Otto, Diesel & Dual Combustion Cycles: Thermodynamics
advertisement
Thermodynamics 1
Otto Cycle, Diesel Cycle & Dual Combustion Cycle
Internal combustion engine is a
heat that derives its power from the
energy liberated by the explosion of a
mixture
gaseous
of
or
some
hydrocarbon,
vaporized
atmospheric air.
form,
in
with
Spark – Ignition (SI) or Gasoline Engine
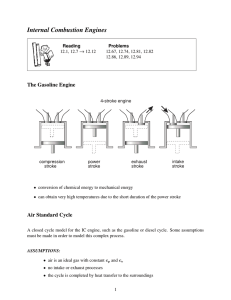
Four-Stroke Cycle Gasoline Engine
Intake stroke - piston moves down the
cylinder and draws in fuel-air mixture
Compression stroke - piston
compresses the mixture in the cylinder
and the spark plug ignites the mixture
Power stroke - burning gases push the
piston down
Exhaust stroke - piston pushes the
burned gases out
Four strokes of the piston and two
revolutions are required to complete a
cycle.
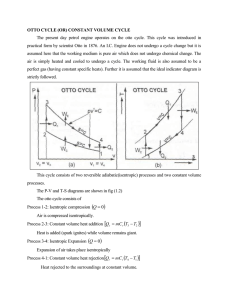
Air-Standard Otto Cycle
1-2: isentropic compression
2-3: constant volume addition of heat
3-4: isentropic expansion
4-1: constant volume rejection of heat
Air-standard cycle means that air alone is the working medium
QA = mcv (T3 – T2)
QR = mcv (T1 –T4) = -mcv (T4 – T1)
W = QA – QR = mcv (T3 – T2) – mcv (T4 – T1)
e=
W
QA
mcv T3 −T2 − mcv (T4 −T1 )
=
mcv (T3 −T2 )
T4 −T1
= 1{1}
T3 −T2
1
=1-
where rk =
x 100
rkk−1
𝑉1
,
𝑉2
the isentropic compression ratio
Derivation of the formula for e:
Process 3-4:
Process 1-2:
•
𝑇2
𝑇1
=
V1
V2
•
k−1
Process 3-4:
•
=
V4 k−1
V3
=
V4 k−1
V3
=
V1 k−1
V2
• T3 = T4rk k−1 {3}
• T2 = T1rk k−1 {2}
𝑇3
𝑇4
𝑇3
𝑇4
=
V1 k−1
V2
• T3 = T4rk k−1 {3}
Substituting equations {2}
and {3} in equation {1}
e = 1e=1-
T4 −T1
T4 rkk−1 −T1 rkk−1
1
rkk−1
Work from the pV plane:
p V −p V
p V −p V
W = ∑W = 2 2 1 1 + 4 4 3 3
1−k
1−k
Clearance volume, percent clearance
rk =
V1
V2
=
VD + V3
V3
=
VD + cVD
cVD
=
1+c
c
Where:
c = percent clearance
V3 = clearance volume
VD = displacement volume
Ideal standard of comparison
Cold-air standard, k = 1.4
Hot air standard, k < 1.4
The thermal efficiency of the theoretical Otto cycle is
• Increased by increase in rk
• Increased by increase in k
• Independent of the heat added
“The average family car has a compression ratio of
about 9:1. The economic life of the average car is 8
years or 80,000 miles of motoring.”
Sample Problem 1: Otto Cycle
1. An Otto cycle operates on 0.1 lb/s of air from 13psia and 130°F at the
beginning of compression. The temperature at the end of
combustion is 5000°R; compression ratio is 5.5; hot-air standard k = 1.3.
(a) Find V1, p2, t2, p3, V3, t4, and p4. (b) Compute QA, QR, W, e, and
the corresponding hp
Sample Problem 1: Otto Cycle
Point 3:
Solution
m = 0.1 lb/s
rk = 5.5
k = 1.3
p1 = 13psia
T1 = 130 + 460 = 590°R
T3 = 5000°R
V1 =
ṁ𝑹𝑻𝟏
𝒑𝟏
p 2 = p1
T2 = T1
=
(𝟎.𝟏)(𝟓𝟑.𝟑𝟒)(𝟓𝟗𝟎)
(𝟏𝟑)(𝟏𝟒𝟒)
= 1.681
𝑽𝟏
𝑽𝟐
𝒌
V2 =
𝑽𝟏
𝒓𝒌
𝒇𝒕𝟑
𝒔
= p1(rk)k = (13)(5.5)1.3
𝑽𝟏 𝒌−𝟏
𝑽𝟐
=
𝒑𝟐
𝑻𝟐
𝒇𝒕𝟑
𝒔
= (5000)
𝟏𝟏𝟗.𝟐
𝟗𝟖𝟑.𝟗
= 605.8psia
𝑽𝟑 𝒌−𝟏
𝟏 𝟏.𝟑−𝟏
T4 = T3
= (5000)
= 2998°R
𝑽𝟒
𝟓.𝟓
𝒑
𝟏𝟑
p4 = T4 𝟏 = (2998)
= 66.1psia
𝑻𝟏
𝟓𝟗𝟎
b) cv =
𝑹
𝒌−𝟏
=
𝟓𝟑.𝟑𝟒
(𝟕𝟕𝟖)(𝟏.𝟑−𝟏)
= 0.2285
𝑩𝒕𝒖
𝒍𝒃𝑹°
QA = mcv(T1 – T4) = (0.1)(0.2285)(5000 – 983.9)
𝟏.𝟔𝟖𝟏
𝟓.𝟓
= T1(rk)k-1 = (590)(5.5)1.3-1
= 0.3056
𝑩𝒕𝒖
= 91.77
𝒔
QR = mcv(T1 – T4) = (0.1)(0.2285)(590 – 2998)
= -55.03
= 983.9°R = 523.9°F
•
p3 = T3
•
= 119.2psia
•
•
•
Point 2:
•
V3 = V2 = 0.3056
Point 4:
a) Point 1:
•
•
Ẇ = QA –QR = 91.77 – 55.03 = 36.75
e=
𝒇𝒕𝟑
𝒔
𝑩𝒕𝒖
𝒔
W=
𝑾
𝑸𝑨
=
𝟑𝟔.𝟕𝟓
𝟗𝟏.𝟕𝟕
= 0.4005 or 40.05%
𝑩𝒕𝒖
𝒔
)(𝟔𝟎
)
𝒔
𝒎𝒊𝒏
𝑩𝒕𝒖
𝟒𝟐.𝟒
(𝒎𝒊𝒏)(𝒉𝒑)
(𝟑𝟔.𝟕𝟓
= 52hp
𝑩𝒕𝒖
𝒔
Sample Problem 2: Otto Engine
2. The conditions at the beginning of compression in an Otto engine
operating on hot-air standard with k = 1.34, are 101.3kPa, 0.038m3
and 32°C. The clearance is 10% and 12.6kJ are added per cycle.
Determine (a) V2, T2, p2, T3, p3, T4 and p4, (b) W, (c) e, and (d) pm.
Sample Problem 2: Otto Engine
Solution
p1 = 101.3kPa
V1 = 0.038m3
T1 = 32°C + 273 = 305K
cv =
𝑹
𝒌−𝟏
=
𝟎.𝟐𝟖𝟕𝟎𝟖
𝟏.𝟑𝟒−𝟏
m=
𝒑𝟏 𝑽𝟏
𝐑𝑻𝟏
=
(𝟏𝟎𝟏.𝟑)(𝟎.𝟎𝟑𝟖)
(𝟎.𝟐𝟖𝟕𝟎𝟖)(𝟑𝟎𝟓)
=
𝒌𝑱
0.8444
𝒌𝒈𝑲
= 0.04396 kg
rk =
𝟏+𝒄
𝒄
=
𝟏 + 𝟎.𝟏𝟎
𝟎.𝟏𝟎
•
=
𝟎.𝟎𝟑𝟖
𝟏𝟏
V2 =
•
•
T2 = T1rkk-1 =
p2 = p1rk k =
= 0.003455m3
(305)(11)1.34-1
= 689 K
(101.3)(11)1.34 = 2518 kPa
𝑻𝟑
𝑻𝟐
= (2518)
𝟏𝟎𝟐𝟖
𝟔𝟖𝟗
= 3757kPa
Point 4:
•
T4 = T3
𝑽𝟑 𝒌−𝟏
𝑽𝟒
= T3
𝟏 𝒌−𝟏
𝒓𝒌
= (1028)
𝟏 𝟏.𝟑𝟒−𝟏
𝟏𝟏
= 455 K
•
•
p3 = p2
= 11
a) Point 2:
𝑽𝟏
𝒓𝒌
Point 3:
QA = mcv (T3 – T2)
12.6 = (0.04396)(0.8444)(T3 – 689)
• T3 = 1028 K
p4 = p3
𝑽𝟑 𝒌
𝑽𝟒
= p3
𝟏 𝒌−𝟏
𝒓𝒌
= (3757)
𝟏 𝟏.𝟑𝟒
𝟏𝟏
= 151 kPa
b) QR = mcv (T1 – T4)
= (0.04396)(0.8444)(305 – 455) = -5.57kJ
W = QA – QR = 12.6 – 5.57 = 7.03 kJ
c) e =
𝑾
𝑸𝑨
d) pm =
=
𝑾
𝑽𝑫
𝟕.𝟎𝟑
𝟏𝟐.𝟔
=
= 0.558 or 55.8%
𝑾
𝑽𝟏 − 𝑽𝟐
=
𝟏𝟐.𝟔
𝟎.𝟎𝟑𝟖−𝟎.𝟎𝟎𝟑𝟒𝟓𝟓
= 364.7kPa
Compression Ignition or Diesel Engine
Four-Stroke Cycle Diesel Engine
Intake stroke - piston moves down
and draws air into the cylinder
Compression stroke - piston rises and
compresses the air to a temperature
of about 900°F (480°C)
Power stroke - oil is injected into the
cylinder, it mixes with the hot air and
burns explosively
Exhaust stroke - gases produced by
combustion push the piston up and
forces the burned gases out of the
cylinder
Air-Standard Diesel Cycle
1-2: isentropic compression
2-3: constant pressure addition of heat
3-4: isentropic expansion
4-1: constant volume rejection of heat
Air-standard cycle means that air alone is the working medium
QA = mcp (T3 – T2)
QR = mcv (T1 – T4) = -mcv (T4 – T1)
W = QA – QR = mcp (T3 – T2) – mcv (T4 –T1)
e=
W
QA
=
mcp (T 3− T2 )− mcv (T4 − T1 )
mcp (T3 − T2 )
=1-
T4 − T1
k(T3 − T2 )
=1-
rck −1
rkk−1 k(rc −1)
{4}
1
V1
V2
V3
V2
where rk = is the compression ratio and rc = is the cutoff ratio and Point 3 is called the cut-off point.
Derivation of the formula for e:
Process 1-2:
•
𝑇2
𝑇1
=
•
V1 k−1
V2
• T2 = T1 (rkk-1)
𝑇3
𝑇2
=
V3
V2
{5}
= rc
• T3 = T1 (rkk-1) (rc)
𝑇4
𝑇3
=
• T4 =
Process 2-3:
•
Process 3-4:
{6}
V3 k−1
V4
T1 (rkk-1)
=
V2 rc k−1
V1
(rc)
=
rck−1
rkk−1
rck−1
rkk−1
• T4 = T1 (rck) {7}
Substituting equations {5}, {6}
and {7} in equation {4}
e=1-
e=1-
T1 (rck )− T1
k T1 rkk−1 rc − T1 (rkk−1 )
rck −1
rkk−1 k(rc −1)
1
Relation among rk, rc and re (expansion ratio)
re =
rk =
𝑉4
𝑉3
𝑉1
𝑉2
=
=
𝑉1
𝑉3
𝑉3
𝑉2
𝑉1
𝑉3
rk = (rc)(re)
The efficiency of the Diesel cycle differs from that of the Otto cycle by
the bracketed factor
rck −1
.
k(rc −1)
This factor is always greater than 1, because rc is
always greater than 1. Thus, for a particular compression ratio rk, the Otto cycle
is more efficient. However, since the Diesel engine compresses air only, the
compression ratio is higher than in an Otto engine.
“An actual Diesel engine with a compression ratio of 15 is more
efficient than an actual Otto engine with a compression ratio of 9.”
Sample Problem 1: Diesel Cycle
1. A Diesel cycle operates with a compression ratio of 13.5 and with a
cut-off occurring at 6% of the stroke. State 1 is defined by 14 psia and
140°F. For the hot-air standard with k = 1.34 and for an initial 1 cu ft.,
Compute (a) t2, p2, V2, t3, V3, p4, and t4, (b) QR, (c) W, (d) e and pm
(e) For a rate of circulation of 1000cfm, compute the horsepower
Sample Problem 1: Diesel Cycle
Solution
a) Point 2:
rk = 13.5
•
T1 = 140+460 = 600°R
V1 = 1 cu ft.
=
𝟓𝟑.𝟑𝟒
(𝟕𝟕𝟖)(𝟏.𝟑𝟒−𝟏)
=
𝑩𝒕𝒖
0.2016
𝐥𝐛𝐑°
cp = kcv = (1.34)(0.2016)
=
m=
𝑩𝒕𝒖
0.2702
𝐥𝐛𝐑°
𝒑𝟏 𝑽𝟏
𝐑𝑻𝟏
=
𝟏
𝟏𝟑.𝟓
= 0.0741 ft3
T2 = T1 (rkk-1) = (600) (13.5)1.34 – 1
= 1454°R = 994°F
• p2 = p1 (rkk-1) = (14) (13.5)1.34 = 457.9psia
Point 3:
• V3 = V2 + 0.06VD = V2 + 0.06(V1 – V2)
• V3 = 0.0741 + (0.06) (1 – 0.0741) = 0.1297 ft3
p1 = 14psia
cv =
𝑽𝟏
𝒓𝒌
•
k = 1.34
𝑹
𝒌−𝟏
V2 =
=
(𝟏𝟒)(𝟏𝟒𝟒)(𝟏)
(𝟔𝟎𝟎)(𝟓𝟑.𝟑𝟒)
•
𝑽𝟑 𝒌−𝟏
𝑽𝟐
= (1454)
𝟎.𝟏𝟐𝟗𝟕
𝟎.𝟎𝟕𝟒𝟏
= 2545°R = 2085°F
Point 4:
•
•
= 0.630lb
T3 = T2
T4 =
p4 =
𝑽𝟑 𝒌−𝟏
T3
𝑽𝟒
𝑽𝟑 𝒌
p3
𝑽𝟒
= (2545)
= (457.9)
𝟎.𝟏𝟐𝟗𝟕 𝟏.𝟑𝟒−𝟏
𝟏
𝟎.𝟏𝟐𝟗𝟕 𝟏.𝟑𝟒
𝟏
= 1271°R = 811°F
= 29.7psia
Sample Problem 1: Diesel Cycle
b) QA = mcp (T3 – T2) = (0.063) (0.2702) (2545 – 1454) = 18.57 Btu
QR = mcv (T1 – T4) = (0.063) (0.2016) (600 – 1271) = 8.52Btu
c) W = QA – QR = 18.57 – 8.52 = 10.05Btu
d) e =
e) W =
𝑾
𝑸𝑨
=
𝟏𝟎.𝟎𝟓
𝟏𝟖.𝟓𝟕
= 0.5412 or 54.12% and pm =
𝒇𝒕𝟑
𝟏𝟎𝟎𝟎𝒎𝒊𝒏
𝑩𝒕𝒖
𝟒𝟐.𝟒𝒎𝒊𝒏 𝒉𝒑
𝑩𝒕𝒖
𝟏𝟎.𝟎𝟓 𝟑
𝒇𝒕
= 237hp
𝟏𝟎.𝟎𝟓 (𝟕𝟕𝟖)
𝟏−𝟎.𝟎𝟕𝟒𝟏 (𝟏𝟒𝟒)
= 58.64psi
Sample Problem 2: Diesel Engine
2. There are supplied 317 kJ/cycle to an ideal Diesel engine operating
on 227 g air: p1 = 97.91kPa, t1 = 48.9°C. At the end of compression,
p2 = 3930kPa. Determine (a) rk, (b) c, (c) rc, (d) W, (e) e, and (f) pm.
Sample Problem 2: Diesel Engine
Solution
m = 0.227kg
p1 = 97.91kPa
T1 = 48.9 + 273 = 321.9 K
p2 = 3930kPa
QA = 317kJ/cycle
•
Point 1:
•
V1 =
Point 2:
•
•
𝐦𝐑𝑻𝟏
𝒑𝟏
V2 = V1
T2 = T1
=
𝟏
𝒑𝟏 𝒌
𝒑𝟐
(𝟎.𝟐𝟐𝟕)(𝟎.𝟐𝟖𝟕𝟎𝟖)(𝟑𝟐𝟏.𝟗)
𝟗𝟕.𝟗𝟏
= (0.2143)
𝒌− 𝟏
𝒑𝟏 𝒌
𝒑𝟐
= (321.9)
𝟗𝟕.𝟗𝟏
𝟑𝟗𝟑𝟎
𝟑𝟗𝟑𝟎
𝟗𝟕.𝟗𝟏
𝟏
𝟏.𝟒
= 0.2143 m3
= 0.0153m3
𝟏.𝟒−𝟏
𝟏.𝟒
•
V3 =
= (0.0153)
𝟐𝟑𝟏𝟐
𝟗𝟐𝟒.𝟒
a) rk =
𝑽𝟏
𝑽𝟐
b) rk =
𝟏+𝒄
;
𝒄
=
= (2312)
𝟎.𝟐𝟏𝟒𝟑
𝟎.𝟎𝟏𝟓𝟑
𝟎.𝟎𝟐𝟖𝟑 𝟏.𝟒−𝟏
𝟎.𝟐𝟏𝟒𝟑
= 1161 K
= 14
𝟏+𝒄
𝒄
14 =
c = 0.0769 or 7.69%
𝑽𝟑
𝑽𝟐
=
𝟎.𝟎𝟑𝟖𝟑
𝟎.𝟎𝟏𝟓𝟑
= 2.50
d) QR = mcv (T1 – T4)
= (0.227) (0.7186) (321.9 – 1161)
= 924.4 K
= 0.0383m3
𝑽𝟑 𝒌−𝟏
𝑽𝟒
T4 = T3
c) rc =
Point 3:
QA = mcp (T3 – T2)
317 = (0.227) (1.0062) (T3 – 924.4)
• T3 = 2312 K
𝑻
V2 𝟑
𝑻𝟐
Point 4:
= -136.9kJ
W = QA – QR = 317 – 136.9 = 180.1 kJ
e) e =
𝑾
𝑸𝑨
f) pm =
𝑾
𝑽𝑫
=
𝟏𝟖𝟎.𝟏
𝟑𝟏𝟕
=
= 0.5681 or 56.81%
𝑾
𝑽𝟏 − 𝑽𝟐
=
𝟏𝟖𝟎.𝟏
𝟎.𝟐𝟏𝟒𝟑− 𝟎.𝟎𝟏𝟓𝟑
= 905kPa
Dual Combustion Engine
“In modern compression ignition engines the pressure
is not constant during the combustion process. The major
part of combustion can be considered to approach a
constant-volume process, and the late burning, a constantpressure process.”
Air-Standard Dual Cycle
1-2: isentropic compression
2-3: constant volume addition of heat
3-4: constant pressure addition of heat
4-5: isentropic expansion
5-1: constant volume rejection of heat
Air-standard cycle means that air alone is the working medium
QA = mcv(T3 – T2) + mcp (T4 – T3)
QR = mcv (T1 – T5) = -mcv (T5 – T1)
W = QA – QR = mcv (T3 – T2) = mcv (T4 – T3) – mcv (T5 – T1)
e=
W
QA
=1–
=1–
=
mcv T3 − T2 +mcp T4 − T3 −mcv (T5 − T1 )
mcv T3 − T2 +mcp (T4 − T3 )
T5 − T1
T3 − T2 + k (T4 − T3 )
1
{8}
rp rkk−1 −1)
rkk−1 rp −1+ rp k (rc − 1)
p
where rp = 3 is the pressure ratio during the constant volume portion of
p2
V
V
combustion, rk = 1 is the compression ratio and rc = 4 is the cut-off ratio
V2
V3
The thermal efficiency of this cycle lies between that of the ideal
Otto and the ideal Diesel.
Derivation of the formula for e: Process 4-5:
Process 1-2:
•
T2
T1
=
• T2 = T1rkk-1
Process 2-3:
•
T3
T2
=
p3
p2
T4
T3
=
V4
V3
{9}
• T5 =
= rp
• T3 = T1rkk-1 (rp)
Process 3-4:
•
•
V1 k−1
V2
{10}
= rc
• T4 = T1rkk-1 (rp)(rc)
V4 k−1
V4 k−1
=
=
V5
V1
V2 rc k−1
rck− 1
= k− 1
V1
rk
T5
T4
T1rkk-1(rp)(rc)
• T5= T1(rp)(rc)
=
V3 rc k−1
V1
=
rck− 1
rkk− 1
{12}
k
Substituting equations {9}, {10}, {11}
and {12} in equation {8}
e=
1-
{11}
e = 1-
𝑇1 𝑟𝑝 𝑟𝑐 𝑘 − 𝑇1
𝑇1 𝑟𝑘𝑘− 1 𝑟𝑝 − 𝑇1 𝑟𝑘𝑘−1 + 𝑘(𝑇1 𝑟𝑘𝑘−1 𝑟𝑝 𝑟𝑐 − 𝑇1 𝑟𝑘𝑘−1 𝑟𝑝 )
1
𝑟𝑝 𝑟𝑐 𝑘 −1
𝑟𝑘𝑘−1 𝑟𝑝 −1 + 𝑟𝑝 𝑘(𝑟𝑐 −1)
Sample Problem 1: Dual Cycle
1. At the beginning of compression in an ideal dual combustion cycle,
the working fluid is 1 lb of air at 14.1 psia and 80°F. The compression
ratio is 9, the pressure at the end of the constant volume addition of
heat is 470psia, and there are added 100Btu during the constant
pressure expansion. Find (a) rp, (b) rc, (c) the percentage clearance,
(d) e and (f) pm
Sample Problem 1: Dual Cycle
Solution
m = 1lb air
p1 = 14.1 psia
T1 = 80 + 460 = 540°R
p3 = 470 psia
rk = 9
Q3-4 = 100Btu
Point 1:
•
V1 =
𝐦𝐑𝑻𝟏
𝒑𝟏
=
𝟏 𝟓𝟑.𝟑𝟒 (𝟓𝟒𝟎)
𝟏𝟒.𝟏 (𝟏𝟒𝟒)
𝑽𝟏
𝒓𝒌
𝟏𝟒.𝟏𝟖𝟔
𝟗
= 14.186ft3
Point 2:
•
V2 =
=
•
T2 = T1
𝑽𝟏 𝒌−𝟏
𝑽𝟐
•
p 2 = p1
𝑽𝟏 𝒌
𝑽𝟐
= 1.576ft3
= (540) (9)1.4 – 1 = 1300°R
= (14.1) (9)1.4 – 1 = 305.6psia
Point 3:
•
T3 = T2
𝒑𝟑
𝒑𝟐
= (1300)
𝟒𝟕𝟎
𝟑𝟎𝟓.𝟔
= 𝟏𝟗𝟗𝟗°R
Point 4:
Q3-4 = (m) (cp) (T4 – T3)
100 = (1) (0.24) (T4 – 1999)
• T4 = 2416°R
•
V4 = V3
𝑻𝟒
𝑻𝟑
= (1.576)
𝟐𝟒𝟏𝟔
𝟏𝟗𝟗𝟗
= 1.905ft3
Point 5:
•
T5 =
𝑽𝟒 𝒌−𝟏
T4
𝑽𝟓
= (2416)
𝟏.𝟗𝟎𝟓 𝟏.𝟒−𝟏
𝟏𝟒.𝟏𝟖𝟔
= 1082°R
Sample Problem 1: Dual Cycle
a) rp =
𝒑𝟑
𝒑𝟐
=
𝟒𝟕𝟎
𝟑𝟎𝟓.𝟔
= 1.54
b) rc =
𝑽𝟒
𝑽𝟑
=
𝟏.𝟗𝟎𝟓
𝟏.𝟓𝟕𝟔
= 1.21
c) rk =
𝟏+𝒄
;
𝒄
9=
𝟏+𝒄
𝒄
c = 0.125 or 12.5%
d) QA = Q2-3 + Q3-4 = (m) (cv) (T3 – T2) + 100
= (1) (0.1714) (1999 – 1300) + 100 = 219.8Btu
QR = (m) (cv) (T1 – T5) = (1) (0.1714) (540 – 1082) = -92.9Btu
e=
𝑾
𝑸𝑨
pm =
𝟐𝟏𝟗−𝟗𝟐.𝟗
𝟐𝟏𝟗.𝟖
=
𝑾
𝑽𝟏 − 𝑽𝟐
=
= 0.5773 or 57.73%
𝟏𝟐𝟔.𝟗 (𝟕𝟕𝟖)
𝟏𝟒.𝟏𝟖𝟔−𝟏.𝟓𝟕𝟔 (𝟏𝟒𝟒)
= 54.37psi
Sample Problem 2: Dual Cycle
2. An ideal dual combustion cycle operates on 454g of air. At the
beginning of compression, the air is at 96.53kPa, 43.3°C. Let rp = 1.5,
rc = 1.60 and rk = 11. Determine (a) the percentage clearance, b) p,
V, and T at each corner of the cycle, (c) QA, (d) e, and (e) pm.
Sample Problem 2: Dual Cycle
Solution
m = 0.454 kg of air
p1 = 96.53 kPa
T1 = 43.3 + 273 = 316.3 K
rp = 1.5
rc = 1.60
rk = 11
a)
b)
𝟏+𝒄
𝟏+𝒄
rk =
; 11 =
;
𝒄
𝒄
𝒎𝑹𝑻𝟏
𝟎.𝟒𝟓𝟒
V1 =
=
𝒑𝟏
V2 =
𝑽𝟏
𝒓𝒌
T2 = T1
=
𝟎.𝟒𝟐𝟕𝟏
𝟏𝟏
𝑽𝟏 𝒌−𝟏
𝑽𝟐
=
c = 0.10 or 10%
𝟎.𝟐𝟖𝟕𝟎𝟖 (𝟑𝟏𝟔.𝟑)
𝟗𝟔.𝟓𝟑
= 0.4271m3
0.03883m3
= T1 (rk) k-1 = (316.3) (11) 1.4-1
= 825.4 K
p2 = p 1
𝑽𝟏 𝒌
𝑽𝟐
= p1 (rk) k = (96.53) (11)1.4
= 2770.8kPa
p3 = (p2) (rp) = (2770.8) (1.5) = 4156.2 kPa
𝟒𝟏𝟓𝟔.𝟐
𝟐𝟕𝟕𝟎.𝟖
𝒑𝟑
𝒑𝟐
= (825.4)
T4 = T3
𝑽𝟒
𝑽𝟑
= (1238.1) (1.6) = 1981 K
T5 = T4
𝑽𝟒 𝒌−𝟏
𝑽𝟓
T3 = T2
= 1238.1 K
V4 = (V3) (rc) = (0.03883) (1.60) = 0.06213m3
p5 = p1
𝑻𝟓
𝑻𝟏
= (1981)
= (96.53)
𝟎.𝟎𝟔𝟐𝟏𝟑 𝟏.𝟒−𝟏
𝟎.𝟒𝟐𝟕𝟏
𝟗𝟏𝟔.𝟐
𝟑𝟏𝟔.𝟑
= 916.2 K
= 279.6kPa
c) QA = (m) (cv) (T3 – T2) + (m) (cp) (T4 – T3)
= (0.454)(0.7186)(1238.1 – 825.4) +
(0.454) (1.0062) (1981 – 1238.1) = 474 kJ
d) QR = (m) (cv) (T1 – T5)
= (0.454)(0.7186)(316.3 – 916.2) = 195.7kJ
W = QA – QR = 474 – 195.7 = 278.3 kJ
e=
𝑾
𝑸𝑨
e) pm =
=
𝟐𝟕𝟖.𝟑
𝟒𝟕𝟒
𝑾
𝑽𝟏 − 𝑽𝟐
=
= 0.5871 or 58.71%
𝟐𝟕𝟖.𝟑
𝟎.𝟒𝟐𝟕𝟏 − 𝟎.𝟎𝟑𝟖𝟖𝟑
= 716.8kPa
Thermodynamics 1
Reference: Sta. Maria, H. B. (1990). Thermodynamics 1. Mandaluyong City, Philippines: National Book Store.
Presentation made by David Anthony C. Manalo & Gino Carlo O. Cadao