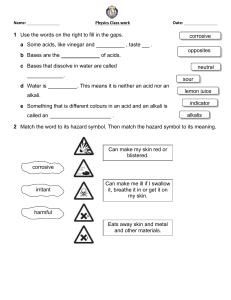
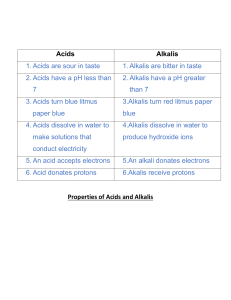
ACIDS BASES AND SALTS 1. ACIDS An acid is a substance that ionizes/ dissociates or break down into ions to give hydrogen ion (H+) AS THE ONLY POSITIVE ION, in solution. An acid is also defined as a proton (hydrogen ion,H+) donor. Eg HCl + H2 O → H+ + Cl- Physical properties of acids - Have a sour taste Turns blue litmus red Have a pH less than 7 Strong acids are corrosive Strong acids (man-made acids) These are acids which completely ionize/dissociate in water. They produce a very high number of hydrogen ions (H+) when dissolved in water. They have a Ph between 1 and 3. Examples Hydrochloric acid – HCl Sulphuric acid – H2SO4 Nitric acid - HNO3 Phosphoric acid- H3PO4 Examples of complete ionization Space for two examples (2 equations) Weak acids (natural acids/organic acids) These are acids which partially/partly ionize/dissociate in water. They produce very few hydrogen ions (H+) in solution. The have a Ph between 4 and 7. Examples Ethanoic acid (found in vinegar) Ascorbic acid (found in vitamin C) Lactic acid (found in sour milk) Citric acid (found in citrius fruits) Example of partial ionization CHEMICAL PROPERTIES OF ACIDS 1. Acids react with metals to form a salt and hydrogen gas. NB: only the MAZIT metals (magnesium, aluminium, zinc, iron and tin) metals are used. Acid + metal → salt + hydrogen HCl + Mg → MgCl2 + H2 Test for hydrogen gas - Use a burning/lighted splint - Result: pop sound is produced 2. Acids react with carbonates to give a salt, carbon dioxide and water. Acid + carbonate → salt + carbon dioxide + water 2HCl + CaCO3 → CaCl2 + CO2 + H2O Test for Carbon dioxide - Bubble/pass the gas in lime water - Result: limewater turns milky/ white precipitate formed 3. Acids react with bases or alkalis to form salt and water. This reaction is called a NEUTRALISATION REACTION. ACID HCl + ALKALI → + NaOH → SALT + NaCl + WATER H 2O 4. Acids react with metal oxides (bases) to form salt and water. This is also a neutralization reaction. Acid + metal oxide → salt + water H2SO4 + CuO → CuSO4 + H2O BASES Bases are the opposite of the acids. A base is defined as a proton acceptor. A BASE WHICH IS SOLUBLE IN WATER IS CALLED AN ALKALI NB: not all bases are alkalis but all alkalis are bases. Some alkalis include NaOH, KOH, NH4OH When dissolved in water , alkalis produce the common ion as hydroxide ion (OH-). An alkali can also be defined as a substance that ionizes/dissociate in water to give hydroxide (OH-) AS THE ONLY NEGATIVE ION. STRONG ALKALIS These are alkalis which completely ionizes/dissociate in water. They produce a high number of hydroxide ions (OH-) in solution. Have a very high Ph range between 12 and 14. Eg NaOH + H2O → Na+ + OH- WEAK ALKALIS These alkalis partially/partly ionizes/dissociate in water. They produce very few hydroxide ions when dissolved in solution. They have a Ph between 8 and 11. Eg NH4OH + H2O → NH4+ + OH- PHYSICAL PROPERTIES OF ALKALIS - They have a bitter taste Turn red litmus blue Have a Ph more than 7 Feel soapy to the touch ie they are slippery when felt Chemical properties 1. Alkalis and bases react with acids forming a salt and water. REMEMBER- This is called a neutralization reaction. NaOH + HCl → NaCl + H 2O 2. Alkalis and bases react with ammonium salts to form a salt, ammonia gas and water. Eg NaOH + NH4Cl → NaCl + NH3 + H2O Test for ammonia gas - Use a wet/damp/moist red litmus paper - Result: moist/damp/wet red litmus paper turn blue. THE DIFFERENCE BETWEEN STRENGTH AND CONCENTRATION Concentration is the amount of acid or alkali that is dissolved in a litre of water. NB; A concentrated solution of an acid/alkali contains a large amount of the acid/alkali in a little amount of water. A dilute solution contains little amount of the acid/alkali in a large amount of water. Strength of an acid/ alkali refers to the extent to which the acid/alkali ionizes in solution (ie degree of ionization). Strength therefore refers to whether the substance is strong or weak. INDICATORS An indicator is a substance used to show whether another substance is acid or alkaline. This is shown by change in colours to acid and alkalis. Examples of indicators indicator phenolpthalein Methyl orange Methyl red Blue litmus Red litmus Universal indicator Colour of indicator colourless orange red blue red green Colour in acid Colourless pink red Red red Colour in alkali Pink Yellow Yellow Blue blue NB: universal indicator shows different colours in strong acids and weak acids. It also shows different colours in strong and weak alkalis depending on the strength. pH (potential hydrogen) This is the measure of the acidity or alkalinity of a solution. The pH is related to the number of hydrogen ions (H+) and hydroxide ions (OH) in solution. NB: Low pH means high number of H+ ions and low number of OHions. High pH means low number of H+ ions and high number of OHions. pH is measured using a pH scale or pH meter. The scale/meter ranges from 1 to 14, and has no units. NB; THE pH METER/scale IS ONLY USED WITH RESULTS FROM THE UNIVERSAL INDICATOR. pH scale diagram APPLICATION OF ACID-BASE REACTIONS IN DAILY LIFE - Brushing teeth with toothpaste: toothpaste is a base, and will neutralize acid formed by bacteria in the mouth. - Treatment of acidic soils: Acidic soils are treated by adding quicklime (CaO) or slaked lime [Ca(OH)2] - Baking with baking powder: Baking powder contains tartaric acid and bicarbonate of soda (sodium hydrogen carbonate). The two solid react together when dissolved in water produce carbon dioxide gases that raise dough. - Treatment of indigestion; Excess acid in the stomach leads to indigestion, this can be neutralized by milk of magnesia, baking soda (NaHCO3). - Treatment of stings; Bees inject acidic liquids into the skin (methanoic/formic acid). This is treated by calamine lotion (ZnCO3), bicarbonate of soda, or baking soda. Wasp stings are alkaline and are treated with vinegar. - Descaling; Removing scales or fur on electric kettles (caused by hard water) using vinegar. OXIDES An oxide is formed when a metal or non-metal react with oxygen. Types of oxides Oxides are classfified into: # Acidic oxide # Basic oxides # Neutral oxides # Amphoteric oxides - 1. Acidic oxides These are non-metallic oxides which dissolve in water forming acidic solutions. Examples: Carbon dioxide (CO2) Sulphur dioxide (SO2) Nitrogen dioxide (NO2) Sulphur trioxide (SO3) CO2 + H2O → H2CO3 (Carbonic acid) SO2 + H2O → H2SO3 (Sulphurous acid) SO3 + H2O → H2SO4 (sulphuric acid) When acidic oxides react with alkali, they are neutralized forming a salt and water solution. Example: CO2 SO3 + + NaOH → Na2CO3 NaOH → Na2SO4 + H2O + H2O 2. Basic oxides (they are alkalis) These are metallic oxides which dissolve in water and form a basic/alkaline solution Examples - Sodium oxide (Na2O) - Calcium oxide (CaO) - Potassium oxide (K2O) Na2O + H2O → NaOH CaO + H2O → Ca(OH)2 Basic oxides react with acids forming salt and water solution. Ie they neutralize acids. CaO + HCl → CaCl2 + H2O 3. Neutral oxides These are the non-metallic oxides which dissolve in water forming a neutral solution. NB: these oxides do not react with acids and alkalis. Examples: - Carbon monoxide (CO) - Nitrogen monoxide (NO) - Hydrogen oxide (water) H2O 4. Amphoteric oxides These are metallic oxides which have/show both acid and basic properties. They react with both acids and bases in the same way, forming a salt and water solution. NB: they can act as both acids and bases Examples: - Zinc oxide (ZnO) - Aluminium oxide (Al2O3) - Lead II oxide (PbO) Reaction with acid (act as a base) ZnO + HCl → Al2O3 + HCl → ZnCl2 + H2O AlCl3 + H2 O Reaction with alkalis (act as an acid) ZnO + NaOH → Na2ZnO2 [Sodium zincate] + H2 O Al2O3 + NaOH → NaAlO2 + [Sodium aluminate] H2 O METHODS OF COLLECTING GASES 1. Collecting over water/downward displacement of water The method is used to collect gases which are insoluble in water, or gases that are slightly soluble in water ie where only a small amount of the gas dissolve in water. Examples: H2, O2, CO2, Cl2, N2 Diagram 2. Upward displacement of air/ downward delivery The method is used for dense gases (high density gases). The gas displaces the air in the gas jar. Examples: Cl2, CO2, SO2 Diagram 3. Downward displacement of air/ upward delivery The method is used for gases of low density (gases less denser than air) Examples: Ammonia (NH3), H2 Diagram 4. Collecting using a gas syringe This method is used for any gas, where the volume of gas collected is to be measured. Diagram SALTS A salt is formed when the hydrogen ion (H+) of an acid is replaced by a metal ion. NB: Salts are named from the acid in which they are obtained from. Salts made from hydrochloric acid are the CHLORIDES Salts made from sulphuric acid are the SULPHATES Salts made from nitric acid are the NITRATES Salts made from phosphoric acid are the PHOSPHATES A good number of salts are soluble in water while other salts are insoluble. NB: knowledge of the solubility rules of salts is important when preparing salts METHODS OF PREPARING SOLUBLE SALTS 1. REACTING ACIDS WITH SOLIDS [Metal, metal carbonate, metal oxide (insoluble base)] NB: only MAZIT metals are used. It is very dangerous to use very reactive metals like potassium and sodium. (a) Reacting an acid with a metal Acid + metal → H2SO4 + Mg → (b) Reacting an acid with a carbonate Acid + carbonate → HCl + CaCO3 → (c) Reacting an acid with an insoluble base Acid + base → HNO3 + CuO → STEP 1: REACTION. Add excess solid (metal, carbonate or insoluble base) to the acid. Excess solid ensures that all the acid react to give a neutral salt solution. NB; when using an insoluble base, the acid is first warmed so as to speed up the reaction. STEP 2: FILTRATION. The unreacted solid is removed from the salt solution by filtration. STEP 3: EVAPORATION. The salt solution is heated to evaporate excess water, leaving the salt saturated. STEP 4: CRYSTALLISATION. When saturated, the salt solution is left to cool and crystallise at room temperature. STEP 5: WASH AND DRY. The crystals are washed with small amounts of cold distilled water and dried with clean tissues or filter papers. (Diagram) 2. ACIDS ARE REACTED WITH ALKALIS , Acid + alkali → salt + water HCl + NaOH → NaCl + H2O In this method, the amount of acid needed to react with an alkali is added with the help of an indicator such as methyl orange. The acid is added slowly until an immediate permanent ORANGE color is obtained indicating neutral products. This is the END POINT. This method is known as TITRATION. END POINT; A point where an acid has completely neutralized the alkali. STEP 1: REACTION. The acid is titrated against the alkali to obtain the salt solution. NB; the salt solution may be filtered through charcoal to remove the indicator. - TITRATION Apparatus and materials used; Burette Pipette Conical flasks (250 ml) Beakers (250 ml) Methyl orange Retort stand White tile Funnel Acid and alkali Procedure 1. 2. 3. 4. Fill the burette with the acid Pipette 25 cm3 of the alkali into a conical flask Add 2-3 drops of the methyl orange indicator Add the acid slowly (drop by drop) into the conical flask with the alkali until an immediate permanent ORANGE colour is obtained. 5. Record results in the table 6. Repeat steps 1-5 until consistent results are obtained Titration Final burette reading (cm3) Initial burette reading (cm3) Volume of acid used (cm3) Tick ( ) best results 1 2 3 4 Step 2: FILTRATION, the solution obtained from titration is then filtered through charcoal to remove the indicator. Step 3: EVAPORATION, the salt solution is then heated to evaporate excess water, until the solution is saturated. Step 4: CRYSTALLISATION, when saturated the salt solution is then left to cool and crystallise at room temperature. PREPARATION OF INSOLUBLE SALTS Insoluble salts are generally prepared by the PRECIPITATION method. This involves mixing two solutions of soluble salts. When these are mixed a reaction takes place in which the salt is formed as a PRECIPITATE (insoluble). Examples: 1. preparation of Barium Sulphate (BaSO4) We need a soluble Barium salt eg, BaCl2, Ba(NO3)2 etc. we also need a soluble sulphate salt eg, Na2SO4, (NH4)2SO4 etc SOLUBLE + SOLUBLE → INSOLUBLE + SOLUBLE barium nitrate + Sodium sulphate Ba(NO3)2 + Na2SO4 → → 2. Preparation of silver chloride (AgCl) (skip two lines) 3. Preparation of lead (II) iodide (PbI2) (skip 2 lines) TEST FOR IONS (Qualitative analysis) These are the tests conducted on unknown samples to determine the positive ions (cations) and the negative ions (anions) in the sample hence the name and formula of an unknown compound. 1. TESTING FOR NEGATIVE IONS (ANIONS): Each negative ion is tested for using a different test reagent. anion test Carbonate Add dilute acid Chloride (in solution) Acidify with dilute nitric acid, then add Result Bubbles/effervescence Carbon dioxide gas produced White precipitate Sulphate (in solution) Nitrate (in solution) Iodide (in solution) aqueous silver nitrate Acidify with dilute nitric acid, then add aqueous barium nitrate or barium chloride Add aqueous sodium hydroxide, then alumnium foil; warm carefully Acidify with dilute nitric acid, then add lead (II) nitrate White precipitate Ammonia gas produced Yellow precipitate 2. TEST FOR POSITIVE IONS (CATIONS): ONLY two test reagents are use to test for positive ions; (i) Aqueous Sodium hydroxide AND/OR (ii) Aqueous ammonia (ammonium hydroxide) cation Aluminium Zinc Calcium Copper (II) Iron (II) Effect of aqueous sodium hydroxide White ppt, soluble in excess giving a colourless solution White ppt, soluble in excess giving a clourless solution White ppt, insoluble in excess Light blue ppt, insoluble in excess Effect of aqueous ammonia/ammonium hydroxide GREEN ppt, insoluble in excess Green ppt, insoluble in excess White ppt, insoluble n excess White ppt, soluble in excess giving a colourless solution No ppt, or very slight ppt Light blue pp, soluble in excess giving a dark blue solution Iron (III) ammonium cation Cu+2 Zn+2 Ca+2 Al+3 Fe+2 Fe+3 RED-BROWN ppt, insoluble in excess Ammonia gas produced on warming Result of adding NaOH(aq) Light blue ppt, insoluble in excess Red-brown ppt, insoluble in excess Result of adding NH4OH/NH3(aq) Light blue ppt, soluble in excess giving a dark blue solution Name and formula of precipitate Copper (II) Hydroxide