrasive wheels, which produce a smooth, close tolerance surface. Bending and Forming Austenitic heat resisting alloys should almost always be bent cold. Heating without adequate temperature control is dangerous because of the narrow working range and the possibility of over or under heating. In the 1200 to 1600°F (650-870°C), or red heat, range, both 18-8 stainless and the nickel heat resisting grades will tear or rupture in forming. It will take more power to form these alloys than it takes to form mild steel, but because of good ductility, the alloys will take a lot of deformation without rupture. Extremely severe forming may require annealing between operations. The ferritic grade RA446 does not form well at room temperature. Plates 1/4” (6.35 mm) and thicker should be preheated 250-400°F (120-205°C) for any bending or forming. Failure to preheat may result in some plates cracking apart, while others may be formed successfully. These alloys always harden on deformation and cannot be worked beyond a limit without rupture. Our stock materials have all been scientifically annealed. A given size will have limits on hardness, elongation, and reduction of area. A typical plate might have a Rockwell B hardness of 84, and elongation of 35% and a reduction of area of 60%. Every lot of RA material is checked for these properties, and the mill certifications of the material delivered to you are kept on file for ten years at Rolled Alloys. Records on your order are kept by RA for six years. After work hardening, but before rupturing, the material can be restored to its original mechanical properties by annealing. The process varies with the alloy, the mass, and the hardness. A piece of 3/16” (4.8 mm) plate, for example, that had been formed into a 4” (100 mm) tube might have had its hardness raised from the original Rockwell B 84 up to RB 96. It could be returned to RB 84 by heating to 1950°F (1065°C) and holding at this temperature for five minutes, then cooling quickly with an air blast. There probably would be little reason for annealing this shape, unless it was to be formed again, and it were required to be soft to permit further cold work. - 90 - Bending and Forming, continued These solid solution strengthened materials, therefore, can be hardened only by cold working, and softened by annealing. Occasionally tooling for aerospace requires to be stress relieved after rough machining, which, in the case of RA330, may be accomplished by heating for one hour per inch (25 mm) of thickness to 1800°F (982°C), and furnace cooling until black, then air cooling. The austenitic alloys will take a bend of 180° with a minimum inside radius equal to twice the thickness of the material. They will sometimes accept a bend flat on themselves, but they are not guaranteed to do so. The fabricator must perform bends with small radii and his own risk and be prepared to weld cracks that may develop. For extreme bends or the harder alloys it is better to bend across the grain, rather than having the grain parallel to the bending axis. The work hardened surface of a sheared or punched edge limits the amount of forming possible befo re cracking. As a minimum precaution, the shear burr or drag should be ground off. If severe forming is anticipated, the work hardened metal must be removed from the edge to be formed. The photo on page 80 shows the effect of edge condition on formability of 1/2” (12.7 mm) RA333 plate. With an as-sheared edge, the plate cracked after only a 40° bend. Removing the shear burr permitted a 90° bend with some cracks. With a ground or bandsawn edge, material from the same plate was bent 180°, nearly flat on itself, with no cracking. The first sign of overstretching is an orange peel appearance. This in itself is seldom detrimental, but the fracture soon to follow with further forming is incurable except by welding. It is far better to avoid a design that makes use of minimum radii. A generous radius is better for keeping the metal solid in service as well as during forming, because it gives the structure freedom to expand and contract, minimizing the thermal stresses created by heating and cooling. A thermal expansion of some 3/16” per lineal foot (16 mm per meter) is going to occur between room temperature and the average service temperature. The resulting stresses are great, and the metal is going to move; so it should be pointed in the right direction. After RA333, by a slight margin RA 353 MA, RA 602 CA, and RA 253 MA are the strongest metals of the group in most temperature ranges; likewise they are slightly tougher to work. RA309 and RA310 are a little weaker. RA600 is somewhat softer and weaker. Its high nickel makes it “gummier” than alloys with more iron. The ferritic RA446 is less ductile and requires preheating before bending. - 91 - About 0.7 Scale RA333 1/2 inch (12.7 mm) plate, formed with different edge preparations. Left - Sheared edge ground. Bent 180° flat on itself, no cracks. Middle - Shear burr removed. Bend 90° before cracking. Right- As-sheared, burr up. Cracked at 40° bend angle. - 92 - SPINNING AND DEEP DRAWING Spinning and deep drawing can be accomplished by taking into consideration the physical properties, work hardening, and annealing. RA330 spins rather well, roughly comparable to 305 stainless. None of the heat resistant alloys will deep draw as well as 304 stainless. Dies for drawing the heat resistant alloys ought not be proofed with 304, as results will be different. Illustrated below is an 11 gage RA85H spun half for a radiant tube return bend. - 93 - MACHINING The alloys described here work harden rapidly during machining and require more power to cut than do the plain carbon steels. The metal is “gummy”, with chips that tend to be stringy and tough. Machine tools should be rigid and used to no more than75% of their rated capacity. Both workplace and tool should be held rigidly; tool overhand should be minimized. Rigidity is particularly important when machining titanium, as titanium has a much lower modulus of esasticity than either steel or nickel alloys. Slender work pieces of titanium tend to deflect under tool pressures causing chatter, tool rubbing and tolerance problems. Make sure that tools are always sharp. Change to sharpened tools at regular intervals rather than out of necessity. Titanium chips in particular tend to gall and weld to the tool cutting edges, speeding up tool wear and failure. Remember—cutting edges, particularly throw-away inserts, are expendable. Don’t trade dollars in machine time for pennies in tool cost. Speed Surface ft/min Material Speed as a % of B1112 Feed rate should be high enough to ensure that the tool cutting edge is getting under the previous cut thus avoiding work-hardened zones. Slow speeds are generally required with heavy cuts. Sulfur-chlorinated petroleum oil lubricants are suggested for all alloys but titanium. Such lubricants may be thinned with paraffin oil for finish cuts at higher speeds. The tool should not ride on the work piece as this will work harden the material and result in early tool dulling or breakage. Use an air jet directed on the tool when dry cutting, to significantly increase tool life. Lubricants or cutting fluids for titanium should be carefully selected. Do not use fluids containing chlorine or other halogens (fluorine, bromine or iodine), in order to avoid risk of corrosion problems. The following speeds are for single point turning operations using high speed steel tools. This information is provided as a guide to relative machineability, higher speeds are used with carbide tooling. Speed Surface ft/min Material ® Speed as a % of B1112 AISI B1112 ® René 41 165 12 100 7 RA 353 MA ® RA 253 MA 40-60 45-60 25-35 28-35 25 (l-605) RA188 N-155 15 15 20 9 9 12 RA2205 ® 20Cb-3 ® AL-6XN 50-65 65 65 30-40 40 40 20 20-40 20 12 12-24 12 RA309 RA310 304 70 70 75 42 42 45 RA X ® RA333 A-286 20 20-25 30 12 12-15 18 RA321 RA446 17-4PH 75 75 45 45 RA601 RA800AT Ti 6Al-4V 25-35 25-35 15-21 15-21 Sol’n treated Aged H1025 303 75 60 100 45 36 60 Sol’n annealed Aged ® RA330 30-40 15-45 30-45 18-30 9-27 18-27 WASPALOY RA718 RA625 TM RA333 is a Registered Trademark of Rolled Alloys RA330 is a Registered Trademark of Rolled Alloys 253MA and 353MA are Registered Trademarks of Outokumpu 20Cb-3 is a Registered Trademark of Carpenter Technology - 94 - FORGING Hot forging should be used only if cold pressing cannot do the job. We know the materials are forgeable, because they all came from large cast ingots; but the working ranges are narrow, and close control of temperature, time, heating atmosphere and reduction are all important. Heat resistant alloys must be heated throughout the section thickness. Typically, forging should begin when the metal is around 2100-2200°F (1150-1200°C), and finish before the metal cools below 1700°F (930°C). The exact temperature ranges vary from alloy to alloy. Forging either too hot or, more likely, too cold may cause cracking. Never, never attempt to bend or form any austenitic alloy in the 1100-1600°F (590870°C) temperature range. Whether 304 stainless or nickel alloy 600, all will tear when formed at these temperatures. Unlike carbon steel, heating locally with a torch to make bending easier just doesn’t work. It is too difficult to heat nickel alloys uniformly hot enough throughout the section. 1/2” (12.7 mm) diameter RA330 scale: 1 7/8 X Torch heated to bend. Although the operator thought it was hot enough, the brown temper color in the crack is typical of about 1200°F (650°C) - 95 - WELDING Welding heat resistant alloys is covered in general in our Bulletin No. 115, and in more detail in No.’s 201 & 207 for RA330, 202 for RA 253 MA, 209 for RA 353 MA, with Bulletin120 covering RA333 welding products. For our corrosion resistant alloys see Bulletins 203 for AL-6XN® , 1071 for RA2205, and Bulletin 205 for 20Cb-3 stainless. Heat resistant alloys are readily welded but they do require more time, and a DIFFERENT approach than stainless, or carbon steel. A few important rules: 1. Make reinforced, stringer beads. Do not weave. Shallow fillet welds or broad, flat weld beads tend to crack down the center as they solidify. Cover or fill in craters, to prevent them from cracking. 2. Keep heat input low. Do not ever preheat, except to dry moisture off of the metal. Keep the temperature of the metal between weld passes low, below 212°F (100°C). 3. For RA330 specifically, use RA330-04 or RA330-80-15 weld fillers. Do not use AWS E330 weld wire, as it will be crack sensitive. Absolutely do not try to weld RA330 with stainless rods such as E308, E309, or E310 as they will crack. E312 electrodes, in particular, are often sold under various tradenames for general shop repair welding and dissimilar metal welds. However, E312 weldments are not suited for high temperature service. They embrittle severely with exposure above 600°F (1100°C). At red heat E312 welds are very weak, as well as brittle. - 96 - 4. Make full penetration weld joints. Incompletely welded areas will open up as cracks during normal heat treat thermal cycles. Incompletely penetrated weld joints are the most common cause of weld failures in service. Weld joints in fans, in particular, must be fully penetrated. Let us back up a bit, and first describe some of the differences between welding carbon steels, and welding either stainless or nickel alloys. Then, we will cover the important differences between stainless (under 20% nickel) and the higher nickel alloys. CARBON STEEL VERSUS STAINLESS Some important differences between welding the carbon or low alloy structural steels and the austenitic stainless and nickel alloys include A. Surface Preparation B. Shielding Gases C. Cold cracking vs Hot Cracking D. Distortion E. Penetration F. Fabrication Time. A. Surface Preparation When fabricating carbon steels it is common practice to weld right over scale (a socalled “mill finish” is a layer of blue-black oxide, or scale, on the metal surface), red rust and even paint. The weld fillers normally contain sufficient deoxidizing agents, such as manganese and silicon, to reduce these surface iron oxides back to metallic iron. The resultant Mn-Si slag floats to the weld surface. Iron oxide, or “scale”, melts at a lower temperature, 2500°F (1371°C)1, than does the steel itself. One can see this in a steel mill when a large ingot is removed from the soaking pit for forging—the molten scale literally drips off of the white hot steel. Stainless steel, by contrast must be clean and free of any black scale from hot rolling, forging or annealing operations. Of course, stainless normally comes from the mill with a white or bright finish. A few users of heat resistant alloys, though, do prefer “black plate”, that is, plate with the mill hot rolling scale intact. This scale is thought to provide additional environmental protection at red heat. - 97 - WELDING, Surface Preparation, continued Stainless steel melts at a lower temperature than does its chromium oxide scale, and the stainless weld filler chemistry is not capable of reducing this scale back to metallic chromium. As a result, with gas shielded processes it is difficult to get the weld bead to even “wet”, or stick to, a scaled piece of stainless. With SMAW a weld of sorts can be made, as the coating fluxes away most of the scale. The need to clean or grind down to bright metal is more likely to cause trouble when stainless is being joined to carbon steel. That is because in this dissimilar metal joint it is necessary to grind that carbon steel to bright metal, on both sides of the joint, free of all rust, mill scale, grease and paint. Incidentally, the appropriate weld fillers for this particular joint, to minimize the hard martensitic layer on the steel side, are alloy 182 covered electrode, ENiCrFe-3, or alloy 82 bare wire, ERNiCr-3. Alloy 62 bare wire, ERNiCrFe-5, and weld metal A, ENiCrFe-2 covered electrodes are also used. E309 electrodes are commonly used but may leave a hard layer on the steel side, which may crack. Both stainless and high nickel alloys which are designed for corrosion resistance are produced to very low carbon contents, less than 0.03% and sometimes less than 0.01% carbon. Any higher carbon will reduce the metal’s corrosion resistance. For this reason it is necessary to clean these alloys thoroughly of all trades of grease and oil before welding. Also the very high nickel alloys, such as 400 alloy (Monel®, UNS N04400), or commercially pure nickel 200/201, are sensitive to weld cracking from the sulphur in oil. Metallic zinc paint is a common way to protect structural steel from corrosion. Even a small amount of that zinc paint overspray on stainless will cause the stainless to crack badly when welded. Consider completing all stainless welding before painting the structural steel in the area. B. Shielding Gases For gas metal arc welding (a.k.a. MIG) carbon steel the shielding gases are usually 95% argon 5% oxygen, 75% argon 25% CO2 (carbon dioxide) or 100% CO2. These are suitable with carbon or low alloy steel welding wire but far, far too oxidizing for use with stainless or nickel alloys. It is not unknown to hear the complaint “. . . clouds of red smoke are coming off when I weld your 310. . . heavy spatter. . .” and then learn that the shielding gas used was 75%Ar 25%CO2. A fine gas for carbon steel but not for stainless. One exception to this high CO2 prohibition is when using flux cored wire, either stainless or nickel alloy. Some of these cored wires are specifically formulated to run best with 75%Ar 25%CO2. - 98 - B. Shielding gases, continued Stainless and nickel alloys are often GMAW spray-arc welded with 100% argon. Weldability is greatly improved by adding from 10 to 20% helium. Helium provides a hotter arc. This helps burn away the stable chromium oxide film which does impair weldability of stainless and chromium-nickel alloys. A very small amount of CO2, about 1%, will stabilize the arc (prevent arc wander). When 75% argon 25% helium is used for GMAW a true arc transfer cannot be obtained. Rather, the arc transfer somewhat resembles the globular transfer mode. There are shops where this is preferred. Shortcircuiting arc welding generally requires the 75%Ar 25%He mix, but a 90%He 7 1/2%Ar 2 1/2%CO2 “tri-mix” is commonly used. C. Cold Cracking versus Hot Cracking Carbon steel weldments may harden, and crack, as they cool from welding. High hardness, and the resulting cracking, are more likely when the steel contains over 0.25% carbon. Alloying elements which increase hardenability, such as manganese, chromium, molybdenum, etc. can make steels of lower carbon content also harden. Hydrogen pickup from moisture in the air causes underbead cracking in steels that harden as they cool from welding. To prevent such cracking the steel is usually preheated before welding, to retard the cooling rate of the weld and avoid martensite formation. Postweld heat treatment, or stress relief, is also applied to some steels, or for certain applications. Austenitic stainless and nickel alloys do NOT harden no matter how fast they cool from welding. So, it is not necessary to preheat stainless, nor to post weld heat treat it. As a matter of fact preheating stainless, beyond what may be necessary to dry it, can be positively harmful. Stress relief 1100-1200°F (600-650°C) as applied to carbon steel is ineffective with stainless or nickel alloys, and may damage the corrosion resistance of some grades. Stainless steel welds generally do not crack unless contaminated, possibly by zinc or copper, more rarely by aluminum. High nickel alloys are susceptible to cracking in restrained joints, or heavy sections. This is a hot tearing, not a cold crack. That is, the weld bead tears rather than stretching, as the bead contracts upon solidifying. This hot tearing/hot cracking has nothing to do with hardness. The faster a nickel alloy weld freezes solid, the less time it spends in the temperature range where it can tear. For this reason preheating, which slows down the cooling rate, is actually harmful, as it permits more opportunity for hot tearing to occur. - 99 - D. Distortion2,3 Stainless steel has poor thermal conductivity, only about one fourth that of A 36 structural steel. This means the welding heat tends to remain concentrated, rather than spread out. Stainless also expands with heat about half again as much as does carbon steel. The combination of these two factors means that stainless or nickel alloy fabrications distort significantly more than similar designs in carbon steel. Among other things, tack welds need to be more closely spaced in stainless/nickel alloy welds. Welds should be sequenced about the neutral axis of the fabrication to balance welding stresses, hence minimize distortion. Back step welding is also helpful. Tack welds should be sequenced. 1 6 4 7 3 8 5 9 2 If the tacks are simply done in order from one end, the plate edges close up and the gap disappears. 1 2 3 4 5 Back step welding helps reduce distortion. - 100 - D. Distortion, continued 3 2 1 E. Penetration The arc will not penetrate a stainless nearly as deeply as it will carbon steel. Penetration is even less in high nickel alloys. Increasing welding current will not solve the problem! Stainless, and especially nickel alloy, joints must be more open, single or double beveled, with a root gap, so that the weld metal may be placed in the joint. Lack of weld penetration is the single most important reason why austenitic alloy weldments fail in high temperature service. F. Fabrication Time Cleanliness, distortion control measures, maintaining low interpass temperatures and even machining add up to more time spent fabricating stainless than carbon steel. A shop experienced with stainless may require one and a half times as long to complete the same fabrication in stainless, as in carbon steel. A good carbon steel shop encountering stainless or nickel alloys for the first time can easily spend twice as long, maybe even three times as long, to do the stainless fabrication, as it would the same job in carbon steel. - 101 - WELDING AUSTENITIC ALLOYS The fundamental problem to be overcome in welding austenitic nickel bearing alloys is the tendency of the weld to hot tear upon solidification. This matter is readily handled in alloys of up to about 15% or so nickel. In these stainless grades the weld metal composition is adjusted, usually by slightly higher chromium and reduced nickel, to form a small amount of ferrite upon solidification. The amount of ferrite in the weld may be measured magnetically, and is reported as a Ferrite Number, FN. This ferrite acts to nullify the effects of the elements responsible for hot cracking in the Ni-Cr-Fe austenitics. These elements are chiefly phosphorus, sulphur, silicon and boron. In higher nickel grades, about 20% nickel and over, it is metallurgically not possible to form any measurable amount of ferrite. Therefor other means of minimizing hot cracking must be used. Foremost among these is to use high purity raw materials in the manufacture of weld fillers. Simply beginning with low phosphorus alloying elements, and reducing the amounts of harmful sulfur and silicon in the weld metal improves its ability to make a sound weld. Phosphorus, in particular, must be kept below 0.015% in the weld wire itself. Certain alloy additions such as manganese, columbium (niobium), molybdenum and carbon serve in one way or another to reduce the austenitic propensity for weld hot cracking. Manganese ranges from about 2% in AWS E310-15 covered electrodes to 5% in RA330-04 wire & electrodes and 8% in alloy 182 (ENiCrFe-3) covered electrodes. Columbium at the 0.5% level, as in 347 stainless, is harmful whereas 2 to 4% columbium is quite beneficial in many nickel base weld fillers. Molybdenum isn’t necessarily a common addition specifically for weldability but it does enhance the properties of RA333-70-16 covered electrodes. High molybdenum is responsible for the popularity of the various “C type” electrodes (15Cr 15Mo balance Ni) in repair welding. 2% Mo contributes to 316 as being the most weldable of the stainless steels. Carbon is slightly elevated in 310 weld fillers, to about 1/10%. The one welding electrode specifically using very high carbon to promote sound welds is the heat resistant grade RA330-80-15 (UNS W88338). Maintaining a weld deposit chemistry of some 0.85% carbon permits this electrode to make sound welds in either wrought or cast 35% Ni high silicon heat resistant alloys. The distinction between the lower nickel stainless grades, which depend upon ferrite to ensure weldability, and the high nickel alloys, which require high purity weld fillers, is an important one to remember. Most ferrite containing (stainless) weld fillers are useless with nickel alloy base metal, as dilution of the weld bead with nickel from the base metal eliminates this ferrite. Likewise a high purity nickel alloy weld filler, such as ER320LR, may be not quite so crack resistant when contaminated by phosphorus from 316L, cast alloy 20 (CN-7M), or carbon steel base metal. With respect to welding there are some distinctions between those alloys intended for use above 1000°F (540°C), and those meant for aqueous corrosion service. One - 102 - Welding austenitic alloys, continued difference is in carbon content. Corrosion resistant grades are generally limited to 0.03% carbon maximum, and typically much lower. They may also have small additions of columbium or titanium. Restriction of carbon, or tying it up with a stabilizing element (Cb or Ti) is necessary to prevent heat affected zone (HAZ) intergranular corrosion and stress corrosion cracking (SCC) due to carbide precipitation. Heat resistant alloys by contrast typically require 0.04 - 0.010% carbon for good hot strength. RA 602 CA is even higher, at the 0.2% level, while RA330HC belt pin stock and the cast heat resistant alloys have a nominal 0.4% carbon. In the absence of a wet corrosive environment a little intergranular carbide precipitation is not particularly harmful to a heat resistant alloy. In both classes of material, incompletely penetrated welds and open crevices must be avoided in fabrication design. Serious aqueous corrosion can begin in crevices. In high temperature carburizing service crevices are where carbon (soot) can deposit, grow, and pry the joint apart like tree roots in rock. For both classes of alloy, weldability alone is not the entire issue. The weld filler must also have the mechanical and environmental resistance required for its intended service. Usually this point is addressed in fabricating corrosion resistant alloys. It is sometimes overlooked in heat resistant alloy fabrication and even less often considered in repair of high temperature alloy fixturing. ALLOYS UNDER 20% NICKEL Most austenitic grades containing less than 20% nickel are joined with weld fillers that utilize perhaps 4-12 FN (Ferrite Number) to ensure weldability. Heat resistant alloys with 20% or less nickel include 304H, 321, RA 253 MA, RA309, RA85H, RA310, and the cast heat resistant alloys HH and HK. All save RA310 and the cast alloys depend upon some level of ferrite in the weld bead to prevent solidification defects. The cast grades are usually welded with high carbon, fully austenitic electrodes of similar or higher nickel. RA310 stands in an odd position between the stainless and the nickel alloys, having neither ferrite nor any particular alloy addition for weldability. Not surprisingly, 310 welds have a reputation for fissuring. In the past it was possible for 310S (UNS N031008) base metal to contain as much as 1.50% silicon in the ASTM/ASME specifications. Heats on the high side of silicon and phosphorus were definitely a problem to weld (Rolled Alloys traditionally limited silicon in RA310 to 0.75% max). With the advent of 310H (UNS S31009) ASTM limited silicon to 0.75% maximum. To avoid melting two chemistries of 310, in practice all 310 varieties now melted in North America have less than 0.75% Si. Phosphorus in the weld wire may still be an issue with some lots of ER310 welding wire. The current AWS limit for ER310 wire is 0.03%P max. This is far too high. For 310 welding wire to be of practical use the phosphorus must be kept under 0.015%P max. - 103 - ALLOYS OVER 20% NICKEL Heat resistant alloys in this category include, but are not limited to, RA800H/AT, RA330, RA 353 MA, 803, alloy X (UNS N06002), RA333, 617, Haynes alloys HR-120, 230 and 214, 601, RA 602 CA, 600, and Nimonic 75. The cobalt alloys N155, 556, 188, L605, and HR-160 may be treated in similar fashion with appropriate weld fillers. Many nickel alloys are joined with matching composition weld fillers, modified only by restrictions on phosphorus, sulphur, silicon and boron. Titanium may be added for deoxidation. Other nickel weld fillers contain manganese, high carbon, columbium or molybdenum to improve resistance to fissuring and hot cracking. Such chemistry modifications are rarely as effective as is the use of ferrite in the lower nickel stainless weld fillers. Welding technique and attention to cleanliness, then, become increasingly important to ensure the soundness of fully austenitic welds. Techniques include reinforced, convex stringer beads and low interpass temperature. Cleanliness includes NOT using oxygen additions to the GMAW shielding gases for nickel alloys. It is worth repeating here that high nickel alloys can not reliably be welded using stainless steel weld fillers. Stainless steel weld metal (308, 309, etc.) depends upon a small amount of deposited ferrite to ensure a sound weld. But when a stainless rod is deposited on a high nickel base metal, the additional nickel melted into the weld bead makes it fully austenitic, with no ferrite at all. Without ferrite, the stainless weld bead may crack. WELDING PROCESSES Five different arc welding processes are generally used with heat resisting alloys. The most common, in North America, is Gas Metal Arc Welding (GMAW), formerly known as MIG (Metal Inert Gas), using spooled bare wire filler. Next in popularity is Shielded Metal Arc Welding (SMAW), or just plain “stick” welding, with covered electrodes. The least volume of work is done by Gas Tungsten Arc Welding (GTAW), formerly called TIG (Tungsten Inert Gas) and originally trade named Heliarc. Two other methods are Plasma Arc Welding (PAW) and Submerged Arc Welding (SAW). In addition resistance welding, particularly cross wire resistance welding, is often used in heat resistant alloy fabrication. There are two basic types of welding machines, Constant Current, and Constant Potential. A constant current machine is used for GTAW (TIG) and SMAW (stick) welding. Practically speaking it won’t work for GMAW (MIG) welding. The dial on a Constant Current machine reads in amperes, and the current is regulated by this dial. Constant Potential (voltage) machines are used for GMAW (MIG) welding. They don’t work well with covered electrodes (SMAW). The dial regulates voltage, and is marked with numbers in the 20-40 range. - 104 - Gas Metal Arc Welding In this process, the weld filler metal is bare wire. The most common size is 0.045” (1.14 mm), though 0.035” (0.89 mm) and 0.0625” (1.59 mm) are also stocked, typically on 2530 pound (11-14 kg) spools. Wire is fed continuously through a hollow cable to the welding gun, where it makes electrical contact. The arc between weld wire and workpiece melts the metal. Molten weld filler transfers as either a spray of fine drops, or as larger globs. The metal is protected from oxidation by a continuous flow of inert shielding gas, usually argon, through the weld torch and around the wire. Current is always Electrode Positive (DCRP, direct current reverse polarity). The GMAW process is fast and well suited to high volume work. It can be automated, as for welding long tubes. Welding with relatively high current, about 190-220 amperes for 0.045” (1.14 mm) wire, and argon shielding is used for the spray-arc transfer mode. In this mode, molten weld metal crosses the arc to the work as a fine spray. At lower current, roughly 100 amperes for 0.035” (0.89 mm) wire, with 75% argon 25% helium shielding, the molten weld metal transfers as large, individual drops. This is known as short-arc, or short-circuiting arc, welding, characterized by a noisy arc and low heat input. Choice of shielding gas is important. First, do not use oxygen additions to the gas when welding nickel alloys and NEVER use 75% argon 25% carbon dioxide for GMAW welding either stainless or nickel alloys. Oxygen above 2% starts burning out major alloying elements. CO2 above 5% adds carbon to the low carbon stainless grades. Although very small amounts of CO2 may be used in argon, at above 15% CO2 in argon the arc transfer mode is no longer spray, but rather a hot globular transfer with a great deal of spatter. For spray-arc welding the most common gas is 100% argon. To improve bead contour and reduce arc wander, respectively, 10 to 20% helium and a very small amount of CO2 may be added to the argon. One such gas from Air Liquide, their BlueShield TM 20 is a nominal 81% argon 18% helium and 1% carbon dioxide. A mix of 75%Ar 25%He is also used, although the transfer mode will then not quite be a true spray-arc. For shortcircuiting arc transfer 75% Ar 25% He is used, as is the commonly available 90%He 7 1/2% Ar 2 1/2% CO2 . Because the welding wire must be pushed through a cable, ranging from 10 to 15 foot (3 to 4 1/2m) long, there may be feeding problems. The result can be a tangle of wire known, appropriately, as a “bird’s nest”. This shuts down the operation until the welder clears it. The care with which the filler metal is wound on the spool affects how smoothly the wire feeds. While the manufacturer is often blamed for feeding problems, more often than not proper attention to machine set up will ensure freedom from “bird’s nests”. - 105 - WELDING, gas metal arc, continued Smooth feeding depends on the cast and helix of the spooled wire. Both AWS A5.9 for stainless, and A5.14 for nickel alloy wire require cast and helix of wire on 12 inch (300mm) spools to be4 “such that a specimen long enough to produce a single loop, when cut from the spool and laid unrestrained on a flat surface, will do the following: 1. Form a circle not less than 15 in. (380mm) in diameter and not more than 50 in. (1.3m) in diameter. 2. Rise above the flat surface no more than 1 in. (25mm) at any location. Our RA 253 MA wire, for example, typically has 36 to 42 inch (915 to 1070mm) cast and 1/2 inch (12.7mm) helix. The following discussion is based on information from Ron Stahura, AvestaPolarit Welding Products, Inc. Many heat resistant alloy weld wires are much higher in strength than stainless wire (e.g., ER308 or ER316L), and therefor require more care to feed smoothly. When tangling, or bird’s nest, occurs the first thing we suggest is to examine machine set-up. Does this problem occur on more than one machine? How long is the cable—the longer the cable, the more tension in the feed rolls. Are the feed rolls, inlet guide and outlet guide all clean? Incidentally, V groove rolls are used with solid stainless/nickel alloy wire, U groove for copper or aluminum and serrated rolls for flux cored wire. Use minimal pressure on the feed rolls—more is not better. A rule of thumb is to hold the wire between the fingers as it enters the feed rolls. If you can hold it back, there is not enough pressure. Adjust the pressure until you just can not hold the wire, then give it another half turn beyond that. For 0.045 inch (1.14mm) wire, use a 1/16 inch (1.6mm) conduit, instead of a 0.045”/1.14mm conduit. The oversize conduit won’t hurt, and will give more room for the wire to flex. A heavy duty contact tip is preferred instead of a standard contact tip. When spray-arc welding the tip runs hot, and the wire may swell into the tip and jam it. The heavy duty tip simply has more copper, and can handle more heat. Flux Cored Arc Welding FCAW is similar to GMAW except that the wire used is tubular, with flux and metal alloy powders inside. Because this wire contains its own flux, gas shielding may be 75% Argon 25% CO2, even with nickel alloys! The advantage of flux cored wire is that welding is easier than when solid wire is used, and the arc is “softer”. As a result there is greater overall productivity with flux cored wire. Flux cored wire is sensitive to moisture pick-up, and should be left in its sealed plastic bag until ready to use. - 106 – Flux Cored Arc Welding, continued Shielded Metal Arc Welding Covered welding electrodes consist of an alloy core wire and a flux coating. The core wire is usually, but not always, about the same composition as the base metal. Often, however, various alloy additions are made in the coating itself, so that the weld bead chemistry will not be the same as the chemistry of the core wire itself. In the case of RA330-80-15 or -16, and RA330-04-15 covered electrodes, a 35%Ni 15%Cr AWS E330 core wire is used. The additional carbon, manganese and chromium required in the weld deposit are added to the flux coating. During welding, these additions melt in and adjust the chemistry of the weld bead to the specified composition. RA333-70-16 electrodes do use RA333 core wire. The electrode coating does four basic jobs: 1. 2. 3. 4. Provides a gas that shields the metal crossing the arc from oxidation Produces a molten slag which further protects the molten weld bead from oxidation, affects out-of-position weldability, and controls the bead shape Adds more alloying elements, such as manganese, carbon or chromium Promotes electrical conductivity across the arc and helps to stabilize the arc, important when alternating current (AC) is used - 107 - Shielded Metal Arc Welding, continued There are three types of coatings used on Rolled Alloys electrodes. Coating type is designated by a “-15”, a “-16”, or, most recently, a “-17” after the alloy number. DC lime-type coatings are designated -15. RA330-04-15 and RA330-80-15 both have DC (Direct Current) lime coatings. This means that these electrodes can ONLY be used with direct current. Normally the current is reverse polarity (DCRP, or Electrode Positive). That is, the electrode is positive and the workpiece is the negative electrical pole of the circuit, electrons are emitted from the work and go toward the electrode. If the welder attempts to use a DC electrode with an AC (alternating current) setting on the welding machine, the electrode simply won’t run. He will not be able to keep the arc going. This is very basic knowledge, but every couple of years someone complains that RA330-04-15 “won’t run”. Well, it will indeed run on DC current, but not on AC. That is, not unless that AC current is turned up so high that the whole electrode glows red and the coating spalls off. The AC/DC titania coated electrodes are designated -16. RA333-70-16 and RA330-8016 both have AC/DC coatings. These electrodes may be used with alternating current (AC). They have compounds of potassium and titanium in the coating which stabilize the arc. This means it will not extinguish itself as the current reverses direction (and goes to zero) 60 times a second on normal 60 cycle current (50 cycle in Europe). AC/DC electrodes may also be used with direct current, DC. In fact, they run better when using DC. Weld repair with RA333-70-16 covered electrodes is best accomplished using direct current, reverse polarity (DCRP). The newer coating designation is -17, which also operates on alternating current, as well as on direct current. At this writing (2001) RA 253 MA-17 are the only electrodes we stock with this coating. The slag from the electrode coating is extremely corrosive at elevated temperatures. After welding, all traces of this slag must be removed, prior to using the fabrication at elevated temperature. Otherwise the slag destroys the protective chromium oxide scale on the metal. Under oxidizing conditions this simply results in excessive loss of metal to oxidation. In a carburizing atmosphere small traces of slag will cause local carburization to proceed rapidly. In any reducing atmosphere the fluoride flux will scavenge enough sulphur from the atmosphere5, even a very low-sulphur atmosphere, to cause sulphidation attack of the base metal. - 108 - Gas Tungsten Arc Welding In GTAW, the arc is struck between the workpiece and a tungsten electrode, which remains unmelted. The argon shielding gas, which protects both the hot tungsten electrode and the molte n weld puddle, is brought in through a nozzle or gas cup which is around the electrode. This process used to be called TIG (Tungsten Inert Gas), and was originally patented as Heliarc ®, a name still used occasionally. For both stainless and nickel alloys the current used is DCSP, direct current straight polarity. The work is electrically positive and the tungsten electrode is the negative electrical pole. The electrode is usually thoriated tungsten, that is, tungsten metal with 1 or 2% thorium oxide added to improve the emissivity of electrons. Rare earth oxides are also used. For aluminum welding the electrode is pure tungsten, used with AC (alternating current). Shielding gas must be pure argon or helium. Argon is used for manual welding. A helium addition may be used for automated welding, where a hotter arc is preferred. No oxygen or carbon dioxide can be tolerated or the tungsten electrode would literally burn up. For some corrosion alloys, such as AL-6XN® or RA2205, up to 4% nitrogen is added. This may cause some erosion of the tungsten electrode but improves weld bead properties in these particular alloys. In the case of RA 602 CA, it is necessary to add 2 to 2 ½% nitrogen to the argon shielding. This is to resist hot cracking. The arc between the tungsten electrode and the work is what melts the workpiece. The weld filler metal is fed by hand into the molten puddle. GTAW weld wire for heat & corrosion resistant alloys is sold as 36” (914 mm) straight lengths of bare wire, in 10 pound (4 1/2 kg) tubes. The welder has the most control when using gas tungsten arc, and this process makes the best quality weld, but it is relatively slow. It may be automated for volume production. In automatic GTAW the wire is fed into the joint from a spool of wire, just like GMAW wire. For faster welding speed helium is added to the argon shielding gas. GTAW is often used to make the root pass in pipes or whenever the joint can only be made from one side. The rest of the weld may be built up with either GMAW or SMAW, both of which are faster. Remember--the core wire of RA330-04-15 covered electrodes is AWS ER330, and not RA330-04 chemistry. Welders sometimes knock the coating off an electrode and use the core wire as GTAW filler. Do not do this with RA330-04-15 or the RA330-80 electrodes. This AWS ER330 will make a crack-sensitive weld, without the benefit of the alloying elements which were in the coating. - 109 - Gas Tungsten Arc Welding, continued Atmospheric contamination, as from strong winds or too long an arc length, is a potential cause of porosity. Look at work to tip distance, shielding gas flow rates, cup size and consider the use of a gas lens. When using a 2—4% nitrogen addition for welding the corrosion alloys, the shielding gas will be just that much more sensitive to atmospheric contamination. Minimize the arc length, no more than 1/4 to 3/8 inch (6-9.5mm). The longer the arc length, the greater the opportunity to entrain air into the shielding gas. Gas cup size depends upon what diameter tungsten electrode is being used. A 3/32” (2.4mm) electrode should use anywhere from a No. 6 to No. 8 cup (9.5-12.7mm cup dia), No. 7 (11mm) being about right. An 1/8 inch (3.2mm) electrode requires a No. 8 (12.7mm) cup. Consider using a gas lens, a wire screen which serves to reduce turbulence of the shielding gas flow. It is this turbulence which causes air to get mixed in with the argon shielding gas. Gas Metal Arc (MIG) Gas Tungsten Arc (TIG) Plasma Arc Welding The plasma arc torch is roughly analogous to a GTAW torch. It generates intense heat in a very narrow zone, and has been used to weld RA330 without added filler (with GTAW this would be extremely difficult). PAW is an excellent welding process for heat resisting alloys. - 110 - Submerged Arc Welding Submerged arc uses a spool of weld wire, much like GMAW. Instead of shielding gas, a hopper feeds granulated flux into the arc to shield the arc and molten weld puddle. While it is possible to use 0.045” (1.14 mm) dia. Wire, larger sizes such as 1/16 or 3/32” (1.6 or 2.4 mm) are generally preferred. For nickel alloys such as RA330 a strongly basic flux must be used, suc h as Avesta Flux 805 or Böhler-Thyssen’s RECORD NiCrW. Absolutely do not use acid fluxes or any flux meant for stainless steel. Heat input must be as low as possible. For this reason 1/8” (3.2 mm) wire is not suggested for submerged arc welding the nickel heat resistant alloys. SAW is a process naturally inclined to high heat input, but this heat must be kept to a minimum to avoid centerbead cracking in fully austenitic alloys. Resistance Welding6 Spot and seam welding parameters for heat resistant alloys will differ from those used with stainlesses such as 304L or 316L, and markedly from those used for carbon steel. Heat resistance alloys may have twice the yield strength of stainless and considerably higher electrical resistivity. Electrode force, welding current and time, and electrode tip contours may all need to be modified accordingly. - 111 – Resistance Welding, continued A restricted-dome electrode is suggested for spot welding. Average dome radius may be 3 inch (76 mm) for material up to 11 gage (3mm). For a larger nugget size in material 16 to 11 gage (1.6 to 3mm) a 5 to 8 inch (127 to 203mm) radius dome is sometimes preferred. In seam welding heat time should be adjusted to ensure that the wheel maintains pressure until the weld nugget has solidified, to avoid porosity and cracking. Likewise cool time should be sufficient that welded areas are not remelted. The metal must be clean and free of all grease, or a sound weld cannot be made. References 1. Thaddeus B. Massalski, Editor-in-Chief, Binary Alloy Phase Diagrams, Volume 1, ISBN 0-87170-262American Society for Metals, Metals Park, Ohio, U.S.A., 1986 2. Avesta handbook for the welding of stainless steel, Inf. 8901, Avesta Welding AB, S-74401 Avesta, Sweden 1989 3. Berthold Lundqvist, SANDVIK Welding Handbook , Sandvik publication 0,34 E, Sandvik AB, Sandviken, Sweden June, 1977 4. Specification for Nickel and Nickel-Alloy Bare Welding Electrodes and Rods, ANSI/AWS A5.14/A5.14M-97, ISBN 0-87171-543-0, American Welding Society, Miami, Florida, U.S.A. 5. G. R. Pease, Corrosion of Nickel-Chromium-Iron Alloys by Welding Slags, Welding Journal Research Supplement, September, 1956 6. Resistance Welding Manual, 4 Edition, ISBN 0-09624382-0-0, Resistance Welder Manufacturers’ Association, 1900 Arch Street, Philadelphia, Pennsylvania 19103 U.S.A. 1989 th The best general reference we know for welding this class of materials is: R. J. Castro & J.J. de Cadenet, Welding Metallurgy of Stainless and Heat-resisting Steels, ISBN 0 521 20431 3, Cambridge University Press, 1975. First published, in French, as: Métallurgie du soudage des aciers inoxydables et esistant à chaud, by Dunod, Paris, 1968. -112 - Suggested Weld Fillers Base Metal bare wire Preferred covered electrodes Alternates RA330® RA330-04 -- RA330-04-15 RA330-80-15 RA333® , RA82 RA333-70-16 RA333 RA333 RA333-70-16 ERNiCrWMo-1 RA 602 CATM S 6025 6225 Al (SG-, EL-NiCr25FeAlY) RA601 RA333 601 RA333-70-16 6225 Al RA600 82 182 RA 353 MA ® RA 353 MA RA 353 MA RA 253 MA ® RA 253 MA RA 253 MA-17 RA333, RA333-70-16 RA800H/AT RA333 556 RA333-70-16 -- ERNiCrCoMo-1 RA330-04, EniCrFe-2 RA309 ER309 E309-16 RA330-04* RA310 ER310 E310-15 RA330-04* RA446 ER309 ER310 E309-16 E310-15 E312-16 HK, HT, HU RA330-80-15 DC lime is the preferred 35% nickel rod for cast heat resistant alloys. Alternates RA333-70-16, RA330-04-15 ERNiCrCoMo-1 (lacks oxidation resistance, use cover pass of S 6025) -- RA330-04 -- General: Do choose the weld filler for its performance under the expected service conditions, as well as for weldability issues. Do not use—any stainless weld filler on nickel alloys (e.g., RA330, RA333, RA600, RA601, RA 353 MA, RA800H/AT). Dilution by nickel will eliminate ferrite, and the welds will crack. It is better not to use alloy X (ERNiCrMo-2, ENiCrMo-2) weld fillers on RA333 base metal. The X weld bead may be subject to catastrophic oxidation at the higher service temperatures where RA333 is commonly used. *Where sulphidation is an issue, do not use high nickel fillers such as RA330-04 - 113 - Dissimilar Metal Joints, Weld Filler Guidelines Considerations in selecting a filler metal for a dissimilar metal weld joint include the expected service conditions at the joint, relative thermal expansion coefficients of the three metals involved, and freedom from weld metal hot cracking. The final selection should be approved by the end user and weld procedures qualified by the fabricator. Base Metals Carbon Stainless Steel (304,316) RA 253 MA RA 602 CA Cast Alloys HK, HT, HP RA330 182 RA800H/AT RA333 RA330-04 RA333 617A RA333B RA330-80-15 RA330-04 RA333 RA330-04 RA333 RA333 RA330-04 617A RA333B RA333-70-16 RA 353 MA 182 RA 353 MA RA 353 MA RA 353 MA 617A RA 353 MA RA330-80-15 RA 602 CA S 6025 6225 Al 617 RA 253 MA E309-16 RA 253 MA RA 253 MA RA333 617 RA333B RA333-70-16 RA600 82 182 82 182 RA333 RA333-70-16 82 182 RA333-70-16 RA330-80-15 RA601 82 182 82 182 RA333 RA333-70-16 S 6025 6225 Al RA333-70-16 RA330-80-15 RA309 E309-16 E309-16 182 ER309 E309-16 RA 253 MA 82C 182C RA330-80-15 RA310 E309-16 E309-16 182 E310-15 RA 253 MA -- 82C 182C RA330-80-15 RA333-70-16 RA446 E309-16 E309-16 E310-15 E310-15 E309-16 ER309 82C 182C RA333-70-16 -- 182 RA333 82 182 82 182 617 RA333* Note: The carbon steel joint must be ground to bright metal. A “mill finish” is not acceptable. All rust, blue-black hot rolling scale and paint must be removed before welding with any stainless or nickel alloy weld wires. These alloys lack the deoxidation characteristics of carbon steel weld wires. A 617 (ERNiCrCoMo-1) lacks the oxidation resistance of RA 602 CA. B The weldability of RA333 weld filler used on RA 602 CA has not yet been determined. C These high nickel fillers are not suggested for sulfur bearing environments. - 114 - - 115 - BRAZING and SOLDERING Heat resistant alloys are normally assembled by welding. Brazing is used on occasion to attach cooling coils or thermocouples. The age hardening aerospace grades, by contrast, are commonly joined with nickel-silicon-boron braze fillers. SOLDERING Copper cooling coils may be lead-tin soldered to heat resistant alloys. Somewhat better strength may be obtained by using a tin base solder. These may be alloyed with about 2% of either silver or antimony. The acid chloride flux is corrosive and should be washed off after soldering. BRAZING One of the issues in brazing Ni-Cr or Ni-Cr-Fe alloys is the furnace atmosphere. This atmosphere must prevent formation of any oxide film which would prevent the braze alloy from flowing. To be effective with stainless, the incoming hydrogen atmosphere should have a dew point1 –80°F (—60°C) or lower. Aluminum Brazing From the standpoint of the heat resistant alloy supplier, the major issue in aluminum brazing is the flux. Temperatures are low enough that aluminum braze muffles are commonly made of 304 or 316L stainless. If too much flux is applied to the aluminum work pieces, that flux may spill onto the muffle. Anything that will flux aluminum oxide will quickly eat holes through stainless. Technically speaking, a high nickel alloy, such as 600, has somewhat better resistance to the fluoride flux. But the nickel alloy is unlikely to last long enough to be worth the higher cost. Keep the flux off of the alloy fixturing. Silver Brazing Often used to join carbon and low alloy steels. Austenitic alloys are prone to crack when silver brazed, from liquid metal embrittlement. Residual stresses are responsible for cracking during furnace brazing, as the braze temperature is not high enough to reduce these stresses. Stressed austenitic alloy, whether stainless or high nickel, in the presence of molten silver braze alloy will crack. In torch brazing the source of stress is the thermal stress caused by the local heating (which is normal practice when brazing steel). To furnace silver braze an austenitic alloy, consider stress relief annealing the assembly prior to brazing. - 116 - Silver Brazing, continued The lower melting silver braze alloys may require the use of flux when atmosphere brazing stainless. Dry hydrogen may not be sufficiently reducing to chromium oxide at brazing temperatures below 1800°F (980°C). Vacuum brazing requires fillers containing neither cadmium nor zinc, which would vaporize. Successful torch silver brazing of austenitic stainless depends upon technique. The metal should be heated uniformly in the area to be brazed. One approach that has been described to us is to play the torch back and forth about 6 inches (150mm) on each side of the area to be brazed. This minimizes thermal stress where the molten silver braze will contact the austenitic alloy. If there is no stress on the stainless, it will not crack. Silver braze cracking is not an issue with ferritic stainless steel. It is the austenitic structure that is sensitive to intergranular cracking by molten braze alloy. Copper Brazing Copper brazing is a common means of joining carbon steel assemblies. It is not usually chosen to join either heat or corrosion resistant alloys. The process temperature for copper brazing is usually 2050°F (1120°C). Pure copper itself melts at 1981°F (1083°C). These temperatures will quickly anneal out residual stresses from the stainless or nickel alloy parts. Even though copper, given time enough, will penetrate austenitic alloys intergranularly it is unlikely to either crack or seriously attack the metal during the brazing cycle. Nickel Brazing Nickel-base braze alloys are used to join age-hardening nickel base alloys for aerospace applications. Addition of as much as 10% silicon, in some alloys with up to 3.5% boron, greatly lowers the melting point. With AMS 4777 the brazing range is 1850—2150°F (1010—1180°C). During the braze operation boron from the filler diffuses into the base metal, raising the remelt temperature of the braze alloy. Boron can react with nitrogen, in the alloy as well as the atmosphere, preventing braze flow. One indication of nitrogen as the brazing problem is an iridescent bluish-gray color to the base metal. Alloys containing more than about 0.03% nitrogen2 can be difficult to braze, without special treatment. Such treatment might include about 0.001 inch (0.025mm) of nickel electroplate. This may be coupled with rapid heating and short process time to prevent diffusion of nitrogen through the nickel plate. - 117 - Nickel Brazing, continued Alloys containing aluminum and titanium require first to be electroplated with nickel. Otherwise, oxides of Al and Ti will form, even in the best of atmospheres, that prevent braze flow. For vacuum brazing 0.001” (0.025mm) is sufficient. Hydrogen brazing requires a slightly thicker plate, 0.001 to 0.0015” (0.025 to 0.038mm). The plating does need to be an electroplate and not electroless nickel. Electroless nickel contains phosphorus, enough so to depress the melting point to 1610°F (877°C)3 . The braze temperature should not exceed the solution annealing temperature for the alloy in question. Effects on Furnace Equipment Excess silver or copper braze alloys dripping onto the nickel alloy muffle or fixturing will attack that alloy intergranularly. Nickel base braze alloys simply lower the alloy melting point, enough spilled braze can melt a hole through the muffle. An oxide coating on the alloy helps minimize this effect, but of course will not be present in vacuum brazing.. Likewise spilled flux is corrosive to the fixturing. Further Information Detailed insight into all manner of brazing issues is available from the Monthly Column “Brazing Q&A”, by R. L. Peaslee, in Welding Journal, American Welding Society, Maimi, Florida U.S.A. This column began in 1989 a nd continues as of this writing, 2002. This is far and above the best source for thorough, in-depth discussion of brazing problems. Brazing Q&A is authored by Dr. Robert L. Peaslee, of Wall Colmonoy. Dr. Peaslee invented the nickel base brazing alloys about 50 years ago. Reference 1. Brazing of Stainless Steels, in ASM Handbook Volume 6, Welding, Brazing and Soldering, ASM International, 1993 2. Brazing Q&A, September 2001 Welding Journal 3. Brazing Q&A, , January 1991 Welding Journal - 118 - APPLICATIONS Bolts Bolts are commonly used at elevated temperature to withstand a shear load. For example, RA330 threaded rod, nuts and washers are used to assemble high temperature equipment where loose joints are desired to accommodate thermal expansion & contraction during thermal cycling. A good discussion of fasteners in the chemical process industry has been presented by Robert Smallwood1. At high temperatures relaxation is the primary limitation to the use of threaded fasteners to maintain a clamping load. The most common alloy choice for applications up to 1150 or 1200°F (620-640°C) is RA718, an age hardening nickel base alloy. A286, a less expensive age hardening stainless, is sometimes suggested but it does not have as high a temperature capability as does RA718, and is not nearly as available in various bar sizes. Above this temperature, to about 1400-1500°F (760-816°C) the choices narrow down to René 41® or WASPALOYTM. More of this high temperature bolting experience has been with WASPALOY. In addition to selecting a strong bolt material it is important to look at the relative expansion coefficients of the alloy to be clamped, and the alloy from which the bolt is made. If the metal to be clamped expands faster than the bolt, that expansion will add to the tensile load in the bolt and may stretch it, so that the assembly is loose once it comes back down to room temperature. What appear to us as fairly liberal alloy selection suggestions are offered by the Industrial Fasteners Institute as2: Below 450°F (230°C), low alloy steel. 450 to 900°F (230 to 480°C), one of the grades in ASTM A 193. From 900 to 1200°F (480 to 650°C), A286 and 718. Above 1200°F up to 1600°F (650 to 870°C), René 41 or WASPALOY. Some cautions. NEVER, NEVER use anti-seize compounds containing copper anywhere near high temperature equipment. If some of that copper gets carried into an area where the metal is operating above 1981°F (1083°C) it will melt and embrittle or eat holes through any austenitic alloy it touches. Zinc, or galvanized coatings embrittle austenitics and can also embrittle steel bolts at moderately elevated temperatures, even without melting the zinc. - 119 - Cast Link Belts The two basic types of cast link belts are pin-bearing, and interlocking. With a pin-bearing link the belt pin is heavily loaded in shear. If the pin is not strong enough it “crankshafts”, that is, deforms until it looks like an automobile engine’s crankshaft3. crankshafted pin,. Inadequate anneal Belt pins for this service have largely been RA330HC for several decades, usually with cast HT links. RA333 combines greater strength with better carburization resistance and gives the longest belt life with minimum downtime. The best belts use RA333 pins with cast Supertherm® links. Where strength but not carburization resistance is needed, alloys X and HR-120® pins are also used. Alloy 120 pins occasionally break after long service at around 1600-1700°F (870-930°C) or so. X grade may not be as consistent, due to a wider range of grain size as produced. At one time Incoloy® 802 was used, based on high published creep-rupture strength. Ultimately 802 lost out to RA330HC for this market. Two RA333SA (top) and one 802 pin (bottom) from the same belt, 1550-1750°F (843954°C) service - 120 - Cast Link Belts, continued Typical belt work loads on the above belt were 25-60 pounds/foot2 (0.17-0.41 N/mm2). The links are usually cast HT (35Ni 17Cr 0.5C), but Supertherm (35Ni 25Cr 15Co 5W) links are also on trial. With HT links and RA330HC pins, the links outlast the pin. Using RA333 or RA333SA pins, the HT link becomes the weak point. One belt supplier gave us the following examples: Pin RA330HC RA333SA RA333SA Link HT HT Supertherm Typical Life 18-22 month ~30 month 57 mo & running Using RA333SA pins can give a 50% increase in belt life with about 25% increase in belt cost. The optimum, state-of-the-art belt uses a 3” (75mm) wide, 4” (100mm) pitch pin bearing link, alloy HT or better, pinned with 3/4” (19 mm) dia. RA333 or RA333SA. Pin life does not necessarily relate directly to conventional rupture strength, which is measured in tension. This may be because the pin is loaded in shear. Grade 10,000 hour rupture strength, 1800°F (982°C), psi (N/mm2 ) RA333 1050 (7.24) RA330HC 690 (4.76) RA330 630 (4.34) 802 1750B (12.07) Typical pin lifeA months 30 20 3 max similar to RA330HC A in a pin-bearing link . RA330 hex bar is used successfully with certain interlocking link designs. B This is the published data. On two occasions Rolled Alloys had samples of production 802 tested at Joliet Metallurgical. The results were not consistent with the published data, although still a relatively strong alloy. Interlocking links are designed to take up all the shear loading on the casting itself. As pin stresses are low, both RA330 round bar and RA330 hexagonal bar are used to pin interlocking link belts. - 121 - Cast link belts, continued interlocking links from a belt misalignment failure For more than 20 years the majority of belt pin failures which we have been called on to examine have been caused by belt misalignment, which fatigues the pin, or occasionally by carbon and/or salt deposits which freeze the belt solid. The following paragraphs are based largely on discussions with Omega Castings, Inc., Battle Creek, Michigan. Contamination by sodium or potassium salts from parts washing operations can cause formation of bulky oxide which shortens the life of a belt by reducing freedom of movement between pin and link. Presence of sodium contamination is indicated by a bright greenish-yellow showing, once the black scale has been scraped away. We understand that another indication is, in a carburizing furnace, the presence of black “cobwebs” hanging off the radiant tubes, coupled with brickwork which is white, and not black as might be expected . When parts are washed in ionized detergents containing sodium, or in sodium hydroxide, borate or phosphate, those parts must be rinsed in water and then dried before running through the furnace. Otherwise sodium chromate will form at high temperatures. This causes a reaction which will oxidize, and continue to oxidize, the alloy components, until the volume of oxide simply freezes up the belt. For maximum belt life the furnace must be designed to minimize stress on the belt. Suggestions include the use of return rolls, rather than skid tiles, and hearth rolls sized to move at the same surface speed as the belt. Hearth rolls must be level and parallel (perpendicular to the belt travel direction). Good furnace design is one reason the 54 inch (1372mm) wide belt example above - 122 - Cast link belts, continued with RA333SA pins & Supertherm links has run 57 months at this writing, having carried some 180 million pounds (81,650 metric tons) of work. Muffles Alloy Selection RA333 - a first choice for copper brazing muffles. RA333 is strong eno ugh to be used in 11 gage (3 mm) wall, for maximum heat transfer. Some 3/16” (4.8 mm) RA333 is used. RA 353 MA - powder metal sintering muffles, also copper brazing and for brazing stainless 2050-2100°F (1120-1150°C). Experience to date has been that muffles of 1/4” RA 353 MA plate outlast those of 601, fabricated in the same shop, same design, used in the same service. For an iron sintering application 2100-2150°F (1150-1180°C) one shop has gotten 3 year minimum life from RA 353 MA, less than a year with the previous alloy. RA330 - the most widespread choice for muffles up into the 2100°F (1150°C) and over range. RA330 muffles are most commonly 3/16 to 1/4” (4.8 to 6.35 mm) thick. Used for applications from copper brazing to powdered iron sintering. RA330 “D” muffle for copper brazing, bottom lined with 430 stainless for protection against copper spills. - 123 - Muffles, continued RA 602 CA - For longer life in powder metal sintering muffles operating over 2150°F (1180°C). Offers greater strength, oxidation and carburization resistance than does 601. RA 253 MA - used for hydrogen and/or nitrogen atmospheres, not suitable for carburizing environments. Large muffles used for iron powder production are of RA 253 MA. May also suit for the bottoms of brazing muffles, being somewhat more tolerant of spilled copper than are the higher nickel heat resistant alloys. RA601 - strong, oxidation resistant. To minimize leaks in weld seams consider welding with RA 602 CA wire, rather than the commonly used 82. RA309—bright annealing, neutral hardening or sintering bronze powder, under 1800°F (982°C). NEVER for a carburizing atmosphere. Can be used for carbon fiber production when sulfur may be a problem. Muffle of 3/16” (4.8 mm) RA309 plate. Gas fired 1200-1600°F (650-870°C), endothermic atmosphere, bright annealing copper, brass and steel. Typical life 45 years. This is an old muffle design. Today we would suggest RA 253 MA here for greater strength, perhaps in 11gage for better heat transfer. RA600—competitive with RA330 for very high temperature iron sintering in strongly reducing or carburizing atmospheres. RA600 is not suited for sulfur bearing environments. - 124 - Muffles, continued Miscellaneous—The muffle wi ll be fixed at one end, but it absolutely must be free to expand in the other direction. Some shops weld a couple of U-bolts on the flange of the free end and run a chain over a pulley to some dead weights. A rule of thumb is to pull on the muffle with about half of its own weight, to aid the lengthening caused by thermal expansion. Replace the thermocouple at least quarterly. A type K thermocouple can drift 25°F (15°C) in 4-6 weeks. Type S, platinum, are preferred. For electrically heated muffles, silicon carbide elements top and bottom are the least problem. The key is to leave them on—it is cheaper to pay the electricity 7 days/week than to replace a muffle which has been periodically shut down to “save” money. Ribbon or rod elements may sag and create a hot spot. Silicon carbide hearth plates can form a eutectic with high nickel alloys such as 600 and 601, melting a hole right through the muffle. Silicon and nickel form a low melting eutectic at 1767°F (964°C). Common preventatives include painting the hearth plate with alumina castable thinned like paint, or separating the SiC plate from the muffle with refractory cloth, such as Refrasil® silica based insulation. Radiant Tubes 11 gage (3mm) RA333 tubes have lasted up to 8 -10 years in captive shops, carburizing. Most fabricated radiant tubes are RA330. RA 253 MA radiant tubes are used in steel mill annealing applications. Where 309 suits for the straight leg, an RA 253 MA return bend may be used to maximize life. Whether wrought or cast, regardless of alloy selection one very common failure mode is at the weld of return bend to straight leg. The reason for this is lack of full penetration welds in this joint. One solution would be to purge the tube with argon or nitrogen and make the root pass with GTAW or GMAW. The balance of the weld bead may then be made with another process, if convenient. One furnace maker who uses cast HT tubes commonly welds the return bends with either RA330-80-15 covered electrodes, or with RA330-04 bare wire. We suggest you consider RA333 weld fillers with RA601 radiant tubes. - 125 - Rotary Retorts & Calciners Alloy selection covers the range of available high temperature grades—RA309, RA 253 MA, RA330, RA601, RA 602 CA, RA 353 MA and RA333. The design and attachment of flites inside is very important. Best success has been to weld only in the cooler zones. The flight will operate cooler than the shell in an externally fired retort or calciner. This means they will not only expand less, but they will be stronger than the shell alloy. For these reasons the flights must be free to move or they will indeed crack the hotter, weaker shell from effects of differential thermal expansion. When such cracking occurs, it may be mistaken for fatigue or rupture failure. Neutral Salt Pots1 The most common industrial use of molten salts is to heat treat steel. Metallic salt pots used to contain neutral heat treating salts may last anywhere from 2 days to 18 months, depending upon maintenance and operating procedures. Alloy selection does matter somewhat. But alloy choice is outweighed in importance perhaps 50 to 1 by how the pot is maintained. The following points are important for good life in a metallic pot for neutral salt heat treating: 1.) Ensure that there is no salt whatsoever in the combustion chamber of a gas-fired pot or about the elements of an electrically heated pot. This is crucial. 2.) Rectify and desludge neutral chloride salts at least daily. 3.) Idle the pot with salt still molten, rather than shutting down completely and letting the salt freeze solid. 4.) Do not put oily work or any foreign matter (no floor sweepings!) into the pot. 5.) In both pots and fixtures, all welded joints must be full penetration welds. Only after these five points have been addressed should alloy selection be reviewed. Let us examine the reasons behind some of these points. Mixtures of potassium, sodium and barium chlorides are widely used as heating media that neither oxidize nor decarburize carbon, engineering alloy and tool steels. Regarded as “neutral” salts, they are actually quite oxidizing to the chromium in the Ni-Cr-Fe alloys used for pots and fixtures. - 126 - Salt Pots, continued Well, the chloride salts themselves are indeed neutral, but the inevitable oxygen content of the bath is quite destructive. Oxygen is present because the surface of the bath is open to the air, and because air is always brought into the bath with the workpieces. The destructive part is that as fast as the alloy forms a protective chromium oxide scale, the alkali chlorides strip or flux that scale, forming potassium, sodium, and/or barium chromates. As fast as chromium from the alloy diffuses to the surface to re-form the oxide scale, the scale is dissolved. The chromium diffuses along grain boundaries orders of magnitude faster than through the grains themselves, and diffusion voids, or pores develop in the grain boundaries2. Eventually the molten salt physically penetrates the grain boundaries, and permeates the entire thickness of the salt pot wall, until the pot begins to leak through to the outside. This is somewhat more likely to occur in coarse grained regions, such as the fusion line of the weld or in the weld bead itself. Eventual failure in or near a weld does not necessarily mean that weld was defective. It is the combination of alkali chloride salts and oxygen that attacks the pot. If a new pot is put into a furnace contaminated with leaked salt from the previous pot, that salt will volatilize when heated. Those alkali chloride fumes will attack the chromium oxide scale on the outside of the pot, and the hot air or products of combustion provide more than enough oxygen to scale right through the pot. This occasionally happens in as little as three days. And that is why it is most important to clean out all the spilled salt from the previous pot, when installing a new one. - 127 - This photomicrograph, from Rolled Alloys Investigation No. 99-66, shows corrosion attack and corrosion assisted cracking in the fusion line between RA330 plate, top, and the RA330-04-15 weld bead, bottom. In this salt pot the attack along weld fusion lines was so bad that entire lengths of the weld bead could be removed with a few blows of a hammer. The failure occurred simply because the firebox refractory still contained the spilled salt from the previous salt failure. In this case the solution was to replace this blanket insulation each time a new pot is installed. Operating temperature was about 1700-1750°F (930950°C) using a non-cyanide carburizing salt. In normal operation, oxygen builds up in the salt itself. To prevent the steel workpieces from decarburizing, that salt must be rectified. That is, the oxygen content of the bath must be reduced to low levels. This may be done by introducing methyl chloride (well away from electrodes and metallic pot sidewalls), which converts the alkali oxides back to chlorides. Solid rectifiers such as powdered silicon, silica, ferrosilicon or dicyandiamide are also used. - 128 - Salt Pots, continued The inorganic rectifiers form a metallic sludge in the bottom of the pot. If the pot is not rectified well and frequently, the oxygen content will shorten the life of the pot by corrosion from the inside. This happens even at oxygen levels which will not harm the steel workpieces. This sludge must be removed frequently, perhaps twice daily, lest it act as an insulator, causing the bottom of the pot to overheat. Leaving the sludge in can overheat the bottom to the point that it fails, usually in or near a weld, while the sidewalls are still in good condition. Alloy Casting Institute 3 studies of salt baths show that corrosion rates in the sludge itself, even without overheating, are increased by nearly a factor of two. The inorganic rectifiers form a metallic sludge in the bottom of the pot. If the pot is not rectified well and frequently, the oxygen content will shorten the life of the pot by corrosion from the inside. This happens even at oxygen levels which will not harm the steel workpieces. This sludge must be removed frequently, perhaps twice daily, lest it act as an insulator, causing the bottom of the pot to overheat. Leaving the sludge in can overheat the bottom to the point that it fails, usually in or near a weld, while the sidewalls are still in good condition. Alloy Casting Institute 3 studies of salt baths show that corrosion rates in the sludge itself, even without overheating, are increased by nearly a factor of two. 3/8” RA309 salt pot bottom 3/4 scale - 129 - Rolled Alloys Report #01-55 Salt Pots, continued The photo above is a classic example of pot failure due to metallic sludge buildup, in this case over two inches (50mm) deep in the bottom. This sample came from a Western US heat treat shop. Neutral salt pots here were failing by leaks at the bottom weld in 4 to 6 weeks of operation, some after as little as 2 weeks. Operation was neutral salt at 1400—1600°F (760—870°C) 24 hours per day, 6 days per week. Pot idled 1200°F (650°C) on Sundays. Etchant: Oxalic Acid Magnification: 25X Crack near the fusion line, inner weld bead of the RA309 pot above. When salt freezes it contracts in volume. If a salt pot is shut down and allowed to freeze solid, that salt will go through a volume increase when remelted on startup. This increase can be about 3/8 to 1/2 inch per foot (31 to 42 mm/metre) of pot depth. If the pot is full of solid salt, there are a couple of unpleasant possibilities. One is that, as the salt melts first on the bottom, it expands and cracks open the pot, either in a weld or along the knuckle radius of a dished head. Alternately, once sufficient salt has melted, the volume expansion may cause it to explode through the remaining frozen layer on top. If one plans a shut-down, it is a good idea to ladle out most of the salt before it freezes. - 130 - Salt Pots, continued Floor sweepings can do interesting things to salt pots. Aluminum foil left over from someone’s lunch, for example, will melt and go right through the bottom of the pot. Sulphur from whatever will attack the nickel in the pot, the higher the nickel alloy, the worse the attack. One of our fabricator customers related an incident in which short life of RA309 pots was indeed traced to the practice of disposing of floor sweepings in the heat treat pots at night. Salt Pot Alloy Selection Since the 1930’s the most popular alloys have been the wrought alloys RA309, RA330 and RA600, or the cast grades HT (17Cr 35Ni) and HW (12Cr 60Ni). The higher chromium content of RA310, or of cast HK, is disadvantageous in salt. Both laboratory studies and observations of fixtures are reasonably consistent in showing that the higher nickel grades usually have better resistance to alkali chloride salts. If that were all there were to it, all metallic salt pots would be RA600 or HW, and all electrodes in ceramic pots RA600 or commercially pure nickel. But in practice the majority of metallic pots today are RA330 or RA309, with RA600 distinctly in the minority. Almost all submerged or over-the-top heating electrodes are RA446, with a very, very few being RA330 or RA600. Almost none are pure nickel. It is the case that the various ills that may befall metallic pots obscure any theoretical advantages of higher nickel to the extent that 35% or 13% nickel grades are considered more cost-effective. With respect to fixtures for automated salt lines, performance often follows the alloy’s resistance to chloride salts. Either RA600 or RA330 is, in our opinion, superior to RA309. In some shops RA600 has the advantage over RA330, in others there is no clear difference. In all cases, full penetration welds are necessary. This alloy selection discussion is for pots containing chloride salts, in which some steel piece will be austenitized. Tempering salts, which are mixtures of sodium nitrate and sodium nitrite, are another matter. We might be inclined to suggest carbon steel for this moderate temperature application. Some people use 304 stainless. More is not better, high nickel alloys have no advantage in containing molten tempering salts. The same pot should not be used to hold both tempering salts for one operation, and neutral chloride salts for another. The combination of the two salts, one being residual, may corrode intergranularly through the pot wall in a rather short time. - 131 - Salt Pots, concluded References 1. James Kelly, Neutral Salt Pot Alloy Life: Maintenance is the Key, Heat Treating, April 1990 2. A.U. Seybolt, Oxidation of Ni-20Cr Alloy and Stainless Steels in the Presence of Chlorides, Oxidation of Metals, Vol. 2, No. 2, 1970 3. J. H. Jackson and M. H. LaChance, Resistance of Cast Fe-Nf-Cr Alloys to Corrosion in Molten Neutral Heat Treating Salts, Transactions of the ASM Vol. 46, 1954, pp 157-183. - 132 - Springs Metals used as springs at elevated temperature are subject to relaxation under load. “A bar loaded to an initial stress of say, 40,000psi (MPa) and then held at a constant strain and temperature may after a time period have a remaining stress of only 30,000psi (MPa). This time-dependent stress reduction of 10,000psi (MPa) is called stress relaxation. The total strain remains fixed but part of the elastic strain is replaced with inelastic strain4. One intentional example of stress relaxation is the reduction of stress in a fabrication due to a stress relief anneal. From the standpoint of availability, RA718 is a practical choice for applications up to 1150-1200°F (620-650°C). Above that, to 1400-1500°F (760-816°C) René 41® or WASPALOYTM are the remaining practical choices. A more complete, but approximate, guide to alloy selection would be: Max. Use temperature Alloys °F 200 °C 100 music wire, AISI 6150 chromium-vanadium steel 500 260 302 stainless cold drawn, 410, K-500 750 400 17-7PH® condition CH900 (50% cold reduction, plus 900°F (482°C) 1 hour age 1000 540 A286 1200 1500 650 816 RA718, X-750 WASPALOY, René 41 Elastic modulus decreases with temperature, about a 1% drop in modulus for every 100°F (56°C) temperature increase. References 1. R.E. Smallwood, Fastener Problems in the Process Industry, Corrosion 91 Paper No. 161, NACE, Houston, Texas 2. Fastener Standards, 6th Edition, available from: Industrial Fasteners Institute, 1505 East Ohio Building, 1717 East Ninth Street, Cleveland, Ohio 44114 U.S.A. 3. Bruce McLeod, Analyzing Belt Pin Failures, Metal Progress August, 1973, ASM, Metals Park, Ohio 4. Compilation of Stress-Relaxation Data for Engineering Alloys, ASTM DS-60, 1982 ASTM, Philadelphia Pennsylvania - 133 - FIELD FAILURES Every now and then we have a customer who uses a material in exactly the same equipment and presumably under the same operating conditions in which it had previously performed very successfully, only to have early failure occur. In an effort to constantly improve our knowledge, we try to investigate every premature failure reported to us. Some of these investigations are fruitless, but we have benefited from certain common denominators found in a series of such failures. We continually hope to add to our knowledge in this respect and thus produce better materials. The metal supplier is always somewhat on the defensive when a premature failure occurs. The user immediately refers it to his fabricator, who in turn refers it to us, because both think they could not have influenced the performance of the material. Actually, both of them might, so we guarantee no performance. We do guarantee any material we furnish to meet all applicable specifications. If the material can be found defective in any way, we are happy to replace it. The fact that it does not last as long as the user thinks it should, however, is not in itself evidence that the material was defective. Sometimes it is difficult to diplomatically point out to the user—or, perhaps more accurately, we should say—sometimes it is difficult to get the user to accept the fact, when pointed out to him diplomatically, that the equipment was improperly operated. It might be interesting to review a few of the types of failures we have encountered, some of which seem to occur periodically and become almost a routine insofar as explaining their occurrence is concerned. The Melted Radiant Tube About once a year someone from somewhere in the United States returns a radiant tube to his fabricator with a request for credit, because the tube did not last long enough. At the fabricator’s request, we examine the tube, and find a generous deposit of soot on the surface and a mass (and a mess) of material looking like stalactites in a cave. This mass is metal that was molten, and well saturated with the soot. It is obvious that a temperature has been reached, which has melted the alloy, and yet the user insists it was never over 1750°F (955°C). He is absolutely correct, because he is referring to the temperature indicated by his thermocouple. Soot or carbon burns at a very high temperature, however, and burns very rapidly under the proper conditions. - 134 - The Melted Radiant Tube, continued When the furnace door was left open, to burn out the soot, a chain reaction resulted. Having absorbed some carbon while in service, the metal had a lower melting point than when it was new. The extremely high localized temperatures from burning soot also accelerated carburization from the soot, further lowering the melting temperature of that surface. The net result was melted metal and there is nothing we can do about making a metal stay together if somebody melts it. The photo below is a typical example of a melted radiant tube. This problem is more common when alloy tubes are used to replace ceramic tubes. The firing and burn-out procedures acceptable with mullite (ceramic) tend to melt metal. About ½ scale. Vertical radiant tube, melted during carbon b urn-out. Similar failures have been encountered on endothermic generator retorts, and pit-type carburizing retorts. These melt on the inside surface only, which is where the soot is. The external surfaces usually show a good, tightly adhering oxide coating but perhaps have a hole or two. The Hole in the Box A very interesting and unusual failure that we had the pleasure of investigating did not concern our material, but it could have. Our primary interest is still solving the problem that exists. A customer sintering powdered metals had boxes that were manually pushed through a furnace operating at 2400°F (1316°C), with a hydrogen atmosphere. The furnace was equipped with silicon carbide skid rails for the boxes to slide on. -135 - The Hole in the Box, continued Because of the high-temperature application, the original fabricator furnished an alloy containing as much chromium and nickel as feasible, which was 600 alloy (76Ni 15Cr). After a few cycles, each box had two holes in the bottom. The rest of the bottom and all four sides were perfect. Visual examination of the boxes indicated that the metal in the holes had been melted; and, as the box had been pushed through, the molten metal had resolidified on solid material. How could a box, whose entire surface area was exposed to the same operating conditions, melt at only one point? Before destroying the box for investigation, we called the local representative of the Carborundum Company and asked if they had had experience with this type of problem. They had, and he knew the answer. He agreed to have their representative call on the user and suggest a change in the skid rail composition. The subsequent laboratory investigation confirmed our suspicions. Where the alloy box contacted the silicon carbide skid rails, sufficient silicon, and carbon, were absorbed from the rails into the alloy to lower its melting temperature below 2400°F (1316°C). The areas of the box not in contact with the rails did not absorb silicon and carbon and therefore were unaffected. This problem was very intriguing, although easily solved, because ordinarily we would think of a hydrogen atmosphere as being inert. Solid compounds do not usually carburize readily, although at the extreme temperature of 2400°F (1316°C), the unexpected happened. We could not solve this problem by offering an alternate alloy. The only solution was to change the skid rails to either a refractory that would not give up silicon & carbon, or to a heat resisting alloy. The Culprit Copper A very common field complaint, which occurs two or three times a year, is that of very quick failure of material used in copper brazing fixtures or trays. Because of these problems, users change sources on wire-mesh belts, change sources on fabrications, change materials, and sometimes even change sources for their materials, The cause of the trouble in this case is very clear-cut, but explaining it to the customer’s satisfaction is more difficult. - 136 - The Culprit Copper, continued Regardless of what the austenitic alloy may be, if molten copper is allowed to contact it, the copper will be dissolved into the alloy. In one case that we recall, some strips of RA310 had been used in a fixture and failed within two days. A brownish band on the top surface of the strip, where the copper had dropped on the alloy, was very plainly visible. By holding the strip between your thumbs and fingers, you could snap the metal at this point without difficulty. Immediately adjacent to the line of demarcation, the material would accept a 180° bend without fracture. By looking at the fracture through the contaminated area, one could see that the copper had penetrated all the way through the alloy and formed a solid layer on the lower surface, where it had been held in the molten state by surface tension. Copper brazing equipment may fail rapidly, within a matter of days. If the equipment does not fail within the first few days, the chances are that it will last a long time and ultimately fail from fatigue. This is the puzzling part to the user, since he will usually point to a tray that has been in service for a couple of years and insist that it and the new ones are receiving the same operating conditions and therefor the metal in the new trays must be bad because they are the only ones failing. The answer, of course, is that the old trays have accumulated an excellent protection in the form of the oxide coating, and the copper dripping on them rolls off without contacting the metal underneath. Had the new trays been allowed to accumulate the same protective coating, they too would not be affected. This assumes that an exothermic or endothermic atmosphere is used for brazing. For in such atmospheres the CO, CO2 and H2O present is sufficient to develop a chromium oxide scale on heat resistant alloys. When dry hydrogen, cracked ammonia or other hydrogen-nitrogen atmospheres are used, the alloy may remain bright and scale-free. Hence, completely unprotected against braze alloy attack. The quickest braze failures we know of tend to be in hydrogen or cracked ammonia. This leads us to the means of prevention. The best means is to prevent copper from contacting (wetting, really) the heat resisting alloy. One way to do this, where the muffle is concerned, is to use a bottom liner of a completely ferritic alloy such as RA446 or 430 stainless. While copper will penetrate the grain boundaries of any austenitic alloy, a ferritic stainless is largely resistant to copper attack. Note that mild steel liners wo n’t work, because at the braze temperature carbon steel is also austenitic, and susceptible to intergranular copper penetration. RA446 is, of course, far too weak to use as the muffle itself. - 137 - The Culprit Copper, continued The second preventative is to have a good oxide coating on the alloy before it is exposed to the copper. The oxide coating is not a foolproof preventative, since fissures do develop in it from expansion and contraction, and copper just might drop on one of the fissures and penetrate to the alloy. The oxide is at least a barrier, however, and a very effective one, even if not 100%. Another common problem is with muffles used to sinter powdered iron parts. Sintering temperatures are 2050°F (1120°C) or higher, with either a nitrogenhydrogen, or an endothermic atmosphere. On one occasion we had a request for assistance from an end user who normally gets two year life from an RA330 muffle. Then in 15 months he went through five muffles, with no change in the product or process. This plant also makes powdered bronze bearings, but sinters these bronze parts in a separate furnace. Only iron parts are to be treated in the muffle that failed. Visual examination of a jagged foot-long (300 mm) crack in the muffle side showed that molten bronze had dripped out of it. So, the muffle which was supposed to process only iron failed, repeatedly, because of a few bronze parts mixed in. Bronze melts well below the 2050°F (1120°C) iron processing temperature. Perhaps we should emphasize again that any austenitic alloy, regardless of composition, will fail from copper contamination. There is a possibility,, though, of getting somewhat better life if the nickel alloy muffle is made with a lower nickel alloy bottom, such as RA 253 MA. We have customers using RA309, RA310 and RA330 for copper brazing tooling. In most cases, RA330 has proved by far the most satisfactory of any of the alloys used. When we find someone using a high nickel alloy, such as 600, for copper brazing fixtures, RA330 has a very easy job proving itself equal or superior to the higher nickel alloy, at less cost. - 138 - THUMBNAIL BIOGRAPHIES OF RA ALLOYS To conclude this discussion of heat resisting alloys, let us briefly summarize the chief characteristics of each RA product. RA330 The work horse of the furnace industry, because it does more jobs better and for less money. Has enough chromium for good oxidation resistance, enough nickel for good ductility, appropriate silicon to resist absorption of carbon and nitrogen. Almost always preferred for carburizing atmospheres. Withstands a lot of thermal shock, yet has above-average strength at operating temperatures. Can be cut, bent and welded without troubles. RA333 A superior product that also costs more. The combination of 3% cobalt, 3% molybdenum and 3% tungsten adds high-temperature strength to a base of 45% nickel, 25% chromium and 1% silicon. Field installations have proven it has excellent resistance to carburization, thermal fatigue and distortion in quenching applications. RA 253 MA High strength, excellent oxidation resistance to 2000°F (1093°C). RA 353 MA Twice the strength of RA330 in the 1800-2200°F (980-1200°C) temperature range. Oxidation resistance comparable to 601 and RA333, melting point about 100°F (56°C) higher. Use for muffles, rotary calciners, coal nozzles. RA 602 CA The strongest and most oxidation resistant high temperature alloy we offer. An upgrade over 601, and potential alternate to alloys 617 or 230. RA309 Preferred for oxidizing atmospheres under 1900°F (1038°C) where resistance to carburizing or nitriding atmospheres is not necessary. Good resistance to sulfidation. RA310 Good oxidation resistance beyond 2000°F (1093°C) under mildly cyclic conditions. Good sulfidation resistance, generally good hot corrosion resistance. RA600 Lower strength but more ductility. Good oxidation, excellent carburization resistance. Resists hot chlorine gas to 1000°F (538°C) RA601 Stronger and more oxidation resistant than RA600, with very good carburization resistance. RA446 Special applications, such as salt bath electrodes, glass molds, copper launders, thermowells, soot blowers, etc., where the hot corrosion resistance of maximum chromium is required. Has the best sulfidation resistance but very, very low strength and ductility. - 139 - CHEMICAL SYMBOLS Al aluminum Ar argon As arsenic B boron C carbon CO carbon monoxide CO2 carbon dioxide CH4 methane Ca calcium Cb columbium (niobium) Ce cerium Cl chlorine (the gas, Cl2) Co cobalt Cr chromium Cu copper F fluorine Fe iron H hydrogen HCl hydrochloric acid He helium H2O water La lanthanum Mn manganese Mo molybdenum N nitrogen (as the gas, N2) NH3 ammonia Nb niobium (columbium) Ni nickel O oxygen (as the gas, O2) P phosphorus Pb lead S sulfur (sulphur) SO2 sulfur dioxide H2S hydrogen sulfide H2SO4 sulfuric acid Sb antimony Si silicon Sn tin Ta tantalum Ti titanium V vanadium W tungsten Y yttrium Zr zirconiuim Hardness is measured by Rockwell or Brinell machines. The Rockwell B scale (Rb, HRB) is most common for our alloys, Rockwell C is for heat treated steel. Brinell is usually abbreviated BHN (Brinell Hardness Number) on mill certifications. Grain size is in ASTM numbers. ASTM 4-7 is about average for RA330. Small numbers (ASTM 0, 2, 3) mean coarse grains. Larger numbers (7, 8, 9) mean finer grain size. Avesta Sheffield report grain size in micrometers, µm. ASTM 3 to 8 grain size would be 125 to 22 µm, typical for RA 253 MA. Disclaimer Clause: The data and information in this printed matter are believed to be reliable. However, this material is not intended as a substitute for competent professional engineering assistance which is a requisite to any specific application. Rolled Alloys makes no warranty and assumes no legal liability or responsibility for results to be obtained in any particular situation, and shall not be liable for any direct, indirect, special or consequential damages therefrom. This material is subject to revision without prior notice. - 140 – BIBLIOGRAPHY A. L. Marsh, Electric Resistance Element, U.S. Patent No. 811,859 Feb. 6, 1906 F. A. Fahrenwald, Metals for High Temperature, Chemical and Metallurgical Engineering, Vol. 28, p 680—681, April 26, 1923 F. A. Fahrenwald, Some Principles Underlying the Successful Use of Metals at High Temperatures, Proc. ASTM, Vol. 24, p 310—347, 1924 J. D. Corfield, Heat Resisting Alloys and Their Use in the Steel Plant, Iron and Steel Engineer, p 157—194, April, 1929 W.P. Rees, B.D. Burns, and A.J. Cook, Constitution of Iron-Nickel-Chromium Alloys at 650 to 800C, JISI July, 1949 Charles Emery and Paul Goetcheus, Added Life for Brazing Fixtures, Steel, June 27, 1955 Ralph H. Moeller, High-Nickel Alloys for High-Temperature Springs, SPRINGS Magazine, October 1965, Vol. 4, Number 2 H. S. Avery, Cast Heat-Resistant Alloys for High—Temperature Weldments, WRC Bulletin 143, August, 1969 This is the best discussion of heat resistant alloys ever printed. Bruce McLeod, Cracking in Type 309 High Temperature Fabrications and How to Combat It, Industrial Heating, September and October 1972 A. Roy, F. A. Hagen, and J. M. Corwin, Performance of Heat—Resistant Alloys in Emission—Control Systems, SAE Paper No. 740093, Automotive Engineering Congress, Detroit, Michigan February 25—March 1, 1974 James Kelly, Understanding Conditions that Affect Performance of Heat Resisting Alloys, Industrial Heating, March & April, 1979 G. R. Rundell, Evaluation of Heat Resistant Alloys in Composite Fixtures, NACE Paper Number 377, Corrosion 86, March 17—21, 1986 James Kelly, Neutral Salt Pot Alloy Life: Maintenance is the Key, Heat Treating , April, 1990 - 141 - BIBLIOGRAPHY, continued George Y. Lai, High-Temperature Corrosion of Engineering Alloys, 1990 ASM International Gene Rundell and James McConnell, Oxidation Resistance of Eight HeatResistant Alloys at 870o, 980o, 1095o, and 1150oC, Oxidation of Metals, Vol. 36, Nos. 3 / 4, 1991 James C. Kelly, Heat Resistant Alloy Corrosion—More Problems than Solutions, NACE Paper Number 166, Corrosion 91, March 11—15, 1991 James Hamer and James McConnell, Influence of Composition and Microstructure on Performance of Wrought Heat Resisting Alloys, Industrial Heating, April, 1992 James Kelly, Heat Resistant Alloy Performance, Heat Treating, July 1993 J. C. Kelly and J. D. Wilson, Oxidation Rates of Some Heat Resistant Alloys, Heat –Resistant Materials II, Conf. Proc. Of the 2 nd International Conference on Heat-Resistant Materials 11—14 September, 1995, Gatlinburg, Tennessee John P. Steward, Flame Straightening Technology, 1981 LaSalle, Quebec James Kelly, Metal dusting in the heat treat industry, Stainless Steel World 1999 Conference, KCI Publishing BV, Zutphen, NL 1999 - 142 - HISTORY Austenitic heat resistant alloys and stainless steels as we know them today were invented by Benno Strauss1 of Friedrich Krupp before World War I. Our 35Ni 19Cr alloy RA330 may trace its roots to Nichrotherm® 4, containing 35% nickel and 13-14% chromium, introduced to Germany in 1910 for high temperature applications 1. U.S. patents for Strauss’ alloys were issued on June 25, 1913. What we now call 310 was developed by Adolf Fry, also at Krupp, in 1926. The electrical resistance wire Nichrome®, nominally 80Ni 20Cr, and the European alloy Nimonic® 75, nominal 76Ni 20Cr, would appear to be developments of A. L. Marsh’s U.S. Patent No. 811,859, Feb. 6, 1906, for a 15-25% Cr, balance nickel electrical resistance alloy. Rolled Alloys’ verbal history says that in the early 1930’s the Misco sales manager, John Johnson, loaded an ingot of the cast alloy HT, at that time 35Ni 15Cr, in the trunk of his Buick. He drove it from Detroit to Lockport, New York, to be rolled to the wrought alloy trademarked Misco Metal. The cost included new springs for the Buick & a couple of replacement mill rolls for Simonds Saw & Steel Co. who did the rolling. We have a folder from The Simonds Saw & Steel Company, dated 1934, which includes data and microstructures for a wrought 15% Cr 35% Ni alloy. The Rolled Products Division of Michigan Steel Casting Company initially developed the market for rolled Misco Metal in the heat treating industry. When Rolled Alloys was founded as an independent company in 1953, this alloy was re-named RA330. In 1958 Rolled Alloys lowered the carbon to 0.08% max and, to maintain the strength, raised chromium to the present 19% Cr. The RA330 silicon range was tightened at that time, to 1.00-1.50%. In that same year work began at Simonds, in conjunction with Rolled Alloys, on the stronger and more carburization resistant grade, RA333. All of the ASTM specifications for RA330 were written by Rolled Alloys technical personnel, and shepherded through the committee meetings. In 1975, after several years of creep-rupture and tensile testing, along with Rolled Alloys’ attendance at numerous committee meetings RA330 was approved by AMSE Case 1654-1 for use to 800°F (427°C). A few years later RA330 was approved for use to 1650°F (899°C). 1. The Sorby Centennial Symposium On The History Of Metallurgy, Volume 27, edited by Cyril Stanley Smith, Cleveland, Ohio October 22-23, 1963 - 143 - TRADEMARKS RA330 and RA333 are registered trademarks of Rolled Alloys 153 MA, 253 MA and 353 MA are registered trademarks of Outokumpu AB 602 CA is a trademark of ThyssenKrupp VDM AL-6XN is a registered trademark of ATI Properties, Inc. 20Cb-3 is a registered trademark of Carpenter Technology Corporation Haynes and Hastelloy are registered trademarks of Haynes International Kanthal is a registered trademark of Kanthal AB Nimonic, Inconel, Incoloy, Monel, MA956 and 800HT are registered trademarks of Special Metals, Incorporated Refrasil is a registered trademark of SGL Carbon Group, Business Unit Fibers and Composites René 41 is a registered trademark of Teledyne Industries Incorporated Stellite is a a registered trademark of Deloro Stellite, Incorporated MO-RE, 22H and Supertherm are registered trademarks of Duraloy Technologies, Inc. Thermax and Thermalloy are registered trademarks of ElectroAlloys Corporation WASPALOY is a trademark of United Technologies Corporation 17-4PH and 18SR are registered trademarks of AK Steel Corporation - 144 - COMPARISON – German & European Standards with American Grade UNS No. Werkstoff Nr. DIN Designation EN Number ferritic stainless 405 S40500 1.4002 X6CrAl13 410 S41000 1.4006 X12Cr13, X10Cr13 410 S41000 1.4024 X15Cr13 410S S41008 1.4000 X6Cr13 416 S41600 1.4005 X 12 CrS 13 430 S43000 1.4016 X6Cr17 446 S44600 1.4763 X8Cr24 duplex stainless 2304 S32304 --2205 S31803 1.4462 X2CrNiMoN22-5-3 2205 S32205 1.4462 X2CrNiMoN22-5-3 2507 S32750 1.4410 X2CrNiMoN25-7-4 austenitic stainless 201 (stainless) S20100 1.4372 X12CrMnNiN 17-7-5 303 S30300 1.4305 X8CrNiS18-9 304L S30403 1.4307 X2CrNi18-9 304 S30400 1.4301 X 5 CrNi 18 10 (X4CrNi18-10) 304H S30409 1.4301 X 5 CrNi 18 10 (X4CrNi18-10) 316 S31600 1.4401 X 5 CrNiMo 17 12 2 316L S31603 1.4404 X2CrNiMo17-12-2 316Ti S31635 1.4571 X6CrNiMo17-12-2 317L S31703 1.4438 X2CrNiMo18-15-4 321 S32100 1.4541, 1.4878 X6CrNiTi18-10, X12CrNiTi18-9 321H S32109 1.4541, 1.4878 X6CrNiTi18-10, X12CrNiTi18-9 347 S34700 1.4550 X6CrNiNb18-10 heat resistant alloys 153 MA® S30415 1.4891 X 4 CrNiSiN 18 10 --1.4828 X15CrNiSi20-12 RA 253 MA® S30815 1.4893 (EN: X9CrNiSiNCe21-11-2) 309S S30908 1.4833 X12CrNi24-12, X 7 CrNi 23 14 309H S30909 1.4833 X12CrNi24-12, X 7 CrNi 23 14 RA85H® S30615 --310S S31008 1.4845 X8CrNi25-21 310H S31009 1.4845 X8CrNi25-21 310 S31000 1.4845 X12CrNi25-21 314 S31400 1.4841 X15CrNiSi25-20 800 N08800 1.4876 X10NiCrAlTi32-20 800H N08810 1.4876 X10NiCrAlTi32-20 ® TM 800HT /AT N08811 (1.4959 similar) (X8NiCrAlTi32-21, similar) Incoloy® DS - -similar to - -1.4864 - -similar to - -X12NiCrSi36 16 RA330® N08330 -(EN: X10NiCrSi35-19) RA 353 MA® S35315 -(EN: X6NiCrSiNCe35-25) 45 TM N06045 2.4889 NiCr28FeSiCe RA333® N06333 2.4608 NiCr26MoW X N06002 2.4665 NiCr 22 Fe 18 Mo 617 N06617 2.4663 NiCr23Co12Mo 601 N06601 2.4851 NiCr 23 Fe 602CA N06025 2.4633 NiCr25FeAlY 603GT N06603 2.4647 NiCr25FeAlYC 600 N06600 2.4816 NiCr 15 Fe Nimonic ® 75 N06075 2.4951 NiCr 20 Ti 1.4002 1.4006 -1.4000 1.4005 1.4016 -1.4362 1.4462 -1.4410 1.4372 1.4305 1.4307 1.4301 -1.4401 1.4404 1.4571 1.4438 1.4541 --1.4818 -1.4835 1.4833 1.4950 -1.4845 1.4951 ------1.4886 1.4854 ---------- weld filler metals —SG designates bare wire, EL is for covered electrodes RA333 X FM 602 CA FM 617 FM 718 -ERNiCrMo-2 -ERNiCrCoMo-1 ERNiFeCr-2 2.4608 2.4613 2.4649 2.4627 2.4667 NiCr26MoW SG-NiCr21Fe18Mo SG-NiCr25FeAlY SG-NiCr22Co12Mo SG-NiCr19NbMoTi - 145 - -----James Kelly 3 February, 2004 COMPARISON—German & European Standards with American Grade UNS No. Werkstoff Nr. DIN Designation EN Number S17400 S66286 N07750 N07718 N07263 N07041 N07001 1.4548 1.4980 2.4669 2.4668 2.4650 2.4973 2.4654 X5CrNiCuNb17-4-4 X5CrNiTi26-15 NiCr15Fe7TiAl NiCr19NbMo NiCo 20 Cr 20 MoTi NiCr19CoMo NiCr 19 Co 14 Mo 4 Ti 1.4542 ------- R30188 R30605 2.4683 2.4964 CoCr22NiW CoCr 20 W 15 Ni --- age hardening alloys ® 17-4PH A-286 X-750 718 C-263 René 41 WASPALOYTM cobalt alloys 188 L-605 corrosion resistant alloys 904L 1925hMo AL-6XN® Sanicro® 28 3127hMo 3033 20Cb-3® 3620Nb 825 G-30 G G-3 625 C-4 C-276 C-22 690 B B-2 B-3 B-4 B-10 FM B-10 200 (nickel) 201 (nickel) 400 N08904 N08926 N08367 N08028 N08031 R20033 N08020 N08020 N08825 N06030 N06007 N06985 N06625 N06455 N10276 N06022 N06690 N10001 N10665 N10675 N10629 N10624 -N02200 N02201 N04400 1.4539 1.4529 -1.4563 1.4562 1.4591 -2.4660 2.4858 -2.4618 2.4619 2.4856 2.4610 2.4819 2.4602 2.4642 -2.4617 -2.4600 2.4710 2.4702 2.4066 2.4068 2.4360 X1NiCrMoCu 25 20 5 X 1 NiCrMoCu 25 20 6 -X1NiCrMoCu31-27-4 X1NiCrMoCu32-28-7 X1CrNiMoCuN33-32-1 -NiCr20CuMo NiCr21Mo -NiCr 22 Mo 6 Cu NiCr 22 Mo 7 Cu NiCr22Mo9Nb NiMo 16 Cr 16 Ti NiMo 16 Cr 15 W NiCr21Mo14W NiCr29Fe -NiMo 28 -NiMo29Cr ?? ?? Ni 99.2 LC-Ni 99 NiCu30Fe --------------------------- K-500 90-10 Cu-Ni N05500 C70600 2.4375 2.0872 NiCu 30 Al CuNi10Fe1Mn --- weld filler metals —SG designates bare wire, EL is for covered electrodes 70-30Cu-Ni K-500 C-276 C-276 C-22 C-22 625 112 82 182 602 CA 602CA ERCuNi -ERNiCrMo-4 EniCrMo-4 ERNiCrMo-10 ENiCrMo-10 ERNiCrMo-3 ENiCrMo-3 ERNiCr-3 ENiCrFe -3 ERNiCrFe -12 ENiCrFe -12 2.0837 2.4373 2.4886 2.4887 2.4635 2.4638 2.4831 2.4621 2.4806 2.4620 2.4649 SG-CuNi30Fe SG-NiCu 30 Al SG-NiMo16Cr16W EL-NiMo15Cr15W SG-NiCr21Mo14W EL-NiCr20Mo14W SG-NiCr21Mo9Nb EL-NiCr20Mo9Nb SG-NiCr20Nb EL-NiCr16FeMn SG-NiCr25FeAlY EL-NiCr25FeAlY ----------- UNS chemistries generally overlap the German standards shown but they are NOT identical. When the customer requires DIN certification of stock material, it can be re-certified by the producing mill. Two exceptions are AL -6XN and 20Cb-3, as they have no direct German equivalents. RA330 does now have an EN spec, designation X10NiCrSi35-19, EN number 1.4886. DIN 50049 3.1.B is a general quality specification which can apply to any alloy. The producing mill can certify to this specification, or Rolled Alloys can provide a certificate of conformance. Many of the EN (European Harmonized Standards) numbers and designations are the same as DIN, others are not. JCKelly June, 2004 - 146 - Midwest Region: Rolled Alloys 125 West Sterns Road Temperance, Michigan 48182 800-521-0332 • 734-847-0561 Fax: 734-847-6917 Email: sales@rolledalloys.com England: Rolled Alloys, Ltd. Walker Industrial Park Guide, Blackburn Lancashire BB1 2QE, United Kingdom 44 (0) 1254 582 999 • Fax: (0) 1254 582666 Email: Blackburn@rolledalloys.co.uk Central Region: Rolled Alloys 9944 Princeton-Glendale Road Cincinnati, Ohio 45246 800-521-0332 Email: sales@rolledalloys.com Rolled Alloys, Ltd. Unit 5, Priory Industrial Park Airspeed Rd., Christchurch, Dorset BH23 4HD, United Kingdom 44 (0) 1425 280 000 • Fax: (0) 1254 280028 E-mail: Christchurch@rolledalloys.co.uk Eastern Region: Rolled Alloys 30 Baker Hollow Windsor, Connecticut 06095 800-521-0332 Email: sales@rolledalloys.com Netherlands: Rolled Alloys Voorerf 16 4824 GN Breda, The Netherlands 31 (0) 76-548 44 44 • Fax: 31 (0) 76-542-98 88 E-Mail: sales@rolledalloys.nl Southern Region: Rolled Alloys 9818 E. Hardy Road Houston, Texas 77093 800-521-0332 Email: sales@rolledalloys.com Singapore: Rolled Alloys International, Ltd. 10 Anson Road #27-07 International Plaza Singapore 07903 65-62272725 • Fax: 65-62272735 Email: railtd@pacific.net.sg Western Region: Harvey Titanium Ltd., Division of Rolled Alloys 291 Coral Circle Drive El Segundo, California 90245 800-321-0909 • 310-343-6000 Fax: 310-606-9322 Email: harveytisales@rolledalloys.com Canada: Rolled Alloys-Canada, Inc. 151 Brunel Road – Unit 23 Mississauga Ontario Canada L4Z 2H6 800-521-0332• 905-501-7552 Fax: 905-5041-7553 Email: racsales@rolledalloys.com China: Rolled Alloys, Ltd. Room 12B03, 12B Floor Suncome Liauw’s Plaza 738 Shangcheng Road Pudong New Area Shanghai, China 200120 86 (0) 21-5835-5329 • Fax: 86 (0) 21-5835-5339 E-Mail: sales@rolledalloys.com http://www.RolledAlloys.com ©2005 Rolled Alloys