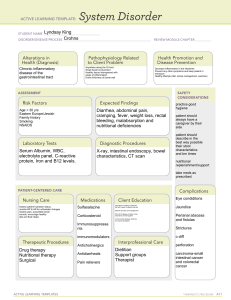
00 Paeds Notes Combined
advertisement

PAEDS SURGERY Middle Clinical Rotation Amalia Geldenhuys Index 1. Resuscitation and fluids 2. Midgut volvulus 3. Oesophageal Atresia 4. Intestinal Atresia 5. Anorectal malformations 6. Hirschprungs disease 7. Abdominal wall defects 8. Surgical umbilicus 9. Infantile hypertrophic pyloric stenosis 10. Intussusception 11. Appendicitis 12. Paediatric Trauma 13. Foreign body ingestion 14. Paediatric oncology (& head and neck masses) 15. Paediatric urology 16. Head and neck 17. Infantile haemangiomas 18. Surgical jaundice 19. Hydatid disease 20. Foreskin & circumcision Intravenous Fluids in Surgical patients: 1. Resuscitation fluids (also known as pre-operative fluid) 2. Maintenance fluid (post-operative fluids) Resuscitation fluid: Fluid resuscitation of surgical patients is one of the most common tasks of junior doctors in Paediatric Surgery. Clinical signs of fluid loss: Tachycardia (the most important sign to assess response to fluid) Dry mucous membrane Reduced skin turgor capillary refill time Sunken eyes or fontanelle Management of dehydration depends on the severity: o Start with a 10 - 20 ml/kg fluid bolus over 15-20 mins. o Reassess clinically and repeat if necessary. Types of resuscitation fluids available: 1. Normal saline [normal saline = 0.9% NaCl is safest]. Always use normal saline as a bolus if available. 2. Ringer’s lactate: 0.85% NaCl, K+, Ca2+, Lactate 3. Rehydration solution: 0.45% NaCl with 5 % dextrose NB! For pyloric stenosis: Initially resuscitate with N/Saline as a bolus, and then change to 0.45% saline + 5% Dextrose (glucose) + 2-3 mmol KCl/litre as maintenance at a 150% of the baby’s fluid requirements. Maintenance fluid: Once surgery is done and fluid loss is minimized Neonates: 10 % neonatalyte Infants and older children: RHS (0.45% NaCL with 5% dextrose) or PMS (paediatric maintenance solution). How much maintenance fluid should be given? Neonates: Weight dependent Babies and toddlers: 100-120 ml/kg/day Children: 1000 ml for 1st 10 kg of body weight 500 ml for next 10 kg of body weight 250 ml for every 10 kg thereafter All maintenance iv fluids should be prescribed on a 24 hour fluid chart as well as hourly rate should be calculated (to reduce errors) and prescribed. If a child has ongoing losses (e.g. from stoma or vomiting), nursing staff must calculate such losses 6 hourly and these losses should be replaced ml for ml with iv resuscitation fluid Blood and blood products: 4ml/kg will raise the HB by 1 g/dl. You need to calculate the total amount and give it over 4 – 6 hours. (Unless the child is actively bleeding of course!). Plasma products and platelets may be given over 30-60 minutes, but they are expensive (>100 times the cost of N.Saline). Ask your supervisors if they are appreciate to an individual case. MIDGUT VOLVULUS (This is a very important chapter) A) DEFINITIONS: Volvulus: An obstruction caused by twisting of the stomach or intestine Midgut volvulus: An obstruction caused by twisting of the midgut: bowel from duodenum (D2/Ampulla of Vater) to mid transverse colon. B) EMBRIOLOGY: The normal development of the midgut includes: Herniation (the midgut herniates through umbilical opening) Rotation (midgut rotates 270o anticlockwise) Retraction (midgut moves back into the abdominal cavity) Fixation (midgut fixes into its normal positon with a wide open mesentery) C) PATHOPOHYSIOLOGY: Incidence: 1. 3% of the population has malrotation of the intestines, predisposing them to midgut volvulus (NB malrotation is not the same as a volvulus) 2. 75% of patients with midgut volvulus are symptomatic within the first month of live Intestinal obstruction may result in a patient with malrotation in three possible ways: 1. Obstruction of the duodenum may result from congenital peritoneal bands (Ladd’s bands) which run over the duodenum from the caecum which is in the right upper quadrant (NOT its normal position in RIF) 2. Midgut volvulus: ABSOLUTE EMERGENCY The entire midgut may twist on its mesentery (supplied by the superior mesenteric artery) due to non-fixation of the mesenteric root during development. Ischaemia and strangulation of the bowel may result if uncorrected. This devastating loss of the midgut is not compatible with long term survival as currently bowel transplant still has poor outcomes 3. Internal hernia in the mesentery D) CLINICAL PRESENTATION: Total bowel necrosis may develop within 6 hours of cut-off of blood supply by twisted mesentery, thus rapid referral and transfer is essential. This is every paediatric surgeon’s nightmare! Early signs: (NB! Make diagnosis ASAP) 75% of patients with midgut volvulus are symptomatic within the first month of life The sudden onset of bile stained vomiting in a previously well child must be regarded as a midgut volvulus until proved otherwise Non-tender, not distended abdomen Late signs: (NB! Intervention needed ASAP) Blood may be passed per rectum Abdominal distension (big abdomen) may result from intestinal obstruction Peritonitis (tender abdomen) may result secondary to ischaemic bowel Malrotation (NOT volvulus): Occasionally, older children may present with: Failure to thrive +/- chronic bilious/ non-bilious vomiting This is usually due to partial or intermittent volvulus (vomiting when volvulus happens and normal in between) Associated abnormalities: Malrotation (NOT VOLVULUS): o Congenital diaphragmatic hernia o Exomphalos o Gastroschisis o Situs inversus: E) DIFFERENTIAL DIAGNOSIS: Bowel atresia Hirschprung’s disease Adhesive small bowel obstruction (usually after surgery) Internal hernia (usually post blunt abdominal trauma) Incarcerated or strangulated hernia F) SPECIAL INVESTIGATIONS: A diagnosis is made: On clinical suspicion Signs of duodenal obstruction on AXR (large stomach) Abnormal C-loop (of duodenum) on contrast meal Abdominal XR NB! XR can be completely normal. Some suspicious signs include: Dilatation (large) of stomach and possibly the duodenum No distal gas Contrast meal and follow-through GOLD Standard and must be done in all patients with suspected midgut volvulus A normal configuration of the duodenum (normal C-loop) excludes malrotation and therefore midgut volvulus in patients with bile stained vomiting. All abnormal C-loops MUST be explored (surgery) ASAP! Birds beak/ Pigtail/ Corkscrew appearance Abdominal ultrasound or CT scan: A doppler ultrasound or CT scan may detect a whorl-pool configuration of the superior mesenteric artery and vein, but this is not a very sensitive sign. G) MANAGEMENT: MAKE DX ASAP!!!!! Primary Mx (ABCDE): ON YOUR WAY TO THEATRE OR ON TABLE – DON’T DELAY SURGERY If a diagnosis of midgut volvulus is confirmed on contrast meal and follow-through, immediate surgical management is essential NGT on free drainage Resuscitation with intravenous fluid & correction Midgut volvulus of metabolic derangement o This should not delay transfer to definitive care o An intra-osseous line should be inserted if intravenous access is difficult o Ongoing fluid resuscitation on the operative table may be necessary Surgical management: (READ ONLY) Can be done by any surgeon if referral will take too long Exploratory laparotomy and derotation of the bowel (Ladd’s derotation procedure): o Transverse supra-umbilical incision o All bowel delivered o Bowel untwisted anticlock wise (NB! Notify anaesthetist before you do this – can have anaest complications due to reperfusion syndrome) o Any abnormal peritoneal bands (called Ladd’s bands) are released o The duodenum is straightened o The mesentery is broadened (like a Spanish fan) and the bowel is placed in the position of non-rotation (this means that the small bowel will be on the right and the colon on the left). o +/- Appendisectomy (The appendix can be removed to prevent confusion later in the event of abdominal pain, as the appendix will now be lying in the left upper quadrant) o Ischaemic bowel must be resected H) COMPLICATIONS: Mortality Short bowel syndrome Malabsorption from ischaemic mucosal injury Take-home message: Sudden-onset bile-stained vomiting in a previously well child = midgut volvulus until proven otherwise (although the majority of children with this situation will prove to have other pathology) OESOPHAGEAL ATRESIA Terminology: OA = oesophageal atresia (born with an interrupted oesophagus) TOF = trachea-oesophageal fistula (A connection between the oesophagus and the trachea) A) PATHOPHYSIOLOGY: Incidence: Incidence of OA with or without TOF is 1 in 2500-3000 live births. Classification: 5 Main types: Most common type is Oesophageal Atresia with a distal Trachea-oesophageal fistula. Elhosny 2019 B) CLINICAL PRESENTATION: Antenatal history: Mother presents with polyhydramnios (too much amniotic fluid) Absent/small stomach bubble (not very sensitive) Postnatal: Prematurity is common Symptoms in the first few hours after birth include: excessive drooling/ frothing of saliva from the mouth respiratory distress especially after feeds (e.g. cough, cyanosis, tachypnoea, aspiration pneumonia); stridor (from tracheomalacia) Unable to pass a naso-gastric tube (NGT) NB! Head to toe examination of the baby is very important Associated Abnormalities: 50 % of OA with/or without TOF will present with associated abnormalities They can include: Any of the VACTERL anomalies o Vertebral abnormalities o Anorectal abnormalities o Cardiac abnormalities (Most common associated abnormality) o Tracheo-oesophageal fistula o Renal abnormalities o Limb abnormalities (Radial dysplasia) Chromosomal anomalies e.g. Trisomy 18 or 21 C) SPECIAL INVESTIGATIONS: 1. Confirm the diagnosis: Babygram (XR of entire baby): OA without a TOF (No air in the abdomen on the XR) o Inability to pass a firm, large NGT o CXR: Curling of NGT in the upper oesophageal pouch (“sigma/delta/ coiled nasogastric tube” sign) OA with a distal TOF: o Unable to pass a firm, large nasogastric tube o XR: Curling of NGT but with air in the stomach below the diaphragm. Air in abdomen indicating the presences of a distal TOF H-type fistula with patent oesophagus NB! Only diagnosed later, usually around 6 weeks of age. o Suspected if patient presents with recurrent aspiration pneumonia and respiratory symptoms associated with feeding o Diagnosed with a special contrast study: (A “TOF study” entails contrast injected via nasogastric tube, which is slowly withdrawn with the patient in a prone position under fluoroscopy, to show the “H-shaped” fistula). 2. Screen for associated abnormalities: o o o o Babygram: Look for vertebral abnormalities, limb abnormalities and other intestinal atresia’s (typically a duodenal atresia, not part of VACTERL). Cardiac echogram Ultrasound of the kidneys, ureters and bladder (KUB) Spinal ultrasound D) MANAGEMENT: Primary care: (ABCDE) Continues suction of the upper oesophagus to avoid aspiration is important! o Large NGT or Reprogle (double-lumen) tube on continuous low pressure suctioning or regular aspiration on the tube is very important especially during transfer of the baby to the referral hospital. o Oxygen (If possible don’t intubate or use CPAP), nasal prongs or high-flow are preferred. o Keep the baby -up to prevent aspiration. IV lines and dextrose containing IVI fluids. Broad-spectrum intravenous antibiotics (e.g. penicillin/ ampicillin, gentamycin and metronidazole) for aspiration pneumonia. Keep baby warm in an incubator. Urgent transfer to tertiary centre with neonatal ICU and paediatric surgical expertise together with any x-rays or blood results. Tertiary care: (READ only) Pre-operative care: o Continue above until surgery o Pre-operative cardiac echogram Surgery: o Repaired through a right lateral thoracotomy, with division and closure of the TOF and end to end anastomosis of the oesophageal ends. o Long gap between ends: A feeding gastrostomy with delayed oesophageal anastomosis after 3-6 weeks may be successful but lung damage from chronic aspiration is problematic. If delayed repair fails, an oesophagostomy must be brought out and oesophageal replacement surgery planned for when the child is older. Post-operative care: o Hospital stay depends on co-morbidities (especially cardiac anomaly) and the ability to put the two ends of oesophagus together. o Long-term follow-up is important to manage any of the following: VACTERL associations if present Gastro-oesophageal reflux disease present in many cases, especially if the distal oesophagus was placed under tension to facilitate anastomosis of the oesophageal ends with a “long gap” Anastomotic stricture requiring dilation Recurrent lung infections Tracheomalacia (a soft trachea); improves with time but rarely may require surgical treatment Psycho-social follow-up of patients and their families E) MORBIDITY AND MORTALITY: Survival >90% is expected with good care The following factors increase morbidity and mortality in patients with oesophageal atresia and trachea-oesophageal fistulae: Patient factors: Premature birth Other congenital abnormalities, e.g. VACTERL associations, especially cardiac lesions: usually ventral septal defect (VSD), patent ductus arteriosus (PDA) or tetralogy of Fallot (ToF); tracheomalacia; Trisomy 21 (Downs syndrome), Trisomy 18 (Edward syndrome) Anatomical variations in length of the atretic oesophageal segments and configuration of the TOF: o “Long-gap” OA: Usually isolated OA (without TOF): higher surgical morbidity INTESTINAL ATRESIA A) DEFINITION: Atresia is a condition in which an orifice or passage in the body is closed or absent (usually a congenital abnormally) Abnormalities of the intestine can be divided into: o Atresia (complete) or stenosis (partial) o Anatomically according to the part of the intestine that is involved B) INCIDENCE: Duodenal atresia occurs in 1 per 5000 – 10 000 live births (boys > girls) Jejunal/ileal atresia occurs in 1 per 5000 live births (boys = girls) Colonic atresia is rare, occurring in 1 per 20 000 live births C) PATHOPHYSIOLOGY: Duodenal atresia: Disruption in normal configuration of duodenum in the fetus Important association with Trisomy 21 and less often, with OA and Ano-Rectal Malformation Ileal Atresia Jejunal/ ileal atresia: Usually secondary to a vascular insult that happens later in pregnancy May be associated with other intestinal atresia’s Not associated with other anomalies Colonic atresia: Rare May be isolated due to vascular insult as small bowel atresia Can be associated with underlying pathology such as Hirschsprung’s disease or other intestinal atresia’s D) CLINICAL PRESENTATION: It is important to get a good antenatal history where possible. Head to toe examination is necessary to look for other abnormalities. Antenatal: Polyhydramnios Antenatal ultrasound: Dilated bowel loops on ultrasound or presence of a double bubble Antenatal detection enables delivery at a tertiary unit with a neonatal intensive care unit (ICU) and paediatric surgical expertise Postnatal: 1. Clinical features of neonatal bowel obstruction (usually within first few days after birth) Vomiting Bile-stained vomitus from birth (first feed) 2. Abdominal distension Upper gastrointestinal tract (GIT) obstruction (duodenal & proximal jejunal atresia) will present with epigastric distension (big stomach) Lower GIT obstruction (ileal/colonic atresia) will present with distension of the entire abdomen 3. Failure to pass meconium >90% of newborns pass meconium (black, sticky newborn stool) within 24 hours Babies with atresia usually do not pass any/only a small amount 4. Dehydration & biochemical derangements Due to ongoing vomiting of gastrointestinal content 5. Jaundice Common esp. in small bowel atresia, which is related to increased bilirubin reabsorption 6. Late presentation Patients with duodenal stenosis (incomplete obstruction) may present much later They can present with: o persistent milk (or bile-stained) vomiting o failure to thrive (poor weight gain) o recurrent lung infection Associated abnormalities: Prematurity: very common Cardiac defects Any of the VACTERL anomalies (especially with Duodenal atresia) Trisomy 21 (Downs syndrome) Cystic fibrosis Hirchsprung’s disease needs to be ruled out in colonic atresia Gastroschisis: around 15% of patients have intestinal atresia(s) E) DIFFERTIAL DIAGNOSIS: Upper GIT obstruction: Gastro-oesophageal reflux causing persistent milk vomiting (does not lead to biochemical abnormalities and failure to thrive) Midgut volvulus (NB!!) In any neonate with bile-stained vomiting, a volvulus must be excluded urgently! Lower GIT obstruction: Hirchsprung’s disease Neonatal small left colon syndrome Meconium plug syndrome and meconium ileus Anorectal malformation (will be identified on perineal inspection) F) SPECIAL INVESTIGATIONS: Radiology: Abdominal x-ray: should be performed in all cases but only 18-24 hours after birth (It can take 12-18 hours after birth for swallowed air to reach the rectum in normal a baby). Duodenal atresia Jejunal atresia Colonic atresia Collapsed proximal small bowel Triple bubble Double bubble Dilated distal bowel Contrast study: a. Meal and follow-through using water-soluble contrast If there is uncertainty in cases of duodenal atresia with distal gas (to exclude a midgut volvulus) b. Enema using water-soluble contrast In all jejenal/ileal atresia’s to rule out colonic atresia For duodenal atresia: investigate for VACTERL anomalies: (see lecture 1) Small unused colon Laboratory tests: U&E is important as electrolytes might shift due to vomiting o Potassium, sodium and chloride might be low o Urea and creatinine might be raised ( if >48 hours after birth) G) MANAGEMENT: 1. Management of neonatal bowel obstruction: a. Nasogastric tube on free drainage and nil by mouth Prevents abdominal distension (overstretching of the bowel wall can lead to gut perforation or it may lead to splinting of the diaphragm & impaired breathing Prevents vomiting of accumulated gut contents and aspiration pneumonia Allows measurement and replacement of GIT losses, preventing dehydration and electrolyte disturbances b. Intravenous fluids (“3 R’s”) Resuscitation fluid: 20ml/kg of 0.9% NaCl bolus may be given and repeated once or twice for dehydration ± hypovolaemic shock due to vomiting and fluid sequestration in obstructed bowel lumen. Regular ongoing maintenance fluid: 10% dextrose containing e.g. Neonatalyte Replacement of ongoing fluid losses c. Broad spectrum intravenous antibiotics if septic or very distended (bacterial translocation) d. Phototherapy for jaundice 2. Optimisation of neonatal physiology: a. Maintain temperature: incubator/ “kangaroo” skin-to-skin with mother if stable/ wrap in plastic or foil during transfer b. Maintain and monitor serum glucose levels c. Respiratory support may be necessary: oxygen (nasal cannula/ intubate) if in respiratory distress d. Monitor cardiovascular, respiratory and metabolic status Pulse rate: tachycardia may indicate depleted intravascular volume (dehydration) Respiratory rate: apnoea and respiratory distress may occur due to aspiration pneumonia, sepsis or elevated diaphragm due to splinting by distended abdomen Blood pressure: Neonates maintain blood pressure by increasing their heart rate to maintain their stroke volume, until advanced hypovolaemic shock is present. At that point they decompensate rapidly, with ensuing bradycardia and cardiac arrest. A low blood pressure is thus a late sign of dehydration or septic shock, and aggressive intravascular fluid replacement (with invasive blood pressure monitoring if possible) is indicated. Serum glucose: Should be monitored routinely in all babies who are nil by mouth; hyperglycaemia may indicate stress and sepsis, while hypoglycaemia is due to low glucose stores (glycogen) in infants who have been vomiting, particularly premature infants Urine output: Should be 1-2ml/kg/hour; another indicator of hydration status Blood gas: serum base excess, lactate and pH should be monitored in cases of delayed presentation to correct metabolic acidosis or alkalosis due to ongoing vomiting through appropriate intravenous fluid and electrolyte replacement. 3. Principles of neonatal transfer: a. Communication with both neonatologist/ paediatrician in charge of neonatal ICU and paediatric surgeon at receiving tertiary institution b. Documentation including x-rays etc. already done should be sent with the patient c. Baby must be stabilised before transfer. All items above must continue during transfer. d. If she herself is stable, mother should ideally be transferred to the same institution to allow bonding, facilitate consent process for surgery etc. Counsel her on baby’s condition. e. A doctor, professional nurse or paramedic trained in neonatal care must accompany the baby. 4. Surgery: (READ ONLY) On the 2nd or 3rd day postnatally once the baby is stable Avoid undue delay to prevent sepsis Immediate surgery in case of a midgut volvulus Duodenal atresia: Duodenoduodenostomy Jejunal & ileal atresia: Approach is by a transverse laparotomy or peri-umbilical incision Deliver all the small bowel and inspect atresia If there is adequate distal bowel length the proximal dilated bowel is resected (as it is dysfunctional with impaired motility) Where short bowel syndrome is a concern, bowel length is preserved and the dilated proximal bowel is tapered The distal bowel should also be cut back End-to-end anastomosis with absorbable sutures Always inspect all the bowel for multiple atresia’s Colonic atresia: Approach is via a transverse laparotomy Either a colostomy (or primary anastomosis) is performed depending on the intraoperative findings 5. Post-operative care: Analgesia and intensive care monitoring Parenteral nutritional (TPN) support may be required for several days post-op Discharge once patient is on full feeds and is growing Regular follow-up at paediatric surgery clinic post discharge ANORECTAL MALFORMATIONS (ARM) A) DEFINITION: Anorectal malformations are abnormalities of the anus and rectum. They include a range of abnormalities from an absent anus (imperforate anus) to a rectal fistula which can appear on the perineum and look like an anal opening in an abnormal place. B) PATHOPHYSIOLOGY: Incidence: 1/ 5000 live births Classification (READ ONLY): According to the level of the fistula BOYS GIRLS Imperforate anus with a rectal fistula: Perineal fistula (most common type of anorectal malformation in boys) Recto-urethral fistula: Recto-bulbar/ Recto-membranous/ Recto-prostatic Recto-vesical fistula (fistula inserts at bladder neck) (<10%) Imperforate anus with a rectal fistula: Recto-vestibular fistula (most common type of anorectal malformation in girls) Perineal fistula Recto-vaginal fistula (<1%): exceptionally rare Cloacal malformation (1 opening instead of 3) urethra, vagina and rectum all drain into single common channel with a single perineal opening instead of three Imperforate anus without rectal fistula (<5%) Imperforate anus without rectal fistula (<5%) C) CLINICAL PRESENTATION: Antenatal ultrasound: Polyhydramnios May see thickened dilated bowel loops May detect associated VACTERL anomalies Inspection of the anus is an essential part of the examination of every newborn prior to discharge from hospital. At birth: No anus (imperforate anus) or an abnormal anus on inspection of the perineum Signs of bowel obstruction: o abdominal distension o vomiting (feeds± bile-stained vomitus) Failure to pass meconium (the black stool passed normally within the first 24-48 hours after birth) NB! Male infant with imperforate anus No anal opening Female infant with cloaca No anal opening Associated abnormalities: Trisomy 21 Genito-urinary abnormalities VACTERL anomalies (VERY COMMON) Vertebral anomalies: butterfly vertebrae, sacral atresia, tethered spinal cord Anorectal malformations Cardiac anomalies Tracheo-(o)esophageal fistulae (+/- OA) Renal: single/ dysplastic kidney, vesico-ureteric reflux, Limb e.g. radial aplasia OA with TOF and an abnormal heart Polydactyly D) MANAGEMENT: Immediate Management: Treat the bowel obstruction (ABCDE) Nasogastric tube decompression Fluid boluses to correct intravascular fluid deficits: 10 – 20 ml/kg N/Saline Bolus Intravenous maintenance fluid: Glucose containing fluids e.g. Neonatalyte Keep patient warm Regular glucose checks Urgent referral to nearest paediatric surgical Decompress the bowel: Transfer to a Paediatric Surgeon ASAP Colostomy (can be done by general surgeon if unable to transfer immediately) Paediatric surgical management: Pre-operatively: VACTERL work-up (NB!) o V: Spinal XR and ultrasound o C: Cardiology consult +/- Echo o TE: Check NGT on babygram o R: KUB (kidney, ureters and bladder ultrasound) +/- MCUG/MAG3 to assess for reflux and kidney function o L: Clinical examination +/- XR of forearms AXR and prone lateral shoot-through (to asses level of fistula) Check if the baby is passing urine; look for meconium in the urine (indicates urethral fistula) by placing gauze over penis in boys Look for an opening or meconium coming out anywhere on the perineum between the expected site of the anus and the urethral meatus MRI Spine and Colostogram: Assess for tethered cord AND define level of the fistula (PLAN for surgery) Surgical management: 1. Double barrel colostomy: Done during the neonatal period = Emergency 2. Definitive surgery: Either at presentation or after colostogram was done (make an anus) Depends on the level of the fistula: o Posterior sagittal anorectoplasty (PSARP) for lower lesions o Laparoscopic assisted anorectoplasty (LAARP) for high lesions (Recto-vesical fistula) o Anoplasty for perineal fistula Sigmoid colostomy Mucus fistula 3. EUA + calibration + electrostimulation Check that the neo-anus is in the correct place and assess the size of the anus (2 weeks after definitive surgery) 4. Colostomy closure: 1- 3 months after anoplasty once the anus is the correct size for age E) COMPLICATIONS: Acute setting: Significant abdominal distension (may develop within 48 hours after birth without relief of the bowel obstruction) leading to: impaired respiration due to abdominal distention bowel wall ischaemia and eventually bowel necrosis bowel perforation severe sepsis, which can cause death if inadequately treated Early post-operative complications: Stoma prolapse Anal stenosis Long-term continence: Soiling or constipation is always a problem Prognosis depends on: o The level of the lesion (The higher the lesion the poorer the prognosis) o Presence of other co-morbidities e.g. tethered cord has a poorer prognosis. These patients should always be follow-up by paediatric surgeons until they are adults in order to help with adequate bowel management. HIRSCHSPRUNG’S DISEASE (HD) A) DEFINITION: Dilated normal bowel Is a congenital functional large bowel obstruction Characterized by the absence of ganglion cells (aganglionosis) in the myenteric and submucosal plexuses of the intestine The affected distal aganglionic bowel is contracted and unable to relax to let faeces pass HD is an abnormal innervation of unknown aetiology affecting the distal bowel leading to inability to pass stool normally; causing distal bowel obstruction Contracted diseased bowel (distal) B) PATHOPHYSIOLOGY: Incidence: 1: 5000 Anatomical distribution: 80% rectosigmoid HD (M:F = 4:1) 10% long segment HD (up to transverse colon; M:F = 2:1) 5 – 10% total colonic HD (M:F = 1:1) C) CLINICAL PRESENTATION: Antenatal US: May see dilated bowel loops of bowel and mom may present with polyhydramnios Postnatal: 1. Clinical features of neonatal distal bowel obstruction (usually within first few days postdelivery) Vomiting Milky vomits increasingly becoming bile stained 2. Failure to pass meconium within 24 hours >90% of normal new-born’s pass meconium within 24 hours (black, tarry sticky stool) Babies with HD typically fail to pass meconium within first 48 hours 3. Abdominal examination Abdominal distention can be significant depending on duration Abdominal redness +/- oedema Tenderness Visible bowel peristalsis 4. Rectal examination (gentle and only by surgeon) Urgent rectal exam is therapeutic! Normal patent anus 5. Dehydration, metabolic & biochemical derangements Severe abdominal distention with visible loops of bowel Due to ongoing vomiting of lower gastrointestinal contents If enterocolitis is present = sepsis with fever, tachycardia and potential septic shock with high mortality Delayed presentation Around 50% of patients with HD present beyond the neonatal period (>28 days old) and can present with: History of delayed passages of meconium at birth Chronic constipation Abdominal distension Malnutrition and failure to thrive Rectum often empty with explosive evacuation of loose stool Associated Abnormalities Trisomy 21 (Down’s syndrome) Other rare neurological disorders Isolated congenital anomalies e.g. congenital heart disease D) DIFFERNTIAL DIAGNOSIS: Neonatal small left colon syndrome Meconium ileus (MI; check for cystic fibrosis) Meconium plug syndrome (MPS) Chronic functional constipation Severe diarrheal disease (?Hirschsprung’s Enterocolitis) E) SPECIAL INVESTIGATIONS: Radiology: 1. Abdominal x-ray: Do an AP and lateral shoot-through To confirm possibility of HD Abdominal distention Large distended loops of bowel (tubes) No air in the rectum Look for any complications of HD Enterocolitis (thickened bowel wall) Pneumoperitoneum Signs of Pneumoperitoneum: o Football sign (Ligamentum Teres outlined with free air from both sides) Liver colour greyish due to overlying air o Rigler’s sign: free air present on both sides of bowel wall 2. Contrast enema: Confirm diagnosis Confirm level of the disease Dilated normal bowel (proximal) Transition zone (between normal and abnormal) Contracted diseased bowel (distal) Histology: Take a piece of the distal rectum via a rectal suction or a full thickness biopsy and send for histology (diagnostic of HD) Histological features include: o Absence of ganglion cells in the intramuscular plexus of Auerbach (myenteric plexus) and the submucosal Meissner plexus. o Proliferation and thickening of peripheral nerves Haematology: U&E: electrolyte disturbances FBC: iron deficiency anaemia is common In summary the diagnosis of HD is made as follows: Clinical suspicion Radiology Histological Confirmation F) MANAGEMENT: Initial management: Primary care for bowel obstruction (see table 1) Urgent bowel decompression: Uncomplicated bowel obstruction: Diagnostic and therapeutic contrast enema Warm saline enemas/ rectal washouts (10-30ml/kg x 3/day) Complicated obstruction: Surgical decompression via colostomy (can be done by general surgeon if not able to transfer to paediatric surgery unit timeously) Primary care for bowel obstruction. 1. Management of neonatal bowel obstruction: a. Nasogastric tube on free drainage (± intermittent aspiration of both swallowed air and gastrointestinal secretions) and nil by mouth Prevents abdominal distension (overstretching of the bowel wall can lead to perforation; splinting of the diaphragm to impair breathing) Prevents vomiting of accumulated GIT contents and aspiration pneumonia Allows measurement and replacement of GIT losses, preventing dehydration and electrolyte disturbances b. Intravenous fluids (“3 R’s”) Resuscitation fluid: 10-20ml/kg of 0.9% NaCl bolus may be given and repeated as necessary for dehydration ± hypovolaemic shock due to vomiting and fluid sequestration in obstructed bowel lumen. Regular ongoing maintenance fluid: 10% dextrose containing e.g. Neonatalyte Replacement of ongoing fluid losses c. Broad spectrum intravenous antibiotics if septic or very distended (bacterial translocation) 2. Optimisation of neonatal physiology: a. Maintain warmth: incubator/ “kangaroo” skin: skin with mother if stable/ wrap in plastic or foil b. Maintain and monitor serum glucose levels c. Respiratory support may be necessary: oxygen (nasal cannulae/ intubate) if distressed d. Monitor cardiovascular, respiratory and metabolic status Pulse rate: tachycardia may indicate depleted intravascular volume (dehydration) Respiratory rate: apnoea and respiratory distress may occur due to aspiration pneumonia, sepsis or impaired diaphragmatic excursion due to splinting by distended abdomen Blood pressure: Neonates maintain blood pressure by increasing their heart rate to maintain their stroke volume until advanced hypovolaemic shock is present and they decompensate, with ensuing to bradycardic cardiac arrest. A low blood pressure is thus a late sign of dehydration or septic shock, and aggressive intravascular fluid replacement (with invasive blood pressure monitoring if possible) is indicated till stabilized. Serum glucose: Should be monitored routinely in all babies who are nil by mouth; hyperglycaemia may indicate stress and sepsis, while hypoglycaemia due to stress response and low glucose stores in infants who have been vomiting as well as particularly premature infants is common. Urine output: Should be 1-2ml/kg/hour; indicator of hydration status Blood gas: serum base excess, lactate and pH should be monitored in cases of delayed presentation to correct metabolic acidosis or alkalosis due to ongoing vomiting through judicious intravenous fluid and electrolyte replacement. 3. Principles of neonatal transfer: a. Communication with both neonatologist/ paediatrician in charge of neonatal ICU and paediatric surgeon at receiving tertiary institution b. Documentation including x-rays etc. already done must be sent with baby c. Baby must be stabilised before transfer. All items above must continue during transfer. d. If she herself is stable, mother should ideally be transferred to the same institution to allow bonding, facilitate consent process for surgery etc. Counsel her on baby’s condition. e. However, the mother alone is not a suitable escort. A doctor, professional nurse or paramedic trained in neonatal care must accompany the baby. Surgery: (READ ONLY) Stoma (colostomy/ Ileostomy) ONLY if unable to decompress with rectal washouts Definitive Treatment Transanal (endo-rectal) pull-through in a well baby that is feeding and growing: # Excision of diseased, aganglionic distal bowel # Pull-through of proximal intestine # Ano-intestinal anastomosis Post-operative care: Analgesia and intensive care monitoring Stoma and wound care as necessary Feeds may be restarted once the post-operative ileus has settled Long-term follow-up by a specialized unit Exclude anastomotic stricture Treatment for recurrent enterocolitis and constipation Might need re-biopsy, re-pull-through if aganglionic intestine was left behind G) COMPLICATIONS: Hirschprung’s Enterocolitis NB All HD patients that present with enteritis must be treated with broad-spectrum antibiotics! This is the main cause of death in HD. Bowel perforation Chronic constipation Malnutrition ABDOMINAL WALL DEFECTS Examphalos and gastroschisis are covered in this lecture OMPHALOCOELE (also known as EXOMPHALOS) A) DEFINITION: Omphalocoele: Is a congenital herniation of intra-abdominal contents through an opening in the umbilical ring (Greek: Omphalos for belly-button and Coele for cyst) B) EMBRYOLOGY: There is failure of fusion of the lateral abdominal wall around the umbilical cord C) PATHOPHYSIOLOGY: Incidence: 1/4000 live births The hernia contents are covered with a transparent membrane which consists of amniotic sac (+Wharton jelly) The umbilical cord arises from the apex of the sac and the umbilical vein and artery runs in the wall of the membranous sac Contents can contain: midgut/liver/spleen/gonads Classification (alters surgical management): Exomphalos minor (< 5cm diameter sheath defect) Exomphalos major (> 5cm sheath defect or contents include liver) D) CLINICAL PRESENTATION: Antenatal: Antenatal ultrasound detection facilitates planned delivery in a tertiary unit as well as consideration of amniocentesis to evaluate for associated chromosomal anomalies Assesses the size and content of omphalocoele Associated abnormalities can be noted, most important are cardiac and central nervous system abnormalities. Severe defects may lead to discussion to terminate pregnancy At birth: Defect through umbilical ring Bowel covered with a membrane Umbilical cord attached Associated abnormalities: Congenital anomalies are important and include: Malrotation is present in 100%. Chromosomal abnormalities (70%) e.g. Trisomy 13, 18, 21 and 45 X Cardiac, central nervous system and genito-urinary abnormalities BECKWITH-WIEDEMAN syndrome : A genetic abnormality related to chromosome short arm of chromosome 10 (also known as EMG syndrome = exomphalos, macroglossia, gigantism) Features include: Congenital abdominal wall defect (omphalocele) Macroglossia (large tongue) Macrosomia/gigantism (big baby) Organomegaly Hypoglycaemia: Severe but temporary hypoglycaemia in neonatal period that can lead to brain damage or death if untreated Mental retardation Embryonal tumours Babies with EMG syndrome also have an increased risk of solid-organ tumours requires regular screening for nephroblastoma and hepatoblastoma for 5 years E) MANAGEMENT: Initial management: Replacing the umbilical clamp with a soft silk tie to prevent tearing the sac. Keep baby warm (temperature control) Administer antibiotics (Penicillin & gentamycin) Assess for associated abnormalities – echo, genetic evaluation & KUB Check blood sugar levels (in suspected cases of Beckwith-Weidemann Syndrome): Surgical management: 1. Reduce bowel safely 2. Close the defect 3. Identify any associated abnormalities 4. Nutritional support 5. Monitor for complications Primary closure (preferred management) The operation depends on: o Size of the defect o Sac contents ( for example the liver) o Associated abnormalities Done under in operating theatre Membrane is removed Contents reduced Sheath mobilized and closed Skin closed Non-operative management (Escharotic agent) Is reserved for Severe associated cardiac abnormality not a candidate for surgery Defect too large to allow safe primary repair Syndromic baby (e.g. trisomy 13) Technique: Apply the chemical as once off The sac is allowed to dry out and granulation tissue forms which is gradually epithelialized The large ventral hernia is closed later, between 1 – 5 years of age Chemical agents that can be used: o Silver sulfadiazine o Povidone-iodine o Gentian violet o 1% Mercurochrome o Silver-impregnated dressings F) COMPLICATIONS: The sac may rupture, leading to drying out and damage to the exposed bowel. o Management: Silo until closure is possible. Infection of the sac and septic complications treated by broad spectrum antibiotics Brain injury from untreated hypoglycaemia GASTROSCHISIS A) DEFINITION: Gastroschisis: Congenital extrusion of bowel through an anterior abdominal wall defect to the right of the umbilicus It is NOT covered with a membrane B) PATHOPHYSIOLOGY: Incidence: 1/ 4000 live births More common in males Risk factors: Young teenage mothers Low socio-economic status Drug abuse including: o Vasoconstrictive drugs e.g. Methamphetamine & pseudoephedrine o Aspirin & ibuprofen o Cigarette smoking C) EMBRYOLOGY: Abdominal wall forms at 4th week of gestation Herniation of midgut into umbilical cord @ 6weeks gestation Elongation & rotation over next 4 weeks Midgut returns to abdominal cavity by week 10 Any interruption of these embryologic processes can result in defects of abdominal wall Theory: o 1 – Involution of a large umbilical vein o 2 – Failure of migration of the right ventral body folds and adhesion to umbilical cord (most widely accepted theory) D) CLINICAL PRESENTATION: Antenatal u/s: Can make the diagnosis Bowel outside of abdominal cavity after 10 weeks gestation No sac covering the bowel Amniocentesis: elevated alpha-fetoprotein and acetylcholinesterase in amniotic fluid Antenatal ultrasound diagnosis facilitates planned delivery in a tertiary hospital Clinical appearance: Bowel protruding through a umbilical defect on the right side The opening is small (1-2cm) No sac covers the bowel Bowel is oedematous and thickened and covered by a fibrinous exudate Can contain: midgut/stomach/gonads Associated abnormalities: In contrast to exomphalos only 1 – 2 % have other associated congenital anomalies (vs 70% of omphalocoele) Usually premature babies Intestinal atresia (in about 10%) E) MANAGEMENT: Initial management includes: ABCDE Nasogastric tube decompression Keep baby warm Intravenous (IV) fluids: Normal maintenance PLUS extra fluid -add 10% extra fluid to maintenance fluid to compensate for evaporative fluid losses from exposed viscera Fluid bolus If presentation is delayed (e.g. baby not born at hospital), dehydration may require 1020ml/kg IV crystalloid boluses (0.9%NaCl) Protect bowel Clear hospital red plastic bag (or cling wrap)-immediately Insert a pre-formed “silo” bag in a tertiary unit NB! NO wet swabs or drapes should be used because it rapidly causes hypothermia and as well as eventual drying out, thus adhering to the bowel and traumatizing it on removal. Avoid bowel ischaemia by ensuring that: the mesentery is not kinked (place baby in right lateral position) Enlargement of the defect may be necessary Urgent transfer to neonatal unit with paediatric surgical expertise Nil by mouth with longline or central line for TPN (start ASA Definitive management: (at the tertiary care unit) 1. Primary reduction and surgical closure of the sheath defect is preferred, this can be achieved At bedside (if the baby is born in a tertiary hospital) No general anaesthesia Monitor for abdominal compartment syndrome 2. Insert a Silo-bag followed by serial reduction (often required if there is significant abdomino-visceral disproportion to prevent abdominal compartment syndrome) o Gentle pressure on the bag and gradually return the bowel to a small abdominal cavity o Delayed secondary closure of the defect is done in 5-10 days o This prevents life-threatening splinting of the diaphragm and impaired abdominal organ blood flow from the sudden rise in intra-abdominal pressure (abdominal compartment syndrome) o Intravenous (parenteral) feeding is required until a prolonged ileus recovers It may take 2-4 weeks for normal intestinal function to return Parenteral nutrition is given through a central line e.g. peripherally inserted central venous catheter or CVC (a central venous catheter made of puly-urethane) or “Broviac” line (a silastic central venous catheter inserted percutaneously or via open venous cut-down and tunnelled subcutaneously from insertion site in vein to decrease infection risk) 3. Repair of an associated atresia o May be delayed to 6 weeks after birth: in cases that a thick inflammatory peel covers the bowel and bowel oedema prevents safe primary repair F) COMPLICATIONS: Risk stratification Complex gastrochisis o Intestinal complications (atresia, ischemia, perforation or necrotizing enterocolitis o Increased mortality, multiple operations, increased rates of sepsis and need for intestinal transplantation EXAMPHALOS VS GASTROSCHISIS OMPHALOCEOLE TERM MORE COMMON IN OLDER MOTHERS NO KNOWN RISK FACTORS DEFECT THROUGH THE UMBILICUS COVERED BY A MEMBRANE SEVERE ASSOCIATED ABNORMALITIES (>70%) SURGERY WHEN BABY IS STABLE PROGNOSIS DEPENDANT ON ASSOCIATED ANOMALIES GASTROSCHISIS Premature More common in teenage mothers High association with drug abuse Defect lateral to umbilicus (almost always to right) Bowel not covered Uncommon associated abnormalities (<10%) (intestinal atresia most common) Surgical management urgent Prognosis dependant on condition of bowel THE SURGICAL UMBILICUS UMBILICAL HERNIA A) DEFINITION: Hernia: Protrusion of an organ from the cavity where it normally resides, through a defect in the wall of that cavity Umbilical hernia: Abdominal contents herniate through a defect in the umbilicus as a result of the incomplete closure of the umbilical ring following the return of the bowel into the abdominal cavity during development. B) PATHOPHYSIOLOGY: Incidence: Common Usually present from around 2 weeks old 5-10% higher incidence in black patients Unknown aetiology C) CLINICAL PRESENTATION: Skin defect does not necessarily reflect size of sheath defect. Considerable variation in diameter (0.5-4cm) Visible mass protruding from the umbilicus Palpable round opening in the umbilicus Usually easily reducible Associated syndromes: (rare) Trisomy 13 or 18 Hurlers syndrome Beckwith Wiedemann syndrome D) MANAGEMENT Indications for surgery In most cases the hernia will close spontaneously without problems(> 50% by 3 years) Surgery is recommended before the child goes to school Earlier surgery for rare complications e.g. incarceration or strangulation E) DIFFERENTIAL DIAGNOSIS: Para-umbilical hernia: The para-umbilical hernia arises from a defect in the linear alba, above the umbilicus The defect is elliptical in shape This hernia seldom closes spontaneously There is a higher risk of omental strangulation and acute pain It is surgically repaired RECTUS-SHEATH DIASTATIS: Normal variant of abdominal wall F) POTENTIAL COMPLICATIONS: Cosmetic concerns Almost no recurrence Incarceration of omentum or bowel is rare. There is an increased risk of bowel incarceration in the following patients: Patients with pica (sand/soil-eaters) During pregnancy (girls) UMBILICAL DISCHARGES (WET UMBILICUS) An umbilical discharge can be one of the following: 1. Pus as a result of infection (Omphalitis) 2. Mucus 3. Faeces 4. Urine (clear fluid) 1. OMPHALITIS A) DEFINITION: Infection of the umbilical cord Can be prevented by daily cleaning of the umbilical cord B) PATHOPHYSIOLOGY: Common in South Africa Causative organisms: Usually Staphylococcus or a haemolytic Streptococcus. Fungal infections may also occur. Tetanus due to use of traditional dressings to newborn umbilical cord e.g. cow dung (less common in recent years) C) CLINICAL PRESENTATION: Foul-smelling umbilicus Purulent discharge Erythema, oedema and cellulitis of the abdominal wall surrounding the umbilicus D) COMPLICATIONS: Infection of the umbilicus is always regarded as a severe infection. The congenital connections to the umbilicus (e.g. via umbilical vein) may result in dissemination of the infection. The innate immunity of the baby is not fully functional and this may result in septicaemia Septicaemia babies have a higher chance of developing septicaemia and must be admitted for intravenous antibiotics administration Cellulitis spreads to the abdominal wall Umbilical gangrene Necrotising fasciitis can spread out of the umbilicus, a life-threatening event which requires aggressive surgical excision of necrotic tissue: Abscesses from infections spreading along congenital tract Umbilical vein thrombosis: From infection; results in long-term extra-hepatic portal vein obstruction and portal hypertension E) MANAGEMENT: IV fluid if needed Correction of electrolytes Broad-spectrum antibiotics Surgical debridement of dead tissue if present Immunoglobulin (passive immunity) if tetanus suspected 2. UMBILICAL GRANULOMA A) DEFENITION: Red granuloma of the umbilicus develops as a result of a low-grade infection. May drain a little pus B) MANAGEMENT: Dehydrating with topical table salt may be tried Usually responds to AgNO3 (Silver nitrate) application May be tied off - if narrow base Surgical excision if not responding to conservative management 3. UMBILICAL MUCOSAL POLYP A) DEFINITION: Mucous discharge results from mucosal remnants or a "cherry tumour" B) CLINICAL PRESENTATION: It is generally redder than the umbilical granuloma C) MANAGEMENT: Treatment is surgical excision 4. VITELLO-INTESTINAL DUCT REMNANTS A) DEFINITION: Intestinal remnant of vitello-intestinal duct (remnant of yolk sac attachment to mid-gut) B) CLINICAL PRESENTATION: Stool draining from the umbilicus Usually diagnosed during the neonatal period C) MANAGEMENT: Surgical exploration of the umbilicus with resection and repair of the bowel wall 5. PATENT URACHUS A) CLINICAL PRESENTATION: A discharge of urine (remnant of allantois) Always exclude posterior urethral valves before surgery B) MANAGEMENT: Surgical excision and repair of the bladder INFANTILE HYPERTROPHIC PYLORIC STENOSIS (IHPS) A) DEFINITION: Pyloric stenosis is a narrowing of the opening from the stomach to the first part of the small intestine (the pylorus). IHPS is one of the most common surgical causes of nonbilious vomiting. B) ANATOMY: C) PATHOPHYSIOLOGY: Incidence: 1 – 4 per 1000 live births Sex distribution: M : F 4 : 1 More common in Caucasian population Risk factors include: o Family history o Gender o Young maternal age o First born D) DIAGNOSIS: Classic presentation: Non-bilious, projectile vomiting in a term neonate who is between 2 – 8 weeks old. Classic arterial blood gas: Hypochloremaemic Metabolic Alkalosis History (from mother): Vomiting: Usually starts two to three weeks after birth NOT bile-stained Associated with feeds Becoming more and more forceful with each feed until eventually vomiting all the feeds Signs and symptoms: 1. Continuous vomiting of stomach contents (non-bile stained) is the most important clinical feature Baby is hungry and cries a lot, feeding eagerly Vomiting becomes progressively more forceful and may be projectile. +/- May have haematemesis (“coffee-ground” vomits) due to gastritis from old milk curds irritating the gastric mucosa as well as oesophagitis from secondary gastro-oesophageal reflux. 2. Dehydration and metabolic derangements follow: Hypochloraemic, Metabolic Alkalosis followed by Hypokaleamia Low Chloride Alkalosis (PH > 7.45) Later low potassium ~1/3 are more than 5% dehydrated 3. Large distended stomach (full epigastrium) may be visualised in cases that present late: Soft abdomen Peristalsis may be visible on the abdominal wall and may run from left to right during a test feed An “Olive” or “tumour” might be palpable in 70 % of patients 4. Additional signs and symptoms that may be present: Malnourished (late presentation) with possible anaemia 2% are jaundiced (due to increased unconjugated bilirubin reabsorption from unused small bowel and impaired glucoronyl transferase activity) Little urine is passed (oliguria) Small hard stools (hunger stools) Palpation of Pyloric Tumour Palpable “olive-shaped tumour” palpated on border of the rectus muscle just above the umbilical level. Special method of palpation: Baby must be relaxed Stomach must be empty Stand on the left side of the patient Feel the liver’s edge Slide fingertips underneath the liver in the midline Feel for pyloric tumour or “olive” If tumour is palpated, the diagnosis is confirm This requires patience! (Sonar has largely replaced palpation) E) SPECIAL INVESTIGATIONS: 1. Arterial blood gas As discussed 2. Radiology: Abdominal ultrasound Standard for diagnosing IHPS Diagnostic criteria: Increased muscle thickness Increased Pyloric channel length Abdominal x-ray- Large distended stomach (extending below 3rd lumbar vertebra level is abnormal); little gas in rest of bowel F) MANAGEMENT: Primary management Correct dehydration & metabolic and electrolyte derangements Nasogastric tube aspiration then leave on free drainage Intravenous fluid therapy: Initial fluid boluses of 0.9% NaCl (“normal saline): 10-20ml/kg given to correct dehydration until normal volume of urine (>1ml/kg/h) is passed Intravenous maintenance fluid and electrolyte replacement “half normal saline” [0.45%NaCl] with 5% dextrose at 130-150% of normal maintenance fluid infusion rate o Add KCL to the maintenance fluid only once a normal urine output is achieved o 0.45% NaCl is usually used rather than normal saline, because alkalosis can take 24-48 hours to correct. Ongoing use of 0.9% NaCl runs the risk of hypernatremia o Do not use lactate containing solution: It is metabolised by the liver to bicarbonate which worsens alkalosis Surgery: Pyloromyotomy (as described by Ramstedt) (Read Only) Surgery follows 24 - 72 hours later once metabolic derangement is corrected Risk of post-operative apnoea, due to respiratory centre dependence on acidosis in triggering inspiration pH>7.5 serum bicarbonate >30mmol/L thus essential to correct first Open/laparoscopic approach o The surgeon can do surgery via a periumbilical/ upper midline/ right upper transverse incision for the open approach o Laparoscopic approach: better cosmesis but higher risk of perforation Division of pyloric channel (hypertrophic muscle): Mucosa/submucosa has its own blood supply and will not perforate Pyloromyotomy Potential complications of surgery: Duodenal perforation by inexperienced surgeon Incomplete myotomy Wound sepsis and wound dehiscence: due to poor wound healing from malnutrition Postoperative Management: Full feeds usually achieved within 24-48 hours after surgery Severely malnourished infants should be monitored for metabolic and electrolyte derangements due to “re-feeding syndrome”: particularly calcium, phosphate and magnesium imbalance Oral zinc and vitamin C supplementation helps with wound healing in malnourished infants, preventing wound dehiscence Usually discharged within 1-5 days depending on pre-operative nutritional status Follow-up for good weight gain and resolution of vomiting INTUSSUSCEPTION A) DEFINITION: Intussusception is the most common cause of bowel obstruction in infants and toddlers Intussuscipiens: “Recipients”: the distal bowel that the proximal bowel telescopes into Intussusceptum: The proximal bowel that telescopes (like a sock) into the distal bowel B) PATHOPHYSIOLOGY: Incidence: Idiopathic intussusception can occur between 3 months and 3 years of age (Peak between 3 and 9 months) Usually heathy, well-nourished infants M>F Natural history: The intussusceptum telescopes into the distal bowel by peristaltic activity A lead point may be present especially in older children Usually ileo-colic but may be colo-colic/ ileo-ileal The mesentery of the proximal bowel is compressed, resulting in venous obstruction and bowel wall oedema If prolonged arterial insufficiency will ultimately lead to ischaemia and bowel wall necrosis. Cause: Idiopathic commonest form lymphoid hyperplasia - due to an increase in the lymphoid tissue mass ("Peyers patches") in the bowel wall of the terminal ileum is thought to be the most likely cause Other possible causes: o ? viral association Usually occurs about 10 days after a respiratory infection or Gastroenteritis o there is a seasonal variation (higher in spring and summer) Ileo-colic (ileum into colon) intussusception is usually found Lead point Much less common Usually seen in the older child and adults (must be ruled out in older children) Lead point include: o Meckel’s diverticulum o Enlarged lymph nodes due to lymphoma o Intestinal polyps Postoperative May occur 2 -5 days postoperatively especially after retroperitoneal surgery e.g. Wilms’ tumour resection Generally painless May present with signs of bowel obstruction (increasing nasogastric aspirates, abdominal distension, inappropriately sick child) Usually small bowel intussusception e.g. ileo-ileal C) CLINICAL PRESENTATION: Symptoms: 1. Sudden onset of colicky abdominal pain The attacks of pain are severe enough to wake the child, who then screams with pain and rolls around. The pain is colicky and intermittent and may then go away only to return every half hour or so, lasting 20 to 30 seconds. The child lies very still in between attacks vomiting occurs in association with the attacks of pain (reflex) 2. The passage of blood and mucus per rectum ("RED CURRANT JELLY" stools) 3. Associated with vomiting Vomiting may initially be milk feeds but becomes bilious 4. Abdomen becomes progressively more distended Signs: Child is restless and irritable during attacks of pain Child is apathetic and looks ill Cries severely with attacks and pulls up legs to the abdomen The child is dehydrated and may be shocked Abdominal mass palpable. The masses typically “sausage” or “cylindrically” shaped – usually it is non-tender (unless presentation is delayed and ischaemia is present) On rectal examination the bloody stool may be identified and a mass may be palpated in the rectum Intussusceptum may prolapse out of the rectum Signs of intestinal obstruction may be present: o Abdominal distension o Bilious/ small bowel contents draining from nasogastric tube o Visible bowel loops in late presentation D) DIFERENTIAL DIAGNOSIS Dysentery Juvenile rectal polyps Rectal prolapse Anal fissure E) SPECIAL INVESTIGATIONS: Abdominal x-ray Signs of intestinal obstruction: tubular dilated loops of bowel; gasless rectum; air-fluid levels at different levels Mass may be seen May be normal early in presentation Abdominal ultrasound Gold Standard to make dx “ target” lesion on transverse view or “pseudo kidney” on longitudinal view Low intestinal obstruction: Multiple air-fluid levels Distended bowel No air in the rectum Target sign F) MANAGEMENT: Initial Management: Prior to special investigations & reduction of intussusception: 1. Management of bowel obstruction: a. Nasogastric tube on free drainage (± intermittent aspiration of both swallowed air and gastrointestinal secretions) and nil by mouth Prevents further abdominal distension (overstretching of the bowel wall can lead to perforation; splinting of the diaphragm impairs breathing) Prevents vomiting of accumulated GIT contents and aspiration pneumonia Allows measurement and replacement of GIT losses, preventing dehydration and electrolyte disturbances. b. Intravenous fluids (“3 R’s”) Resuscitation fluid: 10-20ml/kg of 0.9% NaCl bolus may be given and repeated as necessary for dehydration ± hypovolaemic shock due to vomiting and fluid sequestration in obstructed bowel wall and lumen. o Fluid losses may be significant in intussusception o Repeat as necessary until improving tachycardia, adequate urine output (1ml/kg/hr), improved metabolic acidosis (on blood gas monitoring), wet mucosa & normal capillary refill etc. o Must be well-resuscitated prior to special investigations or pneumatic/ surgical reduction of intussusception Regular ongoing maintenance fluid: 5% dextrose containing e.g. [0.45% NaCl & 5% dextrose] + 20mmol KCl/L Replacement of ongoing fluid losses. Replace small intestinal losses with isotonic crystalloid containing potassium e.g. Balsol/ Plasmalyte B/ Ringers’ lactate/ [0.9% NaCl + 20mmol KCl/L] c. Correct electrolyte abnormalities Blood tests: Check serum urea & serum electrolytes o Concurrent FBC, BC, glucose, XM as per below 2. Prevention/ treatment of septicaemia a. Broad spectrum intravenous antibiotics e.g. stat dose of ceftriaxone to prevent translocation during pneumatic reduction OR treatment course (e.g. ampicillin, gentamycin & metronidazole/ amoxicillin-clavulanate/ cefuroxime & metronidazole) if tender abdomen ± bowel necrosis found at surgery) b. If abnormal vital signs esp. pyrexia: Full blood count (FBC) , blood culture (BC) , serum glucose Blood grouping and cross-match if pneumatic reduction unsuccessful/ contra-indicated Reduction of intussusception: Only once patient has been fluid resuscitated and are stable enough 1. Non-operative reduction: Pneumatic (air enema) reduction under fluoroscopic guidance also known as “Forced Air Reduction Technique” >70% successful reduction and is the preferred method of reduction. hydrostatic (saline enema) reduction under ultrasound guidance done in centres with sonographic expertise Contra-Indication: Absolute: Small bowel intussusception Shock/ incompletely resuscitated Acute abdomen Perforation (pneumoperitoneum) on AXR Relative: Long-standing history >3-4 days Grossly dilated loops of bowel on AXR Bowel >1cm thick with impaired blood flow on Doppler sonar Technique: Child is resuscitated and stable 10 -20 ml/kg N/Saline bolus just before reduction Broad-spectrum antibiotics if not yet given Sedation (optional) with vital sign monitoring Surgeon and radiologist should be present Fluoroscopic (x-ray) screening is employed Air is pumped into the rectum through a Foley catheter while monitoring the pressure used with a gauge and observing the movement of the bowel fluoroscopically The air gives a black rather than white contrast of the bowel lumen Systolic blood pressure should not be exceeded for any length of time o Protocol currently used at TBCH 80 mg for 3 min, rest for 1 min 100 mg for 3 min, rest for 1 min 120 mg for 3 min, rest for 1 min Air should flow into the ileum in a successful reduction If unsuccessful but movement was seen – second attempt 15 min – 6 hours later Complications: Unsuccessful reduction in 20-50% (requires surgical reduction) Higher success in developed countries with early presentation Perforation Should perforation occur with air enema urgent surgery is required and urgent decompression of the pneumoperitoneum with a needle may be necessary as respiratory arrest may occur due to the sudden high pressures in the abdominal cavity Recurrence 5% compared to 2% following surgical reduction 2. Operative reduction: Indications: Unsuccessful pneumatic reduction Peritonitis Small bowel intussusception Perforation Technique (READ ONLY): Approach is usually via a right upper transverse laparotomy is carried out. Laparoscopy (minimal access approach) may be utilized in selected cases without longstanding obstruction. The intussusception is reduced by starting at the top and milking it downwards. Gentle compression of the oedematous bowel may facilitate successful reduction. (See bruised but viable bowel in picture below postreduction) Should this not be possible or necrotic bowel be present, a bowel resection and primary anastomosis must be carried out Lead point may be resected and sent for histology if found e.g. lymphomatous lymph node, Meckel’s diverticulum Post-operative care: Intravenous fluids & analgesia Restart oral feeds once post-operative ileus has resolved (normal bowel sounds, abdominal distension improved, passing flatus, nasogastric aspirates no longer bilious, child hungry) Outcome: 2% risk of recurrence of intussusception (usually during first 10 days post-reduction) after surgery (5% after pneumatic reduction) 10% mortality in developing world due to late presentation; fluid resuscitation and antibiotics essential to treat septic shock in these patients and improve outcome APPENDICITIS IN CHILDREN INTRODUCION: Appendicitis is the most common surgical emergency in childhood and presents more commonly in males. The peak incidence is between 10 – 11 years of age. Incidence rate is variable from 7% life-time risk in Western Europe to 0.5% in Sub-Saharan Africa. Although diagnostic methods and treatment have improved, appendicitis is still associated with a substantial morbidity and although rare, mortality still occurs. The appendix rotates to its final position only in late childhood, the position of its tip is highly variable (e.g. 35% pelvic position). Its blood supply is from the ileocolic artery via the appendiceal branch. FUNCTION OF THE APPENDIX: It serves as a reservoir for normal intestinal flora and has the highest concentration of gut-associated lymphoid tissue in the intestine. It is usually seen in mammals that are herbivores rather than carnivores. PATHOPHYSIOLOGY: Luminal obstruction followed by bacterial infection. Various reasons for luminal obstruction are: faecaloma, lymphoid hyperplasia secondary to Yersinia, Shigella or Salmonella, cystic fibrosis, foreign body or carcinoid tumour. Increased luminal pressure leads to vascular compromise and perforation. CLINICAL PRESENTATION: Symptoms include loss of appetite, fever, vomiting and lower abdominal pain. Abdominal pain starts peri-umbilical (visceral nerve with its origin in 8-10th thoracic dorsal ganglia), and spreads to right lower quadrant when peritonism develops due to somatic sensation. Diarrhoea can develop with a pelvic appendix and renal colic like pain can be present in a retro-caecal appendix. Patients present with different levels of dehydration, tachycardia, pyrexia and according to duration of symptoms. Before palpation of the abdomen, ask the child to point to the area of maximal pain, flexing the hips to relax abdominal muscles also helps prior to palpation. Abdominal distension with local or generalized tenderness or a palpable mass over McBurny’s point can be present on physical examination. McBurny, an American surgeon, mapped out the approximate location of appendix base relative to umbilicus and anterior superior iliac crest by dividing this distance to 3 equal distances (McBurney’s point is 1/3 closer to iliac crest). Children younger than 5 years are usually unable to verbalize pain, clinically difficult to examine and hence present late with perforated appendicitis. SPECIAL INVESTICATIONS: Appendicitis is a clinical diagnosis! Serum Capsular Reactive Protein (CRP) and White Cell Count (WCC) are not sensitive or specific for appendicitis. A few leucocytes in the urine analysis can be due to an inflamed appendix in proximity to the bladder or ureter. Imaging studies 1. Abdominal X-ray may demonstrate faecolith in up to 30% of cases. Other suggestive signs: loss of psoas shadow and presence of scoliosis. Bowel obstruction or free air may also be demonstrated in complicated appendicitis. 2. Ultrasound can help to exclude other pathology when the diagnosis of appendicitis is unclear, this is however operator dependant. Signs suggestive of appendicitis on ultrasound include: a fluid filled non-compressible appendix, diameter > 6mm, appendicolith, peri-appendiceal echogenicity. 3. Computerized tomography (CT) is the imaging study of choice in adults with abdominal pain. Due to risk of 1 in 1000 later malignancies, CT is rarely done for paediatric appendicitis. Children that present with a trauma history and abdominal pain might have a CT done that diagnosis a neglected appendicitis. It might be of value in children <5 years of age. Scoliosis to the right (away from the pain) DIFFERENTIAL DIAGNOSIS: Common: o Right lower lob pneumonia o Right psoas abscess o Diabetic ketoacidosis Other conditions include: Viral mesenteric adenitis, viral gastroenteritis, pancreatitis, cholecystitis, neutropenic colitis and Crohn’s disease. Urinary tract infection, ruptures ovarian cyst, ovarian or testicular torsion, ureteral calculi and salpingitis. MANAGEMENT: Fluid resuscitation: (NB! PRIOR TO SURGERY) 20ml/Kg of intravenous crystalloid (0.9% Saline or Ringers Lactate) to improve tachycardia, prior to general anaesthesia for appendectomy. Repeated boluses of 20ml/Kg may be required to achieve reduction of tachycardia. If inadequate fluid resuscitation prior to induction for general anaesthesia, decreased cardiac output due to further vasodilatation (of anaesthetic agents) can result in cardiac arrest. Antibiotics: Complicated appendicitis requires broad-spectrum intra-venous antibiotics till full ward diet tolerated. Possible regimes include: ampicillin/clavulanic acid, gentamycin & metronidazole, ceftriaxone & metronidazole, amikacin & piperacillin. Surgery: McBurney’s incision is oblique and around the area one third of a line drawn between anterior superior iliac spine (ASIS) and the umbilicus. Lanz incision is a more transverse incision in a skin crease. The operation continues by a muscle splitting i.e. splitting of external and internal oblique and transversalis muscles. Peritoneum is opened and appendix base found and ligated as well as mesenteric vessels. Wound is closed in layers by absorbable sutures. Laparoscopic appendectomy is preferred for adolescent girls or obese patients. Necrotic appendix mobilized laparoscopically Laparoscopic port placement Non-operative management of appendicitis: Treatment of uncomplicated appendicitis in children with antibiotics alone (without surgery) is controversial. Indications include: o Right iliac pain but no guarding with or without demonstration of an appendix mass on clinical examination. Currently, parents’ choice is for non-operative management of appendicitis. The common stated reasons include: o Negative appendectomy rate of 10%, o Associated risks of general anaesthetic o Functional role of the appendix o Low risk of recurrent appendicitis once treated with antibiotics. PAEDIATRIC TRAUMA A) Introduction: Motor vehicle associated injuries are the most common cause of deaths in children of all ages. Non-accidental injuries are the most common cause of deaths in infants. Boys are more commonly affected than girls. Blunt trauma occurs more commonly, penetrating injury less so. Thermal and chemical injury (e.g. burns/scalds) also occur, however, this topic is outside the scope of this lecture. B) Characteristics of Paediatric Patients: Younger children have a proportionately bigger head, consequential in higher incidence of blunt brain injuries. This is the most common cause of fatality in blunt injury. In cases of vehicular accident, due to the smaller body mass of paediatric patients, the impact from a motor vehicle results in a greater force applied per unit of body area, hence higher frequency of multiple injuries compared to adults. This contrasts with softer skeleton structures (and multiple active growth centres) which is more flexible. Internal solid organ damage can present without overlying bony fractures. The presence of skull or rib fractures in a paediatric patient suggests transfer of vast amount of energy. Children are more difficult to intubate in cases where protection of airways is required (usually decreased level of consciousness secondary to head trauma) Non-Accidental injury (NAI) In any child who presents to a trauma unit, a detailed history of the mechanism of injury is very important and should include an accurate timeline of events. There is a high prevalence of NAI in most societies. It is a major cause of death in infants and associated with 2-5% of significant traumatic injuries. ALWAYS THINK OF NAI IN THE FOLLOWING SITUATIONS: 1. 2. 3. 4. Injury not fully explained by the parents/caregivers Contradictory mechanism of injury Delay in seeking medical treatment History of trauma changes in details with time or incompatible with physical findings 5. Multiple other injuries detected in various stages of healing or in unusual locations 6. Fractures in diaphysis of long bones in infants or children too young to walk or spiral appearance on X-ray 7. Unconscious child with history of low impact injury (see point 3) 8. Unusual injuries: retinal haemorrhage, torn frenulum, contact burns, circular (cigarette) burns, bite marks and multiple fractures 9. Care giver unwilling to leave the child alone with medical/nursing staff (to prevent a verbalising child from talking about his/her injuries) 10. Unusual or no interaction between a child and his/her parent or caregiver Traumatic brain injury is the leading cause of death in non-accidental injury. Shaken Baby Syndrome’s classic triad of: bilateral subdural hematoma, retinal haemorrhages and altered mental status Is caused by acceleration and deceleration forces caused by shaking an infant resulting in traumatic, hypoxic-ischaemic encephalopathy. The role of the medical doctor when admitting a child with non-accidental injury is early recognition and adequate documentation (history and thorough physical examination) followed by appropriate referral to social services in case child protection is needed. Special investigation includes: Ophthalmic examination to exclude retinal bleeds, Full skeletal X-rays (+/- bone scan) Computerised Tomography (CT) of the brain. Abdominal Trauma Isolated abdominal injury is common and has a low mortality, however, if combined with head or thoracic injury the mortality rate escalates sharply. A) Aetiology Majority of injuries result from blunt force trauma. This is due to the poor protection offered by the wide-shape and incomplete ossification of the rib cage. Intra-abdominal solid organs are particularly vulnerable to blunt trauma in children. Commonest causes are road traffic accidents, bicycle handle bar injuries and falls. Penetrating injuries (stab or gun-shot) are rare in children. Abdominal injury in children younger than 18-months of age should raise suspicion for NAI. B) Management Hollow visceral injuries and diaphragmatic ruptures require surgical intervention; this occurs when visceral content is forcibly compressed between anterior abdominal wall and the spine; bicycle handlebar, elbow strike to right upper quadrant (by an adult) and seat belt injuries. All patients should be presumed to have a high likelihood of injury to the solid organs (including the diaphragm) and hollow-organs until proven otherwise. Other injuries such as proximal small bowel perforations near ligament of Treitz, ischaemic small bowel perforations from a large mesenteric tear and rupture of a full bladder are more common compared to adults. The liver, spleen, pancreas and kidneys are the solid organs most injured in paediatric patients injured by blunt abdominal trauma. Almost all paediatric trauma deaths in children with blunt abdominal injuries are due to concomitant head injuries. Initial management of the injured paediatric patient follow the basic principles of Advanced Trauma Life Support (ATLS) and Advanced Paediatric Life Support (APLS) courses. Primary survey follows steps in management starting with: control of external bleeding followed by airway and C-spine control, Breathing and ventilation, management of shock, neurological fall out, keeping the child warm and glucose control. Non-operative Management Principles: In general, non-operative treatment of isolated solid organ injuries in paediatric patients is the standard of care. o Haemodynamically unstable children with suspected abdominal injury require fluid administration as part of the initial assessment by the response to fluids administered (e.g. correction of tachycardia). Crystalloid fluids (Normal Saline) 20ml/Kg twice, followed by up to two 20ml/Kg of blood transfusion. o Patients who fail to stabilize, or who are unable to maintain haemoglobin most likely have ongoing haemorrhage and require surgical intervention to stop the bleeding. o Massive blood transfusion may be required in rare occasions. The most common reason for operative management for blunt abdominal trauma for paediatric patients are persistent haemorrhage, bowel injury, pancreatic injury or ruptured diaphragm. Radiology: CT scan is the diagnostic study of choice for the evaluation of blunt abdominal trauma. The main counter arguments is higher cancer risk in adult patients undergoing CT scans as children. Before preceding to this investigation the case should be discussed with a paediatric surgeon. When intra-venous contrast CT is performed, radiation must be kept as low as possible. Abdominal ultrasound may be useful for the initial assessment, but CT remains the gold standard. Peritoneal lavage is no longer advocated in children. Splenic injury The degree of injury ranges from laceration to avulsion as demonstrated by the below grading system (this grading is not relevant to medical student and read-only). Splenectomy is not recommended in children due to: (a) risk of severe post splenectomy sepsis and (b) high success rates of non-operative management for paediatric patients (around 95% of cases). Delayed complications are rare. The decision to continue non-operative management of confirmed solid organ injuries should be made by the surgeon. This is usually done if facilities have: paediatric intensive care, paediatric surgeons and ready access to operating theatre. Successful non-operative management of blunt splenic injury requires a few days of hospitalization and 6-8 weeks of home bed rest by the paediatric patient. Operative management of splenic injury is indicated if: tachycardia persists haemorrhage requires blood transfusion > 40ml/Kg. o Goal of operation is to control bleeding while conserving splenic tissue if possible. Overwhelming post-splenectomy infection (OPSI) is a devastating complication with an incidence of 3% of asplenic patients per life time and a mortality rate above 50% per Infections, typically caused by encapsulated organisms that are filtered out by spleen (in children), e.g. Streptococcus pneumonia, Haemophilus Influenza and Neisseria meningitis. OPSI present with flu-like symptoms and rapidly progression to fulminant sepsis and ultimate death in a few hours. It is a life time risk, but it is at its highest in childhood. Prophylactic antibiotics against encapsulated bacteria e.g. oral amoxycillin daily single dose for 2 years. Parents need to be informed that acute pyrexia episodes should be treated promptly and medical attention sought. Vaccines Recommendation (intra-muscular injections): a) Polyvalent pneumococcal vaccine. All serotypes are not covered with this vaccine. b) Haemophilus influenza vaccine c) Meningococcal vaccine. Only 2/4 serotypes are covered with this vaccine. Repeat vaccination every 5 years until teenage years is usually carried out. Further vaccination can be given if patients request them. Foreign Body Ingestion A) Epidemiology: More commonly ingested in <5-year-old children. Ingested object varies, coins are more commonly ingested because of its widespread availability but cause little damage, in comparison button batteries are dangerous once ingested and requires urgent management. Other potentially dangerous objects ingested include multiple magnets, open safety pins, razor blades or screws/nails. B) Clinical Presentation: Parents present the child to an emergency doctor after witnessed ingestion event Children can be asymptomatic or symptomatic Symptoms include: o Respiratory symptoms: coughing, wheezing, respiratory distress, o GIT symptoms: drooling, dysphagia, refusal of feeds, abdominal pain or vomiting. NB! A history of foreign body ingestion shouldn’t be ignored. Three areas of anatomic narrowing within the oesophagus that render to impaction: cricopharyngeus (70%), aortic arch (15%) lower oesophageal sphincter (15%). Most foreign bodies that pass into stomach will pass through the rest of the gut uneventfully. C) Special Investigations: Radiopaque objects can be demonstrated on anteroposterior or lateral X-rays of chest and abdomen. Radiolucent objects require further investigation either with contrast swallow under fluoroscopy or bronchoscopy/oesophagoscopy. CT scan is useful in rare cases, or if abscess formation is suspected. D) Different Ingested Foreign Bodies: Coin ingestion Occasionally more than one coin can be ingested, this is best confirmed on the lateral view Xray. Commonly impacts in proximal and mid oesophagus, this requires rigid or flexible oesophagoscopy and removal of coin under general anaesthesia in theatre. Impaction in distal oesophagus can be observed for a short time, if still present on repeat CXR, objects need oesophagoscopy to remove. Button batteries Oesophageal button battery ingestion is associated with high morbidity. Button batteries are rounded on the CXR with a double contour rim (a halo effect), this must not be confused with a coin. Tissue injury develops through pressure necrosis, low voltage electric current released which generates high temperature and leakage of alkali chemicals of the battery, causing a liquefaction necrosis. Mucosal injury occurs early and may continue after removal of the battery; therefore, any suspected case of oesophageal battery impaction warrants immediate removal. Early and late complications include: o Oesophageal perforation, stricture formation, tracheoesophageal fistula, erosion through into thoracic aorta and massive bleeding. However, if the battery has passed the oesophagus and is within the stomach or more distal GIT on presentation in an asymptomatic child, the child can be observed since most will pass without intervention within 48hrs. If after 48 hours its persistence within the stomach is demonstrated or fragmented, removal is warranted. Magnet ingestion Ingestion of more than one high-power magnetic toys is associated with significant morbidity. AXR will confirm magnet ingestion. Magnets within the GIT can cause obstruction, volvulus, perforation or fistula formation. Close inpatient observation of these children is required to rule out complications. A single magnet within the stomach can be observed. Multiple magnets within the stomach should be removed via endoscopy. If during inpatient observation of a patient with multiple ingested magnets within the GIT, the patient develops peritonism or pneumoperitoneum a laparoscopy or laparotomy should be performed. Sharp Foreign Bodies Almost all pass uneventfully and can be conservatively managed, parents should be warned of symptoms necessitating re-evaluation. Unusually larger sharp objects have a reported 10% risk of perforation, usually at the ileocecal valve, surgical intervention may be acceptable in these rare cases. Bezoar Collection of undigested material within the GIT Usually causes gastric outlet or more distal bowel obstruction. More common in mentally impaired patients, who usually ingesting their own hair. Frequently due to the size of the bezoar flexible endoscopic removal is unsuccessful and laparotomy is required. Airway Foreign Bodies (Always asked for respiratory symptoms with any FB ingestion) The presence of any respiratory symptoms after ingestion of a foreign body necessitates an URGENT CXR and bronchoscopy. Peanuts aspiration are particularly dangerous because they are not seen on CXR and cause intense inflammatory response. In acute airway obstruction the ingested object should be promptly extracted. E) Complications of foreign object ingestion: Airway obstruction Acquired trachea-oesophageal fistula Gut Perforation Retropharyngeal abscess formation Bowel obstruction Stricture formation PAEDIATRIC ONCOLOGY DIFFERENCES FROM ADULT CANCERS: Mostly embryonal tumours and sarcomas (carcinoma is rare) Etiology is genetic (environmental causes are rear) Most are sensitive to chemotherapy Better cure rates (overall long-term survival rates > 70 %) ROLE OF THE SURGEON: Assist with the diagnosis (clinical examination, fine needle aspiration or biopsy) Central venous access for chemotherapy Resection of the primary tumour Assist with treatment of complications (bowel perforation, pancreatitis etc.) NEPHROBLASTOMA (WILMS’ TUMOUR) A) EPIDEMIOLOGY: 6 % of all paediatric malignancies (1 in 10 000 children) Commonest childhood renal tumour Peak incidence is between 1 – 3 years 5% have associated syndromes Good prognosis B) CLINICAL FEATURES: Most are asymptomatic abdominal masses Mass usually non-tender Moves up with inspirations Extends under the ribs Ballotable (move on manual palpation) Symptoms can include: Hypertension Intermittent abdominal pain Haematuria Systemic symptoms: malaise, lethargy, fever, anemia C) SPECIAL INVESTIGATIONS: RADIOLOGY: Ultrasound is the first-line investigation to make the diagnosis CT Chest needs to be done to exclude pulmonary metastases MRI to assess the lesion, define anatomy and exclude metastases (liver, contra-lateral side, venous invasion) D) MANAGEMENT: Management consists of: Chemotherapy Resection of the tumour Radiotherapy (only for advance stage) NEUROBLASTOMA This is malignant neoplasm of neuroblasts, mostly found in adrenal medulla. Neuroblasts are cells originating from the embryonic neural crest (normally only present in the fetus). A) EPIDEMIOLOGY: 10 % of childhood tumours (1 in 9000 children) Most common solid tumour in children < 1 year old Can be associated with: Hirschprung’s disease Neurofibromatosis Beckwith-Wiedemann syndrome Sites: Adrenal (most common), paraspinal, mediastinal, neck, pelvis B) CLINICAL FEATURES: >50 % present with an abdominal mass Hypertension Horner’s syndrome Systemic symptoms: malaise, weight loss, fever, anemia Symptoms secondary to metastases: Bilateral orbital ecchymosis (raccoon eyes) – secondary to metastases Skull infiltration Multiple skin nodules Hepatomegaly Bone pain Paralysis C) SPECIAL INVESTIGATIONS: LABORATORY TESTS: Raised urinary levels of catecholamine’s (U-VMA), produced by adrenal medulla RADIOLOGY: Ultrasound to confirm diagnosis if abdominal mass MRI to define anatomy of the tumour for adrenal, paraspinal and pelvis tumours CT chest for mediastinal tumour MIBG (Radio-isotope scan), sometimes used and can demonstrate the primary tumour and bony metastases. D) MANAGEMENT: Depends on the stage of the patient: Low risk – complete surgical excision Intermediate risk – surgery and chemotherapy High risk – intensive chemotherapy and surgery +/- radiation therapy. LIVER TUMOURS 2 % of childhood cancers Mostly malignant 1/3 are benign lesions and include haemangio-endothelioma’s. 2 most common malignant liver tumours are: Hepatoblastoma (HPB) Hepatocellular carcinoma (HCC) Hepatoblastoma A) EPIDEMIOLOGY: B) CLINICAL FEATURES: C) SPECIAL INVESTIGATIONS: D) MANAGEMENT: More common (90% of liver tumours) Younger children (peak 2 years) Associated with: Beckwith-Weideman syndrome Low birth weight Better prognosis (80% 5-year survival) Abdominal mass Systemic symptoms: malaise Hepatocellular carcinoma (HCC) Less common (10%) Older children Risk factors: Hepatitis B infection Chronic liver disease 15% 5-year survival LAB TESTS: Anemia (low HB) Thrombocytosis Raised alpha-feto protein levels (tumour marker) RADIOLOGY: Ultrasound MRI CXR for metastases Pre-operative chemotherapy Excision of the tumour if possible HCC has a poor response to chemotherapy and thus worse prognosis RHABDOMYOSARCOMA A malignant tumour arising from mesenchymal cells with the potential for differentiation into skeletal muscle Rare Peak in early childhood an early adolescence Prognosis is best if the mass is <5 cm ANY FIRM, SOFT TISSUE MASS = URGENT REFERAL!! DIAGNOSIS: Imaging (Ultrasound/MRI) Histology (Biopsy) MANAGEMENT: Neo-adjuvant chemotherapy, surgery, chemotherapy +/- Radiation Therapy SACRO-COCCYGEAL TERATOMA Teratomas arise from 2 or 3 germ cell lines. Common locations are: Sacro-coccygeal (most common site in neonates), neck, mediastinal and gonads. The following will only discuss sacro-coccygeal teratoma A) EPIDEMIOLOGY: 2nd most common neonatal tumour F>M B) CLINICAL FEATURES: Different types: Everything outside (see photo) Some inside (pelvis) some outside Pelvic mass only Can be diagnosed antenatal Large lesions can cause problems antenatal which might lead to early “elective” caesarian section Most present with mass after birth – mass can bleed and cause ulcerations Intra-pelvic sub-type may present later C) SPECIAL INVESTIGATIONS: Ultrasound pre-op: Investigate for intra-pelvic extension Alpha-feto Protein – a chemical marker for follow up purposes Pre-operative bloods including a group and screen (blood is needed for the surgery) D) MANAGEMENT: Complete surgical excision is curative Surgery, as soon as possible to prevent bleeding into the tumour and tumour rupture May include surgical reconstruction of the anteriorly displaced anus and surrounding muscle E) PROGNOSIS: Good prognosis May have constipation and/or urinary problems later in life (rare) PAEDIATRIC UROLOGY INGUINAL HERNIA’S A) DEFINITIONS: Hernia: Protrusion of an organ from the cavity where it normally resides, through a defect in the wall of that cavity. Inguinal hernia: Protrusion of abdominal contents into the inguinal canal. Processes vaginalis: Is an embryonic out-pouching of peritoneum within the groin along the path of testicular descent. At birth, 80% of males have a patent processus vaginalis. This decreases to 40% at 2 years and 20% in adults. B) PATHOPHYSIOLOGY: 99% of groin hernias in children are due opened processus vaginalis (PPV) leading to an indirect inguinal hernia. The content of the inguinal hernia sac (which is the PPV) can include: o Small or large bowel (most commonly) o Fallopian tube and, uterus or ovary in girls o Appendix may be present o The bladder (a sliding hernia) A PPV that allows fluid from abdominal cavity to accumulate around the testis within the PPV, is called a fluid hernia or hydrocoele. Incidence: 30% of premature infants; 50% present < 1 year (highest risk < 6 months) Twice as common on the right side 10% bilateral Male: female ratio 5-10:1 Neonates and premature babies have a higher risk of complications. The age and size of the child is not a valid reason to delay surgical repair. C) CLINICAL PRESENTATION: History: There is usually a history of a mass in the inguinal (groin) area which gets bigger with coughing or crying A reliable history from the mother is often the only symptom Symptoms of bowel obstruction (vomiting, constipation ect.) may be present if complicated Clinical findings: Look: Swelling in groin – reducible or not reducible Tender, red, swollen: strangulated hernia (due to decrease in blood supply) Feel: Swelling in the groin and/or scrotum Thickening of the spermatic cord "Silk sign" - feels as if two pieces of silk are rubbed together; indicative of fluid in the processes vaginalis sac. Palpation of bowel in the hernia Cannot get above it mass (differentiates from hydrocoele) intra-abdominal pressure on crying increases size of hernia Position of testis: distinct from hernia mass and able to feel testis(differentiates from other acute scrotal pathology e.g. testicular torsion, hydrocoele) Listen: NB! Presence or absents of bowel sound IS NOT used to make diagnosis in children D) DIFFERENTIAL DIAGNOSIS: Hydrocoele (can get above the mass) Lymphadenitis or inguinal abscess (mass is below the inguinal ligament) Testicular torsion (testicular swelling only, not inguinal-scrotal) Undescended testis Hydrocoele of the cord Buried penis Varicocoele E) COMPLICATIONS: Obstructed (incarcerated) hernia: A hernia with contents persisting outside the abdominal cavity is obstructed If it cannot be manually pushed back into the abdominal cavity it is irreducible The smaller the child the higher the risk. In neonates more than the third become complicated Pathogenesis: The obstructed bowel swells and develops oedema and bowel obstruction develops Should the intra-luminal pressure exceed the systolic blood pressure, it may affect the blood supply of the bowel wall and full thickness necrosis may ensue. Clinical signs: Sudden onset Severe pain Hard mass in inguinal area Intestinal obstruction with vomiting You cannot get above the mass Strangulated hernia: An obstructed hernia in which ischaemia ± necrosis develops Clinical signs: Pain Redness Tenderness Oedema of overlying skin ± abdominal wall Treatment: Requires urgent resuscitation and surgery Intestinal obstruction: Obstructed inguinal hernia is one of the 2 most common causes of small bowel obstruction. Fluid lost by nausea and vomiting as well as into third space may lead to dehydration and shock. An incarcerated hernia which contains an ovary or fallopian tube may strangulate but will not have signs of bowel obstruction Septicaemia and shock: Bacterial translocation may lead to septic shock Full thickness necrosis: Ischaemia and infarcted bowel must be resected and repaired by means of an end to end anastomosis Testicular infarction: Testicular infarction may occur in 10% of obstructed hernias. It appears as the testicular atrophy later. F) MANAGEMENT: Uncomplicated inguinal hernia: “Urgent elective" surgical repair (the chances of spontaneous resolution of a hernia are extremely low and a considerable risk of complications exists). The operation of choice for hernias in children is a herniotomy Incarcerated or obstructed hernia: Manuel reduction in the ward Sedation is given (e.g. 1mg/kg ketamine) The hernia may reduce spontaneously with gentle manipulation of the scrotum or scrotal neck (“gentle taxis”) Surgery is carried out as an emergency if conservative management has been unsuccessful Should the reduction be successful, surgery is carried out up 24 to 48 hours later when the swelling and oedema have subsided Strangulated hernia: A strangulated hernia with necrotic bowel is a surgical emergency as the mortality is high These patients require active resuscitation with intravenous fluid, broad-spectrum antibiotics, and correction of electrolyte deficits. Once resuscitation is completed emergency surgery is carried out At surgery, the surgeon will resect ischaemic bowel if present and perform at primary anastomosis A herniotomy is performed Complications of inguinal hernia repair: Surgical complications (<1%): injury to vas deferens & testicular vessels injury to bladder wound infection haematoma recurrence Anaesthetic complications: high risk of post-operative apnoea in premature infants <60 weeks corrected gestational age (=gestational age + age since birth in weeks); require 24-48h postoperative apnoea monitoring ± caffeine to decrease risk HYDROCOELE A) DEFINITION: A hydrocoele has the same congenital defects as in inguinal hernia, an open processes vaginalis. The opening is so small and only fluid can pass into the scrotum but bowel and omentum are prevented from doing so. It is possible to get above the swelling which itself is round and transilluminates well. B) CLINICAL PRESENTATION: Asymptomatic swelling Cystic Non-tender Usually non-reducible Spermatic cord palpable above Transilluminates well (NOT absolute for dx) C) MANAGEMENT: 50% of congenital hydrocoele's will close in the first two years of life. There is little reason to operate before this time Operation may be indicated if the diagnosis is uncertain for in the event of a secondary hydrocoele under these circumstances testicular pathology must be borne in mind. UNDESCENDED TESTES (UDT) A) DEFINITION: Undescended testis: The testis is not present in the scrotum. They can further be classified into palpable and non-palpable UDT. Palpable testis: The testis is not present in the scrotum, but is palpable in the inguinal canal. Non-palpable testis. The testis is not present in the scrotum and is not palpable in the inguinal canal. They can be either intra-abdominal or there can be no testis (atrophic) on that side. Retractile testis: This is not an undescended testes. They have a normal length of spermatic cord with a strong cremasteric reflex that “pull’s the testis up” out of the scrotum on gentle palpation. You will be able to bring the testis down into the scrotum and they usually have a normal developed hemiscrotum B) CLINICAL PRESENTATION: No testis palpable in the scrotum (can be unilateral or bilateral) Testis may be palpable in the inguinal canal Examine both sides. NB! To examine the entire perineum and to comment on the: o Genitalia (normal/hypospadias/chordee etc.) o Scrotum (undeveloped hemi-scrotum present esp. in not palpable UDT) o The contra-lateral testis o Absence/presence of inguinal hernia’s or hydrocoele’s Always consider DSD (differences in sexual differentiation) in infants with uni/bilateral UDT with abnormal genitalia. Clinical examination is the gold standard. No ultrasounds/MRI are needed to make the diagnosis. If unsure, refer patient. C) MANAGEMENT: Refer patient to a paediatric surgeon if no testis palpable in the scrotum by 6 months. Timing for surgery is still debateable but should be done between 9 – 18 months if possible. Palpable UDT: Open orchidopexy Non-palpable UDT: Laparoscopic localisation first followed by ; o If no testis is present, it is atrophic/vanishing testis o If testis is present, the testis is brought down either via a staged procedure. D) COMPLICATIONS: It is important to bring the testis down into the scrotum for the following reasons: 1. Cosmesis 2. Infertility can occur as a complication (i.e. higher temperature in the abdomen) 3. Cancer can occur as a complication (i.e. easier to palpate in scrotum) 4. Lower risk of torsion TESTICULAR TORSION A) DEFINITION: Testicular torsion is a SURGICAL EMERGENCY! There are two anatomical types: Extravaginal (perinatal): twist is outside the tunica (covering of the testis) at the level of the spermatic cord. Intravaginal (<3 years or > puberty): twist is within the tunica due to the highly mobile testis: “bell-clapper” defect. B) CLINICAL PRESENTATION: Perinatal torsion: Occurs due to events prenatally (75%) or post-natal. Prenatal Torsion Usually noted at birth Hard Non-tender scrotal mass Underlying skin discoloration Mass fixed to the overlying skin Surgery is to remove necrotic tissue Post-natal Torsion Normal scrotum at birth Acutely inflamed scrotum Red (erythema) Tender Surgery ASAP! To detort and fix the testis Torsion outside of neonatal period: Occurs commonly in children < 3 years or after puberty Acute scrotum (red, tender, erythematous) – sudden onset of pain Testis might lie high and horizontal Vomiting Fever might be present C) DIAGNOSIS: 1. Clinical diagnosis justifies an early intervention 2. Doppler ultrasound, might be necessary, if history is > 6 hours If increased Doppler flow: infection e.g. epididimo-orchitis If decreased Doppler flow: Torsion If no Doppler flow present: Testis already necrotic D) MANAGEMENT: Testicular torsion is a surgical emergency. Surgery needed ASAP within 6 hours to “save” the testis. After 6 hours: only Leydig cells might function and continue to produce testosterone Surgery: Detorsion of the affected side. Orchidopexy OR orchidectomy (depending on the testicular viability) Fix of the contralateral side to tunica HYPOSPADIAS Hypospadias (from Greek: hypo – under, spadias – rent) is the most common congenital abnormality of the penis. THE 3 COMPONENTS OF HYPOSPADIAS ARE: 1. URETHRAL MEATUS IN AN ABNORMAL PLACE 2. CHORDEE OF THE PENIS (DOWNWARD CURVATURE) 3. VENTRAL DEFICIENCY OF THE PREPUCE (HOODED PREPUCE/FORESKIN) A) EPIDEMIOLOGY: 1 in 300 males Familial risk More common in Caucasians as well as Jewish families B) CLINICAL FEATURES: Evaluation of hypospadias should include: General examination Antenatal history Specifics of the hypospadias: Position of the meatus Degree of chordee Appearance of the prepuce Appearance of the scrotum Testicular position NB! Neonatal bilateral undescended testis PLUS hypospadias = Emergency Endocrine consult, in case of enzymatic adrenocorticoid inadequacy DISORDERS OF SEXUAL DIFFERENTIATION (DSD) A) DEFINITION: Any abnormal development of chromosomal, gonadal or anatomical sex. Previously known as ambiguous genitalia, intersex or hermaphroditism. B) CLINICAL FEATURES: Abnormal genitalia at birth Bilateral undescended testis Short phallus Hypospadias Labial-scrotal fusion C) SPECIAL INVESTIGATIONS: Electrolytes measurement Karyotype Mullerian inhibiting factor levels Artificial stimulation of testosterone Ultrasound of the pelvis and urogenital tract Referral to Paediatric surgery/Endocrine HEAD AND NECK MASSES 1. THE PHARYNGEAL APPARATUS A) DEFINITION: Congenital remnants of the embryological branchial apparatus that forms most of the structures of the head and neck Branchial anomalies represent 30% of congenital neck masses, present as cysts, sinuses, fistulae or cartilaginous remnants B) EMBRYOLOGY: Head and neck structures develop from the branchial apparatus (“gill” equivalent) which normally develops between 4-8 weeks after fertilization Four pairs of branchial arches dominate the lateral cervicofacial area Fistulas are more common than sinuses in children, cysts appear later in childhood. C) CLINICAL PRESENATION: External opening along the anterior border of sternocleidomastoid muscle, junction of lower & middle thirds Second branchial cleft anomalies represent 95% of all branchial cleft anomalies, 10% of these can be bilateral D) MANAGEMENT: Complete excision: follow the tract with care to protect spinal accessory, hypoglossal and vagus nerves Elective excision only when anomaly is not infected If infected: antibiotics +/- aspiration of abscess 2. THYROGLOSSAL DUCT CYSTS A) DEFINITION: Foramen Caecum Congenital midline cystic neck mass as a result of incomplete closure of thyroglossal duct 7% of population have thyroglossal duct remnants B) EMBRYOLOGY OF THE THYROID GLAND The foramen caecum (in posterior third of tongue) is the site of development of the thyroid diverticulum Thyroglossal As the tongue develops the thyroid diverticulum descends into the neck cyst Thyroid gland Thyroid gland develops between weeks 4 – 7 of gestation, and descends into the pretracehal position The thryoglossal duct passes just in front/ behind/ middle of the hyoid bone Normally the thyroglossal duct disappears by 5 -8 weeks of gestation If this thyroglossal tract fails to obliterate a cyst can develop anywhere along the migratory course C) CLINICAL PRESENTATION: Painless midline neck cyst Usually presents >3 years of age The cyst moves upward on tongue protrusion, as well as with swallowing (due to its lingual and thryoid attachments) One-third of cysts may become infected; if this forms an abscess which drains spontaneously or is incised surgically, a fistula may develop D) DIFFERENTIAL DIAGNOSIS OF A MIDLINE MASS: Thyroid related masses (80%) o thyroglossal duct cyst o ectopic thyroid tissue o nodule in pyramidal lobe of thyroid Dermoid cyst Lymph node o Submental (suprahyoid): look for mouth/ pharynx pathology Thyroglossal E) MANAGEMENT: Sistrunk procedure, which consists of: Complete excision of cystic mass Excision of the middle 1/3 of the hyoid bone Excision of thyroglossal tract upward to base of tongue 3. STERNOCLEIDOMASTOID TUMOUR ("Fibromatosis Colli") A) DEFINITION: Congenital fibrous mass within the sternocleidomastoid muscle It result in torticollis (tilting of the head) B) PATHOPHYSIOLOGY: Unknown Hypothesis that it results from birth trauma (e.g. haematoma after breech birth) is not supported by clinical data Histology suggests it is a fibromatosis (benign, usually self-limiting inflammatory condition starting prenatal since fibrosis already present at birth) C) CLINICAL PRESENTATION: Usually unilateral, firm, non-tender mass within the middle of the sternocleidomastoid(SCM) muscle Usually presents 3 - 6 weeks after birth Leads to torticollis due to fibrosis and shortening of SCM, face & chin rotated away from affected side and head tilted toward the ipsilateral shoulder D) DIFFERENTIAL DIAGNOSIS: Neonatal solid neck mass Neoplasm e.g. rhabdomyosarcoma Torticollis Cervical hemi-vertebrae Neurological disorder Eye pathology (e.g. astigmatism/squint) Gastroesophageal reflux Otolaryngeal infection E) SPECIAL INVESTIGATIONS: Usually clinical diagnosis Ultrasound differentiates it from malignant muscle growths and demonstrates focal/ generalized SCM enlargement F) MANAGEMENT: Physiotherapy o Passive & active stretching exercises (at each diaper change). o Follow up monthly until lesion disappears o Majority (> 90%) improve within 6 months Surgery o Lateral neck incision o Fasciotomy of superficial & deep neck fascia anterior to midline & posterior to anterior border of Trapezius muscle (with continued post-operative physiotherapy) is only rarely needed, if: Facial hemiplasia No improvement with exercises Late diagnosis (>9 months) Progressive fibrosis G) COMPLICATIONS: Torticollis Long-term complication is plagiocephaly (facial asymmetry due to facial hemihypoplasia) from persistent rotation of head with face and chin looking away from the affected side 4. RANULA A) DEFINITION: Mucous retention cyst (mucocele) B) PATHOPHYSIOLOGY: Possibly trauma to salivary gland (usually minor gland) C) CLINICAL PRESENTATION: Cystic lateral mass under tongue in floor of mouth (“simple ranula") Uni-lateral Shiny in appearance Enlarges gradually Cervical ranula (plunging or diving ranula) may present as submandibular swelling D) DIFFERENTIAL DIAGNOSIS: Dermoid cyst Sublingual duplication cyst Cystic teratoma Lymphatic malformation E) MANAGEMENT: Marsupialization of cyst (incision into cyst and suturing the edges of slit to the exterior surface of cyst keeps incision open to drain) 5. DERMOID/ EPIDERMOID CYSTS A) DEFINITION: Congenital cyst containing dermal or epidermal elements B) PATHOPHYSIOLOGY: Inclusion of epidermal/dermal elements under the skin during embryonic development C) CLINICAL PRESENTATION: Most dx before 3 years Slow-growing cystic swelling Common sites: o Lateral angle of eyebrow – “external angular dermoid" o Anterior fontanelle o Midline of face/neck (distinguished from thyroglossal duct cyst when no cephalad movement when tongue protrudes) o Occiput o Over a suture line of scalp, may communicate inside the skull (dumb-bell tumour)) External angular Dermoid cyst D) SPECIAL INVESTIGATION: Ultrasound if fontanelle still open, to exclude intracranial extension MRI or CT will show the intracranial component of a dumb-bell lesion E) MANAGEMENT: Excision of cyst is treatment of choice 6. LYMPHATIC MALFORMATIONS (LYMPHANGIOMA/CYSTIC HYGROMA) A) DEFINITION: Congenital cystic malformation of lymphatic vessels; cervical lesions are traditionally known as “cystic hygromas” B) PATHOPHYSIOLOGY: Failure of developing lymphatic ducts to establish drainage into venous system C) CLINICAL PRESENTATION: Antenatal ultrasound: Many have associated abnormalities e.g. chromosomal defects Cystic swelling: o 75% in neck, usually laterally in region of primary lymphatic sac o 20% in axilla o 5% anywhere in the rest of the body o 50-60% present at birth (90% within first two years) o Mostly isolated lesions but can be associated with capillary vascular malformations On examination: o soft, mobile painless mass o red or blue colour if bleeding occurred (rapid, dangerous increase in size) D) SPECIAL INVESTIGATIONS: o o Chest x-ray to exclude thoracic extension Ultrasound or MRI scan to define anatomy and exclude cystic teratoma E) MANAGEMENT: Surgery: Excision may be difficult as microcytic lesions infiltrate in between vessels and nerves resulting in difficult surgical excision and a high risk of recurrence. Mutilating surgery is avoided for this benign lesion Sclerotherapy: Most common therapy; fluid is aspirated (usually under sonar guidance) and an inflammatory agent in injected into cysts to cause adhesions between cyst walls (e.g. Bleomycin) Usually requires multiple treatments but up to 80% success Complications: Infection common leading to sudden increase in size, fever, cellulitis and oedema of skin Sudden increase in size may result in respiratory distress The tongue may swell and complicate feeding, speech etc. F) PROGNOSIS: generally good recurrence incidence of 10-15% 8. CERVICAL LYMPHADENOPATHY IN CHILDHOOD A) DEFINITION: Pathologically enlarged lymph nodes (LN) in the neck B) CLINICAL PRESENTATION: Incidence: Very common, esp. infective causes History: Reactive lymph node hyperplasia (infectious cause) Usually days to weeks Danger signs: o LN in posterior triangle or supra-clavicular region o Single dominant node present >6weeks o Persistent lymphadenopathy despite appropriate antibiotics and present >6weeks o Node larger than >2cm o Fixed nodes Ask about: Recent upper respiratory tract infection (usually viral) Household tuberculosis (TB) contact Systemic symptoms: night sweats, weight loss (TB) Travel history: cat scratch disease Malignant node Rapid growth Systemic symptoms: fever of unknown origin Short history (days to weeks) Clinical examination: General examination Look for lymphadenopathy in other regions and visceromegaly of other organs esp. of reticulo-endothelial system (liver, spleen): suggestive of viral infection or lymphoma Look for infectious lesions in drainage area of lymph nodes E.g. inflamed tympanic membrane, pharynx or tonsils if anterior cervical triangle nodes esp. submandibular nodes E.g. scalp skin lesions if posterior cervical triangle nodes e.g. eczema, tinea capitis, scratches etc. Local examination Location: Multiple mobile nodes in anterior triangle : usually infectious Large solitary posterior triangle node esp. supraclavicular: suggests lymphoma Multiple matted nodes suggests TB or lymphoma Size >2cm: Possibility of lymphoma Hard painless fixed mass again suggests lymphoma Tenderness indicates infective origin (reactive lymphadenopathy) Lymphadenitis with redness and tenderness of overlying skin often progresses to suppuration (=abscess formation) C) DIFFERENTIAL DIAGNOSIS: Acute suppurative lymphadenitis: Especially submandibular nodes Very common in 3-6 months age group Inflamed overlying skin; very tender with pyrexia Abscess formation occurs within a few days Treatment: Antibiotics for lymphadenitis and cellulitis: Cloxacillin or 1st generation cephalosporin for skin lesions (staphylococcus) Amoxycillin/clavulanic acid or Erythromycin for upper respiratory tract/ ear, nose & throat infections (streptococcus, haemophilus) Drainage of pus once fluctuant (open surgical drainage under conscious sedation in ward usually required) Chronic lymphadenitis: Reactive hyperplasia – virus Granulomatous diseases: TB very common and must be excluded with gastric washings/ induced sputum/ PCR (GeneExpert™ ) & Mantoux skin test in all Other specific infections – toxoplasmosis, cat scratch disease (North America), atypical Mycobacterium (other than tuberculosis) Treatment: Trial of antibiotics (14 days) INFANTILE HAEMANGIOMAS A) DEFINITION: A benign vascular tumour that develop in skin, subcutaneous tissue or an organ. Not visible at birth, but they begin to appear by first two to six weeks of a child’s life. (If it is visible at birth it is not an infantile haemangioma but a congenital haemangioma which is different) All skin haemangiomas will be visible by six months of age. B) PATHOPHYSIOLOGY: Incidence: They are more common in: o Girls (5:1 ration) o Caucasian children o Premature infants Types: There are three general types of infantile haemangiomas: Superficial haemangiomas occur on the outer layers of the skin and are typically bright red to purple in colour. Deep haemangiomas, which grow under the skin in the fat, may be blue, purple or even skin colour (if they are deep enough under the skin surface). Mixed haemangiomas are the most common type of haemangioma. These haemangiomas have both superficial and deep components Cause: The cause is unknown. GLUT-1 (an erythrocyte type glucose transporter) indicates medical response to therapy Contains many oestrogen receptors Natural history: Infantile haemangiomas grow rapidly for the first few weeks or months (rapidly growing phase) They then enter a rest phase by about 8 months of age (plateau phase) And they usually begin to shrink around 1 year of age (involution phase). As the lesion shrinks, the colour may change from red to purple and grey. It may take several years for the haemangioma to go away completely. Larger lesions take a longer time to go away and have a greater chance of scarring. C) CLINICAL PRESENTATION: Most often cutaneous (80%) o Most common in head and neck area, followed by thorax and the extremities Not fully developed at birth, first appear around 2 - 6 weeks of age Multiple lesion in 20 % of cases o Internal haemangioma’s need exclusion (liver or gastrointestinal tract) o Increased risk if > 5 lesions present After Before D) SPECIAL INVESTIGATIONS: Rarely required Doppler US can be useful to check blood flow through the birthmark to help distinguish it from other vascular lesions. An additional benefit is to assess phase of haemangioma (? involution). Skin biopsy MRI could help to make the diagnosis if the lesion is not typical in its appearance E) MANAGEMENT: Most infantile haemangiomas will shrink over time, require no treatment other than reassurance of the parents Regular follow-up and photographic evidence/documentation Whether to treat a haemangioma is determined by a number of factors, including: o Age o Size of the haemangioma o Location of the haemangioma o How rapidly the haemangioma is growing o Complications: ulceration, bleeding or high-output heart failure Treatment options for infantile haemangiomas include: 1. Observation 2. Propranolol A non-blocker The mechanism of action is unclear. It is hypothesized that it induces vasoconstriction, resulting in colour change and palpable softening of the haemangioma, even within 24 hours of treatment, or might cause down-regulation of growth factors, such as vascular endothelial growth factor, and up-regulation of cellular apoptosis. Propranolol is generally safe but occasionally cause adverse events: o Bronchospasm o Bradycardia o Hypotension o Hypoglycemia 3. Oral corticosteroids during growth period blocks oestrogen receptors do not usually shrink haemangioma but control its growth 4. Surgery (scar left from an excision may be worse than results from spontaneous regression) Used most frequently to remove remnants (fibro-fatty tissue) Early excision reasonable option rarely: o where haemangioma threatens life or bodily function o well localized pedunculated tumours complicated by bleeding or ulceration o upper eyelid haemangioma that obstructs vision despite pharmacologic therapy Re-evaluation is recommended 5. Topical beta blockers control growth of small superficial haemangiomas, particularly on the eyelid and around the mouth 6. Intra-lesional corticosteroids Small ulcerating lesions of eyelid, nose, cheek or lip 7. Laser therapy Only for residual telangiectasias that remain after involutes phase is complete F) COMPLICATIONS: Approx. 25 % of haemangiomas will have a complication: Interference in anatomical distribution Rapid growth around eye may cause visual axis obstruction (cortical blindness) and astigmatic amblyopia. Cervicofacial region lesions can lead to airway obstruction Facial lesions cause tissue destruction and cosmetic problems Liver lesions can cause high output congestive heart failure, abdominal compartment syndrome or hypothyroidism (unknown aetiology) Ulceration around diaper area Gastro-intestinal bleeding Ulceration Breakdown of skin overlying the haemangioma. Associated with pan, poor feeding and irritability. Increases the risk of infection and scarring. Haemangiomas that are located around the mouth, nose, ear or the skin under the diaper carry a higher risk of ulceration. Ulcerations heal slowly. Bleeding If a haemangioma is cut or injured, it can bleed or develop a crust or scab. Bleeding tends to be self-limiting. It might be severe in rare cases that the number of platelet number is reduced (Kasabach-Merritt syndrome) SURGICAL JAUNDICE A) Definition: Clinical signs and symptoms of jaundice with conjugated hyperbilirubinemia. Signs and symptoms: Pale stool, dark urine, pruritis and hepatomegaly. Splenomegaly is suggestive of systemic infection, Cirrhosis and portal hypertension will develop later in disease process. Normal values for bilirubin are 3-20umol/L and conjugated < 5umol/L. Pathological jaundice: Prolonged jaundice > 2 weeks in term infants, or > 3 weeks in premature infants. Conjugated hyperbilirubinemia: Conjugated bilirubinaemia > 34umol/L or conjugated fraction >20% of the total bilirubin. B) Bilirubin metabolism: Bilirubin is a metabolic by-product of red cell breakdown. Haemoglobin is converted to bilirubin (enzyme: haem oxygenase via biliverdin) which is transported on albumin to the liver in an unconjugated fat-soluble form. Hepatocyte uptake occurs with conversion to watersoluble form by conjugation with glucuronic acid. This is then excreted into the biliary ducts. Further changes occurs in the ileum to urobilinogen, absorbed and re-excreted in urine and in the colon, stercobilinogen – excreted. C) Causes of unconjugated (medical) jaundice Physiological jaundice from liver enzyme immaturity ABO & Rhesus incompatibility Breast milk jaundice Congenital enzyme defects e.g. Gilbert syndrome D) Causes of conjugated jaundice (surgical or medical): Surgical: Biliary atresia Obstructed cystic choledochal malformation Older children: bile duct worms, stones or tumour compression Medical: Alpha-1-antitrypsin deficiency – a rare liver and lung protein retention Hepatitis (unknown aetiology, cytomegalovirus infection, herpes or congenital syphilis) Toxoplasmosis infection (Cat origin) Cystic fibrosis (inspissated bile in the bile duct) Total Parenteral nutrition(unknown aetiology) Alagille’s syndrome (biliary hypoplasia, abnormal vertebrae, pulmonary stenosis, odd facies) Galactosaemia (defect in enzyme converting galactose into glucose-galactose-1phosphate uridyl transferase E) Special investigation for conjugated jaundice: a. TORCHES: Toxoplasmosis, rubella, cytomegalovirus, herpes simplex, syphilis b. Liver function test: Expect to see high conjugated hyperbilirubin, gamma-glutamyl transferase and alkaline phosphatase c. Serial stool samples: cut stool to look for hypopigmented area in centre of the sample d. Ultrasound: To exclude a choledochal malformation or mass lesions causing extra-hepatic obstruction. e. Intra-operative cholangiogram: This remains the gold standard in excluding biliary atresia. f. Percutaneous liver biopsy: Invasive and often confusing result g. HIDA Scan (hepatobiliary iminodiacetic acid): nuclear scan rarely done F) Management: Conjugated jaundice – surgical causes will be discussed according to the cause in the following pages. Be aware of Vitamin K deficiency and its effect on coagulation pathways, when bile is not present in small bowel to emulsify fatty material. Biliary Atresia A) DEFINITION: Progressive obliterative extra-hepatic cholangiopathy at about 4-weeks of age B) INCIDENCE: Incidence 1:15000 live births, more frequent in Eastern hemisphere C) AETIOLOGY: Uncertain Several distinct groups have been identified. Each group differ in their presentation and progression of disease. o Isolated biliary atresia (+/-75%): Majority of cases, no cause identified. o CMV-associated biliary atresia (Incidence higher in low income countries): Present earlier with poor prognosis despite early Kasai porto-enterostomy. o Cystic Biliary atresia (5%): Present later with overall better prognosis. o BASM/Biliary atresia splenic malformation syndrome (10%): Associated with maternal diabetes and cat-eye syndrome. Found at surgery to have splenic malformations, e.g. asplenia, polysplenia or splenuncles. D) CLASSIFICATION (read only): Classification according to most proximal level of obstruction Type 1 (+/-5%) – common bile duct Type 2 (+/- 3 %) – common hepatic duct Type 3 (>90%) – obstruction within porta hepatis) E) SURGERY – Kasai portoenterostomy: Aim – to excise all obliterated extrahepatic remnants and anastomose jejunal loop onto port hepatis (illustrated schematically) F) PROGNOSIS: 30% will have established good bile drainage following Kasai porto-enterostomy (best change if surgery is performed earlier) 40% will have a bridge to a liver transplant later in life 30% will require early liver transplant G) COMPLICATIONS: Ascending cholangitis (if bile drainage is established by surgery) Poral hypertension Choledochal malformations A) PATHOGENESIS Congenital dilatation of bile ducts. Cause unknown, thought to be due to pancreaticobiliary malunion, this abnormal junction of pancreatic and biliary ducts outside the duodenal wall allows free intermixing of pancreatic juice and bile. This can cause pancreatitis and biliary dilatation and later malignancy. More common in females and in Eastern hemisphere. B) CLINICAL PRESENTATION Older children can present with the classic triad (30% of cases) of jaundice, abdominal pain and a RUQ mass. Half of cases can be diagnosed on antenatal ultrasound. C) INVESTIGATIONS Ultrasound: biliary dilatation and stone formation MRCP anatomical definition ERCP is difficult in paediatric due to size of the bile ducts D) CLASSIFICATION (read only) Classified due to the appearance of the extrahepatic dilatation and presence of intrahepatic ducts are involved. Caroli’s disease: multiple, saccular dilatations of the intrahepatic bile ducts. Caroli’s syndrome choledochal malformation associated with cystic renal pathology and liver fibrosis. Todani classification: Types 1 – 5. E) SURGERY Excision of extra-hepatic biliary tree with a Roux loop reconstruction, usually at the level of the hepatic duct/ hepatico-jejunostomy of hepatico-duodenostomy. Stone should be washed out of intra-hepatic bile ducts before completion of anastomosis. Transduodenal sphincterotomy may be attempted for choledococoeles/type 3. Lilly procedure, anterior incision of cyst with removal of internal lining, may be necessary if complete excision is impossible. F) COMPLICATIONS Recurrent cholangitis or pancreatitis Perforation Stone formation Biliary cirrhosis Portal hypertension Cholangiocarcinoma (later) Biliary Ascaris (rare) A) PRESENTATION Jaundice, biliary colic, ascending cholangitis and micro abscesses of the liver. Ultrasound can demonstrate moving worms within the biliary tree. B) MANAGEMENT Symptomatic treatment during the acute phase includes keeping the child NPO and continuing IV Fluids. This is continued until worms spontaneously leave the biliary tree. Albendzole or Mebandazole is only given after worms have moved, otherwise they die in the bile ducts and require surgical removal. Hydatid disease Echinoccocus granulosis is a 2-3mm flat worm which adheres itself to the mucosa of the proximal foregut of the definitive host (usually dogs). The adult worm, which contains both male and female organs, then releases eggs every few months which get excreted in the faeces. The intermediate host (i.e. sheep) grazing in the vicinity can ingest the ovum. Hatched egg, called oncosphere, are released from the egg and immediately migrate to other organs, via venous or lymphatic vessels, throughout the body after transiting through gut mucosa. Most commonly lodge in the liver or lung and are then called hydatid cyst (derived from Greek word; Hydros, meaning water-like cyst). A carbohydrate wall appears rapidly around the oncosphere which minimizes immune response to the antigen that contains the cellular membrane of the parasite. When the animal is slaughter by humans, dog(s) ingest the infected organs. Hydattis cysts contain pre-scolices (miniature worm prepared to become adult worms). Once ingested, the worms mature rapidly and these scolices adhere to dog mucosa, by hooks and suction cups. Further development occurs and they are ready for egg shedding 6 months later. The life span of the worms is about 2 years but hydatid cyst in sheep can live for many years and hence the evolutionary advantage of a long-term available reservoir in the intermediate host. The parasitic infection is rarely fatal for the dog or the sheep. Humans become incidentally infected by eating food or drinking water that is contaminated with the eggs in dog faeces. The parasitic infection is most often seen in rural South Africa (or other countries) where there is constant contact between sheep and dogs in the area. Deworming of domestic dogs and roaming dogs in the area by farmers is not possible. Human liver hydatid cysts have different appearances depending on their age. Two typical cysts look like the following pictures on a sonar examination of the liver: Management: An enlarging liver cyst causes pain by stretching the liver capsule. First line of treatment is by oral administration of anti-worm medication, such as albendazole, for many months. This class of medication, is cheap and it works by inhibiting glucose transport in the worm. However it also can cause malnutrition in humans and the patients need to be followed up in a clinic on a monthly basis. Calcification of the cyst is considered to be due to the death of the cyst and its inner structure. This rarely occurs and further intervention is often required. If the cyst continues to enlarge despite medical treatment, the next step is percutaneous injection of the cyst by scolicidal agent, such as absolute alcohol or hypertonic saline. The last option, e.g. after failure of injection is laparotomy and open excision of the cyst lining and its content. One must avoid rupturing of the cyst during handling as human can have intense reaction to protein content of the cyst and cause fatal anaphylaxis reaction. Outcome: Overall, prognosis is excellent and rare cases of mortality is due to spontaneous or accidental rupture of the cyst. FORESKIN AND CIRCUMCISION ANATOMY Musculature The prepuce has a sheath of smooth muscle tissue inside the skin which is called the peripenic muscle. The muscle fibers are arranged in a whorl at the end of the foreskin to form a sphincter. The muscle fibers keep the foreskin snugly against the glans penis. Skin and mucosa The outer surface of the prepuce is skin, however the inner surface is mucosal membrane although it resembles skin in appearance. There is a muco-cutaneous boundary just inside the tip of the prepuce. The prepuce normally covers the glans penis and protects it from foreign matter, friction, drying, and injury. Sub-preputial moisture The sub-preputial area is normally slightly moist. There are apocrine glands that produce cathepsin B, lysosyme chymotrypsin, neutrophil elastase, cytokine, and pheromones such an androsterone. Prostatic, vesicular, and urethral secretions also contribute moisture. Moisture may also be exuded through the mucosa of the foreskin. The sub-preputial moisture contains lytic material. Lytic material has an anti-bacterial and anti-viral action. The natural oils lubricate, moisturize, and protect the mucosal covering of the glans penis and inner foreskin. Frenulum The prepuce is usually tethered at the bottom by the frenulum. The frenulum's function is to provide pleasure by stretching during sexual intercourse. The frenulum is known as the "sex nerve" .By destroying this stretching action, circumcision completely destroys this fundamental means of sexual pleasure in the human male. It is hypothesized that stretching of the frenulum during coitus provides a stimulus for ejaculation. Nerve supply The pudendal nerve originates in the sacral plexus; it derives its fibers from the ventral rami of the second, third, and fourth sacral nerves (S2, S3, S4). FUNCTION OF THE FORESKIN Cover and bond with the synechia so as to permit the development of the mucosal surface of the glans and inner foreskin. Protect the infant's glans from faeces and ammonia in diapers. Protect the glans penis from friction and abrasion throughout life. Keep the glans moisturized and soft with emollient oils. Lubricate the glans. Coat the glans with a waxy protective substance. Provide sufficient skin to cover an erection by unfolding. Provide an aid to masturbation and foreplay. Serve as an aid to penetration. Reduce friction and chafing during intercourse. Serve as erogenous tissue because of its rich supply of erogenous receptors. Contact and stimulate the G-spot of the female partner. PENILE STIMULATION The outer foreskin layer contains nerve endings which respond to gentle touching during the early stages of sexual arousal. This triggers an erection. These receptors are stimulated by stretching, or when the foreskin rolls over the surface of the glans during intercourse. The foreskin contains sensory receptors called Meissner corpuscles. These nerves provide pleasure, as well as fine sensory perception. This helps to prevent premature ejaculation, because the man can tell when he is approaching the threshold of orgasm. Stimulation of the frenulum and ridged band results in intense pleasurable feelings during arousal. Stimulation of the glans is most significant in the later stages of sexual intercourse. Sensations from the glans contribute to the quality of the sensual experience. They are capable of triggering orgasm on their own, as would be the case in a circumcised man. If foreskin is removed, the only source of stimulation is the glans rubbing against the wall of the vagina. CIRCUMCISION It was first hypothesised in 1986 that circumcision prevents HIV infection. A review of the surveys conducted primarily in Africa, however, does not support this assertion. Of the 36 published studies examining the relationship between the circumcised penis and HIV infection, 15 found a negative correlation, four found a positive correlation, and 16 found no statistically significant difference. Trials took place in South Africa, Kenya, and Uganda. All three trials were stopped early by their monitoring boards on ethical grounds, because those in the circumcised group had a lower rate of HIV contraction than the control group. The results showed that circumcision reduced vaginal-to-penile transmission of HIV by 60%, 53%, and 51%, respectively. As a result of these findings, the WHO and the Joint United Nations Programme on HIV/AIDS (UNAIDS) stated that male circumcision is an efficacious intervention for HIV prevention but should be carried out by well-trained medical professionals and under conditions of informed consent (parents consent for their infant boys) In South Africa, male circumcision is prohibited under the age of 16, unless it is carried out s